Abstract
Parkinson’s disease (PD) is the second most common neurodegenerative disease and is characterized by a significant decrease in dopamine levels, caused by progressive degeneration of the dopaminergic neurons in the nigrostriatal pathway. Multiple mechanisms have been implicated in its pathogenesis, including oxidative stress, neuroinflammation, protein aggregation, mitochondrial dysfunction, insufficient support for neurotrophic factors and cell apoptosis. The absence of treatments capable of slowing or stopping the progression of PD has increased the interest in the natural antioxidant substances present in the diet, since they have multiple beneficial properties and it is possible that they can influence the mechanisms responsible for the dysfunction and death of dopaminergic neurons. Thus, the purpose of this systematic review is to analyze the results obtained in a set of studies carried out in the last years, which describe the neuroprotective, antioxidant and regenerative functions of some naturally occurring antioxidants in experimental models of PD. The results show that the exogenous no enzymatic antioxidants can significantly modify the biochemical and behavioral mechanisms that contribute to the pathophysiology of Parkinsonism in experimental animals. Therefore, it is possible that they may contribute to effective neuroprotection by providing a significant improvement in neuropathological markers. In conclusion, the results of this review suggest that exogenous antioxidants can be promising therapeutic candidates for the prevention and treatment of PD.
Keywords: Naturally occurring antioxidants, Parkinson’s disease, experimental models of parkinsonism, vitamins, polyphenols, flavonoids
1. INTRODUCTION
Parkinson’s disease (PD) is the second most common neurodegenerative disease worldwide, after Alzheimer’s disease, and affects 1-2% of the population over 60 years [1]. The overall incidence of PD is between 16-19 per 100.000 person-years in the US and European populations [2], which makes this pathological condition a major health problem and a financial burden for healthcare systems. In addition, it is considered that it will experience a notable increase in the coming decades due to the increase in life expectancy [3, 4].
PD is a progressive neurodegenerative disorder, whose motor symptoms include tremor at rest, rigidity, slowness (bradykinesia) or absence of voluntary movement and postural instability [3, 5-7]. The essential neuropathological characteristic of PD, responsible for the emergence of its distinctive symptomatology, is the decrease in dopamine levels in the nigrostriatal pathway. This decrease occurs because of the progressive loss of dopaminergic neurons of the Substantia Nigra Pars Compacta (SNpc) and the degeneration of its axons that project towards the striatum [8]. Moreover, the presence of aggregates of α-synuclein misfolded by the ubiquitin-proteasome system, which form intraneuronal cytoplasmic inclusions called “Lewy bodies”, has also been widely documented. These protein aggregates appear to be fundamental in the death of dopaminergic neurons and in the mitochondrial dysfunction that causes oxidative stress [7, 9-12]. A deficiency in antioxidant defenses, altered iron metabolism, neuroinflammation and apoptosis are also key factors, at the cellular and molecular level, responsible for the development and progression of the disease [6, 11].
Currently, pharmacological treatments for PD are mainly symptomatic and aim to compensate for the loss of dopamine. The standard therapy used to achieve increases in the levels and bioavailability of this neurotransmitter is levodopa (L-DOPA), its direct precursor. Other pharmacological strategies involve the administration of dopamine receptor agonists, treatment with Monoamine Oxidase type B (MAO-B) inhibitors or anticholinergic drugs [5, 8, 13]. In recent years, other therapeutic approaches have also been developed, such as deep brain stimulation of the subthalamic nucleus and globus pallidus through the surgical implantation of electrodes, or the transplantation of cells in the striatum [14].
Nevertheless, to date, there is no effective treatment to reverse the neuronal damage caused by the disease, although some drug can substantially improve the quality of life of patients, but only for a limited period. Therefore, although pharmacological treatments are the only ones proven effective for PD patients, no drug has been able to reduce the progression of the disease.
In addition, frequently the drugs used can produce side effects of varying severity [12, 15]. For that reason, other treatment alternatives have been investigated, including the use of natural products rich in antioxidants. The neuroprotective properties of these substances have been widely documented in a variety of neurological disorders [16], and are a promising approach to the prevention and treatment of neurodegenerative diseases such as PD.
2. THE ANTIOXIDANTS SYSTEMS AND OXIDATIVE STRESS
Oxidative stress is produced by the accumulation of free radicals, such as reactive oxygen species (ROS), either due to excessive production or because their neutralization is insufficient due to a decrease in the effectiveness of antioxidant defense systems [17]. In general, free radicals are toxic because they contain more than one unpaired electron, which makes them unstable and highly reactive, causing damage mainly to lipids, proteins and DNA [16].
ROS like superoxide anion, hydrogen peroxide and hydroxyl radical are produced mainly in the ATP synthesis process, in the mitochondria. ROS production can occur as a consequence of the accumulation of mutations in mitochondrial DNA during the aging process, which cause a deterioration in the efficiency of the electron transport chain [18, 19]. However, factors such as inflammation, phagocytosis, iron accumulation, dopamine metabolism, peroxisome functioning, genetic mutations or exposure to endogenous and exogenous toxins, are some examples of processes that can lead to oxidative stress and that possibly contribute to the development of neurodegenerative diseases such as PD [18, 20].
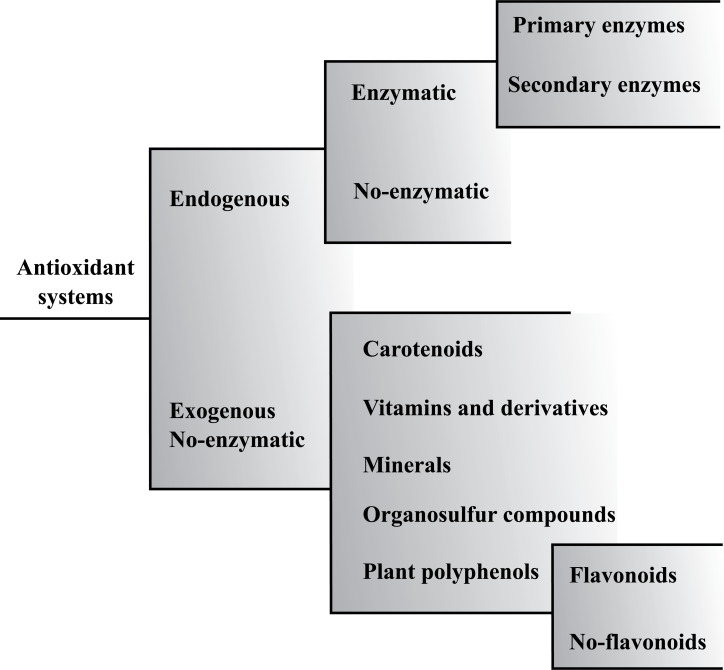
Under physiological conditions, ROS are eliminated by antioxidant defense systems, which have the ability to delay, prevent or eliminate the harmful effects of free radicals and oxidants in the body, protecting it from oxidative damage. Fig. (1) shows a general classification of the different antioxidant substances that can be divided into endogenous or exogenous, depending on whether they come from inside or outside the body [16, 21].
Fig. (1).
General classification of the antioxidant systems that can be divided into endogenous or exogenous.
The endogenous enzymatic antioxidants systems include primary enzymes, such as superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GSH-Px), implicated in the direct ROS elimination. On the other hand, secondary enzymes, such as glutathione reductase (GRx), glutathione-6-phosphate dehydrogenase and glutathione-S-transferase cytosolic, contribute to ROS elimination by decreasing the superoxide anions levels or maintaining a constant supply of metabolic intermediaries necessary for the optimal functioning of primary enzymes. On the other hand, endogenous non-enzymatic antioxidants include vitamins (vitamin A), enzymatic cofactors (Q10), nitrogen compounds (uric acid) and organosulfur compounds (glutathione), which can decrease oxidative stress by disrupting free radical chain reactions [20-23].
Central nervous system (CNS) is highly vulnerable to oxidative damage, which may be due to its poor endogenous antioxidant defenses, which is evidenced by a reduced CAT activity and a lower amount of SOD. Likewise, the susceptibility to oxidative damage is also related to other factors, such as the high oxygen requirement, the high lipid composition or the large iron deposits characteristic of the brain [24, 25]. In particular, it seems that high levels of pro-oxidant iron and low levels of glutathione are responsible for the high vulnerability of dopaminergic neurons of the SNpc to oxidative stress [19].
In this way, despite the marked efficacy of endogenous antioxidant systems, in certain situations they are insufficient to keep free radical concentrations at low levels [21]. Therefore, to achieve this purpose it is essential to obtain antioxidants from the diet. These exogenous non-enzymatic antioxidants, work as a defense barrier against free radicals and oxidants, preventing cell damage induced by them through multiple mechanisms of action [26].
A large number of studies published in recent years show the relevance of antioxidant substances to avoid the formation and prevent the damage that free radicals can produce in cellular components of the CNS and in other systems and organs. Furthermore, in certain situations of stress, the endogenous antioxidant defenses in the CNS are insufficient to prevent oxidative damage efficiently. For this reason, efforts have been increased to study the biochemical mechanisms of exogenous antioxidants and to promote their use in the prevention and reduction of damage caused by oxidative stress [24].
3. EXPERIMENTAL MODELS OF PARKINSONISM
The characteristic heterogeneity of PD has driven the development of a wide variety of experimental models with the aim of investigating the neuropathological mechanisms responsible for the development and progression of the disease and for the development of new drugs for its treatment. Nowadays, there are two main approaches to modeling PD in experimental animals: the systemic or local administration of neurotoxic substances and the transgenic models in which it is intended to replicate the genetic mutations identified in the familial forms of PD [27].
The first neurotoxic substance to be used for the generation of experimental models of PD was 6-hydroxydopamine (6-OHDA). 6-OHDA has a structure similar to dopamine (and noradrenaline), but the presence of an additional hydroxyl group gives this substance a selective toxicity for dopaminergic neurons [27]. However, this molecule is not capable of crossing the blood-brain barrier, so it must be injected directly into de SNpc, the striatum or the bundle of the medial forebrain [28]. This procedure generates models of unilateral Parkinsonism, since bilateral lesions are associated with high mortality rates [12].
After administration, 6-OHDA penetrates into dopaminergic and noradrenergic neurons using the monoamine transporters and accumulates in the cytosol, where it undergoes rapid auto-oxidation that promotes the formation of ROS. In addition, it has also been postulated that 6-OHDA can alter mitochondrial function, since its accumulation in these organelles could inhibit the activity of mitochondrial complex I [29]. Consequently, 6-OHDA induces the selective degeneration of dopaminergic neurons and the depletion of striatal dopamine, as well as the appearance of motor deficits [28]. Nevertheless, it does not lead to the formation of Lewy bodies, the pathological hallmark of PD, which is a limitation of this model [30].
Another of the most commonly used models is that induced by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP), which can be administrated acutely or chronically by different routes [12]. MPTP is mainly used to induce Parkinsonism in mice and primates, but it is unknown why it does not have the same toxic effect in rats [27]. Due to its lipophilic nature, MPTP easily crosses the blood-brain barrier and enters glial cells, where it is converted to 1-methyl-4-phenylpyridinium (MPP+), its active metabolite, in a process catalyzed by the enzyme monoamine oxidase type B (MAO-B). Then, MPP+ enters dopaminergic neurons in the SNpc through the Dopamine Transporter (DAT) and blocks the activity of mitochondrial complex I, resulting in oxidative stress, inflammation and, finally, cell death [12, 29]. This model shows some of the distinctive features of PD, such as degeneration of nigrostriatal dopaminergic neurons, depletion of dopamine, massive ROS generation, inflammation and motor deficits. However, an important limitation is that acute administration of MPTP does not induce Lewy body formation [12, 27].
In recent years, the use of rotenone and paraquat to generate PD models has been emphasized, based on the observation that chronic exposure to pesticides was associated with an increased risk of development of PD in humans [29]. Rotenone crosses the blood-brain barrier easily and can enter dopaminergic neurons, where it induces massive production of ROS by blocking the activity of the mitochondrial complex I [27, 29]. Consequently, it induces the formation of Lewy bodies, degeneration of dopaminergic neurons and the manifestation of motor deficits [12]. Nonetheless, this model has several limitations, among them: the considerable variability in the individual response, its high toxicity for different organs, and its lack of specificity in the production of lesions, since it can cause the degeneration of other neuronal populations different from the dopaminergic [29].
On the other hand, paraquat is an herbicide that shares structural similarities with MPP+, although its mechanisms of action differ considerably. Thus, since paraquat is a charged molecule, it cannot cross the blood-brain barrier freely, so it uses a neutral amino acid transporter. Once in the nervous tissue, it is introduced into the dopaminergic neurons through a transport mechanism independent of the DAT, and generates massive oxidative stress mediated by the redox cycle [31]. However, although the chronic administration of the herbicide in mice can induce some loss of nigrostriatal dopaminergic neurons, it does not produce dopamine depletion or a clear motor phenotype [12].
More recently, a new model has emerged focusing on the role of aggregated and misfolded forms of α-synuclein in the pathogenesis of PD [32]. This model involves the conversion of the recombinant monomeric α-synuclein protein into a fibrillary form, which has a structure similar to the basic components of Lewy bodies [33, 34]. These preformed α-synuclein fibrils are injected into a specific region of the brain (such as the striatum) or they are introduced into the culture media [35]. Therefore, oxidative stress, neuroinflammation and the appearance of phosphorylated α-synuclein aggregates occur. These aggregates are the result of a failed cellular attempt to degrade fibrillary α-synuclein aggregates and spread to synaptically connected brain regions [36-38]. Thus, this model reproduces many clinical characteristics of idiopathic PD such as the progressive loss of dopaminergic neurons, the appearance of behavioral deficits and the development of a Lewy body-like pathology [39-41].
Although PD is primarily a sporadic disorder, it is postulated that approximately 10% of cases are caused by mutations in specific genes [27]. Thus, transgenic models of PD have focused on reproducing mutations or suppressing the expression of genes related to familial PD [42]. These models have allowed the development of many pathological characteristics of the disease, including neuronal loss, motor symptoms and formation of α-synuclein inclusions [12]. However, the main limitation of the genetic models is that they do not produce constant neuronal damage in the cells of the nigrostriatal pathway [29].
In short, there is no ideal model of PD that reproduce all the pathological and phenotypical characteristics of the disease, since both neurotoxic-induced models and transgenic models have their own advantages and limitations, which should be considered in order to select the ideal model for a given study. The present review synthesizes the main results on the protective effects of different substances with antioxidant properties in the two most commonly used rodent models of Parkinsonism: the one induced by MPTP and the 6-OHDA model.
4. METHODS
The present review on exogenous antioxidants and Parkinson’s disease was carried out with the aim of unifying the most recent and relevant studies and identifying possible gaps on this topic. To achieve this purpose, a systematic review was performed following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [43]. The search was made in the specialized databases PubMed, Scopus and MEDLINE in January 2021 with a time restriction limited to studies published in the last 5 years.
Studies were selected using terms referring to the different types of exogenous non-enzymatic antioxidants. Thus, for the identification of articles on the group of vitamins, the following terms were used: “vitamin”, “tocopherol”, “vitamin E”, “vitamin C”, “ascorbic acid”, “vitamin D”, “vitamin A”, “retinol” and “carotenoid”. For the selection of studies concerning the group of polyphenols, the term “polyphenol” was used for a more general search, and the terms “flavonoid”, “flavonol”, “flavanol”, “anthocyanin”, “flavanone”, “flavone” and “isoflavonoid” were used for a more specific search. To find the articles referring to the group of non-flavonoid polyphenolic antioxidants, the terms “curcumin” and “resveratrol” were used. Each one of the terms mentioned above was searched in the databases individually according to the following search strategy: “(type of antioxidant) AND (Parkinson) AND (animal model)”.
4.1. Inclusion and Exclusion Criteria
The articles included in this review met the following inclusion criteria: (1) original studies in article format; (2) in English or Spanish; (3) exclusively in vivo; (4) performed with rats or mice as an animal model; (5) in which Parkinsonism has been induced with MPTP or 6-OHDA; and (6) that the effects of antioxidants have been analyzed on the dopaminergic system. The studies were excluded based on the following exclusion criteria: (1) theoretical articles or reviews; and (2) studies in which the natural antioxidant was administered with other substances or treatments.
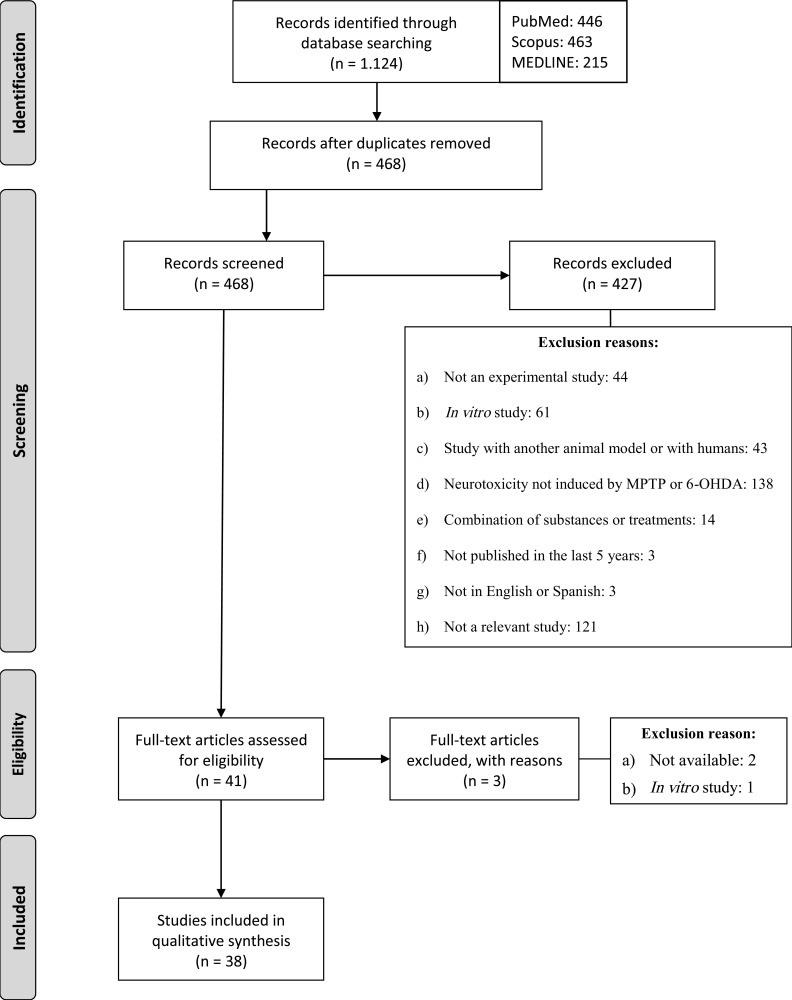
As a result of the searches carried out in the three databases, a total of 1.124 articles published in the last 5 years were identified. The articles were then exported to RefWorks and those that were duplicated were removed. According to the inclusion and exclusion criteria, we screened the 468 titles and abstracts to verify compliance with the inclusion and exclusion criteria. After this procedure, 427 articles were excluded for the reasons described in Fig. (2). Finally, 38 studies were selected to form part of the present review.
Fig. (2).
Flow diagram that reproduces the phases of the search process.
5. RESULTS
5.1. Effects of Naturally Occurring Antioxidants on Dopaminergic System
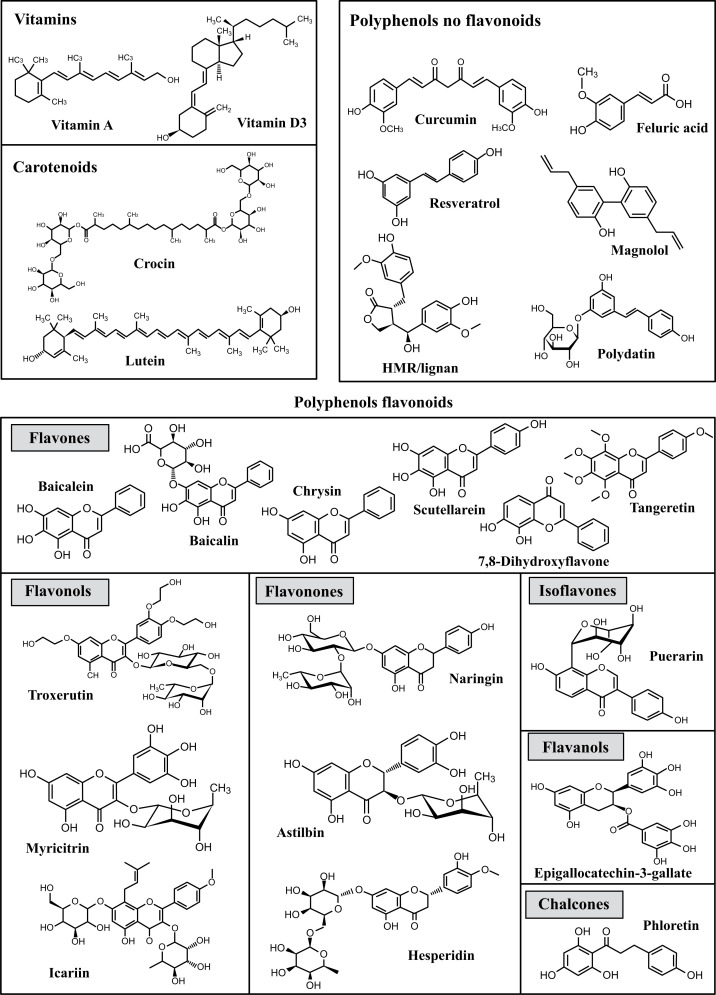
This review collects evidence published over the past 5 years, indicating that naturally occurring antioxidants protect the dopaminergic system from the toxic effects produced by MPTP and 6-OHDA. In addition, it summarizes information on the molecular mechanisms by which antioxidants produce their beneficial effects against oxidative stress, the generation of ROS, neuroinflammation, the accumulation of α-synuclein or apoptosis in nigrostriatal pathway in experimental models of Parkinsonism in rodents. Fig. (3) shows the exogenous antioxidants analyzed in this review and their classification.
