Abstract
Background:
Erenumab is a novel monoclonal calcitonin gene-related peptide receptor antibody that is used for the preventive treatment of migraine.
Objectives:
This study aimed to evaluate the overall safety, efficacy, and dose-response relationship of erenumab in patients with episodic migraine and patients with prior migraine treatment failures.
Methods:
We searched randomized clinical trials on PUBMED, EMBASE database, and Cochrane Library database. A pair-wise meta-analysis and Bayesian network analysis were performed.
Results:
For efficacy outcomes, the network meta-analysis suggests that in comparison to erenumab 70 mg, participants who received erenumab 140 mg reported a significant decrease in monthly acute Migraine-Specific Medication Days (MSMD) and 50% increase in response rate, and erenumab was most likely to be ranked first for Monthly Migraine Days (MMD), MSMD, and 50% response rate. For safety outcomes, the network meta-analysis has found no significant difference between the 70 mg group and the 140 mg group measured by adverse events and serious adverse events. In the 140 mg erenumab group, a significant decreased in MMD and MSMD and 50% and 75% increased in response rate were reported in patients with ≥ 2 treatment failures compared to placebo. For safety outcomes, no significant difference was found between the 140 mg erenumab group and the placebo group.
Conclusion:
Erenumab was effective in patients with episodic migraine. A total of 140 mg erenumab was associated with better efficacy outcomes without any increased risk for developing adverse events compared to 70 mg erenumab. Furthermore, 140 mg erenumab was effective in patients with prior migraine treatment failures.
Keywords: Calcitonin gene-related peptide receptor antagonist, erenumab, migraine, network meta-analysis, MSMD, MMD
1. INTRODUCTION
Migraine is one of the most common neurological disorders, with an estimated global prevalence of 15% [1]. It is manifested with recurrent attacks of unilateral, pulsatile headache that could be triggered by daily activities. A migraine attack usually lasts for 4 to 72 hours and is frequently accompanied by nausea, vomiting, photophobia, and phonophobia. Thus, patients may suffer from significant disabling pain and impaired quality of life.
As an expanding understanding of migraine pathogenesis in the past decades, multiple targets have been identified for migraine therapy. Among them, Calcitonin Gene-Related Peptide (CGRP) is considered one of the most promising targets [2]. CGRP is a 37-amino acid peptide produced through alternative RNA processing of the calcitonin gene, located in the neuronal tissue, especially C sensory fibers [3]. It has two forms, αCGRP and βCGRP. αCGRP is mainly expressed in the central and peripheral nervous system, while βCGRP is primarily found in the enteric nervous system [4]. Dimerization of Calcitonin Receptor-Like Protein (CLR) and Receptor Activity-Modifying Protein1 (RAMP1) creates CGRP receptors [4], which are located in the trigeminal neurons, peripheral intracranial vascular smooth muscle cells, the brainstem, and the dura mater [5].
CGRP receptor antagonists showed efficacy in both acute and preventative treatments of migraine attacks, whereas their use was restraint considering hepatotoxicity [5]. Erenumab is an IgG2 human monoclonal antibody (mAb), which selectively binds to the CGRP receptor, and it has been approved by the Food and Drug Administration for the prevention of migraine [6]. Previous trials have shown the safety and efficacy of erenumab compared to the placebo [1], and higher efficacy of the high erenumab dose in patients with multiple prior preventive treatment failures has been suggested [7].
So far, prior studies have demonstrated that erenumab use was associated with reduced migraine frequency and improved functional outcome compared to placebo; however, no study has been performed to evaluate the dose-response relationship of erenumab for the preventive treatment of migraine. Furthermore, although the effectiveness of erenumab in patients with prior treatment failure has been demonstrated in multiple trials, no systematic approach has been used. The purpose of this study is to conduct a meta-analysis to evaluate the effectiveness and safety of different doses of erenumab in preventive treatment in patients with migraine and patients with prior preventive treatment failures.
2. METHODS
A meta-analysis was performed following the Preferred Reporting Items For Systematic Reviews And Meta-Analyses (PRISMA) guidelines [8]. The study was registered at PROSPERO (http://www.crd.york.ac.uk/PROSPERO; registry number: CRD42020198985).
2.1. Search Strategy
The related articles published before April 1, 2020, were searched through PUBMED, EMBASE database, and Cochrane Library database. The search terms include migraine, erenumab, AMG334, and randomized clinical trials. The full electronic search strategy is listed in Supplement S1 (528.6KB, pdf) .
2.2. Study Selection
The studies included in the meta-analysis met all the following criteria: 1) randomized clinical trials; 2) patients with a history of episode migraine for ≥12 months according to the International Classification of Headache Disorders; 3) using erenumab as an experimental group intervention.
2.3. Data Extraction
The extraction of data was carried out by two investigators independently. The extracted data include (1) literature information: title, author, publication time, sample size; (2) characteristic of the object of the study including region, age, sex ratio, BMI, age at migraine onset, MMD, MSMD, number of patients who have failed previous preventive migraine medication due to insufficient efficacy or (and) unacceptable tolerability; (3) interventions: the dose of erenumab included in any phase III trials; (4) outcome data: the change in MMD, MSMD at month 3, 50% response rate (the proportion of patients achieved a 50% or greater reduction in MMD from baseline) and 75% response rate (the proportion of patients achieved a 75% or greater reduction in MMD from baseline) at month 3, the change in Headache Impact Test (Hit-6) at month 3, Migraine Physical Function Impact Diary-Everyday Activities and Physical Impairment (MPFID-EA and MPFID-PI) at month 3, number of patients with Adverse Events (AE) and Serious Adverse Events (SAE) throughout the study.
2.4. Pair-Wise Meta-Analysis
We conducted a pair-wise meta-analysis to evaluate the overall safety and efficacy of erenumab compared to the placebo and the safety and efficacy of different doses of erenumab in patients with prior treatment failures. Dosages that were assessed in any phase III trials were included. The random-effects model with the Hartung-Knapp-Sidik-Jonkman method was used. Mean Difference (MD) and Risk Ratio (RR) with their 95% Confidence Intervals (CI) were used to evaluate continuous and categorical variables, respectively. The heterogeneity between the included studies was evaluated with I2. R Software version 3.6.2 (R Foundation for Statistical Computing) was used to conduct the analysis.
2.5. Network Meta-Analysis
As studies with direct and indirect comparison exist across different dosage subgroups, we conducted a Bayesian network meta-analysis to compare safety and efficacy outcomes between different dosages using the gemtc package in R software (version 3.6.2). Both fixed-effects and random-effects models were generated with Markov Chain Monte-Carlo simulation for each outcome using default priors with 5000 adaptation iterations with 100,000 iterations of 4 chains. The model with the lowest deviance information criterion was used for further analysis. The potential scale reduction factor was used to assess the model convergence, using a cut-off value of 1.005. The node-split method was used for assessing inconsistency. Log Odds Ratio (LOR) and mean difference with their 95% CI were calculated for binary variables and continuous variables, respectively.
2.6. Risk of Bias
For assessing the risk of bias of RCTs, we used the standard approach developed by the Cochrane collaboration, which included: selection bias, performance bias, detection bias, attrition bias, reporting bias, and other potential biases. The risk of bias plot in individual studies was created using the Review Manager 5.3 software.
