Abstract
The production of reactor-based medical isotopes is fragile, which has meant supply shortages from time to time. This paper reviews alternative production methods in the form of cyclotrons, linear accelerators and neutron generators. Finally, the status of the production of medical isotopes in China is described.
Keywords: medical isotope production, accelerator, nuclear medicine, review
1. Introduction
1.1. Definition of Medical Isotopes
Medical isotopes are radioisotopes that emit positrons or gamma rays for medical diagnosis or particulate radiation, such as alpha or beta particles for medical therapy [1].
1.2. Medical Use
The application process for medical isotopes is depicted in Figure 1 and can be summarized in four steps:
-
(1a)
In a reactor, irradiate a suitable target with neutrons to induce a nuclear reaction;
-
(1b)
In an accelerator, irradiate a suitable target with protons, alpha, or deuteron particles to induce a nuclear reaction;
-
(2)
Separate radioisotopes from the irradiated targets;
-
(3)
Combine the ligands with radioisotopes to prepare radiopharmaceuticals;
-
(4)
Employ the radiopharmaceuticals in nuclear medicine.
Figure 1.
Process for the application of medical isotopes.
Depending on the physical characteristics of the isotopes applied, radiopharmaceuticals have different medical uses in diagnosis, therapy, or both (theranostics) [2], leading to a steady increase in the use of medical isotopes in nuclear medicine over time [3,4].
1.2.1. Radiopharmaceuticals for Diagnosis
Radiopharmaceuticals are generally injected intravenously or, in some cases, taken orally [5,6]. They are transported in the blood throughout the body and, due to their high affinities with specific organs, can target different diseases, especially tumors. The γ rays emitted by radiopharmaceuticals are used for imaging. Currently, there are two main imaging applications for diagnosis in nuclear medicine: Single Photon Emission Computed Tomography (SPECT) [7,8,9] and Positron Emission Tomography (PET) [10,11,12]. The distribution of radiotracers in vivo can be detected using SPECT and PET cameras.
The main advantage of nuclear medicine diagnosis lies in its ability to find lesions earlier since diseased tissues usually first denote functional changes before later evolving into shape and structural changes [13]. Another major feature of nuclear medicine diagnosis is its ability to specifically show the locations and sizes of tumors, especially when combined with Computed Tomography (CT) or Magnetic Resonance Imaging (MRI) [14,15].
1.2.2. Radiopharmaceuticals for Therapy
Therapeutic radiopharmaceuticals accumulate in diseased tissue after entering the human body. Then, their cumulative radioactive emissions can produce biological effects (e.g., killing tumor cells), which makes radiopharmaceuticals particularly suitable for cancer treatment [16]. The applications of radiopharmaceuticals for therapy include α therapy, β therapy, and Auger therapy. This review focuses on α therapy and β therapy.
1.2.3. Radiopharmaceuticals for Theranostics
In theranostics, radiopharmaceuticals can be used to perform diagnostic imaging and medical treatment [17,18,19]. Imaging diagnosis is used to determine an optimal treatment modality and can help monitor and evaluate the medical treatment progress [18,20,21]. Currently, radiopharmaceuticals for theranostics use either the same radiopharmaceutical, which emits γ rays for diagnosis and α or β particles for treatment [22,23], or two different radiopharmaceuticals (one for diagnosis and the other for treatment) [24].
Radiopharmaceuticals for theranostics have developed rapidly in recent years with great progress in treating neuroendocrine tumors, thyroid cancer [20,21,25,26], prostate cancer, breast cancer [27,28], and other diseases.
1.3. The Status of Medical Isotope Production
Radioisotopes are divided into natural and artificial radioisotopes. Currently, there are about 200 radioisotopes in use, most of which are produced artificially [29].
With the widespread usage of radiopharmaceuticals, the stable production and supply of medical isotopes is becoming increasingly important.
Medical isotopes are generally produced via either reactors or accelerators. Typically, reactor-based medical isotopes are neutron-rich isotopes commonly characterized by a long half-life, while accelerator-based medical isotopes tend to offer a shorter half-life and usually emit positrons or γ rays [30]. Reactor irradiation is currently the most commonly used method to produce medical isotopes due to their high yield, low cost, and ease of target preparation. However, this supply is sustained by reactors that were built in the 1950–60s (Table 1). The majority of these reactors will gradually shut down before 2030.
Table 1.
| Country | Reactor | Power [MW] | Year of First Criticality | Estimated Retirement Time |
|---|---|---|---|---|
| Belgium | BR-2 | 100 | 1961 | 2026 |
| Netherlands | HFR | 45 | 1961 | 2024 |
| Czech Republic | LVR-15 | 10 | 1957 | 2028 |
| Poland | MARIA | 20 | 1974 | 2030 |
| South Africa | SAFARI-1 | 20 | 1965 | 2030 |
| Russia | WWR-TS | 15 | 1964 | 2025 |
| United States | HFIR | 100 | 1965 | 2035 |
| Australia | OPAL | 20 | 2006 | 2057 |
| Germany | FRM-II | 20 | 2004 | 2054 |
Moreover, due to their age, and as part of the decommissioning process, reactors can be expected to have longer periods of down time due to maintenance or unplanned shutdown events for safety or technical reasons [35], increasing the risk of supply interruptions or persistent shortages. Additionally, most irradiated targets for 99Mo production in a reactor context use highly enriched uranium (HEU) targets that generate considerable amounts of highly radioactive waste and increase the risk of nuclear proliferation [36,37]. These factors strengthen the argument that medical isotopes produced via reactors should be replaced by accelerator-based production [38,39].
The growing interest and recent improvements in accelerator technologies have already led some medical isotopes produced via reactors to be replaced or partly replaced by accelerator-produced isotopes. There are many advantages to using medical isotopes produced by accelerators:
-
(1)
Supervision is easier, and safety is improved [40];
-
(2)
The maintenance and decommissioning costs are lower [29];
-
(3)
The amount of radioactive waste produced is less than 10% of the amount produced by a reactor, and the radiation levels are lower [41];
-
(4)
It has no risk of nuclear proliferation [42].
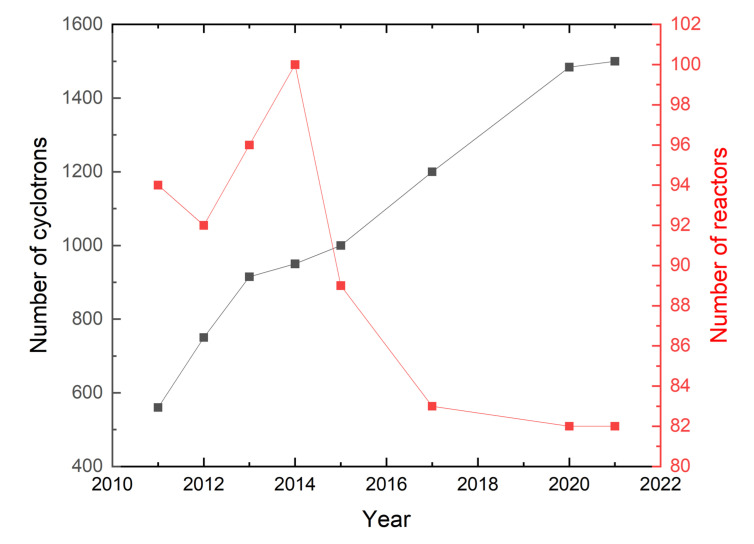
As shown in Figure 2, the number of cyclotrons producing radioisotopes is increasing, while the number of reactors is slowly decreasing.
Figure 2.
A comparison of the number of cyclotrons in the world and the number of reactors reported by the IAEA [43,44,45,46,47,48,49,50,51,52,53,54,55,56,57].
2. Medical Isotopes
This section reviews the medical isotopes produced by cyclotrons, linear accelerators, and neutron generators and lists some of the most commonly used medical isotopes, as well as their characteristics, applications, and production methods.
2.1. Medical Isotopes Produced by Cyclotrons (1–5: PET Radioisotopes, 6–7: SPECT Radioisotopes, 8–10: Therapeutic Radioisotopes)
A cyclotron is a particle accelerator that accelerates charged particles and uses an electromagnetic field to get the particles to follow a spiral path to ever-increasing energies until achieving the energy necessary to produce medical isotopes via nuclear interactions [58]. Compared with linear accelerators, the beams from cyclotrons have characteristically lower beam intensity, but their energy can be higher [59]. Cyclotrons are classified according to the energy of the particles they produce. As shown in Table 2, different types of cyclotrons can produce medical isotopes for a wide range of applications.
Table 2.
Classification of medical cyclotrons [60].
| Type | The Energy of Particles [MeV] | Application |
|---|---|---|
| Small medical cyclotron | <20 | Short-lived radioisotopes for PET |
| Medium-energy cyclotron | 20–35 | Production of SPECT and some PET radioisotopes |
| High-energy cyclotron | >35 | Production of radioisotopes for therapy |
2.1.1. 18F
18F (T1/2 = 109.8 min) decays and emits positrons with an average energy of 0.25 MeV; hence, the distance traveled until reaching positron annihilation in tissues is short. 18F is the most commonly used PET radioisotope. At present, the Food and Drug Administration (FDA) has approved 18F radiopharmaceuticals for use in the diagnosis of a variety of diseases, such as Alzheimer’s disease, infections, and many types of cancer, as well as to evaluate treatment outcomes [61,62]. According to clinical data, [18F]FDG can distinguish between Parkinson’s Disease (PD), MSA with predominant Parkinsonism (MSA-P), and MSA with predominant cerebellar features (MSA-C) [63,64]. PET diagnosis is expensive and can cost over $1000, while doctors can make an early and accurate diagnosis. For that reason, the annual number of PET scans has steadily increased for many years [65]. Most 18F is produced via cyclotrons by exploiting two nuclear reactions:
-
(1)
18O (p, n) 18F: This reaction requires enriched (and more expensive) 18O target materials to produce 18F in a high yield [66]. Technology developments led to improvements in the target system and the production of 18F up to 34 GBq, as well as specific activities of 350–600 GBq/mmol 30 min after the end of bombardment [67]. Subsequently, it was found that with the irradiation of 11 MeV protons, the yield of 18F further increased directly with the proton current. However, the impurities also increased such that for a proton current of 20 μA, the yield of 56Co (4.86 MBq) and 110mAg (1.51 MBq) doubled [68]. Many developing countries do not have medical isotope production facilities. If these countries desire to become self-sufficient in the production of medical isotopes, they could start by installing low-energy cyclotrons to produce 18F [69].
-
(2)
20Ne (d, α) 18F: This is the first production method used to produce 18F. This reaction is characterized by lower yields and low specific activity, so it is gradually being replaced. However, with production improvements, this method could again become an attractive alternative [70].
2.1.2. 68Ga
68Ga (T1/2 = 68 min) is a metal PET radioisotope. Currently, there are about 100 ongoing clinical tests with 68Ga [61], indicating the rapid development of 68Ga-labelled radiotracers. Radiopharmaceuticals labeled with 68Ga are used for the diagnosis of neuroendocrine tumors and are highly accurate when used in patients with suspected but yet not localized neuroendocrine tumors [71]. In addition, 68Ga and 177Lu (T1/2 = 6.7 d) have a similar coordination chemistry, rendering them some of the most promising radiopharmaceuticals for theranostics. For neuroendocrine tumors, both [68Ga]Ga-DOTA-TATE and [177Lu]Lu-DOTA-TATE have been approved by the FDA for clinical PET diagnosis and medical treatment [72,73,74]. [68Ga]Ga-PSMA-11 is the first radiopharmaceutical approved by the FDA for PET imaging of PSMA-positive prostate cancer, and [177Lu]Lu-PSMA-617 has also been used for PSMA-targeted therapy [74,75,76,77].
68Ga is generally available using a 68Ge/68Ga generator and represents a relatively simple and convenient method [78] that can yield up to 1.85 GBq [79]. With the development of technology, the commercial “ionic” generators have made 68Ga clinically successful [80,81]. 68Ga obtained by generators cannot meet the growing demands, however, so the use of accelerators to obtain 68Ga has aroused scientific interest. Moreover, higher yields of 68Ga can be obtained with the 68Zn (p, n) 68Ga reaction using a small cyclotron [82,83]. The yield when using a solid target was reported as 5.032 GBq/μA·h [83]. After 6 h, impurities such as 66Ga and 67Ga only accounted for 0.51% of the total activity [84]. Compared with using a generator, this production method does not require radioactive waste treatment. Although the solid target system is complex, and the separation steps are lengthy, an automated process was developed to separate the solid target and is simpler to operate than alternative methods [85]. This nuclear reaction can also take place in a liquid target, with radiochemical and radionuclidic purities both above 99.9%. However, the yield using a liquid target was found to be significantly lower (192.5 ± 11.0) MBq/μA·h [86]. This production method using the liquid target as an alternative method still needs further optimization to improve the yield.
2.1.3. 64Cu
Upon decay, 64Cu (T1/2 = 12.7 h) emits positrons and electrons that can be utilized for PET diagnosis and have potential applications in β therapy, thus making 64Cu useful as a radiopharmaceutical for theranostics. Furthermore, 64Cu and 67Cu (T1/2 = 61.76 h) can be radiopharmaceuticals for theranostics in order to conduct pre-targeted radioimmunotherapy [87]. Presently, the FDA has approved [64Cu]Cu-DOTA-TATE to localize somatostatin receptor-positive neuroendocrine tumors in adult patients. In clinical experiments, [64Cu]Cu-DOTA-TATE has excellent imaging quality and higher detection rates for lesions [88].
