Abstract
The Broussonetia genus (Moraceae), recognized for its value in many Chinese traditional herbs, mainly includes Broussonetia papyrifera (L.) L’Hér. ex Vent. (BP), Broussonetia kazinoki Siebold (BK), and Broussonetia luzonica (Blanco) Bureau (BL). Hitherto, researchers have found 338 compounds isolated from BP, BK, and BL, which included flavonoids, polyphenols, phenylpropanoids, alkaloids, terpenoids, steroids, and others. Moreover, its active compounds and extracts have exhibited a variety of pharmacological effects such as antitumor, antioxidant, anti-inflammatory, antidiabetic, anti-obesity, antibacterial, and antiviral properties, and its use against skin wrinkles. In this review, the phytochemistry and pharmacology of Broussonetia are updated systematically, after its applications are first summarized. In addition, this review also discusses the limitations of investigations and the potential direction of Broussonetia. This review can help to further understand the phytochemistry, pharmacology, and other applications of Broussonetia, which paves the way for future research.
Keywords: Broussonetia, phytochemistry, pharmacology, applications
1. Introduction
Broussonetia is one of the most significant genera in the Moraceae family, a member of the Urticales order. The genus is composed of eleven species, comprising Broussonetia papyrifera (BP) (see Figure 1) [1], Broussonetia kazinoki (BK) (see Figure 2) [2], Broussonetia zeylanica (Thwaites) Corner (BZ) [3], Broussonetia luzonica (Blanco) Bureau (BL) [4], Broussonetia rupicola F.T. Wang and Tang (BR), Broussonetia kurzii (Hook.f.) Corner (BKU), Broussonetia kaempferi Siebold (BKA) [5], Broussonetia integrifolia Buch.-Ham. (BI), Broussonetia harmandii Gagnep. (BHG), Broussonetia × hanjiana M.Kim (BHM), and Broussonetia greveana (Baill.) C.C.Berg (BG) [6]. The various Broussonetia species have been an excellent source of conventional medicine to treat different diseases. Their roots, barks, fruits, and leaves have all been used in conventional medicine. In China, the leaves have been used to treat chronic prostatitis as a folk medicine [7], as well as for bleeding [8]. The bark could be used for special recipes [8]. The fruits have been confirmed to treat impotence and ophthalmic diseases [7], while the hematochrome from the fruits could be used as a foodstuff in history [9]. In the traditions of Tonga, Fiji, and Samoa, BP, a fibrous tree, was the main raw material used to make tapa cloth [10]. Moreover, one of the Broussonetia species was used by Cai Lun to create paper, one of the four great inventions of ancient China. Broussonetia species were also used as woody forage in ancient history [11].
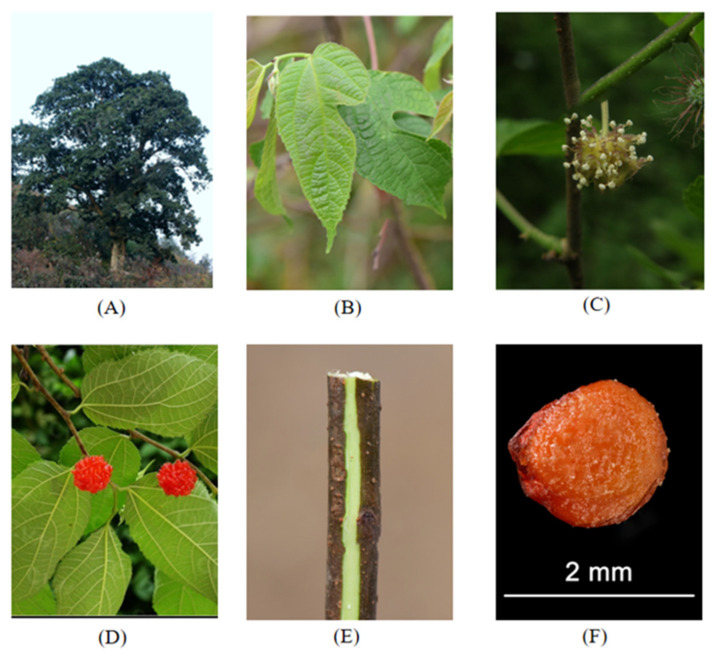
Figure 1.
Broussonetia papyrifera (L.) L’Hér. ex Vent. Images A–F show, respectively: the whole plant (A), leaves (B), flowers (C), fruits (D), twigs (E), and seeds (F).
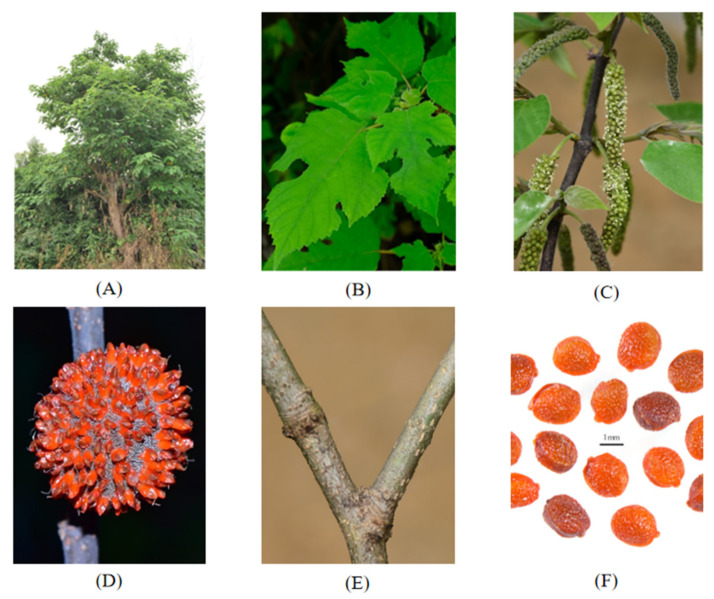
Figure 2.
Broussonetia kazinoki Siebold. Images A–F show, respectively: the whole plant (A), leaves (B), flowers (C), fruits (D), twigs (E) and seeds (F) of Broussonetia kazinoki Siebold.
According to the publication, Flora of China [12], the morphology of the genus is described thus: “Trees or shrubs, or vine-like shrubs; there is emulsion, and the winter buds are small. Leaves are alternate, split or non-divided, with serrated margins, basal veins triangular, lateral pinnate veins, and lateral leaves, detached, ovate lanceolate, early fall. The flowers are hermaphroditic or identical; the male flowers are drooping soft inflorescences or spherical cephalic inflorescences, the flowers are indumental, 4 or 3 lobes, the stamens and the flowers are fissured in the same number, folded inward at the time of flower buds, the degenerated stamens are small; the female flowers are densely spherical head-shaped inflorescences, bracts are stick-like, sustenance, flower tube-shaped, apical 3–4 lobes or full margins, succumbs, ovary hidden, stalked, pedunculate lateral, linear, ovules hanging from the ventricular roof. Polyflora is spherical, the embryo is curved, the cotyledons are rounded, flattened, or folded. The genus is distributed in eastern Asia and Pacific islands”.
The diversity of the chemical structures and pharmacological effects has attracted the interest of a variety of researchers. The genus of Broussonetia has been found to present 338 compounds, including flavonoids, polyphenols, alkaloids, terpenoids, steroids, and others. Active compounds isolated from Broussonetia have been demonstrated to have several biological properties, including antitumor [2], antioxidant [13], anti-inflammation [14], antidiabetic [15], anti-obesity [16], antibacterial [17], and antiviral activities [18], as well as being used for skin whitening [19] and against skin wrinkles, as well as many other uses. In terms of representative applications, Broussonetia species could be used as forage for cattle, Hu rams and lambs, growing goats, and other animals because of the high content of protein and fiber [20,21]. In addition, BP, combined with Lonicera japonica, may be used to treat inflammatory disorders [22]. Liquid bandages that include Styela clava tunics and BK bark cellulose powders could be used to heal cutaneous wounds [23].
This review efforts to provide comprehensive and up-to-date information on the Broussonetia genus, based on published references, focusing on phytochemistry, pharmacology, and several other applications. We also discuss the limitations of current research into Broussonetia. This review may provide a reference for researchers around the world to investigate and explore the potential applications of the Broussonetia genus.
2. Phytochemistry
According to the published references, outstanding results have been acquired by a variety of researchers when studying the stems, leaves, barks, radices, fruits, and whole plants of the Broussonetia genus. In total, 338 compounds have been isolated from BP, BK, and BL of the Broussonetia genus; these compounds consist of 144 flavonoids, 50 penylpropanoids, 38 polyphenols, 35 alkaloids, 17 terpenoids, 5 steroids, and 49 other metabolites. Their chemical structures have been elucidated using the nuclear magnetic resonance spectrometer (NMR) and mass spectrum (MS), along with comparisons with the published data. Their presence might be responsible for the biological properties of the various Broussonetia species. However, bioactive compounds that have been confirmed are scarce, the emphasis being on their crude extracts, leading to limitations in the finding of potential candidates for the treatment of corresponding diseases. Given the fact that abundant phytochemicals have been identified, their structure–activity relationships should be unraveled via numerous assays in the future, which will be conducive to unlocking the answers on how phytochemicals develop as targeted bioactive molecules.
2.1. Flavonoids
Compounds where the basic core is 2-phenylchromone are classified as flavonoids. Flavones are the main compounds in the 144 flavonoids extracted from Broussonetia species. Flavonoids, as primary bioactive compounds, demonstrated the most pharmacological properties, such as antitumor, antioxidant, anti-inflammatory, anti-diabetic, anti-obesity, antibacterial, and antiviral effects, along with skin whitening and anti-wrinkle properties, as well as other activities. In 1994, a new isoprenylated aurone, compound 76, and a novel isoprenylated flavan, compound 18, together with the known compounds 38, 40, 60, and 61, were isolated and characterized from the cortex of BP [24]. In 1995, Fang et al. [25] described how two new isoprenylated flavonols, compounds 49 and 51, were isolated and characterized from the root bark of BP. In 1996, Lin et al. [26] reported the presence of compounds 52, 142, 143, and 144 using two-dimensional techniques. A new prenylflavan, compound 37, and the known compounds 119 and 120 were isolated from the root barks of BK, and these compounds were evaluated for cytotoxic activity against several different cell lines [27]. In 2001, an in vitro aromatase inhibition assay was conducted by Lee et al. [28], and the results showed that the novel compounds 59, 26, 75, and 46, as well as the known compounds 47, 48, 50, 139, and 140, were found to be active as aromatase inhibitors, while compounds 56, 58, and 63 were inactive. Zhang et al. [29] described, for the first time, five new diprenylated flavonols, compounds 45, 100, 136, 137, and 138, which were obtained from an ethanolic extract of the leaves of BK; they evaluated the cytotoxic activities of these compounds. In 2002, two new compounds, 71 and 135, were isolated from the roots of BP and the structures were determined using spectroscopy; moreover, compound 71 exhibited significantly inhibitory activities against the PTP1B enzyme [30]. In 2008, the structure of a novel compound, 126, together with compounds 15, 16, 20, 27, 69, 70, and 110, were identified by the interpretation of MS, 1H NMR, 13C NMR, HMQC, and HMBC data, and compounds 15, 20, and 126 showed high inhibitory activities on mushroom tyrosinase [31]. Ko et al. [32] reported the presence of compounds 4, 5, 133, and 134, which were isolated from the leaves of BP. In 2012, three flavans, compounds 129, 130, and 131, were isolated from the stem barks of BK and, in addition, compound 129 significantly inhibited adipocyte differentiation in 3T3-L1 cells [33]. Guo et al. [34] described the new compounds 82 and 83, together with known compound 128, which were isolated and purified from an ethyl acetate-soluble extract from the bark of BP; moreover, compounds 83 and 128 were found to strongly down-regulate the expression concentrations of estrogen receptor-α and inhibit the growth of the human breast cancer line. In the same year, compound 114 was reported for the first time by Ran et al. [35]; this compound showed definite activities against HepG-2. In 2014, the structures of compounds 11, 12, 22, 74, 78, and 127 were evaluated, on the basis of NMR spectra analysis and chemical evidence, by Yang et al. [36], and compounds 12 and 127 showed strong antioxidant activity against ABTS and DPPH. In 2018, four new flavans, compounds 116, 117, 118, and 121, as well as the known compounds 109, 122, and 123 were obtained from twigs of BK by chiral HPLC resolution; compounds 116, 117, and 118 showed the in vitro inhibition of PTP1B [37]. In 2019, compounds 108, 111, and 112 were reported by Li et al. [38]. A new isoprenylated flavonol, compound 103, and the known compounds 104, 105, and 106, were obtained from Broussonetia for the first time, being isolated from the twigs of BP; compounds 103–107 showed significant inhibitory effects on PTP1B [39]. In 2019, the metabolite investigation of root bark extracts of BP was reported by Ryu et al. [40]. The results showed that the novel compounds 98 and 99 exhibited anti-inflammatory activity by inhibiting NO production in LPS-induced RAW264.7 cells. Four new compounds (89–92) and four known compounds (93–96) were isolated from the root bark of BP [41]. Compound 91 showed inhibitory activity against tyrosinase, while compound 92 exhibited cytotoxic activity against three cancer cell lines (NCIH1975, HepG2, and MCF-7), and compound 89 inhibited the production of IL-2 in Jurkat cells [41]. In 2020, the structures of several compounds (43, 84–88) were elucidated, based on NMR and HRMS data, showing that compounds 43 and 86 could be used for inflammatory diseases [42]. Compounds 53, 54, 55, 57, 62, 64, 65, 66, 67, 68, 72, 77, 79, 80 and 81 were reported by Qureshi et al. [43]. In 2021, six previously undescribed prenylated flavonoids, compounds 32, 33, 34, 36, 39, and 42, and three known compounds, 35, 41, and 44, were isolated from the roots of BK; compounds 32, 33, and 36 showed strong dose-dependent antiosteoclastogenic activities [44]. Compounds 28–31 were isolated from the roots of BK; compounds 29 and 31 showed anti-inflammatory by inhibiting LPS–induced NO production [14]. In addition, Yadav et al. [45] reported compounds 1, 3, 6, 7, 8, 13, 17, 19, 21, 23, 24, and 25.
All flavonoids are summarized in Table 1, and the structures were summarized in Figure S1.
Table 1.
Flavonoids isolated from Broussonetia species.
