Abstract
Background:
Monoclonal antibodies (mAbs) represent the most numerous and significant group of biotherapeutics. While mAbs have undoubtedly improved treatment for many chronic diseases, including inflammatory diseases, they are typically expensive for health care systems and patients. Consequently, access to mAbs has been a problem for many patients especially among Central and Eastern European (CEE) countries. However, biosimilars can potentially help with costs, although there are concerns with their effectiveness and safety. This includes biosimilars for long-acting insulin analogues.
Aim:
Assess the availability and use of biological medicines, including biosimilars within Bosnia and Herzegovina (B&H).
Methods:
Assess the availability of mAbs via the current lists of approved and accessed mAbs versus those licenced in Europe and the United States and their utilisation, as well as specifically insulin glargine and its biosimilars, within B&H.
Results:
The availability of the mAbs in B&H appears satisfactory, which is encouraging. However, current usage is limited to a few mAbs which is a concern for subsequent patient care especially with limited use of biosimilars to address issues of affordability. We also see limited use of biosimilar insulin glargine.
Conclusion
The limited use of mAbs including biosimilars needs to be addressed in B&H to improve the future care of patients within finite resources. We will monitor these developments.
Keywords: Monoclonal antibodies, biosimilars, regulatory approach, demand-side measures, Bosnia and Herzegovina, insulin glargine
Introduction
Monoclonal antibodies (mAbs) represent the most numerous and most significant group of biotherapeutics, with biological medicines for disease areas, such as cancer and inflammatory diseases now dominating medicines expenditure. 1 The importance of mAbs has grown in recent years as they offer treatment options for patients with chronic and often disabling conditions, including autoimmune diseases.2–4 However, mAbs are expensive limiting their prescribing among Central and Eastern European (CEE) countries, including patients with rheumatoid arthritis and inflammatory bowel disease, which needs addressing under solidarity principles.5–7 There are also considerable differences in the availability and use of new oncology medicines across Europe, enhanced by cost issues.8,9 A reduction in the prices of mAbs through biosimilars can result in appreciable savings as well as increasing the number of patients accessing these medicines especially where there are high co-payments, alternatively budget concerns.5,10,11 While price reductions for biosimilars versus pre-patent originator prices have often been limited, this is changing as seen for Humira® (89% price reduction) in the Netherlands and its biosimilar in Denmark (83% price reduction) and the United Kingdom (75% price reduction).10,12,13 This provides hope for the future.
We are aware that regulatory approval for biosimilars across countries is different to the originator, and typically involves abridged non-clinical and clinical data.14,15 However, a lack of trust in biosimilars, coupled with limited government policies enhancing their use, including prescribing targets for new patients and switching, has reduced their prescribing in practice despite numerous publications demonstrating similar effectiveness and safety.10,16–20 Experience with generics in Bosnia and Herzegovina (B&H) and wider has shown that trust among all key stakeholders is essential for savings without compromising care.21,22 The same is true for biosimilars. 10
Consequently, there is a need to document current availability and accessibility of mAbs, including biosimilars, and use the findings to suggest ways forward to improve future care within finite resources to provide direction across countries. The same applies to biosimilars for long-acting insulin analogues given the increasing use of long-acting insulin analogues to reduce rates of hypoglycaemia among insulin-dependent diabetic patients, which can account for up to 30% or more of patients with diabetes, and the increasing cost of care of diabetic patients.23–27 Consequently, the objectives for this study were to assess the availability and use of biological medicines, including biosimilars, within B&H and use the findings to provide future guidance to the authorities in B&H and wider.
Methods
B&H consists of the two constitutive entities, the Republic of Srpska and the Federation of B&H.22,28 Each entity is competent for the health care on its territory, as well as the Brčko District of B&H.