Fig. (3).
Molecular structures of antioxidants analyzed in this review and their classification.
The effects of antioxidants on the dopaminergic neurotransmission, as well as the main mechanisms of their action are illustrated in Table 1.
Table 1.
General effects of the analyzed antioxidants on the dopaminergic neurotransmission.
| Antioxidant Agent | Species | Toxicity Model |
Length of
Exposure |
Dose | Neuroprotective Mechanisms | References |
|---|---|---|---|---|---|---|
| Ferulic acid | Wistar rats | 6-OHDA | 15 days | 100 mg/kg/day | ↑ TH Improved cell morphology |
[48] |
| Lutein | C57BL/6 mice | MPTP | 7 days | 5, 10 or 20 mg/kg/day | Improved motor behavior ↑ DA, DOPAC, HVA |
[49] |
| Troxerutin | Wistar rats | 6-OHDA | 1 week | 150 mg/kg/day | Improved motor behavior ↑ TH |
[50] |
| Astilbin | C57BL/6 mice | MPTP | 1 week | 50 mg/kg/day | Improved motor behavior ↑ TH ↑ DA, DOPAC, HVA |
[52] |
| Chrysin | C57BL/6 mice | 6-OHDA MPTP |
4 weeks; 5 days |
10 mg/kg/day; 50, 100 or 200 mg/kg/day |
Improved cognitive and motor functions ↑ TH ↑ DA, DOPAC, HVA ↑ DAT |
[46] [53] [54] |
| Phloretin | C57BL/6 mice | MPTP | 2 weeks | 5 mg/kg/day | Improved motor behavior ↑ TH ↑ DA |
[71] |
| Baicalein | Sprague-Dawley rats | MPP+ | 1 week | 10 or 30 mg/kg/day | ↑ TH ↑ DA |
[86] |
| Epigallocatechin-3-gallate | C57BL/6 mice | MPTP | 26 days; 1 week |
25 or 50 mg/kg/day; 25 mg/kg/day |
Improved motor behavior ↑ TH ↑ DA |
[47] [87] |
| Hesperidin | C57BL/6 mice | 6-OHDA | 28 days | 50 mg/kg/day | ↑ TH ↑ DA, DOPAC |
[88] |
| Myricitrin | C57BL/6 mice | 6-OHDA | 8 days | 30, 60 or 90 mg/kg/day | ↑ TH | [89] |
| Naringin and naringenin | C57BL/6 mice | 6-OHDA MPTP |
7 days (pre-treatment), 4 or 12 weeks (post-treatment); 5 days |
80 mg/kg/day; 25, 50 or 100 mg/kg/day |
Improved motor behavior ↑ TH ↑ DA, DOPAC, HVA ↑ DAT |
[58] [59] [90] |
| Puerarin | C57BL/6 mice | MPTP | 20 days | Not reported | Improved motor behavior ↑ TH |
[91] |
| Tangeretin | Sprague-Dawley rats | MPTP | 24 days | 50, 100 or 200 mg/kg/day | Improved cognitive and motor functions ↑ TH |
[92] |
| Vitamin D | C57BL/6 mice | MPTP | 10 days | 1 μg/kg/day | ↑ TH | [93] |
| HMR/Lignan | Sprague-Dawley rats | 6-OHDA | 4 weeks | 10 mg/kg/day | Improved motor behavior ↑ dopaminergic terminals TH+ |
[116] |
| Baicalin | Sprague-Dawley rats | 6-OHDA | 4 weeks | 25 mg/kg/day | Improved motor behavior | [119] |
| Icariin | C57BL/6 mice | MPTP | 8 days | 50, 100 or 200 mg/kg/day | Improved motor behavior ↑ TH ↑ DA |
[158] |
| Resveratrol | C57BL/6 mice Sprague- Dawley rats | 6-OHDA MPTP | 33 days; 36 days |
100 mg/kg/day; 15 or 30 mg/kg/day | Improved motor behavior ↑ TH ↑ DA |
[118] [159] |
| Dihydroxyflavone | C57BL/6 mice Sprague-Dawley rats |
6-OHDA MPTP |
2 weeks; 4 weeks (2 weeks pre-treatment and/or 2 weeks post-treatment) |
5 mg/kg/day; 12-16 mg/kg/day |
Improved motor behavior ↑ TH |
[57] [167] |
|
Calcitrol
(Vitamin D metabolite) |
Fischer rats | 6-OHDA | 8 days | 1 μg/kg/day | ↑ DA in the striatum of young and middle-aged rats, but not in aged rats ↑ DA in the SNpc of the three groups |
[242] |
| Crocin | BALB/c mice | MPTP | 15 days | 30 mg/kg/day | Improved motor behavior ↑ TH |
[243] |
| Curcumin | Sprague-Dawley rats C57BL/6 mice |
6-OHDA MPTP |
30 days; 3 weeks; 1 week |
5, 10 or 20 mg/kg/day; 5, 10 or 15 µmol/L/day; 50 mg/kg/day |
Improved motor behavior ↑ TH ↑ DA, ACh ↑ DAT Improved cell morphology |
[45] [55] [56] [244] |
| Magnolol | C57BL/6 mice | MPTP | 6 days | 10 mg/kg/day | ↑ TH ↑ VMAT2 |
[245] |
| Scutellaria baicalensis | C57BL/6 mice | MPTP | 5 days | 5 mg/kg/day | Improved motor behavior ↑ dopaminergic neurons |
[246] |
| Vitamin D3 | Wistar rats C57BL/6 mice |
6-OHDA | 1 week (pre-treatment) or 2 weeks (post-treatment) | 1 μg/kg/day 30 mg/kg/day |
Improved motor behavior ↑ TH ↑ DA, DOPAC ↑ DAT |
[51] [247] |
Abbreviations: 6-OHDA, 6-hydroxydopamine; ACh, acetylcholine; DA, dopamine; DAT, dopamine transporter; DOPAC, 3,4-dihydroxyphenylacetic acid; HVA, homovanillic acid; MPP+, 1-methyl-4-phenylpyridinium; MPTP, 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine; SNpc, substantia nigra pars compacta; TH, tyrosine hydroxylase; VMAT2, vesicular monoamine transporter 2.
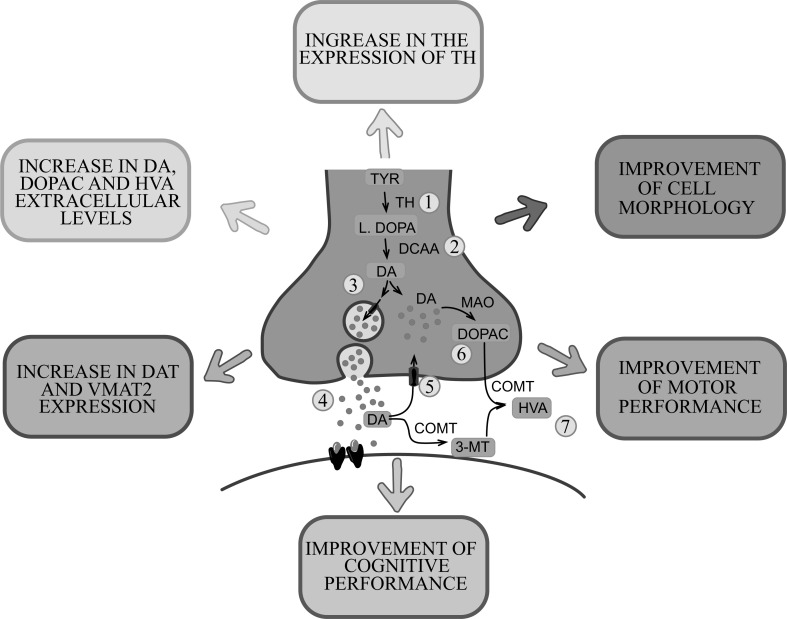
All the studies analyzed show that naturally occurring antioxidants have effective protection against biochemical and behavioral changes in animal models of PD (Fig. 4). The antioxidants induce an increase in dopamine levels and its metabolites, as well as an increase in expression and activity of the tyrosine hydroxylase (TH) in dopaminergic neurons. Other documented changes were the increase in the expression of DAT and vesicular monoamine transporter 2 (VMAT2), responsible for the reuptake of dopamine inside the cell and inside the synaptic vesicles, respectively. These effects produced by antioxidants suggest greater preservation of dopaminergic neurons after treatment with these substances. In addition, these beneficial effects were supported by the results of behavioral tests, which showed a cognitive improvement, as well as a significant recovery of the locomotor activity of Parkinsonism animal models. The exception appears to be vitamin A, which did not produce a significant protective effect on the motor control or expression of TH, although it was able to reverse behavioral alterations in 6-OHDA-induced model [44].
Fig. (4).
Main effects produced by naturally occurring antioxidants on dopaminergic neurotransmission in the experimental models. Main mechanisms of action: increased expression of TH (1) and, with it, dopamine synthesis (2), increased expression of VMAT2 (3), increased levels of extracellular dopamine (4), increased expression of DAT (5), increased levels of DOPAC (6) and HVA (7). These effects are reflected in an improvement in the motor and cognitive performance of the animals. Abbreviations: 3-MT, 3-methoxytyramine; COMT, catechol-O-methyl-transferase; DA, dopamine; DAT, dopamine transporter; AADC, aromatic L-amino acid decarboxylase; DOPAC, 3,4-dihydroxyphenylacetic acid; HVA, homovanillic acid; L-DOPA, levodopa; MAO, monoamine oxidase; TYR, tyrosine; TH, tyrosine hydroxylase; VMAT2, vesicular monoamine transporter 2.
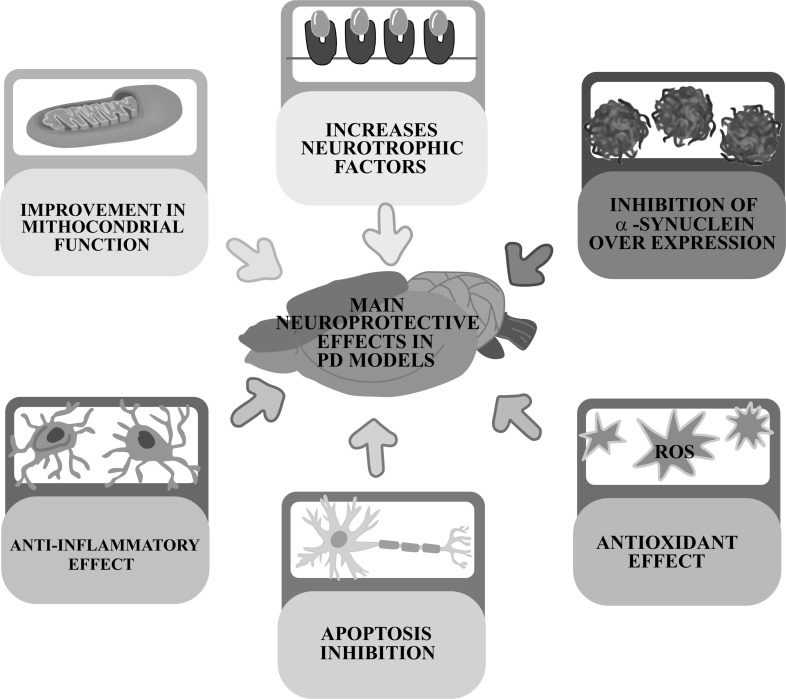
All these effects are achieved through an effective neuroprotective action of antioxidants by acting on the main pathophysiological mechanisms responsible for the development of Parkinsonism: 1) optimization of the cellular antioxidant status; 2) reduction of neuroinflammation; 3) inhibition of overexpression of α-synuclein; 4) improvement in mitochondrial function; 5) increased production of neurotrophic factors and; 6) inhibition of apoptosis (Fig. 5).
Fig. (5).
Main beneficial effects produced by naturally occurring antioxidants in models of experimental parkinsonism.
5.2. Effects on Cellular Antioxidant Status
Studies related to antioxidant effects of the substances tested are summarized in Table 2. As mentioned above, oxidative stress caused by excessive production of free radicals contributes decisively to the pathogenesis of PD by causing damage to lipids, proteins and DNA. This situation can be reversed by decreasing the synthesis of free radicals or by enhancing the effectiveness of cellular antioxidant defense systems [17]. A number of the polyphenols discussed in this review show effective antioxidant properties against oxidative stress produced by 6-OHDA or MPTP. In this way, it has been observed that curcumin, chrysin, epigallocatechin-3-gallate (EGCG), ferulic acid, lutein and troxerutin, significantly reduce intracellular the levels of ROS and, consequently the lipid peroxidation and oxidative damage to proteins and DNA caused by toxics [45-50]. This property has also been reported for the vitamin D3 [51].
Table 2.
Substances with antioxidant effects and their neuroprotective mechanisms.
| Antioxidant Agent | Species | Toxicity Model |
Length of
Exposure |
Dose |
Neuroprotective
Mechanisms |
References |
|---|---|---|---|---|---|---|
| Epigallocatechin-3-gallate | C57BL/6 mice | MPTP | 1 week | 25 mg/kg/day | ↓ Oxidative stress | [47] |
| Ferulic acid | Wistar rats | 6-OHDA | 15 days | 100 mg/kg/day | ↓ ROS, LPO ↑ GSH ↓ DNA damage |
[48] |
| Lutein | C57BL/6 mice | MPTP | 7 days | 5, 10 or 20 mg/kg/day | ↓ LPO ↑ GSH, GSH-Px ↓ CAT, SOD |
[49] |
| Troxerutin | Wistar rats | 6-OHDA | 1 week | 150 mg/kg/day | ↓ LPO, ROS | [50] |
| Vitamin D3 | Wistar rats | 6-OHDA | 1 week (pre-treatment) or 2 weeks (post-treatment) | 1 μg/kg/day | ↓ LPO ↓ Nitrite |
[51] |
| Astilbin | C57BL/6 mice | MPTP | 1 week | 50 mg/kg/day | ↑ SOD, GSH | [52] |
| Chrysin | C57BL/6 mice | 6-OHDA MPTP |
4 weeks; 5 days |
10 mg/kg/day; 50, 100 or 200 mg/kg/day |
↓ ROS, LPO Restoring GSH levels ↑ SOD ↓ NADPH oxidase ↑ Nrf2 |
[46] [53] [54] |
| Curcumin | Sprague-Dawley rats C57BL/6 mice | 6-OHDA MPTP |
30 days; 3 weeks; 1 week |
5, 10 or 20 mg/kg/day; 5, 10 or 15 µmol/L/day; 50 mg/kg/day |
↓ LPO ↓ CAT ↑ GSH-Px ↑↓ SOD (controversy) Restoring GSH levels |
[45] [55] [56] |
| Dihydroxyflavone | C57BL/6 mice | MPTP | 2 weeks | 5 mg/kg/day | ↑ SOD, GSH | [57] |
| Naringenin | C57BL/6 mice | MPTP | 5 days | 25, 50 or 100 mg/kg/day | ↓ LPO ↑ CAT, GSH, SOD, GRx |
[58] [59] |
| Scutellaria baicalensis stem-leaf total flavonoid | C57BL/6 mice | MPTP | 5 days | 5 mg/kg/day | ↓ LPO | [246] |
Abbreviations: 6-OHDA, 6-hydroxydopamine; CAT, catalase; GRx, glutathione reductase; GSH, glutathione; GSH-Px, glutathione peroxidase; LPO, lipid peroxidation; MPTP, 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine; NADPH oxidase, nicotinamide adenine dinucleotide phosphate oxidase; Nrf2, nuclear factor erythroid-derived 2-like 2; ROS, reactive oxygen species; SOD, superoxide dismutase.
Polyphenols also exert a significant antioxidant effect by increasing the expression and/or activity of endogenous antioxidant enzymes, such as SOD, CAT, GSH-Px or GRx, which are the main responsible for neutralizing free radicals in the body. It has been observed that astilbin, chrysin, curcumin, 7,8-dihydroxyflavone and naringenin show an effective ability to reduce the production of free radicals [52-59], and a significant decrease in the cellular stress generated by MPTP [45].
Glutathione is one of the most efficient endogenous antioxidants in the brain and post-mortem samples show that their levels are reduced in SNpc in both people with PD and in experimental models of the disease [60]. For this reason, it should be noted that levels of this antioxidant were significantly increased by some of the polyphenols discussed in this review, such as astilbin, chrysin, curcumin, 7,8-dihydroxyflavone, ferulic acid, lutein or naringenin [45, 46, 48, 49, 52, 54, 57, 58].
In addition to this, other additional mechanisms by which flavonoids can reduce oxidative damage have been identified in this review. On the one hand, numerous studies have reported an excessive deposit of iron in SNpc in PD patients, which may favor the production of hydroxyl radicals through Fenton's reaction and thus oxidative stress [61- 63]. For this reason, the potential of the EGCG to promote the elimination of cellular iron by inducing an increase in the expression of the ferroportin, involved in the export and degradation of this element is of particular importance [47]. On the other hand, another alteration that occurs during PD is the increase in the activity of the enzyme nicotinamide adenine dinucleotide phosphate oxidase (NADPH oxidase), which generates a high amount of ROS and thus contributes to the degeneration of dopaminergic neurons [64]. However, the activity of this enzyme can be decreased by treatment with chrysin, which gives this flavonoid a powerful antioxidant action [46].
The last antioxidant mechanism identified is related to the action of chrysin on the nuclear factor erythroid-derived 2-like 2 (Nrf2). Nrf2 is a transcription factor sensitive to oxidative stress that controls the expression of a wide range of genes responsible for antioxidant homeostasis [65]. In this way, the activation of this factor occurs in states of increased oxidative stress, resulting in the stimulation of the transcription of antioxidant proteins with the aim of protecting cells from oxidative damage [66]. Thus, the increase in the activation of Nrf2 induced by chrysin observed by Krishnamoorthy et al. [54], explains some of the antioxidant effect of this flavonoid. This finding is in line with other research with PD models, in which Nrf2 activation also exerts an important neuroprotective effect and which will be discussed later [67].
5.3. Effects on Neuroinflammation
Studies show that the treatment of animals with naturally sourced antioxidants decreases inflammation caused by MPTP or 6-OHDA through different mechanisms of action. The results obtained are summarized in Table 3.
Table 3.
