Abstract
An increase in the level of cholesterol day by day is easily seen in most people just because of poor life style, food with high cholesterol, lack of physical work, etc. There are lots of molecules available, which lower down the cholesterol level. In this field a new molecule has been introduced that is bimpedoic acid. Researches indicate that bempedoic acid has the same mechanism of action as statins, which means that it also inhibits the HMG-CoA reductase enzyme. This article is my best collection of published scientific data on bempedoic acid till now. It also includes the chemistry, pharmacodynamic and pharmacokinetic parameters of the mentioned new molecule.
Keywords: Cholesterol, bempedoic acid, HMG-CoA reductase enzyme, lipoproteins, gastrointestinal, hypoglycemic activity
1. INTRODUCTION
Now a days, Cardiovascular Diseases (CVDs) are the most important cause of death and according to the survey, it is estimated that 17.9 million are affected by (CVDs) with each year. It is a group of disorders (related to heart) that includes coronary heart disease, cerebrovascular disease, rheumatic heart disease, etc. [1]. Deaths are seen mainly because of heart attacks and strokes whereas one third of these deaths occur prematurely in people under 70 years of age. Risk factors of CVDS include raised blood pressure, glucose, lipids, overweight and obesity. Identifying those at the highest risk of CVDs and ensuring they receive appropriate treatment can prevent premature deaths. Access to essential non-communicable disease medicines and basic health technologies in all primary health care facilities is essential to ensure that those in need receive treatment and counseling [1]. WHO always deals with member states to prevent, manage and monitor CVDs by developing global strategies to reduce the incidence, morbidity and mortality of the diseases. These strategies or factors include reducing risk factors, developing standards of care, enhancing the health system capacity to care for patients with CVDS, and monitoring disease patterns and trends to inform national and global actions. WHO also works with partners and countries to develop cost effective and equitable health care innovations for managing the disease [1].
According to the journal Nature Reviews Cardiology, CVDs are also the leading cause of deaths in China. Dong Zhao has summarized eight important features of the epidemiology of CVDs in China [2].
The American Heart Association (AHA) with the National Institutes of Health and other government agencies, brings together in a single document the most up-to-date statistics related to heart disease, stroke, and the cardiovascular risk factors in the AHA’s My Life Check - Life’s Simple, which include core health behaviors (smoking, physical activity, diet, and weight) and health factors (cholesterol, blood pressure and glucose control) that contribute to cardiovascular health [3].
1.1. Bempedoic Acid
Bempedoic acid is a medication for the treatment of hypercholesterolemia (high blood cholesterol levels). It was approved in the US in February 2020. The European Medicines Agency (EMA) recommended approval in the EU in January 2020 [4, 5]. According to the survey, it reduced low-density lipoproteins (cholesterol) by about 20 mg/dl compared to placebo and had no more side effects than placebo, although a higher percentage of drug receiving subjects had dropped out of the study because of side effects (11% vs. 7% under placebo) [6]. By March 2019, its effects on cardiovascular morbidity and mortality are not clear but studies were under processing [7]. In January 2020, the Committee for Medicinal Products for Human Use (CHMP) in the European Union recommended granting of a marketing authorization for bimpedoic acid as both a standalone drug (Nilemdo) [8] and in combination with ezetimibe (Nustendi) [9]. In February 2020, bempedoic acid was approved for use in the United States [10-12]. The U.S. Food and Drug Administration (FDA) granted the approval of Nexletol to Esperion Therapeutics [4]. On the basis of two clinical trials (Trial 1/ NCT02666664 and Trial 2/NCT02991118) with 3009 subjects with high LDL cholesterol, FDA approved bimpedoic acid [13]. The trials were conducted according to the United States, Canada, and Europe. There were two clinical trials that evaluated the benefits and side effects of bimpedoic acid. The trial designs were similar. All enrolled subjects were on a low cholesterol diet and taking the highest dose of statins (drug commonly used to lower cholesterol) for high cholesterol. In both trials, subjects were randomly assigned to receive bempedoic acid or placebo tablets every day for 52-weeks. Neither the subjects nor the health care providers knew which treatment was being given. The trials measured percent change in LDL-C blood levels from baseline to week 12 and compared bempedoic acid to placebo (Fig. 1) (Table 1) [14].
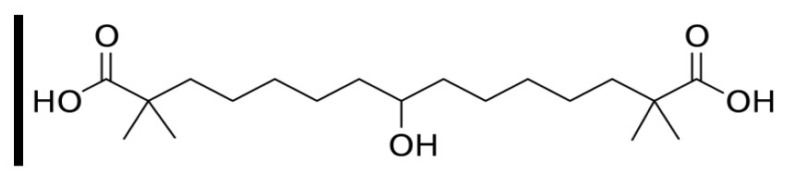
Fig. (1).