64Cu can be produced by small medical cyclotrons via 64Ni (p, n) 64Cu reaction with high specific activity. This production method requires an enriched 64Ni (at least 96%) target to obtain a high yield of 5.89 GBq/μA·h and 64Cu with radionuclidic purity higher than 99% [89]. The disadvantage is that the 64Ni target material has a low isotopic abundance (0.926%) in nature [90], meaning that the target material is expensive and must be recycled to improve its cost-effectiveness [91,92]. Alternative methods of 64Cu production can also be deuteron-zinc reactions such as natZn (d, x) 64Cu, and 66Zn (d, α) 64Cu. Although they have lower costs, their yields are lower, and high-energy deuterons are required [93]. These factors limit actual production through such reactions.
The 64Ni (p, n)64Cu reaction is the preferred choice for 64Cu production in clinical applications. During the past decade, more than 20 countries, including the United States, Japan, Finland, and China, have developed 64Ni (p, n) 64Cu methods for 64Cu production [89,91,94], some of which are shown in Table 3.
Table 3.
| Facility/Location | Nuclear Reaction | Irradiation Parameters | Yield |
|---|---|---|---|
| Fukui Medical University | 64Ni(p, n)64Cu | 12 MeV, (50 ± 3) μA |
2-24 GBq in 2 h |
| The University of Sherbrooke PET Imaging Centre | 64Ni(p, n)64Cu | 15 MeV, 18 μA |
3.9 GBq in 4 h |
| IBA | 64Ni(p, n)64Cu | 10 MeV, 12 μA |
5123 MBq in 3 h |
| Paul Scherrer Institute | 64Ni(p, n)64Cu | 11 MeV, 40–50 μA |
Max 8.2 GBq in 4–5 h |
| Turku PET Centre | 64Ni(p, n)64Cu | 15.7 MeV, < 100 μA |
Max 9.4GBq after purification |
| Sumitomo HM-20 cyclotron | 64Ni(p, n)64Cu | 12.5 MeV, 20 μA |
7.4 GBq in 5–7 h |
| NIRS AVF-930 cyclotron | 64Ni(p, n)64Cu | 24 MeV HH+, 10 eμA | 5.2-13GBq in 1–3 h |
2.1.4. 89Zr
89Zr (T1/2 = 78.4 h) is a positron emitter and a new metal PET radioisotope ideal for immunoimaging [100]. To date, 89Zr-atezolizumab has been studied in renal cell carcinoma (RCC), but some obstacles were encountered, so further research is needed [101]. 89Zr is produced by cyclotrons involving the following nuclear reactions:
-
(1)
89Y (p, n) 89Zr: This reaction only requires low-energy protons (5-15 MeV) and targets with natural abundance 89Y (100%), which reduces the costs significantly. The number of interference nuclear reactions is limited; hence, one can obtain a high specific activity of 89Zr [102,103,104]. The yield of this (p, n) reaction can be as high as 44 MBq/μA·h under irradiation of 14 MeV protons [105]. Various methods for the isolation and purification of 89Zr have been proposed, including solvent extraction, anion exchange chromatography, and weak cation exchange chromatography, which can obtain 89Zr with high specific activity and radionuclidic purity [106]. The proton energy from small medical cyclotrons installed in hospitals can meet the requirements for bombarding the 89Y target, which is the main reason why many hospitals have developed 89Zr production processes.
-
(2)
89Y (d, 2n) 89Zr: This reaction uses low-energy deuterons (also 5–15 MeV) and has the same advantages as the aforementioned production method [102,103,104], as well as offering a higher yield of 58MBq/μA·h. However, one must still factor in the availability of the beam of particles and the costs of these two production methods [105]. Thus, more research is needed.
-
(3)
natSr (α, xn) 89Zr: Besides requiring α beams, if natSr targets are used, abundant quantities of impurities such as 88Zr and 86Zr can easily be produced. For the moment, this production method is only theoretically feasible [107].
2.1.5. 124I
124I (T1/2 = 4.176 d) is a PET nuclide that can provide a higher quality diagnostic image [108]. Currently, 124I is used for the clinical diagnosis of thyroid cancer [109] and neuroblastoma [110]. 124I and 131I can also be combined as radiopharmaceuticals for theranostics to treat thyroid cancer [20].
124I is produced via cyclotrons through two different production methods:
-
(1)
124Te (p, n) 124I: This is the main production method currently employed. Although this method offers a relatively low production rate, it can achieve high currents and use enriched targets to improve the overall yield [108]. The average yield of this reaction is 16 MBq/μA·h, and at the end of bombardment, the impurity content of 123I and 125I only reaches about 1% [111]. Dry distillation is used to extract 124I [112]. On the downside, the enriched 124Te target material costs about 10000$/g, which is relatively expensive [113].
-
(2)
124Te (d, 2n) 124I: Has a high production yield of 17.5 MBq/μA·h, however, this reaction requires a beam of deuterons, which may be difficult to obtain and can result in impurities such as 125I (reaching about 1.7%) [111,114].
2.1.6. 99Mo/99mTc
99mTc (T1/2 = 6.02 h) emits single γ rays with 0.141 MeV and is mostly used in SPECT; for the diagnosis of stroke; and to examine bone, myocardium, kidneys, thyroid, salivary glands, and other organs [61,62]. The proportion of nuclear medicine diagnosis applying 99mTc accounts for approximately 80% of all nuclear medicine procedures, representing around 40 million examinations worldwide every year [115]. 99mTc is mainly produced using a 99Mo/99mTc generator. Currently, 99mTc can be produced by cyclotrons through the following reactions:
-
(1)
100Mo (p, 2n) 99mTc [116,117]: This is the main production method and is optimal with a proton energy range of 19–24 MeV and a highly enriched 100Mo target, such that 98Tc, 97Tc, and other impurities can be reduced to a minimum. According to the experimental data, with a proton beam energy of 24 MeV, the yield of 99mTc is about 592 GBq/mA·h [118]. A target irradiated with a 24 MeV proton beam at 500 μA for 12 h yielded 2.59 TBq of 99mTc [119]. GE PETtrace880 machines have obtained approximately 174 GBq after 6 h [116]. To date, TRIUMF and its partners have successfully verified the feasibility of using a 24 MeV cyclotron to produce 99mTc to supply the needs of all applications in Vancouver by developing a complete process based on 16, 19, and 24 MeV cyclotron production and applied the results to relevant patents [120]. Automated modules to separate 99mTc from irradiated targets of 100Mo are under development [121]. However, the shipped distance should be considered based on the direct product and its half-life [122];
-
(2)
96Zr (α, n) 99Mo→99mTc [123,124]: This production method can produce 99mTc with high specific activity. However, it has a low yield, and a beam with a high current is difficult to obtain, which limits the applicability of this production method.
2.1.7. 123I
123I (T1/2 = 13.2 h) is a γ-ray emitter that can be utilized for SPECT diagnosis. It has especially been used for the diagnosis of Parkinson’s disease, primary and metastatic pheochromocytoma, and neuroblastoma. The sensitivity and specificity of this technology are greater than 90% [125]. It also can be used for diagnosis of the thyroid, brain, and myocardium.
Presently, there are three common production routes yielding 123I:
(1–2) 124Xe (p, 2n) 123Cs→123Xe→123I and 124Xe (p, pn)123Xe→ 123I: These nuclear reactions require a medium-energy cyclotron and can obtain with a high radionuclidic purity. The yield of these reactions simulated by MCNP was 757 MBq/μA·h. Compared with the experimental data, the maximum fluctuation was about 185 MBq/μA·h [126,127]. However, due to the use of enriched 124Xe targets, these methods are costly [128,129].
(3) 123Te (p, n) 123I: This production method can apply a low-energy cyclotron. When enriched targets of 123Te (enrichment of 99.3%) were used, an ultrapure nuclide was obtained, and the yield increased from nearly 18.5 to 37GBq 30 h after EOB (end of the bombardment) [130,131,132]. This production method is also costly because of the enriched target of 123Te. This alternative production method was proven feasible to produce 123I.
2.1.8. 225Ac
225Ac (T1/2 = 9.92 d) has a unique decay chain that can emit four α rays, causing it to be more effective in destroying tumor cells than other isotopes. Presently, the first use of [225Ac]Ac-PSMA-I&T in a clinical context was successful in treating advanced metastatic castration-resistant prostate cancer [133,134,135]. Additionally, the research of [225Ac]Ac-DOTAGA-SP for the treatment of malignant gliomas is ongoing [136].
225Ac can be produced with medium-energy protons via the 226Ra (p, 2n) 225Ac reaction. The yield was only about 2.4 MBq after EOB [137], moreover, its radioactive inventory is difficult to handle [137,138,139]. Production of 225Ac applying high-energy protons (60–140 MeV) through bombarding a 232Th target can produce a high yield of 96 GBq, but this yield requires high intensity and energy [140], which are not readily available. Currently, the U.S. Department of Energy Isotope Program produces 225Ac using a spallation-induced reaction with high-energy protons on natural thorium.
2.1.9. 211At
211At (T1/2 = 7.2 h) emits α particles that can be utilized in α therapy [141]. Currently, 211At in the form of [211At]At-PA and [211At]At-ch81C6 has been studied in glioma and recurrent brain tumors [142,143]. Gothenburg (Sweden) [144] is undergoing a clinical research using [211At]At-MX35(Fab)2 to treat ovarian cancer patients, which is an alpha-emitting radionuclide with great clinical potential [145].
211At is commonly produced by a medium-energy cyclotron bombarding a 209Bi target with α particles, causing a 209Bi (α, 2n) 211At reaction to take place [146,147]. Purifying the 211At from the target material was either done by a wet extraction or a dry distillation. The National Institutes of Health (Bethesda, USA) produced a maximum of 1.71 GBq in one hour, while Sichuan University in China produced a maximum of 200 MBq in 2 h [148]. However, due to the product of toxic impurities such as 210Po, the energy of the α beam needs to be monitored [148,149].
2.1.10. 67Cu
67Cu (T1/2 = 61.76 h) emits γ rays for SPECT diagnosis and β particles that can be used for medical treatment. Thus, 67Cu can be used individually or with 64Cu as a radiopharmaceutical for theranostics. Presently, 67Cu is used for the nuclear medicinal diagnosis of neuroendocrine tumors and lymphomas [150,151] and the medical treatment of lymphoma and colon cancer [152].
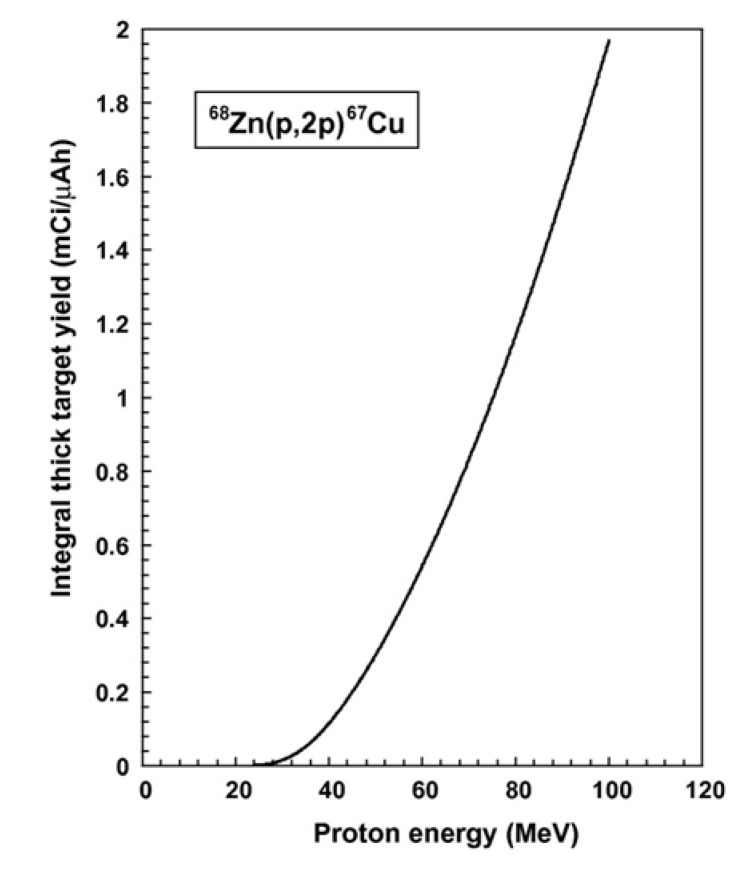
67Cu is generally produced via the 68Zn (p, 2p) 67Cu reaction. This reaction has high recovery and needs both a medium-energy cyclotron and a highly enriched target [153,154,155]. Due to the need for high-energy protons, there are only a few laboratories in the world that can produce 67Cu [156]. The yield of the integral physical thick target was calculated and is shown in Figure 3.
Figure 3.
Integral physical thick target yields for the 68Zn (p, 2p) 67Cu reaction [157].
In addition to the medical isotopes mentioned above, 11C [158], 13N [159], 15O [160], 86Y [161], 44Sc [162,163], 201Tl [164], 47Sc [165,166], 32P [167], 67Ga [168], and other medical isotopes produced by cyclotrons have also been reported.
