Abstract
Sodium oxybate (SO) has been in use for many decades to treat narcolepsy with cataplexy. It functions as a weak GABAB agonist but also as an energy source for the brain as a result of its metabolism to succinate and as a powerful antioxidant because of its capacity to induce the formation of NADPH. Its actions at thalamic GABAB receptors can induce slow-wave activity, while its actions at GABAB receptors on monoaminergic neurons can induce or delay REM sleep. By altering the balance between monoaminergic and cholinergic neuronal activity, SO uniquely can induce and prevent cataplexy. The formation of NADPH may enhance sleep’s restorative process by accelerating the removal of the reactive oxygen species (ROS), which accumulate during wakefulness. SO improves alertness in normal subjects and in patients with narcolepsy. SO may allay severe psychological stress - an inflammatory state triggered by increased levels of ROS and characterized by cholinergic supersensitivity and monoaminergic deficiency. SO may be able to eliminate the inflammatory state and correct the cholinergic/ monoaminergic imbalance.
Keywords: Sleep, narcolepsy, sodium oxybate, sleep homeostasis, depression, oxidative stress
1. INTRODUCTION
Sodium oxybate (SO) has been approved by the United States Food and Drug Administration to treat narcolepsy with cataplexy in adults and children 7-17 years of age. Although it addresses all the symptoms of narcolepsy, its precise mechanism of action remains “obscure” despite decades of use [1, 2].
SO’s use in narcolepsy followed a trial in patients with insomnia and a past history of psychiatric disorders who were given a single dose of SO at bedtime in doses ranging between 1 and 3 grams. Baseline sleep architecture in these patients was normal with an initial period of NREM sleep followed by REM sleep after the expected interval. Given the short approximate 40 minutes half-life of SO, serum levels would have been virtually normal in less than 4 hours [1, 3, 4]. During these 3 to 4 hours, SO produced an early and prolonged REM sleep period followed by a pronounced increase in slow-wave sleep (SWS), reversing the normal sequence of sleep stages at sleep onset. The duration of REM sleep during these initial hours of the night was greatly prolonged, but the total duration of REM sleep during the night was unchanged. SO appeared to have both induced REM sleep and ‘gathered’ the night’s allotment of REM sleep to itself [5]. Could it ‘gather’ together the dissociated fragments of REM sleep distributed around the nycthemeron in narcolepsy, and would this alleviate the symptoms of narcolepsy? Initial trials of SO in patients with narcolepsy proved that this was indeed the case, and these findings were then independently confirmed by other investigators in double-blind controlled studies [6-14].
In this study, we propose to examine the dual actions of SO as a GABAB agonist and as an antioxidant and energy source. SO’s agonistic actions at GABAB receptors generate slow-wave activity, and its effects on neurotransmitter release alter the balance between monoaminergic and cholinergic activity that controls REM sleep. SO, uniquely, can both induce and prevent cataplexy. Its antioxidant effects may explain its capacity to act at night to significantly reduce sleepiness during the day. Gathering evidence suggests that sleep’s restorative properties reflect its capacity to eliminate oxidants formed during wakefulness or in the course of sleep deprivation. SO’s antioxidative effects enhance this restorative process. These dual actions of SO suggest that it may have therapeutic actions in addition to narcolepsy, particularly in the treatment of severe psychological distress where a central cholinergic/monoaminergic imbalance develops together with signs of oxidative stress.
2. SODIUM OXYBATE AND SLOW WAVE SLEEP
SO is a partial agonist at the GABAB receptor, and many of its pharmacological effects can be attributed to its actions at this receptor. GABAB receptors are widely distributed in the adult mammalian brain at both presynaptic terminals and post synaptic sites. Presynaptic GABAB autoreceptors are found on GABAergic terminals and heteroreceptors on non-GABAergic glutamatergic, orexinergic, monoaminergic, and cholinergic neurons [15-18]. Presynaptic GABAergic receptor activation alters the synthesis and/or inhibits the release of neurotransmitters and neuropeptides, while activation of post synaptic GABAB receptors generates the late inhibitory post synaptic potential (IPSP) that results from an increase in membrane potassium conductance. While membrane hyperpolarization with SO has now been demonstrated at many sites in the nervous system, hyperpolarization of the thalamocortical neurons is particularly germane to the genesis of SWS [19]. During wakefulness, under naturally occurring conditions, the thalamocortical membrane potential is greater than -65mV, and neurons tonically fire single action potentials under the influence of cholinergic and other neurotransmitter afferents arising from the brain stem [20]. With the reduced release of these neurotransmitters during sleep, the thalamocortical membrane becomes progressively more hyperpolarized during drowsiness and the early stages of sleep when spindles predominate and then more negatively polarized into the range where SWS prevails [21]. GABAergic reticular thalamic projections set the membrane potential of the thalamocortical neurons at the negative levels which are required for a low threshold calcium current, It, and a slow inwardly rectifying current, Ih, to generate slowly rising and falling rhythms at 0.5-4.0 Hz [21]. Recent work suggests that calcium signaling is involved in the homeostatic regulation of sleep pressure and sleep duration [22-25]. Experimental work in both the rat and cat has demonstrated that slow-wave activity (SWA) can be blocked by GABAB antagonists [26, 27]. Working with brain slices, Williams et al. demonstrated that bath application of SO, in doses starting below 100µM, can also hyperpolarize thalamocortical neurons into the voltage range, -65 to -75 mV, required to generate pacemaker activity at 0.5 to 4Hz [20]. Hyperpolarization appears to depend on actions at the GABAB receptor as it can be blocked by the GABAB antagonist CGP354. Higher concentrations of SO bring the neurons into an even more negative range where intrinsically generated firing ceases [19].
3. SODIUM OXYBATE AND SLEEP HOMEOSTASIS
Despite these elegant neurophysiological observations, questions remain about the nature of the slow-wave activity (SWA) produced by SO and its relationship to the naturally occurring slow waves of sleep. Comprehensive investigative work has defined the homeostatic function of naturally occurring SWS [28, 29]. The duration and electroencephalographic (EEG) power of slow-wave [delta] sleep (0.5 to 4.0 Hz) increase in parallel with the duration of the preceding period of wakefulness. SWA is most prominent early in the night and gradually dissipates during the course of sleep. These characteristics suggest that slow-wave sleep intensifies the homeostatic recovery and restorative functions of sleep, but doubts have been raised about whether the SWA induced by SO has similar restorative properties.
Walsh and his colleagues [30] organized a 5-night study consisting of two baseline nights followed by two nights of sleep deprivation, each followed by a 3-hour nap opportunity between 8 AM and 11 AM during which subjects received a placebo or 3.5 grams of SO. The 2 nights of sleep deprivation were followed by a baseline recovery night. A multiple sleep latency test (MSLT) and a psychomotor vigilance test (PVT) were administered during waking hours. SO significantly increased the SWS time and significantly reduced the REM sleep time during the two-morning naps. In subjects treated with SO, the MSLT revealed significantly prolonged sleep latencies during the night following the first daytime sleep period and during the day following the second daytime sleep period. SO improved the median PVT reaction time, a measure of daytime alertness and attention, following the second day sleep period. On night 5, the recovery night, subjects who received SO had a significantly lower total sleep time and significantly less SWS and less REM sleep than subjects who had received placebo. The reduced impact of sleep loss on alertness and attention together with the reduced recovery sleep response on the final night of the study, suggested a lower homeostatic sleep drive in subjects treated with SO.
In contrast, Vienne and her colleagues [31, 32] arrived at the opposite conclusion in their studies on the homeostatic properties of SO in mice and men. In an initial study in mice, the actions of SO and baclofen, two GABAB agonists, were compared by recording their effects on the EEG of wild-type (WT) mice and mice devoid of both subunits, 1 and 2, of the GABAB receptor, or in mice with one of the two subunits, isoform 1a or 1b, missing. Mice devoid of both units of the GABAB receptor and, to a lesser extent, those missing the 1a subunit displayed spontaneous seizures. The absence of both units of the GABAB receptor altered the distribution of sleep and wakefulness and delayed the onset and the end of the rest period. This was thought to reflect the known role of GABAB receptors in the regulation of circadian phases of depolarization at the suprachiasmatic nucleus [33]. The amplitude and frequency of the EEG in the 1.75 to 10 Hz delta-theta range were significantly reduced in mice devoid of both units of the GABAB receptor compared with that found in wild-type mice. This effect resembled the reduced EEG synchronization observed with GABAB receptor inhibitors and again highlighted the role of GABAB receptors in the generation of thalamocortical neuronal oscillations [27].
In WT mice or in mice missing the 1a or 1b subunit of the GABAB receptor, gamma-butyrolactone (GBL), a precursor of SO, in low doses, induced EEG slow waves even though the mouse remained behaviorally awake with eyes open and able to respond normally to stimuli. At higher doses, spike-like activity was added to the hypersynchronous EEG slow activity. The mice were now immobile but with their eyes open. GBL appeared to have induced a subanesthetic state with little resemblance to normal sleep. At the highest doses, the mice were unresponsive and appeared anaesthetized. Spectral analysis revealed that GBL increased power in the low delta range, 0.75 to1.5 Hz, and suppressed it in the spindle range at 13 Hz. Despite the large increase in spectral power produced by GBL, delta power remained unchanged during subsequent recovery sleep in WT mice and mice lacking either subunits 1a or 1b of the GABAB receptor. Thus, the delta oscillations induced by GBL did not have the homeostatic impact of the naturally occurring delta waves of NREM sleep.
Baclofen, given to WT mice, also induced hypersynchronous SWA and decreased locomotor activity but even at the highest doses failed to induce the spike-like activity induced by high doses of GBL. Baclofen increased the spectral power of the EEG in the high delta range, 4.0 to 5.25 Hz, and suppressed power at higher frequencies, 10.75 to 37.5 Hz. Baclofen produced long-term hypersomnia containing both NREM and REM sleep which was even evident in mice lacking both units, 1 and 2, of the GABAB receptor. In contrast to the acute effects of baclofen on sleep, therefore, long-term hypersomnia may not have been due to actions on the GABAB receptor.
A 6-hour period of sleep deprivation was followed by the expected signs of homeostatic recovery during the next 6 hours in WT mice and mice lacking the 1a or 1b GABAB subunits. There was an increase in NREM sleep and in EEG power. Thus, the homeostatic response to sleep deprivation was not affected in mice lacking either one of these GABAB receptor subunits. The double knockout (KO) mice were too frail to be subjected to sleep deprivation.
Vienne et al. then examined SO’s homeostatic properties in man [32]. They demonstrated that an unmedicated afternoon nap significantly reduced sleep need and sleep intensity the following night. This homeostatic response could be documented by the reduced EEG power in the delta and theta frequency ranges (0.75-7.25 Hz) during the subsequent night. SO, 30 mg/kg, and baclofen, 0.35 mg/kg, both reversed this nap effect. The sleep latency was reduced, and the total sleep time was prolonged on the night following a medicated nap. There was more SWS during the first NREM episode and greater EEG delta and theta power (0.75-7.25 Hz). Vienne and her colleagues found that a drug-free nap in the afternoon also improved memory consolidation and performance on subjective and objective tests of vigilance, while naps enhanced by the use of baclofen or SO did not have these effects. They again concluded that SWA induced by SO and baclofen did not have the homeostatic properties of normal sleep but, at the same time, noted that the number and duration of REM sleep episodes during a nap was greater in subjects treated with baclofen or SO and especially with SO. SO could induce sleep-onset REM periods lasting 30-50 minutes. SO unexpectedly also greatly increased delta and theta power in REM sleep and wakefulness.
In view of the contrary findings of Walsh et al. and Vienne et al., Dornbierer et al. using a different experimental paradigm, again examined whether SWS induced by SO had the same properties as physiological sleep and whether it could account for the improvement in the excessive daytime drowsiness observed with its use in narcolepsy, Parkinson’s disease and fibromyalgia [34-38]. In their study, 50 mg/kg of SO or placebo were given orally to 20 young male volunteers at 2:30 a.m. in a randomized, double-blind, crossover manner, and the drug’s effect on sleep architecture and EEG sleep spectra were examined. SO prolonged SWS at the cost of REM sleep and enhanced delta-theta activity (0.5-8 Hz) in both NREM and REM sleep. Earlier studies were cited, which demonstrated identical changes in sleep architecture during recovery sleep following sleep deprivation [39]. Nocturnal SO also significantly improved morning vigilance quantified on a PVT 3 hours after awakening. It was concluded that there were distinct similarities between sleep augmented by SO and the high voltage sleep that follows the recovery from prolonged wakefulness.
Clinical trials of SO also appear to corroborate the restorative powers of SO. Nocturnal application of SO in patients with narcolepsy, with or without cataplexy, significantly reduces daytime drowsiness whether subjectively measured by questionnaire or objectively with either the MSLT or the multiple wakefulness test (MWT) [40-42]. Remarkably, SO, on its own, is as efficacious for treating excessive daytime sleepiness as the commonly used stimulant, modafinil [43]. In addition to significantly increasing the resistance to sleep on the MWT, SO produced a corresponding reduction in the error rate on a test of sustained attention [42]. The alerting effects of SO cannot be attributed to an increase in total nocturnal sleep time. SO improves the continuity of nocturnal sleep in narcolepsy but not its duration [1, 44, 45].
Despite these data, the exact relationship between naturally occurring SWA and that induced by SO remains unsettled. SO is an anesthetic agent. Its application in low doses produces delta activity similar to natural sleep, which, however, persists if the subject is awakened. Higher doses produce rhythmic and nonrhythmic delta activity during which the subject is comatose and cannot be aroused [46]. What restorative properties can these slow waves have in common with the slow waves of natural sleep? However, studies in rats reveal that SWA produced by anesthetic agents can be restorative. For example, the recovery of NREM and REM sleep following 24 hours of sleep deprivation is the same whether the rats are allowed to recover naturally or are anesthetized for 6 hours with the infusion of propofol [47]. The inhalant halogenated ether anesthetics isoflurane, desflurane, and sevoflurane can also repay the sleep debt but, in contrast to propofol, repay only the NREM debt [48]. These observations suggest that the metabolic mediators of the recovery process differ for the two sleep states [48, 49]. Sevoflurane anesthesia at a level that produces burst suppression activity on the EEG actually accelerates the homeostatic sleep recovery time and reduces it by half. This raises questions about the specific function of slow waves [48]. Is SWA essential to the restorative process?
None of the studies conducted to date illuminate how SWA, whether naturally occurring or drug-induced, increases the restorative powers of sleep. It is difficult to imagine how any drug could exactly duplicate naturally occurring SWA and selectively activate or maintain the ongoing activity of the multiple neuronal circuits that act in concert to produce this activity. This is certainly true for SO, which acts on GABAB receptors widely distributed in the nervous system. Subjects can remain awake following the intake of SO even while the EEG displays high voltage slow activity [46, 50]. Under certain conditions, the same dose that induces SWA induces sleep-onset REM periods with relatively low voltage fast activity and even cataplexy. As will be described below, the effects of SO appear to depend on the time of day of its administration and the state of the nervous system at that moment [45, 51-55].
4. REM SLEEP
While SWA induced by SO has been related to its capacity to hyperpolarize neuronal membranes, the reason for SO’s ability to induce REM sleep remains elusive. The neural circuits generating REM sleep have been carefully scrutinized over many decades, and our current knowledge about them has been described in several excellent recent reviews [56-63]. Defining these circuits has been a daunting task because of their complexity and the great diversity and intermingling of their component cell types, neurotransmitters, and neuropeptides at each pertinent nucleus in the hypothalamus and brain stem. Glutamatergic and GABAergic neurons dominate these nuclei intermingled with monoaminergic and cholinergic neurons. Only a sketch of these circuits will be provided here (Fig. 1). A more detailed description is found in the references cited above.
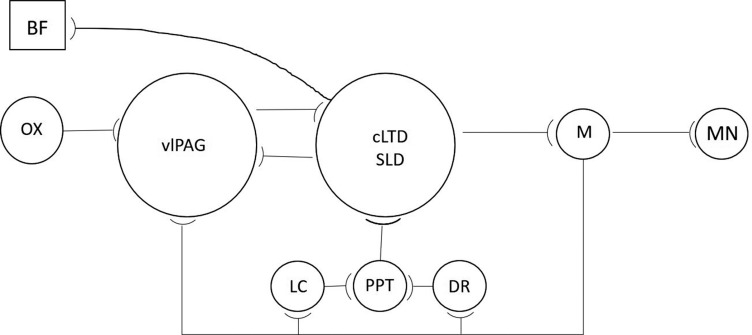
Fig. (1).
A Sketch of the REM Sleep Regulatory Circuit. BF: basal forebrain; cLDT/SLD: caudal lateral dorsal tegmental nucleus/sublateral dorsal nucleus; DR: dorsal raphe; LC: locus coeruleus; M: medulla; MN: motor neuron; OX: orexin; PPT: pedunculopontine tegmentum; vlPAG: ventrolateral periaqueductal grey. During REM sleep, noradrenergic LC and serotonergic DR neurons cease firing and release cholinergic PPT neurons from inhibition. PPT neurons activate glutamatergic neurons in the cLDT/SLD which, in turn, activate gabaergic medullary neurons that inhibit pontine and spinal motor neurons and induce the atonia of REM sleep. REM sleep is maintained by medullary neurons, which feedback to inhibit LC and DR neurons as well as the gabaergic vlPAG neurons. vlPAG neurons inhibit the activity of cLDT/SLD neurons and prevent REM sleep. vlPAG neurons are active during wakefulness, and their activity is enhanced by orexin. A more extensive discussion of REM sleep regulatory mechanisms is found in the text, and more detailed illustrations of the neuronal circuits regulating REM sleep may be found in Herice et al., Peever and Fuller, Scammell et al., (58, 61, 62).
