Purpose of review
Gitelman syndrome is a recessive salt-wasting disorder characterized by hypomagnesemia, hypokalemia, metabolic alkalosis and hypocalciuria. The majority of patients are explained by mutations and deletions in the SLC12A3 gene, encoding the Na+-Cl−-co-transporter (NCC). Recently, additional genetic causes of Gitelman-like syndromes have been identified that should be considered in genetic screening. This review aims to provide a comprehensive overview of the clinical, genetic and mechanistic aspects of Gitelman(-like) syndromes.
Recent findings
Disturbed Na+ reabsorption in the distal convoluted tubule (DCT) is associated with hypomagnesemia and hypokalemic alkalosis. In Gitelman syndrome, loss-of-function mutations in SLC12A3 cause impaired NCC-mediated Na+ reabsorption. In addition, patients with mutations in CLCKNB, KCNJ10, FXYD2 or HNF1B may present with a similar phenotype, as these mutations indirectly reduce NCC activity. Furthermore, genetic investigations of patients with Na+-wasting tubulopathy have resulted in the identification of pathogenic variants in MT-TI, MT-TF, KCNJ16 and ATP1A1. These novel findings highlight the importance of cell metabolism and basolateral membrane potential for Na+ reabsorption in the DCT.
Summary
Altogether, these findings extend the genetic spectrum of Gitelman-like electrolyte alterations. Genetic testing of patients with hypomagnesemia and hypokalemia should cover a panel of genes involved in Gitelman-like syndromes, including the mitochondrial genome.
Keywords: Gitelman syndrome, mitochondria, Na+-Cl−-co-transporter, salt-wasting, tubulopathy
INTRODUCTION
Gitelman syndrome is a recessive salt-wasting disorder characterized by hypomagnesemia, hypokalemia, metabolic alkalosis, hypocalciuria and activation of the renin-angiotensin-aldosterone system (RAAS) [1,2]. Patients often present in late childhood or early adulthood with nonspecific symptoms, including muscle weakness, tetany, hypotension and fatigue [3,4]. Typical complaints may also include salt craving and thirst as a reflection of salt-wasting. Gitelman syndrome is not a benign condition and may cause chondrocalcinosis due to hypomagnesemia, prolonged QTc interval and arrhythmias due to hypokalemia, glucose intolerance and immunodeficiencies [5–8]. The disease was first described in 1966 by Hillel Gitelman as a subtype of Bartter syndrome [2]. However, typical Bartter symptoms such as polyhydramnios, hypercalciuria, nephrocalcinosis, failure to thrive and an antenatal presentation are rare in Gitelman syndrome. Indeed, genetic investigations in the 1990 s revealed that Bartter and Gitelman syndrome are separate clinical entities [9–13].
Classic Gitelman syndrome is caused by biallelic mutations in solute carrier 12 subtype 3 (SLC12A3) encoding the Na+-Cl−-co-transporter (NCC), which is exclusively expressed in the distal convoluted tubule (DCT) [13]. The NCC facilitates apical Na+ and Cl− transport in the DCT and is the therapeutic target of thiazide diuretics. As a consequence of impaired NCC-mediated Na+ reabsorption in the DCT, the Na+ delivery to the collecting duct is increased. Accompanied by RAAS activation, the high Na+ delivery results in increased K+ secretion in the collecting duct explaining the hypokalemia in Gitelman patients. The metabolic alkalosis develops secondary to hypokalemia. The hypomagnesemia is less well understood (extensively reviewed in [14]), but it is generally thought that a reduced DCT mass is a major contributor to this defect [15]. However, human data supporting this hypothesis are scarce.
In recent years, several seminal discoveries have been made to resolve the missing heritability in Gitelman syndrome [16▪,17▪▪]. This review, therefore, provides an overview of all known genetic causes of Gitelman-like syndromes. The differences in clinical presentation, genetic inheritance and molecular disease mechanism will be discussed.
Box 1.
no caption available
SLC12A3 – CLASSIC GITELMAN
In 1996, Simon et al. [2] described homozygous and compound heterozygous loss-of-function mutations in SLC12A3 as cause of Gitelman syndrome. Since then, 133 pathogenic variants have been described (ClinVar, February 2022), including deletions, splice site variants and intronic variants. In most recent screenings, approximately 75% of patients with a Gitelman syndrome presentation are diagnosed with a biallelic mutation in SLC12A3[18,19]. Of them, 20–25% have a homozygous pathogenic variant, 60–70% are compound heterozygous and ±10% have genomic rearrangements (deletion/duplication), which can be picked up by multiplex ligation-dependent probe amplification (MLPA) [18]. Homozygous mutations have been associated with an earlier age of onset and more severe hypocalciuria in a Chinese cohort [19]. In contrast, no phenotypic differences were reported for genomic rearrangements.
In-depth phenotyping of Gitelman patients with SLC12A3 mutations has resulted in the identification of subclinical phenotypes [5,20▪]. In a large European cohort, 20% of patients with Gitelman syndrome had hypoparathyroidism [20▪]. As the parathyroid harmone (PTH) and magnesium concentrations were correlated in this cohort, it has been hypothesized that the hypoparathyroidism is explained by Mg2+-dependent regulation of the calcium-sensing receptor [21]. Alternatively, a positive Ca2+ balance may contribute to hypoparathyroidism in Gitelman syndrome. Several studies reported increased fasting glucose levels and insulin resistance in Gitelman patients [5,22,23]. In a large cohort of 77 patients, the insulin response was almost doubled upon glucose loading, which was associated with a significant increase of the insulin resistance index [5]. Indeed, diabetes mellitus has been reported in one-third of the patients in a Chinese cohort study [24]. Again, hypomagnesemia may (partially) explain the insulin resistance in Gitelman syndrome, as Mg2+ is essential for the insulin signalling pathway [25,26].
Interestingly, only one pathogenic variant is discovered in 10–15% of all Gitelman patients, even after screening for genomic rearrangements [18]. In these cases, mutations may be present in regulatory regions such as promoters and introns. Moreover, two patients were reported with a digenic inheritance pattern consisting of a heterozygous SLC12A3 variant and a heterozygous CLCKNB variant [27,28]. However, it should be noted that it has not been conclusively demonstrated that digenic inheritance can cause Gitelman syndrome. Given that 2–8% of the population are carriers of one pathogenic SLC12A3 variant and the percentage of carriers of one pathogenic CLCKNB variant may be similar, many patients should be affected by such an inheritance pattern [29].
