Abstract
Laccase, a p-diphenol oxidase typical of plants and fungi, has been found recently in a proteobacterium, Azospirillum lipoferum. Laccase activity was detected in both a natural isolate and an in vitro-obtained phase variant that originated from the laccase-negative wild type. In this study, the electron transport systems of the laccase-positive variant and its parental laccase-negative forms were compared. During exponential (but not stationary) growth under fully aerobic (but not under microaerobic) conditions, the laccase-positive variant lost a respiratory branch that is terminated in a cytochrome c oxidase of the aa3 type; this was most likely due to a defect in the biosynthesis of a heme component essential for the oxidase. The laccase-positive variant was significantly less sensitive to the inhibitory action of quinone analogs and fully resistant to inhibitors of the bc1 complex, apparently due to the rearrangements of its respiratory system. We propose that the loss of the cytochrome c oxidase-containing branch in the variant is an adaptive strategy to the presence of intracellular oxidized quinones, the products of laccase activity.
Polyphenol oxidases are a diverse group of copper proteins that catalyze oxidation of aromatic compounds by molecular oxygen. According to the substrate specificity, two classes of phenol oxidases are recognized: tyrosinases and laccases. Tyrosinases have monophenol monooxygenase (EC 1.18.14.1) and o-diphenol:oxygen-oxidoreductase (EC 1.10.3.1) activity and are widely distributed throughout the phylogenetic tree, from bacteria to mammals. Laccases have p-diphenol:oxygen-oxidoreductase (EC 1.10.3.2) activity and have been found exclusively in fungi and plants (44). In contrast to current understanding the redox biochemistry (34, 51) and the structure of laccases (10), relatively little is known of their physiological functions. Laccases play a key role in morphogenesis, development, and lignin metabolism in fungi and plants (22, 31, 44) and are virulence-associated factors in pathogenic fungi (49). Laccases are implicated in the biodegradation of a variety of toxic organic pollutants (4) and thus are potential bioremediation agents.
Recently, laccase activity has been demonstrated in an atypical isolate of the proteobacterium Azospirillum lipoferum (17). Azospirillum spp. are plant root-associated bacteria that stimulate the growth and development of many agriculturally important crops (29). Although laccase has been the subject of study for more than 100 years and the enzyme has been identified in a wide variety of plant and fungal species (44), A. lipoferum remains one of only two prokaryotic organisms in which laccase activity has been demonstrated (36). However, evidence has been obtained that laccases may be widespread in bacteria. Using similarity searches with known plant and fungal laccase gene sequences, we have identified putative laccase genes in several completely and partially sequenced genomes of α- and γ-proteobacteria (3). By initializing the oxidation of plant phenolic compounds (12, 13), the laccase may provide an obvious advantage to plant-associated Azospirillum cells. The physiological role of the enzyme in other bacterial species remains to be seen.
Oxidizing aromatic substrates, laccase generates reactive species, such as semiquinones and quinones, that are powerful inhibitors of the electron transport system in both bacteria (5, 20) and mitochondria (11). It appears that plants and fungi circumvent the problem: where it is known, laccases are extracellular enzymes. In contrast, the A. lipoferum enzyme is located intracellularly (13). Its chemical properties are similar to those of fungal laccases (12). For example, phenolic compounds of the syringic type (aldehyde, acid, and acetophenone) that are typical of plant tissues and exudates are oxidized by the laccase to 2,6-dimethoxy-1,4-benzoquinone (13). In Escherichia coli, derivatives of 1,4-benzoquinone inhibit respiration at concentrations as low as 1 μM by competing for electrons with ubiquinone of the electron transport system (5). How do bacterial cells cope with the intracellular presence of laccase and its toxic by-products? We hypothesized that one way in which the laccase-positive cells adapt to endogenous substituted quinones is by rearranging the electron transport system.
In this study, we compared the arrangement of the electron transport system and its sensitivity to substituted quinones in the laccase-positive variant of A. lipoferum (4VII) and laccase-negative parental forms (4B and 4VI). The laccase-positive variant 4VII emerges from a typical laccase-negative strain 4B via a two-step phase-variation-like process, with atypical laccase-negative variant 4VI being an intermediate form (2).
MATERIALS AND METHODS
Bacterial strains and growth conditions.
A. lipoferum wild-type strain 4B (laccase negative) and its variants 4VI (laccase negative, atypical) and 4VII (laccase positive, atypical) (2) were used in this study. The bacteria were grown in 1-liter Erlenmeyer flasks containing 200 ml of tryptone-yeast extract medium at 30°C. Flasks were incubated on a rotary shaker to achieve either fully aerobic (250 rpm) or microaerobic (100 rpm) conditions. For analysis of the respiratory system, cells were harvested during the exponential growth phase (optical density at 600 nm [OD600] = 0.5 to 0.7 for aerobic cultures and 0.3 to 0.4 for microaerobic cultures) or the stationary phase (OD600 = 1.4 to 1.8 for aerobic cultures and 0.6 to 0.7 for microaerobic cultures). Wild-type Bacillus subtilis OI1085 (47) and a cyd mutant of E. coli GO103 (7) were grown in Luria-Bertani broth to the mid-exponential growth phase. Wild-type E. coli MM335 (6) cells were grown to the stationary phase to achieve a maximal content of cytochrome d.
Polarographic assay.
Oxygen consumption in bacterial suspensions was measured by using a Clark-type electrode and an oxygen monitor (Yellow Springs Instrument Co., Yellow Springs, Ohio). The monitor output was collected in a channel of the MacLab data recording system (Model MK-III; Analog Digital Instruments, Boston, Mass.). The data collected were analyzed and stored in a Macintosh computer by using Chart (version 3.3) software (Analog Digital Instruments). For respiratory measurements, cells were washed twice in 50 mM potassium phosphate buffer (pH 7.0) and suspended in the same buffer containing 1 mM sodium malate. All measurements were performed in a closed 1-ml vessel at 30°C. Inhibitors, electron donors, and redox mediators were added as 10-μl aliquots. Respiration rates were calculated as the O2 uptake per minute per cell, as described previously (50). The number of cells was determined from an equation relating OD (OD600 range of 0.2 to 1.2) to the cell number as follows: no. of cells per milliliter = [0.54 × 10(1.06×OD600)] × 108. The equation was obtained from direct correlation of OD measurements and the cell concentration determined by using a model ZM Coulter Counter (Coulter Electronics, Hialeah, Fla.). As an independent control, the number of cells per milliliter was estimated by using the spectroturbidimetric assay (53). Concentration of dissolved oxygen in a buffer equilibrated with air was assumed to be 250 μM, as previously measured by Shioi et al. (37).
Membrane isolation.
A previously described method (14) was used to prepare membrane fractions. Cells were harvested and suspended in 50 mM potassium phosphate buffer containing 2 mM MgCl2 and 1 mM phenylmethylsulfonyl fluoride (pH 7.0). The suspension was then passed three times through the French press at 1,000 kg/cm2. Unbroken cells were removed by centrifugation at 10,000 × g for 20 min, and the supernatant was centrifuged at 110,000 × g for 2 h. The membrane pellet was frozen and stored at −70°C. Membranes were solubilized in the buffer described above supplemented with 0.5% (wt/vol) sodium dodecyl maltoside (Sigma Chemical Co., St. Louis, Mo.).
