Abstract
The Escherichia coli Ada protein activates ς70-dependent transcription at three different promoters (ada, aidB, and alkA) in response to alkylation damage of DNA. During stationary phase, however, the methylated form of Ada shuts off expression of alkA; this repression is specific for ςS-dependent transcription. Thus, at the alkA promoter, the Ada protein can act as both a positive and negative modulator of the adaptive response to alkylation damage, depending on the cell’s physiological state.
The adaptive response of Escherichia coli to alkylating damage of DNA is regulated by the Ada protein. This response protects cells from the mutagenic and cytotoxic effects of alkylating agents present in the environment and from reactive endogenous metabolites (9, 12, 13). The Ada protein is both a DNA repair protein and a transcriptional regulator. Ada is able to transfer methyl groups from DNA to two of its own cysteine residues (10). Upon self-methylation, Ada stimulates transcription of four genes, ada, aidB, alkA, and alkB (whose transcription is directed by the ada promoter) (10, 15). The target site for Ada activation on RNA polymerase is the ς subunit. However, the determinants in both the Ada protein and RNA polymerase that are required for transcription activation at the ada and aidB promoters differ from the determinants required at alkA. A negatively charged set of amino acids in ς70 is the target for Ada activation at ada and aidB, while a positively charged cluster interacts with Ada at the alkA promoter (6, 7). At the ada and aidB promoters, the methylated form of Ada (meAda) is essential for transcription activation, whereas transcription from the alkA promoter is activated by both the unmethylated and methylated forms (4, 10). Finally, the C-terminal domain of Ada, which is necessary for activation at the ada and aidB promoters, is dispensable at alkA (1). Thus, the Ada protein activates different promoters by different mechanisms.
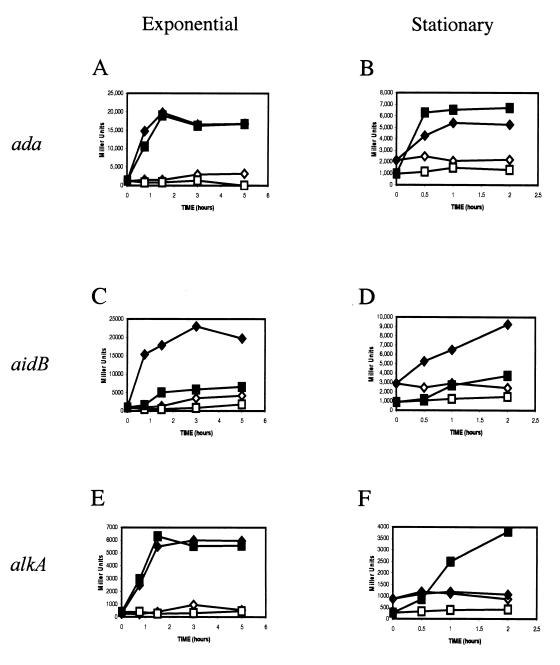
Previous work on Ada-dependent regulation had focused on exponentially growing cells in which ς70 is the major functional ς factor. We have now investigated the regulation of Ada-dependent promoters in stationary-phase cells. During stationary phase, the levels of an alternative ς factor, ςS (8), rise to about 30 to 40% of ς70 levels. In addition, several changes in the cellular environments, such as increased concentrations of glutamate and polyphosphate, a decrease of DNA superhelicity, and increased levels of Rsd, an anti-ς factor specific for ς70, contribute to preferential utilization of ςS (3). Previous in vitro studies had shown that meAda can activate ςS-dependent transcription at ada and aidB but not at alkA (5, 7, 13). To measure Ada-activated, ςS-dependent transcription in vivo, we used either an rpoS-deficient strain (MV2792 [16]) or the corresponding wild type (MV1161) transformed with derivatives of the low-copy-number plasmid pRS1274 carrying the ada, aidB, or alkA promoter regions as previously described (4, 7). The rpoS mutation does not cause detectable variations in pRS1274 copy number in stationary phase nor does it affect the in vivo expression of the Ada-independent lacUV5 promoter (data not shown). Cells were grown in LB medium, and 0.04% of the alkylating agent methyl methanesulfonate (MMS) was added at an optical density at 600 nm (OD600) of 0.04 (to study exponential phase) or 1.0 (to study stationary phase) (Fig. 1). In exponential phase, MMS clearly induces expression from the ada, aidB, and alkA promoters (Fig. 1A, C, and E). Expression from the ada and alkA promoters was not affected by the rpoS mutation, while aidB expression was reduced, consistent with previous observations (5, 16). It is important to note that, when added to exponentially growing cells, 0.04% MMS result in only a slight reduction in the growth rate (to about 75%), which is insufficient to cause a sharp increase in rpoS expression. However, a relatively small increase of ςS might be sufficient for full activation of aidB transcription, due to the strong affinity of RNA polymerase containing ςS (EςS) for the aidB promoter (5).
