Purpose of review
T-cell-engaging antibodies or T-cell engagers (TCEs) can connect a patient's cytotoxic T cells with cancer cells, leading to potent redirected lysis. Until very recently, only one TCE was approved, the CD19/CD3-bispecific blinatumomab. Many new TCEs in late-stage clinical development target various hematopoietic lineage markers like CD20, BCMA, or CD123. Although very compelling single-agent activity of TCEs was observed with various blood-borne cancers, therapy of solid tumor indications has thus far been less successful.
Recent findings
The approval in 2022 of the gp100 peptide-major histocompatibility complex (MHC)/CD3 bispecific TCE tebentafusp in uveal melanoma confirms that TCEs can also efficiently work against solid tumors. TCEs targeting peptide–MHC complexes will expand the target space for solid tumor therapy to intracellular targets. Likewise, early clinical trial data from TCEs targeting DLL3 in small cell lunger cancer showed promising antitumor activity. Various technologies for conditional activation of TCEs in the tumor microenvironment (TME) may expand the scope of conventional surface targets that suffer from a narrow therapeutic window. Finally, pharmacological enhancements for TCE therapies by engagement of certain costimulatory receptors and cytokines, or blockade of checkpoints, are showing promise.
Summary
Targeting peptide–MHC complexes, conditional TCE technologies, and concepts enhancing TCE-activated T cells are paving the way towards overcoming challenges associated with solid tumor therapy.
Keywords: conditional T-cell engager, peptide-MHC targets, T-cell engager, tumor cell lysis
INTRODUCTION
Cytotoxic T cells are the immune cell population in patients with the highest potency to treat cancer. Early clinical evidence for the importance of T cells in cancer therapy was obtained with the approval in 1992 of recombinant IL-2 [1], a T-cell-activating cytokine, and the clinical impact seen with T-cell checkpoint inhibitors like the PD-1 antagonistic antibodies, pembrolizumab and nivolumab [2]. More direct evidence comes from cancer therapy with autologous tumor-infiltrating T cells (TILs), which in combination with lymphodepleting chemotherapy and IL-2 has led to robust responses in melanoma and ovarian cancer patients [3]. Whenever autologous T cells are engineered to express recombinant chimeric antigen receptors (CARs), which allows them major histocompatibility complex (MHC)-independent recognition of cell surface antigens, high complete response rates and durable responses can be observed in liquid tumors, leading to the approval of several CAR-T therapies targeting CD19 or BCMA [4,5]. Likewise, autologous T cells reprogrammed with predefined TCR alpha/beta chains specific for cancer testis antigens, are showing clinical activity [6]. TRuC-T cells, where antibody fragments binding cell surface antigens are directly fused to TCR subunits, can overcome the HLA restriction of such TCR-T cells [7]. Although most CAR-T therapies show limited activity in solid tumors, TCR-T and TRuC-T cells appear to show higher activity [8].
Despite the clinical success of cell therapies in liquid tumors, the treatment can be associated with significant toxicities, responses in solid tumors are limited and manufacturing remains a major challenge [9].
An alternative therapy to cytokines, checkpoint inhibitors and T-cell therapies are T-cell-engaging antibodies or T-cell engagers (TCEs), which are typically based on antibodies or antibody fragments. TCEs form a bispecific adaptor protein able to connect essentially any T cell in the body with cells expressing a select surface marker. TCEs allow for precise control of dose and schedule, helping to minimize side effects, and require much less complex manufacturing processes than cell-based therapies. Compelling complete response rates are observed with TCEs in relapsed/refractory (r/r) ALL, r/r NHL, multiple myeloma and AML patients with TCEs targeting CD19, CD20, BCMA or CD123, respectively [10–13]. Encouraging signs of activity in solid tumors are starting to emerge in some indications, namely uveal melanoma with the approval of tebentafusp and small cell lung cancer with early data from DLL3 targeting TCEs AMG 757 [14] and HPN328 [15]. We here discuss possible challenges for the mode of action of TCEs in solid tumors and highlight recent progress in overcoming these hurdles.