Cyclotrons are the main accelerator-based drivers of medical isotope production. Their output is constantly improving due to advancements in targets [169,170], research on new nuclear reactions [171,172,173], and accelerator technology developments [174,175,176], leading not only to increased yields but also to a reduction in radioactive impurities. Most medical isotopes currently produced by reactors can also alternatively be produced by cyclotrons, and the constant improvements to the medical-isotope-producing abilities of cyclotrons have contributed to the stable supply of medical isotopes.
2.2. Medical Isotopes Produced by Linacs
The charged particles accelerated by a linac pass through the focusing magnetic field and the linear acceleration field once without deflection [58]. Once ejected, these particles irradiate their targets to produce medical isotopes. Linac beams are characterized by high beam intensity and lower energy [59].
In terms of linacs currently used to produce medical isotopes, proton linacs can be relatively easily employed in medical isotope production. For example, proton linacs that produce PET nuclides can reduce the weight of cyclotron magnets, and some high-energy and high-fluxes proton linacs can produce therapeutic nuclides [177,178,179]. While feasibility reports on the ability of electron linacs to produce medical isotopes are common, the pulsed beams and the cross-sections of linacs can create challenges when used in practice [41,180,181,182]. There are other linacs that accelerate other charged particles; however, these linacs will not be described here.
2.2.1. 18F
PET radioisotopes can be produced with proton linacs. The first compact proton linear accelerator in the United States for the generation of medical isotopes produces 18F for a local hospital [183]. Additionally, Hitachi, Ltd. and AccSys Technology, Inc. (Hitachi’s subsidiary company) also developed a proton linac to produce PET nuclides. After bombardment for one hour, 23.5 GBq 18F was produced, indicating that batch production of 18F could be achieved [177].
18F (T1/2 = 109.8 min) can also be produced by electron linacs through a photonuclear reaction 19F (γ, n) 18F, as well as other commonly used PET radioisotopes such as 11C (T1/2 = 20.38 min), 13N (T1/2 = 9.96 min), and 15O (T1/2 = 122 s). When using a photonuclear reaction to produce these PET radioisotopes, the yields are generally lower since the cross-section is 1–2 orders of magnitude lower than that under a proton reaction. However, photonuclear reactions can use a natural target of 19F, thus providing lower costs compared to proton reactions [177]. Many feasibility reports on producing PET nuclides via photonuclear reactions have been published, but actual production still needs further study.
2.2.2. 99Mo
99Mo (T1/2 = 66 h) decays into 99mTc (T1/2 = 6.02 h). An electron linac can be utilized to produce 99Mo via the photonuclear reaction 100Mo (γ, n) 99Mo [184,185,186]. It was reported that the yield of 99Mo obtained after 6.5 days of continuous bombardment of a 6 g high-purity 100Mo target with 36 MeV electrons was 458.8 GBq (average beam power of ~8 kW) [187]. The cost of this production method can be reduced by using a natural target and, although this method will produce the isotopes of Mo, isotopes of Tc will not be produced, making it easy to separate 99Mo via chemical difference or evaporation temperature difference [188]. NorthStar and its partners have studied this production method and listed it as the main 99Mo supply option in their long-term plans [187]. Canadian Light Source (CLS) and TRIUMF also conducted feasibility research on this production method and plan to put it into production [189,190].
In addition to the medical isotopes mentioned above, the production of 67Cu [191,192,193], 64Cu [194], 225Ac [195,196], 68Ga [197], 111In [181], 177Lu [198], 47Sc [199], and other medical isotopes through linacs have been reported.
Overall, linacs have some disadvantages in terms of their design and yields [41,182,200,201]. As a backup method for the production of medical isotopes, linacs still require further research.
2.3. Medical Isotopes Produced by Neutron Generators
A neutron generator is an accelerator-based neutron source device that is capable of delivering neutrons through nuclear fusion reactions. These neutrons will, in turn, irradiate the target to produce medical isotopes. The nuclear fusion reactions commonly used to produce neutrons are shown in Table 4.
Table 4.
| Reaction | Energy [MeV] | The Suitable Reaction of Isotope Production |
|---|---|---|
| D-D reaction | 2–3 | (n, γ) |
| D-T reaction | 14–15 | (n, 2n) (n, p) |
| D-7Li reaction | 10&13 | (n, 2n) (n, p) |
2.3.1. 99Mo/99mTc
99mTc (T1/2 = 6.02h) can be produced by neutron generators [207,208]. After neutron moderation, neutrons with a specific energy can be obtained and then used to produce 99mTc via the nuclear reaction of 235U (n, f) 99Mo→99mTc. The advantages of this production method include both ease of supervision and overall safety, but the yield will be 1–2 orders of magnitude lower than that produced by a reactor [209]. SHINE and Phoenix Laboratory used a DT neutron generator to bombard UO2SO4 to produce 99Mo. After irradiation of a 5 L UO2SO4 solution for about 20 h, the yield of 99Mo was 51.8 GBq [210]. The disadvantage of this production method is that a long-term, stable, and high-intensity beam is difficult to achieve [211].
In addition, 99Mo can be produced via the nuclear reactions of 98Mo (n, γ) 99Mo and 100Mo (n, 2n) 99Mo, both of which use Mo targets instead of U targets. Additionally, sufficient activity of 99Mo can be produced in principle [207,208,212]. The yields of these two nuclear reactions can be increased by improving the fluxes of neutrons and the irradiation time and/or using highly enriched targets, in addition to other methods [213]. However, 99Mo from an irradiated 98Mo/100Mo target is a carrier-added product with a low specific activity. The biggest challenge for this method is how to develop a new type of 99Mo/99mTc generator that meets medical requirements.
2.3.2. 67Cu
67Cu (T1/2 = 61.76 h) is generally produced by cyclotrons. Kin proposed using neutrons to produce 67Cu [212]. Presently, using neutron generators via the D-T reaction in the form of 67Zn (n, p) 67Cu can produce 67Cu. Due to the developments of neutron generators, 67Cu can be produced in the hospital without the need to transport the isotope over long distances. This production method does not produce a large number of impurities [156,214], and the activity can reach hundreds to thousands of MBq [212]. However, when dealing with radioactive isotopes with GBq, the radiation facility will result in higher costs [212].
In addition to the medical isotopes mentioned above, 89Sr [215,216,217], 64Cu [218], 47Sc [219], 132Xe [220], 225Ac [212], and other medical isotopes produced by neutron generators have also been reported.
As a neutron source, a neutron generator is essential to produce neutron-rich medical isotopes. Although such generators have the advantages of low cost and target reusability [212,221], providing continuously high fluxes of neutrons and engaging in separation-extraction of the medical isotopes remain challenging topics [221]. Despite these challenges, generators are presently regarded as a viable alternative to the reactor-production method.
3. The Status of Medical Isotope Production via Accelerators in China
3.1. Available Accelerators for Medical Isotope Production in China
Currently, there are about 160 PET small medical cyclotrons for the routine production of 11C, 18F, and other medical isotopes to meet clinical demands in China [222]. Additionally, there are several medium- and high-energy accelerators used for medical isotope production in China.
The Chinese Institute of Atomic Energy (CIAE) and Shanghai Ansheng Kexing Company each have a C-30 cyclotron with adjustable proton energy of 15.5–30 MeV and beam currents up to 350 µA. These can be used to produce medical isotopes such as 11C, 18F, 64Cu, 68Ge, 89Zr, 123I, 124I, and 201Tl. CIAE has a 100 MeV proton cyclotron (C-100) with a beam current up to 200 µA capable of producing 67Cu, 225Ac, and other medical isotopes of interest.
The Sichuan University owns a cyclotron capable of delivering beams of protons, as well as alpha and deuteron particles (p–26 MeV and α–30 MeV).
The Chinese Academy of Sciences Institute of Modern Physics built a 25 MeV superconducting proton linear accelerator with an intensity in the order of milliamps. At present, the linac can accelerate various beams such as proton beams, 3He2+ beams, and 4He2+ beams. The energy of 3He2+ beams can reach 36 MeV at an intensity of 200 µA, while the energy of 4He2+ beams can reach 32 MeV with a current of 100 µA. The accelerator can meet the needs of medical isotope production and produce various radioisotopes such as 99Mo/99mTc, 117mSn, 211At, 55Fe, 73As, 225Ac, 109Cd, 88Y, and 75Se.
Lanzhou University has been instrumental in the development of advanced ion source selection, ion beam extraction, and acceleration system design, as well as target system design. Additionally, the university independently built a series of neutron generators based on D-D and D-T reactions [223].
3.2. The Status of Medical Isotope Production via Accelerators
There is a solid research foundation for accelerator-based medical isotope production in China. In the 1980s, Sichuan University and others successfully developed production technology for medical isotopes such as 211At, 123I, 111In, and 201Tl by relying on domestic cyclotrons and a CS-30 cyclotron [224]. Since the 1990s, CIAE has produced medical isotopes such as 18F, 111In, and 201Tl using a C-30 cyclotron.
In the last two decades, with the popularization and rapid development of domestic nuclear medicine, the amount of PET equipment increased to 427 by 2019. Today, 117 hospitals equipped with small medical cyclotrons routinely produce 18F to meet clinical needs, with an annual consumption of more than 1850 TBq. Additionally, some emerging isotopes such as 64Cu, 89Zr, and 123/124I have been rapidly developed for medical applications. In 2007, CIAE cooperated with Atom Hitech to carry out research on 123I production using enriched 124Xe gas at 111 GBq for each batch with a C-30 cyclotron. In 2012, Atom Hitech produced carrier-free 64Cu with enriched 64Ni at 37–74 GBq for each batch based on a C-30 cyclotron. In 2016, Sichuan University bombarded an 89Y target with 13 MeV protons and obtained 89Zr with a radionuclidic purity of more than 99% [16]. However, due to the limited availability of high-energy particle accelerators for the production of therapeutic nuclides such as 67Cu, 225Ac, and 223Ra, China is significantly lagging behind the advanced international levels of development. In 2021, for the first time, CIAE obtained around 22.2 MBq of 225Ac with radionuclidic purity greater than 99% using a C-100 cyclotron.
4. Summary
Presently, cyclotrons remain the primary facilities for accelerator-based medical isotope production, although linacs and neutron generators are rapidly becoming a viable alternative.
Cyclotrons with adjustable energy ranges or medium energy can produce various kinds of medical isotopes and can cover most radiopharmaceutical production needs in a region [59]. Yield and purity improvements in medical isotopes and the overall cost of cyclotron production have led researchers to explore further possibilities, including proton linacs, which have significant advantages in providing proton beams in the order of tens to hundreds of MeV [179]. These linacs can be developed in research institutes or laboratories conducting scientific experiments and physical research at the same time. For electron linacs, the cross-section of photonuclear interactions is relatively low, which restricts their practical applications. Other factors, such as impurity products and economic costs, also play major roles when evaluating production techniques and methodologies. Attempts to produce medical isotopes through neutron generators are promising and could theoretically yield the medical isotopes that are currently produced by reactors. However, improving the neutron flux rate remains a major consideration.
As medical isotopes produced by reactors often face supply shortages, interest in the use of accelerator-based techniques to produce medical isotopes will increase. We hope to develop an accelerator with the right energy, right beam types, right location, and good shielding facilities, which will play an important role in the supply of medical isotopes.
Author Contributions
Conceptualization, Y.W. and D.C.; methodology, D.C.; investigation, Y.W., D.C., R.d.S.A., J.L. (Jixin Liang) and Z.L.; resources, Z.L., R.d.S.A., J.L. (Jixin Liang), Z.Q. and J.L. (Juntao Liu); writing—original draft preparation, Y.W.; writing—review and editing, R.d.S.A. and J.L. (Jixin Liang); supervision, Z.Q. and J.L. (Juntao Liu); project administration, Z.L.; funding acquisition, J.L. (Juntao Liu). All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was funded by the National natural Science Foundation of China, grant number 11975115; Special Projects of the Central Government in Guidance of Local Science and Technology Development (Research and development of three-dimensional prospecting technology based on Cosmic-ray muons), grant number YDZX20216200001297; the Research and Development of Medical Isotopes based on High-current Superconducting Linear Accelerator Project, the Fundamental Research Funds for the Central Universities, grant number lzujbky-2019-54; the Science and Technology Planning Project of Gansu, grant number 20JR10RA645; Lanzhou University Talent Cooperation Research Funds sponsored by Lanzhou City, grant number 561121203 and Gansu provincial science and technology plan projects for talents, grant number 054000029.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Radioisotopes in Medicine [EB/OL] [(accessed on 1 June 2022)]. Available online: https://world-nuclear.org/information-library/non-power-nuclear-applications/radioisotopes-research/radioisotopes-in-medicine.aspx.