3. RESULTS
3.1. Baseline Characteristics
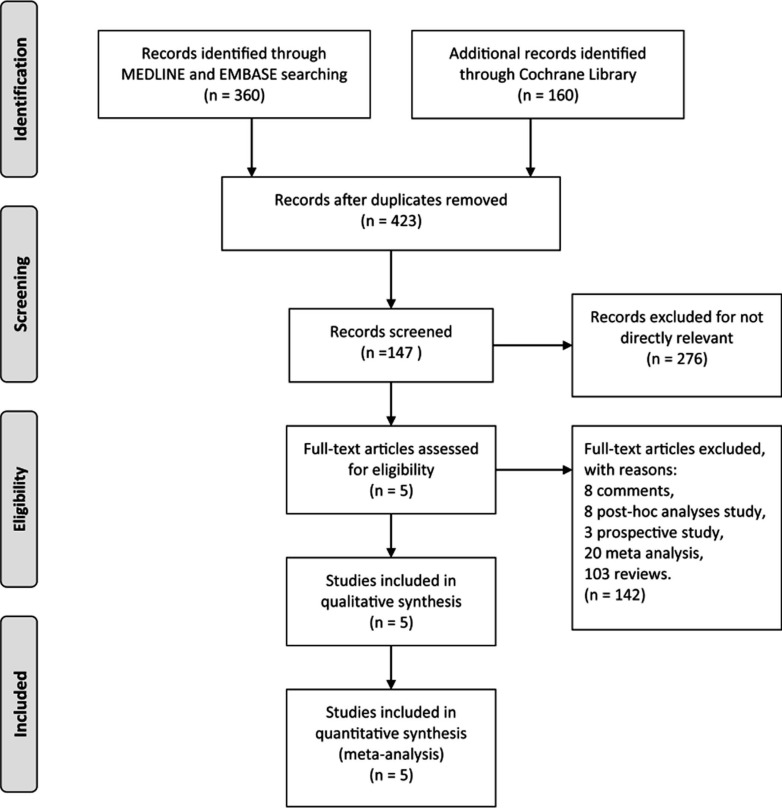
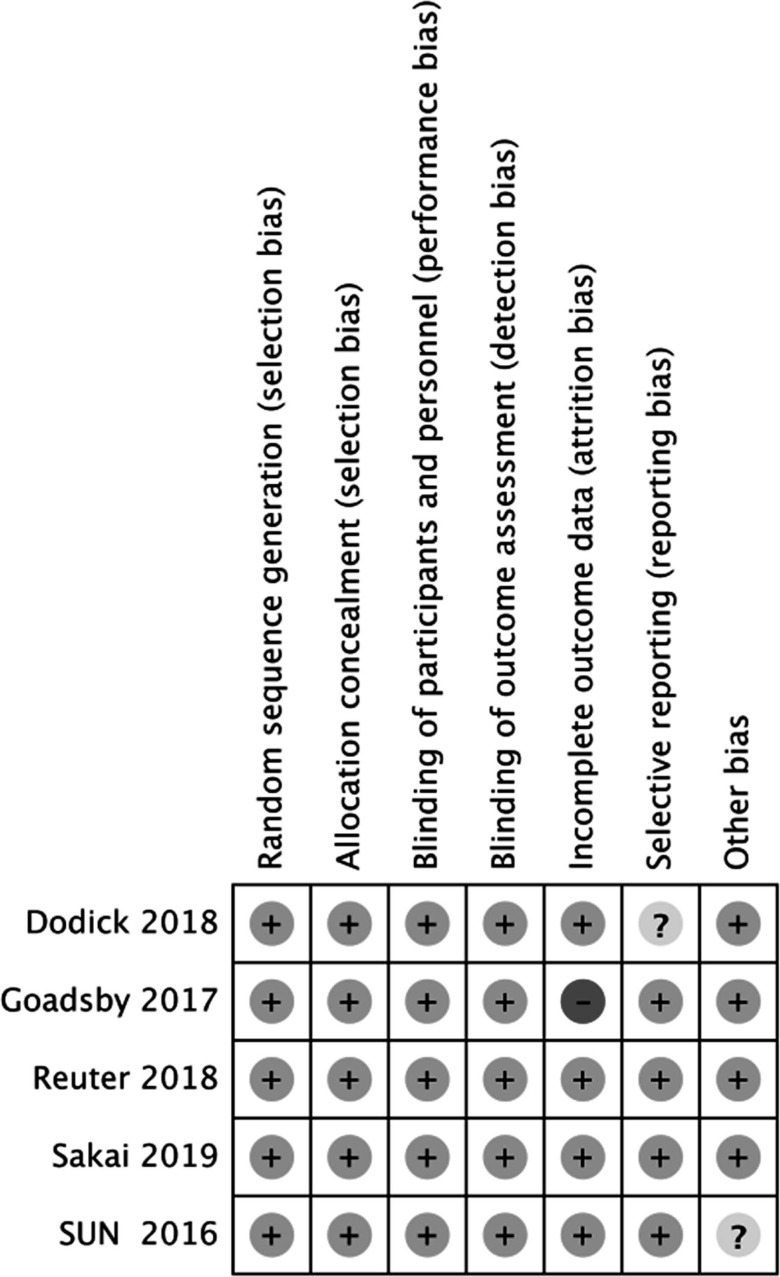
We pooled a total of 2453 patients from five randomized controlled trials to analyze the role of erenumab in episodic migraine [9-13]. Fig. (1) shows the PRISMA flow diagram of the study inclusion process. Characteristics of included trials are listed in Table 1. Among these included trials, we found two studies that reported the effect of erenumab in episodic migraineurs who had preventive treatment failures [11, 14]. The baseline characteristics of each study are shown in Table 2. Overall, 84.2% of patients were female. The baseline MMD of five episodic migraine studies were 8.34±2.56. Our study included the data from erenumab 70 and 140 mg groups, as these dosages were examined in the phase III trials. The risk of bias in each included trial is plotted in Fig. (2).
Fig. (1).
PRISMA flow diagram of the study inclusion process.
Table 1.
Characteristics of included studies.
