Abstract
Background
Acute fulminant myocarditis in children is associated with elevated mortality and morbidity with few advances in its medical management. Here we report a preliminary experience of children treated with IL-1 receptor antagonist associated with rapid myocardial function recovery.
Methods
A retrospective case series of children admitted in the Pediatric Intensive Care Unit of the Bicêtre Hospital (AP–HP Paris Saclay University) between April 2020 and January 2022 with acute myocarditis. Children were treated with subcutaneous anakinra (an IL-1 receptor antagonist). Patients characteristics, and outcome are reported.
Results
Of 10 children admitted with acute fulminant myocarditis, eight were treated with sub-cutaneous anakinra. Seven children had SARS-CoV-2 post-infective myocarditis associated with multisystem inflammatory syndrome in children (MIS-C) and one child Parvovirus B19 myocarditis. In all patients a rapid (< 24 h) improvement in myocardial function was observed with concomitant decrease in myocardial enzymes. All patients survived with full myocardial recovery.
Conclusions
In this pilot study, use of IL-1 receptor antagonist in the initial treatment of acute fulminant myocarditis in children seems to be associated with rapid stabilization and recovery.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13613-022-01054-0.
Keywords: Myocarditis, Children, COVID-19, B19 parvovirus, PIMS–TS, MIS-C, Anakinra, IL-1 receptor antagonist
Introduction
Acute fulminant myocarditis is among the most severe condition in critically ill children for which supportive therapy remain the mainstay. However, aetiology-targeted treatment of myocarditis are missing and may prove to radically improve short and long-term prognosis [1]. Myocarditis has been defined as an inflammatory disease of the myocardium, established with histological, immunological criteria, with or without cardiac failure [2]. In children, myocarditis diagnosis is based on clinical presentation, biological markers (including Troponin T (TnT), NT-pro BNP), cardiac magnetic resonance imaging (MRI), and myocardial biopsy [1]. Myocarditis aetiologies are multiple, but post-infective mechanisms are well-identified as central trigger of the disease. Since the global pandemic of SARS-CoV-2 infection, post-infective myocarditis as part of the multi-inflammatory syndrome in children (MIS-C) was largely described in children and raised the possibility of immunomodulatory therapies with immunoglobulins (IVIg) and steroids [3, 4]. Carter et al., in severe MIS-C with myocarditis, observed high serum concentrations of IL-1β, suggesting a potential role of IL-1 pathways in its pathogenesis [5]. In adults with acute myocarditis, activation of the inflammasome through the production of pro-inflammatory mediators such IL-1β, IL-18 is widely recognized [6]. The benefic effect of an IL-1 receptor antagonist in the treatment of acute myocarditis has been recently suggested [7, 8]. Anakinra, a recombinant human IL-1 receptor antagonist, neutralizes the biological activity of interleukin-1 alpha and beta (IL-1ß) by competitive inhibition of IL-1 binding to its type I receptor (IL-1RI). Thus we report our experience of eight children with acute fulminant myocarditis who received anakinra.
