Abstract
Progressive degeneration and dysfunction of the nervous system because of oxidative stress, aggregations of misfolded proteins, and neuroinflammation are the key pathological features of neurodegenerative diseases. Alzheimer's disease is a chronic neurodegenerative disorder driven by uncontrolled extracellular deposition of β-amyloid (Aβ) in the amyloid plaques and intracellular accumulation of hyperphosphorylated tau protein. Curcumin is a hydrophobic polyphenol with noticeable neuroprotective and anti-inflammatory effects that can cross the blood-brain barrier. Therefore, it is widely studied for the alleviation of inflammatory and neurological disorders. However, the clinical application of curcumin is limited due to its low aqueous solubility and bioavailability. Recently, nano-based curcumin delivery systems are developed to overcome these limitations effectively. This review article discusses the effects and potential mechanisms of curcumin-loaded PLGA nanoparticles in Alzheimer’s disease.
Keywords: Curcuminoids, polymer, PLGA, cognition, inflammation, drug delivery
1. INTRODUCTION
Central Nervous System (CNS) disorders affect nearly 1.5 billion of the world's population [1]. Neurodegenerative diseases cause chronic impairment of sensory, motor, behavioral and cognitive functions due to progressive loss of CNS neurons. Most forms are associated with increased age and are likely due to oxidative stress, aggregations of misfolded proteins [2], and neuroinflammation [3]. Neuroinflammation is the main contributor to the progression of neurodegenerative disorders and is characterized by the breakdown of the integrity of the Blood-Brain Barrier (BBB), morphological changes in glial cells and extensive tissue destruction by invading leukocytes [4]. The enhanced expression of cytokines by lymphocytes and myeloid cells initiates the inflammatory cascade. It is then mediated by secondary messengers (nitric oxide and prostaglandins), ROS and cytokines, such as IL-1B, IL-6, IL-23, TNF-α, granulocyte/macrophage colony-stimulating factor (GM-CSF) and chemokines (like CCL2, CCL5, and CXCL1). The overproduction of the above inflammatory mediators results in neuronal damage and death [5]. Neuro-inflammaging refers to the correlation between aging and neuroinflammation. During this process, activated microglia and astrocytes enhance cyclooxygenase-2 (COX2), nuclear factor-KB (NF-KB), and inducible nitric oxide synthase (iNOS). Subsequently, iNOS induces proinflammatory cytokines (e.g., interleukin (IL)-6, IL-1B) and neurotoxic factors like Reactive Oxidative Species (ROS) and Tumor Necrosis Factor (TNF-B)) which contribute to neuronal damage [6, 7]. Also, Toll-Like Receptors 4 (TLR4) and NF-KB activation by innate immune signal transduction adaptor (MYD88) induce proinflammatory factors (TNF-B, IL-1B, IL-6 and iNOS), which in turn potentiate various inflammatory pathways. A significant contributor to maintain a neuroprotective state against neuroinflammation is the heat shock response [8, 9]. The respective genes involved are known as vitagenes, which are involved in the production of antioxidant and anti-apoptotic molecules and activation of pro-survival pathways [10-12]. The members of the heat shock protein family include heme oxygenase-1 (HO-1), heat shock protein (Hsp70), sirtuins (Sirt-1), γ-glutamyl cysteine synthetase (γ-GCS) and thioredoxin/thioredoxin reductase (Trx/TrxR) [13, 14].
In addition, neurotoxicity could be due to proteotoxicity, which refers to the toxic effect of proteins/peptides misassembly and aggregation in several cell types. The proteotoxicity-associated neurotoxicity mechanisms are inadequately recognized; nevertheless, it is well known that protein aggregation is significantly associated with neurodegenerative disease development [15]. The recognition of proteotoxic insults accompanied protective cellular stress response pathways and chaperone networks associated with preventing protein misfolding and aggregation are required for the adaptation and survival of cells and organisms [16]. Cancer, metabolic and neurodegenerative diseases showed chronic proteotoxic stress where the cell's chaperones capacity and other homeostasis components seem poorly adapted [17]. In this way, the nonnative protein species accumulate, following the dysregulation of protein folding quality that can develop oligomers, aggregates, and compositions characteristic of neurodegenerative disease [18].
Consequently, damage of proteome integrity due to reduction in biosynthetic and repair activities affects protecting genes (vitagenes) that regulate aging, thereby affecting the health and lifespan of the organism [19, 20]. The pharmacologic regulation of pathways involved in cellular-stress response is a potential target for some disease therapies like cancer, cardiovascular and neurodegenerative diseases [21].
The achievement of the therapeutic dose is crucial for any successful medical intervention. Understanding the dose-response nature, especially in the low-dose zone [22-26], is vital for clinical success. However, it is reported that conventional dose-response models (commonly accepted threshold and linear dose-response models) were unsuccessful in accurately predicting responses in the low-dose zone. In contrast, the hormetic dose-response has been reported remarkably powerful [9, 27-33]. Consequently, a hormetic dose-response consideration in the performance, toxicological and pharmacological studies analyses has been proposed to improve the drug development process and chemical hazard/risk assessment [9].
2. ADAPTIVE STRESS RESPONSES/HORMESIS ROLES IN THE NERVOUS SYSTEM
Hormesis is a common toxicologic term that refers to a dose-response phenomenon to a chemical agent or environmental factor identified by low-dose stimulation, zero dose and high-dose inhibition. Therefore, it may be graphically illustrated by a J-shaped or an inverted U-shaped dose-response (the “U” arms are inhibitory or toxic concentrations while the curve zone stimulates a beneficial response) [34, 35]. The normal physiological function of cells and organisms and some natural and synthetic molecules (nutrition) follows a hormetic curve with deficient homeostasis and toxicity regions. Homeostasis is a hormetic zone of physiological concentrations with a safe and beneficial dose and the therapeutic window, a synonym of the hormetic zone in pharmacology [34, 36].
It is reported that a different type of oxidative and other stresses could induce hormesis as an adaptive response that contributes to the resistance of cells/organisms to higher (and normally toxic) doses of the same stressing agent [37]. Studies have shown that reactive oxygen species (ROS) impair cellular homeostasis through complicated and irreversible damage to cellular components [38]. During oxidative damage, the high reactivity of molecular oxygen and its intermediates is produced, resulting in DNA, lipids and proteins oxidative modifications [39]. Mitochondria is one of the main sources of ROS (superoxide anion radical) as unwanted by-products of oxidative phosphorylation. The excessive production of ROS has been involved in several pathological conditions, including inflammatory conditions, such as arthritis, cardiovascular disease and cancers [40-42]. In addition, superoxide production by mitochondria is considered to participate in neuronal damage varying from chronic intermittent cerebral hypoxia [43] to Alzheimer's Disease (AD) [44]. Besides the adverse effects of ROS on cell function and survival, it is now apparent that mitochondrial superoxide and hydrogen peroxide in lower subtoxic levels play critical roles in a variety of cellular functions and can also stimulate signalling pathways that improve cell survival and protect cells against injury and disease [15].
This neuroprotective impact of a subtoxic rise in cellular oxidative stress is known as “preconditioning” [45] but generally named mitochondrial hormesis or mitohormesis [46].
Based on the latest evidence, neuroprotective features of antioxidants, iron-chelating in addition to anti-inflammatory agents with distinct consideration to polyphenols have attracted particular attention [47, 48]. According to the hormesis theory, a stressor agent (drugs, toxins and natural substances), if administered at low doses, may trigger a positive response in the duration of adjustment or protection from the stressors. In contrast, at a higher dose, the toxic effect predominates [49-55]. Based on in vitro evidence, polyphenols stimulate the heat shock protein (Hsp) pathway by applying this paradigm, which represents a critical role in the cellular stress response [56, 57]. Two members of the Hsp family, Heme oxygenase-1 (HO-1) and Hsp70, also remembered as vitagenes, because of their antioxidant activity, have attracted significant attention [56-58].
Various studies on experimental models of cancer [59], neurodegenerative disease [60] and cardiovascular disease [61] reported some phytochemicals through activating adaptive stress response signalling, having favourable effects [62]. These pathways generally involve the kinases and transcription factors activation, including the antioxidant response element (ARE), Nrf-2 (a transcription factor) and its genetic target activation by sulforaphane and curcumin [63]; the transient receptor potential (TRP) calcium channels activation by capsaicin and allicin [64] and histone deacetylases and their target FOXO transcription factors activation by resveratrol [65]. These events finally result in increased cytoprotective protein production, including antioxidant enzymes, phase 2 enzymes, heat-shock proteins, growth factors and proteins required for regulating cellular metabolism [60]. For example, it is reported that Hidrox (HD) is a polyphenol complex from organic olives containing 40-50% of Hydroxytyrosol, which could inhibit the activation of NF-κB and decrease the iNOS levels. This study showed that redox homeostasis regulation by Nrf2 presumably leads to the regulation of NF-kB activity and the inflammatory response characteristic of Parkinson's disease (PD) [66]. Another similar study also revealed that hydroxytyrosol as polyphenols of the olive oil inhibits neurodegeneration (Parkinson’s-like phenotypes) in nematodes and rodents, presumably through the Nrf2 signalling pathway and hormesis response [67].
Consequently, from the viewpoint of hormesis response, the achievement of the right doses of administrated agents like phytochemicals or chemical drugs is required to manage various conditions, particularly neurodegenerative disease, effectively [68, 69]. In recent preclinical studies, natural products derived from plants and herbs, such as curcumin supplementation, have alleviated neuroinflammation progression [70-72].
3. ALZHEIMER’S DISEASE (AD)
Alzheimer’s disease (AD) is a progressive neurodegenerative disease mainly seen in the elderly population [73-76]. However, AD is a significant concern for the 21st century affecting more than 24.3 million people worldwide [77], which is expected to affect around 120 million cases by 2050 [78, 79]. The contributing factors in AD development include uncontrolled extracellular β-amyloid (Aβ) deposition in the amyloid plaques, intracellular tau protein hyperphosphorylation, which forms neurofibrillary tangles mitochondria dysfunction, inflammation and oxidative stress, and eventually cholinergic dysfunction due to progressive degeneration in the basal ganglia [80-85]. Amyloid proteins (Aβ) are a 42 amino acid long cleavage product of amyloid precursor protein (APP), a transmembrane polypeptide with neurotrophic activity. Under non-physiological conditions, the APP processing via β and γ-secretases [86] results in extracellular aggregation of Aβ monomers, which are then modified through phosphorylation forming dimers, oligomers, protofibrils and mature fibrils [87]. These end products can form toxic AGEs (Advanced Glycation endproducts) or amyloid plaques in the parenchyma and blood vessels [88]. These plaques inhibit mitochondrial activity, modify intracellular Ca2+ levels, increase oxidative stress, and stimulate neuroinflammation by impairing proteasome function. In addition, Aβ peptides can induce tau hyperphosphorylation (a microtubule-associated protein) through the interaction with the signaling pathways that disrupt axonal transport and increase neurofibrillary tangles and soluble tau seen in AD. Also, Aβ restricts tau protein degradation [89, 90]. Furthermore, it is demonstrated that Aβs stimulate microglia to secrete proinflammatory cytokines, leading to neuronal damage in AD [91-93].
4. NOVEL THERAPEUTIC STRATEGIES DEVELOPMENT IN ALZHEIMER’S DISEASE
The current AD therapeutic approaches are categorized into mechanism-based strategies, including the Amyloid targeting (Suppressing Aβ Production, stimulating Aβ clearance and preventing Aβ aggregation), Tau targeting (Tau stabilizers and aggregation inhibitors, therapies targeted at Tau post-translational modifications and anti-tau immunotherapy), targeting of apolipoprotein-E (ApoE) function, and neuroprotective therapies (neurotrophins and their receptor-based therapies, therapies targeted at neuroinflammation and oxidative stress). In addition, non-mechanism-based approaches in AD treatment included symptomatic cognitive enhancers, treatments and interventions for AD prevention (secondary AD prevention interventions and primary prevention). Ultimately, lifestyle modifications and risk factor management, including non-pharmacological interventions, have been examined in AD prevention trials [94]. Besides, phytochemical’s efficacy in the treatment of neurodegenerative diseases, including AD and PD, have been investigated by numerous studies. Various studies investigated the probable efficacy of phytochemicals like berberine, epigallocatechin-3-gallate, curcumin, quercetin, resveratrol and limonoids against the most common neurodegenerative diseases, including AD and PD [95].
