Abstract
Coronary Microvascular Dysfunction (CMD) is now considered one of the key underlying pathologies responsible for the development of both acute and chronic cardiac complications. It has been long recognized that CMD contributes to coronary no-reflow, which occurs as an acute complication during percutaneous coronary interventions. More recently, CMD was proposed to play a mechanistic role in the development of left ventricle diastolic dysfunction in heart failure with preserved ejection fraction (HFpEF). Emerging evidence indicates that a chronic low-grade pro-inflammatory activation predisposes patients to both acute and chronic cardiovascular complications raising the possibility that pro-inflammatory mediators serve as a mechanistic link in HFpEF. Few recent studies have evaluated the role of the hyaluronan-CD44 axis in inflammation-related cardiovascular pathologies, thus warranting further investigations. This review article summarizes current evidence for the role of CMD in the development of HFpEF, focusing on molecular mediators of chronic proinflammatory as well as oxidative stress mechanisms and possible therapeutic approaches to consider for treatment and prevention.
Keywords: Heart failure, preserved ejection fraction, microvascular dysfunction, inflammation, oxidative stress, coronary arteries
1. INTRODUCTION
Heart failure is a prevalent disease that affects 22 million people globally every year [1]. It has been long recognized that impaired myocardial perfusion underlies the development of heart failure, which is generally attributed to occlusive lesions in large coronary arteries and is the major cause of heart failure with reduced ejection fraction (HFrEF). A more insidious and poorly defined perfusion deficit can arise from coronary microvascular dysfunction (CMD), which has been recently proposed to play a key mechanistic role in the development of heart failure with preserved ejection fraction (HFpEF, also known as diastolic heart failure). In particular, it is important to understand that occlusive coronary artery disease often coincides with CMD, which can be manifested as coronary no-reflow during unsuccessful percutaneous coronary intervention or as the almost ubiquitous co-occurrence of diastolic and systolic left ventricle dysfunction.
Unlike occlusive coronary artery disease and HFrEF, which is responsive to a variety of pharmacological and non-pharmacological therapeutic approaches, there is no effective treatment for CMD currently available to prevent left ventricle diastolic dysfunction in HFpEF. HFpEF has one of the highest incidences of new cases in the United States, up to 700,000 annually, with a rising mortality rate (5.2% annual death) and is especially prevalent in obese elderly patients, both men and women [2]. These patients are mostly offered treatments for cardiac risk factors and prevention of accompanying symptoms of HFpEF. In spite of these therapeutic attempts, HFpEF patients have a high rate of rehospitalization after their initial diagnosis [3].
While the current understanding of pathological alterations in HFpEF is incomplete, there are key mechanisms that have been considered in the past to contribute to myocardial stiffening and impaired relaxation of the left ventricle, with recent hypotheses focusing on chronic, low-grade inflammation-driven CMD [4, 5]. In particular, landmark works by Paulus and Tschope demonstrated that common comorbidities that cause systemic inflammation and increased oxidative stress uniquely trigger endothelial and cardiomyocyte dysfunction in HFpEF [6, 7]. Elevated inflammatory biomarkers have been associated with a number of cardiovascular risk factors including hypertension and obesity [8], which are comorbidities often seen in HFpEF patients. An inverse correlation between increased levels of inflammatory markers, like CRP and CMD was also found in previous clinical observational studies [9]. CMD was shown to be associated with coronary endothelial dysfunction [5] driven by oxidative stress and pro-inflammatory activation, which typically leads to a worse prognosis in HFpEF patients. Inflammatory cell-mediated activation can lead to endothelial dysfunction, which in turn releases pro-inflammatory cytokines from the activated endothelial cells, further exacerbating the inflammation process and oxidative stress, causing a harmful relationship that would worsen cardiovascular complications. Collectively, these aforementioned preclinical and clinical studies argue that microvascular inflammation could be a primary mechanism linking CMD and HFpEF.
There have been recent successful clinical trials evaluating therapies targeting inflammatory mechanisms in preventing cardiac complications [10, 11], raising the possibility that novel anti-inflammatory agents could also prove to be beneficial and improve outcomes in HFpEF patients. A better understanding of the nature of the molecular mediators in the inflammatory process could drive the development of novel diagnostic biomarkers and therapeutic approaches in HFpEF. In this review, we focus on current evidence that centers on coronary microvascular mechanisms of HFpEF and the role of chronic, low-grade inflammation that can mechanistically link CMD to the development of left ventricular diastolic dysfunction. Ultimately, we summarize studies evaluating therapeutic targets that may prove to be beneficial in preventing and treating CMD and HFpEF.
2. CMD AND HFpEF - PATHOPHYSIOLOGICAL CONSIDERATIONS
The coronary microcirculatory network is comprised of blood vessels of varying sizes with a diameter below 300 μm. The coronary microcirculation matches blood flow with metabolic requirements by coordinating the vascular resistance for the effective delivery of oxygen and nutrients to the working myocardium. This process is governed by distinct mechanisms, including myogenic, flow, and metabolic control of coronary arteriolar diameter [12, 13]. In disease, functional and also structural abnormalities of coronary arterioles can develop, which lead to inadequate flow distribution to the myocardium causing contractile (systolic) and relaxation (diastolic) dysfunction [14]. Abnormal functioning of coronary microvasculature is a pathological condition, commonly referred to as CMD, which includes endothelium-dependent and -independent dysfunction of microvasculature. As proposed by Crea et al., a clinical classification of CMD is based on the following conditions: absence of myocardial diseases and obstructive coronary artery disease, association with myocardial diseases, association with obstructive coronary artery disease, or iatrogenic, such as that caused by coronary no-reflow [15]. In this context, the authors also refer to other comprehensive, excellent review articles regarding CMD, such as by Taqueti et al. [16], classifications and clinical considerations of CMD in cardiomyopathies by Spoladore et al. [17] and Shimokawa et al. [18].
It is of note that in clinical settings, the visualization and evaluation of coronary microcirculation can be achieved to only a limited degree. Myocardial blush rate has been used to assess abnormalities in the coronary microcirculation by detecting opacification of a contrast agent during percutaneous coronary intervention and eventual estimation of the function of the coronary microcirculatory network. More recently, Magnetic Resonance Imaging (MRI) was entered as a clinical tool to help visualize the coronary microcirculatory network and for the assessment of vascular remodeling and pathogenesis in the context of CMD. However, routine cardiac MRI can help to outline the microvascular structure and outline microvascular obstruction, but this is achieved indirectly and at a relatively lower resolution than desired. In spite of carrying some limitations, Coronary Flow Reserve (CFR) is more often used as the mean to assess coronary microcirculation and diagnose CMD. It has been found that CFR is reduced in approximately 75% of HFpEF patients [19]. Interestingly, studies have raised the possibility that CMD manifests early in the pathogenesis of HFpEF. PROMISE-HFpEF is first the multicenter, prospective trial which showed that there is significantly high prevalence of CMD among patients with HFpEF in the absence of significant epicardial coronary stenosis [5]. It should be noted that current diagnosis of HFpEF is difficult due to the incomplete understanding of the underlying mechanisms of the disease. Approaches to diagnose HFpEF include additional measurement of natriuretic peptide levels as a biomarker, but this is often problematic due to the unreliability of the biomarker in HFpEF. Although CMD could be attributable to the comorbidities commonly seen with HFpEF, such as chronic kidney disease, diabetes mellitus, and hypertension, studies have indicated that CMD seen in HFpEF are more severe compared to “comorbidity-matched control subjects” [4]. Comorbid conditions in HFpEF, such as hypertension, obesity, and diabetes, all impose a significantly greater hemodynamic and metabolic burden on the working myocardium, and with a diminishing coronary microvascular reserve this could lead to relative or absolute myocardial ischemia.