Substances with anti-inflammatory effects and their neuroprotective mechanisms.
| Antioxidant Agent | Species | Toxicity Model | Length of Exposure | Dose | Neuroprotective Mechanisms | References |
|---|---|---|---|---|---|---|
| Vitamin A | Wistar rats | 6-OHDA | 28 days | 3000 UI/kg/day | ↓ IL-1β, TNF-α ↓ Iba1 ↑ GFAP |
[44] |
| Troxerutin | Wistar rats | 6-OHDA | 1 week | 150 mg/kg/day | ↓ GFAP | [50] |
| Vitamin D3 | Wistar rats | 6-OHDA | 1 week (pre-treatment) or 2 weeks (post-treatment) |
1 μg/kg/day | ↓ TNF-α | [51] |
| Astilbin | C57BL/6 mice | MPTP | 1 week | 50 mg/kg/day | ↓ Iba1, GFAP | [52] |
| Chrysin | C57BL/6 mice | 6-OHDA MPTP |
4 weeks; 5 days |
10 mg/kg/day; 50, 100 or 200 mg/kg/day |
↓ IL-1β, IL-6, TNF-α, IFN-γ ↑ IL-10 ↓ NO ↓ NF-κB |
[46] [53] [54] |
| Curcumin | Sprague-Dawley rats | 6-OHDA | 3 weeks | 5, 10 or 15 µmol/L/day | ↓ GFAP | [56] |
| Phloretin | C57BL/6 mice | MPTP | 2 weeks | 5 mg/kg/day | ↓ IL-β, IL-6, TNF-α ↓ Iba1, GFAP ↓ iNOS, COX-2 |
[71] |
| Baicalein | Sprague-Dawley rats | MPP+ | 1 week | 10 or 30 mg/kg/day | ↓ IL-1β ↓ ED-1 ↓ Caspase 1, cathepsin B |
[86] |
| Epigallocatechin-3-gallate | C57BL/6 mice | MPTP | 26 days | 25 or 50 mg/kg/day | ↓ IL-6, TNF-α ↑ CD3+ CD4+ and CD3+ CD8+ T cells |
[87] |
| Hesperidin | C57BL/6 mice | 6-OHDA | 28 days | 50 mg/kg/day | ↓ IL-β, IL-2, IL-6, TNF-α, IFN-γ | [88] |
| Myricitrin | C57BL/6 mice | 6-OHDA | 8 days | 30, 60 or 90 mg/kg/day | ↓ TNF-α ↓ Iba1 |
[89] |
| Naringin and naringenin | C57BL/6 mice | 6-OHDA MPTP |
7 days (pre-treatment), 4 or 12 weeks (post-treatment); 5 days |
80 mg/kg/day; 25, 50 or 100 mg/kg/day |
↓ IL-1β, TNF-α ↓ Iba1 ↓ iNOS, NO |
[58] [59] [90] |
| Puerarin | C57BL/6 mice | MPTP | 20 days | Not reported | ↓ GFAP ↓ iNOS |
[91] |
| Tangeretin | Sprague-Dawley rats | MPTP | 24 days | 50, 100 or 200 mg/kg/day | ↓ IL-1β, IL-2, IL-6, TNF-α ↓ Iba1, GFAP ↓ iNOS, COX-2 |
[92] |
| Vitamin D | C57BL/6 mice | MPTP | 10 days | 1 μg/kg/day | ↓ IL-1β, TNF-α ↑ IL-4, IL-10, TGF-β ↓ Iba1, GFAP ↓ iNOS ↓ TLR4 ↑ CD163, CD204, CD206 |
[93] |
| HMR/Lignan | Sprague-Dawley rats | 6-OHDA | 4 weeks | 10 mg/kg/day | ↓ CD11b, GFAP ↓ CD11b+/CD32+ cells ↑ CD11b+/CD206+ cells |
[116] |
Abbreviations: 6-OHDA, 6-hydroxydopamine; COX-2, cyclooxygenase 2; GFAP, glial fibrillary acidic protein; Iba1, ionized calcium-binding adapter molecule 1; IFN-γ, interferon gamma; IL-1β, interleukin 1beta; IL-2, interleukin 2; IL-4, interleukin 4; IL-6, interleukin 6; IL-10, interleukin 10; iNOS, inducible nitric oxide synthase; MPP+, 1-methyl-4-phenylpyridinium; MPTP, 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine; NF-κB, nuclear factor kappa B; NO, nitric oxide; TGF-β, transforming growth factor-beta; TLR4, toll-like receptor 4; TNF-α, tumor necrosis factor alpha.
The inflammatory reaction plays a healthy role in helping the immune system counteract certain pathological states, but when it is unbalanced or prolonged over time it can cause progressive tissue damage, which is particularly evident in pathologies such as PD [68]. In the nervous system, the inflammatory process is mainly mediated by the activation of microglia, whose proliferation and activity appear to be closely related to the onset and progression of PD [69]. As a result of such activation, there is an increase in the release of proinflammatory cytokines, such as interleukin 1β (IL-1 β), interleukin 2 (IL-2) and interleukin 6 (IL-6), tumor necrosis factor alpha (TNF-α) and interferon gamma (IFN-γ), which lead to the degeneration of dopaminergic neurons [64, 70, 71]. A number of studies have shown that serum concentrations of these proinflammatory cytokine are high in patients with PD [72], an increase that could also be seen in Parkinsonism models induced by 6-OHDA or MPTP.
In contrast, proinflammatory cytokines interleukin 4 (IL-4) and interleukin 10 (IL-10) are considered promising candidates for the treatment of PD, as these substances suppress the release of the microglial proinflammatory mediators involved in neurodegeneration [73-75]. Thus, therapies based on the administration of these interleukins have been shown to mitigate damage to dopaminergic neurons in the in vitro and in vivo models of the disease [76-78]. Something similar happens with Transforming Growth Factor-Beta (TGF-β), essential for the survival of mesencephalic dopaminergic neurons, both embryonic and adult [79]. It is considered that this anti-inflammatory cytokine has a prominent role in the regulation of immune system cells, by exerting an important inhibitory action on the proliferation and activation of microglial cells during the inflammatory reaction [80, 81]. In addition, there is evidence pointing to a possible protective effect of TGF-β on the dopaminergic system, as its administration protects against the degeneration of dopaminergic neurons induced by MPP+ and MPTP [79, 82, 83], while its deficiency causes a significant decrease in the number of these cells [84, 85].
In this way, control of microglial activation and inflammation of the nigrostriatal pathway may be paramount for the protection of dopaminergic neurons. In line with this, almost all antioxidant agents analyzed in this review show a significant anti-inflammatory effect by decreasing the activation of glial cells and the production of proinflammatory cytokines, and/or by increasing the release of anti-inflammatory cytokines (Table 3). An example of this would be astilbin, baicalein, chrysin, EGCG, hesperidin, myricitrin, naringenin/naringin, phloretin, puerarin, tangeretin and vitamins A and D [44, 46, 51-53, 58, 71, 86-93]. In these studies, the lower reactivity of microglia has been evidenced by the reduced expression of ionized calcium-binding adapter molecule 1 (Iba1), ED-1 or CD11b, while the decrease in the concentration of Glial Fibrillary Acidic Protein (GFAP) has been used as a marker of lower activation of astrocytes. With regard to vitamin A, it should be noted that, although there has been a decrease in microglial activation and cytokine release, it has led to an increase in the reactivity of astrocytes [44]. This increase could be due to the ability of astrocytes to inhibit microglia through the release of TGF-β, thus limiting neuroinflammation [94]. Therefore, it would be appropriate for a future investigation to check whether vitamin A modulates microglial activation through astrocytes, by analyzing TGF-β.
Another process that takes place during the inflammatory reaction is the activation of the enzymes inducible Nitric Oxide Synthase (iNOS) and Cyclooxygenase-2 (COX-2), whose concomitant activity is related to the damage of dopaminergic neurons. In particular, it is considered that during inflammatory processes the glial cells that express iNOS are activated and, consequently, begin to generate excessive and uncontrolled amounts of Nitric Oxide (NO), which contributes to oxidative damage by becoming free radicals [68, 95]. This hypothesis is supported by several studies that have shown the presence of elevated levels of NO in the post-mortem brains of PD patients [96-98], as well as in experimental studies in which inhibition of iNOS decrease the loss of dopaminergic neurons [99, 100]. In this way, chrysin, phloretin, naringenin, puerarin, tangeretin and vitamin D have been observed to play an important protective role in reducing the activation of iNOS, and/or the consequent release of NO in experimental models of Parkinsonism [46, 58, 59, 71, 91-93]. On the other hand, COX-2 can also cause neuronal death by increasing the production of prostaglandins, which amplify glial response and production of ROS and inflammatory cytokines [95]. Whereas the expression of COX-2 is also elevated in patients with PD [101], the neuroprotection exercised by phloretin and tangeretin, which reduced levels of this enzyme in in vivo models of Parkinsonism is noteworthy [71, 92].
The inflammatory reaction in CNS is usually associated with the activation of toll-like receptors, which play a key role in regulating immune response during infections and damage to nerve tissue. These receptors regulate various signaling pathways that modulate the expression of a wide range of immune response genes, such as proinflammatory cytokines genes [102]. In particular, pathological accumulation of α-synuclein, which takes place during the PD, is considered responsible for inducing proinflammatory responses dependent on toll-like receptor 4 (TLR4) activation [103]. This molecule is expressed by the main types of CNS cells and its activation causes the sustained secretion of proinflammatory cytokines and ROS, which promote the death of dopaminergic neurons in PD [104]. In line with studies such as Campolo et al. [102], in which the absence of TLR-4 in mice suppressed neuroinflammation and avoided the dopaminergic neurodegeneration induced by MPTP, are the results obtained by the Calvello et al. [93] in which vitamin D was able to reduce TLR-4 levels in the MPTP-induced PD model.
In addition, TLR-4 is also considered to be involved in modulating different molecular signaling pathways, including the nuclear factor kappa B (NF-kB) [102]. Thus, it has been observed in both post-mortem brains of patients with PD and in animal models that, when microglial activation occurs, the transcription factor NF-kB by nuclear translocation is also activated [105]. This factor is also related to the upregulation of proinflammatory cytokines and iNOS and COX-2 activities [68, 106]. Therefore, the reduction in the expression of NF-kB induced by flavonoids as chrysin reflects an important anti-inflammatory effect of this substance [53, 54].
Another process closely related to the pathological accumulation of α-synuclein is the activation of inflammasomes, protein complexes present in the cell cytosol that trigger innate immune responses when detecting signs of cellular damage [107]. Thus, cytosol inflammasome of the Nod-Like Receptor Protein 3 (NLRP3) is considered to play a significant role in the physiopathology and progression of PD [108], as direct evidence of its activation in post-mortem brains and cerebrospinal fluid of patients with PD has been provided [109, 110]. In addition, some studies with animal models of PD have shown that pharmacological inhibition of NLRP3 can prevent dopaminergic degeneration in the nigrostriatal pathway [110, 111]. With regard to the activation of NLRP3 in the PD, recent findings suggest that the endocytosis of α-synuclein by lysosomes produces the release of lysosomal protease cathepsin B into the cytoplasm, which in turn causes the assembly of the NLRP3 complex. As a result, activation of caspase 1 occurs, which induces the release of proinflammatory cytokines [112]. Considering this information, it has been shown that the anti-inflammatory effect of baicalein appears to be linked to its ability to inhibit inflammasome in animals with Parkinsonism, as administration of this flavonoid reduced the levels of caspase 1 and cathepsin B [86].
In general, the activation of microglia and astroglia implies a morphological and functional modification of these cells. In relation to microglia, its activation can result in two phenotypes with opposite characteristics: the cytotoxic-proinflammatory phenotype M1-A1 or the cytoprotective-anti-inflammatory phenotype M2-A2 [72]. Each of these microglial phenotypes is characterized by the expression of different cell surface markers. Thus, when microglia acquire the cytotoxic phenotype M1, it mainly expresses the cluster of differentiation 32 receptor (CD32), while when it acquires the cytoprotective phenotype M2 it regulates upwards the expression of the receptors cluster of differentiation 163 (CD163), cluster of differentiation 204 (CD204) and cluster of differentiation 206 (CD206) [113]. Although post-mortem studies suggest that both phenotypes microglial may coexist in the parkinsonian brain, experimental models support a gradual increase in the prevalence of M1 polarization over M2 polarization during chronic PD [114, 115]. In this sense, the research of Calvello et al. [93] and Giuliano et al. [116] demonstrates the potential of vitamin D and HMR/lignan, respectively, to modify microglial polarization, by reducing its neurotoxic activation (phenotype M1) and enhancing anti-inflammatory signaling (phenotype M2).
In recent years, peripheral inflammation has been considered to increase susceptibility to mesencephalic dopaminergic neurodegeneration [117]. Thus, flavonoids such as EGCG can contribute to the anti-inflammatory effect by modulating the peripheral immune response mediated by T cells, as documented in the study by Zhou et al. [87].
5.4. Inhibition of Overexpression of α-synuclein
Table 4 shows which of the antioxidants studied inhibit the overexpression of α-synuclein.
Table 4.
Substances that inhibit α-synuclein overexpression and their neuroprotective mechanisms.
| Antioxidant Agent | Species | Toxicity Model | Length of Exposure | Dose |
Neuroprotective
Mechanisms |
References |
|---|---|---|---|---|---|---|
| Curcumin | C57BL/6 mice | MPTP | 1 week | 50 mg/kg/day | ↓ α-synuclein | [45] |
| Astilbin | C57BL/6 mice | MPTP | 1 week | 50 mg/kg/day | ↓ α-synuclein | [52] |
| Chrysin | C57BL/6 mice | MPTP | 5 days | 50, 100 or 200 mg/kg/day | ↓ α-synuclein | [54] |
| Dihydroxyflavone | C57BL/6 mice | MPTP | 2 weeks | 5 mg/kg/day | ↓ α-synuclein | [57] |
| Naringenin | C57BL/6 mice | MPTP | 5 days | 25, 50 or 100 mg/kg/day | ↓ α-synuclein | [58] |
| Baicalein | Sprague-Dawley rats | MPP+ | 1 week | 10 or 30 mg/kg/day | ↓ α-synuclein | [86] |
| Resveratrol | C57BL/6 mice | MPTP | 33 days | 100 mg/kg/day | ↓ α-synuclein ↑ LC3-II ↓ p62 ↑ SIRT1 |
[118] |
| Baicalin | Sprague-Dawley rats | 6-OHDA | 4 weeks | 25 mg/kg/day | ↓ α-synuclein | [119] |
Abbreviations: LC3-II, microtubule-associated proteins 1A/1B-light chain 3; MPP+, 1-methyl-4-phenylpyridinium; MPTP, 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine, SIRT1: sirtuin 1.
A number of studies have shown that the loss of dopaminergic neurons in PD is associated with overexpression of α-synuclein, which originates the Lewy bodies [11]. Consequently, decreased levels of this protein can rescue dopaminergic neurons from cell death. This property has been demonstrated for polyphenols such as astilbin, baicalein, baicalin, chrysin, curcumin, 7,8-dihydroxyflavone, naringenin and resveratrol [45, 52, 54, 57, 58, 86, 118, 119].
In the study conducted by Guo et al. [118] on the neuroprotective effects of resveratrol, the authors suggest that the elimination of abnormal α-synuclein aggregates could be performed through an improved autophagy process, mediated by activation of sirtuin 1 (SIRT1). In general, the autophagy process is a degradation pathway that eliminates some cytoplasmic components, such as protein aggregates or damaged organelles [120]. There are several regulatory factors of autophagy, including SIRT1, that deacetylate molecules directly related to autophagy, such as microtubule-associated proteins 1A/1B light chain 3 (LC3) [121]. As a result of its deacetylation, LC3 activation occurs, which controls the main steps of the autophagy, and during this process interacts and produces the degradation of the protein p62 [122]. In this regard, it is considered that the deterioration of the autophagy process that takes place during PD causes overexpression and subsequent accumulation of p62 in Lewy bodies, which would be composed of α-synuclein associated with this protein [123-125]. In this way, Guo et al. [118] demonstrated that resveratrol was effective in eliminating α-synuclein by autophagy, as its administration caused a reduction in p62 levels and increased the concentration of LC3-II (a lipidated form of LC3), which is an indicator of autophagy induction.
5.5. Improvement in Mitochondrial Function
Table 5 summarizes the antioxidants that showed the ability to improve mitochondrial function and the mechanisms underlying this effect.
Table 5.
Substances that improve mitochondrial function and their neuroprotective mechanisms.
| Antioxidant Agent | Species | Toxicity Model | Length of Exposure | Dose | Neuroprotective Mechanisms | References |
|---|---|---|---|---|---|---|
| Ferulic acid | Wistar rats | 6-OHDA | 15 days | 100 mg/kg/day | ↑ PGC-1α ↓ Drp1 ↑ Mfn2 |
[48] |
| Curcumin | Sprague-Dawley rats | 6-OHDA | 3 weeks | 5, 10 or 15 µmol/L/day | ↑ Mitochondrial membrane potential | [56] |
Abbreviations: 6-OHDA, 6-hydroxydopamine; Drp1, dynamin-related protein 1; Mfn2, mitofusin 2; PGC-1α, peroxisome proliferator-activated receptor gamma coactivator 1-alpha.
Pathological dysfunction of mitochondria is also a characteristic feature of PD neuropathology, and is associated with oxidative stress and the presence of α-synuclein oligomers [126]. Reduced levels of the peroxisome Proliferator-Activated Receptor Gamma Coactivator 1-alpha (PGC-1α) have also been identified in the brain tissue of PD patients, reflecting mitochondrial biogenesis impaired during the degenerative process [127]. In addition, several studies have linked the overexpression of PGC-1α to the repair of neuronal loss, activation of antioxidant enzymes and protection of dopaminergic neurons against neuroinflammation [128]. Thus, it has been shown that ferulic acid could exert its neuroprotective effects by increasing the levels of PGC-1α, thus improving the mitochondrial functioning [48].
Mitochondria are dynamic organelles that are constantly subjected to fusion (or combination) and fission (or division) processes, whose balance is crucial for the maintenance of their function and for cellular viability [19], being each of these processes orchestrated by different GTPases. Thus, the fusion is directed by mitofusins 1 and 2 (Mfn1 and Mfn2) and optic atrophy protein 1, while the regulation of mitochondrial fission involves the dynamin-related protein 1 (Drp1) [129]. Since the balance between fusion/fission is very sensitive to intracellular physiological conditions, it is considered that its deregulation can be one of the factors that can lead to the development of PD [130].
In line with this, various in vitro and in vivo studies with different Parkinsonism-inducing neurotoxic agents support the hyperactivity of GTPase Drp1 during the progression of the pathology, resulting in an excess of mitochondrial fission [131-134]. In addition, several studies have suggested that the presence of α-synuclein aggregates may cause mitochondrial deficient fusion [135, 136]. In sum, these data suggest an imbalance of mitochondrial dynamics that occurs in PD, characterized by an increase in fission over fusion. Therefore, and considering the data described for ferulic acid, it is possible that the neuroprotective effect exerted by this antioxidant is related to its ability to restore the balance between the processes of mitochondrial dynamics, by reducing the levels of Drp1, involved in the mitochondrial fission, and increasing the concentration of Mfn2, involved in its fusion [48].
The loss of the mitochondrial membrane potential may be another of the consequences arising from a deficiency in the functioning of these organelles and, if not restored, causes the degradation of them [137]. In this way, it has been observed that the treatment of parkinsonized animals with curcumin showed an effective ability to elevate the mitochondrial membrane potential, which could prevent the degradation of these organelles [56].
5.6. Effects on Neurotrophic Factors Levels
The effects of the two antioxidant agents analyzed that have increased the production of neurotrophic factors are summarized in Table 6.
Table 6.
Substances that increase the production of neurotrophic factors and their neuroprotective mechanisms.
| Antioxidant Agent | Species | Toxicity Model | Length of Exposure | Dose | Neuroprotective Mechanisms | References |
|---|---|---|---|---|---|---|
| Chrysin | C57BL/6 mice | 6-OHDA MPTP |
4 weeks; 5 days |
10 mg/kg/day; 50, 100 or 200 mg/kg/day |
↑ BDNF, GDNF, NGF ↓ S100B |
[53] [54] |
| Curcumin | Sprague-Dawley rats | 6-OHDA | 30 days | 5, 10 or 20 mg/kg/day | ↑ NGF ↑ TrkA ↑ bFGF |
[55] |
| Hesperidin | C57BL/6 mice | 6-OHDA | 28 days | 50 mg/kg/day | ↑ BDNF, NGF | [88] |
Abbreviations: 6-OHDA, 6-hydroxydopamine; BDNF, brain-derived neurotrophic factor; bFGF, basic fibroblast growth factor; GDNF, glial cell line-derived neurotrophic factor; MPTP, 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine; NGF, nerve growth factor; S100B, calciumbinding protein B; TrkA, tropomyosin receptor kinase A.
Another of the neuroprotective effects observed after administration of natural antioxidants to animals with Parkinsonism is the increase in the production of neurotrophic factors in the nigrostriatal pathway. Neurotrophic factors are peptides secreted by neurons that play a primary role in promoting phenotypic differentiation that occurs during the development and survival of neurons in the adult nervous system [138]. In line with this, the neuroprotective action exerted by chrysin, curcumin and hesperidin, by raising levels of brain-derived neurotrophic factor (BDNF), the glial cell line-derived neurotrophic factor (GDNF) or nerve growth factor (NGF) is noteworthy [53-55, 88]. In this way, the increases in neurotrophic factors levels induced by these antioxidants could contribute to the survival of dopaminergic neurons in cases of PD, as has been shown in previous studies [139-141].
NGF exerts its effects by binding to tropomyosin receptor kinase A (TrkA). This receptor, depending on NGF levels, shows a pro-survival action by preventing apoptosis, when there is an increase in factor levels or, on the contrary, may exert a pro-apoptotic action when a reduction in such levels occurs [138, 142]. In this regard, in the study of Song et al. [55] curcumin caused a significant increase in the concentration of NGF and its TrkA receptor, resulting in an important neuroprotective action.
The basic fibroblast growth factor (bFGF) is another neurotrophic factor that, although non-neural in nature, is present in high concentrations in CNS [138]. Whereas this factor has a significant neuroprotective effect on the dopaminergic system both in vitro and in vivo [143-145], it is understandable that their levels are considerably reduced in the brain tissue of patients with PD [146]. Therefore, it is remarkable the protection exercised by curcumin by inducing an increase in bFGF levels in SNpc [55].
Calcium binding protein B (S100B), which is expressed and secreted mainly by the astrocytes, is also closely related to the development and maintenance of the CNS [147]. In physiological concentrations (nanomolar), this protein acts as a neurotrophic factor, favoring the survival of neurons, the growth of neurons and the proliferation of astrocytes, while when it is in high concentrations (micromolar) it can lead to astrocytes and neurons to death by apoptosis [148-150]. In line with this, S100B levels have been shown to be elevated in both cerebrospinal fluid and SNpc of PD patients, as well as in brain tissue of animal models of the disease [151-154]. However, chrysin was able to reduce the expression levels of this protein, which could explain, at least in part, the beneficial effects of this antioxidant on the dopaminergic system [53].