Box 1.
no caption available
MODE OF ACTION OF T-CELL ENGAGERS
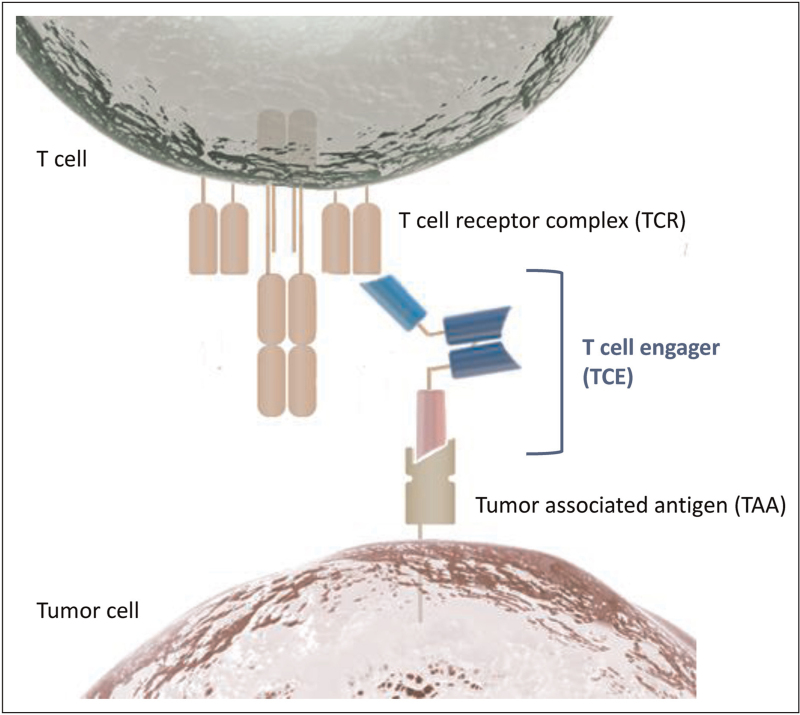
TCEs are a therapeutic modality with outstanding potency and an unusual mode of action. By binding to a tumor-associated cell surface antigen (TAA) with one arm and with a second arm to the invariant CD3 epsilon subunit of the T-cell receptor complex (TCR), TCEs can transiently connect cancer and T cells (Fig. 1). Simultaneous binding promotes the formation of a cytolytic synapse between T and cancer cells by which T cells can inject pore-forming proteins (perforin) and apoptosis-inducing proteases (granzymes) into the attached cancer cells. The synapse formation also leads to TCR crosslinking and T-cell activation, resulting in the release of pro-inflammatory cytokines and induction of T-cell proliferation [16]. Essentially, every cytotoxic T-cell phenotype can be engaged by this modality and made to participate in serial cancer cell lysis, including CD8+ T cells but also CD4+ T cells, gamma/delta T cells and NKT cells [17]. By recognition of a cell surface antigen, T-cell engagement by TCEs is independent of MHC molecules, peptide antigen processing and presentation, and the specificity of the TCR. In principle, every tumor-resident or newly attracted T cell with cytotoxic potential can participate in cancer cell lysis.
FIGURE 1.
T-cell engager mechanism of action. T-cell engagers (TCEs) are therapeutic proteins that can connect a T cell with a tumor cell. Most TCEs have three domains: one domain binds to a component of the T-cell receptor, one domain binds to a tumor-associated antigen, and a third domain provides additional functionality like half-life-extension.
For full activation, T cells typically require two signals. The primary signal 1 comes from activation of the TCR through recognition of its cognate peptide–MHC complex on target cells. Signal 2 serves to coactivate signal 1 and is provided by CD28 stimulation via CD80 [18]. Although both signals 1 and 2 are needed for activation of naive T cells, T cells that have differentiated after initial priming, that is, effector memory T cells, no longer need signal 2 for full activation. As TCEs do not provide costimulatory signal 2, it is likely that TCEs predominantly derive their potent single-agent activity in patients from the engagement of signal 2-independent, effector memory CD8+ and CD4+ T cells. Pharmacodynamic studies have indeed reported that peripheral effector memory T cells get selectively expanded in blinatumomab-treated patients [19].
Both isolated CD4+ T cells and cytotoxic CD8+ T cells can mediate potent redirected lysis by TCEs [20]. Regulatory T cells (T regs) are a subpopulation of CD4+ T cells that can dampen T-cell responses. T regs bear a TCR, and can therefore, also be activated and engaged by TCEs. Although one study reports that levels of T regs are a negative prognostic factor for blinatumomab response [21], other studies find that highly purified T regs, which in part express perforin and granzymes, can likewise support redirected lysis of target cell [22]. Given the high complete response rates of TCEs in liquid tumors, it appears unlikely that TCE-activated T regs can profoundly impede the clinical activity of TCEs.
