Abstract
Birds play a role in maintaining tick-borne diseases by contributing to the multiplication of ticks and pathogens on a local scale during the breeding season. In the present study, we describe the diversity of tick and pathogen species of medical and veterinary importance in Europe hosted by 1040 captured birds (56 species) during their breeding season in France. Of the 3114 ticks collected, Ixodes ricinus was the most prevalent species (89.5%), followed by I. frontalis (0.8%), I. arboricola (0.7%), Haemaphysalis concinna (0.5%), H. punctata (0.5%), Hyalomma spp. (0.2%), and Rhipicephalus spp. (0.06%). Because they may be representative of the bird infection status for some pathogen species, 1106 engorged tick larvae were screened for pathogens. Borrelia burgdorferi sensu lato was the most prevalent pathogen genus in bird-feeding larvae (11.7%), followed by Rickettsia spp. (7.4%), Anaplasma spp. (5.7%), Babesia spp. (2.3%), Ehrlichia spp. (1.4%), and B. miyamotoi (1%). Turdidae birds (Turdus merula and T. philomelos), Troglodytes troglodytes, and Anthus trivialis had a significantly higher prevalence of B. burgdorferi s.l.-infected larvae than other pathogen genera. This suggests that these bird species could act as reservoir hosts for B. burgdorferi s.l. during their breeding season, and thus play an important role in acarological risk.
Keywords: wild bird, breeding season, tick, tick-borne pathogen
1. Introduction
Ticks are the second most important disease vector worldwide in human health, after mosquitoes, and the first in animal health [1]. They are obligate parasites, and their three life stages (larva, nymph and adult) can have their own trophic preferences [2,3]. Ticks can host and transmit a wide variety of pathogens, whether bacteria, viruses or parasites, to a broad spectrum of vertebrate hosts [3]. A better knowledge of the diversity of pathogens that ticks can host and transmit, their different modes of transmission, and the roles of tick hosts in the epidemiological cycle of pathogens is essential to improve the control of tick-borne diseases and reduce the acarological risk [3]. Hosts can participate in tick-borne pathogen dynamics by feeding ticks, thereby allowing them to evolve to their next life stage, and/or by transmitting pathogens to ticks if they are competent reservoir hosts [4]. Birds are important hosts to consider as they can disseminate ticks and their associated pathogens on a large scale during their migration period before and after breeding [5,6,7]. Birds can also participate in the local dynamics of ticks and pathogens during their sedentary periods by feeding ticks (and infecting them or being infected by them) during breeding [8] or wintering [9,10]. In Europe, birds can contribute to the population dynamics of a wide variety of tick species with potential medical and veterinary importance, including Ixodes ricinus, Haemaphysalis concinna, H. punctata, Hyalomma marginatum, and Hy. lusitanicum [11,12,13,14,15,16,17,18]. Birds also feed specialist, ornithophilic ticks such as I. arboricola, I. lividus, I. frontalis, I. festai and I. eldaricus [11,12,15,17,19], some of which also transmit pathogens [20].
Through ticks, birds can participate in the dynamics of pathogens of medical or veterinary importance, whether bacteria (Anaplasma spp., Borrelia spp., Ehrlichia spp., Rickettsia spp., Coxiella spp.) [14,18,21,22,23,24,25,26,27,28], parasites (Babesia spp.) [22,23], or viruses (tick-borne encephalitis virus, Crimean-Congo haemorrhagic fever) [29,30,31].
The objective of this preliminary study was to carry out an inventory of tick and pathogen species (concentrating on bacteria and parasites) of medical and veterinary importance in Europe, that are hosted by common birds in France during their breeding season. This recurring annual event in the life cycle of birds, in spring, is important to consider as it overlaps with the peak activity of ticks (I. ricinus) and the period during which humans do outdoor activities and are more exposed to infectious tick bites. From this inventory, we sought to calculate tick infection rates and prevalence among birds for the pathogen genera identified in order to reveal any evidence of a higher level of infection of certain bird species by specific pathogens.
We therefore evaluated the diversity of tick species hosted by a wide variety of bird species and screened for a broad spectrum of pathogens (27 species from five genera of bacteria, eight species from two genera of parasites) harboured by bird-feeding tick larvae. While co-feeding is negligible, engorged larvae can be considered as an indicator of the infection status of birds for pathogens with negligible transovarial transmission, such as Borrelia burgdorferi sensu lato (Bbsl) and A. phagocytophilum [32,33]. For other pathogens, engorged larvae are considered as proxies of prevalence among birds and transovarial transmission. From the literature, we hypothesised (i) that I. ricinus would be most abundant in the sample, since it is the most common tick in Europe, with an activity peak in the spring [34], (ii) that Borrelia spp. and Rickettsia spp. would be the most frequently detected tick-borne pathogens (TBPs) as they are the most prevalent in bird-feeding ticks [14,23,24,28,35,36], and (iii) that birds belonging to the Turdidae family and Parus major would host a higher proportion of infected larvae, in particular for specific pathogen species such as Bbsl [12,14,28,37].
2. Results
2.1. Bird Capture and Tick Collection
Ticks were collected from a total of 1040 birds belonging to 56 species (491 birds in 2019, 549 birds in 2020) captured from March to September (5% of bird captures before 16 May, 50% before 12 June and 95% before 10 July). Erithacus rubecula, Sylvia atricapilla, Turdus merula and Parus major were the most commonly caught (more than 50%). The 3114 ticks collected belonged to five species, I. ricinus being the most frequently found (89.5% of collected ticks, n = 2787; Table 1 and Table S1). A minor fraction (7.9%) of these ticks could not be identified: 0.4% (n = 14) were identified at genus level only (four Ixodes spp., three Haemaphysalis spp., five Hyalomma spp. and two Rhipicephalus spp.) and 7.5% (n = 233) were too damaged for morphological identification. Molecular techniques failed to confirm identification. As I. ricinus ticks were detected throughout France, Figure S1 represents the geographical distribution respectively of Ixodes ticks other than I. ricinus (I. arboricola and I. frontalis), Haemaphysalis spp. (H. concinna and H. punctata), Hyalomma spp. and Rhipicephalus spp. Among the ticks collected, 55.1% (n = 1715) were nymphs, 41.3% (n = 1285) were larvae—of which 86.1% (n = 1106) were engorged—1.9% (n = 60) were adult females, 0.03% (n = 1) were adult males and 1.7% (n = 53) were too damaged for morphological life-stage identification (Table 1). However, it should be noted that the collection protocol was favourable to nymphs and engorged larvae, so these proportions should not be considered as representative of the actual age structure of ticks feeding on birds.
Table 1.
Number of ticks collected per tick species and tick life stage (percentage calculated out of all the ticks, n = 3114).
| Life Stage | Male | Female | Nymph | Larva (Engorged) | Unidentified | Total (%) | |
|---|---|---|---|---|---|---|---|
| Species | |||||||
| Genus Ixodes | |||||||
| I. ricinus | 0 | 29 | 1591 | 1167 (1039) | 0 | 2787 (89.5%) | |
| I. frontalis | 0 | 16 | 8 | 2 (2) | 0 | 26 (0.8%) | |
| I. arboricola | 0 | 3 | 17 | 2 (0) | 0 | 22 (0.7%) | |
| I. spp. | 0 | 0 | 0 | 4 (2) | 0 | 4 (0.1%) | |
| Genus Haemaphysalis | |||||||
| H. concinna | 0 | 0 | 5 | 11 (11) | 0 | 16 (0.5%) | |
| H. punctata | 0 | 0 | 1 | 15 (15) | 0 | 16 (0.5%) | |
| H. spp. | 0 | 0 | 0 | 3 (3) | 0 | 3 (0.1%) | |
| Genus Hyalomma | |||||||
| H. spp. | 0 | 0 | 5 | 0 | 0 | 5 (0.2%) | |
| Genus Rhipicephalus | |||||||
| R. spp. | 1 | 1 | 0 | 0 | 0 | 2 (0.06%) | |
| Unidentified a | 0 | 11 | 88 | 81 (34) | 53 | 233 (7.5%) | |
| Total (%) | 1 (0.03%) | 60 (1.9%) | 1715 (55.1%) | 1285 (41.3%) | 53 (1.7%) | 3114 | |
a Unidentified because morphologically damaged.
