Abstract
In contrast to that of other monoamine neurotransmitters, the association of the histaminergic system with neuropsychiatric disorders is not well documented. In the last two decades, several clinical studies involved in the development of drugs targeting the histaminergic system have been reported. These include the H3R-antagonist/inverse agonist, pitolisant, used for the treatment of excessive sleepiness in narcolepsy, and the H1R antagonist, doxepin, used to alleviate symptoms of insomnia. The current review summarizes reports from animal models, including genetic and neuroimaging studies, as well as human brain samples and cerebrospinal fluid measurements from clinical trials, on the possible role of the histaminergic system in neuropsychiatric disorders. These studies will potentially pave the way for novel histamine-related therapeutic strategies.
Keywords: Histamine, histidine decarboxylase, histamine receptors, histamine N-methyltransferase, Alzheimer’s disease, Parkinson’s disease, Huntington’s disease, depression, schizophrenia and intellectual disability
1. INTRODUCTION
In human neuropsychiatric disorders, effective treatments have been developed based generally upon changes in neurotransmitter systems affected by the disorder. For instance, dopaminergic neurons in the substantia nigra are largely lost in Parkinson’s disease patients (PD) [1-3], and L-dopa (the precursor of dopamine) was developed as the first-line treatment. In addition, a genetic study showed that serotonin transporter genes are highly associated with depression and anxiety disorders [4], and patients are successfully treated with selective serotonin reuptake inhibitors (SSRIs).
A number of physiological functions are modulated by the neuronal histaminergic system. These include the sleep-wake cycle, sensory and motor functions, cognition and attention, all of which are affected in neuropsychiatric disorders. Significant clinical progress has been made in the use of the H3R-antagonist/inverse agonist for the treatment of excessive sleepiness of narcolepsy [5, 6]. It is time to systematically review the pathophysiology of the histamine system in order to implement novel therapeutic strategies for the treatment of neuropsychiatric disorders. In the present review, we summarize findings from DNA, RNA and protein expression studies, neuroimaging reports, cerebral spinal fluid (CSF) measurements and recent clinical trials to discuss histamine receptors and key enzymes of histamine synthesis and metabolism, and their possible involvement in neuropsychiatric disorders.
2. HISTAMINE SYNTHESIS, METABOLISM AND RECEPTORS IN THE CENTRAL NERVOUS SYSTEM
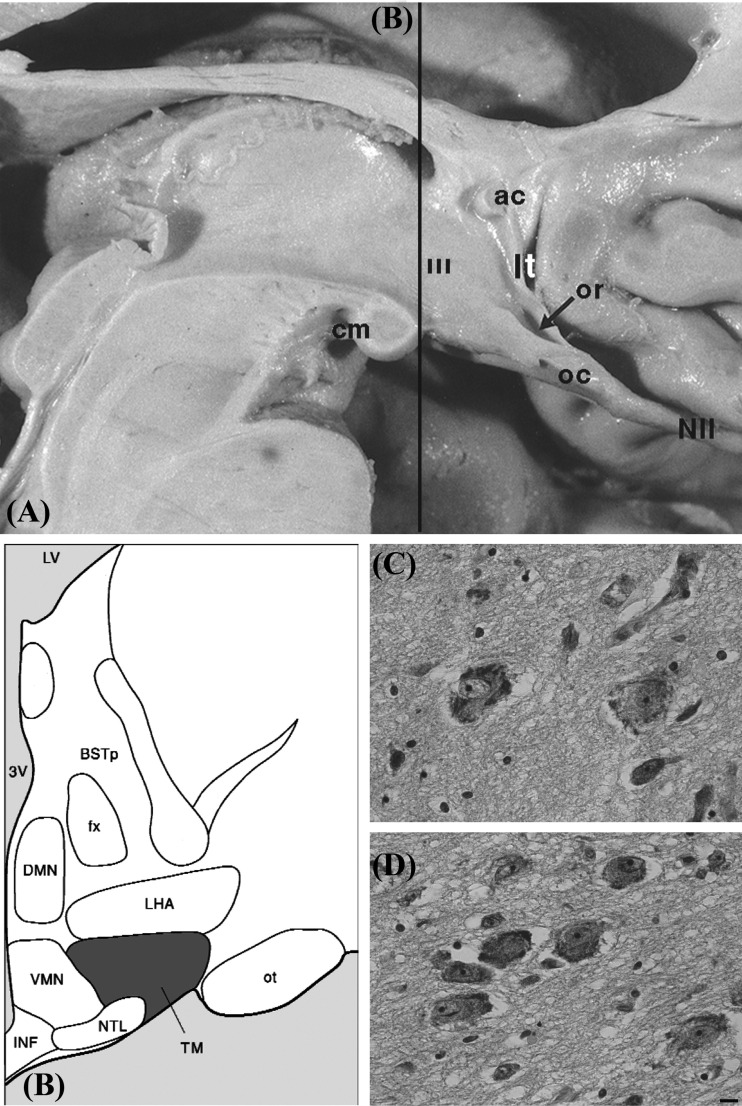
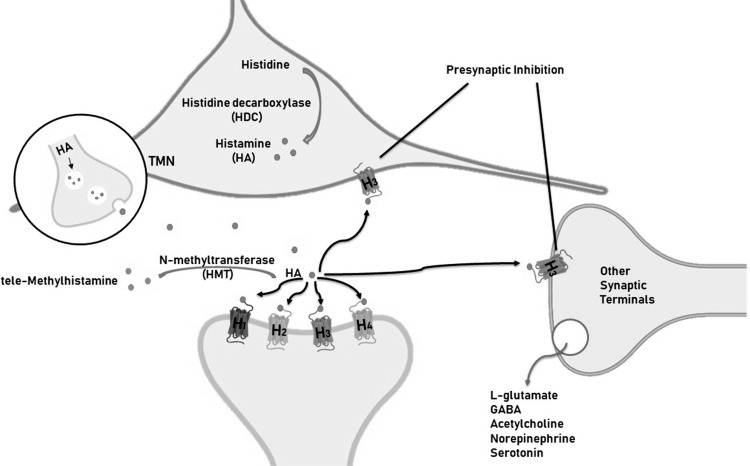
Neuronal histamine is produced in the hypothalamic tuberomamillary nucleus (TMN) [7, 8], where histidine decarboxylase (HDC), involved in the synthesis of histamine, is the key enzyme (Fig. 1). HDC immunoreactivity is often used as a marker of histamine neurons. The TMN innervates several brain areas, including the hypothalamus, basal ganglia, prefrontal cortex, and hippocampus. Histamine is inactivated by conversion to tele-methylhistamine (t-MeHA) by the enzyme histamine N-methyltransferase (HMT) [9-11] (Fig. 2). There are 4 types of G protein-coupled histamine receptors widely expressed in the brain (H1-4R) [9-11]. The H3R is also an auto receptor located pre-synaptic to modulate the release of histamine, glutamate, GABA, acetylcholine, norepinephrine and serotonin.
Fig. (1).
The tuberomamillary nucleus (A). Medial surface of the human hypothalamus. Line b indicating the layer for Figure (B). Abbreviations: ac: anterior commissure, cm: corpus mamillare, lt: lamina terminalis, NII: optic nerve, oc: optic chiasm, or: optic recess, III: third ventricle. B: the human hypothalamus in representative coronal cuts with the tuberomamillary nucleus highlighted (Adapted from [100]; Fig. 2.) Abbreviations: BSTp: bed nucleus of the stria terminalis posterior, DMN: the dorsomedial hypothalamic nucleus, ot: optic tract, oc: optic chiasma, fx: fornix, INF: infundibular nucleus, LHA: Lateral hypothalamus, LV: lateral ventricle, NTL: Lateral tuberal nucleus, TM: tuberomamillary nucleus, VMN: ventromedial hypothalamic nucleus, 3V: third ventricle. (C and D). examples of Nissl staining of TM nucleus neurons w ith typical neuron profiles, scale bar = 5µm ([101] with permission).