Visible light difference spectrophotometry.
Membrane samples in 50 mM potassium phosphate buffer (pH 7.0) containing 2 mM MgCl2 and 1 mM phenylmethylsulfonyl fluoride were oxidized with air or reduced by the addition of a few grains of sodium dithionite. The absorption spectra determined from the difference of the reduced minus oxidized values (reduced-minus-oxidized spectra) were recorded at room temperature on an Aminco DW-2a spectrophotometer (SLM Instruments, Urbana, Ill.) with a 1-nm slit width, a light path of 10 mm, a 0.3-s response time, and a wavelength scanning speed of 0.5 nm s−1. CO-bound reduced-minus-reduced difference absorption spectra were recorded after the membrane samples were reduced with sodium dithionite for 20 min, and then the sample cuvette was flushed with 100% CO for 5 min. Spectra were recorded 15 min after a flushing with CO at room temperature on a Perkin-Elmer Lambda 9 spectrophotometer (Perkin-Elmer and Co., GmbH, Unerlingen, Germany).
Heme extraction and HPLC analysis.
Non-covalently-bound hemes were extracted from membrane samples as described previously (23, 35, 38). Aliquots of membrane preparations were dissolved in 0.5 ml of acetone-HCl (19:1 [vol/vol]) and incubated for 20 min on a rotary mixer. After centrifugation for 2 min at 14,000 × g, 1 ml of ice-cold water and 0.3 ml of ethyl acetate were added to the supernatant, and the sample was vortexed for 30 s and centrifuged again. The ethyl acetate phase was recovered, and the solvent was removed by use of a vacuum concentrator. The residues were dissolved in acetonitrile and stored at −20°C.
The heme composition was analyzed by high-pressure liquid chromatography (HPLC) by using a Jupiter C18 reversed-phase column (Phenomenex, Torrance, Calif.). Hemes were eluted by acetonitrile (0.5% trifluoroacetic acid)-water (0.5% trifluoroacetic acid) gradients and detected spectrophotometrically. Absolute absorption spectra of individual heme species were obtained and analyzed by using the Shimadzu SPD-M10A photodiode array detector (Shimadzu Scientific Instruments, Columbia, Md.) and Shimadzu Class-VP Chromatography laboratory automated software system (version 4.2). Hemes in A. lipoferum membrane samples were identified by comparison with the retention times and spectra of heme standards (A, B, O, and D) prepared from the membranes of B. subtilis (hemes A and B), E. coli GO103 (hemes B and O), and E. coli MM335 (hemes B and D).
RESULTS
Analysis of respiration.
Analysis of respiration with electron transport inhibitors and specific electron donors revealed both cytochrome c oxidase and quinol oxidase activities in aerobically grown A. lipoferum. The respiration rate of A. lipoferum 4B utilizing malate (1 mM) as an electron donor was dependent on the growth stage, decreasing from 2.1 × 10−10 μmol of O2 per min per cell in the mid-exponential phase (OD600 = 0.5) to 0.9 × 10−10 μmol of O2 per min per cell in the stationary phase (OD600 = 1.4) under full aeration. The inhibitory analysis was performed with cells from the mid-exponential phase that had the maximal respiration rate. Myxothiazol is a specific inhibitor of the cytochrome c-dependent branch; it inhibits ubiquinol oxidation by the Rieske iron-sulfur protein, which is specific for the bc1 complex that donates electrons to cytochrome c oxidase (45). Myxothiazol only slightly inhibited respiration of A. lipoferum 4B cells. Maximal inhibition (respiration rate, 1.7 × 10−10 μmol of O2 per min per cell) was achieved with 100 μM myxothiazol. Addition of ascorbate and N,N,N′,N′-tetramethyl-p-phenylenediamine (TMPD; 500 and 250 μM, respectively) that donate electrons directly to cytochrome c, bypassing the bc1 complex, significantly increased the oxygen consumption (respiration rate, 2.6 × 10−10 μmol of O2 per min per cell). Low concentrations of KCN (10 μM) that completely inhibit cytochrome c oxidases inhibited respiration only by 20% (respiration rate, 1.7 × 10−10 μmol of O2 per min per cell). A quinone analog 2-n-heptyl-4-hydroxyquinoline N-oxide (40 μM) inhibited respiration in A. lipoferum 4B by 80% (respiration rate, 0.4 × 10−10 μmol of O2 per min per cell), and 1 mM KCN inhibited respiration by 88% (respiration rate, 0.25 × 10−10 μmol of O2 per min per cell). Taken together, the results are consistent with the presence of the cytochrome c terminated branch and also indicate that quinol oxidase(s) account for most of respiratory activity in aerobically grown A. lipoferum.
Respiration rates of the 4VI and 4VII variants were similar to that of the 4B strain (1.9 and 2.2 × 10−10 μmol of O2 per min per cell, respectively, in the mid-exponential phase under full aeration). Inhibitory respiratory analysis revealed no significant difference between laccase-negative cells of the 4B strain and the 4VI variant. However, respiration in the aerobically grown laccase-positive 4VII variant in the mid-exponential phase was completely insensitive to myxothiazol (100 μM) and not stimulated by ascorbate plus TMPD (500 and 250 μM, respectively), indicating the absence of the bc1 complex and cytochrome c oxidase activity in the variant during the exponential growth. In the stationary phase, however, there was no significant difference between all three strains with respect to the myxothiazol and ascorbate-TMPD effects. For example, the respiration rate of the 4B strain decreased from 0.9 to 0.7 × 10−10 μmol of O2 per min per cell upon the addition of myxothiazol and, similarly, the respiration rate of the 4VII variant decreased from 1.0 to 0.7 × 10−10 μmol of O2 per min per cell. In order to identify individual cytochromes in the respiratory system of A. lipoferum and reveal further possible differences in the respiratory system in the wild type and the variant cells, we used differential spectroscopy and analysis of heme composition.
Difference spectroscopy.
