Abstract
Background:
Rapid and accurate new biomarkers to predict risk of cardiovascular disease (CVD) are essential. The utility of extracellular vesicles in predicting the CVD risk is postulated, yet it remains unknown whether their expression is altered in response to statin therapy.
Methods:
We performed in-vitro studies with human umbilical vein endothelial cells (HUVEC) and vascular smooth muscle cells (hVSMC), and conducted a nested case–control study (nCCS) in hypertensive patients (n = 40) randomized to either atorvastatin or placebo in the ASCOT-LLA. Cases had a major adverse cardiovascular event or death (MACE) during 3.5 years of follow-up (median) from the time of extracellular vesicle characterization while controls, matched for age and duration of treatment, remained event-free. Conditional logistic regression models determined the risk of MACE. Additionally, the relationship of extracellular vesicle levels with statin therapy was assessed.
Results:
Added to HUVEC, extracellular vesicles increased neutrophil recruitment, and to hVSMC, aggravated calcification and proliferation. In the nCCS, compared with controls, cases (i.e. with MACE) had preceding higher levels of CD14+ and CD14+/CD41+ extracellular vesicles (P = 0.009 and P = 0.012, respectively) and a significant reduction in the median size of the vesicles (P = 0.037). On matched analysis, higher CD14+ extracellular vesicles were associated with a 3.7-fold increased risk of MACE (P = 0.032). Patients treated with atorvastatin (vs. placebo) had both reduced size of extracellular vesicles and the proportion of CD146+ extracellular vesicles (P = 0.034 and P = 0.020, respectively).
Conclusion and relevance:
These pilot analyses suggest a mechanistic role for extracellular vesicles in the development of CVD, with significant and differential changes in extracellular vesicles amongst those at risk of MACE, and those on atorvastatin therapy.
Keywords: cardiovascular risk, extracellular vesicles, hypertension, monocyte, platelet
INTRODUCTION
Extracellular vesicles have been proposed as potential biomarkers of cardiovascular risk [1–3]. Compared with other biomarkers, extracellular vesicles present high stability in different bio-fluids, protecting their cargo from external enzymatic activities [4]: these characteristics make them an attractive biomarker for clinical application.
Some studies have explored the relationship of inflammatory biomarkers, such as high sensitivity C-reactive protein (hsCRP), with the risk of CVD [5,6]. The putative role of CRP in the development of CVD and the role of statin therapy in relation to the CRP levels have been tested in clinical trials [7,8]. Findings from these trials suggest that part of the cardiovascular protection associated with statin therapy may be independent of lipid-lowering, and ascribed to anti-inflammatory properties of the statin, monitored by on-treatment reduction of hsCRP levels. However, hsCRP is a nonspecific and possibly unreliable marker of treatment response for other lipid-lowering treatments, including proprotein convertase subtilisin/kexin type 9 enzyme [9], making it rather a nonspecific marker of inflammation [10]. We reasoned that other markers of vascular inflammation and dysfunction, like extracellular vesicles, could have more consistent relationships with different treatment strategies, including statin therapy. Studies have shown already the potential role of extracellular vesicles to predict the occurrence of MACE in primary prevention [11], as well as the effect of statins on circulating extracellular vesicles [12,13].
In a recent study, we have characterized a subset of extracellular vesicles produced from human monocyte/platelet aggregates, evaluating their activities on human vessel cellular components [14]. To explore potential of extracellular vesicles as a biomarker, we used stored bio-specimens from the Lipid-Lowering arm Anglo-Scandinavian Cardiac Outcomes Trial (ASCOT-LLA), to develop a novel nested case–control study (nCCS), using 2×2 factorial design, where we ensured that each participant contributing to the study had similar cardiovascular risk factor [mainly blood pressure (BP)] management and had been cardiovascular event free prior to the start of the study period. Our primary objective was to evaluate differences, if any, in extracellular vesicle levels, size, and characterization between plasma samples from patients with a major adverse cardiovascular event or death (MACE) and those who were event free during 3.5 years of follow-up. Furthermore, we will be able to differentiate changes in extracellular vesicles amongst those who had or had not received statin during preceding 2 years, independently of their subsequent risk of developing a cardiovascular event.
MATERIAL AND METHODS
An extended version of the methods used is provided in the Supplementary file.
In-vitro studies
Monocyte and polymorphonuclear cells (PMN) were isolated from 30 ml anticoagulated blood using RosetteSep kit or dextran gradient enrichment, respectively. Monocytes (1 × 106cells/ml) or PMN (20 × 106 cells/ml) were incubated with TNF-α (50 ng/ml) for 60 or 20 min, respectively, at 37 °C. Time and stimulant concentration were chosen on preliminary experiments and published data [15]. Following cell pelleting, extracellular vesicles were enriched by centrifugation and characterized by Nanosight, Transmission Electron Microscopy (TEM) and Imagestream (see Supplementary Methods).
Human cultured vascular smooth muscle cells (hVSMC) were stimulated with extracellular vesicle subsets on a ratio of 10 extracellular vesicle/cell or 50 ng/ml OxLDL, used as positive control [16]. Calcification and proliferation were then quantified (Supplementary Methods). Human umbilical vein endothelial cells (HUVEC) were stimulated overnight with different subsets of extracellular vesicles at a ratio of 10 extracellular vesicle/cell, or with 10 ng/ml of TNF-α, used as positive control. Concentrations and rations of extracellular vesicles were chosen based on preliminary experiments. Capture, rolling, adhesion, and transmigration of PMN on the endothelial monolayers was then quantified (Supplementary Methods).
