Abstract
Tetrads (or quartets) are arrangements of four nucleobases commonly involved in the stability of four-stranded nucleic acids structures. Four-stranded or quadruplex structures have attracted enormous attention in the last few years, being the most extensively studied guanine quadruplex (G-quadruplex). Consequently, the G-tetrad is the most common and well-known tetrad. However, this is not the only possible arrangement of four nucleobases. A number of tetrads formed by the different nucleobases have been observed in experimental structures. In most cases, these tetrads occur in the context of G-quadruplex structures, either inserted between G-quartets, or as capping elements at the sides of the G-quadruplex core. In other cases, however, non-G tetrads are found in more unusual four stranded structures, such as i-motifs, or different types of peculiar fold-back structures. In this report, we review the diversity of these non-canonical tetrads, and the structural context in which they have been found.
Keywords: DNA structure, RNA structure, quadruplex, tetrad
1. Introduction
Interest in four-stranded nucleic acids structures has been constantly increasing in the last two decades. Although not always the case, four-stranded structures are usually stabilized by arrangements of four nucleobases commonly named as tetrads or quartets. The most extensively studied four-stranded structure is the G-quadruplex [1], the importance of which, in recent times, has been driven by its proven biological relevance and promising therapeutic applications [2,3,4,5].
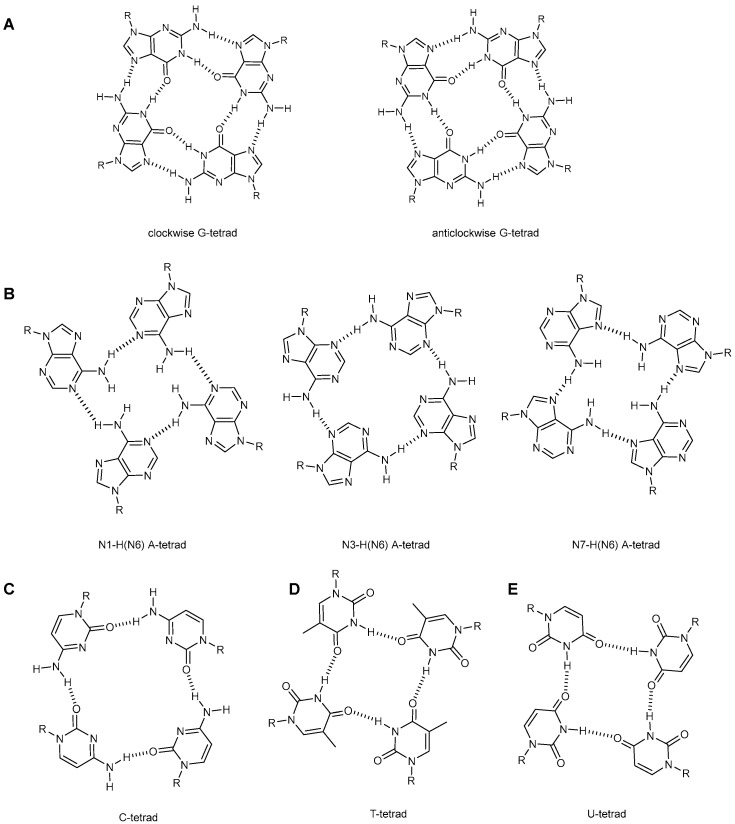
G-quadruplexes exhibit wide structural diversity depending on strands orientation (parallel, antiparallel and hybrid structures) and connecting loop topologies (diagonal, lateral and propeller loops) [6,7,8]. Despite their structural variety, all G-quadruplexes share the same common element: the so-called G-tetrad. G-tetrads are platforms of four guanine nucleobases interacting through their Hoogsteen and Watson–Crick sides, following either a clockwise, or an anticlockwise arrangement (Figure 1A). These tetrads are stabilized by eight hydrogen bonds, and by electrostatic interactions with cations located between two consecutive tetrads. The cation, usually monovalent, compensates the negatively charged oxygens in the center of the tetrad. Among the different monovalent cations, K+ is the most stabilizing, since its atomic radius fits very well in the central position of two consecutive tetrads. G-tetrads are planar, allowing an efficient stacking of multiple layers, and giving rise to extremely thermostable structures.
Figure 1.
Schemes of the different homotetrads discussed in the text. (A) G-tetrads in their two possible orientations (clockwise and anticlokwise); (B) The three most common A-tetrads; (C–E) C-, T-, and U-homotetrads, respectively.
Although the G-quartet is the most common and well-studied tetrad, this is not the only possible arrangement of four nucleobases. In fact, a number of tetrads formed by the different nucleobases has been observed in experimental structures (Table 1). They can be classified in two main groups: (a) those formed by arrangements of the same nucleobase (homotetrads), and (b) those formed by the association of two base pairs. The latter can involve canonical G:C or A:T Watson-Crick base pairs, or a variety of mismatches.
Table 1.
Structures with the different non-G tetrads mentioned in the text with their corresponding references and their PDB codes, when available.
| Type | Tetrad | Cation | PDB | |
|---|---|---|---|---|
| Homo | N1-H(N6) A | - | 1EVM [13], 1NP9 [28] | |
| N3-H(N6) A | Na+ | 6A85 [14], 1MDG [26] | ||
| N7-H(N6) A | -/Na+ | 1EVN [13], 1J6S [19] | ||
| C4 | -/Ba2+/Na+ | 1EVO [9], 4U92 [10], 6A85 [14] | ||
| T4 | -/Na+/K+ | 1EMQ [11], 6A85 [14], 4L0A [23], 1S47 [24] | ||
| U4 | - | 1RAU [15], 6GE1 [18], 1J6S [19], 1MDG [26], 1J8G [54], 2AWE [55], 4RKV [56], 4RJ1 [56], 4RNE [56], 4XK0 [57] |
||
| I4 | K+ | 2GRB [21] | ||
| Base-paired | Major groove |
Direct G:C:G:C | -/Na+ | 1XCE [29], 1A8N [30], 1A6H [31], 3R1E [34], 1JVC [35], 1NYD [58], 6SYK [59], 6SX6 [59] |
| Slipped G:C:G:C | K+ | 1A8W [32], 7CV4 [33] | ||
| Direct A:T:A:T | -/K+ | 1K8P [1] | ||
| Slipped A:T:A:T | -/Na+ | 1XCE [29], 1JVC [35] | ||
| Direct G:T:G:T | - | [36,37] | ||
| Direct G:A:G:A | - | 5M1L, 5M2L [39] | ||
| Minor groove | Direct G:C:G:C | - | 184D [41], 1MF5 [42], 1EU2 [43], 4ZKK [60], 5FHJ [61] | |
| Slipped G:C:G:C | -/divalent | 6MC2, 6MC3, 6MC4, 6N4G [50], 2HK4 [45] | ||
| Slipped C:G:G:C | - | 2K8Z, 2K90, 2K97 [45] | ||
| Direct G:T:G:T | - | -[48] | ||
| Slipped G:T:G:T | - | 1C11 [62], 2LSX [63], 7O5E [64] | ||
| Slipped G:C:G:T | - | 5OGA [65] | ||
| Direct A:T:A:T | Na+ | 1EU6 [43], 284D [46] | ||
| Direct G:C:A:T | Na+ | 1N96 [47] | ||
| Slipped G:A:G:A | divalent | 6MC2, 6MC3, 6MC4, 6N4G [50] | ||
2. Results
2.1. Homotetrads
As in the case of G-tetrads, pure C-[9,10], T-[11,12] and A-tetrads [13] are formed by the arrangement of four identical nucleobases, as shown in Figure 1B–D. These homotetrads have always been observed in the context of parallel G-quadruplex structures, either in internal regions of the quadruplex or as capping elements interacting with terminal G-tetrads. Of particular interest is the case of the oligonucleotide of sequence d (AGAGAGATGGGTGCGTT), which folds as a tetrameric parallel quadruplex. In this case, the four possible DNA homotetrads coexist in the same crystal structure [14]. Homotetrads have also been found in RNA G-quadruplex scaffolds. In this case, U-tetrads (Figure 1E) [15,16,17], usually located at the 3′-end of parallel quadruplexes [18], are the most common. However, RNA homotetrads formed by adenines [19,20] or inosines [21] have also been reported.