The project consists of three elements, including retrospective pricing and utilisation analyses. The first element involved determining the current list of approved mAbs in B&H. The second element involved a comparison between the list of mAbs approved in B&H versus those actually reimbursed, including biosimilars as well as assessing current utilisation patterns. The last part involved retrospectively assessing utilisation patterns for long-acting insulin analogues versus total insulins, as well as utilisation patterns for biosimilar insulin glargine versus total insulin glargine as the first long-acting insulin analogue biosimilar available in B&H and across Europe.
A list of the approved mAbs for B&H market was created by interrogating the data base at the Agency for Medicinal Products and Medical Devices of B&H (ALMBIH), which is the regulatory authority at the state level, 29 until early January 2021. This was undertaken by the principal co-authors (BT and VMP). While B&H is not a member of the EU, and does not apply the European Commission’s marketing authorisation regulations directly, the laws in B&H regarding marketing authorisation including biosimilars have been based on EU regulations, for example, Directive 2001/83/EC.
The availability of mAbs was determined by comparing the list of approved mAbs for B&H versus those listed by the European Medicines Agency (EMA) 30 and the Food and Drug Administration (FDA), based on Lu et al. (2020), again by the principal co-authors (BT and VMP).31,32 Medicines were listed by their anatomical-therapeutic-chemical (ATC) 33 classification to aid comparisons as there could be differences in the names of originators and biosimilars between countries. We included the United States to give a more complete picture as we are aware that a number of new biologics especially for oncology are given accelerated approval in the United States. 34
Affordability in the first instance was assessed by comparing the list of medicines in ALMBIH with those reimbursed within the Health Insurance Fund of Republic of Srpska (HIF-RS), the Health Insurance and Reinsurance Institute of the Federation of B&H (HIRI-FB&H) and the Health Insurance Fund of the Brčko District of B&H (HIF-BD). Subsequently, measuring actual packs dispensed from 2017 to 2019 from the Health Insurance Fund data again via the principal co-authors (BT and VMP). The Health Insurance Fund data are robust and we have used these before in previous research projects.22,35 We chose packs dispensed as the use of defined daily doses (DDDs) is difficult in cancer due to typically multiple indications for oncology medicines. 35 Wholesale prices for the different infliximab preparations were again taken from Health Insurance Fund data.
We also looked specifically at long-acting insulin analogues and their biosimilars, with long-acting insulin analogues typically appreciably more expensive than other forms of insulin.36,37 However, increasingly recognised patient benefits to reduce hypoglycaemia and enhance patient adherence has increased their use across countries, including developing countries,27,36,38,39 although this is not universal. 40 In this situation, we will use DDDs to document utilisation patterns, similar to previous studies, 22 and compare the findings with other countries.27,39,40
In accordance with local legislation neither approval from an ethics committee nor informed consent is required as this study did not deal directly with patients.
Results
There were 96 mAbs approved by the FDA and EMA until early January 2021 (Table 1A in the Appendix). Seventy-six (79.2%) were approved jointly, 19 (19.8%) by the FDA and not by the EMA and 1 (1.04%) solely by EMA. However, several have been withdrawn. Perhaps not surprisingly given rising expenditures for oncology medicines in recent years combined with the high number of new oncology medicines being researched versus other disease areas,8,41,42 the greatest number of approved mAbs were for antineoplastic and immunomodulating agents (ATC–L). These accounted for 63.5% of all mAbs.
Table 1.
Utilisation of different mAbs in B&H broken down by originator and biosimilar 2017–2019.
