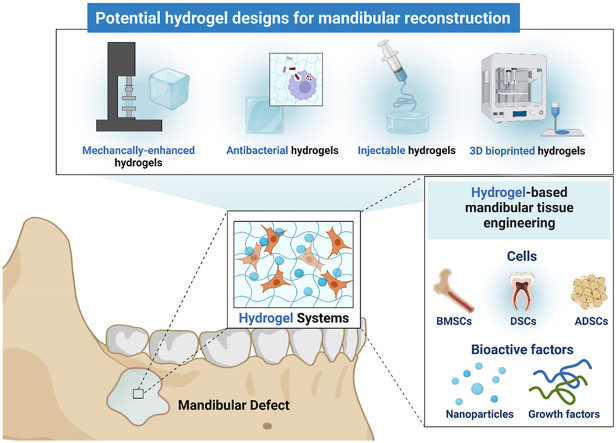
Abstract
Mandibular defect becomes a prevalent maxillofacial disease resulting in mandibular dysfunctions and huge psychological burdens to the patients. Considering the routine presence of oral contaminations and aesthetic restoration of facial structures, the current clinical treatments are however limited, incapable to reconstruct the structural integrity and regeneration, spurring the need for cost-effective mandibular tissue engineering. Hydrogel systems possess great merit for mandibular reconstruction with precise involvement of cells and bioactive factors. In this review, current clinical treatments and distinct mode(s) of mandible formation and pathological resorption are summarized, followed by a review of hydrogel-related mandibular tissue engineering, and an update on the advanced fabrication of hydrogels with improved mechanical property, antibacterial ability, injectable form, and 3D bioprinted hydrogel constructs. The exploration of advanced hydrogel systems will lay down a solid foundation for a bright future with more biocompatible, effective, and personalized treatment in mandibular reconstruction.
Keywords: Hydrogel, Mandibular defect, Tissue engineering, Injectable materials, Bone regeneration
Graphical abstract
Highlights
-
•
The clinical treatments for mandibular reconstruction are limited due to the frequent infection and the donor site morbidity.
-
•
Hydrogel systems with seed cells and bioactive factors possess great regenerative merit in mandibular tissue engineering.
-
•
Advanced hydrogel systems with enhanced mechanical strength, antibacterial ability, and injectability are inspired.
-
•
3D bioprinting will provide more effective and personalized treatment for mandibular reconstruction.
1. Introduction
Mandibular surgery is commonly performed due to tumor (squamous cell carcinoma and osteogenic sarcomas) ablation, traumatic insult, and osteonecrosis (osteoradionecrosis, medication-related osteonecrosis) debridement [1,2]. Acute and chronic osteomyelitis, temporomandibular joint instability, delayed healing, and disfunction are major clinical challenges. The patients may also suffer from such surgical treatment with both psychological and socioeconomic burdens.
The main target of mandibular reconstruction is to restore essential functions, including chewing, phonation, breathing, and normal bone metabolism [3]. Aesthetic restoration is highly desirable [4]. Autologous bone transplantation is the choice of surgical treatment in clinical practice [5]. Free flap transplantation is frequently used in mandibular reconstruction, especially for continuity defect resulting in a loss of mandibular unity. However, the donor site morbidity and the limited resource are the major challenges [6,7], leading to a surge of investigations on the development of bioactive materials as alternatives.
Hydrogel systems exhibit high biocompatibility, controllable degradability, and easy modification to support minimally invasive surgery, with no need for later implant removal surgery [8]. Besides, they are an excellent choice for imitating the extracellular matrix (ECM) and encapsulating various stem cells and bioactive factors, to fill the defect zone and promote bone formation [9]. These advantages make hydrogel systems meet the special demands in mandibular reconstruction. Additional challenges in mandibular reconstruction include the frequent exposure to the oral bacterial environment and aesthetic concerns of facial reconstruction, highlighting the importance of R&D of the advanced hydrogels with antibacterial, injectable and 3D programable properties [[10], [11], [12]]. To date, due to the complexity of mandibular reconstruction, there is few published reviews in the literature elaborating potential of hydrogels on mandibular reconstruction. So, in the present review work, we updated the current research and emerging strategies on hydrogel systems developed for mandibular reconstruction, aiming to inspire more promising research and clinical translation in complex maxillofacial reconstruction.
2. Clinical challenges and drawbacks of the current treatments for mandibular defect
2.1. Causative factors
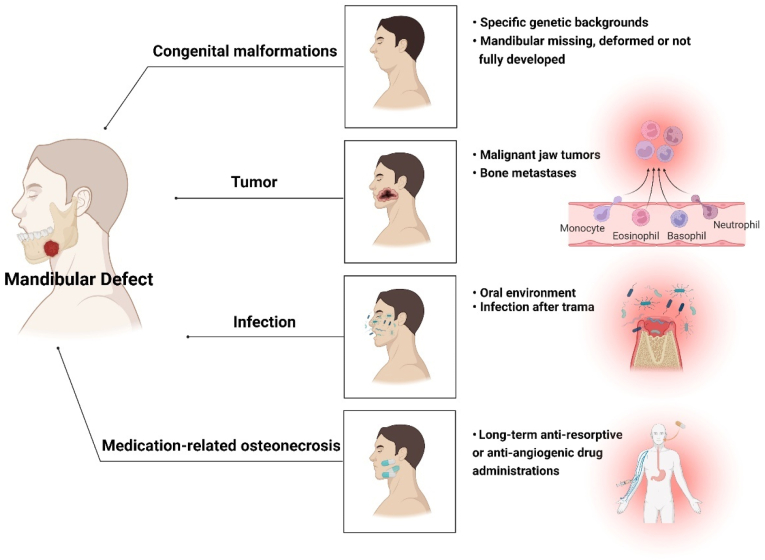
The mandibular defect is defined as the lower jaw with the loss of a portion of the bone with or without damaging facial bone and accessories [13]. Congenital malformations [14], tumors [15], trauma [16], inflammation [17], and medication-related osteonecrosis [18] can all cause mandibular defects (Fig. 1). Additionally, frequent exposure to various disease-inducing agents (cigarettes, drugs, and carbohydrates) increases the probability of periodontal diseases and oral cancers, affecting the cell functions and aggravating the inflammation reaction to destroy the alveolar bone even extended to the mandible [19,20].
Fig. 1.
Causative factors resulting in mandibular defects.
The main causes of mandibular defects are congenital malformations and acquired complications [21]. In the past few decades, mandibular deformities were thought to be common among individuals with specific genetic backgrounds, presenting with the mandibular defect or underdeveloped at birth [22]. Now, the etiologies of the mandibular defects have become more diverse. For example, resection of benign odontogenic tumors and comminution caused by trauma also often results in mandibular defects. Malignant tumor ablation, radiation-induced osteonecrosis, and medication/bisphosphonate (BPs) related osteonecrosis of the jaw (MRONJ/BRONJ) are also main causes in recent years [23].
Of note, MRONJ is a progressive disease of the mandible in which the necrotic bone extends beyond the region of alveolar bone, resulting in pathologic fracture, extra-oral fistula, and osteolysis involving the inferior border of the mandible [24]. In the USA 0.1% of osteoporotic patients treated with oral BPs and 0.8%–12% of patients with multiple myeloma and bone metastases treated with intravenous BPs suffer from the MRONJ [25]. In the late stage of MRONJ, segmental resection is the only method to remove the necrotic bone, resulting in a postoperative defect. Similarly, mandibulectomy is also applied in malignant jaw tumors or bone metastases [26]. Such mandible losses more than 10%, it fails to heal spontaneously [27].
2.2. Unsatisfactory outcome of current clinical therapies
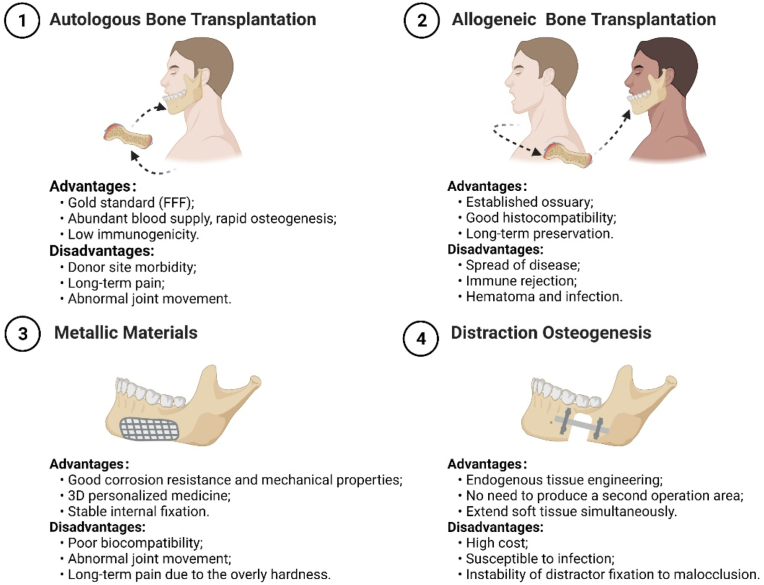
At present, bone transplantation, metallic devices, and distraction osteogenesis have been used to fill or repair the mandibular defect in clinical practice. Among them, the fibula-free flap (FFF) transplantation is the gold standard for mandibular reconstruction [28]. The history of current clinical therapies in mandibular reconstruction is presented in Table 1.
Table 1.
History of current clinical therapies in mandibular reconstruction.
| Clinical Therapies | Years | Key Events or Representative Publications | Outcomes |
|---|---|---|---|
| Autologous and allogeneic bone transplantation | 1949 | Review paper of free bone grafts taken from tibia, rib, and iliac crest for mandibular reconstruction [32] | – |
| 1979 | A report on the use of iliac crest free flap with micro-vessels | The superiority of the deep circumflex iliac vessels [33] | |
| 1979–1997 | A report of 178 mandibular reconstruction cases using microvascular-free flaps | Donor site selection strategies: ilium, fibula, or scapula (lateral bony defect), fibula (anterior bony defect) [34] | |
| 1981 | Combined homologous mandible and autologous bone and bone marrow | Failure in patients who have previous radiation therapy [35] | |
| 1989 | The fibula-free flap (FFF) transplantation has become the gold standard | All osteotomies healed primarily in 12 patients [36]. | |
| 2009 | Non-vascularized bone grafts | Suitable for the condition that the defect is truly lateral and only an extraoral approach. 86% of patients with a successful initial reconstruction [37] | |
| Metallic devices | 1909 | Silver wire [38] | – |
| 1953 | A stainless-steel mesh prosthesis for mandibular replacement | Successful rate of 67/102 patients, failure in patients associated with histories of previous irradiation, extensive resections, and the loss of distant skin flaps [39] | |
| 1990s | Titanium (Ti) and titanium alloys [40] | 71% successful rate. Plate loss occurred in large lateral defects, and pre- or postoperative radiotherapy [41] | |
| 2000 | Titanium mesh wrapping cancellous bone grafts | Exposure of the titanium mesh is 7/16 in maxillary and 16/29 in mandible. The success of the bone grafting procedure was 97.72% [42] | |
| 2010s | Magnesium (Mg)-based Materials [43] | Mg screw was able to distribute the stress to the condyle and ramus region compared to polylactic acid polymer group [44] | |
| 2016 | Finite element simulation and 3D printing technology prefabricated titanium meshes [45] | 21 patients, insufficient bone formation (5 cases), postoperative infection (2 patients), Ti-mesh tray fracture in 2 patients | |
| 2022 | Patient-specific 3D-printed miniplates for free flap fixation | High accuracy of reconstruction (3.64 ± 1.18 mm), Osseous union occurred in all intersegmental gaps [46] | |
| Distraction osteogenesis | 1992 | First applied distraction osteogenesis to the mandibular deformities' reconstruction | Mandibular bone lengthening ranged from 18 to 24 mm [47] |
| 1996 | A report of 5 cases of distraction osteogenesis in maxillofacial surgery | 5 patients, premature consolidation (2 cases), significant relapse (1 case) [48] | |
| 1997 | Case report on a patient received trifocal distraction osteogenesis in oral floor cancer underwent surgery | Infection developed; Free bone transplants were needed for complete continuity [49] | |
| 2000 | Combination of mandibular distraction osteogenesis with electrical stimulation (10 μA) | Significant increase in bone mineral density [50] | |
| 2011 | Electrical stimulation on mandibular distraction osteogenesis conducted in clinical trials | 10 patients, 16 distraction sites, and direct current electrical stimulation promoted bone healing [51] |
The strengths and weaknesses of current clinical approaches are summarized in Fig. 2. The clinical treatments are limited considering the infection with the presence of bacterial contamination and the donor site morbidity. Reconstruction plates demonstrate an infection rate ranging from 7 to 13% [29]. After mandibular distraction osteogenesis, a 12.2% risk of infection related to the distraction device is reported. The patients of 30.5% and 40% suffer from hyperalgesia and neuropraxia, respectively [30]. Besides, the use of metallic devices and distractors for mandible fixation and regeneration inevitably involves multiple invasive operations and extensive presurgical modeling, thus increasing the morbidity and negative aesthetic effects associated with repeated procedures and poor biocompatibility [31].
Fig. 2.
Strengths and weaknesses of current clinical approaches applied in mandibular reconstruction.
3. Unique mode of bone formation and resorption in the mandible
3.1. Bone formation in the mandible
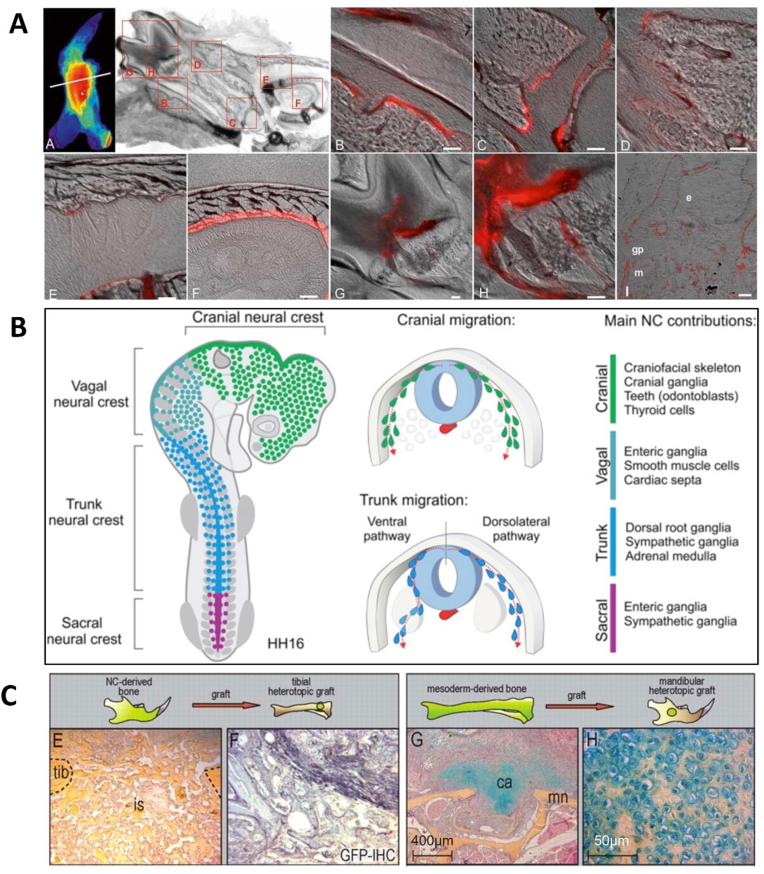
The metabolism and turnover rate of the jaw is considered the most active in the skeletal system due to occlusal mechanical stimulation and odontogenic inflammation. The remodeling rate of the cortical bone of the mandible is 10–20 times higher than the cortex of the human iliac crest in humans [52]. The bone turnover in the adult dog's maxilla and mandible are approximately three- and six-fold higher (19–37%/year) than that of the femoral site (6.4%/year), respectively [53]. Within the mandible, the bone turnover rate of the alveolar bone is the highest. Besides, any bone damage caused by strenuous chewing and surgical intervention can accelerate the turnover rate of the jaw [52]. Researchers have detected the fluorescent-labeled BPs and found that alveolar bone was the most susceptible to the drugs as maximum fluorescence intensity was found at the mandible (Fig. 3A) [54].
Fig. 3.
Osteogenic potential and regeneration in the mandible. (A) The fast turnover rate in the mandible: In-situ fluorescence of far-red fluorescent pamidronate signal (red) labeled BPs localization in the mandible. Scale bar = 50μm. (Copyright © American Society for Bone and Mineral Research, Ref [54]). (B) Schematic diagram showing the location and progression of neural crest subpopulations. The cranial neural crest developed into the craniofacial skeleton (Copyright © 2018 Elsevier Inc, Ref [63]). (C) Placement of neural crest-derived periosteum into a mesoderm injury site resulted in an intramembranous bone formation. However, transplantation of the tibial periosteum initiated an endochondral ossification in the mandibular defect. GFP immunohistochemistry confirmed that the grafted cells were actively committed to the healing response (Copyright © 2022 The Company of Biologists, Ref [62]).
Mesenchymal stem cells (MSCs) derived from the jaw exhibit unique osteogenic potentials. Mandibular bone marrow MSCs (m-BMSCs) originate from the neural crest of the neuroectoderm (Fig. 3B), which differs from appendicular bones in terms of developmental origin, mode of bone formation, and pathological bone resorption [55]. Endochondral ossification stimulates the process of long bone formation generated from the mesoderm, while craniofacial bones are formed primarily by intramembranous ossification. It has been reported that gradual-distraction mandibles exhibited direct intramembranous ossification, while fracture mandibles appeared with a degree of endochondral ossification [56].
Compared with tibial bone marrow MSCs (t-BMSCs), m-BMSCs possess stronger proliferative activity [57], exhibit higher expression of pluripotency markers, and hold stronger osteogenic differentiation potential [58]. In pathologic conditions such as ovariectomized and malnourished rodent models, the mandible loses much less bone than the proximal tibia [59]. Microarray analysis has been used to identify 404 highly abundant genes m-BMSCs and t-BMSCs of pigs, 48 genes of them are differentially expressed for at least 1.5 times between two cell types [60], including higher expression of cranial neural crest-related genes such as BMP-4. m-BMSCs have a phenotypic profile that makes them advantageous for craniofacial bone regeneration.
Based on clinical observation, alveolar cleft repair from mandibular bone grafts yields better results than iliac grafts, mainly due to less graft resorption [61]. Interestingly, a research study tested whether skeletal stem cells from different lineages (neural crest-originated and mesoderm-originated) were functionally interchangeable by grafting neural crest-originated cells into tibia defects and mesoderm-derived cells into mandibular defects, respectively [62]. Surprisingly, the placement of NC-derived periosteum into the tibia injury site resulted in a robust intramembranous bone formation. However, the tibial periosteum failed to new bone formation and underwent an endochondral ossification with fibrotic-like “scar” tissues forming in the mandibular defect site (Fig. 3C) [62]. These differences guide the seed cell selection for the regeneration and reconstruction of site-specific bone defects.
3.2. Bone resorption in the mandible
The mandible possesses a special bone resorption process, owing to the different matrix composition between maxillofacial bones and long bones. The mandible has greater Type I collagen content, a lower number of mature crosslinks, and a lower extent of lysine hydroxylation (posttranslational modifications of collagen), as compared to long bones. The lysine hydroxylation is lower in the mandible (11.9%) than in both humerus (14.8%) and femur (13.7%) [64]. The differences in the number of crosslinks and the concomitant impaired degradability of collagen may explain the observed functional heterogeneity of osteoclasts in proteases used for resorption [65,66].
