Abstract
Gaining a better understanding of the immune cell subsets and molecular factors associated with protective or pathological immunity against SARS-CoV-2 could aid the development of vaccines and therapeutics for coronavirus disease (COVID-19). Single-cell technologies, such as flow cytometry, mass cytometry, single-cell transcriptomics and single-cell multiomic profiling, offer considerable promise in dissecting the heterogeneity of immune responses among individual cells and uncovering the molecular mechanisms of COVID-19 pathogenesis. Particularly noteworthy single-cell immune profiling studies have identified innate and adaptive immune cell subsets that correlate with COVID-19 disease severity, as well as immunological factors/pathways of potential relevance to the development of vaccines and treatments for COVID-19. To facilitate integrative studies and meta-analyses, we provide to the scientific research community in download-ready, standardized form 21 published single-cell sequencing datasets (over 3.2 million cells in total), as well as an interactive visualization portal for data exploration.
High-throughput single-cell technologies such as flow cytometry and mass cytometry, which can measure features on millions of individual cells, and high-dimensional single-cell technologies such as single-cell RNA sequencing (scRNA-seq), which can measure potentially thousands of features in individual cells, are well-suited to support studies for the heterogeneity of immune responses and of how immune cells interact with other host cells and with pathogens. Specific applications of single-cell technologies in the field of immunology include identifying host immunological correlates of disease severity (potentially aiding the design of effective vaccines and therapeutics, as well as allowing for the monitoring of each person’s response to those approaches), elucidating molecular mechanisms of disease and enabling the identification of predictive biomarkers of disease outcome.
The ongoing COVID-19 pandemic has been described as “an explosive pandemic of historic proportions”1, with over 220 million confirmed cases and over 4.5 million confirmed deaths worldwide so far2. In addition to basic measures such as physical distancing and mask wearing, optimal management of the pandemic may involve a diverse armamentarium of scientific tools including effective and safe preventative vaccines3, early therapeutic interventions that can blunt progression to severe disease4,5, and anti-inflammatory treatments to counteract the harmful ‘cytokine storm’ in patients with severe disease6–8. Towards the development of these tools, there is an urgent need to understand severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) interactions with host cells and the host immune response9.
Here we provide an overview of single-cell technologies that have been applied to COVID-19 studies and list the pertinent experimental aspects of each study (e.g., sample size, technology platform, and patient characteristics). We describe our efforts to organize and curate available single-cell sequencing datasets into an easily downloadable format, providing information on how to access these datasets. We also review key insights obtained from single-cell immune profiling and discuss opportunities and challenges of integrative analysis of publicly available datasets.
Single-cell technologies and available datasets
Single-cell technologies that have been used in COVID-19 studies to date are summarized in Table 1. They include 62 published and 2 preprint articles describing studies that applied one or more single-cell technologies in the context of COVID-19. Figure 1 displays the sample size and dimensionality of the studies; Supplementary Table 1 presents relevant experimental details and locations of publicly available data sets (raw fcs files are publicly available for 6 flow cytometry and 3 mass cytometry datasets, and 24 single-cell sequencing datasets are publicly available). Whereas flow cytometry is the most commonly used technology in these studies, scRNA-seq and single-cell multiomic profiling are also increasingly being used. The flow/mass cytometry studies analyzed data from up to ~300 individuals and up to 62 markers with one or multiple panels; 25 out of the 54 datasets include longitudinal data (Fig. 1a). Most single-cell sequencing studies analyzed >50,000 cells from fewer than 150 individuals; only a few of them included longitudinal data (Fig. 1b).
Table 1.
Summary of single-cell technologies that have been applied to the study of COVID-19
| Technology | Measurement | Methodology | Capacity | Pros and cons | Number of published or preprint COVID-19 articles* |
|---|---|---|---|---|---|
| Flow cytometry¶ | Protein expression | Fluorophore-conjugated antibodies; cells are sorted into liquid droplets individually and flowed through a laser beam; the light emitted by each cell informs about marker expression | High throughput (millions of cells); up to ~30 markers | Pros: determination of immune cell subsets by well-defined surface markers and antibody panels; cells can be sorted for further analysis Cons: broad emission spectra of fluorophores |
47 |
| Mass cytometry (CyTOF) | Protein expression | Antibodies conjugated to heavy-metal isotopes; cells are nebulized and the metal-conjugated antibodies are ionized; signals are detected by a time-of-flight mass spectrometer | High throughput (up to millions of cells); many-dimensional (>40 cellular parameters/cell) | Pros: avoids spectral overlap between fluorophores Cons: slower flow rate than flow cytometry; expensive; destructive (not possible to sort cells for further analysis) |
7 |
| scRNA-seq | Gene expression | Single cells are isolated (e.g. through microfluidics, droplet-based methods, or flow cytometry-based sorting), lysed, and their transcripts captured. The subsequent workflow is similar to that of bulk RNA-seq | High dimensional (>10,000 features measured per cell) | Pros: comprehensive and unbiased sequencing | 22 |
| CITE-seq* | Simultaneous surface protein expression and gene expression | Barcoded, oligonucleotide-conjugated antibodies label single-cells that are analyzed by scRNA-seq | High dimensional (>100 proteins can be measured per cell in addition to (>10,000 genes) | Pros: gene expression integrated with multiomic profiling | 4 |
| scBCR/TCR-seq# | Immune antigen receptor repertoire | Single-cell V(D)J enriched libraries are generated utilizing microfluidics, 5′ molecular barcoding, and constant region–specific primers | Paired, full-length receptor sequences from T cells and/or B cells including isotypes. | Pros: comprehensive and unbiased sequencing; combination with multiomic profiling possible (gene and protein expression) | 13 |
Abbreviations: CyTOF, cytometry by time of flight; CITE-seq, Cellular Indexing of Transcriptomes and Epitopes by Sequencing; scBCR/TCR seq, single-cell B-cell receptor/T-cell receptor sequencing; scRNA-seq, single-cell RNA sequencing.
CITE-seq includes scRNA-seq as part of the workflow; to omit redundancy we did not count the CITE-seq studies in the scRNA-seq row.
Includes spectral flow cytometry (1 study), which is based on conventional flow cytometry but uses different optics and detectors.
scBCR/TCR-seq can also be multiomic; here we include 12 multiomic studies that incorporated scBCR/TCR-seq and one scBCR-seq study.
Figure 1.
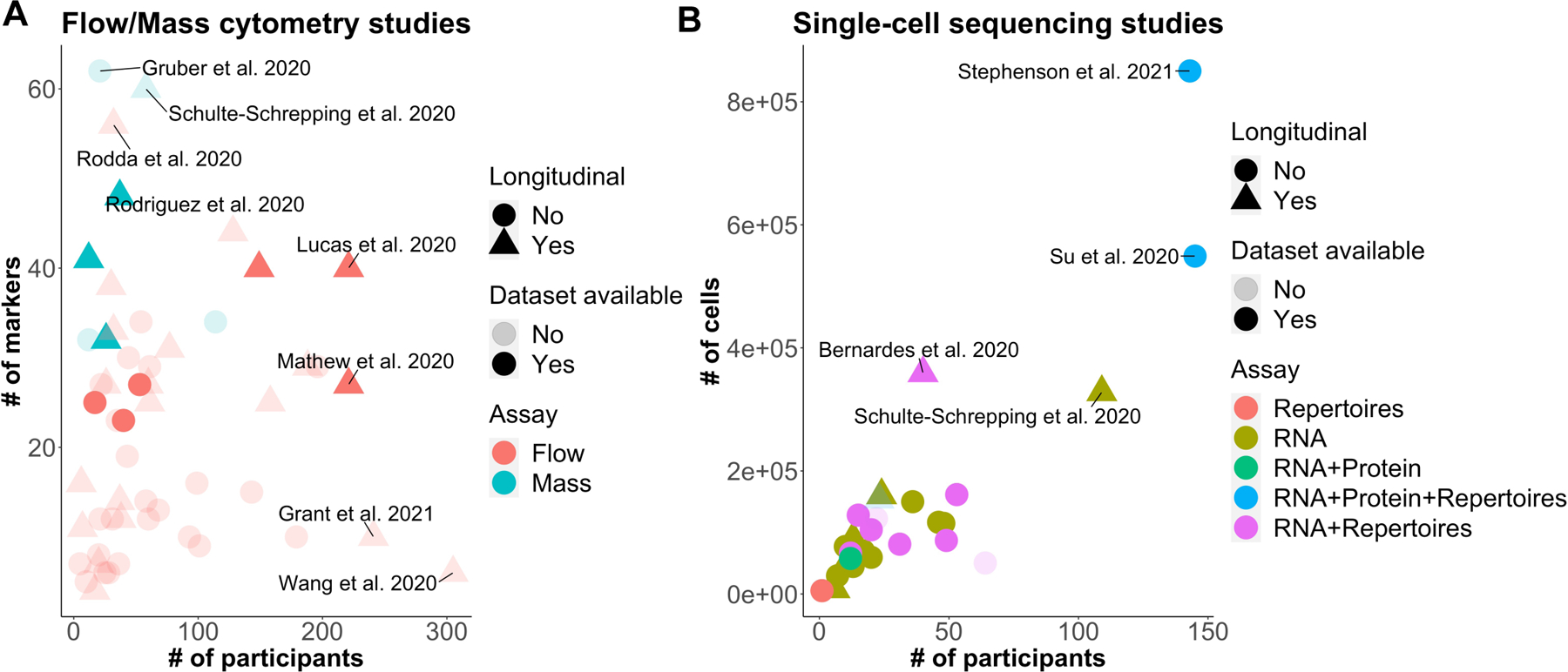
Visual representation of characteristics of the 64 published articles or publicly posted preprints on COVID-19 that have used one or more single-cell technologies (March 2020 – March 2021). (a,b) Scatter plots showing the number of participants versus (a) the number of flow cytometry markers or (b) the number of cells for which data are available in each study. Each symbol represents a dataset using one of the single-cell technologies from a single article/preprint. Opacity indicates dataset availability to the public (light, no; dark, yes), shape indicates whether the dataset has longitudinal data (circle, no; triangle, yes), and color indicates assay type [(a) red, flow cytometry; cyan, mass cytometry; (b) red, repertoires; gold, RNA; green, RNA plus protein; blue, RNA plus protein plus +repertoires; and magenta, RNA plus repertoires].