Nested case–control study in hypertensive patients
Lipid-Lowering arm Anglo-Scandinavian Cardiac Outcomes Trial
In the lipid-lowering arm (LLA) of the ASCOT, hypertensive patients with no previous coronary heart disease were randomized to receive either 10 mg atorvastatin or placebo (ASCOT-LLA). Details of the study are available at http://www.ascotstudy.org.uk/. ASCOT Trial conformed to the guidelines of the Declaration of Helsinki, and its protocol (and other amendments) were reviewed and ratified by various research ethic boards and committees in the UK, Ireland, and Nordic countries.
Nested case–control design
In this 2 × 2 factorial nCCS, plasma samples were obtained at 2 years from the randomizations from cases and controls (Supplementary Figure S1). Cases (n = 20) were patients who had a MACE event (defined as either coronary event or nonfatal stroke, or death) during a median 3.5 years of follow-up after collection of the samples. Controls (n = 20) were matched patients with similar age and duration of in-trial treatment, who did not have a MACE event during the subsequent follow-up. Both case and control groups had the same number of patients who were initially randomized to statin or placebo arm. In these stored plasma samples, we characterized extracellular vesicle concentration, size, and surface markers in a blinded fashion to the case–control allocation. This design allows for us to identify differences between case and control group independent of the influence of statin therapy. Furthermore, the 2-year prior duration of blinded therapy with statin or placebo, allows us to evaluate definitively differences in the two groups, regardless of subsequent development of MACE or not. In all samples, extracellular vesicles were isolated by sequential centrifugation and characterized as indicated above.
Statistical analysis
GraphPad Prism 6 Software (GraphPad Software, San Diego, California, USA) was used for analysis of in-vitro studies. Flow cytometry images and plots were analysed with IDEAS 6.2 software. STATA 15.1 was used for the analysis of nCCs (STATA Corp., College Station, Texas, USA).. Descriptive characteristic statistics are presented with mean and standard deviation (SD) or frequencies and percentages, and descriptive extracellular vesicle statistics are presented with median and interquartile range (IQR). Paired t tests were conducted to compare crude mean differences in extracellular vesicles between cases and controls. The robust Wilcoxon matched-pairs signed-rank test was used to test for distributional differences in extracellular vesicles between cases and controls, while relaxing the assumption of normality of extracellular vesicle distribution. These tests were also repeated, comparing extracellular vesicles between those randomized to atorvastatin or placebo. Conditional logistic regression estimated odds ratios (OR), with accompanying 95% confidence intervals (CI) and P values, comparing the odds of being a case to the odds of being a control. Crude ORs were estimated, as well as adjusted ORs, adjusted for preselected risk factors: sex; baseline SBP; trial treatment allocation and baseline smoking status.
Interaction tests were conducted to see whether associations differed between those allocated to placebo or statin-therapy, by including an interaction term in each model.
RESULTS
In-vitro studies
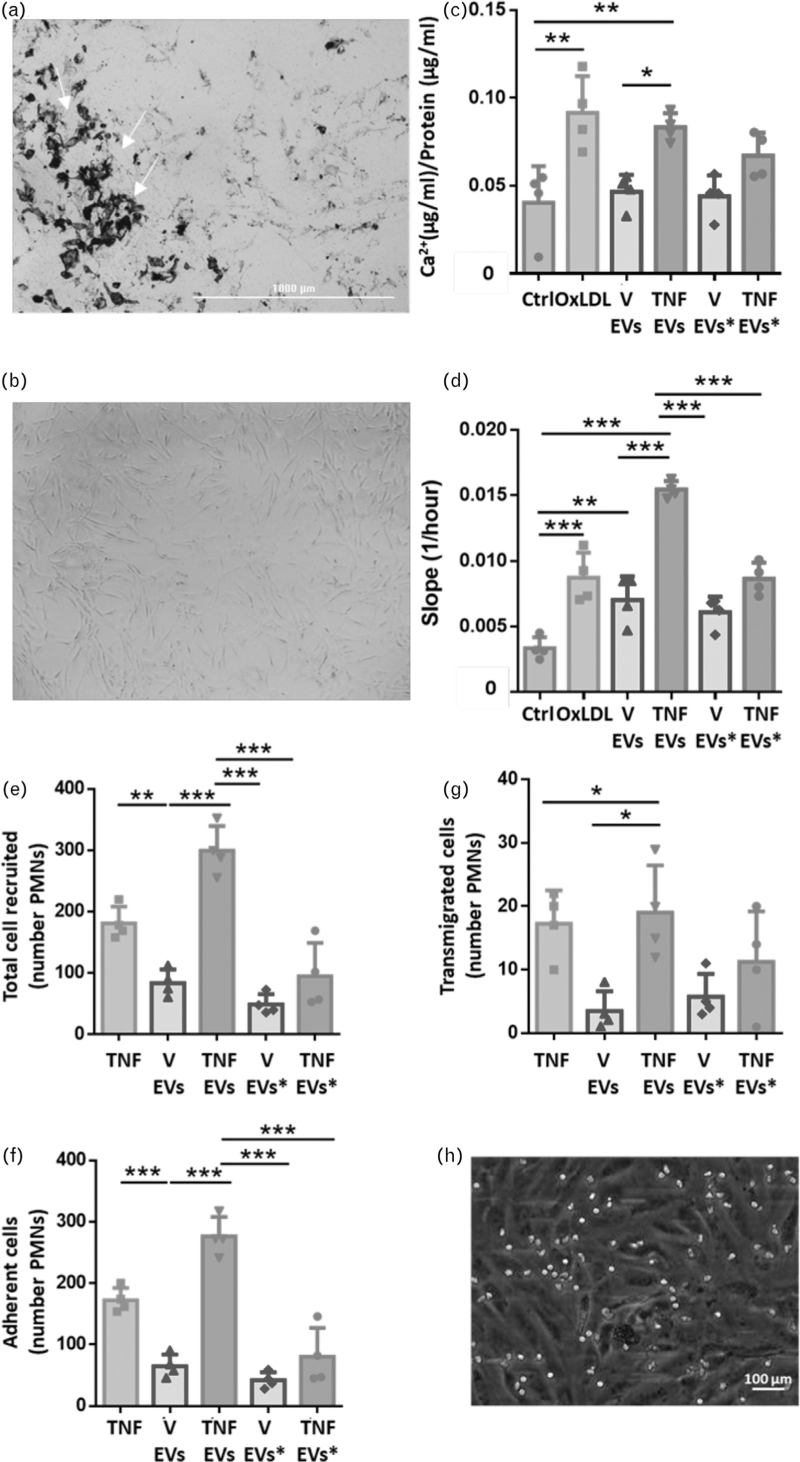