The conformation of the T-tetrad is shown in Figure 1D. T-tetrads were first observed in the solution structure of the tetrameric G-quadruplex formed by the sequence d(TGGTGGC) [11]. This tetrad is mainly stabilized by hydrogen bonds between imino protons H(N3) and O4 atoms of adjacent thymines. However, Liu et al. [14] suggested that weak hydrogen bond interactions between H(C5) and O2 atoms may also contribute to the stability. Similar T-tetrads have been observed in the crystal structure of d(TGGGGT)4 [22] and in an all-LNA G-quadruplex from 5′-TGGGT-3′. In the latter case, a K+ ion bridges the four O4 atoms [23]. Similar hydrogen bonds and K+ interactions have been observed in RNA U-tetrads. Theoretical studies confirm the expected higher stability of U-tetrads due to the absence of methyl-O2 repulsions [24].
In the oligonucleotide d(TGGGCGGT), which also adopts a parallel tetrameric G-quadruplex structure, the central cytosines form the C-tetrad shown in Figure 1C [9]. In this structure, amino protons are at a hydrogen-bond distance from the O2 atoms. However, in the C-tetrad formed in the crystal structure of d(AGAGAGATGGGTGCGTT), the interactions between cytosines do not occur by direct hydrogen bonds, but through a network of highly conserved water molecules located in the middle of the tetrad [14]. In the crystal structure of the DNA decamer, d(CCACNVKGCGTGG) (CNVK, 3-cyanovinylcarbazole), which forms a G-quadruplex structure in the presence of Ba2+, a C-tetrad, is stabilized by water molecules-mediated contacts between the divalent cations and the cytosines, allowing Ba2+ ions to occupy the central ion channel [10]. Computational calculation suggests that C-tetrads in the contexts of G-quadruplex have a high propensity for binding alkaline earth cations [25].
A-tetrads are more common and more diverse (Figure 1B). They have been found in a number of DNA and RNA oligonucleotides derived from telomeric sequences, including the NMR structure of human telomere RNA ORN-1 [20], or the structure of (BrdU)r(GAGGU) [19]. The A-tetrads observed in RNAs usually form hydrogen bonds between their amino H(N6) and N7 atoms (Figure 1B, right), although N3-H(N6) A-tetrads (Figure 1B, middle) have also been observed in the crystal structure of rU(BrdG)r(AGGU) [26]. In the structure of d(AGAGAGATGGGTGCGTT) [14], the hydrogen bonds occur between amino H(N6) and the N3 atoms (Figure 1B, middle), resulting in a bigger central cavity. A third class of A-tetrad, in which hydrogen bonds are formed between H(N6) and N1 atoms (Figure 1B, left), has been reported in the solution structures of d(TGGAGGC)4, a sequence that contains the GGAGG repeat present in the C-MYC oncogene [27], the telomeric repeat sequence [28], and the truncated telomeric sequence d(AG3T) [13]. In this case, a dynamic exchange is observed between this tetrad and the N7-H(N6) A-tetrad [13]. Although in most A-tetrads adenines glycosidic angles are in anti, the syn conformation is also possible [13].
The overall size of T-, C- and A-tetrads, as estimated from diagonal C1′-C1′ distance, is slightly smaller than in the case of the G-tetrad, being this distance around 1–2 Å shorter in T- and A-tetrads, and ~3 Å shorter in the case of the C-tetrad [14]. Due to their similar size, homotetrads provoke only minor distortions in the structure of parallel G-quadruplexes.
2.2. Base-Paired Tetrads
In addition to the homotetrads described above, tetrads can also be formed by the association of two base pairs. In most cases, the base pairs involved are Watson–Crick G:C or A:T base-pairs, although the isomorphous G:T base-pair and other mismatches have also been reported. Base-paired tetrads can be classified in two big groups depending on the relative orientation of the two base pairs: the so-called major groove and minor groove tetrads (Figure 2). Although most of the base-paired tetrads can be classified in these two categories, there are some exceptions of tetrads presenting very unusual nucleobase interactions [29] or water-mediated interactions in complex RNA structures [7].
Figure 2.
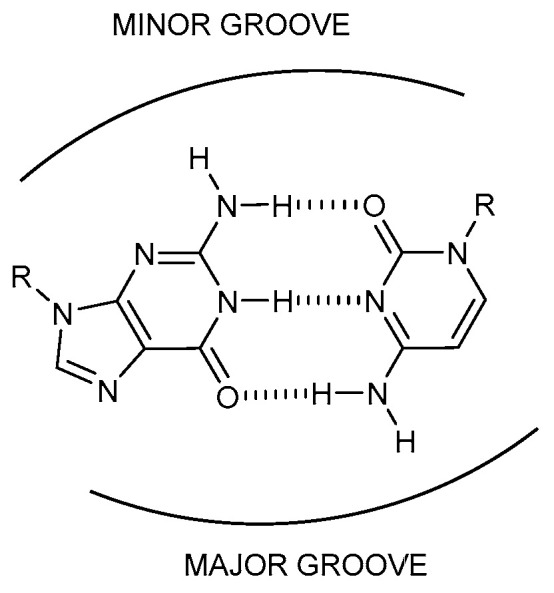
Major and minor groove sides of a G:C Watson-Crick base pair.
2.2.1. Major Groove Tetrads
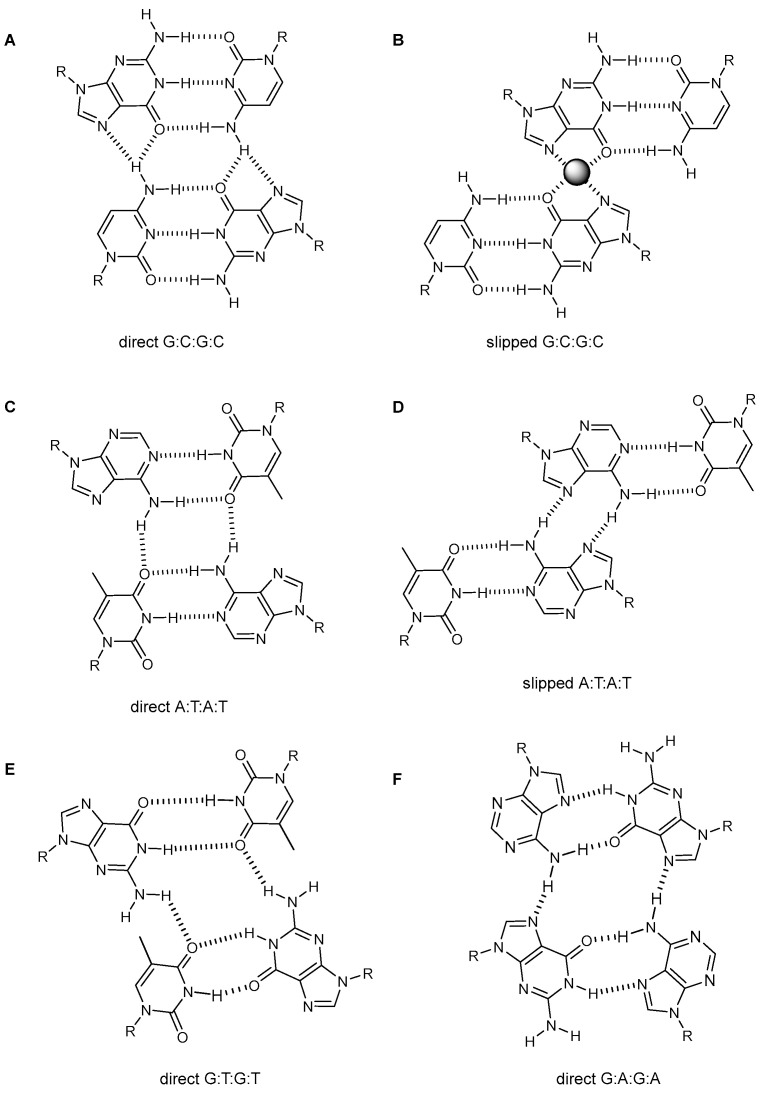
In major groove tetrads, the interaction between base pairs occurs through their major groove side (the base-pair side that would be oriented towards the major groove in a canonical duplex, Figure 2). Among the first cases reported are the major groove G:C:G:C tetrads found in the structure of the fragile X syndrome (CGG)n triplet repeat [16], and in the d(GGGC) repeats of the adeno-associated viral DNA [30]. In both cases, the quadruplex arises from dimerization of two DNA hairpins, stabilized by several G-tetrads and G:C:G:C tetrads. The latter formed by two intra-molecular Watson–Crick base pairs directly facing each other and interacting through intermolecular bifurcated hydrogen bonds between the cytosine H(N4) and the guanine O6 and N7 atoms (Figure 3A). This arrangement gives rise to the so-called “direct” major groove tetrad. These tetrads have also been found in repetitive RNA sequences [31].
Figure 3.