5.7. Inhibition of Apoptosis
The combination of oxidative stress, neuroinflammation, the presence of α-synuclein aggregates, mitochondrial dysfunction and neurotrophic factor deficiency, ultimately leads to the death of dopaminergic neurons by apoptosis. At the onset of apoptosis is involved the protein Bax, which forms heterodimers with other proteins to form a channel in the mitochondrial membrane and enable the release of cytochrome C. When cytochrome C moves to cytoplasm it triggers the caspase 3 reaction, which orchestrates cell apoptosis [155, 156]. The apoptosis process is closely related to neuronal death in PD, as high levels of Bax and caspase 3 have been detected in the brains of patients with it [157]. Several of the polyphenols studied, such as baicalein, baicalin, curcumin, ferulic acid, icariin, lutein, polydatin, puerarin and resveratrol have the ability to inhibit apoptosis in dopaminergic neurons by reducing the expression of proapoptotic factors (Bax, cytochrome C, caspases 3, 8, 9 and 12) and increase levels of the anti-apoptotic protein Bcl-2 [48, 49, 56, 86, 91, 118, 119, 158-160]. These antioxidant substances and their effects are summarized in Table 7.
Table 7.
Substances that inhibit cell death and their neuroprotective mechanisms.
| Antioxidant Agent | Species | Toxicity Model | Length of Exposure | Dose | Neuroprotective Mechanisms | References |
|---|---|---|---|---|---|---|
| Ferulic acid | Wistar rats | 6-OHDA | 15 days | 100 mg/kg/day | ↓ Bax, cytochrome C, caspase 3 ↑ Bcl-2 ↓ p53 |
[48] |
| Lutein | C57BL/6 mice | MPTP | 7 days | 5, 10 or 20 mg/kg/day | ↓ Bax, caspase 3 ↑ Bcl-2 ↓ Caspases 8 and 9 |
[49] |
| Troxerutin | Wistar rats | 6-OHDA | 1 week | 150 mg/kg/day | ↓ DNA fragmentation | [50] |
| Curcumin | Sprague-Dawley rats | 6-OHDA | 3 weeks | 5, 10 or 15 µmol/L/day | ↓ Cell apoptosis | [56] |
| Baicalein | Sprague-Dawley rats | MPP+ | 1 week | 10 or 30 mg/kg/day | ↓ Caspase 12, caspase 9 | [86] |
| Puerarin | C57BL/6 mice | MPTP | 20 days | Not reported | ↓ Bax, caspase 3 | [91] |
| Baicalin | Sprague-Dawley rats | 6-OHDA | 4 weeks | 25 mg/kg/day | ↓ Cell apoptosis | [119] |
| Icariin | C57BL/6 mice | MPTP | 8 days | 50, 100 or 200 mg/kg/day | ↓ Bax, caspase 3 ↑ Bcl-2 |
[158] |
| Resveratrol | C57BL/6 mice Sprague-Dawley rats |
6-OHDA MPTP |
33 days; 36 days |
100 mg/kg/day; 15 or 30 mg/kg/day |
↓ Bax, caspase 3 ↑ Bcl-2 |
[118] [159] |
| Polydatin | BALB/c mice | MPTP | 10 days | 20 or 80 mg/kg/day | ↓ Bax, caspase 3 | [160] |
Abbreviations: 6-OHDA, 6-hydroxydopamine; MPP+, 1-methyl-4-phenylpyridinium; MPTP, 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine.
It has also been shown that the accumulation of toxic α-synuclein during PD promotes the stress of endoplasmic reticulum, which when prolonged over time stimulates specific proapoptotic pathways leading to cell death [161]. Specifically, caspases 12 and 9 are important mediators of cell death produced by endoplasmic reticulum stress in rodents, although the function of caspase 12 in humans is not entirely clear [162, 163]. However, some data suggest that human caspase 4 could play the functional role of rodent caspase 12 by acting as a mediator in the cell death pathway caused by endoplasmic reticulum stress [164]. These data suggest that the rescue of dopaminergic neurons exercised by baicalein in the study by Hung et al. [86] could be related to its ability to inhibit the apoptotic pathway induced by the endoplasmic reticulum stress. This statement is based on the ability of this flavonoid to reduce the levels of two of the mediators of this process: caspases 12 and 9.
On the other hand, the apoptosis process is controlled by a network of proteins whose central role is played by transcription factor p53, called “the guardian of the genome”. Several studies have documented an increase in the level and activity of p53 in the brains of PD patients, as well as in the animal and cellular models of the disease [157]. In line with this, Anis et al. [48] have shown that ferulic acid has a significant effect on inhibition of apoptosis by decreasing the expression of transcription factor p53 in nigrostriatal dopaminergic neurons.
5.8. Signaling Pathways Involved in the Neuroprotective Effect of Antioxidants
Although the work analyzed here has not shown what molecular pathways would be involved in the neuroprotective effects of antioxidants, what is demonstrated is the importance of the phosphoinositide 3-kinase (PI3K)/protein kinase B (Akt) pathway in the neuroprotective effects of astilbin, icariin, resveratrol and troxerutin [50, 52, 158, 159] (Table 8).
Table 8.
Signaling pathways involved in the neuroprotective effect of the analyzed substances.
| Antioxidant Agent | Species | Toxicity Model | Length of Exposure | Dose | Neuroprotective Mechanisms | References |
|---|---|---|---|---|---|---|
| Troxerutin | Wistar rats | 6-OHDA | 1 week | 150 mg/kg/day | Blocking of neuroprotective effects by PHTPP (antagonist ERβ) and/or wortmannin (PI3K inhibitor) Neuroprotective effect mediated by PI3K/Erβ signaling |
[50] |
| Vitamin D3 | Wistar rats | 6-OHDA | 1 week (pre-treatment) or 2 weeks (post-treatment) |
1 μg/kg/day | ↑ VD3R Neuroprotective effect mediated by the VD3R |
[51] |
| Astilbin | C57BL/6 mice | MPTP | 1 week | 50 mg/kg/day | ↑ p-PI3K/PI3K ratio ↑ p-Akt/Akt ratio Neuroprotective effect mediated by PI3K/Akt signaling |
[52] |
| Curcumin | Sprague-Dawley rats | 6-OHDA | 3 weeks | 5, 10 or 15 µmol/L/day | ↑ Wnt3a, β-catenin, c-myc, cyclin D1 Neuroprotective effect mediated by Wnt/β-catenin signaling |
[56] |
| Myricitrin | C57BL/6 mice | 6-OHDA | 8 days | 30, 60 or 90 mg/kg/day | ↑ p-4E-BP1 (mTORC1 substrate) Neuroprotective effect mediated by mTORC1 complex |
[89] |
| Naringin | C57BL/6 mice | 6-OHDA | 1 week (pre-treatment), 4 or 12 weeks (post-treatment) |
80 mg/kg/day | ↑ p-4E-BP1 (mTORC1 substrate) Neuroprotective effect mediated by mTORC1 complex |
[90] |
| Baicalin | Sprague-Dawley rats | 6-OHDA | 4 weeks | 25 mg/kg/day | ↑ mTOR, p-mTOR ↑ Akt, p-Akt ↑ GSK-3β, p-GSK-3β Neuroprotective effect mediated by mTOR/Akt/GSK-3β signaling |
[119] |
| Icariin | C57BL/6 mice | MPTP | 8 days | 50, 100 or 200 mg/kg/day | Blocking of neuroprotective effects by LY294002 (PI3K inhibitor) or PD98059 (MEK inhibitor) Neuroprotective effect mediated by PI3K/Akt and MEK/ERK signaling pathways |
[158] |
| Resveratrol | Sprague-Dawley rats | 6-OHDA | 36 days | 15 or 30 mg/kg/day | ↑ PI3K-110α, p-Akt Ser473 Neuroprotective effect mediated by PI3K/Akt signaling |
[159] |
| Dihydroxyflavone | C57BL/6 mice Sprague-Dawley rats |
MPTP 6-OHDA |
2 weeks; 4 weeks (2 weeks pre-treatment and/or 2 weeks post-treatment) |
5 mg/kg/day; 12-16 mg/kg/day |
↑ p-TrkB Neuroprotective effect mediated by the TrkB |
[57] [167] |
Abbreviations: 6-OHDA, 6-hydroxydopamine; Akt, protein kinase B; ERK, extracellular signalregulated kinase; Erβ, estrogen receptor beta; MEK, mitogen-activated protein kinase kinase; MPTP, 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine; mTORC1, mammalian target of rapamycin complex 1; PI3K, phosphoinositide 3-kinase; p-4E-BP1, phosphorylated eukaryotic translation initiation factor 4E binding protein 1; TrkB, tropomyosin receptor r kinase B; VD3R, vitamin D3 receptor.
It has been widely demonstrated that the PI3K/Akt signaling pathway plays an important protective role in the process of oxidative stress-induced apoptosis and its activation could prevent neuronal death induced by MPTP or 6-OHDA [165, 166]. However, the way in which this pathway is activated to promote the survival of dopaminergic neurons seems to vary in the various studies. On the one hand, Baluchnejadmojarada et al. [50] indicate that troxerutin appears to promote its effects by activating the PI3K pathway by binding to the estrogen receptor beta (Erβ). On the other hand, the 7,8-dihydroxyflavone seems to activate the PI3K/Akt signaling cascade through the activation of the tropomyosin receptor kinase B (TrkB), of which this flavonoid is agonist [57, 167]. In addition, this signaling pathway is associated with the mammalian target of rapamycin complex 1 (mTORC1), indispensable for the survival and neuroprotection of dopaminergic neurons [168], and whose activation was induced by myricitrin and the naringin [89, 90]. Similarly, baicalin treatment was associated with activation of the mTOR/Akt/glycogen synthase kinase-3β (GSK-3β) signaling pathway [119]. GSK-3β is an important gene tightly related to the loss of dopaminergic neurons in PD models and it can be inactivated by Akt [169]. Therefore, activation of this pathway is considered to improve cell survival and protect from the apoptosis.
Additionally, the data analyzed also indicate two other signaling pathways that could be involved in preventing neuronal death. On the other hand, the team of Chen et al. [158] consistently found that the neuroprotective effects of icariin were mediated by the mitogen activated protein kinase (MEK)/extracellular signal-regulated kinase (ERK) signaling pathway, as activation of this pathway has been shown to inhibit apoptosis and therefore promote the survival of dopaminergic neurons [170]. On the other hand, Wang et al. [56] observed that curcumin protects against oxidative stress and cell death by activating the signaling pathway Wnt/β-catenin in the nigrostriatal dopaminergic neurons of animals with parkinsonism. This cascade has been shown to play a key role in the development of the nervous system and in the regeneration of dopaminergic neurons [171, 172], while oxidative stress and neuroinflammation induce its down-regulation, which inhibits the repair of dopaminergic neurons in the brain affected by PD [173]. This highlights the importance of the effects observed after treatment with curcumin, which promotes a significant up-regulation of Wnt/β-catenin signaling and the transcription of its target genes c-myc and cyclin D1, closely related to cell proliferation.
Another important finding has been documented by Lima et al. [51], where the neuroprotective effect exerted by vitamin D3 has been shown to be mediated by its interaction with the vitamin D3 receptor (VDR3). This receptor is a transcription factor that controls the expression of genes responsible for orchestrating various biological responses [174]. It has been shown that this class of receptors is widely distributed in the brain and especially concentrated in dopaminergic neurons of SNpc [175, 176]. This could explain the essential role that vitamin D seems to have in the development and survival of dopaminergic neurons, as well as its influence on the synthesis and metabolism of dopamine [175, 177].
In addition, VDR3 are also expressed in in endothelial cells of the blood-brain barrier and, therefore, vitamin D can also have a direct action on these cells [178-180]. Thus, Kim et al. [181] have shown that treatment with vitamin D3 recovered the expression of P-glycoprotein, which acts as a membrane transporter in endothelial cells of the blood-brain barrier, and whose levels were reduced after exposure to 6-OHDA and preformed α-synuclein fibrils. Therefore, through its interaction with VDR3, vitamin D3 not only supports the survival of dopaminergic neurons, but also restores the function of endothelial cells in the blood-brain barrier.
6. DISCUSSION
The data analyzed in the present review show that antioxidants of natural origin have important neuroprotective effects against morphological, biochemical and molecular alterations observed in the Parkinson’s models generated by 6-OHDA or MPTP in rodents. Taken together, the results show that curcumin, followed by chrysin, nariginin, astilbine and baicalein, are the most studied antioxidants and that they show the most significant neuroprotective effects, since they improved almost all the analyzed variables, exerting a broad action on the multiple pathological mechanisms underlying the development of parkinsonism.
On the other hand, the studies analyzed also show that, although these dietary antioxidants can induce good adaptive responses when administered in certain doses, considered beneficial, they are less effective and can even produce toxicity when administered in higher doses [182-185]. This biphasic pattern of dose response (i.e., low dose stimulation and high dose inhibition) is termed hormesis and could occur for some of the antioxidants included in the present review [186-188].
A hormetic dose response may be mediated by activation of the Kelch-like ECH-associated protein 1 (Keap1)/Nrf2/antioxidant response element (ARE) pathway [184,189, 190]. As mentioned previously, the Nrf2 is a transcription factor that controls the expression of numerous genes related to cell protection and survival under various stress conditions [191,192]. Although under physiological conditions the Nrf2 activity is suppressed by Keap1, it is postulated that exposure to exogenous antioxidants may slightly increase ROS levels, which serves as a hormetic signal to activate the Nrf2, translocate it to the nucleus and bind it to ARE [193-195]. In this way, the activation of the Nrf2/ARE pathway promotes a protective effect by down-regulating oxidative stress, suppressing inflammation, promoting detoxification, and decreasing apoptosis and autophagy [193, 196].
From our point of view, the activation of the Nrf2 induced by several of the antioxidants analyzed here can be considered as a common pathway underlying the protective effects of these substances, particularly baicalein, chrysin, curcumin, EGCG, ferulic acid, hesperidin, naringenin, puerarin and resveratrol [54, 197-207]. Although it must be taken into account that these antioxidants could modulate the activity of the Keap1/Nrf2 system through different molecular mechanisms. Thus, while some of these phytochemicals modulate the activity of the Nrf2 by acting directly on its inhibitor Keap1 [204], other compounds regulate the activity of this protein through epigenetic modifications [208, 209] or through the activation of protein kinases (PI3K, ERK, MAPK, GSK-3) that can phosphorylate and activate the Nrf2 [189, 197, 198, 201, 210-213].
Another signaling pathway that can be highlighted is the PI3K/Akt pathway, which is activated by several of the antioxidants discussed here [50, 52, 158, 159]. Furthermore, since this pathway promotes the activation and nuclear translocation of the Nrf2, it is possible that the neuroprotective effects of antioxidants that activate the PI3K/Akt pathway are also related to the cytoprotective activity of the Nrf2 [210].
Taking this information into account, it can be considered that most of the antioxidants included here could be considered hormetic agents capable of activating the Nrf2 and inducing a wide spectrum of very promising protective responses for the prevention and treatment of PD. However, the biphasic nature of the responses observed for some of the exogenous antioxidants (such as curcumin or resveratrol) suggests the existence of optimal dose ranges that must be carefully considered when these compounds are to be used in therapeutic interventions or public health [182-184]. In this way, possible adverse effects after exposure to higher doses of antioxidants could be avoided.
The evidence presented here indicates that the administration of antioxidants of natural origin promotes a clear neuroprotective effect in experimental models. In contrast, the available data on the use of these substances in patients with PD are more limited, although some studies indicate a putative neuroprotective effect of dietary antioxidants in humans. Moreover, it has been postulated that neurodegeneration in PD could be related to the nutritional deficiency of antioxidant compounds such as vitamins, and some studies show deficient levels of antioxidants in PD patients [214-218]. In the same way, the findings of various investigations support that the intake of antioxidant compounds such as vitamins A, B, C or E reduces the risk of developing PD [219-225].
Despite the fact that some studies have not found a relationship between the consumption of antioxidants and a lower risk of developing PD [226-231], more recent experimental data obtained from in vitro studies with human cells shows that antioxidants reduce oxidative stress and apoptosis, prevent neurotoxicity associated with α-synuclein, and restore mitochondrial activity in human cells [232-238]. Considering these data, it can be concluded that although they demonstrate the potential beneficial effect of antioxidants, the efficacy of nutritional intervention in PD remains controversial, as few human studies have been conducted to date. For this reason, it would be recommended that in the future new observational and interventional studies be carried out to evaluate and confirm the efficacy of the use of antioxidants in patients with PD. Furthermore, given the biphasic response induced by some of the antioxidants, the optimal dose range for each of these compounds should also be clarified.
7. LIMITATIONS
The data analyzed in the present revision show that the information available on the biochemical and molecular mechanisms underlying the protective action of antioxidants is insufficient. Therefore, future research would need to address this question in more detail, in order to draw more rigorous conclusions about the cellular and molecular pathways involved in the neuroprotective action of these substances.
From another point of view, this review has not been without limitations, which should be considered when generalizing the results described. On the one hand, in the studies analyzed, the naturally occurring antioxidants that are present in the diet have been investigated individually, but a single food does not contain a single micronutrient and the effect of the combination of the different molecules that compose it must be considered [3]. On the other hand, in some of the studies analyzed, the administration of antioxidants was carried out intraperitoneally, while it is usual for them to be ingested orally. Consequently, its bioavailability in the body is likely to vary and, with it, so do its effects [239].
Additionally, although no significant safety risks have been documented in the studies with animal models, other studies have reported that ingestion of polyphenols can cause harmful effects. In fact, as the effect of these substance changes depending on the amount consumed and their bioavailability, it is possible that potential toxicities arise when consuming them in excessive quantities [240]. This could be due to the fact that the pro-oxidant activities of polyphenols are linked to their catechol structure, which can lead to the formation of ROS and, consequently, to cell damage [241].
CONCLUSION
The information summarized in the present review indicates that the antioxidants of natural origin show a significant neuroprotective effect on the dopaminergic neurons of the nigrostriatal pathway in the models of parkinsonism induced by MPTP or 6-OHDA. Increases in striatal levels of dopamine, in the number of terminals and dopamine neurons, in the activity of dopamine transporters, are the main effects of these substances that lead to a significant improvement in motor and cognitive function observed in the different studies. These effects are achieved through an effective neuroprotective action of antioxidants by promoting an optimization of the cellular antioxidant status, reducing neuroinflammation, decreasing the overexpression of α-synuclein, improving the mitochondrial function, increasing the levels of neurotrophic factors, and as a consequence of all, inhibiting the apoptosis of dopaminergic neurons of SNpc.
On the other hand, considering its application to humans, these results should be interpreted with caution, since the efficacy of the use of antioxidants in patients with PD is not yet clear. Although numerous studies with cell and animal models support the potential of dietary antioxidants in the treatment of PD, many of these findings cannot be reproduced in human clinical trials. Therefore, future clinical studies are warranted to determine whether antioxidants present in the diet can act individually or in synergy as a strategy for the prevention or treatment of PD.
ACKNOWLEDGEMENTS
The authors thank Dr. Carmen Piñón, Buckingham, England, for the editorial assistance; and Elena Durán, Vigo, Spain, for illustrations.
LIST OF ABBREVIATIONS
- 6-OHDA
6-hydroxydopamine
- ACh
Acetylcholine
- Akt
Protein Kinase B
- ARE
Antioxidant Response Element
- BDNF
Brain-derived Neurotrophic Factor
- bFGF
Basic Fibroblast Growth Factor
- CAT
Catalase
- CD32
Cluster of Differentiation 32 Receptor
- CD163
Cluster of Differentiation 163 Receptor
- CD204
Cluster of Differentiation 204 Receptor
- CD206
Cluster of Differentiation 206 Receptor
- CNS
Central Nervous System
- COX-2
Cyclooxygenase 2
- DA
Dopamine
- DAT
Dopamine Transporter
- DOPAC
3,4-dihydroxyphenylacetic acid
- Drp1
Dynamin-related Protein 1
- EGCG
Epigallocatechin- 3- gallate
- ERK
Extracellular Signal-regulated Kinase
- Erβ
Estrogen Receptor Beta
- GDNF
Glial Cell Line-derived Neurotrophic Factor
- GFAP
Glial Fibrillary Acidic Protein
- GRx
Glutathione Reductase
- GSH
Glutathione
- GSH-Px
Glutathione Peroxidase
- GSK-3β
Glycogen Synthase Kinase-3β
- HVA
Homovanillic Acid
- Iba1
Ionized Calcium-Binding Adapter Molecule 1
- IFN-γ
Interferon Gamma
- IL-1β
Interleukin 1beta
- IL-2
Interleukin 2
- IL-4
Interleukin 4
- IL-6
Interleukin 6
- IL-10
Interleukin 10
- iNOS
Inducible Nitric Oxide Synthase
- Keap1
Kelch-like ECH-associated Protein 1
- LC3
Microtubule-associated Proteins 1A/1B-light chain 3
- L-DOPA
Levodopa
- LPO
Lipid Peroxidation
- MAO-B
Monoamine Oxidase Type B
- MEK
Mitogen-activated Protein Kinase
- Mfn1
Mitofusin 1
- Mfn2
Mitofusin 2
- MPP+
1-methyl-4-phenylpyridinium
- MPTP
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine
- mTORC1
Mammalian Target of Rapamycin Complex 1
- NADPH oxidase
Nicotinamide Adenine Dinucleotide Phosphate Oxidase
- NF-kB
Nuclear Factor Kappa B
- NGF
Nerve Growth Factor
- NLRP3
Nod-like Receptor Protein 3
- NO
Nitric Oxide
- Nrf2
Nuclear Factor Erythroid-derived 2-like 2
- PD
Parkinson’s disease
- p-4E-BP1
Phosphorylated Eukaryotic Translation Initiation Factor 4E Binding Protein 1
- PGC-1α
Peroxisome Proliferator-activated Receptor Gamma Coactivator 1-alpha
- PI3K
Phosphoinositide 3-kinase
- ROS
Reactive Oxygen Species
- S100B
Calcium Binding Protein B
- SIRT1
Sirtuin 1
- SNpc
Substantia Nigra Pars Compacta
- SOD
Superoxide Dismutase
- SQSTM1
Sequestosome-1
- TGF-β
Transforming Growth Factor-beta
- TH
Tyrosine Hydroxylase
- TLR4
Toll-like Receptor 4
- TNF-α
Tumor Necrosis Factor Alpha
- TrkA
Tropomyosin Receptor Kinase A
- TrkB
Tropomyosin Receptor Kinase B
- VD3R
Vitamin D3 Receptor
- VMAT2
Vesicular Monoamine Transporter 2
CONSENT FOR PUBLICATION
Not applicable.
