Abstract
CTXφ is a lysogenic, filamentous bacteriophage. Its genome includes the genes encoding cholera toxin (ctxAB), one of the principal virulence factors of Vibrio cholerae; consequently, nonpathogenic strains of V. cholerae can be converted into toxigenic strains by CTXφ infection. O139 Calcutta strains of V. cholerae, which were linked to cholera outbreaks in Calcutta, India, in 1996, are novel pathogenic strains that carry two distinct CTX prophages integrated in tandem: CTXET, the prophage previously characterized within El Tor strains, and a new CTX Calcutta prophage (CTXcalc). We found that the CTXcalc prophage gives rise to infectious virions; thus, CTXETφ is no longer the only known vector for transmission of ctxAB. The most functionally significant differences between the nucleotide sequences of CTXcalcφ and CTXETφ are located within the phages’ repressor genes (rstRcalc and rstRET, respectively) and their RstR operators. RstRcalc is a novel, allele-specific repressor that regulates replication of CTXcalcφ by inhibiting the activity of the rstAcalc promoter. RstRcalc has no inhibitory effect upon the classical and El Tor rstA promoters, which are instead regulated by their cognate RstRs. Consequently, production of RstRcalc renders a CTXcalc lysogen immune to superinfection by CTXcalcφ but susceptible (heteroimmune) to infection by CTXETφ. Analysis of the prophage arrays generated by sequentially integrated CTX phages revealed that pathogenic V. cholerae O139 Calcutta probably arose via infection of an O139 CTXETφ lysogen by CTXcalcφ.
Cholera is a severe, infectious diarrheal disease caused by the gram-negative bacterium Vibrio cholerae. The principal virulence factor of V. cholerae is cholera toxin (CT), a potent, A-B-type exotoxin that ADP-ribosylates proteins within intoxicated intestinal epithelial cells (19). The CT produced by V. cholerae during the organism’s colonization of its host’s small intestine accounts for a majority of the symptoms that characterize the disease process (11). In 1996, Waldor and Mekalanos discovered that the genes encoding CT (the operon ctxAB) are not integral components of the V. cholerae genome, but instead are elements of the genome of a filamentous bacteriophage, CTXφ, that specifically infects V. cholerae (22). Infection of V. cholerae by CTXφ is frequently followed by integration of the phage genome into the V. cholerae genome, yielding a stable lysogen. Like the filamentous phages of Escherichia coli, CTXφ can also replicate as a plasmid, and it does so in bacterial strains lacking appropriate integration sites; however, most if not all natural isolates of V. cholerae containing ctxAB contain integrated phage DNA (13).
Integration of the CTXφ genome is site specific, but the integration sites and the prophage arrays they contain differ between the two biotypes of V. cholerae O1. Within El Tor biotype strains, which have been used for most analyses of phage genes, CTX prophages are found at a chromosomal site known as attRS (16). Integration of CTXφ DNA into attRS occurs via recombination between an 18-bp sequence (originally designated the end repeat [ER]) in the phage genome and a nearly identical sequence in attRS (16). Some El Tor strains contain a single CTX prophage, while many others contain several in tandem (13). The length of this prophage array can fluctuate (generally expanding) both during the course of an infection and within laboratory cultures, in response to the bacterium’s environment (6, 13). We have found that the CTX prophages in El Tor strains generally give rise to infectious phage particles (10).
V. cholerae strains of the classical biotype, which were the dominant cause of epidemic cholera until 1961 when they were replaced by El Tor strains, contain a more complex arrangement of CTXφ genes. Classical strains have two integration sites, each of which contains a single CTX prophage (13). One site is identical to the attRS integration site found in El Tor strains. The second site has not been well characterized, but it has been localized to a different chromosome than attRS (21). Surprisingly, neither prophage within classical strains apparently gives rise to phage particles (unpublished data). In addition, the DNA of CTXφ derived from El Tor strains does not integrate following CTXφ infection of classical strains. Instead, phage DNA replicates as a plasmid in classical strains, rather than recombining into either of the two classical integration sites (22).
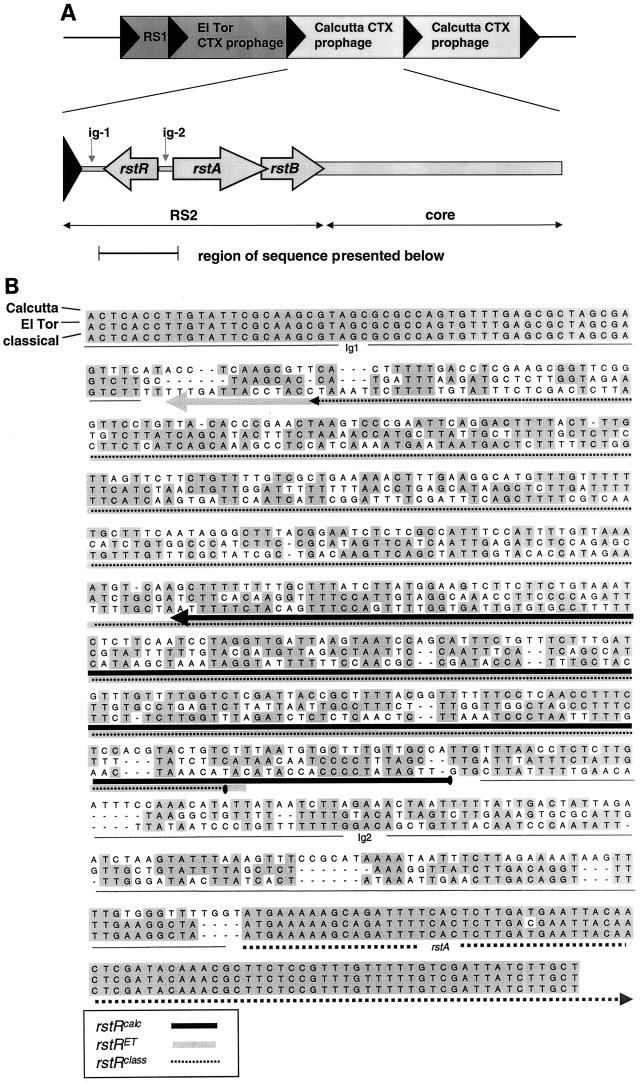
The CTXφ genome is composed of two regions (Fig. 1) (6, 16). The core region contains the genes encoding CT and genes required for phage morphogenesis, including genes that are thought to encode major and minor phage coat proteins and a protein that aids in phage assembly and secretion (24). Some of these morphogenesis genes are similar to genes of E. coli filamentous phages, such as M13 and fd (22). In contrast, the three genes of the other CTXφ region, RS2, are not similar to those of E. coli filamentous phages. Their products control phage replication and site-specific integration (16, 23). RstA is required for phage DNA replication, RstB is required for site-specific integration, and RstR is a repressor of rstA expression (9, 23). RS2 also contains two intergenic regions: ig-1 and ig-2. Ig-2 appears to encompass the rstA promoter and the RstR operator; no role has yet been established for ig-1. These three genes and the intergenic regions are also components of a related genetic element, RS1, which is found adjacent to CTX prophages in many V. cholerae strains (23).