There was no change in size distribution of extracellular vesicles released by monocyte/platelet aggregates upon stimulation with PBS and TNF-α (Figure S2). However, there was an increase in concentration (numbers of extracellular vesicles per 1 ml of plasma, reported as objects/ml) after cytokine stimulation of monocyte (CD14+), platelet (CD41+) and extracellular vesicles positive for both monocyte and platelet markers (CD14+/CD41+). The correct characterization of the vesicles was confirmed by presence of CD9, Annexin A1 and the absence of Calnexin A1 as well as by electron microscopy (Figure S2E and F). Then, the response of hVSMC and HUVEC to monocyte/platelet extracellular vesicle application was investigated.
Addition of extracellular vesicles derived from monocyte/platelet aggregates stimulated with TNF-α to hVSMC increased proliferation and calcification (Fig. 1a–d) and promoted changes in cell shape (Fig. 1b). Notably, the extent of calcification evoked by monocyte/platelet extracellular vesicles was ∼2-fold higher than that obtained with OxLDL (P < 0.01; Fig. 1c). Moreover, PMN extracellular vesicles from either unstimulated or TNF-α-stimulated cells did not affect hVSMC calcification or proliferation (Fig. 1c and d). The same was true for extracellular vesicles obtained from PBS-treated monocytes. Next, we tested the extracellular vesicles on HUVEC.
FIGURE 1.
Extracellular vesicles activate human vascular smooth muscle cells and human umbilical vein endothelial cells. Isolated monocytes (1 × 106/ml; extracellular vesicles) or neutrophils (20 × 106/ml; extracellular vesicles∗) were incubated with vehicle (V) or TNF-α (50 ng/ml) for 60 min. Extracellular vesicles were analysed in cell-free samples following 20 000g centrifugation. hVSMC or HUVEC were treated overnight with extracellular vesicles to achieve a ratio of 10 extracellular vesicle : cell and 50 ng/ml OxLDL or 10 ng/ml of TNF-α were used as positive control. (a) Representative image of calcification of hVSMC treated with extracellular vesicles isolated from TNF-α treated monocytes (calcification is marked by the white arrows). (b) representative image of proliferation of hVSMC treated with extracellular vesicles isolated from TNF-α-treated monocytes. (c) Calcification assay of hVSMC and (d) Proliferation assay of hVSMC following incubation with the reported extracellular vesicle preparations. (e) Total number of recruited neutrophils on HUVEC layer. (f) Adherent neutrophils to HUVEC layer. (g) Transmigrated neutrophil on HUVEC layer. (h) Representative image of neutrophils (white dots) recruited on HUVEC layer. Data are mean ± SEM of three to five distinct experiments. ∗, ∗∗, ∗∗∗P < 0.05, 0.01 and 0.001, respectively. Extracellular vesicles∗ stands for cells treated with neutrophils extracellular vesicles. HUVEC, human umbilical vein endothelial cells; hVSMC, human vascular smooth muscle cells.
The extracellular vesicles obtained from monocyte/platelet aggregates stimulated with TNF-α increased activation of HUVEC as assessed by the extent of neutrophil capture, adhesion and transmigration onto the endothelial monolayer (Fig. 1e–h).
Differences in extracellular vesicle surface marker expression in cases and controls: matched analyses
Table S1 reports the baseline characteristics of age and duration of treatment-matched cases and controls, and the levels of several extracellular vesicle surface markers as well as extracellular vesicle size. Compared with controls, cases were predominantly male, current smokers and with higher baseline SBP and less likely to be assigned to the amlodipine-based treatment.