STANDARDS OF REPORTING
PRISMA guidelines were followed for the study.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
SUPPLEMENTARY MATERIAL
PRISMA checklist is available on the publisher’s website along with the published article.
REFERENCES
- 1.Sandoval-Ávila S., Díaz N.F., Gómez-Pinedo U., Canales-Aguirre A.A., Gutiérrez-Mercado Y.K., Padilla-Camberos E., Márquez-Aguirre A.L., Díaz-Martínez N.E. Efecto neuroprotector de fitoquímicos en cultivo de neuronas dopaminérgicas. Neurologia. 2019;34(2):114–124. doi: 10.1016/j.nrl.2016.04.018. [DOI] [PubMed] [Google Scholar]
- 2.Twelves D., Perkins K.S., Counsell C. Systematic review of incidence studies of Parkinson’s disease. Mov. Disord. 2003;18(1):19–31. doi: 10.1002/mds.10305. [DOI] [PubMed] [Google Scholar]
- 3.Agim Z.S., Cannon J.R. Dietary factors in the etiology of Parkinson’s disease. BioMed Res. Int. 2015;2015:672838. doi: 10.1155/2015/672838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pohl F., Kong T., Lin P. The potential use of plant natural products and plant extracts with antioxidant properties for the prevention/treatment of neurodegenerative diseases: in vitro, in vivo and clinical trials. Molecules. 2018;23(12):3283. doi: 10.3390/molecules23123283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cabreira V., Massano J. Doença de Parkinson: Revisão clínica e atualização. Acta Med. Port. 2019;32(10):661–670. doi: 10.20344/amp.11978. [DOI] [PubMed] [Google Scholar]
- 6.Jankovic J. Parkinson’s disease: clinical features and diagnosis. J. Neurol. Neurosurg. Psychiatry. 2008;79(4):368–376. doi: 10.1136/jnnp.2007.131045. [DOI] [PubMed] [Google Scholar]
- 7.Zhang Y., Dawson V.L., Dawson T.M. Oxidative stress and genetics in the pathogenesis of Parkinson’s disease. Neurobiol. Dis. 2000;7(4):240–250. doi: 10.1006/nbdi.2000.0319. [DOI] [PubMed] [Google Scholar]
- 8.Mhyre T.R., Boyd J.T., Hamill R.W., Maguire-Zeiss K. In: Protein aggregation and fibrillogenesis in cerebral and systemic amyloid disease. Subcellular biochemistry. Harris J.R., editor. Vol. 65. Dordrecht: Springer; 2012. Parkinson’s disease. pp. 389–455. [Google Scholar]
- 9.Dauer W., Przedborski S. Parkinson’s disease: mechanisms and models. Neuron. 2003;39(6):889–909. doi: 10.1016/S0896-6273(03)00568-3. [DOI] [PubMed] [Google Scholar]
- 10.Poewe W., Seppi K., Tanner C.M., Halliday G.M., Brundin P., Volkmann J., Schrag A.E., Lang A.E. Parkinson disease. Nat. Rev. Dis. Primers. 2017;3(1):17013. doi: 10.1038/nrdp.2017.13. [DOI] [PubMed] [Google Scholar]
- 11.Shah S.P., Duda J.E. Dietary modifications in Parkinson’s disease: A neuroprotective intervention? Med. Hypotheses. 2015;85(6):1002–1005. doi: 10.1016/j.mehy.2015.08.018. [DOI] [PubMed] [Google Scholar]
- 12.Stoker T.B., Greenland J.C. Parkinson’s disease: Pathogenesis and clinical aspects. Brisbane: Codon Publications; 2018. [DOI] [PubMed] [Google Scholar]
- 13.Gazewood J.D., Richards D.R., Clebak K. Parkinson disease: an update. Am. Fam. Physician. 2013;87(4):267–273. [PubMed] [Google Scholar]
- 14.Fu W., Zhuang W., Zhou S., Wang X. Plant-derived neuroprotective agents in Parkinson’s disease. Am. J. Transl. Res. 2015;7(7):1189–1202. [PMC free article] [PubMed] [Google Scholar]
- 15.Olanow C.W., Schapira A.H. Therapeutic prospects for Parkinson disease. Ann. Neurol. 2013;74(3):337–347. doi: 10.1002/ana.24011. [DOI] [PubMed] [Google Scholar]
- 16.Ali S.S., Ahsan H., Zia M.K., Siddiqui T., Khan F.H. Understanding oxidants and antioxidants: Classical team with new players. J. Food Biochem. 2020;44(3):e13145. doi: 10.1111/jfbc.13145. [DOI] [PubMed] [Google Scholar]
- 17.Kelsey N.A., Wilkins H.M., Linseman D.A. Nutraceutical antioxidants as novel neuroprotective agents. Molecules. 2010;15(11):7792–7814. doi: 10.3390/molecules15117792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ding Y., Xin C., Zhang C.W., Lim K.L., Zhang H., Fu Z., Li L., Huang W. Natural molecules from Chinese herbs protecting against Parkinson’s disease via anti-oxidative stress. Front. Aging Neurosci. 2018;10:246. doi: 10.3389/fnagi.2018.00246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yan M.H., Wang X., Zhu X. Mitochondrial defects and oxidative stress in Alzheimer disease and Parkinson disease. Free Radic. Biol. Med. 2013;62:90–101. doi: 10.1016/j.freeradbiomed.2012.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Neha K., Haider M.R., Pathak A., Yar M.S. Medicinal prospects of antioxidants: A review. Eur. J. Med. Chem. 2019;178:687–704. doi: 10.1016/j.ejmech.2019.06.010. [DOI] [PubMed] [Google Scholar]
- 21.Carocho M., Ferreira I.C. A review on antioxidants, prooxidants and related controversy: natural and synthetic compounds, screening and analysis methodologies and future perspectives. Food Chem. Toxicol. 2013;51:15–25. doi: 10.1016/j.fct.2012.09.021. [DOI] [PubMed] [Google Scholar]
- 22.Aziz M.A., Diab A.S., Mohammed A.A. In: Antioxidants. Shalaby E., editor. London: IntechOpen; 2019. Antioxidant categories and mode of action. pp. 1–20. [DOI] [Google Scholar]
- 23.He L., He T., Farrar S., Ji L., Liu T., Ma X. Antioxidants maintain cellular redox homeostasis by elimination of reactive oxygen species. Cell. Physiol. Biochem. 2017;44(2):532–553. doi: 10.1159/000485089. [DOI] [PubMed] [Google Scholar]
- 24.Guerra-Araiza C., Álvarez-Mejía A.L., Sánchez-Torres S., Farfan-García E., Mondragón-Lozano R., Pinto-Almazán R., Salgado-Ceballos H. Effect of natural exogenous antioxidants on aging and on neurodegenerative diseases. Free Radic. Res. 2013;47(6-7):451–462. doi: 10.3109/10715762.2013.795649. [DOI] [PubMed] [Google Scholar]
- 25.Lalkovičová M., Danielisová V. Neuroprotection and antioxidants. Neural Regen. Res. 2016;11(6):865–874. doi: 10.4103/1673-5374.184447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lobo V., Patil A., Phatak A., Chandra N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn. Rev. 2010;4(8):118–126. doi: 10.4103/0973-7847.70902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Blesa J., Trigo-Damas I., Quiroga-Varela A., Lopez-Gonzalez del Rey N. In: Challenges in Parkinson’s disease. Dorszewska J., Kozubski W., editors. London: IntechOpen; 2016. Animal models of Parkinson’s disease. pp. 195–216. [DOI] [Google Scholar]
- 28.Blandini F., Armentero M.T., Martignoni E. The 6-hydroxydopamine model: news from the past. Parkinsonism Relat. Disord. 2008;14(2) Suppl. 2:S124–S129. doi: 10.1016/j.parkreldis.2008.04.015. [DOI] [PubMed] [Google Scholar]
- 29.Blandini F., Armentero M.T. Animal models of Parkinson’s disease. FEBS J. 2012;279(7):1156–1166. doi: 10.1111/j.1742-4658.2012.08491.x. [DOI] [PubMed] [Google Scholar]
- 30.Duty S., Jenner P. Animal models of Parkinson’s disease: a source of novel treatments and clues to the cause of the disease. Br. J. Pharmacol. 2011;164(4):1357–1391. doi: 10.1111/j.1476-5381.2011.01426.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fei Q., McCormack A.L., Di Monte D.A., Ethell D.W. Paraquat neurotoxicity is mediated by a Bak-dependent mechanism. J. Biol. Chem. 2008;283(6):3357–3364. doi: 10.1074/jbc.M708451200. [DOI] [PubMed] [Google Scholar]
- 32.Polinski N.K., Volpicelli-Daley L.A., Sortwell C.E., Luk K.C., Cremades N., Gottler L.M., Froula J., Duffy M.F., Lee V.M.Y., Martínez T.N., Dave K.D. Best practices for generating and using alpha-synuclein pre-formed fibrils to model Parkinson’s disease in rodents. J. Parkinsons Dis. 2018;8(2):303–322. doi: 10.3233/JPD-171248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Luk K.C., Song C., O’Brien P., Stieber A., Branch J.R., Brunden K.R., Trojanowski J.Q., Lee V.M.Y. Exogenous α-synuclein fibrils seed the formation of Lewy body-like intracellular inclusions in cultured cells. Proc. Natl. Acad. Sci. USA. 2009;106(47):20051–20056. doi: 10.1073/pnas.0908005106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Luk K.C., Kehm V.M., Zhang B., O’Brien P., Trojanowski J.Q., Lee V.M. Intracerebral inoculation of pathological α-synuclein initiates a rapidly progressive neurodegenerative α-synucleinopathy in mice. J. Exp. Med. 2012;209(5):975–986. doi: 10.1084/jem.20112457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Patterson J.R., Polinski N.K., Duffy M.F., Kemp C.J., Luk K.C., Volpicelli-Daley L.A., Kanaan N.M., Sortwell C.E. Generation of alpha-Synuclein preformed fibrils from monomers and use in vivo. JoVE. 2019; JoVE(148) doi: 10.3791/59758. e59758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grassi D., Howard S., Zhou M., Diaz-Perez N., Urban N.T., Guerrero-Given D., Kamasawa N., Volpicelli-Daley L.A., LoGrasso P., Lasmézas C.I. Identification of a highly neurotoxic α-synuclein species inducing mitochondrial damage and mitophagy in Parkinson’s disease. Proc. Natl. Acad. Sci. USA. 2018;115(11):E2634–E2643. doi: 10.1073/pnas.1713849115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ham S., Yun S.P., Kim H., Kim D., Seo B.A., Kim H., Shin J.Y., Dar M.A., Lee G.H., Lee Y.I., Kim D., Kim S., Kweon H.S., Shin J.H., Ko H.S., Lee Y. Amyloid-like oligomerization of AIMP2 contributes to α-synuclein interaction and Lewy-like inclusion. Sci. Transl. Med. 2020;12(569):eaax0091. doi: 10.1126/scitranslmed.aax0091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang J., Park E.S., Park H.J., Yan R., Grudniewska M., Zhang X., Oh S., Yang X., Baum J., Mouradian M.M. Apoptosis signal regulating kinase 1 deletion mitigates α-synuclein pre-formed fibril propagation in mice. Neurobiol. Aging. 2020;85:49–57. doi: 10.1016/j.neurobiolaging.2019.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chung H.K., Ho H.A., Pérez-Acuña D., Lee S.J. Modeling α-synuclein propagation with preformed fibril injections. J. Mov. Disord. 2019;12(3):139–151. doi: 10.14802/jmd.19046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Earls R.H., Menees K.B., Chung J., Barber J., Gutekunst C.A., Hazim M.G., Lee J.K. Intrastriatal injection of preformed alpha-synuclein fibrils alters central and peripheral immune cell profiles in non-transgenic mice. J. Neuroinflammation. 2019;16(1):250. doi: 10.1186/s12974-019-1636-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kuan W.L., Stott K., He X., Wood T.C., Yang S., Kwok J.C.F., Hall K., Zhao Y., Tietz O., Aigbirhio F.I., Vernon A.C., Barker R.A. Systemic α-synuclein injection triggers selective neuronal pathology as seen in patients with Parkinson’s disease. Mol. Psychiatry. 2021;26(2):556–567. doi: 10.1038/s41380-019-0608-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gamber K.M. Animal models of Parkinson’s disease: New models provide greater translational and predictive value. Biotechniques. 2016;61(4):210–211. doi: 10.2144/000114463. [DOI] [Google Scholar]
- 43.Moher D., Liberati A., Tetzlaff J., Altman D.G. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kunzler A., Ribeiro C.T., Gasparotto J., Petiz L.L., da Rosa Silva H.T., da Silva J.D., Jr, Bortolin R., de Souza P.O., Barreto F., Espitia-Pérez P., Schnorr C.E., Somensi N., Moreira J.C.F., Gelain D.P. The effects of retinol oral supplementation in 6-hydroxydopamine dopaminergic denervation model in Wistar rats. Neurochem. Int. 2019;125:25–34. doi: 10.1016/j.neuint.2019.02.002. [DOI] [PubMed] [Google Scholar]
- 45.Xia X.J., Lian Y.G., Zhao H.Y., Xu Q.L. Curcumin protects from oxidative stress and inhibits α-synuclein aggregation in MPTP induced parkinsonian mice. Int. J. Clin. Exp. Med. 2016;9(2):2654–2665. [Google Scholar]
- 46.Del Fabbro L., Rossito Goes A., Jesse C.R., de Gomes M.G., Cattelan S.L., Lobo L.F.V., Lobo L.A.A.B., Nunes A.R.V., Reis S.A., Oliveira M.S., Furian A.F., Boeira S.P. Chrysin protects against behavioral, cognitive and neurochemical alterations in a 6-hydroxydopamine model of Parkinson’s disease. Neurosci. Lett. 2019;706:158–163. doi: 10.1016/j.neulet.2019.05.036. [DOI] [PubMed] [Google Scholar]
- 47.Xu Q., Langley M., Kanthasamy A.G., Reddy M.B. Epigallocatechin gallate has a neurorescue effect in a mouse model of Parkinson disease. J. Nutr. 2017;147(10):1926–1931. doi: 10.3945/jn.117.255034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Anis E., Zafeer M.F., Firdaus F., Islam S.N., Anees Khan A., Ali A., Hossain M.M. Ferulic acid reinstates mitochondrial dynamics through PGC1α expression modulation in 6-hydroxydopamine lesioned rats. Phytother. Res. 2020;34(1):214–226. doi: 10.1002/ptr.6523. [DOI] [PubMed] [Google Scholar]
- 49.Nataraj J., Manivasagam T., Thenmozhi A.J., Essa M.M. Lutein protects dopaminergic neurons against MPTP-induced apoptotic death and motor dysfunction by ameliorating mitochondrial disruption and oxidative stress. Nutr. Neurosci. 2016;19(6):237–246. doi: 10.1179/1476830515Y.0000000010. [DOI] [PubMed] [Google Scholar]
- 50.Baluchnejadmojarad T., Jamali-Raeufy N., Zabihnejad S., Rabiee N., Roghani M. Troxerutin exerts neuroprotection in 6-hydroxydopamine lesion rat model of Parkinson’s disease: Possible involvement of PI3K/ERβ signaling. Eur. J. Pharmacol. 2017;801:72–78. doi: 10.1016/j.ejphar.2017.03.002. [DOI] [PubMed] [Google Scholar]
- 51.Lima L.A.R., Lopes M.J.P., Costa R.O., Lima F.A.V., Neves K.R.T., Calou I.B.F., Andrade G.M., Viana G.S.B. Vitamin D protects dopaminergic neurons against neuroinflammation and oxidative stress in hemiparkinsonian rats. J. Neuroinflammation. 2018;15(1):249. doi: 10.1186/s12974-018-1266-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhu Y.L., Sun M.F., Jia X.B., Cheng K., Xu Y.D., Zhou Z.L., Zhang P.H., Qiao C.M., Cui C., Chen X., Yang X.S., Shen Y.Q. Neuroprotective effects of Astilbin on MPTP-induced Parkinson’s disease mice: Glial reaction, α-synuclein expression and oxidative stress. Int. Immunopharmacol. 2019;66:19–27. doi: 10.1016/j.intimp.2018.11.004. [DOI] [PubMed] [Google Scholar]
- 53.Goes A.T.R., Jesse C.R., Antunes M.S., Lobo Ladd F.V., Lobo Ladd A.A.B., Luchese C., Paroul N., Boeira S.P. Protective role of chrysin on 6-hydroxydopamine-induced neurodegeneration a mouse model of Parkinson’s disease: Involvement of neuroinflammation and neurotrophins. Chem. Biol. Interact. 2018;279:111–120. doi: 10.1016/j.cbi.2017.10.019. [DOI] [PubMed] [Google Scholar]
- 54.Krishnamoorthy A., Sevanan M., Mani S., Balu M., Balaji S. P, R. Chrysin restores MPTP induced neuroinflammation, oxidative stress and neurotrophic factors in an acute Parkinson’s disease mouse model. Neurosci. Lett. 2019;709:134382. doi: 10.1016/j.neulet.2019.134382. [DOI] [PubMed] [Google Scholar]
- 55.Song S., Nie Q., Li Z., Du G. Curcumin improves neurofunctions of 6-OHDA-induced parkinsonian rats. Pathol. Res. Pract. 2016;212(4):247–251. doi: 10.1016/j.prp.2015.11.012. [DOI] [PubMed] [Google Scholar]
- 56.Wang Y.L., Ju B., Zhang Y.Z., Yin H.L., Liu Y.J., Wang S.S., Zeng Z.L., Yang X.P., Wang H.T., Li J.F. Protective effect of curcumin against oxidative stress-induced injury in rats with Parkinson’s disease through the Wnt/β-catenin signaling pathway. Cell. Physiol. Biochem. 2017;43(6):2226–2241. doi: 10.1159/000484302. [DOI] [PubMed] [Google Scholar]
- 57.Li X.H., Dai C.F., Chen L., Zhou W.T., Han H.L., Dong Z.F. 7,8-dihydroxyflavone ameliorates motor deficits via suppressing α‐synuclein expression and oxidative stress in the MPTP‐induced mouse model of Parkinson’s disease. CNS Neurosci. Ther. 2016;22(7):617–624. doi: 10.1111/cns.12555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mani S., Sekar S., Barathidasan R., Manivasagam T., Thenmozhi A.J., Sevanan M., Chidambaram S.B., Essa M.M., Guillemin G.J., Sakharkar M.K. Naringenin decreases α-synuclein expression and neuroinflammation in MPTP-induced Parkinson’s disease model in mice. Neurotox. Res. 2018;33(3):656–670. doi: 10.1007/s12640-018-9869-3. [DOI] [PubMed] [Google Scholar]
- 59.Sugumar M., Sevanan M., Sekar S. Neuroprotective effect of naringenin against MPTP-induced oxidative stress. Int. J. Neurosci. 2019;129(6):534–539. doi: 10.1080/00207454.2018.1545772. [DOI] [PubMed] [Google Scholar]
- 60.Sian J., Dexter D.T., Lees A.J., Daniel S., Agid Y., Javoy-Agid F., Jenner P., Marsden C.D. Alterations in glutathione levels in Parkinson’s disease and other neurodegenerative disorders affecting basal ganglia. Ann. Neurol. 1994;36(3):348–355. doi: 10.1002/ana.410360305. [DOI] [PubMed] [Google Scholar]
- 61.Dexter D.T., Wells F.R., Lees A.J., Agid F., Agid Y., Jenner P., Marsden C.D. Increased nigral iron content and alterations in other metal ions occurring in brain in Parkinson’s disease. J. Neurochem. 1989;52(6):1830–1836. doi: 10.1111/j.1471-4159.1989.tb07264.x. [DOI] [PubMed] [Google Scholar]
- 62.Riederer P., Sofic E., Rausch W.D., Schmidt B., Reynolds G.P., Jellinger K., Youdim M.B. Transition metals, ferritin, glutathione, and ascorbic acid in parkinsonian brains. J. Neurochem. 1989;52(2):515–520. doi: 10.1111/j.1471-4159.1989.tb09150.x. [DOI] [PubMed] [Google Scholar]