Osteoclasts from specific bone sites differentially respond to certain cytokines. Versus long bone, mandible marrow contains fewer osteoclast precursors, primarily the myeloid blasts, and showed a lower ratio of receptor activator nuclear factor kappa B ligand (RANKL) and osteoprotegerin (OPG) [67]. In contrast, maxillofacial osteoclasts are larger and contain a 25-fold higher level of tartrate-resistant acid phosphatase (TRAP) activity than those of long bones [68], which may contribute to the degradation of noncollagenous protein matrix in maxillofacial bones. Moreover, the previous studies reported that the activation of TRAP was independent of the activities of cathepsin K and L in calvarial osteoclasts (similar to mandible osteoclasts), and TRAP might partially compensate the lower cysteine proteins’ activities [68].
Besides, some antiresorptive and antiangiogenic drugs such as BPs and denosumab target osteoclasts [18], leading to the fragility of mandibular osteoclasts. It has been reported that the mandibular osteoclasts express higher anti-apoptotic genes and capture more BPs [69]. Nowadays, more and more clinical cases of mandibular defects are associated with bone resection and tumor ablation caused by osteoclast-related diseases (BRONJ, periodontitis, cherubism, and suppurative jaw osteomyelitis) [70].
4. Hydrogel-based mandibular tissue engineering
4.1. Advantages of hydrogel systems for mandibular regeneration
Hydrogel systems provide a number of advantages to meet the special demands in mandibular reconstruction [[71], [72], [73]], including 1) More biocompatibility and non-immunogenicity compared to the traditional materials that avoid inflammatory responses; 2) Appropriate pore size and interconnected porosity supporting cell infiltration, adhesion, proliferation, and differentiation; 3) As an ideal delivery carrier of bioactive factors, antibacterial agents, and nanoparticles may enhance therapeutic effects, such as osteogenesis, angiogenesis, and anti-infection; 4) Controllable degradability, synchronizing with rapid mandible ingrowth and elimination of the second removal surgery; 5) Injectability supports minimally invasive surgery, simplifying the administration process and meeting the purpose about aesthetic restoration of facial structures; 6) Various methods are available for improving structural stability and mechanical strength which meet the demands of load-bearing defects; 7) Easy modification suggests that it can be implanted either independently or combination. In the text below, we will describe various kinds of advanced hydrogel designs in detail and highlight their necessity and cutting-edge use in mandibular reconstruction.
4.2. Hydrogel scaffolds
Hydrogels well mimic the extracellular matrix (ECM) of the natural mandible, providing the most similar environment favorable for the adhesion, propagation, and differentiation of osteoblasts, osteoclasts, and osteocytes [9]. The ideal hydrogel is highly biocompatible and nonimmunogenic, which may serve as a carrier for stem cell therapy. It can also provide a 3D network that supports the transportation of nutrients, the discharge of metabolites, and intercellular communications. Besides, the hydrogel can degrade by endogenous enzymes or hydrolysis. Capillaries, perivascular tissues, and osteoblasts can synergistically orchestrate new bones within the pores during degradation [8].
Hydrogels can be divided into two categories, natural and synthetic, according to the sources of their polymer substrates [9,73]. Natural hydrophilic polymers include polysaccharides (alginate, cellulose, heparin, hyaluronic acid (HA), chitosan, etc.) and polypeptides (collagen, gelatin, bovine serum albumin (BSA), poly-l-lysine, poly-l-Glutamic acid, etc.) [74]. Synthetic hydrophilic polymers include alcohol, acrylic acid, and their derivatives (polyethylene glycol (PEG), polyacrylic acid (PAA), polyacrylamide (PAM), etc.) [75]. The hydrogels can be synthesized in a short time through appropriate physical or chemical crosslinking [76]. Physically crosslinked hydrogels can be fabricated by ionic interaction, complementary base pairing, hydrogen bonding, and supramolecular force. Chemical crosslinked hydrogels are produced by Schiff base crosslinking, Diels-Alder reaction, click chemistry, and free radical polymerization (such as photo-initiated, thermal-initiated, and pH-initiated hydrogels) [77].
Hydrogel-based mandibular tissue engineering has been investigated for decades [12]. Natural polysaccharides, chitosan and chondroitin sulfate, are fabricated into 3D porous scaffolds and further modified with apatite coatings and efficient delivery of bone morphogenetic protein 2 (BMP-2) to reconstruct a rat critical-sized (5×5 mm) mandibular defect [78]. In addition, hydrogel made of liposomes takes the advantage of the closed vesicular and amphiphilic structure, improving the delivery efficiency and therapeutic effect of both hydrophilic and hydrophobic pharmaceuticals [79]. A self-assembled liposomal hydrogel is developed to deliver recombinant human BMP-7 for craniomaxillofacial applications [80], a more controlled, linear, and multi-phasic release is demonstrated by this nanogel when compared to the liposome-associated burst and faster release. However, natural hydrogels are sometimes limited by poor mechanical strength and are not suitable for load-bearing sites.
Synthetic hydrogels are more stable. Modification of specific structural units by chemical synthesis can achieve tunned porosity, degradation time, releasing pattern, and mechanical properties [81]. Moreover, stimuli-responsive properties can also be achieved by incorporating specific chemical structures and additives during and after polymerization [82,83]. For example, an injectable thermos-responsive silica nanoparticle (NP)-embedded hydrogel are formulated by adding a specific chemical structure PNIPAAm to the polymer chains, achieving in situ gelation at 37 °C and co-delivery of microRNA-222 and aspirin (ASP) in mandibular defect [84]. A boom in research on hydrogels that exhibit responsiveness with various external control triggering mechanisms-pH, temperature, external chemicals, light, and shear stresses will lead to more applications for mandibular reconstruction in the future.
4.3. Seed cells in mandibular tissue engineering
Cell therapies have been expected to bring substantial benefits to patients [85]. The challenges of cell therapy are mainly divided into two parts: internal and external. Success will rely on how to maintain the retention and viability of seed cells internally, and how to recruit or locate external endogenous cells and control their survival, fate, and function within a certain duration following treatment in vivo. Hydrogels improve stem cell-based therapies “internally and externally”. Firstly, the hydrogel as a carrier can restore the seed cells in the defect site and protect them from immune rejection, which circumvents the challenge that less than 5% of the injected cells are maintained at the defect region after transplantation in clinical practice [86]. Secondly, hydrogels releasing stromal cell-derived factor-1α (SDF-1α) can also recruit endogenous cells [87,88]. Finally, hydrogels may control stem cell fate and function through biophysical and biochemical signals such as topography, degradation, mechanics, and adhesion [88].
Hydrogels combined with cell therapies hold great promise in the treatment of mandibular defects. The stem cells used with hydrogel systems for mandibular regeneration mainly include BMSCs [89], adipose-derived stem cells (ADSCs) [90], and dental stem cells (DSCs) [91]. Bone repairs are advanced by BMSCs that migrate to the injured site and undergo differentiation to realize structural and functional repair. This process also exists in mandibular reconstruction [92]. Liu et al. used a sex-mismatched canine model for systemic BMSCs injection and homing to the periosteum-free mandibular defects [93]. Appendicular BMSCs can migrate to craniofacial defects and lead to 20–40% increase of mineralized bone formation [93]. Besides, combined with hydrogel systems, interleukin-4-loaded (assisting in transforming macrophages from the pro-inflammatory M1 phenotype into the anti-inflammatory M2 phenotype) hydrogel scaffold promotes the osteogenic differentiation of BMSCs via TGF-β1/Smad pathway in mandibular defect of rat [94]; More promisingly, hydrogel incorporation enhances the biocompatibility of metal-based scaffolds. The porous Ti6Al4V scaffolds combined with BMSCs-encapsulated Matrigel promote a large amount of new bone formed around and within the scaffold in rat mandibular defect [95].
ADSCs have also been extensively used attributed to their accessibility, abundance, and less invasive procedure for harvesting tissues [96]. Presenting a great osteogenic differentiation potency in mandibular defects [97,98], ADSCs can be incorporated with polymers for enhancing regenerative applications. For example, ADSCs with tricalcium phosphates (TCP) is used for mandible regeneration by facilitating bone extracellular matrix formation and promoting reparative osteogenesis [99]. Moreover, the regenerative effect of ADSCs containing fibrin scaffold and autologous bone graft in rabbit mandibular defect has also been reported, with a significant increase in the thickness of new cortical bone when fibrin glue scaffold combined with ADSCs are used [100]. Besides, ADSCs can potentially improve the current understanding of disease mechanisms, while minimizing tissue rejection when been transplanted back into the host for organ manufacturing [101]. It has been showed that neither the proliferation ability nor the differentiation behavior of the ADSCs is affected by the 3D bioprinting procedures [102]. Various bioactive polymers such as Polyetherketoneketone (PEKK) can be enhanced in tissue integration by functionalizing with ADSCs in vivo, improving the cell interaction and osteogenesis of a three-dimensional printed PEKK/ADSC implant within the bicortical critical-sized (1.5 cm × 1.0 cm) mandibular defect in a rabbit model [103]. ADSCs encapsulated in the composite structures remain viable within the hydrogel for more than 14 days and show excellent spreading on the 3D printed PLA microstructure could be readily modulated to simulate the stiffness of the human mandibular condyle [104].
Most interestingly, given that DSCs share the same developmental origin as m-BMSCs and are characterized by self-renewal ability, in recent years, they are often used as seed cells in the regeneration of maxillofacial tissues and periodontal tissues. Dental pulp stem cells (DPSCs) [105], stem cells isolated from human exfoliated deciduous teeth (SHEDs) [106], gingival mesenchymal stem/progenitor cells (GMSCs) [107], and periodontal ligament stem cells (PDLSCs) [108] all demonstrate with self-regeneration and multi-differentiation capacity. Their characteristics, cell surface markers, and applications in maxillofacial tissue regeneration are summarized in Table 2.
Table 2.
Properties of dental mesenchymal stem cells.
| Dental stem cells | Dental pulp stem cells | Stem cells isolated from human exfoliated deciduous teeth | Gingival mesenchymal stem/progenitor cells | Periodontal ligament stem cells |
|---|---|---|---|---|
| History (firstly be found or separated) | 2000 | 2003 | 2010 | 2004 |
| Surface markers | Positively express Stro-1, CD29, CD44, CD90, CD105; Negatively express CD34, CD45 | Positively express Oct4, CD13, CD29, CD44, CD73, CD90, CD105, CD146, CD166; Negatively express CD14, CD34, CD45 | Positively express CD90, CD105, CD73, CD44, CD13; Negatively express CD34, CD38, CD45, CD54 | Positively express CD10, CD13, CD29, CD44, CD59, CD73, CD90, CD103; Negatively express CD14, CD34, CD45, CD38, CD54, HLA-DR |
| Multidirectional differentiation ability | Odontoblasts, osteoblasts, endothelial cells, adipocytes, neurons, and chondrocytes | Osteoblasts, neurons, adipocytes, and odontoblasts | Osteoblasts, adipocytes, and chondrocytes | Cementoblasts, adipocytes, osteoblasts, and fibroblasts |
| Superiority | Great Potentials in bone vascularization | Rich sources; great clinical translational potential | Less painful collection | Strong adhesion, rapid growth, and proliferation; ideal material for regenerating the periodontal tissue defects |
| Application in bone tissue engineering | [109,110] | [111,112] | [113,114] | [115,116] |
4.3.1. Dental pulp stem cells (DPSCs)
DPSCs were firstly isolated from the pulp of extracted third molars, identified as undifferentiated cells derived from the neural crest, that is, originated in the same area as the mandible [117]. Under normal circumstances, DPSCs are quiescent and maintain the replacement of new and old pulp cells. When dental pulp suffers from adverse external stimuli and damage, DPSCs differentiate into odontoblasts to support dental pulp tissue reconstruction [118]. Human mandibular reconstruction by the grafting of autologous DPSCs and collagen sponge bio-complexes (similar to lyophilized hydrogel scaffolds) has been conducted in clinical practice for one year. Compared with traditional direct injection, collagen sponges aid the retention of the encapsulated stem cells by providing biological and physical support as same as the hydrogel systems do [119]. As assessed by clinical probing, X-rays, and histological observations, satisfactory bone regeneration is achieved one year after grafting [109].
4.3.2. Stem cells isolated from human exfoliated deciduous teeth (SHEDs)
Monoclonal SHEDs were firstly obtained from the pulp tissue of deciduous teeth. After in vivo transplantation, SHEDs are found to be able to induce bone formation, generate dentin, and survive in the mouse brain along with the expression of neural markers [120]. SHEDs transplantation has an immunosuppressive activity that solves a major concern on stem cell therapies. When human SHEDs from normal exfoliated deciduous teeth are implanted with hydrogel/sponge scaffolds into dog mandibular defects, the new formation of soft tissue, cancellous bone, and compact bone is observed after 12 weeks [111]. SHEDs are noteworthy for easy accessibility from teeth with painless harvest procedures, combined with hydrogel systems for viability and stemness maintenance, which provide a rapid transformation of stem cell-based oral and maxillofacial reconstruction therapy in clinical trials.
4.3.3. Gingival mesenchymal stem/progenitor cells (GMSCs)
Via systemic injection of human GMSCs through the mice tail vein, GMSCs can home to the mandibular defect site and promote bone regeneration by triggering the endogenous MSCs recruitment which is known to be crucial for successful mandibular regeneration [121]. However, up to date, no study has evaluated the contribution of GMSCs migrating from peripheral circulation to mandibular regeneration. To further improve the unmet need for the efficacy of cell therapies and time window, an injectable alginate-based adhesive hydrogel encapsulated GMSCs is developed for bone regeneration around ailing dental implants [113].
4.4. Nanoparticles and growth factors
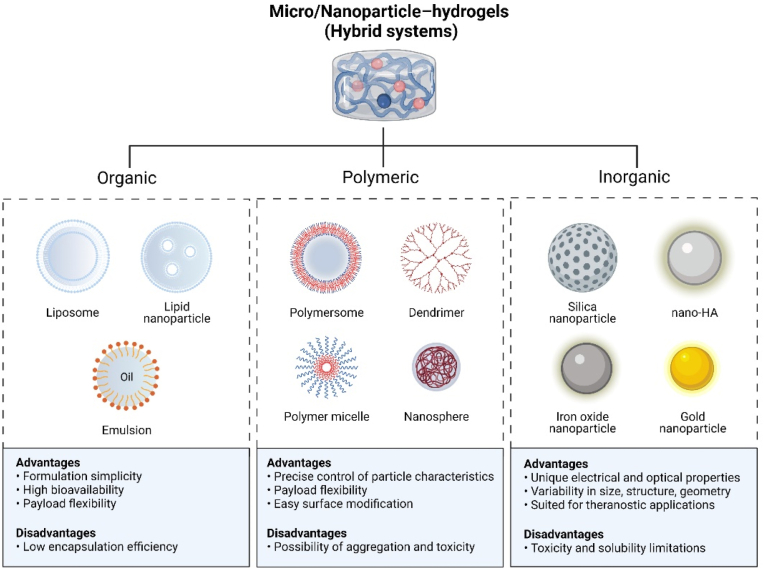
Hydrogels are also considered ideal delivery carriers for NPs, which may not only support the sustainable release of drugs and growth factors but also enhance mechanical properties. The extracellular matrix of bone is a biphasic system, one-third of the component is organic substances, two-thirds are composed of inorganic substances. To simulate this biphasic system of natural bone extracellular matrix, biomaterials are developed based on the strategy of “organic macromolecules network (hydrogels) combined with inorganic NPs [122], which is defined as “hybrid hydrogel”. In hybrid hydrogel, this combination of two different types of materials produces structural diversities and brings a variety of enhancements involving mechanical strength and biological response of osteointegration, osteoconduction, and osteoinduction [122].
Organic liposomal microspheres, polymeric microspheres (e.g., PLGA, PVA, gelatin), and inorganic NPs (e.g., nanohydroxyapatite (nHAp), silica NPs, gold NPs, and bio-ceramics NPs) have been used for integration into hydrogel systems in mandibular tissue engineering (Fig. 4). Inorganic NPs has been reported to provide promising outcome in bone healing applications [123]. Among them, the nHAp is the most widely used, which is regarded as a plentiful source of free calcium and a key factor in mineralization and alveolar [124].
Fig. 4.
Potential micro/nanoparticles used in mandibular tissue engineering.
NP itself has been also successfully applied to deliver growth factors, genes, and drugs either systemically or locally in a controlled and intelligent manner. This versatility is mainly the result of a wide range of NPs functionalization methods and a large surface area [125]. Antibodies, labeled probes, hydrophobic or hydrophilic molecules, DNA, and oligonucleotides can be conjugated to NPs, allowing customized applications for the desired purpose. For instance, contributing to the intracellular delivery of microRNAs (miRNA), nHAp modified with cationic functional groups 3-aminopropyltriethoxysilane shows the optimal efficiency in miRNA delivery and gene regulation in the human mandibular osteoblasts. Plasmid DNA/c-myb (one transcription factor that supports bone formation) conjugated with CS-gold NPs facilitates the osseointegration of dental implants in mandible osteoporosis rats [126]. Following the same approach, CS-gold NPs carrying peroxisome proliferator-activated receptor gamma (PPARγ) plasmid DNA diminishes inflammation and enhances osteogenesis in the mandible around the dental implantation [127]. Besides, tremendous efforts have been made to construct advanced bone fillers with ROS scavenging ability through the functionalization of various types of antioxidants [128]. To endow the gelatin-based hydrogels with high osteoinduction and robust ROS scavenging ability, BMP-2 incorporated polydopamine/heparin nanoparticles have been introduced to the optimized hydrogels. The enhanced ROS-scavenge abilities and sustainable release of bioactive molecules has been reported to augment the mandibular regeneration [129].
More interestingly, NPs like superparamagnetic iron oxide nanoparticles (SPIONs) [130], gold NPs [131], and mesoporous silica NPs [132], can be used to label cells and to achieve continuous cell tracking, including the biodistribution and migration mode of stem cells after transplantation [133]. There is a significant growth of interest in the use of nanoparticles as non-invasive visual monitoring in diagnostics and healing processes [134]. Making full use of the properties such as superparamagnetic, high sensitivity, and easy detection are beneficial for regulating and detecting the healing process. For example, SPIONs loaded gelatin sponge not only enhances the mandible formation but also monitors the scaffold degradation and cell integration by imaging the intensity of scaffolds in incisor sockets [135].
