Abstract
Purpose
To provide the evidence-based recommendations on the role of testosterone (T) on age-related symptoms and signs remains.
Methods
The Italian Society of Andrology and Sexual Medicine (SIAMS) and the and the Italian Society of Endocrinology (SIE) commissioned an expert task force to provide an updated guideline on adult-onset male hypogonadism. Derived recommendations were based on Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) system.
Results
Clinical diagnosis of adult-onset hypogonadism should be based on a combination of clinical and biochemical parameters. Testosterone replacement therapy (TRT) should be offered to all symptomatic subjects with hypogonadism after the exclusion of possible contraindications. T gels and the long-acting injectable T are currently available preparations showing the best efficacy/safety profile. TRT can improve all aspects of sexual function, although its effect is limited in more complicated patients. Body composition (reducing fat mass and increasing lean mass) is improved after TRT, either in subjects with or without metabolic syndrome or type 2 diabetes. Conversely, the role of TRT in improving glycometabolic control is more conflicting. TRT can result in increasing bone mineral density, particularly at lumbar site, but no information on fracture risk is available. Limited data support the use of TRT for improving other outcomes, including mood frailty and mobility.
Conclusions
TRT can improve sexual function and body composition particularly in less complicated adult and in aging subjects with hypogonadism. When hypogonadism is adequately diagnosed, T appropriately prescribed and subjects correctly followed up, no short-term increased risk of adverse events is observed. Longer and larger studies are advisable to better clarify TRT long-term efficacy/safety profile.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40618-022-01859-7.
Keywords: Late-onset hypogonadism, Testosterone, Hypogonadism, Erectile dysfunction, Obesity, Metabolic syndrome, Bone mineral density
Introduction
During the last 2 decades, there was a growing awareness concerning male hypogonadism and its potential treatment. To the classical forms of hypogonadism, the description of an “age-associated testosterone decline”, supported by several epidemiological studies [1–4], widely broadened the number of potentially treatable men. To what extent the decline in testosterone (T) concentrations observed in the aging male contributes to age-related morbidities and symptoms remains under scrutiny [5]. Interestingly, the sales of T-containing medications dramatically increased worldwide [6]. While the global trend reflected an increased awareness toward male hypogonadism, often an underdiagnosed and undertreated condition with a worldwide evenly distributed prevalence in North America reflected the effect of direct-to-consumer advertising (DTCPA). T treatment was inappropriately promoted as a fountain of youth, and exposure to televised DTCPA was associated with greater T testing, new initiation of therapy and, especially, initiation of therapy without prior T testing [7]. To halt T overuse, the US Food and Drug Administration (FDA) cautioned that the benefits of T treatment were not clearly established when T concentrations were low due to aging [8]. The FDA warned that T replacement therapy (TRT) should be considered only for men with “classical hypogonadism”, i.e. due to primary or secondary T deficiency resulting from known problems within the testis, pituitary, or hypothalamus [8]. Later on, this concept was partially incorporated into the clinical practice guidelines of the US Endocrine Society [9] as well as in the Australian one [10] advocating a theoretical dichotomic distinction between organic (classic) and functional hypogonadism, the latter including the age-associated T deficiency.
In Southern Europe, however, the picture is different and DTCPA more often prohibited. The first Italian guidelines covering the issue of male hypogonadism, including age-associated deficiency, were issued in 2015 by the Italian Society of Endocrinology [11]. Important multicenter trials have been published since then, disclosing novel data on the effectiveness of TRT on various outcomes. Considering the different background between the European and North American situation, aim of these guidelines is to provide an evidence-based updated support to clinician operating on male hypogonadism on behalf of the Italian Society of Andrology and Sexual Medicine (SIAMS) and the Italian Society of Endocrinology (SIE).
Methods
The SIAMS and SIE are large national multidisciplinary non-profit scientific societies leading research in this field. The SIAMS and SIE have commissioned an expert task force to provide an updated guideline on male hypogonadism. Following scrutiny and discussion of the best evidence from published literature available in PubMed, the Authors generated a series of consensus recommendations according to the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) system [12]. The GRADE system allows to rate the quality of evidence and the strength of recommendations and to enhance the value of the clinical advices here provided [12] with a consistent language and graphical descriptions for standardizing the grading of both the strength of recommendation and the quality of evidence [12]. Concerning the strength of recommendation, the number 1 indicates a strong recommendation and is associated with the terminology “we recommend”; the number 2 denotes a weak recommendation and is associated with the wording “we suggest”. The four levels grading of the quality of evidence employs the following graphical descriptions: ØOOO denotes “very low-quality evidence”, ØØOO “low quality”, ØØØO “moderate quality”, and ØØØØ “high quality”.
According to SIAMS rulings, these Guidelines have been arranged by a team of experts on the topic coordinated by the senior author and two members of the SIAMS Guideline Committee, then sent to the SIAMS and SIE Executive Committee and to the Directors of all SIAMS Excellence Centers for revisions and/or approval. Guidelines have then been announced by mail and published for two weeks on the SIAMS and SIE Society’s website, siams.info, so that all SIAMS Members could provide further comments and suggest additional minor revisions. Following this last step, the present manuscript has been submitted to the Journal of Endocrinological Investigation for international peer reviewing.
Definitions
Hypogonadism refers to a clinical and biochemical syndrome characterized by the inability of the testes to produce physiological concentrations of T and/or number of normal sperm cells [13–16].
Male hypogonadism can be due to a testicular dysfunction (primary hypogonadism), a pituitary/hypothalamic failure (secondary hypogonadism) or a combination of both (mixed hypogonadism) [13, 14, 16]. Some classifications also consider the onset of symptoms (e.g., late-onset hypogonadism—LOH) or the potential reversibility of the condition. In this respect, “organic hypogonadism” refers to an irreversible form of hypogonadism caused by congenital or acquired (destructive or structural) impairment occurring at any level of the hypothalamic–pituitary–testis axis [17–19], while “functional hypogonadism” refers to a potentially reversible impairment of the hypothalamic–pituitary–testis feedback loop. Functional hypogonadism more often occurs in middle aged or older men (> 40–50 years), is associated to comorbid illnesses (e.g. poor health or obesity), and is associated with a less marked lowering of T concentrations [17–19]. The latter classification is not universally accepted and still a matter of vivid debate.
Clinical presentation
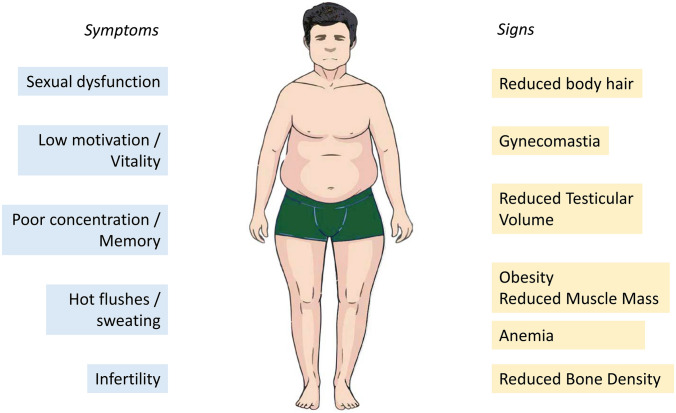
Signs and symptoms of hypogonadism vary according to the age of onset, etiology and severity of T reduction [20, 21]. In adults, the predominant symptoms include sexual dysfunctions (e.g., erectile dysfunction, decreased libido, reduced nocturnal/morning erections), fatigue, impaired concentration, sweating, low wellbeing and depressive mood [13, 14, 20, 21]. Increased body fat, decreased muscle mass and body hair, gynecomastia and reduced testicular volume are common findings at physical examination. Hypogonadism is also associated with anemia, lower prostatic-specific antigen (PSA) concentrations for age, and reduced bone mineral density [13, 14, 20, 21] (Fig. 1).
Fig. 1.
Symptoms and signs frequently associated with adult-onset hypogonadism
Diagnosis
We recommend measuring total testosterone (tT) and luteinizing hormone (LH) in all men with clinical manifestations consistent with hypogonadism and to adopt a threshold of ≤ 12.0 nmol/L to define low total testosterone (1ØØØO).
We suggest measuring sex hormone-binding globulin (SHBG) during diagnostic workup to calculate free testosterone in all men with clinical manifestations consistent with hypogonadism and to adopt a threshold < 220 pmol/L to define low calculated low free testosterone (fT) (2 ØØOO).
A value of LH ≥ 9.4 IU/L, in the presence of low total or calculated free testosterone, suggests primary hypogonadism. For LH concentrations < 9.4 IU/L, measuring follicular stimulating hormone (FSH) is helpful in the differential diagnosis between primary and secondary hypogonadism (2ØOOO).
We suggest a diagnosis of compensated hypogonadism in presence of LH ≥ 9.4 IU/l and normal testosterone or calculated free testosterone concentrations (Expert Opinion).
Evidence
The diagnosis of hypogonadism relies on the concomitant presence of low T concentrations and clinical symptoms and signs of T deficiency [13, 14, 16, 20–22]. Sexual symptoms (erectile dysfunction, low libido, reduced nocturnal erections) are the most frequently complained in adult men with reduced T concentrations [22, 23].
The diagnosis requires the detection of a low T on two separate occasions with blood taken in the morning in standardized conditions, i.e., before 10:00 am and fasting. Liquid chromatography–tandem mass spectrometry (LC–MS/MS) is considered the gold standard for T measurement; however. good-quality immunoassays still provide reliable results in the clinical settings [24]. Conversely, the available immunoassays cannot guarantee accurate values of fT. Hence, fT evaluation by immunoassay is discouraged [25].
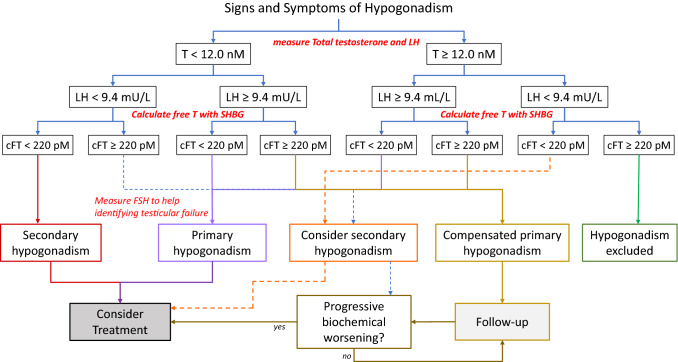
To date, there is no agreement on what should be the T threshold value defining hypogonadism or the lower limit of normal for T distribution in the population, whose values (harmonized to the 2.5th percentile) ranges from 264 ng/ml (9.2 nmol/L) to 348 ng/ml (12 nmol/L), according to large cohort studies on healthy, non-obese, young adult males [26]. However, different thresholds, ranging from 220 (7.6 nmol/L) to 350 ng/dl (12.1 nmol/L), have also been proposed [15]. Data derived from available meta-analyses suggest that TRT is without effects in subjects with baseline T concentrations > 12 nmol/L (3.5 ng/mL) (Fig. 2) [20, 27]. Since some beneficial effects on various outcomes have been documented when T concentrations are below 12 nmol/l [20, 27], the panel reached consensus on adopting the 12 nmol/L as a threshold for considering TRT in symptomatic hypogonadal men.
Fig. 2.
Proposed flowchart to diagnose and manage adult-onset hypogonadism: cFT calculated free, testosterone; FSH follicular-stimulating hormone, LH luteinizing hormone, SHBG sex hormone binding globulin, T testosterone. The dashed lines reflects a lower level of evidence
The EMAS study showed that fT improved the possibility to correctly identify LOH. According to that study, reduced fT (< 220 pmol/L) increased the odds ratio for hypogonadism as compared with the total T level alone, especially for thresholds between 8.0 and 11 nmol per liter [22, 28]. Similar data have been observed in the longitudinal evaluation of the same study [29].
fT can be determined after physical separation from the protein-bound forms, which is achieved through equilibrium dialysis or ultracentrifugation. Equilibrium dialysis is the most accurate method [30]. However, the latter is expensive, time-consuming, and unfeasible in a clinical setting. Several SHBG and albumin level-based calculations have been proposed to estimate fT (cfT). The Vermeulen method [31] is still the most accurate, albeit slightly overestimating fT value [30].
The classification into primary vs. secondary hypogonadism has important clinical and therapeutic implications [13, 14]. According to the baseline data of European Male Aging Study (EMAS), LH concentrations ≥ 9.4 IU/l define primary hypogonadism, whereas low or low-normal LH concentrations, in the presence of reduced T concentrations, define secondary hypogonadism. LH concentrations failing to rise when T is low, suggest they are inappropriately normal [32]. When LH concentrations are close to the 9.4 IU/l threshold value, FSH determination, a marker of Sertoli function [33], may help to identify primary testicular failure, despite there is no consensus on a specific FSH threshold value [13, 14].
Conversely, a suspect of organic secondary hypogonadism requires additional investigations including magnetic resonance imaging (MRI) scanning of the pituitary-hypothalamus, iron saturation as well as prolactin and other anterior pituitary function. To adopt cost-effective strategy, the aforementioned examinations are indicated when a greater suspicion is driven by specific features including—but not limited to—pituitary mass effects symptoms, visual disturbances, headache or significant hyperprolactinemia or severely reduced T concentrations (i.e. < 6 nmol/L or < 175 ng/dL) are detected ([34–36]; see also Fig. 2).