Fig. (2).
Schematic illustration of histamine synthesis, metabolism and receptors in brain. Histamine is synthesized by the enzyme, histidine decarboxylase (HDC) in the tuberomamillary nucleus (TMN). The enzyme histamine N-methyltransferase (HMT) inactivates histamine. There are 4 types of histamine receptors (H1-4R). The H3R is also an auto receptor located pre-synaptic to modulate the release of histamine, glutamate, GABA, acetylcholine, norepinephrine and serotonin.
3. HDC
3.1. Circadian Rhythmicity of HDC Expressing Neurons
Since Dahlstrom and Fuxe’s discovery that monoaminergic cells are segregated in discrete populations [12], it has been assumed that the brain contains a fixed number of each type of monoaminergic neuron. There are about 64000 histaminergic neurons in the TMN [13]. However, the number of HDC expressing neurons fluctuates according to the time of day. Diurnal fluctuations of HDC-mRNA and protein levels have been reported in rodents and humans [14, 15]. In mice, the number of HDC immunoreactive neurons was 34% greater in the active phase than in the rest phase [16]. Some neurons may produce HDC at levels below the detection threshold of immunohistochemistry, becoming detectable only during the active phase. Similarly, histidine decarboxylase-mRNA expression was 37% higher in humans with a clock time of death (the time of the day the case passed away) during the daytime than in those with a clock time of death at night [14]. However, the diurnal HDC-mRNA fluctuations were lost in some patients with Alzheimer’s disease (AD), PD, preclinical PD, and Huntington’s disease [17]. The sleep-wake disturbance of these diseases may thus, at least in part, be caused by the attenuated increase in arousal-induced histamine levels [17, 18]. Furthermore, HDC-mRNA expression and protein levels were altered in mice with a knockdown of BMAL1, a key clock gene, in TMN neurons. These mice also showed altered sleep architecture [15]. The neuronal histamine modulated the output pathway of the circadian system. In the HDC knockout mice, the 24-hour profiles of clock genes in the suprachiasmatic nucleus were intact, whereas the rhythms of mPer-mRNA were altered in other brain areas, such as the cortex and striatum [19]. Conditional knockout CRISPR-Cas 9 mice with neuron-specific deletion of HDC also showed a reduction in circadian activity [20]. Together these findings support the role of HDC in maintaining normal circadian rhythmicity.
3.2. Unaltered HDC Expression in both PD and AD
In PD pathology, Lewy-bodies start accumulating in the TMN at the preclinical stage [21]. However, the HDC-mRNA levels, the number of histaminergic neurons and the enzymatic activity of HDC were found to be stable in PD patients [22-24]. Furthermore, CSF levels of the main metabolite of histamine, t-MeHA, also remained unchanged in PD patients [25]. Despite the significant loss of histaminergic neurons in AD, expression of HDC-mRNA remained unaltered in the TMN [26].
3.3. Increased HDC Immunoreactivity in Narcolepsy Type 1 Patients
The loss of up to 90% of the hypocretin (orexin) neurons in the hypothalamus is a major cause of narcolepsy with cataplexy (narcolepsy type 1) [27, 28]. Narcolepsy is a disabling neurological disease characterized by symptoms of excessive daytime sleepiness, hypnagogic hallucinations, sleep paralysis and disturbed nocturnal sleep [29]. The major clinical symptoms are also exhibited in animal models with disrupted hypocretin pathways [30-32]. The hypothalamic hypocretinergic neurons project to the histaminergic neurons in the TMN. In 2013, two research groups independently observed that HDC immunoreactivity was greatly increased in the TMN of narcoleptic patients [33, 34]. In contrast to the increased number of HDC-positive neurons in humans, decreased [35, 36] or unchanged [37] histamine levels were observed in the lumbar CSF of narcolepsy type 1 cases.
In contrast to the findings in humans, no changes in HDC positive neurons were observed in any of several narcoleptic animal models, including the hypocretin receptor 2 mutant dog, the hypocretin knock-out mouse or the orexin/ ataxin-3 transgenic mouse [33]. The increase in histamine neurons in human narcoleptics was thus not simply a compensation for the loss of hypocretin neurons. In addition, Parkinson’s patients [38, 39] showed a decreased number of hypocretin neurons, but the histamine neurons in the posterior hypothalamus were relatively stable [22, 40]. We, therefore, hypothesised that the increase in histamine neurons in human narcolepsy may be linked to autoimmune response causing new neurons to be generated (as a result of increased histamine production)or ‘transmitter re-specification’ [41-43] (reviewed in [44]).
4. HISTAMINE N-METHYLTRANSFERASE (HMT)
4.1. HMT Mutation Associated with Intellectual Disability and Aggressive Behaviours in Humans
HMT is the key enzyme involved in the metabolism of histamine. Two homozygous HMT mutations (i.e. p.Gly60Asp and p.Leu208Pro) were identified in patients suffering from non-syndromic autosomal recessive intellectual disability, also known as mental retardation autosomal recessive 51 (MRT51; OMIM: 616739), in two unrelated consanguineous families [45]. The p.Gly60Asp mutation affects HMT enzymatic activity and the p.Leu208Pro mutation results in misfolding and rapid degradation of the HMT protein [45]. A recent study reported a severely intellectually disabled Dutch male with a homozygous mutation in the HMT gene, who demonstrated the same behavioural phenotype as HMT-associated MRT51 [46]. Additionally, this case was characterized with autism, dysregulation of sleep-wake states and aggressive behaviours, including self-injury [46]. All these symptoms were found to be associated with high histamine levels because the sleep-wake cycle disturbance and aggressive behaviours were effectively treated with the antihistaminergic compound hydroxyzine, in combination with a histamine-restricted diet [46].
4.2. HMT Knockout Mice Exhibit Sleep-wake Disorders and Aggressive Behaviours
HMT knockout animals showed elevated histamine concentrations in the brain [47]. In line with the augmented histamine concentrations, the HMT knockout mice exhibited prolonged awakening during the light (inactive) period and more sleep during the dark (active) phase [47].
Histamine neurons appear in rats on embryonic day 13; histamine immunoreactive nerve fibres are visible in embryonic day 15 [48]. Histamine facilitates N-methyl-D-aspartate (NMDA) receptor-dependent long-term potentiation (LTP) via H3R receptors during the second postnatal week, but inhibits synaptic plasticity at later developmental stages [49]. Histamine concentration was more than 6-fold higher in HMT knockout neonate mice compared to age-matched wild type mice [47]. Deficiency in the enzyme that inactivates histamine may have a negative influence on brain development, leading to impaired cognitive and behavioural phenotypes.
HMT knockout mice, with higher brain histamine levels displayed high levels of aggression in the resident-intruder and aggressive biting behaviour tests [47]. Conversely, H1R receptor knockout mice with stable brain histamine levels were less aggressive in the resident-intruder test [50, 51]. Lower brain histamine levels were also observed in the H3R knockout mice, where the aggression behaviour phenotypes were unfortunately not studied [52, 53] However, a novel H3R knockout zebrafish exhibited reduced aggression in the mirror-induced aggression behaviour test [54]. Huntington’s disease patients showed increased neuronal histamine production as reflected by elevated HDC-mRNA expression [55] and elevated CSF levels of histamine metabolites [56]. Upregulation of the histamine system in Huntington’s patients may therefore be involved in the aggressive behaviours of this disease.