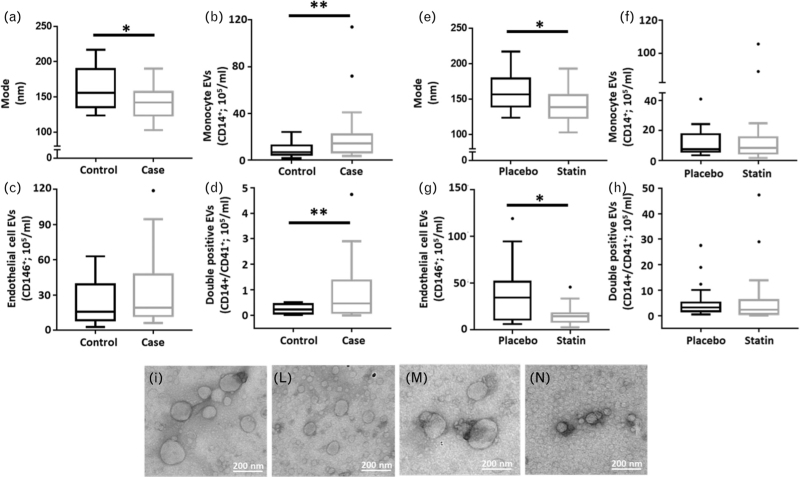
Figure 2 shows box plots of the mode of size of total extracellular vesicles (Fig. 2a) and concentrations of extracellular vesicles from monocytes (CD14+), monocyte/platelet (CD14+/CD41+) and endothelial cells (CD146+) as quantified in the plasma samples (Fig. 2b–d). Table 1 describes the mean difference in size and quantity of the extracellular vesicles. Compared with the control population, those who developed MACE (cases) displayed a 2-to-3-fold increase in plasma extracellular vesicle concentrations, both for CD14+ and CD14+/CD41+ objects (Fig. 2b and c; P = 0.09 and P = 0.012, respectively). there was a significant decrease in the size of extracellular vesicles in cases, compared with controls: 138.7 vs. 156.8 nm (P = 0.025). These data emerged regardless of allocation to either statin or placebo treatment (Fig. 2a) and were confirmed by electron microscopy, where the size of extracellular vesicles in cases was visibly smaller (Fig. 2i–n).
FIGURE 2.
Box plots of extracellular vesicle markers and transmission electron microscopy images of extracellular vesicles, by case status and lipid-lowering allocation status. Extracellular vesicles were isolated with differential centrifugation from plasma samples, re-suspended in PBS and analysed on ImageStream, Nanosight and TEM. (a) Mode and concentrations of CD14+ (b), CD146+ (c) and CD14+/CD41+ (d) extracellular vesicles were analysed in samples by case status. Although (e) mode and concentrations of CD14+ (f), CD146+ (g) and CD14+/CD41+ (h) extracellular vesicles were checked in samples by lipid-lowering allocation status. Representative image of extracellular vesicles analysed by electron microscopy from patient assigned to placebo treatment (i), to statin treatment (l). Representative images of control patients within the follow up period (m) and of patients who experiences a CV event or death in the follow up period (n). Data are from N = 20 patient samples per group and are reported as median ± SEM. CV, cardiovascular; SEM, standard error of the mean; TEM, transmission electron microscopy.
TABLE 1.
Mean difference in the expression of human plasma extracellular vesicle markers between control and cases, when tested on group average and matched paired analysis
| Extracellular vesicle marker | Difference in means control-case (P value from paired t test) | Robust test P value Wilcoxon matched-pairs signed-ranks test |
| Mode (nm) | 19.28 (P = 0.025) | P = 0.037 |
| CD14 (objects ×105/ml) | −12.96 (P = 0.041) | P = 0.009 |
| CD146 (objects ×105/ml) | −9.68 (P = 0.260) | P = 0.332 |
| CD14/CD41 (objects ×105/ml) | −7.56 (P = 0.012) | P = 0.012 |
There is no missing data.
Table 2 shows the results of the conditional logistic regression models: age and duration of treatment-matched analysis (matched ORs), and after adjustments for sex, SBP, BP-treatment allocation, BMI and smoking status (adjusted ORs). Matched analysis suggests that one log unit increase in CD14+ extracellular vesicle concentrations is associated with a 3.7-fold significant increase in the risk of MACE (Table 2 and Fig. 2b; P = 0.032). The effect size increased further to a 4.4-fold after adjustment for other confounders; however, the findings were no longer statistically significant. (Table 2; P = 0.060). When the risk of MACE was stratified by treatment group, the unit increase in CD14+ extracellular vesicle concentrations was associated with 6.4-fold and 2.9-fold increase in the risk of MACE with placebo and statin, albeit not statistically significant (Table S3).
TABLE 2.
Risk (odds) of cardiovascular adverse event based on different levels of human plasma extracellular vesicle markers using conditional logistic regression models
| Extracellular vesicle marker | Matched analysis OR (95% CI)a | P value | Adjusted OR (95% CI)b | P value |
| Mode (per nm) | 0.97 (0.94–1.00) | 0.053 | 0.97 (0.94–1.01) | 0.158 |
| CD14 (log2 objects/ml) | 3.74 (1.12–12.49) | 0.032 | 4.41 (0.94–20.76) | 0.060 |
| CD146 (log2 objects/ml) | 1.41 (0.81–2.48) | 0.228 | 1.61 (0.69–3.79) | 0.273 |
| CD14/CD41 (log2 objects/ml) | 1.39 (0.93–2.06) | 0.104 | 1.49 (0.84–2.63) | 0.172 |
CI, confidence interval.
Matched analysis: matched for age and duration of treatment.
Matched analysis, adjusted for sex, baseline SBP, treatment allocation, and smoking status.
Extracellular vesicle surface markers, statin therapy and cardiovascular events
Table S2 reports baseline characteristics, size and levels of extracellular vesicle surface markers of the age and duration of treatment-matched statin or placebo-treated patients. Compared with statin-treated patients, placebo patients had a higher proportion of female participants but no clear differences in baseline characteristics were registered within the two groups.