Studies also indicated that aging is an independent risk factor for the development of left ventricle diastolic dysfunction in HFpEF. Indeed, a disproportionally large number of the population effected with HFpEF are above the age of 65. Aging is not only associated with an increased risk for left ventricle diastolic dysfunction but also associated with coronary microvascular dysfunction [20-22]. Due to the existing comorbidities in many older HFpEF patients, mechanistic studies on aging-related CMD and left ventricle diastolic dysfunction is often carried out in animal models of healthy, uncomplicated aging. These animal models include the Fischer 344 aged rat, which develops LV diastolic dysfunction and hypertrophy [23]. Other models of aging without comorbidities are the male Friend leukemia virus B inbred (FVB/N) mouse, which displays LV diastolic dysfunction at 12 months of age [23]. Aged dogs with hypertension also exhibit left ventricular diastolic dysfunction and can be used as a model for human HFpEF [24]. The nature of mechanisms and mechanistic interrelationships regarding the normal, healthy aging-associated development of CMD and LV diastolic dysfunction, as well as development of preventive therapeutic strategies in elderly patients require more mechanistic preclinical and clinical studies, respectively, in the future.
In the hearts of HFpEF patients, microvascular remodeling, impaired collateral formation, and vascular pruning leading to impaired angiogenesis have been observed which also favor relative or absolute myocardial perfusion deficits. Evidence for abnormalities in the coronary microcirculation that could lead to myocardial hypoperfusion and consequently developed left ventricle diastolic function was provided by our recent studies combining assessment of human myocardial samples from HFpEF patients and animal models of HFpEF [25, 26]. We have shown that coronary microvascular vasodilator dysfunction develops in HFpEF patients, and that coronary arteriolar dysfunction and myocardial perfusion deficit contributes to the development of left ventricle diastolic dysfunction in obese diabetic Zucker fatty/spontaneously hypertensive heart failure F1 hybrid (ZSF1) rat model of HFpEF [25, 26]. We observed that impaired coronary vasodilation in the obese ZSF1 rats is accompanied by elevated levels of surrogate markers of myocardial hypoxia, including collagen 1 and carbonic anhydrase 9. It is therefore plausible that coronary arteriolar vasodilator dysfunction causes a mismatch between blood supply and metabolic demand, which could lead to relative or absolute hypoperfusion, and as such represents an early manifestation of CMD in HFpEF. The nature of the molecular mechanism(s) that underlie reduced vasodilator function of coronary microvasculature, however remains ill-defined in HFpEF.
3. CMD AND HFpEF - THERAPEUTIC CONSIDERATIONS
In HFpEF, the reduced relaxation and increased stiffening of the myocardium have been primarily attributed to diminished nitric oxide (NO)-induced and protein kinase G (PKG)-mediated phosphorylation of myocardial structural proteins involved in calcium homeostasis and cardiac structure [6, 7]. Indeed, a reduction of bioavailable vascular endothelial NO could underlie vasodilator dysfunction of coronary resistance arteries, [27] a pathology that limits blood supply in the working myocardium resulting in myocardial contractile dysfunction [28, 29]. Clinical trials, such as the NEAT-HFpEF trial of administering Isosorbide Mononitrate, a direct NO donor to HFpEF patients [30], and the RELAX study evaluating the effect of sildenafil, a phosphodiesterase 5A inhibitor, to augment NO-cGMP-PKG signaling, produced somewhat disappointing results [31]. Another recent clinical trial using neladenoson bialanate, a partial adenosine A1 receptor agonist, also produced neutral outcomes on exercise capacity in HFpEF patients [32]. While the underlying mechanisms behind these negative results are not known, clearly therapeutic approaches other than restoring NO levels or directly activating adenosine receptors may be considered. Experimental evidence supports that in the diseased human heart coronary arteriolar vasodilation can be adaptively maintained by mechanisms independent from that of NO, such as achieved by the putative Endothelial Hyperpolarizing Factor (EDHF) [33] and also cyclooxygenase-2-derived vasodilator prostanoids [34]. A previous study by Ohta et al. [35] suggests that endogenously produced adenosine may act as a novel form of EDHF, which was formerly thought to be mediated solely by extracellular potassium, epoxyeicosatrienoic acid or hydrogen peroxide [36]. Our recent studies have also shown that pharmacological inhibition or genetic deletion of the key adenosine metabolizing enzyme, adenosine kinase, via augmenting EDHF-type vasodilation improves both coronary microvascular and left ventricle diastolic function [25, 26]. It is therefore possible that augmenting the endogenous adenosine levels and thereby facilitating EDHF-mediated vasodilation may provide additional therapeutic benefit in HFpEF, which warrants further mechanistic studies.
Furthermore, renin-angiotensin aldosterone system (RAS) and also the role for excess endothelin-1 (ET-1) have been implicated in the pathogenesis of HFpEF. While previous studies have shown upregulation of RAS in both HFrEF and HFpEF, RAS-inhibiting therapies like angiotensin II receptor blockers (ARBs) and angiotensin-converting enzyme (ACE)-inhibitors frequently given to HFrEF patients have not been proven beneficial to HFpEF patients specifically. A very recent meta-analyses of clinical trials reported that RAS inhibitors do not improve clinical outcomes, including cardiovascular mortality, heart failure hospitalization, all- cause mortality, or health-related quality of life in HFpEF patients [37]. For example, the TOPCAT study as well as the PARAGON-HF study have looked at either spironolactone or sacubitril with valsartan, in HFpEF patients, but reported that there were no significant changes in hospitalizations and mortality [38-40].
Moreover, ET-1 has been also linked with endothelial dysfunction, reduced vascular compliance, impaired left ventricle diastolic function and myocardial fibrosis [41]. A study using the ET-A receptor blocker sitaxsentan, while reporting an improved exercise tolerance, did not find any beneficial effects on left ventricular diastolic function or on the mortality in HFpEF patients [42]. Interestingly, a study by Valero-Munoz, et al. suggested that combined inhibition of ET-A and ET-B receptors is needed to exert significant beneficial effects in HFpEF patients, but further studies are needed to confirm this possibility in the future [41].