T-CELL ENGAGERS IN BLOOD BORNE MALIGNANCIES
In blood borne malignancies, the mode of action of TCEs translates into an outstanding single-agent activity. This was first evident by CD19/CD3 bispecific blinatumomab, the first Food and Drug Administration (FDA)-approved TCE [23]. In pivotal trials, r/r ALL patients showed a 40% complete response rate. In ALL patients with minimal residual disease (MRD) – having a much-reduced tumor cell load – an 80% complete response rate was confirmed [24]. Currently, a spade of CD20/CD3-bispecific TCEs is in later stage clinical development mostly in patients with diffuse large B-cell lymphoma (DLBCL) and follicular lymphoma. Their complete response rates as single agent often exceed 50% and favorably compare with CD19-specific CAR-T-cell therapies in these indications [10]. Of note, the design of CD20/CD3-bispecific TCEs can greatly vary. Most are based on an IgG scaffold. This suggests that for treatment of hematological cancers there is a high tolerance for the bispecific design, which may not be the case for solid tumor therapy. TCE targets for blood borne malignancies typically are lineage markers. As exemplified for B-cell antigens CD19, CD20, or BCMA, respective normal B-cell compartments can be ablated alongside cancer cells without deleterious side effects. B cells are simply replenished from hematopoietic stem cells in the bone marrow. The coming years will see a number of TCEs entering the market that can treat most hematologic malignancies.
THE CHALLENGES OF T-CELL ENGAGER THERAPY IN SOLID TUMORS
A key challenge in the treatment of solid tumors with TCEs are treatment-related adverse events, which often limit dose and thereby efficacy. Such adverse events can be on-target and/or off-target. Off-target toxicities are a result of unintentional T-cell activation without target cell binding. It can be caused by aggregated TCEs, or mediated by binding of TCEs to other cell types, for example, to Fc-gamma receptors (FcγR) on antigen-presenting cells. Catumaxomab is an example of a TCE with an intact Fc domain whose utility for systemic administration is limited because of off-target toxicity [25]. Generally, these off-target toxicities present themselves as cytokine release syndrome (CRS), a potentially life-threatening consequence from sudden and extensive activation of the immune system [26].
Compared with TCEs treating liquid tumors, TCEs targeting solid tumors require much higher peripheral exposures in order to achieve efficacious concentrations inside the solid tumor tissue [27]. Higher exposure levels are more prone to off-target toxicities and require particularly careful engineering and dosing. Common approaches include the avoidance of FcγR binding either by removal of FcγR-binding residues from IgG-like TCEs, or by avoiding Fc domains from constructs altogether. Small size and shape – as exemplified by platforms like BiTE, DART, or TriTAC – are thought to be beneficial for solid tumor penetration [28]. If TCEs do not have a long serum half-life, an enhanced diffusion rate into solid tumor tissue and a very high-affinity target binding becomes critical. The stability and propensity to aggregate is another important variable of the binding elements employed for TCE design. Single-chain variable fragments (scFvs) of low melting temperature have less desirable properties than, for example, single-domain antibodies (sdAbs) or Fab fragments as TCE building blocks [29].
On-target toxicities are directly linked to the target specificity of the TCE. Although recent successes like the approval of tebentafusp and the early clinical data from DLL3 targeting TCE demonstrate that certain TAAs are well suited as targets for well engineered TCEs, many other TAAs have issues from their expression on indispensable normal tissues. As shown for tebentafusp, TCEs can recognize extremely low levels of target. As a result, it is not uncommon to observe on-target tissue toxicities, for instance, with TCEs targeting CEA [30] and EpCAM [31] in clinical trials, or TCEs targeting EGFR in nonhuman primate studies [32].
On-target CRS is observed when the target-mediated activation of T cells leads to an overboarding cytokine response. In contrast to on-target tissue toxicity, this effect is generally transient and can sometimes be managed by dose reductions, premedication, and supportive care.