FIG. 1.
Structure and sequence of CTX prophages within AS207, an O139 Calcutta strain of V. cholerae. (A) Within the AS207 chromosome, an RS1 element and a CTXET prophage are followed by two CTXcalc prophages (shown in light grey). The Calcutta prophage is structurally similar to the previously described CTX prophages within El Tor and classical strains, which contain two major domains known as RS2 and core. RS2 contains three genes (rstA, rstB, and rstR) whose transcriptional orientation is indicated by the arrows. The black triangles represent attRS-ER sequences. (B) Alignment of the nucleotide sequences of rstR, ig-2, and parts of rstA and ig-1 from Calcutta, El Tor, and classical prophages. The arrows underneath the sequences depict the ORFs that encode the three variants of RstR.
We recently performed a detailed comparison of the RS2 regions from classical and El Tor CTX prophages (9). We found that rstB and the coding sequence of rstA are highly conserved between the biotypes (94% nucleotide identity), but that rstR and ig-2 (the rstA promoter) sequences diverge considerably (44 and 61% nucleotide identity, respectively). Due to the variations in the sequences and binding sites of both the repressor proteins, each RstR is a biotype-specific repressor of its cognate rstA (9). That is, expression of the classical rstA (rstAclass) reporter construct rstAclass-lacZ is repressed by classical, but not El Tor, RstR, and similarly, expression of the El Tor reporter construct rstAET-lacZ is repressed by El Tor, but not classical, RstR. This repression allows integrated phages to inhibit replication of newly infecting phages of the same biotype, thereby conferring immunity to secondary infection. However, the production of RstRclass by the prophages within classical strains of V. cholerae does not prevent infection of these strains by a Kn-marked El Tor CTXφ, suggesting that classical CTXφ lysogens are heteroimmune to the El Tor CTXφ (9).
Until 1997, only these two forms, El Tor and classical, of CTX prophages had been identified. However, analyses in 1997 of novel O139 strains responsible for severe outbreaks of cholera in Calcutta, India, revealed that they contained prophages with atypical restriction endonuclease sites (3, 20). We reported previously that these Calcutta strains contain sequences within RS2 quite dissimilar to both the classical and El Tor RS2s (8). In this study, we present further analyses of the Calcutta CTX prophage, especially of its RS2 domain. We show that RstRcalc, despite a size and structure dramatically different from previously described repressors, also functions as an allele-specific repressor, capable of repressing rstAcalc expression. In addition, we demonstrate that, unlike the classical prophages, the Calcutta prophage generates infectious phage particles. Thus, CTXcalcφ, as well as the El Tor CTXφ (denoted here as CTXETφ but in earlier works simply as CTXφ), can transmit ctxAB to nonpathogenic strains. Lysogens of CTXcalcφ have immunity to superinfection with CTXcalcφ, similar to the immunity provoked by CTXETφ. Finally, we have used CTXcalcφ and CTXETφ to investigate the potential genesis of multiply lysogenized V. cholerae strains, such as those identified in Calcutta.
MATERIALS AND METHODS
Nucleotide sequence of the CTXcalc prophage RS2 region.
RS2 from the CTXcalc prophage was amplified from strain AS207 using PCR. In this PCR, a forward primer within ctxB (5′ GCGATTGAAAGGATGAAGGATAC 3′) and a reverse primer within cep (5′ AACCCCGAGTGAAAGCGTG 3′) allows amplification of the CTXcalc prophage RS2 region from strains such as AS207, which contain Calcutta prophages downstream of a single El Tor prophage (Fig. 1) (8). This PCR product was cloned into pCR2.1 (Invitrogen, Carlsbad, Calif.) to generate pHK268, which was used as a template for dye terminator cycle sequencing, using an Applied Biosystems 373A DNA sequencer. The BLAST programs (1) were used to compare the Calcutta RS2 nucleotide sequence to sequences in the GenBank databases. Potential repressor and helix-turn-helix (hth) DNA binding domains were evaluated by using the matrix of Dodd and Egan (4) and the motif analysis program fingerPRINTScan (5).
Bacterial strains and culture conditions.