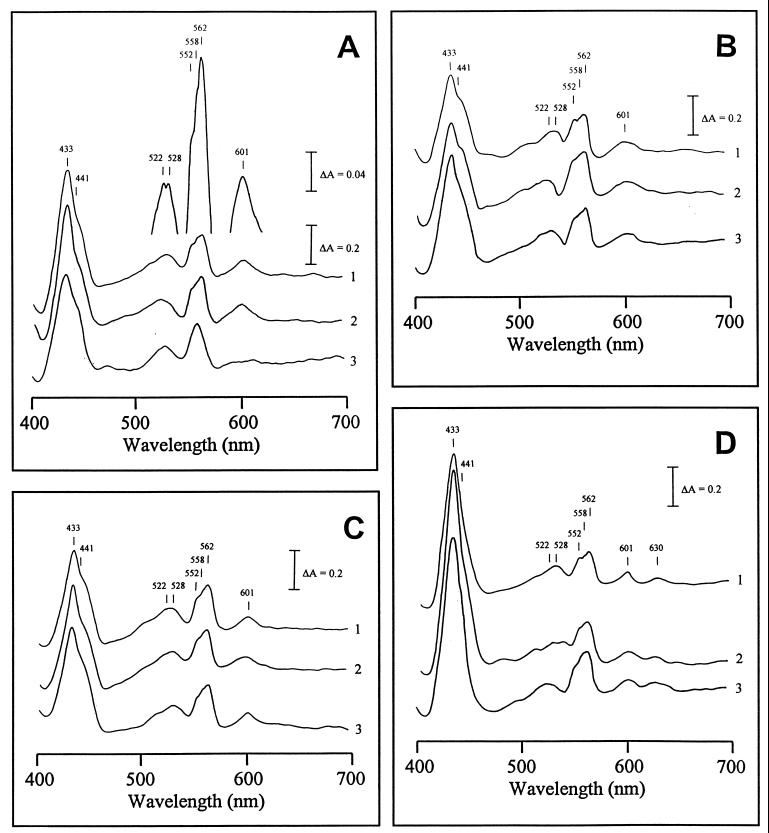
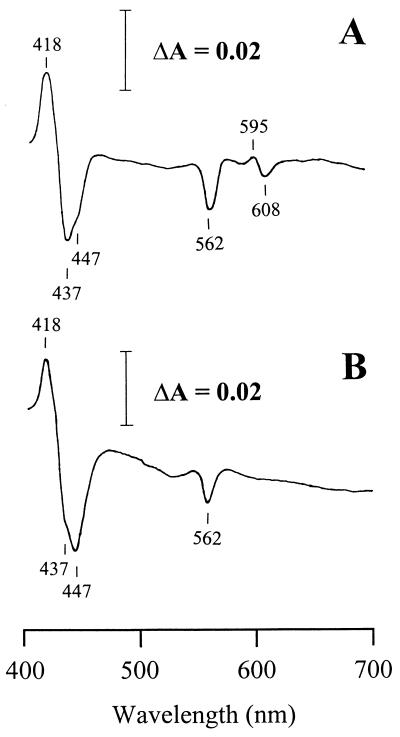
The reduced-minus-oxidized spectra were obtained in parallel experiments for the membrane preparations from wild type (4B), and the variant (4VI and 4VII) cells grown under the following experimental conditions: fully aerated cells, exponential (i) and stationary (ii) growth phases; and low-aerated cells, exponential (iii) and stationary (iv) phases. The following absorption peaks were obtained (Fig. 1A) and assigned to individual cytochromes based on known spectral characteristics (30). An intense Soret band at 433 nm is typical of b-type cytochromes, including heme B-containing terminal oxidases (19, 28, 30, 39). The shoulder at 441 nm and the peak at 601 nm are indicative of cytochrome c oxidase of the aa3 type. The two α peaks at 558 and 562 nm and the β peak at 528 nm are indicative of b-type cytochromes. An α peak at 552 nm and a β peak at 522 nm are indicative of c-type cytochromes. The highly asymmetric pattern of the α and β regions, typical of the cbb-type cytochrome c oxidases (16, 21, 25, 35, 43), has never been observed in membrane preparations from A. lipoferum. These results indicate that (i) the major cytochrome c oxidase in A. lipoferum is most likely of the aa3 and not the cbb type and that (ii) the cells utilize different quinol oxidases. CO-bound reduced-minus-reduced absorption spectra confirmed this conclusion (Fig. 2A). First, the trough at 562 nm is indicative of the bo (bb)-type oxidase. Second, the peak at 595 nm and the troughs at 447 and 608 nm (shifts from the peaks of 441 and 601 nm on the reduced-minus-oxidized spectra, respectively) are indicative of the cytochrome oxidase of the aa3 type.
FIG. 1.
Reduced-minus-oxidized spectra of membrane preparations of laccase-negative (4B and 4VI) and laccase-positive (4VII) A. lipoferum. (A) Fully aerated exponentially grown cells. (B) Fully aerated cells, stationary phase. (C) Low-aerated exponentially grown cells. (D) Low-aerated cells, stationary phase. Bacterial cultures: 1, 4B; 2, 4VI; 3, 4VII.
FIG. 2.
Difference spectra (CO-bound reduced-minus-reduced spectra) of membrane preparations of the laccase-negative strain 4B (A) and the laccase-positive 4VII variant (B) of A. lipoferum. Cells were grown exponentially under full aeration.
No differences between the wild-type and laccase-negative 4VI cells were observed in the difference spectra under any experimental conditions (Fig. 1A to D). A major difference between the wild-type and the laccase-positive 4VII variant cells was observed for the aerobically grown cells at the exponential phase (Fig. 1A). In membrane preparations from the 4VII cells, no peaks at 552, 562, and 601 nm were seen. The spectral pattern suggested that cytochrome c and b562 were present in 4VII in much lower (if any) amounts than in the wild-type cells, whereas cytochrome aa3 (peak at 601 nm) was completely missing. The shoulder at 441 nm was still present in the variant (Fig. 1A), raising the possibility that there is more than one heme A-containing cytochrome in A. lipoferum. No principal differences between the wild-type and 4VII cells were observed under other experimental conditions (Fig. 1B to C). CO-bound reduced-minus-reduced absorption spectra also revealed a difference between the wild-type and the laccase-positive 4VII variant cells grown exponentially under full aeration (Fig. 2), whereas no difference was observed under other growth conditions (data not shown). The peak at 595 nm and the trough at 608 nm were present in the 4B strain but missing in the 4VII variant, confirming the absence of the aa3-type cytochrome in the latter. However, the trough at 447 nm was present in both 4B and 4VII cells (Fig. 2), suggesting the presence of the second heme A-containing terminal oxidase, which has low absorption in the alpha band.
Heme composition.
All of the membrane preparations from A. lipoferum for which differential spectroscopy analysis has been performed (see above) were subjected to heme analysis. First, the control membranes isolated from B. subtilis (containing hemes B and A), E. coli stationary-phase wild-type cells (containing hemes B and D), and E. coli cytochrome d mutant cells (containing hemes B and O) were analyzed to determine the retention times for known heme compounds (Table 1).
TABLE 1.
Heme compounds from A. lipoferum and control membrane preparations
| Heme | Organism | Mean retention time (min) ± SDa |
|---|---|---|
| D | E. coli, A. lipoferum | 17.1 ± 0.6 (15) |
| B | E. coli, B. subtilis, A. lipoferum | 21.1 ± 0.3 (18) |
| R1 | A. lipoferum | 24.8 ± 0.3 (5) |
| A | B. subtilis, A. lipoferum | 29.9 ± 0.4 (14) |
| O | E. coli | 31.9 ± 0.3 (3) |
| R2 | A. lipoferum | 34.7 ± 0.2 (3) |
| R3 | A. lipoferum | 36.4 ± 0.4 (11) |
The results shown are the mean ± standard deviation as calculated from several (number in parentheses) independent experiments.
