Précis:
Short-term overall success rates were high with either SGDD or CPC. However, SGDD was associated with more clinic visits and an increased risk of additional glaucoma surgery. Both treatments were reasonable options for eyes with inadequately controlled IOP after a single GDD.
Purpose:
The purpose of this study is to compare the implantation of a second glaucoma drainage device (SGDD) and transscleral cyclophotocoagulation (CPC) in eyes with inadequately controlled intraocular pressure (IOP), despite the presence of a preexisting glaucoma drainage device.
Methods:
Patients with inadequately controlled IOP, despite the medical therapy and a preexisting glaucoma drainage device, were enrolled at 14 clinical centers and randomly assigned to treatment with a SGDD or CPC.
Main Outcome Measures:
Surgical failure was defined as: (1) IOP ≤5 mm Hg or >18 mm Hg or <20% reduction below baseline on maximum tolerated topical ocular hypotensive therapy, (2) reoperation for glaucoma, or (3) loss of light perception. The primary outcome measure was overall success with or without adjunctive medical therapy.
Results:
Forty-two eyes of 42 participants were randomized to SGDD (n=22) or CPC (n=20). Mean duration of follow-up was 18.6 (±12.1; range: 1.1–38.6) months. The cumulative success rate was 79% for SGDD and 88% for CPC at 1 year (P=0.63). Although the study was underpowered, no significant differences in IOP, postoperative number of IOP-lowering medications, or adverse events were observed. The number of additional glaucoma surgeries (P=0.003), office visits during the first 3 months (P<0.001), and office visits per month after month 3 (P<0.001) were greater in the SGDD group.
Conclusions:
Short-term overall success rates were high with either SGDD or CPC. However, SGDD was associated with more clinic visits and an increased risk of additional glaucoma surgery.
Key Words: glaucoma, aqueous shunt, cyclophotocoagulation, tube shunt, drainage device
BACKGROUND
The frequency of glaucoma drainage device (GDD) implantation has increased 410% between 1994 and 2012.1 Approximately 33%–53% of GDD surgeries fail during the first 5 years,2–4 which suggests that many patients with GDD will require additional glaucoma surgery.
For eyes with a GDD and inadequately controlled intraocular pressure (IOP) despite the adjunctive medical therapy, ophthalmologists usually perform transscleral cyclophotocoagulation (CPC) or implantation of a second GDD (SGDD). In 2015, we conducted a survey of 157 fellowship-trained members of the American Glaucoma Society and found that 66% of surgeons surveyed implant SGDDs in another quadrant, 21% perform CPC, and 13% make decisions based on individual patient factors. Thus, there is no clear consensus regarding which procedure should be performed in this setting. Published retrospective case series provide the only evidence to date that both of these procedures are appropriate options for eyes with inadequate IOP lowering after implantation of a single GDD.5–12 However, additional evidence to guide clinical decision-making in this scenario is needed.
The primary aim of the Second Aqueous Shunt Implant versus Transscleral Cyclophotocoagulation Treatment Study (ASSISTS) was to compare overall success rates of SGDD and CPC for eyes with uncontrolled glaucoma after an initial GDD implant. The secondary aims were to compare safety [ie, complications, loss of best-corrected visual acuity (BCVA), and pain], number of office visits, and participant vision-related quality of life between the 2 treatments.
METHODS
ASSISTS is a prospective, randomized, stratified, controlled multicenter clinical trial comparing cumulative success rates between SGDD and CPC for eyes with uncontrolled glaucoma after an initial GDD implant. In all, 23 sites agreed to participate in the study. Each received Institutional Review Board approval to conduct the study, and informed consent was obtained from each participant before enrollment. The University of Texas Health Science Center and Robert Cizik Eye Clinic served as the headquarters and data-coordinating center (DCC). The study was monitored by an independent Data and Safety Monitoring Board (DSMB). Research adhered to the tenets of the Declaration of Helsinki and was compliant with the Health Insurance Portability and Accountability Act. The study is registered at http://www.clinicaltrials.gov (NCT02691455).
Study Population
Subjects between 40 and 85 years of age with uncontrolled IOP (defined as >18 mm Hg and, in the opinion of the treating physician, in need of further lowering) while on maximally tolerated topical IOP-lowering therapy, despite the presence of 1 GDD in the study eye, were eligible. Exclusion criteria included the presence of more than 1 GDD in the study eye, a history of cyclodestruction in the study eye, the presence of a scleral buckle or scleromalacia in the study eye, a history of scleritis, vision worse than hand motions in the fellow eye, or binocular diplopia. Only 1 eye of each participant was eligible for enrollment. Because of the slow recruitment, the eligible population was increased from a minimum of 40 years of age to 18 years of age after study commencement.
Randomization and Masking
An adaptive block randomization scheme was generated with a block size of 4 and balancing between neovascular glaucoma and non-neovascular glaucoma within each site by the DCC. Upon completion of the screening/baseline visit, sites entered the screening/baseline data into the Research Electronic Data Capture (REDCap) system to confirm eligibility. Once the eligibility of the participant was confirmed, the REDCap system provided the participant randomization assignment, SGDD, or CPC to the site coordinator. Neither the participant nor the investigator was masked to the randomization assignment. The IOP reader, however, was masked.
Sample Size
We anticipated that the types of glaucoma in our study population would be similar to those in the Ahmed Baerveldt Comparison study.3 In this study, the 1-year and 5-year failure rates for the primary Baerveldt and Ahmed GDD groups were 14% and 34%, respectively. Because we were evaluating an SGDD, our anticipated failure rate for the SGDD group was estimated to be 50% more than for the initial GDD5 (21% at 1 y and 50% at 5 y). Setting the power at 80% and significance at 5%, a sample size of 91 participants in each group (182 total) was estimated to have been required to detect a ≥20% difference in the cumulative incidence of failure between groups.
Surgical Procedure
SGDD
A Baerveldt 350-mm2 (Model BG101-350; Johnson & Johnson, Santa Ana, CA) was the implant of choice in participants randomized to the SGDD group, unless there was insufficient space, in which case a smaller implant of the same or different design was permitted. The preferred location of the second implant was approximately diametrically opposite the first GDD. Tube insertion through the anterior chamber angle, ciliary sulcus, or pars plana was acceptable. The surgical procedure was performed with the surgeon’s usual technique, except the tube was required to have been completely occluded in all cases. The method of occlusion was left to the discretion of the surgeon. Fenestration of the tube or wick placement was allowed. The decisions of whether to use a patch graft to cover the tube and the type of allograft were at the discretion of the surgeon. Concurrent procedures (except for repair of an exposed preexisting tube) were not allowed at the time of SGDD implantation. The use of antifibrotic agents (such as 5-fluorouracil, mitomycin-C, or anti-vascular endothelial growth factor agents) was not allowed. Enhancement procedures, such as needling of the bleb over the SGDD plate, were allowed within the first 4 months after the initial study treatment. Manual or laser suture lysis of a ligature or obturator removal was also allowed and considered a planned part of the original surgery.
