Abstract
Background and Objectives: Male hypogonadism is a clinical disorder characterized by reduced serum testosterone in men. Although treatment using herbal medicines, including Eurycoma longifolia, has been investigated, the benefits remain unclear. This study aims to investigate the efficacy of E. longifolia as a sole intervention to increase testosterone levels in males. Materials and Methods: We conducted a systematic review and meta-analysis of randomized clinical trials (RCTs) according to the PRISMA guidelines. Relevant articles were retrieved from the databases PubMed, Scopus, Web of Science, Cochrane, Ovid/Embase, and Google Scholar. Results: After literature screening, a total of nine studies was included in the systematic review. Five RCTs were included in the meta-analysis. A significant improvement in total testosterone levels after E. longifolia treatment was mostly reported in both healthy volunteers and hypogonadal men. The random model effect revealed a significant increase (SMD = 1.352, 95% CI 0.565 to 2.138, p = 0.001) in the total testosterone levels in men receiving E. longifolia supplementation, which was confirmed in the hypogonadism subgroup. Conclusions: This systematic review and meta-analysis of the literature supports the possible use of E. longifolia supplementation for enhancing testosterone production. Although more research is required before its use in clinical practice, this may represent a safe and promising therapeutic option, particularly in hypogonadal men.
Keywords: Eurycoma longifolia, Tongkat ali, testosterone, male, hypogonadism
1. Introduction
Male hypogonadism is a clinical disorder that arises from a failure of the testes to produce adequate levels of testosterone, mainly mediated through a disruption of the hypothalamic–pituitary–gonadal (HPG) axis [1]. Hypogonadism is estimated to affect 1.2–12.8% of middle-aged and older men in the general population, with increasing incidence and burden on healthcare services [2]. The biochemical definition of hypogonadism remains unclear; however, a 300 ng/dL threshold has been generally recommended as the lowest limit of normal [2], with general consensus that testosterone levels above 350 ng/dL do not require treatment. Conversely, patients with testosterone levels less than 230 ng/dL usually benefit from testosterone replacement therapy (TRT) [3,4]. Hypogonadism is further classified based on gonadotropin levels as primary (hypergonadotropic) or secondary (hypogonadotropic) [2].
Common clinical features of hypogonadism include reduced libido and erectile dysfunction, male factor infertility, obesity with reduced lean body mass, reduced bone density, fatigue, and depression [2]. Lower levels of testosterone in healthy men is a predictor of co-morbidities [5,6], such as obesity, metabolic syndrome, type 2 diabetes mellitus, cardiovascular disease, and osteoporosis. Clinically, management of hypogonadism is focused on TRT that can improve sexual dysfunction and well-being, reduce obesity, increase lean body mass, and increase bone density [7]. Although TRT has become a multimillion dollar market, only about 10% of men with hypogonadism in the USA and Europe are being treated with TRT [8]. Furthermore, TRT is contraindicated in patients who want to preserve fertility and those with prostate carcinoma, benign prostatic hyperplasia and/or lower urinary tract symptoms, high serum prostatic specific antigen (PSA) levels, obstructive sleep apnoea, or patients with a history of myocardial or cerebrovascular stroke [1,2]. The benefits compared to risks of long-term TRT remain unclear in men treated for mild hypogonadism or age-related hypogonadism [7]. Hence, many physicians perceive the risks of TRT to be high.
Numerous herbal medicines have shown potential to increase serum testosterone levels and benefit sexual function and fertility [6,9,10]. In particular, Eurycoma longifolia Jack (Tongkat ali or Malaysian ginseng) has been traditionally used for management of male sexual dysfunction and infertility [11,12,13,14]. In addition, E. longifolia also possesses other medical properties, such as positively impacting athletic performance and muscular bulk, reducing adiposity, stimulating appetite, and treating fatigue, malaria, diabetes, anxiety, osteoporosis, cancer, constipation, and peptic ulcers [11,12,13,14,15].
E. longifolia is reported to improve libido and is being used as a common ingredient in more than 700 herbal or nutraceutical products marketed as aphrodisiacs [16,17,18,19]. Recent systematic reviews have summarized the benefits of E. longifolia to men’s reproductive health [20,21]. However, it remains unclear whether E. longifolia improves serum testosterone levels in men. With a high burden of hypogonadism in the general population, E. longifolia may provide benefit in increasing serum testosterone in men. Therefore, this study aims to investigate the efficacy of E. longifolia to increase testosterone in males, using a meta-analysis of available randomized clinical trials (RCTs).
2. Materials and Methods
2.1. Search Strategy and Risk-of-Bias Assessment
A systematic review and meta-analysis was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [22]. The following keyword combinations and Boolean operators were used: (“Tongkat” OR “Eurycoma” OR “longifolia” OR “pasak bumi”) AND (“testosterone”). The literature search was performed on 10 July 2021 to search articles published until that date on the databases PubMed, Scopus, Web of Science, Cochrane, Ovid/Embase, and Google Scholar. These databases were searched to identify clinical trials investigating the use of E. longifolia extraction as a sole intervention in adult males and reporting pre- and post-treatment serum testosterone as an outcome.
Animal studies, in vitro and in silico studies, meta-analyses, reviews, case reports, letters, editorials, comments, and non-English-language publications were excluded. In addition, the duplicate articles retrieved from different databases were removed. The remaining articles were screened independently for titles and abstracts by two authors (R.F. and K.L.) to exclude non-relevant studies, while any disagreement was settled by an additional researcher (M.K.P.S.). Irrelevant articles were removed, and full-text articles were screened for eligibility based on the inclusion and exclusion criteria. Data were extracted by using a precompiled Excel file, and included the study setting, cohort description, details of experimental herbal intervention extraction, dosage and duration of the experimental intervention, total (ng/dL) and free (ng/dL) serum testosterone measurements for cases and controls before and after treatment, along with sex hormone binding globulin (SHBG) (nmol/L) and dehydroepiandrosterone (µg/mL) assessments as secondary outcomes. In case numerical variables were not reported in the manuscripts, respective study authors were contacted via email and requested to share their results for inclusion in the meta-analysis. For the randomized trials included in this meta-analysis, the quality and the risk of bias were assessed independently by two authors (R.F., K.L.) using version 2 of the Cochrane risk-of-bias tool for randomized trials [23]. Both observational and randomized studies were included in the systematic review, whereas the meta-analysis was based on RCTs only.
