Abstract
The gut microbiota is essential for good health. It has also been demonstrated that the gut microbiota can regulate immune responses against respiratory tract infections. Since the outbreak of the COVID-19 pandemic, accumulating evidence suggests that there is a link between the severity of COVID-19 and the alteration of one’s gut microbiota. The composition of gut microbiota can be profoundly affected by COVID-19 and vice versa. Here, we summarize the observations of the mutual impact between SARS-CoV-2 infection and gut microbiota composition. We discuss the consequences and mechanisms of the bi-directional interaction. Moreover, we also discuss the immune cross-reactivity between SARS-CoV-2 and commensal bacteria, which represents a previously overlooked connection between COVID-19 and commensal gut bacteria. Finally, we summarize the progress in managing COVID-19 by utilizing microbial interventions.
Keywords: SARS-CoV-2, gut microbiota, gut–lung axis, cross-reactive immunity
1. Introduction
The gastrointestinal (GI) tract houses a huge micro-ecosystem containing trillions of microorganisms that play essential roles in health and diseases [1,2,3]. Growing evidence shows that the gut microbiota is associated with infectious diseases and inflammation [4,5,6], e.g., multiple studies demonstrate that gut microbiota can regulate immune responses against respiratory tract infections, such as tuberculosis and influenza [7,8,9,10,11,12,13,14]. Moreover, it has also been shown that the gut microbiota is involved with chronic lung diseases via the gut–lung axis [15,16], including asthma, chronic obstructive pulmonary disease, and cystic fibrosis [17,18,19,20,21]. Since the outbreak of COVID-19, the link between gut microbiota and COVID-19 severity has been intensively studied [22,23,24,25,26]. In this review, we will focus on discussing the reciprocal impacts of gut bacteria and COVID-19, thereby characterizing the role of commensal gut bacteria in the pathogenesis of SARS-CoV-2 from a holistic point of view.
2. The Causal Link between COVID-19 and Gut Dysbiosis
Airborne infection is the major route of SARS-CoV-2 transmission [27,28]. However, the respiratory tract is not the only site that SARS-CoV-2 can infect. The intestine can also be an important site of infection, as enterocytes express high levels of ACE2 and TMPRSS2 [29], which have been identified as the two major cell surface molecules mediating SARS-CoV-2 infection [30]. It has been proven by several studies that the virus can replicate in the intestine [31,32,33,34]. Meanwhile, GI complications [24,35,36,37], in addition to respiratory-tract infection symptoms [35], such as fever, fatigue, and dry cough, can be frequently observed among COVID-19 patients. The most common GI symptoms accompanied by SARS-CoV-2 infection are anorexia, diarrhea, nausea, vomiting, and abdominal pain/discomfort [38,39,40,41,42], which can occur in either combination or alone. For example, an analysis of symptoms in 411 COVID-19 patients shows that 42 patients reported gastrointestinal symptoms (10.2%), including nausea (18, 4.3%), vomiting (16, 3.8%), diarrhea (15, 3.6%), or abdominal pain (5, 1.2%) [41]. Although most COVID-19-associated GI symptoms are mild, patients with GI symptoms are reported to have a significantly increased risk of severe COVID-19 compared with patients without GI symptoms [39].
Multiple potential mechanisms might be involved with COVID-19-associated GI symptoms. First, the SARS-CoV-2 infection of gut mucosa results in the endocytosis of the apical ACE2 protein [43,44], a key regulator of dietary amino acid homeostasis and innate immunity in the intestine [45,46], thereby prompting inflammatory responses in the gut. As SARS-CoV-2 infection itself can trigger inflammatory responses in the intestine [47], the dysfunction of the ACE2 may exaggerate the inflammation and lead to the damage of intestinal mucosa [48], as proven by the elevated fecal calprotectin [49] and increased infiltration of lymphocytes in the lamina propria of the GI tract [50]. Second, SARS-CoV-2 infection of the intestinal epithelium can impair the expression of the ACE2, which might cause gut dysbiosis [43,45,51]. The ACE2 forms a heterologous complex with the amino acid transporter B0AT1, which controls the uptake of tryptophan in the intestine [45]. This amino acid regulates the expression of antimicrobial peptides through the mTOR pathway [45]. Downregulation of the ACE2 can cause decreased intestinal absorption of tryptophan and lead to the dysregulation of antimicrobial peptides, intestinal leakage, and dysbiosis [45,52,53]. SARS-CoV-2-mediated intestinal leakage may lead to systemic elevation of bacterial lipopolysaccharide and peptidoglycan, further worsening gastrointestinal inflammation [54]. ACE2 knockout mice exhibited altered gut microbiota and developed more severe dextran sulfate sodium-induced colitis compared to wild-type control mice [45]. Third, SARS-CoV-2 infection of the respiratory tract may regulate the immune status of the digestive tract via the “gut–lung axis”, as exemplified by the observation that respiratory-influenza infection induced CCR9+ CD4+ T cells can be recruited to the small intestine and cause intestinal injury by disturbing the intestinal microbiota composition [55]. In addition, another study using an influenza-infected mouse model demonstrates that influenza-induced IFN-Is, produced in the lungs, can lead to gut dysbiosis and increase host susceptibility to secondary Salmonella transmission, inhibit intestinal immunity, and promote intestinal inflammation [56]. The mechanism underlying the impact of the respiratory-tract infection, from SARS-CoV-2, on the gut microbiota has not been clarified. However, it is suggested that inflammatory cytokines and hypoxia can drive gut dysbiosis during the viral infection [57]. In addition to the above potential mechanisms, some of the COVID-19-associated GI symptoms, such as diarrhea, are related to the use of large quantities of antibiotics [36,58] or reduced food intake [59,60].