Transscleral CPC
CPC was performed with an Iridex laser (Iridex Corp., Mountain View, CA) and G-probe technique. Suggested initial laser settings were 2000 mW for 2 seconds, 1850 mW for 3 seconds, or 1750 mW for 4 seconds, titrating the energy to just below the occurrence of an audible “pop.” Four to 6 spots were recommended per quadrant, for a total of up to 12 spots per 180 degrees (or 2 quadrants). Only 180 degrees (or 2 quadrants) of treatment were permitted during the initial study treatment session. Location of quadrants treated was left to the surgeon’s discretion. An additional CPC session (≤2 quadrants) was allowed in the first 4 months after the initial study treatment if the initial treatment was inadequate.
IOP Measurement
IOP was measured by Goldmann applanation tonometry. Similar to previous studies, measurement was taken by 2 personnel—an operator, who set the tonometer dial, and a masked dial reader, who recorded the IOP measurement.13 Two measurements were obtained for the study eye. If the difference between the 2 measurements for an eye was >2 mm Hg, a third measurement was obtained. The mean of the 2 closer measurements was used for analysis.
Scheduled Follow-up Visits
Study visits were scheduled on postoperative week 1 [postoperative days (POD): 4–14], month 1 (POD: 15–59), month 3 (POD: 60–120), month 6 (POD: 120–270), month 12 (9–18 mo), year 2 (19–30 mo), year 3 (31–48 mo), and year 5 (49–72 mo). If IOP met the treatment failure criteria at a study visit, a confirmation visit was required within 1 month, between 6 months and 1 year postoperatively, or within 3 months, between 1 and 5 years postoperatively.
Outcomes
Surgical failure was defined as: (1) IOP ≤5 mm Hg, >18 mm Hg, or <20% reduction below baseline on maximum tolerated topical IOP-lowering medications on the IOP confirmation visits; (2) reoperation for glaucoma; or (3) loss of light perception.
The primary outcome was the overall success, which was defined as the proportion of eyes that did not meet the failure criteria with or without adjunctive medical therapy. The secondary outcomes were incidence of complications, mean BCVA, incidence of a decrease of ≥0.2 logMAR from baseline after 6 months, mean IOP, change in IOP from baseline, number of IOP-lowering medications, total number of clinic visits during the first 3 months and after 3 months, perioperative surgical pain at week 1 and month 1 (measured on a 1–10 subjective scale), and participant vision-related quality of life, measured by the National Eye Institute Visual Function Questionnaire 25 (VFQ-25) at baseline and month 12. Causes of visual acuity loss ≥0.2 logMAR from baseline were determined by the surgeon.
Data Collection
Study data were collected and managed with REDCap electronic data capture tools hosted at The University of Texas Health Science Center at Houston, School of Biomedical Informatics. The study participant data or documents were entered/uploaded by site coordinators and audited by the study coordinators at the DCC.
Data Analysis
BCVA was measured with a Snellen chart and was converted to logMAR by taking the negative logarithm (base 10) of the decimal equivalent of the Snellen visual acuity. The following adjustments were made: count fingers was coded as 20/1500, hand motion as 20/4000, light perception as 20/8000, and no light perception as 20/20,000. Changes in IOP, number of topical IOP-lowering medications, and logMAR visual acuity were calculated for scheduled follow-up visits.
Data were summarized by frequency (%) and compared between groups with the Fisher exact test for categorical variables. Mean and SD were calculated and compared between groups with 2-sample t tests for continuous variables. The cumulative failure rate and time to failure were estimated by Kaplan-Meier survival analysis for each group and compared between groups with the log-rank test. Cox regression analysis was performed to identify risk factors associated with time to failure. Changes in IOP, number of IOP-lowering medications, and visual acuity from baseline during the follow-up period were compared with mixed-effect models. Poisson regression was used to compare the complication rates, the number of additional procedures, and the number of office visits.
All statistical analyses were performed with SAS for Windows v9.4 (SAS Inc., Cary, NC). A P-value ≤0.05 was considered statistically significant.
RESULTS
Study enrollment began on May 23, 2016, and ended on June 30, 2020. The last date of data collection was September 30, 2020. In all, 14 sites enrolled at least 1 participant. A total of 50 participants were consented, screened, and enrolled. Of those, 5 did not meet eligibility criteria and were excluded. Forty-five participants were randomized, but 3 withdrew consent before undergoing the assigned surgical procedure. Thus, 42 participants underwent the assigned study procedure and were included in the analysis. Twenty-two eyes underwent SGDD, and 20 eyes underwent CPC. Participant follow-up is summarized in Figure 1. One participant was lost to follow-up after treatment. The overall mean follow-up duration was 18.6 (±12.1; range: 1.1–38.6) months, with 17.1 (±12.8; range: 1.1–38.6) months for the SGDD group and 20.3 (±11.4; range: 6.2–37.8) months for the CPC group (P=0.42).
FIGURE 1.
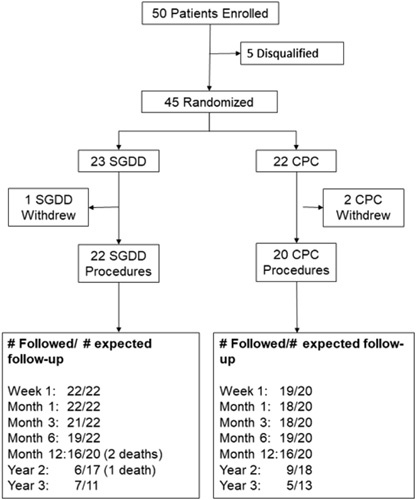
CONSORT diagram and participant retention. CPC indicates cyclophotocoagulation; SGDD, second glaucoma drainage device.
Baseline Characteristics
Demographic and baseline clinical characteristics of study participants are summarized in Table 1. No significant differences in any of the demographic or clinical features were observed between treatment groups at baseline, except there were more right eyes in the SGDD group and more left eyes in the CPC group (P=0.007). The mean BCVA was 0.5 logMAR (±0.8) worse in the SGDD group compared with the CPC group. However, the difference did not reach statistical significance (P=0.06). There were only 29 participants who underwent visual field testing within 6 months before the screening/baseline visit. Overall, the mean deviation was −16.6 dB (±9.8; range: −34.21 to −2.06), and there was no statistically significant difference between groups (P=0.27). However, the mean deviation was numerically worse in the CPC group [−19.0 dB (±11.6; range: −34.2 to −3.6)] compared with the SGDD group [−14.9 dB (±8.2; range: −29.2 to −2.1)].