Case series
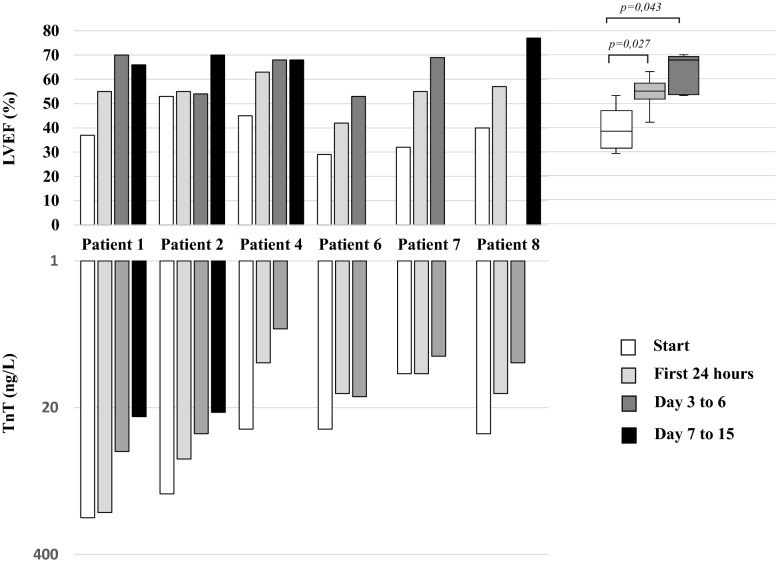
Between April 2020 and January 2022, eight children with acute fulminant myocarditis were admitted to the Paediatric Intensive Care Unit (PICU) of the Bicêtre Hospital and were treated with sub-cutaneous Anakinra. Acute fulminant myocarditis was diagnosed using conventional criteria used in children: acute myocardial dysfunction identified by echocardiography in a previously healthy child without known cardiopathy associated with increased troponin and confirmed by cardiac MRI using revised Lake Louis criterion for diagnosis of myocarditis. Endomyocardial biopsy were not performed. During the same period, two additional children with acute fulminant myocarditis (Covid-19, Mediterranean fever) did not received anakinra. Patients characteristics are described in Table 1. The mean age was 7 years, ranging from 13 months to 14 years. Four of eight children were in cardiogenic shock and needed vasopressor drugs for a median of 1,5 days (1–4 days), one required central extracorporeal membrane oxygenation (ECMO). All but one patient had a Left Ventricular Ejection Fraction (LVEF) of less than 50% at admission in the PICU. The one patient who didn’t have left ventricular (LV) dysfunction was a 1-year-old boy with surgically corrected Tetralogy of Fallot with positive cardiac enzymes (TnT and NT-pro BNP) in the course of an active COVID-19 infection (positive SARS-CoV-2 PCR) associated with hematologically diagnosed macrophage activation syndrome. Two of the six children had ventricular arrhythmia, one evolving toward malignant ventricular fibrillation. Seven patients had post-infective myocarditis, six secondary to SARS-CoV-2 (positive IgG and negative PCR) and one following B19 parvovirus (positive IgG, negative IgM, negative PCR) infection. All six patients with MIS-C experienced a rapid (within 24 h following Anakinra first injection) LVEF improvement, decreased of TnT (Fig. 1) concomitantly to a decrease of blood C-reactive protein (Additional file 1: Figure S1).
Table 1.
Patients characteristics
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 | Patient 7 | Patient 8 | ||
|---|---|---|---|---|---|---|---|---|---|
| Demographics | |||||||||
| Age (years) | 14 | 13 | 1 | 4 | 1 | 9 | 10 | 5 | |
| Weight (kg) | 62 | 86 | 8.2 | 17 | 7.9 | 38.9 | 38 | 24.5 | |
| Sex | Male | Female | Male | Male | Male | Female | Male | Male | |
| Comorbidity | No | Obesity | Fallot tetralogy | No | No | No | No | No | |
| Clinical condition | |||||||||
| Cardiogenic shock | Yes | Yes | No | No | Yes | Yes | No | No | |
| Ventricular arrythmia | Yes | No | No | No | Yes | No | No | No | |
| MAS | No | Yes | Yes | Yes | No | No | No | No | |
| Cardiac evaluation at admission | |||||||||
| LVEF (%) | 37 | 30 | – | 45 | 37 | 29 | 32 | 40 | |
| Subaortic VTI (cm) | 13 | 18 | – | 14 | 8 | 12 | 13 | 14 | |
| LV dilatation | No | No | No | No | No | No | No | No | |
| MI (grade) | Grade I | Grade I | – | Grade I | Grade III | Grade II | Grade II | Grade I | |