Carriers of a single heterozygous pathogenic variant in SLC12A3 were longtime considered healthy. However, recent studies have demonstrated the presence of a subclinical phenotype in heterozygous carriers [5,30▪▪]. Plasma aldosterone was slightly increased in carriers of heterozygous pathogenic SLC12A3 variants [5]. Moreover, heterozygous carriers exhibited a slightly higher plasma Ca2+ concentration and lower plasma PTH concentration compared with controls. A recent study in the Old Order Amish population demonstrated that heterozygous carriers of the pathogenic p.R642G variant had significantly lower serum potassium levels than noncarriers [30▪▪]. These clinical findings are in line with mechanistic studies demonstrating the close connection of NCC and K+ regulation, termed the ‘potassium switch’ [31]. In short, the potassium switch turns on NCC in response to low dietary K+ intake and off in response to high K+ intake (Fig. 1) [32,33]. As such, K+ is currently considered as the main regulator of NCC activity, acting as a natural thiazide diuretic [34].
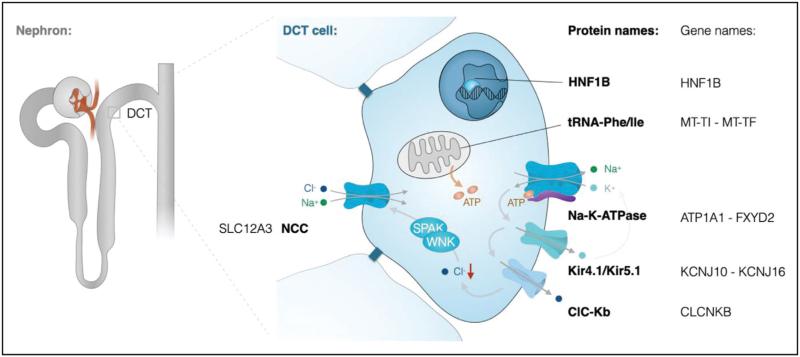
FIGURE 1.
Na+ reabsorption in the distal convoluted tubule. Schematic overview of a distal convoluted tubule cell indicating all genes and proteins that have been associated with Gitelman syndrome. Na+ enters the cell at the luminal membrane via the Na+-Cl− co-transporter (NCC). At the basolateral membrane, Na+ is extruded from the cell by the Na+-K+ ATPase. The ATP production required for Na+-K+ ATPase activity is dependent on mitochondrial function. Basolateral recycling of K+ via Kir4.1/Kir5.1 channels is essential to drive the Na+-K+ ATPase and Cl-extrusion via ClC-Kb Cl− channels. Low intracellular Cl− concentrations activate an intracellular signalling cascade of WNK and SPAK kinases, which results in phosphorylation of NCC.
Altogether, these studies demonstrate that common genetic variants and heterozygous pathogenic variants in SLC12A3 may contribute to subclinical phenotypes in the general population.
MT-TI / MT-TF – MITOCHONDRIAL GITELMAN
In 2004, Lifton et al. [35] first described mutations in the mitochondrial transfer RNA (tRNA) for isoleucine, encoded by the MT-TI gene, in a large family with renal hypomagnesemia, hypokalemia and hypocalciuria. Only recently, these findings were confirmed in 10 additional families with a maternal inheritance pattern [17▪▪]. A large European collaboration demonstrated that mitochondrial DNA variants in MT-TI and MT-TF are causative for a Gitelman-like syndrome [17▪▪]. Interestingly, the MT-TF mutations were also associated with chronic kidney disease, whereas patients with MT-TI mutations showed a preserved kidney function [17▪▪]. Hypertension and dyslipidemia that were originally described to be part of the phenotype were not reported in these additional families, questioning whether this initial association was correct.
The identification of mitochondrial DNA mutations demonstrated the essential role of mitochondria for renal Na+ reabsorption. The DCT cell is the most mitochondria-rich cell type within the kidney in order to meet the high energy demand required for electrolyte transport [36]. In patient fibroblasts, the identified MT-TI and MT-TF mutations were demonstrated to reduce mitochondrial function [17▪▪]. Although the exact mechanisms remain unclear, pharmacological inhibition of complex IV, mimicking the effect of the mtDNA variants, inhibited NCC phosphorylation and NCC-mediated Na+ uptake [17▪▪]. However, it should be noted that only specific MT-TI and MT-TF mutations are associated with a Gitelman-like phenotype. Particularly, the m.591C>T and m.4291T>C variants are hotspot mutations. Other MT-TI and MT-TF mutations also resulting in reduced mitochondrial function have been associated with other syndromes such as mitochondrial encephalomyopathy, lactic acidosis and stroke-like episodes (MELAS) and myoclonic epilepsy with ragged-red fibres (MERRF) [37]. Consequently, one may consider additional pathophysiological mechanisms such as disturbances in tRNA modifications or effects of mitochondrial DNA fragments [38,39].
CLCNKB – BARTTER TYPE 3
Although recessive CLCKNB mutations have originally been described to cause classic Bartter syndrome (type 3), a systematic analysis of a large cohort of patients demonstrates that 25% of all patients present with a Gitelman syndrome phenotype [10,40,41]. In fact, some patients may initially show symptoms of Bartter syndrome and develop a typical Gitelman phenotype in later childhood or adolescence [42]. Consequently, genetic screening of patients with a clinical diagnosis of Gitelman syndrome quite regularly turn out to have CLCKNB mutations upon genetic screening [43,44]. As large deletions account for up to 40% of all cases of Bartter syndrome type 3, testing for structural variations by MLPA or other means is advised [41]. Compared with classic Gitelman syndrome, patients with CLCKNB mutations have generally an earlier age of initial presentations and slightly higher serum Mg2+ and urinary Ca2+ concentrations [45,46]. Patients with CLCKNB mutations may additionally develop chronic kidney disease (up to 25%), nephrocalcinosis (10–20%) or growth retardation [41,46].