2.2. Statistical Analysis
Meta-analysis was performed by using mean and standard deviation for continuous outcome variables (testosterone levels). Based on significance of Cochran’s Q value and I2 (inconsistency) statistics, either fixed or random effects models were used to analyze the pooled data. A subgroup analysis was conducted according to the testosterone levels of study subjects. Studies with subjects having low testosterone levels (<300 ng/dL) prior to treatment with E. longifolia were considered as showing hypogonadism. Furthermore, publication bias among the studies was assessed by Egger’s test [24] and Begg’s rank test [25]. All analyses were performed by using MedCalc Software (version 20.019, Ostend, Belgium).
3. Results
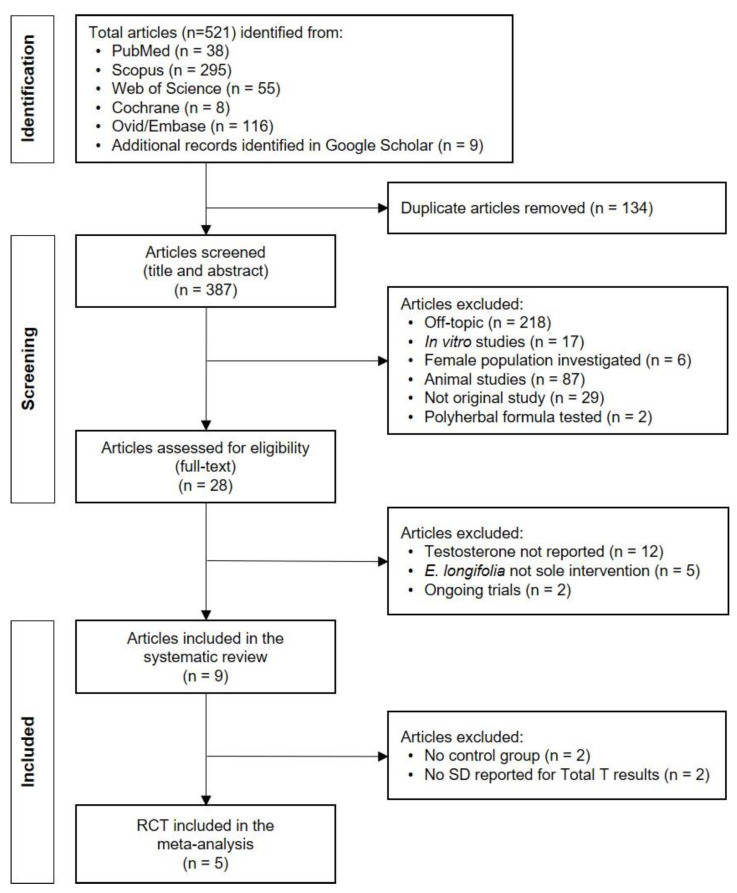
The search strategy identified a total of 521 articles (Figure 1). After removing duplicates (n = 134), the titles and abstracts of 387 articles were screened for inclusion, with a further 359 studies being excluded. The full text of identified articles (n = 28) was assessed based on the inclusion criteria. A total of nine studies was included in this systematic review, while five RCTs were included for meta-analysis.
Figure 1.
Flow diagram reporting the search strategy. SD: standard deviation.
3.1. Systematic Review
The literature screening identified a total of nine studies published between 2012 and 2021 that investigated the effect of E. longifolia on serum testosterone levels in men. Of these, two were comparative (pre- vs. post-) prospective studies, and five were double-blind, controlled trials (of these, four were randomized). Two additional RCTs were published as doctor of philosophy degree (PhD) dissertations. Characteristics of the included studies (n = 9) are reported in Table 1. There were three studies that investigated a male population affected by hypogonadism [26,27,28], while six studies included a population of healthy men with normal testosterone levels [29,30,31,32,33,34]. E. longifolia was investigated primarily as a commercial water-extracted product (Physta®, Biotropics, Berhad, Kuala Lumpur, Malaysia) in seven of the nine studies included [26,27,29,30,31,32,33], with variable dosages from 100 to 600 mg/daily for a minimum of 3 days to a maximum of 6 months (Table 1).
Table 1.
Characteristics of the studies investigating the effect of E. longifolia on serum testosterone levels in men.