| Trials (NCT number) | Phase | Inclusion Criteria | Exclusion Criteria | Group* | Frequency |
|---|---|---|---|---|---|
| Publication | |||||
| Center | |||||
| Sakai 2019(NCT02630459) | Phase 2 | 1. Aged 20-65 years 2. A diagnosis of migraine as defined by ICHD-III beta for at least 1 year prior 3. A history of 4-15 headache days per month on average across the three months prior to screening |
1. Over 50 years old at migraine onset 2. History of cluster headache or hemiplegic migraine 3. Failure to respond to three or more classes of migraine preventive treatments 4. Receiving botulinum toxin within four months before screening 5. The use of interventions or devices for migraine during the two months prior to screening 6. Receiving more than 1 migraine-preventive medication |
Erenumab 28 mg Erenumab 70 mg Erenumab 140 mg Placebo |
Once a month for 6 months |
| Headache | |||||
| Multicenter | |||||
| Dodick 2018(NCT02483585) | Phase 3 | 1. Aged 18-65 years 2. A history of 4-15 migraine days per month and < 15 headache days per month for at least one year prior |
1. Over 50 years old at migraine onset 2. History of cluster headache or hemiplegic migraine Receiving botulinum toxin within four months before screening |
Erenumab 70 mg | Once a month for 3 months |
| Placebo | |||||
| Cephalalgia | |||||
| Multicenter | |||||
| Reuter 2018(NCT03096834) | Phase 3 | 1. Aged 18-65 years 2. A diagnosis of migraine as defined by ICHD-III beta for at least one year prior 3. A history of 4-14 headache days per month on average across the three months prior to screening 4. Have previously been treated unsuccessfully |
1. Over 50 years old at migraine onset 2. History of cluster headache or hemiplegic migraine 3. Use a preventive migraine medication within five times the drug’s half-life before baseline 4. Receiving botulinum toxin within four months before screening 5. The use of interventions or devices for migraine within the month prior to screening |
Erenumab 140 mg | Once a month for 3 months |
| Placebo | |||||
| Lancet | |||||
| Multicenter | |||||
| Goadsby 2017(NCT02456740) | Phase 3 | 1. Aged 18-65 years 2. A diagnosis of migraine as defined by ICHD-III beta for at least one year prior 3. A history of 4-15 migraine days per month and < 15 headache days per month for at least three months prior |
1. Over 50 years old at migraine onset 2. History of cluster headache or hemiplegic migraine 3. Receiving botulinum toxin within four months before screening 4. The use of interventions or devices for migraine within two months prior to screening |
Erenumab 70 mg Erenumab 140 mg Placebo |
Once a month for 6 months |
| New England Journal of Medicine | |||||
| Multicenter | |||||
| Sun 2016(NCT01952574) | Phase 2 | 1. Aged 18-60 years 2. A diagnosis of migraine as defined by ICHD-II for at least one year prior 3. A history of 4-14 migraine days per month and < 15 headache days per month for at least three months prior |
1. Over 50 years old at migraine onset 2. History of cluster headache or hemiplegic migraine 3. Overuse of acute treatment for headache 4. Receiving more than 1 migraine-preventive medication during the two months prior to screening 5. Receiving botulinum toxin within six months before screening |
Erenumab 7 mg Erenumab 21 mg Erenumab 70 mg Placebo |
Once a month for 3 months |
| Lancet Neurology | |||||
| Multicenter |
* We only collected data from patients who received >=70mg erenumab.
Table 2.
Baseline characteristics of included patients.
| Trials | Region | Treatment | Populations | Age (SD) | Female Sex (%) | BMI (SD) | Age at onset (SD) | MMD (SD) | MSMD (SD) | Failed Previous Migraine-Preventive Medications |
|---|---|---|---|---|---|---|---|---|---|---|
| Sakai 2019 | Japan | Erenumab 70 mg | 135 | 44(NA) | 115(85.2%) | 21.6(3.5) | NA | 7.8(2.3) | 5.4(2.9) | 43 |
| Erenumab 140 mg | 137 | 45(NA) | 112(81.8%) | 22(3.5) | NA | 8.1(2.4) | 5.9(2.9) | 54 | ||
| Placebo | 136 | 45(NA) | 118(86.8%) | 22.1(3.5) | NA | 7.7(2.3) | 5.6(2.5) | 44 | ||
| Dodick 2018 | North America and Europe | Erenumab 70 mg | 286 | 42(11) | 245(85.7%) | 27.4(6.3) | 21(10) | 8.1(2.7) | 3.7(3.6) | 117 |
| Placebo | 291 | 42(12) | 247(84.9%) | 27.4(6.1) | 22(11) | 8.4(2.6) | 3.4(3.6) | 115 | ||
| Goadsby 2017 | North America, Europe and Turkey | Erenumab 70 mg | 317 | 41.1(11.3) | 268(84.5%) | 27.3(5.9) | 21.4(11) | 8.3(2.5) | 3.2(3.4) | 127 |
| Erenumab 140 mg | 319 | 40.4(11.1) | 272(85.3%) | 27(6.2) | 20.7(9.9) | 8.3(2.5) | 3.4(3.5) | 116 | ||
| Placebo | 319 | 41.3(11.2) | 274(85.9%) | 27.1(6.3) | 21.2(10.2) | 8.2(2.5) | 3.4(3.4) | 127 | ||
| Sun 2016 | North America and Europe | Erenumab 70 mg | 107 | 42.6(9.9) | 82(76.6%) | 25.8(4.9) | 21.7(11.7) | 8.6(2.5) | 4.3(3.5) | 34 |
| Placebo | 160 | 41.4(10) | 132(82.5%) | 25.9(4.9) | 20.7(11.5) | 8.8(2.7) | 4.5(3.9) | 60 | ||
| Reuter 2018 | Europe and Australia | Erenumab 140 mg | 121 | 44.6(10.5) | 97(80.2) | 25(4.2) | NA | 9.2(2.6) | 4.8(2.9) | 121 |
| Placebo | 125 | 44.2(10.6) | 103(82.4%) | 24.9(5.1) | NA | 9.3(2.7) | 4.4(2.8) | 125 |
NA=not applicable; BMI=Body Mass Index; MMD=Monthly Migraine Days; MSMD=Monthly Acute Migraine-Specific Medication Treatment Days.
Fig. (2).
Summary of risk of bias. Green circles represent a low risk of bias, yellow circles represent an unclear risk of bias, and red circle represents a high risk of bias.
3.2. Overall Safety and Efficacy
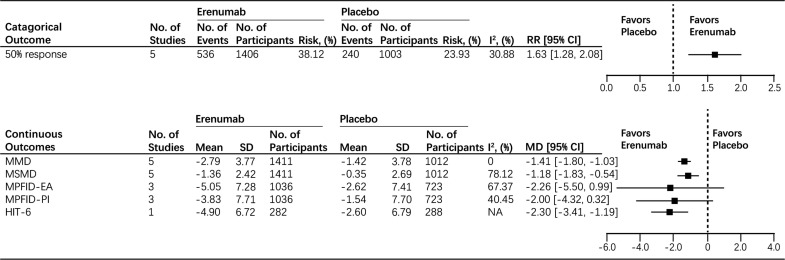
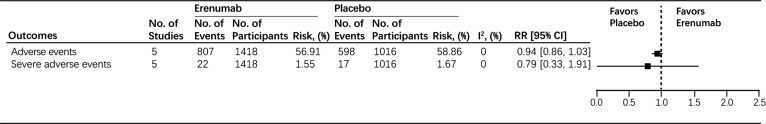
First, we evaluated the overall efficacy of erenumab. The pooled results showed that patients in the erenumab group achieved a greater than 50% reduction in MMD from baseline compared to the placebo group (RR: 1.63, 95% CI: 1.28 to 2.08, I2 = 30.88%) (Fig. 3). Subsequently, our results found that the erenumab group had a significant reduction of MMD from baseline compared to the placebo group (MD: -1.41, 95% CI: -1.80 to -1.03, I2 = 0%) (Fig. 3). Furthermore, in comparison to the placebo group, the use of erenumab was associated with a significant reduction of MSMD (MD: -1.18, 95% CI: -1.83 to -0.54) (Fig. 3); considerable hetero- geneity was found (I2 = 78.12%) (Fig. 3). For functional improvement, no significant difference in measured change of MPFID-EA and MPFID-PI score from baseline was found between the placebo group and the erenumab group (MD: -2.26, 95% CI: -5.50 to 0.99, I2 = 67.37%; MD: -2.00, 95% CI: -4.32 to 0.32, I2 = 40.45%, respectively) (Fig. 3). However, for the HIT-6 score, patients who received erenumab reported significantly improved HIT-6 score from baseline compared to the placebo group (MD: -2.30, 95% CI: -3.41 to -1.19) (Fig. 3). For the safety outcomes, no significant differences were found in adverse events (RR: 0.94, 95% CI: 0.86 to 1.03, I2 = 0%) and serious adverse events (RR: 0.79, 95% CI: 0.33 to 1.91, I2 = 0%) between the placebo and erenumab groups with low heterogeneity (Fig. 4).
