Abstract
The Arabidopsis transcription factor ABSCISIC ACID INSENSITIVE 4 (ABI4) is a key player in the plant hormone abscisic acid (ABA) signaling pathway and is involved in plant response to abiotic stress and development. Expression of the ABI4 gene is tightly regulated, with low basal expression. Maximal transcript levels occur during the seed maturation and early seed germination stages. Moreover, ABI4 is an unstable, lowly expressed protein. Here, we studied factors affecting the stability of the ABI4 protein using transgenic Arabidopsis plants expressing 35S::HA-FLAG-ABI4-eGFP. Despite the expression of eGFP-tagged ABI4 being driven by the highly active 35S CaMV promoter, low steady-state levels of ABI4 were detected in the roots of seedlings grown under optimal conditions. These levels were markedly enhanced upon exposure of the seedlings to abiotic stress and ABA. ABI4 is degraded rapidly by the 26S proteasome, and we report on the role of phosphorylation of ABI4-serine 114 in regulating ABI4 stability. Our results indicate that ABI4 is tightly regulated both post-transcriptionally and post-translationally. Moreover, abiotic factors and plant hormones have similar effects on ABI4 transcripts and ABI4 protein levels. This double-check mechanism for controlling ABI4 reflects its central role in plant development and cellular metabolism.
Keywords: Arabidopsis thaliana, ABI4, MAPK, salinity, ABA, proteasome, transcription factor
1. Introduction
Plant development and response to environmental cues involve signaling pathways in which the last components are often transcription factors (reviewed by [1]). As a result, these signaling pathways affect the transcription of a large number of genes, the expression of which is affected by the respective transcription factors.
The Arabidopsis ABSCISIC ACID INSENSITIVE 4 (ABI4) gene encodes an APETALA 2 (AP2) family transcription factor [2]. APETALA 2 is a plant-specific DNA-binding domain with a length of ~60 amino acids first characterized in the Arabidopsis APETALA2 homeotic gene [3,4]. The ABI4 gene was identified by screening gamma-irradiated Arabidopsis seeds for mutants capable of germination in the presence of inhibitory concentrations of the plant hormone abscisic acid (ABA) [5]. ABI4 alleles were isolated by screening for germination in the presence of high concentrations of salt and sugar [6,7,8,9,10]. ABI4 also plays a central role in other plant signaling pathways, including lipid mobilization, lateral root development, regulation of light-modulated genes, redox signaling, pathogen response, and mitochondrial retrograde signaling (reviewed in [11]). Its role in chloroplast retrograde signaling is disputed [12,13].
ABI4 expression is tightly developmentally regulated; the highest steady-state levels of the ABI4 transcript are found in embryos, maturing pollen, and early germination stages [14,15,16]. The transcript levels of ABI4 are significantly reduced in other developmental stages; its expression is restricted to root phloem companion cells and parenchyma and, to some extent, to the vascular system of the shoot [17,18,19]. In addition, steady-state levels of the ABI4 transcript are enhanced by ABA, NaCl, and glucose and repressed by auxin [17,18,20].
ABI4 is a highly unstable protein [21,22]. Several protein motifs, such as PEST and AP2-associated [21,22], destabilize ABI4 via degradation by the proteasome. Other regions of the protein destabilize it in a proteasome-independent manner [21]. ABI4 is stabilized by high concentrations of salt and sugar [21,22] and by preventing light exposure [23]. COP1 is involved in the light-mediated degradation of ABI4 [23]; levels of ABI4 were enhanced in light-exposed cop1 mutant seedlings and further increased by treating cop1 mutants with the MG132 proteasome inhibitor, suggesting that COP1, as well as additional E3s, modulate ABI4 stability [23].
Being downstream of the signaling pathway cascades, transcription factors are frequently modulated by phosphorylation, resulting in their activation or inhibition. ABI4 was phosphorylated in vitro by MPK3, MPK4, and MPK6 [24,25,26,27]. Phosphorylation of ABI4 by MAPKs repressed the expression of the LHCB gene [25] and inhibited the emergence of adventitious roots [26]. In addition, the phosphorylation of S114 is essential for the biological activity of ABI4, as shown in studies of the complementation of the abi4 mutant phenotype [27].
Here, we studied factors affecting the stability of the ABI4 protein in Arabidopsis plants by expressing HA-FLAG-ABI4-eGFP driven by the constitutive highly active 35S promoter. The tagged ABI4 was detected in embryos rescued from imbibed seeds but not in seedlings. Treatment of the seedlings with NaCl resulted in a transient stabilization of ABI4, peaking at 2–4 h. ABA and high glucose also stabilized ABI4-eGFP but with slower kinetics, reaching lower levels than with NaCl treatment. The phosphomimetic ABI4 (S114E) protein was more stable than the wild-type ABI4 and the phosphorylation-null ABI4 (S114A) mutant in salt-treated plants, suggesting that phosphorylation of ABI4 by MAPKs results in stabilization of ABI4. Interestingly, NaCl, ABA, and glucose are known to similarly affect the steady-state levels of ABI4 transcripts [17,18,20]. We thus propose that the MAPK signaling cascade also activates ABI4 transcription via the phosphorylation of MYB, WRKY, and ABI4 transcription factors known to transactivate the transcription of the ABI4 gene. As a result, similar cues regulate ABI4 in terms of transcriptional and post-transcriptional levels, resulting in a very tight regulation of this key factor.
2. Results
2.1. The 35S::HA-FLAG-ABI4-eGFP Construct Encodes a Biologically Active Protein
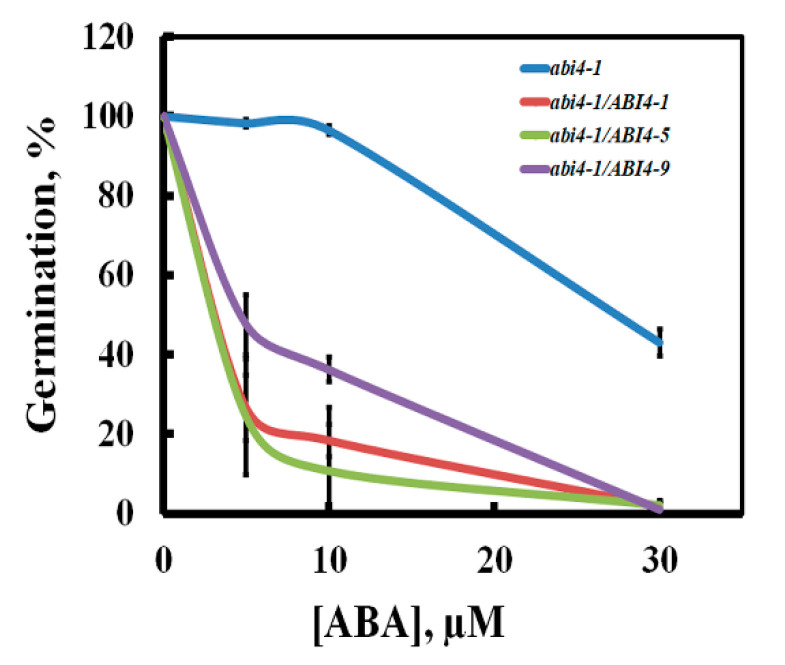
To study ABI4 in planta, we used the enhanced green fluorescent protein (eGFP) [28] fused to the carboxy terminus of ABI4 and transcription driven by the highly active cauliflower mosaic virus constitutive 35S promoter (35S) [29]. We previously found that overexpressing 35S::ABI4 in Arabidopsis resulted in seedling death within three days of germination, whereas fusing the HA3-FLAG3 tag to the N terminus of ABI4 resulted in viable plants [18]. Therefore, constructed 35S::HA-FLAG-ABI4-eGFP and used it for the transformation of Arabidopsis. To determine whether HA-FLAG-ABI4-eGFP protein is biologically active, we tested whether tagged ABI4 can complement the abi4-1 mutant. abi4-1 is a frameshift mutant resulting from a single-bp deletion at codon 157 [2]; the expressed protein has the AP2 DNA binding domain but lacks the transactivation domain. The resulting transgenic plants did not have any visible phenotype when grown on agar plates with 0.5 × MS, 0.5% sucrose medium or in pots containing potting mix. To determine whether the expressed HA-FLAG-ABI4-eGFP (ABI4-eGFP) protein retains the biological activity of ABI4, we examined its ability to complement the phenotype of abi4 mutants by assaying seed germination in the presence of ABA, the most extensively studied phenotype of these mutants [30]. Figure 1 shows that expressing HA-FLAG-ABI4-eGFP in abi4-1 plants restored the ABA sensitivity, indicating that tagging ABI4 at neither its amino- nor carboxy-termini impairs its biological activity.