5. HISTAMINE RECEPTORS
5.1. The H1R Antagonist, Doxepin, Alleviates Symptoms of Insomnia
The over-the-counter antihistamine, diphenhydramine, used to relieve allergies, has H1R antagonistic activity and is able to penetrate the blood-brain barrier [57]. In addition, the antidepressant, doxepin, which affects cholinergic, dopaminergic, serotoninergic and adrenergic receptors, is also a selective H1R antagonist [58]. Both doxepin and diphenhydramine increased non-rapid eye movement sleep in wild type mice but not in H1R knockout mice, suggesting that they act through the H1 receptor [59]. Consistent with this preclinical finding, a randomized, placebo-controlled trial in patients with chronic primary insomnia showed that doxepin prevented early morning awakenings as well as improved sleep in the latter part of the night [60]. Several other studies showed similar improvement in sleep maintenance and sleep duration with doxepin compared to placebo [61-65]. The U.S. food and drug administration (FDA) has approved doxepin for the clinical treatment of insomnia [66].
5.1.1. H1R Modulation of Cognition and Mood
Recently, a comprehensive study showed that selectively knocking out H1R in mouse basal forebrain cholinergic neurons resulted in sensorimotor gating deficit, social impairment and anhedonia-like behaviours (Cheng et al., 2021). This is consistent with the finding that schizophrenic patients who suffer from negative symptoms had lower H1R mRNA in the cholinergic neurons of the nucleus basalis of Meynert (Cheng et al., 2021). This effect is cholinergic cell-specific because targeted deletions of H1R in glutamatergic or dopaminergic neurons did not show sensorimotor gating deficits (Cheng et al., 2021). Indeed, patients with varied neuropsychiatric disorders exhibit reductions of H1R binding in a brain region-specific manner. Positron emission tomography studies showed that H1R binding was much lower in the frontal cerebral cortex of depressed patients compared to matched controls [67, 68]. Interestingly, H1R binding in the frontal cortex and cingulate gyrus decreased in relation to self-rated depressive scale scores [67]. Reduced H1R binding has also been reported in the frontal and temporal brain areas of AD patients [69]. More importantly, there is a correlation between H1R binding and the severity of cognitive deficits [69]. Although changes in H1R binding have been reported in these patients, no changes in H1R mRNA levels were observed in the frontal cortex of depressed (Shan et al., 2013a) or AD patients in our postmortem studies (Shan et al., 2012b).
5.2. H2R has no Effect on Schizophrenia
H2R antagonists are widely used for the treatment of gastric disorders [70]. H2R knockout mice showed cognitive deficits in object recognition and Barnes maze tests, indicating an impairment in hippocampal LTP [71]. Although earlier studies supported the finding that the H2R antagonist, famotidine, exhibited antipsychotic effects and reduced symptoms of schizophrenia in preliminary open-labelled clinical trials [72-75], a meta-analysis, based on 8 double-blind randomized placebo-controlled trials, concluded that H2R-antagonists did not have any effect on schizophrenic symptoms [76]. This is in line with the fact that none of the polymorphisms in H2R has been consistently linked to psychotic symptoms in schizophrenia [77, 78].
5.3. The H3R Antagonist/Inverse Agonist, Pitolisant, Reduces Symptoms of Narcolepsy
By far, the most successful clinical application of the H3R antagonist/inverse agonist, pitolisant, is in the treatment of narcolepsy. As we mentioned, narcoleptic patients lose up to 90% of their hypocretin cells, which results in their inability to stay awake [27, 28]. We speculate that the H3R-antagonist/inverse agonist, pitolisant, used to treat excessive daytime sleepiness in narcoleptics, acts by stimulating adjacent histaminergic neurons, and thus reduces the transition to rapid eye movement sleep [79-81]. PD patients have disrupted circadian rhythmicity of HDC activity [14] as well as loss of hypocretin neurons [38, 82]. We hypothesize that H3R-antagonist/inverse agonists may work in a similar manner to alleviate excessive daytime sleepiness in PD patients [83]. Although patients with preclinical AD, as well as animal models of schizophrenia, showed cognitive improvements with H3R-antagonist/inverse agonists [83, 84], the clinical results were less convincing. Furthermore, H3R-antagonist/inverse agonists failed to reduce the cognitive deficits in patients with AD, in several randomized controlled trials [85-87]. A randomized double-blind placebo-controlled study also showed a lack of cognitive improvement in schizophrenia patients by an H3R-antagonist [88, 89].
5.4. H4R a Promising Therapeutic Target for PD
Loss of dopaminergic neurons in the mesencephalon leads to motor deficits in Parkinson’s disease (PD) [3, 90]. Dr Panula’s group previously observed increased density of histaminergic fibers in the substantia nigra, [91] and enhanced histamine levels in the basal ganglia [92] of postmortem brains of PD patients. Augmented histamine stimulated pro-inflammatory microglial activity and accelerated degeneration of dopamine neurons in the substantia nigra of animal models [93-95]. By inhibiting endogenous neuronal histamine production, dopaminergic neurons in substantia nigra of the 6-hydroxydopamine (6-OHDA)-lesioned rat were partially protected from degeneration [96]. However, to clinically inhibit endogenous neuronal histamine production would be rather difficult. An alternative approach is to determine which histamine receptor is involved in the pro-inflammatory microglial activity process.
Recently, our group showed for the first time that the H4R is a promising therapeutic target in the treatment of PD. Using quantitative PCR (qPCR), our group observed that H4R-mRNA levels were higher in the basal ganglia of 7 PD compared to 7 control postmortem brains [97]. Recently, using unbiased transcriptome wide RNA-sequencing, we confirmed this upregulation of H4R-mRNA levels in the basal ganglia of another 10 PD and 10 control brains [98]. Furthermore, the H4R antagonist, JNJ7777120, has been reported to inhibit dopaminergic neuron degeneration in a rat model of rotenone-induced PD [99]. In addition, we have shown that an H4R antagonist inhibited pro-inflammatory microglia activation in the substantia nigra and striatum, as well as reduced Lewy body-like PD neuropathology [98, 99]. Together these results pave the way for clinical testing of the H4R antagonist in PD.
CONCLUSION
The brain histaminergic system plays a pivotal role in the pathogenesis of several neuropsychiatric disorders, including narcolepsy, schizophrenia, depression, HD, AD and PD. By targeting the histaminergic system in the brain, novel therapeutic interventions may be developed to treat these disorders. Here, we summarized two successful clinical treatments; three preclinical findings to be followed with clinical trials; and several open issues warranting further study.
Two successful clinical treatments: 1) The H1R antagonist, doxepin, has been approved by the FDA for the treatment of insomnia. 2) Both FDA and European medicines agency (EMA) have approved the H3R-antagonist/inverse agonist, pitolisant, for the treatment of excessive daytime sleepiness in narcolepsy.
Three preclinical findings potentially lead to clinical treatments: 1) Loss of diurnal HDC-mRNA rhythmicity in neurodegenerative disorders, including AD, PD, preclinical PD, and HD, may contribute to the restless nights and listless days frequently seen in these patients. The H3R-antagonists/inverse agonists are making advances in the treatment of excessive daytime sleepiness in PD. 2) The negative symptoms of schizophrenia were directly related to lower H1R levels in the basal forebrain cholinergic neurons. 3) The H4R antagonist could be a potential target for PD, by inhibiting inflammation and thereby protecting dopaminergic neurons from degeneration.