As also shown in Table 3, osteoinductive and angiogenic factors like bone morphogenetic protein (BMP), insulin growth factor (IGF), fibroblast growth factor (FGF-2), transforming growth factor-β (TGF-β), stromal cell-derived factor 1 (SDF-1), vascular endothelial growth factor (VEGF), and NGF play crucial roles in mandibular regeneration and have been encapsulated with hydrogel systems [136]. BMP-2 has become the preferred additive for mandibular reconstruction materials [137]. At present, more than 20 kinds of BMPs have been separated, except BMP-1, all belonging to the transforming growth factor β superfamily, among which BMP-2 has the most vital osteogenic ability, which has been extensively used in mandibular reconstruction [[138], [139], [140]]. A review analyzed several cases of BMP-2 used for mandibular reconstruction secondary to tumor resection, trauma, and infection, highlighting the versatility of growth factors-mediated bone regeneration [141]. Moreover, a phase II-randomized controlled study has investigated the safety and efficacy of rhBMP-2 combined with an absorbable collagen sponge versus autograft for maxillary sinus floor augmentation. Such scaffold performed comparable effects to the bone graft in the aspects of maxillary regeneration and functional loading [142], while avoiding the long-term paresthesia, pain, or gait disturbance related to the autograft bone harvest [143]. Clinically, rhBMP-2 sponge collagen or poloxamer-based gel with traditional Ti mesh has achieved better mandibular reconstruction [144,145] (Fig. 5).
Table 3.
Applications of Hydrogel systems with seed cells, nanoparticles, and growth factors for mandibular reconstruction.
| Materials/Hydrogels | Nanoparticles | Agents/Cells | Animal models | Refs. |
|---|---|---|---|---|
| Hyaluronic acid hydrogel | Nano-HA | BMP-2 | Rat mandibular defect | [147] |
| Collagen/alginate hydrogel | Nano-HA | NGF | New Zealand white rabbit mandibular defect with distraction osteogenesis | [148] |
| Collagen sponge/hyaluronic acid hydrogel/PCL outer box | – | rhBMP-2/hBMSCs | Rabbit mandibular defect | [149] |
| PEG hydrogel | HA/β-TCP | rhBMP-2 | Minipig alveolar defect | [150] |
| Chitosan and chondroitin sulfate | – | BMP-2/ADSCs | Rat Mandibular Defect | [78] |
| Chitosan/collagen hydrogel/Gelatin microsphere | – | rhBMP2 | Rabbit mandibular defect | [151] |
| Alginate-fibrin microfibers | Calcium phosphate cement | hBMSCs | Rat mandibular defect | [152] |
| Calcium alginate hydrogels and polylactic acid (PLA) | – | hPDLCs/BMSCs | Rabbit mandibular defect | [153] |
| PLA/gelatin hydrogel | Cyclic RGD conjugated gold NPs | hADSCs | – | [104] |
| Mannuronate (SLM) alginate, high guluronate (SLG) alginate, hyaluronan derivative (HApN) | Collagen-I based recombinant peptide microspheres | BMP-2 | Subcutaneously in rats | [154] |
| Alginate/hyaluronic acid hydrogels | – | BMP-2/hBMSCs | Miniature pig mandibular defects | [155] |
| Alginate/hyaluronic acid hydrogels | – | Vancomycin, BMP-2 | Osteomyelitis rat model | [156] |
| Supramolecular hydrogel (SDF-1/BMP-2/NapFFY) | – | BMP-2, SDF-1 | Rat periodontal bone defect | [157] |
| Gelatin | – | Fibroblast growth factor | Rat BRONJ | [158] |
| PEG–PLGA–PNIPAM hydrogel | Silica NPs | microRNA-222 and ASP | Rat mandibular defects | [159] |
| N-carboxyethyl chitosan (CEC)/hyaluronic acid-aldehyde (HA-ALD)/adipic acid dihydrazide (ADH) | Nano-HA | – | Mandibular incisors of rat | [160] |
| CS scaffolds | Nano-HA/PLGA microspheres | BMP-2/VEGF | Rabbit mandibular defects | [161] |
| Modifying HA with both methacrylate (MA) and 3,4-dihydroxyphenylalanine (Dopa) | – | – | Maxillofacial tissue | [162] |
| Hyaluronic acid hydrogel | β-tricalcium phosphate | rhBMP-2 | Minipig mandibular defect | [163] |
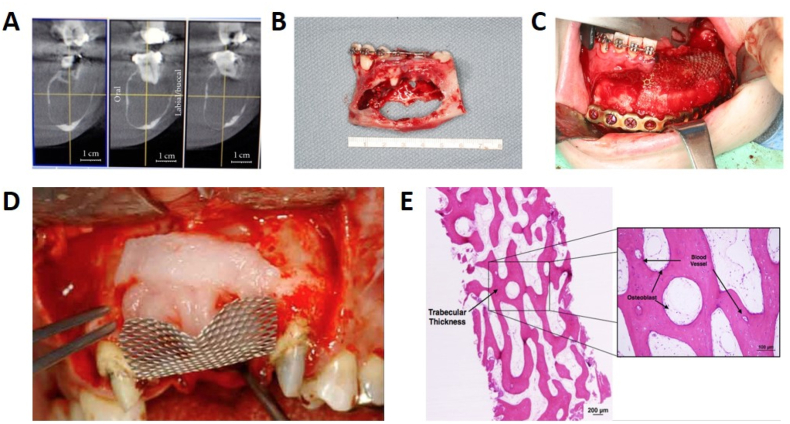
Fig. 5.
Clinical application of hydrogel in mandibular reconstruction. (A) CT image of the mandibular lesion. (B) Tumor specimen. (C) Bioimplant (rhBMP-2/Absorable collage sponge) inserted and covered by titanium mesh (A-C, Copyright © 2011 Alan S. Herford et al. Ref [141]). (D) Another clinical case used absorbable collagen containing rhBMP-2 in the concave area of the titanium mesh. (E) Histology showed a large amount of newly formed bone and bone marrow/connective tissue in the rhBMP-2-containing collagen implanted area (D-E, Copyright © 2022 Elsevier B·V., Ref. [146]).
5. Additive fabrication of novel hydrogels for mandibular reconstruction
5.1. Mechanically enhanced hydrogel designs
The mandible participates in various physical activities such as mastication, articulation, speech, and suppling mechanical support. The ultimate compressive strength of the mandibular bone ranged from 0.22 to 10.44 MPa, with a mean value of 3.9 MPa [164]. However, natural polymers such as collagen, gelatin, and chitosan have poor mechanical strength and structural stability [165]. Natural polymers easily deform after implantation and lead to burst degradation during degradation. Here, we introduce several methods for preparing mechanically enhanced hydrogels as bioactive space fillers, aiming at adequate clinical applications for mandibular regeneration.
Compared with traditional single network hydrogels, the mechanical properties of double network (DN) hydrogels are significantly improved. It is an interpenetrating network formed by two polymers with different properties. One is a polyelectrolyte network structure with high crosslink density performing rigid and brittle, and the other is a neutral network structure with loose crosslink performing soft and tough. The polyelectrolyte network structure provides a “sacrificial bond” for the DN hydrogel to disperse external stress, while the flexible neutral network structure fills in the polyelectrolyte network structure, providing a scaffold and maintaining the shape of the DN hydrogel. It has been almost 20 years since Gong et al. reported the first fabrication of DN hydrogel [166]. Suggesting that the PAMPS-PAAm DN hydrogels hold up to nominal stress of 17.2 MPa, while the PAMPS gel and PAA gel break only at 0.4 MPa and 0.8 MPa, respectively [167]. Given the mechanical enhancement, DN hydrogels are widely used in bone, including mandible tissue engineering.
In order to improve the mechanical strength and stable network of traditional gelatin methacryloyl (GelMA) hydrogel, DN hydrogels doped with magnesium ions have been successfully prepared by two crosslinked including in situ free radical polymerization and magnesium ionic coordination strategies. With the introduction of DN networks and POSS-Mg composite, the maximum compressive strengths are enhanced by about 6-folds, showing a promising bone formation and vascularization in calvarial defect of rats [168]. One novel DN hydrogel is constructed by physical cross-linking of medical-grade PVA (synthetic polymer) and CS (natural polymer). The obtained hydrogel demonstrates excellent tensile strength (0.24 MPa), elongation at break (286%), and high compressive strength (0.11 MPa on the strain of 60%) compared to the single network hydrogels. This mechanically enhanced hydrogel shows excellent biocompatibility in vitro and a significantly accelerated simultaneous regeneration of bone defects in rabbits [169]. Besides, the complex network of the DN hydrogel does not affect the loading and release of seed cells and growth factors. in situ gelling DN Alginate/HA hydrogels can tune the gelling rate to achieve a controllable mechanical strength by supplementing different concentrations of HA. The DN hydrogel also allows BMP-2 encapsulating and sustained releasing, thus greatly enhanced bone regeneration in mandibular defects of miniature pigs [155].
The mechanical properties can be improved by adding nanoparticles and nanostructures into hydrogels, called nanocomposite (NC) hydrogels. Metal NPs, carbon-based NPs, and ceramic NPs all can be incorporated into hydrogels through in situ polymerization, in situ formation of NPs, and physical mixing [170]. Due to the hydrogen bonds or van der Waals interactions between the nanoparticles and polymer chains, the network of hydrogels can be strengthened to obtain nanocomposite hydrogels with stable structure and improved mechanical properties.
The most widely used inorganic NPs in NC hydrogels for bone tissue engineering are calcium phosphates, including hydroxyapatite (HAp), β-TCP, and their mixtures – bidirectional calcium phosphate (BCP). HAp constitutes the inorganic phase of the natural bone and is one of the most biocompatible phosphate molecules. The addition of nHAp in hydrogels exhibits excellent mechanical properties with the tensile stress in the range of 0.21–0.86 MPa, and its compressive strength can reach 35.8 MPa, which has already exceeded the ultimate compressive strength of human mandibular trabecular bone (ranged from 0.22 to 10.44 MPa) [171]. While maintaining high mechanical strength and bioactivity, the hydrogel can still retain its responsiveness and injectability. HA-based hydrogels containing nHAp have been fabricated to achieve mandibular bone augmentation in rats through minimally invasive surgery, holding a great clinical translational potential in oral implantation [172]. To improve the responsiveness, an injectable thermosensitive hydrogel containing Zn-doped CS/nHAps/β-glycerophosphate (Zn-CS/nHAp/β-GP) is prepared and characterized, in which nHAp accelerates bone formation in vivo as evident by the depositions of apatite and collagen. Moreover, to overcome the limitations on inferior mechanical properties as well as the high biomimetic requirement during the 3D bioprinting process, a novel NC hydrogel with bioactive GNPs, reinforcing a 3D printed microstructure via fused deposition modeling has been developed. The stiffness of the composite hydrogel can be readily modulated to simulate the stiffness of the human mandibular condyle [104]. DN and NC hydrogels have also been combined to enhance its mechanical properties. The same mechanical strength can be achieved with a few nano composites. In carbon dots/nano-HA/PVA hydrogels, the results show that 0.6% of solid content nHAp containing could optimize the compression properties to 3.462 MPa of compression strength [153].
Besides, by changing component concentration, molecular weight, or fiber structure of the hydrogel system, the mechanical properties of the hydrogel, such as Young's modulus, stress, deformation, strength, and other indicators, can be improved to varying degrees. However, the mechanical enhancement will inevitably slow down the degradation, and the toxicity of ingredients will be another concern. So, in the future, extensive efforts are needed for preparing mechanical strength-adjustable hydrogels for mandibular tissue engineering, aiming to obtain ideal mechanical properties and restore the hydrogels' advantages.
5.2. Antibacterial hydrogel designs
More than 700 bacterial species or phylotypes have been so far detected in the oral cavity. Once the tooth loses, microorganisms from oral fluids tend to accumulate at the exposed roots and form solid biofilms [173]. These biofilms may cause persistent infection around the implant, and severe alveolar bone resorption [174,175]. Surgical debridement, mandibular decortication, or resections combined with long-term systemic antibiotics are traditionally necessary procedures for adequate removal of osteonecrosis or tumor of the mandible and prevention of recurrent infections [176].
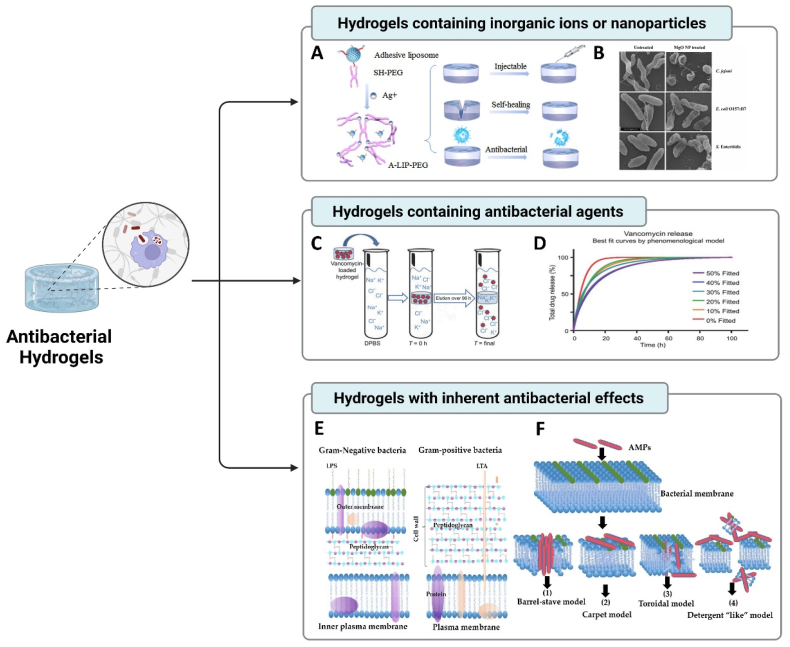
The development and application of antibacterial materials are limited by the problems of drug resistance and short antibacterial effect [177]. Hydrogels can be used as antibacterial agent carriers based on their inherent capabilities such as hydrophilicity and porosity. According to the classification of hydrogel matrix and antibacterial agents, the antibacterial hydrogels are divided into three types: (i) hydrogels containing inorganic ions or metallic oxide NPs, (ii) hydrogels containing antibacterial agents, and (iii) hydrogels with inherent antibacterial effects (Fig. 6).
Fig. 6.
Antibacterial hydrogels for potential oral and maxillofacial applications. (A) Adhesive liposome, SH-PEG, and Ag+ constructed an adhesive, self-healing, and antibacterial hydrogel (Copyright © 2022 Springer Nature Limited, Ref [184]). (B) SEM images of bacterial cells treated (right panel) with MgO NPs (Copyright © 2022 BioMed Central Ltd, Ref [196]). (C) Controlled delivery of Vancomycin via charged hydrogels for combating surgical site infections. (D) Best fit of data calculated by the phenomenological mathematical model described in the text. (C-D, Copyright © PLOS, #C2354500, Ref [197]). Bacterial membrane structures (E) and mechanisms of action of AMPs (F) (Copyright © 1996–2022 MDPI unless otherwise stated, Ref [198]).
In the first category, the hydrogel containing the antibacterial inorganic substance functions by adding metal ions or metallic oxide NPs. Metal ions (e.g., Ag+, Cu2+, Zn2+, Co2+), metal or metallic oxide NPs including silver NPs (Ag-NPs) [178], magnesium oxide (MgO) NPs [179], zinc oxide (ZnO) NPs [180] and Ti dioxide (TiO2) NPs [181] are widely combined with hydrogels refer to their broad-spectrum antibacterial properties and non-resistance to antibiotics. In addition to the local release of the metal ions, metallic oxide NPs also hold an antibacterial effect mainly by (i) oxidative stress via the generation of reactive oxygen species (ROS) on the surfaces of NPs; (ii) physiochemical and morphological properties to bind or damage the bacterial cells [182].
The hydrogel laden with inorganic antibacterial materials enhances the antibacterial performance and maintains antibacterial activity for the long term, thereby reducing the possibility of bacterial resistance. For example, a dynamic supramolecular hydrogel is formed assembly by sodium tetraborate, PVA, Ag-NPs, and tetraethyl orthosilicate, and composited with pTi as an implant system, which serves as an ideal dental implant for contaminated alveolar bone defects [183]. Besides, incorporating adhesive liposomes into PEG hydrogels based on the coordinated cross-linking principle of SH-PEG and Ag+ creates an injectable, antibacterial, and self-healing drug delivery system in bone [184].
In the second category, antibacterial agents-containing hydrogel is most extensively used in mandibular reconstruction. Combining antibiotics or antibacterial drugs (ciprofloxacin, gentamicin, vancomycin, cephalosporin, or other synthetic antibacterial drugs) into hydrogels is the current development mainstream of mandibular substitutes in preclinical studies [185]. For instance, local antibacterial treatment with an injectable gentamicin‐collagen hydrogel shows a higher amount of bone area at 4 weeks post-operation in mandibular osteomyelitis of rabbits [186]. Besides, combined with polymer scaffolds as a functional space maintainer, gelatin microparticles with antibiotic colistin containing are incorporated with porous polymethylmethacrylate constructs for rabbit mandibular defect reconstruction [187]. A clinical study reported that the protective hydrogel (DAC®; Novagenit Srl. Mezzolombardo, Italy) composed of hyaluronic acid and antibiotics performed a beneficial effect in maxillofacial surgery fixation. This protective hydrogel is not only for prevention but also in cases of replacement of infected implants due to biofilm or any other source of infection [188].
The last category, a hydrogel with inherent antibacterial effects, can be mainly divided into two types: containing antibacterial polymers or antibacterial peptides (AMPs) [189]. Specifically, some polymers with antibacterial fragments can also be used to form hydrogels. For example, a new hydrogel composed of thermo-responsive Poly (N-isopropylacrylamide (PNIPAAm) and redox-responsive poly (ferrocenesilane) (PFS) macromolecules exhibits strong antibacterial activity at the same time maintaining good biocompatibility with cells [190].
AMPs-based hydrogels are also widely reported. It is synthesized by antibacterial amino acids or peptides as structural components. AMPs are the primary defense against a broad spectrum of microorganisms, especially for antibiotic-resistant bacterial infections [192]. Generally, AMP has an amphiphilic structure with a hydrophobic core and a polycation surface, promoting interaction with negatively charged bacterial membranes. AMPs destroy bacterial membranes, inhibit cell wall and nucleic acid synthesis, change the formation of cell plasma membrane membranes, inhibit protein synthesis, and cause bacterial death. Based on these characteristics, a modified histatin-5, one antimicrobial peptide, is formulated into a bioadhesive hydrogel to treat oral candidiasis caused by the fungal pathogen Candida albicans [193]. Antibacterial hydrogels by self-assembling the tripeptide (DLeu-Phe-Phe) and the antibiotic ciprofloxacin exhibit a mild antibacterial activity against Gram-negative bacteria [194]. Moreover, to produce an antimicrobial hydrogel that can strongly adhere to hard and soft oral surfaces, an antibacterial adhesive hydrogel based on visible-light-activated gelatin and an AMP (Tet213) is developed for dental peri-implant disease, the adhesion strength and antibacterial effect is stronger than that of the commercially available adhesive product (CoSeal™, Baxter, Deerfield, Ill., USA) to porcine gingiva. It also supports the growth of autologous bone after sealing calvarial bone defects in mice [195].
It is inevitable to cause some contradictions between encapsulating the antibacterial agents and the stem cells in the development of hydrogel systems. In fact, for the mandibular reconstruction secondary to trauma and deformity, highlighting the versatility of stem cell-mediated bone regeneration, wrapped stem cells into hydrogel is to mimic a “bio-tissue” to achieve self-mineralization and recruit endogenous cells to accelerate bone regeneration. However, for the mandibular reconstruction secondary by infections or osteonecrosis under bacteria or verification environment, it has more heed in successfully addressing growing concerns of antimicrobial resistance to conventional therapeutic approaches, so hydrogel systems present as a “bio-carrier” to sustaining-release drugs into the lesions and maximize the efficiency of bone regeneration. Since the latter has no concern for immune response and medical ethics, hydrogel is more frequently applied as a carrier in the development of current clinical products nowadays.