| Number | Compounds | Parts | Source | References |
|---|---|---|---|---|
| 1 | Gancaonin P | Whole plants | BP | [45] |
| 2 | Isolicoflavonol | Whole plants | BP | [27,30,42,44] |
| 3 | Lespedezaflavanone C | Whole plants | BP | [45] |
| 4 | Vitexin | Leaves | BP | [31,35,44] |
| 5 | Apigenin | Leaves | BP/BK | [13,31,35,44] |
| 6 | Pinocembrin | Whole plants | BP | [45] |
| 7 | Isobavachalcone | Whole plants | BP | [45] |
| 8 | 4-Hydroxyisolonchocarpin | Roots | BP | [37,44,45] |
| 9 | Luteolin | Leaves/twigs | BP | [30,35,44,46] |
| 10 | Cosmosiin | Leaves | BP | [35,45] |
| 11 | Isoorientin | Leaves | BP | [36,45] |
| 12 | Orientin | Leaves | BP | [36,45] |
| 13 | 2,4,2′,4′-Tetrahydroxychalcone | Whole plants | BP | [43,45] |
| 14 | Abyssinone II | Whole plants | BP | [28,45] |
| 15 | Uralenol | Roots/twigs/barks | BP | [29,30,33,37,38,44] |
| 16 | Papyriflavonol A | Root barks/twigs | BP | [30,33,40,44,47,48] |
| 17 | Norartocarpanone | Whole plants | BP | [45] |
| 18 | Broussoflavan A | Root barks | BP | [23,39,44,45,49] |
| 19 | Dihydrokaempferol | Whole plants | BP/BK | [14,45] |
| 20 | Quercetin | Twigs | BP | [31,45] |
| 21 | Bavachin | Whole plants | BP | [45] |
| 22 | Isovitexin | Leaves | BP | [36,45] |
| 23 | Broussofluorenone C | Whole plants | BP | [45] |
| 24 | Broussinol | Whole plants | BP | [45] |
| 25 | Sulfuretin | Whole plants | BP | [45] |
| 26 | Isogemichalcone C | Whole plants | BP | [28,45] |
| 27 | Isoliquiritigenin | Twigs | BP | [31,45] |
| 28 | Hesperetin | Roots | BK | [14] |
| 29 | Eriodictyol | Roots | BK | [14] |
| 30 | Chrysoeriol | Roots | BK | [14] |
| 31 | Kaempferol | Roots | BK | [14] |
| 32 | Broussonol F | Roots | BK | [44] |
| 33 | Broussonol G | Roots | BK | [40,44] |
| 34 | Broussonol H | Roots | BK | [44] |
| 35 | Broussonol I | Roots | BK | [44] |
| 36 | Broussonol K | Roots | BK | [44] |
| 37 | Kazinol Q | Root barks/branches and twigs | BP/BK | [26,40,41,43] |
| 38 | Kazinol A | Roots | BP/BK | [23,39,42,43,50] |
| 39 | Broussonol L | Roots | BK | [44] |
| 40 | Kazinol B | Roots/branches and twigs | BP/BK | [24,40,42,43,44] |
| 41 | Daphnegiravan D | Roots | BK | [44] |
| 42 | Broussonol M | Roots | BK | [44] |
| 43 | Broussoflavonol A | Roots/branches and twigs | BK/BP | [42,43,44] |
| 44 | 4,2′-Dihydroxy-4′-methoxychalcone | Roots | BK | [44] |
| 45 | Broussonol C | Roots/leaves | BK | [29,44] |
| 46 | (2S)-2′,4′-Dihydroxy-2″-(1-hydroxy-1-methylethyl)-dihydrofuro-2,3-h flavanone | Whole plants | BP | [28,43] |
| 47 | (2S)-5,7,2′,4′-Tetrahydroxyflavanone | Whole plants | BP | [28,43] |
| 48 | (2S)-Euchrenone | Whole plants | BP | [28,43] |
| 49 | Broussoflavonol F | Root barks/twigs | BP | [24,27,30,42,49] |
| 50 | (2S)-Naringenin | Whole plants | BP | [28,43] |
| 51 | Broussoflavonol E | Root barks/twigs | BP | [24,38,42] |
| 52 | Broussoflavonol G | Root barks/Whole plants | BP | [25,42,49] |
| 53 | Broussoflavonol C | Root barks/Whole plants | BP | [40,43] |
| 54 | Broussoflavonol D | Whole plants | BP | [43] |
| 55 | 4′-O-Methyldavidioside | Whole plants | BP | [43] |
| 56 | 5,7,3′,4′-Tetrahydroxy-3-methoxy-6-geranylflavone | Whole plants/twigs | BP | [27,38,42] |
| 57 | Broussoflavonol B | Whole plants/branches and twigs/root barks | BP | [38,39,41,42] |
| 58 | 5,7,3′,4′-Tetrahydroxy-6-geranylflavonol | Whole plants | BP | [28,43] |
| 59 | 5,7,2′,4′-Tetrahydroxy-3-geranylflavone | Whole plants | BP | [28,43] |
| 60 | Broussochalcone A | Roots/twigs/barks | BP | [23,29,30,33,37,39,42,48] |
| 61 | Broussochalcone B | Roots | BP | [23,42,45,48] |
| 62 | (2S)-Abyssinone II | Whole plants | BP | [43] |
| 63 | (2S)-7,4′-Dihydroxy-3′-prenylflavan | Whole plants/twigs | BP/BK | [27,36,42] |
| 64 | Broussin | Branches and twigs | BP | [42,43] |
| 65 | Isoliquiritigenin 2′-methy ether | Whole plants | BP | [43] |
| 66 | 1,2,4-Dihydroxy-3-(3-methylbut-2-en-1-yl)-phenyl-3-(2,4-dihydroxyphenyl)-propan-1-one | Whole plants | BP | [43] |
| 67 | 2-{5,7-Dihydroxy-2-(4-hydroxyphenyl)-4-oxo-3,4-dihydro-2-H-chromen-8-ylamino}-pentanedioic acid | Whole plants | BP | [43] |
| 68 | Broussofluorenone B | Roots | BP | [42,48,51] |
| 69 | 5,7,3′,5′-Tetrahydroxyflavanone | Twigs | BP | [31,43] |
| 70 | 5,7,3′,4′-Tetrahydroxy-3-methoxyflavone | Twigs | BP | [31,43] |
| 71 | 8-(1,1-Dimethylallyl)-5′-(3-methylbut-2-enyl)-3′,4′,5,7-tetrahydroxyflanvonol | Root barks/roots/twigs | BP | [29,38,39,42,48] |
| 72 | Kazinol E | Roots | BP | [42,45,48] |
| 73 | luteolin-7-O-β-D-glucopyranoside | Leaves | BP | [34,37,42,46] |
| 74 | Apigenin-7-O-β-D-glucoside | Leaves | BP | [36,43] |
| 75 | 3′-γ-Hydroxymethyl-(E)-γ-methylallyl-2,4,2′,4′-tetrahydroxychalcone-11′-O-coumarate | Whole plants | BP | [28,43] |
| 76 | Broussoaurone A | Root barks | BP | [43,46] |
| 77 | Dimethoxy isogemichalcone C | Whole plants | BP | [43] |
| 78 | Chrysoriol-7-O-β-D-glucoside | Leaves | BP | [36,43] |
| 79 | Iuteoloside | Whole plants | BP | [43] |
| 80 | 3,4-Dihydroxyisolonchocarpin | Roots | BP | [42,45,48] |
| 81 | (2S)-2′,4′-Dihydroxy-2″(1-hydroxy-1-methylethyl)-dihydrofurano-2,3-h-flavanone | Whole plants | BP | [43] |
| 82 | 5,7,3′,4′-Tetrahydroxy-3-methoxy-8-geranylflavone | Barks | BP | [33,37,42] |
| 83 | 5,7,3′,4′-Tetrahydroxy-3-methoxy-8,5′-diprenylflavone | Barks/branches and twigs | BP | [33,41,42] |
| 84 | Fipsotwin | Branches and twigs | BP | [42] |
| 85 | Kazinol N | Branches and twigs | BP | [42] |
| 86 | Kazinol M | Branches and twigs | BP | [42] |
| 87 | Threo-dadahol B | Branches and twigs | BP | [42] |
| 88 | Threo-dadahol A | Branches and twigs | BP | [42] |
| 89 | Broussoflavonol H | Root barks | BP | [41] |
| 90 | Broussoflavonol I | Root barks | BP | [41] |
| 91 | Broussoflavonol J | Root barks | BP | [41] |
| 92 | Broussoflavonol K | Root barks | BP | [41] |
| 93 | Glycyrrhiza flavonol A | Root barks | BP | [41] |
| 94 | Isolicofavonol | Root barks | BP | [41] |
| 95 | Broussoflavonol F | Root barks | BP | [41] |
| 96 | Broussoflavonol B | Root barks | BP | [41] |
| 97 | (2R)-7,3′,4′-Trihydroxy-6-prenylflavanone | Root barks | BP | [40] |
| 98 | Broussochalcone C | Root barks | BP | [40] |
| 99 | Broussoflavanonol A | Root barks | BP | [40] |
| 100 | Broussonol D | Root barks/leaves/twigs | BP/BK | [28,38,39] |
| 101 | Daphnegiravan H | Root barks | BP | [40] |
| 102 | (-)-(2S)-Kazinol I | Root barks | BP | [40] |
| 103 | Broupapyrin A | Twigs | BP | [39] |
| 104 | 8-Prenylquercetin-3-methyl ether | Twigs | BP | [39] |
| 105 | 4,2′,4′-Trihydroxychalcone | Twigs | BP | [39] |
| 106 | Butein | Twigs | BP | [39] |
| 107 | Broussonol E | Twigs | BP | [39] |
| 108 | 7,4′-Dihydroxy-3′-prenylflavan | Whole plants | BP | [38] |
| 109 | 7,3′-Dihydroxy-4′-methoxyflavan | Twigs | BP/BK | [37,38] |
| 110 | 3′-(3-Methylbut-2-enyl)-3′,4′,7-trihydroxyflavane | Twigs/roots | BP | [29,30,37,42,48,52] |
| 111 | Brossoflurenone A | Roots | BP | [38,47] |
| 112 | Brossoflurenone B | Roots | BP | [38,47] |
| 113 | Apigenin-7-O-β-D-glucopyranoside | Leaves | BP | [38,48] |
| 114 | Apigenin-6-C-β-D-glucopyranside | Leaves | BP | [34,37,42] |
| 115 | Bropapyrifero | Whole plants | BP | [17] |
| 116 | (−)-Broukazinol A | Twigs | BK | [37] |
| 117 | (+)-Broukazinol A | Twigs | BK | [37] |
| 118 | (2R)-7,4′-Dihydroxy-3′-prenylflavan | Twigs | BK | [37] |
| 119 | (2R)-7,4′-Dihydroxyflavan(tupichinol C) | Twigs/root barks/stem barks | BK | [26,32,36] |
| 120 | (2S)-7,4′-Dihydroxyflavan(demethylbroussin) | Twigs/root barks/stem barks | BK | [26,32,36] |
| 121 | Broussoside F | Twigs | BK | [37] |
| 122 | (2S)-7,3′-Dimethoxy-4′-hydroxyflavan | Twigs | BK | [37] |
| 123 | Kazinol I | Twigs/root barks | BK | [37,49] |
| 124 | Tupichinol C | Root barks | BK | [49,50] |
| 125 | Kazinol U | Root barks | BK | [49,50] |
| 126 | 3,5,7,4′-Tetrahydroxy-3′-(2-hydroxy-3-methylbut-3-enyl) flavone | Twigs | BP | [31,51] |
| 127 | Luteoloside | Leaves | BP | [36] |
| 128 | Broussoflavonol B | Barks | BP | [34] |
| 129 | 3′,7-Dihydroxy-4′-methoxyflavan | Stem barks | BK | [33] |
| 130 | 3,7-Dihydroxy-4′-methoxyflavone | Stem barks | BK | [33] |
| 131 | 3,7,3′-Trihydroxy-4′-methoxyflavone | Stem barks | BK | [33] |
| 132 | (+) − (2R) Kazinol I | Whole plants | BK | [50] |
| 133 | Apigenin-7-O--glucopyranoside | Leaves | BP | [32] |
| 134 | Amentoflavone | Leaves | BP | [32] |
| 135 | 3,3′,4′,5,7-Pentahydroxyflavone | Roots | BP | [30] |
| 136 | Broussonol A | Leaves | BK | [29] |
| 137 | Broussonol B | Leaves | BK | [29] |
| 138 | Broussonol E | Leaves | BK | [29] |
| 139 | 2,4,2′,4′-Tetrahydroxy-3′-prenylchalcone | Whole plants | BP | [28] |
| 140 | (2S)-2′,4′-Dihydroxy-7-methoxy-8-prenylflavan | Whole plants | BP | [28] |
| 141 | Australone A | Root barks | BP | [52] |
| 142 | Cyclomorusin | Whole plants | BP | [26] |
| 143 | Cycloartomunin | Whole plants | BP | [26] |
| 144 | Dihydroisocycloartomunin | Whole plants | BP | [26] |
2.2. Penylpropanoids
Compounds with one or several C6-C3 units are classified as penylpropanoids. Several penylpropanoids, such as compound 145 and compound 150, showed anti-tumor and antioxidant activities, respectively.
A total of 50 penylpropanoids have been isolated from Broussonetia. In 2009, nine new lignans, compounds 154, 155, 172–177, and 184, and three known lignans, compounds 179, 182, and 183, were isolated from the fruits of BP; these compounds exhibited antioxidant activities against H2O2-induced impairment in PC12 cells [53]. In 2010, Zhou et al. [54] reported that compounds 151, 170, 171, 180, 181, and 194 were isolated from BP for the first time; these compounds showed antioxidant activity against H2O2-induced injury in SY5Y cells. In 2014, four compounds (148, 165, 166, and 178) were isolated from an n-butanol extract of BP, and the structures of these compounds were elucidated on the basis of NMR spectra analysis and chemical evidence [36]. In 2019, Li et al. [38] reported compounds 185–192. In 2020, compounds 162 and 167 were isolated from the CHCl3-soluble part of an ethanolic extract of branches and twigs of BP by Malanik et al. [42]. In 2021, Vu et al. [14,44] isolated compounds 156–161 from the roots of BK. In addition, compounds 145, 146, 147, 152, and 153 were reported by Yadav et al. [45].
All penylpropanoids are summarized in Table 2, and the structures were summarized in Figure S2.
Table 2.
Penylpropanoids isolated from Broussonetia species.
| Number | Compounds | Parts | Source | References |
|---|---|---|---|---|
| 145 | Marmesin | Whole plants | BP | [45] |
| 146 | Graveolone | Whole plants | BP | [45] |
| 147 | Sesquineolignan | Whole plants | BP | [45] |
| 148 | Dihydrosyringin | Leaves | BP | [36,45] |
| 149 | Coniferyl alcohol | Fruits | BP | [43,45,54] |
| 150 | Ferulic acid | Fruits | BP | [45,54] |
| 151 | p-Coumaraldehyde | Fruits | BP | [45,54] |
| 152 | Liriodendrin | Leaves | BP | [35,45] |
| 153 | Dihydro-coniferyl alcohol | Whole plants | BP | [45] |
| 154 | Chushizisin G | Fruits | BP | [45,53] |
| 155 | Chushizisin H | Fruits | BP | [45,53] |
| 156 | Pinoresinol | Roots | BK | [14] |
| 157 | 3′-Hydroxymarmesin-1′-O-β-glucopyranosyl | Roots | BK | [14] |
| 158 | Marmesinin | Roots | BK | [14] |
| 159 | Syringaresinol-4-O-β-D-glucopyranoside | Roots | BP/BK | [14,38] |
| 160 | Rutaretin methylether | Roots | BK | [44] |
| 161 | Fipsomin | Roots | BK | [44] |
| 162 | (S)-Marmesin | Branches and twigs | BP | [42] |
| 163 | (+)-Marmesin | Whole plants | BP | [43] |
| 164 | (+)-Pinoresinol-4′-O-β-D-glucopyranosyl-4″-O-β-D-apiofuranoside | Leaves | BP | [35,43] |
| 165 | Syringaresinol-4′-O-β-D-glucoside | Leaves | BP | [36,43] |
| 166 | Pinoresinol-4′-O-β-D-glucopyranoside | Leaves | BP | [36,43] |
| 167 | (S)-8-Methoxymarmesin | Branches and twigs | BP | [42,43] |
| 168 | 7,8-Dihydroxy-6-(3-methylbut-2-en-1yl)-2H-chromen-2-one | Root barks | BP | [41,43] |
| 169 | Broussocoumarin A | Root barks | BP | [41,43] |
| 170 | Cissyringin | Fruits | BP | [43,54] |
| 171 | Cisconiferin | Fruits | BP | [43,54] |
| 172 | Chushizisin A | Fruits | BP | [43,53] |
| 173 | Chushizisin B | Fruits | BP | [43,53] |
| 174 | Chushizisin C | Fruits | BP | [43,53] |
| 175 | Chushizisin D | Fruits | BP | [43,53] |
| 176 | Chushizisin E | Fruits | BP | [43,53] |
| 177 | Chushizisin F | Fruits | BP | [43,53] |
| 178 | p-Coumaric acid | Leaves | BP | [36,43] |
| 179 | Threo-1-(4-hydroxy-3-methoxyphenyl)-2-{4-(E)-3-hydroxy-1-propenyl-2-methoxyphenoxy}-1,3-propanediol | Fruits | BP | [43,53] |
| 180 | Erythro-1-(4-hydroxyphenyl) glycerol | Fruits | BP | [43,54] |
| 181 | Threo-1-(4-hydroxyphenyl) glycerol | Fruits | BP | [43,54] |
| 182 | Erythro-1-(4-hydroxy-3-methoxyphenyl)-2-{4-(E)-3-hydroxy-1-propenyl-2-methoxy-phenoxy}-1,3-propanediol | Fruits | BP | [43,53] |
| 183 | 3-2-(4-Hydroxyphenyl)-3-hydroxymethyl-2,3-dihydro-1-benzofuran-5-ylpropan-1-ol | Fruits | BP | [43,53] |
| 184 | Chushizisin I | Fruits | BP | [43,53] |
| 185 | 6,7-Dimethoxycoumarin | Whole plants | BP | [38] |
| 186 | (+)-(2′S,3′R)-3-Hydroxyl marmesin | Whole plants | BP | [38] |
| 187 | Iariciresinol-9-O-β-D-glucopyranoside | Whole plants | BP | [38] |
| 188 | 3,4′,5′-Trihy- droxy-5-methoxy-6H-benzo [c] chromen-6-one | Whole plants | BP | [38] |
| 189 | Alternariol-4′-O-methyl ether | Whole plants | BP | [38] |
| 190 | Alternariol-5-O-methyl ether | Whole plants | BP | [38] |
| 191 | Alternariol | Whole plants | BP | [38] |
| 192 | Alternuene | Whole plants | BP | [38] |
| 193 | (7S,7′S,7″R,8R,8′R,8″S)-3′-Methoxy-4,4″,9″- trihydroxy- 4′,7″,7,9′,7′,9-triepoxy-5′,8″,8,8″-sesquineolignan | Whole plants | BP | [51] |
| 194 | Dihydroconiferyl alcohol | Fruits | BP | [54] |
2.3. Polyphenols
Compounds with two or more hydroxyl groups that are not flavonoids, phenylpropanoids, terpenes, or alkaloids are classified as polyphenols. The pharmacological effects of polyphenols, apart from skin whitening and anti-wrinkle properties, have been evaluated by researchers, but this is far from the level of research into flavonoids.
In total, 38 polyphenols were isolated from Broussonetia. In 1999, compounds 203 and 226 were isolated from the root bark of BK, and the cytotoxic activity of these compounds was evaluated against several different cell lines [27]. In 2001, Lee et al. [28] reported that compounds 195, 198, 199, 205, 206, 223, and 232 were isolated from the ethyl acetate-soluble extract of the whole plants of BP, and compound 199 exhibited to be active as aromatase inhibitors. In 2009, compounds 204, 207, 224, 225, and 231 were isolated from the methanol extract of BK, and the monophenolase inhibition of compounds 204, 224, and 225 was determined [55]. In 2018, new polyphenols, compounds 221 and 222, and known compounds 196 and 210 were isolated from the twigs of BK; compounds 196 and 222 showed the in vitro inhibition of protein tyrosine phosphatase 1B [37]. In 2019, compounds 219 and 220 were reported by Li et al. [38]. In addition, the structures of compounds 216–218 were elucidated on the basis of spectroscopic data (1D and 2D NMR, MS, MS/MS, and HRMS), and compound 218 exhibited significant inhibitory effects on the NO, iNOS, and pro-inflammatory cytokine production [40]. In 2020, compounds 208, 209, 211, 212, 123, 214, and 215 were reported by Qureshi et al. [43]. In 2021, compounds 197, 200, 201, and 202 were reported by Yadav et al. [45].
All polyphenols are summarized in Table 3, and the structures were summarized in Figure S3.
Table 3.
Polyphenols isolated from Broussonetia species.
| Number | Compounds | Parts | Source | References |
|---|---|---|---|---|
| 195 | Broussonin A | Twigs/stem barks/root barks/whole plants | BP/BK | [28,33,37,40,45,49,50] |
| 196 | Broussonin B | Twigs/stem barks/root barks | BP/BK | [33,37,40,45] |
| 197 | Resveratrol | Whole plants | BP | [45] |
| 198 | Moracin N | Whole plants | BP | [28,43,45] |
| 199 | Demethylmoracin I | Whole plants | BP | [28,45] |
| 200 | Mulberrofuran G | Whole plants | BP | [45] |
| 201 | Curculigoside C | Fruits | BP | [45,54] |
| 202 | Protocatechuic acid | Whole plants | BP | [45] |
| 203 | Kazinol K | Roots/root barks | BK | [27,44] |
| 204 | Kazinol F | Twigs/leaves/root barks | BP/BK | [29,37,40,43,55] |
| 205 | 1-(2,4-Dihydroxyphenyl)-3-(4-hydroxyphenyl)-propane | Whole plants | BP | [28,43] |
| 206 | 1-(4-Hydroxy-2-methoxyphenyl)-3-(4-hydroxy-3-prenylphenyl)-propane | Twigs/whole plants | BP/BK | [28,37,43] |
| 207 | Broussonin C | Root barks | BP/BK | [43,55] |
| 208 | Moracin I | Whole plants | BP | [43] |
| 209 | Moracin D | Whole plants | BP | [43] |
| 210 | Broussonin F | Twigs | BP/BK | [37,43] |
| 211 | Moracin M | Whole plants | BP | [43] |
| 212 | Broussonin E | Roots | BP/BK | [43,44] |
| 213 | 3,5,4′-Trihydroxy-bibenzyl-3-O-β-D-glucoside | Leaves | BP | [35,43] |
| 214 | Broussoside D | Leaves | BP | [36,43] |
| 215 | Broussofluorenone A | Roots | BP | [43,56] |
| 216 | Kazinol V | Roots/root barks | BP/BK | [40,44] |
| 217 | Kazinol J | Root barks/leaves | BP/BK | [29,40] |
| 218 | Kazinol W | Root barks | BP | [40] |
| 219 | Altertoxin IV | Whole plants | BP | [38] |
| 220 | Altertoxin I | Whole plants | BP | [38] |
| 221 | Broukazinol B | Twigs | BK | [37] |
| 222 | Broukazinol C | Twigs | BK | [37] |
| 223 | 1-(2,4-Dihydroxy-3-prenylphenyl)-3-(4-hydroxyphenyl)-propane | Twigs/whole plants | BK/BP | [28,37] |
| 224 | Kazinol S | Twigs/root barks | BK | [37,55] |
| 225 | Kazinol C | Root barks/twigs | BP/BK | [31,49,55] |
| 226 | Kazinol D | Root barks/twigs | BP/BK | [27,31,49,55] |
| 227 | (7′R,8′S) -3-Methoxy-4′,9,9″-trihydroxy-4,7′-epoxy-5,8′-neolignan | Whole plants | BP | [51] |
| 228 | (7R,8S,8′R)-7″,8″-Threo-3′-methoxy-7′-oxo-4,4″,7″,9,9″-pentahydroxy-4′,8″: 7,9′-bis-epoxy-8,8′-sesquineolignan | Fruits | BP | [51,54] |
| 229 | Broussonone A | Stem barks/roots | BP/BK | [33,57] |
| 230 | 3,4-Dihydroxybenzoic acid | Fruits | BP | [54] |
| 231 | Kazinol T | Root barks | BK | [55] |
| 232 | Albanol A | Whole plants | BP | [28] |
2.4. Alkaloids
Nitrogen-containing organic compounds are classified as alkaloids. Until now, there has been little research on the pharmacological effects of alkaloids.