| ATC Code | INN | Brand name | Pharmaceutical form | Dosage and quantity | 2017 | 2018 | 2019 |
|---|---|---|---|---|---|---|---|
| Utilisation | Utilisation | Utilisation | |||||
| L01XC02 | Rituximab | MABTHERA | Concentrate for solution for infusion | 100 mg/10 mL | 1612 | 1261 | 723 |
| Solution for injection | 120 mg/1 mL | 13 | 443 | NM | |||
| Concentrate for solution for infusion | 500 mg/50 mL | 1778 | 1527 | 961 | |||
| Solution for subcutaneous injection | 1400 mg/11.7 mL | NM | NM | 1216 | |||
| Solution for subcutaneous injection | 1600 mg/13.4 mL | NM | NM | 3 | |||
| BLITZIMA | Concentrate for solution for injection/infusion | 10 mg/1 mL | NA | NM | NM | ||
| ACELLBIA | Concentrate for solution for infusion | 10 mg/1 mL | NA | NA | NA | ||
| Concentrate for solution for infusion | 10 mg/1 mL | NA | NA | NA | |||
| RIXATHON | Concentrate for solution for infusion | 10 mg/1 mL (10 mL) | NA | NM | NM | ||
| Concentrate for solution for infusion | 10 mg/1 mL (2 × 10 mL) |
NA | NM | NM | |||
| Concentrate for solution for infusion | 10 mg/1 mL (50 mL) | NA | NM | NM | |||
| Concentrate for solution for infusion | 10 mg/1 mL (2 × 50 mL) |
NA | NM | NM | |||
| L01XC03 | Trastuzumab | HERCEPTIN | Powder for concentrate for solution for infusion | 150 mg | 5969 | 5929 | 4568 |
| HERCEPTIN | Solution for injection | 600 mg/5 mL | 2810 | 3014 | 3885 | ||
| HERTICAD | Powder for concentrate for solution for infusion | 150 mg | NA | NA | NA | ||
| KANJINTI | Powder for concentrate for solution for infusion | 150 mg | NA | NA | NA | ||
| KANJINTI | Powder for concentrate for solution for infusion | 420 mg | NA | NA | NA | ||
| OGIVRI | Powder for concentrate for solution for infusion | 150 mg | NA | NA | NM | ||
| HERZUMA | Powder for concentrate for solution for infusion | 420 mg | NA | NA | NA | ||
| HERZUMA | Powder for concentrate for solution for infusion | 150 mg | NA | NA | NA | ||
| L04AB02 | Infliximab | REMICADE | Powder for concentrate for solution for infusion | 100 mg | 975 | 1180 | 1605 |
| REMSIMA | Powder for concentrate for solution for infusion | 100 mg | 420 | 450 | 520 | ||
| INFLECTRA | Powder for concentrate for solution for infusion | 100 mg | 671 | 81 | 74 | ||
| L04AB04 | Adalimumab | HUMIRA | Solution for injection | 40 mg/0.8 mL | 1152 | 320 | NM |
| HUMIRA | Solution for injection in pre-filled syringe | 40 mg/0.4 mL | 500 | 1899 | 2605 | ||
| AMGEVITA | Solution for injection in pre-filled syringe | 40 mg/0.8 mL | NA | NA | 20 | ||
| AMGEVITA | Solution for injection in pre-filled syringe | 20 mg/0.4 mL | NA | NA | NM | ||
| AMGEVITA | Solution for injection in pre-filled pen | 40 mg/0.8 mL 2 pre-filed pens |
NA | NA | NM | ||
| HULIO | Solution for injection | 40 mg/0.8 mL 1 pre-filled pen |
NA | NA | NA | ||
| HULIO | Solution for injection in pre-filled syringe | 40 mg/0.8 mL 2 pre-filled syringes |
NA | NA | NA | ||
| HULIO | Solution for injection in pre-filled syringe | 40 mg/0.8 mL 1 pre-filled syringe with 2 alcohol pads |
NA | NA | NA | ||
| HULIO | Solution for injection in pre-filled syringe | 40 mg/0.8 mL 2 pre-filled syringes with 2 alcohol pads |
NA | NA | NA |
Shaded: biosimilar; NM: not marketed; NA: not approved.
There were 30 (31.25%) mAbs approved by ALMBIH by early January 2021 out of those approved by the EMA and FDA, with again most, that is, 22 (73.3%), for ATC-L group. These included the latest generation of oncology medicines, which are the checkpoint inhibitors, including pembrolizumab (L01XC18) and atezolizumab (L01XC32). Encouragingly, there appeared to be reasonably equal access to approved mAbs by the ALMBIH for all citizens in the different parts (entities) of B&H (Table 1A), with access to mAbs via HIF-RS and HIRI-FB&H only possible in well-defined therapeutic indications, typically in line with ALMBIH approval.