CLCKNB encodes the ClCKb Cl− channel that is expressed in the TAL, DCT and collecting duct. Loss-of-function mutations in ClCKb result in an increased intracellular Cl− concentration. As Cl− inhibits WNK kinases, an increased Cl− concentration causes reduced NCC activity by inhibition of the WNK-SPAK/OSR1 pathway (Fig. 1) [34,47,48]. A similar regulatory mechanism of NKCC2 exists in the TAL, which explains why ClCKb mutations may result in both Bartter-like and Gitelman-like syndromes [49]. In general, hypochloremia and increased fractional excretions of Na+ and Cl− are more severe in Bartter syndrome type 3 than in Gitelman syndrome, which may reflect that both TAL and DCT are affected by CLCKNB mutations [45].
KCNJ10/ KCNJ16 – EAST / SESAME
The acronym EAST/SeSAME syndrome describes a disease entity with autosomal recessive inheritance combining epilepsy, ataxia, sensorineural deafness and renal tubulopathy with/without mental retardation [50,51]. Patients usually present early in infancy with seizures, developmental delay and ataxia. The renal phenotype closely resembles Gitelman syndrome comprising hypokalemic alkalosis, hypomagnesemia and hypocalciuria. EAST/SeSAME syndrome is caused by loss-of-function mutations in the KCNJ10 gene encoding the inwardly rectifying K+-channel Kir4.1 [50,51]. In the kidney, Kir4.1 is predominantly expressed at the basolateral membrane of cTAL, DCT and CNT cells. Here, it forms heteromers with its close homologue Kir5.1 (KCNJ16). Kir4.1/Kir5.1 potassium channels serve as a K+ sensor of DCT cells [14,34] that allow for a recycling of K+ to drive Na+-K+-ATPase activity [32,52]. Uncoupling of this ‘pump-leak mechanisms’ will result in depolarization of the basolateral membrane and increased intracellular Cl− concentrations, similar to mutations in ClCKb (Fig. 1) [10]. Consequently, the WNK-SPAK/OSR1 signalling cascade is inhibited resulting in reduced NCC activity.
Recently, recessive loss-of-function mutations have also been described in KCNJ16 leading to a tubulopathy with deafness [16▪,53]. Apart from renal salt wasting and hypokalemia, patients may present with opposite changes in acid-base metabolism that are thought to result from a broader expression pattern and more diverse tasks of Kir5.1: In addition to its role in the DCT outlined above, Kir5.1 also forms heteromers with Kir4.2 (KCNJ15) in the proximal tubule that are critical for bicarbonate reabsorption and ammonia excretion [54]. However, if distal tubular salt wasting predominates, patients with KCNJ16 mutations may present with hypokalemic alkalosis and a Gitelman syndrome-like phenotype [16▪].
ATP1A1/ FXYD2 – NA+-K+-ATPASE DYSFUNCTION
More than two decades ago, a missense mutation in FXYD2 encoding the γ-subunit of Na+K+-ATPase was described in two related families. The index patients presented with seizures during childhood and profound hypomagnesemia [55,56]. Laboratory investigations revealed low serum Mg2+ levels also in numerous, apparently healthy family members. In addition, urinary Ca2+ excretion rates were found to be low, a finding reminiscent of patients presenting with Gitelman Syndrome. Later, a careful biochemical workup in members of two additional families with the identical mutation also revealed a tendency towards hypokalemia and metabolic alkalosis. Additional clinical findings in affected members of these families comprised muscle cramps, seizures and chondrocalcinosis [55–57].
Members of the FXYD protein family constitute a third, tissue-specific γ-subunit of Na+-K+-ATPase. FXYD2 is expressed in the distal nephron, especially in the DCT and connecting tubule [58]. Here, the FXYD2 γ-subunit increases the apparent affinity of Na+-K+-ATPase for ATP while decreasing its Na+ affinity providing a mechanism for balancing energy utilization and maintaining appropriate salt gradients [59]. Expression studies of mutant p.G41R-FXYD2 revealed a dominant-negative effect leading to a retention of the γ-subunit in the Golgi complex [60].
Recently, also heterozygous de-novo mutations in the α1-subunit of Na+K+-ATPase (ATP1A1) have been described leading to severe hypomagnesemia due to renal magnesium wasting [61]. Affected children presented in infancy with seizures that were not responsive to antiepileptic medication and did not respond to magnesium supplementation. Unfortunately, all three children developed a significant degree of mental retardation and global developmental delay. In addition, episodes of hypokalemia and elevated bicarbonate levels potentially indicated renal salt wasting [61].
The α1-subunit ATP1A1 represents the exclusive α-subunit of Na+K+-ATPase in kidney. Here, the DCT represents the tubular segment with the highest energy consumption and density of Na+K+-ATPase that generates a favourable electrochemical gradient for transcellular salt and magnesium reabsorption. Moreover, the α1-subunit is ubiquitiously expressed and thought to maintain neuronal housekeeping functions in the central nervous system [62]. The ATP1A1 mutations discovered in hypomagnesemic children were shown to not only lead to a loss of ATPase function, but also to result in abnormal ion permeabilities and leak currents [61].
Whereas in children with ATP1A1 mutations, the severe neurological phenotype is clearly distinct from GS; both entities, FXYD2 and ATP1A1, share a renal GS-like phenotype even though a profound renal magnesium loss prevails.
HNF1B – ADTKD-HNF1B
Hypomagnesemia and hypocalciuria are common in patients with heterozygous HNF1β mutations and deletions [63–66]. In a minor group of patients, these electrolyte disturbances are accompanied by hypokalemia and metabolic alkalosis [67,68] (Table 1). In addition, patients with HNF1β nephropathy often present with symptoms beyond a Gitelman-like phenotype including, but not limited to, tubule interstitial kidney disease (ADTKD), renal cysts, renal hypoplasia, hyperuricemia, hyperparathyroidism, maturity-onset diabetes of the young (MODY5), neurodevelopmental disorders, or genital and urinary tract malformations [64,69–72]. Approximately 50% of ADTKD-HNF1β patients develop chronic kidney disease [67,71,73]. HNF1β defects are therefore among the most common causes of childhood kidney transplantation [74,75]. Interestingly, in some cases, the electrolyte disturbances might represent the first symptom of the disease [63]. Consequently, the initial diagnosis of HNF1β nephropathy has sometimes been Gitelman syndrome, until genetic investigations revealed mutations in the HNF1β gene [43]. Of note, renin-angiotensin-aldosterone system (RAAS) activation is scarce in patients with HNF1β defects, whereas it is one of the main symptoms of Gitelman syndrome. Moreover, hypertension is present in 22% of children with HNF1β nephropathy [76]. Gitelman patients are generally hypotensive compared with healthy family members, though cases with hypertension in later life have been described [6,77]. Several reports noted that young children with HNF1β defects have generally higher serum Mg2+ levels than older patients [63,68,72]. It has, therefore, been proposed that hypomagnesemia developed later in childhood. However, Kolbuc et al. [65] recently showed that serum Mg2+ levels are also higher in early childhood of healthy controls. Consequently, the reference range of 0.7–1.1 mmol/l may not be suitable for young children, resulting in an underestimation of hypomagnesemia in early childhood of ADTKD-HNF1β patients.