To date, 35 alkaloids have been isolated from Broussonetia. In 1997, Shibano et al. isolated eight new pyrrolidine alkaloids, compounds 253–260, from the branches of BK; these compounds demonstrated inhibitory activity on β–galactosidase and β–mannosidase [58,59]. In 1998, the author also isolated four new pyrrolidine piperidine alkaloids, compounds 261–264, from the branches of BK [60,61]. In 1999, three new pyrrolizidine alkaloids, compounds 265–267, were also isolated from the branches of BK; these compounds showed the inhibitory activity of glycosidase [62,63]. In 2000, four new pyrrolidine alkaloids, compounds 249–252, showing the ability to inhibit glycosidase were isolated from the branches of BK [64]. In 2001, Tsukamoto et al. [65] isolated four new pyrrolidine alkaloids, compounds 244, 245, 246, and 248, and a new pyrroline alkaloid, compound 247, from the branches of BK [65]. In 2014, two isoquinonline alkaloids, compounds 241 and 242, were isolated and characterized from the fruits of BP; they showed cytotoxic activities on the BEL-7402 and Hela cell lines [66]. In 2020, compounds 238–241 were reported by Qureshi et al. [43]. In 2021, compounds 233–237 were reported by Yadav et al. [45].
All alkaloids are summarized in Table 4, and the structures were summarized in Figure S4.
Table 4.
Alkaloids isolated from Broussonetia species.
| Number | Compounds | Parts | Source | References |
|---|---|---|---|---|
| 233 | Liriodenine | Fruits | BP | [45,67] |
| 234 | Isoterihanine | Whole plant | BP | [45] |
| 235 | Chelerythrine | Whole plants | BP | [45] |
| 236 | Oxyavicine | Fruits | BP | [45,67] |
| 237 | Broussonpapyrine | Fruits | BP | [45,67] |
| 238 | Nitidine | Fruits | BP | [43,67] |
| 239 | 2′-Deoxyuridine | Whole plants | BP | [43] |
| 240 | 2′-Deoxyadenosine | Whole plants | BP | [43] |
| 241 | Thymidine | Whole plants | BP | [43] |
| 242 | N-Norchelerythrine | Fruits | BP | [66] |
| 243 | Dihydrosanguinarine | Fruits | BP | [66] |
| 244 | Broussonetine R | Branches | BK | [65] |
| 245 | Broussonetine S | Branches | BK | [65] |
| 246 | Broussonetine T | Branches | BK | [65] |
| 247 | Broussonetine U | Branches | BK | [65] |
| 248 | Broussonetine V | Branches | BK | [65] |
| 249 | Broussonetine M | Branches | BK | [64] |
| 250 | Broussonetine O | Branches | BK | [64] |
| 251 | Broussonetine P | Branches | BK | [64] |
| 252 | Broussonetine Q | Branches | BK | [64] |
| 253 | Broussonetine A | Branches | BK | [59,68] |
| 254 | Broussonetinine A | Branches | BK | [59,68] |
| 255 | Broussonetine B | Branches | BK | [59,68] |
| 256 | Broussonetinine B | Branches | BK | [59,68] |
| 257 | Broussonetine C | Branches | BK | [58,68] |
| 258 | Broussonetine E | Branches | BK | [59,68] |
| 259 | Broussonetine D | Branches | BK | [58,68] |
| 260 | Broussonetine F | Branches | BK | [59,68] |
| 261 | Broussonetine G | Branches | BK | [61,68] |
| 262 | Broussonetine H | Branches | BK | [61,68] |
| 263 | Broussonetine I | Branches | BK | [60,68] |
| 264 | Broussonetine J | Branches | BK | [60,68] |
| 265 | Broussonetine K | Branches | BK | [63,68] |
| 266 | Broussonetine L | Branches | BK | [63,68] |
| 267 | Broussonetine N | Branches | BK | [62] |
2.5. Terpenoids and Steroids
Olefins where the molecular formula is an integer multiple of isoprene are classified as terpenoids. Most of the terpenoids are triterpenes.
Until now, a total of 17 triterpenes have been isolated from Broussonetia. Fang et al. reported that two known terpenoids, compounds 283 and 284, were isolated and characterized from BP in 1994 [24] and 1995 [25] respectively. In 2008, three new ent-kaurane type diterpenes, compounds 279–281, were isolated from leaves of BP; these compounds showed mild inhibition of tyrosinase and significant inhibition of xanthine oxidase [32]. In 2011, four new euphane triterpenes, compounds 274–277, were isolated from the bark of BP, and the structures of these compounds were determined by spectroscopic evidence and chemical methods [69]. In addition, compound 270 was a new tirucallane triterpenoid, and compounds 271–273 were isolated from BP for the first time. In 2019, compounds 268 and 269 were reported by Li et al. [38].
All the terpenoids are summarized in Table 5, and the structures were summarized in Figure S5.
Table 5.
Terpenoids isolated from Broussonetia species.
| Number | Compounds | Parts | Source | References |
|---|---|---|---|---|
| 268 | Lupeol acetate | Whole plants | BP | [38] |
| 269 | Augustic acid | Whole plants | BP | [38] |
| 270 | 3β-acetoxy–tirucalla-7-en-24S,25-diol | Barks | BP | [38,70] |
| 271 | Lupeol | Barks | BP | [70] |
| 272 | β-Amyrin | Barks | BP | [70] |
| 273 | α-Amyrin acetate | Barks | BP | [70] |
| 274 | (3β)-3-(acetyloxy)-eupha-7,25-dien-24-one | Barks | BP | [38,69] |
| 275 | (3β,24R)-3-(acetyloxy)–eupha-7,25-dien-24-ol | Barks | BP | [38,69] |
| 276 | (3β,24S)-eupha-7,25-diene-3,24-diol | Barks | BP | [38,69] |
| 277 | (3β,24R)-Eupha-7,25-diene3,24-diol | Barks | BP | [69] |
| 278 | Taraxerol acetate | Leaves | BP | [32] |
| 279 | Broussonetone A | Leaves | BP | [32,51] |
| 280 | Broussonetone B | Leaves | BP | [32,51] |
| 281 | Broussonetone C | Leaves | BP | [32,51] |
| 282 | Oleanolic acid | Root barks | BP/BK | [27,38] |
| 283 | Squalene | Fruits/root barks/leaves | BP/BL | [25,45,53,71] |
| 284 | Butyrospermol acetate | Whole plants | BP | [24] |
All steroids were isolated only from BP. Three compounds (287–289) were reported by Qureshi et al. [43] in 2020, while compounds 285–286 were reported by Yadav et al. [45]. All steroids are summarized in Table 6, and the structures were summarized in Figure S6.
Table 6.
Steroids isolated from Broussonetia species.
2.6. Other Compounds
Apart from the compounds mentioned above, a total of 49 other compounds isolated from Broussonetia species are classified as “others”.
Fang et al. [24,25] showed that compounds 329–332 were isolated and characterized from the root bark of BP. In 2007, two new megastigmane O-glucopyranosides, compounds 327 and 328, were isolated from the leaves of BP; the structures of these compounds were established by chemical methods and spectroscopic techniques, including 2D NMR [72]. In 2010, Zhou et al. [54] established that compounds 323–326, isolated from the fruits of BP for the first time, showed antioxidant activity against H2O2-induced injury in SY5Y cells. In 2011, a novel compound, 322, and a known compound, 321 were isolated from the n-BuOH extract of BP seeds, while their cAMP-regulating activity was evaluated by Mei et al. [73]. In 2014, compounds 316–320 were isolated from the n-butanol extract of BP, and compounds 317, 318, and 320 were found to potently inhibit estrogen biosynthesis in KGN cells [36]. Moreover, compounds 309–314 were reported by Yu et al. [51]. In 2016, compounds 333–338 were isolated from the ethyl acetate leaf extract of BL [71]. In 2019, compounds 293–308 were reported by Li et al. [38]. In 2021, compounds 290 and 291 were reported by Yadav et al. [45].
All these compounds were summarized in Table 7, and the structures were summarized in Figure S7.
Table 7.
Other compounds isolated from Broussonetia species.
| Number | Compounds | Parts | Source | References |
|---|---|---|---|---|
| 290 | Arbutin | Whole plants | BP | [45] |
| 291 | Broussoside B | Whole plants | BP | [45] |
| 292 | D-Galacitol | Whole plants | BP | [43] |
| 293 | Daucosterol palmitate | Whole plants | BP | [38] |
| 294 | Palmitic acid ethyl ester | Whole plants | BP | [38] |
| 295 | Palmitic acid | Whole plants | BP | [38] |
| 296 | Linoleic acid | Whole plants | BP | [38] |
| 297 | 9-Octadecenoic acid | Whole plants | BP | [38] |
| 298 | 8,11-Octadecadienoic acid | Whole plants | BP | [38] |
| 299 | α-Monopalmitin | Whole plants | BP | [38] |
| 300 | Monoheptadecanoin | Whole plants | BP | [38] |
| 301 | Heptadecanoic acid | Whole plants | BP | [38] |
| 302 | Phytol | Whole plants/leaves | BP/BL | [38,71] |
| 303 | Physcion | Whole plants | BP | [38] |
| 304 | Altersolanol A | Whole plants | BP | [38] |
| 305 | Altersolanol C | Whole plants | BP | [38] |
| 306 | δ-Tocopherol | Whole plants | BP | [38] |
| 307 | (4R,5S,10S)-8,9,10-Trihydroxy-4-[3′-methoxy-4′-hydroxyphenyl]-1,6-dioxaspiro [4,5] decan-2-one | Whole plants | BP | [38] |
| 308 | 4-Hydroxyacetophenone | Whole plants | BP | [38] |
| 309 | Erythro-1-(4-hydroxyphenyl)-2-{4-[(E)-3-hydroxy-1-propenyl]-2-methoxyphenoxy}-1,3-propanediol | Whole plants | BP | [51] |
| 310 | Threo-1-(4-hydroxyphenyl)-2-{4-[(E)-3-hydroxy1-propenyl]-2-methoxyphenoxy}-1,3-propanediol | Whole plants | BP | [51] |
| 311 | threo-1-(4-hydroxyphenyl)-2-[4-(3-hydroxy-1-propyl)-2-methoxyphenoxy]-1,3-propanediol | Whole plants | BP | [51] |
| 312 | erythro-1-(4-hydroxyphenyl)-2-[4-(3-hydroxy-1-propyl)-2-methoxyphenoxy]-1,3-propanediol | Whole plants | BP | [51] |
| 313 | (7′R,8′S)-3-Methoxy-7-oxo-4′,9,9″-trihydroxy-4,7′-epoxy-5,8′-neolignan | Whole plants | BP | [51] |
| 314 | (7′R,8′S)-3-Methoxy-4′,9,9″-trihydroxy-4,7′-epoxy-5,8′-neolignan | Whole plants | BP | [51] |
| 315 | Benzyl benzoate-2,6-di-O-β-D-glucopyranoside | Whole plants | BP | [51] |
| 316 | Broussoside A | Twigs/leaves | BP/BK | [36,37,43] |
| 317 | Broussoside C | Leaves | BP | [36,43] |
| 318 | Broussoside E | Leaves | BP | [36,43] |
| 319 | Flacourtin | Leaves | BP | [36,45] |
| 320 | Poliothyrsoside | Leaves | BP | [36,45] |
| 321 | Adenosine | Seeds | BP | [73] |
| 322 | Chushizilactam A | Seeds | BP | [73] |
| 323 | Arbutine | Fruits | BP | [54] |
| 324 | 4-Hydroxybenzaldehyde | Fruits | BP | [43,45,54] |
| 325 | Curculigoside I | Fruits | BP | [43,54] |
| 326 | 2-(4-Hydroxyphenyl) propane-1,3-diol-1-O-β-D-glucopyranoside | Fruits | BP | [43,54] |
| 327 | (2R,3R,5R,6S,9R)-3-Hydroxy-5,6-epoxyb-ionol-2-O-β-D-glucopyranoside | Leaves | BP | [43,72] |
| 328 | (2R,3R,5R,6S,9R)-3-Hydroxyl-5,6-epoxy-acety-b-ionol-2-O-β-D-glucopyranoside | Leaves | BP | [72] |
| 329 | Lignoceric acid | Root barks | BP | [25,45] |
| 330 | Octacosan-1-ol | Root barks | BP | [25,45] |
| 331 | 4′-Hydroxycis-cinnamic acid octacosyl ester | Root barks | BP | [25] |
| 332 | Erythrinasinate | Root barks | BP | [25] |
| 333 | 1,2,3-Propanetriol, monoacetate | Leaves | BL | [71] |
| 334 | 1,2,3-Propanetriol, diacetate | Leaves | BL | [71] |
| 335 | Hexadecanoic acid, ethyl ester | Leaves | BL | [71] |
| 336 | 9,12,15-Octadecatrienoic acid, methyl ester, (Z, Z, Z)- | Leaves | BL | [71] |
| 337 | 9,12,15-Octatrienoic acid, ethyl ester, (Z, Z, Z)- | Leaves | BL | [71] |
| 338 | 3,7,11,15-Tetramethyl-2-hexadecen-1-ol | Leaves | BL | [71] |
3. Pharmacology
Various uses of Broussonetia species have inspired researchers’ interest in exploring pharmacological activities by scientific pharmacological assays including in vitro and in vivo. A variety of crude extracts and purified compounds from Broussonetia species have been evaluated for different biological effects, such as their antitumor, antioxidant, anti-inflammation, antidiabetic, anti-obesity, antibacterial, and antiviral properties, as well as skin whitening, anti-wrinkle, and other activities. Despite the extensive bioactivities that have been identified, the targeted clinical trials that are normally used to evaluate safety and effectiveness for humans are currently absent. Perhaps the addition of clinical trials might be a more comprehensive and scientific way to ascertain the medical role of the Broussonetia genus. All these pharmacological activities are summarized in Table 8.
Table 8.
Pharmacological effects of Broussonetia species.