Figure 1.
Complementation of the abi4-1 mutant by 35S::HA3-FLAG3-ABI4-eGFP. Seeds of the homozygous plants of the indicated genotypes were plated on agar-solidified 0.5 × MS, 0.5% sucrose medium supplemented with the indicated concentrations of ABA. Germination was scored 7 days later. abi4, abi4-1 mutant; abi4/ABI4-1, -5, -9, transgenic lines 1, 5, and 9 of abi4-1 plants transformed with the 35S::HA3-FLAG3-ABI4-eGFP construct. Data represent means ± SE; n = 3 biological replicates.
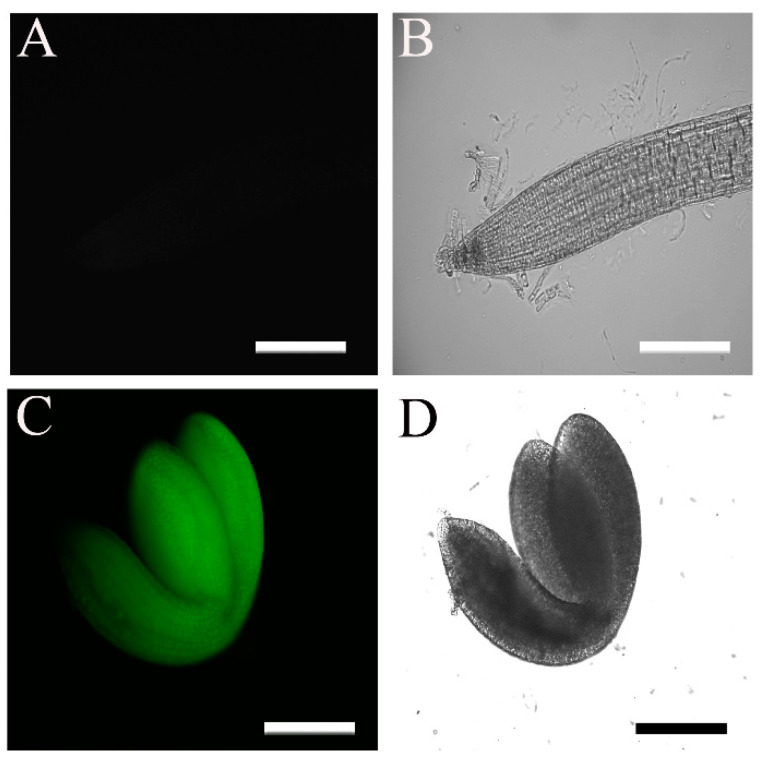
The 35S promoter is a commonly used strong constitutive promoter that is active in most plant tissues [31]. We therefore expected to detect high eGFP fluorescence signals in seedlings of WT plants transformed with the 35S::HA-FLAG-ABI4-eGFP construct (WT/ABI4-eGFP). Surprisingly, we did not detect significant fluorescent signals in these plants (Figure 2A,B). To confirm the construct, we examined the fluorescence in embryos prepared from imbibed seeds and detected a highly fluorescent signal in the entire embryo (Figure 2C,D). These results suggest that ABI4 levels may be subject to post-transcriptional regulation.
Figure 2.
Fluorescence levels of 35S::HA3-FLAG3-ABI4-GFP seedling roots and embryosroots. The fluorescence of plants transformed with the 35S::HA3-FLAG3-ABI4-GFP construct was examined by microscopy. (A,B) Ten-day-old root; (C,D) embryo extracted from a seed imbibed for 24 h; (A,C) fluorescence images; (B,D) bright-field images. Scale bar = 100 µm.
2.2. Accumulation of ABI4-eGFP Is NaCl-Dependent
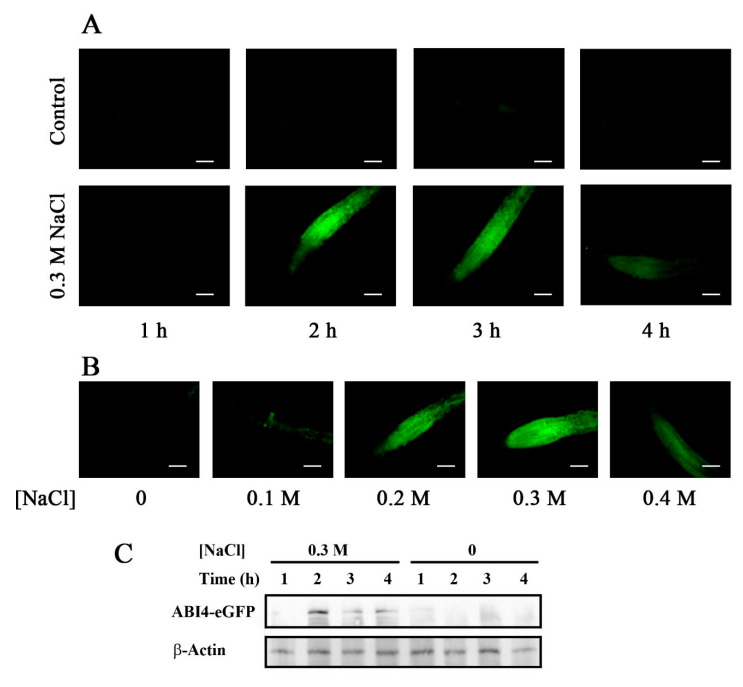
Previous studies showed that environmental signals post-transcriptionally regulate ABI4. To examine whether varying external and internal cues affect the steady-state levels of ABI4, we tested cues known to affect the activity of the ABI4 promoter. The steady-state levels of ABI4 mRNA driven by its endogenous promoter are enhanced by NaCl [17]. We therefore examined whether NaCl also affects protein levels of ABI4 when transcription is driven by the 35S promoter. Exposing WT plants expressing ABI4-eGFP to 0.3 M NaCl resulted in a transient increase in the eGFP fluorescence signal, with the maximal signal observed 2–3 h following seedling exposure to salt (Figure 3A). The signal was NaCl-dose-dependent, with the maximum at 0.3 M NaCl (Figure 3B). No fluorescence was observed in seedlings transferred to fresh 0.5 × MS, 0.5% sucrose medium, suggesting that the transient increase in fluorescence observed in the NaCl-treated seedlings did not result from transferring the seedlings from the agar plates to the buffer-soaked filter paper. To confirm the observed fluorescence signals, protein extracts of the roots of salt-treated WT/ABI4-eGFP seedlings were subjected to western blot analysis using an anti-GFP antibody. The results confirmed that ABI4-eGFP was essentially undetectable in control untreated roots, whereas a transient increase in ABI4-eGFP was observed following exposure to NaCl, peaking 2 h after the application of NaCl (Figure 3C). The protein levels of ABI4-eGFP were low, even at maximal values, and were detected with a high-sensitivity detection assay.
Figure 3.
NaCl treatment transiently enhances ABI4-eGFP protein levels. Ten-day-old Arabidopsis plants expressing the 35S::HA3-FLAG3-ABI4-eGFP construct incubated for the indicated times with 0.5 × MS salts, 0.5% sucrose without (A, control, upper row) or with 0.3 M NaCl (A, lower row) or for 2.5 h with growth medium containing the indicated incubated concentrations of NaCl (B). Roots were examined by fluorescence microscopy. Scale bar = 100 µm. (C) Western blot analysis showing the expression levels of ABI4-eGFP following NaCl treatment. β-actin was used as the loading control.
