Abstract
Delirium and dementia are two frequent causes of cognitive impairment among older adults and have a distinct, complex and interconnected relationship. Delirium is an acute confusional state characterized by inattention, cognitive dysfunction and an altered level of consciousness, whereas dementia is an insidious, chronic and progressive loss of a previously acquired cognitive ability. People with dementia have a higher risk of developing delirium than the general population, and the occurrence of delirium is an independent risk factor for subsequent development of dementia. Furthermore, delirium in individuals with dementia can accelerate the trajectory of the underlying cognitive decline. Delirium prevention strategies can reduce the incidence of delirium and associated adverse outcomes, including falls and functional decline. Therefore, delirium might represent a modifiable risk factor for dementia, and interventions that prevent or minimize delirium might also reduce or prevent long-term cognitive impairment. Additionally, understanding the pathophysiology of delirium and the connection between delirium and dementia might ultimately lead to additional treatments for both conditions. In this Review, we explore mechanisms that might be common to both delirium and dementia by reviewing evidence on shared biomarkers, and we discuss the importance of delirium recognition and prevention in people with dementia.
Subject terms: Dementia, Encephalopathy
In this Review, Fong and Inouye explore mechanisms that might be common to both delirium and dementia. They present delirium as a possible modifiable risk factor for dementia and discuss the importance of delirium prevention strategies in reducing this risk.
Key points
Delirium and dementia are frequent causes of cognitive impairment among older adults and have a distinct, complex and interconnected relationship.
Delirium prevention strategies have been shown to reduce not only the incidence of delirium but also the incidence of adverse outcomes associated with delirium such as falls and functional decline.
Adverse outcomes associated with delirium, such as the onset of dementia symptoms in individuals with preclinical dementia, and/or the acceleration of cognitive decline in individuals with dementia might also be delayed by the implementation of delirium prevention strategies.
Evidence regarding the association of systemic inflammatory and neuroinflammatory biomarkers with delirium is variable, possibly as a result of co-occurring dementia pathology or disruption of the blood–brain barrier.
Alzheimer disease pathology, even prior to the onset of symptoms, might have an effect on delirium risk, with potential mechanisms including neuroinflammation and gene–protein interactions with the APOE ε4 allele.
Novel strategies, including proteomics, multi-omics, neuroimaging, transcranial magnetic stimulation and EEG, are beginning to reveal how changes in cerebral blood flow, spectral power and connectivity can be associated with delirium; further work is needed to expand these findings to patients with delirium superimposed upon dementia.
Introduction
Cognitive impairment is frequent among older adults, with delirium and dementia being two of the most common causes. Delirium refers to a disturbance in attention and awareness that is acute in onset and represents a change from baseline cognitive function1. At least one additional cognitive disturbance is required for diagnosis, for example, disturbance of memory, orientation, language, visuospatial ability or perception; fluctuations in mental status throughout the day are also present. For a diagnosis of delirium, these disturbances cannot be better explained by another neurocognitive disorder and there must be evidence that the disturbances are a consequence of a medical condition, substance intoxication or withdrawal, a toxin, or multiple aetiologies. Often precipitated by illness or hospitalization in older adults, delirium is associated with poor short-term and long-term outcomes, including prolonged length of hospital stay, institutionalization, functional and cognitive decline, and death2. In the United States, more than 2.6 million adults aged 65 years and older develop delirium each year3. The health-care costs associated with delirium are estimated at more than US$ 164 billion per year in the United States4 and over US$ 182 billion per year in 18 European countries combined5,6.
Dementia refers to a progressive loss of a previously acquired cognitive skill and can be used as an umbrella term for many types of progressive cognitive decline. Alzheimer disease (AD), which presents as a progressive loss of cognitive ability, often memory, but including language, orientation and the ability to perform daily tasks, is the most common form of dementia7. An estimated 50 million people have dementia worldwide and this figure is projected to triple by 2050 (ref.8). Associated costs are also expected to rise from the estimated US$ 1 trillion globally in 2018 to US$ 2 trillion by 2030 (ref.8).
Despite the global impact of delirium and dementia, treatment options for these common conditions are limited. For example, although symptomatic treatments for dementia are available, the benefit of these strategies has been modest for most patients9. Much effort has been devoted to finding disease-modifying drugs for dementia, but until 2021 no new dementia treatments had been approved by the FDA since 2003 (ref.10). For delirium, pharmacological treatment strategies, including haloperidol, second-generation antipsychotics11, melatonin12 or cholinesterase inhibitors13, have been tested but were not found to be effective either for prevention or treatment. Current practice guidelines reflect the consensus that drugs should not be used as a treatment for delirium2, with the exception of antipsychotic medications when behaviours pose a safety risk to patients, staff or both, or when there is a risk of interrupting essential medical care3,14.
Delirium and dementia have a complex inter-relationship. Individuals who develop delirium have a higher risk of developing dementia than the general population15,16; however, whether delirium simply serves to unmask unrecognized dementia or an overlap in the pathophysiology of delirium and dementia initiates or accelerates neurodegeneration, remains unclear. Cognitive impairment and dementia are independent risk factors for developing delirium15 and, moreover, delirium has been associated with an acceleration in long-term cognitive decline both in individuals with17–21 and without dementia22,23. Evidence indicates that people with dementia who develop delirium have longer lengths of hospital stays, greater cognitive and functional decline, and a higher risk of institutionalization and mortality than people with dementia who are hospitalized and do not develop delirium24. Delirium prevention strategies have been consistently demonstrated to be successful in reducing the incidence of delirium and adverse outcomes, such as falls, cognitive and functional decline25,26, length of hospital stay27,28, use of sitters29, institutionalization29, readmissions30, and health-care costs (hospital and 1-year), in mixed samples, including persons both with and without dementia31–35. These observations suggest that delirium prevention might also be useful in ameliorating the effect of delirium on the cognitive trajectory in dementia.
Understanding the inter-relationship between delirium and dementia might ultimately lead to more effective treatments for both conditions. Work performed over the last 5 years has led to important advances in delirium research, including harmonization of diagnostic and measurement tools, heightened awareness of delirium, widespread delirium screening, and implementation of clinical guidelines and pathways to optimize care. The development of ultrasensitive assays for the measurement of plasma biomarkers has furthered research into and understanding of delirium pathophysiology. In keeping with the importance of delirium, a number of comprehensive systematic reviews have been published in the past 3 years, most notably on delirium2, delirium prevention in dementia36, delirium in hospitalized older adults24 and delirium biomarkers37. In this Review, we focus on clinical and epidemiological aspects of delirium in people with dementia, examine the evidence for shared mechanisms between delirium and dementia, and discuss delirium prevention in persons with dementia.
Delirium in people with dementia
Clinical features
Dementia and delirium are distinct but inter-related conditions and, at times, are mistaken for each other. The occurrence of delirium in a person with dementia, known as delirium superimposed on dementia (DSD)38, is often unrecognized, making clinical diagnosis challenging. The Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) makes a point of classifying delirium as being distinct from a “pre-existing established, or evolving neurocognitive disorder”, and states that “dementia should not be diagnosed in the face of delirium”1. A key feature in distinguishing delirium from dementia is the presence of an acute change in mentation from baseline or resolution of symptoms with treatment of precipitating factors (for example, infection, dehydration, drugs). A person with AD will experience a decline in memory and cognition that is typically insidious and progressive over months to years and will have preserved consciousness. By contrast, the symptoms suggestive of delirium would include information from a proxy of an acute change in mental status over hours to days characterized by confusion and inattention, fluctuating symptoms, and altered consciousness. Table 1 is a simplified comparison of the symptoms of delirium with the most common symptoms of key types of dementia; however, the concept of DSD, discussed in more detail below, is still applicable broadly to all forms of dementia.
Table 1.
Features of delirium and dementia
| Feature | Delirium | Dementia due to Alzheimer disease | Frontotemporal lobe dementia | Diffuse Lewy body disease | Vascular dementia |
|---|---|---|---|---|---|
| Descriptive features | Inattention, impairment of immediate memory | Memory impairments, plus impairments in multiple other cognitive domains | Behavioural disorder, mental rigidity, distractibility | Fluctuating cognition with variations in attention and alertness | Abrupt deterioration or stepwise progression of cognitive deficits; mood and personality changes |
| Onset | Acute, episodic | Insidious | Insidious | Insidious | Insidious, abrupt or stepwise |
| Duration | Hours to months | Months to years | Months to years | Months to years | Months to years |
| Course | Fluctuating, might be worse at night and on waking | Chronic, progressive | Chronic, progressive | Chronic, progressive | Chronic, progressive |
| Alertness | Altered | Normal | Normal | Fluctuates | Normal |
| Reversibility | Usually | No | No | No | No |
| Attention | Impaired by definition | Usually, normal, but might be impaired in later stages | Might be persistently impaired and early feature | Fluctuates | Might be persistently impaired and early feature |
| Orientation | Fluctuates | Not oriented | Typically intact | Variable | Variable |
| Speech | Incoherent speech | Word-finding difficulties | Altered speech output; stereotypy of speech; echolalia; perseveration; mutism | Hypophonic speech | – |
| Thought | Disorganized and disconnected thoughts, for example, ‘flight of ideas’ | Difficulty with abstract thinking | Poor judgement; impulsivity | – | Abnormal executive function, including mental rigidity and poor insight and judgment |
| Perception | Distorted: illusions, delusions and/or hallucinations (often visual, tactile or poorly formed) | Delusions of theft or persecution, more common in later stages; hallucinations (auditory, distinct) uncommon | Delusions might be paranoid, religious or bizarre in nature | Visual hallucinations are recurrent and typically well-formed and detailed (that is, animals or children); delusions are common | Delusions more common in later stages |
| Psychomotor changes | Frequent | Inconsistent | Hyperorality; utilization behaviour | Parkinsonism | Psychomotor retardation |
| Agitation | Occurs with delirium symptoms, throughout the day | Might occur with sundowning or when resisting activities of daily living | Common | Variable | Variable |
| Sleep–wake cycle | Often reversed | Might be fragmented but circadian rhythmicity retained | Severely fragmented | REM sleep behaviour disorder | Sleep disturbances are common |
Epidemiology
In 2017, a large (n = 1,409) prospective cohort study of hospitalized adults over the age of 60 found the prevalence rate of DSD to be 31%39, whereas a 2021 meta-analysis of 81 studies, including 81,536 people with dementia, reported a pooled DSD prevalence of 48.9% in patients with dementia during hospitalization24. Nevertheless, the reported prevalence rates of DSD across studies are highly variable owing to differences in diagnostic approaches used, the overlap of symptoms between delirium and dementia, and differences in study populations (for example, higher rates are observed in populations with older ages and in medical versus surgical populations), prospective versus retrospective approaches to diagnosis and other aspects of study methodology24,39. By extrapolating from many studies, we previously estimated that delirium will develop in 1 in 2 to 1 in 5 patients with dementia who are hospitalized, which translates to a 3–4 times higher risk of delirium than patients without dementia40. Regardless of the variation in prevalence estimates, DSD is common and, as the global population ages41, the diagnosis and treatment of this condition are likely to emerge as serious challenges. Indeed, in one study, patients with DSD had a 2.6-fold higher risk of in-hospital death than patients without cognitive impairment (neither delirium nor dementia), whereas dementia alone was not associated with a statistically significant increased risk of in-hospital death (1.5-fold higher than patients without cognitive impairment; non-significant)42.
Despite being a common syndrome, DSD often goes undetected or is misattributed to underlying dementia, which contributes, at least in part, to the wide range of prevalence rates reported across studies. Furthermore, these numbers might substantially underestimate the true prevalence, particularly when people have a form of dementia for which the symptom profile overlaps with that of delirium, for example, diffuse Lewy body disease, in which symptom fluctuations are common38. Furthermore, the formalized criteria for the diagnosis of delirium, for example, those given in the DSM-5 (ref.1), do not include specific cognitive tests, criteria, or guidance for diagnosis of delirium in the setting of dementia or pre-existing cognitive impairment, which adds to the difficulty of diagnosing DSD.
Diagnosis
The results of a survey among clinicians who encounter DSD showed that, despite individual clinician confidence in recognizing DSD, global consensus in assessment and diagnosis is lacking43. Baseline cognitive status is often unknown in older adults upon hospital admission. If no established diagnosis of dementia is available in the medical record or from a family member, a screening tool, such as the Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE)44, can be completed by a proxy who has known the patient for the past 10 years. Alternatively, the Eight-item Interview to Differentiate Aging and Dementia (AD8) can be used with a proxy that knows the patient well and can rate change over the past several years45. Although many delirium screening tools are available2, relatively few have been assessed in formal studies that used large cohorts and controlled for dementia subtype or severity46. Standard diagnostic criteria for delirium, such as those in the DSM-5 or the International Classification of Diseases, Tenth Revision (ICD-10), are limited in their application to DSD as neither provides specific recommendations for tests to assess attention, cognition and level of consciousness38. Furthermore, the variability and progressive decline in cognitive impairment among people with dementia present a unique challenge in selecting an appropriate test to measure cognitive impairment; substantial overlap of symptoms in delirium and dementia can also occur (as mentioned above and described in Table 1).