Bacterial strains and plasmids used in this study are described within Table 1. All bacteria were cultured in Luria-Bertani broth (14) at 37°C unless otherwise noted. Antibiotics were used at the following concentrations: ampicillin (AMP), 50 μg/ml (V. cholerae); AMP, 100 μg/ml (E. coli); KAN, 50 μg/ml; streptomycin (STR), 200 μg/ml; chloramphenicol (CMP), 15 μg/ml) (E. coli). Arabinose (ARA)-induced cultures contained 0.05% ARA, and sucrose-resistant (Scr) clones were selected on 10% sucrose.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant characteristics | Source or reference |
|---|---|---|
| Strains | ||
| V. cholerae | ||
| AS207 | O139 Calcutta strain | 20 |
| O395 | O1 classical strain | 15 |
| E7946 | 1978 El Tor clinical isolate from Bahrain | 13 |
| 2740-80 | U.S. Gulf Coast isolate, attRS+, CTXφ− | 16 |
| E. coli CC118 | ΔlacX74 recA1 phoA F− | 12 |
| Plasmids | ||
| pHK101 | pCB192 derivative, rstAclass-lacZ reporter | 9 |
| pHK102 | pCB192 derivative, rstAET-lacZ reporter | 9 |
| pBD40 | pCB192 derivative, rstAcalc-lacZ reporter | This study |
| pHK2 | pBAD33 derivative, ARA-inducible RstRclass | 9 |
| pHK1 | pBAD33 derivative, ARA-inducible RstRET | 9 |
| pBD87 | pBAD33 derivative, ARA-inducible RstRcalc | This study |
| pCTXET-Kn | Replicative form of CTXET-Knφ, previously called CTX-Knφ | 22 |
| pCTXET-Ap | XbaI fragment of pCTXET-Kn inserted into pGP704 | This study |
| pCTXcalc-Kn | Replicative form of CTXcalc-Knφ prophage from AS207 CTXcalc::Kn, contains El Tor rather than Calcutta ig-1 region of CTXφ | This study |
| pCTXcalc-Ap | XbaI fragment of pCTXcalc-Kn inserted into pGP704 | This study |
| pCB192 | β-Galactosidase reporter plasmid, Apr | 18 |
| pBAD33 | ARA-inducible promoter vector, Cmr | 7 |
| pGP704 | oriR6K mobRP4 suicide vector, Apr | 17 |
| pCACTUS | Allele-exchange vector, Temperature-sensitive ori, sacB+, Cmr | Chris Clark |
| pHK260 | pCACTUS derivative containing the SphI/BglII fragment of pCTXET-Kn | This study |
Plasmid and strain construction.
pBD40, which contains an rstAcalc-lacZ fusion, was constructed by first amplifying the rstAcalc promoter and part of the rstAcalc coding sequence with primers rstAcalc proF (5′ GATGTTTGTTTTGGTCTCGATTACCG 3′) and rstA proR (5′ TGAAGCATAAGGAACCGACC 3′). Next, the PCR product was cloned into the TA cloning vector pCRII-TOPO (Invitrogen). An XbaI/HindIII fragment containing the insert was then ligated to XbaI/HindIII-digested pCB192 (18) to generate pBD40. To construct pBD87, a PCR product containing rstRcalc was first amplified with primers rstR-11 (5′ AATAGGGCTTTACGGAATC 3′) and rstR-10 (5′ TGTTTGGAAATCAAGAGAGG 3′). Following subcloning of this product into pCRII-TOPO, a KpnI/XbaI fragment containing the insert was ligated into KpnI/XbaI-digested pBAD33 (7).
AS207 CTXcalc::Kn was made from AS207 with an allele exchange vector derived from the temperature-sensitive, sacB+, counterselectable plasmid pCACTUS. This allele exchange vector, pHK260, was constructed by ligating the SphI/BglII fragment of pCTXET-Kn (22), which spans the Knr cassette, to SphI/BglII-digested pCACTUS. Following electroporation of AS207 with pHK260, plasmid integrants were isolated at 39°C. KAN-resistant colonies were subsequently screened for resistance to sucrose, which results from recombination and excision of the vector sequences. Sucrose- and KAN-resistant colonies were screened by Southern blotting to ascertain which ctxAB gene pair(s) had been replaced by the Knr cassette. In AS207 CTXcalc::Kn, the ctxAB gene pairs of both CTXcalc prophages were replaced. In addition, 408 bp of the Calcutta ig-1 were replaced by El Tor ig-1 sequences, which were present within the targeting vector. CTXcalc-Knφ produced by AS207 CTXcalc::Kn contains only Calcutta sequences for rstR and ig-2 and consequently is expected to have the same replicative and repressive properties as CTXcalcφ. As we have not yet generated a marked version of CTXcalcφ containing only ig-1calc, we used this hybrid CTXcalc-Knφ in experiments requiring a selectable Calcutta phage.
Cell-free supernatant from an AS207 CTXcalc::Kn culture was used to transduce O395 to KAN resistance. pCTXcalc-Kn was then purified from these KAN-resistant O395 cells. Its structure was confirmed by restriction mapping and by sequencing of the ig-1 region. pCTXcalc-Ap was constructed by ligating the XbaI fragment of pCTXcalc-Kn (which lacks only the Knr cassette) to XbaI-digested pGP704 (17). pCTXET-Ap is an equivalent plasmid constructed from pCTXET-Kn and pGP704; pGP704 has the same orientation, relative to the CTXφ genes, in pCTXcalc-Ap and pCTXET-Ap.
Phage transduction assays.
To transfer CTXcalc-Knφ from AS207 CTXcalc::Kn to O395, 50 μl of agglutinated O395 (grown at 30°C to induce expression of the CTXφ receptor, TCP [22]) was mixed with 50 μl of filtered supernatant from a log-phase culture of AS207 CTXcalc::Kn. The mixture was shaken gently at room temperature for 20 min, and transductants were selected on Luria-Bertani plates containing KAN. In order to transfer phages to O395 in the absence of antibiotic selection, 1 μl of agglutinated O395 was mixed with 1.5 ml of filtered log-phase-donor (e.g., AS207) supernatant. The mixture was grown overnight at 30°C, and phage transfer was subsequently detected by Southern blotting of plasmid DNA prepared from the culture.
Molecular biology methods.
Southern hybridization was carried out using horseradish peroxidase-labelled DNA probes, which were prepared and hybridized using the ECL direct nucleic acid labelling and detection system (Amersham Pharmacia, Little Chalfont, Buckinghamshire, England) according to the manufacturer’s instructions. The rstRcalc probe was a PCR product amplified with the primers rstR-10 and rstR-11; the rstRET probe was a PCR product amplified with the primers rstR-3 and rstR-8 (9). The PCR primers used for analysis of pCTX integration sites were TLCF1 (5′ TGTCGGAGCTGCTTGGATTAAG 3′) and RstR Rev (5′ CGACCAAGCAAGATAATCGAC 3′). Other techniques were performed using standard protocols (2).
Nucleotide sequence accession number.
The sequence of the CTXcalcφ RS2 region has been assigned GenBank accession no. AF110029.
RESULTS
Structure and sequence of CTX prophages within Calcutta strains of V. cholerae.