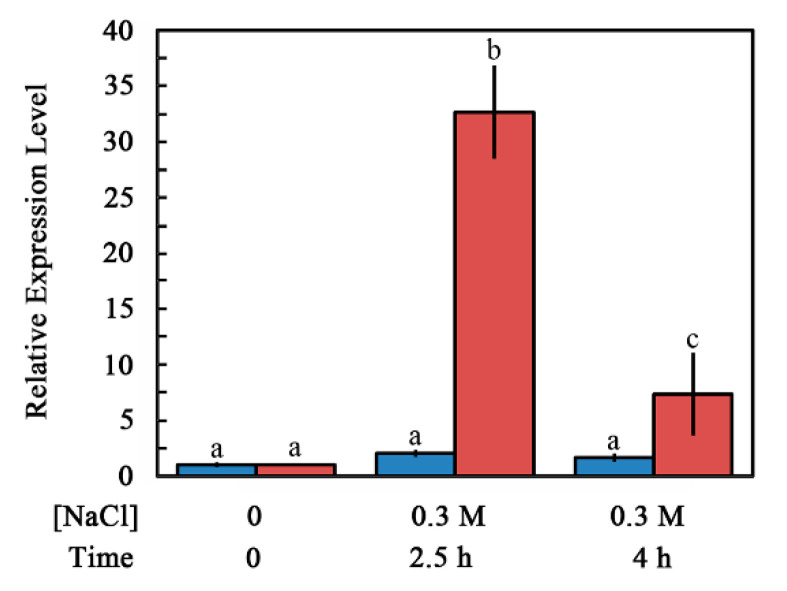
The NaCl-dependent increase in ABI4 protein levels may result from either a change in the transcript levels of the encoding mRNA or regulation of the protein levels. To assess this point, we quantified the ABI4-eGFP transcript and protein levels in roots of untreated and NaCl-treated seedlings. ABI4-eGFP transcript levels were determined by RT-qPCR using amplification primers from the sequence encoding the HA3-FLAG3 tag to avoid assaying the expression of the endogenous ABI4 gene. The steady-state mRNA levels of ABI4-eGFP were increased by 2.0-and 1.6-fold at 2.5 and 4 h, respectively, after seedling exposure to high salt concentration. The ABI4-eGFP protein levels quantified using the fluorescence intensity of the roots were 32.7 and 7.3 times higher for roots of plants exposed to 0.3 M NaCl for 2.5 and 4 h, respectively, compared to control untreated roots (Figure 4). Control fluorescence signals of plants expressing 35S::GFP were not affected by salt treatment (Figure S1). These results indicate that the NaCl-dependent increase in ABI4-GFP protein levels resulted from post-transcriptional regulation of ABI4 rather than from changes in the transcript levels or the effect of salt on the GFP tag.
Figure 4.
Effect of NaCl treatment on steady-state levels of ABI4-eGFP transcript and protein in the roots. Ten-day-old seedlings transformed with the 35S::HA3-FLAG3-ABI4-eGFP construct were transferred onto a filter paper soaked with 0.5 × MS salts, 0.5% sucrose with or without 0.3 M NaCl. Roots were harvested at the indicated times, and the levels of HA3-FLAG3-ABI4-eGFP transcript (blue) or protein (red) were determined by RT-qPCR and fluorescence microscopy, respectively. Data represent mean ± SE. Bars with different letters indicate significant differences according to one-way ANOVA and Tukey’s HSD post hoc test (p ≤ 0.01).
2.3. Subcellular Localization of ABI4-GFP following NaCl Treatment Is Cell-Type-Specific
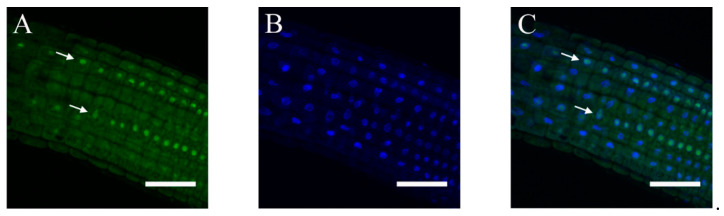
Although NaCl treatment of plants expressing the 35S::HA-FLAG-ABI4-eGFP construct resulted in enhanced protein levels in most root cells, the observed fluorescence pattern of the ABI4-eGFP was diffusive in most cell types. In contrast, it was found in spherical structures mostly in the root stele, suggesting nuclear localization (Figure 5A). Staining nuclei of the roots of NaCl-treated ABI4-eGFP plants with the DNA fluorescence stain 4′,6-diamidino-2-phenylindole (DAPI) (Figure 5B) shows that the DAPI and eGFP fluorescence signals overlap, confirming that ABI4-eGFP is localized in the nuclei of root stele cells (Figure 5C). This pattern is specific to ABI4, as it was not observed in the roots expressing the eGFP tag alone (Figure S1).
Figure 5.
The subcellular localization of ABI4-GFP in the roots is cell-type-dependent. Ten-day old seedlings expressing the 35S::HA3-FLAG3-ABI4-GFP construct were treated with 0.3 M NaCl for 2.5 h. Roots were stained with DAPI and examined by confocal microscopy. (A) GFP fluorescence; (B) DAPI fluorescence; (C) merged images of (A,B). Arrows mark columns of cells expressing ABI4-eGFP in the nuclei. Scale bar = 10 µm.
2.4. ABA and Glucose Treatment Enhance ABI4-eGFP Protein Levels
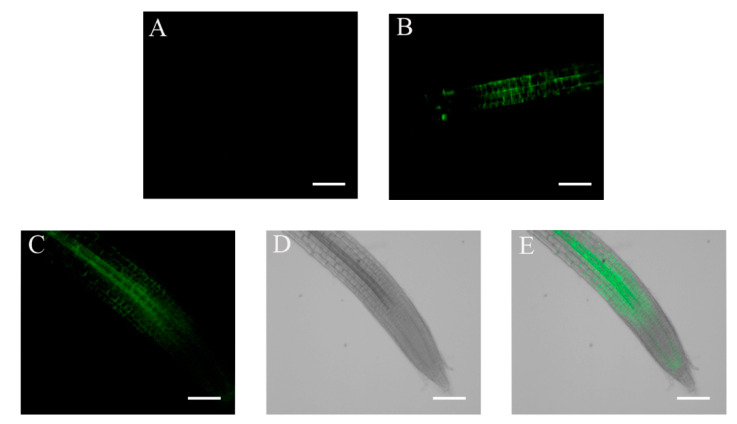
Transcript levels of endogenous ABI4 are also induced by treatment with ABA or high concentrations of glucose [20]. To determine whether these treatments also affect the levels of the ABI4-eGFP protein, ten-day-old 35S::HA-FLAG-ABI4-eGFP transgenic plants were transferred to media containing ABA or glucose, and ABI4-eGFP accumulation was followed by fluorescence microscopy. Enhanced ABI4-eGFP levels were detected in the root stele of seedlings approximately 24 h after treatment with ABA or glucose (Figure 6).
Figure 6.
ABI4 expression is increased following glucose and ABA treatment. Ten-day-old 35S::HA3-FLAG3-ABI4-eGFP-expressing plants were incubated for 24 h in 0.5 × MS, 0.5% sucrose growth medium (A) or in the same medium supplemented with 7% glucose (B) or 30 µM ABA (C). (D,E) Bright-field and merged image of the ABA treated root shown in (C). Scale bar = 100 µm.
2.5. Auxin Counteracts the NaCl-Induced Increase in ABI4-eGFP Levels
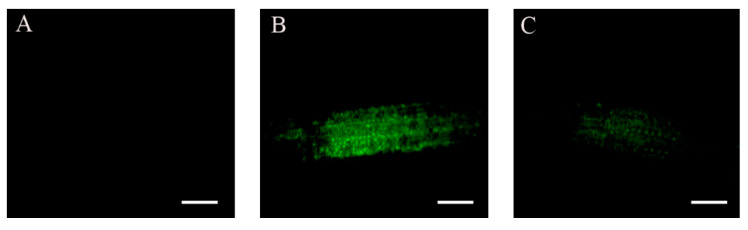
ABI4 mediates cytokinin inhibition of lateral root formation by reducing the polar transport of auxin, a plant hormone known to induce the formation of lateral roots [18]. Exogenous auxin also reduced the steady-state levels of ABI4 transcripts in the roots [18]. To test whether auxin also post-transcriptionally regulates ABI4, we tested whether auxin counteracts the NaCl-induced enhancement of ABI4-eGFP. Figure 7 shows that when added together with NaCl, 3-indole acetic acid (IAA) prevented the NaCl-induced accumulation of ABI4-eGFP, indicating that auxin negatively regulates the steady-state levels of the ABI4 protein.
Figure 7.
Auxin prevents the NaCl-induced increase in ABI4. Ten-day-old seedlings were treated with 0.5 × MS 0.5% sucrose (A) supplemented with 0.3 M NaCl (B) or 0.3 M NaCl and 20 µM IAA (C). Roots were examined 2.5 h later. Scale bar = 100 µm.