One instrument explicitly modified to identify delirium in the presence of dementia is the 4-DSD47, which is a 4-item scale based on the 4-AT (Arousal, Attention, Abbreviated Mental Test-4, Acute change)48. The 4-DSD has a range of 0–12; higher scores suggest the presence of delirium. However, the psychometric properties of the 4-DSD vary depending on the severity of the cognitive impairment47, and high scores on some items in the 4-DSD, such as unawareness, presence of sleep–wake disorder and inattention, might be a result of the underlying dementia process and not the delirium. Furthermore, symptoms such as agitation, aggression or psychomotor retardation, which can occur in both delirium and dementia, are not used in the assessment. Other instruments include the Confusion Assessment Method (CAM)49 and the 3D-CAM50, a brief diagnostic tool derived from the CAM algorithm. These two tools have had relatively limited testing in people with dementia50 but are flexible in the type of assessment used to score the items; for example, attention can be assessed using the months of the year backwards test or simple counting, depending on the severity of cognitive impairment. More recent strategies use brief assessments, which are better tolerated by persons with dementia, to quickly exclude delirium. These strategies include the ultra-brief CAM (UB-CAM)51 or the months of the year backwards test52, where delirium is associated with the inability to engage and complete the task. For all of these tests, the features most crucial to identifying DSD are establishing baseline status and recognizing the presence of an acute change in symptoms or behaviours.
Delirium can also vary in severity. Instruments measuring delirium severity, such as the Memorial Delirium Assessment Scale53, the Delirium Rating Scale–revised-98 (ref.54) or the CAM-S long form55, also face the same challenges as delirium screening tools as symptoms of dementia can affect these severity measurements. Evidence indicates that the CAM-S short form55 is less subject to these biases than the CAM-S version, and might be a better measure of delirium severity in this setting. Ultimately, future research is needed to develop reliable screening tests and reference standards for diagnosing DSD38 and measuring delirium severity in persons with dementia.
Delirium as a modifiable risk factor for dementia
Evidence indicates that delirium is associated with the acceleration of cognitive decline in people with dementia20,21. Results from the Delirium and Cognitive Impact in Dementia (DECIDE) study, published in 2021, found that delirium among adults over the age of 65 years was associated with cognitive decline. This decline consisted of an average 1.8-point reduction on the Mini-Mental State Examination (95% CI –3.5 to –0.2) and an increased risk of new dementia diagnosis at the 12-month follow-up (OR 8.8, 95% CI 1.9–41.4) in individuals with delirium compared with individuals without delirium16. This study also found that greater delirium exposure (that is, recurrent episodes, or episodes of greater severity or longer duration) was associated with a greater risk of dementia and worse cognitive outcomes. A recent meta-analysis also reported a significant association between delirium and long-term cognitive decline (OR 2.30, 95% CI 1.85–2.86)56. These observations further support an association between delirium and subsequent cognitive decline and dementia. However, whether a causal mechanism underlies this observed association is not yet clear. If a causal relationship between delirium and dementia is identified, delirium might be a modifiable risk factor for dementia. An important next step will be to further explore the mechanisms that are shared by both delirium and dementia57.
Delirium and dementia: shared biomarkers
Biomarkers are playing an increasingly important role in advancing the mechanistic understanding of delirium. To date, most of the fluid biomarker studies in delirium and dementia have used cerebrospinal fluid (CSF) or blood (plasma or serum). Although CSF is thought to more accurately represent processes occurring in the brain, the collection of CSF, particularly in people with delirium, over multiple time points or in large-scale studies holds particular challenges58–62. Blood-based biomarkers are more accessible during acute illness, but plasma concentrations of biomarkers are often very low. Fortunately, with the development of ultrasensitive immunoassays, the detection of femtomolar concentrations of protein analytes has made it possible to examine a much wider range of potential biomarkers.
A previous systematic review identified 113 delirium biomarker studies that aligned with the National Institute on Aging – Alzheimer’s Association (NIA-AA) research framework63, which defines AD using biomarkers that reflect underlying pathological processes64. Many of these studies focused on inflammatory cytokines and about 20% of studies explored known AD biomarkers such as amyloid, tau and/or markers of neurodegeneration. A subsequent systematic review of biomarkers of delirium, published in 2021, found insufficient evidence to support the use of any single biomarker as a diagnostic or prognostic marker for delirium37; however, a number of biomarkers did show promise for providing a better understanding of the pathophysiology of delirium.
Our intention for this review is to focus on the interface between delirium and dementia and, in our search for biomarkers shared between delirium and dementia, we opted for a direct approach by reviewing studies of biomarkers in delirium that either involved some participants with AD, including preclinical AD (that is, asymptomatic individuals identified by the presence of AD biomarkers), or that measured traditional AD biomarkers (see review criteria for details). We chose to concentrate on AD as it is the most common type of dementia7 and the one for which the most evidence exists on the inter-relationship with delirium. The underlying pathophysiology of delirium is complex, with evidence supporting the involvement of a number of potential mechanisms, including neurodegeneration and neuronal injury, inflammation, disturbances in brain energy metabolism, disruption in neurotransmitter function, pharmacological effects, and failure of network connectivity2. Other pathways, including cortisol and the stress pathway, melatonin, and disruption of the sleep–wake cycle as well as the dopamine and cholinergic pathways, are also likely to contribute to the pathophysiology of delirium, but the specific contributions of these pathways2,65 to the inter-relationship between delirium and dementia have not been explored in detail and were thus considered to be outside the scope of this Review.
Systemic inflammation
Systemic inflammation is thought to have a key role in the pathogenesis of delirium (see ref.2 published in 2020 for a comprehensive review); however, studies have reported variable associations between plasma concentrations of the inflammatory markers CRP and IL-8 and risk of delirium66–71. A study by McNeil et al., which included participants with and without clinical dementia, found that plasma concentrations of IL-6, IL-8 and plasminogen activator inhibitor-1 (PAI-1) were associated with duration of delirium, but only among participants who did not have clinical dementia66 (Table 2). A possible explanation for this finding is that people with dementia are more vulnerable to delirium than people without dementia. Therefore, people with dementia might become delirious without the occurrence of a substantial increase in inflammatory markers, whereas people without dementia might require a higher degree of systemic inflammation and endothelial dysfunction to develop delirium. In contrast, a single post-mortem study found higher levels of IL-6 in the brains of individuals with delirium than in the brains of individuals without delirium, and the presence of coexisting dementia did not affect these findings72.
Table 2.
Overview of potential plasma biomarkers shared between delirium and dementia
| Biomarker | Cohort size | Findings | Ref. | |||
|---|---|---|---|---|---|---|
| No delirium, no dementia | Dementia only | Delirium only | DSD | |||
| Systemic inflammation | ||||||
| CRP | 421 (80 with APOE ε4; 341 without APOE ε4) | 0 | 132 (26 with APOE ε4; 106 without APOE ε4) | 0 | In presence of APOE ε4, higher CRP was associated with delirium | 68 |
| IL-6 | 69 | 23 | 16 | 48 | Higher IL-6 was associated with longer ED delirium duration, but only in participants without dementia | 66 |
| IL-6, IL-8 | 48 | 10 | 22 | 40 | Higher S100B associated with delirium; S100B correlated with IL-6 and IL-8 | 101 |
| AD biomarkers | ||||||
| Aβ1–40, Aβ1–42, t-tau, p-tau181 | 72 | Excluded | 38 | 0 | Higher plasma tau was associated with delirium incidence and severity | 89 |
| t-tau, p-tau217, p-tau181 | 22 | Not specified | 16 | NA | t-tau, p-tau217 and p-tau181 were elevated after major cardiac surgery; only t-tau was associated with the incidence and severity of postoperative delirium; models adjusted for baseline cognition (MoCA) | 166 |
| Genetic factors | ||||||
| APOE ε4 | 6 (APOE ε4 not reported) | 7 (includes both dementia only and DSD; 2 with APOE ε4) | 47 (APOE ε4 not reported) | Not reported | Presence of at least one APOE ε4 allele was associated with longer delirium duration | 93 |
| 117 (no delirium but might have had dementia) | 82 (includes both dementia only and DSD) | 45 | Not reported | APOE genotyping was performed in 116 participants. At least one APOE ε4 allele was present in 26 participants, with no difference in frequency between delirium and no delirium groups; lower IGF1 and absence of the APOE ε4 allele predicted recovery from delirium; APOE ε4 alone did not show an effect on recovery | 70 | |
| 161 (33 with APOE ε4) | 0 (60 with ‘history of CNS disorder’) | 29 (13 with APOE ε4) | Not reported | Presence of at least one APOE ε4 allele was associated with an increased risk of early postoperative delirium | 94 | |
| Neuronal injury | ||||||
| NfL | 114 | 38 | 46 | 116 | Higher NfL concentration was associated with delirium | 103 |
| S100B | 0 | 20 | 20 | 0 | No association between serum S100B concentration and delirium | 102 |
| 48 | 10 | 22 | 40 | Higher S100B concentration was associated with delirium | 101 | |
| Other | ||||||
| PAI1 | 69 | 23 | 16 | 48 | Higher PAI1 was associated with longer ED delirium duration, but only in participants without dementia | 66 |
| IGF1 | 117 (no delirium but may have had dementia) | 82 (includes both dementia only and DSD) | 45 | Not reported | Delirium associated with lower IGF1 | 70 |
| Diazepam-binding inhibitor | 15 | 30 | 30 | 0 | Higher levels in individuals with dementia than in control individuals; higher levels in individuals with delirium than in individuals with dementia | 111 |
Note that studies differ in methods and reporting standards, definitions and measures for delirium and dementia used, varying study populations, and presence of different comorbidities. Therefore, we report only positive or negative associations and not effect sizes, which were not directly comparable across studies. Aβ, amyloid-β; AD, Alzheimer disease; DSD, delirium superimposed on dementia; ED, emergency department; IGF-1, insulin-like growth factor-1; MoCA, Montreal Cognitive Assessment; NA, not available; NfL, neurofilament light; PAI-1, plasminogen activator inhibitor-1; p-tau, phosphorylated tau; t-tau, total tau.
In animal studies, ample evidence indicates that systemic (peripheral) inflammation contributes to chronic neurodegenerative disorders via the activation of brain microglial cells73,74. In one study, wild-type mice and a mouse model of progressive neurodegeneration (ME7 prion disease) were challenged with the cytokine TNF75. Compared with wild-type mice, ME7 mice had increased sickness behaviour (considered to be an animal variant of delirium), impaired working memory performance, and higher levels of hippocampal and hypothalamic transcription of IL-1β, TNF, and CCL2 and translation of IL-1β. However, TNF did not result in substantial de novo pathology beyond the baseline level of neurodegeneration in ME7 animals75. In the APP/PS1 mouse model of AD, which expresses human amyloid precursor protein and a mutant version of human presenilin-1, secondary inflammatory insults (that is, infection or lipopolysaccharide challenge) were associated with an acute increase in production of IL-1β by microglia, which in turn triggered exaggerated levels of astrocytic chemokines and IL-6 (ref.76).
In sum, the results of these studies suggest that the association between inflammatory markers and delirium is variable and that, in the presence of dementia pathology, the brain might be more vulnerable to developing delirium. The findings also support an association between systemic inflammation and neurodegeneration; however, systemic inflammation might affect the relationship between delirium and dementia in more than one way.
Neuroinflammation
Neuroinflammation is recognized as a prominent feature of AD pathology77. Microglial cells, the resident phagocytic cells of the brain, have many roles, including neuronal support, synaptic modulation and reorganization of neuronal circuitry. A post-mortem case–control study identified an increase in markers of microglial activity (HLA-DR and CD68) and astrocytosis (GFAP) in the brains of individuals with delirium compared with individuals without delirium; coexisting dementia did not affect this relationship72. The concentration of soluble TREM2 (triggering receptor expressed on myeloid cells 2) in the CSF is a surrogate measure of microglial function and has been found to be increased in prodromal and asymptomatic AD, with levels peaking in the mild cognitive impairment (MCI) stage, then declining during clinical dementia stages78. Similar to the IL-6 findings in the study by McNeil et al.66, a study by Henjum et al. found that higher CSF levels of soluble TREM2 were associated with a greater likelihood of delirium, but only among participants without clinical AD79. In this case, Henjum et al. speculated that people with clinical AD have constant activation of microglia by Aβ deposits and tau inclusions, and therefore the effect of delirium on microglial response is attenuated and the association is not observed (Table 3).
Table 3.