| RV dysfunction | Yes | No | No | No | No | No | No | No | |
| ECG | Fleeting ventricular arrhythmia | Normal | Normal | Normal | Vf bursts, st segment elevation | Normal | Normal | Normal | |
| Laboratory findings at admission | |||||||||
| CRP (mg/l) | 310 | 333 | 150 | 94 | 10 | 288 | 248 | 196 | |
| PCT (μg/l) | 84.87 | 15.8 | 28.7 | 2.63 | 0.26 | 6.19 | 161.29 | 3.81 | |
| Wbcs (/mm3) | 19,900 | 4870 | 16,690 | 11,230 | 10,260 | 17,090 | 5980 | 20,000 | |
| Lymphocytes (/mm3) | 1350 | 370 | 1670 | 1680 | 2870 | 1590 | 950 | 1280 | |
| Neutrophils (/mm3) | 17,270 | 4270 | 12,520 | 7640 | 6870 | 14,520 | 4800 | 18,260 | |
| Serum creatinine (μmol/l) | 133 | 79 | 31 | 32 | 37 | 59 | 39 | 30 | |
| NT pro BNP (ng/l) | 16,931 | 20,865 | 4269 | 2231 | 61,079 | 2591 | 7444 | 4105 | |
| PCR SARS-CoV-2 (blood) | Negative | Negative | Positive | Positive | Negative | Negative | Negative | Positive | |
| IgG SARS-CoV-2 | Positive | Positive | Negative | Positive | Negative | Positive | Positive | Negative | |
| Other virus (blood) | No | No | No | No | PB19 (IgG + , IgM -, PCR -) | No | No | No | |
| Treatment | |||||||||
| Immunotherapy |
Anakinra + IVIg |
Anakinra + IVIg + CS |
Anakinra + IVIg+ CS |
Anakinra + IVIg + CS |
Anakinra + IVIg |
Anakinra + CS |
Anakinra + CS |
Anakinra + CS | |
| Reason for initiating anakinra | Poor hemodynamic tolerance of IVIg | Poor hemodynamic tolerance of IVIg | MAS (bone marrow analysis, high blood ferritin and triglycerides) | Poor hemodynamic tolerance of IVIg | Severity of clinical picture | Severity of clinical picture | Major inflammatory state | Major inflammatory state | |
| Time to start anakinra after admission (days) | 0 | 1 | 8 | 0 | 3 | 1 | 0 | 2 | |
| Total anakinra treatment time (days) | 14 | 15 | 11 | 7 | 4 | 1 | 3 | 3 | |
| Anakinra dosage per day | 200 mg | 200 mg | 6 mg/kg | 4 mg/kg | 4 mg/kg | 2 mg/kg | 4 mg/kg | 4 mg/kg | |
| Max VIS score | 55 | 5 | 160 | 0 | 1275 | 20 | 0 | 0 | |
| Total inotropic/ vasopressor support duration (days) | 1 | 1 | 4 | 0 | 4 | 2 | 0 | 0 | |
| Diuretic | Yes | Yes | No | No | Yes | Yes | No | No | |
| Other cardiovascular therapy | No | No | Milrinone | No | Milrinone; amiodarone; levosimendan | No | No | No | |
| Length of stay in ICU (days) | 6 | 7 | 19 | 3 | 23 | 6 | 1 | 3 | |
| Length (days) of non-invasive ventilation | 0 | 4 | 0 | 0 | 1 | 0 | 0 | 0 | |
| Length (days) of invasive ventilation | 0 | 0 | 6 | 0 | 18 | 0 | 0 | 0 | |
Vasoactive-Inotropic Score (VIS) = dopamine dose (μg/Kg/min) + dobutamine dose (μg/Kg/min) + 100 × epinephrine dose (μg/Kg/min) + 10 × milrinone dose (μg/Kg/min) + 10,000 × vasopressin dose (U/Kg/min) + 100 × norepinephrine dose (μg/Kg/min)
BMI body mass index, MI mitral insufficiency, TTE transthoracic echocardiogram, LVEF left venticular ejection fraction, VTI aortic velocity time integral, ECG electrocardiogram, VF ventricular fibrillation, CRP C-reactive protein, PCT procalcitonin, WBCs white blood cells, MAS Macrophage Activation Syndrome, PB19 B19 Parvovirus, IVIg, immunoglobulins, CS corticosteroids
Fig. 1.