4. CHRONIC INFLAMMATION IN CMD AND HFpEF
Several cardiovascular diseases are associated with a state of chronic, low-level inflammation [43, 44]. A Framingham study of 732 elderly subjects revealed that inflammatory mediators, such as Tumor Necrosis Factor (TNF), interleukin-6 and C-reactive protein (CRP), were associated with increased risk of heart failure in the absence of prior myocardial infarction or occlusive coronary artery disease [45]. HFpEF is thought to develop from a chronic systemic inflammatory state which selectively disrupts the function of the coronary microcirculation [6, 7]. Inflammation is recognized as an important cause of coronary artery disease [46], but the exact inflammatory signaling pathways to quell or to regulate in order to prevent cardiovascular complications have not yet been identified [47]. In this context, previous studies have evaluated the interrelationships between chronic autoinflammatory diseases and increased prevalence of cardiovascular complications which found that patients with manifest inflammatory states typically have higher rates of cardiovascular morbidity and mortality [48]. Furthermore, the leading cause of morbidity and mortality in patients with systemic lupus erythematosus was found to be mainly attributed to cardiac and coronary vascular complications [48]. Microvascular impairment and increased systemic inflammation also occur in autoimmune diseases, such as systemic sclerosis and rheumatoid arthritis, resulting in a greater incidence of cardiac complications in these patients [48]. These studies concluded that chronic inflammation is associated with cardiovascular disease and other investigations have revealed that inflammatory biomarkers, such as increased levels of interleukin-6, CRP, and intercellular adhesion molecule-1 [49], can also be useful predictors for the likelihood of the development of coronary microvascular dysfunction and heart failure [50]. There has been a recent landmark clinical trial with therapeutic monoclonal antibody targeting of interleukin-1β in preventing cardiac complications [10], raising the possibility that a specific anti-inflammatory agent could also improve cardiovascular outcomes in HFpEF patients. There are an increasing number of studies raising the possibly of a causative relationship between chronic inflammation and HFpEF [51]. Subsequently, studies have yet to be conducted to identify specific inflammatory pathways and evaluate therapeutic modalities to target specific molecular mediators of chronic inflammation in HFpEF. Of note, chronic, low- grade inflammation and consequent vascular endothelial dysfunction that appears to occur in HFpEF and causes the left ventricular dysfunction does not occur in HFrEF, where eccentric left ventricle remodeling results from cardiomyocyte cell death pathways such as accelerated autophagy, apoptosis, and necrosis [7]. In HFpEF, activated endothelial cells can release inflammatory cytokines, perpetuating the increased myocardial inflammation and leading to the left ventricular diastolic dysfunction [52]. Previous studies have evaluated the role of specific pro-inflammatory mediators, such as soluble vascular cell adhesion molecule-1 and intercellular adhesion molecule-1 in women with CMD and found elevated levels of both as compared to controls [53]. Other studies have found increased levels of CRP that correlated with CMD diagnosis and similarly with decreased CFR. In contrast, studies evaluating other inflammatory biomarkers, including interleukin-6, interleukin-8, and TNF, did not find a causative relationship between inflammation and CMD. When assessing these inflammatory mediators, another recent study has identified a distinct link between inflammatory biomarkers, CMD and left ventricular diastolic function [50], suggesting a relationship between inflammation and CMD in HFpEF. In the RELAX trial, 57% of HFpEF patients had elevated levels of the inflammatory biomarker CRP and the levels progressively increased with additional numbers of comorbidities, [54]. The trial surprisingly found that CRP was not as sensitive biomarker to effectively detect microvascular inflammation, which can be attributed to the wide heterogeneity of patient population with varying level of comorbidities and inflammatory states. Further studies are needed to identify the HFpEF and CMD specific inflammatory mediators and biomarkers that can be used to monitor disease progression and perhaps indicate therapeutic efficiency.
5. INFLAMMATION, OXIDATIVE STRESS AND REDUCED AVAILABILITY OF NITRIC OXIDE IN HFpEF
The nature of molecular mechanisms by which inflammatory endothelial cell activation leads to left ventricle diastolic dysfunction in HFpEF also remains incompletely understood. It is established that many chronic inflammatory processes lead to activation, recruitment, and trans-endothelial migration of leukocytes and monocytes or macrophages into the heart. These inflammatory cells may contribute to left ventricular fibrosis, via an augmented synthesis of inflammatory cytokines, thereby promoting the differentiation of fibroblasts into myofibroblasts [55]. As a result, in HFpEF the chronic inflammatory state can result in significant cardiac fibrosis that potentially causes left ventricle stiffening [55, 56]. In support, increased collagen accumulation in the myocardium was observed in the obese ZSF1 rat model of HFpEF [25, 57]. Whether increased fibrosis, which typically correlates with chronic inflammation, tissue ischemia and tissue damage, underlies left ventricle diastolic dysfunction in human HFpEF, however, remains unclear and has been questioned recently. HFpEF patients are not always found to have increased collagen levels [7] and it appears that a reduced left ventricular relaxation and increased stiffening of the myocardium is independent of increased collagen deposition and has been attributed to diminished vascular endothelial NO and cGMP-PKG-mediated phosphorylation deficit of cardiomyocyte titin in HFpEF patients and rodent models [5-7]. These latter studies raised the possibility that in HFpEF, inflammatory cytokines mainly interfere with NO bioavailability by inducing oxidative stress that triggers vascular endothelial and cardiomyocyte relaxation dysfunction [6, 7]. In this context, it has been long recognized that a reduced bioavailability of vascular endothelium-derived NO could develop as a result of increased production of Reactive Oxygen Species (ROS) [48]. The reduction of available vascular endothelial NO could underlie vasodilator dysfunction of coronary resistance arteries [27], which limits blood supply in the working myocardium, resulting in myocardial contractile dysfunction [28, 29, 58, 59]. Myocardial oxidative stress and increased expression of the oxidant enzyme, such as NAD(P)H-oxidase, was found in the obese ZSF1 rat HFpEF model, but not in HFrEF animal models [7]. It is, therefore, plausible that oxidative stress and reduced NO availability, specifically in the coronary microvascular endothelial cells, leads to cardiomyocyte relaxation dysfunction in HFpEF [3].
Pro-inflammatory cytokines, including TNF, are known to stimulate endothelial ROS via activation of NAD(P)H-oxidases, which can directly interact with and limit NO bioavailability [60-63]. TNF is also an established inhibitor of endothelial function and eNOS activity [61, 64-70]. Previously it was found that the activating phosphorylation of eNOS (P-eNOS S1177) is significantly reduced in mesenteric arteries of diabetic mice with increased TNF levels and along with LV diastolic dysfunction [71]. TNF also induces an inactivating phosphorylation of eNOS at S116 in vascular endothelial cells [72]. This inhibitory phosphorylation is mediated by proline-directed Erk1/2. Increased phosphorylation of S116 reduces eNOS activity via the binding of the prolyl isomerase pin1 to eNOS [73, 74]. Therefore, it is possible that the inflammatory cytokine, TNF plays a central role in the development of CMD and LV diastolic dysfunction in HFpEF, by promoting ROS production (which interacts with NO) and also by inactivating eNOS, ultimately reducing NO availability. Whether anti-TNF therapies, via restoring NO availability, are beneficial in HFpEF patients has yet to be elucidated in future clinical studies.
6. NEW DIRECTIONS: A POTENTIAL ROLE FOR HYALURONAN-CD44 AXIS IN HFpEF
Previous studies have linked the glycoprotein CD44 and its main ligand, the extracellular matrix protein Hyaluronan (HA), to diseases that are accompanied with chronic inflammation and that range from malignant and non-malignant cancers to arthritis [75]. Emerging recent evidence indicates that the interaction between CD44 and HA also contributes to cardiovascular pathologies [76]. HA is a glycosaminoglycan that is a component of the extracellular matrix that degrades during inflammatory states [77, 78]. HA fragments can be found at either high molecular weight HA or low molecular weight HA with the higher molecular weights HA being linked with anti-inflammatory effects where the lower molecular weight HA being linked conversely to proinflammatory changes, and even to CMD [79]. The levels of low versus high-molecular weight HA is thought to be the result of both HA synthases and hyaluronidases [77]. The HA synthases produce different sizes of HA with the HAS1 and HAS2 producing high molecular weight HA and HAS3 producing the lower molecular weight HA [75, 78]. Specifically, the hyaluronidase HYAL2 degrades the high molecular weight HA to smaller fragments, exacerbating an inflammatory state. Low molecular weight HA has been shown to stimulate expression of proinflammatory cytokines and genes [75]. HYAL2 gene expression has been shown to be augmented within patients with an inflammatory coronary artery disease [77]. HYAL2 has also been shown to have a possible role within endothelial dysfunction in the presence of perturbed flow [77]. Recent studies show that HYAL2 protein expression in endothelial cells depends at least in part on the level of wall shear stress and that low shear stress-induced endothelial impairment is partly mediated by increased HYAL2 activity [77].
