Abstract
The production of succinoglycan by Sinorhizobium meliloti Rm1021 is required for successful nodule invasion by the bacterium of its host plant, alfalfa. Rm1021 produces succinoglycan, an acidic exopolysaccharide composed of an octasaccharide repeating unit modified with acetyl, succinyl, and pyruvyl moieties, in both low- and high-molecular-weight forms. Low-molecular-weight (LMW) succinoglycan, previously thought to consist of monomers, trimers, and tetramers of the repeating unit, has been reported as being capable of promoting the formation of nitrogen-fixing nodules by succinoglycan-deficient derivatives of strain Rm1021. We have determined that the three size classes of LMW succinoglycan species are in fact monomers, dimers, and trimers of the repeating unit and that the trimer is the species active in promoting nodule invasion. A detailed structural analysis of the components of LMW succinoglycan by using various chromatographic techniques, along with nuclear magnetic resonance analyses, has revealed that there is considerable heterogeneity within the LMW succinoglycan oligomers in terms of noncarbohydrate substitutions, and we have determined the structural basis of this heterogeneity.
The establishment of the nitrogen-fixing symbiosis between the bacterium Sinorhizobium meliloti (formerly known as Rhizobium meliloti) and its host plant Medicago sativa (alfalfa) involves exchanges of signals between the two partners (9, 23). First, plant flavonoids serve as signals to the bacteria to trigger the expression of nod genes, leading to the synthesis of lipochitooligosaccharide Nod factors. In turn, the Nod factors secreted by the bacteria act as signal molecules to the host plant that elicit root hair curling and formation of root nodules. The bacteria invade the nodules via tubes of plant origin called infection threads and, after being released into cells in the interior of the nodules, differentiate into bacteroids and begin to fix atmospheric nitrogen. Genetic analyses have shown that succinoglycan, the exopolysaccharide (EPS) produced by wild-type S. meliloti Rm1021, is required for successful nodule invasion by the bacteria, as indicated by formation of large, elongated pink nodules (8, 10, 17, 20, 22, 24). Mutants of Rm1021, such as exoY, that are deficient in the production of succinoglycan (27) form primarily ineffective, “empty” white nodules devoid of bacteria or bacteroids (8, 20, 25). Using S. meliloti strains that express green fluorescent protein as an indicator, we have further demonstrated that the defect of nodule invasion of exo mutants is apparently due to the failure of bacteria to elicit and support the formation of infection threads (5). Although it is well established that flavonoids and Nod factors function as signals, the role of EPS in nodule invasion is not yet clear. EPS may function as a signaling molecule, triggering a developmental response in the plant or regulating host defense responses. Alternatively, EPS may play a structural role, benefiting the bacterium by enabling attachment to surfaces, improving nutrient acquisition, or providing protection from environmental stresses and host defenses.
Succinoglycan produced by S. meliloti is an acidic polysaccharide composed of an octasaccharide subunit. Early structural studies of succinoglycan indicated that the octasaccharide repeating unit consists of one galactose (Gal) at the reducing end and seven glucose (Glc) residues. The subunits are polymerized in a fashion that results in a tetrasaccharide backbone with the sequence -4Glcβ-1,4-Glcβ-1,4Glcβ-1,3Galβ-1 and a tetrasaccharide side chain with the sequence Glcβ-1,3-Glcβ-1,3-Glcβ-1,6-Glcβ-1,6-. The repeating unit also carries pyruvyl, succinyl, and acetyl modifications (1, 14–16, 33). Recent analyses with nuclear magnetic resonance (NMR) and mass spectrometry (MS) techniques have confirmed most of the structural details of the octasaccharide subunit and assigned the location of the noncarbohydrate substituents in the molecule (6, 26). Thus, it has been determined that the acetyl group is located at the C-6 position of the third sugar residue from the reducing terminus; the succinyl group is located at the C-6 position of the seventh sugar residue; and the pyruvyl group is linked to the eighth sugar residue through a 4,6-ketal linkage.
S. meliloti Rm1021 produces both high-molecular-weight (HMW) and low-molecular-weight (LMW) succinoglycan (2, 18, 33). The LMW fraction of succinoglycan is of particular interest because past studies have reported that the LMW succinoglycan, rather than the HMW succinoglycan, is able to restore the ability of invasion-deficient S. meliloti mutants to invade nodules (2, 29). Further analysis by Battisti et al. (2) indicated that the largest LMW succinoglycan oligomer, which was estimated to be a tetramer of the octasaccharide subunit, was the symbiotically active species. However, during our recent study of succinoglycan biosynthesis, we found that the trimer of the succinoglycan octasaccharide subunit is the largest LMW oligomer that can be detected (11).
In order to perform further structure-function analyses, we have carried out a detailed structural characterization of the LMW succinoglycan. Using various chromatographic techniques, along with NMR analyses, we have isolated and characterized most of the oligosaccharide components in the LMW fraction. Our new data confirm our previous assessment that the LMW succinoglycan is composed of monomers, dimers, and trimers of the succinoglycan octasaccharide subunit and demonstrate that the trimer is the active species that is active in promoting nodule invasion. Furthermore, our more-detailed analyses of the LMW fraction of succinoglycan have revealed that there is a considerable heterogeneity within the monomer, dimer, and trimer species in terms of noncarbohydrate substitutions, and we have determined the structural basis of this heterogeneity.
MATERIALS AND METHODS
Production of succinoglycan.
S. meliloti Rm1021 was grown in a glutamate–d-mannitol–salts (GMS) medium (pH 7.0) supplemented with biotin, thiamine, and trace elements as described by Zevenhuizen and van Neerven (33). Typically, 1 liter of the GMS medium in a 2-liter Erlenmeyer flask was inoculated with 10 ml of Rm1021 overnight culture and cultivated at 30°C for 5 days with shaking (250 rpm). Cells were centrifuged at 20,000 × g for 30 min, and the clear culture supernatant that contains the secreted exopolysaccharide was lyophilized. For the separation of HMW and LMW succinoglycans, the dried material was suspended in 200 ml of 0.1 M NaCl, to which 3 volumes of ethanol was added with stirring. The HMW succinoglycan came out as a gelatinous precipitate and was collected by centrifugation at 6,000 × g for 30 min. The LMW succinoglycan that remained in the supernatant was then precipitated by the addition of another 7 volumes of ethanol, and the LMW succinoglycan was collected by centrifugation.
Gel filtration chromatography.
The chromatography was performed on a column (1.5 by 90 cm) of Bio-Gel P-6 (fine mesh; Bio-Rad) or a column (1.5 by 90 cm) of Sephadex G-75 (superfine; Sigma), which was preequilibrated and eluted with pyridinium acetate buffer (0.1 M; pH 5.0). Fractions (1.6 ml) were collected with an LKB 2070 Ultrorac II collector. The void volume was determined with blue dextran (2,000 kDa), and the salt volume was determined with glucose. Carbohydrates were assayed by the anthrone-sulfuric acid method (21). Fractions were pooled and lyophilized. The reducing sugar content of the isolated succinoglycan was determined by the neocuproine assay (4).
DEAE anion-exchange chromatography.