Left ventricle ejection fraction and serum troponin T following anakinra therapy. Values are expressed as LVEF (%) at days 0–2, days 3–6, and days 7–15 after Anakinra therapy. Wilcoxon sign rank test was used to test paired values. LVEF, Left Ventricle Ejection Fraction; TnT, Troponin T
The 1-year-old boy with B19 parvovirus acute fulminant myocarditis demonstrated cardiogenic shock and malignant refractory ventricular fibrillation requiring central ECMO. Anakinra was administered on day 0 of ECMO and continued for 4 days in total. ECMO was successfully weaned after 10 days. 2 weeks following the initiation of anakinra, repeat transthoracic echocardiogram (TTE) demonstrated marked improvement in LVEF, from 13 to 55%, with a decrease in left end-diastolic ventricular diameter from 39.9 mm (Z-score 6.47) to 33 mm (Z-score 2.71). A cardiac MRI was performed 27 days following anakinra treatment and showed only a discrete basal and mid-lateral linear subepicardial enhancement. Four of the eight patients (3 MIS-C, 1 B19 parvovirus) had 5 months follow-up. All had normal LVEF and left ventricle longitudinal strain. No treated patients showed any recognized adverse events related to anakinra therapy.
Discussion
Hereby, we report the use of anakinra in eight patients with acute fulminant myocarditis and its benefit on LV function recovery. The possible outcomes of myocarditis in children are sudden death, arrhythmias, myocardial infarcts and heart failure with dilated cardiomyopathy phenotype [1]. An analysis of the Pediatric Cardiomyopathy Registry (PCMR), including 369 children with myocarditis, found that 3 years after presentation approximately 7% of patients had died, 18% had undergone heart transplantation and 53% had normal left ventricular structure and systolic function [10]. In the current case series none of the patient died or were transplanted. All of them had achieved echocardiographic normalization and only two of six children had arrhythmia. Profibrotic cytokines including interleukin IL-1β play an essential role in tissue remodeling by stimulating fibroblast proliferation resulting in greater collagen synthesis and fibrosis [11]. In adults with acute myocarditis, formation of the inflammasome is a well-recognized pathogenic mechanism. Inflammasomes are a group of protein complexes which recognize a diverse set of inflammation-inducing stimuli, including Pathogen- and Damage-Associated Molecular Patterns, and control the production of several proinflammatory cytokines, such as IL-1β [6]. The use of early IL-1 receptor antagonist in patients identified with an inflammatory cardiomyopathy might further reduce progression to chronic dilated cardiomyopathy. In our series, all patient had high C-reactive protein suggestive of significant systemic inflammatory process. A randomized study in adults is currently underway comparing anakinra versus placebo for the treatment of acute myocarditis (ARAMIS, ClinicalTrials.gov Identifier: NCT03018834). Anakinra is a recognized therapy targeting the inflammasome in various systemic inflammatory disease, such as rheumatoid arthritis and juvenile polyarthritis [9]. In children, it is a therapy under investigation for Kawasaki disease that shows many clinical similarities with MIS-C [12, 13]. In our series, in most patients LVEF improvement was noted in the first hours following anakinra injection. The effect of other treatments such as corticosteroids cannot be excluded. Effect of IVIg is questionable. A systematic review of IVIg therapy in presumed viral myocarditis included only one randomized pediatric study of 86 children and found that IVIg did not significantly improve survival or left ventricular function [14]. Three patients did not hemodynamically tolerate large volume load secondary to IVIg infusion and treatment was interrupted. Altogether, currently neither corticosteroids nor IVIg ever showed such immediate recovery of contractile function during acute fulminant myocarditis. As for the treatment of MIS-C, use of IVIg, glucocorticoids, showed conflicting results. The US Overcoming Covid consortium [15] determined that initial treatment of MIS-C with immunoglobulin plus glucocorticoids was associated with a lower risk of cardiovascular dysfunction, requirement of adjunct therapies and vasopressors than with IVIg alone. In contrast, the international Best Available Treatment Study (BATS) consortium found no statistically significant difference for ventilation, inotropic support, death or for improvement on an ordinal clinical severity scale for any of the three treatments: immunoglobulin alone, a combination of immunoglobulin and glucocorticoids, or glucocorticoids alone [16]. This discrepency may be explained by different patient severity, different SARS-CoV-2 variants, but neither of these studies definitively answered the question about effective single or combination of immunomodulatory treatment including steroids, IVIg and biotherapies with monoclonal antibodies, such as anakinra and infliximab [17]. Although it is becoming increasingly clear that rapid immunomodulatory therapies can be lifesaving in patients with MIS-C, the underlying change in the therapeutic paradigm of acute fulminant myocarditis is changing and warrant randomized, controlled trials to evaluate the safety and efficacy of biotherapies in acute myocarditis.