2.2. Tick-Borne Pathogen Infection Rates in Engorged Larvae and Prevalence among Birds
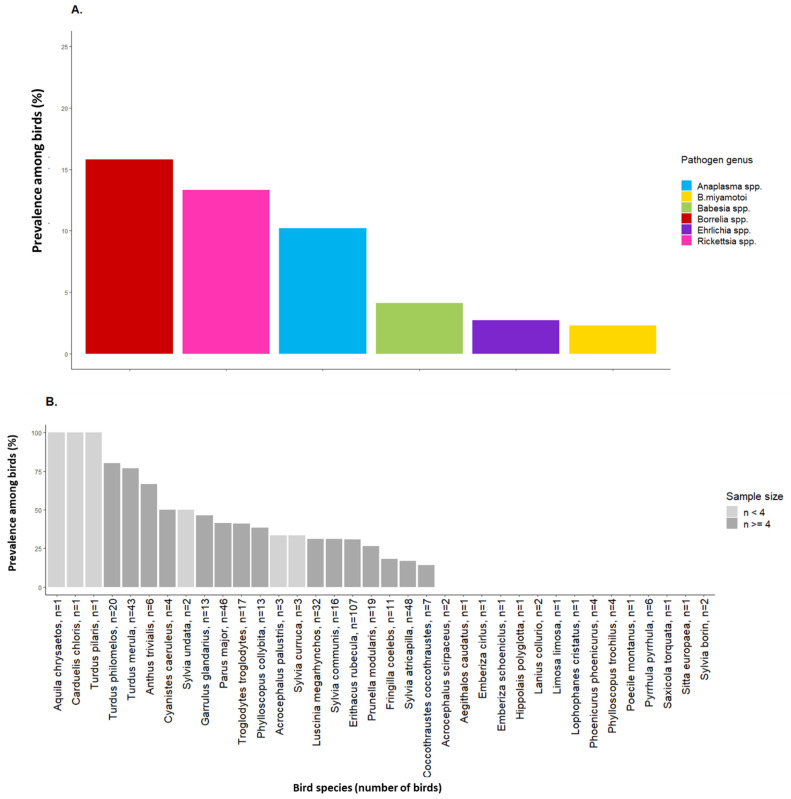
TBPs were detected from the 1106 engorged larvae that were collected from 442 birds belonging to 36 species. Bbsl was the most common pathogen genus found, with a larva infection rate (i.e., the number of TBP-positive engorged larvae out of the total number of engorged larvae collected from birds) of 11.7%. Its prevalence among birds (i.e., the number of tick-infested birds with at least one TBP-positive larva out of the total number of sampled birds) was of 15.8% (Figure 1A, Table 2 and Table S2). Rickettsia spp. (larva infection rate = 7.4%, prevalence = 13.3%) was the second most common pathogen genus detected, followed by Anaplasma spp. (larva infection rate = 5.7%, prevalence = 10.2%), Babesia spp. (larva infection rate = 2.3%, prevalence = 4.1%), Ehrlichia spp. (larva infection rate = 1.4%, prevalence = 2.7%) and B. miyamotoi (larva infection rate = 1%, prevalence = 2.3%) (Figure 1A, Table 2 and Table S2). Bartonella spp., Coxiella spp., Francisella spp. and Theileria spp. were not detected in any engorged larvae. Some pathogen species could not be clearly identified because DNA sequencing failed and did not allow precise identification between several pathogen species (this was the case for 23 larvae positive for Rickettsia spp., 22 larvae positive for Babesia spp., nine larvae positive for Ehrlichia spp. and one larva positive for Borrelia spp., Table 2). The accession numbers of the sequences submitted for tick and TBP species are presented in (Table S3).
Figure 1.
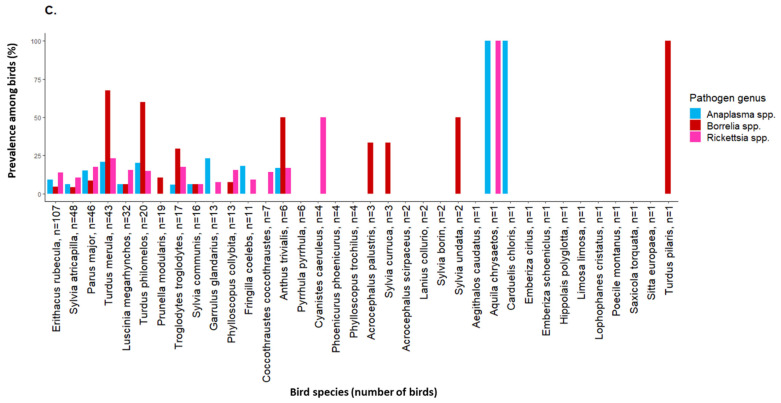
Prevalence among birds according to pathogen genus (A); prevalence according to bird species and sample size, pooling all pathogen genera (B); prevalence for the most prevalent pathogen genera (Bbsl, Rickettsia spp., Anaplasma spp.) according to bird species (C). The number of birds sampled is indicated after the bird species name for Figures (B,C). Bird species are ranked in decreasing order of pathogen prevalence among birds in Figure (B) and from the most frequently to the least frequently sampled bird species in Figure (C).
Table 2.
Engorged larva infection rates (percentage of infected engorged larvae out of the total number of engorged larvae collected from sampled birds) per pathogen and engorged larva species. The number of infected engorged larvae is noted in brackets.
| Pathogen Species | I. ricinus | I. frontalis | Ixodes spp. | H. concinna | H. punctata | Haemaphysalis spp. | Unidentified | Number of Birds with TBP-Positive Larvae | |
|---|---|---|---|---|---|---|---|---|---|
| Pathogen Species | |||||||||
| Genus Anaplasma | |||||||||
| A. phagocytophilum | 5.8 (60) | 0 | 0 | 0 | 6.7 (1) | 0 | 5.9 (2) | 45 | |
| Genus Babesia | 2.3 (24) | 0 | 0 | 0 | 0 | 0 | 2.9 (1) | 18 | |
| B. venatorum | 0.3 (3) | 0 | 0 | 0 | 0 | 0 | 0 | 3 | |
| B. spp. | 2 (21) | 0 | 0 | 0 | 0 | 0 | 2.9 (1) | 15 | |
| Genus Bbsl a | 11.9 (124) | 100 (2) | 50 (1) | 0 | 0 | 33.3 (1) | 2.9 (1) | 70 | |
| B. afzelii | 0.2 (2) | 0 | 0 | 0 | 0 | 0 | 0 | 2 | |
| Bbss b | 0.2 (2) | 0 | 0 | 0 | 0 | 0 | 0 | 2 | |
| B. garinii | 9.2 (96) | 50 (1) | 50 (1) | 0 | 0 | 33.3 (1) | 2.9 (1) | 54 | |
| B. turdi | 0.3 (3) | 50 (1) | 0 | 0 | 0 | 0 | 0 | 3 | |
| B. valaisiana | 1.9 (20) | 0 | 0 | 0 | 0 | 0 | 0 | 15 | |
| B. spp. | 0.1 (1) | 0 | 0 | 0 | 0 | 0 | 0 | 1 | |
| B. miyamotoi | 1.1 (11) | 0 | 0 | 0 | 0 | 0 | 0 | 10 | |
| Genus Ehrlichia | 1.4 (15) | 0 | 0 | 0 | 0 | 0 | 0 | 12 | |
| E. canis | 0.1 (1) | 0 | 0 | 0 | 0 | 0 | 0 | 1 | |
| close to E. chaffeensis | 0.5 (5) | 0 | 0 | 0 | 0 | 0 | 0 | 5 | |
| E. spp. | 0.9 (9) | 0 | 0 | 0 | 0 | 0 | 0 | 9 | |
| Genus Rickettsia | 6.5 (68) | 0 | 0 | 18.2 (2) | 6.7 (1) | 0 | 32.3 (11) | 59 | |
| R. aeschlimannii | 0.4 (4) | 0 | 0 | 0 | 0 | 0 | 0 | 4 | |
| R. helvetica | 4.1 (43) | 0 | 0 | 0 | 0 | 0 | 5.9 (2) | 36 | |
| R. slovaca | 0.1 (1) | 0 | 0 | 0 | 0 | 0 | 26.5 (9) | 2 | |
| R. spp. | 1.9 (20) | 0 | 0 | 18.2 (2) | 6.7 (1) | 0 | 0 | 21 | |
| Total larvae | 1039 | 2 | 2 | 11 | 15 | 3 | 34 | ||
| Total birds | 442 | ||||||||
a Bbsl: Borrelia burgdorferi sensu lato, b Bbss: Borrelia burgdorferi sensu stricto.
Pooling all pathogen genera, prevalence among birds differed between bird species and was significantly higher in T. merula (prevalence = 76.7%) and T. philomelos (prevalence = 80%) than in E. rubecula (prevalence = 30.8%, Figure 1B). Pooling all bird species, prevalence among birds also differed according to the pathogen genus and was significantly lower in Anaplasma spp. (prevalence = 10.2%), Babesia spp. (prevalence = 4.1%), Ehrlichia spp. (prevalence = 2.7%) and B. miyamotoi (prevalence = 2.3%) than in Bbsl (prevalence = 15.8%). There was no significant difference in the prevalence between Bbsl and Rickettsia spp. (prevalence = 13.3%, Figure 1A). Finally, the prevalence among birds for Bbsl differed among bird species: T. merula (prevalence = 67.4%), T. philomelos (prevalence = 60%), Troglodytes troglodytes (prevalence = 29.4%) and Anthus trivialis (prevalence = 50%) were significantly more infected with Bbsl than E. rubecula (prevalence = 4.7%, Figure 1C). There was no significant difference in prevalence according to bird species for Anaplasma spp., Babesia spp., Ehrlichia spp., Rickettsia spp., and B. miyamotoi. Pooling all pathogen genera, prevalence among birds was higher in 2020 than in 2019.
Co-infections of two pathogen genera were detected in 2.9% (n = 32) of the engorged larvae, the most prevalent pathogens (Borrelia spp., Rickettsia spp. and Anaplasma spp.) being represented the most. Moreover, 0.5% of engorged larvae (n = 6) were co-infected with three pathogen genera (Table S4).
3. Discussion
This study characterised the diversity of ticks (five species from two genera plus two species identified at genus level only) and TBPs (13 species from five genera) of veterinary and medical importance hosted by 56 species of European wild birds during their breeding season in spring, in a temperate region (France). As immature ticks of the species collected (I. ricinus, I. frontalis, I. arboricola, H. concinna, H. punctata) mostly feed on small mammals and birds [9,15,17,38,39,40] and their activity peaks during the breeding season of birds [9,34,40,41,42], we mostly collected nymphs and larvae. Pathogens were not equally represented in tick larvae from birds, Bbsl and Rickettsia spp. prevailing. Bbsl was more prevalent in engorged larvae collected from certain bird species (T. merula, T. philomelos, T. troglodytes, A. trivialis), whereas Rickettsia spp. appeared to be equally represented among host bird species. Bird prevalence by all pathogen genera was higher in 2020 than in 2019, a long-term study should be conducted to test whether the infection status of birds varies over time.