The monomers, dimers, and trimers of the succinoglycan octasaccharide subunit separated by Bio-Gel P-6 chromatography were further fractionated on a column (1.5 by 48 cm) of DEAE Sephadex A-25 (Sigma) that was preequilibrated with 5 mM KCl in a MOPS [3-(N-morpholino)propanesulfonic acid] buffer (10 mM; pH 7.0). The individual succinoglycan samples were loaded onto the column and eluted with the following KCl linear gradients: 600 ml of 5 to 500 mM KCl for the trimers, 600 ml of 5 to 400 mM KCl for the dimers, and 400 ml of 5 to 250 mM KCl for the monomers. Fractions (4 ml) were collected and assayed by the anthrone-sulfuric acid method. Peaks were pooled, thoroughly dialyzed against water with a SpectraPor dialysis membrane tube (no. 6; molecular weight cutoff, 1,000; The Spectrum Companies), and lyophilized.
High-performance anion-exchange chromatography coupled with pulsed amperometric detection (HPAEC-PAD).
A Dionex DX500 chromatography system (Dionex Corp., Sunnyvale, Calif.) equipped with a pulsed amperometric detector (ED 40; Dionex Corp.) was used. LMW succinoglycan samples were analyzed on a CarboPac PA-100 column (4 by 250 mm; Dionex Corp.) by using a gradient of NaNO3 in acetate buffer: eluent A, 10 mM sodium acetate (NaOAc) buffer (pH 5); eluent B, 500 mM NaNO3 in 10 mM NaOAc buffer (pH 5). The gradients were 0 to 3 min with 1.5% eluent B and 3 to 30 min with 1.5 to 40% B, and the flow rate was 1 ml/min. The PAD was operated at a 0.1-μC sensitivity by using the following waveforms (potentials and durations): E1 = +0.05 V (T1 = 0 to 0.4 s), E2 = +0.75 V (T2 = 0.41 to 0.6 s), and E3 = −0.15 V (T3 = 0.61 to 1 s). A post-column chromatography addition of 0.4 N NaOH (0.5 ml/min) was applied to ensure highly sensitive detection. The resulting chromatographic data were integrated and plotted by using the Dionex PeakNet system (Dionex Corp.). Monosaccharide analysis was performed on a CarboPac-MA1 column as previously described (11).
NMR analysis.
NMR experiments were performed with a Varian Unity 500 spectrometer with a proton frequency of 500.0 MHz and a 13C frequency of 125.6 MHz. All spectra were acquired in D2O at 70°C. Proton and carbon chemical shifts were referenced relative to the previous assignments (6). 1H-13C heteronuclear single-quantum coherence (HSQC) spectra were recorded in a gradient-selected phase-sensitive mode. Spectral widths in the 1H and 13C dimensions were 7 and 120 ppm, respectively. HSQC spectra were obtained with a total of 512 real points with 16 scans for each point along t1 and 704 points along t2. The data were zero filtered in both dimensions to a 1K×2K matrix before the Fourier transform step and processed by using the Varian software.
Enzymatic degradation of succinoglycan.
Succinoglycan samples were hydrolyzed to the octasaccharide subunits with a specific succinoglycan depolymerase from Cytophaga arvensicola (14). First, 1 mg of the isolated samples was dissolved in 1 ml of an acetate buffer (50 mM; pH 5.6) and treated with 20 μl of the depolymerase preparation (14). Then, the solution was incubated at 30°C for 24 h, and the product was either purified by Bio-Gel P-6 chromatography or directly analyzed by HPAEC.
Deacylation of succinoglycan.
A solution of succinoglycan samples in 10 mM KOH was incubated at 20°C for 6 h. The solution was then neutralized with 0.1 M HCl, dialyzed against water, and lyophilized.
Rescue experiments.
M. sativa cv. Iroquois was obtained from Agway (Plymouth, Ind.). The seedlings were grown on nitrogen-free Jensen’s agar (40 ml per plate). Spot inoculations were performed as described by González et al. (13) and modified as follows: 1 μg of each succinoglycan sample was mixed with about 4,000 Rm7210 (exoY210::Tn5) cells (from an overnight culture in Luria-Bertani broth with 2.5 mM MgSO4 and 2.5 mM CaCl2) in a final volume of 4 μl and applied directly to a recently germinated root in the region of emerging root hairs. At least 30 plants were used for each treatment, including the control. The plants were scored at 5 weeks for the presence of pink nodules. To eliminate any experimenter bias, scoring was performed in a blinded fashion.
RESULTS
LMW succinoglycan consists of monomers, dimers, and trimers of the succinoglycan octasaccharide subunit, and the trimer is the symbiotically active species.
When growing in a glutamic acid-mannitol-salts medium, S. meliloti Rm1021 produces and secretes both HMW succinoglycan, consisting of hundreds to thousands of the octasaccharide subunits, and LMW succinoglycan, which was originally reported to consist of monomers, trimers, and tetramers of the octasaccharide subunit (2). The LMW succinoglycan was shown to be symbiotically active for nodule invasion (2, 29), and Battisti et al. (2) further reported that the active species in the LMW fraction of succinoglycan was a highly charged tetramer of the succinoglycan octasaccharide subunit. However, we recently concluded that the LMW fraction of succinoglycan is instead composed of monomers, dimers, and trimers of the succinoglycan octasaccharide subunit and that no tetramers are present (11). In order to gain further structure-function insights into the biological role(s) of succinoglycan oligosaccharides in promoting nodule invasion, we set out to purify the succinoglycan species in the LMW fraction to determine which species is biologically active and to carry out a detailed structural characterization of that succinoglycan species.
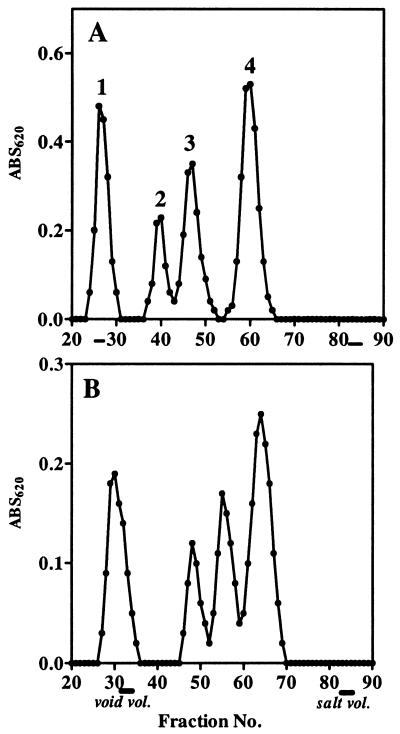
Fractionation of the concentrated supernatant of an S. meliloti Rm1021 culture on a gel filtration column of Bio-Gel P-6 yielded four major carbohydrate peaks (Fig. 1A). Chromatography of the supernatant on a column of Sephadex G-75 also gave a similar fractionation pattern (Fig. 1B). Each of the four carbohydrate peaks represents a succinoglycan species because none of them are produced by the succinoglycan production-deficient S. meliloti exoY mutant, which is blocked at the first step of succinoglycan biosynthesis (27). Compositional analysis of the peaks by acidic hydrolysis and subsequent HPAEC-PAD analyses revealed that peaks 1 to 4 consisted of glucose and galactose in a molar ratio of 7:1, regardless of their molecular size, further confirming that they are succinoglycan species composed of the octasaccharide subunit(s). Considering the fractionation range of the Bio-Gel P-6 (1,000 to 6,000 Da) and Sephadex G-75 (dextrans, 1,000 to 50,000 Da), peak 1, which was eluted in the void volume of each column, was assumed to be the HMW succinoglycan, whereas peaks 2 to 4, which were eluted within the fractionation range of the Bio-Gel P6, should be the LMW succinoglycan. Using the succinoglycan from peaks 2 to 4, we examined the ability of these various molecular weight species to rescue the nodule invasion defect of an S. meliloti exoY mutant, which is deficient in succinoglycan production. Our results indicated that peak 2, which we had previously assigned as the succinoglycan trimer (11), was the only species that clearly showed substantial ability to restore nodule invasion when compared to the exoY background (25).