| Variety | Parts | In Vivo/In Vitro | Model | Active Components | Dosage | Results | References | |
|---|---|---|---|---|---|---|---|---|
| Anti-tumor | BK | - | in vitro | Colon cancer cells | Kazinol C | 0–30 μM | Inducing apoptosis by activating AMPK. | [2] |
| BK | Roots | in vitro | Hela, HL-60, MCF-7 cells | Eriodictyol, apigenin and kaempferol | - | Cytotoxic activity against HL-60 cells (IC50 = 46.43–94.06 μM) and apigenin was cytotoxic against Hela cells (IC50 = 49.26 μM). | [14] | |
| BP | Barks | in vitro | HepG2 cells | Polyphenols | 0–500 μg/mL | Induced mitochondria-mediated apoptosis by inactivating ERK and AKT signaling pathways. | [77] | |
| BK | Stem barks | in vitro | PANC-1 cells | Broussoflavonol B | 0–100 μM | Repressing proliferation by inactivating the ERK/c-Myc/FoxM1 signaling pathway. | [86] | |
| BP | Barks | in vitro | MDA-MB-231 cells | Broussoflavonol B | 0–1 µM | Inducing the arrest of the cell cycle and cell death. | [79] | |
| BP | Barks | in vitro | SK-BR-3 cells | Broussoflavonol B | 0–1 µM | Inhibiting growth and inducing differentiation of stemlike SK-BR-3 cells. | [113] | |
| BP | - | in vitro | Colon and liver cancer cells | Broussochalcone A | 0–20 μM | Cytotoxicity by promoting phosphorylation/ubiquitin-dependent degradation of β-catenin. | [85] | |
| BP | Root barks | in vitro | NCI-H1975, HepG2 and MCF-7 | Broussoflavonol K | - | IC50 = 0.90–2.00 μM | [41] | |
| BP | Barks | in vitro | SGC-7901 cells | Chlorogenic acid-like compounds | 50, 100 and 200 μg/mL | Inducing apoptosis through p38-MAPK and ERK-MAPK signaling pathways. | [76] | |
| BP | - | in vitro | HepG2 and SK-Hep1 cells | Broussochalcone A | 0, 2.5, 5, 10, 20 and 40 µM | Cell cycle arrest by increasing FOXO3 and cell cycle regulatory and pro-apoptotic proteins (IC50 = 20 µM). | [84] | |
| BK | - | in vitro | Esophagus cancer cells | Marmesin | - | Inhibiting the PI3K/Akt pathway. | [87] | |
| BK | - | in vitro | NSCLC cell | Marmesin | 0–10 µM | Abrogating mitogen-stimulated proliferation and invasion. | [83] | |
| BP | Roots | in vitro | T24 and T24R2 cells | Kazinol A | 0–50 µM | Cytotoxicity through G 0/1 arrest mediated by cyclin D1 decrease and p21 increase. | [80] | |
| BK | Roots | in vitro | MCF-7 cells | Kazinol E | 0–50 µM | Blocking EGF-induced ERK activity. | [81] | |
| BK | Root barks | in vitro | MCF-7 cells | Kazinol E | - | Inhibiting Erk activity by binding the ATP-binding pocket of Erk-1. | [82] | |
| BK | Root barks | in vitro | HT-29 colon cells | Kazinol C | 0–120 µM | Promoting AMPK phosphorylation and attenuating HT-29 colon cancer cell growth and viability. | [88] | |
| BP | Fruits | in vitro | A375, BEL-7402 and Hela cells | Total alkaloids and seven individual alkaloids | 50, 10, 5, and 1 mg/mL | IC50 = 6.61–47.41 mg/mL (BEL-7402 cell line) and IC50 = 5.97–40.17 mg/mL (Hela cell line). | [66] | |
| BP | Leaves | in vitro | HepG-2 cells | (+)-pinoresinol-4′-O-β-D-glucopyranosyl-4″-O-β-D-apiofuranoside, liriodendrin, apigenin-6-C-β-Dglucopyranside | 100 mmol/L | IC50 were 17.19, 14.56 and 19.53 μg/mL respectively. | [35] | |
| BP | Fruits | in vitro | MG63 cells | Ethanol extract | 0–7000 µg/mL | Inhibiting the proliferation associated with apoptosis and cell cycle arrest. | [75] | |
| BP | Barks | in vitro | MCF-7 cells | 5,7,3′,4′-Tetrahydroxy-3-methoxy-8,50-diprenylflavone and broussoflavonol B | 0–25 µM | Showing high anti-proliferation activities with IC50 values of 4.41 and 4.19. | [34] | |
| BK | - | in vitro | SCM-1 cells | Kazinol Q | 0–120 µM | Enhancing subsequent cell death due to necrosis. | [78] | |
| BP | Stem Barks | in vitro | HT-29 cells | Dichloromethane Fractions | 50, 100, 150, or 200 μg/mL | Inducing apoptosis through p53-dependent mitochondrial signaling pathway. | [74] | |
| BK | Roots barks | in vitro: Human hepatoma, | PLC/PRF/5, T24 cells, human cervical carcinoma, HT-3, SiHa and CaSki cells | kazinols Q, and R, kazinol D, K, H, 7,4′-dihydroxyflavan | - | Showing the great inhibitory effect to T24, CaSki, PLC/PRF/5, HT3 and SiHa respectively. | [27] | |
| Anti-oxidant activity | BP | Barks | in vitro | - | Ethanol extracts | - | IC50 value was 0.33 ± 0.08 mg/mL | [13] |
| BP | Branches and twigs | in vitro | THP-1 cells | 5,7,3′,4′-tetrahydroxy-3-methoxy-8,5′-diprenylflavone, kazinol M,broussoflavonol A | - | CAA values were 25.9, 6.4, 5.4 respectively. | [42] | |
| BP | Whole plants | in vitro | - | Lignin | 10–100 mg/L | Lignin with more phenolic hydroxyl groups. | [114] | |
| BP | Fruits | in vitro | - | Three purified fractions | 0–2.0 mg/mL | IC50 values of three purified fractions were 0.54, 0.86, and 0.57 mg/mL respectively. | [115] | |
| BP | Leaves | in vitro | KGN cells | Luteolin, luteoloside, orientin, isoorientin | 0.1–3 mg/mL | SC50 values was 19.72, 19.67, 18.86 and 19.33 mmol/L respectively. | [36] | |
| BP | Seeds | in vitro | - | Seed oil | 0.2–0.8 v/v | The hydroxyl radical inhibition rate was 91.21% | [89] | |
| BP | Fruits | in vitro | - | Ethanol extract | 0–400 mg/mL | IC50 for lipid peroxidation inhibition on liver homogenate was 155.7 µg/mL | [90] | |
| BP | Fruits | in vitro | MG63 cells | Ethanolic extract | 0–600 µg/mL | DPPH assay showed IC50 value of 156.3 µg/mL. | [75] | |
| BP | Flowers | in vitro | - | Ethanol and water crude extracts | 5 mg/mL 6 mg/mL |
The ethanol extract showing 62.88% in the DPPH radical scavenging method and 61.15% in chelation Fe2+-activity. | [91] | |
| BP | Fruits | in vitro | - | Ethanol and water crude extracts | DPPH: 0.5–5 mg/mL Fe2+-activity: 0.5–5 mg/mL |
DPPH radicals with a percentage inhibition of 87.17 ± 0.18% to ethanol extract and 58.11 ± 0.11% to aqueous extract.Fe2+-chelating activity of approximately 77.51% of aqueous extract and the ethanol extract showed a chelation capacity of 48.26%. | [7] | |
| BP | Roots | in vitro | - | Broussochalcone A and 3,4-dihydroxyisolonchocarpin | 0.1–1000 µM | IC50 values of 27.6 ± 0.3 µM and 21.8 ± 0.2 µM through DPPH assay, which IC50 values of ABTS were 5.8 ± 0.1 µM and 7.7 ± 0.4 µM as well as IC50 values of XOD were 0.6 ± 0.04 µM and 1.8 ± 0.1 µM. | [57] | |
| BP | Fruits | in vitro | RAW264.7 cells | Petroleum extract | DPPH: 0.31 to 5.0 mg/mL superoxide anion radical scavenging activity: 2.5–40 mg/mL hydroxyl radical scavenging activity: 0.625–10 mg/mL |
IC50 = 8.20 ± 0.003 mg/mL (DPPH). IC50= 89.86 ± 3.40 mg/mL (superoxide anion). IC50 =19.63 ± 0.36 mg/mL (hydrogen peroxide). | [116] | |
| BP | Fruits | in vitro | SY5Y cells | 3,4-dihydroxybenzoic acid, dihydroconiferyl alcohol, ferulic acid and curculigoside C | 0.16–100 mM | The IC50 values were 39.5, 58.9, 65.3, and 65.6 mM respectively through a DPPH assay. | [54] | |
| BP | Barks and woods | in vitro | - | Ethyl acetate fractionhexane fraction | - | The antioxidant activity of bark extract was superior to that of wood. | [94] | |
| BP | Radixes and leaves | in vitro | SH-SY5Y cells | Methanol extract | 0.1–2.5 mg/mL | BP radixes and leaves possessed the best scavenging activities for DPPH, ABTS radical, and H2O2. | [93] | |
| BP | Fruits | in vitro | PC12 cells | Erythro-1-(4-hydroxy-3-methoxyphenyl)-2-{4-[(E)3-hydroxy-1-propenyl]-2-methoxyphenoxy}-1,3-propanediol | 0.16–100 µM | IC50 =60.9 µM (DPPH assay). | [53] | |
| BP | - | in vitro | RAW 264.7 cells | Broussochalcone A | 1–30 µM | IC0.200 was 7.6 ± 0.8 µM (diphenyl-2-picrylhydrazyl assay system). | [92] | |
| BP | Roots | in vitro | - | Broussoflavan A, broussoflavonol F,broussoflavonol G, broussoaurone A | - | Inhibiting the Fe2+-induced formation of TBARS with IC50 values of 2.1, 2.7, 1.0 and 1.2µM respectively. | [46] | |
| Anti-inflammation | BK | Roots | in vitro | RAW264.7 cells | Eriodictyol, apigenin, kaempferol | 0–30 μM | Reducing iNOS expression with IC50 values of 11.98, 10.16, and 24.06 μM. | [14] |
| BP | Branches and twigs | in vitro | THP-1 cells | Kazinol M, broussoflavonol B, broussoflavonol A, 5,7,3′,4′-tetrahydroxy-3-methoxy-8,5′-diprenylflavone and broussofluorenone C | 1 μM | Activating NF-κB/AP-1. | [42] | |
| BP | Root barks | in vitro | NCIH1975, HepG2, and MCF-7 cells | Broussoflavonol H. | - | Inhibiting the production of IL-2 in Jurkat induced by PHA and PMA (IC50 = 9.95 μM). | [41] | |
| BP | Root barks | in vitro | RAW264.7 cells | Flavanone, broussochalcone C, broussoflavanonol A, kazinol V, kazinol W and broussoflavonol B | 1.25–40 μM | Reducing NO production through downregulating iNOS, COX-2, and TNF-α expression and the expression of iNOS protein. | [40] | |
| BK | Barks | in vitro | RAW264.7 cells | Broussonin E | 2.5–20 μM | Inhibiting the ERK and p38 MAPK and enhancing the JAK2-STAT3 signaling pathway. | [96] | |
| BK | Leaves | in vivo | mice | Ethanol extract | 200–1000 μg/mL | Down-regulating the plasma levels of IgE and IL-4 and inhibiting hTARC secretion in HaCaT cells by activated TNF-α/IFN-γ. | [117] | |
| BP | Stem barks | in vitro | RAW 264.7 cells | n-hexane fractionof methanol extract | 10–80 μg/mL | Inhibiting the NO production and proinflammatory cytokines. | [118] | |
| BP | Stem barks | in vitro | RAW 264.7 cells | n-butanol fraction | 0–150 μg/mL | Inhibiting iNOS expression in RAW 264.7 macrophages. | [74] | |
| BK | Heartwood | in vivo | mice | EtOH extract | 50–250 mg/mL | Inhibiting IgE production in β-cell and mast cell infiltration by IL-4 and chemokines by inhibiting Th2-cell activation by allergens. | [98] | |
| BP | - | in vivo | - | Ethanol extract | - | Inhibiting vascular permeability via autocrines and nitric oxide. | [97] | |
| BP | - | in vitro | Bone marrow cells | Papyriflavonol A | 0–250 μM | Inhibiting human group IIA and V sPLA2s with IC50 values of 3.9 and 4.5 mM. | [95] | |
| BP | - | in vitro | RAW 264.7 cells | Broussochalcone A | 1–20 μM | Inhibiting NO production with an IC50 of 11.3 mM via inhibition of IkBa phosphorylation, IkBa degradation, nuclear factor-kappa B activation, and iNOS expression. | [92] | |
| BK | Root barks | in vitro | RAW 264.7 cells | Tupichinol C, kazinol U, kazinol A, kazinol I, broussonin A, kazinol C, kazinol D | 0–20µM | Suppressing the LPS-induced high level of NO with IC50 values of less than 6 µM and attenuating protein and mRNA levels of inducible iNOS. | [49] | |
| Anti-diabetic and Anti-obesity Effects | BK | Fruits | in vivo | mice | Ethanolic extract | - | Inhibiting Erk phosphorylation by preventing STZ-induced oxidative stress and beta cell apoptosis. | [15] |
| BK | Fruits | in vitro | SV40 MES13 cells | Ethanolic extract | 0–40 μg/mL | Ethanolic extract induced the expression of antioxidant enzymes by activating Nrf2 and prevented palmitate-induced lipotoxicity. | [103] | |
| BP | Root barks | in vivo | mice | Broussoflavonol B and kazinol J | 0–100 μg/mL | Suppressing pro-inflammatory responses via activating AMPK. | [102] | |
| BK | Root barks | in vivo | mice | Kazinol C and isokazinol D | 5–25 μM | Blocking the NF-κB pathway and reducing the extent of β-cell damage. | [100] | |
| BK | Stem barks | in vitro | 3T3-L1 cells | Broussonone A together with other isolated phenolic compounds | 100 µM | Inhibitory activity against pancreatic lipase with IC50 of 28.4 µM, and has inhibitory effects on adipocyte differentiation. | [33] | |
| BK | Root barks | in vitro | RINm5F cells | Kazinol U | 0–60 μM | Blocking the NF-kB pathway and reducing cells damage. | [99] | |
| BP | Roots | - | - | Broussochalcone A, broussochalcone B, kazinol A, kazinol B, 8-(1,1-Dimethylallyl)-5′-(3-methylbut-2-enyl)-3′,4′,5,7-tetrahydroxyflanvonol and papyriflavonol A | 0.01–1000 μM | IC50 values of 5.3, 11.1, 12.0, 26.3, 3.6, and 2.1μM respectively. | [56] | |
| BK | Stem barks | in vivo | mice | Stem bark powders | - | Decreasing the serum levels of glucose, fructosamine, triglyceride, and total cholesterol and the activity of ALT, and increasing blood insulin level. | [16] | |
| Antibacterial and Antiviral Effects | BP | - | in vitro | - | Broussochalcone A, papyriflavonol A,3′-(3-methylbut-2-enyl)-3′,4′,7-trihydroxyflavane, broussoflavan A, kazinol F and kazinol J | - | These six polyphenols are more potent Mpro inhibitors than two repurposed drugs (lopinavir and darunavir). | [18] |
| BP | Whole plants | in vitro | - | 5,7,3′,4′-tetrahydroxy-3-methoxy-8,5′-diprenylflavone | 0.12–250 ppm | Suppressing Porphyromonas gingivalis (MIC = 1.95 ppm). | [17] | |
| BP | Roots | in vitro | - | Papyriflavonol A | 1–1000 µM | Inhibitory effect of PLpro with an IC50 value of 3.7 µM. | [105] | |
| BP | Fruits | in vitro | - | BPP-3 | 0.4–2.0 mg/mL | The minimum inhibitory concentration of BPP-3 against E. coli, P. aeruginosa, B. subtilis and S. aureus were 0.3 mg/mL, 0.25 mg/mL, 0.3 mg/mL and 0.25 mg/mL, respectively. | [115] | |
| BP | Seeds | in vitro | - | Hexane extract | 0.25%, 0.5%, 1%, 2%, and 4% (v/v) | The seed oil has an inhibitory effect on Staphylococcus aureus, Proteus vulgaris, Bacillus cereus, and Enterobacter aerogenes. | [104] | |
| Skin whitening and Anti- skin wrinkles Activities | BK | Root barks | in vivoin vitro | Zebrafish/B16F10 cells | Kazinol U | 0–20 μM | Inhibitory activity of MITF and downstream target genes such as tyrosinase, Tyrp1 and Tyrp2. | [19] |
| BK | Stems | in vitro | HEK-293T cells | EtOH extract | 0–100 μg/mL | Maintaining the collagen content of the skin by eliminating reactive oxygen species and inhibiting collagenase activity. | [106] | |
| Others | BK | Roots | in vitro | RAW264.7 cells | Broussonol F, G and K | 10–30 μM | Inhibiting RANKL-induced osteoclast formation. | [44] |
| - | Fruits | in vivo | mice | Chushizi | - | Increasing liver function and alleviating DILI via regulating the TLR3/ JNK/ c-jun/c-fos/JAK/STAT3 pathway. | [107] | |
| BP | - | in vitro | hHFDP cells | Ethanolic extract | 0–20 µg/mL | Regulating β-Catenin and STAT6 target protein. | [108] | |
| BK | Twigs | in vitro | human umbilical vein endothelial cells | Ethanolic extract | 0.1–10 µg/mL | Inhibiting VEGF-A stimulated phosphorylation/activation of ERK, Akt and p70S6K, the downstream targets of the VEGFR-2 signaling pathways, and downregulation of VEGFR-2 and MMP-2. | [109] | |
| BP | Roots | in vitro | - | 8-(1,1-Dimethylallyl)-5′-(3-methylbut-2-enyl)-3′,4′,5,7-tetrahydroxyflanvonol, papyriflavonol A and broussoflavonol B | 0–30 µM | Inhibiting hAChE and BChE with IC50′s ranging from 0.8 to 3.1 μM and from 0.5 to 24.7 μM, respectively. | [47] | |
| BP | Seeds | in vitro | N1E-115 cells | Chushizilactam A and adenosine | 50 µM | Adenosine could obviously increase cAMP. | [73] | |
| BK | Stems | in vivo | mice | Water extract | - | Water extract has immune-stimulating activity by enhancing the Th1 immune response. | [110] | |
| BK | - | in vitro | MCF-7 cells | Broussonin A, tupichinol C kazinol U and (+)-(2R) kazinol I | 10 µM | Modulating the E2-responsive genes as functional ER ligands such as E2. | [50] | |
| BK | Roots | in vitro | C2C12 and 10T1/2 cells | Kazinol P | 1000 nM | Promoting myogenic differentiation through the activation of p38MAPK and MyoD transcription activities. | [111] | |
| BK | Root barks | in vivo | RAW 264.7 cells | Kazinol B | 6.25–50 µM | Inhibiting the NO synthesis with an IC50 of 21.6 mM | [112] |
3.1. Anti-Tumor
In 2010, the dichloromethane fraction extracted from the stem barks of BP was found to induce apoptosis-related DNA fragmentation; it increased sub-G1 accumulation, increased p53, caspase3, and Bax expression, and inhibited the proliferation of human colon cancer HT-29 cells [74]. The ethanol extract of BP exhibited the inhibition of the growth of human osteosarcoma MG63 cells, affected morphological apoptosis, and induced cell-cycle arrest, as found in 2013 [75]. In 2014, seven alkaloids isolated from the ethyl acetate fraction of BP fruits at dosages of 1, 5, 10, and 50 mg/mL showed high cytotoxic activities on the BEL-7402 and Hela cell lines, with IC50 values of 6.61–47.41 mg/mL and 5.97–40.17 mg/mL, respectively [66]. Zhu et al. [76] explored the mechanism of gastric carcinoma cell SGC-7901 apoptosis, as induced by CALCBP, and the results showed that apoptosis might be related to oxidative stress in the cell mitochondria via the p38-MAPK and ERK-MAPK signal pathways. In an in vitro assay, polyphenols showed significant apoptotic activities on HepG2 cells in a dose-dependent and time-dependent manner by inducing cell cycle arrest at the G1 phase, unregulating the ratio of Bax/Bcl-2 and inhibiting the expression of PKB/AKT and ERK [77].
In 1999, the pure compounds kazinol Q, kazinol R, kazinol D, kazinol K, and 7,4′-dihydroxyflavan, isolated from BP root barks, showed strong inhibitory effects on T24, CaSki, PLC/PRF/5, HT3, and SiHa, respectively [27]. In 2011, Wei et al. explored the ability of kazinol Q to induce DNA breakage in the presence of Cu; the results showed that the cell viability of gastric carcinoma SCM-1 cells was significantly decreased [78]. In 2013, (+)-pinoresinol-4′-O-β-D-glucopyranosyl-4″-O-β-D-apiofuranoside, apigenin-6-C-β-D-glucopyranside, and liriodendrin, isolated and purified from BP leaves, exhibited inhibitory effects on HepG-2 cells during the dosage of 100 mmol·L−1; their IC50 values were 17.19, 14.56, and 19.53 μg/mL, respectively [35]. Moreover, broussoflavonol B restricted the growth of breast cancer SK-BR-3 cells and breast cancer MDA-MB-231 cells at sub-micromolar concentrations via inducing cell-cycle arrest at the G0/G1 and G2/M phases and inducing the differentiation of cells [79]. In the same year, broussoflavonol B and 5,7,3′,4′-tetrahydroxy-3-methoxy-8,5′-diprenylflavone were prepared from an ethyl acetate-soluble fraction of BP barks, exerting potent antiproliferation activities on the ER-positive MCF-7 cells, with IC50 values of 4.41 and 4.19 mM, respectively [34]. The two compounds could also inhibit tumor proliferation on BCAP-37 cells in vivo, from a dosage of 0–25 µM [34]. In 2016, kazinol A showed cytotoxicity in T24 and T24R2 cells from a dosage of 0–50 µM via G0/1 arrest, mediated by decreasing cyclin D1 and increasing p21 [80]. In addition, kazinol E was a targeted molecule for breast cancer stem-like cells from a dosage of 0–50 µM, by blocking EGF-induced ERK activity directly [81,82]. Moreover, in 2017, Kim et al. identified that marmesin eliminated mitogen-stimulated proliferation and invasion in both p53 wild-type A549 and p53-deficient H1299 NSCLC cells [83]. In 2018, Park et al. reported that broussochalcone A showed high cytotoxic activities in human hepatoma HepG2 and SK-Hep1 cells, with an IC50 value of 20 µM from a dosage of 0–40 µM; these activities were due to cell-cycle arrest by increasing FOXO3, regulating the cell cycle, and activating pro-apoptotic proteins [84]. In another study conducted by Shin et al. in 2019, broussochalcone A also exerted strong cytotoxic effects upon colon and liver cancer cells with a dosage of 0–20 μM, by promoting the phosphorylation/ubiquitin-dependent degradation of β-catenin [85]. In 2019, broussoflavonol K showed stronger inhibitory effects on NCI-H1975, MCF-7, and HepG2 than isolicofavonol, with IC50 values ranging from 0.90 to 2.00 μM, which were due to cyclization between the isoprenyl moiety and the adjacent phenolic hydroxyl group [41]. A recent study in 2020 investigated the anti-tumor effect of broussoflavonol B; the results showed that it significantly repressed the proliferation of human pancreatic cancer PANC-1 cells, by inactivating the ERK/c-Myc/FoxM1 signaling pathway, with a dosage of 0–100 μM [86]. In 2021, Vu et al. studied the inhibitory effects of eriodictyol, apigenin, and kaempferol against HL-60 cells, with IC50 values ranging from 46.43 to 94.06 μM [14]. In a study in 2022, marmesin also exerted cytotoxicity on esophagus cancer cells via inhibiting the PI3K/Akt pathway [87]. From this evidence, it is clear that AMPK is a major regulator of energy metabolic pathways and plays an important role in the regulation of autophagy. In this work, the active compound kazinol C, isolated from BK whole herbs or root barks, could markedly induce apoptosis in colon cancer cells by activating AMPK phosphorylation [2,88].