Open issues warranting further study: 1) Increased number of histaminergic neurons (marked by HDC immunoreactivity) in narcolepsy patients may be linked with their unique pathogenesis. 2) In addition, human HMT mutations associated with intellectual disability, dysregulation of sleep-wake states and aggressive behaviours, warrant further studies in animal models. 3) HD patients showed elevated HDC-mRNA levels and histamine metabolites, which may be involved in the aggressive behaviours of these patients. 4) Depressed patients showed a reduction of H1R binding in the cerebral cortex, which may imply that H1R availability is associated with mood states. 5) Preliminary results showed that the H2R-antagonist reduced schizophrenic symptoms, but more comprehensive clinical trials failed to show any positive effects.
ACKNOWLEDGEMENTS
The authors are grateful for the valuable discussions with Dr. L Ramanathan from UCLA, Dr. CQ Liu, XY Zhu from Dalian Medical University and Prof. Ai-Min Bao from Zhejiang University School of Medicine.
LIST OF ABBREVIATIONS
- AD
Alzheimer’s Disease
- CSF
Cerebrospinal Fluid
- H1-4R
Histamine 1-4 receptors
- H1R antagonist
Doxepin
- H2R antagonist
Famotidine
- H3R antagonist
Inverse Agonist Pitolisant
- H4R antagonist
JNJ7777120
- HDC
l-histidine decarboxylase
- HMT
Histamine N-methyltransferase
- mRNA
Messenger RNA
- PD
Parkinson’s Disease
- t-MeHA
Tele-methylhistamine
- TMN
Tuberomamillary Nucleus
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
This work was supported by a grant from Stichting ParkinsonFonds (the Dutch Parkinson’s foundation) and Friends of the Netherlands Institute for Neuroscience Foundation. Dr. L Shan has received funding through a Marie Skłodowska-Curie grant from the European Union’s Horizon 2020 research and innovation programme (Agreement No. 707404) and awarded the Young Scientist Award 2020 by the European Narcolepsy Network (supported by the Klaus-Grawe Foundation).
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Hirsch E.C., Graybiel A.M., Agid Y. Selective vulnerability of pigmented dopaminergic neurons in Parkinson’s disease. Acta Neurol. Scand. Suppl. 1989;126:19–22. doi: 10.1111/j.1600-0404.1989.tb01778.x. [DOI] [PubMed] [Google Scholar]
- 2.Damier P., Hirsch E.C., Agid Y. The substantia nigra of the human brain. II. Patterns of loss of dopamine-containing neurons in Parkinson’s disease. Brain. 1999;122(Pt 8):1437–1448. doi: 10.1093/brain/122.8.1437. [DOI] [PubMed] [Google Scholar]
- 3.Damier P., Hirsch E.C., Agid Y. The substantia nigra of the human brain. I. Nigrosomes and the nigral matrix, a compartmental organization based on calbindin D(28K) immunohistochemistry. Brain. 1999;122(Pt 8):1421–1436. doi: 10.1093/brain/122.8.1421. [DOI] [PubMed] [Google Scholar]
- 4.Caspi A., Sugden K., Moffitt T.E. Influence of life stress on depression: Moderation by a polymorphism in the 5-HTT gene. Science (80-) 2003;301:386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- 5.Dauvilliers Y., Bassetti C., Lammers G.J., Arnulf I., Mayer G., Rodenbeck A., Lehert P., Ding C.L., Lecomte J.M., Schwartz J.C. Pitolisant versus placebo or modafinil in patients with narcolepsy: a double-blind, randomised trial. Lancet Neurol. 2013;12(11):1068–1075. doi: 10.1016/S1474-4422(13)70225-4. [DOI] [PubMed] [Google Scholar]
- 6.Kollb-Sielecka M., Demolis P., Emmerich J., Markey G., Salmonson T., Haas M. The European Medicines Agency review of pitolisant for treatment of narcolepsy: summary of the scientific assessment by the Committee for Medicinal Products for Human Use. Sleep Med. 2017;33:125–129. doi: 10.1016/j.sleep.2017.01.002. [DOI] [PubMed] [Google Scholar]
- 7.Panula P., Yang H.Y.T., Costa E. Histamine-containing neurons in the rat hypothalamus. Proc. Natl. Acad. Sci. USA. 1984;81(8):2572–2576. doi: 10.1073/pnas.81.8.2572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Watanabe T., Taguchi Y., Hayashi H., Tanaka J., Shiosaka S., Tohyama M., Kubota H., Terano Y., Wada H. Evidence for the presence of a histaminergic neuron system in the rat brain: an immunohistochemical analysis. Neurosci. Lett. 1983;39(3):249–254. doi: 10.1016/0304-3940(83)90308-7. [DOI] [PubMed] [Google Scholar]
- 9.Haas H.L., Sergeeva O.A., Selbach O. Histamine in the nervous system. Physiol. Rev. 2008;88(3):1183–1241. doi: 10.1152/physrev.00043.2007. [DOI] [PubMed] [Google Scholar]
- 10.Panula P., Chazot P.L., Cowart M., Gutzmer R., Leurs R., Liu W.L., Stark H., Thurmond R.L., Haas H.L. International union of basic and clinical pharmacology. XCVIII histamine receptors. Pharmacol. Rev. 2015;67(3):601–655. doi: 10.1124/pr.114.010249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Panula P., Nuutinen S. The histaminergic network in the brain: basic organization and role in disease. Nat. Rev. Neurosci. 2013;14(7):472–487. doi: 10.1038/nrn3526. [DOI] [PubMed] [Google Scholar]
- 12.Dahlstroem A., Fuxe K. Evidence for the existence of monoamine-containing neurons in the central nervous system. i. demonstration of monoamines in the cell bodies of brain stem neurons. Acta Physiol. Scand. Suppl. 1964;(Suppl. 232):1–55. [PubMed] [Google Scholar]
- 13.Airaksinen M.S., Paetau A., Paljärvi L., Reinikainen K., Riekkinen P., Suomalainen R., Panula P. Histamine neurons in human hypothalamus: anatomy in normal and Alzheimer diseased brains. Neuroscience. 1991;44(2):465–481. doi: 10.1016/0306-4522(91)90070-5. [DOI] [PubMed] [Google Scholar]
- 14.Shan L., Hofman M.A., van Wamelen D.J., Van Someren E.J., Bao A.M., Swaab Dick F. Diurnal fluctuation in histidine decarboxylase expression, the rate limiting enzyme for histamine production, and its disorder in neurodegenerative diseases. Sleep . 2012;35(5):713–715. doi: 10.5665/sleep.1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yu X., Zecharia A., Zhang Z., Yang Q., Yustos R., Jager P., Vyssotski A.L., Maywood E.S., Chesham J.E., Ma Y., Brickley S.G., Hastings M.H., Franks N.P., Wisden W. Circadian factor BMAL1 in histaminergic neurons regulates sleep architecture. Curr. Biol. 2014;24(23):2838–2844. doi: 10.1016/j.cub.2014.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McGregor R., Shan L., Wu M.F., Siegel J.M. Diurnal fluctuation in the number of hypocretin/orexin and histamine producing: Implication for understanding and treating neuronal loss. PLoS One. 2017;12(6):e0178573. doi: 10.1371/journal.pone.0178573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shan L., Fronczek R., Lammers G.J., Swaab D.F. The tuberomamillary nucleus in neuropsychiatric disorders. Handb. Clin. Neurol. 2021;180:389–400. doi: 10.1016/B978-0-12-820107-7.00024-0. [DOI] [PubMed] [Google Scholar]