5.3. Injectable hydrogel designs
Additional consideration includes the special aesthetic challenges posed by the restoration of facial structures and minimally invasive surgery has attracted much attention in maxillofacial reconstruction [199]. Profited by the low-risk infection and high acceptance of patients, the increasing demand for minimally invasive surgery has inspired researchers to focus on the development of injectable biomaterials [200].
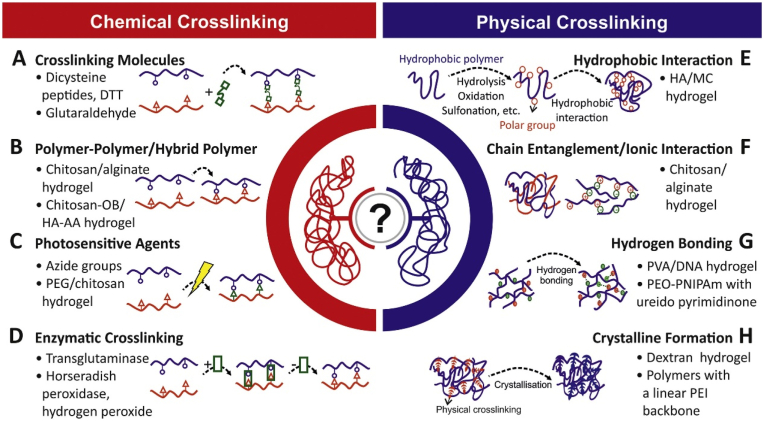
Injectable hydrogels are particularly attractive samples in mandibular reconstruction. Except for delivering the therapeutic cell population and bioactive factors, they can cure under various environmental stimuli which provides a board of design strategies to respond to the in-situ environments. The conventional curing mechanisms can be divided into physical and chemical gelation (Fig. 7). Physical gelation includes ionic crosslinking gelation, thermo-induced phase transition, and self-assembled gelation. Chemical crosslinking includes conjugated effect and free radical polymerized gelation [9]. The physical gelation hydrogels can eliminate the use of crosslinking agents so always maintain good biocompatibility. However, its effectiveness is limited. Chemical crosslinking hydrogels provide greater sustained release kinetics and superior mechanical properties [201]. Here, we review the curing mechanisms and related corresponding injectable hydrogel applications in mandibular tissue engineering.
Fig. 7.
Physical and chemical crosslinking of hydrogel synthesis (Copyright © 2022 Elsevier B·V., Ref [228]).
Ionic crosslinking gelation refers to the interaction of charged polymers with oppositely charged multivalent ions or polymers. Since some natural polysaccharides and their derivatives are polymer polyelectrolytes, they cross-link under normal conditions or in response to pH stimulation. Alginate is a representative polymer of the ionic crosslinking hydrogel. Since its polysaccharide structure consists of 1,4-linked β-d-mannuronic acid and α-homopolymer blocks, it can be crosslinked with calcium, zinc, or other cations at different positions of the alginate chain [202]. Alginate hydrogels can be easily injected and often served as the encapsulating carrier of growth factors. Cao et al. locally injected NGF via alginate containing hydrogel to enhance the bone consolidation and myelinated fiber density of the inferior alveolar nerve during mandibular distraction osteogenesis of rabbits [148]. However, the main limitation of injectable alginate hydrogels attributes to their poor mechanical properties, which cannot ensure the structural shape and mechanical maintenance for a certain period [[203], [204], [205]]. Therefore, alginate is usually chemically modified or combined with other polymer scaffolds or NPs to improve its mechanical and biological behavior. A drug-loadable calcium alginate hydrogels have been developed with a PLA scaffold supporting high plasticity and favorable biological properties to enhance oral bone regeneration [206].
Thermo-induced phase transition refers to the polymer solution that forms a hydrogel due to the manipulation of temperature. When the temperature changes, the physical entanglement of polymer molecular chains forms a polymer network. Such hydrogels can be injected into the body in liquid form, gelation in situ, and their crosslinking point temperature can be adjusted to be close to body temperature around 37 °C [207]. The common feature of thermo-induced phase transition hydrogels is the coexistence of hydrophilic/hydrophobic groups [208]. Hydrophobic groups include methyl, ethyl, and propyl. Hydrogels are generally liquid at low temperatures and possess a low critical solution temperature (LCST). When the temperature is lower than LCST, the enthalpy change plays a leading role, and the polar groups in the molecular chain form hydrogen bonds with water molecules to dissolve the polymer in water. When the temperature is higher than LCST, the entropy change (hydrophobic effect) plays a leading role, the hydrophobic isopropyl group is dehydrated during the transformation, causing polymer molecules to precipitate in the water [209].
The typical representative of thermo-induced phase transition hydrogel is PNIPAAm [210] and Pluronic (PEO-PPO-PEO) [211]. Generally, PNIPAAm and Pluronic can be combined with a degradable polymer in the form of the copolymer or chemical grafting to obtain a temperature-sensitive hydrogel scaffold with controllable injection and biocompatible for the survival of cells [212]. For instance, PNIPAAm-grafted gelatin is synthesized, exhibiting a sol-to-gel transformation at physiological temperature. It also protects cells from harsh shear stress due to the liquid form during the injection and presents an enhanced bone formation incorporating with BMSCs in a cranial defect model [213]. Besides, an injectable thermo-responsive PEG–PLGA–PNIPAAm hydrogel is designed for the microRNA-222 and aspirin minimally invasive surgical delivery, which stimulates bone regeneration in the rat mandibular defect model, displaying the nerve innervation in bone defects with high expression of neuronal protein Tuj1 and glial protein S100 [159].
Self-assembled gelation refers to some amphiphilic polymers displaying gelation properties due to their complexation as a result of desolation, collapse, and intermolecular association of nonpolar segments of the monomers [214]. Supramolecular hydrogels rely on small molecules that self-assemble in water through the cooperative effect of the intermolecular forces including electrostatic, hydrogen bonding, and Van Der Waals forces. Supramolecules are ubiquitous, such as enzymes and their substrates, hormones and their receptors, and inclusion compounds of crown ethers and certain metals [215,216]. The process of those kinds of supramolecular self-assemblies through hierarchical growth to form a hierarchical structure is similar to that of a protein from a primary structure to a tertiary structure [217]. Many studies based on heterodimeric peptide/protein or host macrocyclic interactive systems have demonstrated the revelation of shear-thinning ability and self-healing (rapid network recovery) to develop injectable hydrogels [218].
Among the various types of supramolecular forces, the macrocyclic host-guest recognition force has attracted more attention, because it usually contains a variety of forces and exerts a wealth of environmental responsiveness [219]. This dynamic crosslinking provides mechanical protection for delivered cells from the shear damage during injection, as well as maintains the retention and integrity of the implant in vivo [220]. The previous study prepared a supramolecular injectable hydrogel via the efficient host-guest complexation between the aromatic residues of gelatin and free diffusing photo-crosslinking acylated β-cyclodextrin monomers for the rat calvaria bone defect [221]. This weak crosslinking also facilitates the infiltration and migration of the endogenous cells. So, the self-assembled hydrogel can be used as a combined medical device with metal materials to improve their biocompatibility. 3D inorganic-organic supramolecular bioactive interface consisting of a stiff 3D printing porous metal scaffold and injectable supramolecular polysaccharide hydrogel is constructed for osseointegration enhancement in the osteoporotic microenvironment [222].
The conjugated effect refers to an electronic effect that changes the distribution of π electrons (or p electrons) in the system due to the mutual influence between atoms. The preparation of injectable hydrogels based on this mechanism is a hot topic, mainly including Michael addition (the nucleophilic addition of a carbanion or another nucleophile to an α, β-unsaturated carbonyl compound) [223], Schiff base (aldehyde groups condense with amino groups to form amide bonds) [224], Diels-Alder “click” reaction (conjugated dienes react with substituted olefins to form substituted cyclohexenes), and other click chemistry [225]. These crosslinking processes are carried out under mild conditions, independent of chemical additives. Many natural polymers contain polysaccharides with adjacent hydroxyl groups, such as alginate, chondroitin sulfate, hyaluronic acid, and cellulose, which can be oxidized by periodate to obtain aldehyde groups, when mixed with water-soluble macromolecules containing amino groups like BSA, chitosan, polylysine, an injectable crosslinking hydrogel scaffold can be formed in-situ based on Schiff bases [226]. Diels-Alder click chemistry and dynamic acyl hydrazone bond cross-linking are employed for chondroitin sulfate-based hydrogel formation, aiming to improve the necessary strength and precise mechanical tunability to fit for the maxillofacial bone reconstruction [227].
5.4. 3D bioprinting hydrogel designs
The ideal scaffold resembles the structure and composition of human bone, provides nourishment to graft cells, delivery of bioactive factors, and tube-like structures to allow vascularization, advantages that can positively impact bone graft success (Fig. 8A). Many studies have been dedicated to developing a suitable hydrogel bioink to satisfy the merit of viscosity for fluent printability, the hardness, and stiffness, to support the subsequent layers [229]. During 3D bioprinting, polymer concentration is the key determinant. A high concentration of polymer can improve the viscosity and mechanical properties of the composite. In contrast, a low concentration may offer better efficiency for cell proliferation and differentiation of osteogenesis and chondrogenesis. The viscosity suitable for print is in the range of 300–30000 cps, which requires the printing concentration of alginate should be in the range of 2–4% [230,231]. However, under this concentration, it is challenging to achieve the hardness and strength of implanted bone only using 3D bioprinted hydrogel. Therefore, many studies suggest employing stiff polymers such as PCL, PLGA, and PVA combined with the 3D bioprinted hydrogels [232]. Indeed, hybrid strategies achieve the balance of desired compression modulus for mandible reconstruction while retaining the biological properties of hydrogels.
Fig. 8.
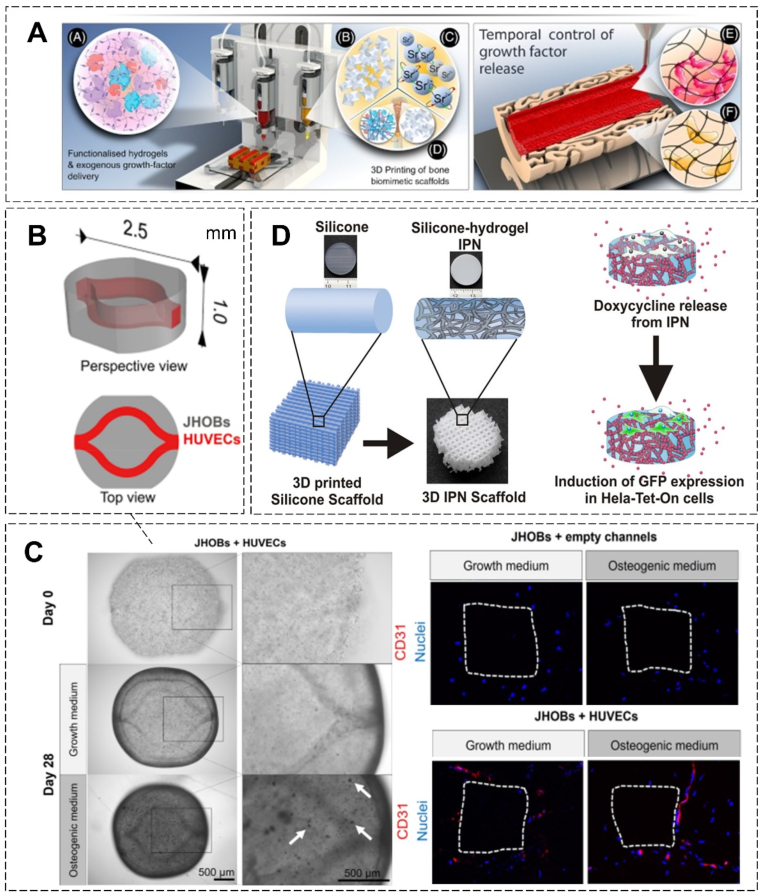
Hydrogel system-based mandibular reconstruction by 3D bioprinting. (A) Multiple-components 3D bioprinting used to generate ‘Print-and-Implant’ scaffold for bone repair (Copyright © 2022 Elsevier Inc., Ref [238]). (B) CAD file of the jawbone model. (C) (Left) Microscopic images of the prints on day 0 and after 28 days of cultivation. (Right) Immunohistochemical staining of CD31 in the bioprinted human jawbone models in vitro, the surface of the constructs is marked with white dotted lines. (B-C, Copyright © 2022 Springer Nature Limited, Ref [235]). (D) 3D Printed silicone–hydrogel interpenetrating polymer network (IPN) with biomolecules delivering and cell fate directing (Copyright © 2022 American Chemical Society, Ref [239]).
3D bioprinting opens new possibilities for site-specific arrangements of multiple cell types and the generation of fine features embedded in structures for mandibular reconstruction [233,234]. This allows for multicellular hydrogel fabrication and different polymers assembling. Constructs are bioprinted containing primary jaw-derived osteoblasts and vasculature-like channel structures harboring primary endothelial cells. The in vitro mandibular construct shows mineralization and hinted at differentiation to an osteocyte phenotype (Fig. 8B–C) [235]. Moreover, 3D printed PCL/hydrogel has been also incorporated with small molecules to target several types of bone cells to fabricate regenerated mandibular constructs. Accompanying with the release of dual drugs (resveratrol and strontium ranelate, RVS, and SrRn), scaffolds significantly promote in vivo mandible formation after 8-week implantation [236]. Besides, the 3D cultural environment provided by the bioprinted hydrogel constructs reflects a more distributed integrin usage and enhanced biological activity, directing the cell fate in proliferation and differentiation (Fig. 8D).
As mentioned, 3D bioprinting also lays down a solid foundation tangible for a promising future of more “personalized” treatment of mandibular reconstruction. Combined with clinical CT imaging and mechanical control technology, a 3D bioprinted biomimetic tissue is fabricated with human amniotic fluid stem cells (hAFSCs)-laden hydrogel for large-scale mandibular reconstruction [237]. Patterning of 3D architecture includes multiple cell-laden hydrogels and supporting PCL polymer. The printing process does not affect cell viability and validates strong osteogenic ability via in vitro experiment [237]. ADSCs encapsulated in the composite structures remains viable within the hydrogel that shows excellent spreading on the 3D printed PLA microstructure and simulates the stiffness of the human mandibular condyle [104]. Descriptions and research outcomes of 3D bioprinted constructs especially with hydrogel involved used for mandibular reconstruction are summarized in Table 4.
Table 4.
3D bioprinted constructs used for mandibular regeneration.
| Type of constructs | Models | Outcome | Ref. |
|---|---|---|---|
| HAp/epoxide acrylate maleic artificial implants | Patients with craniomaxillofacial bone defects | Enhanced aesthetic results and functional recovery | [240] |
| 3D bioprinted PEKK scaffolds combined with ADSCs | Rabbit mandibular defects | Enhanced integration and bone formation | [103] |
| PCL/CS hydrogel with HAp coating custom scaffolds with orthogonal interconnected channels | In vitro | Well mimicking human mandibular condyle | [241] |
| 3D bioprinted bone constructs of PCL/HA and SVF-derived cell (SVFC)-laden hydrogel bioink | In vitro | Successfully microvessel formation in vitro and in vivo. Great promising applications for craniofacial bone defects | [242] |
| 3D bioprinted PCL/TCP scaffolds combined hAFSCs-laden hydrogel with a Pluronic F127 temporary support | In vitro | Successful fabrication of mandible, calvarial bone, cartilage, and skeletal muscle | [237] |
| 3D bioprinted Multicellular (osteoblasts and endothelial cells) GelMA/PEGDA scaffold | In vitro | Successful biofabrication of a human jawbone model | [235] |
| 3D bioprinted PCL/hydrogel scaffold with RVS and SrRn sustained releasing | Rat mandibular defects | Enhanced mandibular bone formation after 8-week implantation | [236] |
6. Challenges and perspectives
Several hydrogel-based applications have received FDA approval and are currently available for clinical use in oral-maxillofacial trauma surgeries. For example, INFUSE® bone graft (Medtronic Sofamor Danek USA, Inc.), a collagen-based hydrogel containing rhBMP-2, has been tested for mandibular reconstruction of structure and function in patients without using iliac crest graft because of the advanced age and comorbidities of the patients [243]. However, some challenges must be overcome before it becomes widely used.
Firstly, there is a conflict between high biocompatibility and poor mechanical strength. Sometimes a compromise in mechanical strength is necessary for using high biocompatible hydrogels as tissue-engineering scaffolds. Although several approaches have been developed to enhance their mechanical properties as we mentioned above, the toxicity of polymers and NCs added during this process, and the inevitable increase of the degradation time, pose risks to bone regeneration. So, hydrogel-related applications are mainly used as independent or composite bone fillers. The load-bearing area still needs to be fixed surgically with metallic materials.
Other concerns are on the uncontrolled release of loaded bioactive factors and stem cells, that is, the burst degradation will lead to burst release. Recently, intelligent hydrogel systems can achieve controllable delivery by sensing stimuli in their external environment and making corresponding changes. In addition to the traditional triggering mechanisms like pH, temperature, and light, hydrogels responsive to biochemical stimuli like enzymes, antigens, and ligands have also been explored [244]. Of note, the more complex functions generate higher cost.
Biosafety and stability should be considered in the whole process of hydrogel production, storage, and transportation [245]. In addition, there is no standardized bone disease model(s) for the current complex hydrogel types to verify their effects, and almost all the hydrogels are not irreplaceable with similar component combinations, gelation mechanisms, and bioactive functions.
Despite the significant challenges that we are facing, the development of hydrogel-based systems for mandibular construction has broadened the scope of craniofacial tissue reconstruction. Such advanced hydrogel systems hold enormous promise for future clinical translations. Based on in-depth understanding of hydrogels, bone regeneration, and their interactions, we will be able to push hydrogel systems toward effective therapy for mandibular reconstruction.
7. Conclusion
Advanced hydrogel systems represent a new series of materials arising from an urgent need in mandibular reconstruction to improve the regenerative capacity and functional recovery stemming from osseous. A comprehensive understanding of the remodeling of mandible and hydrogel design strategies is vital for developing innovative therapeutic strategies with better therapeutic efficacy. The faster bone formation rate of the mandible requires the synchronization of biodegradation and new bone ingrowth as the hydrogels show. This review introduces the hydrogel system designs combined with growth factor delivery, NP enhancement, and cell therapy. Autologous dental stem cells are emphasized as the promising ingredients of hydrogel systems for their easy accessibility and stemness maintenance. Besides, additional considerations include the routine presence of bacterial contamination from the oral and the special aesthetic restoration of facial structures, we highlight that the ideal hydrogels are expected to possess the enhanced mechanical property, antibacterial ability, injectable form via minimally invasive surgical intervention, or especially programmable and 3D bioprinted to suit the mandibular defects under various causative situations. With the continuous progress of material technology and 3D bioprinting manufacturing, we anticipate that in terms of constructing multicellular-meditated osteogenic and irregular bone regeneration, advanced hydrogel systems may lay down the solid foundation tangible for a promising future of more intelligent and personalized treatment in challenging mandibular reconstruction.
Ethics approval
No ethics approvals are needed.