| Reference | Study Design | Study Population | EL Supplement | Dosage | Testosterone (ng/dL) [Mean ± SD] |
p Value * |
|
|---|---|---|---|---|---|---|---|
| Treatment Group | Placebo Group | ||||||
| Chan et al., 2021 [29] | Double-blind, controlled trial | Healthy (18–30 y) men with no diagnosis of hypogonadism (n = 16) vs. controls (n = 16) | Physta, Biotropics | 600 mg/day for 2 weeks | Pre: 802 ± 160 Post: 924 ± 84 |
Pre: 791 ± 150 Post: 769 ± 135 |
N/A |
| Leitão et al., 2021 [28] | Double-blind RCT | Patients with ADAM, 40–59 y (n = 9) vs. controls (n = 12) | Dry extract | 200 mg up to 6 months | Pre: 278.2 ± 20.5 Post: 400.3 ± 38.9 |
Pre: 281.5 ± 17.7 Post: 258.5 ± 33.7 |
p < 0.05 |
| Patients with ADAM, 40–59 y, with concurrent exercise (n = 9) vs. controls (n = 7) | Pre: 253 ± 20.5 Post: 374.5 ± 38.9 |
Pre: 286.7 ± 21.7 Post: 370.8 ± 41.3 |
N/A | ||||
| Chinnappan et al., 2021 [26] | Double-blind, multicenter RCT | Healthy volunteers: 50–70 y (n = 35) vs. controls (n = 35) | Physta, Biotropics | 100 mg/daily up to 12 weeks | Pre: 187.3 ± 46.4 Post: 203.8 ± 54.6 |
Pre: 183.0 ± 37.8 Post: 177.9 ± 43.7 |
p < 0.05 |
| Healthy volunteers: 50–70 y (n = 35) vs. controls (n = 35) | 200 mg/daily up to 12 weeks | Pre: 200.5 ± 46.4 Post: 225.0 ± 49.8 |
Pre: 183.0 ± 37.8 Post: 177.9 ± 43.7 |
p < 0.05 | |||
| Quin, 2021 [34] | Double-blind RCT | Sedentary males (18–30 y) (n = 8) vs. controls (n = 8) | N/A | 600 mg for 2 weeks | Pre: 871 ± 200 Post: 968 ± 70 |
Pre: 863 ± 150 Post: 790 ± 150 |
p < 0.05 |
| Sedentary males (18–30 y) (n = 11) vs. controls (n = 10) | 600 mg for 8 weeks | Pre: 685 ± 240 Post: not reported |
Pre: 725 ± 170 Post: not reported |
N/S | |||
| Lim, 2017 [33] | Double-blind RCT | Men trained at least 3 times/week, 18–30 y, BMI: 18.5–25.0 (n = 9) vs. controls (n = 11) | Physta, Biotropics | 1.7 mg/kg of body weight for 3 days | Pre: 0.63 mmol/L Post: 0.86 mmol/L |
Pre: 0.82 mmol/L Post: 0.59 mmol/L |
p < 0.05 |
| 1.7 mg/kg of body weight for 5 weeks | Pre: 0.63 mmol/L Post: 1.26 mmol/L |
Pre: 0.67 mmol/L Post: 0.83 mmol/L |
p < 0.05 | ||||
| Henkel et al., 2014 [30] | Pre- vs. Post- | Male cyclists (57–72 y), with or without chronic diseases associated with age (n = 13) | Physta, Biotropics | 400 mg for 3 weeks | Pre: 384 ± 79 Post: 409 ± 102 |
N/A | N/A |
| 400 mg for 5 weeks | Pre: 384 ± 79 Post: 442 ± 115 |
N/A | N/A | ||||
| Tambi et al., 2012 [27] | Pre- vs. Post- | Patients with hypogonadism and LOH (n = 76) | Physta, Biotropics | 200 mg up to 1 month | Pre: 163 ± 43.5 Post: 240 ± 71.2 |
N/A | N/A |
| Ismail et al., 2012 [31] | Double-blind RCT | Healthy married men, 30–55 y, with or without stable chronic medical illnesses (n = 54) vs. controls (n = 55) | Physta, Biotropics | 300 mg for 12 weeks | Pre: 476 ± 167 Post: 435 to 479 |
Pre: 542 ± 133 Post: 522 to 549 |
N/S |
| George et al., 2013 [32] | Double-blind RCT | Healthy men (n = 21) vs. controls (n = 19) | Physta, Biotropics | 300 mg for 12 weeks | Pre: 458 ± 152.1 Post: 484 ± 165.3 |
Pre: 540 ± 177 Post: 542 ± 187 |
N/S |
N/A: not available; N/S: not significant. *: p value is reported for treatment vs. placebo group.
Most of the studies (n = 7) reported a significant improvement in total testosterone levels after E. longifolia treatment [26,27,28,29,30,33,34]. However, two studies failed to observe any improvement in testosterone levels when the treatment was stopped after 3 weeks [30] or prolonged to 8 weeks [34]. In addition, Ismail et al. and George et al. did not report any difference when healthy married men were treated [31,32]. Similarly, they did not observe any difference in comparison to the placebo-controlled group [31,32]. Chinnappan et al. observed a significant difference between healthy volunteers with testosterone lower than 300 ng/dL treated with E. longifolia (100 mg/daily or 200 mg/daily) or placebo for 12 weeks [26]. Similar results were also described in the two PhD dissertations, including young, 18–30-year-old participants who were either sedentary males [34] or active males who trained at least 3 times/week [33]. Chan et al. reported a significant intragroup increase in testosterone with 600 mg daily treatment of E. longifolia over 14 days, with no significant change in the placebo group. However, no intergroup statistical analysis was provided [29]. In patients with poor Androgen Deficiency in the Aging Male (ADAM) scores, testosterone significantly increased with 200 mg daily treatment of E. longifolia for up to 6 months compared to placebo [28]. Furthermore, there was a significant intragroup increase in testosterone over 6 months in poor-ADAM-score patients with 200 mg daily E. longifolia treatment alongside concurrent exercise. However, no statistical comparison was provided to compare poor-ADAM-score patients undergoing concurrent exercise with placebo to concurrent exercise with E. longifolia [28].
As secondary data available in some studies, free testosterone, dehydroepiandrosterone, and SHBG results were reported. Six of the included studies analyzed free testosterone as an outcome [26,29,30,31,32,34], whereas three reported a significant intragroup increase in free testosterone with E. longifolia [26,29,30], with no significant improvement reported by other studies [34,35]. Interestingly, six out of nine studies reported no significant variation in SHBG levels after treatment [26,29,30,31,32,34], although Quin observed a significant improvement in SHBG levels (p = 0.022) when sedentary young males were supplemented with E. longifolia (600 mg for 8 weeks) [34]. However, no improvement was reported in comparison with placebo-controlled groups [26,29,31,32,34]. Henkel et al. and George et al. did not observe any change in dehydroepiandrosterone levels after treatment [30,32]. Conversely, Chinnappan et al. observed a slight but significant improvement in the treated group, which disappeared when compared with the placebo-controlled group [26].
Only one study reported adverse effects associated with E. longifolia treatment [26], which included gastrointestinal symptoms and itching, while Ismael et al. observed adverse events in both treated and placebo groups [31].