According to EMAS data, LOH is more frequently characterized by a secondary or mixed, rather than primary or ‘compensated’ hypogonadism [32]. The EMAS consortium proposed to categorize men with a ‘compensated hypogonadism’ those having normal T and elevated LH (≥ 9.4 IU/L) [32]. These subjects report mainly physical symptoms, and they had 16-fold increased risk to progress to genuine primary hypogonadism over time, when compared to eugonadal individuals [37]. For this reason, as for thyroid disorders, it could be named ‘subclinical hypogonadism’ in which ‘decompensation’ occurs when T fell into the hypogonadal range. However, current data on long-term clinical significance of compensated hypogonadism and the impact of TRT in affected subjects are scanty, making advisable a watchful follow-up (Fig. 2) [37–39].
Remarks
Clinical manifestations of hypogonadism can occur at different T thresholds and, owing to inter-individual variability, some patients may experience symptoms having serum T in the range of, rather than at, the punctual, threshold values [22]. For this reason, information coming from LH, SHBG and cfT is much needed. Available data suggest that immunoassays for SHBG evaluation correlate closely with data obtained from LC–MS/MS. However, comparisons of different SHBG immunoassays showed a weaker inconsistent correlation of free T and bioavailable T [40]. On the other hand, several clinical conditions can influence SHBG concentrations (Table 1) limiting the validity of only total T evaluation for a correct clinical diagnosis of hypogonadism.
Table 1.
Main factors associated with an increase or with a reduction of Sex hormone binding globulin (SHBG) circulating levels
| SHBG increase |
- Drugs: anticonvulsant, estrogens, thyroid hormone, antiretroviral drugs - Hyperthyroidism - HIV disease - Cirrhosis and hepatitis - Aging |
| SHBG decrease |
-Drugs: GH, glucocorticoids, testosterone, Anabolic androgenic steroids -Hypothyroidism -Obesity -Acromegaly -Cushing Disease -Insulin resistance -Nephrotic syndrome |
TRT options
We recommend starting TRT in all symptomatic hypogonadal men, after contraindications are excluded, in whom a reversal of the condition cannot be expected in a reasonable time-frame (1 ØØOO).
We suggest using testosterone gels in older hypogonadal men, in particular in case of potentially reversible conditions (Expert Opinion).
We suggest using long-acting injectable T preparations to treat younger hypogonadal men, in particular in case of irreversible conditions (Expert Opinion).
Evidence
T delivered at any age with several preparations, including oral, parental and transdermal T formulations (Table 2), once patients are adequately informed on the advantages and disadvantages of TRT and available formulations. Absolute contraindications should be ruled out and the final decision should be made balancing the clinical situation, available formulations and patient preferences.
Table 2.
Testosterone preparations for hypogonadism treatment available in the Italian market
| Formulation | Chemical structure | Brand name | T 1/2 | Standard dosage | Advantages | Disadvantages |
|---|---|---|---|---|---|---|
| ORAL | ||||||
| Testosterone undecanoate | 17-α-Hydroxyl-ester | Andriol© | 4 h | 120–240 mg 2–3 times daily |
- Reduction of liver involvement -Oral convenience -Modifiable dosage |
-Unpredictable absorption depending of meal fat content -Be taken with meals |
| Mesterolone | 1α-Methyl-4,5α-dihydrotestosterone | Proviron© | 12 h | 50–100 mg 2–3 times daily |
-Oral convenience -Modifiable dosage -Useful in gynecomastia |
-Not aromatizable |
| PARENTAL | ||||||
| Testosterone enanthate | 17-α-Hydroxyl-ester |
Testoenant© Testoviron depot© |
4–5 days | 250 mg every 2–3 weeks |
-Low cost -Short-acting preparation allowing drug withdrawal in case of side-effects |
-Fluctuations in circulating T levels -Multiple injections -Relative risk of polycythemia |
| Testosterone propionate | 17-α-Hydroxyl-ester | Testovis© | 20 h | 100 mg every 2 days |
-Low cost -Very short-acting preparation allowing drug withdrawal in case of side-effects |
-Fluctuations in circulating T levels -Multiple injections -Relative risk of polycythemia |
| Testosterone undecanoate in castor oil | 17-α-Hydroxyl-ester | Nebid© | 34 days |
1000 mg every 10–14 weeks *750 mg every 10 weeks |
-Steady-state testosterone level without fluctuation -Long-lasting -Less frequent administration |
-Pain at injection site -Long-acting preparation not allowing rapid drug withdrawal in case of side-effects |
| Transdermal | ||||||
| Testosterone gel 2% | Native testosterone |
Tostrex© Testavan© |
6 h | 50-100 mg/day | Steady-state testosterone level without fluctuation |
-Possible transfer during intimate contact -Daily administration |
Preparations in bold are supported by the national health service. Nebid is supported only in a limited number of Italian regions including Friuli Venezia-Giulia, Emilia Romagna, Toscana, Marche, Puglia
Compared to primary hypogonadism, LOH can be a reversible condition associated with co-morbidities and medications interfering with T production or activity. Lifestyle modifications and weight loss should be strongly encouraged in all overweight and obese men with hypogonadism since able to increase T levels per se [19]. Similarly, when possible, interfering drugs should be withdrawn [41].
Old oral T undecanoate formulations, although still available in Europe and in Italy are no longer recommended, due to the poor bioavailability [42]. The US FDA approved a new formulation of oral T undecanoate incorporating a liquid-filled hard capsule drug delivery system improving oral availability (https://www.fda.gov/media/110187/download). However, the latter compound is not yet available in several European countries. Very short- or short-acting parental T formulations such as propionate, cypionate and enanthate have been associated with wider fluctuations of T concentrations, often resulting in patient discomfort and more adverse effects, such as polycythemia [42]. At present time, long-acting injectable T undecanoate and T gels appear the most suitable formulations to restore serum T concentrations in the normal range with good safety profile [42, 43]. The implantation of T pellets represents the longest available T formulations lasting from 4 to 7 months. Implants have a good safety profile, but the procedure is invasive, limiting its widespread use [42]. In addition, the latter, like the trans-nasal and trans-buccal formulations, are not available in Italy. Although face-to-face comparisons among different T formulation are lacking, T gels should be preferred in older subjects with a higher risk profile or when a potential reversible condition is suggested. The T gel formulations may also better mimic the circadian variation in T secretion, higher in the morning and lower at bedtime [42]. Conversely, when hypogonadism is irreversible, especially in younger patients, long-acting parental injectable formulation should be considered, as the first option. Accordingly, the daily application of rub-on gel is often considered a time-consuming procedure, reinforcing the suffering from a chronic condition, with possible consequences on long-term compliance. In contrast, long-lasting injectable T preparations, requiring between three to five deliveries per year, can relieve the patient from being remembered daily to suffer from a chronic, irreversible condition [43].
Remarks
Data on the role of TRT in patients with compensated hypogonadism are lacking. This condition represents only a preclinical form of an overt hypogonadism, which might deserve an adequate follow-up. TRT should be started only when overt hypogonadism occurs.
Anti-estrogens or aromatase inhibitors have been frequently used for the treatment of secondary hypogonadism, especially in patients with obesity or with metabolic derangements [44]. However, up to now, the available data are limited and more studies are advisable to better clarify the use of these compounds in patients with LOH.
Contraindications
Prostate and breast contraindications
We recommend against starting TRT in patients with active breast and prostate cancers (Good clinical practice).
We suggest not considering a treated low-risk prostate cancer as an absolute contraindication to TRT (2, ØOOO).
We suggest not considering a mild-to-moderate lower urinary tract symptoms (LUTS) as absolute contraindication to TRT (2, ØØØO).
Evidence
Prostate cancer (PC) growth and development occur in a T-sensitive manner [45]. As a result, hormonal castration is included among the available therapeutic options for advanced and metastatic PC [46]. This justifies the absolute contraindication of TRT in patients with PC. Similarly, patients with a suspicion of PC (e.g. increased/raising PSA concentrations and/or relevant findings at digital rectal examination) deserve further evaluation before commencing TRT. Some meta-analyses reported a very low risk of PC recurrence in patients on TRT after local therapy completion (radical prostatectomy, external beam radiation therapy, brachytherapy, cryotherapy) [47, 48]. However, it should be recognized that the available data are insufficient to address this issue, due to the high heterogeneity and limited follow-up. Hence, risks and benefits of starting TRT in symptomatic hypogonadal men with a treated low-risk PC should be extensively discussed and tailored to the individual condition.
Male breast cancer (MBC) is a rare tumor. Even in men, breast cancer can be hormone sensitive. Considering that breast cancer tissue expresses high levels of P450 aromatase [49], it is conceivable that T administration to an otherwise hypogonadal men with a history of treated mammary cancer could increase the risk of recurrence, although evidence on this topic are very limited [50].
The effects of TRT on benign prostatic hyperplasia (BPH) and low LUTS are a matter of debate. Although some concerns have been raised in patients with BPH-LUTS on TRT, a meta-analysis of 14 RCTs (2029 participants, mean follow-up 34.4 months) reported that TRT does not change the International Prostatic Symptoms Score (IPSS) in patients with LOH [51]. Similarly, another meta-analysis on RCTs (1779 patients) reported no effect of TRT on LUTS [52]. A single study carried out in 52 patients with hypogonadism, mild BPH and LUTS showed an improvement of IPSS, maximum flow rate, and voiding volume and no change in post-voiding residual volume after 12 months compared with an untreated control group [53]. It is important to note that these data refer to patients with mild-to-moderate LUTS since patients with severe LUTS (IPSS > 19) were systematically excluded from RCTs [27].
Values
The evidence places a high value on the unsafe use of TRT in patients with active breast and prostate cancers. The evidence against considering mild-to-moderate LUTS as an absolute contraindication for TRT in patients with hypogonadism is of moderate quality.
Remarks
Limited evidence is available on the effects of TRT in patients with severe LUTS (IPSS > 19), mainly because they are not included in RCTs. Moreover, BPH has not been clearly specified as an inclusion criterion in meta-analytical studies exploring the effects of TRT on LUTS [51, 52, 54].
Cardiovascular contraindications
We suggest not considering mild-to-moderate heart failure as an absolute contraindication for TRT (2, ØØOO).
We suggest not prescribing TRT to patients with a recent major adverse cardiovascular event (2, ØØØO).
We suggest considering the global cardiovascular risk and associated morbidities, including hematocrit level, before prescribing TRT (2, ØØOO).
We suggest collecting a detailed family, personal and clinical history of venous thromboembolism before prescribing TRT (2, ØOOO).
Evidence
In prospective RCTs, analysis of the effects of TRT in patients with mild-to-moderate heart failure (HF) and reduced ejection fraction (rEF) including classes New York Heart Association (NYHA) II and III and left ventricular ejection fraction (LVEF) < 40%,—showed no [55, 56] or positive effects [57] on heart function and no TRT-related major events [56–60]. Accordingly, a meta-analysis of RCTs including 198 patients with heart failure (LVEF < 40%, NHYA II and III) with a duration up to 52 weeks described a significant amelioration of the six-minute-walking test and no adverse cardiovascular events [61]. Conversely, no data are available on the effects of TRT in patients with severe HF and rEF (class NYHA IV), nor in those with recent major adverse cardiovascular events (MACE).
The association between TRT and cardiovascular (CV) risk remains one of the most conflicting issues of the topic. Limited data, mainly derived from pharmaco-epidemiological studies published in the last 10 years, have suggested a possible increased risk of CV mortality and morbidity during TRT, especially in aging and complicated patients (see above; [62, 63].
However, it is important to recognize that several double-blind, placebo-controlled randomized clinical trials (RCTs) have shown that T can delay time to ischemia in patients with coronary artery disease and stable angina followed up from four weeks to 12 months (see for review [62]). In addition, a meta-analysis including 37 observational studies and 43,041 subjects, showed that reduced T concentrations at baseline was associated with an increased CV mortality and morbidity [64]. Data were confirmed when only population-based studies were considered [64]. Similarly, a large epidemiological study including 154,965 men from United Kingdom (UK) Biobank showed that tT and fT concentrations were inversely associated with all-cause and cancer mortality [65]. In line with these data, the largest meta-analysis published so far on CV effect of TRT, including 15 pharmaco-epidemiological and 93 RCTs, documented that TRT reduces overall mortality and CV morbidity in pharmaco-epidemiological studies and had no clear effect, either beneficial or detrimental, on the incidence of CV events when RCTs were considered [66]. The latter study also documented that an increased risk of CV diseases was observed in RCTs when T preparations were prescribed at dosages above those normally recommended, or when frail men were considered [66]. In line wih this evidence a more recent individual patient and aggregate data meta-analysis, including 35 primary studies (5601 participants, mean age 65 years) found no evidence that TRT increased short-term to medium-term CV risks in men with hypogonadism [67]. Accordingly, the conclusion of the European Medicine Agency (EMA) did not align with the FDA opinion and stated the safety of TRT on the cardiovascular profile [68].
Data mainly derived from subjects with an undiagnosed family history of thrombophilia-hypofibrinolysis have emphasized a possible increased risk of venous thromboembolism (VTE) in men under TRT [69]. In addition, a Mendelian randomized study on 3,225 men of European ancestry, 392,038 white British men and women from the UK Biobank, and 171,875 participants of about 77% European descent, reported that endogenous T, genetically predicted by variants in the JMJD1C gene region, was positively associated with VTE in men [70]. However, the most updated meta-analysis, including 13 RCTs and enrolling 5050 subjects with hypogonadism on TRT, does not support the association between VTE and TRT [71].