Overview of potential CSF biomarkers shared between delirium and dementia
| Biomarker | Cohort size | Findings | Ref. | |||
|---|---|---|---|---|---|---|
| No delirium, no dementia | Dementia only | Delirium only | DSD | |||
| Systemic inflammation | ||||||
| IL-8 | 176 | 83 | 19 | 53 | Among patients with HF without dementia, preoperative IL-8 levels were significantly higher in those who developed delirium than in those who did not; both cognitively healthy controls and patients with dementia had significantly lower levels of IL-8 than patients with HF | 167 |
| Total protein | 0 | 20 | 20 | 0 | Higher protein levels in participants with delirium than in participants with dementia | 102 |
| Neuroinflammation | ||||||
| (sTREM2) | 44 | 10 | 15 | 50 | In patients with HF, higher levels of CSF sTREM2 in delirium, but only in participants without pre-existing dementia | 79 |
| AD biomarkers | ||||||
| Aβ1–42 | 242 | 0 | 40 | 0 | Higher CSF Aβ1–42 concentration predicted delirium | 87 |
| Aβ1–42, t-tau, Aβ1–42 to t-tau ratio, Aβ1–42 to p-tau ratio | 49 | 10 | 16 | 54 | Higher t-tau concentration and lower Aβ1–42, Aβ1–42 to t-tau ratio, and Aβ1–42 to p-tau ratio were associated with delirium, but only in individuals without dementia | 88 |
| Aβ1–40, Aβ1–42, t-tau, p-tau181 | 93 | 33 | 26 | 47 | AD biomarkers were not associated with postoperative delirium | 91 |
| Aβ1–42 to t-tau ratio | 122 | Excluded | 31 | NA | Lower Aβ1–42 to t-tau ratio was associated with a greater likelihood of delirium | 90 |
| ATNa | 53 | 0 | 6 | 0 | Preclinical AD biomarkers (presence of amyloid) was associated with greater delirium severity | 168 |
| Neuronal injury | ||||||
| NfL | 114 | 38 | 46 | 116 | Higher NfL concentration was associated with delirium | 103 |
| S100B | 83 (HF); 50 (ES) | 49 (HF); 0 (ES) | 52 (HF); 0 (ES) | 39 | Among patients with pathological levels of p-tau, an increase in S100B concentration was observed in patients with incident delirium compared with patients with no delirium | 99 |
| Lactate | 0 | 20 | 20 | 0 | Patients with delirium had higher lactate levels than patients with dementia | 102 |
| NSE | 0 | 20 | 20 | 0 | Patients with delirium had lower levels of NSE than patients with dementia | 102 |
| FABP3 | 171 | 10 | 16 | 55 | No association with delirium; CSF FABP3 concentration was correlated with t-tau and p-tau levels | 108 |
| Other | ||||||
| Neurogranin | 175 | 10 | 18 | 52 | No association with delirium | 110 |
| Proteomics and metabolomics | ||||||
| Apolipoproteins and chromogranin and secretograninsb | 8 | 17 | 17 | 0 | Discovery proteomics study; identified upregulation of inflammatory proteins and downregulation of apolipoproteins and chromogranin and secretogranins in delirium compared with AD | 112 |
| Spermidine, glutamine, putrescinec | 26 | 0 | 28 | 0 | Targeted metabolomic study; elevated preoperative CSF spermidine, glutamine and putrescine predicted delirium; spermidine concentration was negatively correlated with Aβ1–42 | 113 |
Note that studies differ in methods and reporting standards, definitions and measures for delirium and dementia used, varying study populations, and presence of different comorbidities. Therefore, we report only positive or negative associations and not effect sizes, which were not directly comparable across studies. AD, Alzheimer disease; Aβ, amyloid-β; CSF, cerebrospinal fluid; DSD, delirium superimposed on dementia; ES, elective surgery; FABP3, fatty acid-binding protein 3; HF, hip fracture; NA, not available; NfL, neurofilament light; NSE, neuron-specific enolase; p-tau, phosphorylated tau; sTREM2, soluble fragment of triggering receptor expressed on myeloid cells; t-tau, total tau. aThe ATN biomarker framework is used to distinguish AD from non-AD causes of cognitive impairment with three types of biomarkers: β-amyloid deposition (A), pathological tau (phosphorylated tau, T) and neurodegeneration (total tau, N)64. bSecretogranins associated with neurodegeneration. cPutrescine is elevated in AD and might be involved in amyloid plaque formation.
Disruptions to blood–brain barrier (BBB) integrity allow systemic inflammatory signals to reach the brain and have been observed in individuals with dementia80. One study assessed BBB disruption, as measured by Q-albumin (the ratio of CSF albumin to serum albumin), in a cohort of 120 patients with hip fracture. Ninety-one patients (76%) developed delirium or sub-syndromal delirium, of whom 59 (65%) had underlying dementia. No significant difference in the rate of BBB disruption was observed between the group of participants with dementia and those without dementia. However, all patients with BBB disruption (n = 14) had delirium (n = 11) or sub-syndromal delirium (n = 3), suggesting that this disruption might contribute to the development of delirium81. In a number of animal studies, orthopaedic surgery was associated with increased neuroinflammation, disruption of the BBB and impaired performance on tests of attention82,83. In mice, treatment with a broad spectrum anti-inflammatory agent was found to prevent surgery-induced BBB disruption, microglial activation and memory dysfunction84.
In summary, neuroinflammation is associated with both dementia and delirium separately. Similar to the data regarding systematic inflammation (discussed above), the influence of neuroinflammation on delirium in the presence of dementia pathology seems to be variable. Emerging data also support the hypothesis that BBB disruption is present in both delirium and dementia, which would enable more systemic inflammatory signals to reach the brain and exert their effects.
AD biomarkers and apolipoprotein E
The levels of amyloid-β (Aβ1–42), total tau (t-tau) and phosphorylated tau (p-tau) in the CSF are known to reflect key elements of AD pathology, including the presence of extracellular Aβ plaques and intracellular neurofibrillary tangles (consisting of hyperphosphorylated tau protein) in cortical and limbic areas of the human brain85. AD is associated with lower CSF Aβ1–42 levels and higher CSF tau levels86. These biomarkers have a central position in the 2018 NIA-AA biological definition of AD and AT(N) classification system, which groups biomarkers into those that reflect Aβ deposition (A), pathological tau (T) and neurodegeneration (N)64. Studies that examined these AD biomarkers in individuals with delirium have produced mixed results, but associations between delirium and CSF levels of tau and Aβ have been reported (Table 3).
In one study, low concentrations of Aβ1–42 in CSF after elective hip surgery were predictive of delirium in patients without dementia87. Similarly, in another study of patients with hip fracture without dementia, lower CSF Aβ1–42 and higher CSF t-tau levels were present in participants that developed delirium compared with participants that did not develop delirium88. This observation suggests that preclinical AD brain pathology is relevant to and might have a role in delirium pathophysiology. In a prospective observational cohort of older patients undergoing surgery, plasma p-tau concentrations were higher after surgery than before surgery, and this increase was significantly larger in participants with delirium than in participants without delirium89. After adjusting for age, sex, preoperative cognition and change in plasma IL-8 levels, the association between plasma p-tau concentration and delirium severity remained statistically significant. Another study reported that individuals with a lower CSF Aβ1–42 to tau ratio, which is indicative of greater AD pathology, were more likely to have postoperative delirium than individuals with a higher CSF Aβ1–42 to tau ratio90. One study identified an association between depression and an increased likelihood of postoperative delirium in older adults undergoing hip fracture repair91. In this study, CSF Aβ1–42 to t-tau and Aβ1–42 to p-tau181 ratios were inversely associated with higher depression scores, suggesting that depression is associated with underlying AD pathology and postoperative delirium. In contrast, another study of older adults with hip fracture did not find significant differences in preoperative concentrations of Aβ1–42, t-tau and p-tau in the CSF between participants with and without delirium92 (Tables 2 and 3).
The ε4 allele of the gene encoding apolipoprotein E (APOE) is a known risk factor for AD, but whether it is also a risk factor for delirium is unclear. A number of studies have identified an association between APOE ε4 and the risk of delirium93–95, whereas other studies found no such association70,96. In one study of older adults without dementia undergoing elective surgery, a strong relationship between a high concentration of plasma CRP and delirium incidence was identified in carriers of at least one APOE ε4 allele, but this association was not observed among non-carriers68. This finding suggests that gene–protein interactions might modify inflammation, thereby representing an indirect link between delirium and dementia. A study of patients undergoing surgical repair of hip fracture found that lower plasma levels of IGF-1 and absence of the APOE ε4 allele predicted delirium recovery70 (Table 2).
Animal models have been used to test the hypothesis that postoperative delirium results from disruption of the BBB by neuroinflammation and neurovascular dysfunction and that the brain is more susceptible to such changes in the setting of pre-existing AD pathology. One model that has been used for this purpose is the APPSwDI/NOS2−/− mouse line, which is a cross between a line of mice that develops Aβ protein deposits and a line of mice with nitric oxide synthase 2 (NOS2) knockout. This model, also referred to as the CVN-AD model, has a phenotype of impaired spatial memory, extensive tau pathology, dense microvascular amyloid plaques, and statistically significant neuron loss in the hippocampus and subiculum97. In one study, tibial fracture surgery was performed on 12-month-old CVN-AD mice. Compared with naive mice, mice that had undergone surgery had distinct neuroimmune and vascular impairments, including increased levels of neuroinflammatory markers, such as G‐CSF, IL‐6, IL-1β, TNF, monocyte chemoattractant protein 1 (MCP1) in plasma, acute microgliosis in the hippocampus and cortex, and Aβ deposition in the hippocampus98. Increased expression of aquaporin 4 (AQP4), a marker of neurovascular dysfunction, was also observed in mice that had undergone surgery. These changes were accompanied by impaired performance on a serial reaction time task, a measure of attention and a core feature of delirium-like behaviour in animal models.
The results of the studies discussed here suggest three possible relationships between delirium and dementia. First, most but not all studies support an association between the presence of AD biomarkers and delirium incidence. This suggests that underlying AD pathology, even in the absence of cognitive impairment (that is, preclinical dementia), might influence the development of delirium. Second, work examining the relationship between APOE genotype and delirium suggests that there might be gene–protein interactions that modify inflammation in patients with the APOE ε4 allele who develop delirium. Last, evidence from the CVN-AD animal model further supports a role of neuroinflammation in both delirium and dementia.
Neuronal injury
Emerging data from studies of patients with delirium and patients with DSD indicate an association between neuronal injury markers and delirium, but whether neuronal injury creates a permissive ‘condition’ for delirium occurrence, or is a consequence of delirium, is not yet known. Early work focused on plasma levels of S100B, which is a calcium-binding protein that is found primarily in astrocytes and can easily cross the BBB, and neuron-specific enolase (NSE), which is a cytoplasmic enzyme found in neurons and neuroendocrine cells. This work yielded mixed results, with some studies finding associations between elevated serum S100B and delirium99–101 and others finding no association between CSF S100B and delirium102. One study that did find an association between S100B and delirium observed this association only in participants with p-tau levels consistent with dementia99. In another study, serum S100B levels were associated with delirium but also correlated with levels of IL-6 and IL-8, suggesting that neuroinflammation owing to delirium could be related to neuronal injury101.
In a study published in 2018, CSF levels of neurofilament light (NfL), a marker of neuroaxonal injury, were higher among patients with hip fracture who developed delirium than those who did not develop delirium103. Whether this increase in NfL is a result of underlying neurodegeneration or secondary to a delirium-associated reaction that triggers neuronal injury is unclear. A more recent study of older adults undergoing elective surgery found that plasma NfL levels increased gradually during the 4 days after surgery in all patients, although the increase in NfL was more profound in patients with delirium104. We also performed a study in a cohort of older adults without dementia undergoing elective surgery and found that patients who had higher levels of plasma NfL before surgery were more likely to develop delirium105. Furthermore, those with the highest plasma NfL levels at baseline had more severe delirium than those with the lowest plasma NfL levels. Compared with baseline, plasma NfL levels were increased on postoperative day 1 and this increase was more pronounced in patients with delirium than in patients without delirium. At 1 month after surgery, plasma NfL levels remained elevated, and higher NfL levels were associated with a greater likelihood of delirium during hospitalization and a greater degree of cognitive decline at the 1-month time point105. These findings suggest that NfL could be useful as a predictive biomarker for delirium risk and long-term cognitive decline and, once confirmed, would provide pathophysiological evidence for neuroaxonal injury after delirium. However, these results should be interpreted with caution as increased levels of plasma NfL can also arise from damage to the peripheral nervous system106 as well as general anaesthesia and surgery107.
In a study of patients with hip fracture, increased levels of lactate and reduced levels of NSE in the CSF were observed in individuals with delirium compared with individuals with dementia102. Lactate production is increased by impaired tissue oxygenation, and NSE, an isoform of the glycolytic enzyme enolase, leaks into the extracellular space during cellular injury or death. The authors of the study speculated that, in delirium, changes in metabolism result in increased levels of CSF lactate, which induces secondary suppression of NSE levels, and that lactate has a greater role in delirium than NSE. Fatty acid-binding protein 3 (FABP3) is a cytoplasmic transport protein for fatty acids and other lipophilic substances. FABP3 has been linked to metabolic and inflammatory pathways and is considered a non-specific marker of neurodegeneration. FABP3 is released into the CSF in neurodegenerative disorders and after brain injury, and CSF levels were found to be elevated after hip fracture in older adults108. However, FABP3 levels were not associated with delirium. These results suggest that neuronal injury reflected by FABP3 might be distinct from processes involved in delirium108 (Tables 2 and 3).
In summary, the data on injury markers — neuronal, astrocytic and glial — are mixed. Brain cell injury might increase the risk of delirium, and delirium itself might then lead to additional injury and release of injury markers. The presence of underlying neuroinflammation or AD pathology might also be associated with cellular injury, suggesting that multiple injury pathways are involved in the relationship between delirium and dementia (Table 2). Further studies are needed to establish the presence of a causal relationship between delirium, neuronal injury and cognitive impairment.
Other
In a study published in 2019, PAI-1, which promotes fibrinolysis and contributes to endothelial dysfunction, was measured in the plasma of a group of older adults admitted to hospital. Higher PAI-1 levels were associated with longer duration of delirium, but only among patients without dementia66. This observation suggests that, similar to the findings regarding IL-6, delirium might be precipitated by lower levels of PAI-1 in people with dementia than in people without dementia. Neurogranin is a postsynaptic calmodulin-binding protein commonly found in the hippocampus and cerebral cortex and involved in synaptic regeneration and plasticity. A meta-analysis found that CSF neurogranin concentration can predict cognitive decline in individuals with MCI109; however, in a study of patients with hip fracture with and without delirium and patients with AD, CSF levels of neurogranin were not associated with delirium or with DSD110. Diazepam-binding inhibitor has been proposed as a biomarker of neuroinflammation as it binds to translocator protein (TSPO) and reflects microglial activation and neuroinflammation111. In a small study, the average CSF level of diazepam-binding inhibitor was higher in patients with dementia than in healthy controls and higher in patients with delirium than in patients with dementia111 (Table 3). Further study of these potential mechanisms is required.