FIG. 1.
Gel filtration chromatography of the secreted succinoglycan. (A) Fractionation on Bio-Gel P-6; (B) Fractionation on Sephadex G-75.
Rescue experiments with the succinoglycan species isolated from Bio-Gel P6 showed the percentage of plants with a visible Fix+ phenotype to be as follows: with exoY alone, i.e., strain Rm7210 (exoY210::Tn5), 14%; with exoY plus peak 2, 59%; with exoY plus peak 3, 23%; and with exoY plus peak 4, 23%. A total of 1 μg (glucose equivalent) of each fraction was applied for each treatment as described in Materials and Methods. Plants with a visible Fix+ phenotype had some healthy, dark-green leaves and each formed at least one large, elongated pink nodule. The results presented here are the means of two independent experiments. Background levels of 3 to 22% were previously reported with strain Rm0540, a strain carrying a Tn5 mutation within the exoY gene, at 5 to 6 weeks postinoculation (see reference 25).
Since Battisti et al. (2) previously reported that a succinoglycan tetramer is active in promoting nodule invasion, a result which was contradictory to our previous study (11) and our present activity assay, we felt that it was very important to reexamine the molecular size distribution of LMW succinoglycan. We therefore measured the molar ratio of the total carbohydrate to the reducing sugar of each succinoglycan species, which was the same analytical approach previously used by Battisti et al. (2) in their determination of the degree of polymerization (d.p.) of LMW fractions. Based on the assumption that each molecule of the succinoglycan species contains a single reducing end and that each repeating subunit contains eight sugar residues, our results (Table 1) suggested that peak 1 (ratio, 3,640:1; observed d.p., 455) was the HMW succinoglycan with more than 450 octasaccharide repeating units in the molecule, peak 2 (ratio, 21:1; observed d.p., 2.6) was likely to be the trimer of succinoglycan octasaccharide subunit, peak 3 (ratio, 16:1; observed d.p., 2) was the dimer, and peak 4 (ratio, 7.2:1; observed d.p., 0.9) was the monomer of the succinoglycan octasaccharide subunit. The results we obtained by this method agreed well with our previous HPAEC determination of the d.p., in which we compared the ratios of galactose to galactitol after NaBH4 reduction and subsequent acidic hydrolysis of the various LMW succinoglycan species (11). In our analyses, we have observed that Rm1021 produces and secretes small amounts of cyclic β-1,2-glucans into the medium, which appear as a small, broad shoulder at the right side of peak 3 (Fig. 1). Digestion of peak 3 from the first gel filtration with succinoglycan depolymerase from Cytophaga arvensicola (14) converted peak 3 into monomers but left a small, broad peak at the right side position of the original peak 3 when chromatographed on the Bio-Gel P6 column (data not shown). Compositional analysis of this depolymerase-resistant peak showed that it consisted only of glucose. The composition, together with the size (position) in the Bio-Gel P6 chromatography, strongly suggested that this contaminating peak consisted of cyclic glucans (3). In our analyses, we have rechromatographed the isolated materials on the Bio-Gel P6 twice to ensure that the LMW succinoglycan species used were not contaminated with cyclic glucans. Therefore, it is possible that a small amount of contaminating cyclic glucans, which contributes to the total carbohydrate amount but lacks a reducing end, might have distorted the total carbohydrate/reducing-end ratio obtained by Battisti et al. (2). Interestingly, we did not detect the presence of tetramers or higher oligomers between the trimers and the HMW succinoglycan (Fig. 1). However, dimer is clearly present as a component of the LMW fraction. Taken together, our results indicate that succinoglycan oligosaccharides originally assigned as tetramers (2) are actually trimers of the octasaccharide subunit and that the oligosaccharides originally assigned as trimers (2) are, in fact, dimers of succinoglycan octasaccharide subunits. Thus, the trimer of the succinoglycan octasaccharide subunit is the species that is able to partially restore the ability of an S. meliloti exoY mutant to invade nodules.
TABLE 1.
Measurement of the molar ratio of the total carbohydrate to the reducing sugar of the isolated samples of succinoglycana
| Sample | Total carbohydrate (μg)b | Reducing sugar (μg)c | Molar ratio | Estimated d.p. |
|---|---|---|---|---|
| Peak 1 | 182 | 0.05d | 3,640:1 | 455 |
| Peak 2 | 130 | 6.21 | 21:1 | 2.6 |
| Peak 3 | 123 | 7.56 | 16.3:1 | 2.0 |
| Peak 4 | 123 | 17.3 | 7.1:1 | 0.9 |
Based on 100-μl samples that contain 100 to 200 μg of succinoglycan (glucose equivalent).
Measured by anthrone-sulfuric acid assay.
Measured by neocuproine assay.
Not accurate because the reading is at the lower limit.
The LMW forms of succinoglycan exhibit a great structural heterogeneity in terms of noncarbohydrate substitutions.