As expected, we found that I. ricinus was the predominant tick species hosted by birds, representing 89.5% of all ticks collected. All the life stages of this species were collected from birds, and it was found on 49 out of 56 bird species. As a generalist tick, I. ricinus can carry a wide range of TBPs, such as Bbsl [43], Anaplasma spp. [44], Rickettsia spp. [45], Babesia spp. [46], B. miyamotoi [47] and Ehrlichia spp. [48] as was found in our study, but also Francisella spp. [49] and Coxiella spp. [50] which are occasionally found in bird-feeding ticks [35,51,52]. Only a few other tick species were collected from birds in this study. This was expected for the ornithophilic tick I. frontalis, since nymph and adult I. frontalis are sporadically present on the ground throughout the year, while larvae activity peaks in autumn and decreases in winter [9]. Like other studies, we found I. frontalis to be infected by Bbsl [9], but no A. phagocytophilum contrary to Agoulon et al. [9]. This may be due to the small sample size of I. frontalis engorged larvae (n = 2). Moreover, a few individuals belonging to the ornithophilic and nidicolous tick species I. arboricola [17] were collected. As expected [17], all I. arboricola (whatever their life stage) were found on cavity-nesting bird species: three tit species (P. major, Poecile palustris, Cyanistes caeruleus) and an owl (Athene noctua). No engorged larvae were found, so we could not screen I. arboricola for pathogens, but this tick is known to bear the two most prevalent pathogens, Rickettsia spp. and Bbsl [20,53,54].
Three genera other than Ixodes spp. were found on the collected birds: Haemaphysalis spp., Hyalomma spp. and Rhipicephalus spp. A few individuals (larvae, nymphs) of two tick species belonging to the genus Haemaphysalis spp. (H. concinna and H. punctata) were collected from birds. H. concinna is common in deciduous or mixed forests near the shores of lakes or rivers in Europe and Asia [39]. This could explain the very small number of individuals collected in this study compared to other species like I. ricinus, as this is not the preferred environment for bird capture. In Central Europe, the peak activity of all the life stages of H. concinna overlaps with the bird breeding season [42]. The absence of adult H. concinna found in our study could be explained by the fact that this life stage mostly feeds on deer and farm animals [15]. As found in other studies, H. concinna larvae were infected by Rickettsia spp. [39]. However, we did not find Bbsl, Coxiella spp., Francisella spp. or Babesia spp. [39], possibly due to the small sample size. One H. punctata nymph and a few larvae were collected in our study. The very small number of nymphs and the absence of adults could be explained by the fact that these life stages mainly feed on wild ungulates, domestic animals and medium-sized mammals [40]. As found in other studies, H. punctata larvae were infected by Anaplasma spp. and Rickettsia spp. [55,56], but not by Babesia spp. or Bbsl contrary to Phipps et al. [57]. Finally, five nymphs belonging to Hyalomma spp. were collected from Acrocephalus scirpaceus, a finding already reported in the literature [58], and two adults belonging to Rhipicephalus spp. were collected from Aquila fasciata as reported in [59] for R. bursa.
Turdus merula and T. philomelos were the most infected bird species for all pathogen genera considered. Species belonging to the Turdidae family have been shown to play an important role in TBP circulation [14,28,37]. Bacteria belonging to Bbsl were the most prevalent TBPs (prevalence among birds = 15.8%, larva infection rate = 11.7%) as is the case in many previous studies [14,23,24,28,35,36]. The larva infection rate obtained in our study was similar to that found in a study conducted in Italy (11%; [35]) and was lower than larva infection rates found by three studies conducted in Europe, respectively in Switzerland (15.1%; [28]), the Netherlands and Belgium (19.5%; [14]) and in 11 European countries (20%; [60]). It was higher than that found in Latvia (3%; [23]) and Norway (0% in spring; [36]) perhaps due to the smaller sample tested in these studies (respectively 37 and 52 tested larvae). B. garinii was the most prevalent species (prevalence among birds = 12.2%, larva infection rate = 9%), and is already known to be associated with birds [23,34,61]. Apart from B. garinii, other Bbsl species associated with birds found in our study—B. valaisiana (prevalence = 3.4%, larva infection rate = 1.8%) and B. turdi (prevalence = 0.7%, larva infection rate = 0.4%)—were already known to circulate and multiply mainly in bird hosts [23,34,62]. This last species was, however, nearly as rare as the generalist TBP Borrelia burgdorferi sensu stricto (prevalence = 0.5%, larva infection rate = 0.2%) and the rodent-associated B. afzelii (prevalence = 0.5%, larva infection rate = 0.2%) [63]. It thus appears that we mainly have a community of bacteria belonging to Bbsl essentially linked to bird host communities. The high prevalence of B. garinii among birds could confirm the ability of birds to act as a reservoir for this pathogen [64], taking into account the fact that co-feeding and transovarial transmission could occur sporadically [32,33,65,66]. Bbsl prevalence among birds differed significantly between bird species, with T. merula, T. philomelos, T. troglodytes, and A. trivialis being the most infected. These bird species are known to actively participate in the circulation of Bbsl [14,28,37,67,68]. Contrary to our hypothesis [12], Bbsl was not very prevalent among P. major specimens compared to other bird species. We may conclude that infection by Bbsl has a structuring effect according to the bird species, with some species appearing to be more involved in the circulation of this pathogen.
The second most prevalent TBP genus in larvae collected from birds was Rickettsia spp. (prevalence= 13.3%, larva infection rate = 7.4%). This larva infection rate is similar to rates found in Sweden (6.8%; [25]), Slovakia (5.8%; [21]), and Latvia (5%; [23]). R. helvetica was the most prevalent Rickettsia species (prevalence 8.1%, larva infection rate = 4.1%), and birds have already been identified as participating in its circulation [14,21,23] and acting as potential reservoir hosts [69], although R. helvetica can be transovarially transmitted [70,71]. Unexpectedly, R. aeschlimannii was found in four larvae belonging to I. ricinus collected from four birds (prevalence = 0.9%, larva infection rate = 0.4%), whereas it is usually hosted by Hyalomma spp. ticks [52,72,73]. This implies that I. ricinus (and its bird hosts) could contribute to R. aeschlimannii dynamics as suggested in Mancini et al. [74], where it was detected in a questing I. ricinus and in Wallménius et al. [22], where it was detected in I. frontalis ticks collected from birds. However, it could also suggest that only DNA traces of R. aeschlimannii were found in engorged larvae. Finally, R. slovaca has been sporadically found in birds (prevalence = 0.5%, larva infection rate = 0.9%). Unexpectedly, it was detected in one I. ricinus larva (and nine unidentified larvae), whereas it is usually hosted by Dermacentor marginatus and D. reticulatus in Europe, which are considered as the most important vectors [75]. This implies that I. ricinus ticks hosted by birds could participate in R. slovaca dynamics, as was suggested only once in Mărcuţan et al. [75], where it was detected in an I. ricinus collected on T. merula. R. slovaca can be transmitted transovarially from the female to the larvae in Dermacentor spp. [76]; to our knowledge no such evidence has been found for I. ricinus. The high larva infection rate for Rickettsia spp. could not suggest that birds are competent as reservoir hosts for this pathogen, as transovarial transmission could often occur. According to the statistical analysis, there was no structuring effect of Rickettsia spp. infection depending on bird species, which suggests that there is no particular bird species with a major role in the circulation of this pathogen among sampled bird species.
Anaplasma spp. was the third most prevalent TBP genus in larvae collected from birds, A. phagocytophilum being the only detected species (prevalence = 10.2%, larva infection rate = 5.7%). The larva infection rate in our study was similar to that found in the Netherlands and Belgium (4.6%; [14]) and higher than that found in Latvia (2.7%; [23]).This species has already been found in many ticks collected from birds [36,77,78], which play a role in the species’ circulation by feeding ticks, dispersing infected ticks and/or infecting ticks, as was demonstrated in Johnston et al. and Keesing et al. [79,80]. The relatively high larva infection rate for A. phagocytophilum could suggest that birds are competent as reservoir hosts for this pathogen, as transovarial transmission is negligible in I. ricinus ticks [33]. Moreover, according to our statistical analysis, there is no structuring effect of Anaplasma spp. infection depending on bird species, which suggests that there is no particular bird species with a major role in the circulation of this pathogen among sampled bird species.
Some larvae collected from birds were positive for Babesia spp. (prevalence = 4.1%, larva infection rate = 2.3%). The larva infection rate in our study was lower than that found in Latvia (5%; [23]). B. venatorum (prevalence = 0.7%, larva infection rate = 0.3%) was the only species detected. While the commonly known reservoirs of this species are large domestic and wild ruminants, including cattle and roe deer [81], birds have often been identified as being involved in B. venatorum circulation by hosting infected ticks [23,82,83].
Finally, Ehrlichia spp. was the least prevalent TBP genus in engorged larvae (prevalence = 2.7%, larva infection rate = 1.4%). This genus is not often detected in bird-feeding ticks in Europe [73,84]. E. canis was the most prevalent (prevalence = 0.2%, larva infection rate = 0.1%). The role of birds in the circulation of a species close to E. canis has already been identified in Brazil, where it was detected in the blood of Coragyps atratus [85], Asio clamator and Rupornis magnirostris [86]. Moreover, a species close to E. chaffeensis (prevalence =1.1%, larva infection rate = 0.5%) was also detected in larvae collected from birds in this study. The role of birds in the circulation of this species has already been demonstrated by Hornok et al. [27], who detected it in the blood of a T. philomelos specimen in Hungary, and Machado et al. [85], who detected it in the blood of a Falcos sparverius in Brazil. These results show that birds are involved in Ehrlichia spp. circulation at the very least by feeding potentially infected ticks.