Declaration of competing interest
The authors declare no conflict of interest.
Acknowledgements
The work was supported by the Areas of Excellence Scheme (AoE/M402/20) and Theme-based Research Scheme (T13-402/17 N) under the Research Grant Council of Hong Kong, Mainland-Hong Kong Joint Funding Scheme (MHP/030/20), National Natural Science Foundation of China (81802152 and 32171332), Natural Science Foundation of Guangdong Province (2019A1515012224), Research Grants Council Collaborative Research Fund (C4026-17WF), General Research Fund (14121918 and 14173917), and the Innovation and Technology Commission Funding (Ref No. ITS/208/18FX).
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
Contributor Information
Ling Qin, Email: lingqin@cuhk.edu.hk.
Jiankun Xu, Email: jiankunxu@cuhk.edu.hk.
References
- 1.Hayden R.E., Mullin D.P., Patel A.K. Reconstruction of the segmental mandibular defect: current state of the art. Curr. Opin. Otolaryngol. Head Neck Surg. 2012;20(4):231–236. doi: 10.1097/MOO.0b013e328355d0f3. [DOI] [PubMed] [Google Scholar]
- 2.Paré A., Bossard A., Laure B., Weiss P., Gauthier O., Corre P. Reconstruction of segmental mandibular defects: current procedures and perspectives. Laryngoscope Invest. Otolaryngol. 2019;4(6):587–596. doi: 10.1002/lio2.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kakarala K., Shnayder Y., Tsue T.T., Girod D.A. Mandibular reconstruction. Oral Oncol. 2018;77:111–117. doi: 10.1016/j.oraloncology.2017.12.020. [DOI] [PubMed] [Google Scholar]
- 4.Batstone M. Reconstruction of major defects of the jaws. Aust. Dent. J. 2018;63:S108–S113. doi: 10.1111/adj.12596. [DOI] [PubMed] [Google Scholar]
- 5.Silva A.K., Humphries L.S., Maldonado A.A., Gottlieb L.J. Chimeric vs composite flaps for mandible reconstruction. Head Neck. 2019;41(6):1597–1604. doi: 10.1002/hed.25606. [DOI] [PubMed] [Google Scholar]
- 6.Ling X.F., Peng X. What is the price to pay for a free fibula flap? A systematic review of donor-site morbidity following free fibula flap surgery. Plast. Reconstr. Surg. 2012;129(3):657–674. doi: 10.1097/PRS.0b013e3182402d9a. [DOI] [PubMed] [Google Scholar]
- 7.Momoh A.O., Yu P., Skoracki R.J., Liu S., Feng L., Hanasono M.M. A prospective cohort study of fibula free flap donor-site morbidity in 157 consecutive patients. Plast. Reconstr. Surg. 2011;128(3):714–720. doi: 10.1097/PRS.0b013e318221dc2a. [DOI] [PubMed] [Google Scholar]
- 8.Yue S., He H., Li B., Hou T. Hydrogel as a biomaterial for bone tissue engineering: a review. Nanomaterials. 2020;10(8):1511. doi: 10.3390/nano10081511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bai X., Gao M., Syed S., Zhuang J., Xu X., Zhang X.-Q. Bioactive hydrogels for bone regeneration. Bioact. Mater. 2018;3(4):401–417. doi: 10.1016/j.bioactmat.2018.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tao O., Kort-Mascort J., Lin Y., Pham H.M., Charbonneau A.M., ElKashty O.A., Kinsella J.M., Tran S.D. The applications of 3D printing for craniofacial tissue engineering. Micromachines. 2019;10(7):480. doi: 10.3390/mi10070480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li S., Dong S., Xu W., Tu S., Yan L., Zhao C., Ding J., Chen X. Antibacterial hydrogels. Adv. Sci. 2018;5(5) doi: 10.1002/advs.201700527. 1700527-1700527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mehrotra D., Dwivedi R., Nandana D., Singh R.K. From injectable to 3D printed hydrogels in maxillofacial tissue engineering: a review. J. Oral Biol. Craniofac. Res. 2020;10(4):680–689. doi: 10.1016/j.jobcr.2020.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Akinbami B. Reconstruction of continuity defects of the mandible with non-vascularized bone grafts. Systematic literature review. Craniomaxillofacial Trauma Reconstr. 2016;9(3):195–205. doi: 10.1055/s-0036-1572494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Forbes B.J. Congenital craniofacial anomalies. Curr. Opin. Ophthalmol. 2010;21(5):367–374. doi: 10.1097/ICU.0b013e32833cd422. [DOI] [PubMed] [Google Scholar]
- 15.Thariat J., Julieron M., Brouchet A., Italiano A., Schouman T., Marcy P.-Y., Odin G., Lacout A., Dassonville O., Peyrottes-Birstwisles I. Osteosarcomas of the mandible: are they different from other tumor sites? Crit. Rev. Oncol.-Hematol. 2012;82(3):280–295. doi: 10.1016/j.critrevonc.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 16.Berg B.-I., Juergens P., Soerensen Y., Savic M., Zeilhofer H.-F., Schwenzer-Zimmerer K. Traumatology of the facial skeleton in octogenarian patients: a retrospective analysis of 96 cases. J. Cranio-Maxillofacial Surg. 2014;42(6):870–873. doi: 10.1016/j.jcms.2013.12.007. [DOI] [PubMed] [Google Scholar]
- 17.Zhou R., Shen L., Yang C., Wang L., Guo H., Yang P., Song A. Periodontitis may restrain the mandibular bone healing via disturbing osteogenic and osteoclastic balance. Inflammation. 2018;41(3):972–983. doi: 10.1007/s10753-018-0751-5. [DOI] [PubMed] [Google Scholar]
- 18.Zhu W.-y., Guo J., Yang W.-f., Tao Z.-y., Lan X., Wang L., Xu J., Qin L., Su Y.-x. Biodegradable magnesium implant enhances angiogenesis and alleviates medication-related osteonecrosis of the jaw in rats. J. Orthop.Transl. 2022;33:153–161. doi: 10.1016/j.jot.2022.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seppänen-Kaijansinkko R. 2019. Tissue Engineering in Oral and Maxillofacial Surgery. Springer. [Google Scholar]
- 20.Zhang Y., He J., He B., Huang R., Li M. vol. 17. 2019. (Effect of Tobacco on Periodontal Disease and Oral Cancer, Tobacco Induced Diseases). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nocini P., Saia G., Bettini G., Ragazzo M., Blandamura S., Chiarini L., Bedogni A. Vascularized fibula flap reconstruction of the mandible in bisphosphonate-related osteonecrosis. Eur. J. Surg. Oncol. 2009;35(4):373–379. doi: 10.1016/j.ejso.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 22.Endo T., Ozoe R., Kojima K., Shimooka S. Congenitally missing mandibular incisors and mandibular symphysis morphology. Angle Orthod. 2007;77(6):1079–1084. doi: 10.2319/020106-37.1. [DOI] [PubMed] [Google Scholar]
- 23.Otto S., Abu-Id M.H., Fedele S., Warnke P.H., Becker S.T., Kolk A., Mücke T., Mast G., Köhnke R., Volkmer E. Osteoporosis and bisphosphonates-related osteonecrosis of the jaw: not just a sporadic coincidence–a multi-centre study. J. Cranio-Maxillofacial Surg. 2011;39(4):272–277. doi: 10.1016/j.jcms.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 24.Fliefel R., Tröltzsch M., Kühnisch J., Ehrenfeld M., Otto S. Treatment strategies and outcomes of bisphosphonate-related osteonecrosis of the jaw (BRONJ) with characterization of patients: a systematic review. Int. J. Oral Maxillofac. Surg. 2015;44(5):568–585. doi: 10.1016/j.ijom.2015.01.026. [DOI] [PubMed] [Google Scholar]
- 25.Lo J., O'Ryan F., Gordon N., Yang J., Hui R., Martin D., Hutchinson M., Lathon P., Sanchez G., Silver P. Predicting risk of osteonecrosis of the jaw with oral bisphosphonate exposure (PROBE) investigators. Prevalence of osteonecrosis of the jaw in patients with oral bisphosphonate exposure. J. Oral Maxillofac. Surg. 2010;68(2):243–253. doi: 10.1016/j.joms.2009.03.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.of Thyroid-Head K.S., Force N.S.G.T., Joo Y.-H., Cho J.-K., Koo B.S., Kwon M., Kwon S.K., Kwon S.Y., Kim M.-S., Kim J.K. Guidelines for the surgical management of oral cancer: Korean society of thyroid-head and neck surgery. Clin. Exp. Otorhinolaryngol. 2019;12(2):107. doi: 10.21053/ceo.2018.01816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brierly G.I., Tredinnick S., Lynham A., Woodruff M. Critical sized mandibular defect regeneration in preclinical in vivo models. Curr. Mol. Biol. Rep. 2016;2(2):83–89. [Google Scholar]
- 28.Kumar B.P., Venkatesh V., Kumar K.A.J., Yadav B.Y., Mohan S.R. Mandibular reconstruction: overview. J. Oral Maxillofac. Surg. 2016;15(4):425–441. doi: 10.1007/s12663-015-0766-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bede S.Y.H., Ismael W.K., Hashim E.A. Reconstruction plate-related complications in mandibular continuity defects. Oral Maxillofac. Surg. 2019;23(2):193–199. doi: 10.1007/s10006-019-00762-5. [DOI] [PubMed] [Google Scholar]
- 30.Nørholt S.E., Jensen J., Schou S., Pedersen T.K. Complications after mandibular distraction osteogenesis: a retrospective study of 131 patients. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2011;111(4):420–427. doi: 10.1016/j.tripleo.2010.05.050. [DOI] [PubMed] [Google Scholar]
- 31.Paré A., Bossard A., Laure B., Weiss P., Gauthier O., Corre P. Reconstruction of segmental mandibular defects: current procedures and perspectives. Laryngoscope Invest. Otolaryngol. 2019;4(6):587–596. doi: 10.1002/lio2.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Blocker T., Jr., Stout R.A. Mandibular reconstruction, world war II. Plast. Reconstr. Surg. 1949;4(2):153–156. doi: 10.1097/00006534-194903000-00004. [DOI] [PubMed] [Google Scholar]
- 33.Taylor G.I., Townsend P., Corlett R. Superiority of the deep circumflex iliac vessels as the supply for free groin flaps. Clin. Work. Plast. Reconstr. Surg. 1979;64(6):745–759. doi: 10.1097/00006534-197912000-00001. [DOI] [PubMed] [Google Scholar]
- 34.Takushima A., Harii K., Asato H., Nakatsuka T., Kimata Y. Mandibular reconstruction using microvascular free flaps: a statistical analysis of 178 cases. Plast. Reconstr. Surg. 2001;108(6):1555–1563. doi: 10.1097/00006534-200111000-00018. [DOI] [PubMed] [Google Scholar]
- 35.deFries H.O. Reconstruction of the mandible: use of combined homologous mandible and autologous bone. Otolaryngol. Head Neck Surg.Off. J. Am. Acad. Otolaryngol. Head Neck Surg. 1981;89(4):694–697. doi: 10.1177/019459988108900433. [DOI] [PubMed] [Google Scholar]
- 36.Hidalgo D.A. Fibula free flap: a new method of mandible reconstruction. Plast. Reconstr. Surg. 1989;84(1):71–79. [PubMed] [Google Scholar]
- 37.van Gemert J.T., van Es R.J., Van Cann E.M., Koole R. Nonvascularized bone grafts for segmental reconstruction of the mandible—a reappraisal. J. Oral Maxillofac. Surg. 2009;67(7):1446–1452. doi: 10.1016/j.joms.2008.12.052. [DOI] [PubMed] [Google Scholar]
- 38.White S. The employment of silver wire to bridge the gap after resection of a portion of the lower jaw. Br. Med. J. 1909;2(2552):1525. doi: 10.1136/bmj.2.2552.1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Terz J.J., Bear S.E., Brown P.W., Watkins J., Lawrence W., Jr. Primary reconstruction of the mandible with a wire mesh prosthesis. J. Maxillofac. Surg. 1978;6(2):105–108. doi: 10.1016/s0301-0503(78)80077-0. [DOI] [PubMed] [Google Scholar]
- 40.Dai J., Shen G., Yuan H., Zhang W., Shen S., Shi J. Titanium mesh shaping and fixation for the treatment of comminuted mandibular fractures. J. Oral Maxillofac. Surg. 2016;74(2):337.e1–337.e11. doi: 10.1016/j.joms.2015.10.003. [DOI] [PubMed] [Google Scholar]
- 41.Spencer K.R., Sizeland A., Taylor G.I., Wiesenfeld D. The use of titanium mandibular reconstruction plates in patients with oral cancer. Int. J. Oral Maxillofac. Surg. 1999;28(4):288–290. [PubMed] [Google Scholar]
- 42.Louis P.J., Gutta R., Said-Al-Naief N., Bartolucci A.A. Reconstruction of the maxilla and mandible with particulate bone graft and titanium mesh for implant placement. J. Oral Maxillofac. Surg. 2008;66(2):235–245. doi: 10.1016/j.joms.2007.08.022. [DOI] [PubMed] [Google Scholar]
- 43.Prasadh S., Ratheesh V., Manakari V., Parande G., Gupta M., Wong R. The potential of magnesium based materials in mandibular reconstruction. Metals. 2019;9(3):302. [Google Scholar]
- 44.Lee J.-H., Han H.-S., Kim Y.-C., Lee J.-Y., Lee B.-K. Stability of biodegradable metal (Mg–Ca–Zn alloy) screws compared with absorbable polymer and titanium screws for sagittal split ramus osteotomy of the mandible using the finite element analysis model. J. Cranio-Maxillofacial Surg. 2017;45(10):1639–1646. doi: 10.1016/j.jcms.2017.06.015. [DOI] [PubMed] [Google Scholar]
- 45.Yamada H., Nakaoka K., Sonoyama T., Kumagai K., Ikawa T., Shigeta Y., Harada N., Kawamura N., Ogawa T., Hamada Y. Clinical usefulness of mandibular reconstruction using custom-made titanium mesh tray and autogenous particulate cancellous bone and marrow harvested from tibia and/or ilia. J. Craniofac. Surg. 2016;27(3):586–592. doi: 10.1097/SCS.0000000000002472. [DOI] [PubMed] [Google Scholar]
- 46.Kreutzer K., Steffen C., Koerdt S., Doll C., Ebker T., Nahles S., Flügge T., Heiland M., Beck-Broichsitter B., Rendenbach C. Patient-specific 3D-printed miniplates for free flap fixation at the mandible: a feasibility study. Front. Surg. 2022;9 doi: 10.3389/fsurg.2022.778371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McCarthy J.G., Schreiber J., Karp N., Thorne C.H., Grayson B.H. Lengthening the human mandible by gradual distraction. Plast. Reconstr. Surg. 1992;89(1):1–8. discussion 9-10. [PubMed] [Google Scholar]
- 48.Chin M., Toth B.A. Distraction osteogenesis in maxillofacial surgery using internal devices: review of five cases. J. Oral Maxillofac. Surg. 1996;54(1):45–53. doi: 10.1016/s0278-2391(96)90303-1. discussion 54. [DOI] [PubMed] [Google Scholar]
- 49.Sawaki Y., Hagino H., Yamamoto H., Ueda M. Trifocal distraction osteogenesis for segmental mandibular defect: a technical innovation. J. Cranio-Maxillofacial Surg. 1997;25(6):310–315. doi: 10.1016/s1010-5182(97)80032-7. [DOI] [PubMed] [Google Scholar]
- 50.Hagiwara T., Bell W.H. Effect of electrical stimulation on mandibular distraction osteogenesis. J. Cranio-Maxillofacial Surg. 2000;28(1):12–19. doi: 10.1054/jcms.1999.0104. [DOI] [PubMed] [Google Scholar]
- 51.Khedr M.S., Abdel Al S.E., Osman M.M., Gadala A.S., El-Rakhawy M.M., Eshaq M. The effect of electrical stimulation on mandibular distraction osteogenesis. Egypt. J. Oral Maxillofac. Surg. 2011;2(2) [Google Scholar]
- 52.Ikebe T. Pathophysiology of BRONJ: drug-related osteoclastic disease of the jaw. Oral Sci.Int. 2013;10(1):1–8. [Google Scholar]
- 53.Huja S.S., Fernandez S.A., Hill K.J., Li Y. Remodeling dynamics in the alveolar process in skeletally mature dogs, the anatomical record. Part A Discov. Mol. Cell. Evol. Biol. 2006;288(12):1243–1249. doi: 10.1002/ar.a.20396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kozloff K.M., Volakis L.I., Marini J.C., Caird M.S. Near-infrared fluorescent probe traces bisphosphonate delivery and retention in vivo. J. Bone Miner. Res. : Off. J. Am. Soc. Bone Miner. Res. 2010;25(8):1748–1758. doi: 10.1002/jbmr.66. [DOI] [PubMed] [Google Scholar]
- 55.Li C., Wang F., Zhang R., Qiao P., Liu H. Comparison of proliferation and osteogenic differentiation potential of rat mandibular and femoral bone marrow mesenchymal stem cells in vitro. Stem Cell. Dev. 2020;29(11):728–736. doi: 10.1089/scd.2019.0256. [DOI] [PubMed] [Google Scholar]
- 56.Achilleos A., Trainor P.A. Neural crest stem cells: discovery, properties and potential for therapy. Cell Res. 2012;22(2):288–304. doi: 10.1038/cr.2012.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dong W., Ge J., Zhang P., Fu Y., Zhang Z., Cheng J., Jiang H. Phenotypic characterization of craniofacial bone marrow stromal cells: unique properties of enhanced osteogenesis, cell recruitment, autophagy, and apoptosis resistance. Cell Tissue Res. 2014;358(1):165–175. doi: 10.1007/s00441-014-1927-4. [DOI] [PubMed] [Google Scholar]
- 58.Lee D.J., Kwon J., Current L., Yoon K., Zalal R., Hu X., Xue P., Ko C.C. Osteogenic potential of mesenchymal stem cells from rat mandible to regenerate critical sized calvarial defect. J. Tissue Eng. 2019;10 doi: 10.1177/2041731419830427. 2041731419830427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mavropoulos A., Rizzoli R., Ammann P. Different responsiveness of alveolar and tibial bone to bone loss stimuli. J. Bone Miner. Res. : Off. J. Am. Soc. Bone Miner. Res. 2007;22(3):403–410. doi: 10.1359/jbmr.061208. [DOI] [PubMed] [Google Scholar]
- 60.Lloyd B., Tee B.C., Headley C., Emam H., Mallery S., Sun Z. Similarities and differences between porcine mandibular and limb bone marrow mesenchymal stem cells. Arch. Oral Biol. 2017;77:1–11. doi: 10.1016/j.archoralbio.2017.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Barone A., Covani U. Maxillary alveolar ridge reconstruction with nonvascularized autogenous block bone: clinical results. J. Oral Maxillofac. Surg. 2007;65(10):2039–2046. doi: 10.1016/j.joms.2007.05.017. [DOI] [PubMed] [Google Scholar]
- 62.Leucht P., Kim J.B., Amasha R., James A.W., Girod S., Helms J.A. Embryonic origin and Hox status determine progenitor cell fate during adult bone regeneration. Development (Cambridge, England) 2008;135(17):2845–2854. doi: 10.1242/dev.023788. [DOI] [PubMed] [Google Scholar]