3.2. Study Quality of RCTs
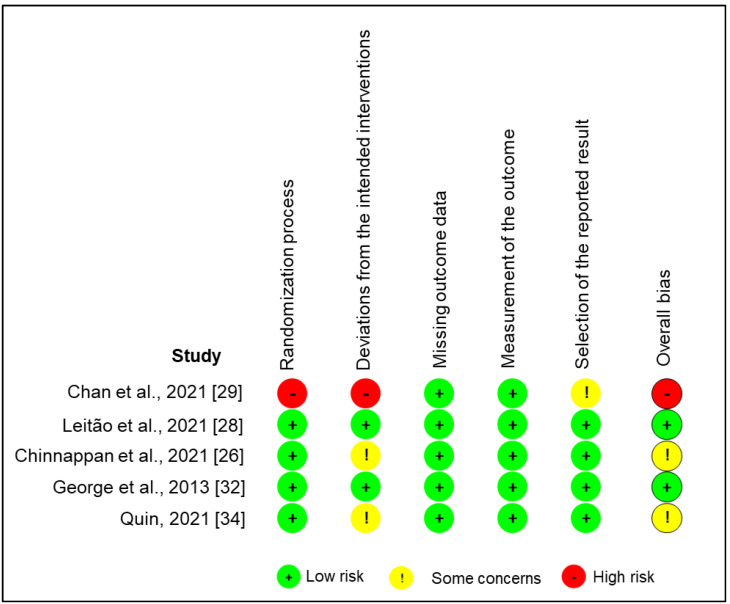
Five eligible RCTs measuring the testosterone levels in men (n = 232) were included in our meta-analysis [26,28,29,32,34]. Of these, some showed low (n = 2) or high (n = 1) risk of bias according to the Cochrane risk-of-bias tool, while others showed some concerns (n = 2) in the domain of deviations from the intended interventions (Figure 2).
Figure 2.
Assessment of quality and risk of bias of the studies included in the meta-analysis, using the version 2 of the Cochrane risk-of-bias tool for randomized trials [23]. Studies included: Chan et al., 2021 [29]; Leitão et al., 2021 [28]; Chinnappan et al., 2021 [26]; George et al., 2013 [32]; Quin, 2021 [34].
3.3. Meta-Analysis
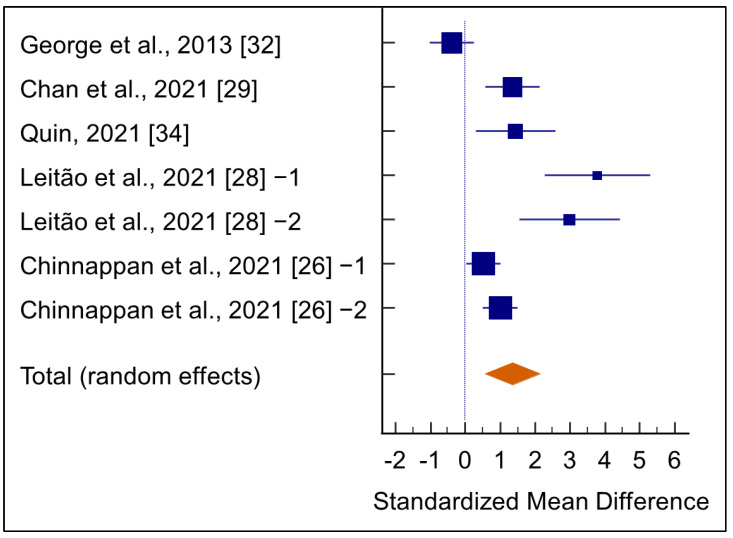
Tests for heterogeneity implicated heterogeneity across the five studies (Q= 47.1472, DF= 6, p < 0.0001) with 87.27% inconsistency (95% CI 76.06 to 93.24). The random model effect revealed a significant increase (SMD = 1.352, 95% CI 0.565 to 2.138, p = 0.001) in the testosterone levels in men receiving E. longifolia supplementation (Figure 3 and Table 2). Furthermore, a low p value (0.0243) was noticed with Begg’s test, indicating publication bias (Supplementary Table S1).
Figure 3.
Average net change in serum testosterone levels of men supplemented with Eurycoma longifolia. The pooled effect size is indicated by the diamond. As different groups were analyzed in their studies after E. longifolia supplementation, Leitão et al., 2021 [28], and Chinnappan et al., 2021 [26], are included twice in our analysis.
Table 2.
Meta-analysis of the outcome variable testosterone in serum.