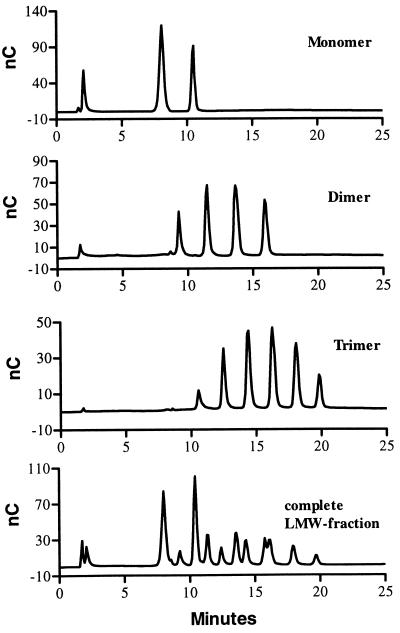
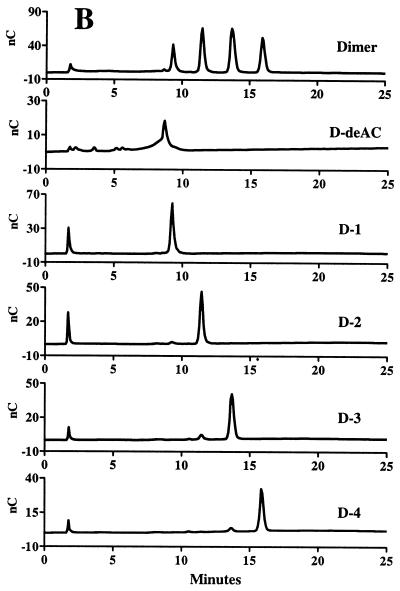
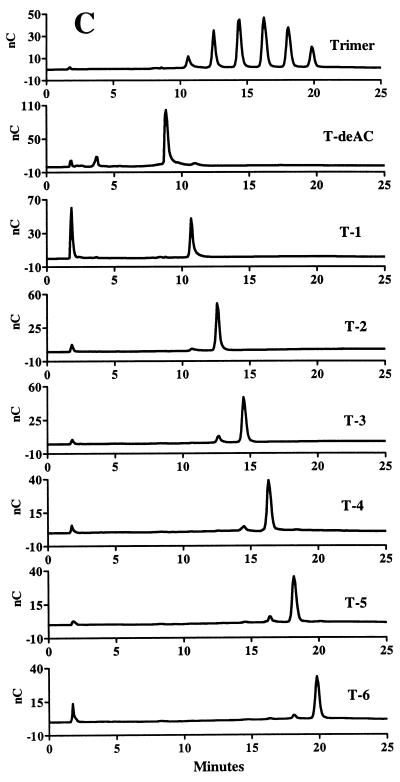
In the course of our investigations, an alternative HPAEC-PAD method was explored for analyzing the LMW succinoglycan. Since succinoglycan contains alkaline-labile succinyl and acetyl groups which are removed during standard alkaline HPAEC conditions, a mild chromatographic condition was developed to preserve these acyl groups. Using a CarboPac PA-100 column, we found that a linear gradient of NaNO3 in an acetate buffer (pH 5.0) was able to resolve the isolated monomers, dimers, and trimers into three, four, and six peaks, respectively (Fig. 2). The observation indicated that there was a great structural heterogeneity within these three size classes of succinoglycan oligosaccharides. Since HPAEC separation is based mainly on the differences in the number of inherent negative charges in the molecules under this mild chromatographic condition, the observation of multiple peaks under the given chromatographic conditions suggested that the structural heterogeneity most likely came from various numbers of succinyl and/or pyruvyl groups, each of which would contribute one negative charge to the molecule.
FIG. 2.
HPAEC analysis of LMW succinoglycan.
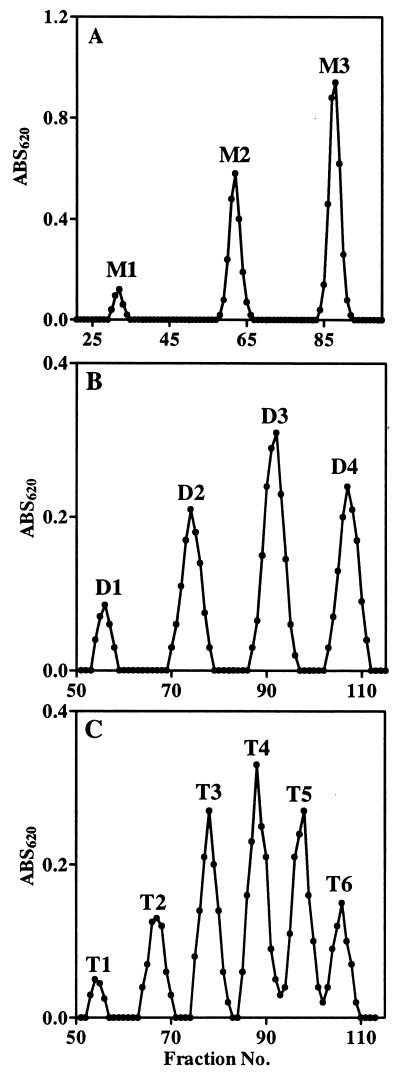
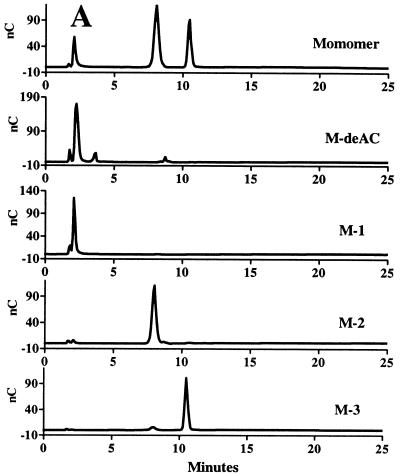
In order to obtain the large quantities of these various species needed for further structural analysis, we isolated the individual species by using preparative anion-exchange chromatography on a DEAE A-25 column. We found that, by using a different linear gradient of KCl in a MOPS buffer (pH 7), most of the species in the LMW fractions of succinoglycan could be separated. As shown in Fig. 3, three (M1, M2, and M3), four (D1 to D4), and six (T1 to T6) succinoglycan species were successfully isolated from the succinoglycan monomers, dimers, and trimers, respectively. Reanalysis of the species isolated from the monomers revealed that M1, M2, and M3 corresponded to the three peaks detected in the HPAEC of monomers (Fig. 4A). Treatment of the unfractionated monomers containing M1, M2, and M3 with KOH to remove the succinyl and acetyl groups in the molecule gave the compound M-deAC, which appeared as a single peak under the chromatographic condition we employed (Fig. 4A). One-dimentional 1H-NMR analyses of the M-deAC (purified by Bio-Gel P6) showed the presence of pyruvyl group at 1.46 ppm as a singlet and the absence of acetyl (supposed at ca. 2.1 ppm) and succinyl (supposed at 2.6 to 2.7 ppm) groups. Integration of the anomeric protons (4.4 to 5.3 ppm) and pyruvyl protons gave a ratio of anomeric to pyruvyl protons of 8:3.43. Because an octasaccharide subunit has eight anomeric protons and one pyruvyl group contains three protons, the observed integration ratio, together with the fact that the ketal linkage of the pyruvyl modification is not base labile, strongly suggested that all of the monomers detected by our chromatographic procedure, M1 to M3, contained one pyruvyl group per molecule and that the structural heterogeneity was most likely a result of a difference in the degree of succinylation. Since M-deAC appeared at the same position as M1 under the same chromatographic conditions (Fig. 4A, M-deAC and M1), this result suggested that the monomer M1 was a form of the monomer that carried no succinyl group. It further suggested that M2 was most likely a form of the monomer carrying one succinyl group and that M3 was most likely a form of the monomer carrying two succinyl groups.
FIG. 3.
Chromatographic separation of LMW succinoglycan on Sephadex DEAE A-25. The column was eluted with a linear gradient of KCl in a MOPS buffer (10 mM; pH 7). Fractions (4 ml) were collected, and carbohydrates were assayed by anthrone-sulfuric acid method. (A) Monomers (elution, 400 ml of 5 to 250 mM KCl); (B) Dimers (elution, 600 ml of 5 to 400 mM KCl); (C) Trimers of succinoglycan (elution, 600 ml of 5 to 500 mM KCl).
FIG. 4.
HPAEC analysis of the isolated succinoglycan oligosaccharides. (A) Monomers; (B) Dimers; (C) Trimers.