Table 1.
Overview of Gitelman(-like) sydromes
| Gene | Protein | Disease | OMIM | Inh. | Onset | Mg2+ | K+ | HCO3− | FECa2+ | RAAS | Other symptoms | Ref |
| SLC12A3 | NCC | Classic Gitelman syndrome | 263800 | R | Childhood Adolescence | ↓ | ↓ | ↑ | ↓ | ↑ | Chondrocalcinosis | [1,2,13,46] |
| MT-TI | Mitochondrial tRNA-Ile | Mitochondrial Gitelman syndrome | M | Adult | ↓ | ↓ | =/↑ | ↓ | =/↑ | [17▪▪,35] | ||
| MT-TF | Mitochondrial tRNA-Phe | Mitochondrial Gitelman syndrome | M | Childhood Adult | ↓ | ↓ | =/↑ | ↓ | =/↑ | CKD | [17▪▪] | |
| CLCNKB | ClCKb | Bartter syndrome type III | 607364 | R | Neonatal Childhood | ↓/= | ↓ | ↑ | ↓/=/↑ | ↑ | CKD | [10,45,46] |
| KCNJ10 | Kir4.1 | SESAME / EAST syndrome | 612780 | R | Neonatal | ↓ | ↓ | ↑ | ↓ | ↑ | Epilepsy, ataxia, sensorineural deafness | [50,51] |
| KCNJ16 | Kir5.1 | 619406 | R | ↓/= | ↓ | ↓/↑ | ↓ | ↑ | Deafness | [16▪,53] | ||
| FXYD2 | γ-subunit of the Na+-K+-ATPase | 154020 | D | Childhood Adult | ↓ | </= | =/↑ | ↓ | Chondrocalcinosis | [56,57] | ||
| ATP1A1 | α-subunit of the Na+-K+-ATPase | 618314 | D | Neonatal | ↓ | ↓/= | = | ↓/=/↑ | = | Intellectual disability | [61] | |
| HNF1B | HNF1β | ADTKD-HNF1B | 137920 | D | Neonatal Childhood | ↓/= | = | = | ↓ | =/↑ | CAKUT MODY5 | [66,68] |
In the DCT, HNF1β acts a transcription factor that regulates the expression of several proteins in the regulatory pathway towards NCC, including FXYD2 and KCNJ16[69,78,79]. Potassium channel Kir4.1/Kir5.1 and the Na+-K+-ATPase activity are both essential components of the ‘pump-leak mechanism’ regulating the membrane potential and basolateral Cl− transport. Disturbed transcription of FXYD2 and KCNJ16 thereby results in reduced NCC activity by the same mechanisms as described above. Clinical studies confirmed that ADTKD-HNF1β patients have reduced NCC activity, as indicated by a diminished response to thiazide [80]. In line with these findings, NCC expression is decreased in kidney-specific HNF1β knock-out mice [79].
OTHER GENES
Several other non-Bartter, non-Gitelman syndromes are associated with salt-wasting, hypomagnesemia and hypokalemic alkalosis. Although these syndromes are independent of NCC dysfunction and therefore do not present as classical Gitelman syndrome, the presentation of individual patient may sometimes be, at least partially, similar.
Hypomagnesemia, hypokalemia and metabolic alkalosis are the cardinal symptoms of patients with mTOR-activating mutations in RRAGD, encoding a small Rag GTPase [81]. These patients often present with nephrocalcinosis and/or cardiomyopathy. As this disorder is often associated with renal Ca2+ wasting, it is hypothesized that RRAGD mutations primarily cause a defect in the TAL [81]. However, DCT defects cannot be excluded as RRAGD is also expressed in this segment of the nephron [81].
Impaired transcellular transport in the TAL is also the cause of salt-wasting in patient with CLDN10 mutations. Patients suffer from hypokalemic hypochloremic alkalosis and RAAS activation, but generally present with hypermagnesemia [82,83]. Additional symptoms of CLDN10 patients include dysfunctional salivary, sweat and lacrimal glands [83].
Hypomagnesemia is frequently associated with hypokalemia. This effect is generally explained by the inhibitory effect of Mg2+ on ROMK-mediated K+ secretion in the distal nephron [84]. In case of Mg2+ deficiency, more K+ is wasted in the urine resulting in hypokalemia. Genetic syndromes of isolated hypomagnesemia, for example by mutations in TRPM6, KCNA1, EGF, CNNM2 or PCBD1 may therefore present with transient episodes of hypokalemia [85–91]. However, these patients are generally without metabolic alkalosis or RAAS activation.
NONGENETIC CAUSES OF GITELMAN-LIKE ELECTROLYTE ABNORMALITIES
Although it goes beyond the scope of this review to discuss all noninherited conditions that can mimic the presentation of Gitelman syndrome, it is important to consider alternative causes of Gitelman syndrome in clinical practice. In particular, abuse of diuretics (most notably thiazides) may result in an identical presentation [92,93]. In addition, chronic use of proton-pump inhibitors, aminoglycosides or laxatives is accompanied by hypokalemia and hypomagnesemia, although metabolic alkalosis is generally absent [92]. Other causes of hypokalemia may include chronic vomiting and primary hyperaldosteronism, but the latter condition is associated with hypertension and a suppressed RAAS [94]. Further guidance on the clinical workup and treatment of Gitelman syndrome is provided by KDIGO [1].