TABLE 1.
Summary of Demographics and Baseline Characteristics
| Variable | Overall (N=42) | SGDD (N=22) | CPC (N=20) |
|---|---|---|---|
| Demographics | |||
| Age [y, mean (±SD)] | 63.6 (±10.4) | 63.1 (±11.2) | 64.2 (±9.8) |
| [range] | [36–83] | [36–83] | [43–78] |
| Sex [males, n (%)] | 14 (57) | 13 (59) | 11 (55) |
| Self-reported race, n (%) | |||
| White | 20 (47.6) | 11 (50) | 9 (45) |
| Black | 17 (41) | 9 (41) | 8 (40) |
| Other | 5 (12) | 2 (9) | 3 (15) |
| Self-reported ethnicity, n (%) | |||
| Hispanic | 13 (31) | 6 (27) | 7 (35) |
| Comorbid conditions | |||
| Systemic hypertension, n (%) | 32 (76) | 17 (77) | 15 (75) |
| Diabetes, n (%) | 17 (40) | 9 (41) | 8 (40) |
| Autoimmune disease, n (%) | 4 (10) | 3 (14) | 1 (5) |
| Ocular characteristics | |||
| Study eye [right, n (%)]** | 20 (48) | 15 (68) | 5 (25) |
| Glaucoma type, n (%) | |||
| Primary open angle | 19 (45) | 10 (41) | 10 (50) |
| Primary angle-closure | 2 (5) | 1 (5) | 1 (5) |
| Mixed mechanism glaucoma | 3 (7) | 2 (9) | 1 (5) |
| Neovascular | 6 (14) | 3 (14) | 3 (15) |
| Uveitic | 5 (12) | 2 (9) | 3 (15) |
| Other secondary glaucoma | 7 (17) | 5 (23) | 2 (10) |
| Intraocular Pressure [mm Hg, mean (±SD)] | 26.9 (±9.1) | 27.6 (±8.7) | 26.2 (±9.7) |
| [range] | [13–55] | [15–46] | [13–55] |
| On IOP-lowering medications, n (%) | 41 (98) | 21 (95) | 20 (100) |
| No. IOP-lowering medications [n, mean, (±SD)] | 3.2 (±0.9) | 3.0 (±1.0) | 3.3 (±1.1) |
| [range] | [0–5] | [0–4] | [1–5] |
| On oral carbonic anhydrase inhibitor, n (%) | 6 (14) | 2 (9) | 4 (20) |
| Best-corrected visual acuity [logMAR, mean (±SD)] | 0.74 (±0.82) | 0.97 (±0.90) | 0.49 (±0.66) |
| [range] | [0.0–2.6] | [0.0–2.3] | [0.0–2.6] |
| Visual field [mean deviation, mean, (±SD)] | −16.6 (±9.8) | −14.9 (±8.2) | −19.0 (±11.6) |
| [range] | [−34.2 to −2.1] | [−29.2 to −2.1] | [−34.2 to −3.6] |
| (n) | (n=29) | (n=17) | (n=12) |
For P<0.05.
For P<0.01.
For P<0.001 Obtained from 2-sample t test or Fisher Exact test for comparing between groups.
CPC indicates cyclophotocoagulation; IOP, intraocular pressure; SGDD, second glaucoma drainage device.
Previous surgeries are summarized in Supplemental Table 2, Supplemental Digital Content 1, http://links.lww.com/IJG/A635. The groups were similar in terms of prior surgeries, including the proportion of eyes that previously underwent cataract surgery. The type of preexisting GDD was not significantly different between groups (P=0.27); however, there was a greater proportion of initial Ahmed GDD implants (55%) in the CPC group and a greater proportion of Baerveldt implants (64%) in the SGDD group.
Treatments
SGDD
A Baerveldt 350-mm2 (Model BG101-350) was utilized as the SGDD in 16 (73%) participants, a Baerveldt 250-mm2 (Model BG101-250) in 4 (18%) participants, and an Ahmed FP-7 Glaucoma Valve (New World Medical, Rancho Cucamonga, CA) in 2 (9%) participants. Thirteen (59%) tubes were inserted into the anterior chamber, 7 (32%) tubes into the posterior chamber through the ciliary sulcus, and 2 (9%) tubes into the posterior segment through the pars plana. All tubes were covered with a corneal allograft, except 1 that was covered with pericardium. There were no enhancements, such as bleb needle revision, performed within the first 120 days postoperatively.
Transscleral CPC
The laser settings are summarized in Table 2. Eight (40%) eyes had >12 spots. One subject underwent repeat treatment within the first 120 days postoperatively.
TABLE 2.
Laser Settings for Cyclophotocoagulation
| CPC Duration (s) | No. Participants | Energy, mW mean (±SD) [Median, Range] | Number of Spots Mean (±SD) [Median, Range] |
|---|---|---|---|
| 2 | 1 | 1750 (NA) | 19 (NA) |
| [1750, 1750–1750] | [19, 19–19] | ||
| 3 | 4 | 1775 (±50) | 15.5 (±4.2) |
| [1750, 1750–1850] | [16, 10–20] | ||
| 4 | 15 | 1712 (±578) | 11.1 (±3.5) |
| [1750, 1000–3000] | [10, 4–18] |
CPC indicates cyclophotocoagulation; mW, millliwatts; NA=not applicable (n=1).
Outcomes
Over a mean of 18.6 (±12.1; range: 1.1–38.6) months of follow-up, only 4 SGDD and 2 CPC eyes failed. Five of 6 failures occurred between month 6 and month 12. After 12 months, there was 1 additional failure in the SGDD group and no additional failures in the CPC group. The cumulative overall success rates were 79% for SGDD and 88% for CPC (P=0.63, log-rank test) at month 12 and 63% and 88% for SGDD and CPC, respectively, at year 3 (P=0.34, log-rank test). Kaplan-Meier curves comparing time to failure in the 2 groups are shown in Figure 2.
FIGURE 2.
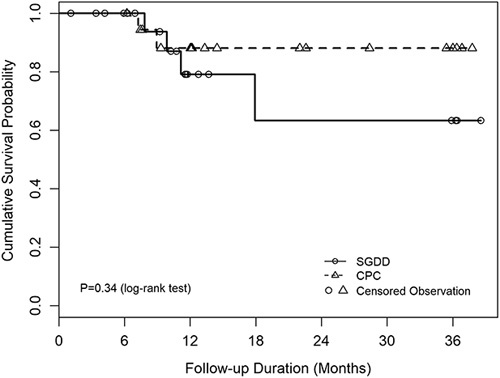
Kaplan-Meier curves comparing time to failure in the 2 groups. CPC indicates cyclophotocoagulation; SGDD, second glaucoma drainage device.