- 2.AMA Manual of Style Committee . AMA manual of style: A guide for authors and editors, 10 th ed. Oxford University Press; New York, NY, USA: 2007. [Google Scholar]
- 3.Reuzé S., Schernberg A., Orlhac F., Sun R., Chargari C., Dercle L., Deutsch E., Buvat I., Robert C. Radiomics in nuclear medicine applied to radiation therapy: Methods, pitfalls, and challenges. Int. J. Radiat. Oncol. Biol. Phys. 2018;102:1117–1142. doi: 10.1016/j.ijrobp.2018.05.022. [DOI] [PubMed] [Google Scholar]
- 4.Langbein T., Weber W.A., Eiber M. Future of theranostics: An outlook on precision oncology in nuclear medicine. J. Nucl. Med. 2019;60:13S–19S. doi: 10.2967/jnumed.118.220566. [DOI] [PubMed] [Google Scholar]
- 5.Kar N.R. Production and applications of radiopharmaceuticals: A review. Int. J. Pharm. Investig. 2019;9:36–42. doi: 10.5530/ijpi.2019.2.8. [DOI] [Google Scholar]
- 6.Vermeulen K., Vandamme M., Bormans G., Cleeren F. Seminars in Nuclear Medicine. Volume 49. WB Saunders; Philadelphia, PA, USA: 2019. Design and challenges of radiopharmaceuticals; pp. 339–356. [DOI] [PubMed] [Google Scholar]
- 7.Holly T.A., Abbott B.G., Al-Mallah M., Calnon D.A., Cohen M.C., DiFilippo F.P., Ficaro E.P., Freeman M.R., Hendel R.C., Jain D., et al. Single photon-emission computed tomography. J. Nucl. Cardiol. 2010;17:941–973. doi: 10.1007/s12350-010-9246-y. [DOI] [PubMed] [Google Scholar]
- 8.Jaszczak R.J., Coleman R.E., Lim C.B. SPECT: Single photon emission computed tomography. IEEE Trans. Nucl. Sci. 1980;27:1137–1153. doi: 10.1109/TNS.1980.4330986. [DOI] [Google Scholar]
- 9.Jaszczak R.J., Coleman R.E. Single photon emission computed tomography (SPECT). Principles and instrumentation. Investig. Radiol. 1985;20:897–910. doi: 10.1097/00004424-198512000-00004. [DOI] [PubMed] [Google Scholar]
- 10.Valk P.E., Delbeke D., Bailey D.L., Townsend D.W., Maisey M.N. Positron Emission Tomography. Springer; London, UK: 2005. [Google Scholar]
- 11.Kubota K. From tumor biology to clinical PET: A review of positron emission tomography (PET) in oncology. Ann. Nucl. Med. 2001;15:471–486. doi: 10.1007/BF02988499. [DOI] [PubMed] [Google Scholar]
- 12.Wagner H.N., Jr. Seminars in Nuclear Medicine. Volume 28. WB Saunders; Philadelphia, PA, USA: 1998. A brief history of positron emission tomography (PET) pp. 213–220. [DOI] [PubMed] [Google Scholar]
- 13.Wheat J.M., Currie G.M., Davidson R., Kiat H. An introduction to nuclear medicine. Radiographer. 2011;58:38–45. doi: 10.1002/j.2051-3909.2011.tb00154.x. [DOI] [Google Scholar]
- 14.Mariani G., Bruselli L., Kuwert T., Kim E.E., Flotats A., Israel O., Dondi M., Watanabe N. A review on the clinical uses of SPECT/CT. Eur. J. Nucl. Med. Mol. Imaging. 2010;37:1959–1985. doi: 10.1007/s00259-010-1390-8. [DOI] [PubMed] [Google Scholar]
- 15.Palmedo H., Bucerius J., Joe A., Strunk H., Hortling N., Meyka S., Roedel R., Wolff M., Wardelmann E., Biersack H.J., et al. Integrated PET/CT in differentiated thyroid cancer: Diagnostic accuracy and impact on patient management. J. Nucl. Med. 2006;47:616–624. [PubMed] [Google Scholar]
- 16.Liqun H., Shufang L., Ge S., Huan L., Jianguo L., Quan A., Zhongwen W. Current Applications and Prospects of Radionuclide for Therapy. J. Isot. 2021;34:412. [Google Scholar]
- 17.Rösch F., Baum R.P. Generator-based PET radiopharmaceuticals for molecular imaging of tumours: On the way to THERANOSTICS. Dalton Trans. 2011;40:6104–6111. doi: 10.1039/c0dt01504k. [DOI] [PubMed] [Google Scholar]
- 18.Notni J., Wester H.J. Re-thinking the role of radiometal isotopes: Towards a future concept for theranostic radiopharmaceuticals. J. Label. Compd. Radiopharm. 2018;61:141–153. doi: 10.1002/jlcr.3582. [DOI] [PubMed] [Google Scholar]
- 19.Qaim S.M., Scholten B., Neumaier B. New developments in the production of theranostic pairs of radionuclides. J. Radioanal. Nucl. Chem. 2018;318:1493–1509. doi: 10.1007/s10967-018-6238-x. [DOI] [Google Scholar]
- 20.Nagarajah J., Janssen M., Hetkamp P., Jentzen W. Iodine symporter targeting with 124I/131I theranostics. J. Nucl. Med. 2017;58((Suppl. S2)):34S–38S. doi: 10.2967/jnumed.116.186866. [DOI] [PubMed] [Google Scholar]
- 21.Eberlein U., Cremonesi M., Lassmann M. Individualized dosimetry for theranostics: Necessary, nice to have, or counterproductive? J. Nucl. Med. 2017;58((Suppl. S2)):97S–103S. doi: 10.2967/jnumed.116.186841. [DOI] [PubMed] [Google Scholar]
- 22.Braccini S., Belver-Aguilar C., Carzaniga T., Dellepiane G., Häffner P., Scampoli P. Novel irradiation methods for theranostic radioisotope production with solid targets at the Bern medical cyclotron; Proceedings of the International Conference on Cyclotrons and their Applications (CYC); Cape Town, South Africa. 22–27 September 2019; pp. 22–27. [Google Scholar]
- 23.Brandt M., Cardinale J., Aulsebrook M.L., Gasser G., Mindt T.L. An overview of PET radiochemistry, part 2: Radiometals. J. Nucl. Med. 2018;59:1500–1506. doi: 10.2967/jnumed.117.190801. [DOI] [PubMed] [Google Scholar]
- 24.Poschenrieder A., Schottelius M., Schwaiger M., Kessler H., Wester H.-J. The influence of different metal-chelate conjugates of pentixafor on the CXCR4 affinity. EJNMMI Res. 2016;6:36. doi: 10.1186/s13550-016-0193-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ahn B.C. Personalized medicine based on theranostic radioiodine molecular imaging for differentiated thyroid cancer. BioMed Res. Int. 2016;2016:1680464. doi: 10.1155/2016/1680464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miller C., Rousseau J., Ramogida C.F., Celler A., Rahmim A., Uribe C.F. Implications of physics, chemistry and biology for dosimetry calculations using theranostic pairs. Theranostics. 2022;12:232. doi: 10.7150/thno.62851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ehlerding E.B., Ferreira C.A., Aluicio-Sarduy E., Jiang D., Lee H.J., Theuer C.P., Engle J.W., Cai W. 86/90Y-based theranostics targeting angiogenesis in a murine breast cancer model. Mol. Pharm. 2018;15:2606–2613. doi: 10.1021/acs.molpharmaceut.8b00133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ferreira C.A., Ehlerding E.B., Rosenkrans Z.T., Jiang D., Sun T., Aluicio-Sarduy E., Engle J.W., Ni D., Cai W. 86/90Y-Labeled monoclonal antibody targeting tissue factor for pancreatic cancer theranostics. Mol. Pharm. 2020;17:1697–1705. doi: 10.1021/acs.molpharmaceut.0c00127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ming-qi L.I., Qi-min D., Zuo-yong C., Mao-liang L.I. Production and application of medical radionuclide: Status and urgent problems to be resolved in China. J. Isot. 2013;26:186. (In Chinese) [Google Scholar]
- 30.Mushtaq A. Reactors are indispensable for radioisotope production. Ann. Nucl. Med. 2010;24:759–760. doi: 10.1007/s12149-010-0425-3. [DOI] [PubMed] [Google Scholar]
- 31.Xoubi N., Primm R.T., III. Modeling of the High Flux Isotope Reactor Cycle 400. Oak Ridge National Laboratory; Oak Ridge, Tennessee, USA: 2005. ORNL/TM-2004/251. [Google Scholar]
- 32.Ruth T.J. The medical isotope crisis: How we got here and where we are going. J. Nucl. Med. Technol. 2014;42:245–248. doi: 10.2967/jnmt.114.144642. [DOI] [PubMed] [Google Scholar]
- 33.Koleška M., Lahodová Z., Šoltés J., Viererbl L., Ernest J., Vinš M., Stehno J. Capabilities of the LVR-15 research reactor for production of medical and industrial radioisotopes. J. Radioanal. Nucl. Chem. 2015;305:51–59. doi: 10.1007/s10967-015-4025-5. [DOI] [Google Scholar]
- 34.OECD-NEA . The Supply of Medical Radioisotopes: 2019 Medical Isotope Supply and Capacity Projection for the 2019–2024 Period. OECD-NEA; Paris, France: 2019. [Google Scholar]
- 35.Gao F., Lin L., Liu Y., Ma X. Production situation and technology prospect of medical isotopes. J. Isot. 2016;29:116–120. (In Chinese) [Google Scholar]
- 36.Kurenkov N.V., Shubin Y.N. Radionuclides for nuclear medicine. Медицинская Радиoлoгия И Радиациoнная Безoпаснoсть. 1996;41:54–63. [Google Scholar]
- 37.IAEA . Nuclear Research Reactors in the World. IAEA; New York, NY, USA: 1997. 120p. [Google Scholar]
- 38.Hoedl S.A., Updegraff W.D. The production of medical isotopes without nuclear reactors or uranium enrichment. Sci. Glob. Secur. 2015;23:121–153. doi: 10.1080/08929882.2015.1037123. [DOI] [Google Scholar]
- 39.Van der Keur H. Medical radioisotopes production without a nuclear reactor. 2010. [(accessed on 20 June 2022)]. Available online: http://www.laka.org/info/publicaties/2010-medical_isotopes.pdf.
- 40.Ziwei L., Yuncheng H., Xiaoyu W., Jiachen Z., Yongfeng W., Qunying H. Production Status and Technical Prospects of Medical Radioisotope 99 Mo/99m Tc. Nucl. Phys. Rev. 2019;36:170–183. (In Chinese) [Google Scholar]
- 41.Starovoitova V.N., Tchelidze L., Wells D.P. Production of medical radioisotopes with linear accelerators. Appl. Radiat. Isot. 2014;85:39–44. doi: 10.1016/j.apradiso.2013.11.122. [DOI] [PubMed] [Google Scholar]
- 42.Kaur C.D., Mishra K.K., Sahu A., Panik R., Kashyap P., Mishra S.P., Kumar A. Theranostics: New era in nuclear medicine and radiopharmaceuticals. In: Naqvi S.A.R., Imrani M.B., editors. Medical Isotopes. IntechOpen; London, UK: 2020. [Google Scholar]
- 43.Zhang T., Fan M., Wei S., Chen S., Yang F. The present situation and the prospect of medical cyclotrons in China. Sci. China Phys. Mech. Astron. 2011;54:260–265. doi: 10.1007/s11433-011-4609-1. [DOI] [Google Scholar]
- 44.Sunderland J., Erdahl C., Bender B., Sensoy L., Watkins G. Considerations, measurements and logistics associated with low-energy cyclotron decommissioning; Proceedings of the AIP Conference Proceedings, Playa del Carmen; Máxico. 26–29 August 2012; New York, NY, USA: American Institute of Physics; 2012. pp. 16–20. [Google Scholar]
- 45.International Atomic Energy Agency . Alternative Radionuclide Production with a Cyclotron. IAEA; Vienna, Austria: 2021. IAEA Radioisotopes and Radiopharmaceuticals Reports No. 4. [Google Scholar]
- 46.Chernyaev A.P., Varzar S.M. Particle accelerators in modern world. Phys. At. Nucl. 2014;77:1203–1215. doi: 10.1134/S1063778814100032. [DOI] [Google Scholar]
- 47.Goethals P.E., Zimmermann R.G. Cyclotrons used in Nuclear Medicine World Market Report & Directory. MEDraysintell; Louvain-la-Neuve, Belgium: 2015. [Google Scholar]
- 48. [(accessed on 1 June 2022)]. Available online: https://www.machinedesign.com/learning-resources/whats-the-difference-between/article/21832184/what-are-the-differences-between-linear-accelerators-cyclotrons-and-synchrotrons.
- 49. [(accessed on 10 July 2022)]. Available online: https://www.iaea.org/newscenter/news/cyclotrons-what-are-they-and-where-can-you-find-them.
- 50. [(accessed on 10 July 2022)]. Available online: https://www.iaea.org/sites/default/files/gc/gc65-inf2.pdf.
- 51. [(accessed on 10 July 2022)]. Available online: https://www.iaea.org/sites/default/files/gc/gc64-inf2.pdf.
- 52. [(accessed on 10 July 2022)]. Available online: https://www.iaea.org/sites/default/files/gc/gc61inf-4_en.pdf.
- 53. [(accessed on 10 July 2022)]. Available online: https://www.iaea.org/sites/default/files/ntr2015.pdf.
- 54. [(accessed on 10 July 2022)]. Available online: https://www.iaea.org/sites/default/files/ntr2014.pdf.
- 55. [(accessed on 10 July 2022)]. Available online: https://www-legacy.iaea.org/OurWork/ST/NE/Pess/assets/13-25751_rep_ntr_2013_web.pdf.
- 56. [(accessed on 10 July 2022)]. Available online: https://www-legacy.iaea.org/OurWork/ST/NE/Pess/assets/ntr2012_web.pdf.
- 57. [(accessed on 10 July 2022)]. Available online: https://www-legacy.iaea.org/OurWork/ST/NE/Pess/assets/ntr2011.pdf.