- 63.Rothstein M., Bhattacharya D., Simoes-Costa M. The molecular basis of neural crest axial identity. Dev. Biol. 2018;444:S170–S180. doi: 10.1016/j.ydbio.2018.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Matsuura T., Tokutomi K., Sasaki M., Katafuchi M., Mizumachi E., Sato H. Distinct characteristics of mandibular bone collagen relative to long bone collagen: relevance to clinical dentistry. BioMed Res. Int. 2014 doi: 10.1155/2014/769414. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.van den Bos T., Speijer D., Bank R.A., Brömme D., Everts V. Differences in matrix composition between calvaria and long bone in mice suggest differences in biomechanical properties and resorption: special emphasis on collagen. Bone. 2008;43(3):459–468. doi: 10.1016/j.bone.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 66.Everts V., de Vries T.J., Helfrich M.H. Osteoclast heterogeneity: lessons from osteopetrosis and inflammatory conditions. Biochim. Biophys. Acta. 2009;1792(8):757–765. doi: 10.1016/j.bbadis.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 67.de Souza Faloni A.P., Schoenmaker T., Azari A., Katchburian E., Cerri P.S., de Vries T.J., Everts V. Jaw and long bone marrows have a different osteoclastogenic potential. Calcif. Tissue Int. 2011;88(1):63–74. doi: 10.1007/s00223-010-9418-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Perez-Amodio S., Jansen D.C., Schoenmaker T., Vogels I.M., Reinheckel T., Hayman A.R., Cox T.M., Saftig P., Beertsen W., Everts V. Calvarial osteoclasts express a higher level of tartrate-resistant acid phosphatase than long bone osteoclasts and activation does not depend on cathepsin K or L activity. Calcif. Tissue Int. 2006;79(4):245–254. doi: 10.1007/s00223-005-0289-z. [DOI] [PubMed] [Google Scholar]
- 69.Omi M., Mishina Y. Role of osteoclasts in oral homeostasis and jawbone diseases. Oral Sci. Int. 2020;18(1):14–27. doi: 10.1002/osi2.1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sharma D., Ivanovski S., Slevin M., Hamlet S., Pop T.S., Brinzaniuc K., Petcu E.B., Miroiu R.I. Bisphosphonate-related osteonecrosis of jaw (BRONJ): diagnostic criteria and possible pathogenic mechanisms of an unexpected anti-angiogenic side effect. Vasc. Cell. 2013;5(1):1. doi: 10.1186/2045-824X-5-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Naahidi S., Jafari M., Logan M., Wang Y., Yuan Y., Bae H., Dixon B., Chen P. Biocompatibility of hydrogel-based scaffolds for tissue engineering applications. Biotechnol. Adv. 2017;35(5):530–544. doi: 10.1016/j.biotechadv.2017.05.006. [DOI] [PubMed] [Google Scholar]
- 72.Lee S.-H., Shin H. Matrices and scaffolds for delivery of bioactive molecules in bone and cartilage tissue engineering. Adv. Drug Deliv. Rev. 2007;59(4–5):339–359. doi: 10.1016/j.addr.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 73.Yue S., He H., Li B., Hou T. Hydrogel as a biomaterial for bone tissue engineering: a review. Nanomaterials. 2020;10(8):1511. doi: 10.3390/nano10081511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhao W., Jin X., Cong Y., Liu Y., Fu J. Degradable natural polymer hydrogels for articular cartilage tissue engineering. J. Chem. Technol. Biotechnol. 2013;88(3):327–339. [Google Scholar]
- 75.Madduma‐Bandarage U.S., Madihally S.V. Synthetic hydrogels: synthesis, novel trends, and applications. J. Appl. Polym. Sci. 2021;138(19) [Google Scholar]
- 76.Bustamante-Torres M., Romero-Fierro D., Arcentales-Vera B., Palomino K., Magaña H., Bucio E. Hydrogels classification according to the physical or chemical interactions and as stimuli-sensitive materials. Gels (Basel, Switzerland) 2021;7(4):182. doi: 10.3390/gels7040182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wang H., Heilshorn S.C. Adaptable hydrogel networks with reversible linkages for tissue engineering. Adv. Mater. 2015;27(25):3717–3736. doi: 10.1002/adma.201501558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fan J., Park H., Lee M.K., Bezouglaia O., Fartash A., Kim J., Aghaloo T., Lee M. Adipose-derived stem cells and BMP-2 delivery in chitosan-based 3D constructs to enhance bone regeneration in a rat mandibular defect model. Tissue Eng. 2014;20(15–16):2169–2179. doi: 10.1089/ten.tea.2013.0523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Koshkaryev A., Sawant R., Deshpande M., Torchilin V. Immunoconjugates and long circulating systems: origins, current state of the art and future directions. Adv. Drug Deliv. Rev. 2013;65(1):24–35. doi: 10.1016/j.addr.2012.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Joo V., Ramasamy T., Haidar Z.S. A novel self-assembled liposome-based polymeric hydrogel for cranio-maxillofacial applications: preliminary findings. Polymers. 2011;3(2):967–974. [Google Scholar]
- 81.Sabir M.I., Xu X., Li L. A review on biodegradable polymeric materials for bone tissue engineering applications. J. Mater. Sci. 2009;44(21):5713–5724. [Google Scholar]
- 82.Stuart M.A., Huck W.T., Genzer J., Müller M., Ober C., Stamm M., Sukhorukov G.B., Szleifer I., Tsukruk V.V., Urban M., Winnik F., Zauscher S., Luzinov I., Minko S. Emerging applications of stimuli-responsive polymer materials. Nat. Mater. 2010;9(2):101–113. doi: 10.1038/nmat2614. [DOI] [PubMed] [Google Scholar]
- 83.Kim S.M., Lee B., Yoon H., Suh K.-Y. Stimuli-responsive hydrogel patterns for smart microfluidics and microarrays. Analyst. 2013;138(21):6230–6242. doi: 10.1039/c3an01119d. [DOI] [PubMed] [Google Scholar]
- 84.Lei L., Liu Z., Yuan P., Jin R., Wang X., Jiang T., Chen X. Injectable colloidal hydrogel with mesoporous silica nanoparticles for sustained co-release of microRNA-222 and aspirin to achieve innervated bone regeneration in rat mandibular defects. J. Mater. Chem. B. 2019;7(16):2722–2735. doi: 10.1039/c9tb00025a. [DOI] [PubMed] [Google Scholar]
- 85.Trounson A., McDonald C. Stem cell therapies in clinical trials: progress and challenges. Cell Stem Cell. 2015;17(1):11–22. doi: 10.1016/j.stem.2015.06.007. [DOI] [PubMed] [Google Scholar]
- 86.Kurtz A. Mesenchymal stem cell delivery routes and fate. Int. J. Stem Cell. 2008;1(1):1–7. doi: 10.15283/ijsc.2008.1.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ying J., Han Z., Pei S., Su L., Ruan D. Effects of stromal cell-derived factor-1α secreted in degenerative intervertebral disc on activation and recruitment of nucleus pulposus-derived stem cells. Stem Cell. Int. 2019:2019. doi: 10.1155/2019/9147835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ko I.K., Lee S.J., Atala A., Yoo J.J. In situ tissue regeneration through host stem cell recruitment. Exp. Mol. Med. 2013;45(11):e57. doi: 10.1038/emm.2013.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Liu X., Bao C., Xu H.H., Pan J., Hu J., Wang P., Luo E. Osteoprotegerin gene-modified BMSCs with hydroxyapatite scaffold for treating critical-sized mandibular defects in ovariectomized osteoporotic rats. Acta Biomater. 2016;42:378–388. doi: 10.1016/j.actbio.2016.06.019. [DOI] [PubMed] [Google Scholar]
- 90.Liu T., Xu J., Pan X., Ding Z., Xie H., Wang X., Xie H. Advances of adipose-derived mesenchymal stem cells-based biomaterial scaffolds for oral and maxillofacial tissue engineering. Bioact. Mater. 2021;6(8):2467–2478. doi: 10.1016/j.bioactmat.2021.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Botelho J., Cavacas M.A., Machado V., Mendes J.J. Dental stem cells: recent progresses in tissue engineering and regenerative medicine. Ann. Med. 2017;49(8):644–651. doi: 10.1080/07853890.2017.1347705. [DOI] [PubMed] [Google Scholar]
- 92.Lin H., Sohn J., Shen H., Langhans M.T., Tuan R.S. Bone marrow mesenchymal stem cells: aging and tissue engineering applications to enhance bone healing. Biomaterials. 2019;203:96–110. doi: 10.1016/j.biomaterials.2018.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Liu X., Liao X., Luo E., Chen W., Bao C., Xu H.H. Mesenchymal stem cells systemically injected into femoral marrow of dogs home to mandibular defects to enhance new bone formation. Tissue Eng. 2014;20(3–4):883–892. doi: 10.1089/ten.tea.2012.0677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhang J., Shi H., Zhang N., Hu L., Jing W., Pan J. Interleukin-4-loaded hydrogel scaffold regulates macrophages polarization to promote bone mesenchymal stem cells osteogenic differentiation via TGF-β1/Smad pathway for repair of bone defect. Cell Prolif. 2020;53(10) doi: 10.1111/cpr.12907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yu L., Wu Y., Liu J., Li B., Ma B., Li Y., Huang Z., He Y., Wang H., Wu Z., Qiu G. 3D culture of bone marrow-derived mesenchymal stem cells (BMSCs) could improve bone regeneration in 3D-printed porous Ti6Al4V scaffolds. Stem Cell. Int. 2018;2018 doi: 10.1155/2018/2074021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mazini L., Rochette L., Amine M., Malka G. Regenerative capacity of adipose derived stem cells (ADSCs), comparison with mesenchymal stem cells (MSCs) Int. J. Mol. Sci. 2019;20(10):2523. doi: 10.3390/ijms20102523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ebrahimian T.G., Pouzoulet F., Squiban C., Buard V., André M., Cousin B., Gourmelon P., Benderitter M., Casteilla L., Tamarat R. Cell therapy based on adipose tissue-derived stromal cells promotes physiological and pathological wound healing. Arterioscler. Thromb. Vasc. Biol. 2009;29(4):503–510. doi: 10.1161/ATVBAHA.108.178962. [DOI] [PubMed] [Google Scholar]
- 98.Wilson S.M., Goldwasser M.S., Clark S.G., Monaco E., Bionaz M., Hurley W.L., Rodriguez-Zas S., Feng L., Dymon Z., Wheeler M.B. Adipose-derived mesenchymal stem cells enhance healing of mandibular defects in the ramus of swine. J. Oral Maxillofac. Surg. 2012;70(3):e193–203. doi: 10.1016/j.joms.2011.10.029. [DOI] [PubMed] [Google Scholar]
- 99.Probst F.A., Fliefel R., Burian E., Probst M., Eddicks M., Cornelsen M., Riedl C., Seitz H., Aszódi A., Schieker M., Otto S. Bone regeneration of minipig mandibular defect by adipose derived mesenchymal stem cells seeded tri-calcium phosphate- poly(D,L-lactide-co-glycolide) scaffolds. Sci. Rep. 2020;10(1):2062. doi: 10.1038/s41598-020-59038-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mehrabani D., Khodakaram-Tafti A., Shaterzadeh-Yazdi H., Zamiri B., Omidi M. Comparison of the regenerative effect of adipose-derived stem cells, fibrin glue scaffold, and autologous bone graft in experimental mandibular defect in rabbit. Dent. Traumatol. : Off. Publ. Int. Assoc. Dent. Traumatol. 2018;34(6):413–420. doi: 10.1111/edt.12435. [DOI] [PubMed] [Google Scholar]
- 101.Xu M., Wang X., Yan Y., Yao R., Ge Y. An cell-assembly derived physiological 3D model of the metabolic syndrome, based on adipose-derived stromal cells and a gelatin/alginate/fibrinogen matrix. Biomaterials. 2010;31(14):3868–3877. doi: 10.1016/j.biomaterials.2010.01.111. [DOI] [PubMed] [Google Scholar]
- 102.Wang X., Liu C. 2018. 3D Bioprinting of Adipose-Derived Stem Cells for Organ Manufacturing, Cutting-Edge Enabling Technologies for Regenerative Medicine; pp. 3–14. [DOI] [PubMed] [Google Scholar]
- 103.Roskies M.G., Fang D., Abdallah M.N., Charbonneau A.M., Cohen N., Jordan J.O., Hier M.P., Mlynarek A., Tamimi F., Tran S.D. Three‐dimensionally printed polyetherketoneketone scaffolds with mesenchymal stem cells for the reconstruction of critical‐sized mandibular defects. Laryngoscope. 2017;127(11):E392–E398. doi: 10.1002/lary.26781. [DOI] [PubMed] [Google Scholar]
- 104.Heo D.N., Castro N.J., Lee S.-J., Noh H., Zhu W., Zhang L.G. Enhanced bone tissue regeneration using a 3D printed microstructure incorporated with a hybrid nano hydrogel. Nanoscale. 2017;9(16):5055–5062. doi: 10.1039/c6nr09652b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Jin Q., Yuan K., Lin W., Niu C., Ma R., Huang Z. Comparative characterization of mesenchymal stem cells from human dental pulp and adipose tissue for bone regeneration potential. Artif. Cell Nanomed. Biotechnol. 2019;47(1):1577–1584. doi: 10.1080/21691401.2019.1594861. [DOI] [PubMed] [Google Scholar]
- 106.Martinez Saez D., Sasaki R.T., Neves A.d.C., da Silva M.C.P. Stem cells from human exfoliated deciduous teeth: a growing literature. Cells Tissues Organs. 2016;202(5–6):269–280. doi: 10.1159/000447055. [DOI] [PubMed] [Google Scholar]
- 107.Fawzy El-Sayed K.M., Dörfer C.E. Gingival mesenchymal stem/progenitor cells: a unique tissue engineering gem. Stem Cell. Int. 2016;2016 doi: 10.1155/2016/7154327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zhu W., Liang M. Periodontal ligament stem cells: current status, concerns, and future prospects. Stem Cell. Int. 2015;2015 doi: 10.1155/2015/972313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.d'Aquino R., De Rosa A., Lanza V., Tirino V., Laino L., Graziano A., Desiderio V., Laino G., Papaccio G. Human mandible bone defect repair by the grafting of dental pulp stem/progenitor cells and collagen sponge biocomplexes. Eur. Cell. Mater. 2009;18:75–83. doi: 10.22203/ecm.v018a07. [DOI] [PubMed] [Google Scholar]
- 110.Gutiérrez-Quintero J.G., Durán Riveros J.Y., Martínez Valbuena C.A., Pedraza Alonso S., Munévar J.C., Viafara-García S.M. Critical-sized mandibular defect reconstruction using human dental pulp stem cells in a xenograft model-clinical, radiological, and histological evaluation. Oral Maxillofac. Surg. 2020;24(4):485–493. doi: 10.1007/s10006-020-00862-7. [DOI] [PubMed] [Google Scholar]
- 111.Behnia A., Haghighat A., Talebi A., Nourbakhsh N., Heidari F. Transplantation of stem cells from human exfoliated deciduous teeth for bone regeneration in the dog mandibular defect. World J. Stem Cell. 2014;6(4):505–510. doi: 10.4252/wjsc.v6.i4.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zheng Y., Liu Y., Zhang C.M., Zhang H.Y., Li W.H., Shi S., Le A.D., Wang S.L. Stem cells from deciduous tooth repair mandibular defect in swine. J. Dent. Res. 2009;88(3):249–254. doi: 10.1177/0022034509333804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hasani-Sadrabadi M.M., Sarrion P., Pouraghaei S., Chau Y., Ansari S., Li S., Aghaloo T., Moshaverinia A. An engineered cell-laden adhesive hydrogel promotes craniofacial bone tissue regeneration in rats. Sci. Transl. Med. 2020;12(534) doi: 10.1126/scitranslmed.aay6853. [DOI] [PubMed] [Google Scholar]
- 114.Qiu J., Wang X., Zhou H., Zhang C., Wang Y., Huang J., Liu M., Yang P., Song A. Enhancement of periodontal tissue regeneration by conditioned media from gingiva-derived or periodontal ligament-derived mesenchymal stem cells: a comparative study in rats. Stem Cell Res. Ther. 2020;11(1):42. doi: 10.1186/s13287-019-1546-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ding G., Liu Y., Wang W., Wei F., Liu D., Fan Z., An Y., Zhang C., Wang S. Allogeneic periodontal ligament stem cell therapy for periodontitis in swine. Stem Cell. 2010;28(10):1829–1838. doi: 10.1002/stem.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Liu Y., Zheng Y., Ding G., Fang D., Zhang C., Bartold P.M., Gronthos S., Shi S., Wang S. Periodontal ligament stem cell-mediated treatment for periodontitis in miniature swine. Stem Cell. 2008;26(4):1065–1073. doi: 10.1634/stemcells.2007-0734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Achilleos A., Trainor P.A. Neural crest stem cells: discovery, properties and potential for therapy. Cell Res. 2012;22(2):288–304. doi: 10.1038/cr.2012.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Nuti N., Corallo C., Chan B., Ferrari M., Gerami-Naini B. Multipotent differentiation of human dental pulp stem cells: a literature review. Stem Cell Rev. Rep. 2016;12(5):511–523. doi: 10.1007/s12015-016-9661-9. [DOI] [PubMed] [Google Scholar]
- 119.Choe G., Park J., Park H., Lee J.Y. Hydrogel biomaterials for stem cell microencapsulation. Polymers. 2018;10(9):997. doi: 10.3390/polym10090997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Vishwanath V.R., Nadig R.R., Nadig R., Prasanna J.S., Karthik J., Pai V.S. Differentiation of isolated and characterized human dental pulp stem cells and stem cells from human exfoliated deciduous teeth: an in vitro study. J. Conserv. Dent.: J. Comput. Dynam. 2013;16(5):423. doi: 10.4103/0972-0707.117509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Xu Q.C., Wang Z.G., Ji Q.X., Yu X.B., Xu X.Y., Yuan C.Q., Deng J., Yang P.S. Systemically transplanted human gingiva-derived mesenchymal stem cells contributing to bone tissue regeneration. Int. J. Clin. Exp. Pathol. 2014;7(8):4922–4929. [PMC free article] [PubMed] [Google Scholar]
- 122.Thoniyot P., Tan M.J., Karim A.A., Young D.J., Loh X.J. Nanoparticle-hydrogel composites: concept, design, and applications of these promising, multi-functional materials. Adv. Sci. 2015;2(1–2) doi: 10.1002/advs.201400010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Burdușel A.C., Gherasim O., Andronescu E., Grumezescu A.M., Ficai A. Inorganic nanoparticles in bone healing applications. Pharmaceutics. 2022;14(4) doi: 10.3390/pharmaceutics14040770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Martin V., Ribeiro I.A., Alves M.M., Gonçalves L., Claudio R.A., Grenho L., Fernandes M.H., Gomes P., Santos C.F., Bettencourt A.F. Engineering a multifunctional 3D-printed PLA-collagen-minocycline-nanoHydroxyapatite scaffold with combined antimicrobial and osteogenic effects for bone regeneration. Mater. Sci. Eng. C. 2019;101:15–26. doi: 10.1016/j.msec.2019.03.056. [DOI] [PubMed] [Google Scholar]