Of 4 SGDD failures, 2 were due to inadequate IOP reduction on maximally tolerated medications, 1 of which went on to receive CPC. One eye failed on implantation of a third GDD and 1 failed on receiving an additional CPC due to IOP being above the clinically determined target. In the CPC group, both failures were due to inadequate IOP reduction on maximally tolerated medication. Of the 2 CPC failures, neither accepted additional surgical intervention, and 1 of these eyes lost light perception. No significant risk factors associated with failure were identified by stepwise Cox regression analysis.
IOP Reduction
Table 3 summarizes baseline and follow-up IOP measurements for the SGDD and CPC groups. Participants who underwent additional glaucoma surgery were censored from analysis after reoperation. No significant difference in mean IOP was observed between treatment groups at any time point (P>0.05), (Table 3). The result from comparing IOP between groups for all scheduled follow-up visits after month 1 with a mixed-effect model showed that there was no significant difference between groups (P=0.94) after adjusting for the number of IOP-lowering medications (P=0.34).
TABLE 3.
Summary of IOP, Reduction in IOP From Baseline and Number of IOP-lowering Medications for Each Study Visit
| No. Participants Analyzed | IOP (mm Hg) | No. IOP-lowering Medications | IOP Reduction From Baseline (mm Hg) [P] | |||||
|---|---|---|---|---|---|---|---|---|
| Visit | SGDD | CPC | SGDD | CPC | SGDD | CPC | SGDD | CPC |
| Baseline | 22 | 20 | 27.6 (±8.7) | 26.2 (±9.7) | 3.0 (±1.0) | 3.3 (±1.1) | — | — |
| [15–46] | [13–55] | [0–4] | [1–5] | |||||
| Week 1 | 22 | 19 | 17.7 (±10.2) | 12.7 (±7.3) | 1.7* (±1.6) | 2.8* (±1.5) | 9.9 (±14.4) | 13.7 (±7.4) |
| [3–46] | [5–40] | [0–4] | [0–4] | [−23.5 to 39] | [−1.5 to 28.5] | |||
| [0.004] | [<0.001] | |||||||
| Month 1 | 22 | 18 | 17.4 (±9.0) | 13.2 (±6.2) | 1.6 (±1.4) | 2.2 (±1.3) | 10.1 (±11.7) | 12.9 (±9.8) |
| [5–36] | [3–27.5] | [0–4] | [0–4] | [−14.5 to 30.5] | [−5 to 31] | |||
| [0.001] | [<0.001] | |||||||
| Month 3 | 21 | 18 | 15.4 (±6.6) | 16.0 (±7.5) | 1.4 (±1.4) | 2.1 (±1.4) | 11.5 (±9.3) | 10.1 (±7.1) |
| [3–31] | [9–42] | [0–4] | [0–4] | [−1.5 to 31] | [−4.5 to 25] | |||
| [<0.001] | [<0.001] | |||||||
| Month 6 | 19 | 19 | 13.5 (±4.2) | 13.6 (±4.0) | 1.9 (±1.3) | 2.2 (±1.2) | 13.7 (±9.1) | 12.8 (±10.7) |
| [4–20] | [5–21] | [0–4] | [0–4] | [2.5–34.5] | [−4.5 to 34] | |||
| [<0.001] | [<0.001] | |||||||
| Month 12 | 16 | 16 | 14.9 (±4.5) | 13.9 (±6.3) | 2.1 (±1.3) | 1.7 (±1.4) | 11.6 (±7.6) | 12.3 (±7.9) |
| [8–24] | [8.5–34.5] | [0–4] | [0–4] | [−3 to 25] | [−5 to 27.5] | |||
| [<0.001] | [<0.001] | |||||||
| Year 2 | 6 | 9 | 14.8 (±6.5) | 14.3 (±4.7) | 1.5 (±1.6) | 1.4 (±1.0) | 16.0 (±6.3) | 13.11 (±10.6) |
| [6.3–25] | [9–24.5] | [0–3] | [0–3] | [7–24] | [3.5–30.5] | |||
| [0.002] | [0.006] | |||||||
| Year 3 | 6 | 5 | 14.6 (±4.4) | 12.8 (±3.0) | 2.2 (±1.7) | 2.2 (±0.8) | 18.5 (±8.1) | 14.50 (±8.8) |
| [9.5–22] | [10–16] | [0–4] | [1–3] | [5–28] | [7–28.5] | |||
| [0.003] | [0.02] | |||||||
For P<0.05.
For P<0.01.
For P<0.001 Obtained from 2-sample t test or Fisher Exact test for comparing between groups.
All data are presented as mean (±SD) [range].
P-value obtained from paired t test for within each group.
CPC indicates cyclophotocoagulation; IOP, intraocular pressure; SGDD, second glaucoma drainage device.
Both treatments produced a significant and sustained reduction in IOP (P<0.02, paired t test). At 1 year, IOP was reduced 11.6 mm Hg (±7.6; range: −3 to 25) in the SGDD group, which was a 40% reduction from baseline, and 12.3 mm Hg (±7.9; range: −5 to 27.5) (P<0.001, paired t test) in the CPC group, a 42% reduction. After month 1, the magnitude of the IOP reduction was not significantly different between groups (P=0.55, mixed-effect model) after adjusting for the effects of the number of IOP-lowering medications.
Medical Therapy
During the first year postoperatively, more than 85% of participants were using at least 1 topical IOP-lowering medication. None were using oral medications. Table 1 and Supplemental Table 5, Supplemental Digital Content 2, http://links.lww.com/IJG/A636 summarize the number of IOP-lowering medications at baseline and follow-up in each treatment group. Participants who underwent additional glaucoma surgery were censored from analysis after reoperation. The mean number of IOP-lowering medications was similar between treatment groups at baseline [3.0 (±1.0; range, 0 to 4) for SGDD and 3.3 (±1.1; range, 1 to 5) for CPC, P=0.44]. At week 1, SGDD participants were using a mean of 1.7 (±1.6; range: 0–4) IOP-lowering medications, which was significantly fewer than CPC participants [2.8 (±1.5; range: 0–4), P=0.03]. There were no significant differences in mean number of IOP-lowering medications after the week 1 visit (P≥0.11). When considering all the scheduled follow-up visits after month 1, there was no significant difference between groups (P=0.30, mixed-effect model).
