Abstract
Background:
Protease inhibitors (PIs) may inhibit Kaposi sarcoma (KS) carcinogenesis. However, PI-based antiretroviral therapy (ART) is rarely a first-line choice in people living with HIV (PLWH) because of cost and toxicities. This is the first systematic review to assess KS incidence stratified by ART type.
Methods:
We searched PubMed to identify original, full research reports of KS incidence in ART-treated adult PLWH, stratified by ART class, published between 1996 and 2017. For overlapping cohorts, we included only the most recent study and supplemented data with earlier relevant analyses. We described study design, sociodemographic characteristics, statistical adjustment factors, and KS incidence.
Results:
We identified three unique retrospective cohort studies, and supplemented one of the studies with results from six prior subgroup reports, which included 242,309 PLWH and 3570 incident KS cases. Overall, KS crude incidence decreased by a factor of 10 between untreated and ART-treated PLWH; CD4-adjusted KS incidence decreased by ~50%, with either non-nucleoside reverse transcriptase inhibitor (NNRTI)- or PI-based ART. A single study measured a cumulative dose-/time-dependent effect of ART, which reported a relative risk reduction in only the cohort receiving boosted PI-based ART. Other studies defined ART categories by first-line therapy only.
Conclusions:
The risk of incident KS was significantly reduced regardless of ART class even after adjusting for CD4 count. The quality of evidence (i.e., most studies categorizing users by first-line ART) does not permit KS risk reduction comparisons across ART types. Given the limited number and retrospective nature of these studies, prospective data is indicated.
Keywords: Oncogenic viruses, KSHV, Acquired Immunodeficiency Syndrome/complications, neoplasms/epidemiology, Protease inhibitors, HIV Infections/drug therapy, Reverse Transcriptase Inhibitors/therapeutic use
Introduction
The introduction of antiretroviral therapy (ART) has dramatically reduced Kaposi sarcoma (KS) incidence in people living with HIV (PLWH) in the last 20 years,1–4 but KS remains the most common malignancy in people living with HIV (PLWH) worldwide,5 with significant morbidity and mortality. Several studies have suggested that protease inhibitors (PIs), a class of antiretroviral therapy, may be effective in controlling KS independently of immune reconstitution and virologic suppression. PIs have both antiviral and anti-neoplastic effects.6 In animal and in vitro studies, ritonavir, saquinavir, nelfinavir, and indinavir appear to inhibit the replication of Kaposi sarcoma-herpesvirus (KSHV), also known as human herpesvirus 8 (HHV8), which is the etiologic agent of all forms of KS.7 The anti-tumor effects of PIs have been seen in a wide range of malignancies, including B-cell lymphomas; lung, brain, and ovarian cancer; as well as KS, attenuating endothelial cell invasion; angiogenesis; and tumor growth.8–10
Clinical studies, however, have not consistently demonstrated a significant reduction in KS incidence associated with PIs compared to other antiretroviral (ARV) classes. For example, PLWH with CD4 count of ≥ 350 cells/μL benefited significantly in the randomized clinical trial (START) by starting ART immediately, with a hazard ratio (HR) for incident KS of 0.09 (95% CI, 0.01–0.71) compared to PLWH who waited until CD4 fell below 350 cells/μL regardless of ART class initiated. Results of phase II trials with indinavir in HIV-negative patients have not been robust enough to stimulate design of phase III trials (NCT01067690, unpublished; and NCT0036231011). Further, in many low- and middle-income countries (LMICs), NNRTIs are utilized first-line because of cost, and recent data demonstrating shorter durability with boosted PIs in comparison to NNRTIs, requiring earlier change of therapy for any reason, whether toxicity, treatment failure, death, or loss to follow-up.12 In high-income countries, PIs are now avoided as first-line therapy due to toxicities. Of note, the U.S. Department of Health and Human Services and World Health Organization (WHO) recommend integrase strand transfer inhibitor (INSTI)- and NNRTI-based regimens for most antiretroviral-naïve people (adults and adolescents) with HIV, and boosted PI is an option for first-line for children only.13–15 An understanding of the effect of specific ART choice on KS risk has the potential to influence policy in LMICs that would help a) decrease KS incidence, which could markedly diminish total cancer burden, and b) decrease the need for anti-neoplastic agents with its associated economic cost. Even in developed countries, lowering chemotherapy requirements is strategic because long-term anti-neoplastic therapy is often associated with cumulative cardiac and hematologic toxicities.7 Our objective was to systematically review the literature evaluating the association between PI use and KS incidence in PLWH. Our hypothesis was that PI-based ART, in comparison to PI-sparing ART, could be associated with lower KS incidence in PLWH.
Methods
We followed Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols (PRISMA-P) guidelines for the conduct and reporting of systematic reviews.16 We searched PubMed to identify original research reports published in English and French in peer-reviewed journals January 1, 1996 – September 27, 2017 that evaluated PI-based ART impact on KS incidence in PLWH during the ART era. We also performed ancestry searches by reviewing the bibliographies of retrieved articles to find additional studies not identified by keyword search. Our search phrase was “Kaposi sarcoma AND (HIV OR AIDS) AND (Protease inhibitors OR HAART OR ART OR Antiretroviral).” We reviewed abstracts based on title relevance.