Another Borrelia spp. species, B. miyamotoi, which does not belong to Bbsl, was detected in larvae feeding on birds (prevalence = 2.3%, larva infection rate = 1%). This species has already been shown to be associated with birds [14,28,36].
To conclude, this study revealed that despite hosting a relatively low diversity of tick species, birds participate in the circulation of a high diversity of TBP species (B. garinii, A. phagocytophilum and R. helvetica being the most prevalent) during their breeding season in France. The higher prevalence of the generalist tick species I. ricinus over more specialist ones could influence TBP circulation. Indeed, infected generalist ticks can increase the acarological risk because they can feed on a wide variety of hosts and thus be infected or infect them, contrary to more specialist ticks that feed on a restricted panel of hosts. Generalist ticks can transmit specialist TBPs to other hosts in the epidemiological system [87,88]. Moreover, birds may play a direct role in local TBP circulation by infecting ticks during their bloodmeal (reservoir-competent hosts), an indirect role by acting as a ‘bridge’ in co-feeding transmission (if they allow the aggregation and simultaneous feeding of ticks at multiple life stages), or an inconsequential role in the case of transovarial transmission. In the latter case, birds participate in TBP circulation by feeding infected ticks, thereby producing infected ticks in the next life stage [89]. Although birds belonging to the Turdidae family would appear to be more involved than others in the circulation of TBP genera (having a higher prevalence among birds than other species), further research is needed to determine which bird-related factors influence their contribution to the circulation of pathogenic species. Indeed, as reservoir hosts, birds may contribute differently than other hosts they live longer and offer a higher species diversity than other reservoir hosts such as rodents, making their role in TBP circulation important both by disseminating ticks over long distances during migration and by producing infected ticks on a local scale (acarological risk) during the breeding season. Further research on the bird compartment (less studied than that of mammals), and in particular on the reservoir host potential of avian species, which depends on their realized reservoir competence, tick production, and density in the environment [4], would clarify the qualitative and quantitative role of birds in the acarological risk of tick-borne diseases.
4. Materials and Methods
4.1. Bird Capture and Tick Collection
Birds were captured by authorised bird-ringers during the breeding season in 2019 and 2020 at 110 sites spread across France (Figure S2). The majority of birds (95%) were sampled at Constant ringing Effort Sites (i.e., fixed plot with a fixed monitoring design), where the three annual capture sessions take place one morning (6 am-12 noon) every two to four weeks between May and early July. The sampling plots cover two to four hectares, across which are spread between ten and twenty 12-metre by 2.5-metre mist nets positioned about 50 m apart. With this method, only birds flying between the ground and about three metres above are sampled. The other sampled birds (5%) were obtained using other bird monitoring designs and were included to increase the range of documented bird species. A maximum of ten ticks feeding on an individual bird was asked to be collected with tweezers (all over the bird’s body) and to be immersed in a single tube filled with ethanol (70%) by the ringers.
4.2. Morphological Tick Identification
Tick stage and species (when possible) were identified morphologically using binocular loupes [90,91]. Only engorged larvae were analysed by molecular methods in the present study, as they can be considered an indicator of bird infection status under the strong assumption of negligible transovarial transmission or co-feeding. Indeed, if co-feeding is negligible, engorged larvae can be considered to indicate the infection status of birds for pathogens with negligible transovarial transmission. For other pathogens, engorged larvae are considered as proxies of prevalence among birds and transovarial transmission. We verified the morphological identification of species of engorged larvae by PCR amplification targeting a fragment of the COI gene (see hereafter) when: (i) the larva was too damaged, (ii) the larva was positive for at least one pathogen genus, and (iii) the identified species was other than I. ricinus, or identified only at genus level.
4.3. DNA Extraction and Pre-Amplification
DNA was extracted from individual larvae using the Nucleopsin tissue kit (Macherey Nagel, Düren, Germany) according to the manufacturer’s instructions (as in Banović et al. [92]). To enhance the detection of pathogen DNA, total DNA was pre-amplified with the PreAmp Master Mix (Fluidigm, San Francisco, CA, USA) according to the manufacturer’s instructions as in Banović et al. [93].
4.4. Detection of Tick-Borne Pathogens: DNA Microfluidic Real-Time PCR
To detect the bacteria and parasites of medical and veterinary importance in Europe (27 bacteria species from five genera, eight parasite protozoa species from two genera), the BioMark™ real-time PCR system (Fluidigm, San Francisco, CA, USA) was used for high-throughput microfluidic real-time PCR amplification using the 48.48 dynamic arrays (Fluidigm, San Francisco, CA, USA) as in Banović et al. and Boularias et al. [93,94]. All the pathogens were confirmed by PCR or nested PCR as in Banović et al. and Boularias et al. [92,94] using the primers presented in Table 3. The PCR products were sequenced by Eurofins Genomics (Cologne, Germany), then assembled using the BioEdit software (Ibis Biosciences, Carlsbad, CA, USA). Our results were compared with the online BLAST (http://www.ncbi.nlm.nih.gov/blast, accessed on 8 April 2022) using the GenBank dataset (https://www.ncbi.nlm.nih.gov/, accessed on 8 April 2022) to identify the sequenced microorganisms. The accession numbers of the sequences submitted for tick and TBP species are given in (Table S3).
Table 3.
List of primers used for confirmation using nested and conventional PCR.
| Pathogen Genus | Target Gene | Primer Name | Sequence (5’-3’) | Amplicon Size (bp) | T | Reference |
|---|---|---|---|---|---|---|
| Borrelia spp. | FlaB | FlaB280F FlaRL flaB_737F FlaLL |
GCAGTTCARTCAGGTAACGG GCAATCATAGCCATTGCAGATTGT GCATCAACTGTRGTTGTAACATTAACAGG ACATATTCAGATGCAGACAGAGGT |
645 407 |
55 59 |
[95] |
| Anaplasma spp./Ehrlichia spp. | 16S rRNA | EHR1 F EHR2 R EHR3 F EHR2 R |
GAACGAACGCTGGCGGCAAGC AGTA(T/C)CG(A/G)ACCAGATAGCCGC TGCATAGGAATCTACCTAGTAG AGTA(T/C)CG(A/G)ACCAGATAGCCGC |
693 629 |
60 55 |
[96] |
| Rickettsia spp. | gltA | Rsfg877 Rsfg1258 |
GGG GGC CTG CTC ACG GCG G ATT GCA AAA AGT ACA GTG AAC A- |
381 | 56 | [97] |
| Babesia spp. | 18S rRNA | BTH 18S 1st F BTH 18S 1st R BTH 18S 2nd F BTH 18S 2nd R |
GTGAAACTGCGAATGGCTCATTAC AAGTGATAAGGTTCACAAAACTTCCC GGCTCATTACAACAGTTATAGTTTATTTG CGGTCCGAATAATTCACCGGAT |
1500 | 58 | [98] |
| B. miyamotoi | IGS | Bospp-IGS-F Bospp-IGS-R Bospp-IGS-Fi Bospp-IGS-Ri | GTATGTTTAGTGAGGGGGGTG GGATCATAGCTCAGGTGGTTAG AGGGGGGTGAAGTCGTAACAAG GTCTGATAAACCTGAGGTCGGA |
1007 388–685 |
56 58 |
[99] |
| Tick species | COI | HCO2198 LCO1490 |
TAA ACT TCA GGG TGA CCA AAA AAT CA GGT CAA CAA ATC ATA AAG ATA TTG G |
710 | 48 | [15] |
F: forward; R: reverse; bp: base pairs; T: hybridisation temperature.
4.5. Statistical Analyses
We calculated larva infection rates as the number of TBP-positive engorged larvae out of the total number of engorged larvae collected from sampled birds. We then calculated the prevalence among birds (i.e., the number of birds hosting ticks with at least one TBP-positive engorged larva out of the total number of sampled birds) for each TBP genus detected and each bird species. Next, we tested the existence of a structuring effect of bird infections by all pathogen genera according to bird species and pathogen genus. This entailed using a binomial generalized linear model to test the prevalence of pathogens among birds for all pathogen genera according to the bird species and the pathogen genus. We then used another generalized linear model to test the prevalence of each pathogen genus among birds separately according to the bird species. Erithacus rubecula was set as the reference bird species because it was the most represented, and Bbsl as the reference pathogen genus because it was found on the greatest number of birds. Finally, we tested the existence of an effect of year on the bird prevalence by all pathogen genera using a generalized linear model. We did not test the effect of tick species on the prevalence among birds because the major tick species was I. ricinus and the samples for other tick species collected were too small. Bird species represented by fewer than four collected birds were removed from these analyses to increase statistical power. Results were considered significant when p < 0.05.