In the following text, we briefly summarize key conclusions from the 64 studies shown in Figure 1, focusing on the insights obtained via single-cell technologies, including studies still at the preprint stage or that have relatively small sample sizes. As the vast majority of studies have been performed in peripheral blood mononuclear cells (PBMCs), only limited conclusions can be drawn about the respiratory tract – the primary site of infection. Most studies focused on transcriptional (as opposed to protein or epigenetic) readouts. In the sections below, we summarize these finding in the context of innate immune cells, B cells and T cells, finally summarizing how these single-cell data may correlate with immune protection.
Innate immune responses
Most flow cytometry-based studies of COVID-19 reported to date that analyzed PBMCs from patients with COVID-19 report reduced frequencies or abundances of circulating basophils10,11, monocytes12–14 (especially CD14lowCD16high non-classical monocytes15), dendritic cells (DCs)10,13,14,16, and NK cells13,14,16–18 when compared with healthy donors, with greater reductions in individuals with severe than with mild COVID-1914–18. Conversely, patients with COVID-19 have shown increased frequencies or abundances of circulating neutrophils, eosinophils, and monocytic myeloid-derived suppressive cells (M-MDSCs) compared with healthy donors, with greater increases in individuals with severe than with mild COVID-1911,13,15,16,18. The neutrophil-to-lymphocyte ratio has also been reported to be associated with severe COVID-1916.
NK cells.
High-dimensional flow cytometry has enabled in-depth characterization of immune cell subsets. A report featuring a 28-color NK-cell-oriented panel described fewer circulating (yet more highly activated and proliferating) NK cells in COVID-19 patients compared to healthy controls17. Worse clinical outcomes were also correlated with increased levels of circulating adaptive NK cells (NKG2C+CD57+CD56dim) and higher levels of perforin expression in CD56bright NK cells17, implicating adaptive and activated NK cells in COVID pathogenesis.
The above study is of particular notice because it incorporated an analysis of publicly available scRNA-seq data (NK cells in bronchoalveolar lavage (BAL) fluid from patients with COVID-1919). The data reveal high activation of NK cells in COVID-19 and corroborate results from flow cytometry17.
Neutrophils.
Neutrophil extracellular traps (NETs) have been implicated in severe COVID-1920,21. A ‘developing neutrophil’ subpopulation, specifically increased in patients with acute respiratory distress syndrome, was identified through scRNA-seq and featured expression of neutrophil granule-related genes and a lack of expression of canonical neutrophil markers14.
Another study featuring scRNA-seq, high-dimensional flow cytometry, and mass cytometry reported that severe COVID-19 was associated with a substantial increase in circulating immature neutrophils and the presence of a neutrophil cluster characterized by upregulated S100A8 and S100A9 (calprotectin) among other genes15.
Dendritic cells.
A study by Arunachalam et al.22 is notable due to its relatively large sample size (n=52 COVID-19 patients and n=62 healthy controls) and its use of a phospho-CyTOF panel to immunophenotype PBMCs in patients with COVID-19 along with CITE-seq to profile gene and protein expression in dendritic cell (DC)-enriched PBMC samples from patients with COVID-19.
One major conclusion from this study included a decrease in the frequency of plasmacytoid DCs (pDCs) in patients with COVID-19. Another key finding was reduced expression of mammalian target of rapamycin (mTOR) signaling proteins in these pDCs, suggesting that pDCs may have deficient interferon (IFN)-α signaling in patients with COVID-19.
Monocytes.
In another important work also distinguished by a relatively large sample size (n=53 COVID-19 patients, n=8 patients with flu-like illness, and n=48 controls, over two independent cohorts), researchers used single-cell transcriptomic (scRNA-seq) and proteomic (CyTOF) interrogation23 to test if distinct innate immune responses are associated with the clinical course of COVID-19. The study revealed that, compared with healthy controls, patients with mild COVID-19 had increased levels of inflammatory huma leukocyte antigen (HLA)-DRhiCD11chiCD14+ monocytes. Conversely, patients with severe COVID-19 were distinguished not only by monocyte populations with low expression of HLA-DR (indicative of monocyte dysfunction24) and enhanced expression of genes related to anti-inflammatory macrophage functions (e.g. CD62L, CD163), but also an abundance of immature neutrophils (including pro- and pre-neutrophils) expressing markers indicative of immunosuppression and/or dysfunction. These findings identify a dysfunctional monocyte response as well as dysregulated myelopoiesis as potentially important processes underlying the development of severe disease.
As discussed below, immune-cell phenotyping via single-cell technologies has yielded potential insights into the immune pathways that may be dysregulated in severe COVID-19 (i.e., COVID-19-associated cytokine storm25) and in multisystem inflammatory syndrome (MIS-C) associated with SARS-CoV-2 infection. These insights could in turn inform ongoing and future development of anti-inflammatory treatment strategies of COVID-19 by targeting immunological factors with therapeutic potential. Evidence that such anti-inflammatory treatment strategies may be effective is provided by the encouraging preliminary results of the RECOVERY trial of dexamethasone in patients who were hospitalized with COVID-1926 (which resulted in ongoing corticosteroid trials27–29 to stop early based on the data and safety monitoring board (DSMB) recommendations), along with the results of meta-analyses of randomized clinical trials of corticosteroids in patients with severe COVID-1930,31. Moreover, flow cytometry, mass cytometry, and scRNA-seq have all demonstrated reduced HLA-DR and CD86 expression on monocytes in patients with severe COVID-1915,22,32 as well as in children with MIS-C associated with SARS-CoV-2 infection33, implying potentially impaired antigen presentation to T cells. The downregulation of HLA-DR on monocytes could potentially be driven by interleukin-6 (IL-6), which has been shown to be elevated in patients with severe COVID-1910,18,34,35 as well as in pediatric patients with MIS-C33,36, as the decreased HLA-DR expression can be partially restored by tocilizumab32 (a humanized IgG1κ monoclonal antibody (mAb) that targets interleukin-6 receptor (IL-6R) and blocks IL-6 signaling37).
Tocilizumab has no efficacy in preventing disease progression38 or intubation or death in hospitalized patients with moderate COVID-1939; however, the latter study39 had wide confidence intervals in the efficacy comparisons, and another report40 suggested potential benefit of tocilizumab in reducing the need for ventilation and/or death. Thus, the question of whether IL-6R blockade can benefit patients with moderate or severe COVID-19 disease remains open40, and other chemokine receptor blockers are also being tested in ongoing clinical trials41,42. Additional applications of single-cell technologies in this context yielded the identification of the chemokine receptor CCR111,43 as a potential therapeutic target based on scRNA-sequencing of nasopharyngeal samples from patients with critical or non-critical COVID-1943 or of nasopharyngeal and bronchial samples from patients with moderate or critical COVID-1911.
Macrophages.
These11,43 and other19,44 single-cell studies of airway and alveolar cells have also yielded particular insight into the role of macrophages in COVID-19, specifically, that a dysregulated macrophage response may drive pathological inflammation45. Patients with critical COVID-19 have manifested increased ligand–receptor interactions between epithelial cells and immune cells, upregulation of pro-inflammatory chemokine and cytokine genes in non-resident macrophages, and CCR1 upregulation in neutrophils, macrophages and CD8+ T cells11. These findings suggest the influence of cycles of recruitment of immune cells to the lung (monocytes that differentiate to inflammatory macrophages, further recruitment and activation of more immune cells) on the epithelial damage seen in severe COVID-19. scRNA-seq analysis of cells in bronchoalveolar lavage fluid (BALF) has revealed that the proportion of bronchoalveolar macrophages and the levels of inflammatory cytokine and chemokine receptors are positively associated with disease severity19. Macrophages in severe COVID-19 were also distinguished by high expression of FCN1 (a member of the complement cascade) and SPP1 (a proinflammatory cytokine), suggesting that alveolar macrophages may drive local inflammation in patients with severe COVID-1919. Finally, flow cytometry and scRNA-seq data of BALF cells from patients with pneumonia caused by SARS-CoV-2 infection suggest that high levels of monocytes, as well as CD4+ and CD8+ T cells, are found in the alveolar space.44 Single-cell RNA-seq identified multiple clusters corresponding to tissue-resident alveolar macrophages and monocyte-derived alveolar macrophages, along with expression of IFNG in T cells from patients with pneumonia caused by SARS-CoV-2 infection. The identification of an interferon response signature by bulk sequencing of flow cytometry-sorted alveolar macrophages, and the finding of SARS-CoV-2 RNA in alveolar macrophages (which suggests that SARS-CoV-2 can replicate in alveolar macrophages), gives strength to the hypothesis that activated T cells in severe COVID-19 release IFN-γ. In turn, this IFN-γ drives an IFN response in alveolar macrophages that leads to the recruitment of monocyte-derived alveolar macrophages, completing an ‘inflammatory signaling loop’44.