We defined ART as drug regimens containing three ARVs, including PIs, NNRTIs (non-nucleoside reverse transcriptase inhibitors), one or more NRTIs (nucleoside reverse transcriptase inhibitors), or INSTIs. Due to lack of available data in any of the cohorts examined, we did not conduct comparisons with INSTIs. We excluded publications: that did not provide outcomes separately for PI users; did not report original research findings (e.g., editorials) or were published as abstracts, letters or case reports; had a sample size ≤5; or included non-HIV patients or pediatric cohorts. When more than one eligible report was available for the same study population, we included the most recent or the largest study unless an earlier study contained more detailed data, as notated within study tables. All searches and data extraction were conducted by at least two independent clinician reviewers (PM, SM, EC) using a structured data collection tool that captured study characteristics such as design, location, time period, and sample size; selective sociodemographic characteristics such as median age, sex; immunologic characteristics including median CD4 counts and median HIV viral load; and KS incidence overall and in subgroups. If results were not available, we calculated them using the reported data where possible. Any discrepancies between the reviewers were resolved with discussion or in consultation with the senior authors (DW and EYC).
Results
Search results
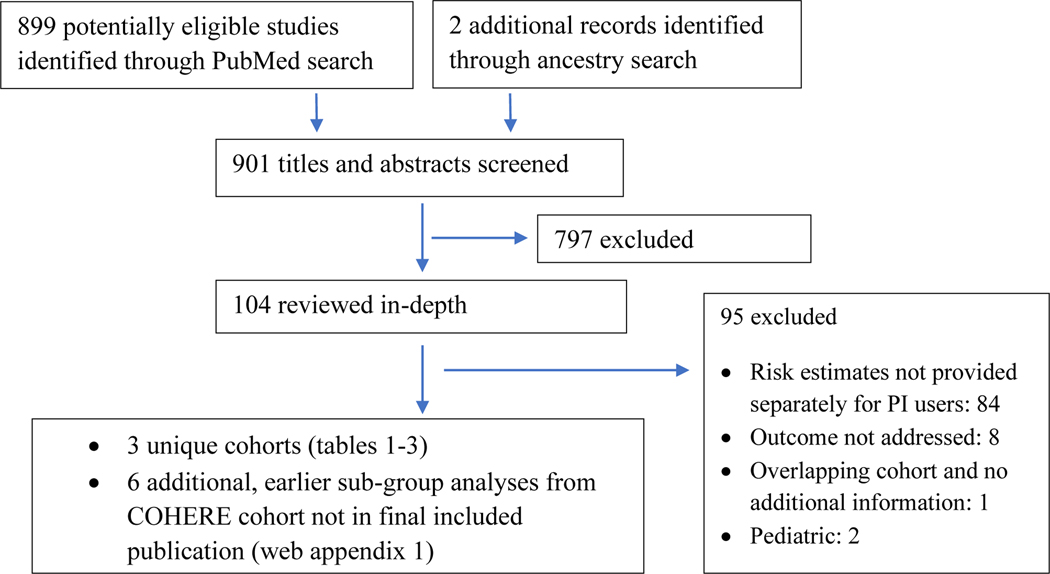
Our combined PubMed keyword and article ancestry searches identified 901 citations (figure 1). Among the 107 full articles reviewed, the most common reason for exclusion was KS incidence or risk estimates not being provided separately for PI users. A total of three articles were eligible as unique cohorts; 4,17,18 six articles provided supplemental data for a single one of the three cohorts (COHERE42).19–24
Figure 1.
Study Flow Diagram
Sociodemographic characteristics:
A total of 242,309 PLWH among three unique study cohorts eligible for our review are included in table 1, with 3570 incident KS cases. 4,17,18 All studies were retrospective analyses. Overall, males represented ~70% of the cumulative PLWH cohort, and 89% of the incident KS cases (Table 2). Black (combining race and geographic origin data, including African American and African) patients comprised at least 13% of the cumulative PLWH (Table 2). Median or mean age was between 33 and 45 years in all studies. At least 20% of PLWH were men who have sex with men (MSM); 14% had unknown MSM status (Table 2).
Table 1.
Characteristics of eligible studies evaluating Kaposi sarcoma incidence in unique cohorts of people living with HIV (PLWH) taking protease inhibitors
| COHORT, Author (year) | Location | Primary data source | Study period | Mean follow-up (years) | PLWH (N); incident KS cases (n) | Crude incidence rate (per 1000 person-years) | Adjusted risk (95% CI) [Referent group]† | Factors adjusted for in multivariable analysis | ||
|---|---|---|---|---|---|---|---|---|---|---|
| No antiretroviral Rx | cART-treated | PI-based cART | NNRTI-based cART | |||||||
| UK, Portsmouth (2003) | United Kingdom | Chelsea and Westminster (single center) | 1996–2001 | NR | N=8640; n=1204 | 30 | 0.03 | aIRR 0.47 (0.38–0.58) [No antiretroviral Rx] | aIRR 0.42 (0.33–0.52) [No antiretroviral Rx] | Age at cohort entry, nadir CD4, gender, race, CD8 count |
|
VA, Kowalkowski (2015) |
US | Veterans Affairs Clinical Case Registry | 1985–2010 | 6.6 | N=25 529; n=341 | NA | 2.0 | aIRR 0.79 (0.69–0.90) [per 10% additional time of PI-based cART]‡ | aIRR 0.98 (0.92–1.04) [per 10% additional time of NNRTI-based cART]‡ | Age, CD4 (nadir and at KS diagnosis),VL, HIV diagnosis era, cART duration (time since start; % time on cART), race, Deyo comorbidity, clinic visits/year |
| COHERE42, COHERE (2017)** | 5 continents (Europe, Asia, N. and S. America, Africa) | 4 IeDEA regions, multiple cohorts (42 cohorts in 57 countries) | 1996–2014 | 4.3§ | N=208 140; n=2046 | NA | 5.2–28.0¶ | aHR 1.12 (1.01–1.24) [NNRTI] | aHR 0.89 (0.81–0.99) [PI-based cART]ǁ | Age, CD4 at KS diagnosis, gender, cART start year, CD4*region, gender*region, age*region |
aHR=adjusted hazard ratio. aIRR=adjusted incident rate ratio. cART=combination antiretroviral therapy. COHERE=Collaboration of Observational HIV Epidemiological Research in Europe. IeDEA=International Epidemiology Databases to Evaluate AIDS. KS=Kaposi sarcoma. N=North. NNRTI=non-nucleotide reverse transcriptase inhibitor. NA=not applicable. NRTI=nucleoside reverse transcriptase inhibitor. PI=protease inhibitor. PLWH=people living with HIV. Rx=medication. S=South. VL=viral load
Bold indicates statistical significance at p<0.05.