Many of the effects by which HA regulates cellular function is mediated through CD44 and RHAMM signaling [80]. CD44 is found throughout the body in various cells, including fibroblasts, macrophages, and endothelial cells [80]. CD44 has many isoforms due to exon splicing within its extracellular region with ten variable regions and can be differently glycosylated dependent on the cell type, causing a wide range of functions [77]. CD44 variants have a wide variety of physiological functions, including cell adhesion, migration, and lymphocyte activation [80] and have been implicated in facilitating proinflammatory conditions, including cancer and arthritis [75, 81]. Different CD44 variants have been implicated in chronic-inflammation induced diseases [82, 83], depending on the disease and the disease severity [75]. CD44 plays an important role in “monocyte/macrophage recruitment to inflammation sites” and when CD44 is overexpressed, the augmented interaction between the proinflammatory low molecular weight HA and CD44 leads to collagen deposition and fibrosis [80]. A previous study has shown that CD44-HA interaction can lead to an impaired cardiac function by hampering the initial inflammatory response after myocardial ischemia [84]. Thus, there is emerging evidence that indicates a potential pathological role for the HA-CD44 axis in chronic inflammation and myocardial dysfunction, a hypothesis has yet to be tested in relation to CMD and HFpEF.
CONCLUSION
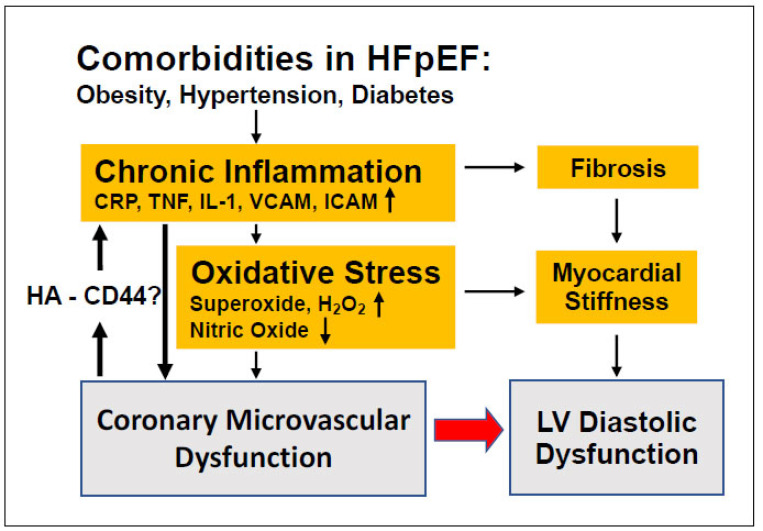
Despite its critical role in regulating myocardial perfusion, the altered function of coronary microvessels and the nature of pathologic mechanism of CMD in relation to HFpEF have not yet been fully characterized. There is an urgent need for better animal/preclinical models, diagnostic and therapeutic strategies that take into account CMD-related, specific pathophysiological mechanisms and also considers the heterogeneity of HFpEF patients [3]. Emerging evidence indicates that chronic, low-grade vascular inflammation mechanistically links CMD and left ventricle diastolic dysfunction in HFpEF. Specific microvascular signaling pathways, including NO and EDHF, proinflammatory mediators, as well as reactive oxygen species producing enzymes that drive CMD should be further evaluated in mechanistic experiments in animal models and clinical studies of HFpEF. As such, recent studies raise the possibility that the extracellular matrix protein, HA degradation and subsequent CD44 interaction with different molecular weight HA fragments could act to amply microvascular pro-inflammatory signaling pathways, which could deteriorate LV diastolic function in HFpEF (Fig. 1). A better understanding of coronary microvascular HA and CD44 signaling could also potentially lead to the identification of novel biomarkers of chronic microvascular inflammation in HFpEF, thereby offering novel avenues for targeted preventive strategies.
Fig. (1).
Comorbidities commonly present in patients with HFpEF cause a chronic inflammatory state, which leads to an increase in fibrosis and myocardial stiffness, two characteristics of left ventricle (LV) dysfunction. Recently, it has been proposed that this chronic inflammatory state could also directly lead to coronary microvascular dysfunction (CMD), which would consequently lead to higher levels of inflammation, which may be further exacerbated by a pathological activation of hyaluronan (HA) - CD44 axis (A higher resolution / colour version of this figure is available in the electronic copy of the article).
ACKNOWLEDGEMENTS
Declared none.
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
The author’s studies are supported by awards from the National Institute of Aging (R01AG054651 and R01 AG065406), National Heart, Lung, and Blood Institute (F31 HL142183).
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Salazar J., Rojas-Quintero J., Cano C., Pérez J.L., Ramírez P., Carrasquero R., Torres W., Espinoza C., Chacín-González M., Bermúdez V. Neprilysin: A potential therapeutic target of arterial hypertension? Curr. Cardiol. Rev. 2020;16(1):25–35. doi: 10.2174/1573403X15666190625160352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oktay A.A., Rich J.D., Shah S.J. The emerging epidemic of heart failure with preserved ejection fraction. Curr. Heart Fail. Rep. 2013;10(4):401–410. doi: 10.1007/s11897-013-0155-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scheffer M., Driessen-Waaijer A., Hamdani N., Landzaat J.W.D., Jonkman N.H., Paulus W.J., van Heerebeek L. Stratified treatment of heart failure with preserved ejection fraction: Rationale and design of the STADIA-HFpEF trial. ESC Heart Fail. 2020;7(6):4478–87. doi: 10.1002/ehf2.13055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dryer K., Gajjar M., Narang N., Lee M., Paul J., Shah A.P., Nathan S., Butler J., Davidson C.J., Fearon W.F., Shah S.J., Blair J.E.A. Coronary microvascular dysfunction in patients with heart failure with preserved ejection fraction. Am. J. Physiol. Heart Circ. Physiol. 2018;314(5):H1033–H1042. doi: 10.1152/ajpheart.00680.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shah S.J., Lam C.S.P., Svedlund S., Saraste A., Hage C., Tan R.S., Beussink-Nelson L., Ljung Faxén U., Fermer M.L., Broberg M.A., Gan L.M., Lund L.H. Prevalence and correlates of coronary microvascular dysfunction in heart failure with preserved ejection fraction: PROMIS-HFpEF. Eur. Heart J. 2018;39(37):3439–3450. doi: 10.1093/eurheartj/ehy531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Paulus W.J., Tschöpe C. A novel paradigm for heart failure with preserved ejection fraction: Comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J. Am. Coll. Cardiol. 2013;62(4):263–271. doi: 10.1016/j.jacc.2013.02.092. [DOI] [PubMed] [Google Scholar]
- 7.Franssen C., Chen S., Unger A., Korkmaz H.I., De Keulenaer G.W., Tschöpe C., Leite-Moreira A.F., Musters R., Niessen H.W., Linke W.A., Paulus W.J., Hamdani N. Myocardial microvascular inflammatory endothelial activation in heart failure with preserved ejection fraction. JACC Heart Fail. 2016;4(4):312–324. doi: 10.1016/j.jchf.2015.10.007. [DOI] [PubMed] [Google Scholar]