Proteomics and metabolomics
Proteomic and metabolomic studies can either use an exploratory, non-hypothesis-driven approach or a targeted approach that assesses the expression of genes or proteins that are already known to be involved in the pathogenesis of a disease. In one exploratory proteomic project, investigators compared CSF from participants with delirium with CSF from participants with mild AD. Delirium was associated with increased levels of proteins involved in neurodegeneration and inflammation, such as α1-acid glycoprotein, α2-macroglobulin and fibrinogen112, and decreased levels of chromogranins and secretogranins. α1-Acid glycoprotein upregulation was confirmed by ELISA. The researchers also compared the control participants with mild AD to those with moderate AD and found that many of the proteins dysregulated in delirium were unaffected in moderate AD, suggesting that there are distinct pathways occurring in delirium and dementia. A targeted metabolomic study of preoperative CSF samples found that polyamines, specifically spermidine, glutamine and putrescine, were elevated in patients who developed postoperative delirium compared with those who did not113. Although this study excluded patients with clinical dementia, other evidence indicates that polyamines are also elevated in AD and might be involved in the formation of Aβ plaques114,115. Future proteomic and metabolomic studies could identify biomarkers and pathways that are associated with the inter-relationship between delirium and dementia (Table 3).
Neuroimaging
Structural and functional neuroimaging biomarkers have been studied extensively in dementia and an increasing number of studies have examined these markers in delirium in older adults without dementia116. A growing number of studies in the past several decades have identified predictors of delirium, such as underlying brain atrophy117 and white matter intensities118,119, or correlates of delirium such as reduced cerebral flood flow120,121 and changes in functional connectivity122. The ‘AD signature’ refers to the reduction in cortical thickness in a specific set of brain regions that has been used as a structural biomarker of AD and is associated with cognitive decline and progression to dementia123. One study examined the link between delirium and the AD signature by performing preoperative MRI scans in a cohort of older adults undergoing elective surgery124. A thinner cortex in the AD signature regions did not predict delirium incidence, although cortical thinning was associated with greater delirium severity among those who developed delirium. This finding suggests that cortical atrophy, possibly as a result of underlying neurodegeneration owing to preclinical AD, might serve as a vulnerability factor that increases severity once delirium occurs.
Functional network connectivity studies have identified lower connectivity strength and network efficiency in patients with AD125 or with amnestic MCI126 compared with healthy controls as well as a loss of efficiency and local clustering in individuals with delirium compared with those without delirium127. These observations support the hypothesis that brain network dysconnectivity is a final common pathway for delirium122,128. A limited number of studies have performed neuroimaging in individuals with DSD. In one study, each participant underwent an [18F]fluoro-2-deoxyglucose PET scan during the delirium episode and again after hospital discharge and resolution of delirium. Global hypometabolism was observed during delirium whereas, after delirium resolution, higher metabolism was observed globally and specifically in the posterior cingulate cortex129. Anatomically, the posterior cingulate cortex is important as a central node in the default network and is involved in diverse brain functions, including cognition, attention and arousal130. Studies have reported early amyloid deposition131 and reduced metabolism132 in this region in individuals with AD. Another study reported increased functional connectivity between the posterior cingulate cortex and dorsolateral prefrontal cortex and reversible reduction of functional connectivity of subcortical regions in participants with ongoing delirium compared with participants without delirium and participants who had recovered from delirium122. In a small study (n = 16) of older patients with hip fracture without dementia, participants who experienced postoperative delirium (n = 5) all had negative PET-amyloid findings and 6 of the 11 participants without postoperative delirium had positive PET-amyloid findings on scans acquired 3–5 months after recovery from surgery133 (Table 4). These findings suggest that preclinical amyloid pathology does not contribute to an increased risk of delirium after non-elective surgery, and further study is warranted.
Table 4.
Overview of potential biomarkers shared between delirium and dementia: neuroimaging and neurophysiological studies
| Biomarker | Cohort size | Findings | Ref. | |||
|---|---|---|---|---|---|---|
| No delirium, no dementia | Dementia only | Delirium only | DSD | |||
| Neuroimaging | ||||||
| 2-[18F]Fluoro-2-deoxyglucose PET | 0 | 4 | 13 | 4 | Global cortical hypometabolism was observed during delirium (n = 13); post-delirium (n = 6) greater glucose metabolism was observed in the whole brain and bilateral PCC compared with during delirium | 129 |
| [18F]Flutemetamol PET | 11 | 0 | 5 | 0 | Participants with delirium had no evidence of amyloidosis, whereas 6 of 11 control participants did have evidence of amyloidosis | 133 |
| Structural MRI | 11 | 0 | 5 | 0 | Compared with controls, participants with delirium had reduced grey matter volumes and white matter integrity in the right temporal and bilateral medial frontal areas | 124 |
| 113 | 0 | 32 | 0 | Patients who had thinner cortex in ‘AD signature’ regions had greater delirium severity | ||
| Resting-state functional MRI | 22 | 0 | 22 (14 scanned again after resolution of delirium) | 0 | Increased functional connectivity between the PCC and DLPFC and reversible reduction of functional connectivity of subcortical regions was observed in delirium | 122 |
| Other imaging | ||||||
| Cerebral hypoperfusion (transcranial doppler) | 14 | 10 | 12 | 8 | Flow velocity was lower in participants with DSD than in participants with acute illness without delirium or dementia; flow velocity was lower in participants with DSD than in participants with either AD or delirium alone | 120 |
Note that studies differ in methods and reporting standards, definitions and measures for delirium and dementia used, varying study populations, and presence of different comorbidities. Therefore, we report only positive or negative associations and not effect sizes, which were not directly comparable across studies. AD, Alzheimer disease; DLPFC, dorsolateral prefrontal cortex; DSD, delirium superimposed on dementia; PCC, posterior cingulate cortex.
In summary, neuroimaging biomarkers have identified both structural and functional predictors of delirium. Some but not all neuroimaging markers of AD were also associated with delirium. The results of a functional imaging study of patients with DSD suggest that changes occur in network connectivity, particularly the posterior cingulate cortex, during an episode of delirium.
Transcranial magnetic stimulation, Doppler and EEG
A number of novel, innovative biomarkers of brain activity have shown promise in understanding the interface of delirium and dementia. Transcranial Doppler is a non-invasive technique that uses ultrasound to measure the velocity of flow through blood vessels in the brain. One study used this approach to measure flow velocity (FV) in the middle cerebral artery in individuals with DSD, dementia or delirium as well as a cognitively healthy control group120. Compared with the other groups, statistically significant reductions in FV were observed in participants with delirium or DSD, with the lowest FV observed in the group of participants with DSD. Of note, among participants with delirium, FV increased once the delirium resolved.
Changes in EEG spectral power and connectivity — specifically, EEG slowing characterized by increases in delta and theta band frequencies and decreases in alpha band frequencies — have been identified in patients with preclinical AD134, MCI135 and AD136,137 in comparison with cognitively healthy controls. A systematic review of the literature on EEG and delirium reported that delirium in adults is consistently associated with EEG slowing and decreased alpha band EEG connectivity127,138. Similarly, a study identified a correlation between intraoperative frontal alpha power and preoperative cognitive function in older adults139, and an intraoperative processed EEG-based measure of lower brain anaesthetic resistance was associated with increased postoperative delirium risk in older patients undergoing surgery140. However, additional research is needed to understand how this finding relates to DSD. One conceptual model theorizes that delirium occurs from disruption of normal brain function secondary to impairments in brain connectivity and plasticity; this hypothesis could be tested using transcranial magnetic stimulation and EEG138,141.
In summary, markers of brain physiology are beginning to reveal how changes in cerebral blood flow, spectral power and connectivity can be associated with delirium; however, further work is needed to expand these findings to patients with DSD.
Delirium prevention in persons with dementia
Multidisciplinary, multicomponent, non-pharmacological interventions have been developed for delirium prevention. These interventions include the Hospital Elder Life Program (HELP)33,142 and the ABCDEF bundle143, which have been found to reduce the incidence and duration of delirium and reduce functional decline in older patients13. The interventions vary in the number of components included, but most include individualized care, education, reorientation and early mobilization. A meta-analysis of 8 studies, involving a total of 2,105 participants, reported that multicomponent interventions reduce the incidence of delirium (RR 0.53, 95% CI 0.41–0.69, I2 = 0). HELP includes interventions specifically for use in persons with dementia (Table 5), but their effectiveness in this population is not yet clear. A systematic review that assessed the effectiveness of delirium prevention in individuals with dementia identified seven studies that met inclusion criteria144. Using the GRADE framework for the evaluation of study quality, three studies were determined to be of moderate quality and four of low quality. Both studies with moderate grade evidence (that is, the true effect is probably close to the estimated effect) — one study using pre-printed delirium-friendly postoperative orders145 and another using a multidisciplinary postoperative intervention146 — reported significant reductions in delirium incidence compared with usual care. In terms of pharmacological intervention, in a single 2-year, open-label study comparing rivastigmine with aspirin in patients with vascular dementia, significantly fewer participants in the rivastigmine group developed delirium147. However, a subsequent multicentre, double-blind, placebo-controlled randomized trial found that rivastigmine did not reduce delirium duration and the trial was halted early due to increased mortality in the rivastigmine group148.
Table 5.
Suggested adaptations to delirium prevention interventions for individuals with dementia
| Targeted risk factor | Interventions | Description | Adaptation for dementia |
|---|---|---|---|
| Cognitive impairment | Orientation protocol | Orientation board with names of care team members and daily schedule; orienting communication once a day | Orientation protocol three times a day; education for staff in special approaches to communication with individuals with dementia |
| Therapeutic activities | Cognitive stimulation activities three times a day (customized selection according to leisure interests and physical impairments) | Additional customization for the selection of activities according to level of cognitive function | |
| Immobility | Early mobilization | Walking or active range-of-motion exercises three times a day; minimizing use of immobilizing equipment and physical restraints | For all tasks, focus on one-step, as opposed to multistep, instructions |
| Vision impairment | Vision protocol | Providing visual aids and adaptive equipment, with daily reinforcement | For all tasks, focus on one-step, as opposed to multistep, instructions |
| Hearing impairment | Hearing protocol | Providing portable amplifying devices; earwax disimpaction; special communication techniques, with daily reinforcement | For all tasks, focus on one-step, as opposed to multistep, instructions |
| Dehydration | Oral volume repletion | Early recognition of dehydration and oral volume repletion; encouragement during meals | For all tasks, focus on one-step, as opposed to multistep, instructions |
| Sleep deprivation | Non-pharmacological sleep protocol | At bedtime, warm drink, relaxation music or sounds, and massage; unit-wide noise reduction programme; rescheduling medications and procedures to allow uninterrupted sleep | Importance of behavioural (for example, avoid caffeine and diuretics after mid-day) and environmental changes to enhance sleep (for example, darkened, quiet room, minimize interruptions) |
| Polypharmacy and inappropriate medications | Psychoactive medications protocol | Screen medications daily; minimize medications listed in AGS Beers Criteria and psychoactive medications; discuss strategies with an interdisciplinary team | Avoidance of psychoactive medications even more important for this high-risk group |
| Other protocols | Nursing interventions | Targeting delirium risk factors (as above) in all patients, with special nursing focus to maintain early mobility, prevent dehydration, avoid psychoactive medications and maximize sleep hygiene; use of non-pharmacological approaches for sleep, anxiety or pain | Daily delirium screens with medical work-up as indicated; minimizing psychoactive medications; non-opioid treatments for pain; educating patients, families and staff about behavioural management in dementia and sundowning |
| Provider education | Educational programme about delirium and delirium prevention | Educational programme about delirium superimposed on dementia; special needs of dementia patients; behavioural management of agitation | |
| Emotional support | Nursing, chaplaincy, social work support | Include family and informal caregivers |
A major limitation of the studies discussed here is the lack of high-grade evidence owing to variability in how delirium and dementia are defined in the study, the inclusion of more than one type of dementia, small sample sizes and the use of case–control study designs144. Another limitation is that a number of delirium prevention trials have been conducted using ‘cognitive impairment’, as defined by performance on a cognitive screening test, instead of a clinical diagnosis of dementia149,150. One study, which found that use of HELP was associated with a significant reduction in delirium incidence, did include persons with dementia, but the effect of the prevention was not examined in this subgroup142. More definitive delirium prevention trials in persons with dementia are needed and are under way. For example, the PREvention Program for Alzheimer’s RElated Delirium (PREPARED) cluster randomized trial, which aims to assess the effect of a multicomponent intervention on the incidence of delirium, severity of delirium episodes, duration of delirium episodes, and number of delirium episodes among persons with dementia and/or cognitive impairment residing in a long-term care facility151.
In summary, most delirium prevention strategies focus on minimizing one or more modifiable delirium risk factors via a non-pharmacological, multidisciplinary approach142,152,153. Whether and how prevention strategies might address specific pathophysiological mechanisms remains unknown and presents an important area for future research.
A hypothetical model
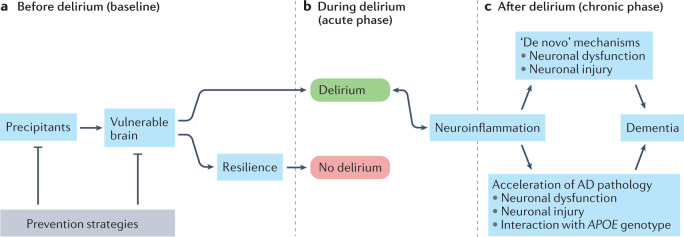
Taken together, the evidence discussed here indicates that the inter-relationship between delirium and dementia is likely to be complex (Fig. 1). Delirium might be the expression of the balance between vulnerability (that is, the factors predisposing towards the development of delirium) and resilience (that is, the ability to maintain function in the setting of an insult or precipitating factors). In this model, patients who are highly vulnerable to delirium because of factors such as underlying neurodegeneration or abnormal neuroinflammation develop delirium only when resilience factors, for example, cognitive reserve, can no longer maintain healthy functioning. The development of delirium might then result in acceleration of the underlying neurodegeneration, perhaps via inflammation or gene interactions with inflammation. Alternatively, in some individuals, delirium itself might be associated with neuronal injury103,105, with ‘de novo’ mechanisms then leading to dementia.