3.2. Anti-Oxidant Activity
Excessive oxidative stress is harmful to cells, protein, DNA, and others, so antioxidants are important molecules that can protect humans from this danger. Various assays of antioxidant activity have been used to test these properties, such as DPPH, ABTS, CAA, hydrogen peroxide scavenging activity assays, hydroxyl radical scavenging activity assays, FRAP, lipid peroxidation inhibitory activity, mitochondrial swelling assays, chelation of metal ions (Fe2+) assays, xanthin oxidase inhibitory activity assays, hydroxyl radical scavenging activity, superoxide anion free radical scavenging activities, superoxide anion radical scavenging activity assays, ferrous ion chelating capacity assays, and TEAC.
The antioxidant activities of the crude extracts of Broussonetia species were measured via the methods mentioned above, of which the most frequently used methods were DPPH, ABTS, FRAP, and hydroxyl radical scavenging activity assays. In 2014, DPPH and pyrogallol autoxidation assays showed that the hydroxyl radical inhibition rate of the seed oil of BP was 91.21% [89]. In the same year, the ethanol extract of BP fruits was revealed to demonstrate antioxidant activity (0–400 mg/mL), with an IC50 value of 155.7 µg/mL for lipid peroxidation inhibition on liver homogenate [90]. In 2013, the ethanolic extract of BP fruits showed maximum antioxidant activity by DPPH assay during the dose of 0–600 µg/mL, with an IC50 value of 156.3 µg/mL [75]. In 2012, Sun et al. indicated that the ethanol extract from BP flowers showed more potent radical scavenging activity than the water extract, which showed 62.88% in the DPPH assay at 5 mg/mL and 61.15% in terms of chelation Fe2+-activity at 6 mg/mL [91]. In another experiment, Sun et al. reported that the ethanol and water extracts of BP fruits showed strong DPPH radical-scavenging activity at 87.17 ± 0.18% and 58.11 ± 0.11%, respectively [7,91]. The Fe2+-chelating activity was approximately 77.51% and 48.26% from an aqueous extract of 5 mg/mL and an ethanol extract of 5 mg/mL [7,91].
Several pure compounds of Broussonetia species also showed antioxidant effects in vitro, apart from the extracts mentioned above. In 2020, broussoflavonol A, 5,7,3′,4′-tetrahydroxy-3-methoxy-8,5′-diprenylflavone and kazinol M isolated from BP branches and twigs have shown good antioxidant activities, with CAA values of 25.9, 6.4, and 5.4, respectively [42]. In 2014, luteolin, luteoloside, orientin, and isoorientin showed strong radical scavenging activities by DPPH from a dosage of 0.1–3 mg/mL, with SC50 values of 19.72, 19.67, 18.86, and 19.33 mmol/L, respectively [36]. In 2012, Ryu et al. indicated that broussochalcone A and 3,4-dihydroxyisolonchocarpin, isolated from BP roots, showed the highest antioxidant activities within a dosage of 0.1–1000 µM, with IC50 values of 27.6 ± 0.3 µM and 21.8 ± 0.2 µM through DPPH and IC50 values of 5.8 ± 0.1 µM and 7.7 ± 0.4 µM through ABTS, as well as IC50 values of 0.6 ± 0.04 µM and 1.8 ± 0.1 µM through an XOD assay [57]. In 2010, curculigoside C, ferulic acid, dihydroconiferyl alcohol, and 3,4-dihydroxybenzoic acid were revealed to have antioxidant activities within a dose of 0.16 to 100 mM, with the IC50 values of 39.5, 58.9, 65.3, and 65.6 mM, respectively [54]. In 2009, MTT and DPPH assays showed that erythro-1-(4-hydroxy-3-methoxyphenyl)-2-{4-[(E)3-hydroxy-1-propenyl]-2-methoxyphenoxy}-1,3-propanediol, isolated from BP fruits (0.16–100 µM), possessed significant antioxidant activities with an IC50 value of 60.9 µM [53]. In a diphenyl-2-picrylhydrazyl assay system, broussochalcone A exerted stronger radical scavenging activity within a dose of 1–30 µM than α-tocopherol at IC0.200 values of 7.6 ± 0.8 µM [92]. Broussoflavonol F, broussoflavan A, broussoaurone A, and broussoflavonol G inhibited the Fe2+-induced formation of TBARS in a concentration-dependent manner, with IC50 values of 2.1, 2.7, 1.0 and 1.2 µM, respectively [46].
From Table 8, it can be seen that a plethora of investigations revealed that the roots and leaves possessed stronger antioxidant activities than other parts [93]. Moreover, many assays indicated that the antioxidant activities of bark extracts were superior to wood extracts [94].
3.3. Anti-Inflammation
In 2001, broussochalcone A, isolated from BP at a dose of 1–20 μM could inhibit NO production in LPS-activated macrophages by inhibiting IkBa phosphorylation, IkBa degradation, nuclear factor-kappa B activation, and iNOS expression, with an IC50 value of 11.3 mM [92]. In 2003, papyriflavonol A, isolated from BP, was demonstrated to inhibit human group IIA and V sPLA2s dose-dependently and reduce IgE-dependent passive cutaneous anaphylaxis in rats from a dose of 0–250 μM with IC50 values of 3.9 to 4.5 mM, suggesting that it could be a novel anti-inflammatory drug in the future [95]. In 2019, Huang et al. demonstrated that broussonin E, isolated from the bark of BK, could treat inflammatory diseases by modulating the activation state of macrophages by suppressing ERK and p38 MAPK and enhancing the JAK2-STAT3 signaling pathway [96]. In the same year, flavanone, broussochalcone C, broussoflavanonol A, kazinol V, kazinol W and broussoflavonol B, isolated from root bark in 100% methanol, were shown to have potent anti-inflammatory effects on LPS-stimulated RAW264.7 cell through downregulating iNOS, COX-2, and TNF-α expression within the dose of 1.25–40 μM [40]. In the same year, the anti-inflammatory effect of broussoflavonol H was studied by Tian et al.; the results showed that the compound could significantly suppress the production of IL-2 in Jurkat induced by PHA and PMA, with an IC50 value of 9.95 μM [41]. Moreover, in 2020, 5,7,3′,4′-tetrahydroxy-3-methoxy-8,5′-diprenylflavone, kazinol M, broussoflavonol B, broussoflavonol A, and broussofluorenone C, isolated from the branches and twigs of BP, showed anti-inflammatory effects by activating NF-κB/AP-1 [42]. In 2021, eriodictyol, apigenin, and kaempferol reduced LPS-induced iNOS expression within the dosage of 0–30 μM in a dose-dependent manner, with IC50 values of 11.98, 10.16, and 24.06 μM, respectively [14].
In 2008, the ethanol extract of BP roots was shown to reduce abdominal Evan’s blue extravasations, including serotonin and sodium nitroprusside, caused by inflammatory mediators; the effects might be related to inhibiting the vascular permeability via autocrines and NO [97]. In 2010, the anti-inflammatory effect of methanol extract of BP heartwood was investigated in NC/Nga mice induced by an extract of the house-dust mite, Dermatophagoides farina; the results showed that the methanol extract could obviously inhibit AD-like skin lesions by decreasing the levels of IgE and IL-4 and inhibiting the induction of TARC/CCL17, MDC/CCL22, and RANTES/CCL5 in HaCaT cells [98]. Furthermore, it was reported that the n-hexane fraction and n-butanol fraction of the methanol extract of BP stem bark at a dosage of 10–80 μg/mL were found to have significant anti-inflammatory activities in RAW 264.7 cells by inhibiting NO and pro-inflammatory cytokine production [74,97]. In 2014, the ethanol extract of BK leaves within a dose of 200–1000 μg/mL could treat Nc/Nga mice that were predisposed to develop AD-like skin lesions induced by D. farinae extract; further study demonstrated that its mechanism might be related to significantly downregulating the plasma levels of IgE and IL-4, as well as inhibiting hTARC secretion in HaCaT cells by activated TNF-α/IFN-γ [97].
In general, the anti-inflammatory activity of Broussonetia species was mainly studied in a murine macrophage RAW264.7 cell model and in mice stimulated with LPS. Moreover, the mechanism of anti-inflammatory activity was mainly concentrated on inhibiting NO production and iNOS expression. iNOS was primarily found in macrophages induced by LPS or cytokines to produce a high level of NO as a pro-inflammatory mediator [49]; therefore, the inhibition of NO production or iNOS expression was a critical strategy for the treatment of inflammatory diseases.
3.4. Anti-Diabetic and Anti-Obesity Effects
Diabetes is a chronic disease that presents as high levels of glucose in the blood, which may be caused by insulin deficiency and insulin resistance. All in vitro and in vivo studies have demonstrated the antidiabetic effects of different extracts and compounds prepared from Broussonetia species.
In 2008, Cha et al. indicated that the ingestion of stem bark powder from BK decreased the serum levels of glucose, fructosamine, triglyceride, and total cholesterol, as well as the activity of ALT in the genetically diabetic OLETF rats; the important regulatory factor would be the increased blood insulin level in the animal model [16]. In 2010, Ryu et al. showed that broussochalcone A, papyriflavonol A, broussochalcone B, kazinol A, kazinol B, and 8-(1,1-dimethylallyl)-5′-(3-methylbut-2-enyl)-3′,4′,5,7-tetrahydroxyflanvonol have inhibitory effects against α-glucosidase with a dose of 0.01–1000 μM; the IC50 values were 5.3, 11.1, 12.0, 26.3, 3.6, and 2.1 μM, respectively [56]. Moreover, kazinol U [99], isokazinol D, and kazinol C [100] showed therapeutic potential in delaying pancreatic β-cell destruction in type 1 diabetes by blocking the NF-kB pathway in pancreatic β-cells and reducing RINm5F cell damage. A report in 2012 indicated the anti-obesity effect of broussonone A, as well as of other isolated phenolic compounds isolated from BK stem barks, the mechanism being related to noncompetitive inhibitory activity on pancreatic lipase, with an IC50 of 28.4 µM and an inhibitory effect of adipocyte differentiation in 3T3-L1 cells [33]. In 2016, antidiabetic activity was also observed in mouse 3T3-L1 preadipocyte cells and C2C12 myoblast cells. Lee et al. demonstrated that kazinol B, isolated from BK roots, within a dose of 0–20 μM could increase insulin sensitivity via improving glucose uptake through the insulin-Akt signaling pathway, along with AMPK activation [101]. In 2020, treatment with broussoflavonol B and kazinol J in HFD-fed C57BL6 male mice within the dosage of 0–100 μg/mL, showed therapeutic potential in obesity and type 2 diabetes via suppressing pro-inflammatory responses by activating AMPK in 3T3-L1 adipocytes [102]. In addition, an ethanolic extract of BK fruits could treat β-cell damage by preventing STZ-induced oxidative stress and suppressing β-cell apoptosis via inhibiting Erk phosphorylation, as found in mice injected with STZ [15], and it could also treat diabetic nephropathy via the activation of Nrf2 and provide protection against PA-induced lipotoxicity in the mesangial cells in diabetes [103].
In a word, the mechanism of anti-diabetic effects is mainly related to blocking the NF-kB pathway and inhibiting α-glucosidase activity. The generation of NO via iNOS and reactive oxygen species plays an important role in pancreatic β-cell damage. The NF-kB transcription factor was activated by oxidative stress due to reactive oxygen species as well as regulating iNOS expression. Thus, the NF-kB pathway can protect the β-cell from damage [99].
3.5. Antibacterial and Antiviral Effects
Some studies have shown that the extracts or pure compounds of Broussonetia species could suppress bacteria. In 2015, N. Naveen Kumar et al. [104] reported that the hexane extract of BP seeds showed high inhibitory activity on Staphylococcus aureus, Proteus vulgaris, Bacillus cereus, and Enterobacter aerogenes, whereas it had no inhibitory effect on fungal strains [104]. In 2017, Park et al. analyzed the antibacterial activity of papyriflavonol A within the dosage of 1–1000 µM; the results showed that the potent inhibitory effect of PLpro, with an IC50 value of 3.7 μM, along with a further study, showed that it may be a potential anti-COVID-19 agent [105]. Geng et al. [17] indicated that 5,7,3′,4′-tetrahydroxy-3-methoxy-8,5′-diprenylflavone, isolated from the BP air-dried aerial part, showed more antibacterial activity in suppressing Actinomyces naeslundii and Porphyromonas gingivalis (MIC = 1.95 ppm) than the positive control, triclosan, at a dosage of 0.12–250 ppm. In 2021, Ghosh et al. [18] found that six polyphenols (broussochalcone A, papyriflavonol A, 3′-(3-methylbut-2-enyl-3′,4′,7-trihydroxyflavane, broussoflavan A, kazinol F, and kazinol J) showed greater Mpro inhibitory effect than two repurposed drugs (lopinavir and darunavir) and may serve as promising anti-COVID-19 drugs.
3.6. Skin Whitening and Anti-Wrinkle Activities
In 2019, Lim et al. [19] reported that kazinol U, a constituent of BK root barks, could attenuate melanogenesis within a dose of 0–20 μM via inhibiting MITF expression, inactivating target genes such as tyrosinase, Tyrp1, and Tyrp2, and activating AMPK and MAPK proteins in both in vitro and in vivo experiments. In the same year, it was reported that collagen is the major structural protein in the extracellular space of the connective tissue of the skin, and BK stem extract could maintain skin collagen content via inactivating the reactive oxygen species and inhibiting collagenase activity [106].
3.7. Other Properties
Out of these pharmacological activities displayed above, Broussonetia species also showed the treatment of bone diseases, liver protection, promoting hair growth, anti-angiogenic activities, anticholinesterase effects, increasing cAMP, immune-stimulating activity, and antinociceptive.
In 2021, Vu et al. observed the antiosteoclastogenic activity of broussonols F, G, and K; the results showed that the compounds significantly inhibited RANKL-induced osteoclast formation with a dosage of 10–30 μM in RAW264.7 cells and they may be the lead compounds against bone diseases in the future [44]. In 2020, CSZ extract increased liver function and alleviated DILI in rats induced in acetaminophen (APAP) via regulating the TLR3/JNK/c-jun/c-fos/JAK/STAT3 pathway [107]. In 2020, Lee et al. [108] showed that ethanolic extract of BP had the capacity of promoting hair growth by regulating β-Catenin and STAT6 target proteins in human hair follicle dermal papilla (hHFDP) cells within a dose of 0–20 µg/mL. In 2014, the ethanolic extract of BK twigs was found to have anti-angiogenic activities with a dosage of 0.1–10 µg/mL; the mechanisms were the inhibition of VEGF-A, stimulated by the phosphorylation/activation of ERK, Akt, and p70S6K, and the downregulation of VEGFR-2 and MMP-2 in human umbilical vein endothelial cells [109]. In 2012, three prenylated flavonols, 8-(1,1-dimethylallyl)-5′-(3-methylbut-2-enyl)-3′,4′,5,7-tetrahydroxyflanvonol,papyriflavonol A, and broussoflavonol B, isolated from BP roots, suppressed two human cholinesterases related to Alzheimer’s disease (AD) in a dose-dependent manner, with IC50 values ranging from 0.8 to 3.1 μM and from 0.5 to 24.7 μM against HAChE and BChE, respectively [47]. In the next year, the immune-stimulating activity of mice that were immunized intraperitoneally with OVA/alum (100 μg/200 μg) was tested; the results showed that mice given BK water extract orally for 21 days could enhance the Th1 immune response and showed no cytotoxicity in the model system [110]. In 2010, Lee et al. [50] explored the estrogenic activity of broussonin A, (+)−(2R) kazinol I, tupichinol C and kazinol U, which showed that these compounds could regulate the E2-responsive genes as functional ER ligands such as E2 in the ER-sensitive MCF-7 cells at 10 µM. Kazinol P, a natural compound isolated from BK, showed the therapeutic effects of improving muscle regeneration and repair with the mechanism of promoting myogenic differentiation through activating p38MAPK and MyoD transcription activities [111]. In a study in 2003, Kazinol B, an isoprenylated flavan, showed significant inhibitory activities of nitric oxide (NO) in lipopolysaccharide-activated macrophages, with an IC50 of 21.6 mM [112].
4. Application
4.1. Supplements to the Diet of Animals
It was reported that a supplement of Broussonetia species in the roughage diet was beneficial to the growth performance, carcass traits, meat quality and color, immune response, digestibility of crude protein, and rumen fermentation in different animals, such as Hu rams and lambs and growing goats [20,119]. Tao et al. [120] demonstrated that beef cattle fed on a diet with 15% BP could enhance their antioxidant functions by decreasing blood 8-OHdG and MDA and increasing blood SOD and TAC; the supplements could strengthen the performance by increasing final BW, ADG, DMI and FCR, improve the meat quality by lowing pH and drip loss and increasing CIE L, and also increase the PUFA and DHA concentrations in meat. A diet supplemented with BP could enhance immune and antioxidant function, as well as increase the polyunsaturated fatty acid concentrations in the milk of Holstein cows [121,122]. A diet supplemented with BP leaf extracts at a certain dosage could increase the growth performance and antioxidant capacity of weaned piglets, enhance immune functions and disease resistance, reduce the occurrence of diarrhea, and affect the composition of fecal microflora [123].
4.2. Phytoremediation of Heavy Metal-Contaminated Soil
The effective phytoremediation of heavy metal-contaminated soil requires species with high metal tolerance. Broussonetia species were excellent choices, owing to their adaptation to drought and to a saline-alkali environment [124]. Zeng et al. [125] showed that BP could effectively alleviate the adverse effects of heavy metal-contaminated soil on plant growth by enhancing the antioxidant enzyme activities in leaves and binding heavy metal-contaminated soil with organic acids, carbohydrates, protein, and amino acids in roots. Huang et al. [126] isolated Bacillus cereus HM5 and Bacillus thuringiensis HM7, and explored their potential to improve the effect of remedying Mn pollution by BP; the results showed that the biomass, total root length, surface area, crossings, tips, forks, and root activity of BP with the two strains were higher than BP without the two strains, so the two strains could promote the accumulation of Mn. Luo et al. [127] indicated that BP could be used for the revegetation and phytostabilization of zinc-smelting slag sites because of the high heavy-metal tolerance and low heavy-metal accumulation. Co-planting was also a sustainable approach for the phytoremediation of the heavy metal-contaminated soil. The hyperaccumulator Pteris vittata L., co-planted with BP, could improve the environmental quality of heavy metal-contaminated soil by promoting the growth and uptake of P. vittata L. and improving the comprehensive extraction of metal [128]. Zeng et al. [129] selected Pteris vittata L., Arundo donax L., Morus alba L. and BP for tree–herb co-planting; the results indicated that the four-herb co-planting system positively affected the soil microbes and had stronger impacts on the composition of soil microorganisms.