EMERGING OPPORTUNITIES FOR T-CELL ENGAGERS FOR TREATING SOLID TUMORS
Peptide–MHC complexes as new surface targets for T-cell engagers
The most significant progress in treating solid tumor by TCEs comes from tebentafusp, which received FDA approval in January 2022 for treatment of uveal melanoma [33]. Tebentafusp is composed of single-chain TCR alpha/beta variable domains fused to an anti-CD3 single-chain antibody. The soluble TCR moiety is binding with low-picomolar affinity to a gp100 peptide–MHC complex present in cancer cells at very low copy number. Albeit only a modest RECIST response rate was seen, the TCE showed a robust increase in overall survival vis-à-vis investigators’ choice [34▪▪]. A key learning is that solid tumors can be treated by TCEs as a single agent, and that peptide–MHC complexes make for functional TCE targets despite their low expression on target cells. Peptide–MHC-specific TCEs allow to essentially target the entire cancer proteome including nuclear, organelle-associated, cytoplasmic, and secreted antigens. An alternative to using soluble TCRs for generation of TCEs are TCR mimetic antibodies (TCRms) that bind HLA-presented peptides very similar to TCRs. A number of recent studies published impressive preclinical data with TCRm-based TCEs targeting pMHC complexes presenting mutant Ras and p53 neoantigens [35]. These targets are tumor-specific and promise a very wide therapeutic window.
Conditionally active T-cell engagers
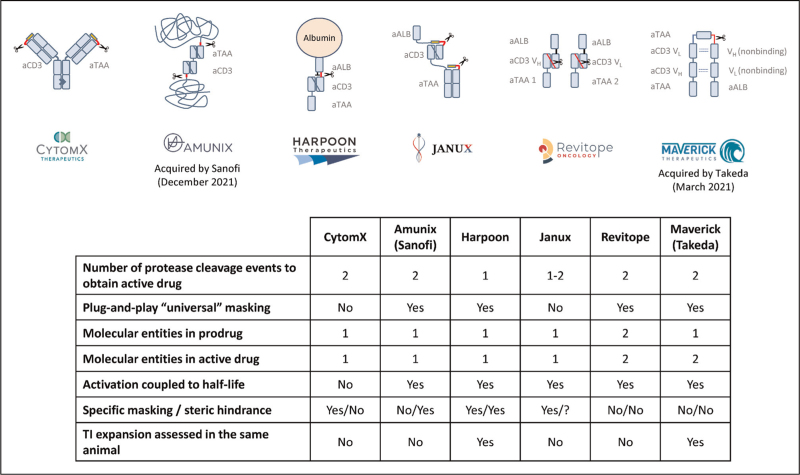
Another exciting development with the potential to increase the target space for TCEs are conditional TCEs. These molecules are administered as inactive prodrugs, and activation is either temporally controlled whereby the active TCE gets slowly formed over time to counter the development of CRS, as Harpoon's TriTAC-XR platform [36], or activation is controlled spatially, leading to activation of the TCE only inside the tumor, which limits off-tumor tissue damage. Several mechanisms for conditional TCE activation are under investigation. BioAtla is proposing to use pH-sensitive antigen-binding antibodies, which are only binding their target antigen in the slightly acidic TME [37]. Most other approaches incorporate proteolytic cleavage sites in the TCE design to take advantage of the enhanced proteolytic activity in the TME for local activation of target and/or CD3 binding, an approach pioneered by CytomX [38]. Several protease activatable TCE formats began clinical testing or are expected to enter the clinic in 2022 (see Fig. 2). These approaches profoundly differ in their design. Most platforms lose the long serum half-life of their prodrug upon activation inside the tumor, which reduces unintended targeting of normal tissues when activated drug is leaving the tumor tissue. Some platforms use specific peptides for masking (CytomX, Janux), others utilize steric blockade by a long repetitive polypeptide (Amunix, now part of Sanofi). One platform has modified the half-life extending albumin-binding domain to also serve as a mask for the T-cell-binding domain, utilizing both specific masking and steric blockade (Harpoon). In principle, conditional TCEs can block either T-cell binding, target cell binding, or both. Most formats in development are blocking both to maximize safety. Harpoon's ProTriTAC is blocking only the T-cell binding, which is still allowing for tumor targeting of the prodrug. It is not yet clear which strategy will lead to the broadest therapeutic index and highest antitumor activity in the clinic.
