A universal feature of the visual system in all previously studied vertebrates is the division of information into pathways specific for the onset and offset of illumination, called ON and OFF pathways [1]. ON and OFF pathways have a central role in the detection of contrast and combine in downstream neurons to produce complicated receptive fields, which detect diverse aspects of the visual world. This ON/OFF division occurs at the first synapse of the visual system between the photoreceptor cells — the rods and cones, and separate groups of ON and OFF bipolar cells [2]. We now show that the separation of visual information into ON and OFF pathways, and the novel metabotropic glutamate receptors which underlie ON bipolar cell light-evoked responses, are both present in the retina of lamprey. The simplest interpretation of this result is that ON and OFF bipolar cells were already present over 500 million years ago when cyclostomes diverged from other vertebrates in the late Cambrian, and that this fundamental organizing principle of the visual system probably arose not long after vertebrates first appeared.
Vertebrate photoreceptors remain depolarized and continuously release glutamate in darkness; light hyperpolarizes their membrane potential and decreases glutamate release. In OFF bipolar cells, the glutamate binds to AMPA/kainate receptors, with the result that the bipolar-cell membrane potential is depolarized in darkness and hyperpolarized by light. ON bipolar cells, in contrast, use a G-protein signal transduction cascade based on the mGluR6 receptor and the TRPM1 channel [2]. Glutamate binds to the mGluR6 receptor in darkness and keeps TRPM1 channels mostly closed; light decreases activation of mGluR6 and allows the channels to open, depolarizing the cell and inverting the photoreceptor signal. The ON bipolar light response is, we believe, the only known example of a metabotropic glutamate receptor acting as the primary postsynaptic mediator of synaptic transmission.
We were curious to know whether ON and OFF pathways and mGluR6 receptors are also present in the retina of lamprey. Lamprey are cyclostomes, a line that separated from all other vertebrates during the late Cambrian [3]. Earlier research has shown that lamprey rods and cones share many of the same transduction proteins as the photoreceptors of other vertebrates [4] and respond to light in a nearly identical manner [5,6], indicating that the mechanisms of phototransduction were established in vertebrates quite early. However, the organization of the remainder of the lamprey retina is rather different from other vertebrates [7]. In the lamprey, the nerve fiber layer runs within the retina between the inner nuclear layer (INL) and the inner plexiform layer (IPL), with retinal ganglion cell bodies distributed in both the ganglion cell layer and the INL. Additionally, some retinal ganglion cells extend dendrites into the outer plexiform layer (OPL), suggesting that they may synapse directly with photoreceptors. These differences indicate that signal processing in the lamprey retina may diverge significantly from other vertebrates.
To explore this possibility, we made patch-clamp recordings from cells in the INL of lamprey retinal slices, using methods we have previously described [8]. We discovered clear examples of both OFF and ON bipolar cells (Figure 1A,B), with responses and morphology similar to those in the retina of other vertebrates [9]. Both kinds of cells had dendritic terminations in the OPL and axonal terminations in the IPL, and some had a process extending beyond the OPL up into the photoreceptor layer (not shown in Figure 1), similar to the Landolt club of lower vertebrates [10]. Light stimulation of the retina produced changes in current in both kinds of bipolar cell. The cell in Figure 1A is representative of 10 recordings for which full-field light produced an initial outward current response in voltage clamp and a hyperpolarization in current clamp; these were therefore OFF cells. The cell in Figure 1B is representative of 35 recordings for which light produced an initial inward current response in voltage clamp and a depolarization in current clamp; these were therefore ON cells. In many cells, the initial inward or outward current was followed by a delayed response producing a rapid inflection in the response waveform. This inflection may be produced in part by the different time courses of rod and cone inputs to the bipolar cell [5,6] and/or surround inhibition from horizontal cells.
Figure 1. Voltage-clamp recordings from lamprey bipolar cells in retinal slices.
