Abstract
lisRK encodes a two-component regulatory system in the food pathogen Listeria monocytogenes LO28. Following identification of the operon in an acid-tolerant Tn917 mutant, a deletion in the histidine kinase component was shown to result in a growth phase variation in acid tolerance, an ability to grow in high ethanol concentrations, and a significant reduction in virulence.
The success of Listeria monocytogenes as a pathogen owes much to its ability to sense and respond to its environment. A number of studies have demonstrated that the ability of L. monocytogenes to respond to stress has consequences in terms of the virulence of the organism (18, 20, 22). In many other bacteria, the link between variations in environmental factors and enhanced or reduced virulence has been shown to result from the sensing and regulatory activities of two-component signal transduction systems (8, 11). A typical two-component system consists of a membrane-associated histidine kinase, which monitors a specific environmental parameter, and a cytoplasmic response regulator, which enables the cell to respond, often via regulation of gene expression, when this parameter varies (21, 27). In this study our aim was to identify mutants of L. monocytogenes with altered abilities to respond to stress and to investigate whether such alterations influence virulence. We describe the identification of a two-component signal transduction system and demonstrate that it functions in stress response and plays a role in in vivo survival. This represents the only description, other than that of the cheA-cheY system (7), of genes encoding a two-component regulatory system in L. monocytogenes.
Isolation and characterization of a mutant of L. monocytogenes with enhanced stationary-phase acid tolerance.
An L. monocytogenes LO28 (serotype 1/2c) Tn917 bank was created by using the temperature-sensitive plasmid pTV1-OK (12). Isolated transformants were grown overnight in tryptic soy broth-yeast extract (TSB-YE) containing kanamycin (50 μg/ml) at 30°C, subcultured (1%) into TSB-YE containing erythromycin (0.04 μg/ml) at 42°C, and selected for kanamycin-sensitive Tn917 integrants on tryptic soy agar-yeast extract containing erythromycin (10 μg/ml). Overnight cultures of the transposon bank were exposed to TSB-YE (adjusted to pH 4) for 36 h. While no survivors were recovered from the LO28 control, some 140 pinpoint and 3 regularly sized colonies were recovered from the bank. Southern hybridization showed that Tn917 had integrated only once and in different locations in each of the three isolates which had given rise to the regular colonies (data not shown). Inverse PCR was used to isolate chromosomal DNA from one isolate, LO28-M9; in this method HpaI was used to generate upstream flanking DNA, and XbaI and HindIII were used to generate downstream DNA. The inverse-PCR products were cloned into pBluescript.
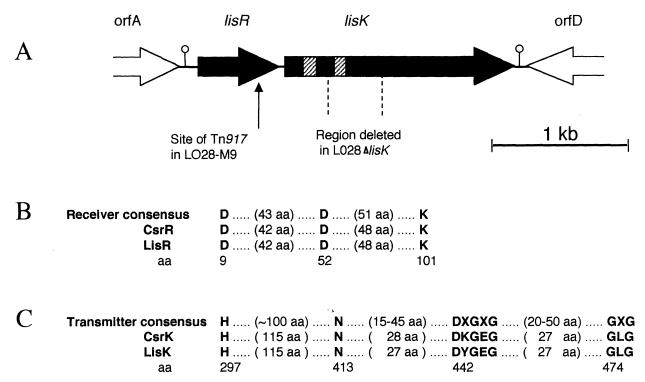
In total, 3,619 bp flanking the point of insertion was sequenced from both strands. Analysis showed that this region contained two partial and two complete open reading frames (ORFs) (Fig. 1A). The ORF into which the transposon had inserted demonstrates a high degree of similarity to regulator components of many bacterial two-component systems. The predicted amino-terminal receiver domain of this ORF includes the invariant aspartate and lysine residues conserved in this family of regulatory proteins (Fig. 1B). Alignments indicated greatest similarity in both the receiver and output domains to the group A Streptococcus regulator of hyaluronic acid capsule synthesis CsrR (17), the uncharacterized Bacillus subtilis putative regulator YkoG (GenBank accession no. AJ002571), and a Synechocystis sp. regulator (accession no. D64002), all of which are members of the OmpR-PhoB subfamily of response regulators. The carboxy termini of members of this subfamily are thought to have DNA-binding roles, and several have been shown to target specific sites upstream from the promoters that they regulate. It is thus probable that the putative protein is also a transcriptional activator. The listerial regulator gene was subsequently assigned the designation lisR. The start codon for this gene can be predicted from the high degree of similarity exhibited at the N termini of response regulators. A putative ribosome-binding site (5′-AGAGG-3′) was identified 9 bp upstream from this location.