During or after year 3 of therapy.
Calculated weighted average of reported medians of 5 continents.
Incidence rate varies depending on geographic region.
Calculated as invert of PI-based results.
Studies in Web Appendix 1 describe some of these cohorts.
Table 2:
Sociodemographic and clinical characteristics of cohorts in KS incidence studies
| COHORT, author (year) | Age in years, median (IQR)* | Male PLWH (%) | Race (%) | MSM† (%) | |||
|---|---|---|---|---|---|---|---|
| PLWH | KS+ | PLWH | KS+ | PLWH | KS+ | ||
| UK, Portsmouth (2003) | Mean, KS+=33 (SD: 8.7) | ≈6048 (70%)§ | 1060 (88%) | NR | African (≈15) | NR | NR |
| VA, Kowalkowski (2015) | Mean=45 (SD: 10.4) | 25 529 (100%) | 341 (100%) | White (37). Black (51). Unknown (5). | White (40). Black (50). Unknown (9) | NR | NR |
| COHERE42, COHERE (2017) | 37 (31–44) | 143 617 (69%) | 1779 (67%) | African (10). Latin American (4) | African (7). Latin American (5). | 36% | 62% |
| FHDH, Grabar (2006) | 37 (30–41) | 71% | 95% | Sub-saharan African (9) | Sub-saharan African (4) | 32% | 74% |
| CASCADE, Lodi (2010) | 36 (32–43) | 100% | 100% | NR | NR | 100% | 100% |
| KAISER, Chao (2012) | Mean=40 (SD: 9.8) | 90% | NR | White (54). Black (20). Hispanic (21). Asian (5). Unknown (5) | NR | 62% | NR |
| FHDH-ANRS, Lacombe (2013) | Age 15–34: 38%; 35–49: 46%; ≥50: 16% | 67% | 94% | NR. Sub-saharan African (14%) | NR | 32% | 70% |
| DAD, Bruyand (2015) | 39 (33–46) | 73% | NR | White (50). Black African (7). Other (2). Unknown (41) | NR | 44% | NR |
| COHERE29, COHERE (2016) | 37 (31–45) | 71% | 88% | European/North American (83). African (15). Other (2). | European/North American (83). African (16). Other (1). | 40% | 64% |
| CD4 (cells/μL), median (IQR) | VL (% patients <500 copies/mL) | |||
|---|---|---|---|---|
| Baseline‡ | At KS diagnosis | Timing of measurement | KS+ | KS− |
| NR | 57 (18–152)¶ | NA | NA | NA |
| CD4KS− <200=44%; ≥200=56% | CD4 <200=54%; ≥200=46% | at KS diagnosis/ censor | 29% | 72% |
| 222 (112–344)ǁ | 165 (53–328)** | at KS diagnosis | 32% | NA |
| 202 (69–349) | 52 (14–181) | NA | NR | NR |
| NR | 195 (33–284)¶ | at KS diagnosis | Median=4.8 (IQR: 3.9–4.3) | NA |
| 433 (281–620) | NR | at KS diagnosis/ censor | Mean, all PLWH=4.7 log10 copies/mL (SD: 5.0) | |
| 202 (69–349) | 52 (14–181) | at KS diagnosis | 9.6% | NA |
| Mean=364 (SD: 279) | NR | at baseline | Median, all PLWH=2.3 log10 copies/mL (IQR: 1.7–4.3) | |
| KS−: 250 (124–370) | 300 (154–460)¶ | At cART initiation | 0.3% | 1.4% |
| KS+: 146 (43–302) | At KS diagnosis | 40% | NA | |
aIRR=adjusted incident rate ratio. CASCADE=Concerted Action on Seroconversion to AIDS and Death in Europe. CD4KS−=CD4 in PLWH who did not develop incident KS. COHERE=Collaboration of Observational HIV Epidemiological Research in Europe. CWA=calculated weighted average. DAD=Data collection on Adverse events of Anti-HIV Drugs. FHDH=French Hospital Database on HIV. FHDH-ANRS=FHDH-Agence Nationale, de Recherches sur le SIDA. KS+=PLWH diagnosed with Kaposi sarcoma during study period. MSM=men who have sex with men. NR=not reported. PLWH=people living with HIV. UK=United Kingdom. VL=viral load.