Currently, Nanomeds for improving traditional therapy have entered the clinical practice of several diseases, especially allergy, cancer and cardiovascular disorders. There are several clinical studies on the use of liposomal, gold and polymeric nanoparticles [96-108]. In addition, a few nano-based products are already used by oncologists. Nanomeds via loading and delivering drugs to the targeted site, specific release profiles (depot effects), preserve the loading drugs from enzymatic degradations and improve bioavailability, provide helpful information at the cellular and tissue scales for designing patient-specific therapeutic interventions in various diseases [109]. As mentioned, curcumin as a phytochemical offers promising safe and inexpensive preventive options for neurodegenerative diseases, particularly AD, because of its actions on several molecular aspects of these diseases [95].
5. BIOLOGIC EFFECTS OF CURCUMIN
Curcumin is a hydrophobic polyphenol produced by Curcuma longa L. [110]. It is dietary safe [111] and has a wide variety of pharmacological activities, including wound healing [112-114], anticoagulant [115], antimicrobial [116], anticancer [117-121], anti-inflammatory [122-125], antioxidant [126], anti-diabetic [127], lipid-modifying [128], anti-amyloid [129, 130] and neuroprotective effects [131]. Curcumin exerts its antioxidant effects through different mechanisms. It can directly scavenge free radicals via its two phenolic sites and suppress ROS and reactive nitrogen species (RNS) production in the cellular environment. It also suppresses protein and DNA oxidation through the reduction of low-density lipoprotein (LDL). The expression of ROS-generating enzymes is inhibited, whereas antioxidant enzymes are upregulated by curcumin [132]. Curcumin modulates neuroinflammation by the downregulation of various inflammatory cytokines [133, 134].
Curcumin has pleiotropic activities through its complex chemistry and its capacity to affect various signalling pathways, including angiogenic and metastatic pathways, survival pathways like those regulated by NF-kB and growth factors Nrf2-dependent cytoprotective pathways [135-140]. It has been demonstrated that curcumin is a hormetic agent via biphasic dose–responses on cells. It is stimulatory at low doses (like activation of the mitogen‐activated protein kinase signalling pathway and an antioxidant function) and inhibitory at high doses (like autophagy and cell death induction). This means that several curcumin effects are dose-dependent, and some effects might be more prominent at lower doses, characteristic of a hormetic response. Curcumin has a modulatory effect in neurological diseases, such as AD,s with a hormetic dose-response [141].
6. CURCUMIN LIMITATIONS
Despite the promising effects of curcumin in numerous clinical trials, it is not yet certified for clinical application. The main obstacles include low oral bioavailability, with an extremely low plasma concentration of 1% [142]. Low structural stability, limited absorption from the gastrointestinal (GI) tract, accelerated metabolism, and rapid systemic clearance is other reasons for curcumin's limited utility in the clinical setting [143, 144]. Limited stability of curcumin at alkaline conditions and light sensitivity are other concerns associated with curcumin [145-149].
7. DEVELOPMENT OF NOVEL CURCUMIN FORMULATIONS
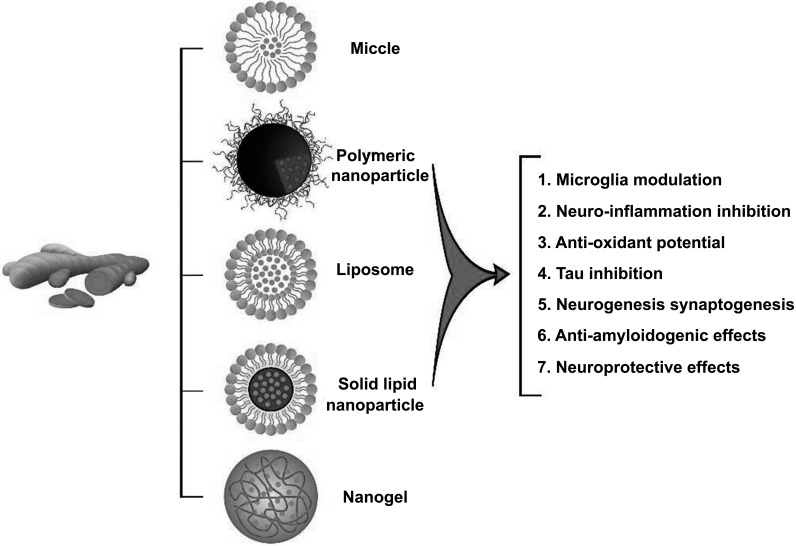
To date, various formulations have been developed to improve curcumin bioavailability and drug delivery. Modifying the solid-state, formulating supersaturated solutions [150] and designing a more soluble compound like artificial analogs to resist in vivo removal and metabolism are some of the methods used to increase bioavailability. Other techniques include reducing particle size, combining curcumin with cellular metabolism and drug efflux suppressors [151]; the addition of adjuvant molecules, such as piperine‚ quercetin or silibinin‚ chemical combination of curcumin with polysaccharides, proteins or phospholipids and bio-conjugation of curcumin with turmeric oil or alanine [152, 153]. Despite the potential effects of these strategies in improving the solubility and bioavailability of curcumin, most of these formulations fail to target curcumin to specific sites of action and preserve its chemical structure, resulting in its rapid metabolization and removal. Nowadays, nanotechnology-based methods are introduced as promising substitutes for conventional formulations [154]. The main categories of nanoformulation-based strategies are the application of stabilizers, adjuvants or polymer conjugates, and the development of liposomes, hydro/micro/nano gels, micelles, and nanoparticles (NPs) [152, 155, 156] (Fig. 1). Curcumin-nanoparti-culated delivery methods represent potent carriers in treating neurodegenerative disorders since the desired size, chemical structure, surface zeta potential charge, and surface functionalization can be modified [156, 157]. It is demonstrated that curcumin encapsulation into nanoparticles remarkably improves its bioavailability, solubility, and chemical stability by protecting it from the outside environment's influence, such as enzymatic and pH degradation [110, 143, 158]. Nanocarriers' toxicity potential is the primary concern of many researchers since their composition and size must be non-invasive for medical purposes. The size of NPs and their elimination and biodegradation determine their safety. The average size of nanoparticles for drug delivery systems is less than 200 nm for brain applications but is conceptually expandable up to 1000 nm. The nanoparticles were developed based on two original opinions; the theory of magical bullets from Paul Ehrlich [159] and the idea of miniaturization from Richard Feynman [160]. The first investigation on drug transport to the brain by nanoparticles was performed in 1969 [161]. Afterwards, functionalized nanoparticles coated with polysorbate 80 facilitate their entry into the CNS. The same application was introduced by Müller et al., [162] using lipid nanoparticles. Subsequently, cellular pharmacokinetic and mechanistic studies were performed to improve vectorization. Recent studies focused on curcumin-loaded nanoparticle delivery systems, specific CNS targeting, and intranuclear levels in neurons [150]. Initially, polymeric nanoparticles were applied for drug delivery to the CNS, which included poly (lactic-co-glycolic acid) (PLGA), chitosan, and poly (butyl cyanoacrylate) (PBCA) [163]. Polymeric nanoparticles are approved by the Food and Drug Administration (FDA), and they are less toxic than the other compounds [164]. PLGA nanoparticles as biodegradable and biocompatible polymers with characteristics, such as the controlled release of various pharmacologically active groups [165, 166] like curcumin [167, 168], are commonly used for drug delivery [169]. The biodegradable and biocompatible properties of PLGA are due to their hydrolytic cleavage into natural metabolites (i.e., lactic acid and glycolic acid), metabolized through the Krebs cycle and are then discharged as carbon dioxide and water [170]. The hydrophobic nature of PLGA guarantees significant entrapment and sustained release of curcumin [170]. It could also cross the lipophilic olfactory and trigeminal nerves [171]. Herein, the current experimental and clinical literature on the effects of curcumin-loaded PLGA particles on Alzheimer’s disease (AD) is reviewed.
Fig. (1).
Different types of curcumin-based nano formulations and their therapeutic effects.
8. CURCUMIN EFFECTS ON AD
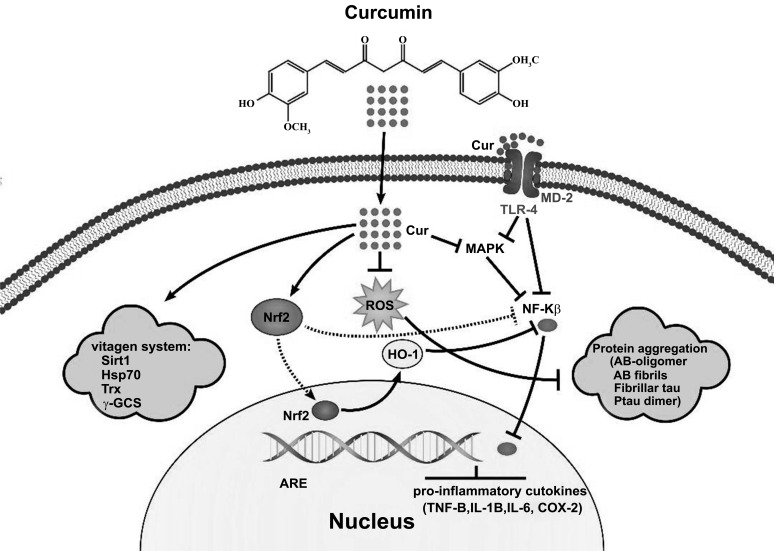
Curcumin inhibits two major pathological changes in AD; it blocks the self-assembly of Aβ plaques [129, 172-174] when binding to them and hinders tau hyperphosphorylation [175]. Also, curcumin has potent neuroprotective effects due to its anti-inflammatory and antioxidant effects. It inhibits the expression of inflammatory cytokines, cyclooxygenase enzyme (COX-2) [174] glycogen synthase-3 [176], and iNOS, possibly through the suppression of NF-κB and JNK/AP-1- mediated gene transcription [177, 178]. An interesting feature of curcumin is its facilitated BBB penetration due to its unique charge and binding capabilities [179]. It is demonstrated that neuronal signaling, membrane homeostasis and cognitive defects following a traumatic brain injury could be improved by curcumin [180], as shown in Fig. (2).
Fig. (2).
The main pleiotropic functions of curcumin in neurodegenerative diseases; Curcumin exerts neuroprotection effects through Nrf2 activation, MAPK inhibition and downregulation of TLR-4 after binding to MD-2, leading to reduced expression of NF-KB and proinflammatory cytokines. Also, curcumin activates the protective systems and removes misfolded proteins by inhibiting ROS production.
Abbreviations: MD-2: myeloid differentiation factor 2, TLR-4: Toll-like receptor 4, MAPK: A mitogen-activated protein kinase, Nf-KB: Nuclear Factor kappa-light-chain-enhancer of activated B cells, ROS: reactive oxygen species, Nrf2: nuclear factor erythroid 2–related factor 2, HO-1: Heme oxygenase-1, ARE: antioxidant response element, COX-2: antioxidant response element, Hsp70: heat shock protein, Sirt-1: sirtuins, Trx: thioredoxin/thioredoxin reductase, γ- γ-GCS: glutamyl cysteine synthetase.