Schemes of the different major groove tetrads discussed in the text. (A) Direct major groove G:C:G:C tetrad; (B) Slipped major groove G:C:G:C tetrad; (C) Direct major groove A:T:A:T tetrad; (D) Slipped major groove G:C:G:C tetrad. (E) Direct major groove G:T:C:T. (F) Direct major groove G:A:G:A tetrad.
In other occasions, the relative position of the two G:C base pairs is shifted with respect to the direct tetrad (Figure 3B). The resulting tetrad has been named as “slipped” major groove tetrad [32]. In this case, the stabilization between base pairs does not arise from additional hydrogen bonds, but through cation coordination with O6(G) and N7(G) atoms from the two guanine residues. Interestingly, the conformations of the G:C:G:C tetrad may depend on the particular cation present in the sample. Thus, the antiparallel quadruplex structure of the d(GGGC)n repeats of the adeno-associated viral DNA in the sodium form contains two direct major groove G:C:G:C tetrads, whereas in the presence of potassium the alignment of the two G:C base pairs is shifted, with the two major groove edges of the guanines coordinating a K+ cation [32]. Slipped major groove G:C:G:C tetrads formation has also been reported in a quadruplex-duplex hybrid structure, in which the slipped mixed tetrad is located between the G-quadruplex core and the duplex [33], and in an unusual quadruplex RNA structure formed by the major groove interaction of two antiparallel duplexes [34].
Direct and slipped major groove tetrads can be also formed by the association of A:T Watson-Crick base pairs (Figure 3C,D). The slipped alignment was found in the dimeric solution structure of the octamer d(GAGCAGGT) [35] and implies the formation of two N7(A)-H(N6)(A) additional hydrogen bonds. In the direct alignment, the inter-base pair hydrogen bonds are O4(T)-H(N6)(A). This tetrad has been observed as crystallographic contacts between unit cells in the dimeric propeller-like quadruplex structure of the human telomeric sequence, d(TAGGGTTAGGGT) [1]. Interestingly, these tetrads are not part of the G-quadruplex core but are formed between loop resides, connecting different quadruplexes and contributing to stabilize the crystal.
Major groove tetrads involving mismatch base pairs have also been found. G:T:G:T major groove tetrads (Figure 3E) have been proposed in the structures of some parallel G-quadruplexes [36] and in the interlocked G-quadruplex structure formed by d(GGGT) and derived sequences [37]. G:A:G:A tetrads involving G:A mismatches have been exhaustively explored by DFT methods [38]. These calculations indicate that squared planar G:A:G:A major groove tetrads are not favored, due to repulsion interactions between lone electron pairs of nitrogen atoms. However, an almost planar G:A:G:A tetrad with similar nucleobase orientation has been observed in the solution structure of d(GCGAGGGAGCGAGGG) [39]. This sequence is a variation in the d(GGGAGCG) repeat, found in the regulatory region of the PLEKHG3 human gene related to autism [40]. This structure is a dimer resulting from the self-association of two fold-back loops, and stabilized by a number of unusual elements, such as two G:A:G:A tetrads (Figure 3F) with all their glycosidic angles in anti, G:G base pairs, and direct minor groove G:C:G:C tetrads (Figure 4A).
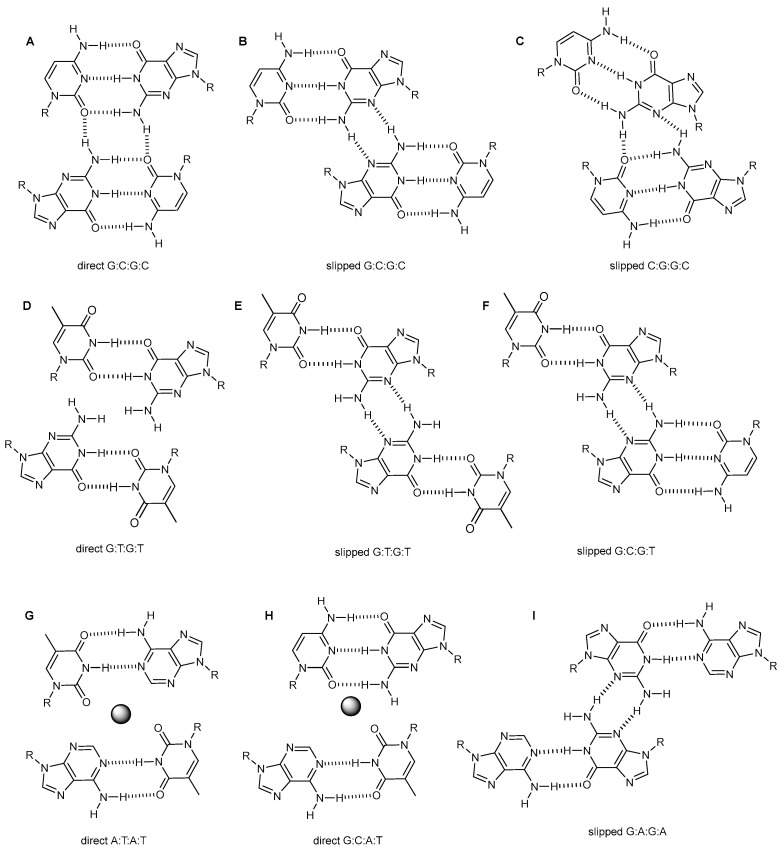
Figure 4.
Schemes of the different minor groove tetrads discussed in the text. Direct (A) and slipped (B) minor groove G:C:G:C tetrads. (C) Minor groove C:G:G:C tetrad. Direct (D) and slipped (E) minor groove G:T:G:T tetrads. (F) Slipped minor groove G:C:G:T. Direct minor groove A:T:A:T (G) and G:C:A:T (H) tetrads stabilized by cation coordination (dark circle). (I) Slipped minor groove G:A:G:A tetrad.
2.2.2. Minor Groove Tetrads
The association between the two base pairs forming the tetrad can also occur through the minor groove side of the two base pairs (the side that would be oriented towards the minor groove in a canonical duplex, Figure 2). As in the previous case, the resulting minor groove tetrad can be direct or slipped, depending on the relative position of the two base pairs involved. G:C:G:C minor groove tetrads were found in the crystallographic structure of d(GCATGCT) [41,42], and in the solution structures of the cyclic oligonucleotides d<pTGCTCGCT> [43] and d<pCCGTCCGT> [44] (the notation d<p(sequence)> indicates cyclic deoxyoligonucleotide). All these structures are dimers stabilized by intermolecular Watson–Crick base-pairs. In the case of d(GCATGCT) and d<pTGCTCGCT>, the minor groove tetrads are direct, forming two bifurcated H(N2)(G)-O2(C) hydrogen bonds between the base pairs (Figure 4A). In contrast, in the structure of d<pCCGTCCGT> the minor groove tetrads are slipped, forming two H(N2)(G)-N3(G) hydrogen bonds (Figure 4B). No cation sensitivity between these two types of tetrads has been observed. However, the order of residues closing the loops seems to be crucial. Thus, in 5′-C-XX-G-3′ turns the tetrad is direct, and in 5′-G-XX-C-3′ slipped [45].
In most cases, the orientation of two Watson-Crick base pairs forming the tetrads is opposite to each other. However, in the dimeric structures of d(TGCTTCGT) and d(TCGTTGCT), and their cyclic analogue d<pCGCTCCGT> [45], the two G:C base pairs are in the same orientation, giving rise to a C:G:G:C tetrad. In this case, the hydrogen bonds between base pairs are H(N2)(G)-N3(G) and H(N2)(G)-O2(C) (Figure 4C).
Minor groove tetrads can also involve A:T base pairs, giving rise to A:T:A:T tetrads, such as in the dimeric structure of d<pCATTCATT> [43,46], or G:C:A:T mixed tetrads, observed in the dimeric structure of d<pCGCTCATT> [47]. In both cases, only the direct alignment has been observed (Figure 4G,H). A:T:A:T and G:C:A:T minor groove tetrads are stabilized by cation coordination, as shown by crystallographic and theoretical studies.
Likewise, minor groove tetrads can involve one or two G:T mismatches. As in the case of G:C:G:C, the two alignments (direct and slipped) have been observed in G:T:G:T tetrads [48] (Figure 4D,E). In mixed G:C:G:T tetrads, however, only the slipped alignment has been reported (Figure 4F) [48,49]. Minor groove tetrads with G:A mismatches (Figure 4I) have been observed in the dimeric fold-back structure of d(CGTAAGGCGTA) [50]. This structure is stabilized by minor groove G:C:G:C and G:A:G:A tetrads, both in the slipped configurations. The glycosidic angle of adenines in this tetrad is in syn.