All PLWH unless otherwise noted.
% overall MSM.
Baseline, nadir, and “at enrollment” were treated synonymously, unless the study included pre-cART era, then nadir is reported rather than “at enrollment.”
Calculated using study reported data.
CWA of reported medians of 2 time periods.
CWA of reported medians of 5 continents.
CWA of reported medians of 4 continents (authors excluded Asia).
Each study provided a multivariable analysis to estimate incident KS risk after ART initiation, adjusted for several risk factors (web appendix 2). All three independent cohorts adjusted for age, CD4 (one for nadir CD4, one for CD4 count at KS diagnosis, and one for both), and ethnicity/geographic origin. Two adjusted for gender; one for HIV viral load (VL); one for HIV diagnosis era and one for ART calendar year start.
KS incidence in patients treated with ART vs. no ARVs
A single study, the United Kingdom (UK) cohort, described PI-based KS risk reduction relative to no ARVs.4 It was the smallest study (8640 PLWH, 1204 incident KS diagnoses) and reported significantly reduced KS incidence (adjusted incidence rate ratio [aIRR] 0.47, 95% CI 0.38–0.58) early in the ART era, with final incidence rates of 0.03 per 1,000 person-years, lower by 100- to 1,000-fold in comparison to studies of other cohorts (table 1). The UK group did not directly compare the effects of PI-based ART to NNRTI-based ART on KS incidence, but the effect estimate for NNRTI (aIRR 0.42 [95% CI, 0.33–0.52]) is similar to the effect estimate for PI, with each compared to no therapy.
KS incidence in patients treated with PI-based ART vs. NNRTI-based ART
One unique cohort (COHERE42, table 1), and four subgroup analyses published earlier within COHERE42 (web appendix 1), compared KS incidence in HIV patients taking PI-based ART directly to incidence in those taking NNRTI-based ART.17,21–24 The unique cohort, COHERE42 (Collaboration of Observational HIV Epidemiological Research Europe, spanning five continents), noted a small increase in risk of incident KS in PI users (adjusted hazard ratio [aHR] = 1.12, p<0.05, Referent = NNRTI-based ART), in contrast to the four earlier analyses, which found no significant difference in KS risk reduction between the types of ART (table 1 and web appendix 1).15–18 All four categorized ART users based on first-line regimen, and three15–17 of the four did not distinguish boosted PI regimens from nonboosted PI regimens (table 1, web appendices 1 and 3). COHERE29 did categorize boosted and nonboosted PI users separately, and showed significantly decreased risk of new KS diagnosis (aHR 0.53 [95% CI 0.32–0.90)] compared to boosted PI) for nonboosted PI users within the first 30–90 days of ART initiation, but this difference became statistically nonsignificant after 3 months (web appendix 3).
KS incidence as a function of time: in patients treated with PI-based ART; and in patients treated with NNRTI-based ART
One unique cohort, and two additional analyses overlapping with COHERE42, evaluating cumulative duration of PI-based ART as a continuous variable reported significantly reduced KS incidence with longer exposure within the same class (VeteransVeterans Health Administration [VA], Kaiser, Data Collection on Adverse Events of Anti-HIV Drugs [DAD]).18–20 The VA found an aIRR of 0.79 (95% CI 0.69–0.90) for every 10% additional exposure time to boosted PI-based ART after 3 years of therapy (table 1) but not to nonboosted PIs, including nelfinavir.19 Additional time with NNRTI-based therapy did not result in significantly lower rates of incident KS. The other two analyses were both subsets of the cohort studied in COHERE42 (Kaiser, DAD; web appendix 1).20,21 Both of these analyses evaluated risk reduction with each additional year of ART by class, and did not exclude KS diagnoses within the first 6 months (web appendix 2), which raises concerns for inclusion of prevalent and IRIS-associated KS. The Kaiser group reported aIRRs of 0.84 (0.75–0.94) and 0.81 (0.67–0.99) for each additional year of PI-based ART and NNRTI-based ART, respectively.19 The DAD group, in a retrospective analysis of a prospectively established cohort across 21 countries, found aIRRs of 0.93 (95% CI 0.87–1.00) and 0.81 (95% CI 0.74–0.90) for each additional year of PI-based and NNRTI-based ART, respectively.21 Of the 3 studies, only the VA group described a method for systematically excluding periods of medication non-adherence in the time-dependent model.19
Effect of other factors besides ART class or duration
Several other factors were associated with KS risk: age, CD4, HIV VL, preexisting AIDS, and MSM status. Five analyses reported on the relationship between current, time-updated CD4 count and incident KS diagnosis; all 5 found significant associations.17–22 Four analyses reported nadir CD4 count. Two4,21 of four studies found that lower nadir CD4 was significantly associated with higher KS incidence rates: The UK study compared <150 vs. ≥150 cells/μL, p<0.05,4 while Grabar with French Hospital Database on HIV (FHDH) used a cutoff of 200 cells/μL.20
COHERE29 demonstrated an increasing effect of HIV viral load on KS risk over time, an interaction not seen between current CD4 count and KS. Patients with detectable (>500 HIV RNA copies/mL) VL did not have a statistically significant increased risk of KS during the first year. However, the risk gradually increased in subsequent years. By 5–8 years after ART initiation, the aHR for ≥100,000 HIV RNA copies/mL was 9.7 (95% CI 5.34–17.7) for incident KS.24 In contrast, while current CD4 count was inversely correlated with KS risk, the relative risk did not change significantly over time.