Further analyses similarly suggested that the different species of the dimers and trimers simply differed in the numbers of succinyl groups that they carried. Reanalysis of the four DEAE-isolated dimers showed that species D1 to D4 corresponded to the four dimer peaks in the HPAEC (Fig. 4B). Treatment of the mixed dimers with KOH converted all four species into a new peak (D-deAC) that was eluted at the position preceding D1 (Fig. 4B), suggesting that D1 to D4 were forms of the dimer carrying one to four succinyl groups in the molecule, respectively. Similarly, the six isolated trimer species matched the six peaks in the HPAEC analysis of the trimers, and removal of the succinyl and acetyl groups by KOH treatment gave a new species (T-deAC) that appeared at the position preceding T1 (Fig. 4C), suggesting that T1 to T6 were forms of the trimer carrying one to six succinyl groups in the molecule, respectively.
1H-NMR analyses of the compounds isolated by the DEAE chromatography of the monomers, dimers, and trimers allowed us to obtain the ratios of pyruvyl to succinyl to acetyl groups in those molecules (Table 2). These NMR data indicated that the various forms of the succinoglycan oligomers that we had separated on the basis of charge did indeed correspond to the numbers of succinyl modifications they carry and that the assignments we had proposed were correct. Interestingly, in all of the succinoglycan species, the level of acetylation was relatively constant at ca. 0.7 per octasaccharide repeating subunit, whereas the degree of succinylation varied markedly. The HPAEC analysis also revealed that only trace amounts of the presumed nonsuccinylated dimer and trimer were produced (Fig. 4B and C). These species were not isolated from the DEAE-A25 because of insufficient quantity.
TABLE 2.
Molar ratios of the noncarbohydrate substituents in the isolated oligosaccharidesa
| Sample | Ratios of substituentsb
|
||
|---|---|---|---|
| Pyr (1.46 ppm) | Ac (2.15 ppm) | Suc (2.63 to 2.70 ppm) | |
| M1 | 1 | 0.68 (0.68)c | 0 (0)d |
| M2 | 1 | 0.67 (0.67) | 1 (1) |
| M3 | 1 | 0.71 (0.71) | 2.1 (2) |
| D1 | 2 | 1.29 (0.65) | 0.85 (1) |
| D2 | 2 | 1.28 (0.64) | 1.8 (2) |
| D3 | 2 | 1.33 (0.66) | 2.9 (3) |
| D4 | 2 | 1.37 (0.69) | 3.9 (4) |
| T1 | 3 | 2.1 (0.70) | 1.1 (1) |
| T2 | 3 | 2.0 (0.70) | 1.7 (2) |
| T3 | 3 | 1.96 (0.65) | 2.9 (3) |
| T4 | 3 | 2.0 (0.66) | 3.7 (4) |
| T5 | 3 | 2.0 (0.66) | 4.8 (5) |
| T6 | 3 | 2.0 (0.66) | 5.9 (6) |
The ratios were determined from the integration of the acetyl, pyruvyl, and succinyl signals in the one-dimentional 1H-NMR spectra of the isolated succinoglycan oligosaccharides. The 1H-NMR spectra were recorded in D2O at 70°C.
Pyr, pyruvyl; Ac, acetyl; Suc, succinyl.
Numbers in the parentheses are the average degree of acetylation per subunit.
Numbers in the parentheses are the numbers of succinyl groups per molecule.
The second succinyl group in the octasaccharide subunit is located at the C-6 of the sixth sugar residue from the reducing end.
Previous MS and NMR analyses were carried out on the monomer of the succinoglycan octasaccharide subunit obtained from HMW succinoglycan through enzymatic degradation (6, 26). The octasaccharide subunit obtained in this fashion was shown to contain one succinyl group, which was assigned to the C-6 of the seventh sugar residue from the reducing end. Although previous MS and NMR studies have implicated the presence of a small amount of disuccinylated octasaccharide subunit in the analytical samples, this species had never been isolated and its structure had not been elucidated. In contrast, our chromatographic analysis clearly demonstrated that within the LMW fraction of succinoglycan, the disuccinylated form of the monomer of the octasaccharide subunit, represented one of the major components (M3 in Fig. 3A).
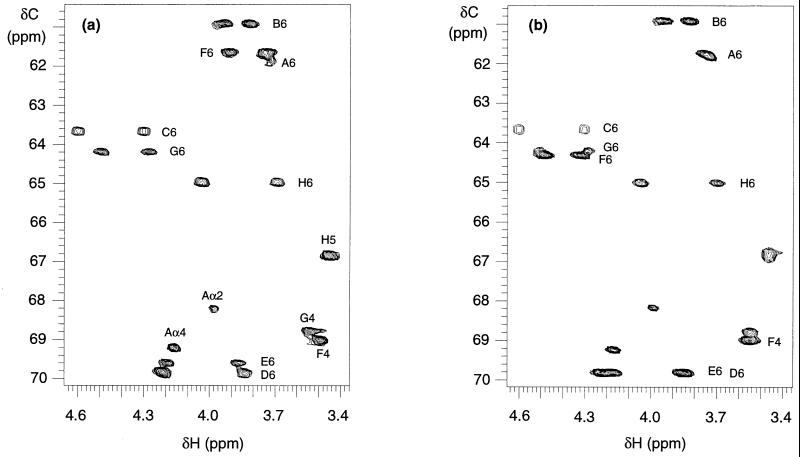
Since the location of the second succinyl group in the octasaccharide subunit had not been assigned, we measured the 1H-13C HSQC spectra of the M2 and M3 species (Fig. 5A and B), comparative analyses of which allowed us to assign the second succinyl group to the C-6 position of the sixth sugar residue (see Fig. 6A for structures). For the ease of comparison, the sugar residues in M2 and M3 were denoted A to H, respectively, starting from the reducing end galactose residue. In the 1H-13C HSQC spectrum of M2, the 1H and 13C chemical shifts matched well with those of the octasaccharide subunit reported previously (6), and the cross-peaks were thus assigned accordingly (Fig. 5A). The 1H-13C HSQC spectrum of M3 showed several differences from that of M2. The most significant difference is the downfield shift of the proton and carbon signals of the F6 position in M3 (here F6 denotes the six-position of sugar F, the sixth sugar residue in the molecule). The proton resonances shifted from 3.91 and 3.74 ppm in M2 to 4.51 and 4.29 ppm in M3, and its carbon signal shifted from 61.66 to 64.24 ppm. These downfield shifts indicated that the C-6 of the sugar residue F in M3 was substituted. Thus, the second succinyl group should be located in this position, that is, the C-6 position of the sixth sugar residue. This conclusion was further supported by the fact that, in the HSQC spectrum of M3 (Fig. 5B), the F6 cross-peaks nearly overlapped with that of G6 (the 6-position of the seventh sugar residue), which carried the first succinyl substitution. In addition, two minor differences were observed at the F4 and E6 positions between M2 and M3. The proton chemical shift of F4 moved to 3.55 ppm in M3, 0.06 ppm downfield shift from that of M2. The carbon signal of E6 showed at 69.83 ppm in M3, ca. 0.2 ppm downfield from that of M2. These minor differences are most likely due to the conformational change caused by the second succinylation in M3. It is worthwhile to note that in M2, the succinyl group was located almost exclusively at the seventh sugar residue (sugar G) instead of at the sixth sugar residue (sugar F), suggesting that succinylation at the sixth sugar residue might require the presence of a succinyl group at the seventh sugar residue.
FIG. 5.
Sections of the 1H-13C HSQC NMR spectra of M2 (A) and M3 (B). Cross-peaks are labeled where B6 denotes the 6-position of residue B. The F6 signals shifted downfield in M3, whereas most of the other signals are about the same in the two spectra.