In addition to the different hydrogen bonds pattern stabilizing the interaction between the two base pairs, there is a fundamental difference between major and minor groove tetrads, whereas in major groove tetrads, the two base-pairs are co-planar and in minor groove tetrads the two base pairs present a relative inclination that ranges from 20° to 40°, depending on the particular structure. The reason of this effect is not clear. It may be related to the close proximity between phosphate groups in structures stabilized by minor groove tetrads. In these cases, a relative inclination between base pairs may alleviate electrostatic repulsion between phosphates. However, it can also be an intrinsic property of purine-purine interactions through their minor groove side. The structures of parallel DNA duplexes stabilized by homopurine base pairs point towards this possibility, since G:G and A:A base pairs in these duplex structures exhibit a similar inclination [51]. Although a number of theoretical studies have shown that coplanarity between G:C base pairs is the most stable configuration in major groove tetrads [52,53], very little is known from a theoretical point of view about the geometry of minor groove G:C:G:C tetrads.
2.3. Structural Context
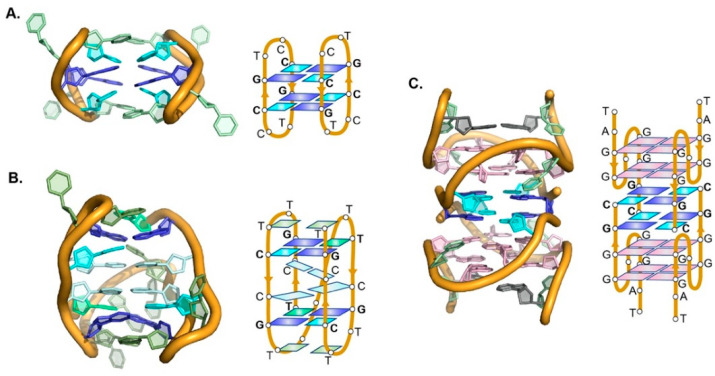
The different structural features between major and minor groove tetrads have important consequences. First, minor grove tetrads are not easily accommodated within G-tetrads scaffolds. In fact, base-paired tetrads found in the context of G-quadruplex structures are always of the major groove type. Secondly, minor groove tetrads cannot be piled up indefinitely due to the mutual inclination between the two base-pairs (between 20–40°, Figure 5A). No structure with more than two consecutive minor groove tetrads has been reported. Two is probably the maximum number of minor groove tetrads that can be assembled together.
Figure 5.
Examples of structures containing non-G tetrads. (A) Dimeric structures formed by two cyclic oligonucleotides <dCCGTCCGT> stabilized by two minor groove C:G:C:G tetrads (PDB 2HK4). (B) Minimal i-motif structure stabilized by two C:C+ base pairs capped by two minor groove G:C:G:T tetrads (PDB 5OGA). (C) Association of two G-quadruplexes through formation of two major groove C:G:C:G tetrads (PDB 1NYD).
Major groove tetrads are planar and, in their direct configuration, are almost isomorphous with G-tetrads, with diagonal C1′C1′ distance between purines almost identical to G-tetrads, and between pyrimidines around 1 Å shorter. This feature makes direct major groove tetrads perfectly suitable to be stacked between G-tetrads (Figure 5C) and, in fact, they are relatively common in G-quadruplex structures [1,16,30,31,32,33,34,35,36,37,39]. In contrast, slipped major groove tetrads are uncommon and only found in terminal positions [32]. The effect of the presence of G:C:G:C tetrads on G-quadruplexes recognition has been explored [66]. However, very few cases of ligands interacting with non-G tetrads has been reported to date [67,68].
On the other hand, minor groove tetrads have always been found in structures in which no G-tetrad is present. These tetrads appear to induce distinct DNA folding (the term “bi-loop” has been suggested for this motif) [46]. This motif has been usually found in homodimers formed by short linear or cyclic oligonucleotides (Figure 5A), in which dimerization occurs through the formation of two minor groove tetrads with intermolecular base pairs [41,42,43,44,45,46,47,48,49,69].
The tetrads are connected by short loops of one to three residues, with two-residue loops being the most stable [70]. The residues in the first position of the loops stack on top of the tetrads forming a cap at both ends of the structure. A common feature of all these structures is the great proximity of the backbones of the two strands in the same subunit. The very short distances between phosphates provokes unfavorable electrostatic interactions, which are partially alleviated by hydrophobic contacts between deoxyriboses.
This peculiar feature is common to other non-canonical DNA structure, the i-motif [71,72]. The i-motif is a four-stranded structure not stabilized by tetrads, but by intercalation of C:C+ base pairs. These structures are formed by the association of two parallel-stranded duplexes through hemiprotonated C:C+ base pairs. The two duplexes are intercalated in opposite orientations. Since the C:C+ base pair requires partial cytosine protonation, i-motif structures are usually more stable at acidic pH. Interestingly, minor groove tetrads have been observed as capping elements in several i-motif structures. The first case reported was that minor grove G:T:G:T tetrad observed in dimeric structures of centromeric sequences [62]. Then, G:T:G:T [63] and G:C:G:T [65] tetrads were observed in other dimeric and monomeric i-motifs. In all cases, the tetrads are in the slipped configuration, and provoke a dramatic thermal and pH stabilization of the i-motif structure. This stabilization is most probably due to the favorable interaction of the charged C:C+ base-pair with the guanines of the tetrad (Figure 5B). The capability of minor groove tetrads to stabilize i-motifs may have profound consequences in biology, since consensus sequences based on these interactions have been found to be prevalent in the human genome, occurring preferentially near regulatory regions [65].
Although several i-motifs have been found to be stable at a neutral pH, to date, the only i-motif structures determined at nearly physiological conditions are structures stabilized by minor groove tetrads. Of particular interest is the structure adopted by oligonucleotides containing several repeats of the sequence 5′-dCCGTTCCGT-3′. At a neutral pH, these sequences fold into i-motif structures stabilized by two C:C+ base pairs and two C:G:C:G minor groove tetrads. This structure is peculiar since it contains neutral and hemiprotonated cytosines under the same experimental conditions. C:G:C:G tetrads are not stable at acidic pH, in which conditions cytosines are partially protonated. When lowering the pH from 7 to 5, these oligonucleotides undergo a large conformational transition towards a different i-motif structure, with four C:C+ base pairs and capped by two G:T:G:T tetrads [73]. This conformational transition was further used to explore the use of a fluorescent cytosine analogue tricyclic 1,3-diaza-2-oxophenoxazine (tCO) as an efficient internal probe. Interestingly, the increased stacking interactions between tCO and the guanine residue of the tetrad, when located in contiguous positions, dramatically enhanced the thermal and pH stability of the i-motif structures [74].
Very recently, minor groove tetrads have been used to stabilize DNA constructs, in which i-motif and B-DNA coexist in the same structure, forming i-motif/duplex junctions [64]. These constructs consist of an i-motif moiety capped by a minor groove G:T:G:T tetrad at one of its ends, and an stem-loop hairpin at the other end of the i-motif. Stabilization conferred by the minor groove tetrad is essential to stabilize the structure at neutral pH, since analogous constructs with sequences unable to form the tetrad are not stable [64].
2.4. Base Paired Tetrads in Higher-Order Structures
In some occasions, major groove G:C:G:C tetrads are found in structures containing multiple interlocked G-quadruplexes in the regions connecting different units [58,75]. The ability to interlock multiple G-quadruplexes through these tetrads has been used to build nanowires [69,76,77]. In the case of sequences containing 5′-GC-3′ in the terminal position, this assembly of multimeric G-quadruplexes is cation dependent [59]. Similar interlocked structures stabilized by major groove G:C:G:C tetrads have been also found in RNA G-quadruplexes [16]. Inclusion of G:C:G:C tetrads in G-quadruplex scaffolds can be also used for avoiding the inherent polymorphism of these structures when designing DNA nanoassemblies [78]. Furthermore, the formation of G:C:G:C tetrads has been proposed in higher order structures formed by multiple GGGGCC repeats found in the sequence of the C9ORF72 gene [79]. Expansion of these repeats is considered the most important genetic cause of the Amyotrophic Lateral Sclerosis (ALS).