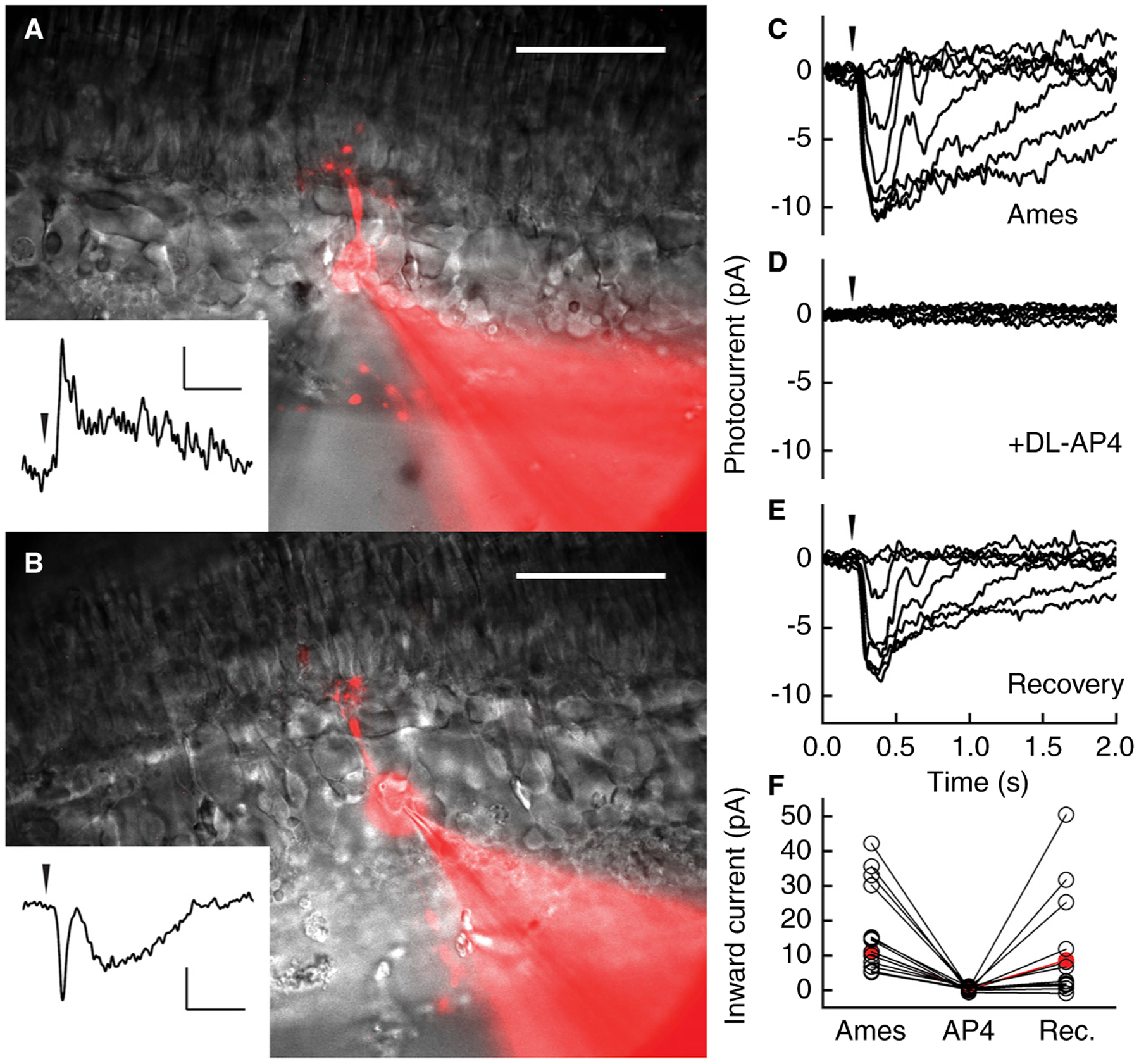
Cells were perfused in Ames’ medium and maintained at a holding potential of −60 mV. (A) OFF and (B) ON bipolar cell filled with Alexa Flour 750 and imaged after the recording. The fluorescent images are overlaid with full-field infrared images. Dendritic and axonal arbors extend above and below the cell bodies. The photoreceptor layer is at the top of each image. Scale bars: 50 μm. Insets show light responses to a single, near-saturating 10-ms light stimulus indicated by arrowheads; flashes were 970 (in A) and 140 (in B) 502-nm photons μm−2. Inset scale bars: 5 pA and 500 ms. (C–F) dl-AP4 blocks light response in an ON bipolar cell. (C) Responses to a series of 10 ms 405-nm flashes indicated by the arrowhead, at the following strengths: 3, 10, 30, 100, 300, 990, 3000, 9900 photons μm−2. (D) Responses to the same set of light stimuli as in (C) after perfusion with Ames’ medium containing 50 μM dl-AP4, which completely blocked the light response. (E) Response family after returning to Ames’ medium. (F) Maximum inward current from 16 ON bipolar cells in Ames’ medium, after perfusion with dl-AP4, and after returning to Ames’ medium (Rec.). dl-AP4 completely blocked the light response in all cells. Ten cells recovered their light responses after returning to Ames’ medium, and 6 cells showed no recovery. In two of the recordings we used l-AP4 (the active isomer) instead of dl-AP4, with results similar to the racemic mixture. The recording presented in panels C–E is highlighted in red.