The O139 strains of V. cholerae that emerged as a cause of widespread disease in Calcutta in 1996 were found by restriction mapping to contain two tandemly arranged copies of a novel CTX prophage (3, 8, 20). These prophages were integrated into the chromosome immediately downstream of an El Tor RS1 element and an El Tor CTX prophage (Fig. 1A). In order to sequence the RS2 region of the Calcutta prophage, we amplified and cloned a PCR product spanning this region from AS207, a representative O139 Calcutta strain (8). Comparison of the putative rstR and ig-2 from the Calcutta prophage with sequences from El Tor and classical prophages revealed striking differences (Fig. 1B). In contrast to rstA and subregions of ig-1, which are highly conserved among the three prophages, the putative Calcutta rstR and ig-2 share no extended sequence identity with the other prophages. In addition, the longest open reading frame (ORF) found within the rstR region of the Calcutta prophage is predicted to encode a protein that is significantly shorter (59 amino acids) than the El Tor and classical RstRs (113 and 112 amino acids, respectively). BLAST searches revealed no significant homology between the Calcutta rstR region and the sequences within the GenBank database, either at the nucleotide or amino acid level. In contrast, both RstRET and RstRclass show sequence similarity to a number of bacteriophage repressors (9), and both are predicted by the Dodd and Egan matrix to contain hth DNA binding motifs that start near their amino termini (4). We could not identify a similar hth domain within RstRcalc with the Dodd and Egan matrix. However, the protein motif analysis program fingerPRINTScan did identify RstRcalc as a repressor containing an hth DNA binding domain (hthrepressr fingerprint), but only if the stringency of analysis was reduced from the default value of 15% to 12%. Unlike in RstRET and RstRclass, the hth motif in RstRcalc is found near the carboxyl terminus of the protein.
Allele-specific repression of rstAcalc by RstRcalc.
We have previously shown that the different RstRs expressed within classical and El Tor CTXφ lysogens repress rstA-lacZ reporters in a biotype-specific manner (9). To assess whether Calcutta strains similarly encode a repressor specific for the novel rstAcalc promoter sequence, we coexpressed the putative RstRcalc with a panel of rstA-lacZ reporters in an E. coli K-12 strain, CC118 (12). In addition, each reporter, rstAET-lacZ, rstAclass-lacZ, and rstAcalc-lacZ, was coexpressed in CC118 with RstRET and RstRclass. Production of the RstRs was controlled by an ARA-inducible promoter (pBAD) (7) as described previously (9). We found that β-galactosidase activity produced from rstAcalc-lacZ was consistently high when this reporter was maintained alone, maintained with a pBAD33 vector control, or maintained with RstRET- and RstRclass-producing plasmids, both under inducing and noninducing conditions (Table 2 and data not shown). However, rstAcalc-lacZ expression decreased 50-fold when production of RstRcalc was induced with ARA (Table 2). Thus, the 59-amino-acid polypeptide encoded by the ORF underlined in Fig. 1B is sufficient to repress rstAcalc expression. No additional repression was observed with a larger repressor construct containing an additional ORF found downstream of rstRcalc, nor was rstAcalc-lacZ expression repressed by the downstream ORF alone (data not shown). The RstRcalc repressor activity was specific; it did not reduce the β-galactosidase activity produced either from rstAclass-lacZ or rstRET-lacZ (Table 2). Specific repression of the reporter constructs was also observed when the reporter plasmids were transformed into V. cholerae strains producing various repressors from their endogenous prophages (data not shown). Thus, endogenous levels of RstR are sufficient to repress expression of RstA; repression is not an artifact of overexpressing the repressors in E. coli.
TABLE 2.
The influence of different inducible RstRs on expression of rstA-lacZ reporters
| Reportera | Repressor constructa | β-Galactosidase activityb
|
Fold repressionc | |
|---|---|---|---|---|
| No ARA | 0.05% ARA | |||
| rstAcalc-lacZ | pBAD33 | 890 | 882 | NS |
| pBADRstRcalc | 1,868 | 39 | 48 | |
| pBADRstRET | 1,563 | 1,641 | NS | |
| pBADRstRclass | 1,256 | 2,315 | NS | |
| rstAET-lacZ | pBAD33 | 100 | 72 | NS |
| pBADRstRcalc | 192 | 133 | NS | |
| pBADRstRET | 133 | 1.8 | 74 | |
| pBADRstRclass | 161 | 114 | NS | |
| rstAclass-lacZ | pBAD33 | 398 | 332 | NS |
| pBADRstRcalc | 946 | 749 | NS | |
| pBADRstRET | 745 | 680 | NS | |
| pBADRstRclass | 616 | 8.8 | 70 | |
Reporter and repressor pairs were introduced into E. coli CC118. The rstAcalc-lacZ reporter was pBD40, the rstAET-lacZ reporter was pHK102, and the rstAclass-lacZ reporter was pHK101. The ARA-inducible Calcutta, El Tor, and classical RstRs were pBD87, pHK1, and pHK2, respectively.
β-Galactosidase activity within overnight cultures is reported in Miller units (14).
Fold repression was calculated by dividing the β-galactosidase activity in the absence of ARA by the β-galactosidase activity in the presence of ARA. Changes in β-galactosidase activity of less than twofold were deemed not significant (NS).
The CTXcalc prophage encodes an infectious bacteriophage.