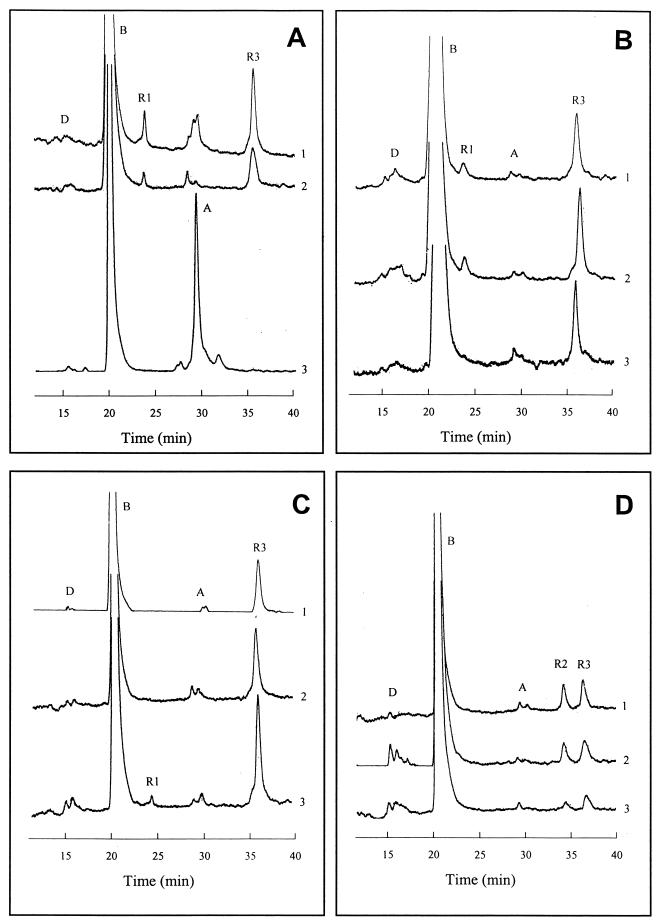
Comparative analysis using membrane fractions from wild type A. lipoferum cells grown under different experimental conditions (described in the previous section) revealed the presence of known and unknown heme compounds. Heme B was present abundantly in all fractions, and traces of hemes A and D were also detected (Fig. 3A to D). No evidence for heme O has been found. Therefore, the oxidase identified on the CO difference spectra (the trough at 562 nm on Fig. 2) is likely that of a bb type and not a bo-type cytochrome. Interestingly, three unknown heme compounds were identified in wild-type A. lipoferum that were designated R1, R2, and R3 according to their retention times (Table 1). Apart from heme B, which was present abundantly in all fractions, heme R3 appears to be a dominant component under all conditions tested. Heme R1 was present only under fully aerobic conditions (Fig. 3A and C), and heme R2 was present only under very low-oxygen concentrations (stationary-phase, microaerobic conditions) (Fig. 3D). Heme R1 had a slightly higher hydrophobicity than heme B and was predicted to be a modified heme B. Hemes R2 and R3 had higher hydrophobicity than hemes A and O.
FIG. 3.
Reversed-phase HPLC chromatograms of the noncovalently membrane-bound hemes from laccase-negative (4B and 4VI) and laccase-positive (4VII) A. lipoferum. (A) Fully aerated exponentially grown cells. (B) Fully aerated cells, stationary phase. (C) Low-aerated exponentially grown cells. (D) Low-aerated cells, stationary phase. Bacterial cultures: 1, 4B; 2, 4VI; 3, 4VII. Positions of peaks corresponding to known (D, B, and A) and unknown (R1, R2, and R3) heme species are indicated.
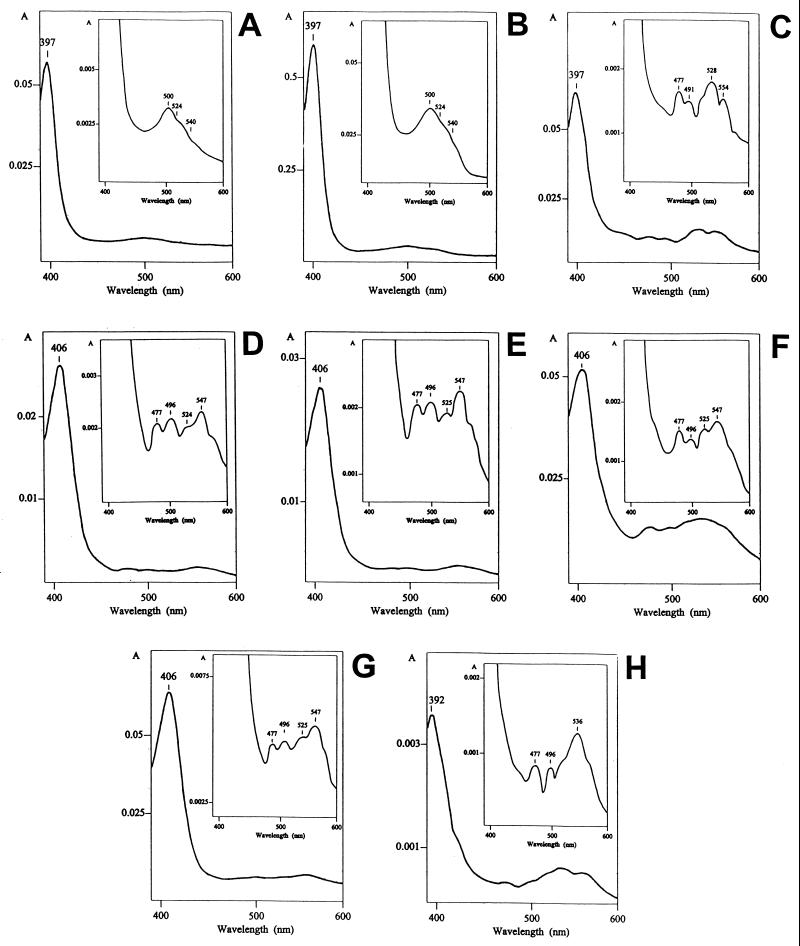
In order to characterize further the unknown heme species, the absolute absorption spectra of individual heme compounds were recorded by using a photodiode array detector (Fig. 4). The spectra confirmed potential relatedness of heme R1 to heme B, and hemes R2 and R3 to heme A based on the maximal absorption peak in the Soret region and a similar pattern of the αβ region. For example, heme R1 had a maximal absorption peak at 397 nm (Fig. 4C); heme B isolated from B. subtilis (Fig. 4A) and A. lipoferum (Fig. 4B) had exactly the same maximal peak. However, both heme R2 (Fig. 4G) and heme R3 (Fig. 4F) had the maximal absorption peak at 406 nm, which is characteristic of heme A isolated from B. subtilis (Fig. 4D) and A. lipoferum (Fig. 4E).
FIG. 4.
Absolute visible absorption spectra of hemes from control and A. lipoferum membranes in acetonitrile solutions obtained by using a photodiode array detector. Heme compounds: heme B from B. subtilis (A) and A. lipoferum 4B (B), heme R1 from A. lipoferum 4B (C), heme A from B. subtilis (D) and A. lipoferum 4VII (E); heme R3 from A. lipoferum 4VII (F), heme R2 from A. lipoferum 4VII (G), and heme D from A. lipoferum 4VII (H).