FIGURE 2.
Conditionally active T-cell engager. Various approaches to conditionally active T-cell engagers are under investigation. Five of the six platforms depicted here are either in clinical development or expected to enter the clinic in 2022.
Other approaches for conditional TCEs are followed by Revitope and Takeda. Revitope has designed TCEs that come in two pieces. Only when they find each other on tumor cells, an active TCE is formed. A similar strategy was pursued by Maverick (now Takeda). An inactive prodrug with long serum half-life is split inside the TME. The halves then unite on the target cell surface to form an active TCE of short serum half-life [39].
For each conditional TCE format, preclinical data show that the inactive prodrugs have orders of magnitude lower in-vitro activity than the proteolytically activated TCEs. However, it is not straightforward to preclinically assess the gained expansion of therapeutic index as often different species are used to assess efficacy and safety, and often different pharmacokinetic properties and dosing schemes hinder a proper comparison. The coming years will provide clinical data with conditional TCEs targeting EGFR, EpCAM, TROP-2, and other TAAs.
ENHANCEMENTS FOR T-CELL ENGAGERS TO OVERCOME THE IMMUNOSUPPRESSIVE TUMOR MICROENVIRONMENT
The low response rates of TCEs in solid tumor indications has prompted the evaluation of checkpoint inhibitors, TNFR family agonists, T-cell costimuli, and cytokines for enhancement of antitumor activity. In most cases, such enhancements are not becoming a component of the TCE but are provided as a separate co-therapy. Combination of TCEs with checkpoint inhibitors, such as anti-PD1 antibodies is a means to overcome exhaustion of TCE-activated T cells. A number of studies have preclinically investigated such combinations and found an enhancement of TCE performance [40]. Another strategy is to combine TCEs with biologics that agonize TNF receptor family members, such as 4-1BB (CD137). Positive results have been reported [41]. In another approach, the activity of the TCE is intentionally weakened so that it requires the second signal again, which is then provided in the form of a CD28 agonistic biologic [42]. This could help to engage naive T cells in addition to effector memory T cells. Engagement of naive T cells may enlarge the available T-cell troop size but can come with safety liabilities from activation and expansion of self-reactive T-cell clones and an enhanced cytokine profile. Lastly, T-cell stimulatory cytokines, such as IL-2 may have utility in promoting TCE activity. This was reported for tebentafusp in a clinical trial [43].
CONCLUSION
TCEs are becoming an important addition to the arsenal of cancer therapies. Widening the target space by peptide–MHC targets and conditional TCEs and the use of T-cell-enhancing strategies are likely to lead to higher response rates in solid tumor indications.
Acknowledgements
None.
Financial support and sponsorship
None.
Conflicts of interest
P.A.B. is an advisor and shareholder of Cullinan Oncology, Inc., and H.W. is an employee and shareholder of Harpoon Therapeutics, Inc.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
REFERENCES
- 1.Rosenberg SA. IL-2: the first effective immunotherapy for human cancer. J Immunol 2014; 192:5451–5458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Morad G, Helmink BA, Sharma P, Wargo JA. Hallmarks of response, resistance, and toxicity to immune checkpoint blockade. Cell 2021; 184:5309–5337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hinrichs CS, Rosenberg SA. Exploiting the curative potential of adoptive T-cell therapy for cancer. Immunol Rev 2014; 257:56–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Singh AK, McGuirk JP. CAR T cells: continuation in a revolution of immunotherapy. Lancet Oncol 2020; 21:e168–e178. [DOI] [PubMed] [Google Scholar]
- 5.June CH, O’Connor RS, Kawalekar OU, et al. CAR T cell immunotherapy for human cancer. Science 2018; 359:1361–1365. [DOI] [PubMed] [Google Scholar]