To investigate the synaptic receptors underlying the ON bipolar-cell responses, we applied dl-AP4, a specific agonist of group III metabotropic glutamate receptors, including mGluR6. In vertebrates, dl-AP4 blocks the responses of ON bipolar cells by activating mGluR6, thereby driving TRPM1 channels further into the closed state and preventing a light-evoked response [2]. In Figure 1C–E, we show the responses of an ON bipolar cell to a series of flashes of increasing intensity. We then perfused the retina with 50 μM dl-AP4, which completely eliminated the light responses (Figure 1D). When dl-AP4 was washed off (Figure 1E), the responses returned. These recordings are representative of 16 similar experiments, 10 of which showed full recovery after washout (Figure 1F). While not every cell recovered, it is notable that the light-evoked response was suppressed in every ON bipolar cell we exposed to dl-AP4.
These experiments indicate that the lamprey retina establishes ON and OFF pathways at the level of the bipolar cells. Furthermore, the ON pathway is mediated by glutamate receptors sensitive to dl-AP4 similar to the ON bipolar cells of other vertebrates, suggesting that the lamprey glutamate receptor is also mGluR6. Because the mechanism of the ON bipolar-cell response is novel and unique to retina, we think it highly unlikely that the formation of this response could have emerged by parallel evolution in the retinas of lamprey and other vertebrates. The more likely explanation is that the fundamental division of retinal processing into ON and OFF pathways and the metabotropic mechanism producing the ON bipolar-cell response first appeared in the Cambrian and have remained relatively unchanged for 500 million years.
Our preliminary findings indicate that both ON and OFF bipolar cells can receive input from rods and cones. It remains to be seen whether separate classes of bipolar cells process distinct aspects of these photoreceptor signals. In addition, preliminary recordings from horizontal cells resemble those in other species. Thus, the OPL of lamprey retina seems to be organized much as in other vertebrates. We have occasionally encountered responses resembling those of vertebrate amacrine and ganglion cells but have not studied them in detail. It will be interesting to establish whether ganglion cells also come in ON and OFF varieties and to explore the organization of the IaPL, given the striking peculiarities of the anatomy of the lamprey inner retina [7]. We hope that continued investigation of the structure and function of lamprey retina will provide further insight into the evolution of vertebrate mechanisms of visual integration and contrast detection.
ACKNOWLEDGMENTS
This research was supported by a grant from the Great Lakes Fishery Commission, an unrestricted grant from Research to Prevent Blindness to the UCLA Department of Ophthalmology, the Stein Eye Institute Core Grant from the National Institutes of Health (EY00331), and the UCLA EyeSTAR program (to E.M.E.).
REFERENCES
- 1.Dowling JE (2012). The Retina : An Approachable Part of the Brain (Cambridge, MA: Harvard University Press; ). [Google Scholar]
- 2.Martemyanov KA, and Sampath AP (2017). The transduction cascade in retinal ON-bipolar cells: signal processing and disease. Annu. Rev. Vis. Sci 3, 25–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kuraku S and Kuratani S (2006). Time scale for cyclostome evolution inferred with a phylogenetic diagnosis of hagfish and lamprey cDNA sequences. Zoolog. Sci 23, 1053–1064. [DOI] [PubMed] [Google Scholar]
- 4.Lamb TD (2019). Evolution of the genes mediating phototransduction in rod and cone photoreceptors. Prog. Retin. Eye Res in press, 100823. [DOI] [PubMed] [Google Scholar]
- 5.Morshedian A and Fain GL (2015). Single-photon sensitivity of lamprey rods with cone-like outer segments. Curr. Biol 25, 484–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Asteriti S, Grillner S, and Cangiano L (2015). A Cambrian origin for vertebrate rods. eLife 4, e07166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jones MR, Grillner S, and Robertson B (2009). Selective projection patterns from subtypes of retinal ganglion cells to tectum and pretectum: distribution and relation to behavior. J. Comp. Neurol 517, 257–275. [DOI] [PubMed] [Google Scholar]
- 8.Ingram NT, Sampath AP, and Fain GL (2019). Voltage-clamp recordings of light responses from wild-type and mutant mouse cone photoreceptors. J. Gen. Physiol 151, 1287–1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu SM, Gao F, and Maple BR (2000). Functional architecture of synapses in the inner retina: segregation of visual signals by stratification of bipolar cell axon terminals. J. Neurosci 20, 4462–4470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Landolt E (1871). Beitrag zur Anatomie der Retina vom Frosch, Salamander und Triton. Arch. Mikr. Anat 7, 81–100. [Google Scholar]