- 63.Stankiewicz J., Panter S.S., Neema M., Arora A., Batt C.E., Bakshi R. Iron in chronic brain disorders: imaging and neurotherapeutic implications. Neurotherapeutics. 2007;4(3):371–386. doi: 10.1016/j.nurt.2007.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Glass C.K., Saijo K., Winner B., Marchetto M.C., Gage F.H. Mechanisms underlying inflammation in neurodegeneration. Cell. 2010;140(6):918–934. doi: 10.1016/j.cell.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ammal Kaidery N., Ahuja M., Thomas B. Crosstalk between Nrf2 signaling and mitochondrial function in Parkinson’s disease. Mol. Cell. Neurosci. 2019;101:103413. doi: 10.1016/j.mcn.2019.103413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kovac S., Angelova P.R., Holmström K.M., Zhang Y., Dinkova-Kostova A.T., Abramov A.Y. Nrf2 regulates ROS production by mitochondria and NADPH oxidase. Biochim. Biophys. Acta. 2015;1850(4):794–801. doi: 10.1016/j.bbagen.2014.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ahuja M., Ammal Kaidery N., Yang L., Calingasan N., Smirnova N., Gaisin A., Gaisina I.N., Gazaryan I., Hushpulian D.M., Kaddour-Djebbar I., Bollag W.B., Morgan J.C., Ratan R.R., Starkov A.A., Beal M.F., Thomas B. Distinct Nrf2 signaling mechanisms of fumaric acid esters and their role in neuroprotection against 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced experimental Parkinson’s-like disease. J. Neurosci. 2016;36(23):6332–6351. doi: 10.1523/JNEUROSCI.0426-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Aquilano K., Baldelli S., Rotilio G., Ciriolo M.R. Role of nitric oxide synthases in Parkinson’s disease: a review on the antioxidant and anti-inflammatory activity of polyphenols. Neurochem. Res. 2008;33(12):2416–2426. doi: 10.1007/s11064-008-9697-6. [DOI] [PubMed] [Google Scholar]
- 69.Marinova-Mutafchieva L., Sadeghian M., Broom L., Davis J.B., Medhurst A.D., Dexter D.T. Relationship between microglial activation and dopaminergic neuronal loss in the substantia nigra: a time course study in a 6-hydroxydopamine model of Parkinson’s disease. J. Neurochem. 2009;110(3):966–975. doi: 10.1111/j.1471-4159.2009.06189.x. [DOI] [PubMed] [Google Scholar]
- 70.Wang Q., Liu Y., Zhou J. Neuroinflammation in Parkinson’s disease and its potential as therapeutic target. Transl. Neurodegener. 2015;4(1):19. doi: 10.1186/s40035-015-0042-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhang G., Yang G., Liu J. Phloretin attenuates behavior deficits and neuroinflammatory response in MPTP induced Parkinson’s disease in mice. Life Sci. 2019;232:116600. doi: 10.1016/j.lfs.2019.116600. [DOI] [PubMed] [Google Scholar]
- 72.Blandini F. Neural and immune mechanisms in the pathogenesis of Parkinson’s disease. J. Neuroimmune Pharmacol. 2013;8(1):189–201. doi: 10.1007/s11481-013-9435-y. [DOI] [PubMed] [Google Scholar]
- 73.Sawada M., Suzumura A., Hosoya H., Marunouchi T., Nagatsu T. Interleukin-10 inhibits both production of cytokines and expression of cytokine receptors in microglia. J. Neurochem. 1999;72(4):1466–1471. doi: 10.1046/j.1471-4159.1999.721466.x. [DOI] [PubMed] [Google Scholar]
- 74.Kwilasz A.J., Grace P.M., Serbedzija P., Maier S.F., Watkins L.R. The therapeutic potential of interleukin-10 in neuroimmune diseases. Neuropharmacology, 2015;96(Pt A):55–69. doi: 10.1016/j.neuropharm.2014.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhao W., Xie W., Xiao Q., Beers D.R., Appel S.H. Protective effects of an anti-inflammatory cytokine, interleukin-4, on motoneuron toxicity induced by activated microglia. J. Neurochem. 2006;99(4):1176–1187. doi: 10.1111/j.1471-4159.2006.04172.x. [DOI] [PubMed] [Google Scholar]
- 76.Arimoto T., Choi D.Y., Lu X., Liu M., Nguyen X.V., Zheng N., Stewart C.A., Kim H.C., Bing G. Interleukin-10 protects against inflammation-mediated degeneration of dopaminergic neurons in substantia nigra. Neurobiol. Aging. 2007;28(6):894–906. doi: 10.1016/j.neurobiolaging.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 77.Hühner L., Rilka J., Gilsbach R., Zhou X., Machado V., Spittau B. Interleukin-4 protects dopaminergic neurons in vitro but is dispensable for MPTP induced neurodegeneration in vivo. Front. Mol. Neurosci. 2017;10:62. doi: 10.3389/fnmol.2017.00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Schwenkgrub J., Joniec-Maciejak I., Sznejder-Pachołek A., Wawer A., Ciesielska A., Bankiewicz K., Członkowska A., Członkowski A. Effect of human interleukin-10 on the expression of nitric oxide synthases in the MPTP-based model of Parkinson’s disease. Pharmacol. Rep. 2013;65(1):44–49. doi: 10.1016/S1734-1140(13)70962-9. [DOI] [PubMed] [Google Scholar]
- 79.Tesseur I., Nguyen A., Chang B., Li L., Woodling N.S., Wyss-Coray T., Luo J. Deficiency in neuronal TGF-β signaling leads to nigrostriatal degeneration and activation of TGF-β signaling protects against MPTP neurotoxicity in mice. J. Neurosci. 2017;37(17):4584–4592. doi: 10.1523/JNEUROSCI.2952-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dobolyi A., Vincze C., Pál G., Lovas G. The neuroprotective functions of transforming growth factor beta proteins. Int. J. Mol. Sci. 2012;13(7):8219–8258. doi: 10.3390/ijms13078219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Makwana M., Jones L.L., Cuthill D., Heuer H., Bohatschek M., Hristova M., Friedrichsen S., Ormsby I., Bueringer D., Koppius A., Bauer K., Doetschman T., Raivich G. Endogenous transforming growth factor β 1 suppresses inflammation and promotes survival in adult CNS. J. Neurosci. 2007;27(42):11201–11213. doi: 10.1523/JNEUROSCI.2255-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Krieglstein K., Unsicker K. Transforming growth factor-β promotes survival of midbrain dopaminergic neurons and protects them against N-methyl-4-phenylpyridinium ion toxicity. Neuroscience. 1994;63(4):1189–1196. doi: 10.1016/0306-4522(94)90583-5. [DOI] [PubMed] [Google Scholar]
- 83.Krieglstein K., Suter-Crazzolara C., Fischer W.H., Unsicker K. TGF-beta superfamily members promote survival of midbrain dopaminergic neurons and protect them against MPP+ toxicity. EMBO J. 1995;14(4):736–742. doi: 10.1002/j.1460-2075.1995.tb07052.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Andrews Z.B., Zhao H., Frugier T., Meguro R., Grattan D.R., Koishi K., McLennan I.S. Transforming growth factor beta2 haploinsufficient mice develop age-related nigrostriatal dopamine deficits. Neurobiol. Dis. 2006;21(3):568–575. doi: 10.1016/j.nbd.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 85.Farkas L.M., Dünker N., Roussa E., Unsicker K., Krieglstein K. Transforming growth factor-β(s) are essential for the development of midbrain dopaminergic neurons in vitro and in vivo. J. Neurosci. 2003;23(12):5178–5186. doi: 10.1523/JNEUROSCI.23-12-05178.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hung K.C., Huang H.J., Wang Y.T., Lin A.M.Y. Baicalein attenuates α-synuclein aggregation, inflammasome activation and autophagy in the MPP+-treated nigrostriatal dopaminergic system in vivo. J. Ethnopharmacol. 2016;194:522–529. doi: 10.1016/j.jep.2016.10.040. [DOI] [PubMed] [Google Scholar]
- 87.Zhou T., Zhu M., Liang Z. (-)-Epigallocatechin-3-gallate modulates peripheral immunity in the MPTP-induced mouse model of Parkinson’s disease. Mol. Med. Rep. 2018;17(4):4883–4888. doi: 10.3892/mmr.2018.8470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Antunes M.S., Cattelan S.L., Ladd F.V.L., Ladd A.A.B.L., Moreira A.L., Bortolotto V.C., Silva M.R.P., Araújo S.M., Prigol M., Nogueira C.W., Boeira S.P. Hesperidin ameliorates anxiety-depressive-like behavior in 6-OHDA model of Parkinson’s disease by regulating striatal cytokine and neurotrophic factors levels and dopaminergic innervation loss in the striatum of mice. Mol. Neurobiol. 2020;57(7):3027–3041. doi: 10.1007/s12035-020-01940-3. [DOI] [PubMed] [Google Scholar]
- 89.Kim H.D., Jeong K.H., Jung U.J., Kim S.R. Myricitrin ameliorates 6-hydroxydopamine-induced dopaminergic neuronal loss in the substantia nigra of mouse brain. J. Med. Food. 2016;19(4):374–382. doi: 10.1089/jmf.2015.3581. [DOI] [PubMed] [Google Scholar]
- 90.Kim H.D., Jeong K.H., Jung U.J., Kim S.R. Naringin treatment induces neuroprotective effects in a mouse model of Parkinson’s disease in vivo, but not enough to restore the lesioned dopaminergic system. J. Nutr. Biochem. 2016;28:140–146. doi: 10.1016/j.jnutbio.2015.10.013. [DOI] [PubMed] [Google Scholar]
- 91.Jiang M., Yun Q., Niu G., Gao Y., Shi F., Yu S. Puerarin prevents inflammation and apoptosis in the neurocytes of a murine Parkinson’s disease model. Genet. Mol. Res. 2016;15(4):1–9. doi: 10.4238/gmr.15047501. [DOI] [PubMed] [Google Scholar]
- 92.Yang J.S., Wu X.H., Yu H.G., Teng L.S. Tangeretin inhibits neurodegeneration and attenuates inflammatory responses and behavioural deficits in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced Parkinson’s disease dementia in rats. Inflammopharmacology. 2017;25(4):471–484. doi: 10.1007/s10787-017-0348-x. [DOI] [PubMed] [Google Scholar]
- 93.Calvello R., Cianciulli A., Nicolardi G., De Nuccio F., Giannotti L., Salvatore R., Porro C., Trotta T., Panaro M.A., Lofrumento D.D. Vitamin D treatment attenuates neuroinflammation and dopaminergic neurodegeneration in an animal model of Parkinson’s disease, shifting M1 to M2 microglia responses. J. Neuroimmune Pharmacol. 2017;12(2):327–339. doi: 10.1007/s11481-016-9720-7. [DOI] [PubMed] [Google Scholar]
- 94.Liu W., Tang Y., Feng J. Cross talk between activation of microglia and astrocytes in pathological conditions in the central nervous system. Life Sci. 2011;89(5-6):141–146. doi: 10.1016/j.lfs.2011.05.011. [DOI] [PubMed] [Google Scholar]
- 95.Okuno T., Nakatsuji Y., Kumanogoh A., Moriya M., Ichinose H., Sumi H., Fujimura H., Kikutani H., Sakoda S. Loss of dopaminergic neurons by the induction of inducible nitric oxide synthase and cyclooxygenase-2 via CD 40: relevance to Parkinson’s disease. J. Neurosci. Res. 2005;81(6):874–882. doi: 10.1002/jnr.20599. [DOI] [PubMed] [Google Scholar]
- 96.Eve D.J., Nisbet A.P., Kingsbury A.E., Hewson E.L., Daniel S.E., Lees A.J., Marsden C.D., Foster O.J. Basal ganglia neuronal nitric oxide synthase mRNA expression in Parkinson’s disease. Brain Res. Mol. Brain Res. 1998;63(1):62–71. doi: 10.1016/S0169-328X(98)00259-9. [DOI] [PubMed] [Google Scholar]
- 97.Hunot S., Boissière F., Faucheux B., Brugg B., Mouatt-Prigent A., Agid Y., Hirsch E.C. Nitric oxide synthase and neuronal vulnerability in Parkinson’s disease. Neuroscience. 1996;72(2):355–363. doi: 10.1016/0306-4522(95)00578-1. [DOI] [PubMed] [Google Scholar]
- 98.Shergill J.K., Cammack R., Cooper C.E., Cooper J.M., Mann V.M., Schapira A.H. Detection of nitrosyl complexes in human substantia nigra, in relation to Parkinson’s disease. Biochem. Biophys. Res. Commun. 1996;228(2):298–305. doi: 10.1006/bbrc.1996.1656. [DOI] [PubMed] [Google Scholar]
- 99.Liberatore G.T., Jackson-Lewis V., Vukosavic S., Mandir A.S., Vila M., McAuliffe W.G., Dawson V.L., Dawson T.M., Przedborski S. Inducible nitric oxide synthase stimulates dopaminergic neurodegeneration in the MPTP model of Parkinson disease. Nat. Med. 1999;5(12):1403–1409. doi: 10.1038/70978. [DOI] [PubMed] [Google Scholar]
- 100.Tieu K., Ischiropoulos H., Przedborski S. Nitric oxide and reactive oxygen species in Parkinson’s disease. IUBMB Life. 2003;55(6):329–335. doi: 10.1080/1521654032000114320. [DOI] [PubMed] [Google Scholar]
- 101.Knott C., Stern G., Wilkin G.P. Inflammatory regulators in Parkinson’s disease: iNOS, lipocortin-1, and cyclooxygenases-1 and -2. Mol. Cell. Neurosci. 2000;16(6):724–739. doi: 10.1006/mcne.2000.0914. [DOI] [PubMed] [Google Scholar]
- 102.Campolo M., Paterniti I., Siracusa R., Filippone A., Esposito E., Cuzzocrea S. TLR4 absence reduces neuroinflammation and inflammasome activation in Parkinson’s diseases in vivo model. Brain Behav. Immun. 2019;76:236–247. doi: 10.1016/j.bbi.2018.12.003. [DOI] [PubMed] [Google Scholar]
- 103.Fellner L., Irschick R., Schanda K., Reindl M., Klimaschewski L., Poewe W., Wenning G.K., Stefanova N. Toll-like receptor 4 is required for α-synuclein dependent activation of microglia and astroglia. Glia. 2013;61(3):349–360. doi: 10.1002/glia.22437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Leitner G.R., Wenzel T.J., Marshall N., Gates E.J., Klegeris A. Targeting toll-like receptor 4 to modulate neuroinflammation in central nervous system disorders. Expert Opin. Ther. Targets. 2019;23(10):865–882. doi: 10.1080/14728222.2019.1676416. [DOI] [PubMed] [Google Scholar]
- 105.Dresselhaus E.C., Meffert M.K. Cellular specificity of NF-κB function in the nervous system. Front. Immunol. 2019;10:1043. doi: 10.3389/fimmu.2019.01043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Liu T., Zhang L., Joo D., Sun S.C. NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2017;2(1):1–9. doi: 10.1038/sigtrans.2017.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lang Y., Chu F., Shen D., Zhang W., Zheng C., Zhu J., Cui L. Role of inflammasomes in neuroimmune and neurodegenerative diseases: a systematic review. Mediators Inflamm. 2018;2018:1549549. doi: 10.1155/2018/1549549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Pellegrini C., Fornai M., Antonioli L., Blandizzi C., Calderone V. Phytochemicals as novel therapeutic strategies for NLRP3 inflammasome-related neurological, metabolic, and inflammatory diseases. Int. J. Mol. Sci. 2019;20(12):2876. doi: 10.3390/ijms20122876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Gordon R., Albornoz E.A., Christie D.C., Langley M.R., Kumar V., Mantovani S., Robertson A.A.B., Butler M.S., Rowe D.B., O’Neill L.A., Kanthasamy A.G., Schroder K., Cooper M.A., Woodruff T.M. Inflammasome inhibition prevents α-synuclein pathology and dopaminergic neurodegeneration in mice. Sci. Transl. Med. 2018;10(465):eaah4066. doi: 10.1126/scitranslmed.aah4066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zhang P., Shao X.Y., Qi G.J., Chen Q., Bu L.L., Chen L.J., Shi J., Ming J., Tian B. Cdk5‐dependent activation of neuronal inflammasomes in Parkinson’s disease. Mov. Disord. 2016;31(3):366–376. doi: 10.1002/mds.26488. [DOI] [PubMed] [Google Scholar]
- 111.Mao Z., Liu C., Ji S., Yang Q., Ye H., Han H., Xue Z. The NLRP3 inflammasome is involved in the pathogenesis of Parkinson’s disease in rats. Neurochem. Res. 2017;42(4):1104–1115. doi: 10.1007/s11064-017-2185-0. [DOI] [PubMed] [Google Scholar]
- 112.Zhou Y., Lu M., Du R.H., Qiao C., Jiang C.Y., Zhang K.Z., Ding J.H., Hu G. MicroRNA-7 targets Nod-like receptor protein 3 inflammasome to modulate neuroinflammation in the pathogenesis of Parkinson’s disease. Mol. Neurodegener. 2016;11(1):28. doi: 10.1186/s13024-016-0094-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Cao L., He C. Polarization of macrophages and microglia in inflammatory demyelination. Neurosci. Bull. 2013;29(2):189–198. doi: 10.1007/s12264-013-1324-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Liu J.Q., Zhao M., Zhang Z., Cui L.Y., Zhou X., Zhang W., Chu S.F., Zhang D.Y., Chen N.H. Rg1 improves LPS-induced Parkinsonian symptoms in mice via inhibition of NF-κB signaling and modulation of M1/M2 polarization. Acta Pharmacol. Sin. 2020;41(4):523–534. doi: 10.1038/s41401-020-0358-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Pisanu A., Lecca D., Mulas G., Wardas J., Simbula G., Spiga S., Carta A.R. Dynamic changes in pro- and anti-inflammatory cytokines in microglia after PPAR-γ agonist neuroprotective treatment in the MPTPp mouse model of progressive Parkinson’s disease. Neurobiol. Dis. 2014;71:280–291. doi: 10.1016/j.nbd.2014.08.011. [DOI] [PubMed] [Google Scholar]
- 116.Giuliano C., Siani F., Mus L., Ghezzi C., Cerri S., Pacchetti B., Bigogno C., Blandini F. Neuroprotective effects of lignan 7-hydroxymatairesinol (HMR/lignan) in a rodent model of Parkinson’s disease. Nutrition. 2020;69:110494. doi: 10.1016/j.nut.2019.04.006. [DOI] [PubMed] [Google Scholar]
- 117.Hernández-Romero M.C., Delgado-Cortés M.J., Sarmiento M., de Pablos R.M., Espinosa-Oliva A.M., Argüelles S., Bández M.J., Villarán R.F., Mauriño R., Santiago M., Venero J.L., Herrera A.J., Cano J., Machado A. Peripheral inflammation increases the deleterious effect of CNS inflammation on the nigrostriatal dopaminergic system. Neurotoxicology. 2012;33(3):347–360. doi: 10.1016/j.neuro.2012.01.018. [DOI] [PubMed] [Google Scholar]
- 118.Guo Y.J., Dong S.Y., Cui X.X., Feng Y., Liu T., Yin M., Kuo S.H., Tan E.K., Zhao W.J., Wu Y.C. Resveratrol alleviates MPTP-induced motor impairments and pathological changes by autophagic degradation of α-synuclein via SIRT1-deacetylated LC3. Mol. Nutr. Food Res. 2016;60(10):2161–2175. doi: 10.1002/mnfr.201600111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zhai H., Kang Z., Zhang H., Ma J., Chen G. Baicalin attenuated substantia nigra neuronal apoptosis in Parkinson’s disease rats via the mTOR/AKT/GSK-3β pathway. J. Integr. Neurosci. 2019;18(4):423–429. doi: 10.31083/j.jin.2019.04.192. [DOI] [PubMed] [Google Scholar]
- 120.Kroemer G., Mariño G., Levine B. Autophagy and the integrated stress response. Mol. Cell. 2010;40(2):280–293. doi: 10.1016/j.molcel.2010.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kitada M., Ogura Y., Koya D. In: Autophagy: cancer, other pathologies, inflammation, immunity, infection, and aging. Hayat M.A., editor. Vol. 8. Academic Press; 2016. Role of SIRT1 as a regulator of autophagy. pp. 89–100. [DOI] [Google Scholar]
- 122.Li X., Wang Y., Xiong Y., Wu J., Ding H., Chen X., Lan L., Zhang H. Galangin induces autophagy via deacetylation of LC3 by SIRT1 in HepG2 cells. Sci. Rep. 2016;6(1):30496. doi: 10.1038/srep30496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Liu H., Dai C., Fan Y., Guo B., Ren K., Sun T., Wang W. From autophagy to mitophagy: the roles of P62 in neurodegenerative diseases. J. Bioenerg. Biomembr. 2017;49(5):413–422. doi: 10.1007/s10863-017-9727-7. [DOI] [PubMed] [Google Scholar]
- 124.Nakaso K., Yoshimoto Y., Nakano T., Takeshima T., Fukuhara Y., Yasui K., Araga S., Yanagawa T., Ishii T., Nakashima K. Transcriptional activation of p62/A170/ZIP during the formation of the aggregates: possible mechanisms and the role in Lewy body formation in Parkinson’s disease. Brain Res. 2004;1012(1-2):42–51. doi: 10.1016/j.brainres.2004.03.029. [DOI] [PubMed] [Google Scholar]