- 8.Prasad M., Matteson E.L., Herrmann J., Gulati R., Rihal C.S., Lerman L.O., Lerman A. Uric acid is associated with inflammation, coronary microvascular dysfunction, and adverse outcomes in postmenopausal women. Hypertension. 2017;69(2):236–242. doi: 10.1161/HYPERTENSIONAHA.116.08436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Long M., Huang Z., Zhuang X., Huang Z., Guo Y., Liao X., Luo C. Association of inflammation and endothelial dysfunction with coronary microvascular resistance in patients with cardiac syndrome X. Arq. Bras. Cardiol. 2017;109(5):397–403. doi: 10.5935/abc.20170149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ridker P.M., Everett B.M., Thuren T., MacFadyen J.G., Chang W.H., Ballantyne C., Fonseca F., Nicolau J., Koenig W., Anker S.D., Kastelein J.J.P., Cornel J.H., Pais P., Pella D., Genest J., Cifkova R., Lorenzatti A., Forster T., Kobalava Z., Vida-Simiti L., Flather M., Shimokawa H., Ogawa H., Dellborg M., Rossi P.R.F., Troquay R.P.T., Libby P., Glynn R.J. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N. Engl. J. Med. 2017;377(12):1119–1131. doi: 10.1056/NEJMoa1707914. [DOI] [PubMed] [Google Scholar]
- 11.Tardif J.C., Kouz S., Waters D.D., Bertrand O.F., Diaz R., Maggioni A.P., Pinto F.J., Ibrahim R., Gamra H., Kiwan G.S., Berry C., López-Sendón J., Ostadal P., Koenig W., Angoulvant D., Grégoire J.C., Lavoie M.A., Dubé M.P., Rhainds D., Provencher M., Blondeau L., Orfanos A., L’Allier P.L., Guertin M.C., Roubille F. Efficacy and safety of low- dose colchicine after myocardial infarction. N. Engl. J. Med. 2019;381(26):2497–2505. doi: 10.1056/NEJMoa1912388. [DOI] [PubMed] [Google Scholar]
- 12.Chilian W.M. Coronary microcirculation in health and disease. Summary of an NHLBI workshop. Circulation. 1997;95(2):522–528. doi: 10.1161/01.CIR.95.2.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jones C.J., Kuo L., Davis M.J., Chilian W.M. Regulation of coronary blood flow: Coordination of heterogeneous control mechanisms in vascular microdomains. Cardiovasc. Res. 1995;29(5):585–596. doi: 10.1016/S0008-6363(96)88626-3. [DOI] [PubMed] [Google Scholar]
- 14.Pries A.R., Badimon L., Bugiardini R., Camici P.G., Dorobantu M., Duncker D.J., Escaned J., Koller A., Piek J.J., de Wit C. Coronary vascular regulation, remodelling, and collateralization: Mechanisms and clinical implications on behalf of the working group on coronary pathophysiology and microcirculation. Eur. Heart J. 2015;36(45):3134–3146. doi: 10.1093/eurheartj/ehv100. [DOI] [PubMed] [Google Scholar]
- 15.Crea F., Camici P.G., Bairey Merz C.N. Coronary microvascular dysfunction: An update. Eur. Heart J. 2014;35(17):1101–1111. doi: 10.1093/eurheartj/eht513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Taqueti V.R., Di Carli M.F. Coronary microvascular disease pathogenic mechanisms and therapeutic options: JACC state-of-the-art review. J. Am. Coll. Cardiol. 2018;72(21):2625–2641. doi: 10.1016/j.jacc.2018.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Spoladore R., Fisicaro A., Faccini A., Camici P.G. Coronary microvascular dysfunction in primary cardiomyopathies. Heart. 2014;100(10):806–813. doi: 10.1136/heartjnl-2013-304291. [DOI] [PubMed] [Google Scholar]
- 18.Shimokawa H., Suda A., Takahashi J., Berry C., Camici P.G., Crea F., Escaned J., Ford T., Yii E., Kaski J.C., Kiyooka T., Mehta P.K., Ong P., Ozaki Y., Pepine C., Rimoldi O., Safdar B., Sechtem U., Tsujita K., Yasuda S., Beltrame J.F., Merz C.N.B. Clinical characteristics and prognosis of patients with microvascular angina: An international and prospective cohort study by the Coronary Vasomotor Disorders International Study (COVADIS) Group. Eur. Heart J. 2021:ehab282. doi: 10.1093/eurheartj/ehab282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hage C., Michaëlsson E., Kull B., Miliotis T., Svedlund S., Linde C., Donal E., Daubert J.C., Gan L.M., Lund L.H. Myeloperoxidase and related biomarkers are suggestive footprints of endothelial microvascular inflammation in HFpEF patients. ESC Heart Fail. 2020;7(4):1534–1546. doi: 10.1002/ehf2.12700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Feher A., Broskova Z., Bagi Z. Age-related impairment of conducted dilation in human coronary arterioles. Am. J. Physiol. Heart Circ. Physiol. 2014;306(12):H1595–H1601. doi: 10.1152/ajpheart.00179.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van de Hoef T.P., Echavarria-Pinto M., Meuwissen M., Stegehuis V.E., Escaned J., Piek J.J. Contribution of age-related microvascular dysfunction to abnormal coronary: Hemodynamics in patients with ischemic heart disease. JACC Cardiovasc. Interv. 2020;13(1):20–29. doi: 10.1016/j.jcin.2019.08.052. [DOI] [PubMed] [Google Scholar]
- 22.Pirmohamed A., Kitzman D.W., Maurer M.S. Heart failure in older adults: Embracing complexity. J. Geriatr. Cardiol. 2016;13(1):8–14. doi: 10.11909/j.issn.1671-5411.2016.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Conceição G., Heinonen I., Lourenço A.P., Duncker D.J., Falcão-Pires I. Animal models of heart failure with preserved ejection fraction. Neth. Heart J. 2016;24(4):275–286. doi: 10.1007/s12471-016-0815-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Munagala V.K., Hart C.Y., Burnett J.C., Jr, Meyer D.M., Redfield M.M. Ventricular structure and function in aged dogs with renal hypertension: A model of experimental diastolic heart failure. Circulation. 2005;111(9):1128–1135. doi: 10.1161/01.CIR.0000157183.21404.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Davila A., Tian Y., Czikora I., Li J., Su H., Huo Y., Patel V., Robinson V., Kapuku G., Weintraub N., Bagi Z. Adenosine kinase inhibition augments conducted vasodilation and prevents left ventricle diastolic dysfunction in heart failure with preserved ejection fraction. Circ. Heart Fail. 2019;12(8):e005762. doi: 10.1161/CIRCHEARTFAILURE.118.005762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dou H., Feher A., Davila A.C., Romero M.J., Patel V.S., Kamath V.M., Gooz M.B., Rudic R.D., Lucas R., Fulton D.J., Weintraub N.L., Bagi Z. Role of adipose tissue endothelial ADAM17 in age-related coronary microvascular dysfunction. Arterioscler. Thromb. Vasc. Biol. 2017;37(6):1180–1193. doi: 10.1161/ATVBAHA.117.309430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cassuto J., Dou H., Czikora I., Szabo A., Patel V.S., Kamath V., Belin de Chantemele E., Feher A., Romero M.J., Bagi Z. Peroxynitrite disrupts endothelial caveolae leading to eNOS uncoupling and diminished flow- mediated dilation in coronary arterioles of diabetic patients. Diabetes. 2014;63(4):1381–1393. doi: 10.2337/db13-0577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Niccoli G., Scalone G., Lerman A., Crea F. Coronary microvascular obstruction in acute myocardial infarction. Eur. Heart J. 2016;37(13):1024–1033. doi: 10.1093/eurheartj/ehv484. [DOI] [PubMed] [Google Scholar]