2.6. Steady-State Levels of ABI4-eGFP Are Controlled by De Novo Translation and Degradation by the 26S Proteasome
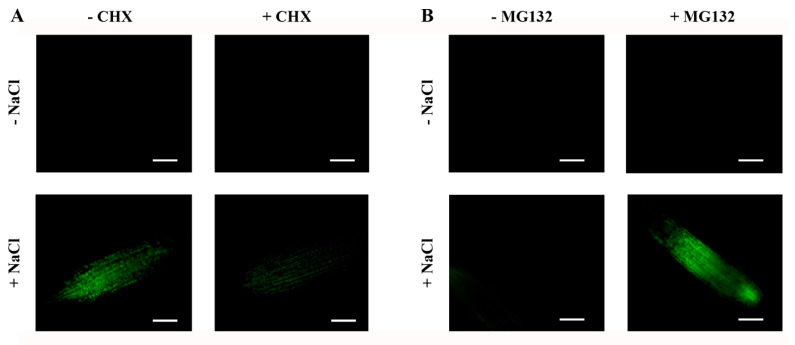
We used the protein synthesis inhibitor cycloheximide (CHX) and the proteasome inhibitor MG132 to further characterize the transient accumulation of ABI4-eGFP following exposure to NaCl. As expected, CHX prevented the NaCl-dependent accumulation of ABI4-eGFP protein (Figure 8A), suggesting that exposure to NaCl enhances de novo translation of ABI4-eGFP. Treatment with a mix of NaCl and MG132 resulted in increased stabilization of ABI4-eGFP, and a high signal was detected, even 6 h after the co-application of NaCl and MG132 (Figure 8B) but not in the roots of plants treated with NaCl alone.
Figure 8.
The NaCl-dependent transient increase in the ABI4-eGFP levels is a result of de novo protein synthesis and its degradation by the 26S proteasome. Ten-day-old ABI4-eGFP-expressing seedlings were incubated in light for 2.5 h (A) or 6 h (B) on filter paper soaked with 0.5 × MS, 0.5% sucrose solution supplemented, as indicated, with 0.3 M NaCl, 20 µg/mL cycloheximide (CHX) or 20 μg/mL MG132. Roots were then examined by fluorescence microscopy. Scale bar = 100 µm.
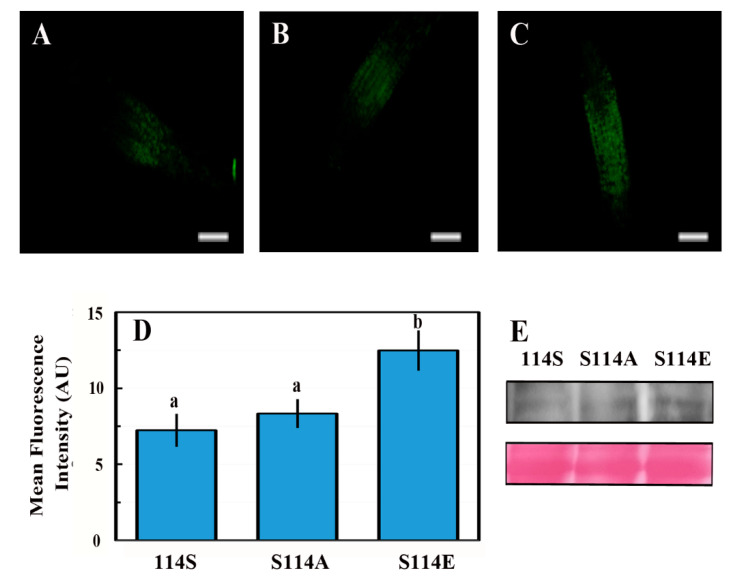
2.7. The Phosphorylation State of Serine 114 Affects the Stability of the ABI4 Protein
We recently showed that phosphorylation of serine 114 of ABI4 by MPK3 or MPK6 is essential for its biological activity [27]. Here, we tested whether the phosphorylation state of S114 of ABI4 also affects its stability; WT Arabidopsis plants were transformed with 35S::HA-FLAG-ABI4-eGFP constructs encoding the ABI4 (S114A), phosphorylation-null mutant or ABI4 (S114E), phosphomimetic mutated proteins. Ten-day-old NaCl-treated seedlings were examined by fluorescent microscopy. Roots expressing WT (114S) ABI4-eGFP showed very low levels of fluorescence (Figure 9A), as shown in Figure 3. Fluorescence levels in roots of plants transformed with the ABI4-eGFP (S114A) phosphorylation-null mutant (Figure 9B) were similar to those of the WT ABI4-eGFP protein. In contrast, the S114E phosphomimetic mutation stabilized the ABI4-eGFP protein, and high levels were observed, even 6 h following NaCl exposure (Figure 9C). The fluorescence signal was quantified (Figure 9D), and the ABI4-eGFP protein levels were also confirmed by western blot analysis using an anti-GFP antibody (Figure 9E). Our data indicate that the phosphorylation of serine 114 by MAPKs stabilizes ABI4, the active form of this transcription factor.
Figure 9.
Phosphorylation of S114 stabilizes the ABI4 protein. Ten-day-old ABI4-eGFP expressing seedlings (A) WT ABI4-eGFP, (B) the phosphorylation-null (S114A) mutant, or (C) the phosphomimetic (S114E) mutant, were incubated for 6 h with 0.5 × MS, 0.5% sucrose and 0.3 M NaCl. The roots were examined by fluorescence microscopy. Scale bar = 100 µm. (D) The fluorescent signals of 70 plants were quantified. Data are expressed as average ± SE. Bars with different letters represent statistically significant differences according to Tukey’s HSD post hoc test (p < 0.05). (E) Western blot analysis of seedling proteins using anti-GFP antibody showing the expression levels of the S114 ABI4-eGFP phosphorylation state mutants following 6 h of NaCl treatment (upper panel). Ponceau-stained RuBisCo large subunit was used as a loading control (lower panel).
3. Discussion
In this study, we showed that ABI4 protein is highly unstable and that it is degraded by the 26S proteasome. In the roots, ABI4 is transiently stabilized by salt, ABA, and high glucose. Phosphorylation of S114 of ABI4, a residue previously shown to be phosphorylated by MAP kinase, increases its stability. ABI4 is a master transcription regulator, acting as both activator and repressor in the regulation of developmental processes, such as seed development, germination, root development, response to stress and hormones, disease resistance, and lipid metabolism [11,32]. ABI4 is evolutionarily conserved and is a single gene in Arabidopsis and in most plant genomes that encode ABI4 [11,33], suggesting that its biological role is non-redundant. As a result, abi4 mutants display pronounced phenotypes, such as insensitivity to ABA inhibition of seed germination and reduced sensitivity to high glucose and salt [6,7,18,30].
3.1. ABI4 Is a Lowly Expressed and Highly Regulated Gene
As expected, as a key regulator, ABI4 levels and activity are tightly regulated. Maximal levels of ABI4 transcripts are detected in developed seeds and in early germination stages, with very low levels present during other developmental stages [14,15,16,19] in which it is expressed in the phloem and parenchyma of the roots [18,19]. ABI4 expression is regulated by plant hormones: enhanced by ABA [14] and cytokinin [34] and reduced by auxin [18]. It is also enhanced in response to high glucose [20], as well as osmotic [20] and salt [17] stresses. Arabidopsis ABI4 is encoded by an intronless gene. Intronless genes are characteristic of highly regulated TFs in both plants and animals [35,36]. Moreover, intronless genes are differentially expressed in response to drought and salt treatment [36].
3.2. ABI4 Is a Post-Transcriptionally Regulated Low-Level Protein
Because the transcription of ABI4 is highly regulated, in order to study the post-transcriptional regulation of ABI4, we expressed HA-FLAG-ABI4-eGFP driven by the constitutive highly expressed CaMV 35S promoter (35S). eGFP is a GFP variant that is 35 times brighter than the original GFP [37], allowing for the detection of lower concentrations of tagged proteins than with the previously used ABI4-GFP [21]. This construct complemented the phenotype of the abi4-1 mutant, indicating that tagging ABI4 at its N and C termini does not eliminate its biological activity (Figure 1). We did not detect recombinant ABI4 protein in ten-day-old seedlings grown on plates under control conditions (Figure 2). In contrast, a high fluorescence signal was observed in imbibed embryos, confirming the performance of the construct (Figure 2). Although we used the highly active constitutive viral 35S CaMV promoter to express ABI4-eGFP, the resulting transgenic plants did not show any significant fluorescence of the eGFP tag (Figure 2). GFP-ABI4 was not detected in Arabidopsis plants transformed with 35S::GFP-ABI4 [21]. GUS activity staining identified the expression of ABI4-GUS recombinant protein driven by the same promoter. In contrast, ABI4-GFP fluorescence was detected in Arabidopsis protoplasts transfected with a 35S::ABI4-GFP construct [22]. This discrepancy may be explained by protoplasts being under stress caused by enzymatic digestion of the cell wall [38].