Acknowledgments
We would like to thank the 79 bird-ringers who made this study possible by voluntarily sampling ticks throughout France: Arnaud SPONGA, Fabien TOULOTTE, Sébastien GAUTIER, Romain PROVOST, Dominique BEAUVAIS, Philippe FONTANILLES, Camille DUPONCHEEL, Gérard CHAUSSI, Pierre MIGOT, Pierre PIOTTE, Maxime LEUCHTMANN, Cyril ERAUD, Claude MAURICE, Pascal BONNIN, Renaud ALLART, Pierre DE BOUET DU PORTAL, Fabien MARTAYAN, Marc BAUMANN, Frédéric VAIDIE, Yves BEAUVALLET, Benoît FONTAINE, Jean JOACHIM, Etienne DUPOUX, Boris JUILLARD, Julien GONIN, Patrice LAVOUÉ, Quentin DUPRIEZ, Yannig COULOMB, Pierre-Yves PERROI, Philippe AUBRY, Aurélie BARBOIRON, Gérard GOUJON, Michel BORIE, Alain RAVAYROL, Jérémy BAUWIN, Olivier BENOIT-GONIN, Julien LAIGNEL, Xavier COMMECY, Marie-Laure TONNELIER, Patrick FREBOURG, Vincent ROUSTANG, Philippe CARRUETTE, Xavier CHAUBY, Sylvain COURANT, Karim GUERBAA, Xavier ROZEC, Jacques BESNAULT, Marc BELLION, Guillaume CHEVRIER, Vincent COHEZ, David LAVOGIEZ, Olivier DELZONS, Brigitte GRAND, Rémi LANDEAU, Nicolas RENOUS, Nicolas PINCZON DU SEL, Lionel FREDERIC, Alain HARDY, David HEMERY, Marko JANKOVIC, Antoine LEONCINI, Albert MILLOT, Patrick MULOT, Bertrand SCAAR, Sylvain CARDONNEL, Jeremy MAINGUENEAU, Philippe OLLIVIER, François STEIMER, Nicolas BOILEAU, Pierre CARON, Nicolas CEBE, Bertrand COUILLENS, Simon DUTILLEUL, Antoine GERGAUD, Samuel HAVET, François HUMBERT, David LOOSE, Stéphanie RONDEL, Yanick JACOB.
Supplementary Materials
The following supporting information can be downloaded from: https://www.mdpi.com/article/10.3390/pathogens11080946/s1, Table S1: Number of ticks per host bird species; Table S2: Number of TBP-positive engorged tick larvae per pathogen and host bird species; Table S3: Accession numbers of submitted sequences; Table S4: Percentage of co-infected larvae; Figure S1: Geographical distribution of tick species other than I. ricinus (I. frontalis, I. arboricola, H. concinna, H. punctata, Hyalomma spp. and Rhipicephalus spp.) collected from breeding birds in 2019 and 2020; Figure S2: Capture sites for breeding birds in France in 2019–2020.
Author Contributions
Conceptualization, M.M., P.-Y.H., S.M. and A.R.; methodology, P.-Y.H., S.M., M.M., A.R. and C.G.; software, M.M. and A.R.; validation, M.M., P.-Y.H., S.M., L.B., C.G. and A.R.; formal analysis, M.M. and A.R.; investigation, S.M., P.-Y.H., M.M. and A.R.; resources, S.M., L.B., P.-Y.H. and M.M.; data curation, P.-Y.H.; writing—original draft preparation, A.R.; writing—review & editing, A.R., M.M., P.-Y.H., S.M., L.B. and C.G.; visualisation, A.R.; Supervision, M.M., S.M. and P.-Y.H.; project administration, M.M.; funding acquisition, P.-Y.H., S.M., M.M. and L.B. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Wild birds were captured by authorised bird-ringers (licensed by the CRBPO Natural History Museum in Paris, which is the scientific bird-ringing platform for France). Tick sampling does not require ethical approval in France.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
We are grateful for the funding provided by the French Agency for Food, Environmental and Occupational Health & Safety (ANSES) and DIM1 HEALTH (Ile-de-France Regional Council).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.de la Fuente J., Estrada-Pena A., Venzal J.M., Kocan K.M., Sonenshine D.E. Overview: Ticks as vectors of pathogens that cause disease in humans and animals. Front. Biosci. 2008;13:6938–6946. doi: 10.2741/3200. [DOI] [PubMed] [Google Scholar]
- 2.Slowik T.J., Lane R.S. Feeding preferences of the immature stages of three western north American ixodid ticks (Acari) for avian, reptilian, or rodent hosts. J. Med. Entomol. 2009;46:115–122. doi: 10.1603/033.046.0115. [DOI] [PubMed] [Google Scholar]
- 3.McCoy K.D., Boulanger N. Tiques Et Maladies à Tiques: BIOLOGIE, Écologie Évolutive, Épidémiologie. IRD Editions (Collection Didactiques); Marseille, France: 2015. [Google Scholar]
- 4.Rataud A., Henry P.Y., Moutailler S., Marsot M. Research effort on birds’ reservoir host potential for Lyme borreliosis: A systematic review and perspectives. Transbound Emerg. Dis. 2021:1–11. doi: 10.1111/tbed.14305. [DOI] [PubMed] [Google Scholar]
- 5.Falchi A., Dantas-Torres F., Lorusso V., Malia E., Lia R.P., Otranto D. Autochthonous and migratory birds as a dispersion source for Ixodes ricinus in southern Italy. Exp. Appl. Acarol. 2012;58:167–174. doi: 10.1007/s10493-012-9571-8. [DOI] [PubMed] [Google Scholar]
- 6.Klitgaard K., Hojgaard J., Isbrand A., Madsen J.J., Thorup K., Bodker R. Screening for multiple tick-borne pathogens in Ixodes ricinus ticks from birds in Denmark during spring and autumn migration seasons. Ticks Tick-Borne Dis. 2019;10:546–552. doi: 10.1016/j.ttbdis.2019.01.007. [DOI] [PubMed] [Google Scholar]
- 7.Mysterud A., Heylen D.J.A., Matthysen E., Garcia A.L., Jore S., Viljugrein H. Lyme neuroborreliosis and bird populations in northern Europe. Proceedings Biol. Sci. 2019;286:20190759. doi: 10.1098/rspb.2019.0759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marsot M., Henry P.Y., Vourc’h G., Gasqui P., Ferquel E., Laignel J., Grysan M., Chapuis J.L. Which forest bird species are the main hosts of the tick, Ixodes ricinus, the vector of Borrelia burgdorferi sensu lato, during the breeding season? Int. J. Parasitol. 2012;42:781–788. doi: 10.1016/j.ijpara.2012.05.010. [DOI] [PubMed] [Google Scholar]
- 9.Agoulon A., Hoch T., Heylen D., Chalvet-Monfray K., Plantard O. Unravelling the phenology of Ixodes frontalis, a common but understudied tick species in Europe. Ticks Tick-Borne Dis. 2019;10:505–512. doi: 10.1016/j.ttbdis.2018.12.009. [DOI] [PubMed] [Google Scholar]
- 10.Kaiser A., Seitz A., Strub O. Prevalence of Borrelia burgdorferi sensu lato in the nightingale (Luscinia megarhynchos) and other passerine birds. Int. J. Med. Microbiol. IJMM. 2002;291 Suppl 33:75–79. doi: 10.1016/S1438-4221(02)80016-9. [DOI] [PubMed] [Google Scholar]
- 11.Ciebiera O., Jerzak L., Nowak-Chmura M., Bochenski M. Ticks (Acari: Ixodida) on birds (Aves) migrating through the Polish Baltic coast. Exp. Appl. Acarol. 2019;77:241–251. doi: 10.1007/s10493-019-00341-z. [DOI] [PubMed] [Google Scholar]
- 12.Norte A.C., da Silva L.P., Tenreiro P.J., Felgueiras M.S., Araujo P.M., Lopes P.B., Matos C., Rosa A., Ferreira P.J., Encarnacao P., et al. Patterns of tick infestation and their Borrelia burgdorferi s.l. infection in wild birds in Portugal. Ticks Tick-Borne Dis. 2015;6:743–750. doi: 10.1016/j.ttbdis.2015.06.010. [DOI] [PubMed] [Google Scholar]
- 13.Norte A.C., de Carvalho I.L., Ramos J.A., Goncalves M., Gern L., Nuncio M.S. Diversity and seasonal patterns of ticks parasitizing wild birds in western Portugal. Exp. Appl. Acarol. 2012;58:327–339. doi: 10.1007/s10493-012-9583-4. [DOI] [PubMed] [Google Scholar]
- 14.Heylen D., Fonville M., Docters van Leeuwen A., Stroo A., Duisterwinkel M., van Wieren S., Diuk-Wasser M., de Bruin A., Sprong H. Pathogen communities of songbird-derived ticks in Europe’s low countries. Parasites Vectors. 2017;10:497. doi: 10.1186/s13071-017-2423-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hornok S., Flaisz B., Takacs N., Kontschan J., Csorgo T., Csipak A., Jaksa B.R., Kovats D. Bird ticks in Hungary reflect western, southern, eastern flyway connections and two genetic lineages of Ixodes frontalis and Haemaphysalis concinna. Parasites Vectors. 2016;9:101. doi: 10.1186/s13071-016-1365-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Keskin A., Erciyas-Yavuz K. A Preliminary Investigation on Ticks (Acari: Ixodidae) Infesting Birds in Kizilirmak Delta, Turkey. J. Med. Entomol. 2016;53:217–220. doi: 10.1093/jme/tjv149. [DOI] [PubMed] [Google Scholar]
- 17.Kocianova E., Rusnakova Taragelova V., Harustiakova D., Spitalska E. Seasonal infestation of birds with immature stages of Ixodes ricinus and Ixodes arboricola. Ticks Tick-Borne Dis. 2017;8:423–431. doi: 10.1016/j.ttbdis.2017.01.006. [DOI] [PubMed] [Google Scholar]