An alternative RNA-seq analysis of PBMCs from four healthy donors, five influenza patients, and eight COVID-19 patients reported that classical monocytes in patients with severe COVID-19 are distinguished by an IFN type I (IFN-I)-related transcriptional signature and an IL-1β−related inflammatory transcriptional signature46. This finding generated the hypothesis that the IFN-I response contributes to detrimental inflammation in severe COVID-19. In a much larger sample size (including 130 patients with COVID-19 from three different centers in the UK), scRNA-seq and quantification of 188 cell surface proteins47 revealed a positive association between the frequency of proliferating monocytes and MKI67- and TOP2A-expressing dendritic cells with COVID-19 disease severity. The same study also describes how platelet expansion was associated with severe COVID-19, along with enhanced interactions of platelets with C1QA/B/C+CD16+ monocytes in COVID-19. These findings lend support to the role of both platelets and monocytes in the tissue thrombosis that has been reported in COVID-1948.
B cell responses
Neutralizing antibodies (nAbs) have been heavily implicated in protection against SARS-CoV-2 infection and COVID-19 disease49–53 and thus intense interest has focused on identifying potent nAbs against SARS-CoV-2. Such analysis requires a single-cell approach, owing to the extensive VDJ recombination and somatic hypermutation in B cells54.
Immunoglobulin sequencing.
A general strategy for identifying relevant antibody sequences has been to sort and process via scRNA-seq individual antigen-specific memory B cells (for example, specific to the receptor-binding domain (RBD) in the spike glycoprotein55 or the spike trimer14,56–58) from convalescent COVID-19 patients. VDJ sequencing performed at the single-cell level (scVDJ-seq33) has led to identification of nAbs with prophylactic and/or therapeutic efficacy against SARS-CoV-2, feeding into the robust pipeline of nAb clinical trials59.
In a study that employed high-throughput scRNA/VDJ-seq, about 9,000 RBD-binding B-cell clonotypes were identified, yielding 14 potent nAbs, one of which was shown to protect against SARS-CoV-2 in a mouse model55. The coupled scRNA-seq data allowed the identification of naïve and memory B cell subsets and helped improve the efficiency of nAb selection by filtering out clonotypes enriched in naïve and exhausted B cells55. A different study used a similar strategy to identify 19 potent nAbs, including RBD-binding and non-RBD binding nAbs, one of which was shown to protect against SARS-CoV-2 in a hamster model60. The integration of two parallel workflows, both of which featured scRNA-seq and one of which incorporated single-cell functional assays along with scVDJ-seq, has led to identification of five major classes of nAbs with different reactivities to the spike glycoprotein and cross-reactivity with SARS-CoV53. Unterman et al.62 performed a multi-omic integration of single-cell analysis, including scRNA-seq and CITE-seq, along with B-cell receptor (BCR) and T cell receptor (TCR) sequencing, on PBMCs from patients with ‘stable’ COVID-19 (who were hospitalized and ultimately discharged) and patients with ‘progressive’ COVID-19 (who were treated in ICU and ultimately succumbed to the disease)61. Their results supported a complex B-cell response in COVID-19, including a high proportion of unmutated immunoglobulin gamma heavy chain (IGHG) B-cell clones present alongside multiple mutated B-cell clones that did not appear to increase levels of somatic hypermutation over time. The latter could potentially be explained by memory B cell cross-reactivity with other coronaviruses or failed formation of robust germinal center reactions58.
B cell markers.
High-dimensional flow cytometry has been used to characterize and compare B-cell responses in patients with different severities of COVID-19 disease. A 24-marker B-cell focused panel designed to identify B cell populations, evaluate their activation status, and assess homing potential62 allowed the identification of a correlation between overactivation of extrafollicular B cells and COVID-19 disease severity, along with greater expansion of antibody-secreting cells in severe versus milder disease. Patients with severe disease had high serum titers of RBD-targeting SARS-CoV-2 nAbs, raising the question of whether this distinct B-cell response in patients with severe COVID-19 is ineffective or potentially even pathogenic. Other studies have described how the humoral immune response (as assessed by BCR clonal expansion and B cell activation) could be correlated with disease severity63; however, it remains an open question whether these positive correlations are simply due to increased initial viral load; this is a particularly complex question, with studies having reported direct64–71, inverse72,73, or no74 correlation. These discrepant findings may be the result of differences in sampling compartment67 (e.g., saliva, blood, or anal), timing of sample collection, and/or population differences. In any case, it is clear that future studies are warranted to define the cellular and molecular determinants that dictate protective versus non-protective humoral responses in infected individuals. The potential of antibody-dependent enhancement of COVID-19 disease must also be considered75.
Convergent antibody clusters — antibodies with highly similar VDJs shared by multiple patients, which generally comprise only a small proportion of the virus-specific B-cell response76 — have also been identified77, with the suggestion that the majority of COVID-19 patients have convergent immunoglobulin heavy chains against the RBD, which may bode well for spike- or RBD-based vaccines.
B-cell phenotypes.
In a study looking at longitudinal samples from individuals who had recovered from mildly symptomatic COVID-19, Rodda et al.78 used RBD tetramer enrichment and flow cytometry to phenotype the rare population of RBD-specific B cells. RBD-specific memory B cells increased from one to three months post-symptom onset and were substantially higher in COVID-19 samples than in healthy controls. Moreover, memory B cells displaying a TbetlowIgG+CD21+CD27+ phenotype (that is, a ‘classical’ memory B cell phenotype) also increased from one to three months post symptom onset, whereas levels of Tbethi memory B cells, which are typically found in chronic infections, remained low. The question of whether SARS-CoV-2 specific memory B cells can produce nAbs after reactivation by secondary infection has also been addressed: sorting and sequencing of RBD-specific single B cells and their BCRs suggested that memory B cells may help protect from secondary infections.
Human tissue-imaging platforms coupled with multi-color immunofluorescence and multispectral imaging via quantitative automated scanning microscopy have been used to study, at the single cell level, thoracic lymph nodes and spleens obtained via from autopsy of patients who succumbed to COVID-1958. Compared with single-cell approaches requiring dissociation of tissue, an advantage of this approach is that tissue architecture could be largely preserved, enabling study of cell–cell interactions. A loss of germinal centers (GCs) in the lymph nodes of these patients, accompanied by substantial reductions in GC B cells and follicular helper T (Tfh) cells in the lymph nodes and spleens, was reported using this approach58. As optimal germinal center reactions are essential for the production of high-affinity antibodies79, these results suggest that the increased proportions of plasmablasts observed in patients with COVID-19 (particularly severe COVID-19)10,14,16,58 are a correlate of suboptimal antiviral humoral immunity and disease rather than protection. Other emerging single-cell imaging-based techniques, such as single-cell spatial transcriptomics, may be particularly relevant to the emerging field of pathological studies in organs from deceased COVID-19 patients80,81.
T-cell responses
Multiple studies using flow cytometry of PBMCs from patients with COVID-19 have reported T lymphopenia: compared with the lower limit of normal or with levels observed in healthy controls, substantially reduced T-cell13,16,18,82,83, CD4+ T-cell16,56,57,82,83, CD8+ T-cell10,16,56,57,82,83, CD8+ mucosal-associated invariant T (MAIT) cells16, γδ T-cell10, αβ T-cell (some subsets)10 and regulatory T-cell83 counts have been observed in patients with mild or severe COVID-19. Similar T-cell lymphopenia has been observed in patients with COVID-19-associated MIS-C33,36,84,85. Whereas Mudd et al.12 reported that the reductions in CD4+ and CD8+ T cells were comparable between patients with COVID-19 and patients with influenza, Giamarellos-Bourboulis et al.32 have observed that the reduction in CD4+ T cells is stronger in patients with COVID-19 than in patients with influenza. Decreased T-cell populations have also been associated with disease severity, as both CD4+56,83 and CD8+56 T cells were shown to be substantially reduced in patients with severe versus moderate or mild COVID-19, or in intensive care unit (ICU) versus non-ICU cases82. An increased CD4+/CD8+ T-cell ratio has also been reported in patients with COVID-1918,57, suggesting that SARS-CoV-2 infection might preferentially impact CD8+ T cells.
T-cell phenotype.
Multiparametric flow cytometry has also revealed phenotypic and functional alterations in T cells of patients with COVID-19, with a general theme emerging of hyperactivation of T cells in COVID-19, as demonstrated by elevated subpopulations expressing activation, proliferation or exhaustion markers. Levels of activated (HLA-DR+CD38+10,13,16,57, CD38+86, HLA-DR+87, or CD25+CD4+/CD8+10), proliferating (Ki67+CD8+57,86 or Ki67+CD4+86), and exhausted (PD-1+CD8+/CD4+82,86, TIGIT+87, or NKG2A+88) T cells have been shown to dramatically increase in patients with COVID-19 or in patients with severe COVID-19 compared with healthy controls, and most activated (CD38+ PD-1+) CD8+ T cells in patients with acute COVID-19 have been shown to be specific for SARS-CoV-286. However, perhaps due to differences in the timing of sampling, healthy controls, patients with influenza, and patients with COVID-19 with similarly low abundances of HLA-DR+CD38+ activated CD8+ T cells have been described. Notably, scRNA-seq and scTCR-seq analysis of CD8+ T cells in BALF samples identified signatures of tissue-resident memory T cells and increased levels of clonal expansion associated with mild disease, whereas elevated proliferative capacity was correlated with severe disease19. Single-cell RNA-seq analysis of cerebrospinal fluid leukocytes isolated from COVID-19 patients with neurological sequelae (‘Neuro-COVID’) has uncovered high levels of CD4+ T cells expressing exhaustion markers (e.g., ICOS, HAVCR2/TIM3 and CD226) compared with those in control patients.