CONCLUSION AND PERSPECTIVES
The discovery of SLC12A3 mutations in the 1990 s established a defective salt reabsorption in the DCT as the underlying pathophysiology of Gitelman syndrome. Genetic heterogeneity of Gitelman syndrome was first demonstrated by the discovery of CLCNKB mutations in patients with a typical Gitelman syndrome-like phenotype. Since then, advances in genetics have led to the discovery of a growing number of hereditary disorders that present with the pathognomonic Gitelman syndrome signature comprising hypokalemic alkalosis, hypomagnesemia and hypocalciuria. Beyond representing important differential diagnoses for the molecular screening of affected patients, these entities not only underline the complex integrative role, but also vulnerability of the Na+ reabsorption machinery in the DCT. Here, transport processes are particularly dependent on cellular electrolyte homeostasis, energy level, respiratory capacity and regulatory pathways. This hereditary and phenotypic complexity will have to be taken into account by NGS-based analytic techniques as well as genetic counselling of the affected families. It appears reasonable to assume that future genetic studies will further expand the spectrum of disorders leading to defective DCT-mediated salt reabsorption or exhibiting the Gitelman syndrome-triad of hypokalemic alkalosis, hypomagnesemia and hypocalciuria as part of a more complex phenotype.
Acknowledgements
None.
Financial support and sponsorship
This work was financially supported by ZonMW under the frame of EJPRD, the European Joint Programme on Rare Diseases (EJPRD2019–40) and by the IMAGEN project, which is co-funded by the PPP Allowance made available by Health∼Holland, Top Sector Life Sciences & Health, to stimulate public-private partnerships (IMplementation of Advancements in GENetic Kidney Disease, LSHM20009) and the Dutch Kidney Foundation (20OP+018). In addition, this project has received funding from the European Union's Horizon 2020 research and innovation programme under the EJP RD COFUND-EJP No. 825575 and the European Research Council (IN-THE-KIDNEY No. 101040682).
Conflicts of interest
There are no conflicts of interest.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
REFERENCES
- 1.Blanchard A, Bockenhauer D, Bolignano D, et al. Gitelman syndrome: consensus and guidance from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int 2017; 91:24–33. [DOI] [PubMed] [Google Scholar]
- 2.Gitelman HJ, Graham JB, Welt LG. A new familial disorder characterized by hypokalemia and hypomagnesemia. Trans Assoc Am Physicians 1966; 79:221–235. [PubMed] [Google Scholar]
- 3.Devuyst O, Belge H, Konrad M. Avner ED, Harmon WE, Niaudet P, Yoshikawa N, Emma F, et al. Renal tubular disorders of electrolyte regulation in children. Pediatric nephrology. Goldstein, SL: Springer Berlin Heidelberg; 2016. 1201–1271. [Google Scholar]
- 4.Seyberth HW, Schlingmann KP. Bartter- and Gitelman-like syndromes: salt-losing tubulopathies with loop or DCT defects. Pediatr Nephrol 2011; 26:1789–1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blanchard A, Vallet M, Dubourg L, et al. Resistance to insulin in patients with Gitelman syndrome and a subtle intermediate phenotype in heterozygous carriers: a cross-sectional study. J Am Soc Nephrol 2019; 30:1534–1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cruz DN, Shaer AJ, Bia MJ, et al. Bartter's Syndrome Collaborative Study G. Gitelman's syndrome revisited: an evaluation of symptoms and health-related quality of life. Kidney Int 2001; 59:710–717. [DOI] [PubMed] [Google Scholar]
- 7.Foglia PE, Bettinelli A, Tosetto C, et al. Cardiac work up in primary renal hypokalaemia-hypomagnesaemia (Gitelman syndrome). Nephrol Dial Transplant 2004; 19:1398–1402. [DOI] [PubMed] [Google Scholar]
- 8.Evans RDR, Antonelou M, Sathiananthamoorthy S, et al. Inherited salt-losing tubulopathies are associated with immunodeficiency due to impaired IL-17 responses. Nat Commun 2020; 11:4368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Birkenhager R, Otto E, Schurmann MJ, et al. Mutation of BSND causes Bartter syndrome with sensorineural deafness and kidney failure. Nat Genet 2001; 29:310–314. [DOI] [PubMed] [Google Scholar]
- 10.Simon DB, Bindra RS, Mansfield TA, et al. Mutations in the chloride channel gene, CLCNKB, cause Bartter's syndrome type III. Nat Genet 1997; 17:171–178. [DOI] [PubMed] [Google Scholar]
- 11.Simon DB, Karet FE, Hamdan JM, et al. Bartter's syndrome, hypokalaemic alkalosis with hypercalciuria, is caused by mutations in the Na-K-2Cl cotransporter NKCC2. Nat Genet 1996; 13:183–188. [DOI] [PubMed] [Google Scholar]
- 12.Simon DB, Karet FE, Rodriguez-Soriano J, et al. Genetic heterogeneity of Bartter's syndrome revealed by mutations in the K+ channel, ROMK. Nat Genet 1996; 14:152–156. [DOI] [PubMed] [Google Scholar]
- 13.Simon DB, Nelson-Williams C, Bia MJ, et al. Gitelman's variant of Bartter's syndrome, inherited hypokalaemic alkalosis, is caused by mutations in the thiazide-sensitive Na-Cl cotransporter. Nat Genet 1996; 12:24–30. [DOI] [PubMed] [Google Scholar]
- 14.Franken GAC, Adella A, Bindels RJM, de Baaij JHF. Mechanisms coupling sodium and magnesium reabsorption in the distal convoluted tubule of the kidney. Acta Physiol (Oxf) 2021; 231:e13528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Loffing J, Vallon V, Loffing-Cueni D, et al. Altered renal distal tubule structure and renal Na(+) and Ca(2+) handling in a mouse model for Gitelman's syndrome. J Am Soc Nephrol 2004; 15:2276–2288. [DOI] [PubMed] [Google Scholar]
- 16▪.Schlingmann KP, Renigunta A, Hoorn EJ, et al. Defects in KCNJ16 cause a novel tubulopathy with hypokalemia, salt wasting, disturbed acid-base homeostasis, and sensorineural deafness. J Am Soc Nephrol 2021; 32:1498–1512. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study identified KCNJ16 mutations in patients with hypokalaemia and salt-wasting. The authors postulate a novel physiological mechanisms explaining different patient phenotypes based on interaction partners of the Kir5.1 (KCNJ16) protein.