- 29.Henderson K.K., Turk J.R., Rush J.W., Laughlin M.H. Endothelial function in coronary arterioles from pigs with early-stage coronary disease induced by high-fat, high-cholesterol diet: Effect of exercise. J. Appl. Physiol. 2004;97(3):1159–1168. doi: 10.1152/japplphysiol.00261.2004. [DOI] [PubMed] [Google Scholar]
- 30.Redfield M.M., Anstrom K.J., Levine J.A., Koepp G.A., Borlaug B.A., Chen H.H., LeWinter M.M., Joseph S.M., Shah S.J., Semigran M.J., Felker G.M., Cole R.T., Reeves G.R., Tedford R.J., Tang W.H., McNulty S.E., Velazquez E.J., Shah M.R., Braunwald E. Isosorbide mononitrate in heart failure with preserved ejection fraction. N. Engl. J. Med. 2015;373(24):2314–2324. doi: 10.1056/NEJMoa1510774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Redfield M.M., Chen H.H., Borlaug B.A., Semigran M.J., Lee K.L., Lewis G., LeWinter M.M., Rouleau J.L., Bull D.A., Mann D.L., Deswal A., Stevenson L.W., Givertz M.M., Ofili E.O., O’Connor C.M., Felker G.M., Goldsmith S.R., Bart B.A., McNulty S.E., Ibarra J.C., Lin G., Oh J.K., Patel M.R., Kim R.J., Tracy R.P., Velazquez E.J., Anstrom K.J., Hernandez A.F., Mascette A.M., Braunwald E., RELAX Trial Effect of phosphodiesterase-5 inhibition on exercise capacity and clinical status in heart failure with preserved ejection fraction: A randomized clinical trial. JAMA. 2013;309(12):1268–1277. doi: 10.1001/jama.2013.2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shah S.J., Voors A.A., McMurray J.J.V., Kitzman D.W., Viethen T., Bomfim Wirtz A., Huang E., Pap A.F., Solomon S.D. Effect of neladenoson bialanate on exercise capacity among patients with heart failure with preserved ejection fraction: A randomized clinical trial. JAMA. 2019;321(21):2101–2112. doi: 10.1001/jama.2019.6717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miura H., Bosnjak J.J., Ning G., Saito T., Miura M., Gutterman D.D. Role for hydrogen peroxide in flow-induced dilation of human coronary arterioles. Circ. Res. 2003;92(2):e31–e40. doi: 10.1161/01.RES.0000054200.44505.AB. [DOI] [PubMed] [Google Scholar]
- 34.Szerafin T., Erdei N., Fülöp T., Pasztor E.T., Edes I., Koller A., Bagi Z. Increased cyclooxygenase-2 expression and prostaglandin-mediated dilation in coronary arterioles of patients with diabetes mellitus. Circ. Res. 2006;99(5):e12–e17. doi: 10.1161/01.RES.0000241051.83067.62. [DOI] [PubMed] [Google Scholar]
- 35.Ohta M., Toyama K., Gutterman D.D., Campbell W.B., Lemaître V., Teraoka R., Miura H. Ecto-5′-nucleotidase, CD73, is an endothelium-derived hyperpolarizing factor synthase. Arterioscler. Thromb. Vasc. Biol. 2013;33(3):629–636. doi: 10.1161/ATVBAHA.112.300600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Félétou M., Vanhoutte P.M. Endothelium-derived hyperpolarizing factor: Where are we now? Arterioscler. Thromb. Vasc. Biol. 2006;26(6):1215–1225. doi: 10.1161/01.ATV.0000217611.81085.c5. [DOI] [PubMed] [Google Scholar]
- 37.Fukuta H., Goto T., Wakami K., Kamiya T., Ohte N. Effect of renin-angiotensin system inhibition on cardiac structure and function and exercise capacity in heart failure with preserved ejection fraction: A meta-analysis of randomized controlled trials. Heart Fail. Rev. 2020 doi: 10.1007/s10741-020-09969-1. [DOI] [PubMed] [Google Scholar]
- 38.Solomon S.D., Claggett B., Desai A.S., Packer M., Zile M., Swedberg K., Rouleau J.L., Shi V.C., Starling R.C., Kozan Ö., Dukat A., Lefkowitz M.P., McMurray J.J. Influence of ejection fraction on outcomes and efficacy of sacubitril/valsartan (LCZ696) in heart failure with reduced ejection fraction: The prospective comparison of ARNI with ACEI to determine impact on global mortality and morbidity in heart failure (PARADIGM-HF) trial. Circ. Heart Fail. 2016;9(3):e002744. doi: 10.1161/CIRCHEARTFAILURE.115.002744. [DOI] [PubMed] [Google Scholar]
- 39.McMurray J.J.V., Jackson A.M., Lam C.S.P., Redfield M.M., Anand I.S., Ge J., Lefkowitz M.P., Maggioni A.P., Martinez F., Packer M., Pfeffer M.A., Pieske B., Rizkala A.R., Sabarwal S.V., Shah A.M., Shah S.J., Shi V.C., van Veldhuisen D.J., Zannad F., Zile M.R., Cikes M., Goncalvesova E., Katova T., Kosztin A., Lelonek M., Sweitzer N., Vardeny O., Claggett B., Jhund P.S., Solomon S.D. Effects of sacubitril-valsartan versus valsartan in women compared with men with heart failure and preserved ejection fraction: Insights from PARAGON-HF. Circulation. 2020;141(5):338–351. doi: 10.1161/CIRCULATIONAHA.119.044491. [DOI] [PubMed] [Google Scholar]
- 40.Solomon S.D., Vaduganathan M., L Claggett B., Packer M., Zile M., Swedberg K., Rouleau J., A Pfeffer M., Desai A., Lund L.H., Kober L., Anand I., Sweitzer N., Linssen G., Merkely B., Luis Arango J., Vinereanu D., Chen C.H., Senni M., Sibulo A., Boytsov S., Shi V., Rizkala A., Lefkowitz M., McMurray J.J.V. Sacubitril/valsartan across the spectrum of ejection fraction in heart failure. Circulation. 2020;141(5):352–361. doi: 10.1161/CIRCULATIONAHA.119.044586. [DOI] [PubMed] [Google Scholar]
- 41.Valero-Munoz M., Li S., Wilson R.M., Boldbaatar B., Iglarz M., Sam F. Dual endothelin-A/endothelin-B receptor blockade and cardiac remodeling in heart failure with preserved ejection fraction. Circ. Heart Fail. 2016;9(11):e003381. doi: 10.1161/CIRCHEARTFAILURE.116.003381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zile M.R., Bourge R.C., Redfield M.M., Zhou D., Baicu C.F., Little W.C. Randomized, double-blind, placebo-controlled study of sitaxsentan to improve impaired exercise tolerance in patients with heart failure and a preserved ejection fraction. JACC Heart Fail. 2014;2(2):123–130. doi: 10.1016/j.jchf.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 43.Libby P., Ridker P.M., Maseri A. Inflammation and atherosclerosis. Circulation. 2002;105(9):1135–1143. doi: 10.1161/hc0902.104353. [DOI] [PubMed] [Google Scholar]