Flow cytometry and scRNA-seq have also identified altered T-cell differentiation and cytotoxicity in COVID-19. Patients with severe COVID-19 had higher proportions of circulating cytotoxic CD8+ T cells compared with healthy controls16, and CD8+ T cells in nasopharyngeal and bronchial samples from patients with severe COVID-19 have increased expression of cytotoxic molecules11. This increased cytotoxicity has been proposed to contribute to epithelial damage11. Regarding differentiation, a decrease in peripheral naïve and central memory CD8+ T cells and an increase in senescent and effector memory CD45RA+ CD8+ T cells in patients with COVID-1918 have been reported, suggesting a skew towards terminal differentiation. In another study58, CD4+ Bcl-6+ germinal center type T follicular helper cells were substantially decreased in thoracic lymph node and spleen autopsy tissue from patients with COVID-19, accompanied by high levels of tumor necrosis factor (TNF)-α at the follicle and increased numbers of T helper type 1 cells (TH1) cells. These findings suggest that COVID-19 impairs T follicular helper cell differentiation, which may explain the lack of germinal centers mentioned above.
T-cell specificity.
SARS-CoV-2-specific T cells recognizing both spike- and non-spike epitopes have been identified in patients with acute COVID-19 and in convalescent patients through various flow cytometry-based techniques such as intracellular cytokine staining (ICS), activation induced marker (AIM) assays (including antigen-reactive T-cell enrichment (ARTE)), and peptide–MHC multimers (Supplementary Table 1), demonstrating the formation of virus-specific memory T cells after infection78,86,89–96. Although patients with severe COVID-19 showed overall higher breadth and magnitude of SARS-CoV-2-specific T-cell responses than patients with mild COVID-19, higher proportions of SARS-CoV-2 specific CD8+ T cells were observed in mild versus severe cases95. SARS-CoV-2-specific CD4+ T cells are predominantly Th1 cells and largely display a central memory T phenotype, whereas SARS-CoV-2-specific CD8+ T cells are more enriched in effector memory and terminally differentiated effector subsets93,96,97. scRNA-seq and scTCR-seq analysis of SARS-CoV-2-reactive CD4+ T cells revealed an association between increased SARS-CoV-2 specific cytotoxic CD4+ T cells and cytotoxic Tfh cells with disease severity, and an inverse association of regulatory T cells with disease severity98. SARS-CoV-2-reactive CD8+ T cells were shown to have enhanced expression of cytotoxic and inflammatory genes, with higher levels of TCR clonal expansion, in patients with severe disease, supporting an association of overactivated antigen-specific T cells with COVID-19 pathogenesis99. As in vitro stimulation can radically alter the gene expression profiles of reactive T cells, further studies using peptide–MHC multimers together with single-cell sequencing should provide complementary and additional insights into the phenotype and function of SARS-CoV-2-specific T cells. In addition, single-cell multiomic technologies, such as scATAC-seq, could be applied to investigate whether SARS-CoV-2-specific T cells retain epigenetic fingerprints that may dictate their recall responses.
Contribution of T cells to COVID-19 immunity.
Many questions remain regarding how prior exposure to endemic coronaviruses and cross-reactive memory T cell immunity shape the immune response to SARS-CoV-2.100,101 Intracellular cytokine staining/flow cytometry, ELISpot, and FluoroSpot assays have been key single-cell technologies in detecting cross-reactive memory CD4+ T cells in SARS-CoV-2-unexposed individuals (ranging from ~20 to 50% of individuals tested, across geographically diverse cohorts), whereas cross-reactive memory CD8+ T cells are much less common86,89–93,102. It has been postulated that cross-reactive memory CD4+ T cells may provide protective immunity against SARS-CoV-2 infection and reduce disease severity by promoting B-cell and antibody responses and/or mediating rapid local antiviral immunity at sites of infection, including the lung and/or upper respiratory tract101. However, Bacher et al.104 reported that pre-existing cross-reactive memory CD4+ T cells may not only have low TCR avidity with reduced clonal expansion, but also exacerbate inflammation and disease severity, especially in the elderly103. Thus, the roles of pre-existing cross-reactive memory T cells in SARS-CoV-2 infection and vaccination in the general population warrant further investigation.
Correlates of immune protection at the single-cell level
With the rapid growth of single-cell datasets on the immunology of COVID-19, one open question is how to best leverage current and emerging public datasets. Ongoing efficacy trials of candidate COVID-19 vaccines are generating single-cell immune-profiling datasets that could be applied to identifying correlates of risk and correlates of protection against primary and secondary endpoints in these trials, for instance as we have done previously for the RV144 HIV vaccine efficacy trial104. Because of limited specimen volumes in COVID-19, vaccine efficacy trials, and the large number of immunological biomarkers that could potentially be evaluated as correlates, it will be critical to perform pilot studies to optimize and identify assays and associated immune signatures with favorable statistical properties for correlates analyses. Important factors in determining the best correlates include high reproducibility, large dynamic range in vaccine recipients, and low response range (low false-positive rate) at baseline in vaccine recipients and in placebo recipients post-placebo. To help expedite correlates analyses, existing single-cell datasets could be explored to optimize and identify highly reproducible single-cell immunological signatures, which will be useful in variable down-selection.
Another major application of these collated datasets is the potential for integrative analyses and meta-analyses, such as the recent meta-analysis of 107 lung single-cell RNA-seq studies that identified additional proteases that may potentially be involved in SARS-CoV-2 infection105.
Box 1 (together with Figs 2 and 3) illustrates one way to organize publicly available single-cell data for use in integrative analysis. Readers should note, however, that a search for biomarkers and signatures associated with COVID-19 disease severity or progression using such published data faces several challenges.
Box 1. Organization and curation of single-cell sequencing datasets.
With the aim of facilitating integrative analyses and meta-analyses, we have organized, curated and standardized a set of publicly available single-cell transcriptomics datasets. Of the 22 scRNA-seq and 4 CITE-seq studies in Table 1, openly accessible and downloadable FASTQ, count matrix, Seurat object, or H5AD data are available for 23. Of these 23, we excluded ref.12, as only raw sequence reads were available, and ref.125, as only four cell populations (monocytes, B cells, megakaryocytes, and cell precursors) were available. Thus, our analysis processed 21 datasets. We first downloaded the datasets from such repositories as Gene Expression Omnibus (GEO), ArrayExpress, and the Genome Sequence Archive. The vast majority of scRNA-seq and CITE-seq studies (22 out of 26) used the Seurat package124,126 for data analysis, whereas three studies used Scanpy127 and one study used Pagoda2128. Thus, for simplicity, we prepared the datasets as Seurat objects in R and performed quality control, normalization, dimensionality reduction, clustering, and cell-type annotation using Seurat v4. Seurat v4 multimodal CITE-seq reference mapping enables not only more accurate resolution of granular cell types that are difficult to distinguish by transcriptomic data alone, but also imputation of the expression of over two hundred surface proteins124. We collected single-cell sequencing data of over 3.2 million single cells across 21 datasets11,14,15,19,22,23,43,44,46,47,98,99,103,118,129–135 and mapped each cell to the human PBMC CITE-seq reference124.
As shown in Figure 2a, substantial diversity was observed with respect to age, and there were more male patients with moderate or severe COVID-19 than female patients. Additionally, the vast majority of the samples were blood as opposed to another tissue type, such as from the airways (Fig. 2b). As only cell types in the Seurat v4 multimodal CITE-seq PBMC reference set can be correctly mapped, for visualization purposes we took only the PBMCs and whole blood datasets (16 in total, the latter minus neutrophils and basophils) and created a ‘merged dataset’ consisting of >2.5 million cells. (We note that as additional references and/or integration methods become available, other tissues could potentially be integrated as well.) Figure 2c presents a visual representation of all cells in the merged dataset in the same Uniform Manifold Approximation and Projection (UMAP) space, along with standardized cell type annotations at three levels of granularity.
We also include standardized cell metadata (e.g. disease severity and time points) from the original publication when available. Briefly, the standardized disease severity categories are based on information provided in datasets and supplementary materials and defined as mild: not hospitalized, moderate: hospitalized (no ICU), and severe: ICU. Standardized time points are defined as the number of days between symptom onset and the sampling time if provided and were estimated for patients where it was not provided (See more details in the Supplementary Text).
To illustrate the usage of the merged dataset, Figure 2d shows the frequencies of CD4+ T cells, CD8+ T cells, mucosal-associated invariant T (MAIT) cells, CD56bright NK cells, CD16+ monocytes, and CD14+ monocytes in samples across 12 datasets grouped by disease severity and time points for COVID-19 samples based on the standardized metadata. Consistent with published results16,32,56,82,83,88,136,137, reductions in CD4+ T cells, CD8+ T cells, MAIT cells, and CD56bright NK cells were each associated with COVID-19 severity. When sampled at an early or intermediate stage of their clinical course, we also observed decreased CD16+ monocytes in patients with severe vs mild COVID-19 (Fig. 2d), which is consistent with published results15,23. Conversely, an increase in CD14+ monocytes was observed in patients with severe versus mild COVID-19, regardless of when in the clinical course the samples were obtained (Fig. 2d). To facilitate standardization and sharing of future datasets by the community, the code used to process the datasets is available at github (https://github.com/RGLab/covid19_sc).
Furthermore, we provide interactive visualization of these datasets on a visualization portal (https://atlas.fredhutch.org/fredhutch/covid). Figure 3 shows an example screenshot of the visualization portal. The processed Seurat objects can be easily downloaded from the visualization portal; to ensure that our resource remains maximally relevant to the research community, we encourage users to request datasets to be added to the portal as new studies emerge. We plan to update the portal as needed to ensure that it includes highly requested studies. We hope that the standardized datasets we present here will facilitate re-analyses and meta-analyses and accelerate much-needed translational discoveries to help stem the COVID-19 crisis.
Figure 2.