- 125.El-Fiqi A., Kim J.H., Kim H.W. Osteoinductive fibrous scaffolds of biopolymer/mesoporous bioactive glass nanocarriers with excellent bioactivity and long-term delivery of osteogenic drug. ACS Appl. Mater. Interfaces. 2015;7(2):1140–1152. doi: 10.1021/am5077759. [DOI] [PubMed] [Google Scholar]
- 126.Takanche J.S., Kim J.-E., Kim J.-S., Lee M.-H., Jeon J.-G., Park I.-S., Yi H.-K. Chitosan-gold nanoparticles mediated gene delivery of c-myb facilitates osseointegration of dental implants in ovariectomized rat, Artificial Cells. Nanomed. Biotechnol. 2018;46(sup3):S807–S817. doi: 10.1080/21691401.2018.1513940. [DOI] [PubMed] [Google Scholar]
- 127.Bhattarai G., Lee Y.H., Yi H.K. Peroxisome proliferator activated receptor gamma loaded dental implant improves osteogenesis of rat mandible. J. Biomed. Mater. Res. B Appl. Biomater. 2015;103(3):587–595. doi: 10.1002/jbm.b.33207. [DOI] [PubMed] [Google Scholar]
- 128.Dulany K., Hepburn K., Goins A., Allen J.B. In vitro and in vivo biocompatibility assessment of free radical scavenging nanocomposite scaffolds for bone tissue regeneration. J. Biomed. Mater. Res. 2020;108(2):301–315. doi: 10.1002/jbm.a.36816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Wu Y., Li X., Sun Y., Tan X., Wang C., Wang Z., Ye L. Multiscale design of stiffening and ROS scavenging hydrogels for the augmentation of mandibular bone regeneration. Bioact. Mater. 2023;20:111–125. doi: 10.1016/j.bioactmat.2022.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Chang Y.K., Liu Y.P., Ho J.H., Hsu S.C., Lee O.K. Amine-surface-modified superparamagnetic iron oxide nanoparticles interfere with differentiation of human mesenchymal stem cells. J. Orthop. Res. 2012;30(9):1499–1506. doi: 10.1002/jor.22088. [DOI] [PubMed] [Google Scholar]
- 131.Meir R., Motiei M., Popovtzer R. Gold nanoparticles for in vivo cell tracking. Nanomedicine (London, England) 2014;9(13):2059–2069. doi: 10.2217/nnm.14.129. [DOI] [PubMed] [Google Scholar]
- 132.Shen Y., Shao Y., He H., Tan Y., Tian X., Xie F., Li L. Gadolinium(3+)-doped mesoporous silica nanoparticles as a potential magnetic resonance tracer for monitoring the migration of stem cells in vivo. Int. J. Nanomed. 2013;8:119–127. doi: 10.2147/IJN.S38213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Bhirde A., Xie J., Swierczewska M., Chen X. Nanoparticles for cell labeling. Nanoscale. 2011;3(1):142–153. doi: 10.1039/c0nr00493f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Li X., Dai B., Guo J., Zheng L., Guo Q., Peng J., Xu J., Qin L. Nanoparticle–Cartilage interaction: pathology-based intra-articular drug delivery for osteoarthritis therapy. Nano-Micro Lett. 2021;13(1):1–48. doi: 10.1007/s40820-021-00670-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Hu S., Zhou Y., Zhao Y., Xu Y., Zhang F., Gu N., Ma J., Reynolds M.A., Xia Y., Xu H.H. Enhanced bone regeneration and visual monitoring via superparamagnetic iron oxide nanoparticle scaffold in rats. J. Tissue Eng. Regen. Med. 2018;12(4):e2085–e2098. doi: 10.1002/term.2641. [DOI] [PubMed] [Google Scholar]
- 136.Dreyer C.H., Kjaergaard K., Ding M., Qin L. Vascular endothelial growth factor for in vivo bone formation: a systematic review. J. Orthop. Translat. 2020;24:46–57. doi: 10.1016/j.jot.2020.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Sheikh Z., Javaid M.A., Hamdan N., Hashmi R. Bone regeneration using bone morphogenetic proteins and various biomaterial carriers. Materials. 2015;8(4):1778–1816. doi: 10.3390/ma8041778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Schliephake H., Weich H.A., Dullin C., Gruber R., Frahse S. Mandibular bone repair by implantation of rhBMP-2 in a slow release carrier of polylactic acid--an experimental study in rats. Biomaterials. 2008;29(1):103–110. doi: 10.1016/j.biomaterials.2007.09.019. [DOI] [PubMed] [Google Scholar]
- 139.Lohse N., Moser N., Backhaus S., Annen T., Epple M., Schliephake H. Continuous delivery of rhBMP2 and rhVEGF165 at a certain ratio enhances bone formation in mandibular defects over the delivery of rhBMP2 alone--An experimental study in rats. J. Contr. Release : Off. J. Control. Release Soc. 2015;220(Pt A):201–209. doi: 10.1016/j.jconrel.2015.10.032. [DOI] [PubMed] [Google Scholar]
- 140.Park J.B., Lee J.Y., Park Y.J., Rhee S.H., Lee S.C., Kim T.I., Seol Y.J., Lee Y.M., Ku Y., Rhyu I.C., Han S.B., Chung C.P. Enhanced bone regeneration in beagle dogs with bovine bone mineral coated with a synthetic oligopeptide. J. Periodontol. 2007;78(11):2150–2155. doi: 10.1902/jop.2007.070108. [DOI] [PubMed] [Google Scholar]
- 141.Herford A.S., Stoffella E., Tandon R. Reconstruction of mandibular defects using bone morphogenic protein: can growth factors replace the need for autologous bone grafts? A systematic review of the literature. Plast. Surg. Int. 2011;2011 doi: 10.1155/2011/165824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Boyne P.J., Lilly L.C., Marx R.E., Moy P.K., Nevins M., Spagnoli D.B., Triplett R.G. De novo bone induction by recombinant human bone morphogenetic protein-2 (rhBMP-2) in maxillary sinus floor augmentation. J. Oral Maxillofac. Surg. 2005;63(12):1693–1707. doi: 10.1016/j.joms.2005.08.018. [DOI] [PubMed] [Google Scholar]
- 143.Triplett R.G., Nevins M., Marx R.E., Spagnoli D.B., Oates T.W., Moy P.K., Boyne P.J. Pivotal, randomized, parallel evaluation of recombinant human bone morphogenetic protein-2/absorbable collagen sponge and autogenous bone graft for maxillary sinus floor augmentation. J. Oral Maxillofac. Surg. 2009;67(9):1947–1960. doi: 10.1016/j.joms.2009.04.085. [DOI] [PubMed] [Google Scholar]
- 144.Tatara A.M., Koons G.L., Watson E., Piepergerdes T.C., Shah S.R., Smith B.T., Shum J., Melville J.C., Hanna I.A., Demian N. Biomaterials-aided mandibular reconstruction using in vivo bioreactors. Proc. Natl. Acad. Sci. USA. 2019;116(14):6954–6963. doi: 10.1073/pnas.1819246116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Herford A.S., Stoffella E., Tandon R. Reconstruction of mandibular defects using bone morphogenic protein: can growth factors replace the need for autologous bone grafts? A systematic review of the literature. Plast. Surg. Int. 2011;2011 doi: 10.1155/2011/165824. 165824-165824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Ribeiro Filho S.A., Francischone C.E., de Oliveira J.C., Ribeiro L.Z., do Prado F.Z.X., Sotto-Maior B.S. Bone augmentation of the atrophic anterior maxilla for dental implants using rhBMP-2 and titanium mesh: histological and tomographic analysis. Int. J. Oral Maxillofac. Surg. 2015;44(12):1492–1498. doi: 10.1016/j.ijom.2015.07.004. [DOI] [PubMed] [Google Scholar]
- 147.Martínez-Sanz E., Varghese O.P., Kisiel M., Engstrand T., Reich K.M., Bohner M., Jonsson K.B., Kohler T., Müller R., Ossipov D.A., Hilborn J. Minimally invasive mandibular bone augmentation using injectable hydrogels. J. Tissue Eng. Regen. Med. 2012;6(Suppl 3):s15–23. doi: 10.1002/term.1593. [DOI] [PubMed] [Google Scholar]
- 148.Cao J., Wang L., Lei D.-l., Liu Y.-P., Du Z.-j., Cui F.-Z. Local injection of nerve growth factor via a hydrogel enhances bone formation during mandibular distraction osteogenesis. J. Surg.Oral Med. Oral Pathol. Oral Radiol. 2012;113(1):48–53. doi: 10.1016/j.tripleo.2011.01.021. [DOI] [PubMed] [Google Scholar]
- 149.Kim J., Yang H.J., Cho T.H., Lee S.E., Park Y.D., Kim H.M., Kim I.S., Seo Y.K., Hwang S.J., Kim S.J. Enhanced regeneration of rabbit mandibular defects through a combined treatment of electrical stimulation and rhBMP-2 application. Med. Biol. Eng. Comput. 2013;51(12):1339–1348. doi: 10.1007/s11517-013-1106-x. [DOI] [PubMed] [Google Scholar]
- 150.Gruber R.M., Krohn S., Mauth C., Dard M., Molenberg A., Lange K., Perske C., Schliephake H. Mandibular reconstruction using a calcium phosphate/polyethylene glycol hydrogel carrier with BMP-2. J. Clin. Periodontol. 2014;41(8):820–826. doi: 10.1111/jcpe.12264. [DOI] [PubMed] [Google Scholar]
- 151.Song W.Y., Liu G.M., Li J., Luo Y.G. Bone morphogenetic protein-2 sustained delivery by hydrogels with microspheres repairs rabbit mandibular defects. J. Tissue Eng. Regen. Med. 2016;13(6):750–761. doi: 10.1007/s13770-016-9123-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Song Y., Zhang C., Wang P., Wang L., Bao C., Weir M.D., Reynolds M.A., Ren K., Zhao L., Xu H.H.K. Engineering bone regeneration with novel cell-laden hydrogel microfiber-injectable calcium phosphate scaffold, Materials science & engineering. C, Mater.Biol. Appl. 2017;75:895–905. doi: 10.1016/j.msec.2017.02.158. [DOI] [PubMed] [Google Scholar]
- 153.Chen L., Shen R., Komasa S., Xue Y., Jin B., Hou Y., Okazaki J., Gao J. Drug-loadable calcium alginate hydrogel system for use in oral bone tissue repair. Int. J. Mol. Sci. 2017;18(5) doi: 10.3390/ijms18050989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Fahmy-Garcia S., Mumcuoglu D., de Miguel L., Dieleman V., Witte-Bouma J., van der Eerden B.C.J., van Driel M., Eglin D., Verhaar J.A.N., Kluijtmans S., van Osch G., Farrell E. Novel in situ gelling hydrogels loaded with recombinant collagen peptide microspheres as a slow-release system induce ectopic bone formation. Adv. Healthc. Mater. 2018;7(21) doi: 10.1002/adhm.201800507. [DOI] [PubMed] [Google Scholar]
- 155.Jung S.W., Byun J.H., Oh S.H., Kim T.H., Park J.S., Rho G.J., Lee J.H. Multivalent ion-based in situ gelling polysaccharide hydrogel as an injectable bone graft. Carbohydr. Polym. 2018;180:216–225. doi: 10.1016/j.carbpol.2017.10.029. [DOI] [PubMed] [Google Scholar]
- 156.Jung S.W., Oh S.H., Lee I.S., Byun J.H., Lee J.H. In situ gelling hydrogel with anti-bacterial activity and bone healing property for treatment of osteomyelitis. J. Tissue Eng. Regen. Med. 2019;16(5):479–490. doi: 10.1007/s13770-019-00206-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Tan J., Zhang M., Hai Z., Wu C., Lin J., Kuang W., Tang H., Huang Y., Chen X., Liang G. Sustained release of two bioactive factors from supramolecular hydrogel promotes periodontal bone regeneration. ACS Nano. 2019;13(5):5616–5622. doi: 10.1021/acsnano.9b00788. [DOI] [PubMed] [Google Scholar]
- 158.Imada M., Yagyuu T., Ueyama Y., Maeda M., Yamamoto K., Kurokawa S., Jo J.I., Tabata Y., Tanaka Y., Kirita T. Prevention of tooth extraction-triggered bisphosphonate-related osteonecrosis of the jaws with basic fibroblast growth factor: an experimental study in rats. PLoS One. 2019;14(2) doi: 10.1371/journal.pone.0211928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Lei L., Liu Z., Yuan P., Jin R., Wang X., Jiang T., Chen X. Injectable colloidal hydrogel with mesoporous silica nanoparticles for sustained co-release of microRNA-222 and aspirin to achieve innervated bone regeneration in rat mandibular defects. J. Mater. Chem. B. 2019;7(16):2722–2735. doi: 10.1039/c9tb00025a. [DOI] [PubMed] [Google Scholar]
- 160.Pan Y., Zhao Y., Kuang R., Liu H., Sun D., Mao T., Jiang K., Yang X., Watanabe N., Mayo K.H., Lin Q., Li J. Injectable hydrogel-loaded nano-hydroxyapatite that improves bone regeneration and alveolar ridge promotion, Materials science & engineering. C, Mater.Biol. Appl. 2020;116 doi: 10.1016/j.msec.2020.111158. [DOI] [PubMed] [Google Scholar]
- 161.Wang T., Guo S., Zhang H., Chen Y., Cai Y. Injectable hydrogel delivering bone morphogenetic protein-2, vascular endothelial growth factor, and adipose-derived stem cells for vascularized bone tissue engineering. J. Drug Deliv. Sci. Technol. 2020;57 [Google Scholar]
- 162.Salzlechner C., Haghighi T., Huebscher I., Walther A.R., Schell S., Gardner A., Undt G., da Silva R.M.P., Dreiss C.A., Fan K., Gentleman E. Adhesive hydrogels for maxillofacial tissue regeneration using minimally invasive procedures. Adv. Healthc. Mater. 2020;9(4) doi: 10.1002/adhm.201901134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Lyu H.Z., Lee J.H. The efficacy of rhBMP-2 loaded hydrogel composite on bone formation around dental implants in mandible bone defects of minipigs. Biomater. Res. 2020;24:5. doi: 10.1186/s40824-020-0183-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Wong R.C., Tideman H., Kin L., Merkx M.A. Biomechanics of mandibular reconstruction: a review. Int. J. Oral Maxillofac. Surg. 2010;39(4):313–319. doi: 10.1016/j.ijom.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 165.Bao Z., Xian C., Yuan Q., Liu G., Wu J. Natural polymer‐based hydrogels with enhanced mechanical performances: preparation, structure, and property. Adv. Healthc. Mater. 2019;8(17) doi: 10.1002/adhm.201900670. [DOI] [PubMed] [Google Scholar]
- 166.Gong J.P., Katsuyama Y., Kurokawa T., Osada Y. Double‐network hydrogels with extremely high mechanical strength. Adv. Mater. 2003;15(14):1155–1158. [Google Scholar]
- 167.Gong J.P. Why are double network hydrogels so tough? Soft Matter. 2010;6(12):2583–2590. [Google Scholar]
- 168.Zhang X., Huang P., Jiang G., Zhang M., Yu F., Dong X., Wang L., Chen Y., Zhang W., Qi Y., Li W., Zeng H. A novel magnesium ion-incorporating dual-crosslinked hydrogel to improve bone scaffold-mediated osteogenesis and angiogenesis. Mater. Sci. Eng. C. 2021;121 doi: 10.1016/j.msec.2021.111868. [DOI] [PubMed] [Google Scholar]
- 169.Bi S., Wang P., Hu S., Li S., Pang J., Zhou Z., Sun G., Huang L., Cheng X., Xing S., Chen X. Construction of physical-crosslink chitosan/PVA double-network hydrogel with surface mineralization for bone repair. Carbohydr. Polym. 2019;224 doi: 10.1016/j.carbpol.2019.115176. [DOI] [PubMed] [Google Scholar]
- 170.Thoniyot P., Tan M.J., Karim A.A., Young D.J., Loh X.J. Nanoparticle-hydrogel composites: concept, design, and applications of these promising, multi-functional materials. Adv. Sci. 2015;2(1–2) doi: 10.1002/advs.201400010. 1400010-1400010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Li Z., Mi W., Wang H., Su Y., He C. Nano-hydroxyapatite/polyacrylamide composite hydrogels with high mechanical strengths and cell adhesion properties. Colloids Surf. B Biointerfaces. 2014;123:959–964. doi: 10.1016/j.colsurfb.2014.10.050. [DOI] [PubMed] [Google Scholar]
- 172.Martínez‐Sanz E., Varghese O.P., Kisiel M., Engstrand T., Reich K.M., Bohner M., Jonsson K.B., Kohler T., Müller R., Ossipov D.A. Minimally invasive mandibular bone augmentation using injectable hydrogels. J. Tissue Eng. Regen. Med. 2012;6(S3):s15–s23. doi: 10.1002/term.1593. [DOI] [PubMed] [Google Scholar]
- 173.Burghardt I., Lüthen F., Prinz C., Kreikemeyer B., Zietz C., Neumann H.-G., Rychly J. A dual function of copper in designing regenerative implants. Biomaterials. 2015;44:36–44. doi: 10.1016/j.biomaterials.2014.12.022. [DOI] [PubMed] [Google Scholar]
- 174.Irie K., Novince C.M., Darveau R.P. Impact of the oral commensal flora on alveolar bone homeostasis. J. Dent. Res. 2014;93(8):801–806. doi: 10.1177/0022034514540173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Chang E., Kobayashi R., Fujihashi K., Komiya M., Kurita-Ochiai T. Impaired salivary SIgA antibodies elicit oral dysbiosis and subsequent induction of alveolar bone loss. Inflamm. Res. 2021;70(1):151–158. doi: 10.1007/s00011-020-01418-x. [DOI] [PubMed] [Google Scholar]
- 176.Marschall J.S., Flint R.L., Kushner G.M., Alpert B. Management of mandibular osteomyelitis with segmental resection, nerve preservation, and immediate reconstruction. J. Oral Maxillofac. Surg. 2019;77(7):1490–1504. doi: 10.1016/j.joms.2019.01.036. [DOI] [PubMed] [Google Scholar]
- 177.Wang L., Hu C., Shao L. The antimicrobial activity of nanoparticles: present situation and prospects for the future. Int. J. Nanomed. 2017;12:1227–1249. doi: 10.2147/IJN.S121956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Qing Y., Cheng L., Li R., Liu G., Zhang Y., Tang X., Wang J., Liu H., Qin Y. Potential antibacterial mechanism of silver nanoparticles and the optimization of orthopedic implants by advanced modification technologies. Int. J. Nanomed. 2018;13:3311–3327. doi: 10.2147/IJN.S165125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Coelho C.C., Araújo R., Quadros P.A., Sousa S.R., Monteiro F.J. Antibacterial bone substitute of hydroxyapatite and magnesium oxide to prevent dental and orthopaedic infections. Mater. Sci. Eng. C. 2019;97:529–538. doi: 10.1016/j.msec.2018.12.059. [DOI] [PubMed] [Google Scholar]
- 180.Chen J., Zhang X., Cai H., Chen Z., Wang T., Jia L., Wang J., Wan Q., Pei X. Osteogenic activity and antibacterial effect of zinc oxide/carboxylated graphene oxide nanocomposites: preparation and in vitro evaluation. Colloids Surf. B Biointerfaces. 2016;147:397–407. doi: 10.1016/j.colsurfb.2016.08.023. [DOI] [PubMed] [Google Scholar]