Discussion
This systematic review is the first to evaluate KS incidence across adult PLWH treated with PI-based and PI-sparing ART. We included three unique cohort studies involving 242,309 PLWH, of which 3570 were diagnosed with KS after ART initiation. We also extracted supplemental data from 6 overlapping cohort studies. Collectively, the results did not provide a universal consensus on the effect of PI-based ART in comparison to NNRTI-based ART, but consistently described statistically significant KS risk reductions associated with both types of ART which were not completely explained by improvement in CD4 count, when compared to no ARVs. The effect metrics and referent groups of each of the three unique cohort studies were not directly comparable to each other, which precluded pooling of results. One reported ART effect on incident KS compared to no ART; one reported an ART-associated effect per unit dose of time; and one reported the effects of ART types on incident KS with PI-based compared to PI-sparing ART.
KS incidence in patients treated with ART vs. no ARVs
Overall, KS crude incidence rates decreased by at least 10-fold when comparing PLWH who received no ARVs to PLWH receiving ART.4,21,22 In the only unique cohort comparing PI-based ART to no ARVs, the CD4-adjusted relative reduction in incident KS was more than 50% in both the PI-treated and NNRTI-treated PLWH.4 This may reflect the role that HIV has in facilitating KSHV lytic replication via proviral insertion and Tat protein activation,25,26 and the equal efficacy of NNRTI- and PI-based regimens in HIV virologic suppression.27 The similar magnitude of protective effects of PI- and NNRTI-based ART in patients at risk of KS corroborates research that identified exposure to chronic HIV-induced immune activation with eventual immune evasion as key pathogenic mechanisms in KS.28 The crude incident KS incident rate in this study was particularly low; at the end of the 5-year study period, it was lower by a factor of 100–1000 in comparison to other reported ART era incident rates.4,27 Possible reasons for this outlier include patient-related factors such as younger age, potentially greater compliance and access to care, as well as provider-related factors, including tighter control of practice and quality of care in a single-center study. Indeed, the median age of participants was younger by 2.6–12 years than the other studies, and increasing age was a significant independent predictor of KS incidence in multivariate analysis, adjusted for nadir CD4 count.4,21
In vitro studies have suggested that PIs can slow oncogenesis by inhibiting angiogenesis and cell invasion, and modulating proteasomes and apoptosis.27 In addition, PIs have direct antiviral effects on KSHV, and some of the PI-associated benefit is thought to be mediated by the inhibition of PI3K/Akt signaling which is upregulated by latent KSHV infection.29,30 It is noteworthy that although the effects of NRTI or NNRTI alone on angiogenesis, proliferation, or KSHV replication have not been studied, NNRTIs have also exhibited anticancer effects in non-KS experimental models.31 In germ line and tumor cells, NNRTIs inhibit endogenous reverse transcriptases, thereby reducing cell proliferation and inducing differentiation. Specifically, nevirapine exposure rescued the differentiation block in acute myeloid leukemia (AML) cell lines from two patients, and high concentrations of efavirenz were anti-proliferative against a pancreatic cancer cell line.29,30 This may explain why PI-based ART and NNRTI-based ART appear to lower KS incidence rates in PLWH in similar magnitude, and also lower B-cell lymphoma rates to similar degree.33
Interestingly, the predominant use of NNRTI-based regimens as first-line ART in sub-Saharan Africa, even in areas where ART has been provided to the vast majority of PLWH, has not been met with the same precipitous decline in HIV-associated KS seen in the USA and Europe.34 Still, the reason for the persistent burden of disease caused by KS in Africa is not necessarily solely ART choice. Other reasonable potential contributing factors include lower CD4 nadir with associated long-term immune dysfunction, a higher incidence of endemic KS prior to the HIV epidemic as well as potential differences in age at and typical mode of infection, and population-level genetic and viral differences. Nonetheless, considering the disproportionate burden of KS in sub-Saharan Africa in comparison to other regions of the world, there is great potential for reducing the burden of disease through even small differences in specific ART regimens on KS incidence.
KS incidence in patients treated with PI-based ART vs. NNRTI-based ART
The majority of studies found that PI-associated protection against KS was not significantly different from the effect of NNRTI-based ART. However, the major methodologic flaw of these studies was that they did not account for the duration of therapy with each specific ART regimen. All four of the analyses that directly compared NNRTI-based risk reduction to PI-based risk reduction categorized ART users by first-line regimen. A more accurate way of representing the degree of PI-associated KS risk reduction would be to quantify the cumulative number of doses received, regardless of first-, second-, or third-line context, as the VA study did.
Another, more minor, limitation refers to the various definitions of “PI-based ART.” Of the four studies comparing NNRTI- to PI-based KS risk reduction, only one, COHERE29, stratified its PI-based group into a nonboosted group and boosted group. The effect estimates were similar, except between the first 30–90 days after ART initiation, when boosted PI-based ART was associated with higher risk of new KS diagnosis, which raises the possibility of PI-associated efficacy increasing the risk of IRIS, manifesting as KS.24 Not only did the three other PI vs. NNRTI studies combine boosted and nonboosted PI users into a single group, but so did three of four other analyses (the UK study, and two of the three reporting KS incidence as a function of time). The results from COHERE29 still indicate a significantly greater NNRTI-associated KS risk reduction when boosted PI users are treated as a referent group, which again may be due to definition and categorization by first-line regimen only.