It remains unresolved which above mechanism plays a major role in the COVID-19-associated GI symptoms. But, it is quite clear that all these mechanisms can lead to gut dysbiosis, which is actually more prevalent than GI symptoms among hospitalized COVID-19 patients [24,61]. The causal relationship between COVID-19 and gut dysbiosis has been verified by animal experiments, which have shown that SARS-CoV-2 infection alters the composition of gut microbiota in mice [62,63], hamsters [64,65], and nonhuman primates [66]. The COVID-19-associated gut dysbiosis is characterized by the enrichment of opportunistic pathogens and the decrease in beneficial symbionts [67]. Compared with healthy persons, patients with COVID-19 have significantly reduced microbial diversity, lower abundances of the anti-inflammatory bacteria (such as Lachnospiraceae, Eubacterium, and Faecalibacterium prausnitzii), and enriched abundances of opportunistic pathogens (such as Streptococcus, Rothia, Veillonella, and Actinomyces) [24,68]. In addition, patients with COVID-19 also have increased proportions of opportunistic fungal pathogens, such as Aspergillus flavus and Aspergillus niger, in feces [61]. Notably, the COVID-19-associated alteration of the gut microbiota occurs irrespective of whether the patient was medicated, and the symptoms can last long after disease resolution [25,69]. The alteration of gut microbiota caused by the SARS-CoV-2 infection is different from that of the H1N1 infection [68]. The abundance of certain common opportunistic bacteria, such as Enterococcus and Enterobacteriaceae, can serve as diagnostic biomarkers for critical COVID-19 [26].
3. The Reverse Impact of Gut Dysbiosis on COVID-19 Disease Progression
The aforementioned evidence shows that SARS-CoV-2 infection impacts the composition of gut microbiota; conversely, the alteration of gut microbiota composition is also found to correlate with increased severity and mortality rates among hospitalized COVID-19 patients [24,25,26,70,71,72,73,74,75]. The abundance of certain bacteria, such as Enterococcus [71] and Bacteroides [75,76], correlates positively with the severity of COVID-19. While, Faecalibacterium, which is suggested to be a marker of health, is negatively correlated with the severity of COVID-19 [24,77]. Moreover, the relatively high incidence of severe COVID-19 found in elderly patients [78,79,80,81] might also be partly explained by the decreased diversity of gut microbiota [82], because there is a possible link between the gut microbiota diversity and the clinical outcome of COVID-19 [23]. This notion is corroborated by a multivariate analysis showing that the Shannon diversity index of gut microbiota is significantly associated with COVID-19 severity [83]. Although the association between gut dysbiosis and the severity of COVID-19 has been observed in different clinical settings (Table 1), the causal impact of the gut microbiota on the severity of COVID-19 has not been fully clarified. A preprint study provides the first evidence that the gut dysbiosis caused by the SARS-CoV-2 infection can lead to a gut-to-blood translocation of microorganisms, suggesting a direct role for gut dysbiosis in enabling secondary bloodstream infections during COVID-19 [71]. Meanwhile, Edwinson et al. suggested that commensal microbiota might play a key role in regulating intestinal ACE2 expression using a humanized mouse model [84].
Table 1.