Fig. 1. A hypothetical model for the inter-relationship between delirium and dementia and potential opportunities for prevention.
a,b | In the setting of precipitating factors, such as hypoxia, metabolic abnormalities, medications, infection or surgery, and in the presence of an existing vulnerability, such as Alzheimer disease (AD) or other neurodegenerative pathology, cerebrovascular disease, or injury, delirium (green) can occur. Alternatively, owing to the presence of resilience factors, such as cognitive reserve, or the implementation of prevention strategies (grey) to minimize one or more modifiable delirium risk factors, delirium does not occur (red). c | The development of delirium and subsequent neuroinflammation might then result in the acceleration of underlying neurodegenerative pathology. Alternatively, in individuals without underlying neurodegenerative pathology, delirium might be associated with neuronal injury, with ‘de novo’ mechanisms leading to dementia.
These hypotheses could be tested by measuring a biomarker of brain vulnerability prior to exposure to a precipitant and determining if biomarker levels are predictive of incident delirium. Next, a biomarker of neuroinflammation could be measured during the delirium episode and an association with incident delirium or delirium severity could be tested for. Last, a biomarker of neuronal injury, such as NfL, could be measured during the delirium episode to test for an association with incident delirium and delirium severity as well as after the delirium episode to test for an association with outcomes of delirium. To date, a causal relationship between delirium and neuronal injury has not been established.
A major challenge in understanding the relationship that exists between delirium and dementia is that the underlying pathology for each condition is complex and involves multiple mechanistic pathways. As discussed above, potential pathophysiological mechanisms in delirium include neurodegeneration and neuronal injury, inflammation, disturbances in brain energy metabolism, disruption in neurotransmitter function, effects of pharmacological agents, and failure of network connectivity. For dementia, although we have focused on studies of AD, possible contributions from mixed dementia pathology (that is, AD and cerebrovascular disease) must also be considered. Furthermore, some patients with AD biomarkers did not develop delirium after surgical treatment of hip fracture91,92, suggesting that the presence of AD pathology does not guarantee the development of delirium in response to this precipitant. Review of the existing literature on delirium biomarkers found that many studies either did not include known AD biomarkers, the quality of biomarker data was moderate or had a high risk of bias, or the cognitive data was limited63.
Adding further complexity to the delirium–dementia inter-relationship is that both delirium and dementia exist along a continuum, with stages of AD ranging from preclinical AD, defined as the presence of pathological AD biomarkers in cognitively healthy individuals, to mild, moderate and severe stages of cognitive impairment. The term sub-syndromal delirium refers to the acute or subacute onset of delirium symptoms, including disturbed attention and other cognitive and/or neuropsychiatric disturbances, in the absence of full syndromal delirium but not better accounted for by another neuropsychiatric condition154. Sub-syndromal delirium sometimes progresses to delirium, which itself varies in severity. Thus, future studies examining the inter-relationship between delirium and dementia must address these issues by incorporating novel biomarkers into thoughtfully designed studies.
Delirium in an ageing population
Our knowledge and understanding of delirium pathophysiology have advanced, but much still needs to be learned about the relationship between delirium and dementia. The number of people with dementia worldwide already exceeds 5 million and, as the global population ages, this figure is expected to reach 152 million by 2050 (ref.8). Likewise, because dementia is such a strong risk factor for delirium and because delirium is common among older adults, delirium and dementia will clearly continue to be substantial public health issues. This increasing burden on health-care systems highlights the imperative for future research. Efforts to refine the consensus definition of delirium, harmonize instruments for measuring delirium and delirium severity, advance our understanding of delirium pathophysiology, and develop novel prevention and treatment strategies155 are ongoing. However, research efforts with the same goals should also be adapted and directed towards the interface between delirium and dementia (Box 1).
One particularly important area for future research is the examination of the effects of delirium prevention on dementia incidence and rate of progression. Our prior work found that, among persons with dementia, an episode of delirium was associated with a threefold increase in the rate of cognitive decline20. Delirium prevention is an intervention that has been shown to be effective and is already readily available; thus, studies that directly quantify the benefit of delirium prevention on the cognitive trajectory in AD are greatly needed.
Box 1 Priorities for advancing the understanding of the interface between delirium and dementia.
Define and measure
Develop consensus approaches, precise diagnostic criteria and comprehensive guidelines for the assessment and diagnosis of delirium superimposed on dementia (DSD)
Use standardized reference criteria for diagnosis
Establish standardized, well-validated DSD measurement instruments for clinical and research use
Define the association between delirium and specific neuropsychological deficits
Identify underlying contributors by incorporating biomarkers and pathophysiological indicators into studies
Develop core outcomes for use in clinical trials
Understand pathophysiology
Develop animal models to test potential pathophysiological mechanisms of DSD
Use standardized preclinical or mechanistic protocols to enable harmonization across studies
Incorporate novel fluid, neuroimaging and neurophysiology biomarkers
Apply innovative approaches, such as systems biology, multi-omics and machine learning to analyse data
Use study designs that consider dementia and delirium mechanisms, both individually and together
Prevent and treat
Implement novel evidence-based approaches
Use multicomponent and sequential treatment approaches. Apply known effective approaches for delirium prevention and test effectiveness for slowing long-term cognitive decline
Follow adaptive trial design to enable modifications in interventions and/or study population
Engage pragmatic trials in multiple settings (that is, acute hospital, rehabilitation, long-term care)
Measure effects of delirium prevention and treatment on dementia severity and progression
Promote awareness
Increase awareness through public education
Prioritize research funding
Define social and economic impact of DSD
Conclusions and future directions
Delirium and dementia are common conditions in older adults, often occurring together as DSD. However, DSD often goes undetected or is assumed to be part of the underlying dementia. Recognition of the presence of an acute change from baseline or of symptoms specific to delirium or dementia might aid in the detection of delirium in a person with dementia. Two major challenges of developing screening and diagnostic instruments for DSD are the varying severity of the underlying cognitive impairment and the lack of a reliable reference standard. In 2020, two working groups published roadmaps of research priorities in delirium57,155, with goals of improving patient care and clinical outcomes, developing better prevention and treatment strategies, and advancing our understanding of the biology of delirium and the inter-relationship between delirium and dementia. As the number of older adults — and thus the number of individuals at risk of delirium — is growing and the evidence supporting delirium as a risk factor and possible trigger for age-related brain disorders is accumulating, there has been a heightened effort to facilitate the recognition and treatment of delirium. For example, the Network for Investigation of Delirium: Unifying Scientists (NIDUS) is a collaborative, interdisciplinary network of investigators across more than 27 institutions worldwide and aims to advance scientific research on the causes, mechanisms, outcomes, diagnosis, prevention and treatment of delirium in older adults. Another global delirium campaign, the International Drive to Illuminate Delirium (IDID)57, aims to advance the field of delirium along the pillars of diagnosis, awareness, burden, biology and policy to ultimately lessen the physical and cognitive burden of delirium. This campaign recognizes that delirium is an important risk factor and a potential trigger for cognitive, motor and mood disorders in older adults and that delirium and accelerated cognitive decline might unmask pre-existing preclinical dementia pathology and thereby reduce the time to onset of clinical dementia. Therefore, delirium is increasingly recognized as an important and unexplored opportunity for dementia prevention57.
With the awareness that delirium is a risk factor for dementia, improving the care of individuals with delirium must become a key focus of public health efforts57. Global public health awareness campaigns that recognize the potential role of delirium as a risk factor and trigger for new dementia and acceleration of cognitive decline will hopefully aid in achieving a better understanding of DSD. Such campaigns could also help identify the extent to which delirium is a potentially modifiable risk factor for dementia and whether delirium and dementia have shared mechanisms. Although delirium has traditionally been considered a geriatric syndrome, re-conceptualizing delirium as a neurological condition and increasing the focus on aetiology and subsequent neuropathophysiology156,157 would help further advance our understanding of the relationship between delirium and dementia.
Evidence from biomarker studies supports a role of systemic inflammation, neuroinflammation and neuronal injury in delirium pathophysiology. Whether underlying factors, such as preclinical dementia, make the brain more vulnerable and thus more likely to lose the ability to function normally in the face of infection or trauma, with the end result being an episode of delirium, or if delirium itself causes neuronal injury and death, remains unknown; however, both of these possibilities are supported by emerging data. Important advances in plasma and CSF biomarkers, animal models of neuroinflammation, functional and structural MRI, and novel neurophysiology markers hold great promise for advancing the field. Future research should use standardized methods for defining and measuring the severity of delirium and dementia, and universal reporting standards for cognitive and functional outcome measures to enable harmonization of the resulting data. Studies should be conducted in a range of study populations and in the presence of different comorbidities to ensure that the findings are generalizable.
The SARS-CoV-2 pandemic has resulted in a substantially increased rate of delirium158,159 both directly, via the effects of COVID-19, and indirectly owing to the subsequent inability to implement normal delirium prevention strategies160. Initial estimates of delirium among patients with COVID-19 ranged from 11% among hospitalizations in Northern Italy158 to 84.3% in two intensive care units in France157; however, a 2021 multi-site, large, international cohort study of more than 2,000 patients who had severe COVID-19 from February to August 2020, found that 54.9% were delirious for a median of 3 days161. Evidence suggests that COVID-19-associated delirium might eventually be more widespread, more severe and associated with more adverse outcomes than previously seen with delirium and post-intensive care unit syndrome (Box 2). An observational cohort study found dementia to be a statistically significant risk factor for COVID-19 (ref.162) and for increased mortality with COVID-19 (ref.163). Delirium can also be the presenting symptom of COVID-19 among individuals with dementia163. Studies of COVID-19-associated delirium should be a priority for future research efforts. These studies should focus on the role of inflammation and neuroinflammation, rates of incident dementia and other cognitive and functional outcomes, and strategies for cognitive rehabilitation and delirium prevention.
Established clinical guidelines focus on improving the diagnosis of delirium and reducing hospital stays and complications14,164,165, but they do not directly address delirium in persons with dementia. Therefore, the development of specific clinical guidelines for the diagnosis and management of DSD is needed. We hope that, through a unified public health approach, these guidelines can effectively reduce the burden, severity and progression of dementia through delirium prevention.
Box 2 Special challenges of COVID-19 in persons with delirium superimposed on dementia.
Individuals with pre-existing cognitive impairment and dementia are at increased risk of serious COVID-19 infection and more severe complications than those without cognitive impairment and dementia. In particular, individuals with dementia have:
Increased risk of delirium with COVID-19 infection169
Increased risk of atypical presentation of COVID-19 infection, such as delirium, falls, functional decline and failure to thrive, in the absence of fever, shortness of breath and cough; this feature can lead to a lack of recognition of COVID-19 infection170,171
Increased risk of COVID-19 causing serious illness and mortality163,172,173
Increased risk of long-term neurocognitive sequela of COVID-19 (refs.174,175)
Recommendations for assessment and management of COVID-19 in individuals with dementia:
Screen for COVID-19 in older adults presenting for urgent medical care, even in the absence of typical symptoms170
Limit use of deliriogenic medications when treating individuals with dementia for COVID-19 (ref.161)
- Apply delirium prevention strategies adapted for persons with dementia; use remote strategies as needed176,177
- Care providers in full personal protective equipment can wear a tag with their name and a photograph visible to patients
- Connect patient with family by teleconferencing
- Enhance mobilization by providing instructions for in-room and bed range-of-motion exercises
Acknowledgements
This work is dedicated to the memory of Joshua Bryan Inouye Helfand. Both authors have received grants from the National Institute on Aging.
Glossary
- Preclinical AD
Asymptomatic condition with biomarkers indicating the presence of Alzheimer disease (AD) pathological changes.
- EEG spectral power
A standard measure for quantifying the electrical signals of the brain, based on extracting different signal frequencies from the electroencephalogram (EEG).
Author contributions
The authors contributed equally to all aspects of the article.
Peer review
Peer review information
Nature Reviews Neurology thanks Leiv Otto Watne, who co-reviewed with Mari Aksnes; Miles Berger; Barbara van Munster; and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Review criteria
A PubMed search was conducted using the keywords: Alzheimer’s disease, dementia, delirium AND biomarkers. Articles were then reviewed to find original studies, which either enrolled persons with dementia, including preclinical dementia (that is, asymptomatic persons identified by the presence of Alzheimer disease biomarkers), or which measured traditional Alzheimer disease biomarkers. Additional original research studies identified from review articles, including Wilson et al.2 and Dunne et al.37, were included. This search was not intended to be a systematic review and it is possible that some articles may have been missed.
Related links
Network for Investigation of Delirium: Unifying Scientists (NIDUS): http://www.deliriumnetwork.org/
References
- 1.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-5 Fifth edn (American Psychiatric Association, 2013).
- 2.Wilson JE, et al. Delirium. Nat. Rev. Dis. Prim. 2020;6:90. doi: 10.1038/s41572-020-00223-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oh ES, Fong TG, Hshieh TT, Inouye SK. Delirium in older persons: advances in diagnosis and treatment. JAMA. 2017;318:1161–1174. doi: 10.1001/jama.2017.12067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leslie DL, Marcantonio ER, Zhang Y, Leo-Summers L, Inouye SK. One-year health care costs associated with delirium in the elderly population. Arch. Intern. Med. 2008;168:27–32. doi: 10.1001/archinternmed.2007.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Health Organization Regional Office for Europe. European Hospital Morbidity Database (World Health Organization, 2012).
- 6.Organization for Economic Co-operation and Development. OECD Health Data 2012 (Organization for Economic Co-operation and Development, 2012).