A population of spinally projecting neurons in the pontine sub-coeruleus region (a.k.a. sub-lateral-dorsal [SLD] nucleus and adjacent caudal lateral-dorsal tegmental nucleus [cLDT] in rodents) fire in anticipation and are maximally active during REM sleep. These REM-ON neurons are glutamatergic and produce atonia by activating interneurons localized in the medullary ventral giganto-cellular reticular nucleus, which synapse with and hyperpolarize the brainstem and spinal motoneurons by releasing glycine and GABA. Motor neurons also receive cholinergic input from local interneurons, and muscle atonia is dependent upon motor neuron hyperpolarization produced by the activation of glycine, GABAA, GABAB, and type 2 muscarinic cholinergic (AchM2) receptors. A homeostatic increase in the density of GABAA, GABAB, and AchM2 receptors on these motor neurons occurs in parallel with the increased duration of wakefulness [64]. There appear to be two sets of glutamatergic neurons in the SLD and cLDT nuclei. Opto-or chemo-genetic activation of one set generates the forebrain features of REM sleep and induces the characteristic cortico-hippocampal theta rhythms, while activation of the other set induces the atonia of REM. Indeed, optogenetic activation of the glutamatergic neurons in the SLD may even elicit cataplexy, i.e., isolated motor atonia during wakefulness, although cataplexy can be elicited even if the glutamatergic neurons are inactivated and, thus, the specific neural circuits engaged in the induction of cataplexy remain to be defined [65].
The discovery that REM sleep could be induced in cats by infusing the muscarinic cholinergic agonist, carbachol, into the pontine brainstem was one of the earliest discoveries of the modern era of sleep research [66, 67]. This work has come full circle with the recent discovery that double knock-out mice missing the Chrm1 and Chrm3 genes for metabotropic muscarinic cholinergic receptors have virtually no REM sleep [68]. Cholinergic neurons cluster in the lateral dorsal tegmental nucleus/pedunculopontine nucleus (LDT/ PPT) region of the pons and in the basal forebrain (BF). High levels of acetylcholine are found in the pons, thalamus, and cortex during REM sleep and wakefulness, although cortical acetylcholine likely derives from the basal forebrain as cortical innervation by LDT/PPT cholinergic neurons is sparse [21, 62, 69-71]. Electrophysiological studies reveal that there are Wake/REM-ON neurons in the LDT/PPT, which are active during both wakefulness, and REM sleep and REM-ON neurons, which are solely active during REM sleep [72]. During wakefulness and NREM sleep, the REM-ON cholinergic neurons are inhibited by the actions of locus coeruleus noradrenergic and dorsal raphe serotonergic neuronal activity. Serotonin 5HT1A agonists, for example, have been shown to inhibit the activity of the REM-ON neurons but not the Wake/REM-ON neurons [72, 73]. Mono-aminergic neurons cease firing during REM sleep, releasing their inhibitory hold on REM-ON cholinergic neurons to make cholinergic neurotransmission and all components of REM sleep possible.
Optogenetic activation of REM-ON GABAergic neurons in the dorsal medullary para-giganto-cellular reticular nucleus reveals that a set of these neurons promotes motor atonia by projecting rostrally to the dorsal serotonergic raphe and noradrenergic locus coeruleus to inhibit the wake-promoting effects of these monoaminergic nuclei. As just noted, this releases the cholinergic REM-ON neurons from inhibition and promotes REM sleep. Inhibiting monoaminergic activity also depresses the excitability of spinal motor neurons and further facilitates motor atonia. The REM-ON GABAergic neurons in the medulla also project to the ventrolateral periaqueductal grey (vlPAG) and inhibit the GABAergic REM-OFF neurons in this region [60, 63, 74] vlPAG GABAergic neurons project to the SLD and cLDT nuclei. Their activity promotes NREM sleep and blocks the initiation and maintenance of REM sleep [75]. By inhibiting vlPAG neuronal activity, the GABAergic neurons projecting rostrally from the medulla release the SLD and cLDT from inhibition and trigger both the cortical activation and atonia of REM sleep. vlPAG neurons cease firing at the onset of REM sleep and start up again at its termination. The activity of these vlPAG GABAergic neurons gradually decreases between REM sleep periods and is reset during REM sleep [76]. The density of GABAA and AchM2 receptors on active vlPAG GABAergic neurons increases progressively with time, and a time-dependent homeostatic recovery occurs during REM sleep. Activation of these receptors by GABAA agonists or AchM2 agonists hyperpolarizes and inhibits the activity of these neurons and elicits REM sleep [77]. The shorter the REM sleep period, the less the homeostatic recovery and the shorter the inter-REM sleep interval [76]. However, during wakefulness, the activation of orexin receptors on vlPAG neurons maintains their activity at a high level and prevents the cycling between NREM and REM sleep [76, 78]. These observations suggest that any condition that increases the density or sensitivity of GABAA or AchM2 receptors on vlPAG neurons may increase the probability of REM sleep and atonia.
5. THE OREXINS
The orexins, 1 and 2, are two small neuropeptides that are produced solely by neurons in the lateral, posterior, and perifornical hypothalamus from the same precursor gene and prepro-orexin protein precursor. These neurons have widespread projections to all levels of the neuraxis aside from the cerebellum and are recognized to integrate arousal, locomotion, and food consumption in the interests of energy balance [79]. Two metabotropic orexin receptors, type 1 and type 2, have been identified with overlapping but distinct distributions within the nervous system and with different affinities for each of the orexins. Acting at these receptors, they produce excitatory effects at pre and post-synaptic sites [80]. Dogs with a mutation in the type 2 receptor gene, KO mice lacking the type 2 receptor and, even more so, lacking both receptors, and rats with a selective loss of hypocretin neurons, are all unable to maintain wakefulness and present with abnormal transitions from wakefulness to REM sleep and with cataplexy-like episodes. HLA DQB1*06:02-positive patients with narcolepsy-cataplexy have low or undetectable cerebrospinal orexin-1 levels [81, 82]. Orexin-2 has been difficult to assay in the cerebrospinal fluid [82].
However, low levels of orexin-1 are not essential for sleep-onset REM periods or for cataplexy. Some HLA DQB1*06:02 negative patients with narcolepsy and cataplexy are not orexin deficient [81, 82]. Early-onset dissociated REM sleep is also common in depressed patients and many other psychiatric disorders and is a prominent feature of antidepressant drug withdrawal [83-86]. Sleep paralysis, like cataplexy, is a dissociated REM sleep phenomenon that consists of motor atonia during wakefulness. It is common in individuals with normal levels of orexin but irregular sleep habits. Scheduled awakenings at night preceding REM sleep in normal subjects have been shown to induce sleep onset REM periods and sleep paralysis [87, 88].
In cats, orexin neurons have been found to be most active during wakefulness when the animal is engaged in exploratory reward-seeking behavior and far less active during quiet wakefulness or SWS [80]. The orexins appear to be involved in the regulation of vigilance and motor control [89, 90]. Although a number of studies reveal that the orexin neurons are quiescent during REM sleep, other work reveals that they fire in conjunction with the phasic twitches of REM sleep likely to facilitate motoneuron activity [80]. Orexin neurons promote wakefulness by exciting the nonspecific thalamocortical projection neurons that stimulate and maintain cortical activation. Dense excitatory projections to brainstem arousal systems such as the noradrenergic locus coeruleus, the dorsal serotonergic raphe, and the histaminergic tuberomammillary nucleus have been identified. The activity of the noradrenergic, serotonergic, histaminergic, and dopaminergic neurons parallels the activity of the orexin neurons with the highest levels during wakefulness, lower levels during SWS, and little or no activity during REM sleep [79, 91]. The decline or absence of hypocretin neuronal activity during NREM sleep and the corresponding decline in monoamine neurotransmitter release have been attributed to signals arising from the suprachiasmatic nucleus controlling the circadian rhythm of sleep and wakefulness [92, 93].
5.1. Orexin and the Monoamines
Changes in monoamine receptor sensitivity have been identified in dogs with mutated orexin -2-receptors and thus with impaired orexin activity. Increased numbers of pre-and post-synaptic α2-adrenoreceptors have been identified in the locus coeruleus, and an increased number of post-synaptic α1-receptors have been found in the amygdala [94, 95]. A significantly increased number of dopamine D2 receptors has also been found in the amygdala, nucleus accumbens, and rostral caudate [96]. Human post-mortem brain studies have confirmed increases in D2 receptor levels in the caudate and putamen but attempts to confirm these findings with SPECT studies in living patients with narcolepsy have met with mixed success [97-102]. The increase in the number of adrenergic and dopaminergic receptors likely reflects the reduction in noradrenergic and dopaminergic neurotransmission in the absence of orexin.
While ascending noradrenergic neurons projecting to the forebrain mediate cortical arousal, descending monoaminergic projections to the spinal cord maintain muscle tone. Motor paralysis, or cataplexy, occurs when motor neurons are hyperpolarized. The monoamines, noradrenaline, and serotonin, acting on α1-adrenergic and 5HT2 receptors, reduce the motor neuron membrane potential and promote increased motor neuron excitability [103]. Orexin, applied to the locus coeruleus, increases noradrenergic neuronal activity and also increases muscle tone while its injection into the cholinoceptive sub-coeruleus region suppresses muscle tone [104]. Orexin also acts peripherally at pre- and post-synaptic receptors to enhance lumbar motor neuron excitability and motor neuron discharge [105]. In canine narcolepsy and in human narcolepsy, in the absence of orexin, cataplexy can be triggered by blocking the effects of noradrenaline with prazosin, an α1-adrenergic receptor antagonist [95, 106-108]. On the other hand, noradrenergic and serotonergic reuptake inhibitors, which increase the availability of these neurotransmitters at the synapse, are commonly used to prevent cataplexy [109-111]. The anti-cataplectic properties of the histamine-H3 inverse agonists may also be attributed to their ability to improve noradrenergic neurotransmission by desensitizing α2-noradrenergic receptors at both the locus coeruleus and at the spinal level [112]. In mice with narcolepsy, inverse histamine-H3 receptor agonists have been shown to increase noradrenergic as well as histaminergic neuronal activity and to decrease abnormal direct transitions from wakefulness to REM sleep, a phenomenon characteristic of cataplexy [113].
5.2. Orexin and Acetylcholine
Orexins also have excitatory effects on cholinergic and noncholinergic neurons in the basal forebrain (BF) and enhance arousal and attention by increasing the cortical release of acetylcholine [114, 115]. Cholinergic neurons in this region discharge in bursts at maximal rates during wakefulness and REM sleep but less frequently during SWS [116]. Cholinergic neurons in the LDT/PPT nucleus are also heavily innervated by orexinergic neurons [117]. Both cholinergic and non-cholinergic neurons in the PPT/LTD have been shown to be excited by orexins, although orexins can also reduce the release of acetylcholine by activating GABAergic interneurons in this region that inhibit cholinergic neuronal activity [90, 118, 119]. Nevertheless, dialysates of the pontine tegmental region reveal that twice as much acetylcholine is released during REM sleep than during SWS or wakefulness [69]. In the absence of effective orexin action, as in dogs lacking the orexin-2 receptor, the reduced cholinergic neuronal activity would be expected to lead to an upregulation of AchM2 receptors and to the development of cholinergic hypersensitivity and, indeed, the infusion of low doses of carbachol into the BF or pons of these animals elicits cataplexy [120]. Normal dogs require much higher doses to elicit this response. A significant increase in the density of AchM2 receptors has been documented in the brainstem of dogs with narcolepsy, and the suggestion of a similar change has been found in the caudate and other regions of the brain post-mortem in patients with narcolepsy [97, 121].
6. DOPAMINE
Spinal dopamine neurons arise primarily in the posterior hypothalamus and project to dopamine receptors, D1R-D5R, which are nonuniformly distributed through the dorsal and ventral horns of the spinal cord [122]. Although there are differences among species, in the mouse, D2-like receptors (D2R, D3R, D4R) are highly expressed in the dorsal horn where they mediate inhibitory effects, although they are also expressed, with less density, in the ventral horn. In contrast, D1-like receptors (D1R and D5R) are highly expressed in the ventral horn, including the motoneurons, where they mediate excitatory effects [123, 124]. The activation of D1-like receptors located on or near cranial somatic motor neurons has also been found to increase muscle tone [125]. Nevertheless, a specific role for spinal dopamine in the genesis of cataplexy has not been identified.
For some time, it was also not clear how the activity of dopamine neurons was related to the vigilance states despite the potent waking effects of drugs like the amphetamines that raise the synaptic levels of dopamine [126]. Early electrophysiological findings suggested that ventral tegmental area (VTA) and substantia nigra pars compacta (SNc) dopaminergic neurons did not change their mean firing rate across sleep-wake states [127]. However, follow-up studies of VTA dopaminergic neuronal firing patterns revealed that VTA neurons had prominent burst firing patterns during REM sleep associated with the extensive release of synaptic dopamine. This activation was similar to the burst firing observed during motivated behaviors like the consumption of food, while slow firing rates with irregular patterns appeared during quiet waking and non-REM (NREM) sleep [128]. Recent chemo-genetic and optogenetic manipulations have provided more definitive evidence that VTA dopaminergic neurons are necessary for arousal and that their inhibition suppresses wakefulness. Opto-genetic stimulation initiates and maintains wakefulness and suppresses sleep [129]. Wake active dopaminergic neurons have also been identified in the ventral periaqueductal gray (vPAG) [130]. Dopamine neurons arising in the VTA and the vPAG innervate areas involved in sleep/wake regulation and include the serotonergic cells of the dorsal raphe, the noradrenergic cells of the locus coeruleus, the cholinergic cells of the LDT/PPT and the BF, the orexinergic neurons of the lateral hypothalamus and the neurons that modulate the behavioural state in the thalamus and the prefrontal cortex. In turn, inputs to the dopaminergic VTA and vPAG cells have been found from most of these anatomical structures [130].
Dopamine neurons also send dense projections to the striatum that are topographically organized. Medially located VTA dopamine neurons project to the ventromedial striatum, while more laterally located SN dopamine neurons project to the dorsolateral striatum [131]. Midbrain dopamine neurons characteristically fire in bursts signalling unexpected reward and reward-related cues. These neurons make the monosynaptic connection with cholinergic interneurons (Chi) and interrupt their firing [132]. Damage to these neurons with 6-OHDOPA appears to result in an increase in acetylcholine release and down regulation of muscarinic receptor sensitivity measured by quinuclidinyl benzilate (3H-QNB) binding [133, 134].
In the narcoleptic canine, local perfusion of the presynaptic D2 dopamine agonist quinpirole into the VTA, substantia nigra, or the globus pallidus/putamen produces a dose-dependent increase in cataplexy without significantly reducing basal muscle tone [135, 136]. Quinpirole inhibits dopamine release. Perfusion with the D2-dopamine antagonist raclopride produces a moderate reduction in cataplexy. In control animals, neither of these drugs induces cataplexy or muscle atonia when perfused into either the VTA or the globus pallidus/putamen. Identical results are found in orexin KO mice in which the intraperitoneal administration of quinpirole increases the incidence of cataplexy, and the intraperitoneal administration of eticlopride, a D2-dopamine antagonist, reduces the incidence of cataplexy [137]. Cataplexy may be induced by the increased release of acetylcholine in the striatum, which follows the inhibition of dopamine release. However, the direct effects of inhibiting dopaminergic neuronal activity on the cholinergic REM-ON neurons in the LDT/PPT await examination.
7. SODIUM OXYBATE
Sodium Oxybate is remarkable in that it can both induce cataplexy and prevent it [2, 13, 55]. It can induce REM sleep and suppress it [41, 45]. It can induce REM sleep and even sleep paralysis in the presence of normal levels of orexin [54]. Its capacity to induce REM sleep, cataplexy, or sleep paralysis is predicated on the pre-existence of increased cholinergic sensitivity and is evident in patients with narcolepsy and in patients suffering from depression and other forms of mental distress as well as following its administration to normal subjects in the early morning hours.
7.1. Sodium Oxybate and Cholinergic Sensitivity
In the rat, the circadian rhythmicity of the cholinergic system is revealed by high levels of cortical acetylcholine (ACH) release during the animal’s active phase with simultaneously enhanced choline-acetyl-transferase activity and reduced ACH esterase activity [138]. In contrast, the number of unbound available muscarinic cholinergic receptors (mACHRs) is highest when ACH neurotransmission is lowest, i.e., during the inactive phase of the animal. The least number of available mAChRs is found when the animal is most active [139]. A comparable pattern has been identified in the post mortem human brain [140]. REM sleep shows a strong circadian distribution in man with an acrophase in the morning. Subjects submitted to a 90-min sleep-wake schedule display REM periods chiefly from 7:30 a.m. to 2:00 p.m. [141]. SO is most likely to induce REM sleep early in the day in healthy subjects when unbound muscarinic receptors are available, and the probability of REM sleep is high [32, 53]. Under normal conditions, unbound mACHRs, generated during the night in response to low ACH levels promote REM sleep in the early morning hours. The tight relationship between cholinergic sensitivity and the onset of REM sleep has been carefully studied in the rat where the density of mACHRs on vlPAG GABAergic neurons has been shown to increase with time until REM sleep is triggered. The density of these receptors then declines in response to the high levels of ACH present during REM [77]. BF cholinergic neurons discharge in bursts at maximal rates during active waking and paradoxical sleep, when gamma and theta electroencephalographic activity is maximal. They virtually cease firing during slow-wave sleep [116].