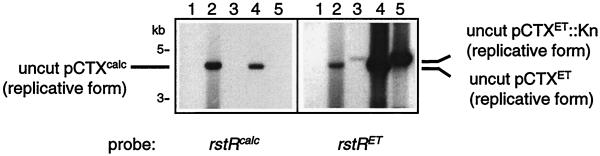
We next ascertained whether the CTXcalc prophage gives rise to transmissible bacteriophage particles, or if, like the classical CTX prophage, it lacks the capacity for independent replication. We hypothesized that CTXcalcφ transmission to the classical strain O395 would result in production of pCTXcalc (the plasmid, or replicative form [RF], of CTXcalcφ) as occurs following the infection of O395 with CTXETφ (22). Therefore, we incubated cell-free supernatants from AS207 with O395, then prepared plasmid DNA from potentially infected cells and used Southern hybridization analysis to assay for transmission of CTXcalcφ from AS207 supernatants to O395. Southern blots were probed sequentially with rstRcalc and then with rstRET. Control experiments revealed that no rstRcalc-hybridizing species could be detected in plasmid DNA prepared from O395 cultures (Fig. 2). However, an rstRcalc-hybridizing plasmid species was detected within plasmid DNA isolated from O395 cultured at 30°C in the presence of filtered supernatants from AS207. An equally sized rstRcalc-hybridizing species was detected in plasmid DNA prepared from AS207. Restriction digests revealed these plasmids to be the RF of CTXcalc, which probably forms in AS207 as a replication intermediate during CTXcalcφ production. Treatment of the AS207 cell-free culture supernatant with DNase I did not prevent the transfer of pCTXcalc to O395 from AS207 supernatants (data not shown), thereby suggesting that AS207 gives rise to a bacteriophage, CTXcalcφ, that is competent to infect and replicate within O395. Rehybridization of these Southern blots with an rstRET probe revealed that AS207 also is capable of transfer of CTXETφ, at a level matching or exceeding that of an El Tor strain with two tandemly arranged KAN-marked El Tor CTX prophages (2740-80 [CTXET-Kn]) (Fig. 2). Thus, the Calcutta strain AS207 of V. cholerae can give rise to two distinct infectious bacteriophages: CTXcalcφ and CTXETφ. Consequently, CTXETφ is not the sole phage capable of conveying the genes encoding CT to nonpathogenic V. cholerae.
FIG. 2.
Detection of transfer of CTXcalcφ and CTXETφ from supernatants of AS207 to O395 using Southern blot analysis of plasmid DNA. Undigested plasmid DNAs were run on agarose gels, transferred to a nylon membrane, and sequentially hybridized with probes for rstRcalc (left panel) and rstRET (right panel). Plasmid DNA was prepared from O395 (lanes 1), AS207 (lanes 2), 2740-80 (CTXET-Kn) (lanes 3), O395 cultured with AS207 supernatant (lanes 4), and O395 cultured with 2740-80 (CTXET-Kn) supernatant (lanes 5). The Knr cassette is slightly larger than the ctxAB genes it replaces in CTXET-Knφ, so pCTXET-Knφ migrates more slowly than pCTXETφ.
Electroporation of the RF of an antibiotic marked version of CTXcalcφ (pCTXcalc-Kn) into CTXφ− strains yielded Knr transformants that produce CTXcalc-Knφ particles (data not shown). This result confirmis that CTXcalcφ is infectious; in addition, it demonstrates that the production of infectious CTXcalcφ particles by AS207 and other Calcutta strains is not dependent upon the CTXET prophage also present in these strains.
Immunity and heteroimmunity of a CTXcalc-Knφ lysogen.
We previously found that strains harboring a CTXET prophage are significantly resistant to further infection with CTXET-Knφ. This immunity results from the repression of rstAET expression by RstRET, as RstA is essential for phage replication. The data presented above indicate that RstRET does not repress RstAcalc production, and similarly, RstRcalc does not repress RstAET production (Table 2). Therefore we tested, with marked CTXcalcφ derivatives, whether a lysogen harboring a CTXcalc prophage is immune to CTXcalcφ superinfection and heteroimmune to CTXETφ infection. Since immunity results from inhibition of phage replication following infection rather than from inhibition of the initial steps of infection, and since El Tor strains cannot be efficiently infected with CTXcalcφ in vitro (due to lack of expression of TCP, the phage receptor), we developed a transformation assay to study the immunity properties of the CTXcalc prophage. For these assays, 2740-80, an El Tor, CTXφ−, attRS+ strain, and 2740-80 lysogens of marked CTXcalcφ and CTXETφ were electroporated with differentially marked CTXcalc or CTXET plasmid DNA.
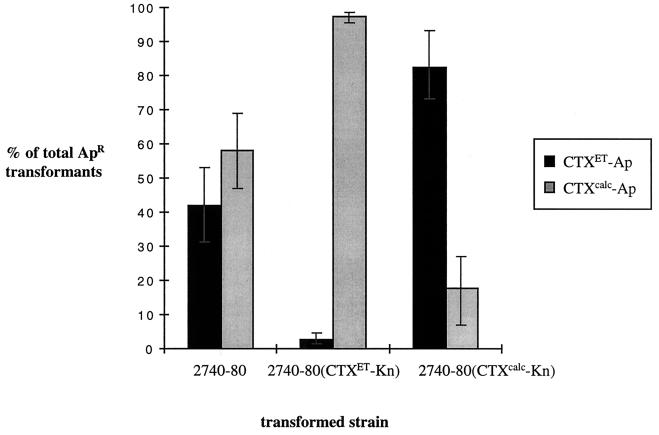
Initially, we electroporated identical amounts of pCTXcalc-Ap and pCTXET-Ap in parallel into 2740-80. A similar number of AMP-resistant colonies was obtained with DNA from each phage, suggesting that these phages replicate and subsequently integrate with comparable efficiency within 2740-80 (Fig. 3). We then electroporated these AMP-marked phage DNAs into 2740-80 (CTXcalc-Kn) and 2740-80 (CTXET-Kn), which contain KAN-marked prophages integrated at the 2740-80 attRS site. Transformation of these strains with pCTXcalc-Ap and pCTXET-Ap did not yield comparable numbers of colonies; instead, the prophages conferred repressor allele-specific resistance to further transformation by CTXφ variants. Thus, pCTXcalc-Ap accounted for 58% of all 2740-80 transformants, but only 19% of 2740-80(CTXcalc-Kn) transformants. Similarly, pCTXET-Ap accounted for 42% of all 2740-80 transformants, but only 3% of 2740-80 (CTXET-Kn) transformants (Fig. 3). These data suggest that CTXcalcφ lysogens are immune to further infection by CTXcalcφ, just as CTXETφ lysogens are immune to further infection by CTXETφ. These data also suggest that CTXcalcφ and CTXETφ lysogens are not immune to infection by CTXET and CTXcalc phages, respectively. Consequently, V. cholerae can be lysogenized by multiple distinct CTX phages; in fact, this process probably accounts for the development of Calcutta strains such as AS207, which contain both El Tor and Calcutta prophages.
FIG. 3.