Intestinal microbial alterations and their effect in COVID-19.
| (Refs.) | Intestinal Microbial Alterations | Effect in COVID-19 |
|---|---|---|
| [24] | Faecalibacterium prausnitzii ↓ | Anti-inflammatory. Inverse correlation between abundance and disease severity. |
| Alistipes onderdonkii ↓ | Involving in the serotonin precursor tryptophan metabolism and maintaining gut immune homeostasis. Negative correlation with COVID-19 severity. | |
|
Bacteroides dorei, Bacteroides thetaiotaomicron, Bacteroides massiliensis, Bacteroides ovatus ↓ |
Downregulating the expression of angiotensin-converting enzyme 2 (ACE2). Correlated inversely with SARS-CoV-2 load in fecal samples. | |
|
Coprobacillus, Clostridium ramosum, Clostridium hathewayi ↑ |
Correlating positively with COVID-19 severity. Coprobacillus bacterium upregulates the expression of ACE2. | |
| [68] |
Streptococcus, Rothia, Veillonella, Actinomyces ↑ |
Opportunistic pathogens. Significantly increased relative abundances in COVID-19 patients compared with those in healthy controls. |
|
Fusicatenibacter, Anaerostipes, Agathobacter, unclassified Lachnospiraceae, Eubacterium hallii ↓ |
Butyrate-producing bacteria. The abundances are dramatically reduced in COVID-19 patients compared with those in healthy controls. | |
| [61] |
Candida albicans, Candida auris, Aspergillus flavus, Aspergillus niger ↑ |
Significantly higher relative abundances in hospitalized COVID-19 patients compared with those in healthy controls. |
| [25] |
Faecalibacterium prausnitzii, Eubacterium rectale, Bifidobacterium adolescentis ↓ |
Anti-inflammatory. These bacteria are depleted in COVID-19 patients. |
|
Bacteroides dorei, Akkermansia muciniphila ↑ |
Correlating positively with IL-1β, IL-6, and CXCL8. Enriched in COVID-19 patients. | |
| [26] |
Faecalibacterium prausnitzii, Clostridium butyricum, Clostridium leptum, Eubacterium rectale ↓ |
Butyrate-producing bacteria. The abundances decreased significantly in COVID-19 patients. |
| Lactobacillus, Bifidobacterium ↓ | Producing lactic acid, regulating immunity, and maintaining intestinal barrier function. Correlating negatively with COVID-19 severity. | |
|
Enterococcus (Ec), Enterobacteriaceae (E) ↑ |
Opportunistic pathogens. Correlating positively with COVID-19 severity and the Ec/E ratio can predict death in critically ill patients. | |
| [71] | Faecalibacterium ↓ | An immunosupportive Clostridiales genus. Correlating negatively with bloodstream infection (BSI). |
| [72] | Bilophila, Citrobacter ↓ | Correlating negatively with COVID-19 severity. |
| Genus: Streptococcus, Clostridium, Lactobacillus, Bifidobacterium, ↑ | The abundances increased significantly in COVID-19 patients compared with those in healthy controls. | |
| [76] |
Genus: Escherichia/Shigella, Citrobacter, Collinsella, Bifidobacterium ↑ |
Correlating positively with COVID-19. |
|
Genus: Bacteroides, Butyricimonas, Odoribacter ↓ |
Short-chain fatty acid (SCFA)-producing bacteria. Markedly reduced in patients with COVID-19 compared to healthy controls. | |
| [75] |
Butyricicoccus pullicaecorum, Clostridium ruminatium, Lachnospira pectinoschiza, Pseudobutyrivibrio xylanivorans, ↓ |
Completely absent in the guts of COVID-19-infected patients. |
|
Roseburia faecis, Lachnospira pectinoschiza, Faecalibacterium prausnitzii ↓ |
Short-chain fatty acid-producing bacteria. Correlating negatively with COVID-19 severity. | |
|
Clostridium hathewayi, arabacteroides distasonis, Ruminococcus gnavus ↑ |
Correlating positively with COVID-19 severity. | |
| [85] |
Bacteroidaceae, Lachnospiraceae, Ruminococcaceae ↓ |
Producing short-chain fatty acids (SCFAs). The abundances decreased significantly in COVID-19 patients compared to those in healthy controls. |
| Enterococcus ↑ | Far overrepresented in COVID-19 patients developing bloodstream infections (BSIs) and admitted to the intensive care unit. | |
|
Enterococcaceae, Coriobacteriaceae, Lactobacillaceae, Veillonellaceae, Porphyromonadaceae Staphylococcaceae ↑ |
The abundance increased significantly in COVID-19 patients compared to those in healthy controls. | |
| [69] |
Ruminococcus gnavus, Bacteroides vulgatus ↑ |
The abundances increased significantly in patients with post-acute COVID-19 syndrome (PACS) than in non-COVID-19 controls. |
|
Bifidobacterium pseudocatenulatum, Faecalibacterium prausnitzii ↓ |
Butyrate-producing bacteria. Correlating negatively with the development of PACS. | |
| [86] |
Genus: Roseburia, Megasphaer Species: Roseburia inulinivorans, Bacteroides faecis, Bifidobacterium bifidum, Parabacteroides goldsteinii, Lachnospiraceae bacterium 9143BFAA, Megasphaera sp. ↓ |
Correlating negatively with COVID-19 severity. |
|
Genus: Paraprevotella, Lachnospiraceae, Erysipelotrichaceae Species: Paraprevotella sp., Streptococcus thermophilus, Clostridium ramosum, Bifidobacterium animalis ↑ |
Correlating positively with COVID-19 severity. | |
| [87] | Genus: Collinsella ↓ | Inhibiting the binding of SARS-CoV-2 to ACE2, suppressing proinflammatory cytokine secretion, antioxidant, and anti-apoptotic. Correlating negatively with the mortality rates of COVID-19. |
| Genus: Dorea, Fusicatenibacter ↓ | Short-chain fatty acid (SCFA)-producing bacteria. Correlating negatively with the mortality rates of COVID-19 |
Notes: ↑, significantly increased; ↓, significantly decreased.