9. EXPERIMENTAL AND CLINICAL STUDIES RELATED TO AD
The in vitro/in vivo studies on curcumin-loaded PLGA NPs formulations and their application in AD are summarized in order of publication year (Table 1). Yin-Meng Tsai evaluated the curcumin-loaded PLGA NPs distribution and showed that Cur-NPs formulation significantly increased curcumin concentration in the spleen and lungs. Compared to free curcumin, Cur-Nps remarkably prolonged the maintenance time of curcumin in the cerebral cortex and hippocampus up to 96% and 83%, respectively [181]. It has been shown that curcumin-loaded PLGA NPs with high water solubility induced potent anti-amyloid effects [182]. Intravenous injection, liposomes, acrylic polymer, and PLGA formulations could pass the BBB and preferentially be concentrated in the hippocampus, striata, and brain stem to exert antioxidant, anti-inflammatory, positive neurogenesis, and neuroplasticity effects [183]. In another study by the same authors, synthetic amyloid-binding aptamer (described as NN2) conjugated with curcumin-loaded PLGA NPs reduced the plasma amyloid levels through its effective attachment to amyloid plaques and their disaggregation [184]. Following this study, curcumin encapsulated-PLGA NPs decorated with Tet-1 peptide with a great affinity for neurons and retrograde transportation characteristics were developed. In vitro results showed that these NPs are non-cytotoxic, destroy amyloid aggregates and display antioxidative features [185]. Another interesting approach to improve curcumin delivery was developed by Marrachea et al. They synthesized mitochondria-targeted curcumin-PLGA-b-PEG-triphenylphosphonium (TPP) to facilitate curcumin entry into mitochondria. These targeted curcumin PLGA-b-PEG-TPP NPS notably enhance the therapeutic drug index for AD compared to nontargeted particles or their free forms [186]. Doggie et al. stated that Cur-PLGA NPs could protect human neuroblastoma SK-N-SH cells from oxidative injury, which is also seen in AD, by preventing H2O2-induced toxicity and inhibiting ROS elevation GSH reduction and activation of Nrf2. They suggested that this formulation is expected to have great potential for pharmaceutical application in neurodegenerative disorders, such as AD [187]. Also, Shashi Kant Tiwari reported that compared to free curcumin, Cur-PLGA-NPs induced endogenous neural stem cells (NSC) proliferation by increasing the expression of cell proliferation genes (reelin, Pax6, and nestin) and improved neuronal differentiation by the upregulation of neuroligin, neurogenin, neuregulin, neuroD1, and Stat3 genes and in vitro activation of Wnt/β-catenin pathway (regulator of neurogenesis) in the rats. Besides, these nanoparticles reduced GSK-3β levels and enhanced TCF/LEF and cyclin-D1 promoter activity. They also improved training and memory impairments in beta-amyloid-induced rat models of AD-like phenotypes by stimulating neurogenesis via activating the canonical Wnt/β-catenin pathway and enhancing a brain self-repair mechanism [188]. Srivastava A et al. also reported that Cur-encapsulated PLGA NPs are potential regulators of gelsolin amyloidogenesis. These NPs increased curcumin's solubility and reduced the effective concentration to modulate amyloid plaques by ~1000 fold compared to their free forms. Consequently, PLGA encapsulation promoted the therapeutic potential of curcumin against amyloid fibrillation and toxicity [189]. Subsequently, Djiokeng Paka et al. developed glutathione-functionalized PLGA-nanoparticles (GSH-NPs) loaded with non-toxic curcumin, and the surface GSH presented a greater neuroprotective effect against acrolein. These GSH-Cur-NPs had a higher and easier neuronal internalization than free curcumin due to a modified internalization route that enabled them to escape uptake via macropinocytosis, thereby avoiding lysosomal degradation [190]. Huang et al. designed NPs encapsulated with curcumin and Aβ generation inhibitor S1 (PQVGHL peptide) to target the harmful factors in AD progression. These NPs were conjugated with brain targeting peptide CRT (cyclic CRTIGPSVC peptide), an iron-mimic peptide that targets transferrin receptors (TfR), for advanced BBB penetration. They showed that these NPs significantly reduced Aβ level, reactive oxygen species (ROS), inflammatory cytokines (e.g., TNF-α and IL-6) and intensified the activity of superoxide dismutase (SOD) and the number of brain synapses, resulting in the improvement of spatial memory and recognition in transgenic AD mice. Consequently, co-delivery of an anti-inflammatory agent like curcumin and Aβ production inhibition (S1) conjugated with brain targeting peptide (CRT) revealed the most favorable effects in which CRT facilitated the BBB permeability of Cur-PLGA NPs, and curcumin decreased Aβ formation, gliosis, and proinflammatory cytokine production in the treatment of AD mice [191]. In a study by Barbara et al., Cur-PLGA NPs conjugated with g7 ligand were formulated to improve BBB crossing. The primary hippocampal cell cultures subjected to these NPs showed no apparent toxicity, a significant reduction of Aβ aggregates and less inflammation, oxidative stress and amyloid plaque load. Hence, brain delivery of curcumin using NPs to cross BBB could be a promising approach in managing AD [192]. Later, Ameruoso et al. developed curcumin-loaded spherical polymeric nano constructs (SPNs) with a size of 200 nm and curcumin-loaded discoidal polymeric nano constructs (DPNs) with a size of 1000 nm using PLGA, polyethylene glycol (PEG) and lipid chains as building blocks. They evaluated specific curcumin delivery to macrophages, previously stimulated by incubation with Amyloid-β fibrils produced in vitro. The cytofluorimetric and confocal microscopic analyses demonstrated that Cur-SPNs are taken up more quickly by macrophages than Cur-DPNs. Also, Cur-SPNs diminished the production of proinflammatory cytokines (IL-1β, IL-6, and TNF-α) in macrophages stimulated via amyloid-β fibers up to 6.5-fold [193]. Huo et al. reported that Cur loaded Selenium-PLGA nanospheres could reduce the amyloid-β load in AD mice's brain specimens and considerably improve their memory deficiency through specific attachments to Aβ plaques [194]. It is demonstrated that other than the brain, peripheral organs like the liver can also produce amyloid proteins [195]. It is safer and easier to reduce peripheral amyloid due to difficulty in BBB penetration of drugs targeted to the CNS and cerebral toxicity [196]. In this regard, Takahashi et al. [197] initially developed amyloid-binding aptamers, preventing the aggregation of amyloid fibrils. In another study, curcumin-loaded PLGA-PEG were conjugated with B6 peptide as a brain target, which showed that these NPs possessed adequate blood compatibility and increased curcumin cellular uptake. Also, Cur-PLGA-PEG-B6 could remarkably improve the spatial training, and the memory ability of APP/PS1 mice versus native Cur. Further experiments confirmed that Cur-PLGA-PEG-B6 could decrease hippocampal β-amyloid formation and deposition and also tau hyperphosphorylation [198]. Recently, Kuo et al. developed polyacrylamide (PAAM)-cardiolipin (CL)-poly(lactide-co-glycolide) (PLGA) NPs grafted with surface 83-14 monoclonal antibody (MAb) to carry rosmarinic acid (RA) and curcumin (CUR), which was named as 83-14 MAb-RA-CUR-PAAM-CL-PLGA NPs. These NPs increased the permeability coefficient of curcumin across the BBB and improved SK-N-MC cells' viability irritated with β-amyloid (Aβ) deposits. Consequently, 83-14 MAb-RA-CUR-PAAM-CL-PLGA NPs may have a great neuroprotective capacity in medication management of AD to prevent neurodegeneration [199].
Table 1.
Studies on the effects of curcumin-loaded-PLGA particles on AD.
| Nanoparticle | Animal Models/Cell Culture | Clinical and Experimental Outcomes | Refs. |
|---|---|---|---|
| Curcumin-loaded PLGA nanoparticles |
Male Sprague-Dawley rats | Increased the concentration and retention time of curcumin in various organs | 2011 [181] |
| Curcumin-loaded PLGA nanoparticles |
Neuroblastoma cell line and glioma cell line | Anti-amyloid effects | 2011 [182] |
| Curcumin formulations composed of liposomes, acrylic polymer, and PLGA | Sprague-Dawley rats | Preferential concentration in hippocampus, striata, and brain stem | 2011 [183] |
| Amyloid-binding aptamer (described as NN2), which conjugated curcumin loaded PLGA NPs | LAG cell line | Reduction in the size of protein aggregation after treating with aptamer bound curcumin nanoparticles | 2012 [184] |
| Tet-1 peptide conjugated-curcumin -encapsulated-PLGA NPs | GI-1 glioma cells | Destroy amyloid aggregates and display antioxidative features |
2012 [185] |
| Mitochondria-targeted curcumin-PLGA-b-PEG-triphenylphosphonium (TPP) | HeLa model cell line | Remarkable enhancement in drug management of AD |
2012 [186] |
| Cur-encapsulated PLGA NPs | Human neuroblastoma SK-N-SH cells | Preventing H2O2-induced toxicity Inhibiting ROS elevation and GSH reduction, and activation of Nrf2 |
2012 [187] |
| Curcumin-loaded PLGA nanoparticles |
Wistar rats | Induced endogenous Neural Stem Cells (NSC) proliferation by: increasing the expression of cell proliferation genes (reelin, Pax6, and nestin) increasing neuronal differentiation by inducing the expression of neuroligin, neurogenin, neuregulin, neuroD1, and Stat3 genes and activating the Wnt/β-catenin pathway (regulator of neurogenesis) in vitro and hippocampus and subventricular zone |
2013 [188] |
| Cur-encapsulated PLGA NPs | Human SH-SY5Y cell line | Promotes the therapeutic potential of curcumin against amyloid fibrillation and prevents toxicity | 2015 [189] |
| Glutathione-functionalized PLGA nanoparticles loaded with curcumin | SK-N-SH cells, a human neuroblastoma cell line | - Non-acrolein toxic - higher and easier neuronal internalization - avoiding lysosomal degradation |
2016 [190] |
| Curcumin and Aβ generation inhibitor S1 (PQVGHL peptide) encapsulated NPS to target the harmful factors in AD progress and conjugating with brain targeting peptide CRT |
Male AD model (APP/PS1dE9) mice (8-month-old) and human neuroblastoma SH-SY5Y cells, mouse microglial BV2 cells and mouse brain capillary endothelial bEnd.3 cells | Significant reduction of Aβ level, ROS, inflammatory cytokines increase the activity of SOD and number of brain synapses in AD mice spatial memory and recognition improvement in transgenic AD mice |
2017 [191] |
| G7- curcumin- PLGA NPs | Primary hippocampal cultures from rat brains (embryonic day 18) | - Attenuated inflammation, oxidative stress, amyloid plaque load significant decrease of Ab aggregates |
2017 [192] |
| Curcumin-loaded SPNs and Curcumin-loaded DPNs |
RAW 264.7 cell line | Reduced production of proinflammatory cytokines | 2017 [193] |
| Cur loaded Selenium-PLGA nanospheres |
AD mice | Improved memory deficiency of AD mice through the reduction of amyloid-β load | 2018 [194] |
| Cur-PLGA-PEG conjugated B6 peptide | HT22 cell line and APP/PS1 Al transgenic mice | - Increasing curcumin cellular uptake - adequate blood compatibility - improving spatial training and memory ability of APP/PS1 -decreasing hippocampal b-amyloid formation and deposition and inhibiting tau hyperphosphorylation |
2018 [198] |
| Rosmarinic acid- and curcumin-loaded polyacrylamide-cardiolipin-PLGA nanoparticles with conjugated 83-14 monoclonal antibody |
SK-N-MC cells, HBMECs, and HAS cells | Increasing the permeability coefficient of curcumin across the BBB and neural membranes improving the viability of SK-N-MC cells irritated with (Aβ) deposits |
2018 [199] |
Abbreviations: PLGA: Poly (lactic-co-glycolic acid), NMPs: Nanomicro particles, PEG:Polyethylene glycol, TPP: Triphenylphosphonium, CRT: Cyclic CRTIGPSVC peptide, BBB: Blood-brain barrier, Aβ: β-amyloid, AD: Alzheimer’s disease.
CONCLUSION
The pleiotropic functions of curcumin, including antioxidant and anti-inflammatory effects as well as protein aggregation inhibition, are the primary contributors to combat neurodegenerative diseases, particularly AD. The main properties of curcumin, which increased its application, are safety, low cost, easy accessibility and effective penetration into the BBB and neuronal membranes. Nevertheless, some curcumin characteristics limited its clinical application, including low water solubility, low bioavailability, and structural instability in the body fluids. Nano-based drug delivery systems are the emerging carriers to enhance medications' efficacy in a controlled target-oriented fashion. In the present review, we summarized the in vitro/in vivo studies and clinical trials on curcumin-loaded PLGA NPs to prevent and treat AD. However, most of the available results have been obtained from in vitro strategies using multiple nano- curcumin technologies that could promote curcumin delivery in the SNC.
Other than multiple nano-curcumin implications, more studies are yet expected to evaluate the toxicity and efficacy of these NPs on a larger group of patients. The main concerns regarding Nanomed-based delivery systems are the possible toxic effects of curcumin-loaded NPs, including neuroinflammation, DNA damage, excitotoxicity, and allergic responses. Some methods, such as combination therapy and specific targeting, can minimize these toxic effects by decreasing the main therapeutic agent's dose and functionalizing the NPs, respectively. Consequently, nano curcumin carriers' preparation and purification methods play a pivotal role in reducing the aggregation and mechanical properties of NPs to mitigate their toxicity.
ACKNOWLEDGEMENTS
The authors are grateful to Omid Izadi (Department of Industrial Engineering, ACECR Institute of Higher Education of Kermanshah, Kermanshah, Iran) for drawing the graphical images.
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
None.
CONFLICT OF INTEREST
Muhammed Majeed is the founder of Sami Labs Ltd. and Sabinsa Corporation, involved in the production and sale of phytonutrients and standardized herbal extracts, including curcumin. The authors have no other conflicting interests to disclose.