- 58.Chao A.W., Chou W., editors. Reviews of Accelerator Science and Technology-Volume 3: Accelerators as Photon Sources. World Scientific; Chiyoda City, Tokyo, Japan: 2011. [Google Scholar]
- 59.Leo K.W.K., Hashim S. Accelerator Selection for Industry and Medical Applications. (This is a preprint article, it offers immediate access but has not been peer reviewed)
- 60.Synowiecki M.A., Perk L.R., Nijsen J.F.W. Production of novel diagnostic radionuclides in small medical cyclotrons. EJNMMI Radiopharm. Chem. 2018;3:3. doi: 10.1186/s41181-018-0038-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zuoyuan D., Bin W. Securities Research Report-In-Depth Discussion Series-Nuclear Medicine. Pacific Securities; Guangdong, China: 2019. [Google Scholar]
- 62.Yuan Z. Foreign Medical Sciences. Volume 24. Section of Radiation Medicine and Nuclear Medicine; Tianjin, China: 2000. FDA approved radiopharmaceuticals; pp. 161–163. [Google Scholar]
- 63.Racette B.A., Antenor J.A., McGee-Minnich L., Moerlein S.M., Videen T.O., Kotagal V., Perlmutter J.S. [18F] FDOPA PET and clinical features in parkinsonism due to manganism. Mov. Disord. 2005;20:492–496. doi: 10.1002/mds.20381. [DOI] [PubMed] [Google Scholar]
- 64.Zhao P., Zhang B., Gao S., Li X. Clinical features, MRI, and 18F-FDG-PET in differential diagnosis of Parkinson disease from multiple system atrophy. Brain Behav. 2020;10:e01827. doi: 10.1002/brb3.1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rahmim A., Zaidi H. PET versus SPECT: Strengths, limitations and challenges. Nucl. Med. Commun. 2008;29:193–207. doi: 10.1097/MNM.0b013e3282f3a515. [DOI] [PubMed] [Google Scholar]
- 66.Ruth T.J., Wolf A.P. Absolute cross sections for the production of 18F via the 18O (p, n) 18F reaction. Radiochim. Acta. 1979;26:21–24. doi: 10.1524/ract.1979.26.1.21. [DOI] [Google Scholar]
- 67.Hess E., Blessing G., Coenen H.H., Qaim S.M. Improved target system for production of high purity [18F] fluorine via the 18O (p, n) 18F reaction. Appl. Radiat. Isot. 2000;52:1431–1440. doi: 10.1016/S0969-8043(99)00248-1. [DOI] [PubMed] [Google Scholar]
- 68.Kambali I., Parwanto, Suryanto H., Huda N., Listiawadi F.D., Astarina H., Ismuha R.R., Kardinah Dependence of 18F Production Yield and Radioactive Impurities on Proton Irradiation Dose. Phys. Res. Int. 2017;2017:2124383. doi: 10.1155/2017/2124383. [DOI] [Google Scholar]
- 69.P Perini E.A., Skopchenko M., Hong T.T., Harianto R., Maître A., Rodríguez M.R.R., de Oliveira Santos N., Guo Y., Qin X., Zeituni C.A., et al. Pre-feasibility study for establishing radioisotope and radiopharmaceutical production facilities in developing countries. Curr. Radiopharm. 2019;12:187–200. doi: 10.2174/1874471012666190328164253. [DOI] [PubMed] [Google Scholar]
- 70.Barnhart T.E., Nickles R.J., Roberts A.D. Revisiting Low Energy Deuteron Production of [18F] Fluoride and Fluorine for PET; Proceedings of the AIP Conference Proceedings; Denton, Texas, USA. 12-16 November 2002; New York, NY, USA: American Institute of Physics; 2003. pp. 1086–1089. [Google Scholar]
- 71.Haug A.R., Cindea-Drimus R., Auernhammer C.J., Reincke M., Wängler B., Uebleis C., Schmidt G.P., Göke B., Bartenstein P., Hacker M. The role of 68Ga-DOTATATE PET/CT in suspected neuroendocrine tumors. J. Nucl. Med. 2012;53:1686–1692. doi: 10.2967/jnumed.111.101675. [DOI] [PubMed] [Google Scholar]
- 72.Kręcisz P., Czarnecka K., Królicki L., Mikiciuk-Olasik E.b., Szymański P. Radiolabeled peptides and antibodies in medicine. Bioconjugate Chem. 2020;32:25–42. doi: 10.1021/acs.bioconjchem.0c00617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Vaughn B.A. Chelation Approaches for the Theranostic Radioisotopes of Copper, Scandium and Lutetium. State University of New York at Stony Brook; York, NE, USA: 2021. [Google Scholar]
- 74.Krebs S., O’Donoghue J.A., Biegel E., Beattie B.J., Reidy D., Lyashchenko S.K., Lewis J.S., Bodei L., Weber W.A., Pandit-Taskar N. Comparison of 68Ga-DOTA-JR11 PET/CT with dosimetric 177Lu-satoreotide tetraxetan (177Lu-DOTA-JR11) SPECT/CT in patients with metastatic neuroendocrine tumors undergoing peptide receptor radionuclide therapy. Eur. J. Nucl. Med. Mol. Imaging. 2020;47:3047–3057. doi: 10.1007/s00259-020-04832-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Maffey-Steffan J., Scarpa L., Svirydenka A., Nilica B., Mair C., Buxbaum S., Bektic J., von Guggenberg E., Uprimny C., Horninger W., et al. The 68Ga/177Lu-theragnostic concept in PSMA-targeting of metastatic castration–resistant prostate cancer: Impact of post-therapeutic whole-body scintigraphy in the follow-up. Eur. J. Nucl. Med. Mol. Imaging. 2020;47:695–712. doi: 10.1007/s00259-019-04583-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Scarpa L., Buxbaum S., Kendler D., Fink K., Bektic J., Gruber L., Decristoforo C., Uprimny C., Lukas P., Horninger W., et al. The 68Ga/177Lu theragnostic concept in PSMA targeting of castration-resistant prostate cancer: Correlation of SUVmax values and absorbed dose estimates. Eur. J. Nucl. Med. Mol. Imaging. 2017;44:788–800. doi: 10.1007/s00259-016-3609-9. [DOI] [PubMed] [Google Scholar]
- 77.Sartor O., Herrmann K. Prostate Cancer Treatment: 177Lu-PSMA-617 Considerations, Concepts, and Limitations. J. Nucl. Med. 2022;63:823–829. doi: 10.2967/jnumed.121.262413. [DOI] [PubMed] [Google Scholar]
- 78.Velikyan I. 68Ga-based radiopharmaceuticals: Production and application relationship. Molecules. 2015;20:12913–12943. doi: 10.3390/molecules200712913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lin M., Waligorski G.J., Lepera C.G. Production of curie quantities of 68Ga with a medical cyclotron via the 68Zn (p, n) 68Ga reaction. Appl. Radiat. Isot. 2018;133:1–3. doi: 10.1016/j.apradiso.2017.12.010. [DOI] [PubMed] [Google Scholar]
- 80.Razbash A.A., Sevastianov Y.u.G., Krasnov N.N., Leonov A.I., Pavlekin V.E. Germanium-68 row of products; Proceedings of the 5th International Conference on Isotopes, 5ICI; Brussels, Belgium. 25–29 April 2005 ; Bologna, Italy: Medimond; p. 147. [Google Scholar]
- 81.Rösch F. Past, present and future of 68Ge/68Ga generators. Appl. Radiat. Isot. 2013;76:24–30. doi: 10.1016/j.apradiso.2012.10.012. [DOI] [PubMed] [Google Scholar]
- 82.Engle J., Lopez-Rodriguez V., Gaspar-Carcamo R., Valdovinos H., Valle-Gonzalez M., Trejo-Ballado F., Severin G.W., Barnhart T., Nickles R., Avila-Rodriguez M.A. Very high specific activity 66/68Ga from zinc targets for PET. Appl. Radiat. Isot. 2012;70:1792–1796. doi: 10.1016/j.apradiso.2012.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sadeghi M., Kakavand T., Rajabifar S., Mokhtari L., Rahimi-Nezhad A. Cyclotron production of 68Ga via proton-induced reaction on 68Zn target. Nukleonika. 2009;54:25–28. [Google Scholar]
- 84.Nelson B.J., Wilson J., Richter S., Duke M.J.M., Wuest M., Wuest F. Taking cyclotron 68Ga production to the next level: Expeditious solid target production of 68Ga for preparation of radiotracers. Nucl. Med. Biol. 2020;80:24–31. doi: 10.1016/j.nucmedbio.2020.01.005. [DOI] [PubMed] [Google Scholar]
- 85.Mardon A., Saleem H., Parish G., Syed M., Inayat E., Henry J., Heinen L., Amiscaray D.E., Dong F., Mak E., et al. What in the World is Medical Isotope Production? Golden Meteorite Press; Edmonton, Alberta, Canada: 2021. [Google Scholar]
- 86.Pandey M.K., Byrne J.F., Jiang H., Packard A.B., DeGrado T.R. Cyclotron production of 68Ga via the 68Zn (p, n) 68Ga reaction in aqueous solution. Am. J. Nucl. Med. Mol. Imaging. 2014;4:303. [PMC free article] [PubMed] [Google Scholar]
- 87.Keinänen O., Fung K., Brennan J.M., Zia N., Harris M., van Dam E., Biggin C., Hedt A., Stoner J., Donnelly P.S., et al. Harnessing 64Cu/67Cu for a theranostic approach to pretargeted radioimmunotherapy. Proc. Natl. Acad. Sci. USA. 2020;117:28316–28327. doi: 10.1073/pnas.2009960117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pfeifer A., Knigge U., Mortensen J., Oturai P., Berthelsen A.K., Loft A., Binderup T., Rasmussen P., Elema D., Klausen T.L., et al. Clinical PET of neuroendocrine tumors using 64Cu-DOTATATE: First-in-humans study. J. Nucl. Med. 2012;53:1207–1215. doi: 10.2967/jnumed.111.101469. [DOI] [PubMed] [Google Scholar]
- 89.Avila-Rodriguez M.A., Nye J.A., Nickles R.J. Simultaneous production of high specific activity 64Cu and 61Co with 11.4 MeV protons on enriched 64Ni nuclei. Appl. Radiat. Isot. 2007;65:1115–1120. doi: 10.1016/j.apradiso.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 90.Szelecsényi F., Kovács Z., Nagatsu K., Zhang M.R., Suzuki K. Excitation function of (p, α) nuclear reaction on enriched 67Zn: Possibility of production of 64Cu at low energy cyclotron. Radiochim. Acta. 2014;102:465–472. doi: 10.1515/ract-2013-2145. [DOI] [Google Scholar]
- 91.Obata A., Kasamatsu S., McCarthy D.W., Welch M.J., Saji H., Yonekura Y., Fujibayashi Y. Production of therapeutic quantities of 64Cu using a 12 MeV cyclotron. Nucl. Med. Biol. 2003;30:535–539. doi: 10.1016/S0969-8051(03)00024-6. [DOI] [PubMed] [Google Scholar]
- 92.McCarthy D.W., Shefer R.E., Klinkowstein R.E., Bass L.A., Margeneau W.H., Cutler C.S., Anderson C.J., Welch M.J. Efficient production of high specific activity 64Cu using a biomedical cyclotron. Nucl. Med. Biol. 1997;24:35–43. doi: 10.1016/S0969-8051(96)00157-6. [DOI] [PubMed] [Google Scholar]
- 93.Hilgers K., Stoll T., Skakun Y., Coenen H.H., Qaim S.M. Cross-section measurements of the nuclear reactions natZn (d, x) 64Cu, 66Zn (d, α) 64Cu and 68Zn (p, αn) 64Cu for production of 64Cu and technical developments for small-scale production of 67Cu via the 70Zn (p, α) 67Cu process. Appl. Radiat. Isot. 2003;59:343–351. doi: 10.1016/S0969-8043(03)00199-4. [DOI] [PubMed] [Google Scholar]
- 94.Elomaa V.V., Jurttila J., Rajander J., Solin O. Automation of 64Cu production at Turku PET Centre. Appl. Radiat. Isot. 2014;89:74–78. doi: 10.1016/j.apradiso.2014.02.010. [DOI] [PubMed] [Google Scholar]
- 95.Zeisler S.K., Pavan R.A., Orzechowski J., Langlois R., Rodrigue S., Van Lier J.E. Production of 64Cu on the Sherbrooke TR-PET cyclotron. J. Radioanal. Nucl. Chem. 2003;257:175–177. doi: 10.1023/A:1024782318219. [DOI] [Google Scholar]
- 96.Thieme S., Walther M., Pietzsch H.J., Henniger J., Preusche S., Mäding P., Steinbach J. Module-assisted preparation of 64Cu with high specific activity. Appl. Radiat. Isot. 2012;70:602–608. doi: 10.1016/j.apradiso.2012.01.019. [DOI] [PubMed] [Google Scholar]
- 97.Van der Meulen N.P., Hasler R., Blanc A., Farkas R., Benešová M., Talip Z., Müller C., Schibli R. Implementation of a new separation method to produce qualitatively improved 64Cu. J. Label. Compd. Radiopharm. 2019;62:460–470. doi: 10.1002/jlcr.3730. [DOI] [PubMed] [Google Scholar]
- 98.Xie Q., Zhu H., Wang F., Meng X., Ren Q., Xia C., Yang Z. Establishing reliable Cu-64 production process: From target plating to molecular specific tumor micro-PET imaging. Molecules. 2017;22:641. doi: 10.3390/molecules22040641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ohya T., Nagatsu K., Suzuki H., Fukada M., Minegishi K., Hanyu M., Fukumura T., Zhang M.-R. Efficient preparation of high-quality 64Cu for routine use. Nucl. Med. Biol. 2016;43:685–691. doi: 10.1016/j.nucmedbio.2016.07.007. [DOI] [PubMed] [Google Scholar]