No differences between the laccase-negative 4B and 4VI cells were observed in heme profiles under any experimental conditions (Fig. 3A to D). A major difference between the laccase-negative forms and the laccase-positive 4VII variant was observed for the cells in the exponential phase under full aeration. Two heme species typical of these experimental conditions, namely, R3 and R1, were absent in the 4VII cells, whereas the amount of heme A drastically increased in comparison with laccase-negative wild-type and 4VI cells (Fig. 3A). Also, heme R1, which was present in laccase-negative parental strains only under fully aerobic conditions, appeared in the 4VII variant cells grown exponentially under microaerobic conditions (Fig. 3C).
Inhibitory effects of substituted quinones on the respiratory system.
Substituted quinones that pass freely across the cytoplasmic membrane are known to be strong respiratory inhibitors for bacteria (5, 20). On the other hand, substituted quinones are products of laccase activity in A. lipoferum (13). The laccase-positive variant apparently lost the cytochrome c oxidase terminated branch of electron transport, which is known to be the most sensitive to respiratory inhibitors in different bacterial species (45). We hypothesized that laccase-positive variant is less sensitive to the inhibitory effect of oxidized quinones. The sensitivity of respiration to substituted quinones of different reduction potentials was tested in the laccase-positive variant and its parental forms. Substituted quinones of high reduction potential decreased the respiration in the strain 4B and the laccase-negative variant 4VI, but to a significantly lesser extent in the laccase-positive variant 4VII (Table 2). The 4VII cells were two times more resistant to the most potent inhibitor, 1,4-benzoquinone, than the parental strains. The laccase-positive variant was practically insensitive to the low-reduction-potential quinones at concentrations that caused a statistically significant inhibitory effect on the laccase-negative strains. As expected (5), there was a direct correlation between the inhibitory effect and the reduction potential (electron affinity) of the quinone: the higher the reduction potential, the more toxic the quinone.
TABLE 2.
Effects of exogenous substituted quinones on respiration of laccase-negative (4B and 4VI) and laccase-positive (4VII) variants of A. lipoferum
| Quinone | Reduction potential (mV)a | Concn (μM) | Oxygen consumption rate (μmol/min per cell [10−11])b
|
||
|---|---|---|---|---|---|
| 4B | 4VI | 4VII | |||
| None | 21 ± 3 | 19 ± 2 | 22 ± 2 | ||
| 1,4-Benzoquinone | +99 | 10 | 5 ± 1 | 4 ± 1 | 11 ± 2 |
| 2-Methyl-1,4-benzoquinone | +23 | 10 | 15 ± 1 | 14 ± 2 | 20 ± 2 |
| 2,6-Dimethyl-1,4-benzoquinone | −80 | 100 | 16 ± 2 | 14 ± 1 | 21 ± 1 |
| Tetramethyl-1,4-benzoquinone | −240 | 100 | 20 ± 1 | 17 ± 2 | 23 ± 3 |
| 2-Hydroxy-1,4-naphtoquinone | −415 | 1,000 | 22 ± 2 | 20 ± 2 | 20 ± 1 |
Data from Wardman (48).
The results shown are the mean ± the standard deviations as calculated from two independent experiments, with three replicates in each.
DISCUSSION
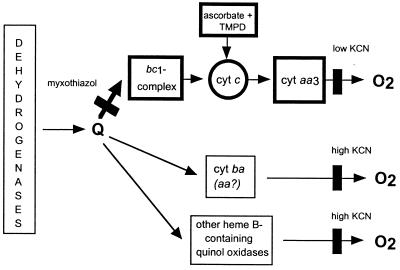
Prior to this investigation, no information was available on the electron transport system of A. lipoferum. Therefore, our first goal was to obtain sufficient data on the arrangement of the respiratory system in A. lipoferum in order to reveal possible differences in laccase-positive and laccase-negative variants. Previous spectral and polarographic studies suggested the presence of cytochrome aa3, cytochrome d, and cytochrome o terminal oxidases in Azospirillum brasilense, the closest relative of A. lipoferum (33). Recent investigation provided genetic and biochemical evidence for the presence of an alternative (cbb3) cytochrome c oxidase in A. brasilense (25). The data obtained during inhibitory respiratory analysis, difference absorption spectroscopy, and heme composition analysis suggest a general scheme for the arrangement of the respiratory system in A. lipoferum 4B and its variants 4VI and 4VII (Fig. 5). Both cytochrome c oxidase and quinol oxidase activities were detected. We refer to the major cytochrome c oxidase of A. lipoferum as the aa3 type based on the spectral characteristics typical of this oxidase. However, without detailed mechanistic studies of the enzyme (which are beyond the scope of this investigation) it is impossible to determine its exact nature. Although the spectral studies did not indicate the presence of the cbb3-type oxidase in A. lipoferum, whereas the cbb3 signature peaks are present in similar membrane preparations from A. brasilense (25), we cannot rule out the presence of an alternative cytochrome c oxidase. The major cytochrome c oxidase terminated branch was present in wild-type A. lipoferum 4B and in its laccase-negative variant 4VI under all growth conditions tested, a result consistent with the notion that it is the most efficient energy generating pathway in bacteria (46). However, it is unusual that the bacteria appear to use this respiratory branch under microaerobic conditions in the stationary stage of growth, where in most species it is not active. In addition to the cytochrome c oxidase terminated branch, A. lipoferum contains quinol oxidases that account for myxothiazol- and cyanide-resistant respiration. Carbon monoxide difference spectra and heme analysis suggest that several quinol oxidases, including those of the bb and ba types, may be present in A. lipoferum.
FIG. 5.
Putative respiratory pathways in A. lipoferum. In the laccase-positive cells during the exponential growth phase under fully aerobic conditions, the pathway shown in bold outlines is not present. Black rectangles indicate the sites of inhibition. Q, ubiquinone and/or menaquinon.
Only four types of noncovalently bound hemes are usually found in bacteria: heme B (protoheme IX) and its derivatives heme D, heme O, and heme A (15, 42). Archaebacteria have prenylated hemes, which are modifications of hemes A and O, as components of their terminal cytochromes (23, 24). A. lipoferum appears to have three known hemes (B, D, and A) and three unknown hemes, designated R1, R2, and R3 (Table 1). Heme R1 is predicted to be a modification of heme B, whereas hemes R2 and R3 are predicted to be modifications of heme A. The following lines of evidence support our suggestion that compounds designated R1, R2, and R3 are indeed heme components of the respiratory complexes. First, the method of Sone and Fujiwara (38), which was used for heme extraction in our study, has been applied to different microbial species and always yielded no compounds other than noncovalently bound hemes of the respiratory complexes. Second, the presence of at least two novel heme species on the HPLC profiles correlated with the presence of characteristic adsorption peaks on the reduced-minus-oxidized spectra obtained from the same membrane preparations. Heme R2 correlated with the 630-nm peak and heme R3 correlated with the 601-nm peak (compare Fig. 1A to D and Fig. 3A to D). Finally, the absolute absorption spectra of R1 and of R2 and R3 compounds were similar to those of heme B and heme A, respectively (Fig. 4).