FIG. 1.
In vivo expression of the adaptive response genes. (A and B) ada; (C and D) aidB; (E and F) alkA. (A, C, and E) cultures in exponential phase at time 0 (OD600, 0.04); (B, D, and F) cultures in stationary phase at time 0 (OD600, 1.0). Symbols: diamonds, wild-type strain; squares, rpoS strain; open symbols, no MMS added (Ada-independent transcription); closed symbols, 0.04% MMS added at time zero. Data shown are from a typical experiment.
A more complex pattern emerged when cells were treated with MMS at the onset of stationary phase. In the absence of MMS induction, levels of Ada-independent expression were two- to threefold higher in the wild type than in rpoS strains. This effect is independent of the presence of a functional ada gene (data not shown). These results are in agreement with previous observations that EςS is able to carry out transcription from the ada and aidB promoters even in the absence of meAda (5, 13) and suggests a similar situation for alkA. MMS treatment had very different effects on transcription at different promoters. With the aidB promoter, clear induction that is strongly dependent on a functional rpoS gene is observed, mirroring the behavior observed in exponential phase. With the ada promoter, induction was found in both the wild-type and rpoS strains, again similar to the situation in exponential phase. In sharp contrast, treatment with MMS failed to induce expression of alkA in the wild-type strain, while in the rpoS strain alkA expression was increased by ca. 7.5-fold (Fig. 1F).
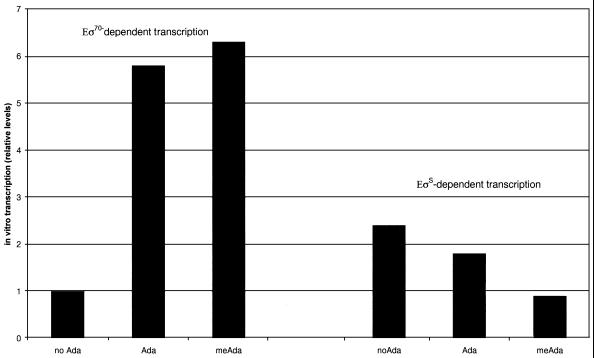
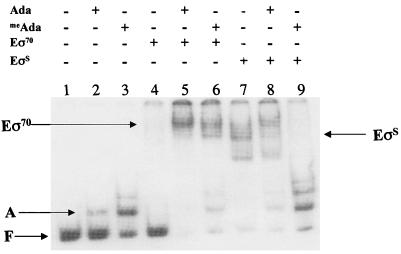
The negative effect of the rpoS gene on Ada-dependent transcription of alkA was surprising. Although the Ada protein is known to be unable to stimulate EςS-dependent transcription from the alkA promoter in vitro (7), we expected some induction in vivo, since Eς70 is also available. We performed in vitro transcription experiments to compare Eς70 and EςS, using the conditions described previously (7). The results summarized in Fig. 2 show that EςS is more efficient than Eς70 in carrying out transcription from the alkA promoter in the absence of Ada and that the methylated form of the Ada protein represses EςS-dependent transcription approximately threefold. Confirmation that meAda acts as a specific repressor for EςS came from gel retardation experiments with Eς70 and EςS forms of RNA polymerase. The results in Fig. 3 show that no interaction between Eς70 and the alkA promoter was detected in the absence of Ada (lane 4). In contrast, incubation with EςS resulted in an almost complete retardation of the promoter fragment (Fig. 3, lane 7); several bands are detectable, possibly a consequence of partial dissociation of the binary complex during electrophoresis or formation of higher orders of EςS-alkA complexes. Addition of either form of the Ada protein stimulated binding of Eς70 to alkA (Fig. 3, lanes 5 and 6). In contrast, addition of meAda, but not of the unmethylated form of the protein, resulted in the dissociation of the EςS-alkA binary complex (Fig. 3, lanes 8 and 9).