- 17▪▪.Viering D, Schlingmann KP, Hureaux M, et al. Gitelman-like syndrome caused by pathogenic variants in mtDNA. J Am Soc Nephrol 2022; 33:305–325. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study describes the identification of mitochondrial DNA mutations as a cause for Gitelman syndrome. This finding highlights the importance of mitochondria for DCT Na+ and Cl− transport.
- 18.Vargas-Poussou R, Dahan K, Kahila D, et al. Spectrum of mutations in Gitelman syndrome. J Am Soc Nephrol 2011; 22:693–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shen Q, Chen J, Yu M, et al. Multicentre study of the clinical features and gene variant spectrum of Gitelman syndrome in Chinese children. Clin Genet 2021; 99:558–564. [DOI] [PubMed] [Google Scholar]
- 20▪.Verploegen MFA, Vargas-Poussou R, Walsh SB, et al. Parathyroid hormone and phosphate homeostasis in patients with Bartter and Gitelman syndrome: an international cross-sectional study. Nephrol Dial Transplant 2022; doi: 10.1093/ndt/gfac029. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study demonstrates that patients with Bartter and Gitelman syndrome often present with disturbances in phosphate homeostasis and PTH levels. It provides further insight in the complex pathophysiology of Gitelman syndrome.
- 21.Zhang C, Zhang T, Zou J, et al. Structural basis for regulation of human calcium-sensing receptor by magnesium ions and an unexpected tryptophan derivative co-agonist. Sci Adv 2016; 2:e1600241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ren H, Qin L, Wang W, et al. Abnormal glucose metabolism and insulin sensitivity in Chinese patients with Gitelman syndrome. Am J Nephrol 2013; 37:152–157. [DOI] [PubMed] [Google Scholar]
- 23.Yuan T, Jiang L, Chen C, et al. Glucose tolerance and insulin responsiveness in Gitelman syndrome patients. Endocr Connect 2017; 6:243–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu T, Wang C, Lu J, et al. Genotype/phenotype analysis in 67 Chinese patients with Gitelman's syndrome. Am J Nephrol 2016; 44:159–168. [DOI] [PubMed] [Google Scholar]
- 25.Gommers LM, Hoenderop JG, Bindels RJ, de Baaij JH. Hypomagnesemia in Type 2 diabetes: a vicious circle? Diabetes 2016; 65:3–13. [DOI] [PubMed] [Google Scholar]
- 26.Kurstjens S, de Baaij JH, Bouras H, et al. Determinants of hypomagnesemia in patients with type 2 diabetes mellitus. Eur J Endocrinol 2017; 176:11–19. [DOI] [PubMed] [Google Scholar]
- 27.Bettinelli A, Borsa N, Syren ML, et al. Simultaneous mutations in the CLCNKB and SLC12A3 genes in two siblings with phenotypic heterogeneity in classic Bartter syndrome. Pediatr Res 2005; 58:1269–1273. [DOI] [PubMed] [Google Scholar]
- 28.Kong Y, Xu K, Yuan K, et al. Digenetic inheritance of SLC12A3 and CLCNKB genes in a Chinese girl with Gitelman syndrome. BMC Pediatr 2019; 19:114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kondo A, Nagano C, Ishiko S, et al. Examination of the predicted prevalence of Gitelman syndrome by ethnicity based on genome databases. Sci Rep 2021; 11:16099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30▪▪.Wan X, Perry J, Zhang H, et al. Heterozygosity for a pathogenic variant in SLC12A3 that causes autosomal recessive Gitelman syndrome is associated with lower serum potassium. J Am Soc Nephrol 2021; 32:756–765. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study demonstrates that heterozygous mutations in SLC12A3 are associated with lower serum potassium and chloride levels. It highlights the presence of subclinical phenotypes in heterozygous carriers of Gitelman syndrome.
- 31.Ellison DH, Welling P. Insights into salt handling and blood pressure. N Engl J Med 2021; 385:1981–1993. [DOI] [PubMed] [Google Scholar]
- 32.Terker AS, Zhang C, McCormick JA, et al. Potassium modulates electrolyte balance and blood pressure through effects on distal cell voltage and chloride. Cell Metab 2015; 21:39–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang MX, Cuevas CA, Su XT, et al. Potassium intake modulates the thiazide-sensitive sodium-chloride cotransporter (NCC) activity via the Kir4.1 potassium channel. Kidney Int 2018; 93:893–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hoorn EJ, Gritter M, Cuevas CA, Fenton RA. Regulation of the renal NaCl cotransporter and its role in potassium homeostasis. Physiol Rev 2020; 100:321–356. [DOI] [PubMed] [Google Scholar]
- 35.Wilson FH, Hariri A, Farhi A, et al. A cluster of metabolic defects caused by mutation in a mitochondrial tRNA. Science 2004; 306:1190–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hansell P, Welch WJ, Blantz RC, Palm F. Determinants of kidney oxygen consumption and their relationship to tissue oxygen tension in diabetes and hypertension. Clin Exp Pharmacol Physiol 2013; 40:123–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Webb BD, Diaz GA, Prasun P. Mitochondrial translation defects and human disease. J Transl Genet Genom 2020; 4:71–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kazuhito T, Wei FY. Posttranscriptional modifications in mitochondrial tRNA and its implication in mitochondrial translation and disease. J Biochem 2020; 168:435–444. [DOI] [PubMed] [Google Scholar]
- 39.Meseguer S, Navarro-Gonzalez C, Panadero J, et al. The MELAS mutation m.3243A>G alters the expression of mitochondrial tRNA fragments. Biochim Biophys Acta Mol Cell Res 2019; 1866:1433–1449. [DOI] [PubMed] [Google Scholar]
- 40.Konrad M, Vollmer M, Lemmink HH, et al. Mutations in the chloride channel gene CLCNKB as a cause of classic Bartter syndrome. J Am Soc Nephrol 2000; 11:1449–1459. [DOI] [PubMed] [Google Scholar]