- 18.Lin J.S. Brain structures and mechanisms involved in the control of cortical activation and wakefulness, with emphasis on the posterior hypothalamus and histaminergic neurons. Sleep Med. Rev. 2000;4(5):471–503. doi: 10.1053/smrv.2000.0116. [DOI] [PubMed] [Google Scholar]
- 19.Abe H., Honma S., Ohtsu H., Honma K. Circadian rhythms in behavior and clock gene expressions in the brain of mice lacking histidine decarboxylase. Brain Res. Mol. Brain Res. 2004;124(2):178–187. doi: 10.1016/j.molbrainres.2004.02.015. [DOI] [PubMed] [Google Scholar]
- 20.Morin F., Singh N., Mdzomba J.B., Dumas A., Pernet V., Vallières L. Conditional deletions of Hdc confirm roles of histamine in anaphylaxis and circadian activity but not in autoimmune encephalomyelitis. J. Immunol. 2021;206(9):2029–2037. doi: 10.4049/jimmunol.2000719. [DOI] [PubMed] [Google Scholar]
- 21.Del Tredici K., Rüb U., De Vos R.A., Bohl J.R., Braak H. Where does parkinson disease pathology begin in the brain? J. Neuropathol. Exp. Neurol. 2002;61(5):413–426. doi: 10.1093/jnen/61.5.413. [DOI] [PubMed] [Google Scholar]
- 22.Shan L., Liu C.Q., Balesar R., Hofman M.A., Bao A.M., Swaab D.F. Neuronal histamine production remains unaltered in Parkinson’s disease despite the accumulation of Lewy bodies and Lewy neurites in the tuberomamillary nucleus. Neurobiol. Aging. 2012;33(7):1343–1344. doi: 10.1016/j.neurobiolaging.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 23.Nakamura S., Ohnishi K., Nishimura M., Suenaga T., Akiguchi I., Kimura J., Kimura T. Large neurons in the tuberomammillary nucleus in patients with Parkinson’s disease and multiple system atrophy. Neurology. 1996;46(6):1693–1696. doi: 10.1212/WNL.46.6.1693. [DOI] [PubMed] [Google Scholar]
- 24.Garbarg M., Javoy-Agid F., Schwartz J.C., Agid Y. Brain histidine decarboxylase activity in Parkinson’s disease. Lancet, 1983;1(8314):74–75. doi: 10.1016/S0140-6736(83)91613-6. [DOI] [PubMed] [Google Scholar]
- 25.Prell G.D., Khandelwal J.K., Burns R.S., Blandina P., Morrishow A.M., Green J.P. Levels of pros-methylimidazoleacetic acid: correlation with severity of Parkinson’s disease in CSF of patients and with the depletion of striatal dopamine and its metabolites in MPTP-treated mice. J. Neural Transm. Park. Dis. Dement. Sect. 1991;3(2):109–125. doi: 10.1007/BF02260886. [DOI] [PubMed] [Google Scholar]
- 26.Shan L., Swaab D.F., Bao A.M. Neuronal histaminergic system in aging and age-related neurodegenerative disorders. Exp. Gerontol. 2013;48(7):603–607. doi: 10.1016/j.exger.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 27.Peyron C., Faraco J., Rogers W., Ripley B., Overeem S., Charnay Y., Nevsimalova S., Aldrich M., Reynolds D., Albin R., Li R., Hungs M., Pedrazzoli M., Padigaru M., Kucherlapati M., Fan J., Maki R., Lammers G.J., Bouras C., Kucherlapati R., Nishino S., Mignot E. A mutation in a case of early onset narcolepsy and a generalized absence of hypocretin peptides in human narcoleptic brains. Nat. Med. 2000;6(9):991–997. doi: 10.1038/79690. [DOI] [PubMed] [Google Scholar]
- 28.Thannickal T.C., Moore R.Y., Nienhuis R., Ramanathan L., Gulyani S., Aldrich M., Cornford M., Siegel J.M. Reduced number of hypocretin neurons in human narcolepsy. Neuron. 2000;27(3):469–474. doi: 10.1016/S0896-6273(00)00058-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bassetti C.L.A., Adamantidis A., Burdakov D., Han F., Gay S., Kallweit U., Khatami R., Koning F., Kornum B.R., Lammers G.J., Liblau R.S., Luppi P.H., Mayer G., Pollmächer T., Sakurai T., Sallusto F., Scammell T.E., Tafti M., Dauvilliers Y. Narcolepsy - clinical spectrum, aetiopathophysiology, diagnosis and treatment. Nat. Rev. Neurol. 2019;15(9):519–539. doi: 10.1038/s41582-019-0226-9. [DOI] [PubMed] [Google Scholar]
- 30.Chemelli R.M., Willie J.T., Sinton C.M., Elmquist J.K., Scammell T., Lee C., Richardson J.A., Williams S.C., Xiong Y., Kisanuki Y., Fitch T.E., Nakazato M., Hammer R.E., Saper C.B., Yanagisawa M. Narcolepsy in orexin knockout mice: molecular genetics of sleep regulation. Cell. 1999;98(4):437–451. doi: 10.1016/S0092-8674(00)81973-X. [DOI] [PubMed] [Google Scholar]
- 31.Hara J., Beuckmann C.T., Nambu T., Willie J.T., Chemelli R.M., Sinton C.M., Sugiyama F., Yagami K., Goto K., Yanagisawa M., Sakurai T. Genetic ablation of orexin neurons in mice results in narcolepsy, hypophagia, and obesity. Neuron. 2001;30(2):345–354. doi: 10.1016/S0896-6273(01)00293-8. [DOI] [PubMed] [Google Scholar]
- 32.Tabuchi S., Tsunematsu T., Black S.W., Tominaga M., Maruyama M., Takagi K., Minokoshi Y., Sakurai T., Kilduff T.S., Yamanaka A. Conditional ablation of orexin/hypocretin neurons: a new mouse model for the study of narcolepsy and orexin system function. J. Neurosci. 2014;34(19):6495–6509. doi: 10.1523/JNEUROSCI.0073-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.John J., Thannickal T.C., McGregor R., Ramanathan L., Ohtsu H., Nishino S., Sakai N., Yamanaka A., Stone C., Cornford M., Siegel J.M. Greatly increased numbers of histamine cells in human narcolepsy with cataplexy. Ann. Neurol. 2013;74(6):786–793. doi: 10.1002/ana.23968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Valko P.O., Gavrilov Y.V., Yamamoto M., Reddy H., Haybaeck J., Mignot E., Baumann C.R., Scammell T.E. Increase of histaminergic tuberomammillary neurons in narcolepsy. Ann. Neurol. 2013;74(6):794–804. doi: 10.1002/ana.24019. [DOI] [PubMed] [Google Scholar]
- 35.Nishino S., Sakurai E., Nevsimalova S., Yoshida Y., Watanabe T., Yanai K., Mignot E. Decreased CSF histamine in narcolepsy with and without low CSF hypocretin-1 in comparison to healthy controls. Sleep. 2009;32(2):175–180. doi: 10.1093/sleep/32.2.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bassetti C.L., Baumann C.R., Dauvilliers Y., Croyal M., Robert P., Schwartz J.C. Cerebrospinal fluid histamine levels are decreased in patients with narcolepsy and excessive daytime sleepiness of other origin. J. Sleep Res. 2010;19(4):620–623. doi: 10.1111/j.1365-2869.2010.00819.x. [DOI] [PubMed] [Google Scholar]