FIG. 2.
In vitro transcription with reconstituted RNA polymerases (50 nM). Experimental conditions were as described previously (5). Values are relative to the ratio of alkA/lacUV5 transcripts for Eς70. When necessary, the Ada protein was present at 0.2 μM. Data are an average from the results of three experiments. Standard deviation was less than 15%.
FIG. 3.
Gel retardation assays performed with reconstituted RNA polymerases. Conditions were as described previously (5) except for heparin, which was omitted from the reactions. Lanes: 1 to 3, no RNA polymerase; 4 to 6, Eς70; 7 to 9, EςS; 1, 4, and 7, no Ada protein; 2, 5, and 8, 0.2 μM unmethylated Ada; 3, 6, and 9, 0.2 μM methylated Ada protein. F indicates the unbound alkA promoter DNA; A indicates the (me)Ada-alkA complex; Eς70 and EςS indicate the different RNA polymerase-alkA complexes.
Our previous study (7) showed that the activation of the alkA promoter is dependent on the interaction between Ada and a positively charged cluster in ς70. The fact that this cluster is not conserved in ςS neatly explains why Ada is unable to activate transcription by EςS at the alkA promoter. Our present results show that meAda negatively regulates EςS-dependent transcription of alkA, and this mechanism is used by E. coli to down-regulate alkA. Since methylation of Ada is irreversible, cells have the problem of shutting off the adaptive response once alkylation damage has been removed. Two different mechanisms have already been proposed: proteolytic cleavage of meAda in the hinge region linking the N-terminal to the C-terminal domain of the protein (1), and negative regulation of the ada gene by the unmethylated form of the Ada protein (11). However, since both the N-terminal fragment of meAda and the unmethylated form of the protein can activate transcription at alkA, it was postulated that the simple dilution of the Ada protein over several growth cycles must eventually result in the return to basal levels of expression from alkA (1, 11). The function of meAda as a negative regulator of EςS-dependent transcription suggests an alternative mechanism: alkA expression is activated as long as transcription is mostly ς70 dependent. When cells reach stationary phase, concentrations of EςS increase. EςS can bind and carry out transcription from the alkA promoter (Fig. 2 and 3); however, meAda prevents EςS from binding to alkA (Fig. 3), thus reducing the amount of RNA polymerase available for alkA transcription. This results in low levels of alkA transcription in stationary phase even upon MMS treatment. Negative regulation by a functional rpoS gene has been observed for another ς70-dependent gene, uspA (2), suggesting that ς factors might indeed compete for a limiting amount of RNA polymerase during stationary phase. The need to express alkA only in the exponential phase of growth might be due to the role of the AlkA protein, whose main function is to remove 3-methyladenine from DNA. Methylation of this base is toxic because of its effects on DNA replication (9). During stationary phase, in the absence of DNA replication, the presence in E. coli of a second 3-methyladenine DNA glycosylase, encoded by the constitutively expressed tag gene (14), might be sufficient for the repair of this lesion, making high-level expression of the alkA gene no longer necessary.
Acknowledgments
We thank Mike Volkert for the gift of strains MV1161 and MV2792 and Annette Keith for technical assistance.
Work in Birmingham, United Kingdom, was supported by a TMR Fellowship from the European Union to P.L.