The other important reason for higher PI-associated KS incidence compared to NNRTI-associated KS in COHERE 42 is the possibility of confounding by indication. The threshold to developing PI resistance is generally higher than the threshold to NNRTI resistance. Therefore, clinicians may prescribe PI-based ART to noncompliant patients, rather than risk losing efficacy to the entire NNRTI class in a patient who may not take medication consistently. A higher risk of incident KS in PI users may reflect less well-controlled HIV due to noncompliance-associated undertreatment. The VA study may have adjusted for this more effectively as it was the only analysis that excluded periods of non-compliance when calculating cumulative drug exposure.
KS incidence as a function of time: in patients treated with PI-based ART; and in patients treated with NNRTI-based ART
Only the VA study measured the effect of actual exposure time to ART class on KS incidence. They found that longer cumulative duration of boosted PI was associated with lower incident KS rates compared to shorter duration of PI use. In the context of the data from the other studies, it is not clear if longer duration of exposure to NNRTI-based ART has the same effect.18–20 In the VA study, only boosted PI regimens resulted in a cumulative dose-/time-dependent KS risk reduction.18 Since COHERE29 showed significantly decreased risk of new KS diagnosis (aHR 0.53 [95% CI 0.32–0.90)] compared to boosted PI) within the first 2–3 months of nonboosted PI-based regimen initiation (web appendix 3),24 these two studies together suggest that boosted PIs may be associated with IRIS-associated KS risk.
Though the nelfinavir group in the VA study did not have significantly better outcomes, its small sample size limits interpretation of this finding. Nelfinavir, the PI reported to have the most potent and broad anti-neoplastic activity,35 is under investigation in a phase II clinical trial, AMC-098, which includes dose escalation for any patients with less than a complete response taking standard dose, and monitoring KSHV viral load.
Effect of other factors besides ART class or duration
As expected, time-updated CD4 count was consistently associated with KS incidence. In contrast, nadir CD4 in the ART era was not consistently predictive of incident KS risk. It is possible that this is due to the inability of included studies to completely adjust for competing death as an outcome. Patients with lower nadir CD4 are at risk of dying from infections prior to developing KS.
HIV viral load was also associated KS incidence, and appears to have an especially prominent role in the ART era. COHERE29 suggests that patients treated with ART for several years but still have high viral loads are vulnerable to increasing risk of incident KS over time and that ART-mediated immune reconstitution is not always sufficient to prevent disease progression in PLWH. This supports earlier evidence that the role of HIV in KS is not limited to immunosuppression, but that HIV proteins themselves are carcinogens that synergize with KSHV in the pathogenesis of KS.25,26,26 The HIV protein Nef promotes KSHV latency, a critical characteristic of an immune-tolerant state conducive to eventual malignant transformation of infected cells.35 However, in all the analyses of included studies in our review, interactions between CD4 or VL and ART class were not studied.
Race was not completely described in the majority of the studies. When described, the effect of race/ethnicity on KS risk was not consistent. The UK study and COHERE42 reported that African origin was prognostic for greater risk of incident KS,4,17 while the VA study found a lower adjusted risk of KS in Black patients in the U.S. (web appendix 2).18 This may be due to high seroprevalence of KSHV in Africa. Black populations in European studies more frequently represent first-generation African immigrants, while Black populations in American studies represent African Americans who typically have been in the U.S. for multiple generations.36 Additionally, none of the studies addressed race as a potential confounder or mediator of observed associations between ART use and KS incidence. FHDH showed no significant difference in adjusted KS risk in patients of sub-Saharan origin in France compared to non-Africans, but the proportion of KS+ patients with sub-Saharan origin progressively increased over time, from 1.4% prior to 1996 to 10% after 1996.21 It is not clear if this trend is due to access to resources or differential responses to ART. Race and ART utilization may need to be described more fully in future studies of KS incidence.
Limitations
Most incidence studies did not utilize methodology to avoid including potential prevalent KS among incident cases. The only study that reported a more significant effect on KS incidence in PI users was also the only study that excluded KS diagnoses within the first 6 months following HIV diagnosis or ART initiation throughout all results and analyses.18 The Kaiser study reported no significant changes after conducting a sensitivity analysis excluding KS diagnoses within 6 months following study enrollment, but it is not clear if incidence in PI users and NNRTI users were compared in the sensitivity analysis.19 Additionally, by including boosted and nonboosted PIs in the same group, their analysis may have missed a potentially significant difference between the rates of IRIS-associated incident KS in the first 3–6 months in boosted PIs, as suggested by COHERE29, compensated by decreased incidence after year 2.24
The small number of eligible studies and the heterogeneity between study populations and outcome metrics limited our ability to compare across studies. Only three studies contained entirely separate cohorts. Follow-up duration varied significantly, with the Kaiser, VA, and DAD groups reporting the longest follow-up durations (4.5, 6.6, and 5.8 years, respectively).18–20 Baseline CD4 counts ranged widely, and crude KS incidence rates in the ART era ranged from 0.03 to 32 per 1,000 person-years.4,21 Some studies took place in North America, where males with KS outnumber females by at least a factor of 10, in contrast to Africa, where females with KS outnumber males slightly. Furthermore, certain subpopulations remained grossly underrepresented. Asians were not described as a group in any of these cohorts. Studies in African populations are particularly needed, where KSHV is endemic, thereby increasing the incidence of KS by 2- to 5-fold compared to Europe, and where KS is the second largest contributor of cancer burden overall.37,38 Prospective studies, to eliminate confounding by indication, are essential, and should enroll representative subgroups of Africans, women, and PLWH treated with integrase-strand transfer inhibitor (INSTI)-based regimens, a treatment exposure group which was not addressed in any of the published studies.