FIG. 1.
Graphic illustration of the lisRK locus. (A) Line drawing of the orientation of ORFs flanking the Tn917 insertion site. The striped regions within lisK indicate the potential membrane-spanning regions, while the lollipops indicate putative rho-independent terminators. The 498-bp region deleted in the mutant LO28ΔlisK is also indicated. (B) Alignment of the predicted receiver domains of both lisR and csrR and the consensus sequence motif for the receiver element of response regulators (27). (C) Alignment of the predicted transmitter domain of both lisR and csrR and the consensus sequence motif for the transmitter element of histidine kinase sensor proteins (27). aa, amino acids.
Immediately downstream from lisR, an ORF encoding a histidine kinase was identified. The carboxy terminus of the predicted histidine kinase, designated LisK, most resembles LlkinA from Lactococcus lactis (19), CsrS (the partner of CsrR) (17), and YkoH (the partner of YkoG)—all of which have transmitters typical of the EnvZ-NarX family of histidine kinases. An alignment (Fig. 1C) also demonstrates the presence of the H, N, G1, and G2 boxes conserved among transmitter domains. In contrast to the carboxy termini, which are usually cytoplasmic in location, the amino termini of histidine kinases are quite often membrane bound, containing at least two membrane-spanning regions with a periplasmic loop. The membrane-spanning regions, which can be identified as stretches of hydrophobic amino acids, are also present in LisK (data not shown). It is not surprising that a procedure designed to identify genes involved in sensing and responding to acid stress should have identified a two-component regulatory system, the most-characterized mechanism by which environmental signals can be sensed in bacteria. There are numerous examples of two-component systems which regulate virulence factor production in response to specific environmental signals (8, 11).
The amino acid sequence deduced from the incomplete upstream orfA showed high identity with the Escherichia coli enzyme 6-phosphogluconate dehydrogenase, while the downstream orfD most closely resembled a variety of inner membrane proteins found in E. coli. However, due to the presence of putative rho-independent terminators (hairpin loops) both upstream of lisR and immediately downstream of lisK, it appears that lisRK constitutes a discrete transcriptional unit.
Creation and analysis of a lisK deletion mutant.
A mutant, with a nonpolar 498-bp deletion in lisK from nucleotide 1872 to 2369, was created to confirm that the Tn917 insertion event was responsible for the enhanced survival at low pHH displayed by LO28-M9. The SOE (splicing by overlap extension) PCR procedure (13) was used to splice two 348-bp PCR products from either side of the sequence to be deleted. This hybrid was subsequently cloned into the temperature-sensitive shuttle vector pKSV7 (25) and transformed into LO28. An allelic exchange between the SOE product on pKSV7 and the intact gene resulted in the removal of a 498-bp sequence encoding one of the hydrophobic regions and the conserved histidine of the histidine kinase (Fig. 1A). The mutation was confirmed by PCR analysis, and the mutant was subsequently designated LO28ΔlisK.