REFERENCES
- 1.Emerit J., Edeas M., Bricaire F. Neurodegenerative diseases and oxidative stress. Biomed. Pharmacother. 2004;58(1):39–46. doi: 10.1016/j.biopha.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 2.Koo E.H., Lansbury P.T., Jr, Kelly J.W. Amyloid diseases: Abnormal protein aggregation in neurodegeneration. Proc. Natl. Acad. Sci. USA. 1999;96(18):9989–9990. doi: 10.1073/pnas.96.18.9989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amor S., Puentes F., Baker D., van der Valk P. Inflammation in neurodegenerative diseases. Immunology. 2010;129(2):154–169. doi: 10.1111/j.1365-2567.2009.03225.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Becher B., Spath S., Goverman J. Cytokine networks in neuroinflammation. Nat. Rev. Immunol. 2017;17(1):49–59. doi: 10.1038/nri.2016.123. [DOI] [PubMed] [Google Scholar]
- 5.DiSabato D.J., Quan N., Godbout J.P. Neuroinflammation: The devil is in the details. J. Neurochem. 2016;139(Suppl. 2):136–153. doi: 10.1111/jnc.13607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Agostinho P., Cunha R.A., Oliveira C. Neuroinflammation, oxidative stress and the pathogenesis of Alzheimer’s disease. Curr. Pharm. Des. 2010;16(25):2766–2778. doi: 10.2174/138161210793176572. [DOI] [PubMed] [Google Scholar]
- 7.Dantzer R., O’Connor J.C., Freund G.G., Johnson R.W., Kelley K.W. From inflammation to sickness and depression: When the immune system subjugates the brain. Nat. Rev. Neurosci. 2008;9(1):46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Trovato Salinaro A., Cornelius C., Koverech G., Koverech A., Scuto M., Lodato F., Fronte V., Muccilli V., Reibaldi M., Longo A., Uva M.G., Calabrese V. Cellular stress response, redox status, and vitagenes in glaucoma: A systemic oxidant disorder linked to Alzheimer’s disease. Front. Pharmacol. 2014;5:129. doi: 10.3389/fphar.2014.00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Calabrese V., Cornelius C., Dinkova-Kostova A.T., Calabrese E.J., Mattson M.P. Cellular stress responses, the hormesis paradigm, and vitagenes: Novel targets for therapeutic intervention in neurodegenerative disorders. Antioxid. Redox Signal. 2010;13(11):1763–1811. doi: 10.1089/ars.2009.3074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Trovato Salinaro A., Pennisi M., Di Paola R., Scuto M., Crupi R., Cambria M.T., Ontario M.L., Tomasello M., Uva M., Maiolino L., Calabrese E.J., Cuzzocrea S., Calabrese V. Neuroinflammation and neurohormesis in the pathogenesis of Alzheimer’s disease and Alzheimer-linked pathologies: Modulation by nutritional mushrooms. Immun. Ageing. 2018;15(1):8. doi: 10.1186/s12979-017-0108-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Calabrese V., Cornelius C., Mancuso C., Barone E., Calafato S., Bates T., Rizzarelli E., Kostova A.T. Vitagenes, dietary antioxidants and neuroprotection in neurodegenerative diseases. Front. Biosci. 2009;14:376–397. doi: 10.2741/3250. [DOI] [PubMed] [Google Scholar]
- 12.Cornelius C., Trovato Salinaro A., Scuto M., Fronte V., Cambria M.T., Pennisi M., Bella R., Milone P., Graziano A., Crupi R., Cuzzocrea S., Pennisi G., Calabrese V. Cellular stress response, sirtuins and UCP proteins in Alzheimer disease: Role of vitagenes. Immun. Ageing. 2013;10(1):41. doi: 10.1186/1742-4933-10-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Trovato A., Siracusa R., Di Paola R., Scuto M., Fronte V., Koverech G., Luca M., Serra A., Toscano M.A., Petralia A., Cuzzocrea S., Calabrese V. Redox modulation of cellular stress response and lipoxin A4 expression by Coriolus versicolor in rat brain: Relevance to Alzheimer’s disease pathogenesis. Neurotoxicology. 2016;53:350–358. doi: 10.1016/j.neuro.2015.09.012. [DOI] [PubMed] [Google Scholar]
- 14.Calabrese V., Scapagnini G., Davinelli S., Koverech G., Koverech A., De Pasquale C., Salinaro A.T., Scuto M., Calabrese E.J., Genazzani A.R. Sex hormonal regulation and hormesis in aging and longevity: Role of vitagenes. J. Cell Commun. Signal. 2014;8(4):369–384. doi: 10.1007/s12079-014-0253-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ruz C., Alcantud J.L., Vives Montero F., Duran R., Bandres-Ciga S., Bandres-Ciga S. Proteotoxicity and neurodegenerative diseases. Int. J. Mol. Sci. 2020;21(16):5646. doi: 10.3390/ijms21165646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morimoto R.I. Regulation of the heat shock transcriptional response: Cross talk between a family of heat shock factors, molecular chaperones, and negative regulators. Genes Dev. 1998;12(24):3788–3796. doi: 10.1101/gad.12.24.3788. [DOI] [PubMed] [Google Scholar]
- 17.Morimoto R.I. Stress, aging, and neurodegenerative disease. Mol. Biol. Cell. 2004;15:657–664. doi: 10.1091/mbc.E03-07-0532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morimoto R.I. Proteotoxic stress and inducible chaperone networks in neurodegenerative disease and aging. Genes Dev. 2008;22(11):1427–1438. doi: 10.1101/gad.1657108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Calabrese V., Mancuso C., Calvani M., Rizzarelli E., Butterfield D.A., Stella A.M.G. Nitric oxide in the central nervous system: Neuroprotection versus neurotoxicity. Nat. Rev. Neurosci. 2007;8(10):766–775. doi: 10.1038/nrn2214. [DOI] [PubMed] [Google Scholar]
- 20.Calabrese V., Cornelius C., Mancuso C., Pennisi G., Calafato S., Bellia F., Bates T.E., Giuffrida S.A.M., Schapira T., Dinkova Kostova A.T., Rizzarelli E. Cellular stress response: A novel target for chemoprevention and nutritional neuroprotection in aging, neurodegenerative disorders and longevity. Neurochem. Res. 2008;33(12):2444–2471. doi: 10.1007/s11064-008-9775-9. [DOI] [PubMed] [Google Scholar]
- 21.Calabrese V., Bates T.E., Mancuso C., Cornelius C., Ventimiglia B., Cambria M.T., Di Renzo L., De Lorenzo A., Dinkova-Kostova A.T. Curcumin and the cellular stress response in free radical-related diseases. Mol. Nutr. Food Res. 2008;52(9):1062–1073. doi: 10.1002/mnfr.200700316. [DOI] [PubMed] [Google Scholar]
- 22.Calabrese E.J. Hormesis and medicine. Br. J. Clin. Pharmacol. 2008;66(5):594–617. doi: 10.1111/j.1365-2125.2008.03243.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cedergreen N., Streibig J.C., Kudsk P., Mathiassen S.K., Duke S.O. The occurrence of hormesis in plants and algae. Dose Response. 2007;5(2):150–162. doi: 10.2203/dose-response.06-008.Cedergreen. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hoffmann G.R. A perspective on the scientific, philosophical, and policy dimensions of hormesis. Dose Response. 2009;7(1):1–51. doi: 10.2203/dose-response.08-023.Hoffmann. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Masoro E.J. Role of hormesis in life extension by caloric restriction. Dose Response. 2007;5(2):163–173. doi: 10.2203/dose-response.06-005.Masoro. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Scott BR .It's time for a new low-dose-radiation risk assessment paradigm—one that acknowledges hormesis. Dose-Response, 2008;6(4):07–005. doi: 10.2203/dose-response.07-005.Scott. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Calabrese E.J., Baldwin L.A. Hormesis: U-shaped dose responses and their centrality in toxicology. Trends Pharmacol. Sci. 2001;22(6):285–291. doi: 10.1016/S0165-6147(00)01719-3. [DOI] [PubMed] [Google Scholar]
- 28.Calabrese E.J., Baldwin L.A. U-shaped dose-responses in biology, toxicology, and public health. Annu. Rev. Public Health. 2001;22(1):15–33. doi: 10.1146/annurev.publhealth.22.1.15. [DOI] [PubMed] [Google Scholar]
- 29.Calabrese E.J. Historical blunders: how toxicology got the dose-response relationship half right. Cell. Mol. Biol. 2005;51(7):643–654. [PubMed] [Google Scholar]
- 30.Calabrese E.J. Hormetic dose-response relationships in immunology: occurrence, quantitative features of the dose response, mechanistic foundations, and clinical implications. Crit. Rev. Toxicol. 2005;35(2-3):89–295. doi: 10.1080/10408440590917044. [DOI] [PubMed] [Google Scholar]
- 31.Calabrese E.J. Getting the dose-response wrong: why hormesis became marginalized and the threshold model accepted. Arch. Toxicol. 2009;83(3):227–247. doi: 10.1007/s00204-009-0411-5. [DOI] [PubMed] [Google Scholar]
- 32.Mattson M.P. Awareness of hormesis will enhance future research in basic and applied neuroscience. Crit. Rev. Toxicol. 2008;38(7):633–639. doi: 10.1080/10408440802026406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Calabrese E.J. Dose-response features of neuroprotective agents: An integrative summary. Crit. Rev. Toxicol. 2008;38(4):253–348. doi: 10.1080/10408440801981965. [DOI] [PubMed] [Google Scholar]
- 34.Mattson M.P. Hormesis defined. Ageing Res. Rev. 2008;7(1):1–7. doi: 10.1016/j.arr.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Calabrese E.J., Bachmann K.A., Bailer A.J., Bolger P.M., Borak J., Cai L., Cedergreen N., Cherian M.G., Chiueh C.C., Clarkson T.W., Cook R.R., Diamond D.M., Doolittle D.J., Dorato M.A., Duke S.O., Feinendegen L., Gardner D.E., Hart R.W., Hastings K.L., Hayes A.W., Hoffmann G.R., Ives J.A., Jaworowski Z., Johnson T.E., Jonas W.B., Kaminski N.E., Keller J.G., Klaunig J.E., Knudsen T.B., Kozumbo W.J., Lettieri T., Liu S.Z., Maisseu A., Maynard K.I., Masoro E.J., McClellan R.O., Mehendale H.M., Mothersill C., Newlin D.B., Nigg H.N., Oehme F.W., Phalen R.F., Philbert M.A., Rattan S.I., Riviere J.E., Rodricks J., Sapolsky R.M., Scott B.R., Seymour C., Sinclair D.A., Smith-Sonneborn J., Snow E.T., Spear L., Stevenson D.E., Thomas Y., Tubiana M., Williams G.M., Mattson M.P. Biological stress response terminology: Integrating the concepts of adaptive response and preconditioning stress within a hormetic dose-response framework. Toxicol. Appl. Pharmacol. 2007;222(1):122–128. doi: 10.1016/j.taap.2007.02.015. [DOI] [PubMed] [Google Scholar]
- 36.Hayes D.P. Nutritional hormesis. Eur. J. Clin. Nutr. 2007;61(2):147–159. doi: 10.1038/sj.ejcn.1602507. [DOI] [PubMed] [Google Scholar]
- 37.Martins I., Galluzzi L., Kroemer G. Hormesis, cell death and aging. Aging. 2011;3(9):821–828. doi: 10.18632/aging.100380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.de Grey A.D. Free radicals in aging: causal complexity and its biomedical implications. Free Radic. Res. 2006;40(12):1244–1249. doi: 10.1080/10715760600913176. [DOI] [PubMed] [Google Scholar]
- 39.Ludovico P., Burhans W.C. Reactive oxygen species, ageing and the hormesis police. FEMS Yeast Res. 2014;14(1):33–39. doi: 10.1111/1567-1364.12070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee S.H., Blair I.A. Oxidative DNA damage and cardiovascular disease. Trends Cardiovasc. Med. 2001;11(3-4):148–155. doi: 10.1016/S1050-1738(01)00094-9. [DOI] [PubMed] [Google Scholar]