| Study | EL Group (n) | CTRL (n) | Tot (n) | SMD | SE | 95% CI | t | p | Random Weight (%) |
|---|---|---|---|---|---|---|---|---|---|
| Studies evaluating testosterone levels in men supplemented with Eurycoma longifolia | |||||||||
| George et al., 2013 [32] | 21 | 19 | 40 | −0.388 | 0.313 | −1.022 to 0.247 | 15.84 | ||
| Chan et al., 2021 [29] | 16 | 16 | 32 | 1.344 | 0.383 | 0.561 to 2.127 | 15.10 | ||
| Quin et al., 2021 [34] | 8 | 8 | 16 | 1.438 | 0.537 | 0.287 to 2.589 | 13.33 | ||
| Leitão et al., 2021-1 * [28] | 9 | 12 | 21 | 3.783 | 0.721 | 2.274 to 5.292 | 11.16 | ||
| Leitão et al., 2021-2 * [28] | 11 | 7 | 18 | 2.983 | 0.678 | 1.546 to 4.419 | 11.66 | ||
| Chinnappan et al., 2021-1 * [26] | 35 | 35 | 70 | 0.518 | 0.240 | 0.0382 to 0.998 | 16.50 | ||
| Chinnappan et al., 2021-2 * [26] | 35 | 35 | 70 | 0.994 | 0.251 | 0.494 to 1.495 | 16.41 | ||
| Total (random effects) | 135 | 132 | 267 | 1.352 | 0.399 | 0.565 to 2.138 | 3.384 | 0.001 | 100.00 |
| Q = 47.1472, DF = 6, p < 0.0001, I2 = 87.27% (95% CI 76.06 to 93.24) | |||||||||
| Studies evaluating testosterone levels in normal healthy men supplemented with Eurycoma longifolia | |||||||||
| George et al., 2013 [32] | 21 | 19 | 40 | −0.388 | 0.313 | −1.022 to 0.247 | 35.39 | ||
| Chan et al., 2021 [29] | 16 | 16 | 32 | 1.344 | 0.383 | 0.561 to 2.127 | 34.02 | ||
| Quin et al., 2021 [34] | 8 | 8 | 16 | 1.438 | 0.537 | 0.287 to 2.589 | 30.59 | ||
| Total (random effects) | 45 | 43 | 88 | 0.760 | 0.654 | −0.540 to 2.060 | 1.162 | 0.249 | 100.00 |
| Q = 15.9216, DF = 2, p = 0.0003, I2 = 87.44% (95% CI 64.47 to 95.56) | |||||||||
| Studies evaluating testosterone levels in men with hypogonadism supplemented with Eurycoma longifolia | |||||||||
| Leitão et al., 2021-1 * [28] | 9 | 12 | 21 | 3.783 | 0.721 | 2.274 to 5.292 | 5.16 | ||
| Leitão et al., 2021-2 * [28] | 11 | 7 | 18 | 2.983 | 0.678 | 1.546 to 4.419 | 5.84 | ||
| Chinnappan et al., 2021-1 * [26] | 35 | 35 | 70 | 0.518 | 0.240 | 0.0382 to 0.998 | 46.40 | ||
| Chinnappan et al., 2021-2 * [26] | 35 | 35 | 70 | 0.994 | 0.251 | 0.494 to 1.495 | 42.60 | ||
| Total (random effects) | 90 | 89 | 179 | 1.861 | 0.579 | 0.719 to 3.002 | 3.217 | 0.002 | 100.00 |
| Q = 27.4384, DF = 3, p < 0.0001, I2 = 89.07% (95% CI 74.70 to 95.27) | |||||||||
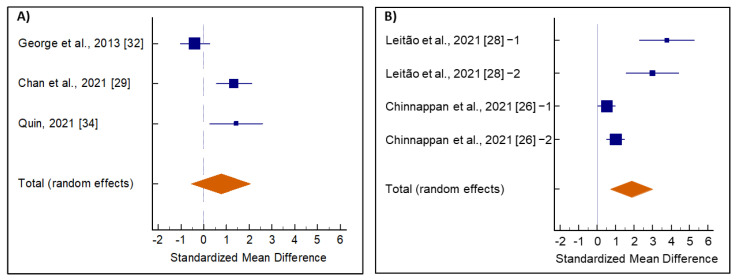
Results of the subgroup analysis are presented in Figure 4 and Table 2. There were increased testosterone levels in both groups of men with (testosterone < 300 ng/dL) and without hypogonadism (testosterone > 300 ng/dL) after E. longifolia supplementation, although the increase was significant in the hypogonadism group. Heterogeneity was noticed among the studies in both groups (Table 2). Furthermore, Egger’s and Begg’s tests revealed significant levels of publication bias among studies included in the hypogonadism group (Supplementary Table S1).
Figure 4.
Average net change in serum testosterone levels of (A) normal healthy men and (B) men with hypogonadism supplemented with Eurycoma longifolia. The pooled effect size is indicated by the diamond.
4. Discussion
E. longifolia is reported to have anti-inflammatory and immune-regulating properties, such as improving osteoporosis, diabetes, and metabolic complications, as well as having anti-malarial, anti-anxiolytic, cytotoxic, and anti-proliferative functions in malignancy. [12,13,14]. Furthermore, E. longifolia may lead to increased osteoblast proliferation and apoptosis of osteoclasts. This reduces bone loss in osteoporosis, and thus, it can be considered as an alternative to TRT in these patients [36]. This systematic review highlights the beneficial use of E. longifolia supplementation to enhance testosterone levels, particularly in those men suffering from hypogonadism. Most of the studies included in this systematic review utilize the same commercial, freeze-dried water extract of E. longifolia root standardized to 0.8–1.5% eurycomanone [26,27,29,30,31,32,33]. E. longifolia, predominantly the roots, contains active constituents such as quassinoids, quassinoid diterpenoids, canthin-6-one alkaloids, β-carboline alkaloids, squalene derivatives, triterpene-type tirucallane, tirucallane-type triterpenes, laurycolactone, and bioactive steroids [11,12,14,15]. Derivatives of quassinoids, a group of physiologically active diterpenoids, are further classified as eurycomanones, eurycomanols, eurycomalactones, eurycolactones, eurycomanosl, and eurycomaosides [12,37]. These molecules have shown inhibitory functions in vivo and in vitro, particularly anti-inflammatory, anti-viral, anti-malarial, and anti-proliferative activities [12,15,38]. Quassinoids present in E. longifolia also contribute towards ergogenic effects, including increased muscle strength and endurance in cycling time, along with anxiolytic properties [37].
Eurycomanone derivatives reportedly increase testosterone levels and have anti-estrogenic activity, improving spermatogenesis [12]. Animal studies reported higher release of luteinizing hormone (LH) and follicle-stimulating hormone (FSH) gonadotropins, resulting in increased testosterone production in the Leydig cells. In addition, there may be inhibition of the aromatase enzyme, which limits its conversion to estrogen after E. longifolia supplementation [12,39,40]. The proprietary extraction of E. longifolia has also been shown to improve semen quality in infertile males over 9 months, alongside an increase in spontaneous pregnancy [41]. Similarly, a polyherbal formulation that included E. longifolia improved semen volume, sperm concentration, and motility after 90 days in oligozoospermic males, alongside improved serum hormone levels compared to placebo [42]. In addition to semen quality, E. longifolia, along with Polygonum minus, improved the erection hardness scale, the aging male symptom scale, and the sexual health inventory as per the diaries of men, suggesting its possible use for the treatment of male infertility [43].
Despite its impact on testosterone production, E. longifolia does not seem to affect the ratio between urinary testosterone glucuronide and epitestosterone glucuronide (T:E ratio), which is frequently used to determine testosterone abuse in sports doping analysis (ratio of > 6 suggests previous abuse) [44]. Therefore, since E. longifolia does not breech the doping policies of international sports for testosterone or precursory abuse in athletes, it is safe for consumption [45,46].