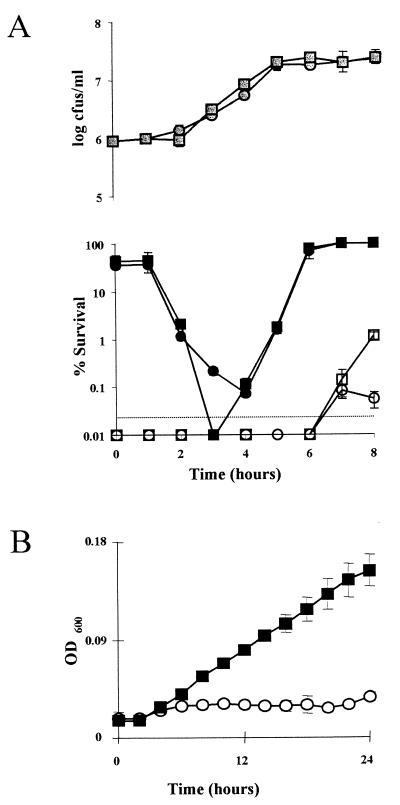
Carbohydrate utilization (as assayed by API-CH50), listeriolysin O production on blood agar plates, and phospholipase production on egg yolk emulsion plates (all at 37°C) were found to be unaffected in LO28ΔlisK (data not shown). As LO28-M9 was isolated as an acid-tolerant mutant, the acid resistance of the deletion mutant was tested. The growth phase acid sensitivities of LO28ΔlisK and the parent strain were tested by inoculating (2%) overnight cultures into TSB-YE, removing aliquots at regular intervals, and inoculating them into TSB-YE adjusted to pH 3.5 with 3 M lactic acid. Survivors were counted after 45- and 140-min periods. With the longer acid exposure time, a significant difference (0.01 < P < 0.02) was observed, in that the mutant was more resistant to acid than the parent during stationary phase (Fig. 2A), thus confirming the M9 phenotype. This mutant was used for further analysis. No difference could be observed at lower cell densities because this treatment regimen reduced bacterial numbers to below detectable levels. However, when the time of exposure to low pH was reduced to 45 min, the mutant was shown to be significantly more sensitive (0.01 < P < 0.02) than the parent strain during early logarithmic growth (Fig. 2A). No difference between stationary-phase cells could be observed due to the reduced exposure to acidified media. These results indicate that the relative resistance of the mutant is growth phase dependent. These results also matched those obtained with the original LO28-M9 mutant, confirming that transposon insertion in lisR was responsible for the mutant’s selection in acidified broth (data not shown). It is tempting to speculate that a signal which would normally stimulate LisRK to induce acid tolerance is missed during the early stages of growth, leading to an extremely sensitive cell. However, this supposes that a separate induction event which overcompensates for the lack of LisRK by inducing even greater tolerance subsequently occurs. In response to the altered acid resistance profile of LO28ΔlisK, the effects of several other stresses were examined. Overnight cultures were inoculated (2%) into TSB adjusted to pH 5.75 with lactic acid, with 7% NaCl, or with 5% ethanol. Growth was determined with a Spectra max 340 spectrophotometer (Molecular Devices) over a 24-h period. The mutant proved able to grow in the presence of 5% ethanol, a concentration which is bacteriostatic for the parent strain (Fig. 2B). This result was representative of a trend that was also apparent for a number of other ethanol concentrations (data not shown). Ethanol stress, and alcohol stress in general, is thought to be exerted by a reduction of cytoplasmic membrane integrity (15, 23). An increase in unsaturated-fatty-acid content has been shown to aid growth and survival when the organisms are exposed to ethanol (14). It may be at the membrane that the two-component system ultimately exerts an influence, as it has been shown that the composition of the cell membrane reflects the relative acid tolerance of a bacterial strain (2, 4, 5).
FIG. 2.
(A) Percent survival of cultures of LO28 and LO28ΔlisK in TSB-YE (pH 3.5) after 45 min (● and ■, respectively) and 120 min (○ and □, respectively), at different points during growth ( and ░⃞, respectively). Points below the dotted line indicate that there were no survivors. (B) Growth of LO28 (○) and LO28ΔlisK (■) in TSB-YE supplemented with 5% ethanol at 37°C. Error bars represent the standard deviations of triplicate experiments. OD600, optical density at 600 nm.