Fig. (3).
Efficacy endpoints in all participants.
Abbreviations: MMD=Monthly Migraine Days; MSMD=Monthly Acute Migraine-Specific Medication Treatment Days; MPFIDEA= Migraine Physical Function Impact Diary-Everyday Activities; MPFID-PI=Migraine Physical Function Impact Diary-Physical Impairment; HIT=Headache Impact Test; CI=Confidence Interval; RR=Risk Radio; MD=Mean Difference; SD=Standard Difference.
Fig. (4).
Safety endpoints in all participants. Abbreviations: CI=Confidence Interval; RR=Risk Radio.
3.3. Network Meta-Analysis Comparing Different Doses of Erenumab
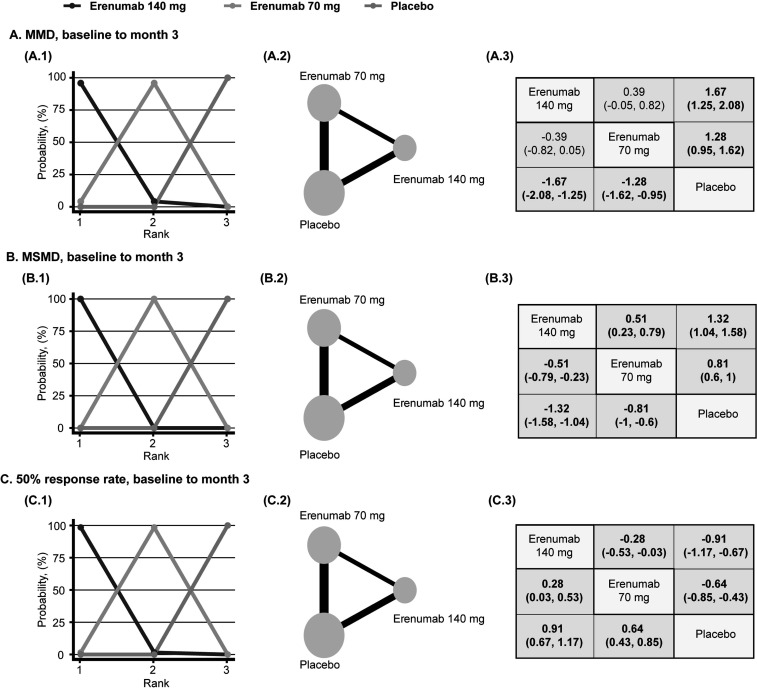
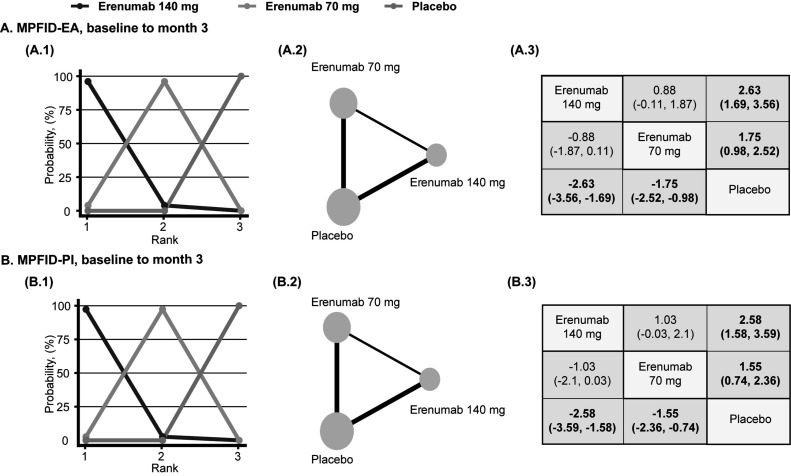
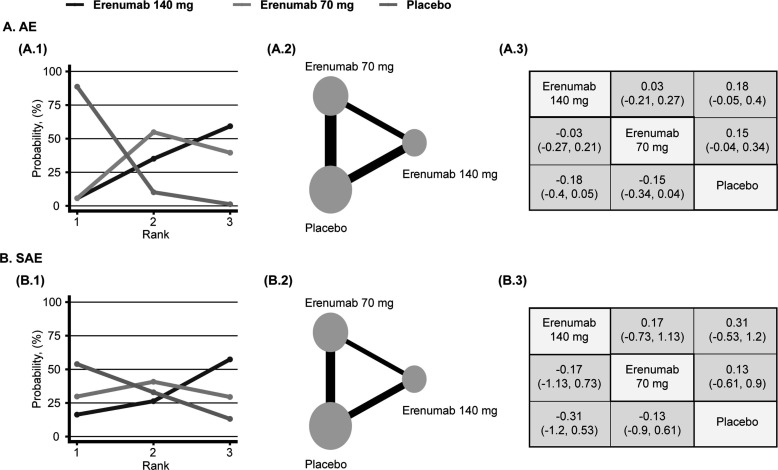
Afterwards, we conducted a Bayesian network meta-analysis assessing the safety and efficacy of different doses of erenumab for the preventive treatment of episodic migraine. In adult patients diagnosed with episodic migraine, the use of 70 mg and 140 mg erenumab was associated with significantly decreased MMD, MSMD, and 50% increased response rate compared to placebo (MMD, 70 mg, MD: -1.28, 95% CI: -1.62 to -0.95, MMD, 140 mg, MD: -1.67, 95% CI: -2.08 to -1.25; MSMD, 70 mg, MD: -0.81, 95% CI: -1 to -0.6; MSMD, 140 mg, MD: -1.32, 95% CI: -1.58 to -1.04; 50% response rate, 70 mg, LOR: 0.64, 95% CI: 0.43 to 0.85; 50% response rate, 140 mg, LOR: 0.91, 95% CI: 0.67 to 1.17) (Fig. 5). Subsequently, compared to erenumab 70 mg, participants who received erenumab 140 mg reported significantly decreased MSMD and 50% increased response rate, and erenumab was most likely to be ranked first for MMD, MSMD, and 50% response rate. (MSMD, MD: -0.51, 95% CI: -0.79 to -0.23; 50% response rate, LOR: 0.28, 95% CI: 0.03 to 0.53) (Fig. 5). For functional improvement, patients who received 70 mg and 140 mg erenumab reported improved MPFID-EA and MPFID-PI at month 3 (MPFID-EA, 70 mg, MD: -1.75, 95% CI: -2.52 to -0.98, MPFID-EA, 140 mg, MD: -2.63, 95% CI: -3.56 to -1.69; MPFID-PI, 70 mg, MD: -1.55, 95% CI: -2.36 to -0.74; MSMD, 140 mg, MD: -2.58, 95% CI: -3.59 to -1.58) (Fig. 6). When comparing 140 mg and 70 mg, although 140 mg erenumab has a high probability to outperform 70 mg, the analysis fails to demonstrate a significant difference in MPFID-EA and MPFID-PI (MPFID-EA, MD: -0.88, 95% CI: -1.87 to 0.11; MPFID-PI, MD: -1.03, 95% CI: -2.1 to 0.03) (Fig. 6). For safety outcomes, the network meta-analysis has found no significantly increased risk for patients received erenumab 70 mg and 140 mg developing adverse events and serious adverse events compared to patients who received placebo (AE, 70 mg, LOR: -0.15, 95% CI: -0.34 to 0.04; AE, 140 mg, LOR: -0.18, 95% CI: -0.4 to 0.05; SAE, 70 mg, LOR: -0.13, 95% CI: -0.9 to 0.61; SAE, 140 mg, LOR: -0.31, 95% CI: -1.2 to 0.53) (Fig. 7). However, the placebo group has the highest probability to be ranked first. Furthermore, no significant difference was found between the 70 mg group and the 140 mg group measured by AE and SAE (AE, LOR: -0.03, 95% CI: -0.27 to 0.21; SAE, LOR: -0.17, 95% CI: -1.13 to 0.73) (Fig. 7). Detailed rank probability chart is listed in Supplement S2 (528.6KB, pdf) , Inconsistency analysis, DIC for random and fixed model, and diagnosis for convergence of each model are listed in Supplement S3 (528.6KB, pdf) -S5 (528.6KB, pdf) .