There were 22 mAbs accessible via HIF-RS (73.3%), although basiliximab is currently not approved by ALMBIH. Basiliximab is reimbursed for prophylaxis of acute organ rejection in de novo allogeneic renal transplantation in patients with panel reactive antibodies less than 80%, or in a triple maintenance immunosuppressive regimen containing cyclosporine for microemulsion, corticosteroids and either azathioprine or mycophenolate mofetil. Eighteen mAbs were accessible via HIRI-FB&H (60.0%), with currently not approved, but reimbursed nivolumab as monotherapy for advanced (unresectable or metastatic) melanoma.
The greatest number of reimbursed mAbs in B&H belong to the L01 group: 10 mAbs (33.3%) in the Republic of Srpska and 12 (40.0%) in the Federation of B&H (Table 2A). In the Brčko District of B&H, three mAbs are reimbursed, adalimumab, secukinumab and vedolizumab from ATC group L04.
Table 2.
Wholesale prices of different infliximab presentations in B&H.
| Name | Pharmaceutical form | Content concentration | Wholesale price per pack (EUR) | ||
|---|---|---|---|---|---|
| 2017 | 2018 | 2019 | |||
| REMSIMA | Powder for concentrate for solution for infusion | 100 mg/1 vial | 421.49 | 377.90 | 153.99 |
| INFLECTRA | Powder for concentrate for solution for infusion | 100 mg/1 vial | 415.22 | 377.90 | 373.81 |
| REMICADE | Powder for concentrate for solution for infusion | 100 mg/1 vial | 529.19 | 454.47 | 441.31 |
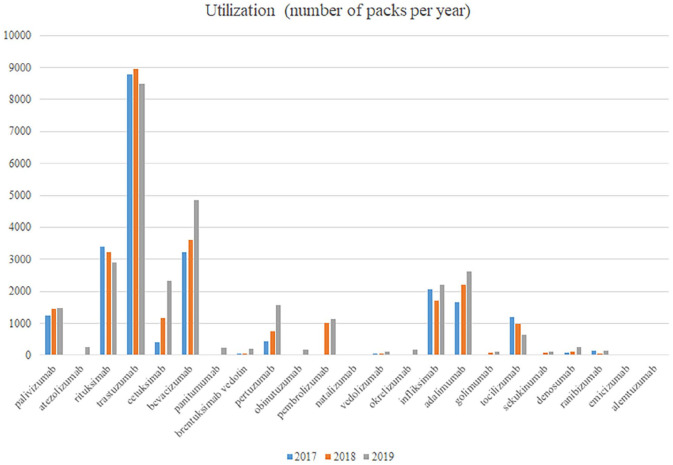
There are currently four approved biosimilars for mAbs in B&H, which are rituximab, trastuzumab, infliximab and adalimumab (Table 2A), with the greatest number of approvals for trastuzumab biosimilars. This may have facilitated their use; however, there appears only limited changes in utilisation patterns for mAbs between 2017, with biosimilars for infliximab launched in 2016, and 2019 (Figure 1) with currently limited use of biosimilars in recent years despite being marketed (Table 1). The exception is bevacizumab, which is currently unavailable as a biosimilar. Limited use of the other mAbs may well reflect issues of affordability despite being listed on the reimbursement lists of the different entities of B&H.
Figure 1.
Utilisation (number of packs) of mAbs per year.
The limited use of biosimilar infliximab is despite an appreciable fall in prices versus the originator in recent years (Table 2).