However, relative growth rates were not affected under other stress conditions, such as media adjusted to various pHs or containing various concentrations of salt (data not shown). These results were similar to those seen in LO28-M9. As it is likely that the Tn917 insertion in LO28-M9 resulted in a polar mutation in lisK, it is possible to attribute the phenotypic changes common in both mutants to lisK mutations.
LO28ΔlisK is affected in virulence compared with LO28.
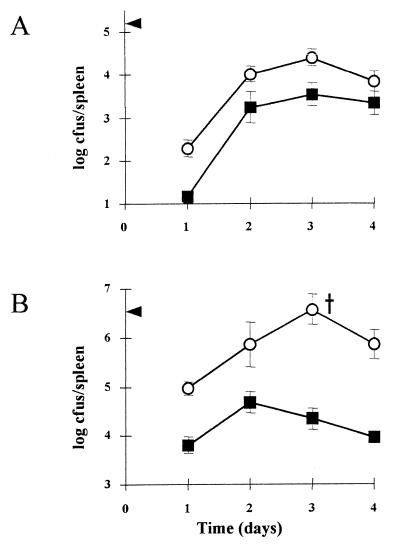
A mouse model was used to measure any possible impact of the ΔlisK mutation on virulence. The number of bacteria in the spleen was recorded, as this reflects the progress of the infection (16). The data show that, regardless of whether a low (1 × 105-CFU) or high (2 × 106-CFU) dose is used, the number of LO28ΔlisK cells in the spleen 1 day postinfection is much lower than that for wild-type-infected mice (Fig. 3). This represents a significant attenuation of the virulence of the bacteria. It is necessary to understand the consequence of intraperitoneal inoculation to appreciate how alterations in stress-induced responses can alter the virulence of Listeria. Initially, the host immune system responds via the arrival of infiltrating polymorphonuclear neutrophils and subsequently of macrophages (9). Following phagocytosis, Listeria produces hemolysin and phosphatidyl inositol phospholipase C to enable its escape from the phagosome (1, 3, 10). It seems to be at this stage of escape that the sensing of and response to stresses in vivo are most important: firstly, to adapt to the presence of toxic radicals, acidification, and degradative enzymes (24) and, secondly, to activate the production of virulence factors, many of which have been shown to be regulated by environmental conditions (6, 26). Thus, there is increasing evidence that stress responses, possibly through a number of pathways, can regulate the virulence of L. monocytogenes.
FIG. 3.
Growth of LO28 (○) and LO28ΔlisK (■) in the spleens of BALB/c mice. Mice were inoculated with 1 × 105 (A) and 2 × 106 (B) Listeria cells by the intraperitoneal route. The arrowheads indicate the numbers inoculated into the peritoneal cavity on day 0. The cross indicates that one mouse died at that time point. Each datum point represents the mean log10 number of Listeria cells per spleen for four mice. Error bars represent the standard deviations of triplicate experiments. (A) Differences were significant on days 1 and 3 (0.1 < P < 0.5). (B) Differences were significant on days 1, 3, and 4 (0.01 < P < 0.02). In all cases, significance was calculated by using the Student t test.
It is thus significant that genes encoding LisRK, initially identified as having a role in stress response, are also important in the virulence of this pathogen. These results contribute to the increasing evidence of the importance of functional stress-responsive mechanisms for a successful infection.
Nucleotide sequence accession number.
The sequence of the 3,619-bp DNA determined in this study has been deposited in GenBank under accession no. AF139908.
Acknowledgments
We thank Kathryn J. Boor for providing plasmid pKSV7 and advice on the SOE PCR technique.
This work has been funded by grant aid under the Food Sub-Programme administered by the Department of Agriculture, Food and Forestry and is supported by national and EU funds.