Relative efficiency of transformation of pCTXcalc-Ap versus pCTXET-Ap into 2740-80, 2740-80 (CTXET-Kn), and 2740-80 (CTXcalc-Kn). Percentage of total Apr transformants = (pCTXcalcAp or pCTXETAp transformants)/(pCTXcalcAp + pCTXETAp transformants).
Integration site preference and evolution of V. cholerae O139 Calcutta.
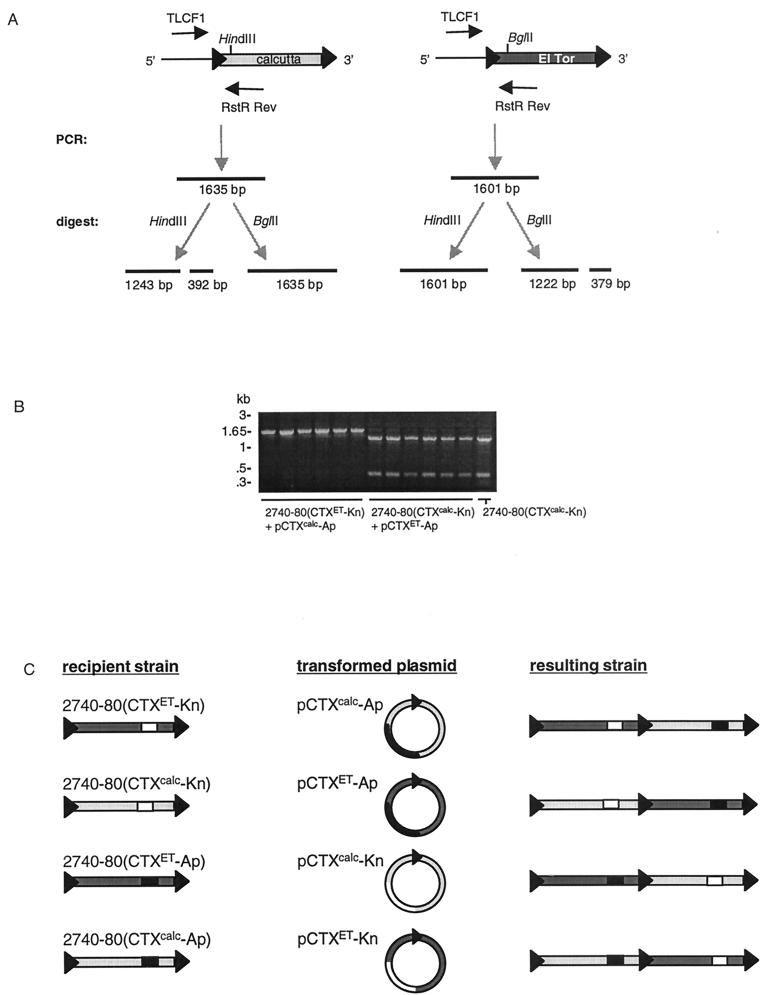
We used 2740-80 harboring either marked El Tor or Calcutta CTX prophages to explore potential steps in the evolution of Calcutta strains such as AS207. In these experiments, we determined where the DNA of CTXETφ and CTXcalcφ integrates on the chromosome in strains harboring either the CTXcalc prophage or the CTXET prophage, respectively. We have found that CTX phage DNA reliably integrates into attRS following infection of attRS+, CTX− El Tor strains, such as 2740-80. However, integration regenerates the 18-bp core of attRS or the very similar ER sequence on both ends of the prophage (16). Thus, a subsequently infecting phage has two or more potential integration targets; the precise number of integration targets is determined by the length of the prophage array. To determine the site of CTXφ integration following infection of a CTXφ lysogen, we developed a PCR and restriction-digest-based assay. For the PCR reaction, one primer was complementary to DNA 5′ of attRS on the 2740-80 chromosome and the other primer was complementary to a conserved sequence within rstA (Fig. 4). PCR products were digested with enzymes that cleave either the El Tor or the Calcutta rstR, thereby allowing us to identify the furthest 5′ prophage within an array of prophages. We found that the initial phage integrated on the 2740-80 chromosome always retained the most 5′ position on the chromosome (Fig. 4); thus, CTXcalc-Kn, CTXET-Kn, CTXcalc-Ap, and CTXET-Ap were each maintained as the most 5′ prophage if their DNA was the first in a series to be electroporated into 2740-80. Subsequent integrations, regardless of which particular phage’s DNA was tested, occurred 3′ of an integrated prophage (Fig. 4 and data not shown). In other words, DNA from the second antibiotic-marked phage always integrated either between tandem prophages or between an integrated prophage and 3′ chromosomal DNA. This unambiguous insertion site preference in sequentially transformed strains (and presumably also in sequentially infected strains) strongly suggests that Calcutta strains such as AS207 arose by infection of a V. cholerae CTXET lysogen by CTXcalcφ.
FIG. 4.
Integration site selection by sequentially integrated plasmids. (A) PCR followed by restriction digestion was used to determine the order of prophages within the chromosome following sequential integration of CTXET and CTXcalc antibiotic-marked plasmids. When the CTXcalc prophage is the furthest 5′, the TLCF1-RstR Rev PCR product contains a HindIII site but not a BglII site. Conversely, when CTXET is upstream, the PCR product contains a BglII site but not a HindIII site. (B) A representative agarose gel containing PCR products digested with HindIII. Template DNA is as follows: 2740-80(CTXET-Kn) transformed with pCTXcalc-Ap (lanes 1 to 6), 2740-80 (CTXcalc-Kn) transformed with pCTXET-Ap (lanes 7 to 12), and 2740-80 (CTXcalc-Kn) (lane 13). (C) Summary and model of integration site selection following sequential CTXφ integration. Phage DNA does not integrate into the 5′ ER (black triangle) if the chromosome already contains a CTX prophage. Instead, the new phage DNA inserts into the 3′ ER.