3.3. ABI4 Is Stabilized by External Signals
Although the 35S promoter is active in most plant tissues, ABI4-GFP is expressed primarily in the roots following stress (Figure 3), confirming observations by Finkelstein et al., who expressed ABI4-GUS fusion protein [21]. ABI4-eGFP was observed mainly in the vascular system of the roots following ABA and glucose treatments (Figure 6). ABI4-eGFP accumulated in the cells in which the endogenous ABI4 promoter was active [18]. Although NaCl treatment resulted in the accumulation of ABI4-eGFP throughout the roots, it was targeted to the nuclei only in the vascular cells (Figure 5), suggesting that both the accumulation and subcellular localization of the ABI4 protein are regulated in a cell-specific manner. This is similar to the transcription factor ABI5, which also accumulated following NaCl and ABA treatments, although increased levels were observed only four days after exposure to 200 mM NaCl [39]. Furthermore, ABA stabilization of ABI5 was restricted to a narrow developmental window 2 days after germination [39]. The difference in kinetics and responsive window suggests that although ABI5 and ABI4 proteins are stabilized by similar agents (ABA and NaCl), each protein has different domains [2,40], and as such, they are likely to be stabilized through other mechanisms. Transient expression of stress-induced genes has been reported for many genes, whereby the steady-state levels of mRNA peak at a given time after application of the stress agent, followed by a decrease. For example, mRNA levels of the stress-induced Arabidopsis transcription factors DREB1A DREB2A and rd29A are transiently induced following exposure to cold, drought, and salt stresses [41].
3.4. Phosphorylation of S114 Stabilizes ABI4
The phosphomimetic S114E form of ABI4 was more stable than the WT or the non-phosphorylated S114A mutant (Figure 9), suggesting that phosphorylation of S114 may decrease its ubiquitylation by a yet unidentified ubiquitin ligase. The S114 residue is included in the serine/threonine (S/T) region motif of ABI4 [2]. Several domains are proposed to contribute to the instability of ABI4: the PEST domain located at the N terminus of ABI4 (amino-acids 22–40) enhances the degradation of ABI4 [21,22]. Furthermore, the N-terminal half of the ABI4 protein, including the PEST, APETALA2 (AP2), serine/threonine rich domain (S/T); the glutamine-rich domain (Q); and the C-terminal half containing the Q and proline-rich (P) domains, were shown to be highly unstable [21]. Degradation of the N-terminal half but not the C-terminal half of ABI4 was suppressed by the MG132 proteasome inhibitor, suggesting that although highly unstable, the C-terminal half of ABI4 may not be degraded by the proteasome [21]. The AP2-associated motif was also shown to destabilize ABI4 [22]. Although the S/T rich region was included in the labile N-terminal half of ABI4 [21], the instability of this region was mainly attributed to the PEST motif. Proteasomal degradation of ABI4 through the PEST motif is modulated by sugar levels [22].
Using the proteomic approach in human cell lines, Wu et al. [42] recently showed that phosphorylation delays the turnover of many proteins in growing cells. Moreover, the phosphomimetic mutated proteins catenin beta-1 (CTNNB1) S191D and the transcriptional receptor protein YY1 S118D were more stable than the WT proteins, and the phosphorylation-null in which the respective serine residues were mutated to alanine were destabilized [42]. In addition, phosphoserine residues had a larger stabilization effect than phosphothreonine, and phosphotyrosine had only a marginal stabilization effect.
Phosphorylation of type-A response regulator 5 (ARR5) by SnRK2s enhanced its stability [43]. Furthermore, overexpressing WT ARR5 but not the non-phosphorylatable mutated protein enhanced ABA hypersensitivity, suggesting that the phosphorylated form of ARR5 is biologically active. ABA suppressed the degradation of ARR5 [43]. Phosphorylation of the rate-limiting enzymes of ethylene biosynthesis, 1-aminocyclopropane-1-carboxylic acid synthase2 and 6 (ACS2 and ACS6), by MPK6 stabilizes the respective ACS proteins. Furthermore, the phosphomimetic ACS6 mutant was constitutively active, suggesting that phosphorylation of ACS6 by MPK6 is essential for its activity [44]. The RNA-binding protein tandem zinc finger 9 (TZF9) is destabilized by MAPK-mediated phosphorylation [45].
3.5. MAPK Regulates ABI4 Both Transcriptionally and Post-Transcriptionally
We showed that phosphorylation of S114 stabilizes ABI4 (Figure 9). We recently demonstrated that MPK3, MPK4, and MPK6 phosphorylate S114 of ABI4 and that this phosphorylation is essential for the biological activity of ABI4 and the complementation of abi4 mutant plants [27]. MPK3, MPK4, and MP6 are involved in the abiotic and biotic stress response (reviewed in [46]). Treatments with NaCl, ABA, and high glucose, which result in stabilization of ABI4 (Figure 3 and Figure 6), also enhance the steady-state levels of the ABI4 transcripts [17,18,20]. Our results indicate that MAPK signaling affects both ABI4 transcription and protein stability.
The kinetics we observed for the transient stabilization of ABI4 following salt treatment (Figure 3) resemble the described transient activation of MKK5 following exposure of Arabidopsis plants to high salt, whereby increased activity of MKK5 was detected within 30 min of the treatment, reaching maximal activity at 2–4 h and declining at 6 h after exposure to NaCl to nearly basal activity levels [47]. MKK5 phosphorylates and activates several MPKs, including MPK3, MPK4, and MPK6. Therefore, the activity of these MPKs is also expected to be transient following salt treatment, resulting in a transient wave of phosphorylation of ABI4. MPK4 and MPK6 are rapidly activated by treatments such as high salt and osmotic stress but not by ABA treatment [48]. ABA activates the transcription of many genes encoding components of the MAPK cascade [49], suggesting that the slow kinetics leading to accumulation of ABI4-eGFP following ABA treatment may result from slow de novo synthesis of the MAPKs rather than fast activation of pre-existing latent enzymes.
MPK3, MPK4, and MPK6 also phosphorylate the transcription factors WRKY and MYB [24,50]. Several WRKY and MYB transcription factors may regulate ABI4 expression [51,52,53,54,55,56,57,58,59,60]. In addition, as ABI4 also activates the transcription of its own gene [61], its phosphorylation by these MAPKs also enhances its transcript levels.
In summary, our results show that phosphorylation of ABI4 by MAPK results in the stabilization of ABI4. Phosphorylation of S114 by MPKs may interfere with its binding to a yet unidentified E3 for proteasomal degradation. Alternatively, the catalytic efficiency of the E3 may be reduced toward phosphorylated ABI4. MAPK signaling also regulates ABI4 transcription. Thus, we suggest that regulation of both the ABI4 transcript and ABI4 protein levels results in the tight regulation of the activity of this key transcription factor in the ABA signaling pathway.
4. Materials and Methods
4.1. Plant Material and Growth Conditions
Arabidopsis thaliana (Col) seeds of the indicated genotypes were surface-sterilized, cold-treated for 3 days, and plated in Petri dishes containing 0.5 × Murashige and Skoog medium (MS), 0.55% plant agar, and 0.5% (w/v) sucrose, as previously described [18]. Plates were incubated at 22–25 °C and 50% humidity under a circadian regime of 12 h light/12 h dark.