Figure 2 shows box plots of the mode of size of total extracellular vesicles (Fig. 2e) and concentrations expressed as objects/ml for CD14+, CD146+ and CD14+/CD41+ extracellular vesicles, stratified by those receiving a placebo or statin therapy (Fig. 2f–h). Compared with those on placebo, patients on atorvastatin have significant reduction in both size and concentration of CD146+ extracellular vesicles (Fig. 2e–g and Table 3; P = 0.02). There was no statistically significant interaction between the two treatment groups but there was a trend to attenuation of the effect size for the risk of MACE amongst those on statin compared with those on placebo (e.g. the odds of MACE with increase in one-unit log of CD14+ on placebo were 6.4-fold, and on statin were 2.9-fold) (Table S3 and Table S4).
TABLE 3.
Level of extracellular vesicle markers in plasma samples of ASCOT patients analysed by statin treatment allocation
| Extracellular vesicle marker | Difference in means placebo-statin (P value from unpaired t test) | Robust test P value Wilcoxon rank-sum test |
| Mode (nm) | 20.39 (P = 0.022) | P = 0.034 |
| CD14+ (objects ×105/ml) | -6.12 (P = 0.347) | P = 0.914 |
| CD146+ (objects ×105/ml) | 20.95 (P = 0.006) | P = 0.020 |
| CD14+/CD41+ (objects ×105/ml) | -1.22 (P = 0.691) | P = 0.441 |
DISCUSSION
In these hypothesis-generating experiments, we have been able to show the biological properties of specific extracellular vesicle subsets on the vasculature, proposing their potential as predictors of cardiovascular risk, and for response to atorvastatin therapy. We have leveraged a unique and more efficient 2 × 2 nCCS design. These findings suggest that extracellular vesicles have a potential role as predictors of CVD, and that these microstructures are altered significantly on atorvastatin, which is independent, and additional to, whether patients subsequently develop a cardiovascular event.
Studies have shown an association of extracellular vesicles with cardiovascular risk factors, such as dyslipidaemia [17,18]. Another study has shown that statins may have a differential effect on LDL-extracellular vesicles compared with the serum proteins [19]. However, all these studies present some flaws either in design or other issues, for example, the treatment response to statins was not compared with cardiovascular outcomes [19]. A few of these studies are cross-sectional [18], or prospective, amongst those with history of previous cardiovascular events [20], thereby making interpretation of their role in cardiovascular risk prediction difficult. Many studies are epidemiological, with no mechanistic underpinning and in some cases, serum samples rather than plasma have been analysed [19] bringing an inherent artefact because of blood cell activation during coagulation [19]. None of these studies have explored the role of statin therapy, or the role of extracellular vesicles in primary prevention settings and/or in hypertensive patients at high risk. Given these issues, we addressed these aspects with in-vitro and patient samples analyses. Our findings provide substantial improvement to existing understanding with a robust and efficient evaluations despite small numbers of samples.
Recently, we have characterized a subset of extracellular vesicles from monocyte and platelet endowed with pro-inflammatory effects in the vasculature [14]. The CD14+/CD41+ extracellular vesicle subset augments adhesion molecule expression in aortic endothelial cells and the release of pro-inflammatory cytokines from human atherosclerotic plaques. Building on this first study [14], the present investigation began by assessing the effects of extracellular vesicles isolated from TNF-α-treated monocyte/platelet aggregates on cells relevant to vascular inflammation. We reported specific and selected activation of HUVEC and hVSMC, effects, which were not produced with extracellular vesicles obtained from neutrophils or from unstimulated monocyte/platelets. The concept that vascular cells release extracellular vesicles in response to different environmental stimuli has been validated for several cells, including platelets [21] and the endothelium [22]. In line with this view, platelet and monocyte extracellular vesicles amplify cellular pro-coagulant responses, thus contributing to aberrant fibrin deposition in vivo[23,24]. It remains to be established how monocyte and platelet extracellular vesicles activated the endothelium monolayers, an issue beyond the remit of this translational study. Notably, extracellular vesicles isolated from monocytic THP-1 cells activate MAP kinases and NF-κB pathways in HUVEC, yielding increased expression of cell adhesion molecules like ICAM-1 and E-selectin [25]: such a mechanism would explain the augmented neutrophil adhesion under flow presented here. In the experiments performed with hVSMC, TNF-α-stimulated monocyte/platelet extracellular vesicles promoted cell proliferation and calcification. Pathological remodelling of the vasculature involves the role of platelet extracellular vesicles on vascular smooth muscle cells, although to date no studies have investigated monocyte extracellular vesicles. Thus, platelet extracellular vesicles induce migration and proliferation of hVSMC in a CXCL4-dependent manner [26]. Together, these in-vitro experiments demonstrate significant activation responses in vascular cells following application of monocyte/platelet extracellular vesicles and suggest potential pathological properties in clinical settings.