- 37.Dauvilliers Y., Delallée N., Jaussent I., Scholz S., Bayard S., Croyal M., Schwartz J.C., Robert P. Normal cerebrospinal fluid histamine and tele-methylhistamine levels in hypersomnia conditions. Sleep . 2012;35(10):1359–1366. doi: 10.5665/sleep.2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fronczek R., Overeem S., Lee S.Y., Hegeman I.M., van Pelt J., van Duinen S.G., Lammers G.J., Swaab D.F. Hypocretin (orexin) loss and sleep disturbances in Parkinson’s Disease. Brain. 2008;131(Pt 1):e88. doi: 10.1093/brain/awm222. [DOI] [PubMed] [Google Scholar]
- 39.Thannickal T.C., Lai Y.Y., Siegel J.M. Hypocretin (orexin) and melanin concentrating hormone loss and the symptoms of Parkinson’s disease. Brain. 2008;131(Pt 1):e87. doi: 10.1093/brain/awm221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shan L., Bossers K., Unmehopa U., Bao A.M., Swaab D.F. Alterations in the histaminergic system in Alzheimer’s disease: a postmortem study. Neurobiol. Aging. 2012;33(11):2585–2598. doi: 10.1016/j.neurobiolaging.2011.12.026. [DOI] [PubMed] [Google Scholar]
- 41.Spitzer N.C. Activity-dependent neurotransmitter respecification. Nat. Rev. Neurosci. 2012;13(2):94–106. doi: 10.1038/nrn3154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee D.A., Blackshaw S. Functional implications of hypothalamic neurogenesis in the adult mammalian brain. Int. J. Dev. Neurosci. 2012;30(8):615–621. doi: 10.1016/j.ijdevneu.2012.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Migaud M., Batailler M., Segura S., Duittoz A., Franceschini I., Pillon D. Emerging new sites for adult neurogenesis in the mammalian brain: a comparative study between the hypothalamus and the classical neurogenic zones. Eur. J. Neurosci. 2010;32(12):2042–2052. doi: 10.1111/j.1460-9568.2010.07521.x. [DOI] [PubMed] [Google Scholar]
- 44.Shan L., Dauvilliers Y., Siegel J.M. Interactions of the histamine and hypocretin systems in CNS disorders. Nat. Rev. Neurol. 2015;11(7):401–413. doi: 10.1038/nrneurol.2015.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Heidari A., Tongsook C., Najafipour R., Musante L., Vasli N., Garshasbi M., Hu H., Mittal K., McNaughton A.J., Sritharan K., Hudson M., Stehr H., Talebi S., Moradi M., Darvish H., Arshad R.M., Mozhdehipanah H., Rashidinejad A., Samiei S., Ghadami M., Windpassinger C., Gillessen-Kaesbach G., Tzschach A., Ahmed I., Mikhailov A., Stavropoulos D.J., Carter M.T., Keshavarz S., Ayub M., Najmabadi H., Liu X., Ropers H.H., Macheroux P., Vincent J.B. Mutations in the histamine N-methyltransferase gene, HNMT, are associated with nonsyndromic autosomal recessive intellectual disability. Hum. Mol. Genet. 2015;24(20):5697–5710. doi: 10.1093/hmg/ddv286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Verhoeven W.M.A., Egger J.I.M., Janssen P.K.C., van Haeringen A. Adult male patient with severe intellectual disability caused by a homozygous mutation in the HNMT gene. BMJ Case Rep. 2020;13(12):235972. doi: 10.1136/bcr-2020-235972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Naganuma F., Nakamura T., Yoshikawa T., Iida T., Miura Y., Kárpáti A., Matsuzawa T., Yanai A., Mogi A., Mochizuki T., Okamura N., Yanai K. Histamine N-methyltransferase regulates aggression and the sleep-wake cycle. Sci. Rep. 2017;7(1):15899. doi: 10.1038/s41598-017-16019-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Auvinen S., Panula P. Development of histamine-immunoreactive neurons in the rat brain. J. Comp. Neurol. 1988;276(2):289–303. doi: 10.1002/cne.902760211. [DOI] [PubMed] [Google Scholar]
- 49.Han S., Márquez-Gómez R., Woodman M., Ellender T. Histaminergic control of corticostriatal synaptic plasticity during early postnatal development. J. Neurosci. 2020;40(34):6557–6571. doi: 10.1523/JNEUROSCI.0740-20.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zlomuzica A., Viggiano D., De Souza Silva M.A., Ishizuka T., Gironi C.U.A., Ruocco L.A., Watanabe T., Sadile A.G., Huston J.P., Dere E. The histamine H1-receptor mediates the motivational effects of novelty. Eur. J. Neurosci. 2008;27(6):1461–1474. doi: 10.1111/j.1460-9568.2008.06115.x. [DOI] [PubMed] [Google Scholar]
- 51.Yanai K., Son L.Z., Endou M., Sakurai E., Nakagawasai O., Tadano T., Kisara K., Inoue I., Watanabe T., Watanabe T. Behavioural characterization and amounts of brain monoamines and their metabolites in mice lacking histamine H1 receptors. Neuroscience. 1998;87(2):479–487. doi: 10.1016/S0306-4522(98)00167-5. [DOI] [PubMed] [Google Scholar]
- 52.Takahashi K., Suwa H., Ishikawa T., Kotani H. Targeted disruption of H3 receptors results in changes in brain histamine tone leading to an obese phenotype. J. Clin. Invest. 2002;110(12):1791–1799. doi: 10.1172/JCI15784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gondard E., Anaclet C., Akaoka H., Guo R.X., Zhang M., Buda C., Franco P., Kotani H., Lin J.S. Enhanced histaminergic neurotransmission and sleep-wake alterations, a study in histamine H3-receptor knock-out mice. Neuropsychopharmacology. 2013;38(6):1015–1031. doi: 10.1038/npp.2012.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Reichmann F., Rimmer N., Tilley C.A., Dalla V.E., Pinion J., Al Oustah A., Carreño G.H., Young A.M.J., McDearmid J.R., Winter M.J., Norton W.H.J. The zebrafish histamine H3 receptor modulates aggression, neural activity and forebrain functional connectivity. Acta Physiol. (Oxf.) 2020;230(4):e13543. doi: 10.1111/apha.13543. [DOI] [PubMed] [Google Scholar]
- 55.van Wamelen D.J., Shan L., Aziz N.A., Anink J.J., Bao A.M., Roos R.A., Swaab D.F. Functional increase of brain histaminergic signaling in Huntington’s disease. Brain Pathol. 2011;21(4):419–427. doi: 10.1111/j.1750-3639.2010.00465.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Prell G.D., Green J.P. Histamine metabolites and pros-methylimidazoleacetic acid in human cerebrospinal fluid. Agents Actions Suppl. 1991;33:343–363. doi: 10.1007/978-3-0348-7309-3_25. [DOI] [PubMed] [Google Scholar]
- 57.Lieberman P. Histamine, antihistamines, and the central nervous system. Allergy Asthma Proc. 2009;30(5):482–486. doi: 10.2500/aap.2009.30.3264. [DOI] [PubMed] [Google Scholar]