In conclusion, our retrospective case series supports further investigation of the role for IL-1 receptor antagonist in the treatment of pediatric acute and fulminant myocarditis. All patients had rapid response to anakinra on contractile dysfunction and cardiac enzymes. Given its safety [18] and rapid onset of action, anakinra may have a place in the pediatric myocarditis treatment.
Take-home message
Anakinra, an IL-1 receptor antagonist, is shown to be safe and may provide a novel approach to treat fulminant acute myocarditis in children.
Supplementary Information
Additional file 1: Figure S1. Blood C-reactive protein response to anakinra.
Acknowledgements
Consortia for The COVID-19 Immune Suppression (CLOVIS) study group: Simon Barreault, Mélissa Beggaz, Emre Belli, Ramy Charbel, Caroline Claude, Philippe Durand, Caroline Galeotti, Sébastien Hascoet, Virginie Lambert, Alice Maltret, Clémence Marais, Louise Maunier, Jordi Miatello, Luc Morin, Louise Othoniel, Bastien Provot, Adrien Schvartz, Pierre Tissieres, Isabelle Van Aershot, Joy Zogby.
Author contributions
Design of the work: LM, VL, PT. Acquisition, analysis, or interpretation of data for the work: LM, RC, VL, PT. Drafting the manuscript: LM, PT. Revising it critically for important intellectual content: all authors. All authors read and approved the final manuscript.
Funding
No funding.
Availability of data and materials
Upon request to corresponding author.
Declarations
Ethical approval and consent to participate
The study (French study classification: MR004) was approved by the local IRB and French Data Protection Authority (CNIL Registration Number: 2219981) waiving the need of written consent. The study was registered at ClinicalTrial.gov Identifier: NCT04544878 registered on September 10, 2020, https://clinicaltrials.gov/ct2/show/NCT04544878.
Consent for publication
All authors approved the final version submitted for publication and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Competing interests
All authors disclose any competing interest related to the manuscript.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Pierre Tissières, Email: pierre.tissieres@aphp.fr.
the CLOVIS study group:
Simon Barreault, Mélissa Beggaz, Emre Belli, Ramy Charbel, Caroline Claude, Philippe Durand, Caroline Galeotti, Sébastien Hascoet, Virginie Lambert, Alice Maltret, Clémence Marais, Louise Maunier, Jordi Miatello, Luc Morin, Louise Othoniel, Bastien Provot, Adrien Schvartz, Pierre Tissieres, Isabelle Van Aershot, and Joy Zogby
References
- 1.Canter CE, Simpson KE. Diagnosis and treatment of myocarditis in children in the current era. Circulation. 2014;129(1):115–128. doi: 10.1161/CIRCULATIONAHA.113.001372. [DOI] [PubMed] [Google Scholar]
- 2.Richardson P, McKenna W, Bristow M, Maisch B, Mautner B, O'Connell J, Olsen E, Thiene G, Goodwin J, Gyarfas I, Martin I, Nordet P. Report of the 1995 world health organization/international society and federation of cardiology task force on the definition and classification of cardiomyopathies. Circulation. 1996;93(5):841–842. doi: 10.1161/01.CIR.93.5.841. [DOI] [PubMed] [Google Scholar]
- 3.Belhadjer Z, et al. Acute heart failure in multisystem inflammatory syndrome in children in the context of global SARS-CoV-2 pandemic. Circulation. 2020 doi: 10.1161/CIRCULATIONAHA.120.048360.(3). [DOI] [PubMed] [Google Scholar]