DISCUSSION
We have investigated the repressive and replicative capabilities of a novel variant of CTXφ, which was found as a prophage within epidemic-linked strains of O139 V. cholerae isolated from Calcutta. This prophage gives rise to CTXcalcφ virions that infect and/or lysogenize both relatively nonpathogenic (lacking ctxAB) V. cholerae strains, such as 2740-80, as well as classical and El Tor strains that already contain CTX prophages. These findings demonstrate that CTXETφ is not the sole viable, CT-encoding, filamentous phage capable of transmitting ctxAB within V. cholerae populations. CTXcalcφ lysogens are immune to infection by CTXcalcφ due to the production by these lysogens of a new repressor, RstRcalc, that inhibits expression from the adjacent rstAcalc promoter. Like RstRET and RstRclass, the transcriptional repressor activity of RstRcalc is sequence specific; RstRcalc does not inhibit the activity of either the rstAclass or the rstAET promoter. Similarly, the rstAcalc promoter is not repressed by RstRclass or RstRET.
Despite the functional similarity of the three CTXφ repressors, the RstRcalc amino acid sequence is unrelated to the sequences of RstRclass, RstRET, and the known repressor proteins of other lysogenic phages. In fact, BLAST searches using the nucleotide and predicted amino acid sequences of this repressor revealed no significant homology to any sequence within the GenBank databases. It is therefore not surprising that most protein analysis algorithms that we tested did not predict any repressor or DNA binding activity for RstRcalc. However, the motif-detecting program fingerPRINTScan does detect a low level of similarity between RstRcalc and the hth domain found in lambdoid repressors and in a subset of homeotic proteins (hthrepressr fingerprint). Interestingly, fingerPRINTScan assigns RstRclass and RstRET to different hth categories (homeobox and hthLysR fingerprints, respectively), suggesting that the three V. cholerae repressors may be more closely related to proteins from other species than they are to each other. The genetic mechanism(s) by which the unrelated RstR-RstR operator pairs became associated with otherwise very similar CTXφs has not been explored. However, recombination between a CTXφ containing a particular repressor-operator pair and repressor-operator sequences within other temperate bacteriophages or even within other genetic elements is clearly one possibility.
Production of RstRcalc enables the CTXcalc prophage both to control its own replication and to inhibit RstA-mediated replication of any newly introduced DNA that relies upon the rstAcalc promoter. This confers upon a lysogen a degree of immunity to secondary infections by identical phages, similar to the immunity produced by λ prophages within E. coli. Also like lambdoid prophages, CTXcalcφ lysogens are susceptible to infection (heteroimmune) by CTXφ with different immunity regions (rstR/RstR operator sequences). In addition, results from our transformation efficiency assay suggest that CTXETφ lysogens are heteroimmune to CTXcalcφ infection. Integrated CTXcalc-Kn does not completely inhibit replication of the related replicon, CTXcalc-Ap; however, it does significantly diminish CTXcalc-Ap replication relative to that of CTXET-Ap. In the transformation efficiency assay system, CTXcalc-Kn is less effective at providing immunity than is CTXET-Kn. This finding could reflect the relative weakness of RstRcalc as a repressor; alternatively, it may indicate the strength of the rstAcalc promoter. When assayed in CC118, an rstAcalc-lacZ reporter fusion has higher activity than either rstAclass-lacZ or rstAET-lacZ reporters, so even if repressed to the same degree as the other promoters, rstAcalc may maintain higher residual activity. Residual production of RstA, either from the prophage or from the RF, presumably enables some plasmids to replicate and subsequently integrate. Although our assay measures immunity indirectly, by monitoring transformation of lysogens with the RF of the CTXφ genomes, these experiments yielded results similar to those obtained when CTXETφ immunity was assayed directly, using an intraintestinal transduction assay (9).
Our results support the hypothesis that AS207 and related Calcutta strains arose via infection of an O139 CTXETφ lysogen by the previously unknown CTXcalcφ. Epidemic O139 V. cholerae strains isolated prior to 1996 contain only El Tor CTX prophages but otherwise are very similar to Calcutta O139 strains (3, 20) and thus are likely AS207 progenitors. Our experiments indicate that CTXcalcφ infection and lysogenization of such a progenitor strain would result in the same arrangement of CTX prophages as is seen in Calcutta O139 strains such as AS207. A recent survey of environmental V. cholerae strains in Calcutta detected a prophage encoding RstRcalc (presumably integrated CTXcalcφ) in a non-O1, non-O139 strain of V. cholerae (3a). This may indicate that CTXcalcφ is transmitted within the estuarine environment as well as within the laboratory; it also reveals a potential source of CTXcalcφ. Thus, it seems probable that AS207 and other Calcutta strains arose via infection of an earlier, epidemic-linked O139 strain with CTXcalcφ. It is also possible that recombination between a CTXET prophage within an O139 strain and a repressor-operator from an unrelated genetic element gave rise to O139 Calcutta strains, but such a mechanism seems less likely to account for the origin of these strains.
When we proposed using rstRET to protect classical and El Tor live-attenuated V. cholerae vaccine strains from reversion to toxigenicity mediated by CTXφ infection (9), the only described infectious CTXφ was CTXETφ. Nucleotide sequence analysis of the rstR/ig-2 immunity regions in multiple El Tor clinical isolates from around the world had revealed that they are identical (9), lending credence to this approach. However, the current description of the infectious CTXcalcφ, which encodes the novel RstRcalc, suggests that rstR-mediated immunity to CTXφ infection may not constitute a useful method of enhancing the biosafety of live-attenuated V. cholerae vaccines. Besides rstRcalc, two additional putative rstRs have been identified recently in environmental, non-O1/O139 V. cholerae isolates (3a, 3b), and more alleles probably remain to be detected. Thus, introduction of a comprehensive immunizing library of rstRs into vaccine strains may not be practical. Ongoing studies are exploring alternative mechanisms for preventing CTXφ-mediated transfer of ctxA and -B to vaccine strains.
ACKNOWLEDGMENTS
We thank K. Moyer, B. Hochhut, and E. F. Boyd for critical reading of this manuscript. We are grateful to G. B. Nair and A. Ghosh for providing Calcutta O139 strains. We thank A. Kane and the New England Medical Center GRASP Center for preparation of the media and M. Byrne of the Tufts Core Facility for carrying out DNA sequencing.
This work was supported by grants AI-42347 to M.K.W. and grant P30DK-34928 for the New England Medical Center GRASP Digestive Center. M.K.W. is a Pew Scholar in the Biomedical Sciences. H.H.K. was supported by grant T32 AI07329.