- 44.Gonzalez M.A., Selwyn A.P. Endothelial function, inflammation, and prognosis in cardiovascular disease. Am. J. Med. 2003;115(Suppl. 8A):99S–106S. doi: 10.1016/j.amjmed.2003.09.016. [DOI] [PubMed] [Google Scholar]
- 45.Vasan R.S., Sullivan L.M., Roubenoff R., Dinarello C.A., Harris T., Benjamin E.J., Sawyer D.B., Levy D., Wilson P.W., D’Agostino R.B. Inflammatory markers and risk of heart failure in elderly subjects without prior myocardial infarction: The Framingham Heart Study. Circulation. 2003;107(11):1486–1491. doi: 10.1161/01.CIR.0000057810.48709.F6. [DOI] [PubMed] [Google Scholar]
- 46.Crea F., Libby P. Acute coronary syndromes: The way forward from mechanisms to precision treatment. Circulation. 2017;136(12):1155–1166. doi: 10.1161/CIRCULATIONAHA.117.029870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Harrington R.A. Targeting inflammation in coronary artery disease. N. Engl. J. Med. 2017;377(12):1197–1198. doi: 10.1056/NEJMe1709904. [DOI] [PubMed] [Google Scholar]
- 48.Zanatta E., Colombo C., D’Amico G., d’Humières T., Dal Lin C., Tona F. Inflammation and coronary microvascular dysfunction in autoimmune rheumatic diseases. Int. J. Mol. Sci. 2019;20(22):E5563. doi: 10.3390/ijms20225563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vaccarino V., Khan D., Votaw J., Faber T., Veledar E., Jones D.P., Goldberg J., Raggi P., Quyyumi A.A., Bremner J.D. Inflammation is related to coronary flow reserve detected by positron emission tomography in asymptomatic male twins. J. Am. Coll. Cardiol. 2011;57(11):1271–1279. doi: 10.1016/j.jacc.2010.09.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Suhrs H.E., Schroder J., Bové K.B., Mygind N.D., Frestad D., Michelsen M.M., Lange T., Gustafsson I., Kastrup J., Prescott E. Inflammation, non-endothelial dependent coronary microvascular function and diastolic function-Are they linked? PLoS One. 2020;15(7):e0236035. doi: 10.1371/journal.pone.0236035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Taqueti V.R., Ridker P.M. Inflammation, coronary flow reserve, and microvascular dysfunction: Moving beyond cardiac syndrome X. JACC Cardiovasc. Imaging. 2013;6(6):668–671. doi: 10.1016/j.jcmg.2013.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ter Maaten J.M., Damman K., Verhaar M.C., Paulus W.J., Duncker D.J., Cheng C., van Heerebeek L., Hillege H.L., Lam C.S., Navis G., Voors A.A. Connecting heart failure with preserved ejection fraction and renal dysfunction: The role of endothelial dysfunction and inflammation. Eur. J. Heart Fail. 2016;18(6):588–598. doi: 10.1002/ejhf.497. [DOI] [PubMed] [Google Scholar]
- 53.Kuruvilla S., Kramer C.M. Coronary microvascular dysfunction in women: An overview of diagnostic strategies. Expert Rev. Cardiovasc. Ther. 2013;11(11):1515–1525. doi: 10.1586/14779072.2013.833854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.DuBrock H.M., AbouEzzeddine O.F., Redfield M.M. High-sensitivity C-reactive protein in heart failure with preserved ejection fraction. PLoS One. 2018;13(8):e0201836. doi: 10.1371/journal.pone.0201836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gallet R., de Couto G., Simsolo E., Valle J., Sun B., Liu W., Tseliou E., Zile M.R., Marbán E. Cardiosphere-derived cells reverse heart failure with preserved ejection fraction (HFpEF) in rats by decreasing fibrosis and inflammation. JACC Basic Transl. Sci. 2016;1(1-2):14–28. doi: 10.1016/j.jacbts.2016.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Packer M., Lam C.S.P., Lund L.H., Maurer M.S., Borlaug B.A. Characterization of the inflammatory-metabolic phenotype of heart failure with a preserved ejection fraction: A hypothesis to explain influence of sex on the evolution and potential treatment of the disease. Eur. J. Heart Fail. 2020;22(9):1551–1567. doi: 10.1002/ejhf.1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nguyen I.T.N., Brandt M.M., van de Wouw J., van Drie R.W.A., Wesseling M., Cramer M.J., de Jager S.C.A., Merkus D., Duncker D.J., Cheng C., Joles J.A., Verhaar M.C. Both male and female obese ZSF1 rats develop cardiac dysfunction in obesity-induced heart failure with preserved ejection fraction. PLoS One. 2020;15(5):e0232399. doi: 10.1371/journal.pone.0232399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.van Heerebeek L., Hamdani N., Falcão-Pires I., Leite-Moreira A.F., Begieneman M.P., Bronzwaer J.G., van der Velden J., Stienen G.J., Laarman G.J., Somsen A., Verheugt F.W., Niessen H.W., Paulus W.J. Low myocardial protein kinase G activity in heart failure with preserved ejection fraction. Circulation. 2012;126(7):830–839. doi: 10.1161/CIRCULATIONAHA.111.076075. [DOI] [PubMed] [Google Scholar]
- 59.Westermann D., Lindner D., Kasner M., Zietsch C., Savvatis K., Escher F., von Schlippenbach J., Skurk C., Steendijk P., Riad A., Poller W., Schultheiss H.P., Tschöpe C. Cardiac inflammation contributes to changes in the extracellular matrix in patients with heart failure and normal ejection fraction. Circ. Heart Fail. 2011;4(1):44–52. doi: 10.1161/CIRCHEARTFAILURE.109.931451. [DOI] [PubMed] [Google Scholar]
- 60.Griendling K.K., Sorescu D., Ushio-Fukai M. NAD(P)H oxidase: Role in cardiovascular biology and disease. Circ. Res. 2000;86(5):494–501. doi: 10.1161/01.RES.86.5.494. [DOI] [PubMed] [Google Scholar]
- 61.Gao X., Belmadani S., Picchi A., Xu X., Potter B.J., Tewari-Singh N., Capobianco S., Chilian W.M., Zhang C. Tumor necrosis factor-alpha induces endothelial dysfunction in Lepr(db) mice. Circulation. 2007;115(2):245–254. doi: 10.1161/CIRCULATIONAHA.106.650671. [DOI] [PubMed] [Google Scholar]
- 62.Muzaffar S., Jeremy J.Y., Angelini G.D., Stuart-Smith K., Shukla N. Role of the endothelium and nitric oxide synthases in modulating superoxide formation induced by endotoxin and cytokines in porcine pulmonary arteries. Thorax. 2003;58(7):598–604. doi: 10.1136/thorax.58.7.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yang B., Rizzo V. TNF-alpha potentiates protein-tyrosine nitration through activation of NADPH oxidase and eNOS localized in membrane rafts and caveolae of bovine aortic endothelial cells. Am. J. Physiol. Heart Circ. Physiol. 2007;292(2):H954–H962. doi: 10.1152/ajpheart.00758.2006. [DOI] [PubMed] [Google Scholar]