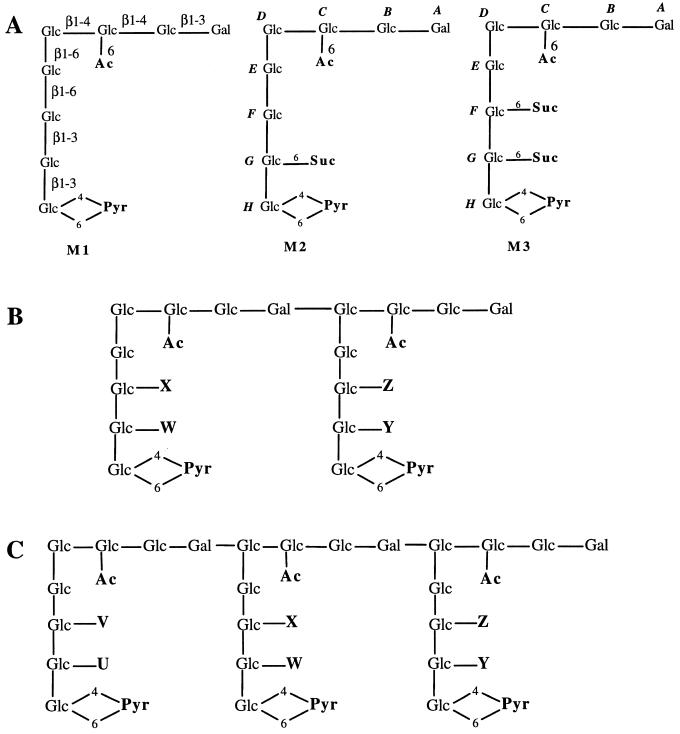
FIG. 6.
Proposed structures of succinoglycan monomers and oligomers. (A) Monomers; (B) Dimers; (C) Trimers. The letters U, V, W, X, Y, and Z indicate the sites for possible succinylation. Abbreviations: Glc, glucose; Gal, galactose; Ac, acetyl; Pyr, pyruvyl; Suc, succinyl.
Analysis of the distribution of succinyl groups within the molecule of dimers or trimers reveals a relatively random succinylation pattern.
As described above, the octasaccharide subunit can carry a succinyl modification at two sites. To determine how the succinyl groups are distributed within those oligosaccharides that consist of more than one octasaccharide subunit but that carry fewer than two succinyl groups per subunit, as in the case of dimers D1 to D3 and trimers T1 to T5, the various dimers and trimers were enzymatically hydrolyzed to octasaccharide subunits, and the relative ratios of the resulting unsuccinylated, monosuccinylated, and disuccinylated subunits (S0, S1, and S2, respectively) were determined by HPAEC. The possible structures for the dimers D1 to D4 and the trimers T1 to T6 were shown in Fig. 6B and C, respectively. The HPAEC analyses (Table 3) indicated that the distribution of succinyl groups within the molecule was relatively random. For example, in the case of dimer D2, which contained two succinyl groups in the molecule, it was a mixture of dimers with the two succinyl groups distributed at the four possible positions. There are on the average 20, 61, and 19% of the octasaccharide subunits which were unsuccinylated, monosuccinylated, and disuccinylated, respectively. Similar results were observed for the other dimers and trimers analyzed (Table 3).
TABLE 3.
HPAEC determination of the succinyl distributions within the oligomersa
| Sample | % Compositional subunitsb
|
||
|---|---|---|---|
| S0 | S1 | S2 | |
| D1 | 50 | 50 | 0 |
| D2 | 20 | 61 | 19 |
| D3 | 0 | 50 | 50 |
| T1 | 69 | 31 | 0 |
| T2 | 43 | 50 | 7 |
| T3 | 25 | 56 | 19 |
| T4 | 5 | 53 | 42 |
| T5 | 3 | 34 | 63 |
Samples D1 to D3 and T1 to T5 were degraded to the compositional octasaccharide subunits by the succinoglycan depolymerase from Cytophaga. The products were analyzed and quantified by Dionex HPAEC.
S0, S1, and S2 represent the octasaccharide subunits containing 0, 1, and 2 succinyl groups, respectively. The percentages were calibrated from standard samples (M1, M2, and M3). The molar responses in PAD detection under the HPAEC-PAD condition for S0, S1, and S2 are 1:1:0.66. The reason for the lower response for S2 is not clear.
The LMW fraction of succinoglycan has a higher degree of succinylation than HMW succinoglycan.
1H NMR analysis of HMW and LMW succinoglycans indicated that the two classes of succinoglycan had substantially different degrees of succinylation (Table 4). While the average degree of succinylation per octasaccharide repeating unit in HMW succinoglycan was 0.93, the succinylation for trimers, dimers, and monomers per repeating unit was 1.25, 1.40, and 1.43, respectively, indicating that the LMW succinoglycan had a higher degree of succinylation than the HMW succinoglycan.
TABLE 4.
Molar ratios of the noncarbohydrate substituents in the LMW and HMW succinoglycan speciesa
| Sample | Noncarbohydrate molar ratios
|
||
|---|---|---|---|
| Pyr | Suc | Ac | |
| Monomer | 1 | 1.43 | 0.71 |
| Dimer | 1 | 1.40 | 0.70 |
| Trimer | 1 | 1.25 | 0.70 |
| HMW-SGb | 1 | 0.93 | 0.70 |
The ratios were determined from the integration of the acetyl (Ac), pyruvyl (Pyr), and succinyl (Suc) signals in the one-dimentional 1H-NMR spectra of the isolated succinoglycan species. The 1H-NMR spectra were recorded in D2O at 70°C.
HMW succinoglycan.
DISCUSSION
Several pieces of evidence suggest that specific LMW forms of S. meliloti EPS or capsular polysaccharides, including succinoglycan, may act as bacterial signaling molecules for nodule invasion (2, 7, 12, 28, 29). Therefore, knowing the identity of the biologically active oligosaccharides is important for further biological and mechanistic studies of this class of molecules. In this study, we have isolated and characterized most of the oligosaccharide species in the LMW fraction of succinoglycan by using various chromatographic techniques combined with NMR studies. Our results indicate that while the HMW succinoglycan consists of hundreds of octasaccharide repeating units, the LMW succinoglycan consists of only three kinds of oligosaccharides in terms of molecular size, namely, the monomers, dimers, and trimers of the octasaccharide subunits (Fig. 1 and Table 1). No tetramers or higher oligomers were detected in the LMW fraction. Furthermore, rescue assays that we have carried out with these three size classes of succinoglycan oligosaccharides indicate that the trimer of the succinoglycan octasaccharide subunit is the active species that can promote nodule invasion (see Results). Strikingly, our more-detailed analyses of these three size classes of succinoglycan oligosaccharides have revealed a great structural heterogeneity in terms of noncarbohydrate substitutions. We found that the acetylation was never complete but was essentially constant (ca. 0.7 acetyl residues per repeating unit) in all three LMW forms of succinoglycan and in the HMW polymer. However, the degree of succinylation varied greatly (Table 2). We isolated three monomer species (M1, M2, and M3), which were shown to contain 0, 1, and 2 succinyl modifications, respectively. Analyses of various dimer and trimer species indicated that repeating units with zero, one, and two succinyl modifications were also contained within the succinoglycan oligomers. Based on the 1H-13C HSQC NMR study, we have determined that the second succinyl group is located at the C-6 position of the sixth sugar residue from the reducing end. In the case of dimers, we isolated four species containing 1 to 4 succinyl groups in the molecule, respectively. Similarly, we isolated six trimers containing 1 to 6 succinyl groups in the molecule, respectively. Since an exoH mutant synthesizes succinoglycan that lacks succinyl modification (19), it is possible that the ExoH protein is responsible for the addition of succinyl groups to both the C-6 positions of the seventh and sixth sugars of the octasaccharide subunit. Alternatively, in the case of monomers, since succinylation of the sixth sugar is almost never seen in the absence of a succinyl group on the seventh sugar, it is possible that an exoH-dependent succinylation of the seventh sugar must precede a succinylation of the sixth sugar that is carried out by an as-yet-unidentified gene product.