Visual Acuity
Table 4 summarizes BCVA and shows that visual impairment was common in the study population, with a baseline BCVA of 0.97 logMAR (~20/200 Snellen visual acuity) in the SGDD group and 0.49 logMAR (~20/60 Snellen visual acuity) in the CPC group. Because baseline visual acuity was marginally better (P=0.06) in the CPC group, the main analysis was the comparison of BCVA in eyes to their own baseline (change in BCVA). In the CPC group, BCVA significantly decreased from baseline during the first 3 months postoperatively, whereas in the SGDD group, there was no significant change from baseline during any follow-up visits. There was no association detected between baseline BCVA and change in BCVA after the first 3 months. The mean difference in change in BCVA (SGDD − CPC) was −0.24 (±0.09) logMAR (about Snellen 2.5 lines, P=0.01, mixed-effect model), indicating a greater decline in BCVA in the CPC group. There was ≥0.2 logMAR deterioration in BCVA from baseline in 3 (14%) participants in the SGDD group and 8 (40%) participants in the CPC group (P=0.08, Fisher exact test). The causes of decreased vision in each group are shown in Supplemental Table 7, Supplemental Digital Content 3, http://links.lww.com/IJG/A637.
TABLE 4.
Summary of Best-corrected Visual Acuity and Change From Baseline at Each Scheduled Follow-up Visit, Mean (±SD) [range]
| No. Participants Analyzed | Best-corrected Visual Acuity | Best-corrected Visual Acuity Change From Baseline [P, paired t test] | ||||
|---|---|---|---|---|---|---|
| Visit | SGDD | CPC | SGDD | CPC | SGDD | CPC |
| Baseline | 22 | 20 | 0.97 (±0.90) | 0.49 (±0.66) | — | — |
| [0–2.3] | [0–2.6] | |||||
| Month 1 | 22 | 15 | 0.94 (±0.86) | 0.68 (±0.72) | −0.03* (±0.18) | 0.12* (±0.20) |
| [0–2.3] | [0–2.6] | [−0.57 to 0.22] | [−0.10 to 0.60] | |||
| [0.49] | [0.035] | |||||
| Month 3 | 21 | 17 | 0.81 (±0.84) | 0.62 (±0.70) | −0.10 * (±0.27) | 0.08 * (±0.13) |
| [0–2.3] | [0–2.6] | [−0.88 to 0.22] | [−0.10 to 0.33] | |||
| [0.11] | [0.017] | |||||
| Month 6 | 19 | 19 | 0.84 (±0.77) | 0.56 (±0.74) | −0.03 (±0.28) | 0.05 (±0.30) |
| [0–2.3] | [0–2.6] | [−0.63 to 0.60] | [−0.49 to 0.70] | |||
| [0.64] | [0.49] | |||||
| Month 12 | 16 | 16 | 0.83 (±0.80) | 0.52 (±0.76) | −0.06 (±0.34) | 0.09 (±0.31) |
| [0–2.3] | [0–2.6] | [−0.88 to 0.57] | [−0.40 to 1.00] | |||
| [0.47] | [0.25] | |||||
| Year 2 | 6 | 9 | 0.68 (±0.88) | 0.81 (±1.03) | −0.28 * (±0.28) | 0.24 * (±0.46) |
| [0–2.3] | [0–3.0] | [−0.70 to 0.00] | [−0.30 to 1.33] | |||
| [0.057] | [0.16] | |||||
| Year 3 | 6 | 5 | 1.02 (1.05) | 0.96 (1.07) | −0.23 (±0.36) | 0.49 (±0.76) |
| [0–2.3] | [0–2.3] | [−0.78 to 0.00] | [−0.40 to 1.33] | |||
| [0.13] | [0.22] | |||||
For P<0.05.
For P<0.01.
For P<0.001 Obtained from 2-sample t test or Fisher Exact test for comparing between groups.
Due to missing data, the mean of the differences in BCVA between baseline and each follow-up visit may not equal the mean changes in BCVA.
P-value obtained from paired t test for within each group.
CPC indicates cyclophotocoagulation; best-corrected visual acuity; SGDD, second glaucoma drainage device.
Complications
There were 14 complications in the SGDD group and 8 in the CPC group (P=0.29, Poisson regression analysis), and these are summarized in Table 5. There were 2 cases of hypotony in the SGDD group that either lasted beyond 3 months or required surgical intervention. One resolved by month 6, simply with a reduction of IOP-lowering medications. The other had drainage of an associated choroidal effusion, with resolution of the hypotony. One eye developed a macular hole after CPC, but this eye retained 20/30 vision without surgical intervention.
TABLE 5.
Summary of Complications
| Complication | SGDD (N=22) | CPC (N=19) |
|---|---|---|
| Hyphema | 2 | 0 |
| Persistent hypotony | 2 | 0 |
| Choroidal effusion | 1 | 0 |
| Cystoid/diabetic macular edema progression | 2 | 3 |
| Primary tube complications | 2 | 0 |
| Cataract progression | 0 | 3 |
| Macular hole | 0 | 1 |
| Herpes simplex keratitis | 1 | 0 |
| Ectropion | 1 | 0 |
| Second tube migration anteriorly | 1 | 0 |
| Vitreous occluding SGDD | 1 | 0 |
| Intraocular lens dislocation | 1 | 0 |
| Phacodonesis | 0 | 1 |
| Total complications (P=0.29*) | 14 | 8 |
Obtained from Poisson regression analysis.
CPC indicates cyclophotocoagulation; SGDD, second glaucoma drainage device.
Additional Surgical Procedures
Table 6 summarizes the additional surgical procedures performed during the follow-up period. There were 9 additional ocular procedures performed in the SGDD group and only 1 additional ocular surgical procedure in the CPC group (P=0.003, Poisson regression analysis). One participant underwent a third GDD implantation 10 months after the SGDD was implanted, and 2 patients underwent additional CPC in the SGDD group. There were 3 participants who had cataract formation/progression after CPC, 1 of whom underwent cataract extraction. No other additional procedures were performed in the CPC group.
TABLE 6.
Additional Surgical Procedures Performed During the Follow-up Period
| Additional Surgical Procedure | SGDD (N=22) | CPC (N=19) |
|---|---|---|
| Third GDD | 1 | 0 |
| CPC | 2 | 0 |
| Cataract extraction | 0 | 1 |
| Vitrectomy | 1 | 0 |
| Anterior chamber washout | 1 | 0 |
| Drainage of choroidal effusion | 1 | 0 |
| Corneal debridement | 1 | 0 |
| Second tube reposition | 1 | 0 |
| Repair of exposed primary GDD | 1 | 0 |
| Repair of exposed SGDD | 1 | 0 |
| Ectropion repair | 1 | 0 |
| Total No additional surgical procedures (P=0.003*) | 11 | 1 |
Obtained from Poisson regression analysis.
CPC indicates cyclophotocoagulation; GDD, glaucoma drainage device; SGDD, second glaucoma drainage device.