7.2. Sodium Oxybate and Depression
SO also induces REM sleep and even sleep-onset REM periods in subjects at risk for depression [5, 52, 53]. Patients with depression suffer from disturbed sleep with difficulty getting to sleep and staying asleep. Sleep studies in patients with depression may reveal REM sleep latencies of less than 20 minutes (normal is about 90 minutes) and even sleep-onset REM sleep periods. The first REM sleep period of the night is often prolonged, and the frequency of rapid eye movements (REM density) during this first period is often high. These changes in REM sleep parameters need not fully remit following the resolution of the depression. Persistent psychological distress appears to induce REM sleep. Studies in rodents show that chronic stress increases the number and duration of REM sleep periods during both the light and dark periods in response to an increased release of corticotropin-releasing hormone (CRH) [142, 143].The increase in REM sleep follows the stress-related rise in the levels of CRH in the amygdala and lateral dorsal tegmentum, which, in turn, activates the release of ACH in these regions and triggers REM sleep [142, 144]. Carbachol, a cholinergic agonist, injected into the central nucleus of the amygdala, is known to promote REM sleep [142, 145].
These observations suggest that clinical depression may be viewed as a stress-induced cholinergic supersensitivity state and that this sensitivity accounts for the short REM sleep latencies found in patients with depression [85, 146, 147]. Reduced REM sleep latencies may persist after the depression remits but may also be found in asymptomatic first-degree relatives suggesting a genetic basis for the excessive cholinergic response to stress [148]. For example, the cholinergic muscarinic 2 receptor gene tends to have distinct structural characteristics in depressed women [149]. Once established, either genetically or perhaps in response to life events, excessive cholinergic sensitivity appears to predispose to the development of depression. This is evident in subjects with a strong family history of affective disorders who may have a normal REM sleep latency at baseline but in whom the induction of a short REM latency with the use of RS-86, a cholinergic agonist, appears predictive for the onset of future major depressive disorders. RS-86 produces a much greater reduction in REM sleep latency in high-risk probands than in normal subjects and may even induce sleep-onset REM periods in these vulnerable individuals [150, 151]. In contrast to the effects of cholinergic agonists, scopolamine, a cholinergic antagonist, increases REM sleep latency and suppresses REM density and duration in depressed patients and shows promising results as a fast-acting antidepressant [146, 152-154]. The early REM sleep periods induced by cholinergic agonists and sodium oxybate may thus identify individuals prone to depression [52, 53]. Rapid REM sleep induction may constitute a depressive trait.
7.3. Sodium Oxybate and Narcolepsy
Narcolepsy may also be viewed as a cholinergic super-sensitivity state. It was the induction of a sleep-onset REM sleep period together with subjective feelings of sleep paralysis in a patient with a history of poor sleep and psychological distress that first linked SO to narcolepsy [54]. In narcolepsy, the immediate application of SO can induce REM sleep and even cataplexy, but its repeated nightly use restores REM sleep latency to normal values and prevents cataplexy. These opposite immediate and long-term effects of SO both appear to depend, at least in part, upon its actions on cholinergic neurotransmission. Although not clearly demonstrated in the human brain, in the absence of effective orexin action, as noted earlier in this essay, a significant increase in the density of AchM2 receptors develops in the pontine reticular formation in narcoleptic canines [97, 121, 155, 156]. In these dogs, local perfusion of the pontine reticular formation and BF with agonists that act on these receptors elicits REM sleep and cataplexy. AchM2 receptor antagonists block these effects [120, 157]. The activation of AchM2 receptors located on vlPAG gabaergic neurons hyperpolarizes and inhibits these neurons and releases their target REM-ON neurons from inhibition [77]. The central role of ACH release and its action on AchM2 receptors in the generation of REM sleep and cataplexy is emphasized by the observation that muscle atonia can even be elicited in dogs with normal levels of orexin using high doses of the AchM2 receptor agonist carbachol [120, 157].
The induction of REM sleep and cataplexy with SO likely reflect its actions on the GABAB receptor with the resulting inhibition of serotonergic, noradrenergic, and dopaminergic neurotransmission leading to an increase in ACH release. GABAB receptors have been identified on all 3 of these monoaminergic neurons [16, 17]. SO, acting on GABAB receptors has been shown to have strong inhibitory actions on dorsal raphe serotonergic cells. Silencing these cells permits REM sleep [18]. GABAB receptors have also been shown to mediate the tonic inhibition of locus coeruleus noradrenergic neurons [158]. In rats, low subcutaneous doses of SO, 40 mg/day, decrease both the spontaneous and evoked burst firing rate of noradrenergic neurons by about 50% [159]. SO withdrawal is followed by a significant 33% increase in spontaneous activity and by a robust 79% increase in burst firing in response to paw pinch. Given at night, the inhibition of noradrenaline release by SO would be expected to promote REM sleep. The tonic increase in noradrenergic neuronal activity upon withdrawal during the day would enhance alertness, and the phasic increase should augment reactivity to goal-relevant sensory stimuli [160]. Substantia nigra dopaminergic neurons appear to be equally sensitive to the effects of SO. A dose-dependent decrease in tonic neuronal activity and burst firing that can be blocked by GABAB antagonists is observed with intravenous SO doses starting as low as 12.5 mg/kg [161]. Studies in our laboratory reveal that the typical 3-gram dose of SO used to treat narcolepsy produces a small, non-significant increase in striatal [C-11] raclopride binding that suggests reduced synaptic dopamine levels (Kish et al., 2021; manuscript submitted for publication). As discussed earlier, inhibition of striatal dopaminergic neuronal activity may trigger REM sleep by promoting the release of acetylcholine.
In the LDT, SO’s inhibitory effect on cholinergic neurons is weak, and it appears to inhibit only a subset of cholinergic cells. It has been proposed that SO selectively suppresses only the ‘wake active’ cholinergic neurons and not the REM-ON cholinergic neurons responsible for generating REM sleep and motor atonia [18].
As it stands, the evidence to date suggests that SO’s capacity to act on GABAB receptors and to simultaneously block serotonergic, noradrenergic, and dopaminergic neurotransmission can account for SO’s capacity to induce REM sleep and cataplexy in patients with narcolepsy. However, SO may also promote cataplexy in these patients by inhibiting neurotransmission directly at pontine and spinal motor neurons. Motor inhibition in man during REM sleep and cataplexy is characterized by an absent or markedly attenuated H-reflex response [162, 163]. Low oral doses of SO, 30 mg/kg, given to normal and narcoleptic subjects similarly inhibit the H-reflex and do so whether or not REM or slow-wave sleep is induced [164]. The effect lasts for about 2 hours. These findings raise the possibility that the prolonged inhibition of motor activity at night by SO is followed by a rebound increase in the threshold for motor inhibition the next day. This may help account for suppression of cataplexy during the day by the nocturnal application of SO. The rebound increase in monoaminergic activity following its suppression at night with SO may further reduce the likelihood of cataplexy during the day.
The repeated and prolonged inhibition of monoaminergic neurotransmission at night and the consequent increase in ACH release gradually downregulates the density of AchM2 receptors. For example, a significant decrease in the number of 3H-QNB binding sites in striatal membranes has been observed in rats exposed to GBL for 3 weeks. The decrease in 3H-QNB binding is not due to a direct effect of GBL on muscarinic receptors since no alteration in binding is produced by high concentrations of GBL or its active metabolite, gamma-hydroxybutyrate (SO). Inhibition of the nigrostriatal dopaminergic pathway produced by chronic GBL treatment leads to persistent activation of the intrastriatal cholinergic interneurons, which, as noted earlier, are normally held in check by dopaminergic nigrostriatal neuronal activity. The activation of these intrastriatal cholinergic interneurons results in a downregulation of muscarinic receptors and reduced sensitivity to ACH [133]. This downregulation takes time and could account for the gradual improvement of cataplexy with the repeated nightly use of SO. Future work will explore whether a similar downregulation can be elicited in the pons where dopaminergic neurons serve the cholinergic cells of the LDT/PPT [130]. Clinically, the reduction in cholinergic sensitivity is reflected in the restoration of the normally high threshold for REM sleep and cataplexy. In narcolepsy, with the repeated nightly use of SO, sleep onset REM periods disappear, and REM latencies gradually return to their normal values [45]. Upon withdrawal, the symptoms of narcolepsy gradually reappear. SO holds a receptor imbalance in check but does not correct the basic pathology of the disorder, the loss of the orexins.
In addition to SO, tricyclic antidepressants (TCA) and selective serotonin and noradrenaline reuptake inhibitors continue to be used for the treatment of cataplexy [1, 2]. These drugs suppress REM sleep and prolong the REM sleep latency and owe their anti-cataplectic effects to their ability to block noradrenaline and serotonin reuptake, but the TCAs, in addition, have anticholinergic properties and can block ACHR2 receptors [110]. Patients with cataplexy withdrawn from antidepressants display a rebound increase in REM sleep and a reduced REM sleep latency, as well as a significant rebound increase in cataplexy. The sudden loss of the agonistic effects of noradrenaline and serotonin on their respective postsynaptic receptors may account for this rebound. Prazosin, for example, an alpha 1 noradrenergic antagonist, has been documented to trigger frequent daily episodes of cataplexy in a patient with narcolepsy [106]. In narcoleptic dogs, a similar increase in cataplexy with prazosin can be blocked by anticholinergic agents [95]. The additional sensitization of AchR2 receptors with the anticholinergic TCAs may explain the greater rebound increase in cataplexy following withdrawal from TCAs than from SSRIs or SNRIs [111].
8. SO: AN ANTIOXIDANT AND AN ENERGY SOURCE
Although the pharmacological actions of SO on the GABAB receptor account for many of its effects on the structure of sleep, this unique molecule has another set of distinct metabolic actions, which may specifically account for its ability to improve the restorative and homeostatic properties of sleep. Its catabolism in both the cytoplasm and mitochondria of the brain generates succinic semialdehyde, which is then rapidly converted to the metabolic intermediate succinate with the concomitant reduction of NAD to NADH [165]. NADH is transformed by mitochondrial nicotinamide nucleotide transhydrogenase to the antioxidant cofactor NADPH. Succinate is metabolized along the electron transport chain to form ATP, but it may also be processed through the malate-pyruvate shuttle to form additional NADPH [166, 167]. SO is also known to shift intermediary metabolism in the direction of the pentose phosphate shunt to generate NADPH [168, 169]. SO is thus both a source of energy to the cell and a powerful antioxidant.
The high metabolic demands and high oxygen consumption of the waking brain favor the formation of free radicals, and many investigators have identified signs of oxidative stress in the rodent brain following prolonged periods of wakefulness [170-174]. For example, a recent study focused on the effects of sleep deprivation on the parvalbumin containing interneurons (PV) in the rat prelimbic prefrontal cortex and orbital frontal cortex [175]. PV interneurons are vulnerable to oxidative stress because they fire at the high rate required to generate the EEG gamma activity (> 30 Hz) that is a prominent feature of the waking brain. In fact, the observed decline in gamma activity in local field potentials of the rodent cerebral cortex across sustained wakefulness is thought to mirror the level of oxidative stress in PV cells as increasing oxidative stress suppresses gamma activity in neural cultures containing these cells. Experimental work has demonstrated that 6 and 12- hour periods of sleep deprivation greatly increase the intensity of the oxidative stress marker 8-oxo-2’-deoxyguanosine in these interneurons. Even more compelling evidence for the intimate relationship between brain energy metabolism, oxidative stress, and sleep comes from a sophisticated body of work using the fruit fly, Drosophila [176]. These flies are active during the day and rest at night. About 2 dozen sleep-inducing neurons in Drosophila that project to its dorsal fan-shaped body (dFB) adjust their electrical activity to the need for sleep by regulating opposing potassium conductance through 2 channels: a voltage-gated A-type current carried by the Shaker channel that fires tonically during sleep; the downregulation of this current and the upregulation of Sandman, a voltage-independent leak current during wakefulness carried by another potassium channel. An NADPH cofactor is bound to Hyperkinetic, the oxidoreductive domain of Shaker’s Kvβ subunit, that keeps Shaker shut during wakefulness. Sleep loss raises the level of mitochondrial reactive oxygen species (ROS) in dFB neurons which register this rise by converting NADPH to NADP. The change in NADPH/NADP ratio opens the Shaker channel increases the frequency of action potentials and puts the fly to sleep. Sleep persists until the NADPH/NADP ratio returns to its baseline level and the channel shuts [177]. In Drosophila, oxidative stress triggers sleep, and sleep then functions as an antioxidant. Preventing oxidative stress reduces the duration of sleep and prolongs wakefulness [178].
Oxidative stress has also been shown to trigger sleep in the mammalian brain. In rats, the intracerebroventricular infusion of low doses of t-butyl hydroperoxide, an organic chemical that generates ROS, significantly increases the duration of NREM and REM sleep. The infusion of t-butyl hydroperoxide into the preoptic/ anterior hypothalamus promotes the release of the sleep-inducing neuromodulators, adenosine, and nitric oxide (NO), without causing neurodegeneration [179]. t-butylhydroperoxide and other oxidants can also increase tissue prostaglandin levels [180, 181]. NO, adenosine, and prostaglandin D2 (PGD2) are the 3 major mediators of the homeostatic sleep response following sleep deprivation. Inhibiting the actions of any one of these mediators prevents this homeostatic response. A naturally occurring increase in oxidative stress may best explain the rise in all 3 of these mediators following sleep deprivation.
8.1. Nitric Oxide
Inducible nitric oxide synthase (iNOS), the generator of nitric oxide, is expressed by BF cholinergic cells and activated microglia and astrocytes. It is found at only low levels during the spontaneous sleep/wake cycle, but its expression increases following prolonged wakefulness, likely in response to its induction by cytokines, specifically IL-1β and TNFα, whose levels rise during sleep deprivation together with signs of oxidative stress. A rise in brain antioxidant enzyme activity with sleep deprivation accompanies signs of increased lipid peroxidation and DNA oxidation [182-190]. The BF concentrations of nitrite and nitrate, indirect measures of NO, double in rats during sleep deprivation. Both a NO scavenger and an iNOS inhibitor will abolish homeostatic NREM recovery sleep, while a NO donor will increase the intensity of NREM recovery sleep. These findings suggest that the elevation of NO produced by induction of iNOS in the BF during prolonged wakefulness is a specific mechanism for producing NREM recovery sleep. In this regard, the application of SO would assist the recovery process by generating NADPH. NOS depends on NADPH to convert arginine to citrulline and NO. This reaction requires tetrahydrobiopterin, among other factors, but tetrahydrobiopterin also requires NADPH for its biosynthesis [191].
Inhibiting iNOS prevents the rise in the level of adenosine in the BF triggered by the release of NO during sleep deprivation. The rise in adenosine levels is thus dependent on the production of NO by iNOS [184, 186, 187]. NO may generate adenosine by inhibiting adenosine kinase (AdK), the enzyme that converts adenosine to AMP and/ or by actions on the electron-transport chain and mitochondrial respiration that reduce ATP production [192, 193]. The exact source of the released adenosine remains uncertain [194]. Destruction of BF cholinergic neurons abolishes the sleep deprivation increase in adenosine release in this region, the homeostatic sleep response, and the sleep-inducing effects of BF adenosine infusion [195]. Evidence for an astrocytic source for adenosine and for glial transmission remains inconclusive; however, an astrocytic source would explain the increase in rebound SWA activity in mice deficient in glial AdK [194, 196-198].
8.2. Prostaglandin D2
Extracellular levels of adenosine also rise in response to prostaglandin D2 (PGD2), the most potent endogenous sleep-promoting agent [199, 200]. The intracerebroventricular infusion of femtomolar (10-15 M/L) quantities of PGD2 profoundly enhances both NREM and REM sleep. The EEG power spectrum of NREM sleep following PGD2 infusion into the monkey brain is identical to that of natural sleep and clearly distinct from benzodiazepine induced sleep which is characterized by reduced power in the theta range and increased fast activity with a peak at 20 Hz. PGD2 is the most abundant prostaglandin in the rat and human brain. Its concentration in the rat cerebrospinal fluid fluctuates with circadian rhythmicity in parallel with the sleep-wake cycle and increases with sleep deprivation, together with a rising need for sleep. PGD2 is synthesized when oxidative stress increases the expression and activity of phospholipase A2, which then acts on membrane phospholipids to produce arachidonic acid (AA) [181, 201]. AA is converted into the unstable intermediate prostaglandin, PGH2, by the action of cyclooxygenase-2 (COX-2), and this step is followed by the synthesis of stable PGD2 by lipocalin-type PGD2 synthase (L-PGDS) [200].
L-PGDS is present in the leptomeninges and produces PGD2 in the arachnoid trabecular cells.PGD2 enters the CSF circulation and activates the leptomeningeal D-type prostanoid receptor 1 (DP1) found almost exclusively on the ventral surface of the rostral BF. Activation of the DP1 receptor increases extracellular adenosine in a dose-dependent manner by an unknown mechanism, and adenosine then acts on the adenosine receptors A1R and A2R to induce sleep. PGD2 infusion fails to induce sleep in KO mice lacking DP1 receptors. Selenium tetrachloride (SeCl4), an L-PGDS inhibitor, produces insomnia and reveals that PGD2 also participates in the generation of baseline physiological sleep. WT mice accumulate PGD2 in their brains during sleep deprivation, and a significant NREM sleep rebound follows. This rebound does not occur in L-PGDS knockout mice [199, 200].