- 58.Krystal A.D. A compendium of placebo-controlled trials of the risks/benefits of pharmacological treatments for insomnia: the empirical basis for U.S. clinical practice. Sleep Med. Rev. 2009;13(4):265–274. doi: 10.1016/j.smrv.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 59.Wang Y.Q., Takata Y., Li R., Zhang Z., Zhang M.Q., Urade Y., Qu W.M., Huang Z.L. Doxepin and diphenhydramine increased non-rapid eye movement sleep through blockade of histamine H1 receptors. Pharmacol. Biochem. Behav. 2015;129:56–64. doi: 10.1016/j.pbb.2014.12.002. [DOI] [PubMed] [Google Scholar]
- 60.Roth T., Rogowski R., Hull S., Schwartz H., Koshorek G., Corser B., Seiden D., Lankford A. Efficacy and safety of doxepin 1 mg, 3 mg, and 6 mg in adults with primary insomnia. Sleep. 2007;30(11):1555–1561. doi: 10.1093/sleep/30.11.1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Krystal A.D., Richelson E., Roth T. Review of the histamine system and the clinical effects of H1 antagonists: basis for a new model for understanding the effects of insomnia medications. Sleep Med. Rev. 2013;17(4):263–272. doi: 10.1016/j.smrv.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 62.Krystal A.D., Durrence H.H., Scharf M., Jochelson P., Rogowski R., Ludington E., Roth T. Efficacy and safety of doxepin 1 mg and 3 mg in a 12-week sleep laboratory and outpatient trial of elderly subjects with chronic primary insomnia. Sleep. 2010;33(11):1553–1561. doi: 10.1093/sleep/33.11.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Krystal A.D., Lankford A., Durrence H.H., Ludington E., Jochelson P., Rogowski R., Roth T. Efficacy and safety of doxepin 3 and 6 mg in a 35-day sleep laboratory trial in adults with chronic primary insomnia. Sleep . 2011;34(10):1433–1442. doi: 10.5665/SLEEP.1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Roth T., Heith Durrence H., Jochelson P., Peterson G., Ludington E., Rogowski R., Scharf M., Lankford A. Efficacy and safety of doxepin 6 mg in a model of transient insomnia. Sleep Med. 2010;11(9):843–847. doi: 10.1016/j.sleep.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 65.Hajak G., Rodenbeck A., Voderholzer U., Riemann D., Cohrs S., Hohagen F., Berger M., Rüther E. Doxepin in the treatment of primary insomnia: a placebo-controlled, double-blind, polysomnographic study. J. Clin. Psychiatry. 2001;62(6):453–463. doi: 10.4088/JCP.v62n0609. [DOI] [PubMed] [Google Scholar]
- 66.Yeung W.F., Chung K.F., Yung K.P., Ng T.H. Doxepin for insomnia: a systematic review of randomized placebo-controlled trials. Sleep Med. Rev. 2015;19:75–83. doi: 10.1016/j.smrv.2014.06.001. [DOI] [PubMed] [Google Scholar]
- 67.Kano M., Fukudo S., Tashiro A., Utsumi A., Tamura D., Itoh M., Iwata R., Tashiro M., Mochizuki H., Funaki Y., Kato M., Hongo M., Yanai K. Decreased histamine H1 receptor binding in the brain of depressed patients. Eur. J. Neurosci. 2004;20(3):803–810. doi: 10.1111/j.1460-9568.2004.03540.x. [DOI] [PubMed] [Google Scholar]
- 68.Yanai K., Tashiro M. The physiological and pathophysiological roles of neuronal histamine: an insight from human positron emission tomography studies. Pharmacol. Ther. 2007;113(1):1–15. doi: 10.1016/j.pharmthera.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 69.Higuchi M., Yanai K., Okamura N., Meguro K., Arai H., Itoh M., Iwata R., Ido T., Watanabe T., Sasaki H. Histamine H(1) receptors in patients with Alzheimer’s disease assessed by positron emission tomography. Neuroscience. 2000;99(4):721–729. doi: 10.1016/S0306-4522(00)00230-X. [DOI] [PubMed] [Google Scholar]
- 70.Tiligada E., Ennis M. Histamine pharmacology: from Sir Henry Dale to the 21st century. Br. J. Pharmacol. 2020;177(3):469–489. doi: 10.1111/bph.14524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dai H., Kaneko K., Kato H., Fujii S., Jing Y., Xu A., Sakurai E., Kato M., Okamura N., Kuramasu A., Yanai K. Selective cognitive dysfunction in mice lacking histamine H1 and H2 receptors. Neurosci. Res. 2007;57(2):306–313. doi: 10.1016/j.neures.2006.10.020. [DOI] [PubMed] [Google Scholar]
- 72.Kaminsky R., Moriarty T.M., Bodine J., Wolf D.E., Davidson M. Effect of famotidine on deficit symptoms of schizophrenia. Lancet. 1990;335(8701):1351–1352. doi: 10.1016/0140-6736(90)91237-5. [DOI] [PubMed] [Google Scholar]
- 73.Oyewumi L.K., Vollick D., Merskey H., Plumb C. Famotidine as an adjunct treatment of resistant schizophrenia. J. Psychiatry Neurosci. 1994;19(2):145–150. [PMC free article] [PubMed] [Google Scholar]
- 74.Rosse R.B., Kendrick K., Fay-McCarthy M., Prell G.D., Rosenberg P., Tsui L.C., Wyatt R.J., Deutsch S.I. An open-label study of the therapeutic efficacy of high-dose famotidine adjuvant pharmacotherapy in schizophrenia: preliminary evidence for treatment efficacy. Clin. Neuropharmacol. 1996;19(4):341–348. doi: 10.1097/00002826-199619040-00007. [DOI] [PubMed] [Google Scholar]
- 75.Meskanen K., Ekelund H., Laitinen J., Neuvonen P.J., Haukka J., Panula P., Ekelund J. A randomized clinical trial of histamine 2 receptor antagonism in treatment-resistant schizophrenia. J. Clin. Psychopharmacol. 2013;33(4):472–478. doi: 10.1097/JCP.0b013e3182970490. [DOI] [PubMed] [Google Scholar]
- 76.Kishi T., Iwata N. Efficacy and tolerability of histamine-2 receptor antagonist adjunction of antipsychotic treatment in schizophrenia: a meta-analysis of randomized placebo-controlled trials. Pharmacopsychiatry. 2015;48(1):30–36. doi: 10.1055/s-0034-1390478. [DOI] [PubMed] [Google Scholar]
- 77.Orange P.R., Heath P.R., Wright S.R., Ramchand C.N., Kolkeiwicz L., Pearson R.C. Individuals with schizophrenia have an increased incidence of the H2R649G allele for the histamine H2 receptor gene. Mol. Psychiatry. 1996;1(6):466–469. [PubMed] [Google Scholar]
- 78.Ito C., Morisset S., Krebs M.O., Olié J.P., Lôo H., Poirier M.F., Lannfelt L., Schwartz J.C., Arrang J.M. Histamine H2 receptor gene variants: lack of association with schizophrenia. Mol. Psychiatry. 2000;5(2):159–164. doi: 10.1038/sj.mp.4000664. [DOI] [PubMed] [Google Scholar]
- 79.Mahoney C.E., Cogswell A., Koralnik I.J., Scammell T.E. The neurobiological basis of narcolepsy. Nat. Rev. Neurosci. 2019;20(2):83–93. doi: 10.1038/s41583-018-0097-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Szakacs Z., Dauvilliers Y., Mikhaylov V., Poverennova I., Krylov S., Jankovic S., Sonka K., Lehert P., Lecomte I., Lecomte J.M., Schwartz J.C. Safety and efficacy of pitolisant on cataplexy in patients with narcolepsy: a randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2017;16(3):200–207. doi: 10.1016/S1474-4422(16)30333-7. [DOI] [PubMed] [Google Scholar]