Comparison of the HMW and LMW succinoglycans has revealed another interesting structural feature. As shown in Table 4, the LMW succinoglycan had a much higher degree of succinylation than the HMW succinoglycan, suggesting that the degree of succinylation may influence the ease of incorporating a succinoglycan octasaccharide subunit into the higher-molecular-weight polymer. Since exoH mutants are able to synthesize unsuccinylated succinoglycan, unsuccinylated subunits can evidently be incorporated into polymer. Similarly, subunits carrying a single succinyl group in the seventh sugar of the subunit are likewise efficiently incorporated into polymer by wild-type S. meliloti. Perhaps the relative absence of disuccinylated subunits in HMW succinoglycan is due to an ability of the protein machinery that synthesizes the HMW polymer to discriminate against such disuccinylated subunits and not incorporate them into polymer, since we observed that more than 90% of the repeating units in the HMW succinoglycan are monosuccinylated (data not shown). If so, the genetically separable system (11) that we have suggested for the synthesis of dimers and trimers must be able to incorporate such disuccinylated subunits. An untested but formal alternative is that disuccinylated subunits are initially incorporated into polymers but are subsequently removed by some type of quality control mechanism, for example, such as that represented by the 3′-5′ proofreading function of many DNA polymerases.
Alternatively, the substantial difference in succinylation may reflect the difference in susceptibility to cleavage of differentially succinylated succinoglycan to the succinoglycan depolymerases, ExoK and ExsH, that have been shown to contribute significantly to the generation of LMW succinoglycan (30, 31). Recently, we have also demonstrated that the succinyl and acetyl modifications influence the susceptibility of succinoglycan to cleavage by ExoK and ExsH. Thus, the acetyl modification decreases the susceptibility of succinoglycan toward ExoK- and ExsH-catalyzed cleavage, whereas the succinyl modification increases the susceptibility of succinoglycan to the ExoK- and ExsH-catalyzed hydrolysis (32).
The results we have reported here raise interesting questions with respect to the relationships between the structure and biological function of succinoglycan oligosaccharides. Specifically, which of the various forms of the trimer of the octasaccharide subunits are required for the interaction with the plant that promotes nodule invasion by succinoglycan-deficient mutants of S. meliloti Rm1021? Similarly, does the presence or the absence of the acetyl modification influence the ability of the trimer to promote this type of nodule invasion? Is the pyruvyl modification necessary for biological activity? Analysis of S. meliloti derivatives labeled with green fluorescent protein have shown that once the parental S. meliloti strain colonizes a curled root hair, the initiation of an infection thread and its extension through the root hair cell into the developing nodule occur with virtually 100% efficiency. In contrast, Rm1021 derivatives that produce unsuccinylated (exoH) or unacetylated (exoZ) succinoglycan are strikingly less efficient, with the exoH mutant having the more severe symbiotic deficiency (5). Thus, the ability to add these specific noncarbohydrate substituents is an important element in fine-tuning succinoglycan for its role in symbiosis. However, it is not yet clear whether the succinyl or the acetyl modification directly affects the interaction of the oligosaccharides with the plant or whether their role might be indirect. The latter possibility must be considered because both the exoH and exoZ mutations have profound effects on the molecular weight distribution of succinoglycan that is produced and apparently because the succinyl and acetyl modifications influence the degradation of succinoglycan by the S. meliloti-secreted succinoglycan-specific glycanases ExoK and ExsH (30–32). Testing the biological activity of the various forms of the trimer of octasaccharide subunits that we have identified should offer insights into whether these noncarbohydrate substituents of succinoglycan are directly required for the interaction with the plant during symbiosis, whether they act in an indirect fashion by influencing molecular weight distribution, or whether they perhaps do both. The very low quantities of oligosaccharides used in our invasion assays suggest that they function as a signal to the plant, so further studies may reveal how a bacterium might generate a signal to another cell by crafting particular oligosaccharides related to one of the extracellular or cell surface polysaccharides.
ACKNOWLEDGMENTS
We thank the members of our laboratory for stimulating discussions and critical reading of the manuscript.
This work was supported by Public Health Service grant GM 31030 (to G.C.W.) from the National Institutes of Health and by National Institutes of Health predoctoral training grant T32GM07287 (to B.P.).