Values
Evidence on the safety of TRT on the cardiovascular profile is of moderate quality and place a high value when TRT is given to restore physiological concentrations. An accurate family history evaluation to rule out thrombophilia-hypofibrinolysis is crucial before initiating TRT.
Remarks
Available data on CV risk of TRT are mainly derived from studies with non-CV primary endpoints and whose design was inadequate to exclude such a risk (limited duration of exposure and insufficient numbers of participants). An industry-supported multicenter RCT is underway to better clarify the CV risk of TRT (clinicaltrials.gov: NCT03518034).
Other contraindications
We recommend against TRT in men desiring fatherhood in the near future (1, ØØØØ).
We suggest not considering a treated obstructive sleep apnea syndrome (OSAS) as an absolute contraindication for testosterone replacement therapy (2, ØØOO).
Evidence
Male hormonal contraceptive trials have clearly shown that T administration suppresses sperm production and sperm cell concentration to various degrees and in 100% of subjects within 24 months [72]. The recovery period from T-induced spermatogenesis suppression is variable, being reported longer when it is consequence of anabolic–androgenic steroids (AAS) abuse [73]. Hence, TRT is contraindicated in all men who desire fatherhood. Specific SIAMS recommendations on this topic were provided elsewhere [74].
The impact of TRT on respiratory parameters in subjects with hypogonadism with OSAS, studied in RCTs, seems to be transient and time-dependent. TRT can worsen saturation index and nocturnal hypoxemia (sleep time with oxygen saturation < 90%) after seven weeks [75, 76], but the effects are neutral after 12–18 weeks [75–77], and eventually resulted in a significant improvement of sleep disturbances after 12 months [78]. Taken together these data suggest that short-term, high-dose TRT may worsen OSAS. However, these adverse effects seem to have disappeared with time, rather than worsen [79].
Remarks
The available evidence regarding the role of TRT in men with OSAS is poor. In addition, the possible role played by obesity in these patients remains unclear.
Outcomes
Sexual function
We recommend using TRT in subjects with hypogonadism with low sexual desire and/or erectile dysfunction (ED) (1 ØØØØ).
Since ED is a multifactorial disorder, we suggest promptly considering combination therapies, in subjects with hypogonadism, whenever needed to fully address the condition (2 ØØØO).
Evidence
As previously reported, sexual dysfunctions are considered a hallmark of T deficiency [22, 23, 80]. Data derived from available meta-analyses documented that TRT can significantly improve all aspects of sexual function, being the magnitude of the outcomes more evident when sexual desire and erectile function were considered ([52, 81–86]; Table 3 and Supplementary Table 1). The same studies [52, 81–86] also excluded beneficial effects of TRT when the mean T of the enrolled subjects exceeded 12 nmol/L. The first meta-analysis of studies reporting International Index of Erectile Function as main outcome suggested that TRT alone is only able to improve mild ED [87]. Similar results were reported by a comparable study [88]. The effect, small, is even attenuated by metabolic impairment, such in obesity and diabetes, most probably because of underlying neuro-vascular alterations [87]. Accordingly, lifestyle modifications and reduction of CV risk is advocated to improve ED [89, 90]. In subjects with hypogonadism with comorbid ED, T should be given prior to phosphodiesterase-5 inhibitors (PDE5i) but if it turns out insufficient to restore sexual function in comorbid vascular patients the add-on of PDE5i should be considered without delay. In this respect, the first meta-analysis of the very few studies on the combination of PDE5i and TRT (mixed enrollment) failed to reveal additive effects [83]. The re-analysis of the latter data, however, documented that the combination therapy (TRT and PDE5i) can produce better outcome in more complicated subjects such as those with type 2 diabetes mellitus [80]. A further meta-analysis including eight studies and 913 patients concluded that combination therapy (TTR plus PDE5-i) is superior to PDE5i monotherapy in restoring erectile function [86]. However, the analysis included also non-placebo-controlled RCTs and paper not published in English language, limiting its generalizability.
Table 3.
Summary of testosterone replacement therapy (TRT) outcomes
| TRT outcomes | Outcome evaluation |
|---|---|
| Sexual function | |
| Erectile dysfunction | ↑ ⊕ ⊕ |
| Libido | ↑ ⊕ ⊕ ⊕ |
| Ejaculation | ↑ ⊕ |
| TRT + PDE5i | |
| Erectile dysfunction | ↔ |
| Body composition | |
| Fat mass | ↓ ⊕ |
| Lean mass | ↑ ⊕ |
| Body mass index | ↔ |
| Waist | ↔ |
| Metabolic control | |
| Glucose metabolism | ↑ ⊕ |
| Lipid profile | ↑ ⊕ ↔ |
| Blood pressure | ↔ |
| Bone | |
| Bone mass | ↑ ⊕ |
| Fracture risk | NA |
| Mood/cognition | |
| Depressive symptoms | ↑ ⊕ |
| Cognition | NA |
| Mobility | |
| ↑ ⊕ |
⊕ = arbitrary unit indicating: ⊕ = mild ⊕ ⊕ = moderate, ⊕ ⊕ ⊕ = strong effect. ↑ = positive effect ↓ = negative effect ↔ = neutral effect. NA = not available; PDE5i, phosphodiesterase type 5 inhibitors
Values
We place a high value for TRT in improving low sexual desire and erectile function in subjects with hypogonadism (tT < 12 nmol/L). We place a lower value on the less consistent effects of TRT on other sexual problems including ejaculatory dysfunctions.
Remarks
At present, the place and timing of combination therapy T plus PDE5i in the management of ED remains unclear, requiring an individually tailored approach. The efficacy of the combined use of TRT and intracavernosal injection of prostaglandin E1 (PGE-1) and/ other drugs is lacking.
Bone
We recommend TRT to improve bone mineral density and prevent bone loss in subjects with hypogonadism (1 ØØØO).
We recommend against TRT as monotherapy to prevent bone fracture in subjects with hypogonadism with high fracture risk (1 ØØOO).
Evidence
T is essential for skeletal development during puberty and bone health maintenance (mass and strength) throughout adult life, by promoting periosteal apposition and endocortical bone resorption, and by slowing bone remodeling rate [91]. Hypogonadism, both in the young men and in aging, is associated with lower bone mineral density (BMD) and represents a major cause of osteoporosis [92, 93]. TRT can improve BMD, particularly at the vertebral level and when T concentrations are very low (Table 3 and Supplementary Table 2; [94–98]. The effect is more evident in younger men with organic hypogonadism, as a meta-analysis showed in men with Klinefelter syndrome [99]. The Bone Trial of the Testosterone Trials (T-Trials), showed that T treatment for one year in older men with low T significantly increased volumetric BMD and estimated bone strength, more in trabecular than peripheral bone and more in the spine than hip [100]. Similarly, the T4 Bone trial, a sub-study of the larger Testosterone for Diabetes Mellitus (T4DM), confirmed that two years of T increased by 3% volumetric bone density over placebo, predominantly acting on cortical bone [101]. However, both trials investigated subjects with relatively normal BMD at baseline, whether similar, lower or higher finding could be expected in osteoporotic men remains to be established.
Values
Hypogonadism should always be considered during the clinical workup of male osteoporosis and, similarly, subjects with hypogonadism should be assessed for bone health [102]. There is growing evidence on the effects of TRT on BMD, although trials exploring its beneficial effect on osteoporosis are lacking. TRT is particularly recommended in young adult subjects with hypogonadism to prevent bone loss and help acquiring peak bone mass [102]. In the other groups of patients, mainly older men with LOH, the benefits and risks of TRT should be accurately discussed with the patients. To date, TRT cannot be recommended as monotherapy and should be associated with antiresorptive drugs when fracture risk is high [102, 103]. In general, we recommend following the European Academy of Andrology clinical guidelines on management of bone health in the andrological outpatient clinic [102].
Remarks
The effect of TRT alone on bone health in subjects with hypogonadism is still not completely defined [93, 102], specifically, no studies with fractures as primary endpoint are yet available. The combined treatment of TRT with drugs approved for the treatment of osteoporosis has not been investigated. To what extent the effects on TRT on bone density are a direct on bone metabolism, or rather an indirect effect through skeletal muscle is still unclear.
Mood
We recommend against TRT as monotherapy for improving major depressive symptoms in subjects with hypogonadism (1 ØØOO).
Evidence
Observational studies found depressive symptoms, including major depressive disorder, and hypogonadism associated [22, 23, 104–107]. A nine-year follow-up study found the risk of depression nearly double in men with low T at baseline [108], confirming previous results of an increased incidence of depression in men with low T concentrations during two-year observation [109]. However, very few placebo-controlled RCTs investigated the role of TRT on depressive symptoms as primary outcome. Overall, they found TRT beneficial, with effect size larger among those subjects with milder symptoms than those who met criteria for major depressive disorder [110]. Data from the Vitality Testosterone Trial [111] showed that TRT in subjects with hypogonadism, who self-reported low vitality and fatigue scores, slightly improved mood and depressive symptoms. A systematic review of six RCTs confirmed that TRT can reduce depressive symptoms in patients with mild depression and in the presence of hypogonadism but not in those with major depressive disorders (Table 3; and Supplementary Table 3; [112–115]).
Values
Although TRT may produce marginal improvement in mood and depressive symptoms in subjects with hypogonadism, we place a high value on the recommendation not to offer TRT with the sole purpose of improving these symptoms. Established anti-depressive treatments should be considered in combination to TRT in all hypogonadal subjects with hypogonadism with major depression.
Remarks
Depressive mood and other non-specific symptoms of hypogonadism are indistinguishable from true psychiatric conditions related to mood and major depressive disorders [110]. Low T can impact on mood in patients with hypogonadal-type depressive conditions [116]. However, the benefit of restoring T concentrations for depressive symptoms and for treatment of major depressive disorder is more controversial. Similarly, it is unclear whether low T is related to the development of major depressive disorders. Studies on the combination of TRT with established anti-depressive therapies have not been performed.
Cognition
We recommend against testosterone replacement therapy in subjects with hypogonadism to specifically improve cognitive function (1 ØØØØ).
Evidence
Some studies suggest that T deficiency might be involved in the pathogenesis of both age-related and Alzheimer disease (AD)-related cognitive impairment [117, 118]. A meta-analysis including 27 studies and 18 599 subjects concluded that low T can predict all-cause dementia or AD [119]. However, that latter study used a fixed model for the analysis which strongly limits the results obtained [119]. The association between LOH and cognitive decline are partially supported by studies on patients undergoing androgen deprivation therapy (ADT), in whom a worse performance in visuomotor tasks, but not in visuospatial tasks not comprising motor components, has been observed when compared to baseline concentrations or to controls [120]. However, the available meta-analyses failed to document a beneficial effect of TRT in improving cognitive function of ageing men ([117, 121, 122]; Table 3 and Supplementary Table 4). A first meta-analysis of 14 RCTs and 1406 patients without definite cognitive impairment, reported a small, albeit significant, improvement in psychomotor speed and executive function in TRT compared to placebo group [122]; but no effects on various cognitive domains were observed in two other larger meta-analyses, even in the subgroup of subjects with hypogonadism only [117, 121].
Remarks
It should be emphasized that the majority of available RCTs and meta-analyses included mixed eugonadal/hypogonadal populations. Only few studies dedicated to subjects with hypogonadism are available, overall reporting no effect of TRT on cognitive function. The current evidence does not allow to draw specific recommendations on the role of TRT on cognitive function in subjects with hypogonadism.
Frailty and mobility
We recommend against prescribing testosterone replacement therapy to improve muscle strength, with a clinically meaningful aim, in frailty of subjects with hypogonadism (1 ØØØ0).
Evidence
Observational studies repeatedly reported an association between low T and frailty [123, 124]. In line with these a meta-analysis including 7 cross-sectional studies and 4 cohort studies showed either reduced tT or fT concentrations were related to an increased risk of frailty in men but not in women [125]. Impairment of mobility and balance, common features in frail individuals, has been frequently found associated with low T concentrations [126]. Accordingly, patients with LOH display an increased risk of falls and functional disabilities [127]. Despite this evidence, the role of TRT in frail patients with low T remains controversial [128–130].
In fact, although TRT was shown to reverse sarcopenia in aging men with LOH [131–133], data derived from available meta-analyses failed to confirm a meaningful improvement of muscle strength and mobility (Table 3, Supplementary Table 5; [94, 127–129]). Data derived from TTrials demonstrated that TRT significantly improved the six minutes walking distance test in the overall cohort and in patients without baseline mobility limitations, but not in patients affected by mobility limitations at baseline (primary outcome). In addition, although TRT was able to significantly improve the parameter of self-reported physical function, the evaluation at the six minutes walking test failed to demonstrate an objective improvement among patients with functional limitation at enrollment [133].
Remarks
The majority of RCTs do not support a beneficial effect of TRT on physical function of frail men; however, some improvement in frailer patients, improvement in body composition and sarcopenia have been consistently reported in aging subjects with hypogonadism. Therefore, a beneficial impact of TRT on these outcomes in frail men cannot be completely ruled out.
Body composition and metabolic parameters
We recommend TRT to improve body composition (reducing fat mass and increasing lean mass) in subjects with hypogonadism with or without metabolic syndrome (MetS) or type 2 diabetes (T2DM) (1 ØØØO).