We have not observed any difference in the respiratory metabolism between the laccase-negative wild-type 4B and the 4VI variant under any experimental conditions, although various changes in carbohydrate and secondary metabolism were observed in the 4VI variant (2). However, we found dramatic differences between the laccase-negative parental forms and the laccase-positive 4VII variant. These changes occurred only under specific growth conditions. During exponential growth under full aeration, the laccase-positive variant cells lost the cytochrome aa3-type oxidase activity, most likely, due to inability to synthesize a heme component essential for the oxidase, and we propose that the novel heme R3 is such a component. The following lines of experimental evidence support this hypothesis.
(i) The absence of the cytochrome c oxidase of the aa3-type is obvious from the loss of a 601-nm peak from the reduced-minus-oxidized spectrum of the 4VII variant grown exponentially under high aeration (Fig. 1A). This peak is characteristic of the high-spin heme A in aa3-type oxidases (30). Similarly, the peak at 595 nm and the trough at 608 nm that are indicative of the oxidase were lost from the carbon monoxide spectrum of the 4VII variant grown under the same conditions (Fig. 2B). Loss of the cytochrome c oxidase activity in the variant grown exponentially under high aeration was also confirmed by respiratory analysis.
(ii) Heme R3 is the only heme compound whose presence or absence in the HPLC profiles was coincident with that of the aa3-oxidase characteristic (601-nm) peak on the reduced-minus-oxidized spectra of the same membrane preparations (compare Fig. 1A to D and Fig. 3A to D).
(iii) Large amounts of unmodified heme A in the laccase-positive variant cells were detected only under exponential growth under full aeration (Fig. 3B), where aa3-type oxidase signature peaks are missing from the reduced-minus-oxidized spectra (601 nm, Fig. 1A) and carbon monoxide spectra (peak at 595 and trough at 608 nm, Fig. 2B). This indicates that unmodified heme A is not a component of the aa3-type cytochrome c oxidase. Most likely, unmodified heme A is a component of another a-type terminal cytochrome (apparently, a quinol oxidase), which is present both in wild type and, to a greater extent (as judged by the difference absorption spectra on Fig. 1A and Fig. 2), in the 4VII variant. This oxidase accounts for the presence of the 441 peak on the reduced-minus-oxidized spectra (Fig. 1A) and the corresponding trough (447 nm) on the carbon monoxide spectra (Fig. 2B) in the cells lacking cytochrome c oxidase of the aa3 type.
(iv) Heme R3 has a higher hydrophobicity than unmodified heme A (Fig. 3); however, its spectral characteristics (absolute absorption spectra) are very similar to those of heme A (Fig. 4D to F), suggesting that R3 is a modified heme A.
Furthermore, our results are supported by previous findings that a modified (prenylated) heme A can substitute heme A as a component of both a cytochrome c oxidase of the aa3 type and the ba3 quinol oxidase in Archaea and Bacteria (23, 24). Spectral characteristics of the aa3-type cytochromes that contain a modified heme A and unmodified heme A are similar (peak in the 600-nm region), whereas hydrophobicity of the modified heme A is higher (23, 24).
Observed changes in the heme and cytochrome content in the laccase-positive variant apparently result from regulation of gene expression since they were specific to a particular growth stage (exponential but not stationary) and oxygen availability (aerobic but not microaerobic conditions). We attribute the difference in regulation to an unspecified mutation that caused the emergence of the laccase-positive variant form (2). Genes for enzymes that are involved in heme modification, such as a protoheme IX farnesyl transferase (cyoE and ctaB), are found in the operons encoding for terminal oxidases in different bacterial species (8, 27, 41). Deficiency in these genes leads to a loss of a correspondent terminal oxidase due to the blockage of the heme O (cyoE) or heme A (ctaA and ctaB) biosynthesis pathway. Where known, expression of genes involved in heme biosynthesis is under environmental control, with oxygen being one of the major factors (9, 18, 26, 52). Interestingly, a loss of a regulatory protein caused changes in B. subtilis that are similar to those observed in the 4VII variant of A. lipoferum. The ResD protein, which is similar to the two-component signal transduction proteins, was shown to be a global regulator of the respiratory system in B. subtilis (40). Mutation in resD leads to a loss of the cytochrome c oxidase of the aa3 type due to defects in heme biosynthesis (ctaB) and some other changes related to oxidative metabolism. Similar changes have been previously reported in the 4VII variant of A. lipoferum; moreover, all changes that occur in the variant are related to oxidative metabolism (1, 2). This allows us to propose that an unspecified mutation in the 4VII variant also affects a regulatory system that controls directly or indirectly the expression of both laccase and enzymes involved in heme biosynthesis.
The exact role of the laccase in A. lipoferum is unknown, although utilization of plant phenolic compounds (12, 13) may be advantageous for the bacterial survival in the rhizosphere. Using a variety of membrane-permeable oxidized quinones, we have demonstrated that the laccase-positive variant was up to two times less sensitive to the inhibitory effect of these compounds on the respiratory system. We attribute the resistance of the variant solely to changes in its electron transport system, i.e., the absence of the cytochrome c oxidase and the preferential use of quinol oxidases. Most interestingly, the loss of the major cytochrome c oxidase and acquisition of resistance to exogenous quinones that are described in this study occurred under conditions (exponential phase, full aeration), where laccase activity reached a high level (1). Such changes in the respiratory metabolism may reflect an adaptive strategy of the bacterium to the presence of intracellular laccase and its toxic quinone by-products. Shutoff of the quinone-sensitive cytochrome c oxidase and the preferential use of quinone-insensitive quinol oxidases would be beneficial for quinone-producing laccase-positive cells. At the same time, a laccase-positive quinone-tolerant variant would have a competitive advantage in the rhizosphere in the presence of quinone compounds, such as sorgoleone, which are naturally occurring inhibitors of the bc1 complex/cytochrome c oxidase-containing branches of the electron transport system (32). Taking into account possible widespread distribution of laccases in bacteria (3), future studies on genetic mechanisms and the environmental control of the expression of laccase and components of the respiratory system in A. lipoferum should be productive.
ACKNOWLEDGMENTS
G.A. was a recipient of a fellowship from the MENESR (France). This work was supported in part by a grant 96-35305-3795 (to I.B.Z.) from the U.S. Department of Agriculture (NRICGP).
We thank R. B. Gennis, G. W. Ordal, and M. D. Manson for bacterial strains and M. S. Johnson for expert advice. We are grateful to the reviewer for helpful suggestions.
REFERENCES
- 1.Alexandre G. Physiological characterization of the phenotypic switching in the rhizospheric bacterium Azospirillum lipoferum 4B. Ph.D. thesis. Lyon, France: Claude Bernard University; 1998. [Google Scholar]
- 2.Alexandre G, Bally R. Emergence of a laccase-positive variant of Azospirillum lipoferum occurs via a two-step phenotypic switching process. FEMS Microbiol Lett. 1999;174:371–378. doi: 10.1111/j.1574-6968.1999.tb13592.x. [DOI] [PubMed] [Google Scholar]
- 3.Alexandre, G., and I. B. Zhulin. Unpublished data.