REFERENCES
- 1.Altschul S F, Madden T L, Schaffer A A, Zhang J, Zhang Z, Miller W, Lipman D J. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidmann J G, Smith J A, Struhl K. Current protocols in molecular biology. New York, N.Y: Greene Publishing and Wiley-Interscience; 1990. [Google Scholar]
- 3.Basu A, Mukhopadhyay A K, Sharma C, Jyot J, Gupta N, Ghosh A, Bhattacharya S K, Takeda Y, Faruque A S, Albert M J, Balakrish Nair G. Heterogeneity in the organization of the CTX genetic element in strains of Vibrio cholerae O139 Bengal isolated from Calcutta, India and Dhaka, Bangladesh and its possible link to the dissimilar incidence of O139 cholera in the two locales. Microb Pathog. 1998;24:175–183. doi: 10.1006/mpat.1997.0186. [DOI] [PubMed] [Google Scholar]
- 3a.Berg, D. Personal communication.
- 3b.Boyd, E. F. Unpublished data.
- 4.Dodd I B, Egan J B. Improved detection of helix-turn-helix DNA-binding motifs in protein sequences. Nucleic Acids Res. 1990;18:5019–5026. doi: 10.1093/nar/18.17.5019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.FingerPRINTScan Website. [Online.] http://www.biochem.ucl.ac.uk/cgi-bin/fingerPRINTScan/fps/PathForm.cgi. [7 May 1999, last date accessed.]
- 6.Goldberg I, Mekalanos J J. Effect of a recA mutation on cholera toxin gene amplification and deletion events. J Bacteriol. 1986;165:723–731. doi: 10.1128/jb.165.3.723-731.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guzman L M, Belin D, Carson M J, Beckwith J. Tight regulation, modulation, and high-level expression by vectors containing the arabinose pBAD promoter. J Bacteriol. 1995;177:4121–4130. doi: 10.1128/jb.177.14.4121-4130.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kimsey H H, Nair G B, Ghosh A, Waldor M K. Diverse CTXφ and evolution of new pathogenic Vibrio cholerae. Lancet. 1998;352:457–458. doi: 10.1016/S0140-6736(05)79193-5. [DOI] [PubMed] [Google Scholar]
- 9.Kimsey H H, Waldor M K. CTXφ immunity: application in the development of cholera vaccines. Proc Natl Acad Sci USA. 1998;95:7035–7039. doi: 10.1073/pnas.95.12.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kimsey H H, Waldor M K. Vibrio cholerae hemagglutinin/protease inactivates CTXφ. Infect Immun. 1998;66:4025–4029. doi: 10.1128/iai.66.9.4025-4029.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Levine M M, Kaper J B, Black R E, Clements M L. New knowledge on pathogenesis of bacterial enteric infections as applied to vaccine development. Microbiol Rev. 1983;47:510–550. doi: 10.1128/mr.47.4.510-550.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Manoil C, Beckwith J. TnphoA: a transposon probe for protein export signals. Proc Natl Acad Sci USA. 1985;82:8129–8133. doi: 10.1073/pnas.82.23.8129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mekalanos J J. Duplication and amplification of toxin genes in Vibrio cholerae. Cell. 1983;35:253–263. doi: 10.1016/0092-8674(83)90228-3. [DOI] [PubMed] [Google Scholar]
- 14.Miller J H. A short course in bacterial genetics: a laboratory manual and handbook for Escherichia coli and relative bacteria. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1992. [Google Scholar]
- 15.Miller V L, Mekalanos J J. A novel suicide vector and its use in construction of insertion mutations: osmoregulation of outer membrane proteins and virulence determinants in Vibrio cholerae requires toxR. J Bacteriol. 1988;170:2575–2583. doi: 10.1128/jb.170.6.2575-2583.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pearson G D, Woods A, Chiang S L, Mekalanos J J. CTX genetic element encodes a site-specific recombination system and an intestinal colonization factor. Proc Natl Acad Sci USA. 1993;90:3750–3754. doi: 10.1073/pnas.90.8.3750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pearson G D N. Ph.D. thesis. Cambridge, Mass: Harvard University Medical School; 1989. [Google Scholar]
- 18.Schneider K, Beck C F. Promoter-probe vectors for the analysis of divergently arranged promoters. Gene. 1986;42:37–48. doi: 10.1016/0378-1119(86)90148-4. [DOI] [PubMed] [Google Scholar]
- 19.Sears C L, Kaper J B. Enteric bacterial toxins: mechanisms of action and linkage to intestinal secretion. Microbiol Rev. 1996;60:167–215. doi: 10.1128/mr.60.1.167-215.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sharma C, Maiti S, Mukhopadhyay A K, Basu A, Basu I, Nair G B, Mukhopadhyaya R, Das B, Kar S, Ghosh R K, Ghosh A. Unique organization of the CTX genetic element in Vibrio cholerae O139 strains which reemerged in Calcutta, India, in September 1996. J Clin Microbiol. 1997;35:3348–3350. doi: 10.1128/jcm.35.12.3348-3350.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Trucksis M, Michalski J, Deng Y K, Kaper J B. The Vibrio cholerae genome contains two unique circular chromosomes. Proc Natl Acad Sci USA. 1998;95:14464–14469. doi: 10.1073/pnas.95.24.14464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Waldor M K, Mekalanos J J. Lysogenic conversion by a filamentous phage encoding cholera toxin. Science. 1996;272:1910–1914. doi: 10.1126/science.272.5270.1910. [DOI] [PubMed] [Google Scholar]
- 23.Waldor M K, Rubin E J, Pearson G D, Kimsey H, Mekalanos J J. Regulation, replication, and integration functions of the Vibrio cholerae CTXφ are encoded by region RS2. Mol Microbiol. 1997;24:917–926. doi: 10.1046/j.1365-2958.1997.3911758.x. [DOI] [PubMed] [Google Scholar]
- 24.Waldor M K, Tschape H, Mekalanos J J. A new type of conjugative transposon encodes resistance to sulfamethoxazole, trimethoprim, and streptomycin in Vibrio cholerae O139. J Bacteriol. 1996;178:4157–4165. doi: 10.1128/jb.178.14.4157-4165.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]