We suggest TRT to improve fasting and post-load glycemic control in subjects with hypogonadism with MetS or pre-diabetic conditions to reduce the risk of developing T2DM (2 ØØOO).
We suggest not considering TRT to control dyslipidemia or to improve glycated hemoglobin in patients with or without T2DM (2 ØØOO).
We suggest TRT to reduce waist circumference in subjects with hypogonadism with MetS (2 ØOOO).
Evidence
The beneficial effects of T on body composition are well studied. Several RCTs have proven that T can reduce fat mass. In detail, TRT has been demonstrated to reduce both visceral [134, 135] and subcutaneous fat mass [136] in subjects with hypogonadism with or without MetS and T2DM. Some double-blind, placebo-controlled studies have shown that TRT can reduce waist circumference and increase lean mass [135–138]. These findings mirror meta-analytic studies [94, 139] and were confirmed by the T4DM trial, where two years of TRT reduced waist circumference (-2.1 cm), total fat mass (-2.7 kg), and abdominal fat mass (-2.3%), while increasing total muscle mass (+ 1.7 kg), over life-style interventions and placebo [140].
There is a well-known relation between low endogenous serum T concentrations and risk of T2DM in observational studies, however data from RTCs are more controversial. Some RCT found TRT positively affecting glycemic control in subjects with MetS and/or T2DM. In the Burntwood Lichfield Atherstone Sutton Tamworth (BLAST) study, 199 men with T2DM were enrolled to study TRT’s effects on glycated hemoglobin (HbA1c). A significant improvement of HbA1c concentrations was achieved after 30 weeks of long-acting injectable T undecanoate administration [141]. However, in the TIMES-2 trial, a larger study on 220 patients with T2DM or MetS treated for six months with 1% T gel or placebo, no significant difference in HbA1c concentrations was found between groups [142]. Similar results were obtained in other double-blind, placebo-controlled studies [136, 143, 144]. The T4DM, the largest (1007 men) RTC, demonstrated that T is able to prevent T2DM, over an intensive lifestyle program, to an extent greater than seen for metformin in the Diabetes Prevention Program. The T4DM trial found a significant TRT-related reduction in fasting glucose and an approximately 40% lowering of the relative risk to have a 2-h glucose on oral glucose tolerance test (OGTT) ≥ 11.1 mmol/L after two years of TRT over lifestyle interventions; however, as previous studies, they found no significant effect on Hb1Ac concentrations advocating the discrepancy—when compared the impressive changes in glucose concentrations—due to a TRT-related change in red blood cell turnover that hampers the possibility to detect Hb1Ac improvements by measuring its circulating concentrations [140].
Results of clinical studies on the effect of T on circulating lipids are contrasting. In some RCTs including subjects with hypogonadism, with or without MetS and T2DM, a reduction of total cholesterol [137, 141–143, 145, 146] and low-density lipoprotein cholesterol [143, 146, 147] was observed in the TRT group. In contrast, other studies reported no change in lipid parameters [136, 138, 144, 148]. Moreover, T seems to have an unfavorable effect on high-density lipoprotein cholesterol concentrations [140, 142, 146, 147]. Unfortunately, the T4DM did not analyzed lipid concentration changes because of budget constraints [140]. A comparison of available meta-analysis is reported in Supplementary Tables 6–8 [5, 94, 96, 139, 149–159].
Value
Although it is unequivocally the effect of TRT in improving body compositions, we place a great value in implementing lifestyle changes needed to control and reduce obesity prior to considering TRT. In those subjects failing to restore normal T concentrations after life style interventions, TRT can prevent the development of T2DM and improve body composition. Dyslipidemia seems not adequately targeted by TRT, suggesting that additional pharmacological interventions might be needed to restore normal lipid concentrations.
Remarks
Hyperinsulinemia, T2DM and MetS are associated with reduced SHBG concentrations; therefore, in the affected subjects, it is recommended to calculate cFT to avoid unnecessary TRT (see Fig. 2). The encouraging data on TRT on diabetes mellitus prevention are limited to a 2-year observation in subjects with borderline hypogonadism who underwent a concomitant lifestyle program, whether the same findings can be expected in all subjects with hypogonadism remains to be established and balanced with long-term safety profile.
Chronic diseases (HIV end-stage renal disease, bowel inflammatory diseases, chronic pulmonary diseases)
We suggest not considering TRT in subjects with hypogonadism with chronic diseases (such as human immunodeficiency virus infection (HIV), chronic obstructive pulmonary diseases (COPD), end-stage renal disease (ESRD), and bowel inflammatory diseases) to improve disease outcomes (2 ØØOO).
Evidence and remarks
Many chronic diseases, such as HIV infection/AIDS, COPD, ESRD, and bowel inflammatory diseases, have been associated with hypogonadism [5, 17, 160, 161]. Data suggest that lean mass may improve in HIV men receiving TRT [155, 161, 162] and COPD patients [155, 163]. However, evidence from RCT is too weak to indicate that TRT may beneficial in this subset of patients and improve their morbidity and mortality. Similar considerations can be drawn for coronavirus disease 19 (COVID-19) infection [164–170]. For this reason, the task force agreed that are the specific outcomes, as detailed in the previous recommendations, to drive decisions and TRT should be individualized based on the signs and symptoms and severity of hypogonadism.
Monitoring
We recommend evaluating clinical outcomes, including biochemical parameters (hematocrit, PSA and testosterone) every 3/6 months during the first year and at least annually thereafter (1 ØØØO).
We suggest further evaluation, if there is: (a) confirmed PSA > 4 ng/mL at any time and (b) detection of a prostatic abnormality on digito-rectal examinations (DRE) or a substantial worsening of LUTS (2 ØOOO).
Evidence
Sexual problems are among the commonest issues complained by subjects with hypogonadism. Data derived from TTrials and meta-analysis have shown that sexual function can be significantly improved after three month of therapy [87, 171]. Hence, three months appear a reasonable time to check, for the first time, TRT outcomes. Given the well-known stimulatory effects of T on erythropoiesis [172], and its potential effects on the prostate [54], the PSA and hematocrit (HCT) evaluation should be mandatory considered before and early checked during TRT. The optimal target concentrations for circulating T upon TRT have not been completely clarified. TTrials showed that maintaining the serum T concentration within the normal range for young men (280–873 ng/dL or 9.6–30 nmol/l; [171]) resulted in a good benefit/risk ratio. However, lower or higher concentrations should be tailored according to individual comorbidities, specific symptoms and HCT levels. In fact, although the data are still not completely clarified, there is a general consensus that a HCT level higher than 54% require phlebotomy, along with TRT withdrawal, to reduce the risk of cardio-vascular complications [15]. However, particularly in the presence of specific conditions, as COPD or OSAS, HCT levels between 48 and 51% should be carefully considered, and handled on an individual basis, before and during TRT [18].
Considering the strong association between LOH and metabolic disturbances, baseline and, at least, annually glycometabolic profile evaluations are suggested. Similarly, bone density scan may be also considered at baseline and 18 to 24 months following TRT, particularly in those subjects with more severe hypogonadism [102].
Overall, complete and andrological clinical evaluation is mandatory before and during TRT patient evaluation. DRE must be performed at any time to rule-out the presence of prostate abnormalities.
Remarks
The correct timing for T concentration evaluation should be managed according to the type of T preparation used (see Table 2). SHBG evaluation and fT calculation should be considered for a better estimation of circulating T levels. Although PSA value above 4 ng/mL still represents a well accepted threshold for further prostate evaluation, it should be recognized that the latter varies according to several other parameters [173]. In particular, the risk of PC is increased in African descent and in those with a first-degree relative with diagnosed PC or previously positive prostate biopsy, and in those with baseline PSA concentrations > 1 ng/mL at age 40 years or > 2 ng/mL at age 60 years [173]. Hence, those subjects deserve particular attention during TRT. The PSA velocity, previously considered a marker for TRT withdrawn and prostate biopsy, has been criticized [174, 175]. Accordingly, the European Association of Urology guidelines suggest that PSA density (PSAD) with a Prostate Imaging Reporting and Data System (PI-RADS) score (≥ 3) might help in decision for further evaluations [173].
Conclusions
Whereas the development of male hypogonadism during fetal and pre-pubertal condition can profoundly affect sexual development and the appearance of sexual secondary characteristics, the underlying pathogenetic mechanisms, and the clinical significance of adult- and late-onset hypogonadism are still not completely clarified. Sexual impairment is the main condition tightly related to the development hypogonadism in adult or aging men. Conversely, the role played by age-associated low T in the characterization of several other symptoms including mood discrepancies, frailty and cognitive impairment is more conflicting. Similarly, metabolic derangements such as obesity T2DM and MetS are frequently associated with low T in aging men. In the latter cases, emerging evidence suggests that TRT can positively influence body composition and metabolic profile in less complicated subjects. Conversely, the role of T substitution in older frailty and more complicated subjects is still contradictory. Available data do not support a role of TRT in increasing CV risk when subjects with hypogonadism are correctly diagnosed, T appropriately prescribed and subjects adequately followed up during the treatment. However, it should be important to recognize that the duration of existing studies is too limited (up to three years) to draw any final conclusions. Hence, further and longer studies are strongly advisable the better clarify the long-term benefit/ratio of TRT in adult and aging men with hypogonadism.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors would like to thanks Marco Zavattaro and Rossella Cannarella for their helpful collaboration during manuscript preparation.
Declarations
Conflict of interest
No conflict of interest was reported by any of the authors.
Ethical approval
This article does not contain any study with human participants or animals performed by any of the authors.
Informed consent
For this type of study informed consent is not required.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ferrini RL, Barrett-Connor E. Sex hormones and age: a cross-sectional study of testosterone and estradiol and their bioavailable fractions in community-dwelling men. Am J Epidemiol. 1998;147(8):750–754. doi: 10.1093/oxfordjournals.aje.a009519. [DOI] [PubMed] [Google Scholar]
- 2.Mohr BA, Guay AT, O'Donnell AB, McKinlay JB. Normal, bound and nonbound testosterone levels in normally ageing men: results from the Massachusetts male ageing study. Clin Endocrinol (Oxf) 2005;62(1):64–73. doi: 10.1111/j.1365-2265.2004.02174.x. [DOI] [PubMed] [Google Scholar]
- 3.Harman SM, Metter EJ, Tobin JD, Pearson J, Blackman MR. Longitudinal effects of aging on serum total and free testosterone levels in healthy men. Baltimore longitudinal study of aging. J Clin Endocrinol Metab. 2001;86(2):724–731. doi: 10.1210/jcem.86.2.7219. [DOI] [PubMed] [Google Scholar]
- 4.Wu FC, Tajar A, Pye SR, Silman AJ, Finn JD, O'Neill TW, et al. Hypothalamic-pituitary-testicular axis disruptions in older men are differentially linked to age and modifiable risk factors: the European male aging study. J Clin Endocrinol Metab. 2008;93(7):2737–2745. doi: 10.1210/jc.2007-1972. [DOI] [PubMed] [Google Scholar]
- 5.Corona G, Maseroli E, Rastrelli G, Francomano D, Aversa A, Hackett GI, et al. Is late-onset hypogonadotropic hypogonadism a specific age-dependent disease, or merely an epiphenomenon caused by accumulating disease-burden? Minerva Endocrinol. 2016;41(2):196–210. [PubMed] [Google Scholar]
- 6.Handelsman DJ. Global trends in testosterone prescribing, 2000–2011: expanding the spectrum of prescription drug misuse. Med J Aust. 2013;199(8):548–551. doi: 10.5694/mja13.10111. [DOI] [PubMed] [Google Scholar]
- 7.Layton JB, Kim Y, Alexander GC, Emery SL. Association between direct-to-consumer advertising and testosterone testing and initiation in the United States, 2009–2013. JAMA. 2017;317(11):1159–1166. doi: 10.1001/jama.2016.21041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.FDA Drug Safety Communication FDA cautions about using T products for low T due to aging requires labeling change to inform of possible increased risk of heart attack and stroke with use. US Food and Drug Administration website March 3rd , 2015 [Available from: https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-cautions-about-using-testosterone-products-low-testosterone-due. [DOI] [PubMed]
- 9.The Endocrine Society. The Risk of Cardiovascular Events in Men Receiving Testosterone Therapy. An Endocrine Society Statement February, 7th, 2014 [Available from: https://www.endocrine.org/~/media/endosociety/Files/Advocacy%20and%20Outreach/Position%20Statements/Other%20Statements/The%20Risk%20of%20Cardiovascular%20Events%20in%20Men%20Receiving%20Testosterone%20Therapy.pdf.
