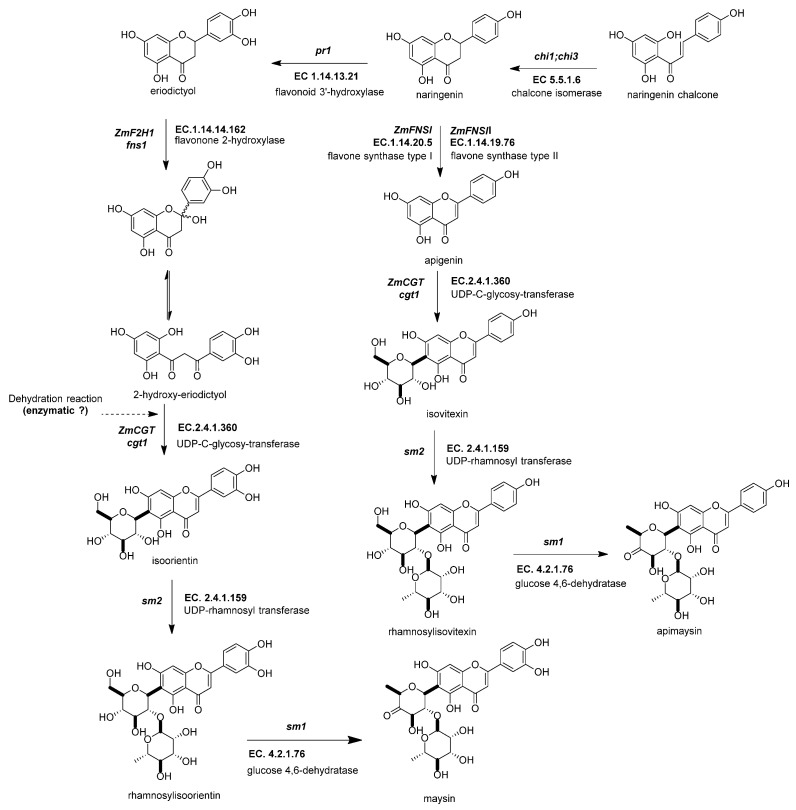
Figure 5.
Biosynthetic genes of flavone C-glycosides. The flavanones naringenin and eriodictyol are the initial substrates for the other flavonoid subgroups. There are two possible ways to generate C-glycosyl flavones, indirectly or directly, from any flavanone. The indirect pathway begins through flavanone-2-hydroxylase (ZmF2H, fnsii1, EC 1.14.14.162) opening the C-ring, producing a 3-oxo-dihydrochalcone. Then, UDP C-glycosyl transferase (ZmCGT, cgt1, EC 2.4.1.360) generates a glycosidic bond in the A-ring. Then, there is a dehydration reaction (spontaneous or enzymatic) that produces the C6-flavone glycoside. The direct pathway firstly involves flavone synthase I (ZmFNSII-2, fnsii2, EC 1.14.20.5) and flavone synthase II (ZmFNSII-1, fnsi2, EC 1.14.19.76) producing the same reaction by the addition of a double bond between C2 and C3 in the flavanone. Then, a flavone functions as a substrate for the UDP C-glycosyl transferase (ZmCGT, cgt1, EC 2.4.1.360). The enzymatic action of UDP-rhamnosyl transferase (ZmCGT, sm2, EC 2.4.1.159) and glucose 4,6 dehydratase (sm1, EC 4.2.1.76) produces either apimaysin or maysin. References: [30,43,90].