We have seen increasing use of long-acting insulins in B&H in recent years rising from 19.6% of total insulin utilisation in 2014 to 35.5% in 2019, with expenditure increasing to 45.1% of total insulin expenditure in 2019, reflecting perceived patient benefits despite increasing costs. Overall costs could have been reduced with the availability of biosimilars. However, this was hampered by only limited differences in prices between the originator and biosimilar insulin glargine 100 IU/ml at 6.8% and 7.9% in 2018 and 2019, respectively. In addition, high use of the patented 300 IU/ml formulation at 52.1% of total insulin glargine in 2019 as a result of promotional activities by the company. Overall, limited use of biosimilar insulin glargine at only 6.17% of total insulin glargine 100 IU/ml in 2019. This again reflects limited demand-side measures instigated by the authorities in B&H to counter-act the activities of the originator company. We have seen a similar situation in a number of other CEE countries, including Estonia, Latvia and Romania resulting in limited or no use of biosimilar insulin glargine. 39
Discussion
Encouragingly, there was reasonable listing of the mAbs among the various entities in B&H given concerns generally with the availability and reimbursement of biologic medicines among CEE countries.5,6 In addition, reasonable usage of medicines for patients with cancer, including rituximab and trastuzumab, and those with immune diseases, such as rheumatoid arthritis, including infliximab and adalimumab. However, limited use of the majority of mAbs (Figure 1) suggests issues with available funding despite being listed on the reimbursement lists in B&H. This is a concern when seeking to improve patient care in these patients. It may be that increased availability of biosimilars at considerably lower prices could help along with increased physician and patient education regarding the regulatory approaches for biosimilars and studies demonstrating similar effectiveness and safety with originators.10,17,20 This builds on examples in other European countries where there have been considerable use of biosimilars and corresponding savings following multiple demand-side measures10,18,43 as well as a number of countries with biosimilars of insulin glargine.27,39
However, physicians and health authorities need to instigate policies to enhance the use of biosimilars in B&H building on successful experiences in other countries.18,44 These include educational policies to address concerns and lack of trust with biosimilars given the impact of the nocebo effect in this area,44,45 alongside prescribing targets and restrictions for more expensive originators.10,43,44 Otherwise, there will continue to be limited use of biosimilars. 16 This is a concern given the potential for appreciable savings with biosimilars as seen with biosimilar infliximab in B&H (Table 2) without compromising care. 17
Lack of trust and use of biosimilars in B&H may be hampered by issues, such as interchangeability and substitutability, with these issues currently not being clearly defined by the ALMBIH. Consequently, there is a need for B&H to learn from other European countries to instigate appropriate educational and other measures to appreciably increase biosimilar use to benefit patients especially given current budgetary issues and competing demands under opportunity cost considerations.10,18,43,46 Increased competition can lower prices of both originator mAbs and biosimilars as seen recently with adalimumab in a number of European markets.12,18 Such approaches may assist in the Brčko District where infliximab is currently not on the list of reimbursed medicines. Lower prices of biosimilars building on existing reductions (Table 2), along with greater patient and physician trust, should enhance their availability and use for the benefit of patients. We will be investigating this further especially with ALMBIH increasingly encouraging physicians to prescribe biosimilars, which should enhance the attractiveness of the biosimilar market and address current concerns with their lack of availability and use (Tables 1 and 2A).
We are aware of a number of limitations with this study. These include the fact that we only included data for 3 years for the mAbs. In addition, we did not contact physicians directly to ascertain the rationale behind the utilisation patterns seen. Despite these limitations, we believe our findings are robust providing direction for the future.
Conclusion
In conclusion, there appeared to be good availability of mAbs in B&H. However, there is currently limited use of a number of these due to issues of affordability, and we also see limited use of biosimilars, including biosimilar insulin glargine. Both can be addressed by enhancing the attractiveness of the market for biosimilars, benefitting all key stakeholder groups. B&H can learn from other European countries.
Supplemental Material
Supplemental material, sj-pdf-1-map-10.1177_23992026211027692 for Availability and accessibility of monoclonal antibodies in Bosnia and Herzegovina: Findings and implications by Biljana Tubic, Vanda Marković-Peković, Saša Jungić, Eleonora Allocati and Brian Godman in Medicine Access @ Point of Care
Footnotes
Author contributions: All authors contributed to developing the concept of the paper and the methodology with S.J. undertaking the majority of the analysis. B.T. and B.G. wrote the first draft, including the literature review, with all authors involved in subsequent revisions. All authors approved the final version.