- 6.Rapoport AP, Stadtmauer EA, Binder-Scholl GK, et al. NY-ESO-1-specific TCR-engineered T cells mediate sustained antigen-specific antitumor effects in myeloma. Nat Med 2015; 21:914–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baeuerle PA, Ding J, Patel E, et al. Synthetic TRuC receptors engaging the complete T cell receptor for potent antitumor response. Nat Commun 2019; 10:2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hong DS, Johnson M, Tanyi JL, et al. Preliminary safety and efficacy of gavocabtagene autoleucel (gavo-cel, TC-210), a T cell receptor fusion construct (TRuC™), in patients with treatment refractory mesothelin overexpressing solid tumors. Cancer Res 2021; 81: (13 Suppl): [abstract CT105]. [Google Scholar]
- 9.Subklewe M. BiTEs better than CAR T cells. Blood Adv 2021; 5:607–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bock AM, Nowakowski GS, Wang Y. Bispecific antibodies for non-Hodgkin lymphoma treatment. Curr Treat Options Oncol 2022; 23:155–170. [DOI] [PubMed] [Google Scholar]
- 11.Yu B, Jiang T, Liu D. BCMA-targeted immunotherapy for multiple myeloma. J Hematol Oncol 2020; 13:125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tabata R, Chi S, Yuda J, Minami Y. Emerging immunotherapy for acute myeloid leukemia. Int J Mol Sci 2021; 22:1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goebeler ME, Bargou RC. T cell-engaging therapies - BiTEs and beyond. Nat Rev Clin Oncol 2020; 17:418–434. [DOI] [PubMed] [Google Scholar]
- 14.Owonikoko TK, Champiat S, Johnson ML, et al. Updated results from a phase 1 study of AMG 757, a half-life extended bispecific T-cell engager (BiTE) immuno-oncology therapy against delta-like ligand 3 (DLL3), in small cell lung cancer (SCLC). J Clin Oncol 2021; 39: (Suppl 15): [abstract 8510]. [Google Scholar]
- 15.Johnson ML, Dy GK, Mamdani H, et al. Interim results of an ongoing phase 1/2a study of HPN328, a tri-specific, half-life extended, DLL3-targeting, T-cell engager, in patients with small cell lung cancer and other neuroendocrine cancers. J Clin Oncol 2022; 40: (Suppl 16): [abstract 8566]. [Google Scholar]
- 16.Dustin ML. The immunological synapse. Cancer Immunol Res 2014; 2:1023–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kischel R, Hausmann S, Baeuerle P, Kufer P. Effector memory T cells make a major contribution to redirected target cell lysis by T cell-engaging BiTE antibody MT110. Cancer Res 2009; 69: (9 Suppl): [abstract 3252]. [Google Scholar]
- 18.Azuma M. Co-signal molecules in T-cell activation: historical overview and perspective. Adv Exp Med Biol 2019; 1189:3–23. [DOI] [PubMed] [Google Scholar]
- 19.Zugmaier G, Gokbuget N, Klinger M, et al. Long-term survival and T-cell kinetics in relapsed/refractory ALL patients who achieved MRD response after blinatumomab treatment. Blood 2015; 126:2578–2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haas C, Krinner E, Brischwein K, et al. Mode of cytotoxic action of T cell-engaging BiTE antibody MT110. Immunobiology 2009; 214:441–453. [DOI] [PubMed] [Google Scholar]
- 21.Sidaway P. Haematological cancer: Treg predict responsiveness to blinatumomab. Nat Rev Clin Oncol 2017; 14:262. [DOI] [PubMed] [Google Scholar]
- 22.Münz M, Murr A, Hoffmann P, et al. Lysis of cancer cells by highly purified T regulatory cells engaged via an EpCAM/CD3-bispecific BiTE antibody. Cancer Res 2012; 72: (8 Suppl): [abstract 4841]. [Google Scholar]
- 23.Przepiorka D, Ko CW, Deisseroth A, et al. FDA approval: blinatumomab. Clin Cancer Res 2015; 21:4035–4039. [DOI] [PubMed] [Google Scholar]
- 24.Burt R, Warcel D, Fielding AK. Blinatumomab, a bispecific B-cell and T-cell engaging antibody, in the treatment of B-cell malignancies. Hum Vaccin Immunother 2019; 15:594–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Seimetz D, Lindhofer H, Bokemeyer C. Development and approval of the trifunctional antibody catumaxomab (anti-EpCAM x anti-CD3) as a targeted cancer immunotherapy. Cancer Treat Rev 2010; 36:458–467. [DOI] [PubMed] [Google Scholar]