Some of the studies included in our analysis reported no or minimal side effects after E. longifolia treatment [26,29,30]. This is of interest because TRT, representing the first therapeutic option in the case of hypogonadism, has been associated with the presence of side effects, such as polycythaemia, fluid retention, testicular atrophy, prostate enlargement, congestive heart failure, and obstructive sleep apnea [47]. Furthermore, in males and females, E. longifolia does not negatively affect AST/ALT or body weight, further supporting safe treatment with E. longifolia [48]. Therefore, E. longifolia extract may represent a promisingly safe treatment option for hypogonadism.
Although this meta-analysis suggests a possible use of E. longifolia supplementation to improve testosterone production, important limitations are to be highlighted. First and foremost, the analysis was based on five studies, which were heterogeneous in terms of study design, included population, dosage and length of treatment, and limited sample sizes. However, the inclusion of randomized controlled trials using E. longifolia as a sole intervention, as well as the Cochrane standardized tool for assessing quality of evidence, may represent the strength of this analysis since conclusions are based on quality publications, with just one study showing high risk of bias. In addition, most of the studies discussed in this systematic analysis used the same commercial product for E. longifolia supplementation, therefore limiting the potential influence of other components on the investigated outcomes. Importantly, the quality of E. longifolia can be determined based on eurycomanone concentrations, with a recommended level of 0.8–1.5 w/v (%) [49]. However, not all products on the market meet these requirements. Out of 41 products containing E. longifolia as a single or compound formulation from Malaysia, 24 products contained eurycomanone: 11/24 reached the recommended levels, while 9 were above the recommended levels (1.6–8.48% w/v). Some products did not contain any eurycomanone [49].
5. Conclusions
In conclusion, this systematic analysis of the literature highlights the possible use of E. longifolia supplementation for enhancing testosterone production. Although more research is required before its use in clinical practice, this may represent a safe and promising therapeutic option, particularly for patients with hypogonadism.
Acknowledgments
The authors thank Alice Erwig Leitão for sharing original data regarding testosterone assessment and for allowing the inclusion of her study (“Exercise associated or not to the intake of Eurycoma longifolia improves strength and cardiorespiratory fitness in men with androgen deficiency”) in our meta-analysis.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/1648-9144/58/8/1047/s1; Table S1: Publication bias across studies included in meta-analysis.
Author Contributions
Conceptualization, M.K.P.S. and K.L.; methodology, R.F.; data curation, R.F. and K.L.; writing—original draft preparation, K.L., R.F., M.K.P.S. and S.C.S.; writing—review and editing, K.L., R.F., M.K.P.S. and S.C.S. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The scientific studies analyzed in this meta-analysis are available in the databases PubMed, Scopus, Web of Science, Cochrane, Ovid/Embase, and Google Scholar.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bhasin S., Brito J.P., Cunningham G.R., Hayes F.J., Hodis H.N., Matsumoto A.M., Snyder P.J., Swerdloff R.S., Wu F.C., Yialamas M.A. Testosterone therapy in men with hypogonadism: An endocrine society. J. Clin. Endocrinol. Metab. 2018;103:1715–1744. doi: 10.1210/jc.2018-00229. [DOI] [PubMed] [Google Scholar]
- 2.Dandona P., Rosenberg M.T. A practical guide to male hypogonadism in the primary care setting. Int. J. Clin. Pract. 2010;64:682–696. doi: 10.1111/j.1742-1241.2010.02355.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang C., Nieschlag E., Swerdloff R., Behre H.M., Hellstrom W.J., Gooren L.J., Kaufman J.M., Legros J.J., Lunenfeld B., Morales A., et al. Investigation, treatment and monitoring of late-onset hypogonadism in males: ISA, ISSAM, EAU, EAA and ASA recommendations. Eur. J. Endocrinol. 2008;159:507–514. doi: 10.1530/EJE-08-0601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhasin S., Pencina M., Jasuja G.K., Travison T.G., Coviello A., Orwoll E., Wang P.Y., Nielson C., Wu F., Tajar A., et al. Reference ranges for testosterone in men generated using liquid chromatography tandem mass spectrometry in a community-based sample of healthy nonobese young men in the framingham heart study and applied to three geographically distinct cohorts. J. Clin. Endocrinol. Metab. 2011;96:2430–2439. doi: 10.1210/jc.2010-3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kasturi S.S., Tannir J., Brannigan R.E. The metabolic syndrome and male infertility. J. Androl. 2008;29:251–259. doi: 10.2164/jandrol.107.003731. [DOI] [PubMed] [Google Scholar]
- 6.Leisegang K., Roychoudhury S., Slama P., Finelli R. The mechanisms and management of age-related oxidative stress in male hypogonadism associated with non-communicable chronic disease. Antioxidants. 2021;10:1834. doi: 10.3390/antiox10111834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Darby E., Anawalt B. Male hypogonadism: An update on diagnosis and treatment. Treat Endocrinol. 2005;4:293–309. doi: 10.2165/00024677-200504050-00003. [DOI] [PubMed] [Google Scholar]
- 8.Zarotsky V., Huang M.-Y., Carman W., Morgentaler A., Singhal P.K., Coffin D., Jones T.H. Systematic literature review of the epidemiology of nongenetic forms of hypogonadism in adult males. J. Horm. 2014;2014:190347. doi: 10.1155/2014/190347. [DOI] [PubMed] [Google Scholar]