Fig. (5).
Network diagrams of comparisons between reduced migraine frequency in the placebo, erenumab 70mg, erenumab 140mg groups of patients with episodic migraine. (1) Rank probability plot. (2) Network diagrams of comparisons. The edges represent direct and indirect comparisons observed in the included trials. The size of the nodes was proportional to the number of patients assigned to the treatment protocol, and the thickness of the edges was proportional to the sample size of the included studies. (3) Mean difference/ log odds ratio (95% credible intervals) between column and row treatment regimens. Statistically significant results, where the 95% credible interval does not include 0, are in bold. (A), monthly migraine days (MMD) (Mean Difference). (B), monthly acute migraine-specific medication treatment days (MSMD) (Mean Difference). (C), 50% response rate (Log Odds Ratio).
Fig. (6).
Network diagrams of comparisons between the improved functional outcome in the placebo, erenumab 70mg, erenumab 140mg groups of patients with episodic migraine. (1) Rank probability plot. (2) Network diagrams of comparisons. The edges represent direct and indirect comparisons observed in the included trials. The size of the nodes was proportional to the number of patients assigned to the treatment protocol, and the thickness of the edges was proportional to the sample size of the included studies. (3) Mean difference (95% credible intervals) between column and row treatment regimens. Statistically significant results, where the 95% credible interval does not include 0, are in bold. (A), migraine physical function impact diary-everyday activities (MPFID-EA) (Mean Difference). (B), migraine physical function impact diary-physical impairment (MPFID-PI) (Mean Difference).
Fig. (7).
Network diagrams of comparisons between safety endpoints in the placebo, erenumab 70mg, erenumab 140mg groups of patients with episodic migraine. (1) Rank probability plot. (2) Network diagrams of comparisons. The edges represent direct and indirect comparisons observed in the included trials. The size of the nodes was proportional to the number of patients assigned to the treatment protocol, and the thickness of the edges was proportional to the sample size of the included studies. (3) Log odds ratio (95% credible intervals) between column and row treatment regimens. Statistically significant results, where the 95% credible interval does not include 0, are in bold. (A), adverse events (AE) (Log Odds Ratio). (B), serious adverse events (SAE) (Log Odds Ratio).
3.4. Safety and Efficacy of Erenumab in Patients with Prior Treatment Failures
We then assessed the safety and efficacy of different doses of erenumab in patients with ≥2 treatment failures. For efficacy endpoints, patients treated with 70 mg erenumab showed no significant difference in all the pooled efficacy endpoints compared to the placebo group (MMD, MD: -0.9, 95% CI: -2.41 to 0.6; MSMD, MD: -0.1, 95% CI: 1.23 to 1.03; 50% response rate, RR: 1.79, 95% CI: 0.65 to 4.95; 75% response rate, RR: 3.86, 95% CI: 0.5 to 29.72) (Table 3). Subsequently, for safety outcomes, the use of 70 mg erenumab is not associated with increased risk for developing adverse events or serious adverse events compared to placebo (adverse events, RR: 0.96, 95% CI: 0.7 to 1.31; serious adverse events, RR: 11.59, 95% CI: 0.02 to 6481.06) (Table 3, Supplement S6 (528.6KB, pdf) -11). As one study was included in the analysis, the heterogeneity could not be assessed. Then, for 140 mg erenumab, patients in the 140 mg erenumab group reported significantly reduced MMD and MSMD (MMD, MD: -1.98, 95% CI: -2.93 to -1.03; MSMD, MD: -1.68, 95% CI: -2.27 to -1.09) (Table 4). As for the 50% and 75% response rate, the use of 140 mg erenumab was related to significantly increased 50% and 75% reduction in MMD from the baseline compared to placebo (50% response rate, RR: 2.39, 95% CI:1.52 to 3.77; 75% response rate, RR: 3.32, 95% CI:1.37 to 8.05) (Table 4). For safety outcomes, we found no significant difference in 140 mg erenumab compared to placebo (AE, RR: 0.96, 95% CI: 0.79 to 1.15; SAE, RR: 2.66, 95% CI: 0.29 to 24.79) (Table 4, Supplement S12 (528.6KB, pdf) -17). Heterogeneity was low in all the included outcomes.
Table 3.
Efficacy and safety of 70mg erenumab in patients with ≥ 2 prior treatment failures.
| Outcomes | Erenumab 70 mg Group (n=49) | Placebo Group (n=27) | Number of Trials Included (Total Patients) | Combined Risk Ratio or Mean Difference (95% CI) | I2 |
|---|---|---|---|---|---|
| MMD | -1.8 (3.09), n=49 | -0.9 (3.29), n=27 | 1 (n=76) | -0.9 (-2.41 to 0.61) # | NA |
| MSMD | -1.1 (2.46), n=49 | -1 (2.38), n=27 | 1 (n=76) | -0.1 (-1.23 to 1.03) # | NA |
| 50% response rate | 13/49 (26.53%) | 4/27 (14.81%) | 1 (n=76) | 1.79 (0.65 to 4.95) * | NA |
| 75% response rate | 7/49 (14.29%) | 1/27 (3.7%) | 1 (n=76) | 3.86 (0.5 to 29.72) * | NA |
| AE | 33/49 (67.35%) | 19/27 (70.37%) | 1 (n=76) | 0.96 (0.7 to 1.31) * | NA |
| SAE | 2/49 (4.08%) | 0/27 (0%) | 1 (n=76) | 11.59 (0.02 to 6481.06) * | NA |
Outcome data are mean (SD) or n/N (%). NA= not applicable; MMD=Monthly Migraine Days; MSMD=Monthly Acute Migraine-Specific Medication Treatment Days; AE=Adverse Events; SAE=Serious Adverse Events; CI=Confidence Interval. *Risk ratio (binary outcomes). #Mean difference (quantitative outcomes).
Table 4.