4.3. Combination Using
The pharmacological effects of Broussonetia genus are limited but, when combined with other plants, it can exert more meaningful pharmacological effects. A liquid bandage was made from Styela clava tunics and BK barks cellulose powders; it could accelerate wound-healing in the surgical skin wounds of Sprague-Dawley rats by stimulating re-epithelialization and connective tissue formation, without liver or kidney toxicities [23]. A new phytoformula containing BP and Lonicera japonica was revealed to have therapeutic potential for systemic septic inflammation, as well as chronic bronchitis, and against LPS-induced septic inflammation in mice by reducing the induction of some important proinflammatory cytokines, at a dosage of 200–400 mg/kg [22]. Phellinus linteus, cultured with BK, could inhibit melanogenesis by activating the phosphatidylinositol 3-kinase/Akt/glycogen synthase kinase-3beta signaling pathway and down-regulating the microphthalmia-associated transcription factor [130].
4.4. Inhibition of Target Enzymes
Broussonetia species showed inhibitory effects on target enzymes, such as monophenolase and diphenolase of tyrosinase [55], mushroom tyrosinase [31], xanthine oxidase, and protein tyrosine phosphatase 1B (PTP1B) [37]. Four compounds, kazinol S, kazinol C, broussonin C, and kazinol F, isolated from BK roots, showed inhibitory effects on the monophenolase of tyrosinase, with IC50 values ranging between 0.43 and 17.9 µM [55]. They also showed reversible slow-binding inhibitory effects on the diphenolase of tyrosinase, with IC50 values of 22.8, 1.7, 0.57, and 26.9 µM, respectively [55]. Zheng et al. [31] revealed the inhibitory activities of mushroom tyrosinase, using L-tyrosine as a substrate of quercetin, broussoflavonol F, 3,5,7,4′-tetrahydroxy-3′-(2-hydroxy-3-methylbut-3-enyl) flavone, and uralenol, with IC50 values of 96.6, 49.5, 57.8, and 82.3 µM, respectively; the results were better than arbutin, a well-known tyrosinase inhibitor [31]. Broussonetones A-C, three new ent-kaurane-type diterpenes, showed marginal inhibitory effect against tyrosinase and xanthine oxidase, as indicated by Ko et al. [32]. 3,3′,4′,5,7-pentahydroxyflavone, 8-(1,1-dimethylallyl)-5′-(3-methylbut-2-enyl)-3′,4′,5,7-tetrahydroxyflanvonol, uralenol and broussochalcone A showed the in vitro inhibition of PTP1B, as reported by Chen et al. [30]. Broukazinol A, 7,4′-dihydroxy-3′-prenylflavan, Broukazinol C, (2S)-7,3′-dimethoxy-4′-hydroxyflavan, broussonin B, 1-(4-hydroxy-2-methoxyphenyl)-3-(4-hydroxy-3-prenylphenyl) propane and 1-(2,4-dihydroxy-3-prenylphenyl)-3-(4-hydroxyphenyl) propane, isolated from BK twigs, also showed the inhibition of PTP1B, as reported by Xue et al. [37].
4.5. Papermaking and Cloth-Making
The barks of the Broussonetia species were excellent materials for the production of papers and clothes, which can be attributed to the high fiber content of the phloem. Cai Lun papermaking was one of the four great inventions of ancient China; this great invention used BP barks at that time. Notably, the first banknote in the world was made from BP. Papers made by BP barks were once used for making books and for writing in the Tang dynasty and their use continued during the Ming and Qing dynasties, as evidenced by many Dunhuang and Turfan manuscripts [21]. The bark of BP can also be used to make ancient tapa clothes; in this process, the barks were peeled from the cut stems to obtain a long strip, then the manufacturer removed the inner bark and washed and pounded the fibers to flatten them, felted them together with sheets in the sun, and finally printed them with native dyes to produce the finished traditional tapa cloth [10].
4.6. Others
In addition to the applications mentioned above, the remaining applications are classified as “others”. A study conducted by Qiu et al. explored their application in cleaning up phosphorus pollution; they showed that BP biochar, when combined with phosphate in the forms of exchangeable phosphorus, Al-bound phosphorus, and Fe-bound phosphorus, could be used to treat eutrophic bodies of water [131]. Zhang et al. [132] measured the ability of BP to resist drought due to the electrophysiological characteristics of the plants; the results showed that the relative tensity of BP and M. alba were 3.965 and 2.624, respectively. The author also demonstrated that the minimal fluorescence efficiency and maximal photochemical efficiency were 5.496 and 7.640 for BP and 6.577 and 5.359 for M. alba, respectively; therefore, the drought-resistance ability of BP was greater than that of M. alba. Mo et al. [133] studied the ability of BP to control air pollution; the results showed that BP was efficient in capturing small particles and showed high levels of PM accumulation.
5. Conclusions and Further Perspectives
This review mainly summarized the phytochemistry, pharmacology, and applications of the Broussonetia genus. In this work, we present a list of 338 compounds that have been isolated from the herbs of Broussonetia, including 144 flavonoids, 50 phenylpropanoids, 38 polyphenols, 35 alkaloids, 17 terpenoids, 5 steroids, and 49 other metabolites, which indicated that flavonoids were the main constituent in the genus of Broussonetia. A variety of pharmacological activities have been demonstrated in vivo or in vitro assays, including anti-tumor, antioxidant, anti-inflammatory, antidiabetic, anti-obesity, antibacterial, and antiviral properties, as well as skin whitening and anti-wrinkle activities. Nevertheless, some studies of the Broussonetia genus are limited at present.
First, phytochemistry clarified 338 compounds isolated from BP, BK, and BL, but only 5 steroids and 17 terpenoids were involved. Undoubtedly, we can make more effort toward the exploration of steroids and terpenoids by the targeted phytochemical methods. Second, the Broussonetia genus consists of 11 species, but the investigations of phytochemistry, pharmacology and applications were only studied in BP, BK, and BL. Therefore, it is extremely important for researchers to conduct a comprehensive evaluation of other species to extend the available source domain. Third, pharmacological studies have uncovered anti-tumor, antioxidant, anti-inflammatory, anti-diabetic, anti-obesity, antibacterial, and antiviral properties, as well as skin whitening and anti-wrinkle activities. Notably, the relevant bioactive compounds only include flavonoids, penylpropanoids, and polyphenols; therefore, the pharmacological activities of the remaining compounds need to be further explored by researchers. Furthermore, the pharmacological activities of the concrete compounds of crude extracts need to be confirmed in the future, which would be conducive to finding new candidates for corresponding diseases. Fourth, mechanism analysis indicated that active compounds and extracts mainly showed anti-tumor effects by inducing cell apoptosis and triggering cell cycle arrest; they showed anti-inflammatory activity mainly by inhibiting NO production and iNOS expression and showed anti-diabetic effects mainly via blocking the NF-kB pathway and α-glucosidase. Undoubtedly, we can see that mechanism studies of the active compounds and extracts isolated from Broussonetia species were mainly concentrated on the typical targets and pathways. Hence, new targets and pathways should be detected in the future. Fifth, in vitro models were considered to be the main conditions; therefore, more in vivo models should be used to investigate properties in the future. Sixth, although the applications indicated that Broussonetia species could be used to supplement the diet of beef cattle, growing goats, cows, and piglets, and showed multiple beneficial effects on their growth performance, carcass traits, meat quality, and immune response, the concrete clinical safety, toxicity and pharmacokinetics studies for animals and humans were extremely limited; thus, exploring these aspects is the top priority in future research.
Overall, this updated review on the plants of the Broussonetia genus can provide an important and valuable reference for researchers interested in Broussonetia and promote scientific development and utilization of the Broussonetia genus.
Abbreviations
| CALCBP | Chlorogenic acid-like compounds extracted from BP |
| MS | Mass Spectrum |
| NMR | Nuclear Magnetic Resonance Spectrometer |
| EC | Esophagus cancer |
| NSCLC | Non-small cell lung cancer cell |
| ERK | Extracellular signal-regulated kinase |
| AMPK | Adenosine 5-monophosphate [134]-activated protein kinase |
| HepG | Human hepatoma carcinoma |
| CAA | Cellular antioxidant activity |
| IC50 | Half maximal inhibitory concentration |
| XOD | Xanthine oxidase |
| TFC | Determination of total flavonoid content |
| TPC | Determination of total phenolic contents |
| MTT | 3-(4,5)-dimethylthiahiazo (-z-y1)-3,5-di- phenytetrazoliumromide |
| TBARS | Thiobarbituric acid-reactive substance |
| PMA | Phorbol 12-myristate 13-acetate |
| PHA | Phytohemagglutinin |
| iNOS | Inducible nitric oxide synthas |
| LPS | Lipopolysaccharide |
| PA- | Palmitate- |
| STZ | Streptozotocin |
| HFD | High fat diet |
| MLDS | Multiple low-dose streptozotocin |
| ALT | Alanine aminotransferase |
| OLETF | Otsuka Long-Evans Tokushima fatty |
| MIC | Minimum inhibitory concentration |
| APAP | Acetaminophen |
| hHFDP | Human hair follicle dermal papilla |
| BChE | Butylcholinesterase |
| hAChE | Human acetylcholinesterase |
| OVA | Ovalbumin |
| BW | Body weight |
| ADG | Average daily gain |
| DMI | Dry matter intake |
| FCR | Feed conversion ratio |
| MDA | Malondialdehyde |
| SOD | Superoxide dismutase |
| TAC | Total antioxidant capacity |
| PUFA | Polyunsaturated fatty acids |
| DHA | Docosahexaenoic acid |
| LPS | Lipopolysaccharide |
| PTP1B | Tyrosine phosphatase 1B protein |
| MAPK | Mitogen-activated protein kinase |
| STAT | Signal transducer and activator of transcription |
| JAK | Janus kinase |
| AD | Atopic dermatitis |
| T24 | Human bladder cancer cells |
| DPPH | 1,1′-diphenyl-2-picryl-hydrazyl radical |
| ABTS | 2,2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid |
| FRAP | Ferric reducing activity power assay |
| TEAC | Trolox equivalent antioxidant capacity |
| TNF-alpha | Tumor necrosis factor-alpha |
| IFN-gamma | Interferongamma |
| TARC/CCL17 | Thymus-and-activation regulated chemokine |
| MDC/CCL22 | Macrophagederived chemokine |
| RANTES/CCL5 | Regulated-onactivation-normal T cell-expressed-and-secreted chemokine |
| IL-4 | Interleukin-4 |
| IgE | Plasma immunoglobulin E |
| RINm5F cells | Rat pancreatic β-cell line |
| HFD | Ten-week-high fat diet |
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/molecules27165344/s1, Figure S1: Chemical structures of the Flavonoids in Broussonetia species, Figure S2: Chemical structures of the Penylpropanoids in Broussonetia species, Figure S3: Chemical structures of the Polyphenols in Broussonetia species, Figure S4: Chemical structures of the Alkaloids in Broussonetia species, Figure S5: Chemical structures of the Terpenoids in Broussonetia species, Figure S6: Chemical structures of the Steroids in Broussonetia species, Figure S7: Chemical structures of the Others in Broussonetia species.
Author Contributions
Y.C. and L.W. contributed equally to this work. Y.C. and L.W.: data curation, writing-original draft and review & editing, methodology. F.W., X.L., Y.A., W.Z. (Wei Zhao), J.T., D.K.: data curation, investigation, formal analysis. W.Z. (Wenru Zhang), Y.X., Y.B.: investigation, methodology, data curation. H.Z. provided the financial support for this manuscript. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was funded by the Project of the Shandong Province Key Research and Development Program (2021CXGC010511), the project of Shandong postgraduate education quality curriculum (SDYKC21051), and the innovation training program for college students of Shandong University of Traditional Chinese Medicine (2021047, 202255) of Honglei Zhou.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Wang F.F., Su Y.L., Chen N.Z., Shen S.H. Genome-Wide analysis of the UGT gene family and identification of flavonoids in Broussonetia papyrifera. Molecules. 2021;26:3449. doi: 10.3390/molecules26113449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee Y., Kwon J., Jeong J.H., Ryu J.H., Kim K. Kazinol C from Broussonetia kazinoki stimulates autophagy via endoplasmic reticulum stress-mediated signaling. Anim. Cells Syst. 2022;26:28–36. doi: 10.1080/19768354.2021.2023628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gunatilaka A.L., Uvais M., Sultanbawa S., Surendrakumar S., Somanathan R. Structure of a bipyridine alkaloid from Broussonetia zeylanica. Phytochemistry. 1983;22:2847–2850. doi: 10.1016/S0031-9422(00)97711-2. [DOI] [Google Scholar]
- 4.Casuga F.P., Castillo A.L., Corpuz M. GC–MS analysis of bioactive compounds present in different extracts of an endemic plant Broussonetia luzonica (Blanco)(Moraceae) leaves. Asian Pac. J. Trop. Biomed. 2016;6:957–961. doi: 10.1016/j.apjtb.2016.08.015. [DOI] [Google Scholar]
- 5.Chung K.F., Kuo W.H., Hsu Y.H., Li Y.H., Rubite R.R., Xu W.B. Molecular recircumscription of Broussonetia (Moraceae) and the identity and taxonomic status of B. kaempferi var. australis. Bot. Stud. 2017;58:11. doi: 10.1186/s40529-017-0165-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lemmens R. Broussonetia greveana (Baill.) CC Berg. Plant. Resour. Trop. Afr. 1977;47:356. [Google Scholar]
- 7.Sun J., Liu S.F., Zhang C.S., Yu L.N., Bi J., Zhu F., Yang Q.L. Chemical composition and antioxidant activities of Broussonetia papyrifera fruits. PLoS ONE. 2012;7:e32021. doi: 10.1371/journal.pone.0032021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang G.W., Huang B.K., Qin L.P. The genus Broussonetia: A review of its phytochemistry and pharmacology. Phytother. Res. 2012;26:1–10. doi: 10.1002/ptr.3575. [DOI] [PubMed] [Google Scholar]
- 9.Luo X., Tian T., Tan X., Yang X.J. First report of lasiodiplodia theobromae causing brown leaf spot on Broussonetia papyrifera in Southwestern China. Plant Dis. 2020;104:2024. doi: 10.1094/PDIS-02-20-0254-PDN. [DOI] [Google Scholar]
- 10.Whistler W.A. Broussonetia papyrifera (paper mulberry) Tradit. Tree Initiat. 2006;9:1–3. [Google Scholar]
- 11.Lin J., Zou J., Zhang B., Que Q., Zhang J., Chen X., Zhou W. An efficient in vitro propagation protocol for direct organo genesis from root explants of a multi-purpose plant, Broussonetia papyrifera (L.) L’Hér. ex Vent. Woody Forage. 2021;170:113686. [Google Scholar]
- 12. [(accessed on 1 July 2022)]. Available online: https://www.plantplus.cn/info/Broussonetia.