- 125.Shin W.H., Park J.H., Chung K.C. The central regulator p62 between ubiquitin proteasome system and autophagy and its role in the mitophagy and Parkinson’s disease. BMB Rep. 2020;53(1):56–63. doi: 10.5483/BMBRep.2020.53.1.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Park J.S., Davis R.L., Sue C.M. Mitochondrial dysfunction in Parkinson’s disease: new mechanistic insights and therapeutic perspectives. Curr. Neurol. Neurosci. Rep. 2018;18(5):21. doi: 10.1007/s11910-018-0829-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Eschbach J., von Einem B., Müller K., Bayer H., Scheffold A., Morrison B.E., Rudolph K.L., Thal D.R., Witting A., Weydt P., Otto M., Fauler M., Liss B., McLean P.J., Spada A.R., Ludolph A.C., Weishaupt J.H., Danzer K.M. Mutual exacerbation of peroxisome proliferator-activated receptor γ coactivator 1α deregulation and α-synuclein oligomerization. Ann. Neurol. 2015;77(1):15–32. doi: 10.1002/ana.24294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Chen C., Turnbull D.M., Reeve A.K. Mitochondrial Dysfunction in Parkinson’s Disease-Cause or Consequence? Biology (Basel) 2019;8(2):38. doi: 10.3390/biology8020038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Franco-Iborra S., Vila M., Perier C. The Parkinson disease mitochondrial hypothesis: where are we at? Neuroscientist. 2016;22(3):266–277. doi: 10.1177/1073858415574600. [DOI] [PubMed] [Google Scholar]
- 130.Hu Q., Wang G. Mitochondrial dysfunction in Parkinson’s disease. Transl. Neurodegener. 2016;5(1):14. doi: 10.1186/s40035-016-0060-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Gómez-Lázaro M., Bonekamp N.A., Galindo M.F., Jordán J., Schrader M. 6-Hydroxydopamine (6-OHDA) induces Drp1-dependent mitochondrial fragmentation in SH-SY5Y cells. Free Radic. Biol. Med. 2008;44(11):1960–1969. doi: 10.1016/j.freeradbiomed.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 132.Rahimmi A., Khosrobakhsh F., Izadpanah E., Moloudi M.R., Hassanzadeh K. N-acetylcysteine prevents rotenone-induced Parkinson’s disease in rat: An investigation into the interaction of parkin and Drp1 proteins. Brain Res. Bull. 2015;113:34–40. doi: 10.1016/j.brainresbull.2015.02.007. [DOI] [PubMed] [Google Scholar]
- 133.Rappold P.M., Cui M., Grima J.C., Fan R.Z., de Mesy-Bentley K.L., Chen L., Zhuang X., Bowers W.J., Tieu K. Drp1 inhibition attenuates neurotoxicity and dopamine release deficits in vivo. Nat. Commun. 2014;5(1):5244. doi: 10.1038/ncomms6244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wang X., Su B., Liu W., He X., Gao Y., Castellani R.J., Perry G., Smith M.A., Zhu X. DLP1-dependent mitochondrial fragmentation mediates 1-methyl-4-phenylpyridinium toxicity in neurons: implications for Parkinson’s disease. Aging Cell. 2011;10(5):807–823. doi: 10.1111/j.1474-9726.2011.00721.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kamp F., Exner N., Lutz A.K., Wender N., Hegermann J., Brunner B., Nuscher B., Bartels T., Giese A., Beyer K., Eimer S., Winklhofer K.F., Haass C. Inhibition of mitochondrial fusion by α-synuclein is rescued by PINK1, Parkin and DJ-1. EMBO J. 2010;29(20):3571–3589. doi: 10.1038/emboj.2010.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Xie W., Chung K.K. Alpha-synuclein impairs normal dynamics of mitochondria in cell and animal models of Parkinson’s disease. J. Neurochem. 2012;122(2):404–414. doi: 10.1111/j.1471-4159.2012.07769.x. [DOI] [PubMed] [Google Scholar]
- 137.Grünewald A., Kumar K.R., Sue C.M. New insights into the complex role of mitochondria in Parkinson’s disease. Prog. Neurobiol. 2019;177:73–93. doi: 10.1016/j.pneurobio.2018.09.003. [DOI] [PubMed] [Google Scholar]
- 138.Siegel G.J., Chauhan N.B. Neurotrophic factors in Alzheimer’s and Parkinson’s disease brain. Brain Res. Brain Res. Rev. 2000;33(2-3):199–227. doi: 10.1016/S0165-0173(00)00030-8. [DOI] [PubMed] [Google Scholar]
- 139.Frim D.M., Uhler T.A., Galpern W.R., Beal M.F., Breakefield X.O., Isacson O. Implanted fibroblasts genetically engineered to produce brain-derived neurotrophic factor prevent 1-methyl-4-phenylpyridinium toxicity to dopaminergic neurons in the rat. Proc. Natl. Acad. Sci. USA. 1994;91(11):5104–5108. doi: 10.1073/pnas.91.11.5104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Gash D.M., Zhang Z., Ovadia A., Cass W.A., Yi A., Simmerman L., Russell D., Martin D., Lapchak P.A., Collins F., Hoffer B.J., Gerhardt G.A. Functional recovery in parkinsonian monkeys treated with GDNF. Nature. 1996;380(6571):252–255. doi: 10.1038/380252a0. [DOI] [PubMed] [Google Scholar]
- 141.Tomac A., Lindqvist E., Lin L.F., Ögren S.O., Young D., Hoffer B.J., Olson L. Protection and repair of the nigrostriatal dopaminergic system by GDNF in vivo. Nature. 1995;373(6512):335–339. doi: 10.1038/373335a0. [DOI] [PubMed] [Google Scholar]
- 142.Sampaio T.B., Savall A.S., Gutierrez M.E.Z., Pinton S. Neurotrophic factors in Alzheimer’s and Parkinson’s diseases: implications for pathogenesis and therapy. Neural Regen. Res. 2017;12(4):549–557. doi: 10.4103/1673-5374.205084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Cai P., Ye J., Zhu J., Liu D., Chen D., Wei X., Johnson N.R., Wang Z., Zhang H., Cao G., Xiao J., Ye J., Lin L. Inhibition of endoplasmic reticulum stress is involved in the neuroprotective effect of bFGF in the 6-OHDA-induced Parkinson’s disease model. Aging Dis. 2016;7(4):336–449. doi: 10.14336/AD.2016.0117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Engele J., Bohn M.C. The neurotrophic effects of fibroblast growth factors on dopaminergic neurons in vitro are mediated by mesencephalic glia. J. Neurosci. 1991;11(10):3070–3078. doi: 10.1523/JNEUROSCI.11-10-03070.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Hsuan S.L., Klintworth H.M., Xia Z. Basic fibroblast growth factor protects against rotenone-induced dopaminergic cell death through activation of extracellular signal-regulated kinases 1/2 and phosphatidylinositol-3 kinase pathways. J. Neurosci. 2006;26(17):4481–4491. doi: 10.1523/JNEUROSCI.4922-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Tooyama I., Kawamata T., Walker D., Yamada T., Hanai K., Kimura H., Iwane M., Igarashi K., McGeer E.G., McGeer P.L. Loss of basic fibroblast growth factor in substantia nigra neurons in Parkinson’s disease. Neurology. 1993;43(2):372–376. doi: 10.1212/WNL.43.2.372. [DOI] [PubMed] [Google Scholar]
- 147.Hu J., Ferreira A., Van Eldik L.J. S100β induces neuronal cell death through nitric oxide release from astrocytes. J. Neurochem. 1997;69(6):2294–2301. doi: 10.1046/j.1471-4159.1997.69062294.x. [DOI] [PubMed] [Google Scholar]
- 148.Bianchi R., Adami C., Giambanco I., Donato R. S100B binding to RAGE in microglia stimulates COX-2 expression. J. Leukoc. Biol. 2007;81(1):108–118. doi: 10.1189/jlb.0306198. [DOI] [PubMed] [Google Scholar]
- 149.Michetti F., D’Ambrosi N., Toesca A., Puglisi M.A., Serrano A., Marchese E., Corvino V., Geloso M.C. The S100B story: from biomarker to active factor in neural injury. J. Neurochem. 2019;148(2):168–187. doi: 10.1111/jnc.14574. [DOI] [PubMed] [Google Scholar]
- 150.Sorci G., Bianchi R., Riuzzi F., Tubaro C., Arcuri C., Giambanco I., Donato R. S100B protein, a damage-associated molecular pattern protein in the brain and heart, and beyond. Cardiovasc. Psychiatry Neurol. 2010;2010:1–13. doi: 10.1155/2010/656481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Muramatsu Y., Kurosaki R., Watanabe H., Michimata M., Matsubara M., Imai Y., Araki T. Expression of S-100 protein is related to neuronal damage in MPTP-treated mice. Glia. 2003;42(3):307–313. doi: 10.1002/glia.10225. [DOI] [PubMed] [Google Scholar]
- 152.Papuć E., Rejdak K. Increased cerebrospinal fluid S100B and NSE reflect neuronal and glial damage in Parkinson’s disease. Front. Aging Neurosci. 2020;12:156. doi: 10.3389/fnagi.2020.00156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Sathe K., Maetzler W., Lang J.D., Mounsey R.B., Fleckenstein C., Martin H.L., Schulte C., Mustafa S., Synofzik M., Vukovic Z., Itohara S., Berg D., Teismann P. S100B is increased in Parkinson’s disease and ablation protects against MPTP-induced toxicity through the RAGE and TNF-α pathway. Brain. 2012;135(Pt 11):3336–3347. doi: 10.1093/brain/aws250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Viana S.D., Valero J., Rodrigues-Santos P., Couceiro P., Silva A.M., Carvalho F., Ali S.F., Fontes-Ribeiro C.A., Pereira F.C. Regulation of striatal astrocytic receptor for advanced glycation end-products variants in an early stage of experimental Parkinson’s disease. J. Neurochem. 2016;138(4):598–609. doi: 10.1111/jnc.13682. [DOI] [PubMed] [Google Scholar]
- 155.Westphal D., Dewson G., Czabotar P.E., Kluck R.M. Molecular biology of Bax and Bak activation and action. Biochim. Biophys. Acta. 2011;1813(4):521–531. doi: 10.1016/j.bbamcr.2010.12.019. [DOI] [PubMed] [Google Scholar]
- 156.Youle R.J., Strasser A. The BCL-2 protein family: opposing activities that mediate cell death. Nat. Rev. Mol. Cell Biol. 2008;9(1):47–59. doi: 10.1038/nrm2308. [DOI] [PubMed] [Google Scholar]
- 157.Szybińska A., Leśniak W. P53 dysfunction in neurodegenerative diseases-the cause or effect of pathological changes? Aging Dis. 2017;8(4):506–518. doi: 10.14336/AD.2016.1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Chen W.F., Wu L., Du Z.R., Chen L., Xu A.L., Chen X.H., Teng J.J., Wong M.S. Neuroprotective properties of icariin in MPTP-induced mouse model of Parkinson’s disease: Involvement of PI3K/Akt and MEK/ERK signaling pathways. Phytomedicine. 2017;25:93–99. doi: 10.1016/j.phymed.2016.12.017. [DOI] [PubMed] [Google Scholar]
- 159.Huang N., Zhang Y., Chen M., Jin H., Nie J., Luo Y., Zhou S., Shi J., Jin F. Resveratrol delays 6-hydroxydopamine-induced apoptosis by activating the PI3K/Akt signaling pathway. Exp. Gerontol. 2019;124:110653. doi: 10.1016/j.exger.2019.110653. [DOI] [PubMed] [Google Scholar]
- 160.Zhang S., Wang S., Shi X., Feng X. Polydatin alleviates parkinsonism in MPTP-model mice by enhancing glycolysis in dopaminergic neurons. Neurochem. Int. 2020;139:104815. doi: 10.1016/j.neuint.2020.104815. [DOI] [PubMed] [Google Scholar]
- 161.Colla E. Linking the endoplasmic reticulum to Parkinson’s disease and alphasynucleinopathy. Front. Neurosci. 2019;13:560. doi: 10.3389/fnins.2019.00560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Burke R.E. Programmed cell death and new discoveries in the genetics of parkinsonism. J. Neurochem. 2008;104(4):875–890. doi: 10.1111/j.1471-4159.2007.05106.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Chaudhry Z.L., Ahmed B.Y. The role of caspases in Parkinson’s disease pathogenesis: a brief look at the mitochondrial pathway. Austin Alzheimers Parkinsons Dis. 2014;1(3):1–5. [Google Scholar]
- 164.Hitomi J., Katayama T., Eguchi Y., Kudo T., Taniguchi M., Koyama Y., Manabe T., Yamagishi S., Bando Y., Imaizumi K., Tsujimoto Y., Tohyama M. Involvement of caspase-4 in endoplasmic reticulum stress-induced apoptosis and Abeta-induced cell death. J. Cell Biol. 2004;165(3):347–356. doi: 10.1083/jcb.200310015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Jha S.K., Jha N.K., Kar R., Ambasta R.K., Kumar P. p38 MAPK and PI3K/AKT signalling cascades in Parkinson’s disease. Int. J. Mol. Cell. Med. 2015;4(2):67–86. [PMC free article] [PubMed] [Google Scholar]
- 166.Nakanishi A., Wada Y., Kitagishi Y., Matsuda S. Link between PI3K/AKT/PTEN pathway and NOX proteinin diseases. Aging Dis. 2014;5(3):203–211. doi: 10.14336/AD.2014.0500203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Luo D., Shi Y., Wang J., Lin Q., Sun Y., Ye K., Yan Q., Zhang H. 7,8-dihydroxyflavone protects 6-OHDA and MPTP induced dopaminergic neurons degeneration through activation of TrkB in rodents. Neurosci. Lett. 2016;620:43–49. doi: 10.1016/j.neulet.2016.03.042. [DOI] [PubMed] [Google Scholar]
- 168.Kim S.R., Chen X., Oo T.F., Kareva T., Yarygina O., Wang C., During M., Kholodilov N., Burke R.E. Dopaminergic pathway reconstruction by Akt/Rheb-induced axon regeneration. Ann. Neurol. 2011;70(1):110–120. doi: 10.1002/ana.22383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Huang Y., Sun L., Zhu S., Xu L., Liu S., Yuan C., Guo Y., Wang X. Neuroprotection against Parkinson’s disease through the activation of Akt/GSK3β signaling pathway by Tovophyllin A. Front. Neurosci. 2020;14:723. doi: 10.3389/fnins.2020.00723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Smith K.M., Dahodwala N. Sex differences in Parkinson’s disease and other movement disorders. Exp. Neurol. 2014;259:44–56. doi: 10.1016/j.expneurol.2014.03.010. [DOI] [PubMed] [Google Scholar]
- 171.Chen L.W. In: Trends in cell signaling pathways in neuronal fate decision. Wislet S., editor. London: IntechOpen; 2013. Roles of Wnt/β-catenin signaling in controlling the dopaminergic neuronal cell commitment of midbrain and therapeutic application for Parkinson’s disease. pp. 141–151. [DOI] [Google Scholar]
- 172.Marchetti B. Wnt/β-catenin signaling pathway governs a full program for dopaminergic neuron survival, neurorescue and regeneration in the MPTP mouse model of Parkinson’s disease. Int. J. Mol. Sci. 2018;19(12):3743. doi: 10.3390/ijms19123743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Marchetti B., Tirolo C., L’Episcopo F., Caniglia S., Testa N., Smith J.A., Pluchino S., Serapide M.F. Parkinson’s disease, aging and adult neurogenesis: Wnt/β-catenin signalling as the key to unlock the mystery of endogenous brain repair. Aging Cell. 2020;19(3):e13101. doi: 10.1111/acel.13101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Pike J.W., Meyer M.B. The vitamin D receptor: new paradigms for the regulation of gene expression by 1,25-dihydroxyvitamin D(3). Endocrinol. Metab. Clin. North Am. 2010;39(2):255–269. doi: 10.1016/j.ecl.2010.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Cui X., Pelekanos M., Liu P.Y., Burne T.H.J., McGrath J.J., Eyles D.W. The vitamin D receptor in dopamine neurons; its presence in human substantia nigra and its ontogenesis in rat midbrain. Neuroscience. 2013;236:77–87. doi: 10.1016/j.neuroscience.2013.01.035. [DOI] [PubMed] [Google Scholar]
- 176.Eyles D.W., Smith S., Kinobe R., Hewison M., McGrath J.J. Distribution of the vitamin D receptor and 1 α-hydroxylase in human brain. J. Chem. Neuroanat. 2005;29(1):21–30. doi: 10.1016/j.jchemneu.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 177.Pertile R.A.N., Cui X., Hammond L., Eyles D.W. Vitamin D regulation of GDNF/Ret signaling in dopaminergic neurons. FASEB J. 2018;32(2):819–828. doi: 10.1096/fj.201700713R. [DOI] [PubMed] [Google Scholar]
- 178.Kim D.H., Meza C.A., Clarke H., Kim J.S., Hickner R.C. Vitamin D and endothelial function. Nutrients. 2020;12(2):575. doi: 10.3390/nu12020575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Takahashi S., Maeda T., Sano Y., Nishihara H., Takeshita Y., Shimizu F., Kanda T. Active form of vitamin D directly protects the blood–brain barrier in multiple sclerosis. Clin. Exp. Neuroimmunol. 2017;8(3):244–254. doi: 10.1111/cen3.12398. [DOI] [Google Scholar]
- 180.Won S., Sayeed I., Peterson B.L., Wali B., Kahn J.S., Stein D.G. Vitamin D prevents hypoxia/reoxygenation-induced blood-brain barrier disruption via vitamin D receptor-mediated NF-kB signaling pathways. PLoS One. 2015;10(3):e0122821. doi: 10.1371/journal.pone.0122821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Kim H., Shin J.Y., Lee Y.S., Yun S.P., Maeng H.J., Lee Y. Brain endothelial P-glycoprotein level is reduced in Parkinson’s disease via a vitamin D receptor-dependent pathway. Int. J. Mol. Sci. 2020;21(22):8538. doi: 10.3390/ijms21228538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Calabrese E.J., Mattson M.P., Calabrese V. Resveratrol commonly displays hormesis: occurrence and biomedical significance. Hum. Exp. Toxicol. 2010;29(12):980–1015. doi: 10.1177/0960327110383625. [DOI] [PubMed] [Google Scholar]
- 183.Calabrese E.J., Dhawan G., Kapoor R., Mattson M.P., Rattan S.I. Curcumin and hormesis with particular emphasis on neural cells. Food Chem. Toxicol. 2019;129:399–404. doi: 10.1016/j.fct.2019.04.053. [DOI] [PubMed] [Google Scholar]
- 184.Calabrese E.J., Kozumbo W.J. The phytoprotective agent sulforaphane prevents inflammatory degenerative diseases and age-related pathologies via Nrf2-mediated hormesis. Pharmacol. Res. 2021;163:105283. doi: 10.1016/j.phrs.2020.105283. [DOI] [PubMed] [Google Scholar]
- 185.Inoue H., Akiyama S., Maeda-Yamamoto M., Nesumi A., Tanaka T., Murakami A. High-dose green tea polyphenols induce nephrotoxicity in dextran sulfate sodium-induced colitis mice by down-regulation of antioxidant enzymes and heat-shock protein expressions. Cell Stress Chaperones. 2011;16(6):653–662. doi: 10.1007/s12192-011-0280-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Brunetti G., Di Rosa G., Scuto M., Leri M., Stefani M., Schmitz-Linneweber C., Calabrese V., Saul N. Health span maintenance and prevention of Parkinson’s-like phenotypes with hydroxytyrosol and oleuropein aglycone in C. elegans. Int. J. Mol. Sci. 2020;21(7):2588. doi: 10.3390/ijms21072588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Calabrese V., Santoro A., Trovato Salinaro A., Modafferi S., Scuto M., Albouchi F., Monti D., Giordano J., Zappia M., Franceschi C., Calabrese E.J. Hormetic approaches to the treatment of Parkinson’s disease: Perspectives and possibilities. J. Neurosci. Res. 2018;96(10):1641–1662. doi: 10.1002/jnr.24244. [DOI] [PubMed] [Google Scholar]
- 188.Miquel S., Champ C., Day J., Aarts E., Bahr B.A., Bakker M., Bánáti D., Calabrese V., Cederholm T., Cryan J., Dye L., Farrimond J.A., Korosi A., Layé S., Maudsley S., Milenkovic D., Mohajeri M.H., Sijben J., Solomon A., Spencer J.P.E., Thuret S., Vanden Berghe W., Vauzour D., Vellas B., Wesnes K., Willatts P., Wittenberg R., Geurts L. Poor cognitive ageing: Vulnerabilities, mechanisms and the impact of nutritional interventions. Ageing Res. Rev. 2018;42:40–55. doi: 10.1016/j.arr.2017.12.004. [DOI] [PubMed] [Google Scholar]