In line with the in-vitro work, our nCCS findings revealed significant differences in the expression of specific extracellular vesicle subsets between patients who did and did not have a MACE. Indeed, one unit (in log scale) rise in CD14+ expression on extracellular vesicles was associated with a 3.7-fold significant increase in the risk of MACE; and the effect size remained similar (yet higher in magnitude) after adjusting for the confounders (albeit with borderline significance). These findings are remarkable, given the sample size and are apparent despite that. The relationship between the increased risk of MACE and rise in CD14+ expression was also apparent, when analysis was stratified by treatment allocation. However, in the statin-treated patients, the odds risk was substantially attenuated when compared with that seen in placebo-treated patients, suggesting the potential efficacy of the statin treatment in reducing this excess cardiovascular risk. The role of statins in reducing CVDs is well documented [27,28] and as analysed samples were collected from patients up to 3.5 years prior to the MACE, the findings that these relationships are significant despite the relatively small number in the pilot study, and consistent with sub-group stratified analysis, suggest the potential role of CD14+ extracellular vesicles as a predictor of cardiovascular events in hypertensive patients regardless of their treatment allocation. Interestingly, the results also showed increased levels of extracellular vesicles bearing markers of both the monocyte and platelet (CD14+/CD41+ vesicles), suggesting that the cross-talk between monocyte and platelets may play a pivotal role in the adverse outcomes amongst hypertensive patients. Monocyte/platelet aggregates are associated with the development of cardiovascular pathologies and Ashman et al.[29], for instance, have described aggregates in patients with end-stage renal disease, also accompanied by hypertension [30], which was associated with an increased risk of CVD. We acknowledge that 3.5 years could be considered a short period of time to follow-up MACE, thus further study will investigate longer time points. Nevertheless, another interesting aspect that we are unable to comment in this study but that can be explored in further studies, is the comparison of extracellular vesicles and hsCRP as biomarker for cardiovascular risk.
A novel and intriguing observation made here relates to the size of extracellular vesicles, modulated not only by the treatment with atorvastatin but also in patients who have a subsequent cardiovascular event. The reduction of extracellular vesicle diameter, a ‘shrinking effect’, after atorvastatin treatment may be consequent to lipid depletion, in particular of cholesterol. Like all statins, atorvastatin inhibits the enzyme hydroxymethylglutaryl-coenzyme A reductase, pivotal for the synthesis of cholesterol. In vascular cells, cholesterol levels can be reduced up to 40% by statins [31]. Lower cellular cholesterol would explain reduced incorporation of this structural lipid in the budding extracellular vesicles with consequent impact on the structure/size of the microstructure [32]. In support to this, a study, which analysed levels of extracellular vesicles in patients from the METEOR trial showed a significant increase of levels of extracellular vesicles containing LDL [19]. Although researchers did not investigate the extracellular vesicle size, release of cholesterol could lead to a reduction in lipid membrane enrichment within the cells. However, this mechanistic explanation may require further validation.
Finally, a secondary observation emerged from analyses of the whole extracellular vesicle profiles in the ASCOT-LLA patient samples: whenever analysed by treatment, a significant decrease of endothelial CD146+ extracellular vesicles was quantified in patients treated with atorvastatin. extracellular vesicle levels have been investigated, to some degree, in patients affected by hypertension. Atorvastatin improves endothelial function and cardiac performance in patients with dilated cardiomyopathy, inhibiting inflammation by significantly decreasing serum circulating soluble intercellular adhesion molecule-1, Von Willebrand factor and CRP, and positively regulating the flow-mediated dilatation of the brachial artery, the left ventricular ejection fraction and the 6-min walk-test in the patients [33]. Our findings add to this evidence and raise a possibility of evaluating the protective efficacy of statin therapy, and possibly antihypertensive therapies, by characterizing extracellular vesicle subsets in blood, including endothelial extracellular vesicles.
Although exciting, there are limitations for the present study, which ought to be stressed. First, our design did not include isolation of extracellular vesicles at the time of sample collection, and therefore, it cannot be ruled out that handling of stored samples might have affected vesicle numbers and content. However, extracellular vesicles are stable and cycles of freeze/thawing do not influence extracellular vesicle structural features and their concentrations [18]. Moreover, any hypothetical influence on numbers would apply to all samples used in the study. Second, the small sample with 20 MACE may raise issues of statistical power; however, the nCCS and 2 × 2 factorial design provides for an opportunity to establish the impact of statin therapy given for 2 years (vs. placebo), and the nested design provides for more efficient design. It was unsurprising that we found significant differences, with same trends on further adjustments and stratification. However, we recognize that we can only adjust for limited number of confounders, and there may be some residual confounding despite matched analysis, and adjustments such as that of baseline diabetes and BMI. Whilst there is no denying that we have limited power to explore various sub-group comparisons, the significant findings (despite power concerns) should encourage and stimulate further exploration using different datasets. From a different angle, the study benefits from adjudicated events and reliable drug exposure in the trial setting for a period of time to assess for both the relationship with cardiovascular risk, and with the statin therapy. Furthermore, it is important to acknowledge the gender imbalance of the samples analysed; however, when we adjusted for gender and other confounders, our findings remained essentially same. Altogether, we are confident the consistency of our findings in different domains and analysis, when both adjusted and unadjusted for different confounders, highlight the potential of extracellular vesicles as novel biomarkers of CVDs and response to statin therapy.
In conclusion, whilst there is the need to confirm these novel and exciting results in larger and more stratified study, we present evidence of the potential for vesicles released by monocytes and platelets to propagate vascular inflammation and exploit this biology for diagnostic and prognostic purposes. If confirmed, these findings identify a robust biomarker that potentially may help to further improve cardiovascular risk prediction, enable identification of patients who are likely to respond to the statin therapy, and may allow further mechanistic insights on the role of extracellular vesicles in relation to the development of CVD.