Terao et al., 1998 found that IL-1β could induce NREM sleep by acting at the PGD2-sensitive sleep-promoting zone and that the induced sleep could be completely inhibited by pre-treating the rats with NS398, an inhibitor of COX-2 [202]. As various cytokines, such as IL-1β and TNFα induce cyclooxygenase in a variety of cells and increase the production of prostaglandins, they proposed that IL-1β-induced NREM sleep was dependent upon the production of prostaglandins, most likely PGD2. However, Zhang et al., 2017, using the same inhibitor, failed to suppress the induction of NREM sleep in WT mice or in DP1R KO mice, and they concluded that the somnogenic effect of IL-1β was independent of the PGD2/DP1R system [203]. They suggested that IL-1β may act directly on sleep-active neurons of the preoptic area of the hypothalamus and inhibit the wake-active neurons in the BF and pons. They noted that IL-1β decreased the delta power of NREM sleep, whereas PGD2 did not and, therefore, that the brain mechanisms for the sleep-inducing effects of IL-1β and PGD2 were likely different.
8.3. Adenosine Triphosphate: ATP
ATP co-released with neurotransmitters into the extracellular space from multiple brain regions, and glia has been shown to act on glial purine P2 receptors to induce the release of IL-1β and TNFα. A P2 receptor agonist, benzoyl-benzoyl ATP, increases NREM sleep and EEG delta power in rats, while two different P2 receptor antagonists, acting by different mechanisms, reduce spontaneous NREM sleep. Compared to wild-type mice, mice lacking functional P2 receptors have attenuated NREM and delta power responses to sleep deprivation but not to IL-1β [204].
8.4. Adenosine
Adenosine levels in the BF fluctuate as a function of sleep-wake states. In the cat, adenosine levels decline by 15 to 20% during episodes of spontaneous sleep relative to wakefulness but levels increase significantly in the cholinergic region of the BF (to 140% of baseline) and, to a lesser extent in the cortex, during 6 hours of sleep deprivation. This increase is not observed in other regions such as the thalamus, preoptic hypothalamus, and pons. Following sleep deprivation, BF adenosine levels decline very slowly, remaining significantly elevated throughout a 3-hour period of recovery sleep [205, 206].
Adenosine regulates neuronal activity at two major receptors: the inhibitory A1R that are distributed throughout the brain and the excitatory A2R that are mainly localized in the striatum, nucleus accumbens, and the olfactory tubercle [200, 207]. In A2R KO mice, caffeine (an A1R and A2R antagonist) fails to promote wakefulness, and the deprivation-induced NREM sleep rebound is reduced. Activation of A2R directly excites ventrolateral preoptic (VLPO) neurons which, in turn, excite VLPO neurons that indirectly induce sleep by sending inhibitory signals to the histaminergic wake-promoting neurons in the tuberomammillary nucleus. On the other hand, A1R mediate inhibitory effects of adenosine on wake-active BF cholinergic neurons, hypothalamic orexinergic and histaminergic neurons, and locus coeruleus adrenergic neurons. The sleep deprivation-induced adenosine increases in BF and cortical adenosine would be expected to both reduce the release of cortical acetylcholine and promote slow-wave sleep as adenosine presynaptically inhibits the cortical release of acetylcholine as well as the activity of cholinergic and other BF neurons [206, 208]. Adenosine may also be able to elicit the slow oscillations of slow-wave sleep by acting on thalamic AIR to increase potassium conductance and hyperpolarize thalamic relay neurons. However, the potassium currents generated by near maximal stimulation of adenosine and GABAB receptors are not additive [209, 210]. The rebound in SWA induced by sleep deprivation is attenuated in A1R KO mice [211].
Reducing extracellular adenosine by knocking out 5’-ectonucleotidase, an enzyme that converts AMP to adenosine, or by reducing the glial release of ATP, which may be also be converted to adenosine, reduces homeostatic sleep rebound [198, 212]. Thus, both extracellular adenosine and extracellular ATP appear to be involved in the regulation of NREM sleep.
Adenosine is not involved in the mechanism that determines the set point of the amount of sleep; rather, it induces high voltage delta (0.5-4.0 Hz) sleep, a measure of NREM sleep intensity, in response to the toxic inflammatory state which develops in response to sleep deprivation [211].
9. SLEEP RESTORATION
But how does delta sleep resolve this toxic inflammatory state? A generalized reduction in neural activity is recorded in many regions of the brain during sleep that may be attributed to the loss of cholinergic input [213]. Activation of the PPT cholinergic nucleus transforms the slowly oscillating cortical cells of sleep into tonically firing neurons. Even the long-lasting hyperpolarization separating the prolonged depolarization of the slow cortical rhythms (< 1.0 Hz) are selectively blocked by stimulation of the PPT nucleus [21, 214]. Neural activity accounts for close to 90% of the energy used by the brain, and the decline in neural activity during sleep may account for the concomitant reduction in oxygen consumption and glucose utilization [215]. Compared with wakefulness, a 25% reduction in the cerebral metabolic rate of oxygen (CMRO2) has been found during slow-wave sleep together with a striking 44% reduction in cerebral glucose utilization and a corresponding reduction in cerebral blood flow [216]. Indeed, there appears to be an inverse relationship between the EEG power in delta sleep and the CMRO2. The greater the power, the lower the brain’s oxygen consumption [217]. The reduction in CMRO2 and in glucose utilization may promote metabolic recovery by reducing ROS formation, and thus the reduction in cerebral metabolism may have an antioxidant effect, although this has not been clearly demonstrated [218].
As noted earlier in this essay, propofol, and the inhalant halogenated ether anesthetics, isoflurane, desflurane and sevoflurane have also been shown able to repay the sleep debt following sleep deprivation [47, 49]. These agents reduce cerebral cholinergic neurotransmission and neuronal activity [219-222], and they reduce the cerebral metabolic rate of oxygen and glucose [223-227]. Propofol has definite antioxidant properties, as do the inhalant anesthetics, particularly sevoflurane, the most potent homeostatic anesthetic, capable of restoring sleep loss in about half the time required by physiological sleep [228-230].
However, metabolic depression may not even be a necessary feature of the restorative process. Recovery may occur in the absence of this depression. While SO, like other anesthetic agents, diffusely depresses neuronal activity in the brain, SO does not reduce cerebral oxygen consumption or cerebral blood flow [19, 231]. SO reduces cerebral glucose utilization but supplies the brain with succinate in its stead. In fact, SO increases oxygen consumption in brain tissue slices [169]. Thus, it maintains or even augments the brain’s supply of energy while at the same time raising the concentration of the brain’s essential antioxidant cofactor, NADPH. As noted earlier, SO may do so by activating the pentose phosphate shunt and supplying NADH to nicotinamide nucleotide transhydrogenase and succinate to the malate shuttle [166-168]. SO’s capacity to improve the restorative capacity of sleep may rest largely upon its ability to remove the products of oxidative metabolism, which accumulate during the day in response to the high metabolic rate of the waking brain. This may also be a key function of naturally occurring sleep in man and fruit fly. [Curiously, propofol, despite its antioxidant properties, is toxic to Drosophila and does not satisfy their homeostatic need for sleep [232]].
CONCLUSION
SO’s unique biochemical properties may explain its capacity to both induce and prevent cataplexy and to improve attentiveness and daytime alertness in normal subjects and patients in whom sleep’s restorative process is inefficient or incomplete, such as in narcolepsy, fibromyalgia, and Parkinson’s disease [35-38]. SO may have a place in the management of shift-work. This remains to be tested. Future work will also determine whether it can be used to correct the inflammatory state and metabolic imbalance which develops in the brain in response to severe mental distress. Signs of oxidative stress accompanied by increased cytokine release have been demonstrated in the brains of laboratory animals in response to psychological distress as well as in human brains post-mortem [170, 233-237]. In mice, for example, chronic psychosocial stress, a model for anxiety and depression, activates microglia, increases the release of proinflammatory substances, and elevates ROS. Antioxidants prevent the behavioral effects of this stress [238, 239]. Severe stress also activates REM sleep in animals and induces premature REM sleep in humans. Severe stress produces a central monoaminergic /cholinergic imbalance with deficient monoaminergic activity coupled to cholinergic supersensitivity [84, 85, 146, 147]. The repeated use of SO at night may antagonize the development of this pathological inflammatory/metabolic state. By generating high levels of NADPH, SO could mitigate oxidative stress and the inflammation it brings about. SO would suppress noradrenergic and serotonergic tone at night during sleep, but a rebound during the day would enhance the release of these two mood-enhancing neurotransmitters [159, 240]. The repeated use of SO at night would correct the monoaminergic/cholinergic imbalance that is a marker of the stressed brain.
SO may have a key role to play in the management of depression and other disorders of severe psychological distress.
ACKNOWLEDGEMENTS
Declared none.
LIST OF ABBREVIATIONS
- 6-OHDOP
6-Hydroxydopamine
- A1R
Type 1 Adenosine Receptor
- A2R
Type 2 Adenosine Receptor
- AA
Arachidonic Acid
- AchM2
Type 2 Muscarinic Cholinergic Receptor
- AMP
Adenosine Monophosphate
- ATP
Adenosine Triphosphate
- BF
Basal Forebrain
- Chrm
Muscarinic Cholinergic Receptors
- cLDT
Caudal Lateral Dorsal Tegmental Nucleus
- CMRO2
Cerebral Metabolic Rate of Oxygen
- COX-2
Cyclo-oxygenase Type 2
- CRH
Corticotropin-Releasing Hormone
- D-1
Type 1 Dopamine Receptor
- D-2
Type 2 Dopamine Receptor
- dFB
Dorsal Fan Shaped Body
- DNA
Deoxyribonucleic Acid
- DP-1
D-Type Prostanoid Receptor-1
- EEG
Electroencephalogram
- GABAA
GABAA Receptor
- GABAB
GABAB Receptor
- GBL
Gammabutyrolactone
- Hz
Hertz: Cycles/Second
- IL-1β
Interleukin 1β
- iNOS
Inducible Nitric Oxide Synthase
- IPSP
Inhibitory Post Synaptic Potential
- KO
Knock Out
- L-PGDS
Lopocalin Type Prostaglandin D2 Synthase
- LDT/PPT
Lateral Dorsal Tegmental Nucleus/Pedunculopontine Tegmental Nucleus
- MSLT
Multiple Sleep Latency Test
- MWT
Multiple Wakefulness Test
- NADP
Nicotinamide Adenine Dinucleotide Phosphate
- NO
Nitric Oxide
- NREM
Non-Rapid Eye Movement Sleep
- P2
Type P2 Purine receptors
- PGD2
Prostaglandin D2
- PGH2
Prostaglandin H2
- PV
Parvalbumin
- PVT
Psychomotor Vigilance Test
- QNB
Quinuclidinyl Benzilate
- REM
Rapid Eye Movement Sleep
- ROS
Reactive Oxygen Species
- SLD
Sublateral Dorsal Nucleus
- SNc
Substantia Nigra pars Compacta
- SO
Sodium Oxybate
- SWA
Slow Wave Activity
- SWS
Slow Wave Sleep
- TNFα
Tumor Necrosis Factor α
- vlPAG
Ventrolateral Periaqueductal Grey
- VLPO
Ventrolateral Preoptic
- vPAG
Ventral Periaqueductal Grey
- VTA
Ventral Tegmental Area
- WT
Wild Type
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Abad V.C. An evaluation of sodium oxybate as a treatment option for narcolepsy. Expert Opin. Pharmacother. 2019;20(10):1189–1199. doi: 10.1080/14656566.2019.1617273. [DOI] [PubMed] [Google Scholar]
- 2.Paul R. Cataplexy. Pract. Neurol. 2019;19(1):21–27. doi: 10.1136/practneurol-2018-002001. [DOI] [PubMed] [Google Scholar]
- 3.Borgen L.A., Okerholm R.A., Lai A., Scharf M.B. The pharmacokinetics of sodium oxybate oral solution following acute and chronic administration to narcoleptic patients. J. Clin. Pharmacol. 2004;44(3):253–257. doi: 10.1177/0091270003262795. [DOI] [PubMed] [Google Scholar]
- 4.Scharf M.B., Lai A.A., Branigan B., Stover R., Berkowitz D.B. Pharmacokinetics of gammahydroxybutyrate (GHB) in narcoleptic patients. Sleep. 1998;21(5):507–514. doi: 10.1093/sleep/21.5.507. [DOI] [PubMed] [Google Scholar]
- 5.Mamelak M., Escriu J.M., Stokan O. Sleep-inducing effects of gammahydroxybutyrate. Lancet. 1973;2(7824):328–329. doi: 10.1016/S0140-6736(73)90839-8. [DOI] [PubMed] [Google Scholar]
- 6.Ahmed M., Bassetti C., Becker P., Michael B., Black J., Bogan R. Further evidence supporting the use of sodium oxybate for the treatment of cataplexy: a double-blind, placebo-controlled study in 228 patients. Sleep Med. 2005;6(5):415–421. doi: 10.1016/j.sleep.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 7.Broughton R., Mamelak M. Guilleminault, C.; Dement, W.C.; Passouant, P., Eds.; Narcolepsy, New York, Spectrum, Gamma hydroxy butyrate in the treatment of compound narcolepsy: a preliminary report; 1976. pp. 659–666. [Google Scholar]
- 8.Broughton R., Mamelak M. The treatment of narcolepsy-cataplexy with nocturnal gamma-hydroxybutyrate. Can. J. Neurol. Sci. 1979;6(1):1–6. doi: 10.1017/S0317167100119304. [DOI] [PubMed] [Google Scholar]
- 9.Broughton R., Mamelak M. Effects of nocturnal gamma-hydroxybutyrate on sleep/waking patterns in narcolepsy-cataplexy. Can. J. Neurol. Sci. 1980;7(1):23–31. [PubMed] [Google Scholar]
- 10.Scharf M.B., Brown D., Woods M., Brown L., Hirschowitz J. The effects and effectiveness of γ-hydroxybutyrate in patients with narcolepsy. J. Clin. Psychiatry. 1985;46(6):222–225. [PubMed] [Google Scholar]
- 11.Scrima L., Hartman P.G., Johnson F.H., Jr, Hiller F.C. Efficacy of gamma-hydroxybutyrate versus placebo in treating narcolepsy-cataplexy: double-blind subjective measures. Biol. Psychiatry. 1989;26(4):331–343. doi: 10.1016/0006-3223(89)90048-6. [DOI] [PubMed] [Google Scholar]
- 12.Scrima L., Hartman P.G., Johnson F.H., Jr, Thomas E.E., Hiller F.C. The effects of γ-hydroxybutyrate on the sleep of narcolepsy patients: a double-blind study. Sleep. 1990;13(6):479–490. doi: 10.1093/sleep/13.6.479. [DOI] [PubMed] [Google Scholar]
- 13.A randomized, double blind, placebo-controlled multicenter trial comparing the effects of three doses of orally administered sodium oxybate with placebo for the treatment of narcolepsy. Sleep. 2002;25(1):42–49. [PubMed] [Google Scholar]
- 14.Lammers G.J., Arends J., Declerck A.C., Ferrari M.D., Schouwink G., Troost J. Gammahydroxybutyrate and narcolepsy: a double-blind placebo-controlled study. Sleep. 1993;16(3):216–220. doi: 10.1093/sleep/16.3.216. [DOI] [PubMed] [Google Scholar]
- 15.Bernasconi R., Mathivet P., Otten U., Bettler B., Bischoff S., Marescaux C. Eds.; Lonon New York, Part of the pharmacological actions of gamma-hydroxybutyrate are mediated by GABAB receptors. Gamma-hydroxybutyrate Mol. Funct. Clin. Asp.; Tunnicliff, G.; Cash, C.D.; Taylor, Fr. 2002. pp. 28–63. [Google Scholar]