Chemical structure of bempedoic acid (A higher resolution / colour version of this figure is available in the electronic copy of the article).
Table 1.
Bempedoic acid chemical.
| Bempedoic Acid Chemical Data [14] | |||
|---|---|---|---|
| Molecular Formula | C19H36O5 | Molecular Weight | 344.5 g/mol |
| Synonyms | Bempedoic acid ETC-1002 738606-46-7 8-Hydroxy-2,2,14,14-tetramethylpentadecanedioic acid ESP-55016 |
IUPAC name | 8-hydroxy-2,2,14,14-tetramethylpentadecanedioic acid |
| XLogP3-AA | 4.8 | Topological Polar Surface Area | 94.8 Å2 |
| Hydrogen Bond Donor Count | 3 | Heavy Atom Count | 24 |
| Hydrogen Bond Acceptor Count | 5 | Formal Charge | 0 |
| Rotatable Bond Count | 14 | Complexity | 351 |
| Exact Mass | 344.256274 g/mol | Isotope Atom Count | 0 |
| Monoisotopic Mass | 344.256274 g/mol | Defined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 | Undefined Atom Stereocenter Count | 0 |
2. PHARMACOLOGY OF BEMPEDOIC ACID
It is clear that it is an excellent replacement of statins due to the high level of side effects, so it cannot be given with statins on behalf of available scientific data (further studies are needed for the exploration of detailed pharmacology). The detailed genetics of bempedoic acid is not available now [15-17].
2.1. Mechanism of Action
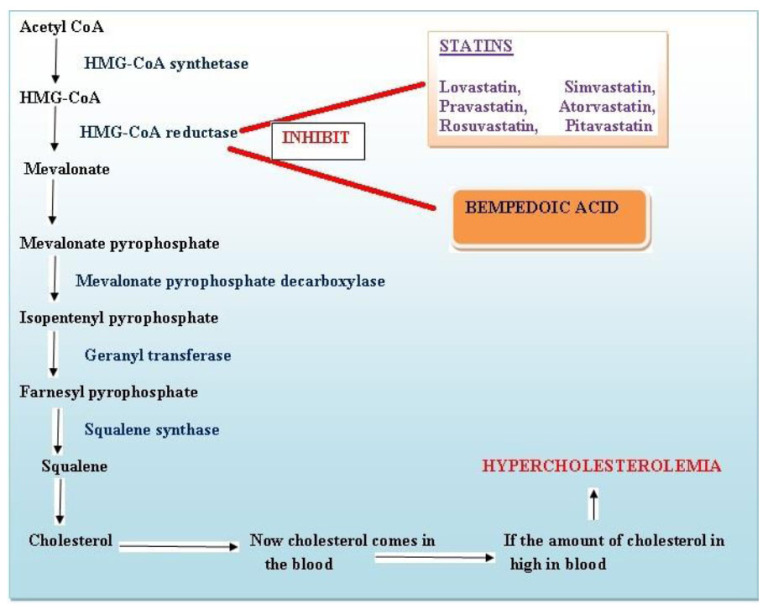
According to Dr. Kausik Ray (Cardiologist, lead author of the study and professor at Royal College, London School of Public Health), Bempedoic acid works in the same pathways as statins and it blocks the key enzyme, which is used to produce cholesterol (Fig. 2).
Fig. (2).
Mechanism of action of bempedoic acid and statins (A higher resolution / colour version of this figure is available in the electronic copy of the article).
2.2. Pharmacokinetic Data
The pharmacokinetic data of bempedoic acid is presented in Table 2.
| Route of administration oral |
| Protein binding 99.3% |
| Metabolism via liver (Glucuronidation) |
| Half life 21±11 hrs |
| Exertion 70% urine, 30% feces |
2.3. Side Effects
Common adverse effects were muscle spasms, pain in the back or in a limb, gout and gastrointestinal problems such as diarrhea (seen in clinical trials). A less common but more serious adverse effect was tendon rupture in the rotator cuff of the shoulder, the biceps tendon or achilles tendon [4].
CONCLUSION
This article clearly reflects the pharmacology of bempedoic acid. Bempedoic acid has passed many phases of clinical trials. It also has hypoglycemic activity. The side effects are low in clinical trials. More research is needed for the development of more formulations, biochemistry of product, etc. Further detailed research is needed for the complete exploration of pharmacology.