Pain
There were no significant differences in postoperative pain between the 2 treatment groups (P>0.05). At postoperative week 1, the mean participant self-reported pain score was 1.6 (±2.0; range: 0–7) and 1.4 (±1.5; range: 0–5) for the SGDD and CPC groups, respectively (P=0.83). At 1 month after the assigned treatment, the mean pain score was 1.4 (±2.3; range: 0–8) and 1.3 (±1.4; range: 0–4) for the SGDD and CPC groups, respectively (P=0.84).
Number of Office Visits
During the first 3 months, participants in the SGDD group had more frequent office visits [3.7 (±2.5; range: 1–9)] compared with the CPC group [0.9 (±1.2; range: 0–4), P<0.001, Poisson regression analysis]. After adjusting for the follow-up duration, the rate of office visits per month after month 3 was also significantly higher in the SGDD group [0.3 per month (±0.3; range: 0–1.3)] compared with the CPC group [0.1 (±0.1; range: 0–0.4), P<0.001, Poisson regression analysis].
VFQ-25
VFQ-25 results at baseline and month 12 are summarized in Supplemental Table 10, Supplemental Digital Content 4, http://links.lww.com/IJG/A638. One participant did not complete the VFQ-25 at baseline. At baseline, general health in participants assigned to the CPC group [48.7 (±21.2; range: 25–100)] was significantly worse than participants in the SGDD group [62.5 (±21.5; range: 25–100), P=0.05]. General vision [64.5 (±23.0; range: 25–100) for SGDD and 63.2 (±15.3; range: 25–100), P=0.82] and other visual function subscales were similar at the baseline (P>0.25).
In all, 27 participants (14 SGDD and 13 CPC) completed the VFQ-25 at the month 12 visit, at which time all visual function subscales were not different between groups (P≥0.15). Changes from baseline in visual function subscales were statistically similar in each group, except near vision in the SGDD group, which improved 9.6 (±11.7; range: −8.3 to 33.3) points (P=0.01).
DISCUSSION
The 1-year success rate in this study was 79% for SGDD and 88% for CPC (P=0.63), with no significant differences in IOP, postoperative number of IOP-lowering medications, or adverse events. Both groups achieved a significant and sustained reduction in IOP from baseline to 1 year postoperatively to a mean IOP <15 mm Hg. The number of additional glaucoma procedures, office visits during the first 3 months postoperatively, and office visits per month after month 3 were greater in the SGDD group.
Fewer IOP-lowering medications were used in the SGDD group early in the study through month 6. However, the difference was not statistically significant. By 6 months, the reduction in the number of medications from baseline seems similar between groups. This may represent the fact that the surgeons chose to leave patients on IOP-lowering medications immediately after CPC, and slowly taper off medication but immediately stop IOP-lowering medications after SGDD—and add them back as clinically needed. This is the result of the SGDD procedure, generally including a method of immediate IOP lowering (ie, vents or fenestrations) while clinicians may not be able to determine the extent of IOP lowering after CPC until later.
Three retrospective studies previously compared CPC with SGDD.14–16 These studies also showed that both the treatments were efficacious in lowering IOP and reducing the number of IOP-lowering medications. In addition, Schaefer et al and Wang et al showed that eyes treated with CPC had a higher early failure rate, whereas all the 3 studies showed that the SGDD group had more complications.14–16
Although this study did not reach enrollment targets, we believe it provides the highest level of evidence available comparing CPC versus SGDD because study subjects were randomized to treatment assignment and the clinical data were collected prospectively, in a rigorous and standardized manner. Randomization is particularly important because various baseline factors, such as age and visual acuity, can differentially influence a surgeon’s decision to recommend 1 of the 2 treatments, thereby, potentially confounding the results of a retrospective case series or prospective, nonrandomized clinical trial. A high failure rate of SGDD may have been expected because failure of the first GDD was likely due to the presence of an overly exuberant fibrotic capsule. As a thick fibrotic capsule was the likely cause of inadequate IOP reduction after the initial GDD implantation, it might be expected that a SGDD would also result in the formation of a similarly over exuberant fibrotic capsule and inadequate IOP lowering. The high success rate in the SGDD group in this study suggests that this is not the case and, possibly, that the increased total surface area of filtration can overcome the presence of a thick capsule.
Eyes in the CPC group had a nonstatistically significant decline in BCVA from baseline. Conversely, there was a nonstatistically significant improvement from baseline BCVA in the SGDD group. The reasons for the higher risk of late vision loss in the CPC group are unclear. Many of the underlying causes of BCVA loss observed in the study, such as macular edema, hypotony, and cataract progression, may have been directly attributable to the study procedures. The risk of a reduction in BCVA has long been known to be associated with diode laser CPC.17 This may occur even with modern, slow-burn laser settings,18,19 as well as with micropulse CPC.20,21 In a large retrospective series of 300 eyes, loss of 2 or more lines of Snellen visual acuity occurred in more than half of eyes, with at least 2 years of follow-up.22 Other studies, however, found no statistically significant change in visual acuity attributable to CPC, either compared with baseline23 or, in the case of a prospective clinical trial, to medical therapy.24 Overall, surgeons should counsel patients about the risk of vision loss with either procedure, though the risk may be higher with CPC.
The incidence of complications was higher in the SGDD group compared with the CPC group, although not statistically significant. Furthermore, the eyes in the SGDD group were more likely to require additional ocular procedures (P=0.003). This is consistent with previously published studies.2–4,14–16
There were no cases of prolonged hypotony in the CPC group. In addition, no eyes required retreatment in the early postoperative period. The lack of hypotony and retreatment may be related to the study treatment parameters for CPC and, perhaps, to residual outflow of an existing GDD that allowed a single session of CPC to control IOP.
The number of office visits was higher in the SGDD group during the first 3 months after surgery and higher overall. This represented almost 3 more visits over the first 3 months. A higher number of office visits may be important to consider in patients who cannot travel easily because of distance, level of visual impairment, or other factors. Finally, the assessment of quality of life with VFQ-25 revealed no statistically significant difference between groups at the month 12 visit (Table 10, Supplemental Digital Content 4, http://links.lww.com/IJG/A638).
Limitations
The major limitation of the study is the failure to achieve recruitment goals, resulting in decreased power to detect differences between groups. After 4 years, the Steering Committee and the DSMB agreed to halt recruitment. Given the small differences in success rates between groups in an interim analysis, the DSMB determined that completing enrollment was unlikely to alter the results. One challenge to recruitment may have been the introduction of a new method of performing CPC called micropulse CPC technique. This was excluded as a method of performing CPC, as it was too new with unclear efficacy. In addition, the recruitment centers had difficulty recruiting patients who met inclusion criteria. Finally, the study did not have funding for typical study costs such as for coordinator and testing fees, which may have placed financial constraints on recruitment. Despite these challenges, this study has a sample size that provides a higher level of evidence to compare these procedures. However, the sample size and limited follow-up duration make it more difficult to interpret results beyond 1 year. In contrast, extrapolation of the data is not suggesting differences over a longer time period—and there is no data in the study trending toward, or suggesting, any later differences.