- 13.Sirita J., Chomsawan B., Yodsoontorn P., Kornochalert S., Lapinee C., Jumpatong K. Antioxidant activities, phenolic and tannin contents of paper mulberry (Broussonetia papyrifera) extract. Med. Plants. 2020;12:371–375. doi: 10.5958/0975-6892.2020.00046.5. [DOI] [Google Scholar]
- 14.Vu N.K., Le T.T., Woo M.H., Min B. Anti-inflammatory and cytotoxic activities of phenolic compounds from Broussonetia kazinoki. Phytochemistry. 2021;27:176–182. [Google Scholar]
- 15.Kim H.J., Kim D., Yoon H., Choi C.S., Oh Y.S., Jun H.S. Prevention of oxidative stress-induced pancreatic Beta cell damage by Broussonetia kazinoki Siebold fruit extract via the ERK-Nox4 pathway. Antioxidants. 2020;9:406. doi: 10.3390/antiox9050406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cha J.Y., Kim Y.T., Kim H.S., Cho Y.S. Antihyperglycemic effect of stem bark powder from paper mulberry (Broussonetia kazinoki sieb.) in type 2 diabetic Otsuka long-evans Tokushima fatty rats. J. Med. Food. 2008;11:499–505. doi: 10.1089/jmf.2007.0028. [DOI] [PubMed] [Google Scholar]
- 17.Geng C.A., Yan M.H., Zhang X.M., Chen J.J. Anti-oral microbial flavanes from Broussonetia papyrifera under the guidance of bioassay. Nat. Prod. Bioprospect. 2019;9:139–144. doi: 10.1007/s13659-019-0197-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ghosh R., Chakraborty A., Biswas A., Chowdhuri S. Identification of polyphenols from Broussonetia papyrifera as SARS-CoV-2 main protease inhibitors usingin silicodocking and molecular dynamics simulation approaches. J. Biomol. Struct. Dyn. 2021;39:6747–6760. doi: 10.1080/07391102.2020.1802347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lim J., Nam S., Jeong J.H., Kim M.J., Yang Y., Lee M.S., Lee H.G., Ryu J.H., Lim J.S. Kazinol U inhibits melanogenesis through the inhibition of tyrosinase-related proteins via AMP kinase activation. Br. J. Pharmacol. 2019;176:737–750. doi: 10.1111/bph.14560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sheng P., He L., Ji S.S., Huang J.L., Zhang Z.H., Wang D.S., Liu J.P., Zhang H.Q. Effect of Broussonetia papyrifera L. (paper mulberry) on growth performance, carcase traits, meat quality and immune performance in Hu ram lambs. Ital. J. Anim. Sci. 2021;20:691–697. doi: 10.1080/1828051X.2021.1904795. [DOI] [Google Scholar]
- 21.Peng X., Liu H., Chen P., Tang F., Hu Y., Wang F., Pi Z., Zhao M., Chen N., Chen H. A chromosome-scale genome assembly of paper mulberry (Broussonetia papyrifera) provides new insights into its forage and papermaking usage. Mol. Plant. 2019;12:661–677. doi: 10.1016/j.molp.2019.01.021. [DOI] [PubMed] [Google Scholar]
- 22.Ko H.J., Kwon O.S., Jin J.H., Son K.H., Kim H.P. Inhibition of experimental systemic inflammation (septic inflammation) and chronic bronchitis by new phytoformula BL containing Broussonetia papyrifera and Lonicera japonica. Biomol. Ther. 2013;21:66–71. doi: 10.4062/biomolther.2012.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Park J.J., Kim J.E., Yun W.B., Lee M.R., Choi J.Y., Song B.R., Son H.J., Lim Y., Kang H.G., An B.S., et al. Therapeutic effects of a liquid bandage prepared with cellulose powders from Styela clava tunics and Broussonetia kazinoki bark: Healing of surgical wounds on the skin of Sprague Dawley rats. Mol. Med. Rep. 2019;19:452–460. doi: 10.3892/mmr.2018.9668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fang S.C., Shieh B.J., Lin C.N. Phenolic constituents of formosan Broussonetia-papyrifera. Phytochemistry. 1994;37:851–853. [Google Scholar]
- 25.Fang S.C., Shieh B.J., Wu R.R., Lin C.N. Isoprenylated flavonols of formosan Broussonetia-papyrifera. Phytochemistry. 1995;38:535–537. doi: 10.1016/0031-9422(94)00594-J. [DOI] [Google Scholar]
- 26.Lin C.N., Chiu P.H., Fang S.C., Shieh B.J., Wu R.R. Revised structure of broussoflavonol G and the 2D NMR spectra of some related prenylflavonoids. Phytochemistry. 1996;41:1215–1217. [Google Scholar]
- 27.Ko H.H., Yen M.H., Wu R.R., Won S.J., Lin C.N. Cytotoxic isoprenylated flavans of Broussonetia kazinoki. J. Nat. Prod. 1999;62:164–166. doi: 10.1021/np980281c. [DOI] [PubMed] [Google Scholar]
- 28.Lee D., Bhat K.P.L., Fong H.H.S., Farnsworth N.R., Pezzuto J.M., Kinghorn A.D. Aromatase inhibitors from Broussonetia papyrifera. J. Nat. Prod. 2001;64:1286–1293. doi: 10.1021/np010288l. [DOI] [PubMed] [Google Scholar]
- 29.Zhang P.C., Wang S., Wu Y., Chen R.Y., Yu D.Q. Five new diprenylated flavonols from the leaves of Broussonetia kazinoki. J. Nat. Prod. 2001;64:1206–1209. doi: 10.1021/np010283o. [DOI] [PubMed] [Google Scholar]
- 30.Chen R.M., Hu L.H., An T.Y., Li J., Shen Q. Natural PTP1B inhibitors from Broussonetia papyrifera. Bioorg. Med. Chem. Lett. 2002;12:3387–3390. doi: 10.1016/S0960-894X(02)00757-6. [DOI] [PubMed] [Google Scholar]
- 31.Zheng Z.P., Cheng K.W., Chao J.F., Wu J.J., Wang M.F. Tyrosinase inhibitors from paper mulberry (Broussonetia papyrifera) Food Chem. 2008;106:529–535. doi: 10.1016/j.foodchem.2007.06.037. [DOI] [Google Scholar]
- 32.Ko H.H., Chang W.L., Lu T.M. Antityrosinase and antioxidant effects of ent-kaurane diterpenes from leaves of Broussonetia papyrifera. J. Nat. Prod. 2008;71:1930–1933. doi: 10.1021/np800564z. [DOI] [PubMed] [Google Scholar]
- 33.Ahn J.H., Liu Q., Lee C., Ahn M.J., Yoo H.S., Hwang B.Y., Lee M.K. A new pancreatic lipase inhibitor from Broussonetia kanzinoki. Bioorg. Med. Chem. Lett. 2012;22:2760–2763. doi: 10.1016/j.bmcl.2012.02.088. [DOI] [PubMed] [Google Scholar]
- 34.Guo F.J., Feng L., Huang C., Ding H.X., Zhang X.T., Wang Z.Y., Li Y.M. Prenylflavone derivatives from Broussonetia papyrifera, inhibit the growth of breast cancer cells in vitro and in vivo. Phytochem. Lett. 2013;6:331–336. doi: 10.1016/j.phytol.2013.03.017. [DOI] [Google Scholar]
- 35.Ran X.K., Wang X.T., Liu P.P., Chi Y.X., Wang B.J., Dou D.Q., Kang T.G., Xiong W. Cytotoxic constituents from the leaves of Broussonetia papyrifera. Chin. J. Nat. Med. 2013;11:269–273. doi: 10.1016/S1875-5364(13)60027-8. [DOI] [PubMed] [Google Scholar]
- 36.Yang C.Y., Li F., Du B.W., Chen B., Wang F., Wang M.K. Isolation and characterization of new phenolic compounds with estrogen biosynthesis-inhibiting and antioxidation activities from Broussonetia papyrifera leaves. PLoS ONE. 2014;9:e94198. doi: 10.1371/journal.pone.0094198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xue J.J., Lei C., Wang P.P., Kim K.Y., Li J.Y., Li J., Hou A.J. Flavans and diphenylpropanes with PTP1B inhibition from Broussonetia kazinoki. Fitoterapia. 2018;130:37–42. doi: 10.1016/j.fitote.2018.08.001. [DOI] [PubMed] [Google Scholar]
- 38.Li F., Wen T., Liu J. Progress in chemical constituents and bioactivities of Broussonetia papyrifera (L.) Vent. Med. Res. 2019;3:190005. doi: 10.21127/yaoyimr20190005. [DOI] [Google Scholar]
- 39.Lou Y., Su S.Y., Li Y.N., Lei C., Li J.Y. Flavonoids with PTP1B inhibition from Broussonetia papyrifera. China J. Chin. Mater. Med. 2019;44:88–94. doi: 10.19540/j.cnki.cjcmm.2019.0002. [DOI] [PubMed] [Google Scholar]
- 40.Ryu H.W., Park M.H., Kwon O.K., Kim D.Y., Hwang J.Y., Jo Y.H., Ahn K.S., Hwang B.Y., Oh S.R. Anti-inflammatory flavonoids from root bark of Broussonetia papyrifera in LPS-stimulated RAW264.7 cells. Bioorg. Chem. 2019;92:103233. doi: 10.1016/j.bioorg.2019.103233. [DOI] [PubMed] [Google Scholar]
- 41.Tian J.L., Liu T.L., Xue J.J., Hong W., Zhang Y., Zhang D.X., Cui C.C., Liu M.C., Niu S.L. Flavanoids derivatives from the root bark of Broussonetia papyrifera as a tyrosinase inhibitor. Ind. Crops Prod. 2019;138:111445. doi: 10.1016/j.indcrop.2019.06.008. [DOI] [Google Scholar]
- 42.Malanik M., Treml J., Lelakova V., Nykodymova D., Oravec M., Marek J., Smejkal K. Anti-inflammatory and antioxidant properties of chemical constituents of Broussonetia papyrifera. Bioorg. Chem. 2020;104:104298. doi: 10.1016/j.bioorg.2020.104298. [DOI] [PubMed] [Google Scholar]
- 43.Qureshi H., Anwar T., Khan S., Fatimah H., Waseem M. Phytochemical constituents of Broussonetia papyrifera (L.) L’He’r. ex Vent: An overview. J. of the Indian Chem. Soc. 2020;97:55–65. [Google Scholar]
- 44.Vu N.K., Ha M.T., Kim C.S., Gal M., Kim J.A., Woo M.H., Lee J.H., Min B.S. Structural characterization of prenylated compounds from Broussonetia kazinoki and their antiosteoclastogenic activity. Phytochemistry. 2021;188:112791. doi: 10.1016/j.phytochem.2021.112791. [DOI] [PubMed] [Google Scholar]
- 45.Yadav A. Brousssonetia Papyrifera linn: Deep insights into its dominant pharmacological perspectives. World J. Pharm. Res. 2021;10:577–590. [Google Scholar]
- 46.Ko H.H., Yu S.M., Ko F.N., Teng C.M., Lin C.N. Bioactive constituents of Morus australis and Broussonetia papyrifera. J. Nat. Prod. 1997;60:1008–1011. doi: 10.1021/np970186o. [DOI] [PubMed] [Google Scholar]
- 47.Ryu H.W., Curtis-Long M.J., Jung S., Jeong I.Y., Kim D.S., Kang K.Y., Park K.H. Anticholinesterase potential of flavonols from paper mulberry (Broussonetia papyrifera) and their kinetic studies. Food Chem. 2012;132:1244–1250. doi: 10.1016/j.foodchem.2011.11.093. [DOI] [PubMed] [Google Scholar]
- 48.Ma Y.M., Zhang Z.W., Feng C.L. Flavonoids of Broussonetia papyrifera. Chem. Nat. Compd. 2009;45:881–882. doi: 10.1007/s10600-010-9480-1. [DOI] [Google Scholar]
- 49.Lee D.Y., Lee H.J., Ryu J.H. Prenylated polyphenols from Broussonetia kazinoki as inhibitors of Nitric Oxide production. Molecules. 2018;23:639. doi: 10.3390/molecules23030639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lee D.Y., Kim D.H., Lee H.J., Lee Y., Ryu K.H., Jung B.I., Song Y.S., Ryu J.H. New estrogenic compounds isolated from Broussonetia kazinoki. Bioorg. Med. Chem. Lett. 2010;20:3764–3767. doi: 10.1016/j.bmcl.2010.04.064. [DOI] [PubMed] [Google Scholar]
- 51.Yu D., Jing Q., Yu X. Research progress on new chemical constituents and biological activities of Broussonetia papyrifera. Nat. Prod. Res. Dev. 2014;26:1327. [Google Scholar]
- 52.Venkatesh K.R., Chauhan S. Mulberry: Life enhancer. J. Med. Plants Res. 2008;2:271–278. [Google Scholar]
- 53.Mei R.Q., Wang Y.H., Du G.H., Liu G.M., Zhang L., Cheng Y.X. Antioxidant lignans from the fruits of Broussonetia papyrifera. J. Nat. Prod. 2009;72:621–625. doi: 10.1021/np800488p. [DOI] [PubMed] [Google Scholar]
- 54.Zhou X.J., Mei R.Q., Zhang L., Lu Q., Zhao J., Adebayo A.H., Cheng Y.X. Antioxidant phenolics from Broussonetia papyrifera fruits. J. Asian Nat. Prod. Res. 2010;12:399–406. doi: 10.1080/10286020.2010.481260. [DOI] [PubMed] [Google Scholar]
- 55.Baek Y.S., Ryu Y.B., Curtis-Long M.J., Ha T.J., Rengasamy R., Yang M.S., Park K.H. Tyrosinase inhibitory effects of 1,3-diphenylpropanes from Broussonetia kazinoki. Bioorg. Med. Chem. 2009;17:35–41. doi: 10.1016/j.bmc.2008.11.022. [DOI] [PubMed] [Google Scholar]
- 56.Ryu H.W., Lee B.W., Curtis-Long M.J., Jung S., Ryu Y.B., Lee W.S., Park K.H. Polyphenols from Broussonetia papyrifera displaying potent alpha-glucosidase inhibition. J. Agric. Food Chem. 2010;58:202–208. doi: 10.1021/jf903068k. [DOI] [PubMed] [Google Scholar]
- 57.Ryu H.W., Lee J.H., Kang J.E., Jin Y.M., Park K.H. Inhibition of Xanthine Oxidase by phenolic phytochemicals from Broussonetia papyrifera. J. Korean Soc. Appl. Biol. Chem. 2012;55:587–594. doi: 10.1007/s13765-012-2143-0. [DOI] [Google Scholar]
- 58.Shibano M., Kitagawa S., Kusano G. Studies on the constituents of Broussonetia species. 1. Two new pyrrolidine alkaloids, broussonetines C and D, as beta-galactosidase and beta-mannosidase inhibitors from Broussonetia kazinoki SIEB. Chem. Pharm. Bull. 1997;45:505–508. doi: 10.1248/cpb.45.505. [DOI] [Google Scholar]
- 59.Shibano M., Kitagawa S., Nakamura S., Akazawa N., Kusano G. Studies on the constituents of Broussonetia species. 2. Six new pyrrolidine alkaloids, Broussonetine A, B, E, F and Broussonetinine A and B, as inhibitors of glycosidases from Broussonetia kazinoki SIEB. Chem. Pharm. Bull. 1997;45:700–705. doi: 10.1248/cpb.45.700. [DOI] [PubMed] [Google Scholar]
- 60.Shibano M., Nakamura S., Kubori M., Minoura K., Kusano G. Studies on the constituents of Broussonetia species. IV. Two new pyrrolidinyl piperidine alkaloids, broussonetines I and J, from Broussonetia kazinoki SIEB. Chem. Pharm. Bull. 1998;46:1416–1420. doi: 10.1248/cpb.46.1416. [DOI] [PubMed] [Google Scholar]
- 61.Shibano M., Nakamura S., Akazawa N., Kusano G. Studies on the constituents of Broussonetia species. III. Two new pyrrolidine alkaloids, broussonetines G and H, as inhibitors of glycosidase, from Broussonetia kazinoki SIEB. Chem. Pharm. Bull. 1998;46:1048–1050. doi: 10.1248/cpb.46.1048. [DOI] [PubMed] [Google Scholar]
- 62.Shibano M., Tsukamoto D., Kusano G. A new pyrrolizidine alkaloid, broussonetine N, as an inhibitor of glycosidase, from Broussonetia kazinoki SIEB and absolute stereostructures of broussonetines A and B. Chem. Pharm. Bull. 1999;47:907–908. doi: 10.1248/cpb.47.907. [DOI] [Google Scholar]
- 63.Shibano M., Nakamura S., Motoya N., Kusano G. Studies on the constituents of Broussonetia species. V. Two new pyrrolidine alkaloids, broussonetines K and L, as inhibitors of glycosidase, from Broussonetia kazinoki SIEB. Chem. Pharm. Bull. 1999;47:472–476. doi: 10.1248/cpb.47.472. [DOI] [PubMed] [Google Scholar]
- 64.Shibano M., Tsukamoto D., Fujimoto R., Masui Y., Sugimoto H., Kusano G. Studies on the constituents of Broussonetia species. VII. Four new pyrrolidine alkaloids, broussonetines M, O, P, and Q, as inhibitors of glycosidase, from Broussonetia kazinoki Sieb. Chem. Pharm. Bull. 2000;48:1281–1285. doi: 10.1248/cpb.48.1281. [DOI] [PubMed] [Google Scholar]
- 65.Tsukamoto D., Shibano M., Okamoto R., Kusano G. Studies on the constituents of Broussonetia species VIII. Four new pyrrolidine alkaloids, broussonetines R, S, T, and V and a new pyrroline alkaloid, broussonetine U, from Broussonetia kazinoki Sieb. Chem. Pharm. Bull. 2001;49:492–496. doi: 10.1248/cpb.49.492. [DOI] [PubMed] [Google Scholar]
- 66.Pang S.Q., Wang G.Q., Lin J.S., Diao Y., Xu R.A. Cytotoxic activity of the alkaloids from Broussonetia papyrifera fruits. Pharm. Biol. 2014;52:1315–1319. doi: 10.3109/13880209.2014.891139. [DOI] [PubMed] [Google Scholar]
- 67.Pang S.Q., Wang G.Q., Huang B.K., Zhang Q.Y., Qin L.P. Isoquinoline alkaloids from Broussonetia papyrifera fruits. Chem. Nat. Compd. 2007;43:100–102. doi: 10.1007/s10600-007-0042-0. [DOI] [Google Scholar]
- 68.O’Hagan D. Pyrrole, pyrrolidine, pyridine, piperidine and tropane alkaloids. Nat. Prod. Rep. 2000;17:435–446. doi: 10.1039/a707613d. [DOI] [PubMed] [Google Scholar]
- 69.Zhong H.T., Li F., Chen B., Wang M.K. Euphane triterpenes from the bark of Broussonetia papyrifera. Helv. Chim. Acta. 2011;94:2061–2065. doi: 10.1002/hlca.201100136. [DOI] [Google Scholar]
- 70.Liu F., Liu Y., Yan Z., Ma Y., Zhai X.Q. Triterpenes from the bark of Broussonetia papyrifera (L.) Vent. Nat. Prod. Res. Dev. 2011;40:120–122. [Google Scholar]
- 71.Casuga F.P., Castillo A.L., Corpuz M.J. Bioactive compounds and cytotoxicity of ethyl acetate extract from Broussonetia luzonica (Moraceae) blanco leaves against hepatocellular carcinoma (Hepg2) cell lines. Pharmacogn. J. 2016;8:497–501. doi: 10.5530/pj.2016.5.15. [DOI] [Google Scholar]
- 72.Feng W.S., Li H.W., Zheng X.K., Chen S.Q. Two new megastigmane O-glucopyranosides from the leaves of Broussonetia papyrifera. Chin. Chem. Lett. 2007;18:1518–1520. doi: 10.1016/j.cclet.2007.10.028. [DOI] [Google Scholar]
- 73.Mei R.Q., Wang X.T., Chen R., Luo H.R., Cheng Y.X. N-containing compounds from Broussonetia papyrifera seeds and their cAMP regulatory activity in N1E-115 cells. Chem. Nat. Compd. 2011;47:783–785. doi: 10.1007/s10600-011-0058-3. [DOI] [Google Scholar]
- 74.Wang L., Son H.J., Xu M.L., Hu J.H., Wang M.H. Anti-inflammatory and anticancer properties of dichloromethane and butanol fractions from the stem bark of Broussonetia papyrifera. J. Korean Soc. Appl. Biol. Chem. 2010;53:297–303. doi: 10.3839/jksabc.2010.046. [DOI] [Google Scholar]
- 75.Li Y., Li H.L., Zhang Y., Li L., Qin C.G. In vitro antioxidant and anticancer activities of the extract from Paper Mulberry (Broussonetia papyrifera L.) fruit. Asian J. Chem. 2013;25:5453–5456. doi: 10.14233/ajchem.2013.14594. [DOI] [Google Scholar]
- 76.Zhu Z.X., Zhao Y.T. Mechanism of gastric carcinoma cell apoptosis induced by chlorogenic acid-like compounds extracted from Broussonetia papyrifera. Chin. Tradit. Herb. Drugs. 2018;24:5345–5351. [Google Scholar]