4.2. Constructs and Plant Transformation
The pGA-eGFP2 vector was constructed by replacing the sequences of the MCS and 35S::mGFP5 (9640–1038) in the pCAMBIA1302 vector (www.cambia.org accessed on 1 April 2010) with the 2 × 35S-MCS-eGFP DNA sequence (405–2332) from the pSAT4-eGFP-N1 plasmid using Gibson assembly cloning [62]. The DNA sequence encoding HA3-FLAG3-ABI4 was isolated by digesting the pJIM19-ABI4 plasmid [17] with restriction enzymes NcoI and PstI and subcloning into the respective sites in pGA-eGFP2 to yield the pGA-HA3-FLAG3-ABI4 plasmid. To construct plasmids encoding ABI4 (S114A) and ABI4 (S114E) mutant proteins, the respective DNA sequences were amplified from the respective pRSET-ABI4 plasmid [27] using gene-specific primers flanked by the SalI restriction sites and digesting the amplified sequences with SalI. The DNA sequence encoding WT-ABI4 was removed from the pGA-HA3-FLAG3-ABI4 plasmid by digestion with SalI, followed by subcloning of the DNA sequences encoding mutated ABI4 protein. Primers used for the construction of plasmids are shown in Table S1. The resulting plasmids were verified by PCR and DNA sequencing and were introduced into Agrobacterium tumefaciens strain GV3101. The transformed bacteria were used to transform WT Col or abi4-1 Arabidopsis plants by the floral dip method [63]. Transgenic plants were selected on plates containing hygromycin and transferred to pots. Plant were grown at 22–25 °C and 50% humidity with 16 h light/8 h dark. Homozygous T2 and T3 generation plants were used in this study.
4.3. Germination Assay
Sterilized cold-treated seeds were plated on agar-solidified 0.5 × MS, 0.5% sucrose medium supplemented with the indicated concentrations of the phytohormone ABA. Germination was scored 7 days later.
4.4. Plant Treatment
For the various treatments, 10-day-old seedlings were transferred to Petri dishes containing Whatman No.1 filter papers soaked with 0.5 × MS medium and 0.5% (w/v) sucrose supplemented with the indicated stress agent, plant hormone, or inhibitors. Plants were incubated at room temperature in under light for the indicated times.
4.5. Microscopy
The indicated tissues were examined using a fluorescent microscope (ECLIPSE Ci-L; Nikon) with filters set for GFP. The images reflect GFP signals in all the cells of the examined tissue, thus representing the expression levels in all cell types. All images in each experimental repeat were taken using the same microscope, camera setup, and exposure times. Each experiment was repeated at least three times using at least four independent lines of the transgenic plants. Fluorescent signals were quantified using ImageJ software [64], with the black background set as zero for the measurement of the fluorescent intensity of the image. Subcellular localization images were taken with a Zeiss LSM-880 confocal microscope
4.6. Embryo Excision
Arabidopsis seeds imbibed for 24 h in water at room temperature were pressed gently between two microscope slides. Embryos released from seed coats were collected and rinsed briefly in water.
4.7. Protein Extraction, SDS-PAGE, and Western Blot Analysis
Ten-day-old seedlings were harvested into a 1.5 mL microcentrifuge tube, and their fresh weight was determined. Next, 2:1 (v/w) 4 × SDS-PAGE sample buffer [65] was added, and the seedlings were homogenized with a microcentrifuge pestle. To ensure efficient solubilization of plant proteins, homogenates were passed through 2 cycles of freezing in liquid nitrogen andboiling for 5 min. Tubes were centrifuged for 10 min at 12,000× g at room temperature, and supernatant samples were resolved by SDS PAGE. Proteins were electroblotted onto nitrocellulose membranes. ABI4-eGFP and β-actin were detected using the primary antibodies anti-GFP (Abcam, ab1218, Cambridge, UK) and anti-β-actin (Sigma, A4700, Saint Louis, MO, USA), respectively, and secondary peroxidase-coupled anti-mouse IgG antibody (Sera Care 5450–0011). Membranes were incubated in reaction mixes prepared from with a highly sensitive SuperSignal West Dura extended substrate kit (Thermo scientific, Waltham, MA, USA), and chemiluminescent signals were recorded using ImageQuant RT ECL Imager (GE Healthcare, Chicago, IL, USA).
4.8. Quantitative RT-PCR Analysis
Total RNA was isolated from roots using a ZR Plant RNA MiniPrep kit (Zymo research). The RNA concentration was estimated spectrally (Nano Drop ND-1000; Nano Drop Technologies). cDNA was synthesized using a qScript cDNA synthesis kit (Quanta). The reaction mixture contained 700 ng total RNA and random primers. Primer design and RT-qPCR assays for determining relative steady-state transcript levels were as previously described [17]. Primers are described in Table S1.
Acknowledgments
We thank Guy Adler for constructing the pGA-eGFP vector. Dudy Bar-Zvi is the incumbent of The Israel and Bernard Nichunsky Chair in Desert Agriculture, Ben-Gurion University of the Negev.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/plants11162179/s1, Figure S1: eGFP expression in roots of salt-treated 35s::eGFP plants; Table S1: Primers used in this study.
Author Contributions
Conceptualization, T.M. and D.B.-Z.; methodology, T.M. and N.E.; writing, T.M. and D.B.-Z.; supervision, D.B.-Z.; funding acquisition, D.B.-Z. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was funded by the US Israel Binational Science Foundation (BSF), grant number 2011097.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Chen X., Ding Y., Yang Y., Song C., Wang B., Yang S., Guo Y., Gong Z. Protein kinases in plant responses to drought, salt, and cold stress. J. Integr. Plant Biol. 2021;63:53–78. doi: 10.1111/jipb.13061. [DOI] [PubMed] [Google Scholar]
- 2.Finkelstein R.R., Wang M.L., Lynch T.J., Rao S., Goodman H.M. The Arabidopsis abscisic acid response locus ABI4 encodes an APETALA 2 domain protein. Plant Cell. 1998;10:1043–1054. doi: 10.1105/tpc.10.6.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jofuku K.D., Den Boer B., Van Montagu M., Okamuro J.K. Control of Arabidopsis flower and seed development by the homeotic gene APETALA2. Plant Cell. 1994;6:1211–1225. doi: 10.1105/tpc.6.9.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Okamuro J.K., Caster B., Villarroel R., Van Montagu M., Jofuku K.D. The AP2 domain of APETALA2 defines a large new family of DNA binding proteins in Arabidopsis. Proc. Natl. Acad. Sci. USA. 1997;94:7076–7081. doi: 10.1073/pnas.94.13.7076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Finkelstein R.R. Mutations at two new Arabidopsis ABA response loci are similar to the abi3 mutations. Plant J. 1994;6:765–771. doi: 10.1046/j.1365-313X.1994.5060765.x. [DOI] [Google Scholar]
- 6.Quesada V., Ponce M.R., Micol J.L. Genetic analysis of salt-tolerant mutants in Arabidopsis thaliana. Genetics. 2000;154:421–436. doi: 10.1093/genetics/154.1.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arenas-Huertero F., Arroyo A., Zhou L., Sheen J., León P. Analysis of Arabidopsis glucose insensitive mutants, gin5 and gin6, reveals a central role of the plant hormone ABA in the regulation of plant vegetative development by sugar. Genes Dev. 2000;14:2085–2096. doi: 10.1101/gad.14.16.2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Laby R.J., Kincaid M.S., Kim D., Gibson S.I. The Arabidopsis sugar-insensitive mutants sis4 and sis5 are defective in abscisic acid synthesis and response. Plant J. 2000;23:587–596. doi: 10.1046/j.1365-313x.2000.00833.x. [DOI] [PubMed] [Google Scholar]
- 9.Huijser C., Kortstee A., Pego J., Weisbeek P., Wisman E., Smeekens S. The Arabidopsis SUCROSE UNCOUPLED-6 gene is identical to ABSCISIC ACID INSENSITIVE-4: Involvement of abscisic acid in sugar responses. Plant J. 2000;23:577–585. doi: 10.1046/j.1365-313x.2000.00822.x. [DOI] [PubMed] [Google Scholar]