- 181.Subhapriya S., Gomathipriya P. Green synthesis of titanium dioxide (TiO2) nanoparticles by Trigonella foenum-graecum extract and its antimicrobial properties. Microb. Pathog. 2018;116:215–220. doi: 10.1016/j.micpath.2018.01.027. [DOI] [PubMed] [Google Scholar]
- 182.Moritz M., Geszke-Moritz M. The newest achievements in synthesis, immobilization and practical applications of antibacterial nanoparticles. Chem. Eng. J. 2013;228:596–613. [Google Scholar]
- 183.Qiao S., Wu D., Li Z., Zhu Y., Zhan F., Lai H., Gu Y. The combination of multi-functional ingredients-loaded hydrogels and three-dimensional printed porous titanium alloys for infective bone defect treatment. J. Tissue Eng. 2020;11 doi: 10.1177/2041731420965797. 2041731420965797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Liu L., Xiang Y., Wang Z., Yang X., Yu X., Lu Y., Deng L., Cui W. Adhesive liposomes loaded onto an injectable, self-healing and antibacterial hydrogel for promoting bone reconstruction. NPG Asia Mater. 2019;11(1):81. [Google Scholar]
- 185.Yang K., Han Q., Chen B., Zheng Y., Zhang K., Li Q., Wang J. Antimicrobial hydrogels: promising materials for medical application. Int. J. Nanomed. 2018;13:2217–2263. doi: 10.2147/IJN.S154748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Eltawila A.M., Hassan M.N., Safaan S.M., Abd El‐Fattah A., Zakaria O., El‐Khordagui L.K., Kandil S. Local treatment of experimental mandibular osteomyelitis with an injectable biomimetic gentamicin hydrogel using a new rabbit model. J. Biomed. Mater. Res. B Appl. Biomater. 2021;109(11):1677–1688. doi: 10.1002/jbm.b.34824. [DOI] [PubMed] [Google Scholar]
- 187.Shi M., Kretlow J.D., Spicer P.P., Tabata Y., Demian N., Wong M.E., Kasper F.K., Mikos A.G. Antibiotic-releasing porous polymethylmethacrylate/gelatin/antibiotic constructs for craniofacial tissue engineering. J. Contr. Release. 2011;152(1):196–205. doi: 10.1016/j.jconrel.2011.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Grillo R., Pedrosa G., Teixeira R.G., Mandic L. Protective hydrogel to heal post maxillofacial surgery paresthesia? Ann. Oral Maxillofac Surg. 2021;2 [Google Scholar]
- 189.Carpa R., Remizovschi A., Culda C.A., Butiuc-Keul A.L. Inherent and composite hydrogels as promising materials to limit antimicrobial resistance. Gels. 2022;8(2):70. doi: 10.3390/gels8020070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Sui X., Feng X., Di Luca A., van Blitterswijk C.A., Moroni L., Hempenius M.A., Vancso G.J. Poly (N-isopropylacrylamide)–poly (ferrocenylsilane) dual-responsive hydrogels: synthesis, characterization and antimicrobial applications. Polym. Chem. 2013;4(2):337–342. [Google Scholar]
- 192.Shen W., He P., Xiao C., Chen X. From antimicrobial peptides to antimicrobial poly (α‐amino acid) s. Adv. Healthc. Mater. 2018;7(20) doi: 10.1002/adhm.201800354. [DOI] [PubMed] [Google Scholar]
- 193.Sultan A.S., Vila T., Hefni E., Karlsson A.J., Jabra-Rizk M.A. Evaluation of the antifungal and wound-healing properties of a novel peptide-based bioadhesive hydrogel formulation. Antimicrob. Agents Chemother. 2019;63(10) doi: 10.1128/AAC.00888-19. e00888-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Marchesan S., Qu Y., Waddington L.J., Easton C.D., Glattauer V., Lithgow T.J., McLean K.M., Forsythe J.S., Hartley P.G. Self-assembly of ciprofloxacin and a tripeptide into an antimicrobial nanostructured hydrogel. Biomaterials. 2013;34(14):3678–3687. doi: 10.1016/j.biomaterials.2013.01.096. [DOI] [PubMed] [Google Scholar]
- 195.Sani E.S., Lara R.P., Aldawood Z., Bassir S.H., Nguyen D., Kantarci A., Intini G., Annabi N. An antimicrobial dental light curable bioadhesive hydrogel for treatment of peri-implant diseases. Matter. 2019;1(4):926–944. doi: 10.1016/j.matt.2019.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.He Y., Ingudam S., Reed S., Gehring A., Strobaugh T.P., Irwin P. Study on the mechanism of antibacterial action of magnesium oxide nanoparticles against foodborne pathogens. J. Nanobiotechnol. 2016;14(1):54. doi: 10.1186/s12951-016-0202-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Gustafson C.T., Boakye-Agyeman F., Brinkman C.L., Reid J.M., Patel R., Bajzer Z., Dadsetan M., Yaszemski M.J. Controlled delivery of vancomycin via charged hydrogels. PLoS One. 2016;11(1) doi: 10.1371/journal.pone.0146401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Zhang C., Yang M. Antimicrobial peptides: from design to clinical application. Antibiotics. 2022;11(3):349. doi: 10.3390/antibiotics11030349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Matichescu A., Ardelean L.C., Rusu L.-C., Craciun D., Bratu E.A., Babucea M., Leretter M. Advanced biomaterials and techniques for oral tissue engineering and regeneration—a review. Materials. 2020;13(22):5303. doi: 10.3390/ma13225303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Kretlow J.D., Young S., Klouda L., Wong M., Mikos A.G. Injectable biomaterials for regenerating complex craniofacial tissues. Adv. Mater. 2009;21(32‐33):3368–3393. doi: 10.1002/adma.200802009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Imran A.B., Seki T., Takeoka Y. Recent advances in hydrogels in terms of fast stimuli responsiveness and superior mechanical performance. Polym. J. 2010;42(11):839–851. [Google Scholar]
- 202.Zou Z., Zhang B., Nie X., Cheng Y., Hu Z., Liao M., Li S. A sodium alginate-based sustained-release IPN hydrogel and its applications. RSC Adv. 2020;10(65):39722–39730. doi: 10.1039/d0ra04316h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Hernández-González A.C., Téllez-Jurado L., Rodríguez-Lorenzo L.M. Alginate hydrogels for bone tissue engineering, from injectables to bioprinting: a review. Carbohydr. Polym. 2020;229 doi: 10.1016/j.carbpol.2019.115514. [DOI] [PubMed] [Google Scholar]
- 204.Raucci M.G., D'Amora U., Ronca A., Ambrosio L. Injectable functional biomaterials for minimally invasive surgery. Adv. Healthc. Mater. 2020;9(13) doi: 10.1002/adhm.202000349. [DOI] [PubMed] [Google Scholar]
- 205.Bidarra S.J., Barrias C.C., Granja P.L. Injectable alginate hydrogels for cell delivery in tissue engineering. Acta Biomater. 2014;10(4):1646–1662. doi: 10.1016/j.actbio.2013.12.006. [DOI] [PubMed] [Google Scholar]
- 206.Chen L., Shen R., Komasa S., Xue Y., Jin B., Hou Y., Okazaki J., Gao J. Drug-loadable calcium alginate hydrogel system for use in oral bone tissue repair. Int. J. Mol. Sci. 2017;18(5):989. doi: 10.3390/ijms18050989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Singh N.K., Lee D.S. In situ gelling pH-and temperature-sensitive biodegradable block copolymer hydrogels for drug delivery. J. Contr. Release. 2014;193:214–227. doi: 10.1016/j.jconrel.2014.04.056. [DOI] [PubMed] [Google Scholar]
- 208.Kim Y.-J., Matsunaga Y.T. Thermo-responsive polymers and their application as smart biomaterials. J. Mater. Chem. B. 2017;5(23):4307–4321. doi: 10.1039/c7tb00157f. [DOI] [PubMed] [Google Scholar]
- 209.Yang L., Fan X., Zhang J., Ju J. Preparation and characterization of thermoresponsive poly(N-isopropylacrylamide) for cell culture applications. Polymers (Basel) 2020;12(2) doi: 10.3390/polym12020389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Li X., Zhou J., Liu Z., Chen J., Lü S., Sun H., Li J., Lin Q., Yang B., Duan C. A PNIPAAm-based thermosensitive hydrogel containing SWCNTs for stem cell transplantation in myocardial repair. Biomaterials. 2014;35(22):5679–5688. doi: 10.1016/j.biomaterials.2014.03.067. [DOI] [PubMed] [Google Scholar]
- 211.Rey-Rico A., Cucchiarini M. PEO-PPO-PEO tri-block copolymers for gene delivery applications in human regenerative medicine—an overview. Int. J. Mol. Sci. 2018;19(3):775. doi: 10.3390/ijms19030775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Dou Q.Q., Liow S.S., Ye E., Lakshminarayanan R., Loh X.J. Biodegradable thermogelling polymers: working towards clinical applications. Adv. Healthc. Mater. 2014;3(7):977–988. doi: 10.1002/adhm.201300627. [DOI] [PubMed] [Google Scholar]
- 213.Ren Z., Wang Y., Ma S., Duan S., Yang X., Gao P., Zhang X., Cai Q. Effective bone regeneration using thermosensitive poly(N-isopropylacrylamide) grafted gelatin as injectable carrier for bone mesenchymal stem cells. ACS Appl. Mater. Interfaces. 2015;7(34):19006–19015. doi: 10.1021/acsami.5b02821. [DOI] [PubMed] [Google Scholar]
- 214.Appel E.A., Tibbitt M.W., Webber M.J., Mattix B.A., Veiseh O., Langer R. Self-assembled hydrogels utilizing polymer–nanoparticle interactions. Nat. Commun. 2015;6(1):1–9. doi: 10.1038/ncomms7295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Zhang Z., Ai S., Yang Z., Li X. Peptide-based supramolecular hydrogels for local drug delivery. Adv. Drug Deliv. Rev. 2021;174:482–503. doi: 10.1016/j.addr.2021.05.010. [DOI] [PubMed] [Google Scholar]
- 216.Li J. Self-assembled supramolecular hydrogels based on polymer–cyclodextrin inclusion complexes for drug delivery. NPG Asia Mater. 2010;2(3):112–118. [Google Scholar]
- 217.Sun H., Li Y., Yu S., Liu J. Hierarchical self-assembly of proteins through rationally designed supramolecular interfaces. Front. Bioeng. Biotechnol. 2020;8:295. doi: 10.3389/fbioe.2020.00295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Uman S., Dhand A., Burdick J.A. Recent advances in shear‐thinning and self‐healing hydrogels for biomedical applications. J. Appl. Polym. Sci. 2020;137(25) [Google Scholar]
- 219.Beatty M.A., Hof F. Host–guest binding in water, salty water, and biofluids: general lessons for synthetic, bio-targeted molecular recognition. Chem. Soc. Rev. 2021;50(8):4812–4832. doi: 10.1039/d0cs00495b. [DOI] [PubMed] [Google Scholar]
- 220.Yu L., Ding J. Injectable hydrogels as unique biomedical materials. Chem. Soc. Rev. 2008;37(8):1473–1481. doi: 10.1039/b713009k. [DOI] [PubMed] [Google Scholar]
- 221.Feng Q., Wei K., Lin S., Xu Z., Sun Y., Shi P., Li G., Bian L. Mechanically resilient, injectable, and bioadhesive supramolecular gelatin hydrogels crosslinked by weak host-guest interactions assist cell infiltration and in situ tissue regeneration. Biomaterials. 2016;101:217–228. doi: 10.1016/j.biomaterials.2016.05.043. [DOI] [PubMed] [Google Scholar]
- 222.Bai H., Zhao Y., Wang C., Wang Z., Wang J., Liu H., Feng Y., Lin Q., Li Z., Liu H. Enhanced osseointegration of three-dimensional supramolecular bioactive interface through osteoporotic microenvironment regulation. Theranostics. 2020;10(11):4779–4794. doi: 10.7150/thno.43736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Rodell C.B., MacArthur J.W., Dorsey S.M., Wade R.J., Wang L.L., Woo Y.J., Burdick J.A. Shear-thinning supramolecular hydrogels with secondary autonomous covalent crosslinking to modulate viscoelastic properties in vivo. Adv. Funct. Mater. 2015;25(4):636–644. doi: 10.1002/adfm.201403550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Cao Z., Bai X., Wang C., Ren L., Ma D. A simple polysaccharide based injectable hydrogel compositing nano-hydroxyapatite for bone tissue engineering. Mater. Lett. 2021;293 [Google Scholar]
- 225.Hermann C.D., Wilson D.S., Lawrence K.A., Ning X., Olivares-Navarrete R., Williams J.K., Guldberg R.E., Murthy N., Schwartz Z., Boyan B.D. Rapidly polymerizing injectable click hydrogel therapy to delay bone growth in a murine re-synostosis model. Biomaterials. 2014;35(36):9698–9708. doi: 10.1016/j.biomaterials.2014.07.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Xu J., Liu Y., Hsu S.-H. Hydrogels based on Schiff base linkages for biomedical applications. Molecules. 2019;24(16):3005. doi: 10.3390/molecules24163005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Lü S., Bai X., Liu H., Ning P., Wang Z., Gao C., Ni B., Liu M. An injectable and self-healing hydrogel with covalent cross-linking in vivo for cranial bone repair. J. Mater. Chem. B. 2017;5(20):3739–3748. doi: 10.1039/c7tb00776k. [DOI] [PubMed] [Google Scholar]
- 228.George J., Hsu C.-C., Nguyen L.T.B., Ye H., Cui Z. Neural tissue engineering with structured hydrogels in CNS models and therapies. Biotechnol. Adv. 2020;42 doi: 10.1016/j.biotechadv.2019.03.009. [DOI] [PubMed] [Google Scholar]
- 229.Genova T., Roato I., Carossa M., Motta C., Cavagnetto D., Mussano F. Advances on bone substitutes through 3D bioprinting. Int. J. Mol. Sci. 2020;21(19) doi: 10.3390/ijms21197012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.He Y., Yang F., Zhao H., Gao Q., Xia B., Fu J. Research on the printability of hydrogels in 3D bioprinting. Sci. Rep. 2016;6 doi: 10.1038/srep29977. 29977-29977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Mancha Sánchez E., Gómez-Blanco J.C., López Nieto E., Casado J.G., Macías-García A., Díaz Díez M.A., Carrasco-Amador J.P., Torrejón Martín D., Sánchez-Margallo F.M., Pagador J.B. Hydrogels for bioprinting: a systematic review of hydrogels synthesis, bioprinting parameters, and bioprinted structures behavior. Front. Bioeng. Biotechnol. 2020;8:776. doi: 10.3389/fbioe.2020.00776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Lee J.-S., Hong J.M., Jung J.W., Shim J.-H., Oh J.-H., Cho D.-W. 3D printing of composite tissue with complex shape applied to ear regeneration. Biofabrication. 2014;6(2) doi: 10.1088/1758-5082/6/2/024103. [DOI] [PubMed] [Google Scholar]
- 233.Gaspar V.M., Lavrador P., Borges J., Oliveira M.B., Mano J.F. Advanced bottom‐up engineering of living architectures. Adv. Mater. 2020;32(6) doi: 10.1002/adma.201903975. [DOI] [PubMed] [Google Scholar]
- 234.Zheng F., Xiao Y., Liu H., Fan Y., Dao M. Patient‐specific organoid and organ‐on‐a‐chip: 3D cell‐culture meets 3D printing and numerical simulation. Advanced Biology. 2021;5(6) doi: 10.1002/adbi.202000024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Amler A.-K., Thomas A., Tüzüner S., Lam T., Geiger M.-A., Kreuder A.-E., Palmer C., Nahles S., Lauster R., Kloke L. 3D bioprinting of tissue-specific osteoblasts and endothelial cells to model the human jawbone. Sci. Rep. 2021;11(1):4876. doi: 10.1038/s41598-021-84483-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Zhang W., Shi W., Wu S., Kuss M., Jiang X., Untrauer J.B., Reid S.P., Duan B. 3D printed composite scaffolds with dual small molecule delivery for mandibular bone regeneration. Biofabrication. 2020;12(3) doi: 10.1088/1758-5090/ab906e. 035020-035020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Kang H.-W., Lee S.J., Ko I.K., Kengla C., Yoo J.J., Atala A. A 3D bioprinting system to produce human-scale tissue constructs with structural integrity. Nat. Biotechnol. 2016;34(3):312–319. doi: 10.1038/nbt.3413. [DOI] [PubMed] [Google Scholar]
- 238.Freeman F.E., Burdis R., Kelly D.J. Printing new bones: from print-and-implant devices to bioprinted bone organ precursors. Trends Mol. Med. 2021;27(7):700–711. doi: 10.1016/j.molmed.2021.05.001. [DOI] [PubMed] [Google Scholar]
- 239.Mohanty S., Alm M., Hemmingsen M., Dolatshahi-Pirouz A., Trifol J., Thomsen P., Dufva M., Wolff A., Emnéus J. 3D printed silicone–hydrogel scaffold with enhanced physicochemical properties. Biomacromolecules. 2016;17(4):1321–1329. doi: 10.1021/acs.biomac.5b01722. [DOI] [PubMed] [Google Scholar]
- 240.Zhang L., Shen S., Yu H., Shen S.G., Wang X. Computer-aided design and computer-aided manufacturing hydroxyapatite/epoxide acrylate maleic compound construction for craniomaxillofacial bone defects. J. Craniofac. Surg. 2015;26(5):1477–1481. doi: 10.1097/SCS.0000000000001410. [DOI] [PubMed] [Google Scholar]
- 241.Lee J.-Y., Choi B., Wu B., Lee M. Customized biomimetic scaffolds created by indirect three-dimensional printing for tissue engineering. Biofabrication. 2013;5(4) doi: 10.1088/1758-5082/5/4/045003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Kuss M.A., Harms R., Wu S., Wang Y., Untrauer J.B., Carlson M.A., Duan B. Short-term hypoxic preconditioning promotes prevascularization in 3D bioprinted bone constructs with stromal vascular fraction derived cells. RSC Adv. 2017;7(47):29312–29320. doi: 10.1039/c7ra04372d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Castro-Núñez J., Cunningham L.L., Van Sickels J.E. Atrophic mandible fractures: are bone grafts necessary? An update. J. Oral Maxillofac. Surg. 2017;75(11):2391–2398. doi: 10.1016/j.joms.2017.06.023. [DOI] [PubMed] [Google Scholar]
- 244.Anjum F., Lienemann P.S., Metzger S., Biernaskie J., Kallos M.S., Ehrbar M. Enzyme responsive GAG-based natural-synthetic hybrid hydrogel for tunable growth factor delivery and stem cell differentiation. Biomaterials. 2016;87:104–117. doi: 10.1016/j.biomaterials.2016.01.050. [DOI] [PubMed] [Google Scholar]
- 245.Haugen H.J., Lyngstadaas S.P., Rossi F., Perale G. Bone grafts: which is the ideal biomaterial? J. Clin. Periodontol. 2019;46:92–102. doi: 10.1111/jcpe.13058. [DOI] [PubMed] [Google Scholar]