- 189.Andreadi C.K., Howells L.M., Atherfold P.A., Manson M.M. Involvement of Nrf2, p38, B-Raf, and nuclear factor-kappaB, but not phosphatidylinositol 3-kinase, in induction of hemeoxygenase-1 by dietary polyphenols. Mol. Pharmacol. 2006;69(3):1033–1040. doi: 10.1124/mol.105.018374. [DOI] [PubMed] [Google Scholar]
- 190.Calabrese V., Cornelius C., Dinkova-Kostova A.T., Calabrese E.J., Mattson M.P. Cellular stress responses, the hormesis paradigm, and vitagenes: novel targets for therapeutic intervention in neurodegenerative disorders. Antioxid. Redox Signal. 2010;13(11):1763–1811. doi: 10.1089/ars.2009.3074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Calkins M.J., Johnson D.A., Townsend J.A., Vargas M.R., Dowell J.A., Williamson T.P., Kraft A.D., Lee J.M., Li J., Johnson J.A. The Nrf2/ARE pathway as a potential therapeutic target in neurodegenerative disease. Antioxid. Redox Signal. 2009;11(3):497–508. doi: 10.1089/ars.2008.2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Kim S., Indu Viswanath A.N., Park J.H., Lee H.E., Park A.Y., Choi J.W., Kim H.J., Londhe A.M., Jang B.K., Lee J., Hwang H., Lim S.M., Pae A.N., Park K.D. Nrf2 activator via interference of Nrf2-Keap1 interaction has antioxidant and anti-inflammatory properties in Parkinson’s disease animal model. Neuropharmacology. 2020;167:107989. doi: 10.1016/j.neuropharm.2020.107989. [DOI] [PubMed] [Google Scholar]
- 193.Calabrese E.J., Kozumbo W.J. The hormetic dose-response mechanism: Nrf2 activation. Pharmacol. Res. 2021;167:105526. doi: 10.1016/j.phrs.2021.105526. [DOI] [PubMed] [Google Scholar]
- 194.Itoh K., Wakabayashi N., Katoh Y., Ishii T., Igarashi K., Engel J.D., Yamamoto M. Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domain. Genes Dev. 1999;13(1):76–86. doi: 10.1101/gad.13.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Joshi G., Johnson J.A. The Nrf2-ARE pathway: a valuable therapeutic target for the treatment of neurodegenerative diseases. Recent Patents CNS Drug Discov. 2012;7(3):218–229. doi: 10.2174/157488912803252023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Wakabayashi N., Slocum S.L., Skoko J.J., Shin S., Kensler T.W. When NRF2 talks, who’s listening? Antioxid. Redox Signal. 2010;13(11):1649–1663. doi: 10.1089/ars.2010.3216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Chen C.Y., Jang J.H., Li M.H., Surh Y.J. Resveratrol upregulates heme oxygenase-1 expression via activation of NF-E2-related factor 2 in PC12 cells. Biochem. Biophys. Res. Commun. 2005;331(4):993–1000. doi: 10.1016/j.bbrc.2005.03.237. [DOI] [PubMed] [Google Scholar]
- 198.Chen M.C., Ye Y.Y., Ji G., Liu J.W. Hesperidin upregulates heme oxygenase-1 to attenuate hydrogen peroxide-induced cell damage in hepatic L02 cells. J. Agric. Food Chem. 2010;58(6):3330–3335. doi: 10.1021/jf904549s. [DOI] [PubMed] [Google Scholar]
- 199.Cheng D., Li W., Wang L., Lin T., Poiani G., Wassef A., Hudlikar R., Ondar P., Brunetti L., Kong A.N. Pharmacokinetics, pharmacodynamics, and PKPD modeling of curcumin in regulating antioxidant and epigenetic gene expression in healthy human volunteers. Mol. Pharm. 2019;16(5):1881–1889. doi: 10.1021/acs.molpharmaceut.8b01246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Ghanim H., Sia C.L., Korzeniewski K., Lohano T., Abuaysheh S., Marumganti A., Chaudhuri A., Dandona P. A resveratrol and polyphenol preparation suppresses oxidative and inflammatory stress response to a high-fat, high-carbohydrate meal. J. Clin. Endocrinol. Metab. 2011;96(5):1409–1414. doi: 10.1210/jc.2010-1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Hwang Y.P., Jeong H.G. Mechanism of phytoestrogen puerarin-mediated cytoprotection following oxidative injury: estrogen receptor-dependent up-regulation of PI3K/Akt and HO-1. Toxicol. Appl. Pharmacol. 2008;233(3):371–381. doi: 10.1016/j.taap.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 202.Kode A., Rajendrasozhan S., Caito S., Yang S.R., Megson I.L., Rahman I. Resveratrol induces glutathione synthesis by activation of Nrf2 and protects against cigarette smoke-mediated oxidative stress in human lung epithelial cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 2008;294(3):L478–L488. doi: 10.1152/ajplung.00361.2007. [DOI] [PubMed] [Google Scholar]
- 203.Ma Z.C., Hong Q., Wang Y.G., Liang Q.D., Tan H.L., Xiao C.R., Tang X.L., Shao S., Zhou S.S., Gao Y. Ferulic acid induces heme oxygenase-1 via activation of ERK and Nrf2. Drug Discov. Ther. 2011;5(6):299–305. doi: 10.5582/ddt.2011.v5.6.299. [DOI] [PubMed] [Google Scholar]
- 204.Qin S., Chen J., Tanigawa S., Hou D.X. Gene expression profiling and pathway network analysis of hepatic metabolic enzymes targeted by baicalein. J. Ethnopharmacol. 2012;140(1):131–140. doi: 10.1016/j.jep.2011.12.046. [DOI] [PubMed] [Google Scholar]
- 205.Seyyedebrahimi S., Khodabandehloo H., Nasli Esfahani E., Meshkani R. The effects of resveratrol on markers of oxidative stress in patients with type 2 diabetes: a randomized, double-blind, placebo-controlled clinical trial. Acta Diabetol. 2018;55(4):341–353. doi: 10.1007/s00592-017-1098-3. [DOI] [PubMed] [Google Scholar]
- 206.Yang H., Xu W., Zhou Z., Liu J., Li X., Chen L., Weng J., Yu Z. Curcumin attenuates urinary excretion of albumin in type II diabetic patients with enhancing nuclear factor erythroid-derived 2-like 2 (Nrf2) system and repressing inflammatory signaling efficacies. Exp. Clin. Endocrinol. Diabetes. 2015;123(6):360–367. doi: 10.1055/s-0035-1545345. [DOI] [PubMed] [Google Scholar]
- 207.Yu C.L., Zhao X.M., Niu Y.C. Ferulic acid protects against lead acetate-induced inhibition of neurite outgrowth by upregulating HO-1 in PC12 cells: involvement of ERK1/2-Nrf2 pathway. Mol. Neurobiol. 2016;53(9):6489–6500. doi: 10.1007/s12035-015-9555-x. [DOI] [PubMed] [Google Scholar]
- 208.Khor T.O., Huang Y., Wu T.Y., Shu L., Lee J., Kong A.N.T. Pharmacodynamics of curcumin as DNA hypomethylation agent in restoring the expression of Nrf2 via promoter CpGs demethylation. Biochem. Pharmacol. 2011;82(9):1073–1078. doi: 10.1016/j.bcp.2011.07.065. [DOI] [PubMed] [Google Scholar]
- 209.Shankar S., Kumar D., Srivastava R.K. Epigenetic modifications by dietary phytochemicals: implications for personalized nutrition. Pharmacol. Ther. 2013;138(1):1–17. doi: 10.1016/j.pharmthera.2012.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Fainstein M.K. Nrf2: La historia de un nuevo factor de transcripción que responde a estrés oxidativo. Rev. Educ. Bioquímica. 2007;26(1):18–25. [Google Scholar]
- 211.Hui Y., Chengyong T., Cheng L., Haixia H., Yuanda Z., Weihua Y. Resveratrol attenuates the cytotoxicity induced by amyloid-β1–42 in PC12 cells by upregulating heme oxygenase-1 via the PI3K/Akt/Nrf2 pathway. Neurochem. Res. 2018;43(2):297–305. doi: 10.1007/s11064-017-2421-7. [DOI] [PubMed] [Google Scholar]
- 212.Qin S., Hou D.X. The biofunctions of phytochemicals and their applications in farm animals: the Nrf2/Keap1 system as a target. Engineering. 2017;3(5):738–752. doi: 10.1016/J.ENG.2017.03.011. [DOI] [Google Scholar]
- 213.Zhang Y., Liu B., Chen X., Zhang N., Li G., Zhang L.H., Tan L.Y. Naringenin ameliorates behavioral dysfunction and neurological deficits in a d-galactose-induced aging mouse model through activation of PI3K/Akt/Nrf2 pathway. Rejuvenation Res. 2017;20(6):462–472. doi: 10.1089/rej.2017.1960. [DOI] [PubMed] [Google Scholar]
- 214.Lister T. Nutrition and lifestyle interventions for managing Parkinson’s disease: a narrative review. J. Mov. Disord. 2020;13(2):97–104. doi: 10.14802/jmd.20006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Medeiros M.S., Schumacher-Schuh A., Cardoso A.M., Bochi G.V., Baldissarelli J., Kegler A., Santana D., Chaves C.M.M.B.S., Schetinger M.R.C., Moresco R.N., Rieder C.R.M., Fighera M.R. Iron and oxidative stress in Parkinson’s disease: an observational study of injury biomarkers. PLoS One. 2016;11(1):e0146129. doi: 10.1371/journal.pone.0146129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Paraskevas G.P., Kapaki E., Petropoulou O., Anagnostouli M., Vagenas V., Papageorgiou C. Plasma levels of antioxidant vitamins C and E are decreased in vascular parkinsonism. J. Neurol. Sci. 2003;215(1-2):51–55. doi: 10.1016/S0022-510X(03)00184-9. [DOI] [PubMed] [Google Scholar]
- 217.Percário S., da Silva Barbosa A., Varela E.L.P., Gomes A.R.Q., Ferreira M.E.S., de Nazaré Araújo Moreira T., Dolabela M.F. Oxidative stress in Parkinson’s disease: potential benefits of antioxidant supplementation. Oxid. Med. Cell. Longev. 2020;2020:2360872. doi: 10.1155/2020/2360872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Sudha K., Rao A.V., Rao S., Rao A. Free radical toxicity and antioxidants in Parkinson’s disease. Neurol. India. 2003;51(1):60–62. [PubMed] [Google Scholar]
- 219.Etminan M., Gill S.S., Samii A. Intake of vitamin E, vitamin C, and carotenoids and the risk of Parkinson’s disease: a meta-analysis. Lancet Neurol. 2005;4(6):362–365. doi: 10.1016/S1474-4422(05)70097-1. [DOI] [PubMed] [Google Scholar]
- 220.Hantikainen E., Trolle Lagerros Y., Ye W., Serafini M., Adami H.O., Bellocco R., Bonn S. Dietary antioxidants and the risk of Parkinson disease: the Swedish national march cohort. Neurology. 2021;96(6):e895–e903. doi: 10.1212/WNL.0000000000011373. [DOI] [PubMed] [Google Scholar]
- 221.Miyake Y., Fukushima W., Tanaka K., Sasaki S., Kiyohara C., Tsuboi Y., Yamada T., Oeda T., Miki T., Kawamura N., Sakae N., Fukuyama H., Hirota Y., Nagai M. Dietary intake of antioxidant vitamins and risk of Parkinson’s disease: a case-control study in Japan. Eur. J. Neurol. 2011;18(1):106–113. doi: 10.1111/j.1468-1331.2010.03088.x. [DOI] [PubMed] [Google Scholar]
- 222.de Rijk M.C., Breteler M.M., den Breeijen J.H., Launer L.J., Grobbee D.E., van der Meché F.G., Hofman A. Dietary antioxidants and Parkinson disease. The Rotterdam Study. Arch. Neurol. 1997;54(6):762–765. doi: 10.1001/archneur.1997.00550180070015. [DOI] [PubMed] [Google Scholar]
- 223.Rimmelzwaan L.M., van Schoor N.M., Lips P., Berendse H.W., Eekhoff E.M. Systematic review of the relationship between vitamin D and Parkinson’s disease. J. Parkinsons Dis. 2016;6(1):29–37. doi: 10.3233/JPD-150615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Schirinzi T., Martella G., Imbriani P., Di Lazzaro G., Franco D., Colona V.L., Alwardat M., Sinibaldi Salimei P., Mercuri N.B., Pierantozzi M., Pisani A. Dietary vitamin E as a protective factor for Parkinson’s disease: clinical and experimental evidence. Front. Neurol. 2019;10:148. doi: 10.3389/fneur.2019.00148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Yang F., Wolk A., Håkansson N., Pedersen N.L., Wirdefeldt K. Dietary antioxidants and risk of Parkinson’s disease in two population-based cohorts. Mov. Disord. 2017;32(11):1631–1636. doi: 10.1002/mds.27120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Lee D.H., Kim C.S., Lee Y.J. Astaxanthin protects against MPTP/MPP+-induced mitochondrial dysfunction and ROS production in vivo and in vitro. Food Chem. Toxicol. 2011;49(1):271–280. doi: 10.1016/j.fct.2010.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Lin T.K., Chen S.D., Chuang Y.C., Lin H.Y., Huang C.R., Chuang J.H., Wang P.W., Huang S.T., Tiao M.M., Chen J.B., Liou C.W. Resveratrol partially prevents rotenone-induced neurotoxicity in dopaminergic SH-SY5Y cells through induction of heme oxygenase-1 dependent autophagy. Int. J. Mol. Sci. 2014;15(1):1625–1646. doi: 10.3390/ijms15011625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Öz A., Çelik Ö. Curcumin inhibits oxidative stress-induced TRPM2 channel activation, calcium ion entry and apoptosis values in SH-SY5Y neuroblastoma cells: Involvement of transfection procedure. Mol. Membr. Biol. 2016;33(3-5):76–88. doi: 10.1080/09687688.2017.1318224. [DOI] [PubMed] [Google Scholar]
- 229.Pandareesh M.D., Shrivash M.K., Naveen K.H.N., Misra K., Srinivas B.M.M. Curcumin monoglucoside shows improved bioavailability and mitigates rotenone induced neurotoxicity in cell and Drosophila models of Parkinson’s disease. Neurochem. Res. 2016;41(11):3113–3128. doi: 10.1007/s11064-016-2034-6. [DOI] [PubMed] [Google Scholar]
- 230.Shen D.F., Qi H.P., Ma C., Chang M.X., Zhang W.N., Song R.R. Astaxanthin suppresses endoplasmic reticulum stress and protects against neuron damage in Parkinson’s disease by regulating miR-7/SNCA axis. Neurosci. Res. 2021;165:51–60. doi: 10.1016/j.neures.2020.04.003. [DOI] [PubMed] [Google Scholar]
- 231.van der Merwe C., van Dyk H.C., Engelbrecht L., van der Westhuizen F.H., Kinnear C., Loos B., Bardien S. Curcumin rescues a PINK1 knock down SH-SY5Y cellular model of Parkinson’s disease from mitochondrial dysfunction and cell death. Mol. Neurobiol. 2017;54(4):2752–2762. doi: 10.1007/s12035-016-9843-0. [DOI] [PubMed] [Google Scholar]
- 232.Wu H.C., Hu Q.L., Zhang S.J., Wang Y.M., Jin Z.K., Lv L.F., Zhang S., Liu Z.L., Wu H.L., Cheng O.M. Neuroprotective effects of genistein on SH-SY5Y cells overexpressing A53T mutant α-synuclein. Neural Regen. Res. 2018;13(8):1375–1383. doi: 10.4103/1673-5374.235250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Anderson C., Checkoway H., Franklin G.M., Beresford S., Smith-Weller T., Swanson P.D. Dietary factors in Parkinson’s disease: the role of food groups and specific foods. Mov. Disord. 1999;14(1):21–27. doi: 10.1002/1531-8257(199901)14:1<21:AID-MDS1006>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 234.Hughes K.C., Gao X., Kim I.Y., Rimm E.B., Wang M., Weisskopf M.G., Schwarzschild M.A., Ascherio A. Intake of antioxidant vitamins and risk of Parkinson’s disease. Mov. Disord. 2016;31(12):1909–1914. doi: 10.1002/mds.26819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.King D., Playfer J.R., Roberts N.B. Concentrations of vitamins A, C and E in elderly patients with Parkinson’s disease. Postgrad. Med. J. 1992;68(802):634–637. doi: 10.1136/pgmj.68.802.634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Morens D.M., Grandinetti A., Waslien C.I., Park C.B., Ross G.W., White L.R. Case-control study of idiopathic Parkinson’s disease and dietary vitamin E intake. Neurology. 1996;46(5):1270–1274. doi: 10.1212/WNL.46.5.1270. [DOI] [PubMed] [Google Scholar]
- 237.Scheider W.L., Hershey L.A., Vena J.E., Holmlund T., Marshall J.R., Freudenheim J.L. Dietary antioxidants and other dietary factors in the etiology of Parkinson’s disease. Mov. Disord. 1997;12(2):190–196. doi: 10.1002/mds.870120209. [DOI] [PubMed] [Google Scholar]
- 238.Takeda A., Nyssen O.P., Syed A., Jansen E., Bueno-de-Mesquita B., Gallo V. Vitamin A and carotenoids and the risk of Parkinson’s disease: a systematic review and meta-analysis. Neuroepidemiology. 2014;42(1):25–38. doi: 10.1159/000355849. [DOI] [PubMed] [Google Scholar]
- 239.Pogačnik L., Ota A., Ulrih N.P. An overview of crucial dietary substances and their modes of action for prevention of neurodegenerative diseases. Cells. 2020;9(3):576. doi: 10.3390/cells9030576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Silva R.F.M., Pogačnik L. Polyphenols from food and natural products: Neuroprotection and safety. Antioxidants. 2020;9(1):61. doi: 10.3390/antiox9010061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Hajieva P. The effect of polyphenols on protein degradation pathways: implications for neuroprotection. Molecules. 2017;22(1):159. doi: 10.3390/molecules22010159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Cass W.A., Peters L.E. Reduced ability of calcitriol to promote augmented dopamine release in the lesioned striatum of aged rats. Neurochem. Int. 2017;108:222–229. doi: 10.1016/j.neuint.2017.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Haeri P., Mohammadipour A., Heidari Z., Ebrahimzadeh-Bideskan A. Neuroprotective effect of crocin on substantia nigra in MPTP-induced Parkinson’s disease model of mice. Anat. Sci. Int. 2019;94(1):119–127. doi: 10.1007/s12565-018-0457-7. [DOI] [PubMed] [Google Scholar]
- 244.El Nebrisi E., Javed H., Ojha S.K., Oz M., Shehab S. Neuroprotective effect of curcumin on the nigrostriatal pathway in a 6-hydroxydopmine-induced rat model of Parkinson’s disease is mediated by α7-nicotinic receptors. Int. J. Mol. Sci. 2020;21(19):7329. doi: 10.3390/ijms21197329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Weng C.C., Chen Z.A., Chao K.T., Ee T.W., Lin K.J., Chan M.H., Hsiao I.T., Yen T.C., Kung M.P., Hsu C.H., Wey S.P. Quantitative analysis of the therapeutic effect of magnolol on MPTP-induced mouse model of Parkinson’s disease using in vivo 18F-9-fluoropropyl-(+)-dihydrotetrabenazine PET imaging. PLoS One. 2017;12(3):e0173503. doi: 10.1371/journal.pone.0173503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Li X.L., Xu X.F., Bu Q.X., Jin W.R., Sun Q.R., Feng D.P., Zhang Q.J., Wang L.X. Effect of total flavonoids from Scutellaria baicalensis on dopaminergic neurons in the substantia nigra. Biomed. Rep. 2016;5(2):213–216. doi: 10.3892/br.2016.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Bayo-Olugbami A., Nafiu A.B., Amin A., Ogundele O.M., Lee C.C., Owoyele B.V. Vitamin D attenuated 6-OHDA-induced behavioural deficits, dopamine dysmetabolism, oxidative stress, and neuro-inflammation in mice. Nutr. Neurosci. 2020:1–12. doi: 10.1080/1028415X.2020.1815331. [Epub a head of Print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
PRISMA checklist is available on the publisher’s website along with the published article.