On the other hand, G:C:G:C tetrads, usually in their minor groove conformation, are common between symmetry-related molecules in crystallographic structures. On many occasions, they are formed between terminal G:C base pairs in the crystal structures of DNA duplexes, contributing to stable crystal packing [80]. Slipped tetrads found in the context of a crystallographic network are more common between DNA duplexes that crystallize in B-form [81,82]. In contrast, direct minor groove tetrads are found in A-form DNA structures. The direct arrangement may be facilitated by the wider minor-groove characteristic of A-form double helices [83]. Although not so common, these interactions do not involve terminal residues. This is the case of the DNA-RNA chimeric duplex of sequence d(CCGGC)r(G)d(CCGG). Interestingly, direct minor grove G:C:G:C tetrads connecting different duplexes involve the single 2′-hydroxyl group per strand. This result indicates that minor groove tetrads can be formed with at least one of the four nucleotides being ribo-. However, studies on the association between cyclic and linear oligonucleotides suggest that minor groove tetrads containing ribonucleotides are much less stable than those formed by four dexoyribonucleotides [49].
Minor groove G:C:G:C tetrads have also been found involving terminal G and C residues that do not form base-pairs with their own duplex but with symmetry related ones, forming junction-like quadruplexes. Such structures have been found in pure DNA crystals and in several bisintercalative complexes involving acridine derivatives. In these complexes, the acridine derivatives do not intercalate in the usual way, but interact with the terminal nucleotides of four DNA duplexes forming a large intercalation platform between two G:C:G:C tetrads [84,85,86,87,88]. The structures of these complexes have attracted significant interest because some of the intercalating drugs involved in them are potent topoisomerase inhibitors. Interestingly, in most cases, these structures have been found in crystals containing Co2+ ions. Such counterions might be relevant to increase the stability of minor groove tetrads, since cobalt hexamine residues have also been found to stabilize the crystal lattice in one of the crystallographic structures of the dimeric linear heptamer d(GCATGCT) [41,42,60,61].
Base-paired tetrads have also been observed in ordered nanostructures based on G:C pairing. High resolution scanning tunnelling microscopy studies in adlayers formed by coadsorption of guanine and cytosine at Au(111) [89], and at graphite surfaces [90] have revealed the formation of well-ordered periodic structures based on G:C:G:C tetrads in the solid/liquid interface. Such structures are formed by alternate arrangements of G:C base pairs though their major and minor groove sides. Similar supramolecular nanopatterns based on A:T:A:T have also been reported [90]. More recently, base paired C:G:G:C, G:C:G:C and A:U:A:U tetrads have been used as building blocks for the formation of 2D-nanoporous networks [91]. Finally, G-quartets and other homotetrads have been also found in template-assembled synthetic supramolecular structures [92,93,94].
3. Conclusions
In summary, the number of arrangements of four nucleobases found in experimental structures amply exceeds the canonical G-tetrads. In this review we have classified these non-guanine tetrads in two main groups: homotetrads, formed by the arrangement of identical nucleobase, and base-paired tetrads, formed by the association of two base pairs (G:C, A:T or mismatches). Most of the tetrads in the second group can be classified in major groove or minor groove tetrads, depending on the side of the two base pairs interacting with each other. The structural contexts in which these tetrads occur is conditioned by their specific geometry. Thus, whereas homo- and major groove tetrads are mainly formed in the context of G-quadruplex structures, minor groove tetrads occur in i-motifs and, in peculiar, fold-back DNA structures. Interestingly, non-canonical tetrads are involved in different DNA-DNA recognition events, such as loop-loop association, formation of G-quadruplex assemblies, or DNA duplex packing. These tetrad-mediated interactions may be involved in biological processes and may have useful applications in DNA nanotechnology.
Author Contributions
Conceptualization, N.E. and C.G.; writing—review and editing, N.E., B.M., M.G. and C.G. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Funding Statement
This research was funded by Ministerio de Ciencia e Innovación, grant number PID2020-116620GB-I00. B. M. acknowledges a “Margarita Salas” contract from the Spanish Ministerio de Ciencia e Innovación, and M. G. a European Union Marie Sklodowska Curie Action (799693).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Parkinson G.N., Lee M.P.H., Neidle S. Crystal Structure of Parallel Quadruplexes from Human Telomeric DNA. Nature. 2002;417:876–880. doi: 10.1038/nature755. [DOI] [PubMed] [Google Scholar]
- 2.Spiegel J., Adhikari S., Balasubramanian S. The Structure and Function of DNA G-Quadruplexes. Trends Chem. 2020;2:123–136. doi: 10.1016/j.trechm.2019.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Varshney D., Spiegel J., Zyner K., Tannahill D., Balasubramanian S. The Regulation and Functions of DNA and RNA G-Quadruplexes. Nat. Rev. Mol. Cell Biol. 2020;21:459–474. doi: 10.1038/s41580-020-0236-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Frasson I., Pirota V., Richter S.N., Doria F. Multimeric G-Quadruplexes: A Review on Their Biological Roles and Targeting. Int. J. Biol. Macromol. 2022;204:89–102. doi: 10.1016/j.ijbiomac.2022.01.197. [DOI] [PubMed] [Google Scholar]
- 5.Kosiol N., Juranek S., Brossart P., Heine A., Paeschke K. G-Quadruplexes: A Promising Target for Cancer Therapy. Mol. Cancer. 2021;20:40. doi: 10.1186/s12943-021-01328-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lightfoot H.L., Hagen T., Tatum N.J., Hall J. The Diverse Structural Landscape of Quadruplexes. FEBS Lett. 2019;593:2083–2102. doi: 10.1002/1873-3468.13547. [DOI] [PubMed] [Google Scholar]
- 7.Banco M.T., Ferré-D’Amaré A.R. The Emerging Structural Complexity of G-Quadruplex RNAs. RNA. 2021;27:390–402. doi: 10.1261/rna.078238.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Malgowska M., Czajczynska K., Gudanis D., Tworak A., Gdaniec Z. Overview of the RNA G-Quadruplex Structures. Acta Biochim. Pol. 2016;63:609–621. doi: 10.18388/abp.2016_1335. [DOI] [PubMed] [Google Scholar]
- 9.Patel P.K., Bhavesh N.S., Hosur R.V. NMR Observation of a Novel C-Tetrad in the Structure of the SV40 Repeat Sequence GGGCGG. Biochem. Biophys. Res. Commun. 2000;270:967–971. doi: 10.1006/bbrc.2000.2479. [DOI] [PubMed] [Google Scholar]
- 10.Zhang D., Huang T., Lukeman P.S., Paukstelis P.J. Crystal Structure of a DNA/Ba2+ G-Quadruplex Containing a Water-Mediated C-Tetrad. Nucleic Acids Res. 2014;42:13422–13429. doi: 10.1093/nar/gku1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Patel P.K., Hosur R. V NMR Observation of T-Tetrads in a Parallel Stranded DNA Quadruplex Formed by Saccharomyces Cerevisiae Telomere Repeats. Nucleic Acids Res. 1999;27:2457–2464. doi: 10.1093/nar/27.12.2457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oliviero G., Amato J., Borbone N., Galeone A., Varra M., Piccialli G., Mayol L. Synthesis and Characterization of DNA Quadruplexes Containing T-Tetrads Formed by Bunch-Oligonucleotides. Biopolymers. 2006;81:194–201. doi: 10.1002/bip.20399. [DOI] [PubMed] [Google Scholar]