- 81.Lin J.S., Dauvilliers Y., Arnulf I., Bastuji H., Anaclet C., Parmentier R., Kocher L., Yanagisawa M., Lehert P., Ligneau X., Perrin D., Robert P., Roux M., Lecomte J.M., Schwartz J.C. An inverse agonist of the histamine H(3) receptor improves wakefulness in narcolepsy: studies in orexin-/- mice and patients. Neurobiol. Dis. 2008;30(1):74–83. doi: 10.1016/j.nbd.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 82.Thannickal T.C., Lai Y.Y., Siegel J.M. Hypocretin (orexin) cell loss in Parkinson’s disease. Brain. 2007;130(Pt 6):1586–1595. doi: 10.1093/brain/awm097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Passani M.B., Blandina P. Histamine receptors in the CNS as targets for therapeutic intervention. Trends Pharmacol. Sci. 2011;32(4):242–249. doi: 10.1016/j.tips.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 84.Sadek B., Saad A., Sadeq A., Jalal F., Stark H. Histamine H3 receptor as a potential target for cognitive symptoms in neuropsychiatric diseases. Behav. Brain Res. 2016;312:415–430. doi: 10.1016/j.bbr.2016.06.051. [DOI] [PubMed] [Google Scholar]
- 85.Egan M., Yaari R., Liu L., Ryan M., Peng Y., Lines C., Michelson D. Pilot randomized controlled study of a histamine receptor inverse agonist in the symptomatic treatment of AD. Curr. Alzheimer Res. 2012;9(4):481–490. doi: 10.2174/156720512800492530. [DOI] [PubMed] [Google Scholar]
- 86.Grove R.A., Harrington C.M., Mahler A., Beresford I., Maruff P., Lowy M.T., Nicholls A.P., Boardley R.L., Berges A.C., Nathan P.J., Horrigan J.P. A randomized, double-blind, placebo-controlled, 16-week study of the H3 receptor antagonist, GSK239512 as a monotherapy in subjects with mild-to-moderate Alzheimer’s disease. Curr. Alzheimer Res. 2014;11(1):47–58. doi: 10.2174/1567205010666131212110148. [DOI] [PubMed] [Google Scholar]
- 87.Kubo M., Kishi T., Matsunaga S., Iwata N. Histamine H3 Receptor Antagonists for Alzheimer’s Disease: A systematic review and meta-analysis of randomized placebo-controlled trials. J. Alzheimers Dis. 2015;48(3):667–671. doi: 10.3233/JAD-150393. [DOI] [PubMed] [Google Scholar]
- 88.Haig G.M., Bain E., Robieson W., Othman A.A., Baker J., Lenz R.A. A randomized trial of the efficacy and safety of the H3 antagonist ABT-288 in cognitive impairment associated with schizophrenia. Schizophr. Bull. 2014;40(6):1433–1442. doi: 10.1093/schbul/sbt240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jarskog L.F., Lowy M.T., Grove R.A., Keefe R.S., Horrigan J.P., Ball M.P., Breier A., Buchanan R.W., Carter C.S., Csernansky J.G., Goff D.C., Green M.F., Kantrowitz J.T., Keshavan M.S., Laurelle M., Lieberman J.A., Marder S.R., Maruff P., McMahon R.P., Seidman L.J., Peykamian M.A. A Phase II study of a histamine H3 receptor antagonist GSK239512 for cognitive impairment in stable schizophrenia subjects on antipsychotic therapy. Schizophr. Res. 2015;164(1-3):136–142. doi: 10.1016/j.schres.2015.01.041. [DOI] [PubMed] [Google Scholar]
- 90.Surmeier D.J., Obeso J.A., Halliday G.M. Selective neuronal vulnerability in Parkinson disease. Nat. Rev. Neurosci. 2017;18(2):101–113. doi: 10.1038/nrn.2016.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Anichtchik O.V., Rinne J.O., Kalimo H., Panula P. An altered histaminergic innervation of the substantia nigra in Parkinson’s disease. Exp. Neurol. 2000;163(1):20–30. doi: 10.1006/exnr.2000.7362. [DOI] [PubMed] [Google Scholar]
- 92.Rinne J.O., Anichtchik O.V., Eriksson K.S., Kaslin J., Tuomisto L., Kalimo H., Röyttä M., Panula P. Increased brain histamine levels in Parkinson’s disease but not in multiple system atrophy. J. Neurochem. 2002;81(5):954–960. doi: 10.1046/j.1471-4159.2002.00871.x. [DOI] [PubMed] [Google Scholar]
- 93.Rocha S.M., Saraiva T., Cristóvão A.C., Ferreira R., Santos T., Esteves M., Saraiva C., Je G., Cortes L., Valero J., Alves G., Klibanov A., Kim Y.S., Bernardino L. Histamine induces microglia activation and dopaminergic neuronal toxicity via H1 receptor activation. J. Neuroinflammation. 2016;13(1):137. doi: 10.1186/s12974-016-0600-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Rocha S.M., Pires J., Esteves M., Graça B., Bernardino L. Histamine: a new immunomodulatory player in the neuron-glia crosstalk. Front. Cell. Neurosci. 2014;8:120. doi: 10.3389/fncel.2014.00120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Vizuete M.L., Merino M., Venero J.L., Santiago M., Cano J., Machado A. Histamine infusion induces a selective dopaminergic neuronal death along with an inflammatory reaction in rat substantia nigra. J. Neurochem. 2000;75(2):540–552. doi: 10.1046/j.1471-4159.2000.0750540.x. [DOI] [PubMed] [Google Scholar]
- 96.Liu C.Q., Chen Z., Liu F.X., Hu D.N., Luo J.H. Involvement of brain endogenous histamine in the degeneration of dopaminergic neurons in 6-hydroxydopamine-lesioned rats. Neuropharmacology. 2007;53(7):832–841. doi: 10.1016/j.neuropharm.2007.08.014. [DOI] [PubMed] [Google Scholar]
- 97.Shan L., Bossers K., Luchetti S., Balesar R., Lethbridge N., Chazot P.L., Bao A.M., Swaab D.F. Alterations in the histaminergic system in the substantia nigra and striatum of Parkinson’s patients: a postmortem study. Neurobiol. Aging. 2012;33(7):1488.e1–1488.e13. doi: 10.1016/j.neurobiolaging.2011.10.016. [DOI] [PubMed] [Google Scholar]
- 98.Fang Q., Xicoy H., Shen J. Histamine-4 receptor antagonist ameliorates Parkinson-like pathology in the striatum. Brain Behav. Immun. 2021;92:127–138. doi: 10.1016/j.bbi.2020.11.036. [DOI] [PubMed] [Google Scholar]
- 99.Zhou P., Homberg J.R., Fang Q., Wang J., Li W., Meng X., Shen J., Luan Y., Liao P., Swaab D.F., Shan L., Liu C. Histamine-4 receptor antagonist JNJ7777120 inhibits pro-inflammatory microglia and prevents the progression of Parkinson-like pathology and behaviour in a rat model. Brain Behav. Immun. 2019;76:61–73. doi: 10.1016/j.bbi.2018.11.006. [DOI] [PubMed] [Google Scholar]
- 100.Ferna A., Kruijver F.P., Fodor M. Sex differences in the distribution of androgen receptors in the human hypothalamus. J. Comp. Neurol. 2000;425(3):422–435. doi: 10.1002/1096-9861(20000925)425:3422:aid-cne7>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 101.Shan L., Bao A-M., Swaab D.F. Changes in histidine decarboxylase, histamine n-methyltransferase and histamine receptors in neuropsychiatric disorders. Handb. Exp. Pharmacol. 2017;241:259–276. doi: 10.1007/164_2016_125. [DOI] [PubMed] [Google Scholar]