Another limitation may be that the study procedures allowed a variation in technique (while including suggested parameters for treatment). For example, CPC had differences in the number of applications, power, and duration. Similarly, the SGDD group also included smaller glaucoma tube implants. It is unclear how these individual differences in CPC and SGDD treatment altered the results. However, these allowances in differences in treatment may provide more clinical translation. In addition, we were unable to determine whether the treatment or another etiology was the cause of a complication, such as macular edema (ie, diabetic vs. uveitic vs. postoperative).
CONCLUSIONS
Short-term overall success rates were high with either SGDD or CPC. However, SGDD was associated with more clinic visits and an increased risk of additional glaucoma surgery. Both treatments were reasonable options for eyes with inadequately controlled IOP after a single GDD.
Supplementary Material
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website, www.glaucomajournal.com.
ASSISTS Group
Steering Committee: Lauren S. Blieden, Nicholas P. Bell, Robert M. Feldman, David S. Greenfield, Ronald L. Gross, Jeffrey M. Liebmann, Steve L. Mansberger, Louis R. Pasquale, Robert N. Weinreb
Data Coordinating Center: Lauren S. Blieden and Alice Z. Chuang
Data and Safety Monitoring Board: Dale K. Heuer, Rebecca Lundstroth, Mark B. Sherwood, and Martha M. Wright
Study Managers: Laura A. Baker and Kimberly A. Mankiewicz.
Editorial Assistance: Kimberly A. Mankiewicz
Sites
Bascom Palmer Eye Institute—Miami, Miami, FL: Principal Investigator: Steven J. Gedde; Sub-Investigators: Ta Chen (Peter) Chang, Alana Grajewski, Richard Lee, Richard Parrish, Sarah Wellik, Luis Vazquez; Study Coordinator: Ruth Vandenbroucke.
Bascom Palmer Eye Institute—Palm Beach, Palm Beach Gardens, FL: Principal Investigator: Krishna Kishor; Sub-Investigators: David S. Greenfield, Arindel S. Maharaj; Study Coordinator: Chantey Robert
Devers Eye Institute/Legacy Health, Portland, OR: Principal Investigator: Steven L. Mansberger; Sub-Investigators: Claude Burgonye, Emily Jones, Robert Kinast; Study Coordinator: Gordon Barker, Casie Goldman
Feinberg School of Medicine, Northwestern University, Chicago, IL: Principal Investigator: Angelo P. Tanna; Sub-Investigator: Anupama Anchala; Study Coordinator: Crystal Santillanes, Nicole Seddon
Glaucoma Consultants of St. Louis, Chesterfield, MO: Principal Investigator: Paul M. Tesser; Study Coordinators: Jeremy Beatty, Angela Walters
Glaucoma Institute of Northern NJ, Rochelle Park, NJ: Principal Investigator: Paul Lama; Sub-Investigator: Linda Huang; Study Coordinator: Vanessa M. Mora
Massachusetts Eye and Ear Infirmary, Boston, MA: Principal Investigator: Ambika Hoguet; Sub-Investigators: Louis Pasquale; Study Coordinator: Ai Ren
New York Eye and Ear Infirmary of Mount Sinai, New York, NY: Principal Investigator: Joseph Panarelli; Sub-Investigators: Reena Garg, Paul Sidoti; Study Coordinators: Lorena Dominguez, Katy Tai, Meliza Unson
Robert Cizik Eye Clinic, Ruiz Department of Ophthalmology and Visual Science, McGovern Medical School at The University of Texas Health Science Center at Houston, Houston, TX: Principal Investigator: Nicholas P. Bell; Sub-Investigators: Lauren S. Blieden, Robert M. Feldman, and David A. Lee; Study Coordinators: Theodore Baker and Ephrem Melese
Stiles Eyecare Excellence and Glaucoma Institute, Overland Park, KS: Principal Investigator: Michael Stiles; Sub-Investigators: Ann Stechschulte and Amanda Strom; Study Coordinator: Jessica Punjabi
University of North Carolina Kittner Eye Center, Chapel Hill, NC: Principal Investigator: David Fleischman; Sub-Investigators: Donald L. Budenz and O’Rese Knight; Study Coordinators: Cassandra Barnhart and Elizabeth DuBose
Vanderbilt Eye Institute, Nashville, TN: Principal Investigator: Rachel Kuchtey; SubInvestigator: Eric Brown; Study Coordinator: Scott Ruark
West Virginia University Eye Institute, Morgantown, WV: Principal Investigator: Ronald L. Gross; Sub-Investigator: Brian McMillan
Wills Eye Hospital, Philadelphia, PA: Principal Investigator: Anand Mantravadi; Sub-Investigators: Scott Fudemberg, Daniel Lee, L. Jay Katz, and Jonathan Myers; Study Coordinator: Jeanne Molineaux, Lillian Nguyen, and Kamran Rahmatnejad
Footnotes
Presented in part at the 2021 American Academy of Ophthalmology Annual Meeting, New Orleans, LA, November 12–15, 2021.
This work was supported by an American Glaucoma Society Challenge Grant (San Francisco, CA); Research to Prevent Blindness (New York, NY); NIH/NEI Vision Core Grants P30EY028102 and P30EY010608 (Bethesda, MD); NIH/NCATS grants UL1 TR000371, UL1 TR000445, and UL1 TR001105 (Bethesda, MD); and NIH/NCATS Colorado CTSA Grant Number UL1TR002535 (REDCap).
The sponsors or funding organizations had no role in the design or conduct of this research. The contents of this publication are the authors’ sole responsibility and do not necessarily represent official NIH views.
Disclosure: Dr R.L.G. reports personal fees from Aerie during the conduct of the study; membership of Data and Safety Monitoring Board for Glauckos and consultant and stockholder for Intelligent Retinal Imaging Systems, outside the submitted work. Dr D.S.G. reports personal fees (consultant) from Allergan, Alcon, Aerie, and Eyenovia outside the submitted work. Dr L.R.P. reports grants from NIH, The Glaucoma Foundation, and Research to Prevent Blindness, as well as personal fees from Eyenovia, Twenty-Twenty, and Skye Biosciences outside the submitted work. Dr S.L.M. reports grants from AbbVie outside the submitted work. Dr A.P.T. reports grants or contracts from Google and Research to Prevent Blindness (to Northwestern University); consulting fees from Ivantis, Sandoz, and Zeiss; and payment for expert testimony from Ivantis. Dr R.M.F. reports personal fees from Bausch and Lomb and Catawba; stock in 4DMD; and grants from Santen and Ivantis outside the submitted work. The remaining authors declare no conflict of interest.