Declaration of conflicting interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship and/or publication of this article: The authors declare that they have no competing interests although B.T. works for Agency for Medicinal Products and Medical Devices of Bosnia and Herzegovina.
Funding: The author(s) received no financial support for the research, authorship and/or publication of this article.
ORCID iD: Brian Godman  https://orcid.org/0000-0001-6539-6972
https://orcid.org/0000-0001-6539-6972
Supplemental material: Supplemental material for this article is available online.
References
- 1. IQVIA. The global use of medicine in 2019 and outlook to 2023 – forecasts and areas to watch, 2019, https://www.iqvia.com/-/media/iqvia/pdfs/institute-reports/the-global-use-of-medicine-in-2019-and-outlook-to-2023.pdf (accessed 14 March 2021).
- 2. Scott D, Ibrahim F, Hill H, et al. The clinical effectiveness of intensive management in moderate established rheumatoid arthritis: the titrate trial. Semin Arthritis Rheum 2020; 50(5): 1182–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wilson FR, Coombes ME, Brezden-Masley C, et al. Herceptin® (trastuzumab) in HER2-positive early breast cancer: a systematic review and cumulative network meta-analysis. Syst Rev 2018; 7(1): 191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kaegi C, Wuest B, Schreiner J, et al. Systematic review of safety and efficacy of rituximab in treating immune-mediated disorders. Front Immunol 2019; 10: 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Baumgart DC, Misery L, Naeyaert S, et al. Biological therapies in immune-mediated inflammatory diseases: can biosimilars reduce access inequities. Front Pharmacol 2019; 10: 279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pentek M, Lakatos PL, Oorsprong T, et al. Access to biologicals in Crohn’s disease in ten European countries. World J Gastroenterol 2017; 23(34): 6294–6305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kostic M, Djakovic L, Sujic R, et al. Inflammatory bowel diseases (Crohn’s Disease and Ulcerative Colitis): cost of treatment in Serbia and the implications. Appl Health Econ Health Policy 2017; 15(1): 85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Godman B, Hill A, Simoens S, et al. Potential approaches for the pricing of cancer medicines across Europe to enhance the sustainability of healthcare systems and the implications. Expert Rev Pharmaco Outcome Res. Epub ahead of print 11 March 2021:1–14. DOI: 10.1080/14737167.2021.1884546. [DOI] [PubMed] [Google Scholar]
- 9. Wilking N, Bucsics A, Kandolf Sekulovic L, et al. Achieving equal and timely access to innovative anticancer drugs in the European Union (EU): summary of a multidisciplinary CECOG-driven roundtable discussion with a focus on Eastern and South-Eastern EU countries. ESMO Open 2019; 4(6): e000550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Godman B. Biosimilars are becoming indispensable in the management of multiple diseases although concerns still exist. Bangladesh J Med Sci 2021; 20(1): 5–10. [Google Scholar]
- 11. Putrik P, Ramiro S, Kvien TK, et al. Variations in criteria regulating treatment with reimbursed biologic DMARDs across European countries. Ann Rheum Dis 2014; 73(11): 2010–2021. [DOI] [PubMed] [Google Scholar]
- 12. Jensen TB, Kim SC, Jimenez-Solem E, et al. Shift from Adalimumab originator to biosimilars in Denmark. JAMA Internal Medicine 2020; 180(6): 902–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sagonowsky E. AbbVie’s massive Humira discounts are stifling Netherlands biosimilars: report, 2019, https://www.fiercepharma.com/pharma/abbvie-stifling-humira-biosim-competition-massive-discounting-dutch-report (accessed 13 March 2021).
- 14. European Medicines Agency. Biosimilar medicines: overview, 2020, https://www.ema.europa.eu/en/human-regulatory/overview/biosimilar-medicines-overview (accessed 15 January 2021).