- 4.Amitai G, Adani R, Sod-Moriah G, Rabinovitz I, Vincze A, Leader H, Chefetz B, Leibovitz-Persky L, Friesem D, Hadar Y. Oxidative biodegradation of phosphorothiolates by fungal laccase. FEBS Lett. 1998;438:195–200. doi: 10.1016/s0014-5793(98)01300-3. [DOI] [PubMed] [Google Scholar]
- 5.Bespalov V A, Zhulin I B, Taylor B L. Behavioral responses of Escherichia coli to changes in redox potential. Proc Natl Acad Sci USA. 1996;93:10084–10089. doi: 10.1073/pnas.93.19.10084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brass J M, Manson M D. Reconstitution of maltose chemotaxis in Escherichia coli by addition of maltose-binding protein to calcium-treated cells of maltose regulon mutants. J Bacteriol. 1984;157:881–890. doi: 10.1128/jb.157.3.881-890.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Calhoun M W, Oden K L, Gennis R B, de Mattos M J T, Neijssel O M. Energetic efficiency of Escherichia coli: effects of mutations in components of the aerobic respiratory chain. J Bacteriol. 1993;175:3020–3025. doi: 10.1128/jb.175.10.3020-3025.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clements M O, Watson S P, Poole R P, Foster S J. CtaA of Staphylococcus aureus is required for starvation survival, recovery, and cytochrome biosynthesis. J Bacteriol. 1999;181:501–507. doi: 10.1128/jb.181.2.501-507.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Darie S, Gunsalus R P. Effect of heme and oxygen availability on hemA gene expression in Escherichia coli: role of the fnr, arcA, and himA gene products. J Bacteriol. 1994;176:5270–5276. doi: 10.1128/jb.176.17.5270-5276.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ducros V, Brzozowski A M, Wilson K S, Brown S H, Ostergaard P, Schneider P, Yaver D S, Pedersen A H, Davies G J. Crystal structure of the type-2 Cu depleted laccase from Coprinus cinereus at 2.2 Å resolution. Nat Struct Biol. 1998;5:310–316. doi: 10.1038/nsb0498-310. [DOI] [PubMed] [Google Scholar]
- 11.Esposti M D, Ngo A, Ghelli A, Benelli B, Carelli V, McLennan H, Linnane A W. The interaction of Q analogs, particularly hydroxydecyl benzoquinone (idebenone), with the respiratory complexes of heart mitochondria. Arch Biochem Biophys. 1996;330:395–400. doi: 10.1006/abbi.1996.0267. [DOI] [PubMed] [Google Scholar]
- 12.Faure D, Bouillant M-L, Bally R. Comparative study of substrates and inhibitors of Azospirillum lipoferum and Pyricularia oryzae laccases. Appl Environ Microbiol. 1995;61:1144–1146. doi: 10.1128/aem.61.3.1144-1146.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Faure D, Bouillant M L, Jacoud C, Bally R. Phenolic derivatives related to lignin metabolism as substrates for Azospirillum laccase activity. Phytochemistry. 1996;42:357–359. [Google Scholar]
- 14.Ferber D M, Maier R J. Incorporation of a bacterial membrane-bound hydrogenase into proteoliposomes. Anal Biochem. 1992;203:235–244. doi: 10.1016/0003-2697(92)90308-t. [DOI] [PubMed] [Google Scholar]
- 15.Garcia-Horsman J A, Barquera B, Rumbley J, Ma J, Gennis R B. The superfamily of heme-copper respiratory oxidases. J Bacteriol. 1994;176:5587–5600. doi: 10.1128/jb.176.18.5587-5600.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garcia-Horsman J A, Berry E, Shapleigh J P, Alben J O, Gennis R B. A novel cytochrome c oxidase from Rhodobacter sphaeroides that lacks CuA. Biochemistry. 1994;33:3113–3119. doi: 10.1021/bi00176a046. [DOI] [PubMed] [Google Scholar]
- 17.Givaudan A, Effosse A, Faure D, Potier P, Bouillant M-L, Bally R. Polyphenol oxidase from Azospirillum lipoferum isolated from rice rhizosphere: evidence for laccase activity in non-motile strains of Azospirillum lipoferum. FEMS Microbiol Lett. 1993;108:205–210. [Google Scholar]
- 18.Gunsalus R P. Control of electron flow in Escherichia coli: coordinated transcription of respiratory pathway genes. J Bacteriol. 1992;174:7069–7074. doi: 10.1128/jb.174.22.7069-7074.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hicks D B, Plass R J, Quirk P G. Evidence for multiple terminal oxidases, including cytochrome d, in facultative alkaliphilic Bacillus firmus OF4. J Bacteriol. 1991;173:5010–5016. doi: 10.1128/jb.173.16.5010-5016.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Imlay J, Fridovich I. Exogenous quinones directly inhibit the respiratory NADH dehydrogenase in Escherichia coli. Arch Biochem Biophys. 1992;296:337–346. doi: 10.1016/0003-9861(92)90581-g. [DOI] [PubMed] [Google Scholar]
- 21.Keefe K G, Maier R J. Purification and characterization of an O2-utilizing cytochrome-c oxidase complex from Bradyrhizobium japonicum bacteroid membranes. Biochim Biophys Acta. 1993;1183:91–104. doi: 10.1016/0005-2728(93)90008-4. [DOI] [PubMed] [Google Scholar]
- 22.Liu L, Dean J F D, Friedman W E, Eriksson K-E L. A laccase-like phenol oxidase is correlated with lignin biosynthesis in Zinnia elegans stem tissues. Plant J. 1994;6:213–224. [Google Scholar]
- 23.Lübben M, Morand K. Novel prenylated hemes as cofactors of cytochrome oxidases: Archaea have modified hemes A and O. J Biol Chem. 1994;269:21473–21479. [PubMed] [Google Scholar]
- 24.Lübben M, Warne A, Albracht S P J, Saraste M. The purified SoxABCD quinol oxidase complex of Sulfolobus acidocaldarius contains a novel haem. Mol Microbiol. 1994;13:327–335. doi: 10.1111/j.1365-2958.1994.tb00426.x. [DOI] [PubMed] [Google Scholar]
- 25.Marchal K, Sun J, Keijers V, Haaker H, Vanderleyden J. A cytochrome cbb3 (cytochrome c) terminal oxidase in Azospirillum brasilense Sp7 supports microaerobic growth. J Bacteriol. 1998;180:5689–5696. doi: 10.1128/jb.180.21.5689-5696.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McNicholas P M, Javor G, Darie S, Gunsalus R P. Expression of the heme biosynthetic pathway genes hemCD, hemH, hemM, and hemA of Escherichia coli. FEMS Microbiol Lett. 1997;146:143–148. doi: 10.1111/j.1574-6968.1997.tb10184.x. [DOI] [PubMed] [Google Scholar]
- 27.Mogi T, Saiki K, Anraku Y. Biosynthesis and functional role of heam O and haem A. Mol Microbiol. 1994;189:391–398. doi: 10.1111/j.1365-2958.1994.tb02174.x. [DOI] [PubMed] [Google Scholar]