- 16.Burman K.J., Ige A.O., White J.H., Marshall F.H., Pangalos M.N., Emson P.C., Minson J.B., Llewellyn-Smith I.J. GABAB receptor subunits, R1 and R2, in brainstem catecholamine and serotonin neurons. Brain Res. 2003;970(1-2):35–46. doi: 10.1016/S0006-8993(02)04269-5. [DOI] [PubMed] [Google Scholar]
- 17.Madden T.E., Johnson S.W. Gamma-hydroxybutyrate is a GABAB receptor agonist that increases a potassium conductance in rat ventral tegmental dopamine neurons. J. Pharmacol. Exp. Ther. 1998;287(1):261–265. [PubMed] [Google Scholar]
- 18.Kohlmeier K.A., Vardar B., Christensen M.H. γ-Hydroxybutyric acid induces actions via the GABAB receptor in arousal and motor control-related nuclei: implications for therapeutic actions in behavioral state disorders. Neuroscience. 2013;248:261–277. doi: 10.1016/j.neuroscience.2013.06.011. [DOI] [PubMed] [Google Scholar]
- 19.Crunelli V., Leresche N. Eds.; Lonon New York, Action of gamma-hydroxybutyrate on neuronal excitability and underlying membrane conductances. Gamma-hydroxybutyrate Mol Funct Clin Asp; Tunnicliff, G.; Cash, C.D.; Taylor, Fr. 2002. pp. 75–110. [Google Scholar]
- 20.Williams S.R., Turner J.P., Crunelli V. Gamma-hydroxybutyrate promotes oscillatory activity of rat and cat thalamocortical neurons by a tonic GABAB, receptor-mediated hyperpolarization. Neuroscience. 1995;66(1):133–141. doi: 10.1016/0306-4522(94)00604-4. [DOI] [PubMed] [Google Scholar]
- 21.Steriade M., McCormick DA., Sejnowski TJ. Thalamocortical oscillations in the sleeping and aroused brain. Science. 1993;262(5134):679–685. doi: 10.1126/science.8235588. [DOI] [PubMed] [Google Scholar]
- 22.Cui S.Y., Li S.J., Cui X.Y., Zhang X.Q., Yu B., Sheng Z.F., Huang Y.L., Cao Q., Xu Y.P., Lin Z.G., Yang G., Song J.Z., Ding H., Wang Z.J., Zhang Y.H. Phosphorylation of CaMKII in the rat dorsal raphe nucleus plays an important role in sleep-wake regulation. J. Neurochem. 2016;136(3):609–619. doi: 10.1111/jnc.13431. [DOI] [PubMed] [Google Scholar]
- 23.Shi S., Ueda H.R. Ca2+ -Dependent Hyperpolarization Pathways in Sleep Homeostasis and Mental Disorders. BioEssays. 2018;40(1):1–15. doi: 10.1002/bies.201700105. [DOI] [PubMed] [Google Scholar]
- 24.Tatsuki F., Sunagawa G.A.A., Shi S., Susaki E.A.A., Yukinaga H., Perrin D., Sumiyama K., Ukai-Tadenuma M., Fujishima H., Ohno R., Tone D., Ode K.L., Matsumoto K., Ueda H.R. Involvement of Ca(2+)-dependent hyperpolarization in sleep duration in mammals. Neuron. 2016;90(1):70–85. doi: 10.1016/j.neuron.2016.02.032. [DOI] [PubMed] [Google Scholar]
- 25.Tatsuki F., Ode K.L., Ueda H.R. Ca2+-dependent hyperpolarization hypothesis for mammalian sleep. Neurosci. Res. 2017;118:48–55. doi: 10.1016/j.neures.2017.03.012. [DOI] [PubMed] [Google Scholar]
- 26.Gauthier P., Arnaud C., Gandolfo G., Gottesmann C. Influence of a GABA(B) receptor antagonist on the sleep-waking cycle in the rat. Brain Res. 1997;773(1-2):8–14. doi: 10.1016/S0006-8993(97)00643-4. [DOI] [PubMed] [Google Scholar]
- 27.Juhász G., Emri Z., Kékesi K.A., Salfay O., Crunelli V. Blockade of thalamic GABAB receptors decreases EEG synchronization. Neurosci. Lett. 1994;172(1-2):155–158. doi: 10.1016/0304-3940(94)90685-8. [DOI] [PubMed] [Google Scholar]
- 28.Borbély A.A., Daan S., Wirz-Justice A., Deboer T. The two-process model of sleep regulation: a reappraisal. J. Sleep Res. 2016;25(2):131–143. doi: 10.1111/jsr.12371. [DOI] [PubMed] [Google Scholar]
- 29.Borbély A.A. A two process model of sleep regulation. Hum. Neurobiol. 1982;1(3):195–204. [PubMed] [Google Scholar]
- 30.Walsh J.K., Hall-Porter J.M., Griffin K.S., Dodson E.R., Forst E.H., Curry D.T., Eisenstein R.D., Schweitzer P.K. Enhancing slow wave sleep with sodium oxybate reduces the behavioral and physiological impact of sleep loss. Sleep. 2010;33(9):1217–1225. doi: 10.1093/sleep/33.9.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vienne J., Bettler B., Franken P., Tafti M. Differential effects of GABAB receptor subtypes, γ-hydroxybutyric Acid, and Baclofen on EEG activity and sleep regulation. J. Neurosci. 2010;30(42):14194–14204. doi: 10.1523/JNEUROSCI.3145-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vienne J., Lecciso G., Constantinescu I., Schwartz S., Franken P., Heinzer R., Tafti M. Differential effects of sodium oxybate and baclofen on EEG, sleep, neurobehavioral performance, and memory. Sleep (Basel) 2012;35(8):1071–1083. doi: 10.5665/sleep.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Albers H.E., Walton J.C., Gamble K.L., McNeill J.K., IV, Hummer D.L. The dynamics of GABA signaling: Revelations from the circadian pacemaker in the suprachiasmatic nucleus. Front. Neuroendocrinol. 2017;44:35–82. doi: 10.1016/j.yfrne.2016.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dornbierer D.A., Baur D.M., Stucky B., Quednow B.B., Kraemer T., Seifritz E., Bosch O.G., Landolt H.P. Neurophysiological signature of gamma-hydroxybutyrate augmented sleep in male healthy volunteers may reflect biomimetic sleep enhancement: a randomized controlled trial. Neuropsychopharmacology. 2019;44(11):1985–1993. doi: 10.1038/s41386-019-0382-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Büchele F., Hackius M., Schreglmann S.R., Omlor W., Werth E., Maric A., Imbach L.L., Hägele-Link S., Waldvogel D., Baumann C.R. Sodium oxybate for excessive daytime sleepiness and sleep disturbance in Parkinson disease: A randomized clinical trial. JAMA Neurol. 2018;75(1):114–118. doi: 10.1001/jamaneurol.2017.3171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang Y.S., Guilleminault C. Narcolepsy: action of two γ-aminobutyric acid type B agonists, baclofen and sodium oxybate. Pediatr. Neurol. 2009;41(1):9–16. doi: 10.1016/j.pediatrneurol.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 37.Ondo W.G., Perkins T., Swick T., Hull K.L., Jr, Jiminez J. Ernesto MEd, G., Tippy, S., Pardi D. Sodium oxybate for excessive daytime sleepiness in Parkinson’s disease: An open label polysomnigraphic study. Arch. Neurol. 2008;65(10):1337–1340. doi: 10.1001/archneur.65.10.1337. [DOI] [PubMed] [Google Scholar]
- 38.Swick T.J. Sodium oxybate: a potential new pharmacological option for the treatment of fibromyalgia syndrome. Ther. Adv. Musculoskelet. Dis. 2011;3(4):167–178. doi: 10.1177/1759720X11411599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marzano C., Ferrara M., Curcio G., De Gennaro L. The effects of sleep deprivation in humans: topographical electroencephalogram changes in non-rapid eye movement (NREM) sleep versus REM sleep. J. Sleep Res. 2010;19(2):260–268. doi: 10.1111/j.1365-2869.2009.00776.x. [DOI] [PubMed] [Google Scholar]
- 40.Black J., Swick T., Bogan R., Lai C., Carter L.P. Impact of sodium oxybate, modafinil, and combination treatment on excessive daytime sleepiness in patients who have narcolepsy with or without cataplexy. Sleep Med. 2016;24:57–62. doi: 10.1016/j.sleep.2016.07.010. [DOI] [PubMed] [Google Scholar]
- 41.Plazzi G., Pizza F., Vandi S., Aricò D., Bruni O., Dauvilliers Y., Ferri R. Impact of acute administration of sodium oxybate on nocturnal sleep polysomnography and on multiple sleep latency test in narcolepsy with cataplexy. Sleep Med. 2014;15(9):1046–1054. doi: 10.1016/j.sleep.2014.04.020. [DOI] [PubMed] [Google Scholar]
- 42.van Schie M.K.M., Werth E., Lammers G.J., Overeem S., Baumann C.R., Fronczek R. Improved vigilance after sodium oxybate treatment in narcolepsy: a comparison between in-field and in-laboratory measurements. J. Sleep Res. 2016;25(4):486–496. doi: 10.1111/jsr.12386. [DOI] [PubMed] [Google Scholar]
- 43.Black J., Houghton W.C. Sodium oxybate improves excessive daytime sleepiness in narcolepsy. Sleep. 2006;29(7):939–946. doi: 10.1093/sleep/29.7.939. [DOI] [PubMed] [Google Scholar]
- 44.Boscolo-Berto R., Viel G., Montagnese S., Raduazzo D.I., Ferrara S.D., Dauvilliers Y. Narcolepsy and effectiveness of gamma-hydroxybutyrate (GHB): a systematic review and meta-analysis of randomized controlled trials. Sleep Med. Rev. 2012;16(5):431–443. doi: 10.1016/j.smrv.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 45.Mamelak M., Black J., Montplaisir J., Ristanovic R. A pilot study on the effects of sodium oxybate on sleep architecture and daytime alertness in narcolepsy. Sleep. 2004;27(7):1327–1334. doi: 10.1093/sleep/27.7.1327. [DOI] [PubMed] [Google Scholar]
- 46.Metcalf D.R., Emde R.N., Stripe J.T. An EEG-behavioral study of sodium hydroxybutyrate in humans. Electroencephalogr. Clin. Neurophysiol. 1966;20(5):506–512. doi: 10.1016/0013-4694(66)90107-6. [DOI] [PubMed] [Google Scholar]
- 47.Tung A., Bergmann B.M., Herrera S., Cao D., Mendelson W.B. Recovery from sleep deprivation occurs during propofol anesthesia. Anesthesiology. 2004;100(6):1419–1426. doi: 10.1097/00000542-200406000-00014. [DOI] [PubMed] [Google Scholar]
- 48.Pal D., Lipinski W.J., Walker A.J., Turner A.M., Mashour G.A. State-specific effects of sevoflurane anesthesia on sleep homeostasis: selective recovery of slow wave but not rapid eye movement sleep. Anesthesiology. 2011;114(2):302–310. doi: 10.1097/ALN.0b013e318204e064. [DOI] [PubMed] [Google Scholar]
- 49.Nelson A.B., Faraguna U., Tononi G., Cirelli C. Effects of anesthesia on the response to sleep deprivation. Sleep. 2010;33(12):1659–1667. doi: 10.1093/sleep/33.12.1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yamada Y., Yamamoto J., Fujiki A., Hishikawa Y., Kaneko Z. Effect of butyrolactone and gamma-hydroxybutyrate on the EEG and sleep cycle in man. Electroencephalogr. Clin. Neurophysiol. 1967;22(6):558–562. doi: 10.1016/0013-4694(67)90064-8. [DOI] [PubMed] [Google Scholar]
- 51.Godbout R., Montplaisir J. Eds.; Lonon New York, Effects of gamma-hydroxybutyrate on sleep. Gamma-hydroxybutyrate Mol Funct Clin Asp; Tunnicliff, G.; Cash, C.D.; Taylor, Fr. 2002. pp. 120–131. [Google Scholar]
- 52.Lapierre O., Montplaisir J., Poirier G. GHB REM-induction test: a possible biological marker for depression. Sleep Res. 1989;18:368. [Google Scholar]
- 53.Lapierre O., Montplaisir J., Lamarre M., Bedard M.A. The effect of gamma-hydroxybutyrate on nocturnal and diurnal sleep of normal subjects: further considerations on REM sleep-triggering mechanisms. Sleep. 1990;13(1):24–30. doi: 10.1093/sleep/13.1.24. [DOI] [PubMed] [Google Scholar]
- 54.Mamelak M., Escriu J.M., Stokan O. The effects of gamma-hydroxybutyrate on sleep. Biol. Psychiatry. 1977;12(2):273–288. [PubMed] [Google Scholar]
- 55.Price P.A., Schachter M., Smith S.J., Baxter R.C., Parkes J.D. Gamma-Hydroxybutyrate in narcolepsy. Ann. Neurol. 1981;9(2):198. doi: 10.1002/ana.410090217. [DOI] [PubMed] [Google Scholar]
- 56.Brown R.E., Basheer R., McKenna J.T., Strecker R.E., McCarley R.W. Control of sleep and wakefulness. Physiol. Rev. 2012;92(3):1087–1187. doi: 10.1152/physrev.00032.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Burgess C.R., Scammell T.E. Narcolepsy: neural mechanisms of sleepiness and cataplexy. J. Neurosci. 2012;32(36):12305–12311. doi: 10.1523/JNEUROSCI.2630-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Héricé C., Patel A.A., Sakata S. Circuit mechanisms and computational models of REM sleep. Neurosci. Res. 2019;140:77–92. doi: 10.1016/j.neures.2018.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jones BE. Principal cell types of sleep–wake regulatory circuits. Curr. Opin. Neurobiol. 2017;44:101–109. doi: 10.1016/j.conb.2017.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Luppi P.H., Gervasoni D., Verret L., Goutagny R., Peyron C., Salvert D., Leger L., Fort P. Paradoxical (REM) sleep genesis: the switch from an aminergic-cholinergic to a GABAergic-glutamatergic hypothesis. J. Physiol. Paris. 2006;100(5-6):271–283. doi: 10.1016/j.jphysparis.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 61.Peever J., Fuller P.M. The Biology of REM Sleep. Curr. Biol. 2017;27(22):R1237–R1248. doi: 10.1016/j.cub.2017.10.026. [DOI] [PubMed] [Google Scholar]
- 62.Scammell T.E., Arrigoni E., Lipton J.O. Neural circuitry of wakefulness and sleep. Neuron. 2017;93(4):747–765. doi: 10.1016/j.neuron.2017.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Weber F., Chung S., Beier K.T., Xu M., Luo L., Dan Y. Control of REM sleep by ventral medulla GABAergic neurons. Nature. 2015;526(7573):435–438. doi: 10.1038/nature14979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Toossi H., Del Cid-Pellitero E., Jones B.E. Homeostatic regulation through GABA and acetylcholine muscarinic receptors of motor trigeminal neurons following sleep deprivation. Brain Struct. Funct. 2017;222(7):3163–3178. doi: 10.1007/s00429-017-1392-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Torontali Z.A., Fraigne J.J., Sanghera P., Horner R., Peever J. The Sublaterodorsal Tegmental Nucleus Functions to Couple Brain State and Motor Activity during REM Sleep and Wakefulness. Curr. Biol. 2019;29(22):3803–3813.e5. doi: 10.1016/j.cub.2019.09.026. [DOI] [PubMed] [Google Scholar]
- 66.Kashiwagi M., Hayashi Y. Life without dreams: muscarinic receptors are required to regulate REM sleep in mice. Cell Rep. 2018;24(9):2211–2212. doi: 10.1016/j.celrep.2018.08.044. [DOI] [PubMed] [Google Scholar]
- 67.Jouvet M. Recherches sur les strutures nerveuse et les mecanismes responsables des differentes phases du sommeil physioligique. Arch. Ital. Biol. 1962;100:125–206. [PubMed] [Google Scholar]
- 68.Niwa Y., Kanda G.N., Yamada R.G., Shi S., Sunagawa G.A., Ukai-Tadenuma M., Fujishima H., Matsumoto N., Masumoto K.H., Nagano M., Kasukawa T., Galloway J., Perrin D., Shigeyoshi Y., Ukai H., Kiyonari H., Sumiyama K., Ueda H.R. Muscarinic Acetylcholine Receptors Chrm1 and Chrm3 Are Essential for REM Sleep. Cell Rep. 2018;24(9):2231–2247.e7. doi: 10.1016/j.celrep.2018.07.082. [DOI] [PubMed] [Google Scholar]
- 69.Kodama T., Takahashi Y., Honda Y. Enhancement of acetylcholine release during paradoxical sleep in the dorsal tegmental field of the cat brain stem. Neurosci. Lett. 1990;114(3):277–282. doi: 10.1016/0304-3940(90)90576-U. [DOI] [PubMed] [Google Scholar]
- 70.Jasper H.H., Tessier J. Acetylcholine liberation from cerebral cortex during paradoxical (REM) sleep. Science. 1971;172(3983):601–602. doi: 10.1126/science.172.3983.601. [DOI] [PubMed] [Google Scholar]
- 71.Williams J.A., Comisarow J., Day J., Fibiger H.C., Reiner P.B. State-dependent release of acetylcholine in rat thalamus measured by in vivo microdialysis. J. Neurosci. 1994;14(9):5236–5242. doi: 10.1523/JNEUROSCI.14-09-05236.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Thakkar M.M., Strecker R.E., McCarley R.W. Behavioral state control through differential serotonergic inhibition in the mesopontine cholinergic nuclei: a simultaneous unit recording and microdialysis study. J. Neurosci. 1998;18(14):5490–5497. doi: 10.1523/JNEUROSCI.18-14-05490.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Goutagny R., Luppi P.H., Salvert D., Lapray D., Gervasoni D., Fort P. Role of the dorsal paragigantocellular reticular nucleus in paradoxical (rapid eye movement) sleep generation: a combined electrophysiological and anatomical study in the rat. Neuroscience. 2008;152(3):849–857. doi: 10.1016/j.neuroscience.2007.12.014. [DOI] [PubMed] [Google Scholar]