REFERENCES
- 1.Bielecki J, Youngman P, Connelly P, Portnoy D A. Bacillus subtilis expressing a haemolysin gene from Listeria monocytogenescan grow in mammalian cells. Nature. 1990;345:175–176. doi: 10.1038/345175a0. [DOI] [PubMed] [Google Scholar]
- 2.Brown J L, Ross T, McMeekin T A, Nichols P D. Acid habituation of Escherichia coliand the potential role of cyclopropane fatty acids in low pH tolerance. Int J Food Microbiol. 1997;37:163–173. doi: 10.1016/s0168-1605(97)00068-8. [DOI] [PubMed] [Google Scholar]
- 3.Camilli A, Goldfine H, Portnoy D A. Listeria monocytogenesmutants lacking phosphatidyl-specific phospholipase C are avirulent. J Exp Med. 1991;173:751–754. doi: 10.1084/jem.173.3.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chou L S, Weimer B. Isolation and characterization of acid- and bile-tolerant isolates from strains of Lactobacillus acidophilus. J Dairy Sci. 1999;82:23–31. doi: 10.3168/jds.S0022-0302(99)75204-5. [DOI] [PubMed] [Google Scholar]
- 5.Correa O S, Rivas E A, Barneix A J. Cellular envelopes and tolerance to acid pH in Mesorhizobium loti. Curr Microbiol. 1999;38:329–334. doi: 10.1007/pl00006812. [DOI] [PubMed] [Google Scholar]
- 6.Datta A R, Kothary M H. Effects of glucose, growth temperature, and pH on listeriolysin O production in Listeria monocytogenes. Appl Environ Microbiol. 1993;59:3495–3497. doi: 10.1128/aem.59.10.3495-3497.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dons L, Olsen J E, Rasmussen O F. Characterization of two putative Listeria monocytogenesgenes encoding polypeptides homologous to the sensor protein CheA and the response regulator CheY of chemotaxis. DNA Sequence. 1994;4:301–311. doi: 10.3109/10425179409020856. [DOI] [PubMed] [Google Scholar]
- 8.Forst S A, Roberts D L. Signal transduction by the EnvZ-OmpR phosphotransfer system in bacteria. Res Microbiol. 1994;145:363–373. doi: 10.1016/0923-2508(94)90083-3. [DOI] [PubMed] [Google Scholar]
- 9.Gahan C G M, Collins J K. Non-dystrophic 129 REJ mice are susceptible to i.p. infection with Listeria monocytogenesdespite an ability to recruit inflammatory neutrophils to the peritoneal cavity. Microb Pathog. 1995;18:355–364. doi: 10.1006/mpat.1995.0032. [DOI] [PubMed] [Google Scholar]
- 10.Gaillard J-L, Berche P, Mounier J, Richard S, Sansonetti P. In vitro model of penetration and intracellular growth of Listeria monocytogenesin the human enterocyte-like cell line Caco-2. Infect Immun. 1987;55:2822–2829. doi: 10.1128/iai.55.11.2822-2829.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garcia Vescovi E, Soncini F C, Groisman E A. The role of the PhoP/PhoQ regulon in Salmonellavirulence. Res Microbiol. 1994;145:473–480. doi: 10.1016/0923-2508(94)90096-5. [DOI] [PubMed] [Google Scholar]
- 12.Gutierrez J A, Crowley P J, Brown D P, Hillman J D, Youngman P, Bleiweis A S. Insertional mutagenesis and recovery of interrupted genes of Streptococcus mutans by using transposon Tn917: preliminary characterization of mutants displaying acid sensitivity and nutritional requirements. J Bacteriol. 1996;178:4166–4175. doi: 10.1128/jb.178.14.4166-4175.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Horton R M, Cai Z, Ho S N, Pease L R. Gene splicing by overlap extension: tailor-made genes using the polymerase chain reaction. BioTechniques. 1990;8:528–534. [PubMed] [Google Scholar]