ACKNOWLEDGEMENTS
Funding: EVOluTION has received funding from the European Union's Horizon 2020 research and innovation programme under the Marie Sklodowska-Curie grant agreement No. 675111 (S.O., M.P.). The ImageStream used was funded by the Wellcome Trust (infrastructure grant 101604/Z/13/Z). This work has been facilitated by the National Institute for Health Research Biomedical Research Centre at Barts Hospital NHS Trust. A.K.G. has been supported by a grant from Barts Charity.
Conflicts of interest
There are no conflicts of interest.
Supplementary Material
Supplementary Material
Present address: Wolfson Centre for Age-related Diseases, King's College, London, UK.
M.P. and A.G. contributed equally to this study and are joint senior authors.
Abbreviations: ASCOT-LLA, Lipid-Lowering arm Anglo-Scandinavian Cardiac Outcomes Trial; EV, extracellular vesicles; IS, xImageStreamX; MACE, major adverse cardiovascular event or death; PMN, polymorphonuclear cells; TEM, transmission electron microscopy
Supplemental digital content is available for this article.
Contributor Information
Silvia Oggero, Email: s.oggero@qmul.ac.uk.
Thomas Godec, Email: t.godec@qmul.ac.uk.
Rick van Gorp, Email: rickvangorp@gmail.com.
Adreia L. Pinto, Email: A.Pinto@rbht.nhs.uk.
Leon J. Schurgers, Email: l.schurgers@maastrichtuniversity.nl.
Chris Reutelingsperger, Email: c.reutelingsperger@maastrichtuniversity.nl.
Peter Sever, Email: p.sever@imperial.ac.uk.
Lucy V. Norling, Email: l.v.norling@qmul.ac.uk.
REFERENCES
- 1.Dickhout A, Koenen RR. Extracellular vesicles as biomarkers in cardiovascular disease; chances and risks. Front Cardiovasc Med 2018; 5:113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Emanueli C, Shearn AI, Laftah A, Fiorentino F, Reeves BC, Beltrami C, et al. Coronary artery-bypass-graft surgery increases the plasma concentration of exosomes carrying a cargo of cardiac microRNAs: an example of exosome trafficking out of the human heart with potential for cardiac biomarker discovery. PLoS One 2016; 11:e0154274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jansen F, Yang X, Proebsting S, Hoelscher M, Przybilla D, Baumann K, et al. MicroRNA expression in circulating microvesicles predicts cardiovascular events in patients with coronary artery disease. J Am Heart Assoc 2014; 3:e001249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cypryk W, Nyman TA, Matikainen S. From inflammasome to exosome-does extracellular vesicle secretion constitute an inflammasome-dependent immune response? Front Immunol 2018; 9:2188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kistorp C, Raymond I, Pedersen F, Gustafsson F, Faber J, Hildebrandt P. N-terminal pro-brain natriuretic peptide, C-reactive protein, and urinary albumin levels as predictors of mortality and cardiovascular events in older adults. JAMA 2005; 293:1609–1616. [DOI] [PubMed] [Google Scholar]
- 6.Li Y, Zhong X, Cheng G, Zhao C, Zhang L, Hong Y, et al. Hs-CRP and all-cause, cardiovascular, and cancer mortality risk: a meta-analysis. Atherosclerosis 2017; 259:75–82. [DOI] [PubMed] [Google Scholar]
- 7.Ridker PM, Danielson E, Fonseca FA, Genest J, Gotto AM, Jr, Kastelein JJ, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med 2008; 359:2195–2207. [DOI] [PubMed] [Google Scholar]
- 8.Sever PS, Poulter NR, Chang CL, Thom SA, Hughes AD, Welsh P, et al. Evaluation of C-reactive protein before and on-treatment as a predictor of benefit of atorvastatin: a cohort analysis from the Anglo-Scandinavian Cardiac Outcomes Trial lipid-lowering arm. J Am Coll Cardiol 2013; 62:717–729. [DOI] [PubMed] [Google Scholar]
- 9.Murphy SA, Pedersen TR, Gaciong ZA, Ceska R, Ezhov MV, Connolly DL, et al. Effect of the PCSK9 inhibitor evolocumab on total cardiovascular events in patients with cardiovascular disease: a prespecified analysis from the FOURIER Trial. JAMA Cardiol 2019; 4:613–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sproston NR, Ashworth JJ. Role of C-reactive protein at sites of inflammation and infection. Front Immunol 2018; 9:754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Christersson C, Thulin A, Siegbahn A. Microparticles during long-term follow-up after acute myocardial infarction. Association to atherosclerotic burden and risk of cardiovascular events. Thromb Haemost 2017; 117:1571–1581. [DOI] [PubMed] [Google Scholar]
- 12.Suades R, Padro T, Vilahur G, Badimon L. Circulating and platelet-derived microparticles in human blood enhance thrombosis on atherosclerotic plaques. Thromb Haemost 2012; 108:1208–1219. [DOI] [PubMed] [Google Scholar]
- 13.Mobarrez F, Egberg N, Antovic J, Broijersen A, Jorneskog G, Wallen H. Release of endothelial microparticles in vivo during atorvastatin treatment; a randomized double-blind placebo-controlled study. Thromb Res 2012; 129:95–97. [DOI] [PubMed] [Google Scholar]