- 13.Patel P.K., Koti A.S.R., Hosur R.V. NMR Studies on Truncated Sequences of Human Telomeric DNA: Observation of a Novel A-Tetrad. Nucleic Acids Res. 1999;27:3836–3843. doi: 10.1093/nar/27.19.3836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu H., Wang R., Yu X., Shen F., Lan W., Haruehanroengra P., Yao Q., Zhang J., Chen Y., Li S., et al. High-Resolution DNA Quadruplex Structure Containing All the A-, G-, C-, T-Tetrads. Nucleic Acids Res. 2018;46:11627–11638. doi: 10.1093/nar/gky902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheong C., Moore P.B. Solution Structure of an Unusually Stable RNA Tetraplex Containing G-and U-Quartet Structures. Biochemistry. 1992;31:8406–8414. doi: 10.1021/bi00151a003. [DOI] [PubMed] [Google Scholar]
- 16.Malgowska M., Gudanis D., Kierzek R., Wyszko E., Gabelica V., Gdaniec Z. Distinctive Structural Motifs of RNA G-Quadruplexes Composed of AGG, CGG and UGG Trinucleotide Repeats. Nucleic Acids Res. 2014;42:10196–10207. doi: 10.1093/nar/gku710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu Y., Ishizuka T., Kimura T., Komiyama M. A U-Tetrad Stabilizes Human Telomeric RNA G-Quadruplex Structure. J. Am. Chem. Soc. 2010;132:7231–7233. doi: 10.1021/ja909708a. [DOI] [PubMed] [Google Scholar]
- 18.Andrałojć W., Małgowska M., Sarzyńska J., Pasternak K., Szpotkowski K., Kierzek R., Gdaniec Z. Unraveling the Structural Basis for the Exceptional Stability of RNA G-Quadruplexes Capped by a Uridine Tetrad at the 3′ Terminus. RNA. 2019;25:121–134. doi: 10.1261/rna.068163.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pan B., Xiong Y., Shi K., Deng J., Sundaralingam M. Crystal Structure of an RNA Purine-Rich Tetraplex Containing Adenine Tetrads: Implications for Specific Binding in RNA Tetraplexes. Structure. 2003;11:815–823. doi: 10.1016/S0969-2126(03)00107-2. [DOI] [PubMed] [Google Scholar]
- 20.Xiao C.D., Ishizuka T., Zhu X.Q., Li Y., Sugiyama H., Xu Y. Unusual Topological RNA Architecture with an Eight-Stranded Helical Fragment Containing A-, G-, and U-Tetrads. J. Am. Chem. Soc. 2017;139:2565–2568. doi: 10.1021/jacs.6b12274. [DOI] [PubMed] [Google Scholar]
- 21.Pan B., Shi K., Sundaralingam M. Crystal Structure of an RNA Quadruplex Containing Inosine Tetrad: Implications for the Roles of NH2 Group in Purine Tetrads. J. Mol. Biol. 2006;363:451–459. doi: 10.1016/j.jmb.2006.08.022. [DOI] [PubMed] [Google Scholar]
- 22.Cáceres C., Wright G., Gouyette C., Parkinson G., Subirana J.A. A Thymine Tetrad in d(TGGGGT) Quadruplexes Stabilized with Tl+/Na+ Ions. Nucleic Acids Res. 2004;32:1097. doi: 10.1093/nar/gkh269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Russo Krauss I., Parkinson G.N., Merlino A., Mattia C.A., Randazzo A., Novellino E., Mazzarella L., Sica F. A Regular Thymine Tetrad and a Peculiar Supramolecular Assembly in the First Crystal Structure of an All-LNA G-Quadruplex. Acta Crystallogr. Sect. D Biol. Crystallogr. 2014;70:362–370. doi: 10.1107/S1399004713028095. [DOI] [PubMed] [Google Scholar]
- 24.Gu J., Leszczynski J. A Theoretical Study of Thymine and Uracil Tetrads: Structures, Properties, and Interactions with the Monovalent K+ Cation. J. Phys. Chem. A. 2001;105:10366–10371. doi: 10.1021/jp004550v. [DOI] [Google Scholar]
- 25.Milovanović B., Petković M., Popov I., Etinski M. Water-Mediated Interactions Enhance Alkaline Earth Cation Chelation in Neighboring Cavities of a Cytosine Quartet in the DNA Quadruplex. J. Phys. Chem. B. 2021;125:11996–12005. doi: 10.1021/acs.jpcb.1c05598. [DOI] [PubMed] [Google Scholar]
- 26.Pan B., Xiong Y., Shi K., Sundaralingam M. An Eight-Stranded Helical Fragment in RNA Crystal Structure: Implications for Tetraplex Interaction. Structure. 2003;11:825–831. doi: 10.1016/S0969-2126(03)00108-4. [DOI] [PubMed] [Google Scholar]
- 27.Searle M.S., Williams H.E.L., Gallagher C.T., Grant R.J., Stevens M.F.G. Structure and K Ion-Dependent Stability of a Parallel-Stranded DNA Quadruplex Containing a Core A-Tetrad. Org. Biomol. Chem. 2004;2:810–812. doi: 10.1039/b314559j. [DOI] [PubMed] [Google Scholar]
- 28.Gavathiotis E., Searle M.S. Structure of the Parallel-Stranded DNA Quadruplex d(TTAGGGT) 4 Containing the Human Telomeric Repeat: Evidence for A-Tetrad Formation from NMR and Molecular Dynamics Simulations. Org. Biomol. Chem. 2003;1:1650–1656. doi: 10.1039/b300845m. [DOI] [PubMed] [Google Scholar]
- 29.Da Silva M.W. Experimental Demonstration of T:(G:G:G:G):T Hexad and T:A:A:T Tetrad Alignments within a DNA Quadruplex Stem. Biochemistry. 2005;44:3754–3764. doi: 10.1021/bi0478190. [DOI] [PubMed] [Google Scholar]
- 30.Kettani A., Bouaziz S., Gorin A., Zhao H., Jones R.A., Patel D.J. Solution Structure of a Na Cation Stabilized DNA Quadruplex Containing G·G·G·G· and G·C·G·C· Tetrads Formed by G-G-G-C Repeats Observed in Adeno-Associated Viral DNA. J. Mol. Biol. 1998;282:619–636. doi: 10.1006/jmbi.1998.2030. [DOI] [PubMed] [Google Scholar]
- 31.Kettani A., Kumar R.A., Patel D.J. Solution Structure of a DNA Quadruplex Containing the Fragile X Syndrome Triplet Repeat. J. Mol. Biol. 1995;254:638–656. doi: 10.1006/jmbi.1995.0644. [DOI] [PubMed] [Google Scholar]
- 32.Bouaziz S., Kettani A., Patel D.J. A K Cation-Induced Conformational Switch within a Loop Spanning Segment of a DNA Quadruplex Containing G-G-G-C Repeats. J. Mol. Biol. 1998;282:637–652. doi: 10.1006/jmbi.1998.2031. [DOI] [PubMed] [Google Scholar]
- 33.Tan D.J.Y., Winnerdy F.R., Lim K.W., Tû An Phan A. Coexistence of Two Quadruplex-Duplex Hybrids in the PIM1 Gene. Nucleic Acids Res. 2020;48:11162–11171. doi: 10.1093/nar/gkaa752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gudanis D., Popenda L., Szpotkowski K., Kierzek R., Gdaniec Z. Structural Characterization of a Dimer of RNA Duplexes Composed of 8-Bromoguanosine Modified CGG Trinucleotide Repeats: A Novel Architecture of RNA Quadruplexes. Nucleic Acids Res. 2016;44:2409–2416. doi: 10.1093/nar/gkv1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang N., Gorin A., Majumdar A., Kettani A., Chernichenko N., Skripkin E., Patel D.J. Dimeric DNA Quadruplex Containing Major Groove-Aligned A·T·A·T and G·C·G·C Tetrads Stabilized by Inter-Subunit Watson-Crick A·T and G·C Pairs. J. Mol. Biol. 2001;312:1073–1088. doi: 10.1006/jmbi.2001.5002. [DOI] [PubMed] [Google Scholar]
- 36.Jing N., Hogan M.E. Structure-Activity of Tetrad-Forming Oligonucleotides as a Potent Anti- HIV Therapeutic Drug. J. Biol. Chem. 1998;273:34992–34999. doi: 10.1074/jbc.273.52.34992. [DOI] [PubMed] [Google Scholar]
- 37.Krishnan-Ghosh Y., Liu D., Balasubramanian S. Formation of an Interlocked Quadruplex Dimer by d(GGGT) J. Am. Chem. Soc. 2004;126:11009–11016. doi: 10.1021/ja049259y. [DOI] [PubMed] [Google Scholar]