- 100.Verel I., Visser GW M., Boellaard R., Stigter-van Walsum M., Snow G.B., Van Dongen G.A. 89Zr immuno-PET: Comprehensive procedures for the production of 89Zr-labeled monoclonal antibodies. J. Nucl. Med. 2003;44:1271–1281. [PubMed] [Google Scholar]
- 101.Vento J., Mulgaonkar A., Woolford L., Nham K., Christie A., Bagrodia A., de Leon A.D., Hannan R., Bowman I., McKay R.M., et al. PD-L1 detection using 89Zr-atezolizumab immuno-PET in renal cell carcinoma tumorgrafts from a patient with favorable nivolumab response. J. Immunother. Cancer. 2019;7:144. doi: 10.1186/s40425-019-0607-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Taghilo M., Kakavand T., Rajabifar S., Sarabadani P. Cyclotron production of 89Zr: A potent radionuclide for positron emission tomography. Int. J. Phys. Sci. 2012;7:1321–1325. doi: 10.5897/IJPS11.1394. [DOI] [Google Scholar]
- 103.Ciarmatori A., Cicoria G., Pancaldi D., Infantino A., Boschi S., Fanti S., Marengo M. Some experimental studies on 89Zr production. Radiochim. Acta. 2011;99:631–634. doi: 10.1524/ract.2011.1822. [DOI] [Google Scholar]
- 104.Sadeghi M., Enferadi M., Bakhtiari M. Accelerator production of the positron emitter zirconium-89. Ann. Nucl. Energy. 2012;41:97–103. doi: 10.1016/j.anucene.2011.11.014. [DOI] [Google Scholar]
- 105.Tang Y., Li S., Yang Y., Chen W., Wei H., Wang G., Yang J., Liao J., Luo S., Liu N. A simple and convenient method for production of 89Zr with high purity. Appl. Radiat. Isot. 2016;118:326–330. doi: 10.1016/j.apradiso.2016.09.024. [DOI] [PubMed] [Google Scholar]
- 106.Deri M.A., Zeglis B.M., Francesconi L.C., Lewis J.S. PET imaging with 89Zr: From radiochemistry to the clinic. Nucl. Med. Biol. 2013;40:3–14. doi: 10.1016/j.nucmedbio.2012.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kandil S.A., Spahn I., Scholten B., Saleh Z.A., Saad S.M.M., Coenen H.H., Qaim S.M. Excitation functions of (α, xn) reactions on natRb and natSr from threshold up to 26 MeV: Possibility of production of 87Y, 88Y and 89Zr. Appl. Radiat. Isot. 2007;65:561–568. doi: 10.1016/j.apradiso.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 108.Liqiang L., Feng W., Teli L., Hua Z., Zhi Y. Production of Iodine-124 and Its Application in PET Molecular Imaging. J. Isot. 2018;31:188. (In Chinese) [Google Scholar]
- 109.Freudenberg L.S., Jentzen W., Stahl A., Bockisch A., Rosenbaum-Krumme S.J. Clinical applications of 124I-PET/CT in patients with differentiated thyroid cancer. Eur. J. Nucl. Med. Mol. Imaging. 2011;38:48–56. doi: 10.1007/s00259-011-1773-5. [DOI] [PubMed] [Google Scholar]
- 110.Aboian M.S., Huang S.-y., Hernandez-Pampaloni M., Hawkins R.A., VanBrocklin H.F., Huh Y., Vo K.T., Gustafson W.C., Matthay K.K., Seo Y. 124I-MIBG PET/CT to monitor metastatic disease in children with relapsed neuroblastoma. J. Nucl. Med. 2021;62:43–47. doi: 10.2967/jnumed.120.243139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lewis J.S. Production, Use and Applications of 124I. PowerPoint Slides. Memorial–Sloan Kettering Cancer Center; New York, NY, USA: 2020. [Google Scholar]
- 112.Braghirolli AM S., Waissmann W., da Silva J.B., dos Santos G.R. Production of iodine-124 and its applications in nuclear medicine. Appl. Radiat. Isot. 2014;90:138–148. doi: 10.1016/j.apradiso.2014.03.026. [DOI] [PubMed] [Google Scholar]
- 113.Bzowski P., Borys D., Gorczewski K., Chmura A., Daszewska K., Gorczewska I., Kastelik-Hryniewiecka A., Szydło M., d’Amico A., Sokół M. Efficiency of 124I radioisotope production from natural and enriched tellurium dioxide using 124Te (p, xn) 124I reaction. EJNMMI Phys. 2022;9:41. doi: 10.1186/s40658-022-00471-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Bastian T., Coenen H.H., Qaim S.M. Excitation functions of 124Te (d, xn) 124,125 I reactions from threshold up to 14 MeV: Comparative evaluation of nuclear routes for the production of 124I. Appl. Radiat. Isot. 2001;55:303–308. doi: 10.1016/S0969-8043(01)00079-3. [DOI] [PubMed] [Google Scholar]
- 115.Payolla F.B., Massabni A.C., Orvig C. Radiopharmaceuticals for diagnosis in nuclear medicine: A short review. Eclética Química. 2019;44:11–19. [Google Scholar]
- 116.Schaffer P., Bénard F., Bernstein A., Buckley K., Celler A., Cockburn N., Corsaut J., Dodd M., Economou C., Eriksson T., et al. Direct production of 99mTc via 100Mo (p, 2n) on small medical cyclotrons. Phys. Procedia. 2015;66:383–395. doi: 10.1016/j.phpro.2015.05.048. [DOI] [Google Scholar]
- 117.Takacs S., Hermanne A., Ditroi F., Tárkányi F., Aikawa M. Reexamination of cross sections of the 100Mo (p, 2n) 99mTc reaction. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2015;347:26–38. doi: 10.1016/j.nimb.2015.01.056. [DOI] [Google Scholar]
- 118.Scholten B., Lambrecht R.M., Cogneau M., Ruiz H.V., Qaim S.M. Excitation functions for the cyclotron production of 99mTc and 99Mo. Appl. Radiat. Isot. 1999;51:69–80. doi: 10.1016/S0969-8043(98)00153-5. [DOI] [Google Scholar]
- 119.Rodrigue S., van Lier J.E., van Lier M.A.S.E. Cyclotron production of 99mTc: An approach to the medical isotope crisis. J. Nucl. Med. 2010;51:13N. [PubMed] [Google Scholar]
- 120.Hoehr C., Bénard F., Buckley K., Crawford J., Gottberg A., Hanemaayer V., Kunz P., Ladouceur K., Radchenko V., Ramogida C., et al. Medical isotope production at TRIUMF–from imaging to treatment. Phys. Procedia. 2017;90:200–208. doi: 10.1016/j.phpro.2017.09.059. [DOI] [Google Scholar]
- 121.Pillai M.R.A., Dash A., Knapp F.F.R. Sustained availability of 99mTc: Possible paths forward. J. Nucl. Med. 2013;54:313–323. doi: 10.2967/jnumed.112.110338. [DOI] [PubMed] [Google Scholar]
- 122.Lebeda O., van Lier E.J., Štursa J., Ráliš J., Zyuzin A. Assessment of radionuclidic impurities in cyclotron produced 99mTc. Nucl. Med. Biol. 2012;39:1286–1291. doi: 10.1016/j.nucmedbio.2012.06.009. [DOI] [PubMed] [Google Scholar]
- 123.Pupillo G., Esposito J., Gambaccini M., Haddad F., Michel N. Experimental cross section evaluation for innovative 99Mo production via the (α, n) reaction on 96Zr target. J. Radioanal. Nucl. Chem. 2014;302:911–917. doi: 10.1007/s10967-014-3321-9. [DOI] [Google Scholar]
- 124.Hagiwara M., Yashima H., Sanami T., Yonai S. Measurement of the excitation function of 96Zr (α, n) 99Mo for an alternative production source of medical radioisotopes. J. Radioanal. Nucl. Chem. 2018;318:569–573. doi: 10.1007/s10967-018-6118-4. [DOI] [Google Scholar]
- 125.Jacobson A.F., Deng H., Lombard J., Lessig H.J., Black R.R. 123I-meta-iodobenzylguanidine scintigraphy for the detection of neuroblastoma and pheochromocytoma: Results of a meta-analysis. J. Clin. Endocrinol. Metab. 2010;95:2596–2606. doi: 10.1210/jc.2009-2604. [DOI] [PubMed] [Google Scholar]
- 126.Eslami M., Kakavand T., Mirzaii M. Simulation of Proton beam using the MCNPX code; A prediction for the production of 123 I via 124 Xe (p, x) 123 I reaction; Proceedings of the DAE-BRNS symposium on nuclear physics; Mumbai, India. 2–6 December 2013; p. 860. [Google Scholar]
- 127.EXFOR Experimental Nuclear Reaction Data. 2011. [(accessed on 15 June 2022)]. Available online: http://www-nds.iaea.org/exfor.
- 128.Kakavand T., Sadeghi M., Kamali Moghaddam K., Shokri Bonab S., Fateh B. Computer simulation techniques to design Xenon-124 solid target for iodine-123 production. Iran. J. Radiat. Res. 2008;5:207–212. [Google Scholar]
- 129.Tárkányi F., Qaim S.M., Stöcklin G., Sajjad M., Lambrecht R.M., Schweickert H. Excitation functions of (p, 2n) and (p, pn) reactions and differential and integral yields of 123I in proton induced nuclear reactions on highly enriched 124Xe. Int. J. Radiat. Appl. Instrumentation. Part A Appl. Radiat. Isot. 1991;42:221–228. doi: 10.1016/0883-2889(91)90080-K. [DOI] [Google Scholar]
- 130.Hupf H.B., Beaver J.E., Armbruster J.M., Pendola J.P. Production of ultra-pure I-123 from the 123 Te (p, n) 123 I reaction. AIP Conf Proc. 2001;576:845–848. [Google Scholar]
- 131.Mertens J. New Development in Radio-Iodinated Radiopharmaceuticals for SPECT and Radionuclide Therapy; Proceedings of the IAEA-CN-130 International Symposium on Trends in Radiopharmaceuticals ISTR-2005; Vienna, Austria. 14–18 November 2005; New York, NY, USA: IAEA; pp. 101–103. [Google Scholar]
- 132.Scholten B., Qaim S.M., Stöcklin G. Excitation functions of proton induced nuclear reactions on natural tellurium and enriched 123Te: Production of 123I via the 123Te (p, n) 123I-process at a low-energy cyclotron. Int. J. Radiat. Appl. Instrumentation. Part A Appl. Radiat. Isot. 1989;40:127–132. doi: 10.1016/0883-2889(89)90187-1. [DOI] [Google Scholar]
- 133.Kratochwil C., Haberkorn U., Giesel F.L. Seminars in Nuclear Medicine. Volume 50. WB Saunders; Philadelphia, PA, USA: 2020. 225Ac-PSMA-617 for therapy of prostate cancer; pp. 133–140. [DOI] [PubMed] [Google Scholar]
- 134.Kratochwil C., Bruchertseifer F., Giesel F.L., Weis M., Verburg F.A., Mottaghy F., Kopka K., Apostolidis C., Haberkorn U., Morgenstern A. 225Ac-PSMA-617 for PSMA-targeted α-radiation therapy of metastatic castration-resistant prostate cancer. J. Nucl. Med. 2016;57:1941–1944. doi: 10.2967/jnumed.116.178673. [DOI] [PubMed] [Google Scholar]
- 135.Zacherl M.J., Gildehaus F.J., Mittlmeier L., Böning G., Gosewisch A., Wenter V., Unterrainer M., Schmidt-Hegemann N., Belka C., Kretschmer A. First clinical results for PSMA-targeted α-therapy using 225Ac-PSMA-I&T in advanced-mCRPC patients. J. Nucl. Med. 2021;62:669–674. doi: 10.2967/jnumed.120.251017. [DOI] [PubMed] [Google Scholar]
- 136.Królicki L., Kunikowska J., Bruchertseifer F., Koziara H., Królicki B., Jakuciński M., Pawlak D., Rola R., Morgenstern A., Rosiak E., et al. Seminars in Nuclear Medicine. Volume 50. WB Saunders; Philadelphia, PA, USA: 2020. 225Ac-and 213Bi-substance P analogues for glioma therapy; pp. 141–151. [DOI] [PubMed] [Google Scholar]
- 137.Nagatsu K., Suzuki H., Fukada M., Ito T., Ichinose J., Honda Y., Minegishi K., Higashi T., Zhang M.-R. Cyclotron production of 225Ac from an electroplated 226Ra target. Eur. J. Nucl. Med. Mol. Imaging. 2021;49:279–289. doi: 10.1007/s00259-021-05460-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Lee K.C. 225Ac production at KIRAMS; Proceedings of the IAEA Workshop on the Supply of 225Ac; Vienna, Austria. 9–10 October 2018; (unpublished) [Google Scholar]
- 139.Bruchertseifer F., Kellerbauer A., Malmbeck R., Morgenstern A. Targeted alpha therapy with bismuth-213 and actinium-225: Meeting future demand. J. Label. Compd. Radiopharm. 2019;62:794–802. doi: 10.1002/jlcr.3792. [DOI] [PubMed] [Google Scholar]
- 140.Ermolaev S., Zhuikov B., Kokhanyuk V., Matushko V., Kalmykov S.N., Aliev R.A., Tananaev I.G., Myasoedov B.F. Production of actinium, thorium and radium isotopes from natural thorium irradiated with protons up to 141 MeV. Radiochim. Acta. 2012;100:223–229. doi: 10.1524/ract.2012.1909. [DOI] [Google Scholar]