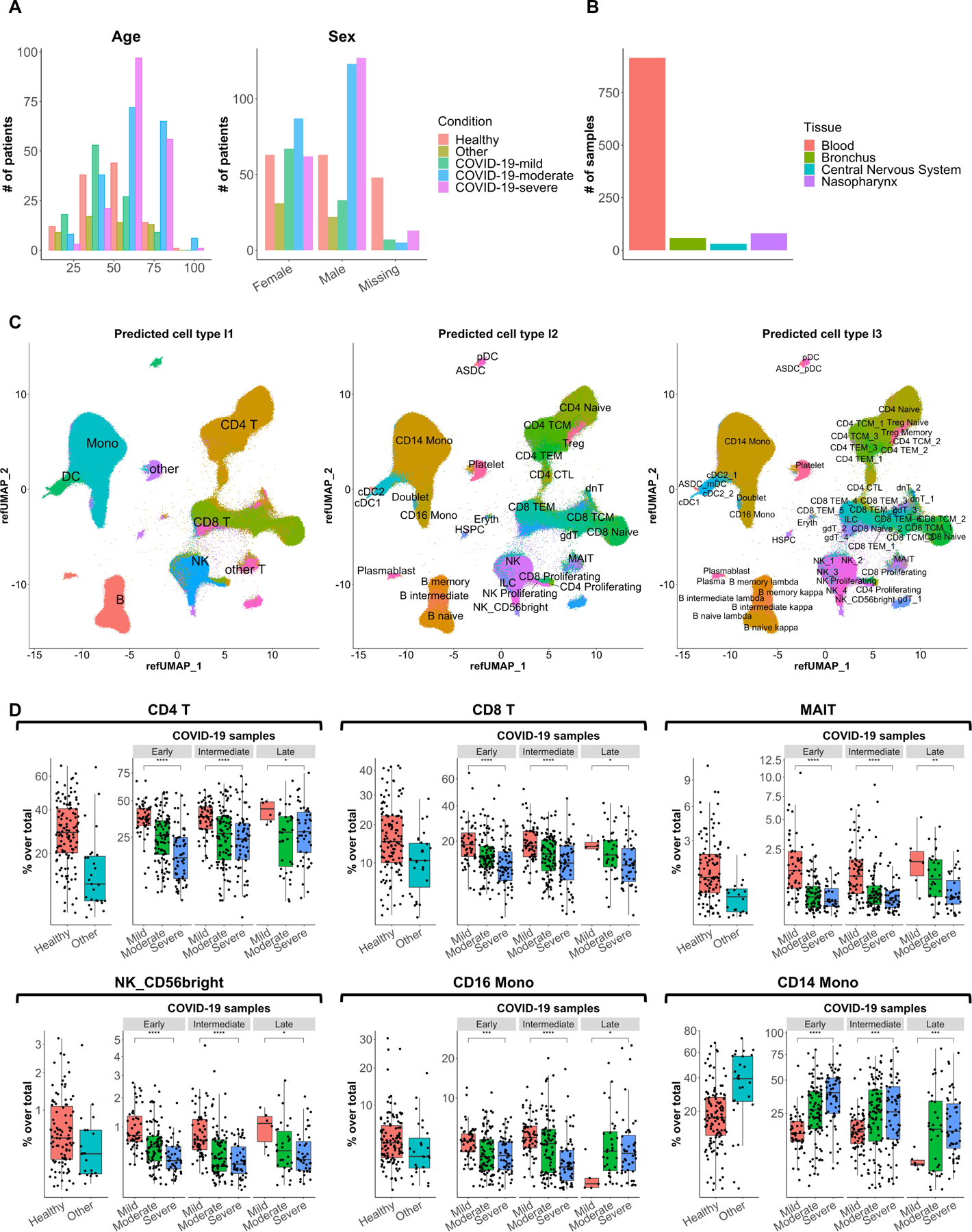
Visual representation of single-cell transcriptomics data. (a) Plots showing the distribution of age (left panel) and sex (right panel) among individuals included in the collected 21 datasets. (b) Bar plot showing the number of samples per tissue type among the collected 21 datasets. (c) Uniform manifold approximation and projection (UMAP) plots showing the projection of over 2.5 million single cells from 16 PBMC and whole blood datasets (the latter minus neutrophils and basophils) mapped to the Seurat CITE-seq reference, colored and labeled by reference-defined cell type annotations at level 1 (left panel), level 2 (middle panel), and level 3 (right panel) granularity. (D) Box plots showing the frequencies of CD4+ T cells, CD8+ T cells, mucosal-associated invariant T (MAIT) cells, CD56bright NK cells, CD16+ monocytes, and CD14+ monocytes among samples grouped by disease severity and time points for COVID-19 samples. Samples from Arunachalam et al.22 (enriched for DCs), Meckiff et al.98 (enriched for antigen-specific CD4+ T cells), Kusnadi et al.99 (enriched for antigen-specific CD8+ T cells), and Bacher et al.103 (enriched for antigen-specific CD4+ T cells) were excluded. Early, <= 8 days post symptom onset; intermediate, > 8 and <= 15 days post symptom onset; late, > 15 days post symptom onset. Statistical significance between mild and severe was determined by Wilcoxon test. *: p <= 0.05, **: p <= 0.01, ***: p <= 0.001, ****: p <= 0.0001.
Figure 3.
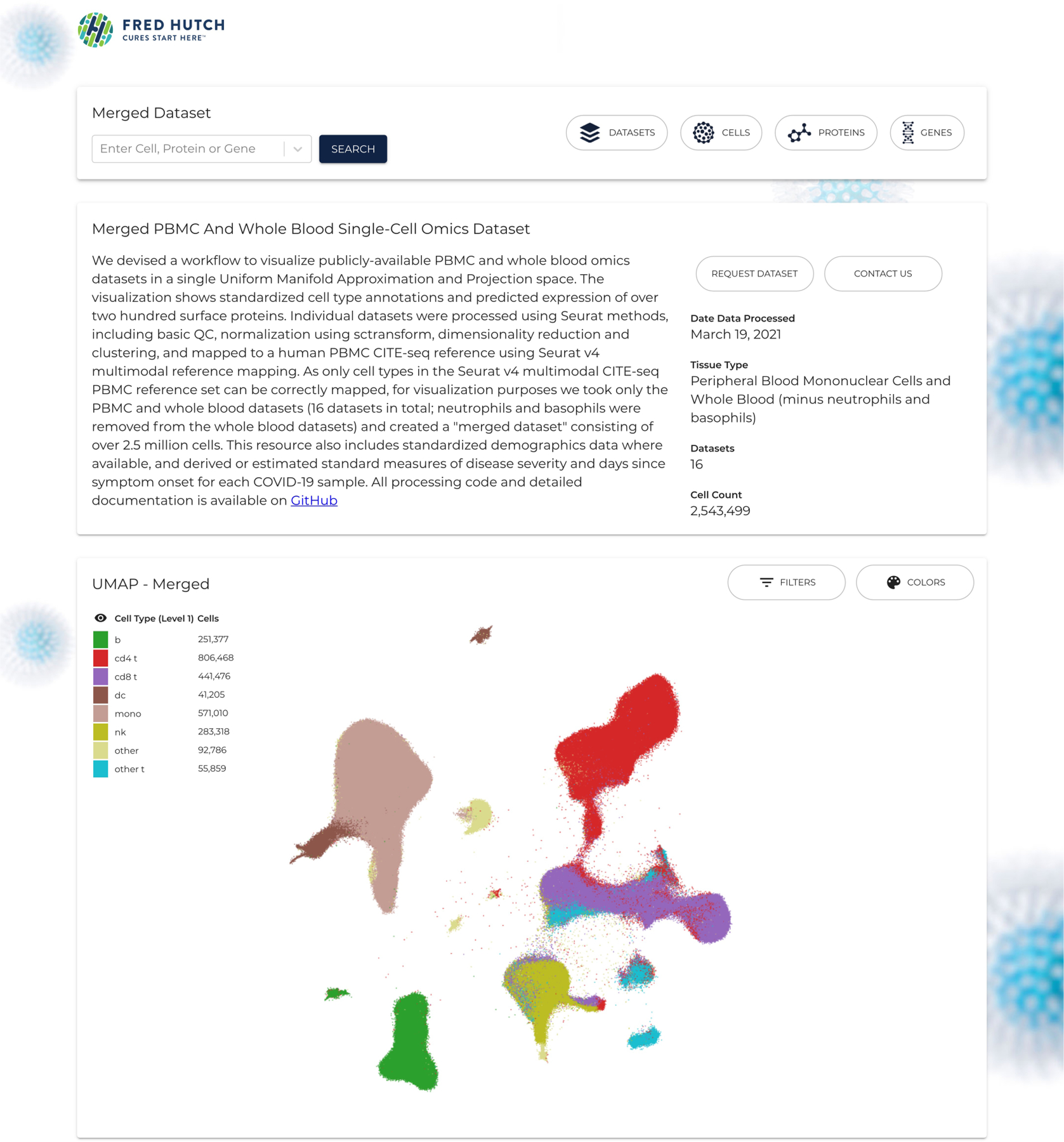
An example screenshot of the visualization portal. The website provides visualization of 21 individual datasets and the merged dataset consisting of 16 datasets. The UMAP plot showing the merged dataset consisting of over 2.5 million cells mapped to the human PBMC CITE-seq reference124. The processed datasets can also be downloaded from the website at https://atlas.fredhutch.org/fredhutch/covid.
One challenge is whether and how to account for trial-participant demographics, as ethnic106–109, sex108,110, and age111–115 differences in clinical presentation, immune responses, and outcomes of SARS-CoV-2 infection have been identified, even when adjusting for potential confounders.