- 4.Grimaud M, Starck J, Levy M, et al. Acute myocarditis and multisystem inflammatory emerging disease following SARS-CoV-2 infection in critically ill children. Ann Intensive Care. 2020;10(1):69. doi: 10.1186/s13613-020-00690-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carter MJ, Fish M, Jennings A, et al. Peripheral immunophenotypes in children with multisystem inflammatory syndrome associated with SARS-CoV-2 infection. Nat Med. 2020;26(11):1701–1707. doi: 10.1038/s41591-020-1054-6. [DOI] [PubMed] [Google Scholar]
- 6.Strowig T, Henao-Mejia J, Elinav E, Flavell R. Inflammasomes in health and disease. Nature. 2012;481(7381):278–286. doi: 10.1038/nature10759. [DOI] [PubMed] [Google Scholar]
- 7.Cavalli G, et al. Treating life-threatening myocarditis by blocking interleukin-1*. Crit Care Med. 2016 doi: 10.1097/CCM.0000000000001654. [DOI] [PubMed] [Google Scholar]
- 8.Trpkov C, et al. Rapid response to cytokine storm inhibition using Anakinra in a patient with COVID-19 Myocarditis. CJC Open. 2020 doi: 10.1016/j.cjco.2020.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Furst DE. Anakinra: review of recombinant human interleukin-I receptor antagonist in the treatment of rheumatoid arthritis. Clin Ther. 2004;26(12):1960–1975. doi: 10.1016/j.clinthera.2004.12.019. [DOI] [PubMed] [Google Scholar]
- 10.Foerster SR, Canter CE, Cinar A, et al. Ventricular remodeling and survival are more favorable for myocarditis than for idiopathic dilated cardiomyopathy in childhood: an outcomes study from the pediatric cardiomyopathy registry. Circ Heart Fail. 2010;3(6):689–697. doi: 10.1161/CIRCHEARTFAILURE.109.902833. [DOI] [PubMed] [Google Scholar]
- 11.Fairweather D, Frisancho-Kiss S, Yusung SA, et al. Interferon-γ protects against chronic viral myocarditis by reducing mast cell degranulation, fibrosis, and the profibrotic cytokines transforming growth factor-β1, Interleukin-1β, and interleukin-4 in the heart. Am J Pathol. 2004;165(6):1883–1894. doi: 10.1016/S0002-9440(10)63241-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Toubiana J, Poirault C, Corsia A, et al. Kawasaki-like multisystem inflammatory syndrome in children during the covid-19 pandemic in Paris, France: prospective observational study. BMJ. 2020 doi: 10.1136/bmj.m2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koné-Paut I, Tellier S, Belot A, et al. Phase II open label study of Anakinra in intravenous immunoglobulin-resistant kawasaki disease. Arthritis Rheumatol. 2021;73(1):151–161. doi: 10.1002/art.41481. [DOI] [PubMed] [Google Scholar]
- 14.Robinson J, Hartling L, Vandermeer B, Sebastianski M, Klassen TP. Intravenous immunoglobulin for presumed viral myocarditis in children and adults. Cochrane Database Syst Rev. 2020 doi: 10.1002/14651858.CD004370.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Son MBF, Murray N, Friedman K, et al. Multisystem inflammatory syndrome in children—initial therapy and outcomes. N Engl J Med. 2021;385(1):23–34. doi: 10.1056/NEJMoa2102605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McArdle AJ, Vito O, Patel H, et al. Treatment of multisystem inflammatory syndrome in children. N Engl J Med. 2021;385(1):11–22. doi: 10.1056/NEJMoa2102968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DeBiasi RL. Immunotherapy for MIS-C - IVIG, glucocorticoids, and biologics. N Engl J Med. 2021;385(1):74–75. doi: 10.1056/NEJMe2108276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Singh JA, Wells GA, Christensen R, et al. Adverse effects of biologics: a network meta-analysis and Cochrane overview. Cochrane Database Syst Rev. 2011 doi: 10.1002/14651858.CD008794.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Figure S1. Blood C-reactive protein response to anakinra.
Data Availability Statement
Upon request to corresponding author.