- 41.Seys E, Andrini O, Keck M, et al. Clinical and genetic spectrum of Bartter syndrome type 3. J Am Soc Nephrol 2017; 28:2540–2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jeck N, Konrad M, Peters M, et al. Mutations in the chloride channel gene, CLCNKB, leading to a mixed Bartter-Gitelman phenotype. Pediatr Res 2000; 48:754–758. [DOI] [PubMed] [Google Scholar]
- 43.Ashton EJ, Legrand A, Benoit V, et al. Simultaneous sequencing of 37 genes identified causative mutations in the majority of children with renal tubulopathies. Kidney Int 2018; 93:961–967. [DOI] [PubMed] [Google Scholar]
- 44.Hureaux M, Ashton E, Dahan K, et al. High-throughput sequencing contributes to the diagnosis of tubulopathies and familial hypercalcemia hypocalciuria in adults. Kidney Int 2019; 96:1408–1416. [DOI] [PubMed] [Google Scholar]
- 45.Matsunoshita N, Nozu K, Shono A, et al. Differential diagnosis of Bartter syndrome, Gitelman syndrome, and pseudo-Bartter/Gitelman syndrome based on clinical characteristics. Genet Med 2016; 18:180–188. [DOI] [PubMed] [Google Scholar]
- 46.Walsh PR, Tse Y, Ashton E, et al. Clinical and diagnostic features of Bartter and Gitelman syndromes. Clin Kidney J 2018; 11:302–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen JC, Lo YF, Lin YW, et al. WNK4 kinase is a physiological intracellular chloride sensor. Proc Natl Acad Sci U S A 2019; 116:4502–4507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Piala AT, Moon TM, Akella R, et al. Chloride sensing by WNK1 involves inhibition of autophosphorylation. Sci Signal 2014; 7:ra41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu Z, Xie J, Wu T, et al. Downregulation of NCC and NKCC2 cotransporters by kidney-specific WNK1 revealed by gene disruption and transgenic mouse models. Hum Mol Genet 2011; 20:855–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bockenhauer D, Feather S, Stanescu HC, et al. Epilepsy, ataxia, sensorineural deafness, tubulopathy, and KCNJ10 mutations. N Engl J Med 2009; 360:1960–1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Scholl UI, Choi M, Liu T, et al. Seizures, sensorineural deafness, ataxia, mental retardation, and electrolyte imbalance (SeSAME syndrome) caused by mutations in KCNJ10. Proc Natl Acad Sci U S A 2009; 106:5842–5847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Palygin O, Levchenko V, Ilatovskaya DV, et al. Essential role of Kir5.1 channels in renal salt handling and blood pressure control. JCI Insight 2017; 2:e92331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Webb BD, Hotchkiss H, Prasun P, et al. Biallelic loss-of-function variants in KCNJ16 presenting with hypokalemic metabolic acidosis. Eur J Hum Genet 2021; 29:1566–1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bignon Y, Pinelli L, Frachon N, et al. Defective bicarbonate reabsorption in Kir4.2 potassium channel deficient mice impairs acid-base balance and ammonia excretion. Kidney Int 2020; 97:304–315. [DOI] [PubMed] [Google Scholar]
- 55.Geven WB, Monnens LA, Willems HL, et al. Renal magnesium wasting in two families with autosomal dominant inheritance. Kidney Int 1987; 31:1140–1144. [DOI] [PubMed] [Google Scholar]
- 56.Meij IC, Koenderink JB, van Bokhoven H, et al. Dominant isolated renal magnesium loss is caused by misrouting of the Na(+),K(+)-ATPase gamma-subunit. Nat Genet 2000; 26:265–266. [DOI] [PubMed] [Google Scholar]
- 57.de Baaij JH, Dorresteijn EM, Hennekam EA, et al. Recurrent FXYD2 p.Gly41Arg mutation in patients with isolated dominant hypomagnesaemia. Nephrol Dial Transplant 2015; 30:952–957. [DOI] [PubMed] [Google Scholar]
- 58.Arystarkhova E, Wetzel RK, Sweadner KJ. Distribution and oligomeric association of splice forms of Na(+)-K(+)-ATPase regulatory gamma-subunit in rat kidney. Am J Physiol Renal Physiol 2002; 282:F393–F407. [DOI] [PubMed] [Google Scholar]
- 59.Arystarkhova E, Wetzel RK, Asinovski NK, Sweadner KJ. The gamma subunit modulates Na(+) and K(+) affinity of the renal Na,K-ATPase. J Biol Chem 1999; 274:33183–33185. [DOI] [PubMed] [Google Scholar]
- 60.Cairo ER, Friedrich T, Swarts HG, et al. Impaired routing of wild type FXYD2 after oligomerisation with FXYD2-G41R might explain the dominant nature of renal hypomagnesemia. Biochim Biophys Acta 2008; 1778:398–404. [DOI] [PubMed] [Google Scholar]
- 61.Schlingmann KP, Bandulik S, Mammen C, et al. Germline de novo mutations in ATP1A1 cause renal hypomagnesemia, refractory seizures, and intellectual disability. Am J Hum Genet 2018; 103:808–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Munzer JS, Daly SE, Jewell-Motz EA, et al. Tissue- and isoform-specific kinetic behavior of the Na,K-ATPase. J Biol Chem 1994; 269:16668–16676. [PubMed] [Google Scholar]
- 63.van der Made CI, Hoorn EJ, de la Faille R, et al. Hypomagnesemia as first clinical manifestation of ADTKD-HNF1B: a case series and literature review. Am J Nephrol 2015; 42:85–90. [DOI] [PubMed] [Google Scholar]
- 64.Raaijmakers A, Corveleyn A, Devriendt K, et al. Criteria for HNF1B analysis in patients with congenital abnormalities of kidney and urinary tract. Nephrol Dial Transplant 2015; 30:835–842. [DOI] [PubMed] [Google Scholar]
- 65.Kolbuc M, Lessmeier L, Salamon-Slowinska D, et al. Hypomagnesemia is underestimated in children with HNF1B mutations. Pediatr Nephrol 2020; 35:1877–1886. [DOI] [PubMed] [Google Scholar]
- 66.Adalat S, Woolf AS, Johnstone KA, et al. HNF1B mutations associate with hypomagnesemia and renal magnesium wasting. J Am Soc Nephrol 2009; 20:1123–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Faguer S, Chassaing N, Bandin F, et al. The HNF1B score is a simple tool to select patients for HNF1B gene analysis. Kidney Int 2014; 86:1007–1015. [DOI] [PubMed] [Google Scholar]