- 14.Ingram L O, Vreeland N S, Eaton L C. Alcohol tolerance in Escherichia coli. Pharmacol Biochem Behav. 1980;13:191–195. doi: 10.1016/s0091-3057(80)80030-x. [DOI] [PubMed] [Google Scholar]
- 15.Ingram L O, Buttke T M. Effects of alcohols on micro-organisms. Adv Microb Physiol. 1984;25:253–300. doi: 10.1016/s0065-2911(08)60294-5. [DOI] [PubMed] [Google Scholar]
- 16.Kongshavn P A L. Genetic control of the murine listeriosis expressed in the macrophage response. Immunol Lett. 1985;11:181–188. doi: 10.1016/0165-2478(85)90166-x. [DOI] [PubMed] [Google Scholar]
- 17.Levin J L, Wessels M R. Identification of csrR/csrS, a genetic locus that regulates hyaluronic acid capsule synthesis in group A Streptococcus. Mol Microbiol. 1998;30:209–219. doi: 10.1046/j.1365-2958.1998.01057.x. [DOI] [PubMed] [Google Scholar]
- 18.Marron L, Emerson N, Gahan C G M, Hill C. A mutant of Listeria monocytogenesLO28 unable to induce an acid tolerance response displays diminished virulence in a murine model. Appl Environ Microbiol. 1997;63:4945–4947. doi: 10.1128/aem.63.12.4945-4947.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.O’Connell-Motherway M, Fitzgerald G F, van Sinderen D. Cloning and sequence analysis of putative histidine protein kinases isolated from Lactococcus lactisMG1363. Appl Environ Microbiol. 1997;63:2454–2459. doi: 10.1128/aem.63.6.2454-2459.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O’Driscoll B, Gahan C G M, Hill C. Adaptive acid tolerance response in Listeria monocytogenes: isolation of an acid-tolerant mutant which demonstrates increased virulence. Appl Environ Microbiol. 1996;62:1693–1698. doi: 10.1128/aem.62.5.1693-1698.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Parkinson J S. Signal transduction schemes of bacteria. Cell. 1993;73:857–871. doi: 10.1016/0092-8674(93)90267-t. [DOI] [PubMed] [Google Scholar]
- 22.Rouquette C, Ripio M T, Pellegrini E, Bolla J M, Tascon R L, Vasquez-Boland J A, Berche P. Identification of a ClpC ATPase required for stress tolerance and in vivo survival of Listeria monocytogenes. Mol Microbiol. 1996;21:977–987. doi: 10.1046/j.1365-2958.1996.641432.x. [DOI] [PubMed] [Google Scholar]
- 23.Sikkema J, de Bont J A M, Poolman B. Mechanisms of membrane toxicity of hydrocarbons. Microbiol Rev. 1995;59:201–222. doi: 10.1128/mr.59.2.201-222.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Small P L C, Ramakrishanan L, Falkow S. Remodeling schemes of intracellular pathogens. Science. 1994;263:637–639. doi: 10.1126/science.8303269. [DOI] [PubMed] [Google Scholar]
- 25.Smith K, Youngman P. Use of a new integrational vector to investigate compartment-specific expression of the Bacillus subtilisspoIIM gene. Biochimie. 1992;74:705–711. doi: 10.1016/0300-9084(92)90143-3. [DOI] [PubMed] [Google Scholar]
- 26.Sokolovic Z, Riedel J, Wuenscher M, Goebel W. Surface-associated, PrfA-regulated proteins of Listeria monocytogenessynthesized under stress conditions. Mol Microbiol. 1993;8:219–227. doi: 10.1111/j.1365-2958.1993.tb01566.x. [DOI] [PubMed] [Google Scholar]
- 27.Stock J B, Ninfa A J, Stock A M. Protein phosphorylation and regulation of adaptive responses in bacteria. Microbiol Rev. 1989;53:450–490. doi: 10.1128/mr.53.4.450-490.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]