- 7.Lopez OL, Kuller LH. Epidemiology of aging and associated cognitive disorders: prevalence and incidence of Alzheimer’s disease and other dementias. Handb. Clin. Neurol. 2019;167:139–148. doi: 10.1016/B978-0-12-804766-8.00009-1. [DOI] [PubMed] [Google Scholar]
- 8.Patterson, C. & Alzheimer’s Disease International. World Alzheimer Report 2018 (Alzheimer’s Disease International, 2018).
- 9.Marucci G, et al. Efficacy of acetylcholinesterase inhibitors in Alzheimer’s disease. Neuropharmacology. 2021;190:108352. doi: 10.1016/j.neuropharm.2020.108352. [DOI] [PubMed] [Google Scholar]
- 10.PhRMA. Researching Alzheimer’s Medicines: Setbacks and Stepping Stones (PhRMA, 2018).
- 11.Oh ES, et al. Antipsychotics for preventing delirium in hospitalized adults: a systematic review. Ann. Intern. Med. 2019;171:474–484. doi: 10.7326/M19-1859. [DOI] [PubMed] [Google Scholar]
- 12.Asleson DR, Chiu AW. Melatonin for delirium prevention in acute medically ill, and perioperative geriatric patients. Aging Med. 2020;3:132–137. doi: 10.1002/agm2.12112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Siddiqi N, et al. Interventions for preventing delirium in hospitalised non-ICU patients. Cochrane Database Syst. Rev. 2016;3:CD005563. doi: 10.1002/14651858.CD005563.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Soiza RL, Myint PK. The Scottish Intercollegiate Guidelines Network (SIGN) 157: guidelines on risk reduction and management of delirium. Medicina. 2019;55:491. doi: 10.3390/medicina55080491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fong TG, Davis D, Growdon ME, Albuquerque A, Inouye SK. The interface between delirium and dementia in elderly adults. Lancet Neurol. 2015;14:823–832. doi: 10.1016/S1474-4422(15)00101-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Richardson SJ, et al. Recurrent delirium over 12 months predicts dementia: results of the delirium and cognitive impact in dementia (DECIDE) study. Age Ageing. 2021;50:914–920. doi: 10.1093/ageing/afaa244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Davis DH, et al. Delirium is a strong risk factor for dementia in the oldest-old: a population-based cohort study. Brain. 2012;135:2809–2816. doi: 10.1093/brain/aws190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Davis DH, et al. Association of delirium with cognitive decline in late life: a neuropathologic study of 3 population-based cohort studies. JAMA Psychiatry. 2017;74:244–251. doi: 10.1001/jamapsychiatry.2016.3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Davis DHJ, et al. Worsening cognitive impairment and neurodegenerative pathology progressively increase risk for delirium. Am. J. Geriatr. Psychiatry. 2015;23:403–415. doi: 10.1016/j.jagp.2014.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fong TG, et al. Delirium accelerates cognitive decline in Alzheimer disease. Neurology. 2009;72:1570–1575. doi: 10.1212/WNL.0b013e3181a4129a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gross AL, et al. Delirium and long-term cognitive trajectory among persons with dementia. Arch. Intern. Med. 2012;172:1324–1331. doi: 10.1001/archinternmed.2012.3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Inouye SK, et al. The short-term and long-term relationship between delirium and cognitive trajectory in older surgical patients. Alzheimers Dement. 2016;12:766–775. doi: 10.1016/j.jalz.2016.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pandharipande PP, et al. Long-term cognitive impairment after critical illness. N. Engl. J. Med. 2013;369:1306–1316. doi: 10.1056/NEJMoa1301372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Han QYC, Rodrigues NG, Klainin-Yobas P, Haugan G, Wu XV. Prevalence, risk factors, and impact of delirium on hospitalized older adults with dementia: a systematic review and meta-analysis. J. Am. Med. Dir. Assoc. 2022;23:23–32. doi: 10.1016/j.jamda.2021.09.008. [DOI] [PubMed] [Google Scholar]
- 25.Hshieh TT, Yang T, Gartaganis SL, Yue J, Inouye SK. Hospital elder life program: systematic review and meta-analysis of effectiveness. Am. J. Geriatr. Psychiatry. 2018;26:1015–1033. doi: 10.1016/j.jagp.2018.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Inouye SK, Bogardus ST, Jr., Baker DI, Leo-Summers L, Cooney LM., Jr. The Hospital Elder Life Program: a model of care to prevent cognitive and functional decline in older hospitalized patients. Hospital Elder Life Program. J. Am. Geriatr. Soc. 2000;48:1697–1706. doi: 10.1111/j.1532-5415.2000.tb03885.x. [DOI] [PubMed] [Google Scholar]
- 27.Rubin FH, Neal K, Fenlon K, Hassan S, Inouye SK. Sustainability and scalability of the hospital elder life program at a community hospital. J. Am. Geriatr. Soc. 2011;59:359–365. doi: 10.1111/j.1532-5415.2010.03243.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rubin FH, et al. Replicating the Hospital Elder Life Program in a community hospital and demonstrating effectiveness using quality improvement methodology. J. Am. Geriatr. Soc. 2006;54:969–974. doi: 10.1111/j.1532-5415.2006.00744.x. [DOI] [PubMed] [Google Scholar]
- 29.Caplan GA, Harper EL. Recruitment of volunteers to improve vitality in the elderly: the REVIVE study. Intern. Med. J. 2007;37:95–100. doi: 10.1111/j.1445-5994.2007.01265.x. [DOI] [PubMed] [Google Scholar]
- 30.Rubin FH, Bellon J, Bilderback A, Urda K, Inouye SK. Effect of the Hospital Elder Life Program on risk of 30-day readmission. J. Am. Geriatr. Soc. 2018;66:145–149. doi: 10.1111/jgs.15132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Akunne A, Davis S, Westby M, Young J. The cost-effectiveness of multi-component interventions to prevent delirium in older people undergoing surgical repair of hip fracture. Eur. J. Orthop. Surg. Traumatol. 2014;24:187–195. doi: 10.1007/s00590-013-1170-9. [DOI] [PubMed] [Google Scholar]
- 32.Chen CC, et al. Modified hospital elder life program: effects on abdominal surgery patients. J. Am. Coll. Surg. 2011;213:245–252. doi: 10.1016/j.jamcollsurg.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 33.Hshieh TT, et al. Effectiveness of multicomponent nonpharmacological delirium interventions: a meta-analysis. JAMA Intern. Med. 2015;175:512–520. doi: 10.1001/jamainternmed.2014.7779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Khan A, et al. Preventing delirium takes a village: systematic review and meta-analysis of delirium preventive models of care. J. Hosp. Med. 2019;14:E1–E7. doi: 10.12788/jhm.3212. [DOI] [PubMed] [Google Scholar]
- 35.Kratz T, Heinrich M, Schlauß E, Diefenbacher A. Preventing postoperative delirium. Dtsch. Arztebl Int. 2015;112:289–296. doi: 10.3238/arztebl.2015.0289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schnitker L, et al. Prevention of delirium in older adults with dementia: a systematic literature review. J. Gerontol. Nurs. 2020;46:43–54. doi: 10.3928/00989134-20200820-02. [DOI] [PubMed] [Google Scholar]
- 37.Dunne SS, et al. Biomarkers in delirium: a systematic review. J. Psychosom. Res. 2021;147:110530. doi: 10.1016/j.jpsychores.2021.110530. [DOI] [PubMed] [Google Scholar]
- 38.Morandi A, et al. The diagnosis of delirium superimposed on dementia: an emerging challenge. J. Am. Med. Dir. Assoc. 2017;18:12–18. doi: 10.1016/j.jamda.2016.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Avelino-Silva TJ, Campora F, Curiati JA, Jacob-Filho W. Association between delirium superimposed on dementia and mortality in hospitalized older adults: a prospective cohort study. PLoS Med. 2017;14:e1002264. doi: 10.1371/journal.pmed.1002264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Inouye SK, Westendorp RG, Saczynski JS. Delirium in elderly people. Lancet. 2014;383:911–922. doi: 10.1016/S0140-6736(13)60688-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.He, W., Goodkind, D. & Kowal, P. An Aging World: 2015 (U.S. Government Publishing Office, 2016).
- 42.Morandi A, et al. Delirium, dementia, and in-hospital mortality: the results from the Italian delirium day 2016, a national multicenter study. J. Gerontol. A Biol. Sci. Med. Sci. 2019;74:910–916. doi: 10.1093/gerona/gly154. [DOI] [PubMed] [Google Scholar]
- 43.Richardson S, et al. Delirium superimposed on dementia: a survey of delirium specialists shows a lack of consensus in clinical practice and research studies. Int. Psychogeriatr. 2016;28:853–861. doi: 10.1017/S1041610215002288. [DOI] [PubMed] [Google Scholar]
- 44.Jorm AF, Jacomb PA. The informant questionnaire on cognitive decline in the elderly (IQCODE): socio-demographic correlates, reliability, validity and some norms. Psychol. Med. 1989;19:1015–1022. doi: 10.1017/s0033291700005742. [DOI] [PubMed] [Google Scholar]
- 45.Galvin JE, et al. The AD8: a brief informant interview to detect dementia. Neurology. 2005;65:559–564. doi: 10.1212/01.wnl.0000172958.95282.2a. [DOI] [PubMed] [Google Scholar]
- 46.Morandi A, et al. Tools to detect delirium superimposed on dementia: a systematic review. J. Am. Geriatr. Soc. 2012;60:2005–2013. doi: 10.1111/j.1532-5415.2012.04199.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Morandi A, et al. The 4-DSD: a new tool to assess delirium superimposed on moderate to severe dementia. J. Am. Med. Dir. Assoc. 2021;22:1535–1542. doi: 10.1016/j.jamda.2021.02.029. [DOI] [PubMed] [Google Scholar]
- 48.Bellelli G, et al. Validation of the 4AT, a new instrument for rapid delirium screening: a study in 234 hospitalised older people. Age Ageing. 2014;43:496–502. doi: 10.1093/ageing/afu021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Inouye SK, et al. Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann. Intern. Med. 1990;113:941–948. doi: 10.7326/0003-4819-113-12-941. [DOI] [PubMed] [Google Scholar]
- 50.Marcantonio ER, et al. 3D-CAM: derivation and validation of a 3-minute diagnostic interview for CAM-defined delirium: a cross-sectional diagnostic test study. Ann. Intern. Med. 2014;161:554–561. doi: 10.7326/M14-0865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Motyl CM, et al. Comparative accuracy and efficiency of four delirium screening protocols. J. Am. Geriatr. Soc. 2020;68:2572–2578. doi: 10.1111/jgs.16711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hasemann W, et al. Comparing performance on the months of the year backwards test in hospitalised patients with delirium, dementia, and no cognitive impairment: an exploratory study. Eur. Geriatr. Med. 2021;12:1257–1265. doi: 10.1007/s41999-021-00521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Breitbart W, et al. The memorial delirium assessment scale. J. Pain. Symptom Manag. 1997;13:128–137. doi: 10.1016/s0885-3924(96)00316-8. [DOI] [PubMed] [Google Scholar]
- 54.Trzepacz PT, et al. Validation of the Delirium Rating Scale-revised-98: comparison with the delirium rating scale and the cognitive test for delirium. J. Neuropsychiatry Clin. Neurosci. 2001;13:229–242. doi: 10.1176/jnp.13.2.229. [DOI] [PubMed] [Google Scholar]
- 55.Inouye SK, et al. The CAM-S: development and validation of a new scoring system for delirium severity in 2 cohorts. Ann. Intern. Med. 2014;160:526–533. doi: 10.7326/M13-1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Goldberg TE, et al. Association of delirium with long-term cognitive decline: a meta-analysis. JAMA Neurol. 2020;77:1373–1381. doi: 10.1001/jamaneurol.2020.2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Khachaturian AS, et al. International drive to illuminate delirium: a developing public health blueprint for action. Alzheimers Dement. 2020;16:711–725. doi: 10.1002/alz.12075. [DOI] [PubMed] [Google Scholar]
- 58.Berger M, et al. Postoperative changes in cognition and cerebrospinal fluid neurodegenerative disease biomarkers. Ann. Clin. Transl. Neurol. 2022;9:155–170. doi: 10.1002/acn3.51499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Berger M, et al. The INTUIT study: investigating neuroinflammation underlying postoperative cognitive dysfunction. J. Am. Geriatr. Soc. 2019;67:794–798. doi: 10.1111/jgs.15770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Browndyke JN, et al. Perioperative neurocognitive and functional neuroimaging trajectories in older APOE4 carriers compared with non-carriers: secondary analysis of a prospective cohort study. Br. J. Anaesth. 2021;127:917–928. doi: 10.1016/j.bja.2021.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Richardson S, et al. Delirium and delirium severity predict the trajectory of the hierarchical assessment of balance and mobility in hospitalized older people: findings from the DECIDE study. J. Gerontol. A Biol. Sci. Med. Sci. 2022;77:529–533. doi: 10.1093/gerona/glab081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.VanDusen KW, et al. The MARBLE study protocol: modulating ApoE signaling to reduce brain inflammation, DeLirium, and PostopErative cognitive dysfunction. J. Alzheimers Dis. 2020;75:1319–1328. doi: 10.3233/JAD-191185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang S, et al. A systematic review of delirium biomarkers and their alignment with the NIA-AA research framework. J. Am. Geriatr. Soc. 2021;69:255–263. doi: 10.1111/jgs.16836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jack CR, Jr., et al. NIA-AA research framework: toward a biological definition of Alzheimer’s disease. Alzheimers Dement. 2018;14:535–562. doi: 10.1016/j.jalz.2018.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Maldonado JR. Delirium pathophysiology: an updated hypothesis of the etiology of acute brain failure. Int. J. Geriatr. Psychiatry. 2018;33:1428–1457. doi: 10.1002/gps.4823. [DOI] [PubMed] [Google Scholar]