- 41.Afonso V., Champy R., Mitrovic D., Collin P., Lomri A. Reactive oxygen species and superoxide dismutases: role in joint diseases. Joint Bone Spine. 2007;74(4):324–329. doi: 10.1016/j.jbspin.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 42.McCord J.M. Superoxide dismutase in aging and disease: An overview. Methods in Enzymology. Academic Press; 2002. pp. 331–341. [DOI] [PubMed] [Google Scholar]
- 43.Shan X., Chi L., Ke Y., Luo C., Qian S., Gozal D., Liu R. Manganese superoxide dismutase protects mouse cortical neurons from chronic intermittent hypoxia-mediated oxidative damage. Neurobiol. Dis. 2007;28(2):206–215. doi: 10.1016/j.nbd.2007.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Keller J.N., Kindy M.S., Holtsberg F.W., St Clair D.K., Yen H.C., Germeyer A., Steiner S.M., Bruce-Keller A.J., Hutchins J.B., Mattson M.P. Mitochondrial manganese superoxide dismutase prevents neural apoptosis and reduces ischemic brain injury: suppression of peroxynitrite production, lipid peroxidation, and mitochondrial dysfunction. J. Neurosci. 1998;18(2):687–697. doi: 10.1523/JNEUROSCI.18-02-00687.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dirnagl U., Meisel A. Endogenous neuroprotection: mitochondria as gateways to cerebral preconditioning? Neuropharmacology. 2008;55(3):334–344. doi: 10.1016/j.neuropharm.2008.02.017. [DOI] [PubMed] [Google Scholar]
- 46.Ristow M., Zarse K. How increased oxidative stress promotes longevity and metabolic health: The concept of mitochondrial hormesis (mitohormesis). Exp. Gerontol. 2010;45(6):410–418. doi: 10.1016/j.exger.2010.03.014. [DOI] [PubMed] [Google Scholar]
- 47.Mandel S., Amit T., Reznichenko L., Weinreb O., Youdim M.B. Green tea catechins as brain-permeable, natural iron chelators-antioxidants for the treatment of neurodegenerative disorders. Mol. Nutr. Food Res. 2006;50(2):229–234. doi: 10.1002/mnfr.200500156. [DOI] [PubMed] [Google Scholar]
- 48.Mandel S., Weinreb O., Reznichenko L., Kalfon L., Amit T. Green tea catechins as brain-permeable, non toxic iron chelators to “iron out iron” from the brain. J. Neural Transm. Suppl. 2006;(71):249–257. doi: 10.1007/978-3-211-33328-0_26. [DOI] [PubMed] [Google Scholar]
- 49.Rattan S.I. Hormesis in aging. Ageing Res. Rev. 2008;7(1):63–78. doi: 10.1016/j.arr.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 50.Calabrese E.J. Astrocytes: Adaptive responses to low doses of neurotoxins. Crit. Rev. Toxicol. 2008;38(5):463–471. doi: 10.1080/10408440802004023. [DOI] [PubMed] [Google Scholar]
- 51.Calabrese E.J. Pharmacological enhancement of neuronal survival. Crit. Rev. Toxicol. 2008;38(4):349–389. doi: 10.1080/10408440801981973. [DOI] [PubMed] [Google Scholar]
- 52.Calabrese E.J. Neuroscience and hormesis: overview and general findings. Crit. Rev. Toxicol. 2008;38(4):249–252. doi: 10.1080/10408440801981957. [DOI] [PubMed] [Google Scholar]
- 53.Cook R., Calabrese E.J. The importance of hormesis to public health. Environ. Health Perspect. 2006;114(11):1631–1635. doi: 10.1289/ehp.8606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Calabrese E.J. Converging concepts: Adaptive response, preconditioning, and the Yerkes-Dodson Law are manifestations of hormesis. Ageing Res. Rev. 2008;7(1):8–20. doi: 10.1016/j.arr.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 55.Calabrese V., Renis M., Calderone A., Russo A., Reale S., Barcellona M.L., Rizza V. Stress proteins and SH-groups in oxidant-induced cellular injury after chronic ethanol administration in rat. Free Radic. Biol. Med. 1998;24(7-8):1159–1167. doi: 10.1016/S0891-5849(97)00441-3. [DOI] [PubMed] [Google Scholar]
- 56.Mancuso C., Scapagini G., Currò D. Mitochondrial dysfunction, free radical generation and cellular stress response in neurodegenerative disorders. Front. Biosci. 2007;12:1107–1123. doi: 10.2741/2130. [DOI] [PubMed] [Google Scholar]
- 57.Calabrese V., Guagliano E., Sapienza M., Panebianco M., Calafato S., Puleo E., Pennisi G., Mancuso C., Butterfield D.A., Stella A.G. Redox regulation of cellular stress response in aging and neurodegenerative disorders: role of vitagenes. Neurochem. Res. 2007;32(4-5):757–773. doi: 10.1007/s11064-006-9203-y. [DOI] [PubMed] [Google Scholar]
- 58.Mancuso C., Perluigi M., Cini C., De Marco C., Giuffrida S.A.M., Calabrese V. Heme oxygenase and cyclooxygenase in the central nervous system: A functional interplay. J. Neurosci. Res. 2006;84(7):1385–1391. doi: 10.1002/jnr.21049. [DOI] [PubMed] [Google Scholar]
- 59.Soobrattee M.A., Bahorun T., Aruoma O.I. Chemopreventive actions of polyphenolic compounds in cancer. Biofactors. 2006;27(1-4):19–35. doi: 10.1002/biof.5520270103. [DOI] [PubMed] [Google Scholar]
- 60.Mattson M.P., Cheng A. Neurohormetic phytochemicals: Low-dose toxins that induce adaptive neuronal stress responses. Trends Neurosci. 2006;29(11):632–639. doi: 10.1016/j.tins.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 61.Wu L., Noyan A.M.H., Facci M., Wang R., Paterson P.G., Ferrie A., Juurlink B.H. Dietary approach to attenuate oxidative stress, hypertension, and inflammation in the cardiovascular system. Proc. Natl. Acad. Sci. USA. 2004;101(18):7094–7099. doi: 10.1073/pnas.0402004101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Miquel S., Champ C. Day, J Poor cognitive ageing: Vulnerabilities, mechanisms and the impact of nutritional interventions. Ageing Res. Rev. 2018;42:40–55. doi: 10.1016/j.arr.2017.12.004. [DOI] [PubMed] [Google Scholar]
- 63.Lee J.S., Surh Y.J. Nrf2 as a novel molecular target for chemoprevention. Cancer Lett. 2005;224(2):171–184. doi: 10.1016/j.canlet.2004.09.042. [DOI] [PubMed] [Google Scholar]
- 64.Bautista D.M., Movahed P., Hinman A., Axelsson H.E., Sterner O., Högestätt E.D., Julius D., Jordt S.E., Zygmunt P.M. Pungent products from garlic activate the sensory ion channel TRPA1. Proc. Natl. Acad. Sci. USA. 2005;102(34):12248–12252. doi: 10.1073/pnas.0505356102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Frescas D., Valenti L., Accili D. Nuclear trapping of the forkhead transcription factor FoxO1 via Sirt-dependent deacetylation promotes expression of glucogenetic genes. J. Biol. Chem. 2005;280(21):20589–20595. doi: 10.1074/jbc.M412357200. [DOI] [PubMed] [Google Scholar]
- 66.Siracusa R., Scuto M., Fusco R., Trovato A., Ontario M.L., Crea R., Di Paola R., Cuzzocrea S., Calabrese V. Anti-inflammatory and Anti-oxidant Activity of Hidrox® in Rotenone-Induced Parkinson’s Disease in Mice. Antioxidants. 2020;9(9):824. doi: 10.3390/antiox9090824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Brunetti G., Di Rosa G., Scuto M., Leri M., Stefani M., Schmitz-Linneweber C., Calabrese V., Saul N. Healthspan maintenance and prevention of parkinson’s-like phenotypes with hydroxytyrosol and oleuropein aglycone in C. elegans. Int. J. Mol. Sci. 2020;21(7):E2588. doi: 10.3390/ijms21072588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Calabrese E.J., Mattson M.P., Dhawan G., Kapoor R., Calabrese V., Giordano J., Söderbom G., Esterline R., Oscarsson J., Mattson M.P. Hormesis: A potential strategic approach to the treatment of neurodegenerative disease. Int. Rev. Neurobiol. 2020;155:271–301. doi: 10.1016/bs.irn.2020.03.024. [DOI] [PubMed] [Google Scholar]
- 69.Calabrese E.J., Calabrese V., Giordano J. Demonstrated hormetic mechanisms putatively subserve riluzole-induced effects in neuroprotection against amyotrophic lateral sclerosis (ALS): Implications for research and clinical practice. Ageing Res. Rev. 2021;67:101273. doi: 10.1016/j.arr.2021.101273. [DOI] [PubMed] [Google Scholar]
- 70.Bassani T.B., Turnes J.M., Moura E.L.R., Bonato J.M., Cóppola-Segovia V., Zanata S.M., Oliveira R.M.M.W., Vital M.A.B.F. Effects of curcumin on short-term spatial and recognition memory, adult neurogenesis and neuroinflammation in a streptozotocin-induced rat model of dementia of Alzheimer’s type. Behav. Brain Res. 2017;335:41–54. doi: 10.1016/j.bbr.2017.08.014. [DOI] [PubMed] [Google Scholar]
- 71.Ray B., Lahiri D.K. Neuroinflammation in Alzheimer’s disease: different molecular targets and potential therapeutic agents including curcumin. Curr. Opin. Pharmacol. 2009;9(4):434–444. doi: 10.1016/j.coph.2009.06.012. [DOI] [PubMed] [Google Scholar]
- 72.Xiao L., Ding M., Fernandez A., Zhao P., Jin L., Li X. Curcumin alleviates lumbar radiculopathy by reducing neuroinflammation, oxidative stress and nociceptive factors. Eur. Cell. Mater. 2017;33:279–293. doi: 10.22203/eCM.v033a21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pardridge W.M. Re-engineering therapeutic antibodies for Alzheimer’s disease as blood-brain barrier penetrating bi-specific antibodies. Expert Opin. Biol. Ther. 2016;16(12):1455–1468. doi: 10.1080/14712598.2016.1230195. [DOI] [PubMed] [Google Scholar]
- 74.Yang L., Yin T., Liu Y., Sun J., Zhou Y., Liu J. Gold nanoparticle-capped mesoporous silica-based H2O2-responsive controlled release system for Alzheimer’s disease treatment. Acta Biomater. 2016;46:177–190. doi: 10.1016/j.actbio.2016.09.010. [DOI] [PubMed] [Google Scholar]
- 75.Amiri H., Saeidi K., Borhani P., Manafirad A., Ghavami M., Zerbi V. Alzheimer’s disease: Pathophysiology and applications of magnetic nanoparticles as MRI theranostic agents. ACS Chem. Neurosci. 2013;4(11):1417–1429. doi: 10.1021/cn4001582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Melchels F.P., Feijen J., Grijpma D.W. A review on stereolithography and its applications in biomedical engineering. Biomaterials. 2010;31(24):6121–6130. doi: 10.1016/j.biomaterials.2010.04.050. [DOI] [PubMed] [Google Scholar]
- 77.Ferri C.P., Prince M., Brayne C., Brodaty H., Fratiglioni L., Ganguli M., Hall K., Hasegawa K., Hendrie H., Huang Y., Jorm A., Mathers C., Menezes P.R., Rimmer E., Scazufca M. Global prevalence of dementia: A Delphi consensus study. Lancet. 2005;366(9503):2112–2117. doi: 10.1016/S0140-6736(05)67889-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Qin J., Park J.S., Jo D.G., Cho M., Lee Y. Curcumin-based electrochemical sensor of amyloid-β oligomer for the early detection of Alzheimer’s disease. Sens. Actuators B Chem. 2018;273:1593–1599. doi: 10.1016/j.snb.2018.07.078. [DOI] [Google Scholar]
- 79.Yang C-C., Yang S-Y., Chieh J-J., Horng H.E., Hong C.Y., Yang H.C., Chen K.H., Shih B.Y., Chen T.F., Chiu M.J. Biofunctionalized magnetic nanoparticles for specifically detecting biomarkers of Alzheimer’s disease in vitro. ACS Chem. Neurosci. 2011;2(9):500–505. doi: 10.1021/cn200028j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cheng K.K., Chan P.S., Fan S., Kwan S.M., Yeung K.L., Wáng Y.X., Chow A.H., Wu E.X., Baum L. Curcumin-conjugated magnetic nanoparticles for detecting amyloid plaques in Alzheimer’s disease mice using magnetic resonance imaging (MRI). Biomaterials. 2015;44:155–172. doi: 10.1016/j.biomaterials.2014.12.005. [DOI] [PubMed] [Google Scholar]
- 81.Cheng K.K., Wang Y.X., Chow A.H., Baum L. Amyloid plaques binding curcumin conjugated magnetic nanoparticles for diagnosis in Alzheimer’s disease Tg2576 mice. Alzheimers Dement. 2014;10(4):152–P153. doi: 10.1016/j.jalz.2014.04.122. [DOI] [Google Scholar]