- 15. Knezevic I, Griffiths E. WHO standards for biotherapeutics, including biosimilars: an example of the evaluation of complex biological products. Ann N Y Acad Sci 2017; 1407(1): 5–16. [DOI] [PubMed] [Google Scholar]
- 16. Kim Y, Kwon H-Y, Godman B, et al. Uptake of biosimilar Infliximab in the UK, France, Japan, and Korea: budget savings or market expansion across countries. Front Pharmacol 2020; 11: 970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jorgensen KK, Olsen IC, Goll GL, et al. Switching from originator infliximab to biosimilar CT-P13 compared with maintained treatment with originator infliximab (NOR-SWITCH): a 52-week, randomised, double-blind, non-inferiority trial. Lancet 2017; 389(10086): 2304–2316. [DOI] [PubMed] [Google Scholar]
- 18. Moorkens E, Godman B, Huys I, et al. The expiry of Humira ® market exclusivity and the entry of adalimumab biosimilars in Europe: an overview of pricing and national policy measures. Front Pharmacol 2021; 11: 591134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Blevins TC, Barve A, Raiter Y, et al. Efficacy and safety of MYL-1501D versus insulin glargine in people with type 1 diabetes mellitus: results of the INSTRIDE 3 phase 3 switch study. Diabetes Obes Metab 2020; 22(3): 365–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pivot X, Bondarenko I, Nowecki Z, et al. A phase III study comparing SB3 (a proposed trastuzumab biosimilar) and trastuzumab reference product in HER2-positive early breast cancer treated with neoadjuvant-adjuvant treatment: final safety, immunogenicity and survival results. Eur J Cancer 2018; 93: 19–27. [DOI] [PubMed] [Google Scholar]
- 21. Godman B, Wettermark B, van Woerkom M, et al. Multiple policies to enhance prescribing efficiency for established medicines in Europe with a particular focus on demand-side measures: findings and future implications. Front Pharmacol 2014; 5: 106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Markovic-Pekovic V, Skrbic R, Godman B, et al. Ongoing initiatives in the Republic of Srpska to enhance prescribing efficiency: influence and future directions. Expert Rev Pharmaco Outcome Res 2012; 12(5): 661–671. [DOI] [PubMed] [Google Scholar]
- 23. Bommer C, Sagalova V, Heesemann E, et al. Global economic burden of diabetes in adults: projections from 2015 to 2030. Diabetes Care 2018; 41(5): 963–970. [DOI] [PubMed] [Google Scholar]
- 24. Basu S, Yudkin JS, Kehlenbrink S, et al. Estimation of global insulin use for type 2 diabetes, 2018-30: a microsimulation analysis. Lancet Diabetes Endocrinol 2019; 7(1): 25–33. [DOI] [PubMed] [Google Scholar]
- 25. Sharma M, Nazareth I, Petersen I. Trends in incidence, prevalence and prescribing in type 2 diabetes mellitus between2000 and 2013 in primary care: a retrospective cohort study. BMJ open. 2016;6(1):e010210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Venkataraman AP, Laxminarayana K, Samhita S. Knowledge, attitude and practice of insulin use of diabetic patients in India. Pharmacol Clin Pharmacy Res 2020; 5(1): 23–32. [Google Scholar]
- 27. Haque M, Islam S, Kamal ZM, et al. Ongoing efforts to improve the management of patients with diabetes in Bangladesh and the implications. Hosp Pract. Epub ahead of print 28 April 2021. DOI: 10.1080/21548331.2021.1906083. [DOI] [PubMed] [Google Scholar]
- 28. Bojanic L, Markovic-Pekovic V, Skrbic R, et al. Recent initiatives in the Republic of Srpska to enhance appropriate use of antibiotics in ambulatory care; Their influence and implications. Front Pharmacol 2018; 9: 442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Agency for Medicines Medical Devices of Bosnia Herzegovina. List of medicinal products with marketing authorization, 2020, http://lijekovi.almbih.gov.ba:8090/SpisakLijekova.aspx (accessed 15 January 2021).