- 9.Alahmadi B. Effect of herbal medicine on fertility potential in experimental animals—An update review. Mater. Socio Medica. 2020;32:140. doi: 10.5455/msm.2020.32.140-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smith S.J., Lopresti A.L., Teo S.Y.M., Fairchild T.J. Examining the effects of herbs on testosterone concentrations in men: A systematic review. Adv. Nutr. 2021;12:744–765. doi: 10.1093/advances/nmaa134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bhat R., Karim A.A. Tongkat Ali (Eurycoma longifolia Jack): A review on its ethnobotany and pharmacological importance. Fitoterapia. 2010;81:669–679. doi: 10.1016/j.fitote.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 12.Rehman S.U., Choe K., Yoo H.H. Review on a traditional herbal medicine, eurycoma longifolia Jack (Tongkat Ali): Its traditional uses, chemistry, evidence-based pharmacology and toxicology. Molecules. 2016;21:331. doi: 10.3390/molecules21030331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.George A., Henkel R. Phytoandrogenic properties of Eurycoma longifolia as natural alternative to testosterone replacement therapy. Andrologia. 2014;46:708–721. doi: 10.1111/and.12214. [DOI] [PubMed] [Google Scholar]
- 14.Mohamed A.N., Vejayan J., Yusoff M.M. Review on Eurycoma longifolia pharmacological and phytochemical properties. J. Appl. Sci. 2015;15:831–844. doi: 10.3923/jas.2015.831.844. [DOI] [Google Scholar]
- 15.Han Y.M., Jang M., Kim I.S., Kim S.H., Yoo H.H. Simultaneous quantitation of six major quassinoids in Tongkat Ali dietary supplements by liquid chromatography with tandem mass spectrometry. J. Sep. Sci. 2015;38:2260–2266. doi: 10.1002/jssc.201500207. [DOI] [PubMed] [Google Scholar]
- 16.Cui T., Kovell R.C., Brooks D.C., Terlecki R.P. A urologist’s guide to ingredients found in top-selling nutraceuticals for men’s sexual health. J. Sex. Med. 2015;12:2105–2117. doi: 10.1111/jsm.13013. [DOI] [PubMed] [Google Scholar]
- 17.Corazza O., Martinotti G., Santacroce R., Chillemi E., Di Giannantonio M., Schifano F., Cellek S. Sexual enhancement products for sale online: Raising awareness of the psychoactive effects of yohimbine, maca, horny goat weed, and ginkgo biloba. Biomed Res. Int. 2014;2014:841798. doi: 10.1155/2014/841798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Balasubramanian A., Thirumavalavan N., Srivatsav A., Yu J., Hotaling J.M., Lipshultz L.I., Pastuszak A.W. An analysis of popular online erectile dysfunction supplements. J. Sex. Med. 2019;16:843–852. doi: 10.1016/j.jsxm.2019.03.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuchakulla M., Narasimman M., Soni Y., Leong J.Y., Patel P., Ramasamy R. A systematic review and evidence-based analysis of ingredients in popular male testosterone and erectile dysfunction supplements. Int. J. Impot. Res. 2021;33:311–317. doi: 10.1038/s41443-020-0285-x. [DOI] [PubMed] [Google Scholar]
- 20.Jayusman P.A., Mohamed I.N., Thu H.E., Shuid A.N. Effect of Eurycoma longifolia on sexual behavior in sexually dysfunctional male: A systematic review. Int. J. Pharm. Pharm. Sci. 2017;9:46–52. doi: 10.22159/ijpps.2017v9i12.21812. [DOI] [Google Scholar]
- 21.Thu H.E., Mohamed I.N., Hussain Z., Jayusman P.A., Shuid A.N. Eurycoma Longifolia as a potential adoptogen of male sexual health: A systematic review on clinical studies. Chin. J. Nat. Med. 2017;15:71–80. doi: 10.1016/S1875-5364(17)30010-9. [DOI] [PubMed] [Google Scholar]
- 22.Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., Shamseer L., Tetzlaff J.M., Akl E.A., Brennan S.E., et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ. 2021;10:89. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sterne J.A.C., Savović J., Page M.J., Elbers R.G., Blencowe N.S., Boutron I., Cates C.J., Cheng H.Y., Corbett M.S., Eldridge S.M., et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 24.Egger M., Smith G.D., Schneider M., Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Begg C., Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101. doi: 10.2307/2533446. [DOI] [PubMed] [Google Scholar]
- 26.Chinnappan S.M., George A., Pandey P., Narke G., Choudhary K. Effect of Eurycoma longifolia standardised aqueous root extract—Physta® on testosterone levels and quality of life in ageing male subjects: A randomised, double-blind, placebo-controlled multicentre study. Food Nutr. Res. 2021;65:5647. doi: 10.29219/fnr.v65.5647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tambi M.I.B.M., Imran M.K., Henkel R.R. Standardised water-soluble extract of Eurycoma longifolia, Tongkat ali, as testosterone booster for managing men with late-onset hypogonadism? Andrologia. 2012;44:226–230. doi: 10.1111/j.1439-0272.2011.01168.x. [DOI] [PubMed] [Google Scholar]
- 28.Leitão A.E., de Souza Vieira M.C., Pelegrini A., da Silva E.L., de Azevedo Guimarães A.C. A 6-month, double-blind, placebo-controlled, randomized trial to evaluate the effect of Eurycoma longifolia (Tongkat Ali) and concurrent training on erectile function and testosterone levels in androgen deficiency of aging males (ADAM) Maturitas. 2021;145:78–85. doi: 10.1016/j.maturitas.2020.12.002. [DOI] [PubMed] [Google Scholar]
- 29.Chan K.Q., Stewart C., Chester N., Hamzah S.H., Yusof A. The effect of Eurycoma longifolia on the regulation of reproductive hormones in young males. Andrologia. 2021;53:e14001. doi: 10.1111/and.14001. [DOI] [PubMed] [Google Scholar]
- 30.Henkel R.R., Wang R., Bassett S.H., Chen T., Liu N., Zhu Y., Tambi M.I. Tongkat Ali as a potential herbal supplement for physically active male and female seniors—A pilot study. Phyther. Res. 2014;28:544–550. doi: 10.1002/ptr.5017. [DOI] [PubMed] [Google Scholar]