- 18.Pascucci I., Di Domenico M., Capobianco Dondona G., Di Gennaro A., Polci A., Capobianco Dondona A., Mancuso E., Camma C., Savini G., Cecere J.G., et al. Assessing the role of migratory birds in the introduction of ticks and tick-borne pathogens from African countries: An Italian experience. Ticks Tick-Borne Dis. 2019;10:101272. doi: 10.1016/j.ttbdis.2019.101272. [DOI] [PubMed] [Google Scholar]
- 19.Nowak-Chmura M. Ixodes eldaricus Djaparidze, 1950 (Ixodidae) on migrating birds—Reported first time in Poland. Vet. Parasitol. 2012;186:399–402. doi: 10.1016/j.vetpar.2011.11.029. [DOI] [PubMed] [Google Scholar]
- 20.Heylen D., Tijsse E., Fonville M., Matthysen E., Sprong H. Transmission dynamics of Borrelia burgdorferi s.l. in a bird tick community. Environ. Microbiol. 2013;15:663–673. doi: 10.1111/1462-2920.12059. [DOI] [PubMed] [Google Scholar]
- 21.Berthová L., Slobodník V., Slobodník R., Olekšák M., Sekeyová Z., Svitálková Z., Kazimírová M., Špitalská E. The natural infection of birds and ticks feeding on birds with Rickettsia spp. and Coxiella burnetii in Slovakia. Exp. Appl. Acarol. 2016;68:299–314. doi: 10.1007/s10493-015-9975-3. [DOI] [PubMed] [Google Scholar]
- 22.Wallménius K., Barboutis C., Fransson T., Jaenson T.G., Lindgren P.E., Nyström F., Olsen B., Salaneck E., Nilsson K. Spotted fever Rickettsia species in Hyalomma and Ixodes ticks infesting migratory birds in the European Mediterranean area. Parasites Vectors. 2014;7:318. doi: 10.1186/1756-3305-7-318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Capligina V., Salmane I., Keiss O., Vilks K., Japina K., Baumanis V., Ranka R. Prevalence of tick-borne pathogens in ticks collected from migratory birds in Latvia. Ticks Tick-Borne Dis. 2014;5:75–81. doi: 10.1016/j.ttbdis.2013.08.007. [DOI] [PubMed] [Google Scholar]
- 24.Dubska L., Literak I., Kverek P., Roubalova E., Kocianova E., Taragelova V. Tick-borne zoonotic pathogens in ticks feeding on the common nightingale including a novel strain of Rickettsia sp. Ticks Tick-Borne Dis. 2012;3:265–268. doi: 10.1016/j.ttbdis.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 25.Elfving K., Olsen B., Bergström S., Waldenström J., Lundkvist A., Sjöstedt A., Mejlon H., Nilsson K. Dissemination of spotted fever rickettsia agents in Europe by migrating birds. PLoS ONE. 2010;5:e8572. doi: 10.1371/journal.pone.0008572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Graham R.I., Mainwaring M.C., Du Feu R. Detection of spotted fever group Rickettsia spp. from bird ticks in the U.K. Med. Vet. Entomol. 2010;24:340–343. doi: 10.1111/j.1365-2915.2010.00886.x. [DOI] [PubMed] [Google Scholar]
- 27.Hornok S., Boldogh S.A., Takács N., Juhász A., Kontschán J., Földi D., Koleszár B., Morandini P., Gyuranecz M., Szekeres S. Anaplasmataceae closely related to Ehrlichia chaffeensis and Neorickettsia helminthoeca from birds in Central Europe, Hungary. Antonie Van Leeuwenhoek. 2020;113:1067–1073. doi: 10.1007/s10482-020-01415-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lommano E., Dvorak C., Vallotton L., Jenni L., Gern L. Tick-borne pathogens in ticks collected from breeding and migratory birds in Switzerland. Ticks Tick-Borne Dis. 2014;5:871–882. doi: 10.1016/j.ttbdis.2014.07.001. [DOI] [PubMed] [Google Scholar]
- 29.Csank T., Bhide K., Bencúrová E., Dolinská S., Drzewnioková P., Major P., Korytár Ľ., Bocková E., Bhide M., Pistl J. Detection of West Nile virus and tick-borne encephalitis virus in birds in Slovakia, using a universal primer set. Arch. Virol. 2016;161:1679–1683. doi: 10.1007/s00705-016-2828-5. [DOI] [PubMed] [Google Scholar]
- 30.Kazarina A., Japina K., Keiss O., Salmane I., Bandere D., Capligina V., Ranka R. Detection of tick-borne encephalitis virus in I. ricinus ticks collected from autumn migratory birds in Latvia. Ticks Tick-Borne Dis. 2015;6:178–180. doi: 10.1016/j.ttbdis.2014.11.011. [DOI] [PubMed] [Google Scholar]
- 31.Mancuso E., Toma L., Polci A., d’Alessio S.G., Di Luca M., Orsini M., Di Domenico M., Marcacci M., Mancini G., Spina F., et al. Crimean-Congo hemorrhagic fever virus genome in tick from migratory bird, Italy. Emerg. Infect. Dis. 2019;25:1418–1420. doi: 10.3201/eid2507.181345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Richter D., Debski A., Hubalek Z., Matuschka F.-R. Absence of Lyme disease spirochetes in larval Ixodes ricinus ticks. Vector-Borne Zoonotic Dis. 2012;12:21–27. doi: 10.1089/vbz.2011.0668. [DOI] [PubMed] [Google Scholar]
- 33.Hauck D., Jordan D., Springer A., Schunack B., Pachnicke S., Fingerle V., Strube C. Transovarial transmission of Borrelia spp., Rickettsia spp. and Anaplasma phagocytophilum in Ixodes ricinus under field conditions extrapolated from DNA detection in questing larvae. Parasites Vectors. 2020;13:176. doi: 10.1186/s13071-020-04049-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kurtenbach K., Hanincová K., Tsao J.I., Margos G., Fish D., Ogden N.H. Fundamental processes in the evolutionary ecology of Lyme borreliosis. Nat. Rev. Microbiol. 2006;4:660–669. doi: 10.1038/nrmicro1475. [DOI] [PubMed] [Google Scholar]
- 35.Pajoro M., Pistone D., Boccazzi I.V., Mereghetti V., Bandi C., Fabbi M., Scattorin F., Sassera D., Montagna M. Molecular screening for bacterial pathogens in ticks (Ixodes ricinus) collected on migratory birds captured in northern Italy. Folia Parasitol. 2018;65:008. doi: 10.14411/fp.2018.008. [DOI] [PubMed] [Google Scholar]
- 36.Pedersen B.N., Jenkins A., Kjelland V. Tick-borne pathogens in Ixodes ricinus ticks collected from migratory birds in southern Norway. PLoS ONE. 2020;15:e0230579. doi: 10.1371/journal.pone.0230579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dubska L., Literak I., Kocianova E., Taragelova V., Sverakova V., Sychra O., Hromadko M. Synanthropic birds influence the distribution of Borrelia species: Analysis of Ixodes ricinus ticks feeding on passerine birds. Appl. Environ. Microbiol. 2011;77:1115–1117. doi: 10.1128/AEM.02278-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gray J., Kahl O., Zintl A. What do we still need to know about Ixodes ricinus? Ticks Tick-Borne Dis. 2021;12:101682. doi: 10.1016/j.ttbdis.2021.101682. [DOI] [PubMed] [Google Scholar]
- 39.Paulauskas A., Sakalauskas P., Kaminskienė E., Šimkevičius K., Kibiša A., Radzijevskaja J. First record of Haemaphysalis concinna (Acari: Ixodidae) in Lithuania. Ticks Tick-Borne Dis. 2020;11:101460. doi: 10.1016/j.ttbdis.2020.101460. [DOI] [PubMed] [Google Scholar]
- 40.Stanko M., Derdáková M., Špitalská E., Kazimírová M. Ticks and their epidemiological role in Slovakia: From the past till present. Biologia. 2021;77:1575–1610. doi: 10.1007/s11756-021-00845-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Heylen D.J., Van Oosten A.R., Devriendt N., Elst J., De Bruyn L., Matthysen E. Seasonal feeding activity of the tree-hole tick, Ixodes arboricola. Parasitology. 2014;141:1044–1051. doi: 10.1017/S0031182014000225. [DOI] [PubMed] [Google Scholar]
- 42.Rubel F., Brugger K., Walter M., Vogelgesang J.R., Didyk Y.M., Fu S., Kahl O. Geographical distribution, climate adaptation and vector competence of the Eurasian hard tick Haemaphysalis concinna. Ticks Tick-Borne Dis. 2018;9:1080–1089. doi: 10.1016/j.ttbdis.2018.04.002. [DOI] [PubMed] [Google Scholar]
- 43.Stanek G., Wormser G.P., Gray J., Strle F. Lyme borreliosis. Lancet. 2012;379:461–473. doi: 10.1016/S0140-6736(11)60103-7. [DOI] [PubMed] [Google Scholar]
- 44.Ben I., Lozynskyi I. Prevalence of Anaplasma phagocytophilum in Ixodes ricinus and Dermacentor reticulatus and coinfection with Borrelia burgdorferi and Tick-Borne Encephalitis virus in western Ukraine. Vector Borne Zoonotic Dis. 2019;19:793–801. doi: 10.1089/vbz.2019.2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stańczak J., Biernat B., Racewicz M., Zalewska M., Matyjasek A. Prevalence of different Rickettsia spp. in Ixodes ricinus and Dermacentor reticulatus ticks (Acari: Ixodidae) in north-eastern Poland. Ticks Tick-Borne Dis. 2018;9:427–434. doi: 10.1016/j.ttbdis.2017.12.010. [DOI] [PubMed] [Google Scholar]