- 10.Yeap BB, Grossmann M, McLachlan RI, Handelsman DJ, Wittert GA, Conway AJ, et al. Endocrine society of Australia position statement on male hypogonadism (part 1): assessment and indications for testosterone therapy. Med J Aust. 2016;205(4):173–178. doi: 10.5694/mja16.00393. [DOI] [PubMed] [Google Scholar]
- 11.Isidori AM, Balercia G, Calogero AE, Corona G, Ferlin A, Francavilla S, et al. Outcomes of androgen replacement therapy in adult male hypogonadism: recommendations from the Italian society of endocrinology. J Endocrinol Invest. 2015;38(1):103–112. doi: 10.1007/s40618-014-0155-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Swiglo BA, Murad MH, Schünemann HJ, Kunz R, Vigersky RA, Guyatt GH, et al. A case for clarity, consistency, and helpfulness: state-of-the-art clinical practice guidelines in endocrinology using the grading of recommendations, assessment, development, and evaluation system. J Clin Endocrinol Metab. 2008;93(3):666–673. doi: 10.1210/jc.2007-1907. [DOI] [PubMed] [Google Scholar]
- 13.Bhasin S, Brito JP, Cunningham GR, Hayes FJ, Hodis HN, Matsumoto AM, et al. Testosterone therapy in men with hypogonadism: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2018;103(5):1715–1744. doi: 10.1210/jc.2018-00229. [DOI] [PubMed] [Google Scholar]
- 14.Salonia A, Bettocchi C, Boeri L, Capogrosso P, Carvalho J, Cilesiz NC, et al. European association of urology guidelines on sexual and reproductive health-2021 update: male sexual dysfunction. Eur Urol. 2021;80(3):333–357. doi: 10.1016/j.eururo.2021.06.007. [DOI] [PubMed] [Google Scholar]
- 15.Giagulli VA, Castellana M, Lisco G, Triggiani V. Critical evaluation of different available guidelines for late-onset hypogonadism. Andrology. 2020;8(6):1628–1641. doi: 10.1111/andr.12850. [DOI] [PubMed] [Google Scholar]
- 16.Lunenfeld B, Mskhalaya G, Zitzmann M, Corona G, Arver S, Kalinchenko S, et al. Recommendations on the diagnosis, treatment and monitoring of testosterone deficiency in men. Aging Male. 2021;24(1):119–138. doi: 10.1080/13685538.2021.1962840. [DOI] [PubMed] [Google Scholar]
- 17.Grossmann M, Matsumoto AM. A perspective on middle-aged and older men with functional hypogonadism: focus on holistic management. J Clin Endocrinol Metab. 2017;102(3):1067–1075. doi: 10.1210/jc.2016-3580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Corona G, Goulis DG, Huhtaniemi I, Zitzmann M, Toppari J, Forti G, et al. European academy of andrology (EAA) guidelines on investigation, treatment and monitoring of functional hypogonadism in males: endorsing organization: European society of endocrinology. Andrology. 2020;8(5):970–987. doi: 10.1111/andr.12770. [DOI] [PubMed] [Google Scholar]
- 19.Corona G, Rastrelli G, Morelli A, Sarchielli E, Cipriani S, Vignozzi L, et al. Treatment of functional hypogonadism besides pharmacological substitution. World J Mens Health. 2020;38(3):256–270. doi: 10.5534/wjmh.190061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Salonia A, Rastrelli G, Hackett G, Seminara SB, Huhtaniemi IT, Rey RA, et al. Paediatric and adult-onset male hypogonadism. Nat Rev Dis Primers. 2019;5(1):38. doi: 10.1038/s41572-019-0087-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morelli A, Corona G, Filippi S, Ambrosini S, Forti G, Vignozzi L, et al. Which patients with sexual dysfunction are suitable for testosterone replacement therapy? J Endocrinol Invest. 2007;30(10):880–888. doi: 10.1007/BF03349232. [DOI] [PubMed] [Google Scholar]
- 22.Wu FC, Tajar A, Beynon JM, Pye SR, Silman AJ, Finn JD, et al. Identification of late-onset hypogonadism in middle-aged and elderly men. N Engl J Med. 2010;363(2):123–135. doi: 10.1056/NEJMoa0911101. [DOI] [PubMed] [Google Scholar]
- 23.Rastrelli G, Corona G, Tarocchi M, Mannucci E, Maggi M. How to define hypogonadism? Results from a population of men consulting for sexual dysfunction. J Endocrinol Invest. 2016;39(4):473–484. doi: 10.1007/s40618-015-0425-1. [DOI] [PubMed] [Google Scholar]
- 24.Taylor AE, Keevil B, Huhtaniemi IT. Mass spectrometry and immunoassay: how to measure steroid hormones today and tomorrow. Eur J Endocrinol. 2015;173(2):D1–12. doi: 10.1530/EJE-15-0338. [DOI] [PubMed] [Google Scholar]
- 25.Rosner W, Vesper H. Toward excellence in testosterone testing: a consensus statement. J Clin Endocrinol Metab. 2010;95(10):4542–4548. doi: 10.1210/jc.2010-1314. [DOI] [PubMed] [Google Scholar]
- 26.Travison TG, Vesper HW, Orwoll E, Wu F, Kaufman JM, Wang Y, et al. Harmonized reference ranges for circulating testosterone levels in men of four cohort studies in the United States and Europe. J Clin Endocrinol Metab. 2017;102(4):1161–1173. doi: 10.1210/jc.2016-2935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Corona G, Torres LO, Maggi M. Testosterone therapy: what we have learned from trials. J Sex Med. 2020;17(3):447–460. doi: 10.1016/j.jsxm.2019.11.270. [DOI] [PubMed] [Google Scholar]
- 28.Antonio L, Wu FC, O'Neill TW, Pye SR, Ahern TB, Laurent MR, et al. Low free testosterone is associated with hypogonadal signs and symptoms in men with normal total testosterone. J Clin Endocrinol Metab. 2016;101(7):2647–2657. doi: 10.1210/jc.2015-4106. [DOI] [PubMed] [Google Scholar]
- 29.Rastrelli G, O'Neill TW, Ahern T, Bártfai G, Casanueva FF, Forti G, et al. Symptomatic androgen deficiency develops only when both total and free testosterone decline in obese men who may have incident biochemical secondary hypogonadism: prospective results from the EMAS. Clin Endocrinol (Oxf) 2018;89(4):459–469. doi: 10.1111/cen.13756. [DOI] [PubMed] [Google Scholar]
- 30.Fiers T, Wu F, Moghetti P, Vanderschueren D, Lapauw B, Kaufman JM. Reassessing free-testosterone calculation by liquid chromatography-tandem mass spectrometry direct equilibrium dialysis. J Clin Endocrinol Metab. 2018;103(6):2167–2174. doi: 10.1210/jc.2017-02360. [DOI] [PubMed] [Google Scholar]
- 31.Vermeulen A, Verdonck L, Kaufman JM. A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab. 1999;84(10):3666–3672. doi: 10.1210/jcem.84.10.6079. [DOI] [PubMed] [Google Scholar]
- 32.Tajar A, Forti G, O'Neill TW, Lee DM, Silman AJ, Finn JD, et al. Characteristics of secondary, primary, and compensated hypogonadism in aging men: evidence from the European male ageing study. J Clin Endocrinol Metab. 2010;95(4):1810–1818. doi: 10.1210/jc.2009-1796. [DOI] [PubMed] [Google Scholar]
- 33.Oduwole OO, Peltoketo H, Huhtaniemi IT. Role of follicle-stimulating hormone in spermatogenesis. Front Endocrinol (Lausanne) 2018;9:763. doi: 10.3389/fendo.2018.00763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dalvi M, Walker BR, Strachan MW, Zammitt NN, Gibb FW. The prevalence of structural pituitary abnormalities by MRI scanning in men presenting with isolated hypogonadotrophic hypogonadism. Clin Endocrinol (Oxf) 2016;84(6):858–861. doi: 10.1111/cen.13015. [DOI] [PubMed] [Google Scholar]
- 35.Cipriani S, Todisco T, Ghiandai N, Vignozzi L, Corona G, Maggi M, et al. Biochemical predictors of structural hypothalamus-pituitary abnormalities detected by magnetic resonance imaging in men with secondary hypogonadism. J Endocrinol Invest. 2021;44(12):2785–2797. doi: 10.1007/s40618-021-01586-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Das G, Surya A, Okosieme O, Vali A, Tennant BP, Geen J, et al. Pituitary imaging by MRI and its correlation with biochemical parameters in the evaluation of men with hypogonadotropic hypogonadism. Endocr Pract. 2019;25(9):926–934. doi: 10.4158/EP-2018-0609. [DOI] [PubMed] [Google Scholar]
- 37.Eendebak R, Ahern T, Swiecicka A, Pye SR, O'Neill TW, Bartfai G, et al. Elevated luteinizing hormone despite normal testosterone levels in older men-natural history, risk factors and clinical features. Clin Endocrinol (Oxf) 2018;88(3):479–490. doi: 10.1111/cen.13524. [DOI] [PubMed] [Google Scholar]
- 38.Corona G, Rastrelli G, Dicuio M, Concetti S, Minnetti M, Pivonello R, et al. Subclinical male hypogonadism. Minerva Endocrinol (Torino) 2021;46(3):252–261. doi: 10.23736/S2724-6507.20.03208-3. [DOI] [PubMed] [Google Scholar]
- 39.Corona G, Maseroli E, Rastrelli G, Sforza A, Forti G, Mannucci E, et al. Characteristics of compensated hypogonadism in patients with sexual dysfunction. J Sex Med. 2014;11(7):1823–1834. doi: 10.1111/jsm.12549. [DOI] [PubMed] [Google Scholar]
- 40.Veldhuis JD, Bondar OP, Dyer RB, Trushin SA, Klee EW, Singh RJ, et al. Immunological and mass spectrometric assays of SHBG: consistent and inconsistent metabolic associations in healthy men. J Clin Endocrinol Metab. 2014;99(1):184–193. doi: 10.1210/jc.2013-2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Coluzzi F, Billeci D, Maggi M, Corona G. Testosterone deficiency in non-cancer opioid-treated patients. J Endocrinol Invest. 2018;41(12):1377–1388. doi: 10.1007/s40618-018-0964-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rastrelli G, Maggi M, Corona G. Pharmacological management of late-onset hypogonadism. Expert Rev Clin Pharmacol. 2018;11(4):439–458. doi: 10.1080/17512433.2018.1445969. [DOI] [PubMed] [Google Scholar]
- 43.Corona G, Maseroli E, Maggi M. Injectable testosterone undecanoate for the treatment of hypogonadism. Expert Opin Pharmacother. 2014;15(13):1903–1926. doi: 10.1517/14656566.2014.944896. [DOI] [PubMed] [Google Scholar]
- 44.Awouters M, Vanderschueren D, Antonio L. Aromatase inhibitors and selective estrogen receptor modulators: unconventional therapies for functional hypogonadism? Andrology. 2020;8(6):1590–1597. doi: 10.1111/andr.12725. [DOI] [PubMed] [Google Scholar]
- 45.Corona G, Baldi E, Maggi M. Androgen regulation of prostate cancer: where are we now? J Endocrinol Invest. 2011;34(3):232–243. doi: 10.1007/BF03347072. [DOI] [PubMed] [Google Scholar]
- 46.Cornford P, van den Bergh RCN, Briers E, Van den Broeck T, Cumberbatch MG, De Santis M, et al. EAU-EANM-ESTRO-ESUR-SIOG guidelines on prostate cancer. Part II-2020 update: treatment of relapsing and metastatic prostate cancer. Eur Urol. 2021;79(2):263–282. doi: 10.1016/j.eururo.2020.09.046. [DOI] [PubMed] [Google Scholar]
- 47.Teeling F, Raison N, Shabbir M, Yap T, Dasgupta P, Ahmed K. Testosterone therapy for high-risk prostate cancer survivors: a systematic review and meta-analysis. Urology. 2019;126:16–23. doi: 10.1016/j.urology.2018.07.064. [DOI] [PubMed] [Google Scholar]
- 48.Kardoust Parizi M, Abufaraj M, Fajkovic H, Kimura S, Iwata T, D’Andrea D, et al. Oncological safety of testosterone replacement therapy in prostate cancer survivors after definitive local therapy: a systematic literature review and meta-analysis. Urol Oncol. 2019;37(10):637–646. doi: 10.1016/j.urolonc.2019.06.007. [DOI] [PubMed] [Google Scholar]
- 49.Chen S. Aromatase and breast cancer. Front Biosci. 1998;3:d922–d933. doi: 10.2741/A333. [DOI] [PubMed] [Google Scholar]
- 50.Ray A, Fernstrum A, Mahran A, Thirumavalavan N. Testosterone therapy and risk of breast cancer development: a systematic review. Curr Opin Urol. 2020;30(3):340–348. doi: 10.1097/MOU.0000000000000763. [DOI] [PubMed] [Google Scholar]
- 51.Kohn TP, Mata DA, Ramasamy R, Lipshultz LI. Effects of testosterone replacement therapy on lower urinary tract symptoms: a systematic review and meta-analysis. Eur Urol. 2016;69(6):1083–1090. doi: 10.1016/j.eururo.2016.01.043. [DOI] [PubMed] [Google Scholar]
- 52.Ponce OJ, Spencer-Bonilla G, Alvarez-Villalobos N, Serrano V, Singh-Ospina N, Rodriguez-Gutierrez R, et al. The efficacy and adverse events of testosterone replacement therapy in hypogonadal men: a systematic review and meta-analysis of randomized, placebo-controlled trials. J Clin Endocrinol Metab. 2018 doi: 10.1210/jc.2018-00404. [DOI] [PubMed] [Google Scholar]