- 31.Ismail S.B., Wan Mohammad W.M.Z., George A., Nik Hussain N.H., Musthapa Kamal Z.M., Liske E. Randomized clinical trial on the use of PHYSTA freeze-dried water extract of Eurycoma longifolia for the improvement of quality of life and sexual well-being in men. Evidence Based Complement. Altern. Med. 2012;2012:429268. doi: 10.1155/2012/429268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.George A., Liske E., Chen C., Ismail S. The Eurycoma longifolia freeze-dried water extract-Physta does not change normal ratios of testosterone to epitestosterone in healthy males. J. Sports Med. Doping Stud. 2013;3:1000127. [Google Scholar]
- 33.Lim P. Ph.D. Thesis. University of Malaya; Kuala Lumpur, Malaysia: 2017. [(accessed on 10 July 2021)]. Use of complementary and alternative medicine in sports and effects of Eurycoma longifolia Jack on aerobic metabolism in collegiate athletes. Available online: http://studentsrepo.um.edu.my/11511/1/Samsuddin.pdf. [Google Scholar]
- 34.Quin C. Effects of Eurycoma Longifolia Supplementation: An Evaluation of Cell Growth, Exercise Performance and Wellbeing in Adult Males. Liverpool John Moores University; Liverpool, UK: 2021. [(accessed on 10 July 2021)]. p. 285. Available online: https://researchonline.ljmu.ac.uk/id/eprint/15104/ [Google Scholar]
- 35.Faghihimani E., Aminorroaya A., Rezvanian H., Adibi P., Ismail-Beigi F., Amini M. Reduction of insulin resistance and plasma glucose level by salsalate treatment in persons with prediabetes. Endocr. Pract. 2012;18:826–833. doi: 10.4158/EP12064.OR. [DOI] [PubMed] [Google Scholar]
- 36.Mohd Effendy N., Mohamed N., Muhammad N., Naina Mohamad I., Shuid A.N. Eurycoma longifolia: Medicinal plant in the prevention and treatment of male osteoporosis due to androgen deficiency. Evid. Based Complement. Altern. Med. 2012;2012:125761. doi: 10.1155/2012/125761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Khanijo T., Jiraungkoorskul W. Review ergogenic effect of long jack, Eurycoma Longifolia. Pharmacogn. Rev. 2016;10:139. doi: 10.4103/0973-7847.194041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fiaschetti G., A Grotzer M., Shalaby T., Castelletti D., Arcaro A. Quassinoids: From traditional drugs to new cancer therapeutics. Curr. Med. Chem. 2012;18:316–328. doi: 10.2174/092986711794839205. [DOI] [PubMed] [Google Scholar]
- 39.Low B.S., Choi S.B., Abdul Wahab H., Kumar Das P., Chan K.L. Eurycomanone, the major quassinoid in Eurycoma longifolia root extract increases spermatogenesis by inhibiting the activity of phosphodiesterase and aromatase in steroidogenesis. J. Ethnopharmacol. 2013;149:201–207. doi: 10.1016/j.jep.2013.06.023. [DOI] [PubMed] [Google Scholar]
- 40.Low B.S., Das P.K., Chan K.L. Standardized quassinoid-rich Eurycoma longifolia extract improved spermatogenesis and fertility in male rats via the hypothalamic-pituitary- gonadal axis. J. Ethnopharmacol. 2013;145:706–714. doi: 10.1016/j.jep.2012.11.013. [DOI] [PubMed] [Google Scholar]
- 41.Bin Mohd Tambi M.I., Imran M.K. Eurycoma longifolia Jack in managing idiopathic male infertility. Asian J. Androl. 2010;12:376. doi: 10.1038/aja.2010.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hussain S.A., Hameed A., Nasir F., Wu Y., Suleria H.A.R., Song Y. Evaluation of the spermatogenic activity of polyherbal formulation in oligospermic males. Biomed Res. Int. 2018;2018:2070895. doi: 10.1155/2018/2070895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Udani J.K., George A.A., Musthapa M., Pakdaman M.N., Abas A. Effects of a proprietary freeze-dried water extract of Eurycoma longifolia (Physta) and Polygonum minus on sexual performance and well-being in men: A randomized, double-blind, placebo-controlled study. Evid. Based Complement. Altern. Med. 2014;2014:179529. doi: 10.1155/2014/179529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Van De Kerkhof D.H., De Boer D., Thijssen J.H.H., Maes R.A.A. Evaluation of testosterone/epitestosterone ratio influential factors as determined in doping analysis. J. Anal. Toxicol. 2000;24:102–115. doi: 10.1093/jat/24.2.102. [DOI] [PubMed] [Google Scholar]
- 45.Chen C.K., Wan Mohamad W.M.Z., Ooi F.K., Ismail S.B., Abdullah M.R., George A. Supplementation of Eurycoma longifolia Jack extract for 6 weeks does not affect urinary testosterone: Epitestosterone ratio, liver and renal functions in male recreational athletes. Int. J. Prev. Med. 2014;5:728. [PMC free article] [PubMed] [Google Scholar]
- 46.Chen C., Ooi F., Abu Kasim N., Asari M. Effects of Eurycoma longifolia Jack supplementation combined with resistance training on isokinetic muscular strength and power, anaerobic power, and urinary testosterone: Epitestosterone ratio in young males. Int. J. Prev. Med. 2019;10:118. doi: 10.4103/ijpvm.IJPVM_404_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Leung K.M.Y.B., Alrabeeah K., Carrier S. Update on testosterone replacement therapy in hypogonadal men. Curr. Urol. Rep. 2015;16:57. doi: 10.1007/s11934-015-0523-9. [DOI] [PubMed] [Google Scholar]
- 48.Talbott S.M., Talbott J.A., George A., Pugh M. Effect of Tongkat Ali on stress hormones and psychological mood state in moderately stressed subjects. J. Int. Soc. Sports Nutr. 2013;10:28. doi: 10.1186/1550-2783-10-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Norhidayah A., Vejayan J., Yusoff M.M. Detection and quantification of Eurycomanone levels in Tongkat ali herbal products. J. Appl. Sci. 2015;15:999. doi: 10.3923/jas.2015.999.1005. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The scientific studies analyzed in this meta-analysis are available in the databases PubMed, Scopus, Web of Science, Cochrane, Ovid/Embase, and Google Scholar.