- 74.Sapin E., Lapray D., Bérod A., Goutagny R., Léger L., Ravassard P., Clément O., Hanriot L., Fort P., Luppi P.H. Localization of the brainstem GABAergic neurons controlling paradoxical (REM) sleep. PLoS One. 2009;4(1):e4272. doi: 10.1371/journal.pone.0004272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhong P., Zhang Z., Barger Z., Ma C., Liu D., Ding X., Dan Y. Control of non-rem sleep by midbrain neurotensinergic neurons. Neuron. 2019;104(4):795–809.e6. doi: 10.1016/j.neuron.2019.08.026. [DOI] [PubMed] [Google Scholar]
- 76.Weber F., Hoang Do J.P., Chung S., Beier K.T., Bikov M., Saffari Doost M., Dan Y. Regulation of REM and Non-REM Sleep by Periaqueductal GABAergic Neurons. Nat. Commun. 2018;9(1):354. doi: 10.1038/s41467-017-02765-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Toossi H., Del Cid-Pellitero E., Jones B.E. ENEURO.0269-17.2017. Homeostatic changes in GABA and acetylcholine muscarinic receptors on GABAergic neurons in the mesencephalic reticular formation following sleep deprivation. eNeuro, 2018;4(6) doi: 10.1523/ENEURO.0269-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kaur S., Thankachan S., Begum S., Liu M., Blanco-Centurion C., Shiromani P.J. Hypocretin-2 saporin lesions of the ventrolateral periaquaductal gray (vlPAG) increase REM sleep in hypocretin knockout mice. PLoS One. 2009;4(7):e6346. doi: 10.1371/journal.pone.0006346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sakurai T. The neural circuit of orexin (hypocretin): maintaining sleep and wakefulness. Nat. Rev. Neurosci. 2007;8(3):171–181. doi: 10.1038/nrn2092. [DOI] [PubMed] [Google Scholar]
- 80.Torterolo P., Chase M.H. The hypocretins (orexins) mediate the “phasic” components of REM sleep: A new hypothesis. Sleep Sci. 2014;7(1):19–29. doi: 10.1016/j.slsci.2014.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mignot E., Lammers G.J., Ripley B., Okun M., Nevsimalova S., Overeem S., Vankova J., Black J., Harsh J., Bassetti C., Schrader H., Nishino S. The role of cerebrospinal fluid hypocretin measurement in the diagnosis of narcolepsy and other hypersomnias. Arch. Neurol. 2002;59(10):1553–1562. doi: 10.1001/archneur.59.10.1553. [DOI] [PubMed] [Google Scholar]
- 82.Taheri S., Zeitzer J.M., Mignot E. The role of hypocretins (orexins) in sleep regulation and narcolepsy. Annu. Rev. Neurosci. 2002;25:283–313. doi: 10.1146/annurev.neuro.25.112701.142826. [DOI] [PubMed] [Google Scholar]
- 83.Kluge M., Schüssler P., Dresler M., Yassouridis A., Steiger A. Sleep onset REM periods in obsessive compulsive disorder. Psychiatry Res. 2007;152(1):29–35. doi: 10.1016/j.psychres.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 84.Mamelak M. Narcolepsy and depression and the neurobiology of gammahydroxybutyrate. Prog. Neurobiol. 2009;89(2):193–219. doi: 10.1016/j.pneurobio.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 85.Steiger A., Pawlowski M. Depression and sleep. Int. J. Mol. Sci. 2019;20(3):1–14. doi: 10.3390/ijms20030607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wilson S., Argyropoulos S. Antidepressants and sleep: a qualitative review of the literature. Drugs. 2005;65(7):927–947. doi: 10.2165/00003495-200565070-00003. [DOI] [PubMed] [Google Scholar]
- 87.Denis D., French C.C., Gregory A.M. A systematic review of variables associated with sleep paralysis. Sleep Med. Rev. 2018;38:141–157. doi: 10.1016/j.smrv.2017.05.005. [DOI] [PubMed] [Google Scholar]
- 88.Takeuchi T., Miyasita A., Sasaki Y., Inugami M., Fukuda K. Isolated sleep paralysis elicited by sleep interruption. Sleep. 1992;15(3):217–225. doi: 10.1093/sleep/15.3.217. [DOI] [PubMed] [Google Scholar]
- 89.Kiyashchenko L.I., Mileykovskiy B.Y., Maidment N., Lam H.A., Wu M.F., John J., Peever J., Siegel J.M. Release of hypocretin (orexin) during waking and sleep states. J. Neurosci. 2002;22(13):5282–5286. doi: 10.1523/JNEUROSCI.22-13-05282.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Takakusaki K., Takahashi K., Saitoh K., Harada H., Okumura T., Kayama Y., Koyama Y. Orexinergic projections to the cat midbrain mediate alternation of emotional behavioural states from locomotion to cataplexy. J. Physiol. 2005;568(Pt 3):1003–1020. doi: 10.1113/jphysiol.2005.085829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Li S.B., de Lecea L. The hypocretin (orexin) system: from a neural circuitry perspective. Neuropharmacology. 2020;167(January):107993. doi: 10.1016/j.neuropharm.2020.107993. [DOI] [PubMed] [Google Scholar]
- 92.Saper C.B. The central circadian timing system. Curr. Opin. Neurobiol. 2013;23(5):747–751. doi: 10.1016/j.conb.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Saper C.B., Cano G., Scammell T.E. Homeostatic, circadian, and emotional regulation of sleep. J. Comp. Neurol. 2005;493(1):92–98. doi: 10.1002/cne.20770. [DOI] [PubMed] [Google Scholar]
- 94.Fruhstorfer B., Mignot E., Bowersox S., Nishino S., Dement W.C., Guilleminault C. Canine narcolepsy is associated with an elevated number of α 2-receptors in the locus coeruleus. Brain Res. 1989;500(1-2):209–214. doi: 10.1016/0006-8993(89)90315-6. [DOI] [PubMed] [Google Scholar]
- 95.Mignot E., Guilleminault C., Bowersox S., Rappaport A., Dement W.C. Role of central alpha-1 adrenoceptors in canine narcolepsy. J. Clin. Invest. 1988;82(3):885–894. doi: 10.1172/JCI113694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bowersox S.S., Kilduff T.S., Faull K.F., Zeller-DeAmicis L., Dement W.C., Ciaranello R.D. Brain dopamine receptor levels elevated in canine narcolepsy. Brain Res. 1987;402(1):44–48. doi: 10.1016/0006-8993(87)91045-6. [DOI] [PubMed] [Google Scholar]
- 97.Aldrich M.S., Hollingsworth Z., Penney J.B. Autoradiographic studies of post-mortem human narcoleptic brain. Neurophysiol. Clin. 1993;23(1):35–45. doi: 10.1016/S0987-7053(05)80281-3. [DOI] [PubMed] [Google Scholar]
- 98.Eisensehr I., Linke R., Tatsch K., von Lindeiner H., Kharraz B., Gildehaus F.J., Eberle R., Pollmacher T., Schuld A., Noachtar S. Alteration of the striatal dopaminergic system in human narcolepsy. Neurology. 2003;60(11):1817–1819. doi: 10.1212/01.WNL.0000069608.84542.46. [DOI] [PubMed] [Google Scholar]
- 99.Khan N., Antonini A., Parkes D., Dahlitz M.J., Meier-Ewert K., Weindl A., Leenders K.L. Striatal dopamine D2 receptors in patients with narcolepsy measured with PET and 11C-raclopride. Neurology. 1994;44(11):2102–2104. doi: 10.1212/WNL.44.11.2101. [DOI] [PubMed] [Google Scholar]
- 100.Kish S.J., Mamelak M., Slimovitch C., Dixon L.M., Lewis A., Shannak K., DiStefano L., Chang L.J., Hornykiewicz O. Brain neurotransmitter changes in human narcolepsy. Neurology. 1992;42(1):229–234. doi: 10.1212/WNL.42.1.229. [DOI] [PubMed] [Google Scholar]
- 101.MacFarlane J.G., List S.J., Moldofsky H., Firnau G., Chen J.J., Szechtman H., Garnett S., Nahmias C. Dopamine D2 receptors quantified in vivo in human narcolepsy. Biol. Psychiatry. 1997;41(3):305–310. doi: 10.1016/S0006-3223(96)00003-0. [DOI] [PubMed] [Google Scholar]
- 102.Rinne J.O., Hublin C., Partinen M., Ruottinen H., Ruotsalainen U., Någren K., Lehikoinen P., Laihinen A. Positron emission tomography study of human narcolepsy: no increase in striatal dopamine D2 receptors. Neurology. 1995;45(9):1735–1738. doi: 10.1212/WNL.45.9.1735. [DOI] [PubMed] [Google Scholar]
- 103.Heckman C.J., Mottram C., Quinlan K., Theiss R., Schuster J. Motoneuron excitability: the importance of neuromodulatory inputs. Clin. Neurophysiol. 2009;120(12):2040–2054. doi: 10.1016/j.clinph.2009.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kiyashchenko L.I., Mileykovskiy B.Y., Lai Y.Y., Siegel J.M. Increased and decreased muscle tone with orexin (hypocretin) microinjections in the locus coeruleus and pontine inhibitory area. J. Neurophysiol. 2001;85(5):2008–2016. doi: 10.1152/jn.2001.85.5.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Yamuy J., Fung S.J., Xi M., Chase M.H. Hypocretinergic control of spinal cord motoneurons. J. Neurosci. 2004;24(23):5336–5345. doi: 10.1523/JNEUROSCI.4812-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Aldrich M.S., Rogers A.E. Exacerbation of human cataplexy by prazosin. Sleep. 1989;12(3):254–256. doi: 10.1093/sleep/12.3.254. [DOI] [PubMed] [Google Scholar]
- 107.Mignot E., Guilleminault C., Bowersox S., Rappaport A., Dement W.C. Effect of α 1-adrenoceptors blockade with prazosin in canine narcolepsy. Brain Res. 1988;444(1):184–188. doi: 10.1016/0006-8993(88)90927-4. [DOI] [PubMed] [Google Scholar]
- 108.Nishino S., Fruhstorfer B., Arrigoni J., Guilleminault C., Dement W.C., Mignot E. Further characterization of the alpha-1 receptor subtype involved in the control of cataplexy in canine narcolepsy. J. Pharmacol. Exp. Ther. 1993;264(3):1079–1084. [PubMed] [Google Scholar]
- 109.Lopez R., Dauvilliers Y. Pharmacotherapy options for cataplexy. Expert Opin. Pharmacother. 2013;14(7):895–903. doi: 10.1517/14656566.2013.783021. [DOI] [PubMed] [Google Scholar]
- 110.Nishino S., Mignot E. Pharmacological aspects of human and canine narcolepsy. Prog. Neurobiol. 1997;52(1):27–78. doi: 10.1016/S0301-0082(96)00070-6. [DOI] [PubMed] [Google Scholar]
- 111.Ristanovic R.K., Liang H., Hornfeldt C.S., Lai C. Exacerbation of cataplexy following gradual withdrawal of antidepressants: manifestation of probable protracted rebound cataplexy. Sleep Med. 2009;10(4):416–421. doi: 10.1016/j.sleep.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 112.Chaumette T., Chapuy E., Berrocoso E., Llorca-Torralba M., Bravo L., Mico J.A., Chalus M., Eschalier A., Ardid D., Marchand F., Sors A. Effects of S 38093, an antagonist/inverse agonist of histamine H3 receptors, in models of neuropathic pain in rats. Eur. J. Pain. 2018;22(1):127–141. doi: 10.1002/ejp.1097. [DOI] [PubMed] [Google Scholar]
- 113.Lin J.S., Dauvilliers Y., Arnulf I., Bastuji H., Anaclet C., Parmentier R., Kocher L., Yanagisawa M., Lehert P., Ligneau X., Perrin D., Robert P., Roux M., Lecomte J.M., Schwartz J.C. An inverse agonist of the histamine H(3) receptor improves wakefulness in narcolepsy: studies in orexin-/- mice and patients. Neurobiol. Dis. 2008;30(1):74–83. doi: 10.1016/j.nbd.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 114.Eggermann E., Serafin M., Bayer L., Machard D., Saint-Mleux B., Jones B.E., Mühlethaler M. Orexins/hypocretins excite basal forebrain cholinergic neurones. Neuroscience. 2001;108(2):177–181. doi: 10.1016/S0306-4522(01)00512-7. [DOI] [PubMed] [Google Scholar]
- 115.Fadel J., Burk J.A. Orexin/hypocretin modulation of the basal forebrain cholinergic system: Role in attention. Brain Res. 2010;1314:112–123. doi: 10.1016/j.brainres.2009.08.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lee M.G., Hassani O.K., Alonso A., Jones B.E. Cholinergic basal forebrain neurons burst with theta during waking and paradoxical sleep. J. Neurosci. 2005;25(17):4365–4369. doi: 10.1523/JNEUROSCI.0178-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Inutsuka A. Yamanaka, A The physiological role of orexin / hypocretin neurons in the regulation of sleep / wakefulness and neuroendocrine functions. 2013;4:1–10. doi: 10.3389/fendo.2013.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Burlet S., Tyler C.J., Leonard C.S. Direct and indirect excitation of laterodorsal tegmental neurons by Hypocretin/Orexin peptides: implications for wakefulness and narcolepsy. J. Neurosci. 2002;22(7):2862–2872. doi: 10.1523/JNEUROSCI.22-07-02862.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kim J., Nakajima K., Oomura Y., Wayner M.J., Sasaki K. Electrophysiological effects of orexins/hypocretins on pedunculopontine tegmental neurons in rats: an in vitro study. Peptides. 2009;30(2):191–209. doi: 10.1016/j.peptides.2008.09.023. [DOI] [PubMed] [Google Scholar]
- 120.Nishino S, Tafti M, Reid MS. Muscle atonia is triggered by cholinergic stimulation of the basal forebrain: Implication for the pathophysiology of canine narcolepsy. J Neurosci. 1995;15(7 I):4806–4814. doi: 10.1523/JNEUROSCI.15-07-04806.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Boehme R.E., Baker T.L., Mefford I.N., Barchas J.D., Dement W.C., Ciaranello R.D. Narcolepsy: cholinergic receptor changes in an animal model. Life Sci. 1984;34(19):1825–1828. doi: 10.1016/0024-3205(84)90675-1. [DOI] [PubMed] [Google Scholar]
- 122.Sharples S.A., Koblinger K., Humphreys J.M., Whelan P.J. Dopamine: a parallel pathway for the modulation of spinal locomotor networks. Front. Neural Circuits. 2014;8(JUNE):55. doi: 10.3389/fncir.2014.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Han P., Nakanishi S.T., Tran M.A., Whelan P.J. Dopaminergic modulation of spinal neuronal excitability. J. Neurosci. 2007;27(48):13192–13204. doi: 10.1523/JNEUROSCI.1279-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Rivera-Oliver M., Moreno E., Álvarez-Bagnarol Y., Ayala-Santiago C., Cruz-Reyes N., Molina-Castro G.C., Clemens S., Canela E.I., Ferré S., Casadó V., Díaz-Ríos M. Adenosine A1-Dopamine D1 receptor heteromers control the excitability of the spinal motoneuron. Mol. Neurobiol. 2019;56(2):797–811. doi: 10.1007/s12035-018-1120-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Schwarz P.B., Peever J.H. Dopamine triggers skeletal muscle tone by activating D1-like receptors on somatic motoneurons. J. Neurophysiol. 2011;106(3):1299–1309. doi: 10.1152/jn.00230.2011. [DOI] [PubMed] [Google Scholar]
- 126.Wisor J.P., Nishino S., Sora I., Uhl G.H., Mignot E., Edgar D.M. Dopaminergic role in stimulant-induced wakefulness. J. Neurosci. 2001;21(5):1787–1794. doi: 10.1523/JNEUROSCI.21-05-01787.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Trulson M.E., Preussler D.W., Howell G.A. Activity of substantia nigra units across the sleep-waking cycle in freely moving cats. Neurosci. Lett. 1981;26(2):183–188. doi: 10.1016/0304-3940(81)90346-3. [DOI] [PubMed] [Google Scholar]
- 128.Dahan L., Astier B., Vautrelle N., Urbain N., Kocsis B., Chouvet G. Prominent burst firing of dopaminergic neurons in the ventral tegmental area during paradoxical sleep. Neuropsychopharmacology. 2007;32(6):1232–1241. doi: 10.1038/sj.npp.1301251. [DOI] [PubMed] [Google Scholar]
- 129.Eban-Rothschild A., Rothschild G., Giardino W.J., Jones J.R., de Lecea L. VTA dopaminergic neurons regulate ethologically relevant sleep-wake behaviors. Nat. Neurosci. 2016;19(10):1356–1366. doi: 10.1038/nn.4377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Lu J., Jhou T.C., Saper C.B. Identification of wake-active dopaminergic neurons in the ventral periaqueductal gray matter. J. Neurosci. 2006;26(1):193–202. doi: 10.1523/JNEUROSCI.2244-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Chuhma N., Mingote S., Yetnikoff L., Kalmbach A., Ma T., Ztaou S., Sienna A.C., Tepler S., Poulin J.F., Ansorge M., Awatramani R., Kang U.J., Rayport S. Dopamine neuron glutamate cotransmission evokes a delayed excitation in lateral dorsal striatal cholinergic interneurons. eLife. 2018;7:1–29. doi: 10.7554/eLife.39786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Chuhma N., Mingote S., Moore H., Rayport S. Dopamine neurons control striatal cholinergic neurons via regionally heterogeneous dopamine and glutamate signaling. Neuron. 2014;81(4):901–912. doi: 10.1016/j.neuron.2013.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Giorgi O., Rubio M.C. Decreased 3H-L-quinuclidinyl benzilate binding and muscarine receptor subsensitivity after chronic gamma-butyrolactone treatment. Naunyn Schmiedebergs Arch. Pharmacol. 1981;318(1):14–18. doi: 10.1007/BF00503306. [DOI] [PubMed] [Google Scholar]