Conclusion
Studies in PLWH consistently demonstrate that PI-based ART significantly reduces the incidence of KS compared to no ART. However, the design of the published studies does not allow for accurate comparison of the effects of PI-based to NNRTI-based ART. Prospective studies are necessary to define more precisely the impact of boosted PIs on KS incidence in the ART era, particularly in comparison to newer regimens with INSTIs and in African populations.
Supplementary Material
Sources of funding:
• VA Health Services Research & Development Center of Innovation grant CIN 13-413
• NIH grant 1R01 CA206476
• NIH grant T32 CA174647
• AIDS Malignancy Consortium grant 2U01CA121947-04
• P30 CA125123 (Dan L Duncan Comprehensive Cancer Center)
• Dr. White receives salary support from the U.S. Department of Veterans Affairs Clinical Science Research and Development program (CX001430)
References
- 1.International Collaboration on HIV and Cancer. Highly active antiretroviral therapy and incidence of cancer in human immunodeficiency virus-infected adults. J Natl Cancer Inst. 2000;92(22):1823–30. [DOI] [PubMed] [Google Scholar]
- 2.Clifford GM, Polesel J, Rickenbach M, et al. Cancer risk in the Swiss HIV Cohort Study: associations with immunodeficiency, smoking, and highly active antiretroviral therapy. J Natl Cancer Inst. 2005;97(6):425–32. [DOI] [PubMed] [Google Scholar]
- 3.Jacobson LP, Yamashita TE, Detels R, et al. Impact of potent antiretroviral therapy on the incidence of Kaposi’s sarcoma and non-Hodgkin’s lymphomas among HIV-1-infected individuals. Multicenter AIDS Cohort Study. J Acquir Immune Defic Syndr. 1999;21 Suppl 1:S34–41. [PubMed] [Google Scholar]
- 4.Portsmouth S, Stebbing J, Gill J, et al. A comparison of regimens based on non-nucleoside reverse transcriptase inhibitors or protease inhibitors in preventing Kaposi’s sarcoma. AIDS. 2003;17(11):F17–22. [DOI] [PubMed] [Google Scholar]
- 5.Robey RC, Bower M. Facing up to the ongoing challenge of Kaposi’s sarcoma. Curr Opin Infect Dis. 2015;28(1):31–40. [DOI] [PubMed] [Google Scholar]
- 6.INSIGHT START Study Group. Initiation of Antiretroviral Therapy in Early Asymptomatic HIV Infection. N Engl J Med. 2015;373:795–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yarchoan R, Uldrick TS. HIV-associated cancers and related diseases. N Engl J Med. 2018;378(11): 1029–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gantt S, Carlsson J, Ikoma M, et al. The HIV protease inhibitor nelfinavir inhibits Kaposi’s sarcoma-associated herpesvirus replication in vitro. Antimicrob Agents Chemother. 2011;55(6):2696–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pati S, Pelser CB, Dufraine J, Bryant JL, Reitz MS Jr., Weichold FF. Antitumorigenic effects of HIV protease inhibitor ritonavir: inhibition of Kaposi sarcoma. Blood. 2002;99(10):3771–9. [DOI] [PubMed] [Google Scholar]
- 10.Eatemadi A, Aiyelabegan HT, Negahdari B, et al. Role of protease and protease inhibitors in cancer pathogenesis and treatment. Biomed Pharmacother. 2017;85:221–231. [DOI] [PubMed] [Google Scholar]
- 11.Monini P, Sgadari C, Grosso MG, et al. Clinical Course of classic Kaposi’s sarcoma in HIV-negative patients treated with the HIV protease inhibitor indinavir. AIDS. 2009;23:534–538. [DOI] [PubMed] [Google Scholar]
- 12.Caro-Vega Y, Belaunzaran-Zamudio PF, Crabtree-Ramirez BE, et al. Durability of Efavirenz Compared with Boosted Protease Inhibitor-Based Regimens in Antiretroviral-Naïve Patients in the Caribbean and Central and South America. OFID. 2018;5(3):ofy004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.AIDSinfo. What to Start: Initial Combination Regimens for the Antiretroviral-Naive Patient. Available at: https://aidsinfo.nih.gov/guidelines/html/1/adult-and-adolescent-arv/11/what-to-start. Accessed May 8, 2018.
- 14.AIDSinfo. Guidelines for the Use of Antiretroviral Agents in Pediatric HIV Infection. Available at: https://aidsinfo.nih.gov/guidelines/html/2/pediatric-arv/444/regimens-recommended-for-initial-therapy-of-antiretroviral-naive-children. Accessed May 7, 2018.
- 15.Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection (Second edition): Summary of recommendations. 2016;xxxii–xxxiii. Available at: http://www.who.int/hiv/pub/arv/summary-recommendations.pdf?ua=1. Accessed May 12, 2018.