A second major challenge is the substantial heterogeneity thus far in the ordinal scales that have been used for assessing coronavirus disease severity116: out of the 44 studies that categorized disease severity, 7 used WHO scores, 5 used National Health Commission of China guidelines, 3 used NIH scores, 1 used German Robert Koch Institute symptom classification, 1 used National Early Warning Score, 4 did not provide relevant information on how disease severity was defined, and 23 used custom scoring (Supplementary Table 1). Moreover, for studies that have obtained samples at ‘early’ and ‘late’ disease stages, there is substantial heterogeneity in how the times post-symptom onset are defined. The incorporation of standardized definitions of disease severity into future single-cell immune profiling studies would facilitate such integrative and meta-analyses. As one approach to overcome this problem, Zheng et al.117 have defined seven disease severity categories and manually assigned standardized categories in an integrated analysis of 4,780 PBMC transcriptomic samples from patients infected with a different virus (16 in total, across 26 datasets) — an approach that may help mitigate the problem caused by non-standardized definitions of disease severity. Integrative analysis of bulk sequencing data has supported the hypothesis that there is a conserved pan-viral response associated with disease severity, Zheng et al.118 also have performed an integrative analysis of three single-cell data sets (two CITE-seq22,118 and one scRNA-seq14; 264,224 cells from 71 PBMC samples overall) from three independent cohorts including healthy controls14,22,118, patients with SARS-CoV-2 infection14,22,118, and patients with influenza or RSV infection22, reporting that a ‘Meta-Virus Signature’ score in single myeloid cells is positively correlated with viral infection severity across different virus strains.
The lack of standardized experimental protocols and analysis pipelines is an additional challenge in integrating single-cell datasets. As a result, published results and datasets can be difficult to compare and integrate due to experimental and computational variation on how the cells and datasets were processed. Fortunately, the research community has worked hard to provide computational approaches that can be used to reduce technical variation and produce standardized cell annotations that be compared, visualized and modeled across datasets119,120. These approaches have already been used in many of the COVID single-cell studies published to date to correct for batch effects118,118. The Human Cell Atlas121 initiative has also been working on standardization of the different aspects of single-cell sequencing, and the Human Cell Atlas Data Portal provides publicly available data sets processed by standardized pipelines122.
Conclusions
Single-cell analyses have held up to their promise of overcoming certain limitations of bulk methods and enabling a deep dive into the cellular heterogeneity of antiviral immune responses. Multiple single-cell immune profiling studies of COVID-19 patients have identified distinct cell subsets of the innate and adaptive immune systems that correlate with disease severity; this body of evidence supports the hypothesis that such subsets may have important functions in blunting (or even enhancing) COVID-19 disease severity. There is also evidence to suggest that targeting certain immunological factors, such as cytokine/chemokine receptors (e.g. IL-6R and CCR1), might curb pathogenic responses and/or improve protective immunity. In the near-term future, studies applying single-cell multiomics technologies such as CITE-seq and single-cell BCR-seq/TCR-seq with peptide–MHC multimers are needed to further characterize the phenotypes and functions of immune cell subsets implicated in COVID-19 protection and of those implicated in progression. Application of multiomic technologies such as scATAC-seq to understand the epigenetic changes associated with SARS-CoV-2 infection in immune cells, especially in antigen-specific T and B cells, may help identify new avenues to pursue for COVID-19 therapies. Moreover, additional multiomic spatial immune profiling studies are needed to further dissect local immune responses against SARS-CoV-2 in tissues such as lung. In the longer-term, single-cell immune profiling studies with sufficiently large sample sizes and participant diversity will be valuable for helping investigate potential sex-related and age-related differences in COVID-19, e.g. whether the immune cell subsets of interest described above vary in frequency or in function in males vs females. Application of single-cell immune profiling to better understand the mechanisms driving the range of post-COVID conditions (e.g. long COVID) is also a relatively underexplored area with many unanswered questions.
The scientific community has mobilized in unprecedented fashion in response to the ongoing COVID-19 pandemic. Large collaborative efforts such as the US National Institutes of Health (NIH)-funded Immunophenotyping Assessment in a COVID-19 Cohort (IMPACC) study (NCT04378777) and the COVID-19 Cell Atlas123 (Wellcome Sanger Institute/Chan Zuckerberg Initiative), to name a few, are generating freely available, open access datasets at the single-cell level and work is being done on standardizing protocols and metadata as well. These datasets have been derived from patients with COVID-19 of varying severity and include different tissues, cohort features, and time points. Looking ahead, we anticipate that the amount and complexity of single-cell datasets will rapidly grow, including in important populations, such as pediatric patients (for whom little single-cell data currently exists), and that re-analyses and meta-analyses will become more common as standards become available.
Supplementary Material
Acknowledgments
We thank Dev A. Nambi and Gretchen K. Krenn for their help with building the data visualization portal. R.G. was funded by the NIH Human Immunology Project Consortium (U19AI128914) and the Vaccine and Immunology Statistical Center (Bill and Melinda Gates Foundation OPP1032317). E.W.N was funded by the Fred Hutchinson Cancer Research Center New Development funds, The Andy Hill Endowment Distinguished Researcher CARE fund, and the NIH Human Immunology Project Consortium (U19AI128914). We also acknowledge the Scientific Computing Infrastructure at Fred Hutch funded by ORIP grant S10OD028685.
Footnotes
Competing interests
R.G. has received consulting income from Juno Therapeutics, Takeda, Infotech Soft, Celgene, Merck and has received research support from Janssen Pharmaceuticals and Juno Therapeutics, and declares ownership in CellSpace Biosciences. E.W.N is a co-founder, advisor and shareholder of ImmunoScape Pte. Ltd. and is an advisor for Neogene Therapeutics and Nanostring Technologies.
References
- 1.Joszt L. Fauci and Panel Discuss Ongoing Challenges of the COVID-19 Pandemic, and Lessons Learned. AJMC. [18 Feb 2021]. https://www.ajmc.com/view/fauci-and-panel-discuss-ongoing-challenges-of-the-covid-19-pandemic-and-lessons-learned. Quote from the 2020 CHEST Annual Meeting Keynote Address by Dr. Anthony Fauci. 19 Oct 2020. Access date.
- 2.World Health Organization Coronavirus disease (COVID-19) pandemic https://www.who.int/emergencies/diseases/novel-coronavirus-2019 Last updated 6 Sept, 2021. Access date 6 Sept, 2021.
- 3.Dai L & Gao GF Viral targets for vaccines against COVID-19. Nat Rev Immunol 21, 73–82 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim PS, Read SW & Fauci AS Therapy for Early COVID-19: A Critical Need. JAMA 324, 2149–2150 (2020). [DOI] [PubMed] [Google Scholar]
- 5.Weinreich DM et al. REGN-COV2, a Neutralizing Antibody Cocktail, in Outpatients with Covid-19. N Engl J Med 384, 238–251 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Prescott HC & Rice TW Corticosteroids in COVID-19 ARDS: Evidence and Hope During the Pandemic. JAMA 324, 1292–1295 (2020). [DOI] [PubMed] [Google Scholar]
- 7.Zhang Y, Chen Y & Meng Z Immunomodulation for Severe COVID-19 Pneumonia: The State of the Art. Front Immunol 11, 577442 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lariccia V et al. Challenges and Opportunities from Targeting Inflammatory Responses to SARS-CoV-2 Infection: A Narrative Review. J Clin Med 9 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grifoni A et al. A Sequence Homology and Bioinformatic Approach Can Predict Candidate Targets for Immune Responses to SARS-CoV-2. Cell Host Microbe 27, 671–680 e672 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Laing AG et al. A dynamic COVID-19 immune signature includes associations with poor prognosis. Nat Med (2020). [DOI] [PubMed] [Google Scholar]
- 11.Chua RL et al. COVID-19 severity correlates with airway epithelium-immune cell interactions identified by single-cell analysis. Nat Biotechnol 38, 970–979 (2020). [DOI] [PubMed] [Google Scholar]
- 12.Mudd PA et al. Targeted Immunosuppression Distinguishes COVID-19 from Influenza in Moderate and Severe Disease. medRxiv (2020).
- 13.Zhou R et al. Acute SARS-CoV-2 Infection Impairs Dendritic Cell and T Cell Responses. Immunity (2020). [DOI] [PMC free article] [PubMed]
- 14.Wilk AJ et al. A single-cell atlas of the peripheral immune response in patients with severe COVID-19. Nat Med 26, 1070–1076 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Silvin A et al. Elevated Calprotectin and Abnormal Myeloid Cell Subsets Discriminate Severe from Mild COVID-19. Cell 182, 1401–1418 e1418 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuri-Cervantes L et al. Comprehensive mapping of immune perturbations associated with severe COVID-19. Sci Immunol 5 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maucourant C et al. Natural killer cell immunotypes related to COVID-19 disease severity. Sci Immunol 5 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mazzoni A et al. Impaired immune cell cytotoxicity in severe COVID-19 is IL-6 dependent. J Clin Invest 130, 4694–4703 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liao M et al. Single-cell landscape of bronchoalveolar immune cells in patients with COVID-19. Nat Med 26, 842–844 (2020). [DOI] [PubMed] [Google Scholar]
- 20.Barnes BJ et al. Targeting potential drivers of COVID-19: Neutrophil extracellular traps. J Exp Med 217 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zuo Y et al. Neutrophil extracellular traps in COVID-19. JCI Insight 5 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arunachalam PS et al. Systems biological assessment of immunity to mild versus severe COVID-19 infection in humans. Science 369, 1210–1220 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schulte-Schrepping J et al. Severe COVID-19 Is Marked by a Dysregulated Myeloid Cell Compartment. Cell 182, 1419-+ (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Venet F, Demaret J, Gossez M & Monneret G Myeloid cells in sepsis-acquired immunodeficiency. Ann N Y Acad Sci (2020). [DOI] [PubMed] [Google Scholar]
- 25.Fajgenbaum DC & June CH Cytokine Storm. N Engl J Med 383, 2255–2273 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Group RC et al. Dexamethasone in Hospitalized Patients with Covid-19 - Preliminary Report. N Engl J Med (2020).