- 46.Welc-Falęciak R., Bajer A., Paziewska-Harris A., Baumann-Popczyk A., Siński E. Diversity of Babesia in Ixodes ricinus ticks in Poland. Adv. Med. Sci. 2012;57:364–369. doi: 10.2478/v10039-012-0023-9. [DOI] [PubMed] [Google Scholar]
- 47.Wagemakers A., Jahfari S., de Wever B., Spanjaard L., Starink M.V., de Vries H.J.C., Sprong H., Hovius J.W. Borrelia miyamotoi in vectors and hosts in The Netherlands. Ticks Tick-Borne Dis. 2017;8:370–374. doi: 10.1016/j.ttbdis.2016.12.012. [DOI] [PubMed] [Google Scholar]
- 48.Špitalská E., Literák I., Sparagano O.A.E., Golovchenko M., Kocianová E. Ticks (Ixodidae) from passerine birds in the Carpathian region. Wien. Klin. Wochenschr. 2006;118:759–764. doi: 10.1007/s00508-006-0729-4. [DOI] [PubMed] [Google Scholar]
- 49.Gehringer H., Schacht E., Maylaender N., Zeman E., Kaysser P., Oehme R., Pluta S., Splettstoesser W.D. Presence of an emerging subclone of Francisella tularensis holarctica in Ixodes ricinus ticks from south-western Germany. Ticks Tick-Borne Dis. 2013;4:93–100. doi: 10.1016/j.ttbdis.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 50.Hildebrandt A., Straube E., Neubauer H., Schmoock G. Coxiella burnetii and coinfections in Ixodes ricinus ticks in Central Germany. Vector Borne Zoonotic Dis. 2011;11:1205–1207. doi: 10.1089/vbz.2010.0180. [DOI] [PubMed] [Google Scholar]
- 51.Franke J., Fritzsch J., Tomaso H., Straube E., Dorn W., Hildebrandt A. Coexistence of pathogens in host-seeking and feeding ticks within a single natural habitat in Central Germany. Appl. Environ. Microbiol. 2010;76:6829–6836. doi: 10.1128/AEM.01630-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Toma L., Mancini F., Di Luca M., Cecere J.G., Bianchi R., Khoury C., Quarchioni E., Manzia F., Rezza G., Ciervo A. Detection of microbial agents in ticks collected from migratory birds in central Italy. Vector Borne Zoonotic Dis. 2014;14:199–205. doi: 10.1089/vbz.2013.1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Spitalska E., Literak I., Kocianova E., Taragel’ova V. The importance of Ixodes arboricola in transmission of Rickettsia spp., Anaplasma phagocytophilum, and Borrelia burgdorferi sensu lato in the Czech Republic, Central Europe. Vector Borne Zoonotic Dis. 2011;11:1235–1241. doi: 10.1089/vbz.2010.0210. [DOI] [PubMed] [Google Scholar]
- 54.Van Oosten A.R., Heylen D.J., Matthysen E. Host specificity of a bird-specialised endophilic ectoparasite, the tree-hole tick Ixodes arboricola. Parasitol. Res. 2014;113:4397–4405. doi: 10.1007/s00436-014-4116-1. [DOI] [PubMed] [Google Scholar]
- 55.Palomar A.M., Portillo A., Santibáñez P., Mazuelas D., Roncero L., García-Álvarez L., Santibáñez S., Gutiérrez Ó., Oteo J.A. Detection of tick-borne Anaplasma bovis, Anaplasma phagocytophilum and Anaplasma centrale in Spain. Med. Vet. Entomol. 2015;29:349–353. doi: 10.1111/mve.12124. [DOI] [PubMed] [Google Scholar]
- 56.Tijsse-Klasen E., Hansford K.M., Jahfari S., Phipps P., Sprong H., Medlock J.M. Spotted fever group rickettsiae in Dermacentor reticulatus and Haemaphysalis punctata ticks in the UK. Parasites Vectors. 2013;6:212. doi: 10.1186/1756-3305-6-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Phipps L.P., Hansford K.M., Hernández-Triana L.M., Golding M., McGinley L., Folly A.J., Vaux A.G.C., de Marco M.F., Carter D.P., Medlock J.M., et al. Detection of Borrelia and Babesia species in Haemaphysalis punctata ticks sampled in Southern England. Ticks Tick-Borne Dis. 2022;13:101902. doi: 10.1016/j.ttbdis.2022.101902. [DOI] [PubMed] [Google Scholar]
- 58.Capek M., Literak I., Kocianova E., Sychra O., Najer T., Trnka A., Kverek P. Ticks of the Hyalomma marginatum complex transported by migratory birds into Central Europe. Ticks Tick-Borne Dis. 2014;5:489–493. doi: 10.1016/j.ttbdis.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 59.Pereira A., Parreira R., Cotão A.J., Nunes M., Vieira M.L., Azevedo F., Campino L., Maia C. Tick-borne bacteria and protozoa detected in ticks collected from domestic animals and wildlife in central and southern Portugal. Ticks Tick-Borne Dis. 2018;9:225–234. doi: 10.1016/j.ttbdis.2017.09.008. [DOI] [PubMed] [Google Scholar]
- 60.Norte A.C., Margos G., Becker N.S., Albino Ramos J., Nuncio M.S., Fingerle V., Araujo P.M., Adamik P., Alivizatos H., Barba E., et al. Host dispersal shapes the population structure of a tick-borne bacterial pathogen. Mol. Ecol. 2019;29:485–501. doi: 10.1111/mec.15336. [DOI] [PubMed] [Google Scholar]
- 61.Mtierová Z., Derdáková M., Chvostáč M., Didyk Y.M., Mangová B., Rusňáková Tarageľová V., Selyemová D., Šujanová A., Václav R. Local population structure and seasonal variability of Borrelia garinii genotypes in Ixodes ricinus ticks, Slovakia. Int. J. Environ. Res. Public Health. 2020;17:3607. doi: 10.3390/ijerph17103607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Norte A.C., Ramos J.A., Gern L., Nuncio M.S., Lopes de Carvalho I. Birds as reservoirs for Borrelia burgdorferi s.l. in Western Europe: Circulation of B. turdi and other genospecies in bird-tick cycles in Portugal. Environ. Microbiol. 2013;15:386–397. doi: 10.1111/j.1462-2920.2012.02834.x. [DOI] [PubMed] [Google Scholar]
- 63.Kurtenbach K., De Michelis S., Etti S., Schäfer S.M., Sewell H.-S., Brade V., Kraiczy P. Host association of Borrelia burgdorferi sensu lato–the key role of host complement. Trends Microbiol. 2002;10:74–79. doi: 10.1016/S0966-842X(01)02298-3. [DOI] [PubMed] [Google Scholar]
- 64.Humair P.F., Postic D., Wallich R., Gern L. An avian reservoir (Turdus merula) of the Lyme borreliosis spirochetes. Zent. Fur Bakteriol. Int. J. Med. Microbiol. 1998;287:521–538. doi: 10.1016/S0934-8840(98)80194-1. [DOI] [PubMed] [Google Scholar]
- 65.Gern L., Rais O. Efficient transmission of Borrelia burgdorferi between cofeeding Ixodes ricinus ticks (Acari: Ixodidae) J. Med. Entomol. 1996;33:189–192. doi: 10.1093/jmedent/33.1.189. [DOI] [PubMed] [Google Scholar]
- 66.Randolph S., Gern L., Nuttall P. Co-feeding ticks: Epidemiological significance for tick-borne pathogen transmission. Parasitol. Today. 1996;12:472–479. doi: 10.1016/S0169-4758(96)10072-7. [DOI] [PubMed] [Google Scholar]
- 67.Gryczynska A., Zgodka A., PLoSki R., Siemiatkowski M. Borrelia burgdorferi sensu lato infection in passerine birds from the Mazurian Lake region (Northeastern Poland) Avian Pathol. 2004;33:69–75. doi: 10.1080/03079450310001636309. [DOI] [PubMed] [Google Scholar]
- 68.Wilhelmsson P., Jaenson T.G.T., Olsen B., Waldenström J., Lindgren P.E. Migratory birds as disseminators of ticks and the tick-borne pathogens Borrelia bacteria and tick-borne encephalitis (TBE) virus: A seasonal study at Ottenby Bird Observatory in South-eastern Sweden. Parasites Vectors. 2020;13:607. doi: 10.1186/s13071-020-04493-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hornok S., Kováts D., Csörgő T., Meli M.L., Gönczi E., Hadnagy Z., Takács N., Farkas R., Hofmann-Lehmann R. Birds as potential reservoirs of tick-borne pathogens: First evidence of bacteraemia with Rickettsia helvetica. Parasites Vectors. 2014;7:128. doi: 10.1186/1756-3305-7-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Biernat B., Stańczak J., Michalik J., Sikora B., Wierzbicka A. Prevalence of infection with Rickettsia helvetica in Ixodes ricinus ticks feeding on non-rickettsiemic rodent hosts in sylvatic habitats of west-central Poland. Ticks Tick-Borne Dis. 2016;7:135–141. doi: 10.1016/j.ttbdis.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 71.Socolovschi C., Mediannikov O., Raoult D., Parola P. The relationship between spotted fever group Rickettsiae and ixodid ticks. Vet. Res. 2009;40:34. doi: 10.1051/vetres/2009017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hornok S., Csörgő T., de la Fuente J., Gyuranecz M., Privigyei C., Meli M.L., Kreizinger Z., Gönczi E., Fernández de Mera I.G., Hofmann-Lehmann R. Synanthropic birds associated with high prevalence of tick-borne rickettsiae and with the first detection of Rickettsia aeschlimannii in Hungary. Vector Borne Zoonotic Dis. 2013;13:77–83. doi: 10.1089/vbz.2012.1032. [DOI] [PubMed] [Google Scholar]