REFERENCES
- 1.Akimaru H, Sakumi K, Yoshikai T, Anai M, Sekiguchi M. Positive and negative regulation of transcription by a cleavage product of Ada protein. J Mol Biol. 1990;216:261–273. doi: 10.1016/S0022-2836(05)80318-3. [DOI] [PubMed] [Google Scholar]
- 2.Farewell A, Kvint K, Nyström T. Negative regulation by RpoS: a case of sigma factor competition. Mol Microbiol. 1998;29:1039–1051. doi: 10.1046/j.1365-2958.1998.00990.x. [DOI] [PubMed] [Google Scholar]
- 3.Jishage M, Ishihama A. A stationary phase protein in Escherichia coli with binding activity to the major ς subunit of RNA polymerase. Proc Natl Acad Sci USA. 1998;95:4953–4958. doi: 10.1073/pnas.95.9.4953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Landini P, Volkert M R. Transcriptional activation of the Escherichia coli adaptive response gene aidB is mediated by binding of methylated Ada protein. Evidence for a new consensus sequence for Ada-binding sites. J Biol Chem. 1995;270:8285–8289. doi: 10.1074/jbc.270.14.8285. [DOI] [PubMed] [Google Scholar]
- 5.Landini P, Hajec L I, Nguyen L H, Burgess R R, Volkert M R. The leucine-responsive regulatory protein (Lrp) acts as a specific repressor for ςS-dependent transcription of the Escherichia coli aidB gene. Mol Microbiol. 1996;20:947–955. doi: 10.1111/j.1365-2958.1996.tb02536.x. [DOI] [PubMed] [Google Scholar]
- 6.Landini P, Brown J A, Volkert M R, Busby S J W. Ada protein-RNA polymerase ς subunit interaction and α subunit-promoter DNA interaction are necessary at different steps in transcription initiation at the Escherichia coli ada and aidB promoters. J Biol Chem. 1998;273:13307–13312. doi: 10.1074/jbc.273.21.13307. [DOI] [PubMed] [Google Scholar]
- 7.Landini P, Busby S J W. The Escherichia coli Ada protein can interact with two distinct determinants in the ς70 subunit of RNA polymerase according to promoter architecture: identification of the target of Ada activation at the alkA promoter. J Bacteriol. 1999;181:1524–1529. doi: 10.1128/jb.181.5.1524-1529.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lange R, Hengge-Aronis R. Identification of a central regulator of stationary-phase gene expression in Escherichia coli. Mol Microbiol. 1991;5:49–59. doi: 10.1111/j.1365-2958.1991.tb01825.x. [DOI] [PubMed] [Google Scholar]
- 9.Lindahl T, Sedgwick B, Sekiguchi M, Nakabeppu Y. Regulation of the adaptive response to alkylating agents. Annu Rev Biochem. 1988;57:133–157. doi: 10.1146/annurev.bi.57.070188.001025. [DOI] [PubMed] [Google Scholar]
- 10.Nakabeppu Y, Sekiguchi M. Regulatory mechanisms for induction of synthesis of repair enzymes in response to alkylating agents: Ada acts as a transcriptional regulator. Proc Natl Acad Sci USA. 1986;83:6297–6301. doi: 10.1073/pnas.83.17.6297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saget B M, Walker G C. The Ada protein acts as both a positive and negative modulator of Escherichia coli’s response to methylating agents. Proc Natl Acad Sci USA. 1994;91:9730–9734. doi: 10.1073/pnas.91.21.9730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Samson L, Cairns J. A new pathway for DNA repair in Escherichia coli. Nature (London) 1977;267:281–283. doi: 10.1038/267281a0. [DOI] [PubMed] [Google Scholar]
- 13.Taverna P, Sedgwick B. Generation of an endogenous DNA-methylating agent by nitrosation in Escherichia coli. J Bacteriol. 1996;178:5105–5111. doi: 10.1128/jb.178.17.5105-5111.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Volkert M R. Adaptive response of Escherichia coli to alkylation damage. Environ Mol Mutagen. 1988;11:241–255. doi: 10.1002/em.2850110210. [DOI] [PubMed] [Google Scholar]
- 15.Volkert M R, Nguyen D C. Induction of specific Escherichia coli genes by sublethal treatments with alkylating agents. Proc Natl Acad Sci USA. 1984;81:4110–4114. doi: 10.1073/pnas.81.13.4110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Volkert M R, Hajec L I, Matijasevic Z, Fang F C, Prince R. Induction of the Escherichia coli aidB gene under oxygen-limiting conditions requires a functional rpoS (katF) gene. J Bacteriol. 1994;176:7638–7645. doi: 10.1128/jb.176.24.7638-7645.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]