- 134.Nomura Y., Kajiyama H., Nakata Y., Segawa T. Muscarinic cholinergic binding in striatal and mesolimbic areas of the rat: reduction by 6-hydroxydopa. Eur. J. Pharmacol. 1979;58(2):125–131. doi: 10.1016/0014-2999(79)90003-7. [DOI] [PubMed] [Google Scholar]
- 135.Honda K., Riehl J., Mignot E., Nishino S. Dopamine D3 agonists into the substratia nigra aggravates cataplexy but does not modify sleep. Neuroreport. 1999;10(14):3111–3118. doi: 10.1097/00001756-199909290-00043. [DOI] [PubMed] [Google Scholar]
- 136.Reid M.S., Tafti M., Nishino S., Sampathkumaran R., Siegel J.M., Mignot E. Local administration of dopaminergic drugs into the ventral tegmental area modulates cataplexy in the narcoleptic canine. Brain Res. 1996;733(1):83–100. doi: 10.1016/0006-8993(96)00541-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Burgess C.R., Tse G., Gillis L., Peever J.H. Dopaminergic regulation of sleep and cataplexy in a murine model of narcolepsy. Sleep. 2010;33(10):1295–1304. doi: 10.1093/sleep/33.10.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Hut R.A., Van der Zee E.A. The cholinergic system, circadian rhythmicity, and time memory. Behav. Brain Res. 2011;221(2):466–480. doi: 10.1016/j.bbr.2010.11.039. [DOI] [PubMed] [Google Scholar]
- 139.Mash D.C., Flynn D.D., Kalinoski L., Potter L.T. Circadian variations in radioligand binding to muscarine receptors in rat brain dependent upon endogenous agonist occupation. Brain Res. 1985;331(1):35–38. doi: 10.1016/0006-8993(85)90712-7. [DOI] [PubMed] [Google Scholar]
- 140.Perry E.K., Perry R.H., Tomlinson B.E. Circadian variations in cholinergic enzymes and muscarinic receptor binding in human cerebral cortex. Neurosci. Lett. 1977;4(3-4):185–189. doi: 10.1016/0304-3940(77)90136-7. [DOI] [PubMed] [Google Scholar]
- 141.Carskadon M.A., Dement W.C. Sleep studies on a 90-minute day. Electroencephalogr. Clin. Neurophysiol. 1975;39(2):145–155. doi: 10.1016/0013-4694(75)90004-8. [DOI] [PubMed] [Google Scholar]
- 142.Kimura M., Curzi M.L., Romanowsi C.P. REM sleep alteration and depression. Arch. Ital. Biol. 2014;152(2-3):111–117. doi: 10.12871/000298292014236. [DOI] [PubMed] [Google Scholar]
- 143.Nollet M., Hicks H., McCarthy A.P., Wu H., Möller-Levet C.S., Laing E.E., Malki K., Lawless N., Wafford K.A., Dijk D.J., Winsky-Sommerer R. REM sleep’s unique associations with corticosterone regulation, apoptotic pathways, and behavior in chronic stress in mice. Proc. Natl. Acad. Sci. USA. 2019;116(7):2733–2742. doi: 10.1073/pnas.1816456116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Fernandez S.P., Broussot L., Marti F., Contesse T., Mouska X., Soiza-Reilly M., Marie H., Faure P., Barik J. Mesopontine cholinergic inputs to midbrain dopamine neurons drive stress-induced depressive-like behaviors. Nat. Commun. 2018;9(1):4449. doi: 10.1038/s41467-018-06809-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Calvo J.M., Simón-Arceo K., Fernández-Mas R. Prolonged enhancement of REM sleep produced by carbachol microinjection into the amygdala. Neuroreport. 1996;7(2):577–580. doi: 10.1097/00001756-199601310-00048. [DOI] [PubMed] [Google Scholar]
- 146.Dulawa S.C., Janowsky D.S. Cholinergic regulation of mood: from basic and clinical studies to emerging therapeutics. Mol. Psychiatry. 2019;24(5):694–709. doi: 10.1038/s41380-018-0219-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Palagini L., Baglioni C., Ciapparelli A., Gemignani A., Riemann D. REM sleep dysregulation in depression: state of the art. Sleep Med. Rev. 2013;17(5):377–390. doi: 10.1016/j.smrv.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 148.Giles D.E., Kupfer D.J., Roffwarg H.P., Rush A.J., Biggs M.M., Etzel B.A. Polysomnographic parameters in first-degree relatives of unipolar probands. Psychiatry Res. 1989;27(2):127–136. doi: 10.1016/0165-1781(89)90128-5. [DOI] [PubMed] [Google Scholar]
- 149.Comings DE., Wu S., Rostamkhani M., McGue M., Iacono WG., MacMurray JP. Association of the muscarinic cholinergic 2 receptor (CHRM2) gene with major depression in women. Am. J. Med. Genet. 2002;114(5):527–529. doi: 10.1002/ajmg.10406. [DOI] [PubMed] [Google Scholar]
- 150.Lauer C.J., Modell S. Schreiber,w., Krieg, J-C., Holsbauer F. Prediction of the 0development of a first major depressive episode with a rapid eye movement induction test 0using the cholinergic agonist RS-86. J. Clin. Psychopharmacol. 2004;24(3):356–357. doi: 10.1097/01.jcp.0000125744.22031.3a. [DOI] [PubMed] [Google Scholar]
- 151.Schreiber W., Lauer C.J., Krumrey K., Holsboer F., Krieg J.C. Cholinergic REM sleep induction test in subjects at high risk for psychiatric disorders. Biol. Psychiatry. 1992;32(1):79–90. doi: 10.1016/0006-3223(92)90144-O. [DOI] [PubMed] [Google Scholar]
- 152.Furey M.L., Drevets W.C. Antidepressant efficacy of the antimuscarinic drug scopolamine: a randomized, placebo-controlled clinical trial. Arch. Gen. Psychiatry. 2006;63(10):1121–1129. doi: 10.1001/archpsyc.63.10.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Poland R.E., McCracken J.T., Lutchmansingh P., Lesser I.M., Tondo L., Edwards C., Boone K.B., Lin K.M. Differential response of rapid eye movement sleep to cholinergic blockade by scopolamine in currently depressed, remitted, and normal control subjects. Biol. Psychiatry. 1997;41(9):929–938. doi: 10.1016/S0006-3223(96)00183-7. [DOI] [PubMed] [Google Scholar]
- 154.Witkin J.M., Overshiner C., Li X., Catlow J.T., Wishart G.N., Schober D.A., Heinz B.A., Nikolayev A., Tolstikov V.V., Anderson W.H., Higgs R.E., Kuo M.S., Felder C.C. M1 and m2 muscarinic receptor subtypes regulate antidepressant-like effects of the rapidly acting antidepressant scopolamine. J. Pharmacol. Exp. Ther. 2014;351(2):448–456. doi: 10.1124/jpet.114.216804. [DOI] [PubMed] [Google Scholar]
- 155.Sudo Y., Suhara T., Honda Y., Nakajima T., Okubo Y., Suzuki K., Nakashima Y., Yoshikawa K., Okauchi T., Sasaki Y., Matsushita M. Muscarinic cholinergic receptors in human narcolepsy: a PET study. Neurology. 1998;51(5):1297–1302. doi: 10.1212/WNL.51.5.1297. [DOI] [PubMed] [Google Scholar]
- 156.Kilduff T.S., Bowersox S.S., Kaitin K.I., Baker T.L., Ciaranello R.D., Dement W.C. Muscarinic cholinergic receptors and the canine model of narcolepsy. Sleep. 1986;9(1 Pt 2):102–106. doi: 10.1093/sleep/9.1.102. [DOI] [PubMed] [Google Scholar]
- 157.Reid M.S., Nishino S., Tafti M., Siegel J.M., Dement W.C., Mignot E. Neuropharmacological characterization of basal forebrain cholinergic stimulated cataplexy in narcoleptic canines. Exp. Neurol. 1998;151(1):89–104. doi: 10.1006/exnr.1998.6787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Wang H.Y., Kuo Z.C., Fu Y.S., Chen R.F., Min M.Y., Yang H.W. GABAB receptor-mediated tonic inhibition regulates the spontaneous firing of locus coeruleus neurons in developing rats and in citalopram-treated rats. J. Physiol. 2015;593(1):161–180. doi: 10.1113/jphysiol.2014.281378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Szabo S.T., Gold M.S., Goldberger B.A., Blier P. Effects of sustained gamma-hydroxybutyrate treatments on spontaneous and evoked firing activity of locus coeruleus norepinephrine neurons. Biol. Psychiatry. 2004;55(9):934–939. doi: 10.1016/j.biopsych.2003.12.013. [DOI] [PubMed] [Google Scholar]
- 160.Aston-Jones G., Cohen J.D. An integrative theory of locus coeruleus-norepinephrine function: adaptive gain and optimal performance. Annu. Rev. Neurosci. 2005;28:403–450. doi: 10.1146/annurev.neuro.28.061604.135709. [DOI] [PubMed] [Google Scholar]
- 161.Erhardt S., Andersson B., Nissbrandt H., Engberg G. Inhibition of firing rate and changes in the firing pattern of nigral dopamine neurons by γ-hydroxybutyric acid (GHBA) are specifically induced by activation of GABA(B) receptors. Naunyn Schmiedebergs Arch. Pharmacol. 1998;357(6):611–619. doi: 10.1007/PL00005215. [DOI] [PubMed] [Google Scholar]
- 162.Hishikawa Y., Sumitsuji N., Matsumoto K., Kaneko Z. H-reflex and EMG of the mental and hyoid muscles during sleep, with special reference to narcolepsy. Electroencephalogr. Clin. Neurophysiol. 1965;18(5):487–492. doi: 10.1016/0013-4694(65)90129-X. [DOI] [PubMed] [Google Scholar]
- 163.Hodes R., Dement W.C. Depression of electrically induced (‘H-reflexes”) in man during low voltage EEG ’sleep. Electroencephalogr. Clin. Neurophysiol. 1964;17(6):617–629. doi: 10.1016/0013-4694(64)90229-9. [DOI] [PubMed] [Google Scholar]
- 164.Mamelak M., Sowden K. The effect of gammahydroxybutyrate on the H-reflex: pilot study. Neurology. 1983;33(11):1497–1500. doi: 10.1212/WNL.33.11.1497. [DOI] [PubMed] [Google Scholar]
- 165.Kaufman E.E. In: Metabolism and distribution of gammahydroxybutyrate in the brain. Gammahydroxybutyrate: Molecular, functional and clinival aspects; Tunnicliff, G. Cash C.D., editor. London, and New York: Taylor and Francis; 2002. pp. 1–16. [Google Scholar]
- 166.Bukato G., Kochan Z., Swierczyński J. Different regulatory properties of the cytosolic and mitochondrial forms of malic enzyme isolated from human brain. Int. J. Biochem. Cell Biol. 1995;27(10):1003–1008. doi: 10.1016/1357-2725(95)00080-9. [DOI] [PubMed] [Google Scholar]
- 167.Vogel R., Wiesinger H., Hamprecht B., Dringen R. The regeneration of reduced glutathione in rat forebrain mitochondria identifies metabolic pathways providing the NADPH required. Neurosci. Lett. 1999;275(2):97–100. doi: 10.1016/S0304-3940(99)00748-X. [DOI] [PubMed] [Google Scholar]
- 168.Laborit H. Sodium-4-hydroxybutyrate. Int. J. Neuropharmacol. 1964;3:433–451. doi: 10.1016/0028-3908(64)90074-7. [DOI] [PubMed] [Google Scholar]
- 169.Taberner P.V., Rick J.T., Kerkut G.A. The action of gamma-hydroxybutyric acid on cerebral glucose metabolism. J. Neurochem. 1972;19(2):245–254. doi: 10.1111/j.1471-4159.1972.tb01334.x. [DOI] [PubMed] [Google Scholar]
- 170.Andrabi M., Andrabi M.M., Kunjunni R., Sriwastva M.K., Bose S., Sagar R., Srivastava A.K., Mathur R., Jain S., Subbiah V. Lithium acts to modulate abnormalities at behavioral, cellular, and molecular levels in sleep deprivation-induced mania-like behavior. Bipolar Disord. 2020;22(3):266–280. doi: 10.1111/bdi.12838. [DOI] [PubMed] [Google Scholar]
- 171.Alzoubi K.H., Al Mosabih H.S., Mahasneh A.F. The protective effect of edaravone on memory impairment induced by chronic sleep deprivation. Psychiatry Res. 2019;281(June):112577. doi: 10.1016/j.psychres.2019.112577. [DOI] [PubMed] [Google Scholar]
- 172.Ramanathan L., Hu S., Frautschy S.A., Siegel J.M. Short-term total sleep deprivation in the rat increases antioxidant responses in multiple brain regions without impairing spontaneous alternation behavior. Behav. Brain Res. 2010;207(2):305–309. doi: 10.1016/j.bbr.2009.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Silva R.H., Abílio V.C., Takatsu A.L., Kameda S.R., Grassl C., Chehin A.B., Medrano W.A., Calzavara M.B., Registro S., Andersen M.L., Machado R.B., Carvalho R.C. Ribeiro, Rde.A.; Tufik, S.; Frussa-Filho, R. Role of hippocampal oxidative stress in memory deficits induced by sleep deprivation in mice. Neuropharmacology. 2004;46(6):895–903. doi: 10.1016/j.neuropharm.2003.11.032. [DOI] [PubMed] [Google Scholar]
- 174.Villafuerte G, Miguel-Puga A, Murillo Rodríguez E, Machado S, Manjarrez E, Arias-Carrión O. Oxid Med Cell Longev, 2015, Sleep deprivation and oxidative stress in animal models: A systematic review. 2015. [DOI] [PMC free article] [PubMed]
- 175.Harkness J.H., Bushana P.N., Todd R.P., Clegern W.C., Sorg B.A., Wisor J.P. Sleep disruption elevates oxidative stress in parvalbumin-positive cells of the rat cerebral cortex. Sleep (Basel) 2019;42(1):1–15. doi: 10.1093/sleep/zsy201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Pimentel D., Donlea J.M., Talbot C.B., Song S.M., Thurston A.J.F., Miesenböck G. Operation of a homeostatic sleep switch. Nature. 2016;536(7616):333–337. doi: 10.1038/nature19055. http://www.nature.com/authors/editorial_policies/license.html#terms [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Kempf A., Song S.M., Talbot C.B., Miesenböck G. A potassium channel β-subunit couples mitochondrial electron transport to sleep. Nature. 2019;568(7751):230–234. doi: 10.1038/s41586-019-1034-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Hill V.M., O’Connor R.M., Sissoko G.B., Irobunda I.S., Leong S., Canman J.C., Stavropoulos N., Shirasu-Hiza M. A bidirectional relationship between sleep and oxidative stress in Drosophila. PLoS Biol. 2018;16(7):e2005206. doi: 10.1371/journal.pbio.2005206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Ikeda M., Ikeda-Sagara M., Okada T., Clement P., Urade Y., Nagai T., Sugiyama T., Yoshioka T., Honda K., Inoué S. Brain oxidation is an initial process in sleep induction. Neuroscience. 2005;130(4):1029–1040. doi: 10.1016/j.neuroscience.2004.09.057. [DOI] [PubMed] [Google Scholar]
- 180.Sakamoto H., Kitahara J., Nakagawa Y. Effect of intracellular glutathione on the production of prostaglandin D2 in RBL-2H3 cells oxidized by tert-butyl hydroperoxide. J. Biochem. 1999;125(1):90–95. doi: 10.1093/oxfordjournals.jbchem.a022274. [DOI] [PubMed] [Google Scholar]
- 181.Sun G.Y., Xu J., Jensen M.D., Yu S., Wood W.G., González F.A., Simonyi A., Sun A.Y., Weisman G.A. Phospholipase A2 in astrocytes: responses to oxidative stress, inflammation, and G protein-coupled receptor agonists. Mol. Neurobiol. 2005;31(1-3):27–41. doi: 10.1385/MN:31:1-3:027. [DOI] [PubMed] [Google Scholar]
- 182.Bredow S., Guha-Thakurta N., Taishi P., Obál F., Jr, Krueger J.M. Diurnal variations of tumor necrosis factor alpha mRNA and alpha-tubulin mRNA in rat brain. Neuroimmunomodulation. 1997;4(2):84–90. doi: 10.1159/000097325. [DOI] [PubMed] [Google Scholar]
- 183.Kalinchuk A.V., Porkka-Heiskanen T., McCarley R.W., Basheer R. Cholinergic neurons of the basal forebrain mediate biochemical and electrophysiological mechanisms underlying sleep homeostasis. Eur. J. Neurosci. 2015;41(2):182–195. doi: 10.1111/ejn.12766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Kalinchuk A.V., McCarley R.W., Porkka-Heiskanen T., Basheer R. The time course of adenosine, nitric oxide (NO) and inducible NO synthase changes in the brain with sleep loss and their role in the non-rapid eye movement sleep homeostatic cascade. J. Neurochem. 2011;116(2):260–272. doi: 10.1111/j.1471-4159.2010.07100.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Kalinchuk A.V., McCarley R.W., Porkka-Heiskanen T., Basheer R. Sleep deprivation triggers inducible nitric oxide-dependent nitric oxide production in wake-active basal forebrain neurons. J. Neurosci. 2010;30(40):13254–13264. doi: 10.1523/JNEUROSCI.0014-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Kalinchuk A.V., Lu Y., Stenberg D., Rosenberg P.A., Porkka-Heiskanen T. Nitric oxide production in the basal forebrain is required for recovery sleep. J. Neurochem. 2006;99(2):483–498. doi: 10.1111/j.1471-4159.2006.04077.x. [DOI] [PubMed] [Google Scholar]
- 187.Kalinchuk A.V., Stenberg D., Rosenberg P.A., Porkka-Heiskanen T. Inducible and neuronal nitric oxide synthases (NOS) have complementary roles in recovery sleep induction. Eur. J. Neurosci. 2006;24(5):1443–1456. doi: 10.1111/j.1460-9568.2006.05019.x. [DOI] [PubMed] [Google Scholar]