- 38.Megger D.A., Lax P.M., Paauwe J., Fonseca Guerra C., Lippert B. Mixed Guanine, Adenine Base Quartets: Possible Roles of Protons and Metal Ions in Their Stabilization. J. Biol. Inorg. Chem. 2018;23:41–49. doi: 10.1007/s00775-017-1507-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kocman V., Plavec J. Tetrahelical Structural Family Adopted by AGCGA-Rich Regulatory DNA Regions. Nat. Commun. 2017;8:15355. doi: 10.1038/ncomms15355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kocman V., Plavec J. A Tetrahelical DNA Fold Adopted by Tandem Repeats of Alternating GGG and GCG Tracts. Nat. Commun. 2014;5:5831. doi: 10.1038/ncomms6831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Leonard G.A., Zhang S., Peterson M.R., Harrop S.J., Helliwell J.R., Cruse W.B., Langlois d’Estaintot B., Kennard O., Brown T., Hunter W.N. Self-Association of a DNA Loop Creates a Quadruplex: Crystal Structure of d(GCATGCT) at 1.8 å Resolution. Structure. 1995;3:335–340. doi: 10.1016/S0969-2126(01)00165-4. [DOI] [PubMed] [Google Scholar]
- 42.Thorpe J.H., Teixeira S.C.M., Gale B.C., Cardin C.J. Crystal Structure of the Complementary Quadruplex Formed by d(GCATGCT) at Atomic Resolution. Nucleic Acids Res. 2003;31:844–849. doi: 10.1093/nar/gkg168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Escaja N., Pedroso E., Rico M., González C. Dimeric Solution Structure of Two Cyclic Octamers: Four-Stranded DNA Structures Stabilized by A:T:A:T and G:C:G:C Tetrads. J. Am. Chem. Soc. 2000;122:12732–12742. doi: 10.1021/ja002778q. [DOI] [Google Scholar]
- 44.Viladoms J., Escaja N., Frieden M., Gómez-Pinto I., Pedroso E., González C. Self-Association of Short DNA Loops through Minor Groove C:G:G:C Tetrads. Nucleic Acids Res. 2009;37:3264–3275. doi: 10.1093/nar/gkp191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Escaja N., Gómez-Pinto I., Pedroso E., González C. Four-Stranded DNA Structures Can Be Stabilized by Two Different Types of Minor Groove G:C:G:C Tetrads. J. Am. Chem. Soc. 2007;129:2004–2014. doi: 10.1021/ja066172z. [DOI] [PubMed] [Google Scholar]
- 46.Salisbury S.A., Wilson S.E., Powell H.R., Kennard O., Lubini P., Sheldrick G.M., Escaja N., Alazzouzi E., Grandas A., Pedroso E. The Bi-Loop, a New General Four-Stranded DNA Motif. Proc. Natl. Acad. Sci. USA. 1997;94:5515–5518. doi: 10.1073/pnas.94.11.5515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Escaja N., Gelpí J.L., Orozco M., Rico M., Pedroso E., González C. Four-Stranded DNA Structure Stabilized by a Novel G:C:A:T Tetrad. J. Am. Chem. Soc. 2003;125:5654–5662. doi: 10.1021/ja0344157. [DOI] [PubMed] [Google Scholar]
- 48.Viladoms J., Escaja N., Pedroso E., González C. Self-Association of Cyclic Oligonucleotides through G:T:G:T Minor Groove Tetrads. Bioorganic Med. Chem. 2010;18:4067–4073. doi: 10.1016/j.bmc.2010.04.018. [DOI] [PubMed] [Google Scholar]
- 49.Escaja N., Gómez-Pinto I., Viladoms J., Rico M., Pedroso E., González C. Induced-Fit Recognition of DNA by Small Circular Oligonucleotides. Chemistry. 2006;12:4035–4042. doi: 10.1002/chem.200600050. [DOI] [PubMed] [Google Scholar]
- 50.Chu B., Zhang D., Hwang W., Paukstelis P.J. Crystal Structure of a Tetrameric DNA Fold-Back Quadruplex. J. Am. Chem. Soc. 2018;140:16291–16298. doi: 10.1021/jacs.8b10153. [DOI] [PubMed] [Google Scholar]
- 51.Luteran E.M., Paukstelis P.J. The Parallel-Stranded d(CGA) Duplex Is a Highly Predictable Structural Motif with Two Conformationally Distinct Strands. Acta Cryst. 2022;D78:299–309. doi: 10.1107/S2059798322000304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Meyer M., Schneider C., Brandl M., Sühnel J. Cyclic Adenine-, Cytosine-, Thymine-, and Mixed Guanine—Cytosine-Base Tetrads in Nucleic Acids Viewed from a Quantum-Chemical and Force Field Perspective. J. Phys. Chem. A. 2001;105:11560–11573. doi: 10.1021/jp012546t. [DOI] [Google Scholar]
- 53.Gu J., Leszczynski J. Structures and Properties of the Planar G·C·G·C Tetrads: Ab Initio HF and DFT Studies. J. Phys. Chem. A. 2000;104:7353–7358. doi: 10.1021/jp000741m. [DOI] [Google Scholar]
- 54.Deng J., Xiong Y., Sundaralingam M. X-Ray Analysis of an RNA Tetraplex (UGGGGU)4 with Divalent Sr2+ Ions at Subatomic Resolution (0.61 Å) Proc. Natl. Acad. Sci. USA. 2001;98:13665. doi: 10.1073/pnas.241374798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pan B., Shi K., Sundaralingam M. Base-Tetrad Swapping Results in Dimerization of RNA Quadruplexes: Implications for Formation of the i-Motif RNA Octaplex. Proc. Natl. Acad. Sci. USA. 2006;103:3130–3134. doi: 10.1073/pnas.0507730103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fyfe A.C., Dunten P.W., Martick M.M., Scott W.G. Structural Variations and Solvent Structure of r(UGGGGU) Quadruplexes Stabilized by Sr2 + Ions. J. Mol. Biol. 2015;427:2205–2219. doi: 10.1016/j.jmb.2015.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen M.C., Murat P., Abecassis K., Ferre-D’Amare A.R., Balasubramanian S. Insights into the Mechanism of a G-Quadruplex-Unwinding DEAH-Box Helicase. Nucleic Acids Res. 2015;43:2223–2231. doi: 10.1093/nar/gkv051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Da Silva M.W. Association of DNA Quadruplexes through G:C:G:C Tetrads. Solution Structure of d(GCGGTGGAT) Biochemistry. 2003;42:14356–14365. doi: 10.1021/bi0355185. [DOI] [PubMed] [Google Scholar]
- 59.Pavc D., Wang B., Spindler L., Drevenšek-Olenik I., Plavec J., Šket P. GC Ends Control Topology of DNA G-Quadruplexes and Their Cation-Dependent Assembly. Nucleic Acids Res. 2020;48:2749–2761. doi: 10.1093/nar/gkaa058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Thirugnanasambandam A., Karthik S., Mandal P.K., Gautham N. The Novel Double-Folded Structure of d(GCATGCATGC): A Possible Model for Triplet-Repeat Sequences. Acta Crystallogr. Sect. D Biol. Crystallogr. 2015;71:2119–2126. doi: 10.1107/S1399004715013930. [DOI] [PubMed] [Google Scholar]
- 61.Thirugnanasambandam A., Karthik S., Artheswari G., Gautham N. DNA Polymorphism in Crystals: Three Stable Conformations for the Decadeoxynucleotide d(GCATGCATGC): Three. Acta Crystallogr. Sect. D Struct. Biol. 2016;72:780–788. doi: 10.1107/S2059798316006306. [DOI] [PubMed] [Google Scholar]
- 62.Gallego J., Chou S.H., Reid B.R. Centromeric Pyrimidine Strands Fold into an Intercalated Motif by Forming a Double Hairpin with a Novel T:G:G:T Tetrad: Solution Structure of the d(TCCCGTTTCCA) Dimer. J. Mol. Biol. 1997;273:840–856. doi: 10.1006/jmbi.1997.1361. [DOI] [PubMed] [Google Scholar]
- 63.Escaja N., Viladoms J., Garavís M., Villasante A., Pedroso E., González C. A Minimal I-Motif Stabilized by Minor Groove G:T:G:T Tetrads. Nucleic Acids Res. 2012;40:11737–11747. doi: 10.1093/nar/gks911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Serrano-Chacón I., Mir B., Escaja N., González C. Structure of I-Motif/Duplex Junctions at Neutral PH. J. Am. Chem. Soc. 2021;143:12919–12923. doi: 10.1021/jacs.1c04679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mir B., Serrano I., Buitrago D., Orozco M., Escaja N., González C. Prevalent Sequences in the Human Genome Can Form Mini I-Motif Structures at Physiological PH. J. Am. Chem. Soc. 2017;139:13985–13988. doi: 10.1021/jacs.7b07383. [DOI] [PubMed] [Google Scholar]
- 66.Cao Y., Yang L., Ding P., Li W., Pei R. Ligand Selectivity by Inserting GCGC-Tetrads into G-Quadruplex Structures. Chem. A Eur. J. 2020;26:14730–14737. doi: 10.1002/chem.202003004. [DOI] [PubMed] [Google Scholar]