Efficacy and safety of 140mg erenumab in patients with ≥ 2 prior treatment failures.
| Outcomes | Erenumab 140 mg Group (n=177) | Placebo Group (n=151) | Number of Trials Included (Total Patients) | Combined Risk Ratio or Mean Difference (95% CI) | I2 |
|---|---|---|---|---|---|
| MMD | -2.36 (4.05), n=176 | -0.33 (4.2), n=147 | 2 (n=323) | -1.98 (-2.93 to -1.03) # | 11.43 |
| MSMD | -1.66 (2.3), n=176 | 0.22 (3.19), n=147 | 2 (n=323) | -1.68 (-2.27 to -1.09) # | 0 |
| 50% response rate | 63/177 (35.59%) | 21/151 (13.91%) | 2 (n=328) | 2.39 (1.52 to 3.77) * | 0 |
| 75% response rate | 26/177 (14.69%) | 6/151 (3.97%) | 2 (n=328) | 3.32 (1.37 to 8.05) * | 0 |
| AE | 100/177 (56.5%) | 86/151 (56.95%) | 2 (n=328) | 0.96 (0.79 to 1.15) * | 0 |
| SAE | 5/177 (2.82%) | 1/151 (0.66%) | 2 (n=328) | 2.66 (0.29 to 24.79) * | 0 |
Outcome data are mean (SD) or n/N (%). NA= not applicable; MMD=Monthly Migraine Days; MSMD=Monthly Acute Migraine-Specific Medication Treatment Days; AE=Adverse Events; SAE=Serious Adverse Events; CI=Confidence Interval. *Risk ratio (binary outcomes). #Mean difference (quantitative outcomes).
4. DISCUSSION
Our study pooled five randomized clinical trials evaluating the safety and efficacy of erenumab for the treatment of episodic migraine. Our analysis showed that erenumab was efficacious and safe for the treatment of migraine. Furthermore, our study pooled both direct and indirect evidence using the Bayesian network meta-analysis method and demonstrated that 140 mg erenumab outperforms 70 mg erenumab in multiple efficacy endpoints while related to the same risk for developing adverse events. In addition, our study demonstrated that 70 mg and 140 mg erenumab were associated with significantly reduced migraine frequency and improved functional outcomes in patients with prior migraine treatment failures. The heterogeneity was low across all the primary outcomes, indicating a high level of clinical evidence. It is the first meta-analysis that compares the safety and efficacy of different doses of erenumab and the first meta-analysis to demonstrate the safety and efficacy of erenumab in patients with ≥2 prior migraine treatment failures.
The origin of the mechanisms is unclear. However, the involvement of the activation of the trigeminovascular system in migraine is widely accepted. The activation of trigeminal ganglion stimulates the trigeminal nerve fibers. These nerve fibers, releasing CGRP, project to intracranial and extracranial structures, including the pial, arachnoid, dural blood vessels, and the spinal cord trigeminocervical complex (TCC) [15]. Then, the neurons from the TCC project to the brainstem and even to higher-order regions. Some interesting discoveries have been found in the past decades. Serum CGRP levels are elevated during migraine attacks [16]. Meanwhile, the infusion of human CGRP could induce headaches and migraine in migraineurs [17]. CGRP receptors are widely distributed in both the central and peripheral nervous systems [18]. During this process, CGRP is thought to be involved in the transmission of pain information [15]. Perivascular release of CGRP from the trigeminal nerve induces vasodilation, dural mast cell degranulation, and satellite glial activation, leading to peripheral sensitization. The role of CGRP in central sensitization has also been demonstrated.
Although current evidence suggests that the effectiveness of CGRP monoclonal antibodies in migraine treatment is not dramatically increased compared to traditional therapy, the monoclonal antibodies targeting CGRP and its receptors have their unique advantages in migraine treatment. Low compliance in patients with migraine with chronic prophylactic medication use is frequently reported [19]. Erenumab has been designed and modified to extend its circulating half-lives [20]. In clinical practice, it could be given once per month, which could potentially increase compliance among migraineurs. As a preventive therapy, erenumab is likely to be prescribed for long-term use. Thus, the cross-talk of erenumab with other commonly-used drugs is another concern for physicians. A study conducted in 2017 found that subcutaneous erenumab did not influence the effect of estrogen/progestin combination oral contraceptives among healthy females [21]. A placebo-controlled trial investigating co-administration of erenumab 140 mg and sumatriptan 12 mg has found no additional effect on averages of mean arterial pressure or the pharmacokinetics of sumatriptan [22]. Furthermore, evidence suggests that the long-term use of erenumab increased the conversion from chronic migraine to episode migraine [23], especially 140 mg dose [24]. Besides, a previous study reported that erenumab use was associated with a higher response rate in patients who had a high susceptibility to migraine induction by CGRP [25], which suggested its potential applicable role. Moreover, results from clinical trials have demonstrated that erenumab is effective in patients with medication overuse [26-28]. In a posthoc analysis, erenumab showed comparable efficacy between medication over-users and the non-medication overuse group [29]. A cost-analysis based on US societal perspective Markov health state transition model has demonstrated that the use of erenumab was associated with reduced migraine-related direct and indirect costs compared to supportive care [30].
As CGRP ligand could dilate blood vessels, the potential risk on the cardiovascular system caused by CGRP targeted therapies attracts much attention. A study conducted on human isolated cranial arteries has found that the use of erenumab was not associated with direct vasoconstriction and did not influence the effect of endogenous or exogenous vasoactive compounds [31]. A 12-week trial conducted by Tepper et al. confirmed that erenumab did not affect the blood pressure and 24-hour blood pressure changes in healthy volunteers [32]. No significant difference was found in the emergence of vascular adverse events between the erenumab group and the placebo group in the short-term migraine treatment phase [33]. In addition, a study conducted on patients with stable angina has found that the use of erenumab had no effect on exercise time [34]. Although these studies have demonstrated that CGRP targeted therapies are not likely to induce severe adverse events caused by vasoconstriction, CGRP may play a more critical role in the change of vascular tension in hypertensive rats than in normotensive rats [35]. Hence, future researches are still needed to evaluate the long-term use of erenumab in patients with cardiovascular risk or hypertension.
Our study mainly pooled short-term placebo-controlled trials for episodic migraineurs. Erenumab also showed its effect on chronic migraine patients [36]. So far, a limited number of studies have been conducted to evaluate the long-term use of erenumab. An open-label study evaluating long-term safety and efficacy of erenumab in patients with episodic migraine found that erenumab use is related to improved function and favorable safety and tolerability profile [37]. The long-term adverse effects of erenumab include injection-site reactions, constipation, and muscle spasm [38]. In another similar long-term study, erenumab was found to be safe and well-tolerated, and the adverse events were considered consistent with shorter-term placebo treatment [39].
Our study had a few limitations. First, although our study included five multicenter randomized trials that have a low risk for bias, the heterogeneity of overall functional improvement in the pair-wise meta-analysis was considerably high, indicating a low level of clinical evidence. Second, all the included clinical trials were limited to short-term use; further pooled analyses based on long-term, or real-world studies are needed. Third, our analysis on 70 mg erenumab in patients with previous treatment failure only includes one trial with insufficient sample size. Further studies are still needed to provide more robust evidence.
CONCLUSION
Our results showed that compared to 70mg erenumab, 140 mg erenumab might be a better choice for patients with episodic migraine, especially for those with prior migraine treatment failures. So far, no direct comparison between using 140 mg directly and switching 70 mg to 140 mg, if poorly responded, has been made. Further studies need to be carried out if possible.
ACKNOWLEDGEMENTS
The authors are thankful to all participants of the neurosurgical study.