- 82.Sehlin D., Fang X.T., Cato L., Antoni G., Lannfelt L., Syvänen S. Antibody-based PET imaging of amyloid beta in mouse models of Alzheimer’s disease. Nat. Commun. 2016;7:10759. doi: 10.1038/ncomms10759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Moreira P.I., Carvalho C., Zhu X., Smith M.A., Perry G. Mitochondrial dysfunction is a trigger of Alzheimer’s disease pathophysiology. Biochim. Biophys. Acta. 2010;1802(1):2–10. doi: 10.1016/j.bbadis.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 84.Markesbery W.R. Oxidative stress hypothesis in Alzheimer’s disease. Free Radic. Biol. Med. 1997;23(1):134–147. doi: 10.1016/S0891-5849(96)00629-6. [DOI] [PubMed] [Google Scholar]
- 85.Auld D.S., Kornecook T.J., Bastianetto S., Quirion R. Alzheimer’s disease and the basal forebrain cholinergic system: relations to β-amyloid peptides, cognition, and treatment strategies. Prog. Neurobiol. 2002;68(3):209–245. doi: 10.1016/S0301-0082(02)00079-5. [DOI] [PubMed] [Google Scholar]
- 86.Vassar R., Bennett BD. Babu-Khan, S β-Secretase cleavage of Alzheimer’s amyloid precursor protein by the transmembrane aspartic protease BACE. Science. 1999;286(5440):735–741. doi: 10.1126/science.286.5440.735. [DOI] [PubMed] [Google Scholar]
- 87.Kumar S., Walter J. Phosphorylation of amyloid beta (Aβ) peptides - a trigger for formation of toxic aggregates in Alzheimer’s disease. Aging (Albany NY) 2011;3(8):803–812. doi: 10.18632/aging.100362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Butterfield D.A. β-Amyloid-associated free radical oxidative stress and neurotoxicity: implications for Alzheimer’s disease. Chem. Res. Toxicol. 1997;10(5):495–506. doi: 10.1021/tx960130e. [DOI] [PubMed] [Google Scholar]
- 89.Mathew A., Yoshida Y., Maekawa T., Kumar D.S. Alzheimer’s disease: cholesterol a menace? Brain Res. Bull. 2011;86(1-2):1–12. doi: 10.1016/j.brainresbull.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 90.Ghiso J., Frangione B. Amyloidosis and Alzheimer’s disease. Adv. Drug Deliv. Rev. 2002;54(12):1539–1551. doi: 10.1016/S0169-409X(02)00149-7. [DOI] [PubMed] [Google Scholar]
- 91.Heppner F.L.R., Becher B. Immune attack: the role of inflammation in Alzheimer disease. 358r372. Nat. Rev. Neurosci. 2015;16(6) doi: 10.1038/nrn3880. [DOI] [PubMed] [Google Scholar]
- 92.Akiyama H., Barger S., Barnum S., Bradt B., Bauer J., Cole G.M., Cooper N.R., Eikelenboom P., Emmerling M., Fiebich B.L., Finch C.E., Frautschy S., Griffin W.S., Hampel H., Hull M., Landreth G., Lue L., Mrak R., Mackenzie I.R., McGeer P.L., O’Banion M.K., Pachter J., Pasinetti G., Plata-Salaman C., Rogers J., Rydel R., Shen Y., Streit W., Strohmeyer R., Tooyoma I., Van Muiswinkel F.L., Veerhuis R., Walker D., Webster S., Wegrzyniak B., Wenk G., Wyss-Coray T. Inflammation and Alzheimer’s disease. Neurobiol. Aging. 2000;21(3):383–421. doi: 10.1016/S0197-4580(00)00124-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mandrekar-Colucci S., Landreth G.E. Microglia and inflammation in Alzheimer’s disease. CNS Neurol. Disord. Drug Targets. 2010;9(2):156–167. doi: 10.2174/187152710791012071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cao J., Hou J., Ping J., Cai D. Advances in developing novel therapeutic strategies for Alzheimer’s disease. Mol. Neurodegener. 2018;13(1):64. doi: 10.1186/s13024-018-0299-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Velmurugan B.K., Rathinasamy B., Lohanathan B.P., Thiyagarajan V., Weng C.F. Neuroprotective role of phytochemicals. Molecules. 2018;23(10):E2485. doi: 10.3390/molecules23102485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Keshavarz Shahbaz S., Varasteh A-R., Koushki K. Sublingual dendritic cells targeting by aptamer: Possible approach for improvement of sublingual immunotherapy efficacy. Int. Immunopharmacol. 2020;85:106603. doi: 10.1016/j.intimp.2020.106603. [DOI] [PubMed] [Google Scholar]
- 97.Koushki K., Varasteh A-R., Shahbaz S.K. Dc-specific aptamer decorated gold nanoparticles: A new attractive insight into the nanocarriers for allergy epicutaneous immunotherapy. Int. J. Pharm. 2020;584:119403. doi: 10.1016/j.ijpharm.2020.119403. [DOI] [PubMed] [Google Scholar]
- 98.Xiao Z., Ji C., Shi J., Pridgen E.M., Frieder J., Wu J., Farokhzad O.C. DNA self-assembly of targeted near-infrared-responsive gold nanoparticles for cancer thermo-chemotherapy. Angew. Chem. Int. Ed. Engl. 2012;51(47):11853–11857. doi: 10.1002/anie.201204018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wang X., Cheng R., Zhong Z. Facile fabrication of robust, hyaluronic acid-surfaced and disulfide-crosslinked PLGA nanoparticles for tumor-targeted and reduction-triggered release of docetaxel. Acta Biomater. 2021;125:280–289. doi: 10.1016/j.actbio.2021.02.044. [DOI] [PubMed] [Google Scholar]
- 100.Deng W., Kautzka Z., Chen W., Goldys E.M. PLGA nanocomposites loaded with verteporfin and gold nanoparticles for enhanced photodynamic therapy of cancer cells. RSC Advances. 2016;6(113):112393–112402. doi: 10.1039/C6RA21997G. [DOI] [Google Scholar]
- 101.Khan N.H., Mir M., Ngowi E.E., Zafar U., Khakwani M.M.A.K., Khattak S., Zhai Y.K., Jiang E.S., Zheng M., Duan S.F., Wei J.S., Wu D.D., Ji X.Y. Nanomed: a promising way to manage Alzheimer’s Disease. Front. Bioeng. Biotechnol. 2021;9:630055. doi: 10.3389/fbioe.2021.630055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Jia L., Nie X-Q., Ji H-M., Yuan Z-X., Li R-S. Multiple-Coated PLGA nanoparticles loading triptolide attenuate injury of a cellular model of Alzheimer’s Disease. BioMed Res. Int. 2021;2021:8825640. doi: 10.1155/2021/8825640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Abbas M. Potential role of nanoparticles in treating the accumulation of amyloid-beta peptide in Alzheimer’s Patients. Polymers (Basel) 2021;13(7):1051. doi: 10.3390/polym13071051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Del Amo L., Cano A., Ettcheto M. Surface Functionalization of PLGA Nanoparticles to Increase Transport across the BBB for Alzheimer’s Disease. Appl. Sci. (Basel) 2021;11(9):4305. doi: 10.3390/app11094305. [DOI] [Google Scholar]
- 105.Gao C., Chu X., Gong W., Zheng J., Xie X., Wang Y., Yang M., Li Z., Gao C., Yang Y. Neuron tau-targeting biomimetic nanoparticles for curcumin delivery to delay progression of Alzheimer’s disease. J. Nanobiotechnol. 2020;18(1):71. doi: 10.1186/s12951-020-00626-1. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 106.Sadeghi M., Koushki K., Mashayekhi K. DC-targeted gold nanoparticles as an efficient and biocompatible carrier for modulating allergic responses in sublingual immunotherapy. Int. Immunopharmacol. 2020;86:106690. doi: 10.1016/j.intimp.2020.106690. [DOI] [PubMed] [Google Scholar]
- 107.Hasanpour A., Esmaeili F., Hosseini H., Amani A. Use of mPEG-PLGA nanoparticles to improve bioactivity and hemocompatibility of streptokinase: In-vitro and in-vivo studies. Materials Sci. Eng. 2021;118:111427. doi: 10.1016/j.msec.2020.111427. [DOI] [PubMed] [Google Scholar]
- 108.Esfandyari-Manesh M., Abdi M., Talasaz A.H., Ebrahimi S.M., Atyabi F., Dinarvand R. S2P peptide-conjugated PLGA-Maleimide-PEG nanoparticles containing Imatinib for targeting drug delivery to atherosclerotic plaques. DARU. 2020;28(1):131–138. doi: 10.1007/s40199-019-00324-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Patra J.K., Das G., Fraceto L.F., Campos E.V.R., Rodriguez-Torres M.D.P., Acosta-Torres L.S., Diaz-Torres L.A., Grillo R., Swamy M.K., Sharma S., Habtemariam S., Shin H.S. Nano based drug delivery systems: recent developments and future prospects. J. Nanobiotechnol. 2018;16(1):71. doi: 10.1186/s12951-018-0392-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Maiti P., Dunbar G.L. Use of curcumin, a natural polyphenol for targeting molecular pathways in treating age-related neurodegenerative diseases. Int. J. Mol. Sci. 2018;19(6):1637. doi: 10.3390/ijms19061637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Soleimani V., Sahebkar A., Hosseinzadeh H. Turmeric (Curcuma longa) and its major constituent (curcumin) as nontoxic and safe substances. Review Phytother. Res. 2018;32(6):985–995. doi: 10.1002/ptr.6054. [DOI] [PubMed] [Google Scholar]
- 112.Gopinath D., Ahmed M.R., Gomathi K., Chitra K., Sehgal P.K., Jayakumar R. Dermal wound healing processes with curcumin incorporated collagen films. Biomaterials. 2004;25(10):1911–1917. doi: 10.1016/S0142-9612(03)00625-2. [DOI] [PubMed] [Google Scholar]
- 113.Mohanty C., Das M., Sahoo S.K. Sustained wound healing activity of curcumin loaded oleic acid based polymeric bandage in a rat model. Mol. Pharm. 2012;9(10):2801–2811. doi: 10.1021/mp300075u. [DOI] [PubMed] [Google Scholar]
- 114.Tummalapalli M., Berthet M., Verrier B., Deopura B.L., Alam M.S., Gupta B. Composite wound dressings of pectin and gelatin with aloe vera and curcumin as bioactive agents. Int. J. Biol. Macromol. 2016;82:104–113. doi: 10.1016/j.ijbiomac.2015.10.087. [DOI] [PubMed] [Google Scholar]
- 115.Shah B.H., Nawaz Z., Pertani S.A., Roomi A., Mahmood H., Saeed S.A., Gilani A.H. Inhibitory effect of curcumin, a food spice from turmeric, on platelet-activating factor- and arachidonic acid-mediated platelet aggregation through inhibition of thromboxane formation and Ca2+ signaling. Biochem. Pharmacol. 1999;58(7):1167–1172. doi: 10.1016/S0006-2952(99)00206-3. [DOI] [PubMed] [Google Scholar]
- 116.Zorofchian M.S., Abdul K.H., Hassandarvish P., Tajik H., Abubakar S., Zandi K. A review on antibacterial, antiviral, and antifungal activity of curcumin. BioMed Res. Int. 2014;2014:186864. doi: 10.1155/2014/186864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Shanmugam M.K., Rane G., Kanchi M.M., Arfuso F., Chinnathambi A., Zayed M.E., Alharbi S.A., Tan B.K., Kumar A.P., Sethi G. The multifaceted role of curcumin in cancer prevention and treatment. Molecules. 2015;20(2):2728–2769. doi: 10.3390/molecules20022728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ahmadi F., Ghasemi-Kasman M., Ghasemi S., Gholamitabar Tabari M., Pourbagher R., Kazemi S., Alinejad-Mir A. Induction of apoptosis in HeLa cancer cells by an ultrasonic-mediated synthesis of curcumin-loaded chitosan-alginate-STPP nanoparticles. Int. J. Nanomed. 2017;12:8545–8556. doi: 10.2147/IJN.S146516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Moustapha A., Pérétout P.A., Rainey N.E., Sureau F., Geze M., Petit J.M., Dewailly E., Slomianny C., Petit P.X. Curcumin induces crosstalk between autophagy and apoptosis mediated by calcium release from the endoplasmic reticulum, lysosomal destabilization and mitochondrial events. Cell Death Discov. 2015;1(1):15017. doi: 10.1038/cddiscovery.2015.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Teymouri M., Pirro M., Johnston T.P., Sahebkar A. Curcumin as a multifaceted compound against human papilloma virus infection and cervical cancers: A review of chemistry, cellular, molecular, and preclinical features. Biofactors. 2017;43(3):331–346. doi: 10.1002/biof.1344. [DOI] [PubMed] [Google Scholar]