- 10.Rook F., Corke F., Card R., Munz G., Smith C., Bevan M.W. Impaired sucrose-induction mutants reveal the modulation of sugar-induced starch biosynthetic gene expression by abscisic acid signalling. Plant J. 2001;26:421–433. doi: 10.1046/j.1365-313X.2001.2641043.x. [DOI] [PubMed] [Google Scholar]
- 11.Wind J.J., Peviani A., Snel B., Hanson J., Smeekens S.C. ABI4: Versatile activator and repressor. Trends Plant Sci. 2013;18:125–132. doi: 10.1016/j.tplants.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 12.Koussevitzky S., Nott A., Mockler T.C., Hong F., Sachetto-Martins G., Surpin M., Lim J., Mittler R., Chory J. Signals from chloroplasts converge to regulate nuclear gene expression. Science. 2007;316:715–719. doi: 10.1126/science.1140516. [DOI] [PubMed] [Google Scholar]
- 13.Kacprzak S.M., Mochizuki N., Naranjo B., Xu D., Leister D., Kleine T., Okamoto H., Terry M.J. Plastid-to-nucleus retrograde signalling during chloroplast biogenesis does not require ABI4. Plant Physiol. 2019;179:18–23. doi: 10.1104/pp.18.01047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Söderman E.M., Brocard I.M., Lynch T.J., Finkelstein R.R. Regulation and function of the Arabidopsis ABA-insensitive4 gene in seed and abscisic acid response signaling networks. Plant Physiol. 2000;124:1752–1765. doi: 10.1104/pp.124.4.1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nakabayashi K., Okamoto M., Koshiba T., Kamiya Y., Nambara E. Genome-wide profiling of stored mRNA in Arabidopsis thaliana seed germination: Epigenetic and genetic regulation of transcription in seed. Plant J. 2005;41:697–709. doi: 10.1111/j.1365-313X.2005.02337.x. [DOI] [PubMed] [Google Scholar]
- 16.Penfield S., Li Y., Gilday A.D., Graham S., Graham I.A. Arabidopsis ABA INSENSITIVE4 regulates lipid mobilization in the embryo and reveals repression of seed germination by the endosperm. Plant Cell. 2006;18:1887–1899. doi: 10.1105/tpc.106.041277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shkolnik-Inbar D., Adler G., Bar-Zvi D. ABI4 downregulates expression of the sodium transporter HKT1;1 in Arabidopsis roots and affects salt tolerance. Plant J. 2013;73:993–1005. doi: 10.1111/tpj.12091. [DOI] [PubMed] [Google Scholar]
- 18.Shkolnik-Inbar D., Bar-Zvi D. ABI4 mediates abscisic acid and cytokinin inhibition of lateral root formation by reducing polar auxin transport in Arabidopsis. Plant Cell. 2010;22:3560–3573. doi: 10.1105/tpc.110.074641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shkolnik-Inbar D., Bar-Zvi D. Expression of ABSCISIC ACID INSENSITIVE 4 (ABI4) in developing Arabidopsis seedlings. Plant Signal. Behav. 2011;6:694–696. doi: 10.4161/psb.6.5.14978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arroyo A., Bossi F., Finkelstein R.R., León P. Three genes that affect sugar sensing (Abscisic Acid Insensitive 4, Abscisic acid Insensitive 5, and Constitutive Triple Response 1) are differentially regulated by glucose in Arabidopsis. Plant Physiol. 2003;133:231–242. doi: 10.1104/pp.103.021089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Finkelstein R., Lynch T., Reeves W., Petitfils M., Mostachetti M. Accumulation of the transcription factor ABA-insensitive (ABI)4 is tightly regulated post-transcriptionally. J. Exp. Bot. 2011;62:3971–3979. doi: 10.1093/jxb/err093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gregorio J., Hernández-Bernal A.F., Cordoba E., León P. Characterization of evolutionarily conserved motifs involved in activity and regulation of the ABA-INSENSITIVE (ABI) 4 transcription factor. Mol. Plant. 2014;7:422–436. doi: 10.1093/mp/sst132. [DOI] [PubMed] [Google Scholar]
- 23.Xu X., Chi W., Sun X., Feng P., Guo H., Li J., Lin R., Lu C., Wang H., Leister D., et al. Convergence of light and chloroplast signals for de-etiolation through ABI4-HY5 and COP1. Nat. Plants. 2016;2:16066. doi: 10.1038/nplants.2016.66. [DOI] [PubMed] [Google Scholar]
- 24.Popescu S.C., Popescu G.V., Bachan S., Zhang Z., Gerstein M., Snyder M., Dinesh-Kumar S.P. MAPK target networks in Arabidopsis thaliana revealed using functional protein microarrays. Genes Dev. 2009;23:80–92. doi: 10.1101/gad.1740009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guo H., Feng P., Chi W., Sun X., Xu X., Li Y., Ren D., Lu C., Rochaix J.D., Leister D. Plastid-nucleus communication involves calcium-modulated MAPK signalling. Nat. Commun. 2016;7:12173. doi: 10.1038/ncomms12173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bai Z., Zhang J., Ning X., Guo H., Xu X., Huang X., Wang Y., Hu Z., Lu C., Zhang L. A kinase–phosphatase–transcription factor module regulates adventitious root emergence in Arabidopsis root–hypocotyl junctions. Mol. Plant. 2020;13:1162–1177. doi: 10.1016/j.molp.2020.06.002. [DOI] [PubMed] [Google Scholar]
- 27.Eisner N., Maymon T., Sanchez E.C., Bar-Zvi D., Brodsky S., Finkelstein R., Bar-Zvi D. Phosphorylation of serine 114 of the transcription factor ABSCISIC ACID INSENSITIVE 4 is essential for activity. Plant Sci. 2021;305:110847. doi: 10.1016/j.plantsci.2021.110847. [DOI] [PubMed] [Google Scholar]
- 28.Cinelli R.A., Ferrari A., Pellegrini V., Tyagi M., Giacca M., Beltram F. The enhanced green fluorescent protein as a tool for the analysis of protein dynamics and localization: Local fluorescence study at the single-molecule level. Photochem. Photobiol. 2000;71:771–776. doi: 10.1562/0031-8655(2000)0710771TEGFPA2.0.CO2. [DOI] [PubMed] [Google Scholar]
- 29.Odell J.T., Nagy F., Chua N.H. Identification of DNA sequences required for activity of the cauliflower mosaic virus 35S promoter. Nature. 1985;313:810–812. doi: 10.1038/313810a0. [DOI] [PubMed] [Google Scholar]
- 30.Finkelstein R.R. Abscisic acid-insensitive mutations provide evidence for stage-specific signal pathways regulating expression of an Arabidopsis late embryogenesis-abundant (lea) gene. Mol. Gen. Genet. 1993;238:401–408. doi: 10.1007/BF00291999. [DOI] [PubMed] [Google Scholar]
- 31.Amack S.C., Antunes M.S. CaMV35S promoter—A plant biology and biotechnology workhorse in the era of synthetic biology. Curr. Plant Biol. 2020;24:100179. doi: 10.1016/j.cpb.2020.100179. [DOI] [Google Scholar]
- 32.Chandrasekaran U., Luo X.F., Zhou W.G., Shu K. Multifaceted signaling networks mediated by Abscisic Acid Insensitive 4. Plant Commun. 2020;1:100040. doi: 10.1016/j.xplc.2020.100040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xie Z., Nolan T.M., Jiang H., Yin Y. AP2/ERF transcription factor regulatory networks in hormone and abiotic stress responses in Arabidopsis. Front. Plant Sci. 2019;10:228. doi: 10.3389/fpls.2019.00228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huang X., Zhang X., Gong Z., Yang S., Shi Y. ABI4 represses the expression of type-A ARRs to inhibit seed germination in Arabidopsis. Plant J. 2017;89:354–365. doi: 10.1111/tpj.13389. [DOI] [PubMed] [Google Scholar]
- 35.Aviña-Padilla K., Ramírez-Rafael J.A., Herrera-Oropeza G.E., Muley V.Y., Valdivia D.I., Díaz-Valenzuela E., García-García A., Varela-Echavarría A., Hernández-Rosales M. Evolutionary perspective and expression analysis of intronless genes highlight the conservation of their regulatory role. Front. Genet. 2021;12:654256. doi: 10.3389/fgene.2021.654256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu H., Lyu H.-M., Zhu K., Van de Peer Y., Cheng Z.-M. The emergence and evolution of intron-poor and intronless genes in intron-rich plant gene families. Plant J. 2021;105:1072–1082. doi: 10.1111/tpj.15088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang G., Gurtu V., Kain S.R. An enhanced green fluorescent protein allows sensitive detection of gene transfer in mammalian cells. Biochem. Biophys. Res. Commun. 1996;227:707–711. doi: 10.1006/bbrc.1996.1573. [DOI] [PubMed] [Google Scholar]