Contributor Information
Robert M. Feldman, Email: Robert.M.Feldman@uth.tmc.edu.
Alice Z. Chuang, Email: Alice.Z.Chuang@uth.tmc.edu.
Steve L. Mansberger, Email: SMansberger@deverseye.org.
Angelo P. Tanna, Email: atanna@northwestern.edu.
Lauren S. Blieden, Email: Lauren.Blieden@bcm.edu.
Nicholas P. Bell, Email: npbell.work@gmail.com.
Ronald L. Gross, Email: rongrossmd@gmail.com.
Louis R. Pasquale, Email: louis.pasquale@gmail.com.
David S. Greenfield, Email: d.greenfield@med.miami.edu.
Jeffrey M. Liebmann, Email: jml2314@cumc.columbia.edu.
Robert N. Weinreb, Email: rweinreb@health.ucsd.edu.
REFERENCES
- 1. Arora KS, Robin AL, Corcoran KJ, et al. Use of various glaucoma surgeries and procedures in Medicare beneficiaries from 1994 to 2012. Ophthalmology. 2015;122:1615–1624. [DOI] [PubMed] [Google Scholar]
- 2. Gedde SJ, Schiffman JC, Feuer WJ, et al. Treatment outcomes in the Tube Versus Trabeculectomy (TVT) study after five years of follow-up. Am J Ophthalmol. 2012;153:789–803; e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Budenz DL, Barton K, Gedde SJ, et al. Five-year treatment outcomes in the Ahmed Baerveldt comparison study. Ophthalmology. 2015;122:308–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Christakis PG, Kalenak JW, Tsai JC, et al. The Ahmed Versus Baerveldt study: five-year treatment outcomes. Ophthalmology. 2016;123:2093–2102. [DOI] [PubMed] [Google Scholar]
- 5. Anand A, Tello C, Sidoti PA, et al. Sequential glaucoma implants in refractory glaucoma. Am J Ophthalmol. 2010;149:95–101. [DOI] [PubMed] [Google Scholar]
- 6. Burgoyne JK, WuDunn D, Lakhani V, et al. Outcomes of sequential tube shunts in complicated glaucoma. Ophthalmology. 2000;107:309–314. [DOI] [PubMed] [Google Scholar]
- 7. Godfrey DG, Krishna R, Greenfield DS, et al. Implantation of second glaucoma drainage devices after failure of primary devices. Ophthalmic Surg Lasers. 2002;33:37–43. [PubMed] [Google Scholar]
- 8. Jimenez-Roman J, Gil-Carrasco F, Costa VP, et al. Intraocular pressure control after the implantation of a second Ahmed glaucoma valve. Int Ophthalmol. 2016;36:347–353. [DOI] [PubMed] [Google Scholar]
- 9. Ko SJ, Hwang YH, Ahn SI, et al. Surgical outcomes of additional Ahmed glaucoma valve implantation in refractory glaucoma. J Glaucoma. 2016;25:e620–e624. [DOI] [PubMed] [Google Scholar]
- 10. Ness PJ, Khaimi MA, Feldman RM, et al. Intermediate term safety and efficacy of transscleral cyclophotocoagulation after tube shunt failure. J Glaucoma. 2012;21:83–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Semchyshyn TM, Tsai JC, Joos KM. Supplemental transscleral diode laser cyclophotocoagulation after aqueous shunt placement in refractory glaucoma. Ophthalmology. Jun 2002;109:1078–1084. [DOI] [PubMed] [Google Scholar]
- 12. Smith M, Buys YM, Trope GE. Second Ahmed valve insertion in the same eye. J Glaucoma. 2009;18:336–340. [DOI] [PubMed] [Google Scholar]
- 13. Gordon MO, Kass MA. The Ocular Hypertension Treatment Study: design and baseline description of the participants. Arch Ophthalmol. 1999;117:573–583. [DOI] [PubMed] [Google Scholar]
- 14. Levinson JD, Giangiacomo AL, Beck AD, et al. A comparison of sequential glaucoma drainage device implantation versus cyclophotocoagulation following failure of a primary drainage device. J Glaucoma. 2017;26:311–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Schaefer JL, Levine MA, Martorana G, et al. Failed glaucoma drainage implant: long-term outcomes of a second glaucoma drainage device versus cyclophotocoagulation. Br J Ophthalmol. 2015;99:1718–1724. [DOI] [PubMed] [Google Scholar]
- 16. Wang MY, Patel K, Blieden LS, et al. Comparison of efficacy and complications of cyclophotocoagulation and second glaucoma drainage device after initial glaucoma drainage device failure. J Glaucoma. 2017;26:1010–1018. [DOI] [PubMed] [Google Scholar]
- 17. Kosoko O, Gaasterland DE, Pollack IP, et al. Long-term outcome of initial ciliary ablation with contact diode laser transscleral cyclophotocoagulation for severe glaucoma. The Diode Laser Ciliary Ablation Study Group. Ophthalmology. 1996;103:1294–1302. [DOI] [PubMed] [Google Scholar]
- 18. Duerr ER, Sayed MS, Moster S, et al. Transscleral diode laser cyclophotocoagulation: a comparison of slow coagulation and standard coagulation techniques. Ophthalmol Glaucoma. 2018;1:115–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Quigley HA. Improved outcomes for transscleral cyclophotocoagulation through optimized treatment parameters. J Glaucoma. 2018;27:674–681. [DOI] [PubMed] [Google Scholar]
- 20. Sarrafpour S, Saleh D, Ayoub S, et al. Micropulse transscleral cyclophotocoagulation: a look at long-term effectiveness and outcomes. Ophthalmol Glaucoma. 2019;2:167–171. [DOI] [PubMed] [Google Scholar]
- 21. Zaarour K, Abdelmassih Y, Arej N, et al. Outcomes of micropulse transscleral cyclophotocoagulation in uncontrolled glaucoma patients. J Glaucoma. 2019;28:270–275. [DOI] [PubMed] [Google Scholar]
- 22. Rasmuson E, Linden C, Lundberg B, et al. Efficacy and safety of transscleral cyclophotocoagulation in Swedish glaucoma patients. Acta Ophthalmol. 2019;97:764–770. [DOI] [PubMed] [Google Scholar]
- 23. Ansari E, Gandhewar J. Long-term efficacy and visual acuity following transscleral diode laser photocoagulation in cases of refractory and non-refractory glaucoma. Eye (Lond). 2007;21:936–940. [DOI] [PubMed] [Google Scholar]
- 24. Egbert PR, Fiadoyor S, Budenz DL, et al. Diode laser transscleral cyclophotocoagulation as a primary surgical treatment for primary open-angle glaucoma. Arch Ophthalmol. 2001;119:345–350. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website, www.glaucomajournal.com.