- 14.Oggero S, de Gaetano M, Marcone S, Fitzsimons S, Pinto AL, Ikramova D, et al. Extracellular vesicles from monocyte/platelet aggregates modulate human atherosclerotic plaque reactivity. J Extracell Vesicles 2021; 10:12084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rhys HI, Dell’Accio F, Pitzalis C, Moore A, Norling LV, Perretti M. Neutrophil microvesicles from healthy control and rheumatoid arthritis patients prevent the inflammatory activation of macrophages. EBioMedicine 2018; 29:60–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chahine MN, Dibrov E, Blackwood DP, Pierce GN. Oxidized LDL enhances stretch-induced smooth muscle cell proliferation through alterations in nuclear protein import. Can J Physiol Pharmacol 2012; 90:1559–1568. [DOI] [PubMed] [Google Scholar]
- 17.Amabile N, Cheng S, Renard JM, Larson MG, Ghorbani A, McCabe E, et al. Association of circulating endothelial microparticles with cardiometabolic risk factors in the Framingham Heart Study. Eur Heart J 2014; 35:2972–2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Hoog VC, Timmers L, Schoneveld AH, Wang JW, van de Weg SM, Sze SK, et al. Serum extracellular vesicle protein levels are associated with acute coronary syndrome. Eur Heart J Acute Cardiovasc Care 2013; 2:53–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Verbree-Willemsen L, Zhang YN, Gijsberts CM, Schoneveld AH, Wang JW, Lam CSP, et al. LDL extracellular vesicle coagulation protein levels change after initiation of statin therapy. Findings from the METEOR trial. Int J Cardiol 2018; 271:247–253. [DOI] [PubMed] [Google Scholar]
- 20.Kanhai DA, Visseren FL, van der Graaf Y, Schoneveld AH, Catanzariti LM, Timmers L, et al. Microvesicle protein levels are associated with increased risk for future vascular events and mortality in patients with clinically manifest vascular disease. Int J Cardiol 2013; 168:2358–2363. [DOI] [PubMed] [Google Scholar]
- 21.Wiedmer T, Sims PJ. Participation of protein kinases in complement C5b-9-induced shedding of platelet plasma membrane vesicles. Blood 1991; 78:2880–2886. [PubMed] [Google Scholar]
- 22.Leeuwenberg JF, Smeets EF, Neefjes JJ, Shaffer MA, Cinek T, Jeunhomme TM, et al. E-selectin and intercellular adhesion molecule-1 are released by activated human endothelial cells in vitro. Immunology 1992; 77:543–549. [PMC free article] [PubMed] [Google Scholar]
- 23.Berckmans RJ, Lacroix R, Hau CM, Sturk A, Nieuwland R. Extracellular vesicles and coagulation in blood from healthy humans revisited. J Extracell Vesicles 2019; 8:1688936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Satta N, Toti F, Feugeas O, Bohbot A, Dachary-Prigent J, Eschwege V, et al. Monocyte vesiculation is a possible mechanism for dissemination of membrane-associated procoagulant activities and adhesion molecules after stimulation by lipopolysaccharide. J Immunol 1994; 153:3245–3255. [PubMed] [Google Scholar]
- 25.Ou ZJ, Chang FJ, Luo D, Liao XL, Wang ZP, Zhang X, et al. Endothelium-derived microparticles inhibit angiogenesis in the heart and enhance the inhibitory effects of hypercholesterolemia on angiogenesis. Am J Physiol Endocrinol Metab 2011; 300:E661–E668. [DOI] [PubMed] [Google Scholar]
- 26.Vajen T, Benedikter BJ, Heinzmann ACA, Vasina EM, Henskens Y, Parsons M, et al. Platelet extracellular vesicles induce a pro-inflammatory smooth muscle cell phenotype. J Extracell Vesicles 2017; 6:1322454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee MMY, Sattar N, McMurray JJV, Packard CJ. Statins in the prevention and treatment of heart failure: a review of the evidence. Curr Atheroscler Rep 2019; 21:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gupta A, Mackay J, Whitehouse A, Godec T, Collier T, Pocock S, et al. Long-term mortality after blood pressure-lowering and lipid-lowering treatment in patients with hypertension in the Anglo-Scandinavian Cardiac Outcomes Trial (ASCOT) Legacy study: 16-year follow-up results of a randomised factorial trial. Lancet 2018; 392:1127–1137. [DOI] [PubMed] [Google Scholar]
- 29.Ashman N, Macey MG, Fan SL, Azam U, Yaqoob MM. Increased platelet-monocyte aggregates and cardiovascular disease in end-stage renal failure patients. Nephrol Dial Transplant 2003; 18:2088–2096. [DOI] [PubMed] [Google Scholar]
- 30.Martinez-Maldonado M. Hypertension in end-stage renal disease. Kidney Int Suppl 1998; 68:S67–72. [DOI] [PubMed] [Google Scholar]
- 31.Argmann CA, Edwards JY, Sawyez CG, O’Neil CH, Hegele RA, Pickering JG, et al. Regulation of macrophage cholesterol efflux through hydroxymethylglutaryl-CoA reductase inhibition: a role for RhoA in ABCA1-mediated cholesterol efflux. J Biol Chem 2005; 280:22212–22221. [DOI] [PubMed] [Google Scholar]
- 32.Kuklenyik Z, Jones JI, Gardner MS, Schieltz DM, Parks BA, Toth CA, et al. Core lipid, surface lipid and apolipoprotein composition analysis of lipoprotein particles as a function of particle size in one workflow integrating asymmetric flow field-flow fractionation and liquid chromatography-tandem mass spectrometry. PLoS One 2018; 13:e0194797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu M, Wang F, Wang Y, Jin R. Atorvastatin improves endothelial function and cardiac performance in patients with dilated cardiomyopathy: the role of inflammation. Cardiovasc Drugs Ther 2009; 23:369–376. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.