- 66.McNeil JB, et al. Plasma biomarkers of inflammation, coagulation, and brain injury as predictors of delirium duration in older hospitalized patients. PLoS One. 2019;14:e0226412. doi: 10.1371/journal.pone.0226412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dillon ST, et al. Higher C-reactive protein levels predict postoperative delirium in older patients undergoing major elective surgery: a longitudinal nested case-control study. Biol. Psychiatry. 2017;81:145–153. doi: 10.1016/j.biopsych.2016.03.2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vasunilashorn SM, et al. Apolipoprotein E genotype and the association between C-reactive protein and postoperative delirium: Importance of gene-protein interactions. Alzheimers Dement. 2020;16:572–580. doi: 10.1016/j.jalz.2019.09.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Slor CJ, et al. The trajectory of C-reactive protein serum levels in older hip fracture patients with postoperative delirium. Int. J. Geriatr. Psychiatry. 2019;34:1438–1446. doi: 10.1002/gps.5139. [DOI] [PubMed] [Google Scholar]
- 70.Adamis D, et al. APOE and cytokines as biological markers for recovery of prevalent delirium in elderly medical inpatients. Int. J. Geriatr. Psychiatry. 2007;22:688–694. doi: 10.1002/gps.1732. [DOI] [PubMed] [Google Scholar]
- 71.van den Boogaard M, et al. Biomarkers associated with delirium in critically ill patients and their relation with long-term subjective cognitive dysfunction; indications for different pathways governing delirium in inflamed and noninflamed patients. Crit. Care. 2011;15:R297. doi: 10.1186/cc10598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Munster BC, et al. Neuroinflammation in delirium: a postmortem case-control study. Rejuvenation Res. 2011;14:615–622. doi: 10.1089/rej.2011.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Calsolaro V, Edison P. Neuroinflammation in Alzheimer’s disease: current evidence and future directions. Alzheimers Dement. 2016;12:719–732. doi: 10.1016/j.jalz.2016.02.010. [DOI] [PubMed] [Google Scholar]
- 74.Hensley K. Neuroinflammation in Alzheimer’s disease: mechanisms, pathologic consequences, and potential for therapeutic manipulation. J. Alzheimers Dis. 2010;21:1–14. doi: 10.3233/JAD-2010-1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hennessy E, et al. Systemic TNF-alpha produces acute cognitive dysfunction and exaggerated sickness behavior when superimposed upon progressive neurodegeneration. Brain Behav. Immun. 2017;59:233–244. doi: 10.1016/j.bbi.2016.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lopez-Rodriguez AB, et al. Acute systemic inflammation exacerbates neuroinflammation in Alzheimer’s disease: IL-1β drives amplified responses in primed astrocytes and neuronal network dysfunction. Alzheimers Dement. 2021;17:1735–1755. doi: 10.1002/alz.12341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Leng F, Edison P. Neuroinflammation and microglial activation in Alzheimer disease: where do we go from here? Nat. Rev. Neurol. 2021;17:157–172. doi: 10.1038/s41582-020-00435-y. [DOI] [PubMed] [Google Scholar]
- 78.Liu D, et al. Soluble TREM2 changes during the clinical course of Alzheimer’s disease: a meta-analysis. Neurosci. Lett. 2018;686:10–16. doi: 10.1016/j.neulet.2018.08.038. [DOI] [PubMed] [Google Scholar]
- 79.Henjum K, et al. CSF sTREM2 in delirium-relation to Alzheimer’s disease CSF biomarkers Aβ42, t-tau and p-tau. J. Neuroinflammation. 2018;15:304. doi: 10.1186/s12974-018-1331-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zeevi N, Pachter J, McCullough LD, Wolfson L, Kuchel GA. The blood-brain barrier: geriatric relevance of a critical brain-body interface. J. Am. Geriatr. Soc. 2010;58:1749–1757. doi: 10.1111/j.1532-5415.2010.03011.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hov KR, et al. Blood-cerebrospinal fluid barrier integrity in delirium determined by Q-albumin. Dement. Geriatr. Cogn. Disord. 2016;41:192–198. doi: 10.1159/000443789. [DOI] [PubMed] [Google Scholar]
- 82.Velagapudi R, et al. Orthopedic surgery triggers attention deficits in a delirium-like mouse model. Front. Immunol. 2019;10:2675. doi: 10.3389/fimmu.2019.02675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Xiong C, Zhang Z, Baht GS, Terrando N. A mouse model of orthopedic surgery to study postoperative cognitive dysfunction and tissue regeneration. J. Vis. Exp. 2018 doi: 10.3791/56701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Miller-Rhodes P, et al. The broad spectrum mixed-lineage kinase 3 inhibitor URMC-099 prevents acute microgliosis and cognitive decline in a mouse model of perioperative neurocognitive disorders. J. Neuroinflammation. 2019;16:193. doi: 10.1186/s12974-019-1582-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Blennow K, Zetterberg H. Biomarkers for Alzheimer’s disease: current status and prospects for the future. J. Intern. Med. 2018;284:643–663. doi: 10.1111/joim.12816. [DOI] [PubMed] [Google Scholar]
- 86.Sunderland T, et al. Decreased beta-amyloid1-42 and increased tau levels in cerebrospinal fluid of patients with Alzheimer disease. JAMA. 2003;289:2094–2103. doi: 10.1001/jama.289.16.2094. [DOI] [PubMed] [Google Scholar]
- 87.Cunningham EL, et al. CSF beta-amyloid 1-42 concentration predicts delirium following elective arthroplasty surgery in an observational cohort study. Ann. Surg. 2019;269:1200–1205. doi: 10.1097/SLA.0000000000002684. [DOI] [PubMed] [Google Scholar]
- 88.Idland AV, et al. Preclinical amyloid-β and axonal degeneration pathology in delirium. J. Alzheimers Dis. 2017;55:371–379. doi: 10.3233/JAD-160461. [DOI] [PubMed] [Google Scholar]
- 89.Ballweg T, et al. Association between plasma tau and postoperative delirium incidence and severity: a prospective observational study. Br. J. Anaesth. 2021;126:458–466. doi: 10.1016/j.bja.2020.08.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Xie Z, et al. Preoperative cerebrospinal fluid beta-Amyloid/Tau ratio and postoperative delirium. Ann. Clin. Transl. Neurol. 2014;1:319–328. doi: 10.1002/acn3.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chan CK, et al. Association of depressive symptoms with postoperative delirium and CSF biomarkers for Alzheimer’s disease among hip fracture patients. Am. J. Geriatr. Psychiatry. 2021;29:1212–1221. doi: 10.1016/j.jagp.2021.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Witlox J, et al. Cerebrospinal fluid β-amyloid and tau are not associated with risk of delirium: a prospective cohort study in older adults with hip fracture. J. Am. Geriatr. Soc. 2011;59:1260–1267. doi: 10.1111/j.1532-5415.2011.03482.x. [DOI] [PubMed] [Google Scholar]
- 93.Ely EW, et al. Apolipoprotein E4 polymorphism as a genetic predisposition to delirium in critically ill patients. Crit. Care Med. 2007;35:112–117. doi: 10.1097/01.CCM.0000251925.18961.CA. [DOI] [PubMed] [Google Scholar]
- 94.Leung JM, et al. Apolipoprotein E e4 allele increases the risk of early postoperative delirium in older patients undergoing noncardiac surgery. Anesthesiology. 2007;107:406–411. doi: 10.1097/01.anes.0000278905.07899.df. [DOI] [PubMed] [Google Scholar]
- 95.van Munster BC, Korevaar JC, Zwinderman AH, Leeflang MM, de Rooij SE. The association between delirium and the apolipoprotein E epsilon 4 allele: new study results and a meta-analysis. Am. J. Geriatr. Psychiatry. 2009;17:856–862. doi: 10.1097/JGP.0b013e3181ab8c84. [DOI] [PubMed] [Google Scholar]
- 96.Vasunilashorn SM, et al. Cytokines and postoperative delirium in older patients undergoing major elective surgery. J. Gerontol. A Biol. Sci. Med. Sci. 2015;70:1289–1295. doi: 10.1093/gerona/glv083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wilcock DM, et al. Progression of amyloid pathology to Alzheimer’s disease pathology in an amyloid precursor protein transgenic mouse model by removal of nitric oxide synthase 2. J. Neurosci. 2008;28:1537–1545. doi: 10.1523/JNEUROSCI.5066-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wang P, et al. Neurovascular and immune mechanisms that regulate postoperative delirium superimposed on dementia. Alzheimers Dement. 2020;16:734–749. doi: 10.1002/alz.12064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hov KR, et al. Cerebrospinal fluid S100B and Alzheimer’s disease biomarkers in hip fracture patients with delirium. Dement. Geriatr. Cogn. Dis. Extra. 2017;7:374–385. doi: 10.1159/000481853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.van Munster BC, et al. Markers of cerebral damage during delirium in elderly patients with hip fracture. BMC Neurol. 2009;9:21. doi: 10.1186/1471-2377-9-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.van Munster BC, et al. Cortisol, interleukins and S100B in delirium in the elderly. Brain Cogn. 2010;74:18–23. doi: 10.1016/j.bandc.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 102.Caplan GA, et al. Cerebrospinal fluid in long-lasting delirium compared with Alzheimer’s dementia. J. Gerontol. A Biol. Sci. Med. Sci. 2010;65:1130–1136. doi: 10.1093/gerona/glq090. [DOI] [PubMed] [Google Scholar]
- 103.Halaas NB, et al. Neurofilament light in serum and cerebrospinal fluid of hip fracture patients with delirium. Dement. Geriatr. Cogn. Disord. 2018;46:346–357. doi: 10.1159/000494754. [DOI] [PubMed] [Google Scholar]
- 104.Casey CP, et al. Postoperative delirium is associated with increased plasma neurofilament light. Brain. 2020;143:47–54. doi: 10.1093/brain/awz354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Fong TG, et al. Association of plasma neurofilament light with postoperative delirium. Ann. Neurol. 2020;88:984–994. doi: 10.1002/ana.25889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Körtvelyessy P, et al. Ratio and index of neurofilament light chain indicate its origin in Guillain-Barré syndrome. Ann. Clin. Transl. Neurol. 2020;7:2213–2220. doi: 10.1002/acn3.51207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Evered L, Silbert B, Scott DA, Zetterberg H, Blennow K. Association of changes in plasma neurofilament light and tau levels with anesthesia and surgery: results from the CAPACITY and ARCADIAN studies. JAMA Neurol. 2018;75:542–547. doi: 10.1001/jamaneurol.2017.4913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Neerland BE, et al. Fatty acid-binding protein 3 in cerebrospinal fluid of hip fracture patients with delirium. J. Alzheimers Dis. 2020;77:183–190. doi: 10.3233/JAD-200364. [DOI] [PubMed] [Google Scholar]
- 109.Yoong SQ, et al. The prognostic utility of CSF neurogranin in predicting future cognitive decline in the Alzheimer’s disease continuum: A systematic review and meta-analysis with narrative synthesis. Ageing Res. Rev. 2021;72:101491. doi: 10.1016/j.arr.2021.101491. [DOI] [PubMed] [Google Scholar]
- 110.Halaas NB, et al. Cerebrospinal fluid concentration of neurogranin in hip fracture patients with delirium. J. Alzheimers Dis. 2021;81:667–677. doi: 10.3233/JAD-201341. [DOI] [PubMed] [Google Scholar]
- 111.Conti E, et al. Serum DBI and biomarkers of neuroinflammation in Alzheimer’s disease and delirium. Neurol. Sci. 2021;42:1003–1007. doi: 10.1007/s10072-020-04608-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Poljak A, et al. Quantitative proteomics of delirium cerebrospinal fluid. Transl. Psychiatry. 2014;4:e477. doi: 10.1038/tp.2014.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Pan X, et al. Cerebrospinal fluid spermidine, glutamine and putrescine predict postoperative delirium following elective orthopaedic surgery. Sci. Rep. 2019;9:4191. doi: 10.1038/s41598-019-40544-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Luo J, et al. Cellular polyamines promote amyloid-beta (Aβ) peptide fibrillation and modulate the aggregation pathways. ACS Chem. Neurosci. 2013;4:454–462. doi: 10.1021/cn300170x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Polis B, Karasik D, Samson AO. Alzheimer’s disease as a chronic maladaptive polyamine stress response. Aging. 2021;13:10770–10795. doi: 10.18632/aging.202928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Nitchingham A, Kumar V, Shenkin S, Ferguson KJ, Caplan GA. A systematic review of neuroimaging in delirium: predictors, correlates and consequences. Int. J. Geriatr. Psychiatry. 2018;33:1458–1478. doi: 10.1002/gps.4724. [DOI] [PubMed] [Google Scholar]
- 117.Brown CHT, et al. The association of brain MRI characteristics and postoperative delirium in cardiac surgery patients. Clin. Ther. 2015;37:2686–2699. doi: 10.1016/j.clinthera.2015.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hatano Y, et al. White-matter hyperintensities predict delirium after cardiac surgery. Am. J. Geriatr. Psychiatry. 2013;21:938–945. doi: 10.1016/j.jagp.2013.01.061. [DOI] [PubMed] [Google Scholar]
- 119.Omiya H, et al. Preoperative brain magnetic resonance imaging and postoperative delirium after off-pump coronary artery bypass grafting: a prospective cohort study. Can. J. Anaesth. 2015;62:595–602. doi: 10.1007/s12630-015-0327-x. [DOI] [PubMed] [Google Scholar]
- 120.Caplan GA, et al. Transcranial Doppler to measure cerebral blood flow in delirium superimposed on dementia. A cohort study. J. Am. Med. Dir. Assoc. 2014;15:355–360. doi: 10.1016/j.jamda.2013.12.079. [DOI] [PubMed] [Google Scholar]
- 121.Fong TG, et al. Cerebral perfusion changes in older delirious patients using 99mTc HMPAO SPECT. J. Gerontol. A Biol. Sci. Med. Sci. 2006;61:1294–1299. doi: 10.1093/gerona/61.12.1294. [DOI] [PubMed] [Google Scholar]