- 16.Moher D, Shamseer L, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Systematic Reviews. 2015;4:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.AIDS-defining Cancer Project Working Group for IeDEA and COHERE in EuroCoord. Comparison of Kaposi sarcoma risk in HIV-positive adults across five continents: a multiregional multicohort study. Clin Infect Dis. 2017;65(8):1316–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kowalkowski MA, Kramer JR, Richardson PR, Suteria I, Chiao EY. Use of boosted protease inhibitors reduces Kaposi sarcoma incidence among male veterans with HIV infection. Clin Infect Dis. 2015;60(9):1405–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chao C, Leyden WA, Xu L, et al. Exposure to antiretroviral therapy and risk of cancer in HIV-infected persons. AIDS. 2012;26(17):2223–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bruyand M, Ryom L, Shepherd L, et al. Cancer risk and use of protease inhibitor or nonnucleoside reverse transcriptase inhibitor-based combination antiretroviral therapy: the D:A:D study. J Acquir Immune Defic Syndr. 2015;68(5):568–77. [DOI] [PubMed] [Google Scholar]
- 21.Grabar S, Abraham B, Mahamat A, Del Giudice P, Rosenthal E, Costagliola D. Differential impact of combination antiretroviral therapy in preventing Kaposi’s sarcoma with and without visceral involvement. J Clin Oncol. 2006;24(21):3408–14. [DOI] [PubMed] [Google Scholar]
- 22.Lacombe JM, Boue F, Grabar S, et al. Risk of Kaposi sarcoma during the first months on combination antiretroviral therapy. AIDS. 2013;27(4):635–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lodi S, Guiguet M, Costagliola D, Fisher M, de Luca A, Porter K. Kaposi sarcoma incidence and survival among HIV-infected homosexual men after HIV seroconversion. J Natl Cancer Inst. 2010;102(11):784–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cancer Project Working Group for the Collaboration of Observational HIV Epidemiological Research Europe (COHERE) study in EuroCoord. Changing incidence and risk factors for Kaposi sarcoma by time since starting antiretroviral therapy: collaborative analysis of 21 European cohort studies. Clin Infect Dis. 2016;63(10):1373–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ensoli B, Barillari G, Salahuddin SZ, Gallo RC, Wong-Staal F. Tat protein of HIV-1 stimulates growth of cells derived from Kaposi’s sarcoma lesions of AIDS patients. Nature. 1990;345(6270):84–6. [DOI] [PubMed] [Google Scholar]
- 26.Ensoli B, Gendelman R, Markham P, et al. Synergy between basic fibroblast growth factor and HIV-1 Tat protein in induction of Kaposi’s sarcoma. Nature. 1994;371(6499):674–80. [DOI] [PubMed] [Google Scholar]
- 27.Borges ÁH, Lundh A,Tendal B, et al. Nonnucleoside Reverse-transcriptase Inhibitor- vs Ritonavir-boosted Protease Inhibitor–based Regimens for Initial Treatment of HIV Infection: A Systematic Review and Metaanalysis of Randomized Trials. Clin Infect Dis. 2016;63(2):268–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stebbing J, Portsmouth S, Gazzard B. How does HAART lead to the resolution of Kaposi’s sarcoma? J. Antimicrob Chemother. 2003;51:1095–1098. [DOI] [PubMed] [Google Scholar]
- 29.Sgadari C, Monini P, Barillari G, Ensoli B. Use of HIV protease inhibitors to block Kaposi’s sarcoma and tumour growth. Lancet Oncol. 2003;4(9):537–47. [DOI] [PubMed] [Google Scholar]
- 30.Hecht M, Erber S, Harrer T, et al. Efavirenz has the highest anti-proliferative effect of non-nucleoside reverse transcriptase inhibitors against pancreatic cancer cells. PLoS One. 2015;10(6):e0130277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mangiacasale R, Pittoggi C, Sciamanna I, et al. Exposure of normal and transformed cells to nevirapine, a reverse transcriptase inhibitor, reduces cell growth and promotes differentiation. Oncogene. 2003;22(18):2750–61. [DOI] [PubMed] [Google Scholar]
- 32.Kennedy AR. Chemopreventive agents: protease inhibitors. Pharmacology & Therapeutics. 1998;78(3):167–209. [DOI] [PubMed] [Google Scholar]
- 33.Stebbing J, Gazzard B, Mandalia S, et al. Antiretroviral treatment regimens and immune parameters in the prevention of systemic AIDS-related non-Hodgkin’s lymphoma. J Clin Oncol. 2004;22(11):2177–83. [DOI] [PubMed] [Google Scholar]
- 34.Gantt S, Casper C. Human herpesvirus 8-associated neoplasms: the roles of viral replication and antiviral treatment. Curr Opin Infect Dis. 2011;24(4):295–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gantt S, Casper C, Ambinder RF. Insights into the broad cellular effects of nelfinavir and the HIV protease inhibitors supporting their role in cancer treatment and prevention. Curr Opin Oncol. 2013;25(5):495–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Totonchy J, Cesarman E. Does persistent HIV replication explain continued lymphoma incidence in the era of effective antiretroviral therapy? Curr Opin Virol. 2016;20:71–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dedicoat M, Newton R. Review of the distribution of Kaposi’s sarcoma-associated herpesvirus (KSHV) in Africa in relation to the incidence of Kaposi’s sarcoma. British Journal of Cancer. 2003;88(1):1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Plummer M, de Martel C, Vignat J, Ferlay J, Bray F, Franceschi S. Global burden of cancers attributable to infections in 2012: a synthetic analysis. Lancet Glob Health. 2016;4(9):e609–16. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.