REFERENCES
- 1.Aman P, McNeil M, Franzen L-E, Darvill A G, Albersheim P. Structural elucidation, using HPLC-MS and GLC-MS, of the acidic exopolysaccharide secreted by Rhizobium meliloti strain Rm1021. Carbohydr Res. 1981;95:263–282. [Google Scholar]
- 2.Battisti L, Lara J C, Leigh J A. Specific oligosaccharide form of the Rhizobium meliloti exopolysaccharide promotes nodule invasion in alfalfa. Proc Natl Acad Sci USA. 1992;89:5625–5629. doi: 10.1073/pnas.89.12.5625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Breedveld M W, Miller K J. Cyclic β-glucans of members of the family Rhizobiaceae. Microbiol Rev. 1994;58:145–161. doi: 10.1128/mr.58.2.145-161.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chaplin M F. Reducing sugar-neocuproine assay, page 3. In: Chaplin M F, Kennedy J F, editors. Carbohydrate analysis—a practical approach. Oxford, United Kingdom: IRL; 1986. [Google Scholar]
- 5.Cheng H-P, Walker G C. Succinoglycan is required for initiation and elongation of infection threads during nodulation of alfalfa by Rhizobium meliloti. J Bacteriol. 1998;180:5183–5191. doi: 10.1128/jb.180.19.5183-5191.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chouly C, Colquhuon I J, Jodelet A, York G, Walker G C. NMR studies of succinoglycan repeating-unit octasaccharides from Rhizobium meliloti and Agrobacterium radiobacter. Int J Biol Macromol. 1995;17:357–363. doi: 10.1016/0141-8130(96)81846-0. [DOI] [PubMed] [Google Scholar]
- 7.Djordjevic S P, Chen H, Batley M, Redmond J W, Rolfe B G. Nitrogen fixation ability of exopolysaccharide synthesis mutants of Rhizobium sp. strain NGR234 and Rhizobium trifolii is restored by the addition of homologous exopolysaccharides. J Bacteriol. 1987;169:53–60. doi: 10.1128/jb.169.1.53-60.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Finan T M, Hirsch A M, Leigh J A, Johansen E, Kuldau G A, Deegan S, Walker G C, Signer E R. Symbiotic mutants of Rhizobium meliloti that uncouple plant from bacterial differentiation. Cell. 1985;40:869–877. doi: 10.1016/0092-8674(85)90346-0. [DOI] [PubMed] [Google Scholar]
- 9.Fisher R F, Long S R. Rhizobium-plant signal exchange. Nature. 1992;357:655–660. doi: 10.1038/357655a0. [DOI] [PubMed] [Google Scholar]
- 10.Glazebrook J, Walker G C. A novel exopolysaccharide can function in place of the Calcofluor-binding exopolysaccharide in nodulation of alfalfa by Rhizobium meliloti. Cell. 1989;56:661–672. doi: 10.1016/0092-8674(89)90588-6. [DOI] [PubMed] [Google Scholar]
- 11.González J E, Semino C E, Wang L X, Castellano-Torres L E, Walker G C. Biosynthetic control of molecular weight in the polymerization of the octasaccharide subunits of succinoglycan, a symbiotically important exopolysaccharide of Rhizobium meliloti. Proc Natl Acad Sci USA. 1998;95:13477–13482. doi: 10.1073/pnas.95.23.13477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.González J E, Reuhs B L, Walker G C. Low molecular weight EPS II of Rhizobium meliloti allows nodule invasion in Medicago sativa. Proc Natl Acad Sci USA. 1996;93:8636–8641. doi: 10.1073/pnas.93.16.8636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.González J E, York G M, Walker G C. Rhizobium meliloti exopolysaccharides: synthesis and symbiotic function. Gene. 1996;179:141–146. doi: 10.1016/s0378-1119(96)00322-8. [DOI] [PubMed] [Google Scholar]
- 14.Harada T. Determination of the structure of β-d-glycans from strains of Agrobacterium and Rhizobium, 155–163. In: BeMiller J N, Manners D J, Sturgeon R J, editors. Methods in carbohydrate chemistry. New York, N.Y: John Wiley & Sons, Inc.; 1994. [Google Scholar]
- 15.Hisamatsu M, Abe J, Amemura A, Harada T. Structural elucidation of succinoglycan and related polysaccharides from Agrobacterium and Rhizobium by fragmentation with two special β-d-glycanases and methylation analysis. Agric Biol Chem. 1980;44:1049–1055. [Google Scholar]
- 16.Jansson P-E, Kenne L, Lindberg B, Ljunggren H, Ruden U, Svensson S. Demonstration of an octasaccharide repeating unit in the extracellular polysaccharide of R. meliloti by sequential degradation. J Am Chem Soc. 1977;99:3812–3815. doi: 10.1021/ja00453a049. [DOI] [PubMed] [Google Scholar]
- 17.Keller M, Müller P, Simon R, Pühler A. Rhizobium meliloti genes for exopolysaccharide synthesis and nodule infection located on megaplasmid 2 are actively transcribed during symbiosis. Mol Plant-Microbe Interact. 1988;1:267–274. [Google Scholar]
- 18.Leigh J A, Lee C C. Characterization of polysaccharides of Rhizobium meliloti exo mutants that form ineffective nodules. J Bacteriol. 1988;170:3327–3332. doi: 10.1128/jb.170.8.3327-3332.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leigh J A, Reed J W, Hanks J F, Hirsch A M, Walker G C. Rhizobium meliloti mutants that fail to succinylate their Calcofluor-binding exopolysaccharide are defective in nodule invasion. Cell. 1987;51:579–587. doi: 10.1016/0092-8674(87)90127-9. [DOI] [PubMed] [Google Scholar]
- 20.Leigh J A, Signer E R, Walker G C. Exopolysaccharide-deficient mutants of Rhizobium meliloti that form ineffective nodules. Proc Natl Acad Sci USA. 1985;82:6231–6235. doi: 10.1073/pnas.82.18.6231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Loewus F A. Improvement in the anthrone method for determination of carbohydrates. Anal Chem. 1952;24:219. [Google Scholar]
- 22.Long S, Reed J W, Himawan J, Walker G C. Genetic analysis of a cluster of genes required for synthesis of the Calcofluor-binding exopolysaccharide of Rhizobium meliloti. J Bacteriol. 1988;170:4239–4248. doi: 10.1128/jb.170.9.4239-4248.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Long S R. Rhizobium symbioses: nod factors in perspective. Plant Cell. 1996;8:1885–1898. doi: 10.1105/tpc.8.10.1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Müller P, Hynes M, Kapp D, Niehaus K, Pühler A. Two classes of Rhizobium meliloti infection mutants differ in exopolysaccharide production and in coinoculation properties with nodulation mutants. Mol Gen Genet. 1988;211:17–26. [Google Scholar]
- 25.Niehaus K, Kapp D, Pühler A. Plant defense and delayed infection of alfalfa pseudonodules induced by an exopolysaccharide (EPS I)-deficient Rhizobium meliloti. Planta. 1993;190:415–425. [Google Scholar]
- 26.Reinhold B B, Chan S Y, Reuber T L, Marra A, Walker G C, Reinhold V N. Detailed structural characterization of succinoglycan, the major symbiotically important exopolysaccharide of Rhizobium meliloti strain Rm1021. J Bacteriol. 1994;176:1997–2002. doi: 10.1128/jb.176.7.1997-2002.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reuber T L, Walker G C. Biosynthesis of succinoglycan, a symbiotically important exopolysaccharide of Rhizobium meliloti. Cell. 1993;74:269–280. doi: 10.1016/0092-8674(93)90418-p. [DOI] [PubMed] [Google Scholar]
- 28.Reuhs B L, Williams M N V, Kim J S, Carlson R W, Côté F. Suppression of the Fix− phenotype of Rhizobium meliloti exoB by lpsZ is correlated to a modified expression of the K polysaccharide. J Bacteriol. 1995;177:4289–4296. doi: 10.1128/jb.177.15.4289-4296.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Urzainqui A, Walker G C. Exogenous suppression of the symbiotic deficiencies of Rhizobium meliloti exo mutants. J Bacteriol. 1992;174:3403–3406. doi: 10.1128/jb.174.10.3403-3406.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.York G M, Walker G C. The Rhizobium meliloti exoK gene and prsD/prsE/exsH genes are components of independent degradative pathways which contribute to production of low-molecular-weight succinoglycan. Mol Microbiol. 1997;25:117–134. doi: 10.1046/j.1365-2958.1997.4481804.x. [DOI] [PubMed] [Google Scholar]
- 31.York G M, Walker G C. The Rhizobium meliloti ExoK and ExsH glycanases specifically depolymerize nascent succinoglycan chains. Proc Natl Acad Sci USA. 1998;95:4912–4917. doi: 10.1073/pnas.95.9.4912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.York G M, Walker G C. The succinyl and acetyl modifications of succinoglycan influence susceptibility of succinoglycan to cleavage by the Rhizobium meliloti glycanases ExoK and ExsH. J Bacteriol. 1998;180:4184–4191. doi: 10.1128/jb.180.16.4184-4191.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zevenhuizen L P T M, van Neerven A R W. (1,2)-β-d-Glucan and acidic oligosaccharides produced by Rhizobium meliloti. Carbohydr Res. 1983;118:127–134. [Google Scholar]