- 121.Mohajeri M., Bianconi V., Ávila-Rodriguez M.F., Barreto G.E., Jamialahmadi T., Pirro M., Sahebkar A. Curcumin: A phytochemical modulator of estrogens and androgens in tumors of the reproductive system. Pharmacol. Res. 2020;156:104765. doi: 10.1016/j.phrs.2020.104765. [DOI] [PubMed] [Google Scholar]
- 122.Mukhopadhyay A., Basu N., Ghatak N., Gujral P.K. Anti-inflammatory and irritant activities of curcumin analogues in rats. Agents Actions. 1982;12(4):508–515. doi: 10.1007/BF01965935. [DOI] [PubMed] [Google Scholar]
- 123.Bianconi V., Sahebkar A., Atkin S.L., Pirro M. The regulation and importance of monocyte chemoattractant protein-1. Curr. Opin. Hematol. 2018;25(1):44–51. doi: 10.1097/MOH.0000000000000389. [DOI] [PubMed] [Google Scholar]
- 124.Ghandadi M., Sahebkar A. Curcumin: An effective inhibitor of interleukin-6. Curr. Pharm. Des. 2017;23(6):921–931. doi: 10.2174/1381612822666161006151605. [DOI] [PubMed] [Google Scholar]
- 125.Mollazadeh H., Cicero A.F.G., Blesso C.N., Pirro M., Majeed M., Sahebkar A. Immune modulation by curcumin: The role of interleukin-10. Crit. Rev. Food Sci. Nutr. 2019;59(1):89–101. doi: 10.1080/10408398.2017.1358139. [DOI] [PubMed] [Google Scholar]
- 126.Menon V.P., Sudheer A.R. Antioxidant and anti-inflammatory properties of curcumin. The molecular targets and therapeutic uses of curcumin in health and disease. Springer; 2007. pp. 105–125. [DOI] [PubMed] [Google Scholar]
- 127.Panahi Y., Khalili N., Sahebi E., Namazi S., Simental-Mendía L.E., Majeed M., Sahebkar A. Effects of curcuminoids plus piperine on glycemic, hepatic and inflammatory biomarkers in patients with type 2 diabetes mellitus: a randomized double-blind placebo-controlled trial. Drug Res. (Stuttg.) 2018;68(7):403–409. doi: 10.1055/s-0044-101752. [DOI] [PubMed] [Google Scholar]
- 128.Panahi Y., Ahmadi Y., Teymouri M., Johnston T.P., Sahebkar A. Curcumin as a potential candidate for treating hyperlipidemia: A review of cellular and metabolic mechanisms. J. Cell. Physiol. 2018;233(1):141–152. doi: 10.1002/jcp.25756. [DOI] [PubMed] [Google Scholar]
- 129.Yang F., Lim G.P., Begum A.N., Ubeda O.J., Simmons M.R., Ambegaokar S.S., Chen P.P., Kayed R., Glabe C.G., Frautschy S.A., Cole G.M. Curcumin inhibits formation of amyloid β oligomers and fibrils, binds plaques, and reduces amyloid in vivo. J. Biol. Chem. 2005;280(7):5892–5901. doi: 10.1074/jbc.M404751200. [DOI] [PubMed] [Google Scholar]
- 130.Aggarwal B.B., Sundaram C., Malani N., Ichikawa H. Curcumin: the Indian solid gold. The molecular targets and therapeutic uses of curcumin in health and disease. Springer; 2007. pp. 1–75. [DOI] [PubMed] [Google Scholar]
- 131.Thiyagarajan M., Sharma S.S. Neuroprotective effect of curcumin in middle cerebral artery occlusion induced focal cerebral ischemia in rats. Life Sci. 2004;74(8):969–985. doi: 10.1016/j.lfs.2003.06.042. [DOI] [PubMed] [Google Scholar]
- 132.Shen L., Ji H-F. The pharmacology of curcumin: is it the degradation products? Trends Mol. Med. 2012;18(3):138–144. doi: 10.1016/j.molmed.2012.01.004. [DOI] [PubMed] [Google Scholar]
- 133.Karlstetter M., Lippe E., Walczak Y., Moehle C., Aslanidis A., Mirza M., Langmann T. Curcumin is a potent modulator of microglial gene expression and migration. J. Neuroinflam. 2011;8(1):125. doi: 10.1186/1742-2094-8-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Tizabi Y., Hurley L.L., Qualls Z., Akinfiresoye L. Relevance of the anti-inflammatory properties of curcumin in neurodegenerative diseases and depression. Molecules. 2014;19(12):20864–20879. doi: 10.3390/molecules191220864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Chen J., Tang XQ, Zhi JL. Curcumin protects PC12 cells against 1-methyl-4-phenylpyridinium ion-induced apoptosis by bcl-2-mitochondria-ROS-iNOS pathway. Apoptosis. 2006;11(6):943–953. doi: 10.1007/s10495-006-6715-5. [DOI] [PubMed] [Google Scholar]
- 136.Divya C.S., Pillai M.R. Antitumor action of curcumin in human papillomavirus associated cells involves downregulation of viral oncogenes, prevention of NFkB and AP-1 translocation, and modulation of apoptosis. Mol. Carcinog. 2006;45(5):320–332. doi: 10.1002/mc.20170. [DOI] [PubMed] [Google Scholar]
- 137.Pérez-Arriaga L., Mendoza-Magaña M.L., Cortés-Zárate R., Corona-Rivera A., Bobadilla-Morales L., Troyo-Sanromán R., Ramírez-Herrera M.A. Cytotoxic effect of curcumin on Giardia lamblia trophozoites. Acta Trop. 2006;98(2):152–161. doi: 10.1016/j.actatropica.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 138.Ramsewak R.S., DeWitt D.L., Nair M.G. Cytotoxicity, antioxidant and anti-inflammatory activities of curcumins I-III from Curcuma longa. Phytomedicine. 2000;7(4):303–308. doi: 10.1016/S0944-7113(00)80048-3. [DOI] [PubMed] [Google Scholar]
- 139.Goel A., Kunnumakkara A.B., Aggarwal B.B. Curcumin as “Curecumin”: from kitchen to clinic. Biochem. Pharmacol. 2008;75(4):787–809. doi: 10.1016/j.bcp.2007.08.016. [DOI] [PubMed] [Google Scholar]
- 140.Calabrese V., Cornelius C., Trovato A., Cavallaro M., Mancuso C., Di Rienzo L., Condorelli D., De Lorenzo A., Calabrese E.J. The hormetic role of dietary antioxidants in free radical-related diseases. Curr. Pharm. Des. 2010;16(7):877–883. doi: 10.2174/138161210790883615. [DOI] [PubMed] [Google Scholar]
- 141.Moghaddam N.S.A., Oskouie M.N., Butler A.E., Petit P.X., Barreto G.E., Sahebkar A. Hormetic effects of curcumin: What is the evidence? J. Cell. Physiol. 2019;234(7):10060–10071. doi: 10.1002/jcp.27880. [DOI] [PubMed] [Google Scholar]
- 142.Yang K-Y., Lin L-C., Tseng T-Y., Wang S-C., Tsai T-H. Oral bioavailability of curcumin in rat and the herbal analysis from Curcuma longa by LC-MS/MS. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2007;853(1-2):183–189. doi: 10.1016/j.jchromb.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 143.Anand P., Kunnumakkara A.B., Newman R.A., Aggarwal B.B. Bioavailability of curcumin: Problems and promises. Mol. Pharm. 2007;4(6):807–818. doi: 10.1021/mp700113r. [DOI] [PubMed] [Google Scholar]
- 144.Lopresti A.L. The problem of curcumin and its bioavailability: could its gastrointestinal influence contribute to its overall health-enhancing effects? Adv. Nutr. 2018;9(1):41–50. doi: 10.1093/advances/nmx011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kurien B.T., Singh A., Matsumoto H., Scofield R.H. Improving the solubility and pharmacological efficacy of curcumin by heat treatment. Assay Drug Dev. Technol. 2007;5(4):567–576. doi: 10.1089/adt.2007.064. [DOI] [PubMed] [Google Scholar]
- 146.Tønnesen H.H., Másson M., Loftsson T. Studies of curcumin and curcuminoids. XXVII. Cyclodextrin complexation: solubility, chemical and photochemical stability. Int. J. Pharm. 2002;244(1-2):127–135. doi: 10.1016/S0378-5173(02)00323-X. [DOI] [PubMed] [Google Scholar]
- 147.Wang Y-J., Pan M-H., Cheng A-L., Lin L.I., Ho Y.S., Hsieh C.Y., Lin J.K. Stability of curcumin in buffer solutions and characterization of its degradation products. J. Pharm. Biomed. Anal. 1997;15(12):1867–1876. doi: 10.1016/S0731-7085(96)02024-9. [DOI] [PubMed] [Google Scholar]
- 148.Aggarwal B.B., Surh Y-J., Shishodia S. The molecular targets and therapeutic uses of curcumin in health and disease. Vol. 595. Springer Science & Business Media; 2007. [DOI] [Google Scholar]
- 149.Souza C.R., Osme S.F., Glória M.B.A. stability of curcuminoib pigments in model systems. J. Food Process. Preserv. 1997;21(5):353–363. doi: 10.1111/j.1745-4549.1997.tb00789.x. [DOI] [Google Scholar]
- 150.Del Prado-Audelo M.L., Caballero-Florán I.H., Meza-Toledo J.A., Mendoza-Muñoz N., González-Torres M., Florán B., Cortés H., Leyva-Gómez G. Formulations of curcumin nanoparticles for brain diseases. Biomolecules. 2019;9(2):56. doi: 10.3390/biom9020056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Mahran R.I., Hagras M.M., Sun D., Brenner D.E. Bringing curcumin to the clinic in cancer prevention: A review of strategies to enhance bioavailability and efficacy. AAPS J. 2017;19(1):54–81. doi: 10.1208/s12248-016-0003-2. [DOI] [PubMed] [Google Scholar]
- 152.Naksuriya O., Okonogi S., Schiffelers R.M., Hennink W.E. Curcumin nanoformulations: A review of pharmaceutical properties and preclinical studies and clinical data related to cancer treatment. Biomaterials. 2014;35(10):3365–3383. doi: 10.1016/j.biomaterials.2013.12.090. [DOI] [PubMed] [Google Scholar]
- 153.Yallapu M.M., Nagesh P.K.B., Jaggi M., Chauhan S.C. Therapeutic applications of curcumin nanoformulations. AAPS J. 2015;17(6):1341–1356. doi: 10.1208/s12248-015-9811-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Mouhieddine T.H., Itani M.M., Nokkari A., Ren C., Daoud G., Zeidan A., Mondello S., Kobeissy F.H. Nanotheragnostic applications for ischemic and hemorrhagic strokes: improved delivery for a better prognosis. Curr. Neurol. Neurosci. Rep. 2015;15(1):505. doi: 10.1007/s11910-014-0505-1. [DOI] [PubMed] [Google Scholar]
- 155.Ghalandarlaki N, Alizadeh AM, Ashkani-Esfahani S. Nanotechnology-applied curcumin for different diseases therapy.BioMed. Res. Intl.2014. 2014. [DOI] [PMC free article] [PubMed]
- 156.Yallapu M.M., Jaggi M., Chauhan S.C. Curcumin nano formulations: A future Nanomed for cancer. Drug Discov. Today. 2012;17(1-2):71–80. doi: 10.1016/j.drudis.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Sun M., Su X., Ding B., He X., Liu X., Yu A., Lou H., Zhai G. Advances in nanotechnology-based delivery systems for curcumin. Nanomedicine (Lond.) 2012;7(7):1085–1100. doi: 10.2217/nnm.12.80. [DOI] [PubMed] [Google Scholar]
- 158.Szymusiak M., Hu X., Leon Plata P.A., Ciupinski P., Wang Z.J., Liu Y. Bioavailability of curcumin and curcumin glucuronide in the central nervous system of mice after oral delivery of nano-curcumin. Int. J. Pharm. 2016;511(1):415–423. doi: 10.1016/j.ijpharm.2016.07.027. [DOI] [PubMed] [Google Scholar]
- 159.Kreuter J. Nanoparticles--a historical perspective. Int. J. Pharm. 2007;331(1):1–10. doi: 10.1016/j.ijpharm.2006.10.021. [DOI] [PubMed] [Google Scholar]
- 160.Leson A. There is plenty of room at the Bottom. Vakuum in Forschung und Praxis. 2005;17(3):123–123. doi: 10.1002/vipr.200590035. [DOI] [Google Scholar]
- 161.Khanna S.C., Soliva M., Speiser P. Epoxy resin beads as a pharmaceutical dosage form. II. Dissolution studies of epoxy-amine beads and release of drug. J. Pharm. Sci. 1969;58(11):1385–1388. doi: 10.1002/jps.2600581120. [DOI] [PubMed] [Google Scholar]
- 162.Müller R.H., Maassen S., Weyhers H., Mehnert W. Phagocytic uptake and cytotoxicity of solid lipid nanoparticles (SLN) sterically stabilized with poloxamine 908 and poloxamer 407. J. Drug Target. 1996;4(3):161–170. doi: 10.3109/10611869609015973. [DOI] [PubMed] [Google Scholar]