- 68.Adalat S, Hayes WN, Bryant WA, et al. HNF1B mutations are associated with a Gitelman-like tubulopathy that develops during childhood. Kidney Int Rep 2019; 4:1304–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Verhave JC, Bech AP, Wetzels JF, Nijenhuis T. Hepatocyte nuclear factor 1beta-associated kidney disease: more than renal cysts and diabetes. J Am Soc Nephrol 2016; 27:345–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kolbuc M, Bienias B, Habbig S, et al. Hyperuricemia is an early and relatively common feature in children with HNF1B nephropathy but its utility as a predictor of the disease is limited. J Clin Med 2021; 10: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Faguer S, Decramer S, Chassaing N, et al. Diagnosis, management, and prognosis of HNF1B nephropathy in adulthood. Kidney Int 2011; 80:768–776. [DOI] [PubMed] [Google Scholar]
- 72.Clissold R, Shields B, Ellard S, et al. Assessment of the HNF1B score as a tool to select patients for HNF1B genetic testing. Nephron 2015; 130:134–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Okorn C, Goertz A, Vester U, et al. HNF1B nephropathy has a slow-progressive phenotype in childhood-with the exception of very early onset cases: results of the German Multicenter HNF1B Childhood Registry. Pediatr Nephrol 2019; 34:1065–1075. [DOI] [PubMed] [Google Scholar]
- 74.Verbitsky M, Westland R, Perez A, et al. The copy number variation landscape of congenital anomalies of the kidney and urinary tract. Nat Genet 2019; 51:117–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Weber S, Moriniere V, Knuppel T, et al. Prevalence of mutations in renal developmental genes in children with renal hypodysplasia: results of the ESCAPE study. J Am Soc Nephrol 2006; 17:2864–2870. [DOI] [PubMed] [Google Scholar]
- 76.Seeman T, Weigel F, Blahova K, et al. Blood pressure in children with renal cysts and diabetes syndrome. Eur J Pediatr 2021; 180:3599–3603. [DOI] [PubMed] [Google Scholar]
- 77.Berry MR, Robinson C, Karet Frankl FE. Unexpected clinical sequelae of Gitelman syndrome: hypertension in adulthood is common and females have higher potassium requirements. Nephrol Dial Transplant 2013; 28:1533–1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ferre S, Veenstra GJ, Bouwmeester R, et al. HNF-1B specifically regulates the transcription of the gammaa-subunit of the Na+/K+-ATPase. Biochem Biophys Res Commun 2011; 404:284–290. [DOI] [PubMed] [Google Scholar]
- 79.Kompatscher A, de Baaij JHF, Aboudehen K, et al. Loss of transcriptional activation of the potassium channel Kir5.1 by HNF1beta drives autosomal dominant tubulointerstitial kidney disease. Kidney Int 2017; 92:1145–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bech AP, Wetzels JF, Bongers EM, Nijenhuis T. Thiazide responsiveness testing in patients with renal magnesium wasting and correlation with genetic analysis: a diagnostic test study. Am J Kidney Dis 2016; 68:168–170. [DOI] [PubMed] [Google Scholar]
- 81.Schlingmann KP, Jouret F, Shen K, et al. mTOR-activating mutations in RRAGD are causative for kidney tubulopathy and cardiomyopathy. J Am Soc Nephrol 2021; 32:2885–2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bongers E, Shelton LM, Milatz S, et al. A novel hypokalemic-alkalotic salt-losing tubulopathy in patients with CLDN10 mutations. J Am Soc Nephrol 2017; 28:3118–3128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hadj-Rabia S, Brideau G, Al-Sarraj Y, et al. Multiplex epithelium dysfunction due to CLDN10 mutation: the HELIX syndrome. Genet Med 2018; 20:190–201. [DOI] [PubMed] [Google Scholar]
- 84.Huang CL, Kuo E. Mechanism of hypokalemia in magnesium deficiency. J Am Soc Nephrol 2007; 18:2649–2652. [DOI] [PubMed] [Google Scholar]
- 85.Ferre S, de Baaij JH, Ferreira P, et al. Mutations in PCBD1 cause hypomagnesemia and renal magnesium wasting. J Am Soc Nephrol 2014; 25:574–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Glaudemans B, van der Wijst J, Scola RH, et al. A missense mutation in the Kv1.1 voltage-gated potassium channel-encoding gene KCNA1 is linked to human autosomal dominant hypomagnesemia. J Clin Invest 2009; 119:936–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Schlingmann KP, Weber S, Peters M, et al. Hypomagnesemia with secondary hypocalcemia is caused by mutations in TRPM6, a new member of the TRPM gene family. Nat Genet 2002; 31:166–170. [DOI] [PubMed] [Google Scholar]
- 88.Stuiver M, Lainez S, Will C, et al. CNNM2, encoding a basolateral protein required for renal Mg2+ handling, is mutated in dominant hypomagnesemia. Am J Hum Genet 2011; 88:333–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Walder RY, Landau D, Meyer P, et al. Mutation of TRPM6 causes familial hypomagnesemia with secondary hypocalcemia. Nat Genet 2002; 31:171–174. [DOI] [PubMed] [Google Scholar]
- 90.Groenestege WM, Thebault S, van der Wijst J, et al. Impaired basolateral sorting of pro-EGF causes isolated recessive renal hypomagnesemia. J Clin Invest 2007; 117:2260–2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Franken GAC, Muller D, Mignot C, et al. The phenotypic and genetic spectrum of patients with heterozygous mutations in cyclin M2 (CNNM2). Hum Mutat 2021; 42:473–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bosman W, Hoenderop JGJ, de Baaij JHF. Genetic and drug-induced hypomagnesemia: different cause, same mechanism. Proc Nutr Soc 2021; 80:327–338. [DOI] [PubMed] [Google Scholar]
- 93.Hollifield JW. Thiazide treatment of hypertension. Effects of thiazide diuretics on serum potassium, magnesium, and ventricular ectopy. Am J Med 1986; 80:8–12. [DOI] [PubMed] [Google Scholar]
- 94.Burrello J, Monticone S, Losano I, et al. Prevalence of hypokalemia and primary aldosteronism in 5100 patients referred to a tertiary hypertension unit. Hypertension 2020; 75:1025–1033. [DOI] [PubMed] [Google Scholar]