- 53.Shigehara K, Sugimoto K, Konaka H, Iijima M, Fukushima M, Maeda Y, et al. Androgen replacement therapy contributes to improving lower urinary tract symptoms in patients with hypogonadism and benign prostate hypertrophy: a randomised controlled study. Aging Male. 2011;14(1):53–58. doi: 10.3109/13685538.2010.518178. [DOI] [PubMed] [Google Scholar]
- 54.Rastrelli G, Vignozzi L, Corona G, Maggi M. Testosterone and benign prostatic hyperplasia. Sex Med Rev. 2019;7(2):259–271. doi: 10.1016/j.sxmr.2018.10.006. [DOI] [PubMed] [Google Scholar]
- 55.Tao J, Liu X, Bai W. Testosterone supplementation in patients with chronic heart failure: a meta-analysis of randomized controlled trials. Front Endocrinol (Lausanne) 2020;11:110. doi: 10.3389/fendo.2020.00110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Navarro-Peñalver M, Perez-Martinez MT, Gómez-Bueno M, García-Pavía P, Lupón-Rosés J, Roig-Minguell E, et al. Testosterone replacement therapy in deficient patients with chronic heart failure: a randomized double-blind controlled pilot study. J Cardiovasc Pharmacol Ther. 2018;23(6):543–550. doi: 10.1177/1074248418784020. [DOI] [PubMed] [Google Scholar]
- 57.Mirdamadi A, Garakyaraghi M, Pourmoghaddas A, Bahmani A, Mahmoudi H, Gharipour M. Beneficial effects of testosterone therapy on functional capacity, cardiovascular parameters, and quality of life in patients with congestive heart failure. Biomed Res Int. 2014;2014:392432. doi: 10.1155/2014/392432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wallis CJ, Lo K, Lee Y, Krakowsky Y, Garbens A, Satkunasivam R, et al. Survival and cardiovascular events in men treated with testosterone replacement therapy: an intention-to-treat observational cohort study. Lancet Diabetes Endocrinol. 2016;4(6):498–506. doi: 10.1016/S2213-8587(16)00112-1. [DOI] [PubMed] [Google Scholar]
- 59.Dos Santos MR, Sayegh AL, Bacurau AV, Arap MA, Brum PC, Pereira RM, et al. Effect of exercise training and testosterone replacement on skeletal muscle wasting in patients with heart failure with testosterone deficiency. Mayo Clin Proc. 2016;91(5):575–586. doi: 10.1016/j.mayocp.2016.02.014. [DOI] [PubMed] [Google Scholar]
- 60.Stout M, Tew GA, Doll H, Zwierska I, Woodroofe N, Channer KS, et al. Testosterone therapy during exercise rehabilitation in male patients with chronic heart failure who have low testosterone status: a double-blind randomized controlled feasibility study. Am Heart J. 2012;164(6):893–901. doi: 10.1016/j.ahj.2012.09.016. [DOI] [PubMed] [Google Scholar]
- 61.Toma M, McAlister FA, Coglianese EE, Vidi V, Vasaiwala S, Bakal JA, et al. Testosterone supplementation in heart failure: a meta-analysis. Circ Heart Fail. 2012;5(3):315–321. doi: 10.1161/CIRCHEARTFAILURE.111.965632. [DOI] [PubMed] [Google Scholar]
- 62.Sesti F, Pofi R, Minnetti M, Tenuta M, Gianfrilli D, Isidori AM. Late-onset hypogonadism: reductio ad absurdum of the cardiovascular risk-benefit of testosterone replacement therapy. Andrology. 2020;8(6):1614–1627. doi: 10.1111/andr.12876. [DOI] [PubMed] [Google Scholar]
- 63.Khera M, Miner M, Jaffe J, Pastuszak AW. Testosterone therapy and cardiovascular risk: a critical analysis of studies reporting increased risk. J Sex Med. 2021;18(1):83–98. doi: 10.1016/j.jsxm.2020.10.019. [DOI] [PubMed] [Google Scholar]
- 64.Corona G, Rastrelli G, Di Pasquale G, Sforza A, Mannucci E, Maggi M. Endogenous testosterone levels and cardiovascular risk: meta-analysis of observational studies. J Sex Med. 2018;15(9):1260–1271. doi: 10.1016/j.jsxm.2018.06.012. [DOI] [PubMed] [Google Scholar]
- 65.Wang J, Fan X, Yang M, Song M, Wang K, Giovannucci E, et al. Sex-specific associations of circulating testosterone levels with all-cause and cause-specific mortality. Eur J Endocrinol. 2021;184(5):723–732. doi: 10.1530/EJE-20-1253. [DOI] [PubMed] [Google Scholar]
- 66.Corona G, Rastrelli G, Di Pasquale G, Sforza A, Mannucci E, Maggi M. Testosterone and cardiovascular risk: meta-analysis of interventional studies. J Sex Med. 2018;15(6):820–838. doi: 10.1016/j.jsxm.2018.04.641. [DOI] [PubMed] [Google Scholar]
- 67.Hudson J, Cruickshank M, Quinton R, Aucott L, Aceves-Martins M, Gillies K, et al. Adverse cardiovascular events and mortality in men during testosterone treatment: an individual patient and aggregate data meta-analysis. Lancet Healthy Longev. 2022;3(6):e381–e393. doi: 10.1016/S2666-7568(22)00096-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.European Medicines Agency. No consistent evidence of an increased risk of heart problems with testosterone medicines [cited 2014 November, 21th]. Available from: https://www.ema.europa.eu/en/news/no-consistent-evidence-increased-risk-heart-problems-testosterone-medicines.
- 69.Corona G, Dicuio M, Rastrelli G, Maseroli E, Lotti F, Sforza A, et al. Testosterone treatment and cardiovascular and venous thromboembolism risk: what is 'new'? J Investig Med. 2017;65(6):964–973. doi: 10.1136/jim-2017-000411. [DOI] [PubMed] [Google Scholar]
- 70.Luo S, Au Yeung SL, Zhao JV, Burgess S, Schooling CM. Association of genetically predicted testosterone with thromboembolism, heart failure, and myocardial infarction: mendelian randomisation study in UK biobank. BMJ. 2019;364:l476. doi: 10.1136/bmj.l476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ayele HT, Brunetti VC, Renoux C, Tagalakis V, Filion KB. Testosterone replacement therapy and the risk of venous thromboembolism: a systematic review and meta-analysis of randomized controlled trials. Thromb Res. 2021;199:123–131. doi: 10.1016/j.thromres.2020.12.029. [DOI] [PubMed] [Google Scholar]
- 72.Long JE, Lee MS, Blithe DL. Update on novel hormonal and nonhormonal male contraceptive development. J Clin Endocrinol Metab. 2021;106(6):e2381–e2392. doi: 10.1210/clinem/dgab034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Corona G, Rastrelli G, Marchiani S, Filippi S, Morelli A, Sarchielli E, et al. Consequences of anabolic-androgenic steroid abuse in males; sexual and reproductive perspective. World J Mens Health. 2021 doi: 10.5534/wjmh.210021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ferlin A, Calogero AE, Krausz C, Lombardo F, Paoli D, Rago R, et al. Management of male factor infertility: position statement from the Italian society of andrology and sexual medicine (SIAMS): endorsing organization: Italian society of embryology, reproduction, and research (SIERR) J Endocrinol Invest. 2022 doi: 10.1007/s40618-022-01741-6. [DOI] [PubMed] [Google Scholar]
- 75.Killick R, Wang D, Hoyos CM, Yee BJ, Grunstein RR, Liu PY. The effects of testosterone on ventilatory responses in men with obstructive sleep apnea: a randomised, placebo-controlled trial. J Sleep Res. 2013;22(3):331–336. doi: 10.1111/jsr.12027. [DOI] [PubMed] [Google Scholar]
- 76.Hoyos CM, Killick R, Yee BJ, Grunstein RR, Liu PY. Effects of testosterone therapy on sleep and breathing in obese men with severe obstructive sleep apnoea: a randomized placebo-controlled trial. Clin Endocrinol (Oxf) 2012;77(4):599–607. doi: 10.1111/j.1365-2265.2012.04413.x. [DOI] [PubMed] [Google Scholar]
- 77.Melehan KL, Hoyos CM, Yee BJ, Wong KK, Buchanan PR, Grunstein RR, et al. Increased sexual desire with exogenous testosterone administration in men with obstructive sleep apnea: a randomized placebo-controlled study. Andrology. 2016;4(1):55–61. doi: 10.1111/andr.12132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Shigehara K, Konaka H, Sugimoto K, Nohara T, Izumi K, Kadono Y, et al. Sleep disturbance as a clinical sign for severe hypogonadism: efficacy of testosterone replacement therapy on sleep disturbance among hypogonadal men without obstructive sleep apnea. Aging Male. 2018;21(2):99–105. doi: 10.1080/13685538.2017.1378320. [DOI] [PubMed] [Google Scholar]
- 79.Payne K, Lipshultz LI, Hotaling JM, Pastuszak AW. Obstructive sleep apnea and testosterone therapy. Sex Med Rev. 2021;9(2):296–303. doi: 10.1016/j.sxmr.2020.04.004. [DOI] [PubMed] [Google Scholar]
- 80.Corona G, Rastrelli G, Vignozzi L, Maggi M. Androgens and male sexual function. Best Pract Res Clin Endocrinol Metab. 2022 doi: 10.1016/j.beem.2022.101615. [DOI] [PubMed] [Google Scholar]
- 81.Isidori AM, Giannetta E, Gianfrilli D, Greco EA, Bonifacio V, Aversa A, et al. Effects of testosterone on sexual function in men: results of a meta-analysis. Clin Endocrinol (Oxf) 2005;63(4):381–394. doi: 10.1111/j.1365-2265.2005.02350.x. [DOI] [PubMed] [Google Scholar]
- 82.Boloña ER, Uraga MV, Haddad RM, Tracz MJ, Sideras K, Kennedy CC, et al. Testosterone use in men with sexual dysfunction: a systematic review and meta-analysis of randomized placebo-controlled trials. Mayo Clin Proc. 2007;82(1):20–28. doi: 10.1016/S0025-6196(11)60963-4. [DOI] [PubMed] [Google Scholar]
- 83.Corona G, Isidori AM, Buvat J, Aversa A, Rastrelli G, Hackett G, et al. Testosterone supplementation and sexual function: a meta-analysis study. J Sex Med. 2014;11(6):1577–1592. doi: 10.1111/jsm.12536. [DOI] [PubMed] [Google Scholar]
- 84.Elliott J, Kelly SE, Millar AC, Peterson J, Chen L, Johnston A, et al. Testosterone therapy in hypogonadal men: a systematic review and network meta-analysis. BMJ Open. 2017;7(11):e015284. doi: 10.1136/bmjopen-2016-015284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Algeffari M, Jayasena CN, MacKeith P, Thapar A, Dhillo WS, Oliver N. Testosterone therapy for sexual dysfunction in men with type 2 diabetes: a systematic review and meta-analysis of randomized controlled trials. Diabet Med. 2018;35(2):195–202. doi: 10.1111/dme.13553. [DOI] [PubMed] [Google Scholar]
- 86.Zhu J, Zhang W, Ou N, Song Y, Kang J, Liang Z, et al. Do testosterone supplements enhance response to phosphodiesterase 5 inhibitors in men with erectile dysfunction and hypogonadism: a systematic review and meta-analysis. Transl Androl Urol. 2020;9(2):591–600. doi: 10.21037/tau.2020.01.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Corona G, Rastrelli G, Morgentaler A, Sforza A, Mannucci E, Maggi M. Meta-analysis of results of testosterone therapy on sexual function based on international index of erectile function scores. Eur Urol. 2017;72(6):1000–1011. doi: 10.1016/j.eururo.2017.03.032. [DOI] [PubMed] [Google Scholar]
- 88.Taniguchi H, Shimada S, Kinoshita H. Testosterone therapy for late-onset hypogonadism improves erectile function: a systematic review and meta-analysis. Urol Int. 2021;2021:1–14. doi: 10.1159/000520135. [DOI] [PubMed] [Google Scholar]
- 89.Allen MS, Walter EE. Health-related lifestyle factors and sexual dysfunction: a meta-analysis of population-based research. J Sex Med. 2018;15(4):458–475. doi: 10.1016/j.jsxm.2018.02.008. [DOI] [PubMed] [Google Scholar]
- 90.Corona G, Rastrelli G, Isidori AM, Pivonello R, Bettocchi C, Reisman Y, et al. Erectile dysfunction and cardiovascular risk: a review of current findings. Expert Rev Cardiovasc Ther. 2020;18(3):155–164. doi: 10.1080/14779072.2020.1745632. [DOI] [PubMed] [Google Scholar]
- 91.Almeida M, Laurent MR, Dubois V, Claessens F, O'Brien CA, Bouillon R, et al. Estrogens and androgens in skeletal physiology and pathophysiology. Physiol Rev. 2017;97(1):135–187. doi: 10.1152/physrev.00033.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ferlin A, Selice R, Carraro U, Foresta C. Testicular function and bone metabolism–beyond testosterone. Nat Rev Endocrinol. 2013;9(9):548–554. doi: 10.1038/nrendo.2013.135. [DOI] [PubMed] [Google Scholar]
- 93.Porcelli T, Maffezzoni F, Pezzaioli LC, Delbarba A, Cappelli C, Ferlin A. Management of endocrine disease: male osteoporosis: diagnosis and management - should the treatment and the target be the same as for female osteoporosis? Eur J Endocrinol. 2020;183(3):R75–r93. doi: 10.1530/EJE-20-0034. [DOI] [PubMed] [Google Scholar]