- 188.Mackiewicz M., Sollars P.J., Ogilvie M.D., Pack A.I. Modulation of IL-1 β gene expression in the rat CNS during sleep deprivation. Neuroreport. 1996;7(2):529–533. doi: 10.1097/00001756-199601310-00037. [DOI] [PubMed] [Google Scholar]
- 189.Manchanda S., Singh H., Kaur T., Kaur G. Low-grade neuroinflammation due to chronic sleep deprivation results in anxiety and learning and memory impairments. Mol. Cell. Biochem. 2018;449(1-2):63–72. doi: 10.1007/s11010-018-3343-7. [DOI] [PubMed] [Google Scholar]
- 190.Valvassori S.S., Resende W.R., Dal-Pont G., Sangaletti-Pereira H., Gava F.F., Peterle B.R., Carvalho A.F., Varela R.B., Dal-Pizzol F., Quevedo J. Lithium ameliorates sleep deprivation-induced mania-like behavior, hypothalamic-pituitary-adrenal (HPA) axis alterations, oxidative stress and elevations of cytokine concentrations in the brain and serum of mice. Bipolar Disord. 2017;19(4):246–258. doi: 10.1111/bdi.12503. [DOI] [PubMed] [Google Scholar]
- 191.Stanton R.C. Glucose-6-phosphate dehydrogenase, NADPH, and cell survival. IUBMB Life. 2012;64(5):362–369. doi: 10.1002/iub.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Brorson J.R., Schumacker P.T., Zhang H. Nitric oxide acutely inhibits neuronal energy production. J. Neurosci. 1999;19(1):147–158. doi: 10.1523/JNEUROSCI.19-01-00147.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Rosenberg P.A., Li Y., Le M., Zhang Y. Nitric oxide-stimulated increase in extracellular adenosine accumulation in rat forebrain neurons in culture is associated with ATP hydrolysis and inhibition of adenosine kinase activity. J. Neurosci. 2000;20(16):6294–6301. doi: 10.1523/JNEUROSCI.20-16-06294.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Lazarus M., Oishi Y., Bjorness T.E., Greene R.W. Gating and the need for sleep: Dissociable effects of adenosine a1and a2a receptors. Front. Neurosci. 2019;13:740. doi: 10.3389/fnins.2019.00740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Kalinchuk A.V., McCarley R.W., Stenberg D., Porkka-Heiskanen T., Basheer R. The role of cholinergic basal forebrain neurons in adenosine-mediated homeostatic control of sleep: lessons from 192 IgG-saporin lesions. Neuroscience. 2008;157(1):238–253. doi: 10.1016/j.neuroscience.2008.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Bjorness T.E., Dale N., Mettlach G., Sonneborn A., Sahin B., Fienberg A.A., Yanagisawa M., Bibb J.A., Greene R.W. An adenosine-mediated glial-neuronal circuit for homeostatic sleep. J. Neurosci. 2016;36(13):3709–3721. doi: 10.1523/JNEUROSCI.3906-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Fujita T., Chen M.J., Li B., Smith N.A., Peng W., Sun W., Toner M.J., Kress B.T., Wang L., Benraiss A., Takano T., Wang S., Nedergaard M. Neuronal transgene expression in dominant-negative SNARE mice. J. Neurosci. 2014;34(50):16594–16604. doi: 10.1523/JNEUROSCI.2585-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Halassa M.M., Florian C., Fellin T., Munoz J.R., Lee S.Y., Abel T., Haydon P.G., Frank M.G. Astrocytic modulation of sleep homeostasis and cognitive consequences of sleep loss. Neuron. 2009;61(2):213–219. doi: 10.1016/j.neuron.2008.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Ahmad A.S., Ottallah H., MacIel C.B., Strickland M., Doré S. Role of the L-PGDS-PGD-DP1 receptor axis in sleep regulation and neurologic outcomes. Sleep (Basel) 2019;42(6):1–16. doi: 10.1093/sleep/zsz073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Urade Y., Hayaishi O. Prostaglandin D2 and sleep/wake regulation. Sleep Med. Rev. 2011;15(6):411–418. doi: 10.1016/j.smrv.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 201.Yui D., Nishida Y., Nishina T., Mogushi K., Tajiri M., Ishibashi S., Ajioka I., Ishikawa K., Mizusawa H., Murayama S., Yokota T. Enhanced phospholipase A2 group 3 expression by oxidative stress decreases the insulin-degrading enzyme. PLoS One. 2015;10(12):e0143518. doi: 10.1371/journal.pone.0143518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Terao A., Matsumura H., Saito M. Interleukin-1 induces slow-wave sleep at the prostaglandin D2-sensitive sleep-promoting zone in the rat brain. J. Neurosci. 1998;18(16):6599–6607. doi: 10.1523/JNEUROSCI.18-16-06599.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Zhang B.J., Shao S.R., Aritake K., Takeuchi A., Urade Y., Huang Z.L., Lazarus M., Qu W.M. Interleukin-1β induces sleep independent of prostaglandin D2 in rats and mice. Neuroscience. 2017;340:258–267. doi: 10.1016/j.neuroscience.2016.09.053. [DOI] [PubMed] [Google Scholar]
- 204.Krueger J.M., Taishi P., De A., Davis C.J., Winters B.D., Clinton J., Szentirmai E., Zielinski M.R. ATP and the purine type 2 X7 receptor affect sleep. J. Appl. Physiol. 2010;109(5):1318–1327. doi: 10.1152/japplphysiol.00586.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Porkka-Heiskanen T, Strecker RE, Thakkar M, Bjørkum AA, Greene RW, McCarley RW. Adenosine: A mediator of the sleep-inducing effects of prolonged wakefulness. Science (80-) 1997;276(5316):1265–1267. doi: 10.1126/science.276.5316.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Porkka-Heiskanen T., Strecker R.E., McCarley R.W. Brain site-specificity of extracellular adenosine concentration changes during sleep deprivation and spontaneous sleep: an in vivo microdialysis study. Neuroscience. 2000;99(3):507–517. doi: 10.1016/S0306-4522(00)00220-7. [DOI] [PubMed] [Google Scholar]
- 207.Urade Y., Eguchi N., Qu W.M., Sakata M., Huang Z.L., Chen J.F., Schwarzschild M.A., Fink J.S., Hayaishi O. Sleep regulation in adenosine A2A receptor-deficient mice. Neurology. 2003;61(11) Suppl. 6:S94–S96. doi: 10.1212/01.WNL.0000095222.41066.5E. [DOI] [PubMed] [Google Scholar]
- 208.Rainnie D.G., Grunze H.C.R., Mccarley R.W., Robert W., Rainnie D.G., Grunze H.C.R. Adenosine Inhibition of Mesopontine Cholinergic Neurons: Implications for EEG Arousal Published by: American Association for the Advancement of Science Stable URL. American Associationfor the Advancement of Science. 1994;263(5147):689–692. doi: 10.1126/science.8303279. http://www.jstor.com/stable/2883094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Fukumitsu N., Ishii K., Kimura Y., Oda K., Sasaki T., Mori Y., Ishiwata K. Adenosine A1 receptor mapping of the human brain by PET with 8-dicyclopropylmethyl-1-11C-methyl-3-propylxanthine. J. Nucl. Med. 2005;46(1):32–37. [PubMed] [Google Scholar]
- 210.Pape H-C. Adenosine promotes burst activity in guinea-pig geniculocortical neurones through two different ionic mechanisms. J. Physiol. 1992;447:729–753. doi: 10.1113/jphysiol.1992.sp019026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Bjorness T.E., Kelly C.L., Gao T., Poffenberger V., Greene R.W. Control and function of the homeostatic sleep response by adenosine A1 receptors. J. Neurosci. 2009;29(5):1267–1276. doi: 10.1523/JNEUROSCI.2942-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Zielinski M.R., Taishi P., Clinton J.M., Krueger J.M. 5′-Ectonucleotidase-knockout mice lack non-REM sleep responses to sleep deprivation. Eur. J. Neurosci. 2012;35(11):1789–1798. doi: 10.1111/j.1460-9568.2012.08112.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Steriade M., Hobson J.A. Neuronal activity during the sleep-waking cycle. Prog. Neurobiol. 1976;157:157–376. doi: 10.1016/0301-0082(76)90013-7. [DOI] [PubMed] [Google Scholar]
- 214.Descarries L., Gisiger V., Steriade M. Diffuse transmission by acetylcholine in the CNS. Prog. Neurobiol. 1997;53(5):603–625. doi: 10.1016/S0301-0082(97)00050-6. [DOI] [PubMed] [Google Scholar]
- 215.Harris J.J., Jolivet R., Attwell D. Synaptic energy use and supply. Neuron. 2012;75(5):762–777. doi: 10.1016/j.neuron.2012.08.019. [DOI] [PubMed] [Google Scholar]
- 216.Madsen P.L., Schmidt J.F., Wildschiødtz G., Friberg L., Holm S., Vorstrup S., Lassen N.A. Cerebral O2 metabolism and cerebral blood flow in humans during deep and rapid-eye-movement sleep. J. Appl. Physiol. 1991;70(6):2597–2601. doi: 10.1152/jappl.1991.70.6.2597. [DOI] [PubMed] [Google Scholar]
- 217.Caporale A., Lee H., Lei H., Rao H., Langham M.C., Detre J.A. Cerebral metabolic rate of oxygen during transition from wakefulness to sleep measured with high temporal resolution OxFlow MRI with concurrent EEG. J. Cereb. Blood Flow Metab. 2021;41(4):780–792. doi: 10.1177/0271678X20919287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Turrens J.F. Mitochondrial formation of reactive oxygen species. J. Physiol. 2003;552(Pt 2):335–344. doi: 10.1113/jphysiol.2003.049478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Aksenov D.P., Miller M.J., Dixon C.J., Wyrwicz A.M. The effect of sevoflurane and isoflurane anesthesia on single unit and local field potentials. Exp. Brain Res. 2019;237(6):1521–1529. doi: 10.1007/s00221-019-05528-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Andrada J., Livingston P., Lee B.J., Antognini J. Propofol and etomidate depress cortical, thalamic, and reticular formation neurons during anesthetic-induced unconsciousness. Anesth. Analg. 2012;114(3):661–669. doi: 10.1213/ANE.0b013e3182405228. [DOI] [PubMed] [Google Scholar]
- 221.Kikuchi T., Wang Y., Sato K., Okumura F. In vivo effects of propofol on acetylcholine release from the frontal cortex, hippocampus and striatum studied by intracerebral microdialysis in freely moving rats. Br. J. Anaesth. 1998;80(5):644–648. doi: 10.1093/bja/80.5.644. [DOI] [PubMed] [Google Scholar]
- 222.Shichino T., Murakawa M., Adachi T., Arai T., Miyazaki Y., Mori K. Effects of inhalation anaesthetics on the release of acetylcholine in the rat cerebral cortex in vivo. Br. J. Anaesth. 1998;80(3):365–370. doi: 10.1093/bja/80.3.365. [DOI] [PubMed] [Google Scholar]
- 223.Alkire M.T., Haier R.J., Barker S.J., Shah N.K., Wu J.C., Kao Y.J. Cerebral metabolism during propofol anesthesia in humans studied with positron emission tomography. Anesthesiology. 1995;82(2):393–403. doi: 10.1097/00000542-199502000-00010. [DOI] [PubMed] [Google Scholar]
- 224.Laaksonen L., Kallioinen M., Långsjö J., Laitio T., Scheinin A., Scheinin J., Kaisti K., Maksimow A., Kallionpää R.E., Rajala V., Johansson J., Kantonen O., Nyman M., Sirén S., Valli K., Revonsuo A., Solin O., Vahlberg T., Alkire M., Scheinin H. Comparative effects of dexmedetomidine, propofol, sevoflurane, and S-ketamine on regional cerebral glucose metabolism in humans: a positron emission tomography study. Br. J. Anaesth. 2018;121(1):281–290. doi: 10.1016/j.bja.2018.04.008. [DOI] [PubMed] [Google Scholar]
- 225.Mielck F., Stephan H., Buhre W., Weyland A., Sonntag H. Effects of 1 MAC desflurane on cerebral metabolism, blood flow and carbon dioxide reactivity in humans. Br. J. Anaesth. 1998;81(2):155–160. doi: 10.1093/bja/81.2.155. [DOI] [PubMed] [Google Scholar]
- 226.Oshima T., Karasawa F., Satoh T. Effects of propofol on cerebral blood flow and the metabolic rate of oxygen in humans. Acta Anaesthesiol. Scand. 2002;46(7):831–835. doi: 10.1034/j.1399-6576.2002.460713.x. [DOI] [PubMed] [Google Scholar]
- 227.Oshima T., Karasawa F., Okazaki Y., Wada H., Satoh T. Effects of sevoflurane on cerebral blood flow and cerebral metabolic rate of oxygen in human beings: a comparison with isoflurane. Eur. J. Anaesthesiol. 2003;20(7):543–547. doi: 10.1097/00003643-200307000-00005. [DOI] [PubMed] [Google Scholar]
- 228.Berdili N., Bagriacik E.V., Yilmaz G., Ozkose Z., Kavutcu M., Bayraktar A.C., Bedirli A. Signaling pathway Sevoflurane exerts brain-protective effects against sepsis-associated encephalopathy and memory impirment through caspase 3/9 and Bax/Bd signaling pathway in a rat model of sepsis. J. Int. Med. Res. 2018;46(7):2828–2842. doi: 10.1177/0300060518773265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Pal D., Dean J.G., Liu T., Li D., Watson C.J., Hudetz A.G., Mashour G.A. Differential role of prefrontal and parietal cortices in controlling level of consciousness. Curr. Biol. 2018;28(13):2145–2152.e5. doi: 10.1016/j.cub.2018.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Volti L.G., Murabito P., Attaguile G., Rodella L.F., Astuto M., Di Giacomo C. Antioxidant properties of propofol: when oxidative stress sleeps with patients. EXCLI J. 2006;5:25–32. [Google Scholar]
- 231.Haller C., Mende M., Schuier F., Schuh R., Schröck H., Kuschinsky W. Effect of γ-hydroxybutyrate on local and global glucose metabolism in the anesthetized cat brain. J. Cereb. Blood Flow Metab. 1990;10(4):493–498. doi: 10.1038/jcbfm.1990.91. [DOI] [PubMed] [Google Scholar]
- 232.Gardner B., Strus E., Meng Q.C., Coradetti T., Naidoo N.N., Kelz M.B., Williams J.A. Sleep homeostasis and general anesthesia: are fruit flies well rested after emergence from propofol? Anesthesiology. 2016;124(2):404–416. doi: 10.1097/ALN.0000000000000939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Miller A.H., Raison C.L. The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nat. Rev. Immunol. 2016;16(1):22–34. doi: 10.1038/nri.2015.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Pitsillou E., Bresnehan S.M., Kagarakis E.A., Wijoyo S.J., Liang J., Hung A., Karagiannis T.C. The cellular and molecular basis of major depressive disorder: towards a unified model for understanding clinical depression. Mol. Biol. Rep. 2020;47(1):753–770. doi: 10.1007/s11033-019-05129-3. [DOI] [PubMed] [Google Scholar]
- 235.Rao J.S., Harry G.J., Rapoport S.I., Kim H.W. Increased excitotoxicity and neuroinflammatory markers in postmortem frontal cortex from bipolar disorder patients. Mol. Psychiatry. 2010;15(4):384–392. doi: 10.1038/mp.2009.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Steiner J., Bielau H., Brisch R., Danos P., Ullrich O., Mawrin C., Bernstein H.G., Bogerts B. Immunological aspects in the neurobiology of suicide: elevated microglial density in schizophrenia and depression is associated with suicide. J. Psychiatr. Res. 2008;42(2):151–157. doi: 10.1016/j.jpsychires.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 237.Torres-Platas S.G., Cruceanu C., Chen G.G., Turecki G., Mechawar N. Evidence for increased microglial priming and macrophage recruitment in the dorsal anterior cingulate white matter of depressed suicides. Brain Behav. Immun. 2014;42:50–59. doi: 10.1016/j.bbi.2014.05.007. [DOI] [PubMed] [Google Scholar]
- 238.Guevara C.A., Del Valle P., Mercedes C.R. Microglia and reactive oxygen species are required for behavioral susceptibility to chronic social defeat stress. J. Neurosci. 2020;40(7):1370–1372. doi: 10.1523/JNEUROSCI.2175-19.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Lehmann M.L., Weigel T.K., Poffenberger C.N., Herkenham M. The behavioral sequelae of social defeat require microglia and are driven by oxidative stress in mice. J. Neurosci. 2019;39(28):5594–5605. doi: 10.1523/JNEUROSCI.0184-19.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Vandermaelen C.P., Aghajanian G.K. Electrophysiological and pharmacological characterization of serotonergic dorsal raphe neurons recorded extracellularly and intracellularly in rat brain slices. Brain Res. 1983;289(1-2):109–119. doi: 10.1016/0006-8993(83)90011-2. [DOI] [PubMed] [Google Scholar]