- 38.Birnbaum K., Shasha D.E., Wang J.Y., Jung J.W., Lambert G.M., Galbraith D.W., Benfey P.N. A gene expression map of the Arabidopsis root. Science. 2003;302:1956–1960. doi: 10.1126/science.1090022. [DOI] [PubMed] [Google Scholar]
- 39.Lopez-Molina L., Mongrand S., Chua N.H. A postgermination developmental arrest checkpoint is mediated by abscisic acid and requires the ABI5 transcription factor in Arabidopsis. Proc. Natl. Acad. Sci. USA. 2001;98:4782–4787. doi: 10.1073/pnas.081594298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Finkelstein R.R., Lynch T.J. The Arabidopsis abscisic acid response gene ABI5 encodes a basic leucine zipper transcription factor. Plant Cell. 2000;12:599–609. doi: 10.1105/tpc.12.4.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu Q., Kasuga M., Sakuma Y., Abe H., Miura S., Yamaguchi-Shinozaki K., Shinozaki K. Two transcription factors, DREB1 and DREB2, with an EREBP/AP2 DNA binding domain separate two cellular signal transduction pathways in drought- and low-temperature-responsive gene expression, respectively, in Arabidopsis. Plant Cell. 1998;10:1391–1406. doi: 10.1105/tpc.10.8.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wu C., Ba Q., Lu D., Li W., Salovska B., Hou P., Mueller T., Rosenberger G., Gao E., Di Y., et al. Global and site-specific effect of phosphorylation on protein turnover. Dev. Cell. 2021;56:111–124. doi: 10.1016/j.devcel.2020.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huang X., Hou L., Meng J., You H., Li Z., Gong Z., Yang S., Shi Y. The Antagonistic action of abscisic acid and cytokinin signaling mediates drought stress response in Arabidopsis. Mol. Plant. 2018;11:970–982. doi: 10.1016/j.molp.2018.05.001. [DOI] [PubMed] [Google Scholar]
- 44.Liu Y., Zhang S. Phosphorylation of 1-aminocyclopropane-1-carboxylic acid synthase by MPK6, a stress-responsive mitogen-activated protein kinase, induces ethylene biosynthesis in Arabidopsis. Plant Cell. 2004;16:3386–3399. doi: 10.1105/tpc.104.026609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Maldonado-Bonilla L.D., Eschen-Lippold L., Gago-Zachert S., Tabassum N., Bauer N., Scheel D., Lee J. The Arabidopsis tandem zinc finger 9 protein binds RNA and mediates pathogen-associated molecular pattern-triggered immune responses. Plant Cell Physiol. 2014;55:412–425. doi: 10.1093/pcp/pct175. [DOI] [PubMed] [Google Scholar]
- 46.Bigeard J., Hirt H. Nuclear Signaling of Plant MAPKs. Front. Plant Sci. 2018;9:469. doi: 10.3389/fpls.2018.00469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xing Y., Chen W.H., Jia W., Zhang J. Mitogen-activated protein kinase kinase 5 (MKK5)-mediated signalling cascade regulates expression of iron superoxide dismutase gene in Arabidopsis under salinity stress. J. Exp. Bot. 2015;66:5971–5981. doi: 10.1093/jxb/erv305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ichimura K., Mizoguchi T., Yoshida R., Yuasa T., Shinozaki K. Various abiotic stresses rapidly activate Arabidopsis MAP kinases ATMPK4 and ATMPK6. Plant J. 2000;24:655–665. doi: 10.1046/j.1365-313x.2000.00913.x. [DOI] [PubMed] [Google Scholar]
- 49.Menges M., Dóczi R., Ökrész L., Morandini P., Mizzi L., Soloviev M., Murray J.A., Bögre L. Comprehensive gene expression atlas for the Arabidopsis MAP kinase signalling pathways. New Phytol. 2008;179:643–662. doi: 10.1111/j.1469-8137.2008.02552.x. [DOI] [PubMed] [Google Scholar]
- 50.Sheikh A.H., Eschen-Lippold L., Pecher P., Hoehenwarter W., Sinha A.K., Scheel D., Lee J. Regulation of WRKY46 transcription factor function by mitogen-activated protein kinases in Arabidopsis thaliana. Front. Plant Sci. 2016;7:61. doi: 10.3389/fpls.2016.00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ma Q., Xia Z., Cai Z., Li L., Cheng Y., Liu J., Nian H. GmWRKY16 enhances drought and salt tolerance through an ABA-mediated pathway in Arabidopsis thaliana. Front. Plant Sci. 2019;9:1979. doi: 10.3389/fpls.2018.01979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen Y.-S., Chao Y.-C., Tseng T.-W., Huang C.-K., Lo P.-C., Lu C.-A. Two MYB-related transcription factors play opposite roles in sugar signaling in Arabidopsis. Plant Mol. Biol. 2017;93:299–311. doi: 10.1007/s11103-016-0562-8. [DOI] [PubMed] [Google Scholar]
- 53.Huang Y., Feng C.Z., Ye Q., Wu W.H., Chen Y.F. Arabidopsis WRKY6 transcription factor acts as a positive regulator of abscisic acid signaling during seed germination and early seedling development. PLoS Genet. 2016;12:e1005833. doi: 10.1371/journal.pgen.1005833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ding Z.J., Yan J.Y., Li C.X., Li G.X., Wu Y.R., Zheng S.J. Transcription factor WRKY46 modulates the development of Arabidopsis lateral roots in osmotic/salt stress conditions via regulation of ABA signaling and auxin homeostasis. Plant J. 2015;84:56–69. doi: 10.1111/tpj.12958. [DOI] [PubMed] [Google Scholar]
- 55.Li X., Zhong M., Qu L., Yang J., Liu X., Zhao Q., Liu X., Zhao X. AtMYB32 regulates the ABA response by targeting ABI3, ABI4 and ABI5 and the drought response by targeting CBF4 in Arabidopsis. Plant Sci. 2021;310:110983. doi: 10.1016/j.plantsci.2021.110983. [DOI] [PubMed] [Google Scholar]
- 56.Guo Z., Xu H., Lei Q., Du J., Li C., Wang C., Yang Y., Yang Y., Sun X. The Arabidopsis transcription factor LBD15 mediates ABA signaling and tolerance of water-deficit stress by regulating ABI4 expression. Plant J. 2020;104:510–521. doi: 10.1111/tpj.14942. [DOI] [PubMed] [Google Scholar]
- 57.Feng C.Z., Chen Y., Wang C., Kong Y.H., Wu W.H., Chen Y.F. Arabidopsis RAV1 transcription factor, phosphorylated by SnRK2 kinases, regulates the expression of ABI3, ABI4, and ABI5 during seed germination and early seedling development. Plant J. 2014;80:654–668. doi: 10.1111/tpj.12670. [DOI] [PubMed] [Google Scholar]
- 58.Shang Y., Yan L., Liu Z.-Q., Cao Z., Mei C., Xin Q., Wu F.-Q., Wang X.-F., Du S.-Y., Jiang T. The Mg-chelatase H subunit of Arabidopsis antagonizes a group of WRKY transcription repressors to relieve ABA-responsive genes of inhibition. Plant Cell. 2010;22:1909–1935. doi: 10.1105/tpc.110.073874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lee K., Seo P.J. Coordination of seed dormancy and germination processes by MYB96. Plant Signal. Behav. 2015;10:e1056423. doi: 10.1080/15592324.2015.1056423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Reeves W.M., Lynch T.J., Mobin R., Finkelstein R.R. Direct targets of the transcription factors ABA-Insensitive(ABI)4 and ABI5 reveal synergistic action by ABI4 and several bZIP ABA response factors. Plant Mol. Biol. 2011;75:347–363. doi: 10.1007/s11103-011-9733-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bossi F., Cordoba E., Dupré P., Mendoza M.S., Román C.S., León P. The Arabidopsis ABA-INSENSITIVE (ABI) 4 factor acts as a central transcription activator of the expression of its own gene, and for the induction of ABI5 and SBE2.2 genes during sugar signaling. Plant J. 2009;59:359–374. doi: 10.1111/j.1365-313X.2009.03877.x. [DOI] [PubMed] [Google Scholar]
- 62.Gibson D.G., Young L., Chuang R.Y., Venter J.C., Hutchison C.A., 3rd, Smith H.O. Enzymatic assembly of DNA molecules up to several hundred kilobases. Nat. Methods. 2009;6:343–345. doi: 10.1038/nmeth.1318. [DOI] [PubMed] [Google Scholar]
- 63.Clough S.J., Bent A.F. Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 1998;16:735–743. doi: 10.1046/j.1365-313x.1998.00343.x. [DOI] [PubMed] [Google Scholar]
- 64.Schneider C.A., Rasband W.S., Eliceiri K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods. 2012;9:671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Laemmli U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.