- 94.Isidori AM, Giannetta E, Greco EA, Gianfrilli D, Bonifacio V, Isidori A, et al. Effects of testosterone on body composition, bone metabolism and serum lipid profile in middle-aged men: a meta-analysis. Clin Endocrinol (Oxf) 2005;63(3):280–293. doi: 10.1111/j.1365-2265.2005.02339.x. [DOI] [PubMed] [Google Scholar]
- 95.Tracz MJ, Sideras K, Boloña ER, Haddad RM, Kennedy CC, Uraga MV, et al. Testosterone use in men and its effects on bone health. A systematic review and meta-analysis of randomized placebo-controlled trials. J Clin Endocrinol Metab. 2006;91(6):2011–2016. doi: 10.1210/jc.2006-0036. [DOI] [PubMed] [Google Scholar]
- 96.Junjie W, Dongsheng H, Lei S, Hongzhuo L, Changying S. Testosterone replacement therapy has limited effect on increasing bone mass density in older men: a meta-analysis. Curr Pharm Des. 2019;25(1):73–84. doi: 10.2174/1381612825666190206223244. [DOI] [PubMed] [Google Scholar]
- 97.Zhang Z, Kang D, Li H. The effects of testosterone on bone health in males with testosterone deficiency: a systematic review and meta-analysis. BMC Endocr Disord. 2020;20(1):33. doi: 10.1186/s12902-020-0509-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Corona G, Vena W, Pizzocaro A, Giagulli VA, Francomano D, Rastrelli G, et al. Testosterone supplementation and bone parameters: a systematic review and meta-analysis study. J Endocrinol Invest. 2022 doi: 10.1007/s40618-021-01702-5. [DOI] [PubMed] [Google Scholar]
- 99.Pizzocaro A, Vena W, Condorelli R, Radicioni A, Rastrelli G, Pasquali D, et al. Testosterone treatment in male patients with Klinefelter syndrome: a systematic review and meta-analysis. J Endocrinol Invest. 2020;43(12):1675–1687. doi: 10.1007/s40618-020-01299-1. [DOI] [PubMed] [Google Scholar]
- 100.Snyder PJ, Kopperdahl DL, Stephens-Shields AJ, Ellenberg SS, Cauley JA, Ensrud KE, et al. Effect of testosterone treatment on volumetric bone density and strength in older men with low testosterone: a controlled clinical trial. JAMA Intern Med. 2017;177(4):471–479. doi: 10.1001/jamainternmed.2016.9539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ng Tang Fui M, Hoermann R, Bracken K, Handelsman DJ, Inder WJ, Stuckey BGA, et al. Effect of testosterone treatment on bone microarchitecture and bone mineral density in men: a 2-year RCT. J Clin Endocrinol Metab. 2021;106(8):3143–3158. doi: 10.1210/clinem/dgab149. [DOI] [PubMed] [Google Scholar]
- 102.Rochira V, Antonio L, Vanderschueren D. EAA clinical guideline on management of bone health in the andrological outpatient clinic. Andrology. 2018;6(2):272–285. doi: 10.1111/andr.12470. [DOI] [PubMed] [Google Scholar]
- 103.Watts NB, Adler RA, Bilezikian JP, Drake MT, Eastell R, Orwoll ES, et al. Osteoporosis in men: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2012;97(6):1802–1822. doi: 10.1210/jc.2011-3045. [DOI] [PubMed] [Google Scholar]
- 104.Barrett-Connor E, Von Mühlen DG, Kritz-Silverstein D. Bioavailable testosterone and depressed mood in older men: the Rancho Bernardo study. J Clin Endocrinol Metab. 1999;84(2):573–577. doi: 10.1210/jcem.84.2.5495. [DOI] [PubMed] [Google Scholar]
- 105.Giltay EJ, van der Mast RC, Lauwen E, Heijboer AC, de Waal MWM, Comijs HC. Plasma testosterone and the course of major depressive disorder in older men and women. Am J Geriatr Psychiatry. 2017;25(4):425–437. doi: 10.1016/j.jagp.2016.12.014. [DOI] [PubMed] [Google Scholar]
- 106.McIntyre RS, Mancini D, Eisfeld BS, Soczynska JK, Grupp L, Konarski JZ, et al. Calculated bioavailable testosterone levels and depression in middle-aged men. Psychoneuroendocrinology. 2006;31(9):1029–1035. doi: 10.1016/j.psyneuen.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 107.Westley CJ, Amdur RL, Irwig MS. High rates of depression and depressive symptoms among men referred for borderline testosterone levels. J Sex Med. 2015;12(8):1753–1760. doi: 10.1111/jsm.12937. [DOI] [PubMed] [Google Scholar]
- 108.Ford AH, Yeap BB, Flicker L, Hankey GJ, Chubb SA, Handelsman DJ, et al. Prospective longitudinal study of testosterone and incident depression in older men: the health in men study. Psychoneuroendocrinology. 2016;64:57–65. doi: 10.1016/j.psyneuen.2015.11.012. [DOI] [PubMed] [Google Scholar]
- 109.Shores MM, Sloan KL, Matsumoto AM, Moceri VM, Felker B, Kivlahan DR. Increased incidence of diagnosed depressive illness in hypogonadal older men. Arch Gen Psychiatry. 2004;61(2):162–167. doi: 10.1001/archpsyc.61.2.162. [DOI] [PubMed] [Google Scholar]
- 110.Smith JB, Rosen J, Colbert A. Low serum testosterone in outpatient psychiatry clinics: addressing challenges to the screening and treatment of hypogonadism. Sex Med Rev. 2018;6(1):69–76. doi: 10.1016/j.sxmr.2017.08.007. [DOI] [PubMed] [Google Scholar]
- 111.Snyder PJ, Bhasin S, Cunningham GR, Matsumoto AM, Stephens-Shields AJ, Cauley JA, et al. Effects of testosterone treatment in older men. N Engl J Med. 2016;374(7):611–624. doi: 10.1056/NEJMoa1506119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Vartolomei MD, Kimura S, Vartolomei L, Shariat SF. Systematic review of the impact of testosterone replacement therapy on depression in patients with late-onset testosterone deficiency. Eur Urol Focus. 2020;6(1):170–177. doi: 10.1016/j.euf.2018.07.006. [DOI] [PubMed] [Google Scholar]
- 113.Zarrouf FA, Artz S, Griffith J, Sirbu C, Kommor M. Testosterone and depression: systematic review and meta-analysis. J Psychiatr Pract. 2009;15(4):289–305. doi: 10.1097/01.pra.0000358315.88931.fc. [DOI] [PubMed] [Google Scholar]
- 114.Amanatkar HR, Chibnall JT, Seo BW, Manepalli JN, Grossberg GT. Impact of exogenous testosterone on mood: a systematic review and meta-analysis of randomized placebo-controlled trials. Ann Clin Psychiatry. 2014;26(1):19–32. [PubMed] [Google Scholar]
- 115.Walther A, Breidenstein J, Miller R. Association of testosterone treatment with alleviation of depressive symptoms in men: a systematic review and meta-analysis. JAMA Psychiat. 2019;76(1):31–40. doi: 10.1001/jamapsychiatry.2018.2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Bhasin S, Seidman S. Testosterone treatment of depressive disorders in men: too much smoke, not enough high-quality evidence. JAMA Psychiat. 2019;76(1):9–10. doi: 10.1001/jamapsychiatry.2018.2661. [DOI] [PubMed] [Google Scholar]
- 117.Corona G, Guaraldi F, Rastrelli G, Sforza A, Maggi M. Testosterone deficiency and risk of cognitive disorders in aging males. World J Mens Health. 2021;39(1):9–18. doi: 10.5534/wjmh.200017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Geerlings MI, Strozyk D, Masaki K, Remaley AT, Petrovitch H, Ross GW, et al. Endogenous sex hormones, cognitive decline, and future dementia in old men. Ann Neurol. 2006;60(3):346–355. doi: 10.1002/ana.20918. [DOI] [PubMed] [Google Scholar]
- 119.Zhang Z, Kang D, Li H. Testosterone and cognitive impairment or dementia in middle-aged or aging males: causation and intervention, a systematic review and meta-analysis. J Geriatr Psychiatry Neurol. 2021;34(5):405–417. doi: 10.1177/0891988720933351. [DOI] [PubMed] [Google Scholar]
- 120.McGinty HL, Phillips KM, Jim HS, Cessna JM, Asvat Y, Cases MG, et al. Cognitive functioning in men receiving androgen deprivation therapy for prostate cancer: a systematic review and meta-analysis. Support Care Cancer. 2014;22(8):2271–2280. doi: 10.1007/s00520-014-2285-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Buskbjerg CR, Gravholt CH, Dalby HR, Amidi A, Zachariae R. Testosterone supplementation and cognitive functioning in men-a systematic review and meta-analysis. J Endocr Soc. 2019;3(8):1465–1484. doi: 10.1210/js.2019-00119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Tan S, Sohrabi HR, Weinborn M, Tegg M, Bucks RS, Taddei K, et al. Effects of testosterone supplementation on separate cognitive domains in cognitively healthy older men: a meta-analysis of current randomized clinical trials. Am J Geriatr Psychiatry. 2019;27(11):1232–1246. doi: 10.1016/j.jagp.2019.05.008. [DOI] [PubMed] [Google Scholar]
- 123.Mailliez A, Guilbaud A, Puisieux F, Dauchet L, Boulanger É. Circulating biomarkers characterizing physical frailty: CRP, hemoglobin, albumin, 25OHD and free testosterone as best biomarkers. Results of a meta-analysis. Exp Gerontol. 2020;139:111014. doi: 10.1016/j.exger.2020.111014. [DOI] [PubMed] [Google Scholar]
- 124.Clegg A, Hassan-Smith Z. Frailty and the endocrine system. Lancet Diabetes Endocrinol. 2018;6(9):743–752. doi: 10.1016/S2213-8587(18)30110-4. [DOI] [PubMed] [Google Scholar]
- 125.Peng X, Hou L, Zhao Y, Lin T, Wang H, Gao L, et al. Frailty and testosterone level in older adults: a systematic review and meta-analysis. Eur Geriatr Med. 2022 doi: 10.1007/s41999-022-00614-8. [DOI] [PubMed] [Google Scholar]
- 126.Szulc P, Claustrat B, Marchand F, Delmas PD. Increased risk of falls and increased bone resorption in elderly men with partial androgen deficiency: the MINOS study. J Clin Endocrinol Metab. 2003;88(11):5240–5247. doi: 10.1210/jc.2003-030200. [DOI] [PubMed] [Google Scholar]
- 127.Ottenbacher KJ, Ottenbacher ME, Ottenbacher AJ, Acha AA, Ostir GV. Androgen treatment and muscle strength in elderly men: a meta-analysis. J Am Geriatr Soc. 2006;54(11):1666–1673. doi: 10.1111/j.1532-5415.2006.00938.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Skinner JW, Otzel DM, Bowser A, Nargi D, Agarwal S, Peterson MD, et al. Muscular responses to testosterone replacement vary by administration route: a systematic review and meta-analysis. J Cachexia Sarcopenia Muscle. 2018;9(3):465–481. doi: 10.1002/jcsm.12291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Parahiba SM, Ribeiro ÉCT, Corrêa C, Bieger P, Perry IS, Souza GC. Effect of testosterone supplementation on sarcopenic components in middle-aged and elderly men: a systematic review and meta-analysis. Exp Gerontol. 2020;142:111106. doi: 10.1016/j.exger.2020.111106. [DOI] [PubMed] [Google Scholar]
- 130.Kenny AM, Kleppinger A, Annis K, Rathier M, Browner B, Judge JO, et al. Effects of transdermal testosterone on bone and muscle in older men with low bioavailable testosterone levels, low bone mass, and physical frailty. J Am Geriatr Soc. 2010;58(6):1134–1143. doi: 10.1111/j.1532-5415.2010.02865.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Snyder PJ, Peachey H, Hannoush P, Berlin JA, Loh L, Lenrow DA, et al. Effect of testosterone treatment on body composition and muscle strength in men over 65 years of age. J Clin Endocrinol Metab. 1999;84(8):2647–2653. doi: 10.1210/jcem.84.8.5885. [DOI] [PubMed] [Google Scholar]
- 132.Svartberg J, Agledahl I, Figenschau Y, Sildnes T, Waterloo K, Jorde R. Testosterone treatment in elderly men with subnormal testosterone levels improves body composition and BMD in the hip. Int J Impot Res. 2008;20(4):378–387. doi: 10.1038/ijir.2008.19. [DOI] [PubMed] [Google Scholar]
- 133.Bhasin S, Ellenberg SS, Storer TW, Basaria S, Pahor M, Stephens-Shields AJ, et al. Effect of testosterone replacement on measures of mobility in older men with mobility limitation and low testosterone concentrations: secondary analyses of the testosterone trials. Lancet Diabetes Endocrinol. 2018;6(11):879–890. doi: 10.1016/S2213-8587(18)30171-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Allan CA, Strauss BJ, Burger HG, Forbes EA, McLachlan RI. Testosterone therapy prevents gain in visceral adipose tissue and loss of skeletal muscle in nonobese aging men. J Clin Endocrinol Metab. 2008;93(1):139–146. doi: 10.1210/jc.2007-1291. [DOI] [PubMed] [Google Scholar]
- 135.Aversa A, Bruzziches R, Francomano D, Spera G, Lenzi A. Efficacy and safety of two different testosterone undecanoate formulations in hypogonadal men with metabolic syndrome. J Endocrinol Invest. 2010;33(11):776–783. doi: 10.1007/BF03350341. [DOI] [PubMed] [Google Scholar]