- 64.Aoki N., Siegfried M., Lefer A.M. Anti-EDRF effect of tumor necrosis factor in isolated, perfused cat carotid arteries. Am. J. Physiol. 1989;256(5 Pt 2):H1509–H1512. doi: 10.1152/ajpheart.1989.256.5.H1509. [DOI] [PubMed] [Google Scholar]
- 65.Czikora I., Alli A., Bao H.F., Kaftan D., Sridhar S., Apell H.J., Gorshkov B., White R., Zimmermann A., Wendel A., Pauly-Evers M., Hamacher J., Garcia-Gabay I., Fischer B., Verin A., Bagi Z., Pittet J.F., Shabbir W., Lemmens-Gruber R., Chakraborty T., Lazrak A., Matthay M.A., Eaton D.C., Lucas R. A novel tumor necrosis factor-mediated mechanism of direct epithelial sodium channel activation. Am. J. Respir. Crit. Care Med. 2014;190(5):522–532. doi: 10.1164/rccm.201405-0833OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Myers P.R., Wright T.F., Tanner M.A., Adams H.R. EDRF and nitric oxide production in cultured endothelial cells: Direct inhibition by E. coli endotoxin. Am. J. Physiol. 1992;262(3 Pt 2):H710–H718. doi: 10.1152/ajpheart.1992.262.3.H710. [DOI] [PubMed] [Google Scholar]
- 67.Neumann P., Gertzberg N., Johnson A. TNF-alpha induces a decrease in eNOS promoter activity. Am. J. Physiol. Lung Cell. Mol. Physiol. 2004;286(2):L452–L459. doi: 10.1152/ajplung.00378.2002. [DOI] [PubMed] [Google Scholar]
- 68.Seidel M., Billert H., Kurpisz M. Regulation of eNOS expression in HCAEC cell line treated with opioids and proinflammatory cytokines. Kardiol. Pol. 2006;64(2):153–158. [PubMed] [Google Scholar]
- 69.Valerio A., Cardile A., Cozzi V., Bracale R., Tedesco L., Pisconti A., Palomba L., Cantoni O., Clementi E., Moncada S., Carruba M.O., Nisoli E. TNF-alpha downregulates eNOS expression and mitochondrial biogenesis in fat and muscle of obese rodents. J. Clin. Invest. 2006;116(10):2791–2798. doi: 10.1172/JCI28570.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yan G., You B., Chen S.P., Liao J.K., Sun J. Tumor necrosis factor-alpha downregulates endothelial nitric oxide synthase mRNA stability via translation elongation factor 1-alpha 1. Circ. Res. 2008;103(6):591–597. doi: 10.1161/CIRCRESAHA.108.173963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Huang A., Yang Y.M., Feher A., Bagi Z., Kaley G., Sun D. Exacerbation of endothelial dysfunction during the progression of diabetes: Role of oxidative stress. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2012;302(6):R674–R681. doi: 10.1152/ajpregu.00699.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kennard S., Ruan L., Buffett R.J., Fulton D., Venema R.C. TNFα reduces eNOS activity in endothelial cells through serine 116 phosphorylation and Pin1 binding: Confirmation of a direct, inhibitory interaction of Pin1 with eNOS. Vascul. Pharmacol. 2016;81:61–68. doi: 10.1016/j.vph.2016.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kou R., Greif D., Michel T. Dephosphorylation of endothelial nitric-oxide synthase by vascular endothelial growth factor. Implications for the vascular responses to cyclosporin A. J. Biol. Chem. 2002;277(33):29669–29673. doi: 10.1074/jbc.M204519200. [DOI] [PubMed] [Google Scholar]
- 74.Ruan L., Torres C.M., Qian J., Chen F., Mintz J.D., Stepp D.W., Fulton D., Venema R.C. Pin1 prolyl isomerase regulates endothelial nitric oxide synthase. Arterioscler. Thromb. Vasc. Biol. 2011;31(2):392–398. doi: 10.1161/ATVBAHA.110.213181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Misra S., Hascall V.C., Markwald R.R., Ghatak S. Interactions between hyaluronan and its receptors (CD44, RHAMM) regulate the activities of inflammation and cancer. Front. Immunol. 2015;6:201. doi: 10.3389/fimmu.2015.00201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Farb A., Kolodgie F.D., Hwang J.Y., Burke A.P., Tefera K., Weber D.K., Wight T.N., Virmani R. Extracellular matrix changes in stented human coronary arteries. Circulation. 2004;110(8):940–947. doi: 10.1161/01.CIR.0000139337.56084.30. [DOI] [PubMed] [Google Scholar]
- 77.Pedicino D., Vinci R., Giglio A.F., Pisano E., Porto I., Vergallo R., Russo G., Ruggio A., D’Aiello A., Flego D., Annibali G., Trotta F., Piacentini R., Niccoli G., Liuzzo G., Crea F. Alterations of hyaluronan metabolism in acute coronary syndrome: Implications for plaque erosion. J. Am. Coll. Cardiol. 2018;72(13):1490–1503. doi: 10.1016/j.jacc.2018.06.072. [DOI] [PubMed] [Google Scholar]
- 78.Slevin M., Krupinski J., Gaffney J., Matou S., West D., Delisser H., Savani R.C., Kumar S. Hyaluronan-mediated angiogenesis in vascular disease: Uncovering RHAMM and CD44 receptor signaling pathways. Matrix Biol. 2007;26(1):58–68. doi: 10.1016/j.matbio.2006.08.261. [DOI] [PubMed] [Google Scholar]
- 79.Litwiniuk M., Krejner A., Speyrer M.S., Gauto A.R., Grzela T. Hyaluronic acid in inflammation and tissue regeneration. Wounds. 2016;28(3):78–88. [PubMed] [Google Scholar]
- 80.Jordan A.R., Racine R.R., Hennig M.J., Lokeshwar V.B. The role of CD44 in disease pathophysiology and targeted treatment. Front. Immunol. 2015;6:182. doi: 10.3389/fimmu.2015.00182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Krolikoski M., Monslow J., Puré E. The CD44-HA axis and inflammation in atherosclerosis: A temporal perspective. Matrix Biol. 2019;78-79:201–218. doi: 10.1016/j.matbio.2018.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Suwannakul N., Ma N., Thanan R., Pinlaor S., Ungarreevittaya P., Midorikawa K., Hiraku Y., Oikawa S., Kawanishi S., Murata M. Overexpression of CD44 variant 9: A novel cancer stem cell marker in human cholangiocarcinoma in relation to inflammation. Mediators Inflamm. 2018;2018:4867234. doi: 10.1155/2018/4867234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wittig B., Seiter S., Schmidt D.S., Zuber M., Neurath M., Zöller M. CD44 variant isoforms on blood leukocytes in chronic inflammatory bowel disease and other systemic autoimmune diseases. Lab. Invest. 1999;79(6):747–759. [PubMed] [Google Scholar]
- 84.Suleiman M., Abdulrahman N., Yalcin H., Mraiche F. The role of CD44, hyaluronan and NHE1 in cardiac remodeling. Life Sci. 2018;209:197–201. doi: 10.1016/j.lfs.2018.08.009. [DOI] [PubMed] [Google Scholar]