- 30. European Medicines Agency. Download medicine data, 2021, https://www.ema.europa.eu/en/medicines/download-medicine-data (accessed 15 January 2021).
- 31. Lu RM, Hwang YC, Liu IJ, et al. Development of therapeutic antibodies for the treatment of diseases. J Biomed Sci 2020; 27(1): 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Food Drug Administration. Novel Drug Approvals for 2020, 2020, https://www.fda.gov/drugs/new-drugs-fda-cders-new-molecular-entities-and-new-therapeutic-biological-products/novel-drug-approvals-2020 (accessed 16 January 2021). [Google Scholar]
- 33. WHO. WHO collaborating centre for drug statistics methodology. ATC/ DDD Index, https://www.whocc.no/
- 34. Pontes C, Zara C, Torrent-Farnell J, et al. Time to review authorisation and funding for new cancer medicines in Europe? Inferences from the case of Olaratumab. Appl Health Econ Health Policy 2020; 18(1): 5–16. [DOI] [PubMed] [Google Scholar]
- 35. Godman B, Hill A, Simoens S, et al. Pricing of oral generic cancer medicines in 25 European countries; findings and implications. Generic Biosimilar Initiat J 2019; 8(2): 49–70. [Google Scholar]
- 36. Caires de, Souza AL, de Assis Acurcio F, Guerra Junior AA, et al. Insulin glargine in a Brazilian state: should the government disinvest? An assessment based on a systematic review. Appl Health Econ Health Policy 2014; 12(1): 19–32. [DOI] [PubMed] [Google Scholar]
- 37. Ewen M, Joosse HJ, Beran D, et al. Insulin prices, availability and affordability in 13 low-income and middle-income countries. BMJ Glob Health 2019; 4(3): e001410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chan JCN, Lim LL, Wareham NJ, et al. The Lancet commission on diabetes: using data to transform diabetes care and patient lives. Lancet 2021; 396(10267): 2019–2082. [DOI] [PubMed] [Google Scholar]
- 39. Godman B, Haque M, Leong T, et al. The current situation regarding long-acting insulin analogues including biosimilars among selected African, Asian, European and South American countries: findings and implications for the future. Front Public Health 2021;9: 671961. 10.3389/fpubh.2021.671961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Opanga S, Njeri LW, Kimonge D, et al. Assessing utilisation and expenditure on long-acting insulin analogues in Kenya; Findings and implications for the future. Sch Acad J Pharm 2021; 10(4): 63–70. [Google Scholar]
- 41. IQVIA Institute for Human Data Science. Global Oncology Trends 2018, https://www.iqvia.com/institute/reports/global-oncology-trends-2018 (accessed 10 March 2021).
- 42. Lee M, Ly H, Möller CC, et al. Innovation in regulatory science is meeting evolution of clinical evidence generation. Clin Pharmacol Ther 2019; 105(4): 886–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Godman B, Allocati E, Moorkens E, et al. Can local policies on biosimilars optimize the use of freed resources – experiences from Italy. Generic Biosimilar Initiat J 2020; 9(4)): 183–187. [Google Scholar]
- 44. Moorkens E, Vulto AG, Huys I, et al. Policies for biosimilar uptake in Europe: an overview. PLoS ONE 2017; 12(12): e0190147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Colloca L, Panaccione R, Murphy TK. The clinical implications of nocebo effects for biosimilar therapy. Front Pharmacol 2019; 10: 1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Barrett A, Roques T, Small M, et al. How much will herceptin really cost? BMJ 2006; 333(7578): 1118–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-map-10.1177_23992026211027692 for Availability and accessibility of monoclonal antibodies in Bosnia and Herzegovina: Findings and implications by Biljana Tubic, Vanda Marković-Peković, Saša Jungić, Eleonora Allocati and Brian Godman in Medicine Access @ Point of Care