- 141.Chen D., Liu W., Huang Q., Cao S., Tian W., Yin X., Tan C., Wang J., Chu J., Jia Z., et al. Accelerator Production of the Medical Isotope 211At and Monoclonal Antibody Labeling. Acta Chim. Sin. 2021;79:1376–1384. doi: 10.6023/A21060266. [DOI] [Google Scholar]
- 142.Watabe T., Kaneda-Nakashima K., Shirakami Y., Liu Y., Ooe K., Teramoto T., Toyoshima A., Shimosegawa E., Nakano T., Kanai Y., et al. Targeted alpha therapy using astatine (211At)-labeled phenylalanine: A preclinical study in glioma bearing mice. Oncotarget. 2020;11:1388. doi: 10.18632/oncotarget.27552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Zalutsky M.R., Reardon D.A., Akabani G., Coleman R.E., Friedman A.H., Friedman H.S., McLendon R.E., Wong T.Z., Bigner D.D. Clinical experience with α-particle–emitting 211At: Treatment of recurrent brain tumor patients with 211At-labeled chimeric antitenascin monoclonal antibody 81C6. J. Nucl. Med. 2008;49:30–38. doi: 10.2967/jnumed.107.046938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Lindegren S., Albertsson P., Bäck T., Jensen H., Palm S., Aneheim E. Realizing clinical trials with astatine-211: The chemistry infrastructure. Cancer Biother. Radiopharm. 2020;35:425–436. doi: 10.1089/cbr.2019.3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Cederkrantz E., Andersson H., Bernhardt P., Bäck T., Hultborn R., Jacobsson L., Jensen H., Lindegren S., Ljungberg M., Magnander T., et al. Absorbed doses and risk estimates of 211At-MX35 F (ab’) 2 in intraperitoneal therapy of ovarian cancer patients. Int. J. Radiat. Oncol. Biol. Phys. 2015;93:569–576. doi: 10.1016/j.ijrobp.2015.07.005. [DOI] [PubMed] [Google Scholar]
- 146.Washiyama K., Oda T., Sasaki S., Aoki M., Gomez F.L.G., Taniguchi M., Nishijima K.-i., Takahashi K. At-211 production using the CYPRIS MP-30. J. Med. Imaging Radiat. Sci. 2019;50:S42. doi: 10.1016/j.jmir.2019.03.128. [DOI] [Google Scholar]
- 147.Alfarano A., Abbas K., Holzwarth U., Bonardi M., Groppi F., Alfassi Z., Menapace E., Gibson P. Journal of Physics: Conference Series. Volume 41. IOP Publishing; Bristol, UK: 2006. Thick target yield measurement of 211At through the nuclear reaction 209Bi (α, 2n) p. 009. [Google Scholar]
- 148.Feng Y., Zalutsky M.R. Production, purification and availability of 211At: Near term steps towards global access. Nucl. Med. Biol. 2021;100:12–23. doi: 10.1016/j.nucmedbio.2021.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Guérard F., Gestin J.F., Brechbiel M.W. Production of [211At]-astatinated radiopharmaceuticals and applications in targeted α-particle therapy. Cancer Biother. Radiopharm. 2013;28:1–20. doi: 10.1089/cbr.2012.1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Cullinane C., Jeffery C.M., Roselt P.D., van Dam E.M., Jackson S., Kuan K., Jackson P., Binns D., van Zuylekom J., Harris M., et al. Peptide receptor radionuclide therapy with 67Cu-CuSarTATE is highly efficacious against a somatostatin-positive neuroendocrine tumor model. J. Nucl. Med. 2020;61:1800–1805. doi: 10.2967/jnumed.120.243543. [DOI] [PubMed] [Google Scholar]
- 151.DeNardo S.J., DeNardo G.L., Kukis D.L., Shen S., Kroger L.A., DeNardo D.A., Goldstein D.S., Mirick G.R., Salako Q., Mausner L.F., et al. 67Cu-21T-BAT-Lym-1 pharmacokinetics, radiation dosimetry, toxicity and tumor regression in patients with lymphoma. J. Nucl. Med. 1999;40:302–310. [PubMed] [Google Scholar]
- 152.Pupillo G., Sounalet T., Michel N., Mou L., Esposito J., Haddad F. New production cross sections for the theranostic radionuclide 67Cu. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2018;415:41–47. doi: 10.1016/j.nimb.2017.10.022. [DOI] [Google Scholar]
- 153.Katabuchi T., Watanabe S., Ishioka N.S., Iida Y., Hanaoka H., Endo K., Matsuhashi S. Production of 67Cu via the 68Zn (p, 2p) 67Cu reaction and recovery of 68Zn target. J. Radioanal. Nucl. Chem. 2008;277:467–470. doi: 10.1007/s10967-007-7144-9. [DOI] [Google Scholar]
- 154.Mou L., Martini P., Pupillo G., Cieszykowska I., Cutler C.S., Mikołajczak R. 67Cu production capabilities: A mini review. Molecules. 2022;27:1501. doi: 10.3390/molecules27051501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Hovhannisyan G.H., Stepanyan A.V., Saryan E.R., Amirakyan L.A. Methods of Production the Isotope 67Cu. J. Contemp. Phys. (Armen. Acad. Sci.) 2020;55:183–190. doi: 10.3103/S106833722003010X. [DOI] [Google Scholar]
- 156.Kin T., Nagai Y., Iwamoto N., Minato F., Iwamoto O., Hatsukawa Y., Segawa M., Harada H., Konno C., Ochiai K., et al. New production routes for medical isotopes 64Cu and 67Cu using accelerator neutrons. J. Phys. Soc. Jpn. 2013;82:034201. doi: 10.7566/JPSJ.82.034201. [DOI] [Google Scholar]
- 157.Szelecsényi F., Steyn G.F., Dolley S.G., Kovács Z., Vermeulen C., Van der Walt T.N. Investigation of the 68Zn (p, 2p) 67Cu nuclear reaction: New measurements up to 40 MeV and compilation up to 100 MeV. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2009;267:1877–1881. doi: 10.1016/j.nimb.2009.03.097. [DOI] [Google Scholar]
- 158.Pandey M.K., DeGrado T.R. Cyclotron production of PET radiometals in liquid targets: Aspects and prospects. Curr. Radiopharm. 2021;14:325–339. doi: 10.2174/1874471013999200820165734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Deng X., Rong J., Wang L., Vasdev N., Zhang L., Josephson L., Liang S.H. Chemistry for positron emission tomography: Recent advances in 11C-, 18F-, 13N-, and 15O-labeling reactions. Angew. Chem. Int. Ed. 2019;58:2580–2605. doi: 10.1002/anie.201805501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.McQuade P., Rowland D.J., Lewis J.S., Welch M.J. Positron-emitting isotopes produced on biomedical cyclotrons. Curr. Med. Chem. 2005;12:807–818. doi: 10.2174/0929867053507397. [DOI] [PubMed] [Google Scholar]
- 161.Schmitz J. The production of [124I] iodine and [86Y] yttrium. Eur. J. Nucl. Med. Mol. Imaging. 2011;38:4–9. doi: 10.1007/s00259-011-1782-4. [DOI] [PubMed] [Google Scholar]
- 162.van der Meulen N.P., Bunka M., Domnanich K.A., Müller C., Haller S., Vermeulen C., Türler A., Schibli R. Cyclotron production of 44Sc: From bench to bedside. Nucl. Med. Biol. 2015;42:745–751. doi: 10.1016/j.nucmedbio.2015.05.005. [DOI] [PubMed] [Google Scholar]
- 163.van der Meulen N.P., Hasler R., Talip Z., Grundler P.V., Favaretto C., Umbricht C.A., Müller C., Dellepiane G., Carzaniga T.S., Braccini S. Developments toward the implementation of 44Sc production at a medical cyclotron. Molecules. 2020;25:4706. doi: 10.3390/molecules25204706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Lagunas-Solar M.C., Jungerman J.A., Paulson D.W. Cyclotron production of Thallium-201 via the 205 Tl (p, 5n) 201 Pb→ 201 Tl reaction; Proceedings of the International Symposium on Radiopharmaceuticals; Seattle, WA, USA. 18–23 March 1979; New York, NY, USA: Society of Nuclear Medicine; pp. 779–789. [Google Scholar]
- 165.Misiak R., Walczak R., Wąs B., Bartyzel M., Mietelski J.W., Bilewicz A. 47Sc production development by cyclotron irradiation of 48Ca. J. Radioanal. Nucl. Chem. 2017;313:429–434. doi: 10.1007/s10967-017-5321-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Abel E.P., Domnanich K., Clause H.K., Kalman C., Walker W., Shusterman J.A., Greene J., Gott M., Severin G.W. Production, collection, and purification of 47Ca for the generation of 47Sc through isotope harvesting at the national superconducting cyclotron laboratory. ACS Omega. 2020;5:27864–27872. doi: 10.1021/acsomega.0c03020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Lawrence J.H. Nuclear physics and therapy: Preliminary report on a new method for the treatment of leukemia and polycythemia. Radiology. 1940;35:51–60. doi: 10.1148/35.1.51. [DOI] [Google Scholar]
- 168.Hupf H.B., Beaver J.E. Cyclotron production of carrier-free gallium-67. Int. J. Appl. Radiat. Isot. 1970;21:75–76. doi: 10.1016/0020-708X(70)90014-1. [DOI] [PubMed] [Google Scholar]
- 169.do Carmo S.J.C., Scott P.J.H., Alves F. Production of radiometals in liquid targets. EJNMMI Radiopharm. Chem. 2020;5:2. doi: 10.1186/s41181-019-0088-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Skliarova H., Cisternino S., Cicoria G., Marengo M., Cazzola E., Gorgoni G., Palmieri V. Medical Cyclotron Solid Target Preparation by Ultrathick Film Magnetron Sputtering Deposition. Instruments. 2019;3:21. doi: 10.3390/instruments3010021. [DOI] [Google Scholar]
- 171.McNeil B.L., Robertson A.K., Fu W., Yang H., Hoehr C., Ramogida C.F., Schaffer P. Production, purification, and radiolabeling of the 203Pb/212Pb theranostic pair. EJNMMI Radiopharm. Chem. 2021;6:6. doi: 10.1186/s41181-021-00121-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Gracheva N., Carzaniga T.S., Schibli R., Braccini S., van der Meulen N.P. 165Er: A new candidate for Auger electron therapy and its possible cyclotron production from natural holmium targets. Appl. Radiat. Isot. 2020;159:109079. doi: 10.1016/j.apradiso.2020.109079. [DOI] [PubMed] [Google Scholar]
- 173.Nelson B.J.B., Wilson J., Andersson J.D., Wuest F. High yield cyclotron production of a novel 133/135La theranostic pair for nuclear medicine. Sci. Rep. 2020;10:22203. doi: 10.1038/s41598-020-79198-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Dey M.K., Gupta A.D., Chakrabarti A. Design of ultra-light superconducting proton cyclotron for production of isotopes for medical applications. Proc. Cyclotr. 2013;2013:447–450. [Google Scholar]
- 175.Waites L.H., Alonso J.R., Conrad J. IsoDAR: A cyclotron-based neutrino source with applications to medical isotope production. AIP Conf. Proc. 2019;2160:040001. [Google Scholar]
- 176.Waites L.H., Alonso J., Conrad J.M., Koser D., Winklehner D. Tools for the Development and Applications of the IsoDAR Cyclotron. Energy (MeV/Nucl.) 2021;60:30. [Google Scholar]
- 177.Pramudita A. Proceeding on the scientific meeting and presentation on accelerator technology and its applications: Physics, nuclear reactor, Yogyakarta, Indonesia, 13 December 2011. Volume 47. National Nuclear Energy Agency; Tangerang, Jawa Barat, Indonesia: 2012. Linacs for medical isotope production; pp. 11–16. [Google Scholar]
- 178.Griswold J.R., Medvedev D.G., Engle J.W., Copping R., Fitzsimmons J., Radchenko V., Cooley J., Fassbender M., Denton D., Murphy K., et al. Large scale accelerator production of 225Ac: Effective cross sections for 78–192 MeV protons incident on 232Th targets. Appl. Radiat. Isot. 2016;118:366–374. doi: 10.1016/j.apradiso.2016.09.026. [DOI] [PubMed] [Google Scholar]
- 179.Zhuikov B.L., Ermolaev S.V. Radioisotope research and development at the Linear Accelerator of the Institute for Nuclear Research of RAS. Phys.-Uspekhi. 2021;64:1311. doi: 10.3367/UFNe.2021.07.039010. [DOI] [Google Scholar]
- 180.Antipov K., Ayzatsky M., Akchurin Y.I., Boriskin V., Beloglasov V., Biller E., Demidov N., Dikiy N., Dovbnya A., Dovbush L., et al. Electron linacs in NSC KIPT: R&D and application. Вoпрoсы Атoмнoй Науки И Техники. 2001;37:40–47. [Google Scholar]