- 27.Dequin PF et al. Effect of Hydrocortisone on 21-Day Mortality or Respiratory Support Among Critically Ill Patients With COVID-19 A Randomized Clinical Trial. Jama-J Am Med Assoc 324, 1298–1306 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tomazini BM et al. Effect of Dexamethasone on Days Alive and Ventilator-Free in Patients With Moderate or Severe Acute Respiratory Distress Syndrome and COVID-19: The CoDEX Randomized Clinical Trial. JAMA 324, 1307–1316 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Angus DC et al. Effect of Hydrocortisone on Mortality and Organ Support in Patients With Severe COVID-19: The REMAP-CAP COVID-19 Corticosteroid Domain Randomized Clinical Trial. JAMA 324, 1317–1329 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sterne JAC et al. Association Between Administration of Systemic Corticosteroids and Mortality Among Critically Ill Patients With COVID-19 A Meta-analysis. Jama-J Am Med Assoc 324, 1330–1341 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Siemieniuk RA et al. Drug treatments for covid-19: living systematic review and network meta-analysis. BMJ 370, m2980 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Giamarellos-Bourboulis EJ et al. Complex Immune Dysregulation in COVID-19 Patients with Severe Respiratory Failure. Cell Host Microbe 27, 992–1000 e1003 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Carter MJ et al. Peripheral immunophenotypes in children with multisystem inflammatory syndrome associated with SARS-CoV-2 infection. Nat Med (2020). [DOI] [PubMed] [Google Scholar]
- 34.Zhou Y et al. Pathogenic T-cells and inflammatory monocytes incite inflammatory storms in severe COVID-19 patients. National Science Review 7, 998–1002 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mann ER et al. Longitudinal immune profiling reveals key myeloid signatures associated with COVID-19. Sci Immunol 5 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gruber CN et al. Mapping Systemic Inflammation and Antibody Responses in Multisystem Inflammatory Syndrome in Children (MIS-C). Cell (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hennigan S & Kavanaugh A Interleukin-6 inhibitors in the treatment of rheumatoid arthritis. Ther Clin Risk Manag 4, 767–775 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Salvarani C et al. Effect of Tocilizumab vs Standard Care on Clinical Worsening in Patients Hospitalized With COVID-19 Pneumonia: A Randomized Clinical Trial. Jama Intern Med 181, 24–31 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stone JH et al. Efficacy of Tocilizumab in Patients Hospitalized with Covid-19. N Engl J Med 383, 2333–2344 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huang E & Jordan SC Tocilizumab for Covid-19 - The Ongoing Search for Effective Therapies. N Engl J Med 383, 2387–2388 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.ClinicalTrials.gov. Charité Trial of Cenicriviroc (CVC) Treatment for COVID-19 Patients https://clinicaltrials.gov/ct2/show/NCT04500418 Last updated 26 Aug, 2020. Access date 19 Feb, 2020.
- 42.ClinicalTrials.gov. Maraviroc in Patients With Moderate and Severe COVID-19 https://clinicaltrials.gov/ct2/show/NCT04435522 Last update 4 Feb, 2021. Access date 19 Feb, 2021.
- 43.Trump S et al. Hypertension delays viral clearance and exacerbates airway hyperinflammation in patients with COVID-19. Nat Biotechnol (2020). [DOI] [PubMed]
- 44.Grant RA et al. Circuits between infected macrophages and T cells in SARS-CoV-2 pneumonia. 10.1038/s41586-020-03148-w. Nature (2021). [DOI] [PMC free article] [PubMed]
- 45.Merad M & Martin JC Pathological inflammation in patients with COVID-19: a key role for monocytes and macrophages. Nat Rev Immunol 20, 355–362 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee JS et al. Immunophenotyping of COVID-19 and influenza highlights the role of type I interferons in development of severe COVID-19. Sci Immunol 5 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stephenson E et al. The cellular immune response to COVID-19 deciphered by single cell multi-omics across three UK centres. 10.1101/2021.01.13.21249725 Posted 15 Jan, 2021. medRxiv, 2021.2001.2013.21249725 (2021). [DOI]
- 48.Levi M, Thachil J, Iba T & Levy JH Coagulation abnormalities and thrombosis in patients with COVID-19. Lancet Haematol 7, e438–e440 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Addetia A et al. Neutralizing antibodies correlate with protection from SARS-CoV-2 in humans during a fishery vessel outbreak with high attack rate. J Clin Microbiol (2020). [DOI] [PMC free article] [PubMed]
- 50.Klasse PJ & Moore JP Antibodies to SARS-CoV-2 and their potential for therapeutic passive immunization. Elife 9 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Alsoussi WB et al. A Potently Neutralizing Antibody Protects Mice against SARS-CoV-2 Infection. J Immunol 205, 915–922 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kreye J et al. A Therapeutic Non-self-reactive SARS-CoV-2 Antibody Protects from Lung Pathology in a COVID-19 Hamster Model. Cell (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zost SJ et al. Potently neutralizing and protective human antibodies against SARS-CoV-2. Nature 584, 443–449 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bassing CH, Swat W & Alt FW The mechanism and regulation of chromosomal V(D)J recombination. Cell 109 Suppl, S45–55 (2002). [DOI] [PubMed] [Google Scholar]
- 55.Cao Y et al. Potent Neutralizing Antibodies against SARS-CoV-2 Identified by High-Throughput Single-Cell Sequencing of Convalescent Patients’ B Cells. Cell 182, 73–84 e16 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang F et al. Characteristics of Peripheral Lymphocyte Subset Alteration in COVID-19 Pneumonia. J Infect Dis 221, 1762–1769 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mathew D et al. Deep immune profiling of COVID-19 patients reveals distinct immunotypes with therapeutic implications. Science 369 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kaneko N et al. Loss of Bcl-6-Expressing T Follicular Helper Cells and Germinal Centers in COVID-19. Cell 183, 143–157 e113 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Renn A, Fu Y, Hu X, Hall MD & Simeonov A Fruitful Neutralizing Antibody Pipeline Brings Hope To Defeat SARS-Cov-2. Trends Pharmacol Sci (2020). [DOI] [PMC free article] [PubMed]
- 60.Liu L et al. Potent neutralizing antibodies against multiple epitopes on SARS-CoV-2 spike. Nature 584, 450–456 (2020). [DOI] [PubMed] [Google Scholar]
- 61.Unterman A et al. Single-Cell Omics Reveals Dyssynchrony of the Innate and Adaptive Immune System in Progressive COVID-19. medRxiv, 2020.2007.2016.20153437 (2020). [DOI] [PMC free article] [PubMed]
- 62.Woodruff MC et al. Extrafollicular B cell responses correlate with neutralizing antibodies and morbidity in COVID-19. Nature Immunology (2020). [DOI] [PMC free article] [PubMed]
- 63.Zhang F et al. Adaptive immune responses to SARS-CoV-2 infection in severe versus mild individuals. Signal Transduct Target Ther 5, 156 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang Y et al. Kinetics of viral load and antibody response in relation to COVID-19 severity. J Clin Invest 130, 5235–5244 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chen WL et al. Detectable 2019-nCoV viral RNA in blood is a strong indicator for the further clinical severity. Emerg Microbes Infec 9, 469–473 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zheng SF et al. Viral load dynamics and disease severity in patients infected with SARS-CoV-2 in Zhejiang province, China, January-March 2020: retrospective cohort study. Bmj-Brit Med J 369 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Silva J et al. Saliva viral load is a dynamic unifying correlate of COVID-19 severity and mortality. 10.1101/2021.01.04.21249236v1 Posted 6 Jan, 2021. Access date 17 Feb, 2021. medRxiv (2021). [DOI]
- 68.Pujadas E et al. SARS-CoV-2 viral load predicts COVID-19 mortality. Lancet Respir Med 8, e70 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Liu Y et al. Viral dynamics in mild and severe cases of COVID-19. Lancet Infect Dis 20, 656–657 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fajnzylber J et al. SARS-CoV-2 viral load is associated with increased disease severity and mortality. Nat Commun 11 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Maltezou HC et al. Association between upper respiratory tract viral load, comorbidities, disease severity and outcome of patients with SARS-CoV-2 infection. J Infect Dis (2021). [DOI] [PMC free article] [PubMed]
- 72.Argyropoulos KV et al. Association of Initial Viral Load in Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Patients with Outcome and Symptoms. Am J Pathol 190, 1881–1887 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hasanoglu I et al. Higher viral loads in asymptomatic COVID-19 patients might be the invisible part of the iceberg. Infection 49, 117–126 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lee S et al. Clinical Course and Molecular Viral Shedding Among Asymptomatic and Symptomatic Patients With SARS-CoV-2 Infection in a Community Treatment Center in the Republic of Korea. Jama Intern Med 180, 1447–1452 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lee WS, Wheatley AK, Kent SJ & DeKosky BJ Antibody-dependent enhancement and SARS-CoV-2 vaccines and therapies. Nat Microbiol 5, 1185–1191 (2020). [DOI] [PubMed] [Google Scholar]
- 76.Davis CW et al. Longitudinal Analysis of the Human B Cell Response to Ebola Virus Infection. Cell 177, 1566–1582 e1517 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nielsen SCA et al. Human B Cell Clonal Expansion and Convergent Antibody Responses to SARS-CoV-2. Cell Host Microbe 28, 516–525 e515 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rodda LB et al. Functional SARS-CoV-2-specific immune memory persists after mild COVID-19. Cell 184, 169–183 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Victora GD & Nussenzweig MC Germinal centers. Annu Rev Immunol 30, 429–457 (2012). [DOI] [PubMed] [Google Scholar]
- 80.Maiese A et al. Autopsy findings in COVID-19-related deaths: a literature review. Forensic Sci Med Pathol (2020). [DOI] [PMC free article] [PubMed]