- 122.Choi SH, et al. Neural network functional connectivity during and after an episode of delirium. Am. J. Psychiatry. 2012;169:498–507. doi: 10.1176/appi.ajp.2012.11060976. [DOI] [PubMed] [Google Scholar]
- 123.Dickerson BC, et al. The cortical signature of Alzheimer’s disease: regionally specific cortical thinning relates to symptom severity in very mild to mild AD dementia and is detectable in asymptomatic amyloid-positive individuals. Cereb. Cortex. 2009;19:497–510. doi: 10.1093/cercor/bhn113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Racine AM, et al. Alzheimer’s-related cortical atrophy is associated with postoperative delirium severity in persons without dementia. Neurobiol. Aging. 2017;59:55–63. doi: 10.1016/j.neurobiolaging.2017.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Daianu M, et al. Breakdown of brain connectivity between normal aging and Alzheimer’s disease: a structural k-core network analysis. Brain Connect. 2013;3:407–422. doi: 10.1089/brain.2012.0137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wang L, et al. Amnestic mild cognitive impairment: topological reorganization of the default-mode network. Radiology. 2013;268:501–514. doi: 10.1148/radiol.13121573. [DOI] [PubMed] [Google Scholar]
- 127.van Montfort SJT, et al. Resting-state fMRI reveals network disintegration during delirium. Neuroimage Clin. 2018;20:35–41. doi: 10.1016/j.nicl.2018.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.van Montfort SJT, et al. Brain network disintegration as a final common pathway for delirium: a systematic review and qualitative meta-analysis. Neuroimage Clin. 2019;23:101809. doi: 10.1016/j.nicl.2019.101809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Haggstrom LR, Nelson JA, Wegner EA, Caplan GA. 2-(18)F-fluoro-2-deoxyglucose positron emission tomography in delirium. J. Cereb. Blood Flow. Metab. 2017;37:3556–3567. doi: 10.1177/0271678X17701764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Leech R, Sharp DJ. The role of the posterior cingulate cortex in cognition and disease. Brain. 2014;137:12–32. doi: 10.1093/brain/awt162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Buckner RL, et al. Molecular, structural, and functional characterization of Alzheimer’s disease: evidence for a relationship between default activity, amyloid, and memory. J. Neurosci. 2005;25:7709–7717. doi: 10.1523/JNEUROSCI.2177-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Johnson KA, et al. Preclinical prediction of Alzheimer’s disease using SPECT. Neurology. 1998;50:1563–1571. doi: 10.1212/wnl.50.6.1563. [DOI] [PubMed] [Google Scholar]
- 133.Rolandi E, et al. Association of postoperative delirium with markers of neurodegeneration and brain amyloidosis: a pilot study. Neurobiol. Aging. 2018;61:93–101. doi: 10.1016/j.neurobiolaging.2017.09.020. [DOI] [PubMed] [Google Scholar]
- 134.Gaubert S, et al. EEG evidence of compensatory mechanisms in preclinical Alzheimer’s disease. Brain. 2019;142:2096–2112. doi: 10.1093/brain/awz150. [DOI] [PubMed] [Google Scholar]
- 135.Zheng LL, Jiang ZY, Yu EY. Alpha spectral power and coherence in the patients with mild cognitive impairment during a three-level working memory task. J. Zhejiang Univ. Sci. B. 2007;8:584–592. doi: 10.1631/jzus.2007.B0584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Han Y, Wang K, Jia J, Wu W. Changes of EEG spectra and functional connectivity during an object-location memory task in Alzheimer’s disease. Front. Behav. Neurosci. 2017;11:107. doi: 10.3389/fnbeh.2017.00107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Wang R, et al. Power spectral density and coherence analysis of Alzheimer’s EEG. Cogn. Neurodyn. 2015;9:291–304. doi: 10.1007/s11571-014-9325-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Boord MS, et al. Investigating how electroencephalogram measures associate with delirium: a systematic review. Clin. Neurophysiol. 2021;132:246–257. doi: 10.1016/j.clinph.2020.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Giattino CM, et al. Intraoperative frontal alpha-band power correlates with preoperative neurocognitive function in older adults. Front. Syst. Neurosci. 2017;11:24. doi: 10.3389/fnsys.2017.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Cooter Wright M, et al. A processed electroencephalogram-based brain anesthetic resistance index is associated with postoperative delirium in older adults: a dual center study. Anesth. Analg. 2022;134:149–158. doi: 10.1213/ANE.0000000000005660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Shafi MM, et al. Advancing the neurophysiological understanding of delirium. J. Am. Geriatr. Soc. 2017;65:1114–1118. doi: 10.1111/jgs.14748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Inouye SK, et al. A multicomponent intervention to prevent delirium in hospitalized older patients. N. Engl. J. Med. 1999;340:669–676. doi: 10.1056/NEJM199903043400901. [DOI] [PubMed] [Google Scholar]
- 143.Marra A, Ely EW, Pandharipande PP, Patel MB. The ABCDEF bundle in critical care. Crit. Care Clin. 2017;33:225–243. doi: 10.1016/j.ccc.2016.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Schnitker L, et al. Prevention of delirium in older adults with dementia: a systematic literature review. J. Gerontol. Nurs. 2020;46:43–54. doi: 10.3928/00989134-20200820-02. [DOI] [PubMed] [Google Scholar]
- 145.Freter S, Koller K, Dunbar M, MacKnight C, Rockwood K. Translating delirium prevention strategies for elderly adults with hip fracture into routine clinical care: a pragmatic clinical trial. J. Am. Geriatr. Soc. 2017;65:567–573. doi: 10.1111/jgs.14568. [DOI] [PubMed] [Google Scholar]
- 146.Stenvall M, Berggren M, Lundström M, Gustafson Y, Olofsson B. A multidisciplinary intervention program improved the outcome after hip fracture for people with dementia-subgroup analyses of a randomized controlled trial. Arch. Gerontol. Geriatr. 2012;54:e284–e289. doi: 10.1016/j.archger.2011.08.013. [DOI] [PubMed] [Google Scholar]
- 147.Moretti R, Torre P, Antonello RM, Cattaruzza T, Cazzato G. Cholinesterase inhibition as a possible therapy for delirium in vascular dementia: a controlled, open 24-month study of 246 patients. Am. J. Alzheimers Dis. Other Demen. 2004;19:333–339. doi: 10.1177/153331750401900607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.van Eijk MM, et al. Effect of rivastigmine as an adjunct to usual care with haloperidol on duration of delirium and mortality in critically ill patients: a multicentre, double-blind, placebo-controlled randomised trial. Lancet. 2010;376:1829–1837. doi: 10.1016/S0140-6736(10)61855-7. [DOI] [PubMed] [Google Scholar]
- 149.Chan MT, Cheng BC, Lee TM, Gin T. BIS-guided anesthesia decreases postoperative delirium and cognitive decline. J. Neurosurg. Anesthesiol. 2013;25:33–42. doi: 10.1097/ANA.0b013e3182712fba. [DOI] [PubMed] [Google Scholar]
- 150.Radtke FM, et al. Monitoring depth of anaesthesia in a randomized trial decreases the rate of postoperative delirium but not postoperative cognitive dysfunction. Br. J. Anaesth. 2013;110(Suppl. 1):i98–i105. doi: 10.1093/bja/aet055. [DOI] [PubMed] [Google Scholar]
- 151.Wilchesky M, et al. The prevention program for Alzheimer’s related delirium (PREPARED) cluster randomized trial: a study protocol. BMC Geriatr. 2021;21:645. doi: 10.1186/s12877-021-02558-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Boockvar KS, Teresi JA, Inouye SK. Preliminary data: an adapted hospital elder life program to prevent delirium and reduce complications of acute illness in long-term care delivered by certified nursing assistants. J. Am. Geriatr. Soc. 2016;64:1108–1113. doi: 10.1111/jgs.14091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Marcantonio ER, Flacker JM, Wright RJ, Resnick NM. Reducing delirium after hip fracture: a randomized trial. J. Am. Geriatr. Soc. 2001;49:516–522. doi: 10.1046/j.1532-5415.2001.49108.x. [DOI] [PubMed] [Google Scholar]
- 154.Cole MG, Ciampi A, Belzile E, Dubuc-Sarrasin M. Subsyndromal delirium in older people: a systematic review of frequency, risk factors, course and outcomes. Int. J. Geriatr. Psychiatry. 2013;28:771–780. doi: 10.1002/gps.3891. [DOI] [PubMed] [Google Scholar]
- 155.Oh ES, et al. A roadmap to advance delirium research: recommendations from the NIDUS Scientific Think Tank. Alzheimers Dement. 2020;16:726–733. doi: 10.1002/alz.12076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Oldham MA. Delirium disorder: unity in diversity. Gen. Hosp. Psychiatry. 2022;74:32–38. doi: 10.1016/j.genhosppsych.2021.11.007. [DOI] [PubMed] [Google Scholar]
- 157.Oldham MA, Holloway RG. Delirium disorder: integrating delirium and acute encephalopathy. Neurology. 2020;95:173–178. doi: 10.1212/WNL.0000000000009949. [DOI] [PubMed] [Google Scholar]
- 158.Helms J, et al. Delirium and encephalopathy in severe COVID-19: a cohort analysis of ICU patients. Crit. Care. 2020;24:491. doi: 10.1186/s13054-020-03200-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Ticinesi A, et al. Delirium in COVID-19: epidemiology and clinical correlations in a large group of patients admitted to an academic hospital. Aging Clin. Exp. Res. 2020;32:2159–2166. doi: 10.1007/s40520-020-01699-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.O’Hanlon S, Inouye SK. Delirium: a missing piece in the COVID-19 pandemic puzzle. Age Ageing. 2020;49:497–498. doi: 10.1093/ageing/afaa094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Pun BT, et al. Prevalence and risk factors for delirium in critically ill patients with COVID-19 (COVID-D): a multicentre cohort study. Lancet Respir. Med. 2021;9:239–250. doi: 10.1016/S2213-2600(20)30552-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Zhou J, Liu C, Sun Y, Huang W, Ye K. Cognitive disorders associated with hospitalization of COVID-19: results from an observational cohort study. Brain Behav. Immun. 2021;91:383–392. doi: 10.1016/j.bbi.2020.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Bianchetti A, et al. Clinical presentation of COVID19 in dementia patients. J. Nutr. Health Aging. 2020;24:560–562. doi: 10.1007/s12603-020-1389-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.American Geriatrics Society Expert Panel on Postoperative Delirium in Older Adults. American Geriatrics Society abstracted clinical practice guideline for postoperative delirium in older adults. J. Am. Geriatr. Soc. 2015;63:142–150. doi: 10.1111/jgs.13281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.National Institute for Health and Care Excellence. Delirium: Prevention, Diagnosis and Management (NICE, 2019). [PubMed]
- 166.McKay TB, et al. Tau as a serum biomarker of delirium after major cardiac surgery: a single centre case-control study. Br. J. Anaesth. 2022;129:e13–e16. doi: 10.1016/j.bja.2022.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Sajjad MU, et al. Cerebrospinal fluid levels of interleukin-8 in delirium, dementia, and cognitively healthy patients. J. Alzheimers Dis. 2020;73:1363–1372. doi: 10.3233/JAD-190941. [DOI] [PubMed] [Google Scholar]
- 168.Fong TG, et al. Association of CSF Alzheimer’s disease biomarkers with postoperative delirium in older adults. Alzheimers Dement. 2021;7:e12125. doi: 10.1002/trc2.12125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Emmerton D, Abdelhafiz AH. Care for older people with dementia during COVID-19 pandemic. SN Compr. Clin. Med. 2021;3:437–443. doi: 10.1007/s42399-020-00715-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Kennedy M, et al. Delirium in older patients with COVID-19 presenting to the emergency department. JAMA Netw. Open. 2020;3:e2029540. doi: 10.1001/jamanetworkopen.2020.29540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.McLoughlin BC, et al. Functional and cognitive outcomes after COVID-19 delirium. Eur. Geriatr. Med. 2020;11:857–862. doi: 10.1007/s41999-020-00353-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Marengoni A, Zucchelli A, Grande G, Fratiglioni L, Rizzuto D. The impact of delirium on outcomes for older adults hospitalised with COVID-19. Age Ageing. 2020;49:923–926. doi: 10.1093/ageing/afaa189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Poloni TE, et al. Prevalence and prognostic value of delirium as the initial presentation of COVID-19 in the elderly with dementia: an Italian retrospective study. EClinicalMedicine. 2020;26:100490. doi: 10.1016/j.eclinm.2020.100490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Ismail II, Kamel WA, Al-Hashel JY. Association of COVID-19 pandemic and rate of cognitive decline in patients with dementia and mild cognitive impairment: a cross-sectional study. Gerontol. Geriatr. Med. 2021;7:23337214211005223. doi: 10.1177/23337214211005223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Tondo G, Sarasso B, Serra P, Tesser F, Comi C. The impact of the COVID-19 pandemic on the cognition of people with dementia. Int. J. Env. Res. Public Health. 2021;18:4825. doi: 10.3390/ijerph18084285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.LaHue SC, et al. Collaborative delirium prevention in the age of COVID-19. J. Am. Geriatr. Soc. 2020;68:947–949. doi: 10.1111/jgs.16480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Inouye SK. The importance of delirium and delirium prevention in older adults during lockdowns. JAMA. 2021;325:1779–1780. doi: 10.1001/jama.2021.2211. [DOI] [PMC free article] [PubMed] [Google Scholar]