- 73.Santos-Silva M.M., Sousa R., Santos A.S., Melo P., Encarnação V., Bacellar F. Ticks parasitizing wild birds in Portugal: Detection of Rickettsia aeschlimannii, R. helvetica and R. massiliae. Exp. Appl. Acarol. 2006;39:331–338. doi: 10.1007/s10493-006-9008-3. [DOI] [PubMed] [Google Scholar]
- 74.Mancini F., Ciccozzi M., Lo Presti A., Cella E., Giovanetti M., Di Luca M., Toma L., Bianchi R., Khoury C., Rezza G., et al. Characterization of spotted fever group Rickettsiae in ticks from a city park of Rome, Italy. Ann. Dell’istituto Super. Di Sanita. 2015;51:284–290. doi: 10.4415/ann_15_04_07. [DOI] [PubMed] [Google Scholar]
- 75.Mărcuţan I.D., Kalmár Z., Ionică A.M., D’Amico G., Mihalca A.D., Vasile C., Sándor A.D. Spotted fever group rickettsiae in ticks of migratory birds in Romania. Parasites Vectors. 2016;9:294. doi: 10.1186/s13071-016-1565-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Martello E., Selmi M., Ragagli C., Ambrogi C., Stella M.C., Mannelli A., Tomassone L. Rickettsia slovaca in immature Dermacentor marginatus and tissues from Apodemus spp. in the northern Apennines, Italy. Ticks Tick-Borne Dis. 2013;4:518–521. doi: 10.1016/j.ttbdis.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 77.Hoffman T., Wilhelmsson P., Barboutis C., Fransson T., Jaenson T.G.T., Lindgren P.E., Von Loewenich F.D., Lundkvist Å., Olsen B., Salaneck E. A divergent Anaplasma phagocytophilum variant in an Ixodes tick from a migratory bird; Mediterranean basin. Infect. Ecol. Epidemiol. 2020;10:1729653. doi: 10.1080/20008686.2020.1729653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Palomar A.M., Santibanez P., Mazuelas D., Roncero L., Santibanez S., Portillo A., Oteo J.A. Role of birds in dispersal of etiologic agents of tick-borne zoonoses, Spain, 2009. Emerg. Infect. Dis. 2012;18:1188–1191. doi: 10.3201/eid1807.111777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Johnston E., Tsao J.I., Muñoz J.D., Owen J. Anaplasma phagocytophilum infection in American robins and gray catbirds: An assessment of reservoir competence and disease in captive wildlife. J. Med. Entomol. 2013;50:163–170. doi: 10.1603/ME12141. [DOI] [PubMed] [Google Scholar]
- 80.Keesing F., Hersh M.H., Tibbetts M., McHenry D.J., Duerr S., Brunner J., Killilea M., LoGiudice K., Schmidt K.A., Ostfeld R.S. Reservoir competence of vertebrate hosts for Anaplasma phagocytophilum. Emerg. Infect. Dis. 2012;18:2013–2016. doi: 10.3201/eid1812.120919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Karshima S.N., Karshima M.N., Ahmed M.I. Animal reservoirs of zoonotic Babesia species: A global systematic review and meta-analysis of their prevalence, distribution and species diversity. Vet. Parasitol. 2021;298:109539. doi: 10.1016/j.vetpar.2021.109539. [DOI] [PubMed] [Google Scholar]
- 82.Baráková I., Derdáková M., Selyemová D., Chvostáč M., Špitalská E., Rosso F., Collini M., Rosà R., Tagliapietra V., Girardi M., et al. Tick-borne pathogens and their reservoir hosts in northern Italy. Ticks Tick-Borne Dis. 2018;9:164–170. doi: 10.1016/j.ttbdis.2017.08.012. [DOI] [PubMed] [Google Scholar]
- 83.Wilhelmsson P., Pawełczyk O., Jaenson T.G.T., Waldenström J., Olsen B., Forsberg P., Lindgren P.E. Three Babesia species in Ixodes ricinus ticks from migratory birds in Sweden. Parasites Vectors. 2021;14:183. doi: 10.1186/s13071-021-04684-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Monks D., Fisher M., Forbes N. Ixodes frontalis and avian tick—related syndrome in the United Kingdom. J. Small Anim. Pract. 2006;47:451–455. doi: 10.1111/j.1748-5827.2006.00031.x. [DOI] [PubMed] [Google Scholar]
- 85.Machado R.Z., André M.R., Werther K., de Sousa E., Gavioli F.A., Alves Junior J.R. Migratory and carnivorous birds in Brazil: Reservoirs for Anaplasma and Ehrlichia species? Vector Borne Zoonotic Dis. 2012;12:705–708. doi: 10.1089/vbz.2011.0803. [DOI] [PubMed] [Google Scholar]
- 86.Sacchi A.B.V., André M.R., Calchi A.C., de Santi M., Guimarães A., Pires J.R., Baldani C.D., Werther K., Machado R.Z. Molecular and serological detection of arthropod-borne pathogens in carnivorous birds from Brazil. Vet. Parasitol. Reg. Stud. Rep. 2021;23:100539. doi: 10.1016/j.vprsr.2021.100539. [DOI] [PubMed] [Google Scholar]
- 87.Heylen D., De Coninck E., Jansen F., Madder M. Differential diagnosis of three common Ixodes spp. ticks infesting songbirds of Western Europe: Ixodes arboricola, I. frontalis and I. ricinus. Ticks Tick-Borne Dis. 2014;5:693–700. doi: 10.1016/j.ttbdis.2014.05.006. [DOI] [PubMed] [Google Scholar]
- 88.Heylen D., Krawczyk A., Lopes de Carvalho I., Nuncio M.S., Sprong H., Norte A.C. Bridging of cryptic Borrelia cycles in European songbirds. Environ. Microbiol. 2017;19:1857–1867. doi: 10.1111/1462-2920.13685. [DOI] [PubMed] [Google Scholar]
- 89.Tsao J.I., Hamer S.A., Han S., Sidge J.L., Hickling G.J. The contribution of wildlife hosts to the rise of ticks and tick-borne diseases in North America. J. Med. Entomol. 2021;58:1565–1587. doi: 10.1093/jme/tjab047. [DOI] [PubMed] [Google Scholar]
- 90.Estrada-Peña A., Mihalca A.D., Petney T.N. Ticks of Europe and North Africa: A guide to species identification. Springer; Berlin/Heidelberg, Germany: 2018. [Google Scholar]
- 91.Pérez-Eid C. Les Tiques: Identification, Biologie, Importance médicale Et Vétérinaire. Lavoisier; Paris, France: 2007. [Google Scholar]
- 92.Banović P., Díaz-Sánchez A.A., Galon C., Simin V., Mijatović D., Obregón D., Moutailler S., Cabezas-Cruz A. Humans infested with Ixodes ricinus are exposed to a diverse array of tick-borne pathogens in Serbia. Ticks Tick-Borne Dis. 2021;12:101609. doi: 10.1016/j.ttbdis.2020.101609. [DOI] [PubMed] [Google Scholar]
- 93.Banović P., Díaz-Sánchez A.A., Galon C., Foucault-Simonin A., Simin V., Mijatović D., Papić L., Wu-Chuang A., Obregón D., Moutailler S. A One Health approach to study the circulation of tick-borne pathogens: A preliminary study. One Health. 2021;13:100270. doi: 10.1016/j.onehlt.2021.100270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Boularias G., Azzag N., Galon C., Šimo L., Boulouis H.J., Moutailler S. High-throughput microfluidic real-time PCR for the detection of multiple microorganisms in Ixodid cattle ticks in northeast Algeria. Pathogens. 2021;10:362. doi: 10.3390/pathogens10030362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Loh S.-M., Gofton A.W., Lo N., Gillett A., Ryan U.M., Irwin P.J., Oskam C.L. Novel Borrelia species detected in echidna ticks, Bothriocroton concolor, in Australia. Parasites Vectors. 2016;9:339. doi: 10.1186/s13071-016-1627-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Rar V.A., Fomenko N.V., Dobrotvorsky A.K., Livanova N.N., Rudakova S.A., Fedorov E.G., Astanin V.B., Morozova O.V. Tickborne pathogen detection, western Siberia, Russia. Emerg. Infect. Dis. 2005;11:1708. doi: 10.3201/eid1111.041195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Regnery R.L., Spruill C.L., Plikaytis B. Genotypic identification of rickettsiae and estimation of intraspecies sequence divergence for portions of two rickettsial genes. J. Bacteriol. 1991;173:1576–1589. doi: 10.1128/jb.173.5.1576-1589.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Masatani T., Hayashi K., Andoh M., Tateno M., Endo Y., Asada M., Kusakisako K., Tanaka T., Gokuden M., Hozumi N. Detection and molecular characterization of Babesia, Theileria, and Hepatozoon species in hard ticks collected from Kagoshima, the southern region in Japan. Ticks Tick-Borne Dis. 2017;8:581–587. doi: 10.1016/j.ttbdis.2017.03.007. [DOI] [PubMed] [Google Scholar]
- 99.Bunikis J., Garpmo U., Tsao J., Berglund J., Fish D., Barbour A.G. Sequence typing reveals extensive strain diversity of the Lyme borreliosis agents Borrelia burgdorferi in North America and Borrelia afzelii in Europe. Microbiology. 2004;150:1741–1755. doi: 10.1099/mic.0.26944-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.