- 81.Deshmukh V, Motwani R, Kumar A, Kumari C & Raza K Histopathological observations in COVID-19: a systematic review. J Clin Pathol 74, 76–83 (2021). [DOI] [PubMed] [Google Scholar]
- 82.Diao B et al. Reduction and Functional Exhaustion of T Cells in Patients With Coronavirus Disease 2019 (COVID-19). Front Immunol 11, 827 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chen G et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest 130, 2620–2629 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Consiglio CR et al. The Immunology of Multisystem Inflammatory Syndrome in Children with COVID-19. Cell (2020). [DOI] [PMC free article] [PubMed]
- 85.Vella L et al. Deep Immune Profiling of MIS-C demonstrates marked but transient immune activation compared to adult and pediatric COVID-19. medRxiv (2020). [DOI] [PMC free article] [PubMed]
- 86.Sekine T et al. Robust T Cell Immunity in Convalescent Individuals with Asymptomatic or Mild COVID-19. Cell 183, 158–168 e114 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zheng H-Y et al. Elevated exhaustion levels and reduced functional diversity of T cells in peripheral blood may predict severe progression in COVID-19 patients. Cellular & Molecular Immunology 17, 541–543 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zheng M et al. Functional exhaustion of antiviral lymphocytes in COVID-19 patients. Cellular & Molecular Immunology 17, 533–535 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Grifoni A et al. Targets of T Cell Responses to SARS-CoV-2 Coronavirus in Humans with COVID-19 Disease and Unexposed Individuals. Cell 181, 1489–1501 e1415 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Braun J et al. SARS-CoV-2-reactive T cells in healthy donors and patients with COVID-19. Nature (2020). [DOI] [PubMed]
- 91.Le Bert N et al. SARS-CoV-2-specific T cell immunity in cases of COVID-19 and SARS, and uninfected controls. Nature 584, 457–462 (2020). [DOI] [PubMed] [Google Scholar]
- 92.Mateus J et al. Selective and cross-reactive SARS-CoV-2 T cell epitopes in unexposed humans. Science 370, 89–94 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Weiskopf D et al. Phenotype and kinetics of SARS-CoV-2-specific T cells in COVID-19 patients with acute respiratory distress syndrome. Sci Immunol 5 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ni L et al. Detection of SARS-CoV-2-Specific Humoral and Cellular Immunity in COVID-19 Convalescent Individuals. Immunity 52, 971–977 e973 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Peng Y et al. Broad and strong memory CD4(+) and CD8(+) T cells induced by SARS-CoV-2 in UK convalescent individuals following COVID-19. Nat Immunol (2020). [DOI] [PMC free article] [PubMed]
- 96.Neidleman J et al. SARS-CoV-2-Specific T Cells Exhibit Phenotypic Features of Helper Function, Lack of Terminal Differentiation, and High Proliferation Potential. Cell Rep Med 1, 100081 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kared H et al. CD8+ T cell responses in convalescent COVID-19 individuals target epitopes from the entire SARS-CoV-2 proteome and show kinetics of early differentiation. bioRxiv (2020).
- 98.Meckiff BJ et al. Imbalance of regulatory and cytotoxic SARS-CoV-2-reactive CD4+ T cells in COVID-19. Cell (2020). [DOI] [PMC free article] [PubMed]
- 99.Kusnadi A et al. Severely ill COVID-19 patients display augmented functional properties in SARS-CoV-2-reactive CD8+ T cells. bioRxiv, 2020.2007.2009.194027 (2020). [DOI] [PMC free article] [PubMed]
- 100.Sette A & Crotty S Pre-existing immunity to SARS-CoV-2: the knowns and unknowns. Nat Rev Immunol 20, 457–458 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lipsitch M, Grad YH, Sette A & Crotty S Cross-reactive memory T cells and herd immunity to SARS-CoV-2. Nature Reviews Immunology (2020). [DOI] [PMC free article] [PubMed]
- 102.Ferretti AP et al. Unbiased screens show CD8+ T cells of COVID-19 patients recognize shared epitopes in SARS-CoV-2, most of which are not located in the Spike protein. DOI: 10.1016/j.immuni.2020.10.006. Immunity (2020). [DOI] [PMC free article] [PubMed]
- 103.Bacher P et al. Pre-existing T cell memory as a risk factor for severe 1 COVID-19 in the elderly. medRxiv, 2020.2009.2015.20188896 (2020).
- 104.Lin L et al. COMPASS identifies T-cell subsets correlated with clinical outcomes. Nat Biotechnol 33, 610–616 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Muus C et al. Single-cell meta-analysis of SARS-CoV-2 entry genes across tissues and demographics. Nature Medicine (2021). [DOI] [PMC free article] [PubMed]
- 106.Sze S et al. Ethnicity and clinical outcomes in COVID-19: A systematic review and meta-analysis. EClinicalMedicine 29, 100630 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Benitez J, Courtemanche C & Yelowitz A Racial and ethnic disparities in Covid-19: Evidence from six large cities. Journal of Economics, Race, and Policy 3, 243–261 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kopel J et al. Racial and Gender-Based Differences in COVID-19. Front Public Health 8, 418 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Gu T et al. Characteristics Associated With Racial/Ethnic Disparities in COVID-19 Outcomes in an Academic Health Care System. JAMA Netw Open 3, e2025197 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Bhopal SS & Bhopal R Sex differential in COVID-19 mortality varies markedly by age. Lancet 396, 532–533 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Starke KR et al. The Age-Related Risk of Severe Outcomes Due to COVID-19 Infection: A Rapid Review, Meta-Analysis, and Meta-Regression. Int J Env Res Pub He 17 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ho FK et al. Is older age associated with COVID-19 mortality in the absence of other risk factors? General population cohort study of 470,034 participants. Plos One 15 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Pierce CA et al. Immune responses to SARS-CoV-2 infection in hospitalized pediatric and adult patients. Sci Transl Med 12 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Weisberg SP et al. Distinct antibody responses to SARS-CoV-2 in children and adults across the COVID-19 clinical spectrum. Nature Immunology 22 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hobbs CV, Khaitan A, Kirmse BM & Borkowsky W COVID-19 in Children: A Review and Parallels to Other Hyperinflammatory Syndromes. Front Pediatr 8 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zarin DA & Rosenfeld S Lack of harmonization of coronavirus disease ordinal scales. Clin Trials, 1740774520972082 (2020). [DOI] [PubMed]
- 117.Zheng H et al. Multi-cohort analysis of host immune response identifies conserved protective and detrimental modules associated with severity irrespective of virus. medRxiv, 2020.2010.2002.20205880 (2020). [DOI] [PMC free article] [PubMed]
- 118.Su Y et al. Multi-omics resolves a sharp disease-state shift between mild and moderate COVID-19. Published online October 28. DOI: 10.1016/j.cell.2020.10.037. Cell (2020). [DOI] [PMC free article] [PubMed]
- 119.Amezquita RA et al. Orchestrating single-cell analysis with Bioconductor. Nat Methods 17, 137–145 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Stuart T et al. Comprehensive Integration of Single-Cell Data. Cell 177, 1888–1902 e1821 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Regev A et al. The Human Cell Atlas. Elife 6 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Human Cell Atlas Data Portal https://data.humancellatlas.org/about Access date 17 Feb 2021. .
- 123.Ballestar E et al. Single cell profiling of COVID-19 patients: an international data resource from multiple tissues. medRxiv 10.1101/2020.11.20.20227355 Access date: 23 Nov, 2020. [DOI]
- 124.Hao Y et al. Integrated analysis of multimodal single-cell data. 10.1101/2020.10.12.335331 Access date 17 Mar, 2021. bioRxiv (2020). [DOI] [PMC free article] [PubMed]
- 125.Bernardes JP et al. Longitudinal Multi-omics Analyses Identify Responses of Megakaryocytes, Erythroid Cells, and Plasmablasts as Hallmarks of Severe COVID-19. Immunity 53, 1296–1314 e1299 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Butler A, Hoffman P, Smibert P, Papalexi E & Satija R Integrating single-cell transcriptomic data across different conditions, technologies, and species. Nat Biotechnol 36, 411–420 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Wolf FA, Angerer P & Theis FJ SCANPY: large-scale single-cell gene expression data analysis. Genome Biol 19, 15 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Lake BB et al. Integrative single-cell analysis of transcriptional and epigenetic states in the human adult brain. Nat Biotechnol 36, 70–80 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Wen W et al. Immune cell profiling of COVID-19 patients in the recovery stage by single-cell sequencing. Cell Discov 6, 31 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zhu L et al. Single-Cell Sequencing of Peripheral Mononuclear Cells Reveals Distinct Immune Response Landscapes of COVID-19 and Influenza Patients. Immunity 53, 685–696 e683 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Yu K et al. Dysregulated adaptive immune response contributes to severe COVID-19. Cell Res 30, 814–816 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Yao C et al. Cell-Type-Specific Immune Dysregulation in Severely Ill COVID-19 Patients. Cell Rep 34, 108590 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Bost P et al. Deciphering the state of immune silence in fatal COVID-19 patients. Nat Commun 12, 1428 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Combes AJ et al. Global absence and targeting of protective immune states in severe COVID-19. Nature 591, 124–130 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Heming M et al. Neurological Manifestations of COVID-19 Feature T Cell Exhaustion and Dedifferentiated Monocytes in Cerebrospinal Fluid. Immunity 54, 164–175 e166 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Varchetta S et al. Unique immunological profile in patients with COVID-19. Cell Mol Immunol (2020). [DOI] [PMC free article] [PubMed]
- 137.Osman M et al. Impaired natural killer cell counts and cytolytic activity in patients with severe COVID-19. Blood Adv 4, 5035–5039 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