- 181.Danagulyan A.S., Hovhannisyan G.H., Bakhshiyan T.M., Avagyan R.H., Avetisyan A.E., Kerobyan I.A., Dallakyan R.K. Formation of medical radioisotopes 111In, 117m Sn, 124Sb, and 177Lu in photonuclear reactions. Phys. At. Nucl. 2015;78:447–452. doi: 10.1134/S1063778815030035. [DOI] [Google Scholar]
- 182.Courtney W., Sowder K., McGyver M., Stevenson N., Brown D. The challenges of commercial isotope production on a linear accelerator. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2007;261:739–741. doi: 10.1016/j.nimb.2007.04.209. [DOI] [Google Scholar]
- 183.Matthews M., Saey P., Bowyer T., Vandergrift G., Cutler N.R.C., Ponsard B., Mikolajczak R., Tsipenyuk Y., Solin L., Fisher D., et al. Workshop on Signatures of Medical and Industrial Isotope Production: A Review. Pacific Northwest National Laboratory; Richland, Washington, USA: 2010. [Google Scholar]
- 184.Dikiy N.P., Dovbnya A.N., Medvedyeva E.P., Tur Y.D. Experience of Technetium-99m Generation for Nuclear Medicine on Electron Linac. VANT; Kharkov, Ukraine: 1997. pp. 165–167. [Google Scholar]
- 185.Uvarov V.L., Dikiy N.P., Dovbnya A.N., Medvedyeva Y.P., Pugachov G.D., Tur Y.D. Electron Accelerator’s Based Production of Technetium-99m for Nuclear Medicine. Bull. Amer. Phys. Soc. 1997;42:1338. [Google Scholar]
- 186.De Jong M. Producing medical isotopes using X-rays. Sin Proc. IPAC. 2012;12:3177. [Google Scholar]
- 187.Chemerisov S., Bailey J., Heltemes T., Jonah C., Makarashvili V., Tkac P., Rotsch D., Virgo M., Vandegrift G. Results of the Six-and-a-Half Day Electron-Accelerator Irradiation of Enriched Mo-100 Targets for the Production of Mo-99. Argonne National Lab.(ANL); Argonne, IL, USA: 2016. [Google Scholar]
- 188.Takeda T., Fujiwara M., Kurosawa M., Takahashi N., Tamura M., Kawabata T., Fujikawa Y., Suzuki K.N., Abe N., Kubota T., et al. 99mTc production via the (γ, n) reaction on natural Mo. J. Radioanal. Nucl. Chem. 2018;318:811–821. doi: 10.1007/s10967-018-6078-8. [DOI] [Google Scholar]
- 189.Szpunar B., Rangacharyulu C., Date S., Ejiri H. Estimate of production of medical isotopes by photo-neutron reaction at the Canadian light source. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2013;729:41–50. doi: 10.1016/j.nima.2013.06.106. [DOI] [Google Scholar]
- 190.Babcock C., Goodacre T.D., Amani P., Au M., Bricault P., Brownell M., Cade B., Chen K., Egoriti L., Johnson J., et al. Offline target and ion source studies for TRIUMF-ARIEL. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2020;463:464–467. doi: 10.1016/j.nimb.2019.04.023. [DOI] [Google Scholar]
- 191.Danon Y., Block R.C., Testa R., Testa R., Moore H. Medical isotope production using a 60 MeV linear electron accelerator. Trans.-Am. Nucl. Soc. 2008;98:894. [Google Scholar]
- 192.Yagi M., Kondo K. Preparation of carrier-free 67Cu by the 68Zn (γ, p) reaction. Int. J. Appl. Radiat. Isot. 1978;29:757–759. doi: 10.1016/0020-708X(78)90127-8. [DOI] [Google Scholar]
- 193.Hovhannisyan G.H., Bakhshiyan T.M., Dallakyan R.K. Photonuclear production of the medical isotope 67Cu. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2021;498:48–51. doi: 10.1016/j.nimb.2021.04.016. [DOI] [Google Scholar]
- 194.Gopalakrishna A., Suryanarayana S., Naik H., Nayak B., Patil B., Devraju S., Upreti R., Kinhikar R., Deshpande D., Maletha P., et al. Production of 99Mo and 64Cu in a mixed field of photons and neutrons in a clinical electron linear accelerator. J. Radioanal. Nucl. Chem. 2018;317:1409–1417. doi: 10.1007/s10967-018-6016-9. [DOI] [Google Scholar]
- 195.Maslov O.D., Sabel’nikov A.V., Dmitriev S.N. Preparation of 225Ac by 226Ra (γ, n) photonuclear reaction on an electron accelerator, MT-25 microtron. Radiochemistry. 2006;48:195–197. doi: 10.1134/S1066362206020184. [DOI] [Google Scholar]
- 196.Robertson A.K.H., Ramogida C.F., Schaffer P., Radchenko V. Development of 225Ac radiopharmaceuticals: TRIUMF perspectives and experiences. Curr. Radiopharm. 2018;11:156–172. doi: 10.2174/1874471011666180416161908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Inagaki M., Sekimoto S., Tanaka W., Tadokoro T., Ueno Y., Kani Y., Ohtsuki T. Production of 47Sc, 67Cu, 68Ga, 105Rh, 177Lu, and 188Re using electron linear accelerator. J. Radioanal. Nucl. Chem. 2019;322:1703–1709. doi: 10.1007/s10967-019-06904-z. [DOI] [Google Scholar]
- 198.Radel R., Sengbusch E., Piefer G. Recent Progress on the PNL Accelerator-Based Intense Fusion Neutron Source. Trans. Am. Nucl. Soc. 2016;114:11–12. [Google Scholar]
- 199.Rotsch D.A., Brown M.A., Nolen J.A., Brossard T., Henning W.F., Chemerisov S.D., Gromov R.G., Greene J. Electron linear accelerator production and purification of scandium-47 from titanium dioxide targets. Appl. Radiat. Isot. 2018;131:77–82. doi: 10.1016/j.apradiso.2017.11.007. [DOI] [PubMed] [Google Scholar]
- 200.Melville G., Allen B.J. Cyclotron and linac production of Ac-225. Appl. Radiat. Isot. 2009;67:549–555. doi: 10.1016/j.apradiso.2008.11.012. [DOI] [PubMed] [Google Scholar]
- 201.Deshpande A., Dixit T., Bhat S., Jadhav P., Kottawar A., Krishnan R., Thakur K., Vidwans M., Waingankar A. Design of High Energy Linac for Generation of Isotopes for Medical Applications; Proceedings of the IPAC 2021-12th International Particle Accelerator Conference; Campinas, SP, Brazi. 24-28 May 2021; Geneva, Switzerland: JACoW Publishing; 2021. pp. 2472–2474. [Google Scholar]
- 202.Leung K.N. New compact neutron generator system for multiple applications. Nucl. Technol. 2020;206:1607–1614. doi: 10.1080/00295450.2020.1719800. [DOI] [Google Scholar]
- 203.Kononov V.N., Bokhovko M.V., Kononov O.E., Soloviev N.A., Chu W.T., Nigg D. Accelerator-based fast neutron sources for neutron therapy. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2006;564:525–531. doi: 10.1016/j.nima.2006.03.043. [DOI] [Google Scholar]
- 204.Cloth P., Conrads H. Neutronics of a dense-plasma focus—An investigation of a fusion plasma. Nucl. Sci. Eng. 1977;62:591–600. doi: 10.13182/NSE77-A15203. [DOI] [Google Scholar]
- 205.Csikai J., Dóczi R. Applications of neutron generators. Handb. Nucl. Chem. 2011;3:363. [Google Scholar]
- 206.Reijonen J. Compact neutron generators for medical, home land security, and planetary exploration; Proceedings of the 2005 Particle Accelerator Conference; Knoxville, TN, USA. 16–20 May 2005; pp. 49–53. [Google Scholar]
- 207.Dovbnya A.N., Kuplennikov E.L., Tsymba V.A., Krasil’nikov V.V. Possibility of 99mT c production at neutron generator. Вoпрoсы Атoмнoй Науки И Техники. 2009;5:64–66. [Google Scholar]
- 208.Pagdon K., Gentile C., Cohen A., Ascione G., Baker G. Production of Tc-99m from naturally occurring molybdenum absent uranium; Proceedings of the 2011 IEEE/NPSS 24th Symposium on Fusion Engineering; Chicago, IL, USA. 26–30 June 2011; pp. 1–4. [Google Scholar]
- 209.Mausolf E.J., Johnstone E.V., Mayordomo N., Williams D.L., Guan E.Y.Z., Gary C.K. Fusion-Based Neutron Generator Production of Tc-99m and Tc-101: A Prospective Avenue to Technetium Theranostics. Pharmaceuticals. 2021;14:875. doi: 10.3390/ph14090875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.National Academies of Sciences, Engineering, and Medicine . Molybdenum-99 for Medical Imaging. National Academies Press; Washington, DC, USA: 2016. [PubMed] [Google Scholar]
- 211.Youker A.J., Chemerisov S.D., Tkac P., Kalensky M., Heltemes T.A., Rotsch D.A., Vandegrift G.F., Krebs J.F., Makarashvili V., Stepinski D.C. Fission-produced Mo-99 without a nuclear reactor. J. Nucl. Med. 2017;58:514–517. doi: 10.2967/jnumed.116.181040. [DOI] [PubMed] [Google Scholar]
- 212.Leung K.N., Leung J.K., Melville G. Feasibility study on medical isotope production using a compact neutron generator. Appl. Radiat. Isot. 2018;137:23–27. doi: 10.1016/j.apradiso.2018.02.026. [DOI] [PubMed] [Google Scholar]
- 213.Badwar S., Ghosh R., Lawriniang B.M., Vansola V., Sheela Y., Naik H., Naik Y., Suryanarayana S.V., Jyrwa B., Ganesan S. Measurement of formation cross-section of 99Mo from the 98Mo (n, γ) and 100Mo (n, 2n) reactions. Appl. Radiat. Isot. 2017;129:117–123. doi: 10.1016/j.apradiso.2017.08.019. [DOI] [PubMed] [Google Scholar]
- 214.Auditore L., Amato E., Baldari S. Theoretical estimation of 64Cu production with neutrons emitted during 18F production with a 30 MeV medical cyclotron. Appl. Radiat. Isot. 2017;122:229–234. doi: 10.1016/j.apradiso.2017.02.002. [DOI] [PubMed] [Google Scholar]
- 215.Pandit-Taskar N., Batraki M., Divgi C.R. Radiopharmaceutical therapy for palliation of bone pain from osseous metastases. J. Nucl. Med. 2004;45:1358–1365. [PubMed] [Google Scholar]
- 216.Kim S.K., Choi H.D. New technique for Producing Therapeutic Radioisotope 89Sr; Proceedings of the Korean Nuclear Society Conference; jeju, Korea. 26–26 May 2005; Seoul, Korea: Korean Nuclear Society; pp. 751–752. [Google Scholar]
- 217.Molla N.I., Basunia S., Miah M.R., Hossain S.M., Rahman M.M., Spellerberg S., Qaim S.M. Radiochemical Study of 45Sc (n, p) 45Ca and 89Y (n, p) 89Sr Reactions in the Neutron Energy Range of 13.9 to 14.7 MeV. Radiochim. Acta. 1998;80:189–192. doi: 10.1524/ract.1998.80.4.189. [DOI] [Google Scholar]
- 218.Capogni M., Capone M., Pietropaolo A., Fazio A., Dellepiane G., Falconi R., Colangeli A., Palomba S., Valentini G., Fantuzi M., et al. 64Cu production by 14 MeV neutron beam. J. Neutron Res. 2020;22:257–264. doi: 10.3233/JNR-190140. [DOI] [Google Scholar]
- 219.Voyles A., Basunia M., Batchelder J., Bauer J., Becker T., Bernstein L., Matthews E., Renne P., Rutte D., Unzueta M., et al. Measurement of the 64Zn, 47Ti (n, p) cross sections using a DD neutron generator for medical isotope studies. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2017;410:230–239. doi: 10.1016/j.nimb.2017.08.021. [DOI] [Google Scholar]
- 220.Mellard S.C., Biegalski S.R. MCNP based simulations for the optimization of radioxenon via DD and DT neutron generators from 132Xe. J. Radioanal. Nucl. Chem. 2018;318:313–322. doi: 10.1007/s10967-018-6112-x. [DOI] [Google Scholar]
- 221.Weicheng Z. Short-lived medical isotopes produced by 14MeV neutron generator. . At. Energy Sci. Technol. 1981;03:366–368. (In Chinese) [Google Scholar]
- 222.Yuntao L., Shizhong A., Jixin L. Current Situation and Development Trend of Nuclear Technology Application. Sci. Technol. Rev. 2022;40:88–97. (In Chinese) [Google Scholar]
- 223.Huang Z., Wang J., Ma Z., Lu X., Wei Z., Zhang S., Liu Y., Zhang Z., Zhang Y., Yao Z. Design of a compact D–D neutron generator. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2018;904:107–112. doi: 10.1016/j.nima.2018.07.005. [DOI] [Google Scholar]
- 224.Jixin L., Yuqing C., Guang L., Xuesong D., Yijia S., Laicheng Q., Yuping L., Hua J., Guiqun L. Annual Report for China Institute of Atomic Energy. China Institute of Atomic Energy; Beijing, China: 2013. Production process of 64Cu by C-30 cyclotron. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.