- 136.Dhindsa S, Ghanim H, Batra M, Kuhadiya ND, Abuaysheh S, Sandhu S, et al. Insulin resistance and inflammation in hypogonadotropic hypogonadism and their reduction after testosterone replacement in men with type 2 diabetes. Diabetes Care. 2016;39(1):82–91. doi: 10.2337/dc15-1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kapoor D, Goodwin E, Channer KS, Jones TH. Testosterone replacement therapy improves insulin resistance, glycaemic control, visceral adiposity and hypercholesterolaemia in hypogonadal men with type 2 diabetes. Eur J Endocrinol. 2006;154(6):899–906. doi: 10.1530/eje.1.02166. [DOI] [PubMed] [Google Scholar]
- 138.Kalinchenko SY, Tishova YA, Mskhalaya GJ, Gooren LJ, Giltay EJ, Saad F. Effects of testosterone supplementation on markers of the metabolic syndrome and inflammation in hypogonadal men with the metabolic syndrome: the double-blinded placebo-controlled Moscow study. Clin Endocrinol (Oxf) 2010;73(5):602–612. doi: 10.1111/j.1365-2265.2010.03845.x. [DOI] [PubMed] [Google Scholar]
- 139.Corona G, Giagulli VA, Maseroli E, Vignozzi L, Aversa A, Zitzmann M, et al. Therapy of endocrine disease: testosterone supplementation and body composition: results from a meta-analysis study. Eur J Endocrinol. 2016;174(3):R99–116. doi: 10.1530/EJE-15-0262. [DOI] [PubMed] [Google Scholar]
- 140.Wittert G, Bracken K, Robledo KP, Grossmann M, Yeap BB, Handelsman DJ, et al. Testosterone treatment to prevent or revert type 2 diabetes in men enrolled in a lifestyle programme (T4DM): a randomised, double-blind, placebo-controlled, 2-year, phase 3b trial. Lancet Diabetes Endocrinol. 2021;9(1):32–45. doi: 10.1016/S2213-8587(20)30367-3. [DOI] [PubMed] [Google Scholar]
- 141.Hackett G, Cole N, Bhartia M, Kennedy D, Raju J, Wilkinson P. Testosterone replacement therapy improves metabolic parameters in hypogonadal men with type 2 diabetes but not in men with coexisting depression: the BLAST study. J Sex Med. 2014;11(3):840–856. doi: 10.1111/jsm.12404. [DOI] [PubMed] [Google Scholar]
- 142.Jones TH, Arver S, Behre HM, Buvat J, Meuleman E, Moncada I, et al. Testosterone replacement in hypogonadal men with type 2 diabetes and/or metabolic syndrome (the TIMES2 study) Diabetes Care. 2011;34(4):828–837. doi: 10.2337/dc10-1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Gianatti EJ, Dupuis P, Hoermann R, Strauss BJ, Wentworth JM, Zajac JD, et al. Effect of testosterone treatment on glucose metabolism in men with type 2 diabetes: a randomized controlled trial. Diabetes Care. 2014;37(8):2098–2107. doi: 10.2337/dc13-2845. [DOI] [PubMed] [Google Scholar]
- 144.Gopal RA, Bothra N, Acharya SV, Ganesh HK, Bandgar TR, Menon PS, et al. Treatment of hypogonadism with testosterone in patients with type 2 diabetes mellitus. Endocr Pract. 2010;16(4):570–576. doi: 10.4158/EP09355.OR. [DOI] [PubMed] [Google Scholar]
- 145.Malkin CJ, Pugh PJ, Jones RD, Kapoor D, Channer KS, Jones TH. The effect of testosterone replacement on endogenous inflammatory cytokines and lipid profiles in hypogonadal men. J Clin Endocrinol Metab. 2004;89(7):3313–3318. doi: 10.1210/jc.2003-031069. [DOI] [PubMed] [Google Scholar]
- 146.Mohler ER, 3rd, Ellenberg SS, Lewis CE, Wenger NK, Budoff MJ, Lewis MR, et al. The effect of testosterone on cardiovascular biomarkers in the testosterone trials. J Clin Endocrinol Metab. 2018;103(2):681–688. doi: 10.1210/jc.2017-02243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Basaria S, Coviello AD, Travison TG, Storer TW, Farwell WR, Jette AM, et al. Adverse events associated with testosterone administration. N Engl J Med. 2010;363(2):109–122. doi: 10.1056/NEJMoa1000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Basaria S, Harman SM, Travison TG, Hodis H, Tsitouras P, Budoff M, et al. Effects of testosterone administration for 3 years on subclinical atherosclerosis progression in older men with low or low-normal testosterone levels: a randomized clinical trial. JAMA. 2015;314(6):570–581. doi: 10.1001/jama.2015.8881. [DOI] [PubMed] [Google Scholar]
- 149.Whitsel EA, Boyko EJ, Matsumoto AM, Anawalt BD, Siscovick DS. Intramuscular testosterone esters and plasma lipids in hypogonadal men: a meta-analysis. Am J Med. 2001;111(4):261–269. doi: 10.1016/S0002-9343(01)00833-6. [DOI] [PubMed] [Google Scholar]
- 150.Haddad RM, Kennedy CC, Caples SM, Tracz MJ, Boloña ER, Sideras K, et al. Testosterone and cardiovascular risk in men: a systematic review and meta-analysis of randomized placebo-controlled trials. Mayo Clin Proc. 2007;82(1):29–39. doi: 10.1016/S0025-6196(11)60964-6. [DOI] [PubMed] [Google Scholar]
- 151.Fernández-Balsells MM, Murad MH, Lane M, Lampropulos JF, Albuquerque F, Mullan RJ, et al. Clinical review 1: adverse effects of testosterone therapy in adult men: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2010;95(6):2560–2575. doi: 10.1210/jc.2009-2575. [DOI] [PubMed] [Google Scholar]
- 152.Neto WK, Gama EF, Rocha LY, Ramos CC, Taets W, Scapini KB, et al. Effects of testosterone on lean mass gain in elderly men: systematic review with meta-analysis of controlled and randomized studies. Age (Dordr) 2015;37(1):9742. doi: 10.1007/s11357-014-9742-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Guo C, Gu W, Liu M, Peng BO, Yao X, Yang B, et al. Efficacy and safety of testosterone replacement therapy in men with hypogonadism: a meta-analysis study of placebo-controlled trials. Exp Ther Med. 2016;11(3):853–863. doi: 10.3892/etm.2015.2957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Corona G, Monami M, Rastrelli G, Aversa A, Sforza A, Lenzi A, et al. Type 2 diabetes mellitus and testosterone: a meta-analysis study. Int J Androl. 2011;34(6 Pt 1):528–540. doi: 10.1111/j.1365-2605.2010.01117.x. [DOI] [PubMed] [Google Scholar]
- 155.Corona G, Rastrelli G, Maggi M. Diagnosis and treatment of late-onset hypogonadism: systematic review and meta-analysis of TRT outcomes. Best Pract Res Clin Endocrinol Metab. 2013;27(4):557–579. doi: 10.1016/j.beem.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 156.Cai X, Tian Y, Wu T, Cao CX, Li H, Wang KJ. Metabolic effects of testosterone replacement therapy on hypogonadal men with type 2 diabetes mellitus: a systematic review and meta-analysis of randomized controlled trials. Asian J Androl. 2014;16(1):146–152. doi: 10.4103/1008-682X.122346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Corona G, Rastrelli G, Vignozzi L, Barbonetti A, Sforza A, Mannucci E, et al. The role of testosterone treatment in patients with metabolic disorders. Expert Rev Clin Pharmacol. 2021;14(9):1091–1103. doi: 10.1080/17512433.2021.1938548. [DOI] [PubMed] [Google Scholar]
- 158.Corona G, Monami M, Rastrelli G, Aversa A, Tishova Y, Saad F, et al. Testosterone and metabolic syndrome: a meta-analysis study. J Sex Med. 2011;8(1):272–283. doi: 10.1111/j.1743-6109.2010.01991.x. [DOI] [PubMed] [Google Scholar]
- 159.Zhang J, Yang B, Xiao W, Li X, Li H. Effects of testosterone supplement treatment in hypogonadal adult males with T2DM: a meta-analysis and systematic review. World J Urol. 2018;36(8):1315–1326. doi: 10.1007/s00345-018-2256-0. [DOI] [PubMed] [Google Scholar]
- 160.Nasser M, Haider A, Saad F, Kurtz W, Doros G, Fijak M, et al. Testosterone therapy in men with Crohn's disease improves the clinical course of the disease: data from long-term observational registry study. Horm Mol Biol Clin Investig. 2015;22(3):111–117. doi: 10.1515/hmbci-2015-0014. [DOI] [PubMed] [Google Scholar]
- 161.Santi D, Spaggiari G, Vena W, Pizzocaro A, Maggi M, Rochira V, et al. The prevalence of hypogonadism and the effectiveness of androgen administration on body composition in HIV-infected men: a meta-analysis. Cells. 2021;10(8):2067. doi: 10.3390/cells10082067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Zhou T, Hu ZY, Zhang HP, Zhao K, Zhang Y, Li Y, et al. Effects of Testosterone supplementation on body composition in HIV patients: a meta-analysis of double-blinded randomized controlled trials. Curr Med Sci. 2018;38(1):191–198. doi: 10.1007/s11596-018-1864-7. [DOI] [PubMed] [Google Scholar]
- 163.Atlantis E, Fahey P, Cochrane B, Wittert G, Smith S. Endogenous testosterone level and testosterone supplementation therapy in chronic obstructive pulmonary disease (COPD): a systematic review and meta-analysis. BMJ Open. 2013;3(8):e003127. doi: 10.1136/bmjopen-2013-003127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Corona G, Pizzocaro A, Vena W, Rastrelli G, Semeraro F, Isidori AM, et al. Diabetes is most important cause for mortality in COVID-19 hospitalized patients: systematic review and meta-analysis. Rev Endocr Metab Disord. 2021;22(2):275–296. doi: 10.1007/s11154-021-09630-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Salonia A, Corona G, Giwercman A, Maggi M, Minhas S, Nappi RE, et al. SARS-CoV-2, testosterone and frailty in males (PROTEGGIMI): a multidimensional research project. Andrology. 2021;9(1):19–22. doi: 10.1111/andr.12811. [DOI] [PubMed] [Google Scholar]
- 166.Rastrelli G, Di Stasi V, Inglese F, Beccaria M, Garuti M, Di Costanzo D, et al. Low testosterone levels predict clinical adverse outcomes in SARS-CoV-2 pneumonia patients. Andrology. 2021;9(1):88–98. doi: 10.1111/andr.12821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Pivonello R, Auriemma RS, Pivonello C, Isidori AM, Corona G, Colao A, et al. Sex disparities in COVID-19 severity and outcome: are men weaker or women stronger? Neuroendocrinology. 2021;111(11):1066–1085. doi: 10.1159/000513346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Corona G, Vena W, Pizzocaro A, Pallotti F, Paoli D, Rastrelli G, et al. Andrological effects of SARS-Cov-2 infection: a systematic review and meta-analysis. J Endocrinol Invest. 2022 doi: 10.1007/s40618-022-01801-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Vena W, Pizzocaro A, Maida G, Amer M, Voza A, Di Pasquale A, et al. Low testosterone predicts hypoxemic respiratory insufficiency and mortality in patients with COVID-19 disease: another piece in the COVID puzzle. J Endocrinol Invest. 2022;45(4):753–762. doi: 10.1007/s40618-021-01700-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Sansone A, Mollaioli D, Ciocca G, Limoncin E, Colonnello E, Vena W, et al. Addressing male sexual and reproductive health in the wake of COVID-19 outbreak. J Endocrinol Invest. 2021;44(2):223–231. doi: 10.1007/s40618-020-01350-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Snyder PJ, Ellenberg SS, Farrar JT. Testosterone treatment in older men. N Engl J Med. 2016;375(1):90. doi: 10.1056/NEJMc1603665. [DOI] [PubMed] [Google Scholar]
- 172.Ohlander SJ, Varghese B, Pastuszak AW. Erythrocytosis following testosterone therapy. Sex Med Rev. 2018;6(1):77–85. doi: 10.1016/j.sxmr.2017.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Mottet N, van den Bergh RCN, Briers E, Van den Broeck T, Cumberbatch MG, De Santis M, et al. EAU-EANM-ESTRO-ESUR-SIOG guidelines on prostate cancer-2020 update. Part 1: screening, diagnosis, and local treatment with curative intent. Eur Urol. 2021;79(2):243–262. doi: 10.1016/j.eururo.2020.09.042. [DOI] [PubMed] [Google Scholar]
- 174.Nelson TJ, Javier-DesLoges J, Deka R, Courtney PT, Nalawade V, Mell L, et al. Association of prostate-specific antigen velocity with clinical progression among African American and non-hispanic white men treated for low-risk prostate cancer with active surveillance. JAMA Netw Open. 2021;4(5):e219452. doi: 10.1001/jamanetworkopen.2021.9452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Morales A. Effect of testosterone administration to men with prostate cancer is unpredictable: a word of caution and suggestions for a registry. BJU Int. 2011;107(9):1369–1373. doi: 10.1111/j.1464-410X.2011.10193.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.