- 26.Fajgenbaum DC, June CH. Cytokine Storm. N Engl J Med 2020; 383:2255–2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dreher MR, Liu W, Michelich CR, et al. Tumor vascular permeability, accumulation, and penetration of macromolecular drug carriers. J Natl Cancer Inst 2006; 98:335–344. [DOI] [PubMed] [Google Scholar]
- 28.Austin RJ, Lemon BD, Aaron WH, et al. TriTACs, a novel class of T-cell-engaging protein constructs designed for the treatment of solid tumors. Mol Cancer Ther 2021; 20:109–120. [DOI] [PubMed] [Google Scholar]
- 29.Jovcevska I, Muyldermans S. The therapeutic potential of nanobodies. BioDrugs 2020; 34:11–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tabernero J, Melero I, Ros W, et al. Phase Ia and Ib studies of the novel carcinoembryonic antigen (CEA) T-cell bispecific (CEA CD3 TCB) antibody as a single agent and in combination with atezolizumab: preliminary efficacy and safety in patients with metastatic colorectal cancer (mCRC). J Clin Oncol 2017; 35:3002–13002.28644773 [Google Scholar]
- 31.Kebenko M, Goebeler ME, Wolf M, et al. A multicenter phase 1 study of solitomab (MT110, AMG 110), a bispecific EpCAM/CD3 T-cell engager (BiTE(R)) antibody construct, in patients with refractory solid tumors. Oncoimmunology 2018; 7:e1450710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lutterbuese R, Raum T, Kischel R, et al. T cell-engaging BiTE antibodies specific for EGFR potently eliminate KRAS- and BRAF-mutated colorectal cancer cells. Proc Natl Acad Sci U S A 2010; 107:12605–12610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.First drug approved for rare eye cancer. Cancer Discov 2022; 12:879–880. [DOI] [PubMed] [Google Scholar]
- 34▪▪.Nathan P, Hassel JC, Rutkowski P, et al. IMCgp100-202 Investigators. Overall survival benefit with tebentafusp in metastatic uveal melanoma. N Engl J Med 2021; 385:1196–1206. [DOI] [PubMed] [Google Scholar]; First ever study reporting that a TCE can elicit an overall survival benefit in a solid tumor indication.
- 35.Chang AY, Gejman RS, Brea EJ, et al. Opportunities and challenges for TCR mimic antibodies in cancer therapy. Expert Opin Biol Ther 2016; 16:979–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kwant K, Rocha S, Stephenson K, et al. 867|TriTAC-XR is an extended-release T cell engager platform designed to minimize cytokine release syndrome by reducing Cmax in systemic circulation. J ImmunoTher Cancer 2021; 9:A908–A1908. [Google Scholar]
- 37.Chang HW, Frey G, Liu H, et al. Generating tumor-selective conditionally active biologic anti-CTLA4 antibodies via protein-associated chemical switches. Proc Natl Acad Sci U S A 2021; 118:e2020606118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Autio KA, Boni V, Humphrey RW, Naing A. Probody therapeutics: an emerging class of therapies designed to enhance on-target effects with reduced off-tumor toxicity for use in immuno-oncology. Clin Cancer Res 2020; 26:984–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Panchal A, Seto P, Wall R, et al. COBRA: a highly potent conditionally active T cell engager engineered for the treatment of solid tumors. MAbs 2020; 12:1792130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Krupka C, Kufer P, Kischel R, et al. Blockade of the PD-1/PD-L1 axis augments lysis of AML cells by the CD33/CD3 BiTE antibody construct AMG 330: reversing a T-cell-induced immune escape mechanism. Leukemia 2016; 30:484–491. [DOI] [PubMed] [Google Scholar]
- 41.Belmontes B, Sawant DV, Zhong W, et al. Immunotherapy combinations overcome resistance to bispecific T cell engager treatment in T cell-cold solid tumors. Sci Transl Med 2021; 13:eabd1524. [DOI] [PubMed] [Google Scholar]
- 42.Skokos D, Waite JC, Haber L, et al. A class of costimulatory CD28-bispecific antibodies that enhance the antitumor activity of CD3-bispecific antibodies. Sci Transl Med 2020; 12:eaaw7888. [DOI] [PubMed] [Google Scholar]
- 43.Khanolkar R, Naidoo R, Güç E, et al. 571|IL-2 combination with ImmTAC overcomes CD163+ TAM-like M2 macrophage inhibition of ImmTAC-mediated T cell killing of tumor cells. J ImmunoTher Cancer 2021; 9:A600–A1600. [Google Scholar]