ACKNOWLEDGEMENTS
I would like to express my hearty thanks to Smt. Madhu Kulshreshtha [My loving mother and respected Principal, Juniour High School, Judawai (U.P.), India] for valuable suggestions and technical advises and Dr. Ch.V.Rao, Scientist, National Botanical Research Institute, Lucknow, India for Help me to provide articles. I would also thank full to my various professors in different countries for sent me the different views.
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.World Health Organization. Cardiovascular diseases. Available from: https://www.who.int/health- topics/cardiovascular-diseases/#tab=tab_1 (Accessed on Feb 15, 2021).
- 2.Zhao D., Liu J., Wang M., Zhang X., Zhou M. Epidemiology of cardiovascular disease in China: current features and implications. Nat. Rev. Cardiol. 2019;16(4):203–212. doi: 10.1038/s41569-018-0119-4. [DOI] [PubMed] [Google Scholar]
- 3.Benjamin E.J., Muntner P., Alonso A., Bittencourt M.S., Callaway C.W., Carson A.P., Chamberlain A.M., Chang A.R., Cheng S., Das S.R., Delling F.N., Djousse L., Elkind M.S.V., Ferguson J.F., Fornage M., Jordan L.C., Khan S.S., Kissela B.M., Knutson K.L., Kwan T.W., Lackland D.T., Lewis T.T., Lichtman J.H., Longenecker C.T., Loop M.S., Lutsey P.L., Martin S.S., Matsushita K., Moran A.E., Mussolino M.E., O’Flaherty M., Pandey A., Perak A.M., Rosamond W.D., Roth G.A., Sampson U.K.A., Satou G.M., Schroeder E.B., Shah S.H., Spartano N.L., Stokes A., Tirschwell D.L., Tsao C.W., Turakhia M.P., VanWagner L.B., Wilkins J.T., Wong S.S., Virani S.S. Heart disease and stroke statistics-2019 update: A report from the American heart association. Circulation. 2019;139(10):e56–e528. doi: 10.1161/CIR.0000000000000659. [DOI] [PubMed] [Google Scholar]
- 4.Nexletol- Bempedoic Acid Tablet, Film Coated. Daily Med. Available from: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=88d06d89-a3da-40b4-b273-8f4f7d56c4c9 (Accessed on Feb 15, 2021).
- 5.Nexletol: FDA-Approved drugs. US Food and Drug Administration (FDA) 2020. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2020/211616Orig1s000TOC.cfm (Accessed on Feb 15, 2021).
- 6.Ray K.K., Bays H.E., Catapano A.L., Lalwani N.D., Bloedon L.T., Sterling L.R., Robinson P.L., Ballantyne C.M. Safety and efficacy of bempedoic acid to reduce LDL cholesterol. N. Engl. J. Med. 2019;380(11):1022–1032. doi: 10.1056/NEJMoa1803917. [DOI] [PubMed] [Google Scholar]
- 7.Bempedoic acid. Esperion Therapeutics. Available from: https://adisinsight.springer.com/drugs/800031255 (Accessed on Feb 15, 2021).
- 8.European Medicines Agencey. Nustendi. Available from: https://www.ema.europa.eu/en/medicines/human/summaries-opinion/nustendi (Accessed on Feb 15, 2021).
- 9.Lipids. Available from: https://www.britannica.com/science/lipid (Accessed on Feb 15, 2021).
- 10.Bempedoic Acid and Ezetimibe. Available from: https://www.esperion.com/investors-media/press-releases/?reques (Accessed on Feb 15, 2021).
- 11.Nexletol. Available from: https://www.nytimes.com/aponline/2020/02/21/health/ap-us-med-fda-cholesterol-drug.html (Accessed on Feb 15, 2021).
- 12.McGinley L. FDA approves first non-statin pill to treat high cholesterol in almost two decades. The Washington Post. Available from: https://www.washingtonpost.com/health/2020/02/21/fda-approves-first-non-statin-pill-treat-high-cholesterol-almost-two-decades/ (Accessed on Feb 15, 2021).
- 13.Nexletol. Available from: https://www.fda.gov/drugs/resources-information-approved-drugs/drug-trials-snapshots-nexletol (Accessed on Feb 15, 2021).
- 14.Bempedoic acid Classification. Available from: https://pubchem.ncbi.nlm.nih.gov/compound/Bempedoic-acid#section=MeSH-Pharmacological-Classification .
- 15.Bempedoic acid. Available from: https://www.ncbi.nlm.nih.gov/mesh/68004791 (Accessed on Feb 15, 2021).
- 16.Bempedoic acid. Available from: https://www.ncbi.nlm.nih.gov/mesh/68000960 (Accessed on Feb 15, 2021).
- 17.Bempedoic acid. Available from: https://www.ncbi.nlm.nih.gov/mesh/68007004 (Accessed on Feb 15, 2021).