- 67.Gavathiotis E., Heald R.A., Stevens M.F.G., Searle M.S. Drug Recognition and Stabilisation of the Parallel-Stranded DNA Quadruplex d(TTAGGGT)4 Containing the Human Telomeric Repeat. J. Mol. Biol. 2003;334:25–36. doi: 10.1016/j.jmb.2003.09.018. [DOI] [PubMed] [Google Scholar]
- 68.Kotar A., Kocman V., Plavec J. Intercalation of a Heterocyclic Ligand between Quartets in a G-Rich Tetrahelical Structure. Chem. A Eur. J. 2020;26:814–817. doi: 10.1002/chem.201904923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Troha T., Drevenšek-Olenik I., Webba Da Silva M., Spindler L. Surface-Adsorbed Long G-Quadruplex Nanowires Formed by G:C Linkages. Langmuir. 2016;32:7056–7063. doi: 10.1021/acs.langmuir.6b01222. [DOI] [PubMed] [Google Scholar]
- 70.Escaja N., Gómez-Pinto I., Viladoms J., Pedroso E., González C. The Effect of Loop Residues in Four-Stranded Dimeric Structures Stabilized by Minor Groove Tetrads. Org. Biomol. Chem. 2013;11:4804–4810. doi: 10.1039/c3ob40741a. [DOI] [PubMed] [Google Scholar]
- 71.Assi H.A., Garavís M., González C., Damha M.J. I-Motif DNA: Structural Features and Significance to Cell Biology. Nucleic Acids Res. 2018;46:8038–8056. doi: 10.1093/nar/gky735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Benabou S., Aviñó A., Eritja R., González C., Gargallo R. Fundamental Aspects of the Nucleic Acid I-Motif Structures. RSC Adv. 2014;4:26956. doi: 10.1039/C4RA02129K. [DOI] [Google Scholar]
- 73.Serrano-Chacón I., Mir B., Orozco M., Escaja N., González C. PH-Induced Largescale Structural Transitions in i-Motifs. J. Am. Chem. Soc. 2022 doi: 10.1021/jacs.2c13043. submitted . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mir B., Serrano-Chacón I., Terrazas M., Garavís M., Orozco M., Escaja N., González C. Site-Specific Incorporation of TCo Results into Highly Stable i-Motifs Amenable to in Vivo Detection. Nucleic Acids Res. 2022 doi: 10.1093/nar/gkae106. submitted . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mergny J.L., De Cian A., Amrane S., da Silva M.W. Kinetics of Double-Chain Reversals Bridging Contiguous Quartets in Tetramolecular Quadruplexes. Nucleic Acids Res. 2006;34:2386–2397. doi: 10.1093/nar/gkl098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ma’Ani Hessari N., Spindler L., Troha T., Lam W.C., Drevenšek-Olenik I., Webba Da Silva M. Programmed Self-Assembly of a Quadruplex DNA Nanowire. Chem. A Eur. J. 2014;20:3626–3630. doi: 10.1002/chem.201300692. [DOI] [PubMed] [Google Scholar]
- 77.Ilc T., Šket P., Plavec J., Webba Da Silva M., Drevenšek-Olenik I., Spindler L. Formation of G-Wires: The Role of G:C-Base Pairing and G-Quartet Stacking. J. Phys. Chem. C. 2013;117:23208–23215. doi: 10.1021/jp4019348. [DOI] [Google Scholar]
- 78.Zheng J., Du Y., Wang H., Peng P., Shi L., Li T. Ultrastable Bimolecular G-Quadruplexes Programmed DNA Nanoassemblies for Reconfigurable Biomimetic DNAzymes. ACS Nano. 2019;13:11947–11954. doi: 10.1021/acsnano.9b06029. [DOI] [PubMed] [Google Scholar]
- 79.Zamiri B., Mirceta M., Bomsztyk K., Macgregor R.B., Pearson C.E. Quadruplex Formation by Both G-Rich and C-Rich DNA Strands of the C9orf72 (GGGGCC)8•(GGCCCC)8 Repeat: Effect of CpG Methylation. Nucleic Acids Res. 2015;43:10055–10064. doi: 10.1093/NAR/GKV1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Subirana J.A., Abrescia N.G.A. Extra-Helical Guanine Interactions in DNA. Biophys. Chem. 2000;86:179189. doi: 10.1016/S0301-4622(00)00168-X. [DOI] [PubMed] [Google Scholar]
- 81.Tereshko V., Subirana J.A. Influence of Packing Interactions on the Average Conformation of B-DNA in Crystalline Structures. Acta Cryst. 1999;55:810–819. doi: 10.1107/S0907444999000591. [DOI] [PubMed] [Google Scholar]
- 82.Grzechowiak M., Ruszkowska A., Sliwiak J., Urbanowicz A., Jaskolski M., Ruszkowski M. New Aspects of DNA Recognition by Group II WRKY Transcription Factor Revealed by Structural and Functional Study of AtWRKY18 DNA Binding Domain. Int. J. Biol. Macromol. 2022;213:589–601. doi: 10.1016/j.ijbiomac.2022.05.186. [DOI] [PubMed] [Google Scholar]
- 83.Wahl M.C., Sundaralingam M. A-DNA duplexes in the crystal. In: Neidle S., editor. Oxford Handbook of Nucleic Acid Sructures. Oxford University Press; New York, NY, USA: 1999. pp. 389–453. [Google Scholar]
- 84.Adams A., Guss J.M., Denny W.A., Wakelin L.P.G. Structure of 9-Amino-[N-(2-Dimethylamino)Propyl]-Acridine-4-Carboxamide Bound to d(CGTACG)2: A Comparison of Structures of d(CGTACG) 2 Complexed with Intercalators in the Presence of Cobalt. Acta Crystallogr. Sect. D Biol. Crystallogr. 2004;60:823–828. doi: 10.1107/S0907444904003907. [DOI] [PubMed] [Google Scholar]
- 85.Adams A., Guss J.M., Collyer C.A., Denny W.A., Wakelin L.P.G. A Novel Form of Intercalation Involving Four DNA Duplexes in an Acridine-4-Carboxamide Complex of d(CGTACG)2. Nucleic Acids Res. 2000;28:4244–4253. doi: 10.1093/nar/28.21.4244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Thorpe J.H., Hobbs J.R., Todd A.K., Denny W.A., Charlton P., Cardin C.J. Guanine Specific Binding at a DNA Junction Formed by d[CG(5-BrU)ACG]2 with a Topoisomerase Poison in the Presence of Co2+ Ions. Biochemistry. 2000;39:15055–15061. doi: 10.1021/bi001749p. [DOI] [PubMed] [Google Scholar]
- 87.Teixeira S.C.M., Thorpe J.H., Todd A.K., Powell H.R., Adams A., Wakelin L.P.G., Denny W.A., Cardin C.J. Structural Characterisation of Bisintercalation in Higher-Order DNA at a Junction-like Quadruplex. J. Mol. Biol. 2002;323:167–171. doi: 10.1016/S0022-2836(02)00923-3. [DOI] [PubMed] [Google Scholar]
- 88.Yang X.L., Robinson H., Gao Y.G., Wang A.H.J. Binding of a Macrocyclic Bisacridine and Ametantrone to CGTACG Involves Similar Unusual Intercalation Platforms. Biochemistry. 2000;39:10950–10957. doi: 10.1021/bi001319z. [DOI] [PubMed] [Google Scholar]
- 89.Ding Y., Xie L., Zhang C., Xu W. Real-Space Evidence of the Formation of the GCGC Tetrad and Its Competition with the G-Quartet on the Au(111) Surface. Chem. Commun. 2017;53:9846–9849. doi: 10.1039/C7CC05548J. [DOI] [PubMed] [Google Scholar]
- 90.Xu S., Dong M., Rauls E., Otero R., Linderoth T.R., Besenbacher F. Coadsorption of Guanine and Cytosine on Graphite: Ordered Structure Based on GC Pairing. Nano Lett. 2006;6:1434–1438. doi: 10.1021/nl060563u. [DOI] [PubMed] [Google Scholar]
- 91.Bilbao N., Destoop I., De Feyter S., González-Rodríguez D. Two-Dimensional Nanoporous Networks Formed by Liquid-to-Solid Transfer of Hydrogen-Bonded Macrocycles Built from DNA Bases. Angew. Chem. Int. Ed. 2016;55:659–663. doi: 10.1002/anie.201509233. [DOI] [PubMed] [Google Scholar]
- 92.Nikan M., Sherman J. Template-Assembled Synthetic G-Quartets (TASQs) Angew. Chem. Int. Ed. 2008;47:4900–4902. doi: 10.1002/anie.200704199. [DOI] [PubMed] [Google Scholar]
- 93.Hui B.W., Sherman J.C. Synthesis and characterization of a template-assembled synthetic U-quartet. Chem. Commun. 2012;48:109–111. doi: 10.1039/C1CC15608J. [DOI] [PubMed] [Google Scholar]
- 94.Murat P., Bonnet R., Van der Heyden A., Spinelli N., Labbé P., Monchaud D., Teulade-Fichou M.P., Dumy P., Defrancq E. Template-Assembled Synthetic G-Quadruplex (TASQ): A Useful System for Investigating the Interactions of Ligands with Constrained Quadruplex Topologies. Chem. A Eur. J. 2010;16:6106–6114. doi: 10.1002/chem.200903456. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.