LIST OF ABBREVIATIONS
- CGRP
Calcitonin Gene-related Peptide
- CI
Confidence Intervals
- HIT
Headache Impact Test
- MMD
Monthly Migraine Days
- MPFID-EA
Migraine Physical Function Impact Diary-Everyday Activities
- MPFID-PI
Migraine Physical Function Impact Diary-physical Impairment
- MSMD
Monthly Acute Migraine-specific Medication Treatment Days
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-analyses
- RCTs
Randomized Clinical Trials
- RR
Relative Risk
- SAE
Serious Adverse Events
- TEAE
Treatment-Emergent Adverse Events
CONSENT FOR PUBLICATION
Not applicable.
STANDARDS OF REPORTING
PRISMA guidelines were followed for the study.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
SUPPLEMENTARY MATERIAL
Supplementary material and PRISMA checklist are available on the publisher’s website along with the published article.
REFERENCES
- 1.Lattanzi S., Brigo F., Trinka E., Vernieri F., Corradetti T., Dobran M., Silvestrini M. Erenumab for preventive treatment of migraine: a systematic review and meta-analysis of efficacy and safety. Drugs. 2019;79(4):417–431. doi: 10.1007/s40265-019-01069-1. [DOI] [PubMed] [Google Scholar]
- 2.Goadsby P.J., Edvinsson L., Ekman R. Vasoactive peptide release in the extracerebral circulation of humans during migraine headache. Ann. Neurol. 1990;28(2):183–187. doi: 10.1002/ana.410280213. [DOI] [PubMed] [Google Scholar]
- 3.Amara S.G., Jonas V., Rosenfeld M.G., Ong E.S., Evans R.M. Alternative RNA processing in calcitonin gene expression generates mRNAs encoding different polypeptide products. Nature. 1982;298(5871):240–244. doi: 10.1038/298240a0. [DOI] [PubMed] [Google Scholar]
- 4.Ho T.W., Edvinsson L., Goadsby P.J. CGRP and its receptors provide new insights into migraine pathophysiology. Nat. Rev. Neurol. 2010;6(10):573–582. doi: 10.1038/nrneurol.2010.127. [DOI] [PubMed] [Google Scholar]
- 5.Tiseo C., Ornello R., Pistoia F., Sacco S. How to integrate monoclonal antibodies targeting the calcitonin gene-related peptide or its receptor in daily clinical practice. J. Headache Pain. 2019;20(1):49. doi: 10.1186/s10194-019-1000-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Traynor K. FDA approves licensing of erenumab-aooe to prevent migraine. J. Health Syst. 2018;75(13):929–930. doi: 10.2146/news180044. [DOI] [PubMed] [Google Scholar]
- 7.Ornello R., Tiseo C., Frattale I., Perrotta G., Marini C., Pistoia F., Sacco S. The appropriate dosing of erenumab for migraine prevention after multiple preventive treatment failures: A critical appraisal. J. Headache Pain. 2019;20(1):99. doi: 10.1186/s10194-019-1054-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liberati A., Altman D.G., Tetzlaff J., Mulrow C., Gøtzsche P.C., Ioannidis J.P., Clarke M., Devereaux P.J., Kleijnen J., Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sakai F., Takeshima T., Tatsuoka Y., Hirata K., Lenz R., Wang Y., Cheng S., Hirama T., Mikol D.D. A Randomized phase 2 study of erenumab for the prevention of episodic migraine in japanese adults. Headache. 2019;59(10):1731–1742. doi: 10.1111/head.13652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dodick D.W., Ashina M., Brandes J.L., Kudrow D., Lanteri-Minet M., Osipova V., Palmer K., Picard H., Mikol D.D., Lenz R.A. ARISE: A Phase 3 randomized trial of erenumab for episodic migraine. Cephalalgia. 2018;38(6):1026–1037. doi: 10.1177/0333102418759786. [DOI] [PubMed] [Google Scholar]
- 11.Reuter U., Goadsby P.J., Lanteri-Minet M., Wen S., Hours-Zesiger P., Ferrari M.D., Klatt J. Efficacy and tolerability of erenumab in patients with episodic migraine in whom two-to-four previous preventive treatments were unsuccessful: A randomised, double-blind, placebo-controlled, phase 3b study. Lancet. 2018;392(10161):2280–2287. doi: 10.1016/S0140-6736(18)32534-0. [DOI] [PubMed] [Google Scholar]
- 12.Goadsby P.J., Reuter U., Hallström Y., Broessner G., Bonner J.H., Zhang F., Sapra S., Picard H., Mikol D.D., Lenz R.A. A controlled trial of erenumab for episodic migraine. N. Engl. J. Med. 2017;377(22):2123–2132. doi: 10.1056/NEJMoa1705848. [DOI] [PubMed] [Google Scholar]
- 13.Sun H., Dodick D.W., Silberstein S., Goadsby P.J., Reuter U., Ashina M., Saper J., Cady R., Chon Y., Dietrich J., Lenz R. Safety and efficacy of AMG 334 for prevention of episodic migraine: A randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Neurol. 2016;15(4):382–390. doi: 10.1016/S1474-4422(16)00019-3. [DOI] [PubMed] [Google Scholar]
- 14.Goadsby P.J., Paemeleire K., Broessner G., Brandes J., Klatt J., Zhang F., Picard H., Lenz R., Mikol D.D. Efficacy and safety of erenumab (AMG334) in episodic migraine patients with prior preventive treatment failure: A subgroup analysis of a randomized, double-blind, placebo-controlled study. Cephalalgia. 2019;39(7):817–826. doi: 10.1177/0333102419835459. [DOI] [PubMed] [Google Scholar]
- 15.Goadsby P.J., Holland P.R., Martins-Oliveira M., Hoffmann J., Schankin C., Akerman S. Pathophysiology of migraine: a disorder of sensory processing. Physiol. Rev. 2017;97(2):553–622. doi: 10.1152/physrev.00034.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goadsby P.J., Edvinsson L., Ekman R. Release of vasoactive peptides in the extracerebral circulation of humans and the cat during activation of the trigemino vascular system. Ann. Neurol. 1988;23(2):193–196. doi: 10.1002/ana.410230214. [DOI] [PubMed] [Google Scholar]
- 17.Lassen L.H., Haderslev P.A., Jacobsen V.B., Iversen H.K., Sperling B., Olesen J. CGRP may play a causative role in migraine. Cephalalgia. 2002;22(1):54–61. doi: 10.1046/j.1468-2982.2002.00310.x. [DOI] [PubMed] [Google Scholar]
- 18.Diener H.C. CGRP as a new target in prevention and treatment of migraine. Lancet Neurol. 2014;13(11):1065–1067. doi: 10.1016/S1474-4422(14)70228-5. [DOI] [PubMed] [Google Scholar]
- 19.Hepp Z., Dodick D.W., Varon S.F., Gillard P., Hansen R.N., Devine E.B. Adherence to oral migraine-preventive medications among patients with chronic migraine. Cephalalgia. 2015;35(6):478–488. doi: 10.1177/0333102414547138. [DOI] [PubMed] [Google Scholar]
- 20.Jain S., Yuan H., Spare N., Silberstein S.D. Erenumab in the treatment of migraine. Pain Manag. (Lond.) 2018;8(6):415–426. doi: 10.2217/pmt-2018-0037. [DOI] [PubMed] [Google Scholar]