- 28.Oertling W A, Surerus K K, Einsrsdottir O, Fee J A, Dyer R B, Woodruff W H. Spectroscopic characterization of cytochrome ba3, a terminal oxidase from Thermus thermophilus: comparison of the a3/CuB site to that of bovine cytochrome aa3. Biochemistry. 1994;33:3128–3141. doi: 10.1021/bi00176a048. [DOI] [PubMed] [Google Scholar]
- 29.Okon Y, Vanderleyden J. Root-associated Azospirillum species can stimulate plants. ASM News. 1997;63:366–370. [Google Scholar]
- 30.Poole R K. Bacterial cytochrome oxidases: a structurally and functionally diverse group of electron-transfer proteins. Biochim Biophys Acta. 1983;726:205–243. doi: 10.1016/0304-4173(83)90006-x. [DOI] [PubMed] [Google Scholar]
- 31.Ranocha P, McDougall G, Hawkins S, Sterjiades R, Borderies G, Stewart D, Cabanes-Macheteau M, Boudet A M, Goffner D. Biochemical characterization, molecular cloning and expression of laccases—a divergent gene family—in poplar. Eur J Biochem. 1999;259:485–495. doi: 10.1046/j.1432-1327.1999.00061.x. [DOI] [PubMed] [Google Scholar]
- 32.Rasmussen J A, Hejl A M, Einhellig F A, Thomas J A. Sorgoleone from root exudate inhibits mitochondrial functions. J Chem Ecol. 1992;18:197–207. doi: 10.1007/BF00993753. [DOI] [PubMed] [Google Scholar]
- 33.Reiner O, Okon Y. Oxygen recognition in aerotactic behaviour of Azospirillum brasilense Cd. Can J Microbiol. 1985;32:829–834. [Google Scholar]
- 34.Reinhammar B. Laccase. In: Lontie R, editor. Copper proteins and copper enzymes. Vol. 3. Boca Raton, Fla: CRC Press; 1984. pp. 1–35. [Google Scholar]
- 35.Reinhold-Hurek B, Zhulin I B. Terminal oxidases of Azoarcus sp. BH72, a strictly respiratory diazotroph. FEBS Lett. 1997;404:143–147. doi: 10.1016/s0014-5793(97)00111-7. [DOI] [PubMed] [Google Scholar]
- 36.Sanchez-Amat A, Solano F. A pluripotent polyphenol oxidase from the melanogenic marine Alteromonas sp. shares catalytic capabilities of tyrosinases and laccases. Biochem Biophys Res Commun. 1997;240:787–792. doi: 10.1006/bbrc.1997.7748. [DOI] [PubMed] [Google Scholar]
- 37.Shioi J, Dang C V, Taylor B L. Oxygen as attractant and repellent in bacterial chemotaxis. J Bacteriol. 1987;169:3118–3123. doi: 10.1128/jb.169.7.3118-3123.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sone N, Fujiwara Y. Haem O2 can replace haem A in the active site of cytochrome c oxidase from thermophilic bacterium PS3. FEBS Lett. 1991;288:154–158. doi: 10.1016/0014-5793(91)81024-3. [DOI] [PubMed] [Google Scholar]
- 39.Sturr M G, Krulwich T A, Hicks D B. Purification of a cytochrome bd terminal oxidase encoded by the Escherichia coli app locus from a delta cyo delta cyd strain complemented by genes from Bacillus firmus OF4. J Bacteriol. 1996;176:1747–1749. doi: 10.1128/jb.178.6.1742-1749.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sun G, Sharkova E, Chesnut R, Birkey S, Duggan M F, Sorokin A, Pujic P, Ehrlich S D, Hulett F M. Regulators of aerobic and anaerobic respiration in Bacillus subtilis. J Bacteriol. 1996;178:1374–1385. doi: 10.1128/jb.178.5.1374-1385.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Svensson B, Hederstedt L. Bacillus subtilis CtaA is a heme-containing membrane protein involved in heme A biosynthesis. J Bacteriol. 1994;176:6663–6671. doi: 10.1128/jb.176.21.6663-6671.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thöny-Meyer L. Biogenesis of respiratory cytochromes in bacteria. Microbiol Mol Biol Rev. 1997;61:337–376. doi: 10.1128/mmbr.61.3.337-376.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thöny-Meyer L, Beck C, Preisig O, Hennecke H. The ccoNOQP gene cluster codes for a cb-type cytochrome oxidase that functions in aerobic respiration of Rhodobacter capsulatus. Mol Microbiol. 1994;14:705–716. doi: 10.1111/j.1365-2958.1994.tb01308.x. [DOI] [PubMed] [Google Scholar]
- 44.Thurston C F. The structure and function of fungal laccases. Microbiology. 1994;140:19–26. [Google Scholar]
- 45.Trumpower B L. Cytochrome bc1 complexes in microorganisms. Microbiol Rev. 1990;54:101–129. doi: 10.1128/mr.54.2.101-129.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Trumpower B L, Gennis R B. Energy transduction by cytochrome complexes in mitochondrial and bacterial respiration: the enzymology of coupling electron transfer reactions to transmembrane proton translocation. Annu Rev Biochem. 1994;63:675–716. doi: 10.1146/annurev.bi.63.070194.003331. [DOI] [PubMed] [Google Scholar]
- 47.Ullah A H, Ordal G W. In vivo and in vitro chemotactic methylation in Bacillus subtilis. J Bacteriol. 1981;145:958–965. doi: 10.1128/jb.145.2.958-965.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wardman P. Reduction potentials of one-electron couples involving free radicals in aqueous solution. J Phys Chem Ref Data. 1989;18:1637–1755. [Google Scholar]
- 49.Williamson P R, Wakamatsu K, Ito S. Melanin biosynthesis in Cryptococcus neoformans. J Bacteriol. 1998;180:1570–1572. doi: 10.1128/jb.180.6.1570-1572.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wong L S, Johnson M S, Zhulin I B, Taylor B L. Role of methylation in aerotaxis in Bacillus subtilis. J Bacteriol. 1995;177:3985–3991. doi: 10.1128/jb.177.14.3985-3991.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xu F. Oxidation of phenols, anilines and benzenethiols by fungal laccases: correlation between activity and redox potentials as well as halide inhibition. Biochemistry. 1996;35:7608–7614. doi: 10.1021/bi952971a. [DOI] [PubMed] [Google Scholar]
- 52.Zeilstra-Ryalls J H, Kaplan S. Control of hemA expression in Rhodobacter sphaeroides 2.4.1: regulation through alterations in the cellular redox state. J Bacteriol. 1996;178:985–993. doi: 10.1128/jb.178.4.985-993.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhulin I B, Panasenko V I, Stupnikova S K, Shchegolev S Y. Study of agglutination of microorganisms in suspension by a spectro-turbidimetric method. Biophysics (Moscow) 1984;29:935–940. [Google Scholar]