- 77.Dou C.Z., Liu Y.F., Zhang L.L., Chen S.H., Hu C.Y., Liu Y., Zhao Y.T. Polyphenols from Broussonetia papyrifera induce apoptosis of HepG2 cells via inactivation of ERK and AKT signaling pathways. J. Evid. Based Complementary Altern. Med. 2021;2021:8841706. doi: 10.1155/2021/8841706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wei B.L., Chen Y.C., Hsu H.Y. Kazinol Q from Broussonetia kazinoki enhances cell death induced by Cu (II) through increased reactive oxygen species. Molecules. 2011;16:3212–3221. doi: 10.3390/molecules16043212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Guo M.X., Wang M.L., Zhang X.T., Deng H., Wang Z.Y. Broussoflavonol B restricts growth of ER-negative breast cancer stem-like cells. Anticancer Res. 2013;33:1873–1879. [PubMed] [Google Scholar]
- 80.Park S., Fudhaili A., Oh S.S., Lee K.W., Madhi H., Kim D.H., Yoo J., Ryu H.W., Park K.H., Kim K.D. Cytotoxic effects of kazinol A derived from Broussonetia papyrifera on human bladder cancer cells, T24 and T24R2. Phytomedicine. 2016;23:1462–1468. doi: 10.1016/j.phymed.2016.08.005. [DOI] [PubMed] [Google Scholar]
- 81.Jung Y.C., Han S., Hua L., Ahn Y.H., Cho H., Lee C.J., Lee H., Cho Y.Y., Ryu J.H., Jeon R., et al. Kazinol-E is a specific inhibitor of ERK that suppresses the enrichment of a breast cancer stem-like cell population. Biochem. Biophys. Res. Commun. 2016;470:294–299. doi: 10.1016/j.bbrc.2016.01.066. [DOI] [PubMed] [Google Scholar]
- 82.Jung Y.C., Han S., Hua L., Zhao H.Y., Lee C.J., Cho Y.Y., Jeon R., Ryu J.H., Kim W.Y. Targeting cancer stem cell through blocking the Erk pathway with Kazinol-E from Broussonetia kazinoki. Cancer Res. 2015;75:2600. doi: 10.1158/1538-7445.AM2015-2600. [DOI] [Google Scholar]
- 83.Kim J.H., Kim M.S., Lee B.H., Kim J.K., Ahn E.K., Ko H.J., Cho Y.R., Lee S.J., Bae G.U., Kim Y.K., et al. Marmesin-mediated suppression of VEGF/VEGFR and integrin beta 1 expression: Its implication in non-small cell lung cancer cell responses and tumor angiogenesis. Oncol. Rep. 2017;37:91–97. doi: 10.3892/or.2016.5245. [DOI] [PubMed] [Google Scholar]
- 84.Park S.H., Lee J., Shon J.C., Phuc N.M., Jee J.G., Liu K.H. The inhibitory potential of Broussochalcone A for the human cytochrome P450 2J2 isoform and its anti-cancer effects via FOXO3 activation. Phytomedicine. 2018;42:199–206. doi: 10.1016/j.phymed.2018.03.032. [DOI] [PubMed] [Google Scholar]
- 85.Shin S., Son Y., Liu K.H., Kang W., Oh S. Cytotoxic activity of broussochalcone a against colon and liver cancer cells by promoting destruction complex-independent beta-catenin degradation. Food Chem. 2019;131:110550. doi: 10.1016/j.fct.2019.05.058. [DOI] [PubMed] [Google Scholar]
- 86.Jeong J.H., Ryu J.H. Broussoflavonol B from Broussonetia kazinoki Siebold exerts anti-pancreatic cancer activity through downregulating FoxM1. Molecules. 2020;25:2328. doi: 10.3390/molecules25102328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wang Q., Zhong S., Wu H., Wu Q.J.E. In vitro anti-cancer effect of marmesin by suppression of PI3K/Akt pathway in esophagus cancer cells. Esophagus. 2022;19:163–174. doi: 10.1007/s10388-021-00872-8. [DOI] [PubMed] [Google Scholar]
- 88.Kim H.S., Lim J., Lee D.Y., Ryu J.H., Lim J.S. Kazinol C from Broussonetia kazinoki activates AMP-activated protein kinase to induce antitumorigenic effects in HT-29 colon cancer cells. Oncol. Rep. 2015;33:223–229. doi: 10.3892/or.2014.3601. [DOI] [PubMed] [Google Scholar]
- 89.Xiao J.Q., Fang F.P., Ying F.Y., Ying Y.T. Ultrasonic extraction and antioxidant activity investigation of Broussonetia papyrifer seed oil. Nat. Prod. Res. Dev. 2014;26:1685. [Google Scholar]
- 90.Li Y., Shang X.Y., Niu W.N., Xu C.L., Qin C.G. Inhibitory Activity of the Extract from Broussonetia papyrifera Fruits to Cellular Lipid Peroxidation in vitro. Asian J. Chem. 2014;26:201–204. doi: 10.14233/ajchem.2014.15518. [DOI] [Google Scholar]
- 91.Sun J., Zhang C.S., Yu L.N., Bi J., Liu S.F., Zhu F., Yang Q.L. Antioxidant activity and total phenolics of Broussonetia papyrifera flower extracts. Appl. Mech. Mater. 2012;140:263–267. doi: 10.4028/www.scientific.net/AMM.140.263. [DOI] [Google Scholar]
- 92.Cheng Z.J., Lin C.N., Hwang T.L., Teng C.M. Broussochalcone A, a potent antioxidant and effective suppressor of inducible nitric oxide synthase in lipopolysaccharide-activated macrophages. Biochem. Pharmacol. 2001;61:939–946. doi: 10.1016/S0006-2952(01)00543-3. [DOI] [PubMed] [Google Scholar]
- 93.Tsai F.H., Lien J.C., Lin L.W., Chen H.Y., Ching H., Wu C.R. Protective effect of Broussonetia papyrifera against Hydrogen Peroxide-induced oxidative stress in SH-SY5Y cells. Biosci. Biotechnol. Biochem. 2009;73:1933–1939. doi: 10.1271/bbb.90080. [DOI] [PubMed] [Google Scholar]
- 94.Xu M.L., Wang L., Hu J.H., Lee S.K., Wang M.H. Antioxidant activities and related polyphenolic constituents of the methanol extract fractions from Broussonetia papyrifera stem bark and wood. Food Sci. Biotechnol. 2010;19:677–682. doi: 10.1007/s10068-010-0095-x. [DOI] [Google Scholar]
- 95.Kwak W.J., Moon T.C., Lin C.X., Rhyn H.G., Jung H.J., Lee E., Kwon D.Y., Son K.H., Kim H.P., Kang S.S., et al. Papyriflavonol A from Broussonetia papyrifera inhibits the passive cutaneous anaphylaxis reaction and has a secretory phospholipase A (2)-inhibitory activity. Biol. Pharm. Bull. 2003;26:299–302. doi: 10.1248/bpb.26.299. [DOI] [PubMed] [Google Scholar]
- 96.Huang S.P., Guan X., Kai G.Y., Xu Y.Z., Xu Y., Wang H.J., Pang T., Zhang L.Y., Liu Y. Broussonin E suppresses LPS-induced inflammatory response in macrophages via inhibiting MAPK pathway and enhancing JAK2-STAT3 pathway. Chin. J. Nat. Med. 2019;17:372–380. doi: 10.1016/S1875-5364(19)30043-3. [DOI] [PubMed] [Google Scholar]
- 97.Lin L.W., Chen H.Y., Wu C.R., Liao P.M., Lin Y.T., Hsieh M.T., Ching H. Comparison with various parts of Broussonetia papyrifera as to the antinociceptive and anti-inflammatory activities in rodents. Biosci. Biotechnol. Biochem. 2008;72:2377–2384. doi: 10.1271/bbb.80276. [DOI] [PubMed] [Google Scholar]
- 98.Lee J.K., Ha H., Lee H.Y., Park S.J., Jeong S.I., Choi Y.J., Shin H.K. Inhibitory effects of heartwood extracts of Broussonetia kazinoki Sieb on the development of atopic dermatitis in NC/Nga mice. Biosci. Biotechnol. Biochem. 2010;74:1802–1806. doi: 10.1271/bbb.100138. [DOI] [PubMed] [Google Scholar]
- 99.Bae U.J., Lee D.Y., Song M.Y., Lee S.M., Park J.W., Ryu J.H., Park B.H. A prenylated flavan from Broussonetia kazinoki prevents cytokine-induced beta-cell death through suppression of nuclear factor-kappa B activity. Biol. Pharm. Bull. 2011;34:1026–1031. doi: 10.1248/bpb.34.1026. [DOI] [PubMed] [Google Scholar]
- 100.Bae U.J., Jang H.Y., Lim J.M., Hua L., Ryu J.H., Park B.H. Polyphenols isolated from Broussonetia kazinoki prevent cytokine-induced beta-cell damage and the development of type 1 diabetes. Exp. Mol. Med. 2015;47:739. doi: 10.1038/emm.2015.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lee H., Li H., Jeong J.H., Noh M., Ryu J.H. Kazinol B from Broussonetia kazinoki improves insulin sensitivity via Akt and AMPK activation in 3T3-L1 adipocytes. Fitoterapia. 2016;112:90–96. doi: 10.1016/j.fitote.2016.05.006. [DOI] [PubMed] [Google Scholar]
- 102.Lee J.M., Choi S.S., Park M.H., Jang H., Lee Y.H., Khim K.W., Oh S.R., Park J., Ryu H.W., Choi J.H. Broussonetia papyrifera root bark extract exhibits A7 anti-inflammatory effects on adipose tissue and improves insulin sensitivity potentially via AMPK activation. Nutrients. 2020;12:773. doi: 10.3390/nu12030773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kim D., Kim H.J., Cha S.H., Jun H.S. Protective effects of Broussonetia kazinoki Siebold fruit extract against palmitate-induced lipotoxicity in mesangial cells. J. Evid. Based Complementary Altern. Med. 2019;2019:4509403. doi: 10.1155/2019/4509403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kumar N.N., Ramakrishnaiah H., Krishna V., Deepalakshmi A.P. GC-MS analysis and antimicrobial activity of seed oil of Broussonetia papyrifera (L.) VENT. J. Pharm. Sci. Res. 2015;6:3954. [Google Scholar]
- 105.Park J.Y., Yuk H.J., Ryu H.W., Lim S.H., Kim K.S., Park K.H., Ryu Y.B., Lee W.S. Evaluation of polyphenols from Broussonetia papyrifera as coronavirus protease inhibitors. J. Enzym. Inhib. Med. Chem. 2017;32:504–512. doi: 10.1080/14756366.2016.1265519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kwon B., Kim M.H., Park I.S., Choo Y.M., Jeong S.I., Yu K.Y., Kim J., Kim K.S., Kim M.S. The potential efficacy of Broussonetia kazinoki stem extract to show antioxidant property or suppress collagenase activity. Biomed. J. Sci. Tech. Res. 2019;17:12802–12804. [Google Scholar]
- 107.Zhang Y.D., Biao Y.N., Chu X.Q., Hao L., Shi C., Liu Y., Zhang Y.X., Wang X. Protective effect of Chushizi (Fructus Broussonetiae) on acetaminophen-induced rat hepatitis by inhibiting the Toll-like receptor 3/c-Jun N-terminal kinase/c-jun/c-fos/janus protein tyrosine kinase/activators of transcription 3 pathway. J. Tradit. Chin. Med. 2020;40:965–973. doi: 10.19852/j.cnki.jtcm.2020.06.008. [DOI] [PubMed] [Google Scholar]
- 108.Lee Y.H., Nam G., Kim M.K., Cho S.C. Broussonetia papyrifera promotes hair growth through the regulation of β-catenin and STAT6 target proteins: A phototrichogram analysis of clinical samples. Cosmetics. 2020;7:40. doi: 10.3390/cosmetics7020040. [DOI] [Google Scholar]
- 109.Cho Y.R., Kim J.H., Kim J.K., Ahn E.K., Ko H.J., In J.K., Lee S.J., Bae G.U., Kim Y.K., Oh J.S., et al. Broussonetia kazinoki modulates the expression of VEGFR-2 and MMP-2 through the inhibition of ERK, Akt and p70(S6K)-dependent signaling pathways: Its implication in endothelial cell proliferation, migration and tubular formation. Oncol. Rep. 2014;32:1531–1536. doi: 10.3892/or.2014.3380. [DOI] [PubMed] [Google Scholar]
- 110.Jung D.Y., Ha H.K., Lee H.Y., Lee J.A., Jeong S.I., Choi Y.J., Shin H.K. Broussonetia kazinoki Siebold stimulates immune response in ovalbumin-immunized mice. J. Korean Orient. Med. 2011;32:10–17. [Google Scholar]
- 111.Hwang J., Lee S.J., Yoo M., Go G.Y., Lee D.Y., Kim Y.K., Seo D.W., Kang J.S., Ryu J.H., Bae G.U. Kazinol-P from Broussonetia kazinoki enhances skeletal muscle differentiation via p38MAPK and MyoD. Biochem. Biophys. Res. Commun. 2015;456:471–475. doi: 10.1016/j.bbrc.2014.11.109. [DOI] [PubMed] [Google Scholar]
- 112.Ryu J.H., Ahn H., Lee H.J. Inhibition of nitric oxide production on LPS-activated macrophages by kazinol B from Broussonetia kazinoki. Fitoterapia. 2003;74:350–354. doi: 10.1016/S0367-326X(03)00062-5. [DOI] [PubMed] [Google Scholar]
- 113.Guo M.X., Wang M.L., Deng H., Zhang X.T., Wang Z.Y. A novel anticancer agent Broussoflavonol B downregulates estrogen receptor (ER)-alpha 36 expression and inhibits growth of ER-negative breast cancer MDA-MB-231 cells. Eur. J. Pharmacol. 2013;714:56–64. doi: 10.1016/j.ejphar.2013.05.047. [DOI] [PubMed] [Google Scholar]
- 114.Yao L., Xiong L., Yoo C.G., Dong C.Y., Meng X.Z., Dai J., Ragauskas A.J., Yang C.L., Yu J., Yang H.T., et al. Correlations of the physicochemical properties of organosolv lignins from Broussonetia papyrifera with their antioxidant activities. Sustain. Energy Fuels. 2020;4:5114–5119. doi: 10.1039/D0SE00940G. [DOI] [Google Scholar]
- 115.Han Q.H., Wu Z.L., Huang B., Sun L.Q., Ding C.B., Yuan S., Zhang Z.W., Chen Y.E., Hu C., Zhou L.J., et al. Extraction, antioxidant and antibacterial activities of Broussonetia papyrifera fruits polysaccharides. Int. J. Biol. Macromol. 2016;92:116–124. doi: 10.1016/j.ijbiomac.2016.06.087. [DOI] [PubMed] [Google Scholar]
- 116.Huang L.Z., Qin L.P., Huang B.K. Antioxidative and anti-inflammatory properties of Chushizi oil from fructus Broussonetiae. J. Med. Plants Res. 2011;5:6407–6412. [Google Scholar]
- 117.Lee H., Ha H., Lee J.K., Park S.J., Jeong S.I., Shin H.K. The leaves of Broussonetia kazinoki Siebold inhibit atopic dermatitis-like response on mite allergen-treated Nc/Nga mice. Biomol. Ther. 2014;22:438–444. doi: 10.4062/biomolther.2014.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wu W.T. Evaluation of anti-inflammatory effects of Broussonetia papyrifera stem bark. Indian J. Pharmacol. 2012;44:26–30. doi: 10.4103/0253-7613.91862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hua J.L., Xu T.F., Shen Q.W., Liu Y., Huang G.J., Rao D.J., Song C.M., Wang J.K. Productive and metabolic increments of the inclusion of Broussonetia papyrifera to replace maize silage in growing goats. Czech J. Anim. Sci. 2020;65:303–310. doi: 10.17221/10/2020-CJAS. [DOI] [Google Scholar]
- 120.Tao H., Si B., Xu W., Tu Y., Diao Q.Y. Effect of Broussonetia papyrifera L. silage on blood biochemical parameters, growth performance, meat amino acids and fatty acids compositions in beef cattle. Asian Australas. J. Anim. Sci. 2020;33:732. doi: 10.5713/ajas.19.0150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hao Y.Y., Huang S., Si J.F., Zhang J., Gaowa N., Sun X.G., Lv J.Y., Liu G.K., He Y.Q., Wang W., et al. Effects of Paper Mulberry Silage on the milk production, apparent digestibility, antioxidant capacity, and fecal bacteria composition in holstein dairy cows. Animals. 2020;10:1152. doi: 10.3390/ani10071152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Si B.W., Tao H., Zhang X.L., Guo J.P., Cui K., Tu Y., Diao Q.Y. Effect of Broussonetia papyrifera L. (paper mulberry) silage on dry matter intake, milk composition, antioxidant capacity and milk fatty acid profile in dairy cows. Asian Australas. J. Anim. Sci. 2018;31:1259–1266. doi: 10.5713/ajas.17.0847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Chen G.S., Shui S.Z., Chai M.J., Wang D., Su Y.Y., Wu H.B., Sui X.D., Yin Y.L. Effects of Paper Mulberry (Broussonetia papyrifera) leaf extract on growth performance and fecal microflora of weaned piglets. BioMed. Res. Int. 2020;2020:6508494. doi: 10.1155/2020/6508494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Huang H.M., Zhao Y.L., Xu Z.G., Zhang W., Jiang K.K. Physiological responses of Broussonetia papyrifera to manganese stress, a candidate plant for phytoremediation. Ecotoxicol. Environ. Saf. 2019;181:18–25. doi: 10.1016/j.ecoenv.2019.05.063. [DOI] [PubMed] [Google Scholar]
- 125.Zeng P., Guo Z., Xiao X., Zhou H., Gu J., Liao B. Tolerance capacities of Broussonetia papyrifera to heavy metal (loid) s and its phytoremediation potential of the contaminated soil. Int. J. Phytorem. 2021;24:580–589. doi: 10.1080/15226514.2021.1958746. [DOI] [PubMed] [Google Scholar]
- 126.Huang H., Zhao Y., Fan L., Jin Q., Yang G., Xu Z. Improvement of manganese phytoremediation by Broussonetia papyrifera with two plant growth promoting (PGP) Bacillus species. Chemosphere. 2020;260:127614. doi: 10.1016/j.chemosphere.2020.127614. [DOI] [PubMed] [Google Scholar]
- 127.Luo Y.F., Wu Y.G., Qiu J., Wang H., Yang L. Suitability of four woody plant species for the phytostabilization of a zinc smelting slag site after 5 years of assisted revegetation. J. Soils Sediments. 2019;19:702–715. doi: 10.1007/s11368-018-2082-4. [DOI] [Google Scholar]
- 128.Zeng P., Guo Z.H., Xiao X.Y., Peng C., Huang B., Feng W.L. Complementarity of co-planting a hyperaccumulator with three metal(loid)-tolerant species for metal(loid)-contaminated soil remediation. Ecotoxicol. Environ. Saf. 2019;169:306–315. doi: 10.1016/j.ecoenv.2018.11.017. [DOI] [PubMed] [Google Scholar]
- 129.Zeng P., Guo Z.H., Xiao X.Y., Peng C. Effects of tree-herb co-planting on the bacterial community composition and the relationship between specific microorganisms and enzymatic activities in metal(loid)-contaminated soil. Chemosphere. 2019;220:237–248. doi: 10.1016/j.chemosphere.2018.12.073. [DOI] [PubMed] [Google Scholar]
- 130.Cha J.Y., Yang H.J., Moon H.I., Cho Y.S. Inhibitory effect and mechanism on melanogenesis from fermented herbal composition for medical or food uses. Food Res. Int. 2012;45:225–231. doi: 10.1016/j.foodres.2011.11.002. [DOI] [Google Scholar]
- 131.Qiu G.Q., Zhao Y.L., Wang H., Tan X.F., Chen F.X., Hu X.J. Biochar synthesized via pyrolysis of Broussonetia papyrifera leaves: Mechanisms and potential applications for phosphate removal. Environ. Sci. Pollut. Res. 2019;26:6565–6575. doi: 10.1007/s11356-018-04095-w. [DOI] [PubMed] [Google Scholar]
- 132.Zhang M., Wu Y., Xing D., Zhao K., Yu R. Rapid measurement of drought resistance in plants based on electrophysiological properties. ASABE. 2015;58:1441–1446. [Google Scholar]
- 133.Mo L., Ma Z.Y., Xu Y.S., Sun F.B., Lun X.X., Liu X.H., Chen J.G., Yu X.X. Assessing the capacity of plant species to accumulate particulate matter in Beijing, China. PLoS ONE. 2015;10:e0140664. doi: 10.1371/journal.pone.0140664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Zerrifi S.E., Kasrati A., Redouane E., Tazart Z., El Khalloufi F., Abbad A., Oudra B., Campos A., Vasconcelos V. Essential oils from Moroccan plants as promising ecofriendly tools to control toxic cyanobacteria blooms. Ind. Crops Prod. 2020;143:111922. doi: 10.1016/j.indcrop.2019.111922. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.