- 163.Xu Y., Kim C.S., Saylor D.M., Koo D. Polymer degradation and drug delivery in PLGA-based drug-polymer applications: A review of experiments and theories. J. Biomed. Mater. Res. B Appl. Biomater. 2017;105(6):1692–1716. doi: 10.1002/jbm.b.33648. [DOI] [PubMed] [Google Scholar]
- 164.Chen Y., Lin X., Park H., Greever R. Study of artemisinin nanocapsules as anticancer drug delivery systems. Nanomedicine, 2009;5(3):316–322. doi: 10.1016/j.nano.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 165.Nguyen H.T., Tran T.H., Kim J.O., Yong C.S., Nguyen C.N. Enhancing the in vitro anti-cancer efficacy of artesunate by loading into poly-D,L-lactide-co-glycolide (PLGA) nanoparticles. Arch. Pharm. Res. 2015;38(5):716–724. doi: 10.1007/s12272-014-0424-3. [DOI] [PubMed] [Google Scholar]
- 166.Keshavarz S.S., Foroughi F., Soltaninezhad E., Jamialahmadi T., Penson P.E., Sahebkar A. Application of PLGA nano/microparticle delivery systems for immunomodulation and prevention of allotransplant rejection. Expert Opin. Drug Deliv. 2020;17(6):767–780. doi: 10.1080/17425247.2020.1748006. [DOI] [PubMed] [Google Scholar]
- 167.Yallapu M.M., Gupta B.K., Jaggi M., Chauhan S.C. Fabrication of curcumin encapsulated PLGA nanoparticles for improved therapeutic effects in metastatic cancer cells. J. Colloid Interface Sci. 2010;351(1):19–29. doi: 10.1016/j.jcis.2010.05.022. [DOI] [PubMed] [Google Scholar]
- 168.Luz P.P., Magalhães L.G., Pereira A.C., Cunha W.R., Rodrigues V., Andrade E., Silva M.L. Curcumin-loaded into PLGA nanoparticles: Preparation and in vitro schistosomicidal activity. Parasitol. Res. 2012;110(2):593–598. doi: 10.1007/s00436-011-2527-9. [DOI] [PubMed] [Google Scholar]
- 169.Danhier F., Ansorena E., Silva J.M., Coco R., Le Breton A., Préat V. PLGA-based nanoparticles: An overview of biomedical applications. J. Control. Release. 2012;161(2):505–522. doi: 10.1016/j.jconrel.2012.01.043. [DOI] [PubMed] [Google Scholar]
- 170.Arshad A., Yang B., Bienemann A.S., Barua N.U., Wyatt M.J., Woolley M., Johnson D.E., Edler K.J., Gill S.S. Convection-enhanced delivery of carboplatin PLGA nanoparticles for the treatment of glioblastoma. PLoS One. 2015;10(7):e0132266. doi: 10.1371/journal.pone.0132266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Mourtas S., Lazar A.N., Markoutsa E., Duyckaerts C., Antimisiaris S.G. Multifunctional nanoliposomes with curcumin-lipid derivative and brain targeting functionality with potential applications for Alzheimer disease. Eur. J. Med. Chem. 2014;80:175–183. doi: 10.1016/j.ejmech.2014.04.050. [DOI] [PubMed] [Google Scholar]
- 172.Ringman J.M., Frautschy S.A., Cole G.M., Masterman D.L., Cummings J.L. A potential role of the curry spice curcumin in Alzheimer’s disease. Curr. Alzheimer Res. 2005;2(2):131–136. doi: 10.2174/1567205053585882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Garcia-Alloza M., Borrelli L.A., Rozkalne A., Hyman B.T., Bacskai B.J. Curcumin labels amyloid pathology in vivo, disrupts existing plaques, and partially restores distorted neurites in an Alzheimer mouse model. J. Neurochem. 2007;102(4):1095–1104. doi: 10.1111/j.1471-4159.2007.04613.x. [DOI] [PubMed] [Google Scholar]
- 174.Mishra S., Palanivelu K. The effect of curcumin (turmeric) on Alzheimer’s disease: An overview. Ann. Indian Acad. Neurol. 2008;11(1):13–19. doi: 10.4103/0972-2327.40220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Park S-Y., Kim H-S., Cho E-K., Kwon B.Y., Phark S., Hwang K.W., Sul D. Curcumin protected PC12 cells against beta-amyloid-induced toxicity through the inhibition of oxidative damage and tau hyperphosphorylation. Food Chem. Toxicol. 2008;46(8):2881–2887. doi: 10.1016/j.fct.2008.05.030. [DOI] [PubMed] [Google Scholar]
- 176.Bustanji Y., Taha M.O., Almasri I.M., Al-Ghussein M.A., Mohammad M.K., Alkhatib H.S. Inhibition of glycogen synthase kinase by curcumin: Investigation by simulated molecular docking and subsequent in vitro/in vivo evaluation. J. Enzyme Inhib. Med. Chem. 2009;24(3):771–778. doi: 10.1080/14756360802364377. [DOI] [PubMed] [Google Scholar]
- 177.Wang H-M., Zhao Y-X., Zhang S., Liu G.D., Kang W.Y., Tang H.D., Ding J.Q., Chen S.D. PPAR gamma agonist curcumin reduces the amyloid-β-stimulated inflammatory responses in primary astrocytes. J. Alzheimers Dis. 2010;20(4):1189–1199. doi: 10.3233/JAD-2010-091336. [DOI] [PubMed] [Google Scholar]
- 178.Aggarwal BB, Kumar A, Bharti AC. Anticancer potential of curcumin: Preclinical and clinical studies. Anticancer Res. 2003;23(1/A):363–398. [PubMed] [Google Scholar]
- 179.Balasubramanian K. Molecular orbital basis for yellow curry spice curcumin’s prevention of Alzheimer’s disease. J. Agric. Food Chem. 2006;54(10):3512–3520. doi: 10.1021/jf0603533. [DOI] [PubMed] [Google Scholar]
- 180.Sharma S., Ying Z., Gomez-Pinilla F. A pyrazole curcumin derivative restores membrane homeostasis disrupted after brain trauma. Exp. Neurol. 2010;226(1):191–199. doi: 10.1016/j.expneurol.2010.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Tsai Y-M., Chien C-F., Lin L-C., Tsai T-H. Curcumin and its nano-formulation: the kinetics of tissue distribution and blood-brain barrier penetration. Int. J. Pharm. 2011;416(1):331–338. doi: 10.1016/j.ijpharm.2011.06.030. [DOI] [PubMed] [Google Scholar]
- 182.Mathew A., Aravind A., Fukuda T. Curcumin nanoparticles-a gateway for multifaceted approach to tackle Alzheimer’s disease. IEEE; 2011. pp. 833–836. [Google Scholar]
- 183.Chiu S.S., Lui E., Majeed M., Vishwanatha J.K., Ranjan A.P., Maitra A., Pramanik D., Smith J.A., Helson L. Differential distribution of intravenous curcumin formulations in the rat brain. Anticancer Res. 2011;31(3):907–911. [PMC free article] [PubMed] [Google Scholar]
- 184.Mathew A., Aravind A., Brahatheeswaran D. Amyloid-binding aptamer conjugated curcumin–PLGA nanoparticle for potential use in Alzheimer’s disease. Bionanoscience. 2012;2(2):83–93. doi: 10.1007/s12668-012-0040-y. [DOI] [Google Scholar]
- 185.Mathew A., Fukuda T., Nagaoka Y., Hasumura T., Morimoto H., Yoshida Y., Maekawa T., Venugopal K., Kumar D.S. Curcumin loaded-PLGA nanoparticles conjugated with Tet-1 peptide for potential use in Alzheimer’s disease. PLoS One. 2012;7(3):e32616. doi: 10.1371/journal.pone.0032616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Marrache S., Dhar S. Engineering of blended nanoparticle platform for delivery of mitochondria-acting therapeutics. Proc. Natl. Acad. Sci. USA. 2012;109(40):16288–16293. doi: 10.1073/pnas.1210096109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Doggui S., Sahni J.K., Arseneault M., Dao L., Ramassamy C. Neuronal uptake and neuroprotective effect of curcumin-loaded PLGA nanoparticles on the human SK-N-SH cell line. J. Alzheimers Dis. 2012;30(2):377–392. doi: 10.3233/JAD-2012-112141. [DOI] [PubMed] [Google Scholar]
- 188.Tiwari S.K., Agarwal S., Seth B., Yadav A., Nair S., Bhatnagar P., Karmakar M., Kumari M., Chauhan L.K., Patel D.K., Srivastava V., Singh D., Gupta S.K., Tripathi A., Chaturvedi R.K., Gupta K.C. Curcumin-loaded nanoparticles potently induce adult neurogenesis and reverse cognitive deficits in Alzheimer’s disease model via canonical Wnt/β-catenin pathway. ACS Nano. 2014;8(1):76–103. doi: 10.1021/nn405077y. [DOI] [PubMed] [Google Scholar]
- 189.Srivastava A., Arya P., Goel S., Kundu B., Mishra P., Fnu A. Gelsolin amyloidogenesis is effectively modulated by curcumin and emetine conjugated PLGA nanoparticles. PLoS One. 2015;10(5):e0127011. doi: 10.1371/journal.pone.0127011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Paka G.D., Ramassamy C. Optimization of curcumin-loaded PEG-PLGA nanoparticles by GSH functionalization: investigation of the internalization pathway in neuronal cells. Mol. Pharm. 2017;14(1):93–106. doi: 10.1021/acs.molpharmaceut.6b00738. [DOI] [PubMed] [Google Scholar]
- 191.Huang N., Lu S., Liu X-G., Zhu J., Wang Y-J., Liu R-T. PLGA nanoparticles modified with a BBB-penetrating peptide co-delivering Aβ generation inhibitor and curcumin attenuate memory deficits and neuropathology in Alzheimer’s disease mice. Oncotarget. 2017;8(46):81001–81013. doi: 10.18632/oncotarget.20944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Barbara R., Belletti D., Pederzoli F., Masoni M., Keller J., Ballestrazzi A., Vandelli M.A., Tosi G., Grabrucker A.M. Novel Curcumin loaded nanoparticles engineered for Blood-Brain Barrier crossing and able to disrupt Abeta aggregates. Int. J. Pharm. 2017;526(1-2):413–424. doi: 10.1016/j.ijpharm.2017.05.015. [DOI] [PubMed] [Google Scholar]
- 193.Ameruoso A., Palomba R., Palange A.L., Cervadoro A., Lee A., Di Mascolo D., Decuzzi P. Ameliorating amyloid-β fibrils triggered inflammation via curcumin-loaded polymeric nanoconstructs. Front. Immunol. 2017;8:1411. doi: 10.3389/fimmu.2017.01411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Huo X., Zhang Y., Jin X., Li Y., Zhang L. A novel synthesis of selenium nanoparticles encapsulated PLGA nanospheres with curcumin molecules for the inhibition of amyloid β aggregation in Alzheimer’s disease. J. Photochem. Photobiol. B. 2019;190:98–102. doi: 10.1016/j.jphotobiol.2018.11.008. [DOI] [PubMed] [Google Scholar]
- 195.Sutcliffe J.G., Hedlund P.B., Thomas E.A., Bloom F.E., Hilbush B.S. Peripheral reduction of β-amyloid is sufficient to reduce brain β-amyloid: implications for Alzheimer’s disease. J. Neurosci. Res. 2011;89(6):808–814. doi: 10.1002/jnr.22603. [DOI] [PubMed] [Google Scholar]
- 196.Matsuoka Y., Saito M., LaFrancois J., Saito M., Gaynor K., Olm V., Wang L., Casey E., Lu Y., Shiratori C., Lemere C., Duff K. Novel therapeutic approach for the treatment of Alzheimer’s disease by peripheral administration of agents with an affinity to β-amyloid. J. Neurosci. 2003;23(1):29–33. doi: 10.1523/JNEUROSCI.23-01-00029.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Takahashi T., Tada K., Mihara H. RNA aptamers selected against amyloid β-peptide (Abeta) inhibit the aggregation of Abeta. Mol. Biosyst. 2009;5(9):986–991. doi: 10.1039/b903391b. [DOI] [PubMed] [Google Scholar]
- 198.Fan S., Zheng Y., Liu X., Fang W., Chen X., Liao W., Jing X., Lei M., Tao E., Ma Q., Zhang X., Guo R., Liu J. Curcumin-loaded PLGA-PEG nanoparticles conjugated with B6 peptide for potential use in Alzheimer’s disease. Drug Deliv. 2018;25(1):1091–1102. doi: 10.1080/10717544.2018.1461955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Kuo Y-C., Tsai H-C. Rosmarinic acid- and curcumin-loaded polyacrylamide-cardiolipin-poly(lactide-co-glycolide) nanoparticles with conjugated 83-14 monoclonal antibody to protect β-amyloid-insulted neurons. Mater. Sci. Eng. C. 2018;91:445–457. doi: 10.1016/j.msec.2018.05.062. [DOI] [PubMed] [Google Scholar]