- 21.Xu Y., Gabriel K., Wang Y., Zhou Y., Eisele O., Vutikullird A., Mikol D.D., Lee E., Multi-Center A. A multi-center, open-label, pharmacokinetic drug interaction study of erenumab and a combined oral contraceptive in healthy females. CNS Drugs. 2019;33(5):513–522. doi: 10.1007/s40263-019-00626-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Hoon J., Van Hecken A., Vandermeulen C., Herbots M., Kubo Y., Lee E., Eisele O., Vargas G., Gabriel K. Phase 1, randomized, parallel-group, double-blind, placebo-controlled trial to evaluate the effects of erenumab (AMG 334) and concomitant sumatriptan on blood pressure in healthy volunteers. Cephalalgia. 2019;39(1):100–110. doi: 10.1177/0333102418776017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lipton R.B., Tepper S.J., Silberstein S., Kudrow D., Ashina M., Reuter U., Dodick D., Zhang F., Rippon G.A., Mikol D.D. Conversion from chronic to episodic migraine with erenumab, a specific inhibitor of the calcitonin gene-related peptide receptor. J. Headache Pain. 2018:19. [Google Scholar]
- 24.Lipton R., Tepper S., Silberstein S., Kudrow D., Ashina M., Reuter U., Dodick D., Cheng S., Rippon G., Zhang F. Conversion from chronic migraine (CM) to episodic migraine (EM) with long-term erenumab treatment. Neurology. 2019;92(15) Suppl [Google Scholar]
- 25.Christensen C.E., Younis S., Deen M., Khan S., Ghanizada H., Ashina M. Migraine induction with calcitonin gene-related peptide in patients from erenumab trials. J. Headache Pain. 2018;19(1):105. doi: 10.1186/s10194-018-0927-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Diener H.C., Ashina M., Brandes J., Friedman D., Reuter U., Tepper S., Cheng S., Leonardi D., Lenz R., Mikol D. Efficacy of erenumab for the treatment of patients with chronic migraine in presence of medication overuse. Eur. J. Neurol. 2017;24:472. [Google Scholar]
- 27.Dodick D., Tepper S., Diener H.C., Tassorelli C., Lucas S., Evers S., Zhang F., Chou D., Tenenbaum N., Klatt J. Efficacy of erenumab in chronic migraine patients with medication overuse and prior preventive treatment failure. Eur. J. Neurol. 2019;26:811. [Google Scholar]
- 28.Tepper S., Diener H.C., Ashina M., Brandes J.L., Friedman D.T., Reuter U., Cheng S., Leonardi D., Lenz R., Mikol D. Efficacy of erenumab for the treatment of patients with chronic migraine in presence of medication overuse. Schmerz. 2017;31(2):S66. [Google Scholar]
- 29.Tepper S.J., Diener H.C., Ashina M., Brandes J.L., Friedman D.I., Reuter U., Cheng S., Nilsen J., Leonardi D.K., Lenz R.A., Mikol D.D. Erenumab in chronic migraine with medication overuse: Subgroup analysis of a randomized trial. Neurology. 2019;92(20):e2309–e2320. doi: 10.1212/WNL.0000000000007497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lipton R.B., Brennan A., Palmer S., Hatswell A.J., Porter J.K., Sapra S., Villa G., Shah N., Tepper S., Dodick D. Estimating the clinical effectiveness and value-based price range of erenumab for the prevention of migraine in patients with prior treatment failures: A US societal perspective. J. Med. Econ. 2018;21(7):666–675. doi: 10.1080/13696998.2018.1457533. [DOI] [PubMed] [Google Scholar]
- 31.Ohlsson L., Haanes K.A., Kronvall E., Xu C., Snellman J., Edvinsson L. Erenumab (AMG 334), a monoclonal antagonist antibody against the canonical CGRP receptor, does not impair vasodilatory or contractile responses to other vasoactive agents in human isolated cranial arteries. Cephalalgia. 2019;39(14):1745–1752. doi: 10.1177/0333102419867282. [DOI] [PubMed] [Google Scholar]
- 32.Tepper S.J., Pascual J., Reuter U., Picard H., Hong F., Trotman M.L., Xue F., Mikol D., Klatt J. Analysis of blood pressure following short-term and long-term treatment with erenumab. J. Headache Pain. 2018;90(15) Suppl. [Google Scholar]
- 33.Kudrow D., Pascual J., Winner P.K., Dodick D.W., Tepper S.J., Reuter U., Hong F., Klatt J., Zhang F., Cheng S., Picard H., Eisele O., Wang J., Latham J.N., Mikol D.D. Vascular safety of erenumab for migraine prevention. Neurology. 2020;94(5):e497–e510. doi: 10.1212/WNL.0000000000008743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Depre C., Antalik L., Starling A., Koren M., Eisele O., Lenz R.A., Mikol D.D.A. Randomized, double-blind, placebo-controlled study to evaluate the effect of erenumab on exercise time during a treadmill test in patients with stable angina. Headache. 2018;58(5):715–723. doi: 10.1111/head.13316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Balfagón G., Márquez-Rodas I., Alvarez Y., Alonso M.J., Cachofeiro V., Salaices M., Lahera V. Aldosterone modulates neural vasomotor response in hypertension: role of calcitonin gene-related peptide. Regul. Pept. 2004;120(1-3):253–260. doi: 10.1016/j.regpep.2004.03.016. [DOI] [PubMed] [Google Scholar]
- 36.Tepper S., Ashina M., Reuter U., Brandes J.L., Doležil D., Silberstein S., Winner P., Leonardi D., Mikol D., Lenz R. Safety and efficacy of erenumab for preventive treatment of chronic migraine: A randomised, double-blind, placebo-controlled phase 2 trial. Lancet Neurol. 2017;16(6):425–434. doi: 10.1016/S1474-4422(17)30083-2. [DOI] [PubMed] [Google Scholar]
- 37.Ashina M., Dodick D., Goadsby P.J., Reuter U., Silberstein S., Zhang F., Gage J.R., Cheng S., Mikol D.D., Lenz R.A. Erenumab (AMG 334) in episodic migraine: Interim analysis of an ongoing open-label study. Neurology. 2017;89(12):1237–1243. doi: 10.1212/WNL.0000000000004391. [DOI] [PubMed] [Google Scholar]
- 38.Ashina M., Kudrow D., Reuter U., Dolezil D., Silberstein S., Tepper S.J., Xue F., Picard H., Zhang F., Wang A., Zhou Y., Hong F., Klatt J., Mikol D.D. Long-term tolerability and nonvascular safety of erenumab, a novel calcitonin gene-related peptide receptor antagonist for prevention of migraine: A pooled analysis of four placebo-controlled trials with long-term extensions. Cephalalgia. 2019;39(14):1798–1808. doi: 10.1177/0333102419888222. [DOI] [PubMed] [Google Scholar]
- 39.Ashina M., Goadsby P. J., Reuter U., Silberstein S., Dodick D., Rippon G. A., Klatt J., Xue F., Chia V., Zhang F., Cheng S., Mikol D. D. Long-term safety and tolerability of erenumab: Threeplus year results from a five-year open-label extension study in episodic migraine. Cephalalgia, Cephalalgia. 2019;39(1455):1455–1464. doi: 10.1177/0333102419854082. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material and PRISMA checklist are available on the publisher’s website along with the published article.