In addition to microbial translocation, gut microbiota can alleviate or aggravate viral infections via other mechanisms [88,89]. On the one hand, microbial products at the entry site can bind with viruses to enhance their stability and infectivity; on the other hand, the microbiota can inhibit viral entry via regulating local pH and host immune responses [90]. Given that gut microbiota or their products are less likely to interact directly with SARS-CoV-2 in the respiratory tract, their impact on the infection in the lung is more likely driven by the indirect modulation of host immunity. The alteration of gut microbial composition can influence inflammatory responses outside the gastrointestinal tract through diverse mechanisms [91]. First, it has been found that the perturbation of gut microbiota can stimulate the immune system to release cytokines such as IL-1β, IL-2, IL-10, TNF-α, and IFN-γ, which may exacerbate the severity of COVID-19 [92,93,94]. As it has been suggested, mortality associated with COVID-19 is mainly caused by enhanced cytokine and chemokine production. This contributes to virally induced hyper-inflammation, referred to as the “cytokine storm” [95,96], where the overproduction of proinflammatory cytokines may exacerbate the severity of COVID-19 [97]. Mechanistically, the reduced abundance of probiotics, such as butyrate-producing bacteria [26,87,98], may undermine the anti-inflammatory effects mediated by regulatory T cells [99]. At the same time, the enrichment of Escherichia and Shigella could lead to systemic inflammation [98]. Additionally, the downregulation of the ACE2 by the SARS-CoV-2 infection may lead to the increased activation of the renin-angiotensin system (RAS), which may cause systemic vasoconstriction and systemic inflammatory response syndrome (SIRS) [100]. Conversely, blocking the renin-angiotensin pathway has been shown to be able to alleviate the SARS-CoV-2 spike protein-induced acute lung failure in mice [101].
Second, gut microbes can affect physiological and pathological immune responses in the airways through neural, endocrine, immune, humoral, and metabolic pathways [102,103,104], which are collectively described as the gut–lung axis [105]. The gut–lung axis is bidirectional, but most of the current evidence suggests that gut microbiota could most likely regulate lung homeostasis, which is exemplified in patients with chronic gastrointestinal diseases, such as irritable bowel syndrome (IBS) and inflammatory bowel disease (IBD), who have a higher prevalence of pulmonary diseases [106,107,108]. Via the gut–lung axis, the gut microbiota can impact the production of type I interferons (IFNs) in the lung [12,13,14], which are well known to control viral infections, including SARS-CoV-2 [109,110,111]. Microbial metabolites such as deaminated tyrosine (DAT, derived from flavonoid and amino acid metabolism) and short-chain fatty acids (SCFAs, the end products of dietary fiber fermentation by commensal bacteria) have been shown to be critical in regulating the anti-virial immunities in the respiratory tract [12,13]. SCFAs exert anti-inflammatory, anti-antitumor, and antibacterial effects by inhibiting histone deacetylase (HDAC) and activating the G protein-coupled receptor (GPCR) [112,113]. They can also strongly reduce the release of several proinflammatory chemokines through regulatory T cells, including CCL3, CCL4, CCL5, CXCL9, CXCL10, and CXCL11 [114]. In addition, butyrate and propionate can inhibit the expression of lipopolysaccharide (LPS)-induced cytokines such as IL-6 and IL-12p40, displaying a strong anti-inflammatory effect [114,115]. The reduced abundance of SCFA-producing bacteria observed in the gut microbiota of COVID-19 patients may be one of the key mechanisms leading to severe clinical outcomes, according to previous studies [26,87,98,116]. Moreover, the impaired capacity to synthesize short-chain fatty acids and L-isoleucine by the gut microbiome of COVID-19 patients continues even after the remission of the disease [94]. Additionally, it has also been found that the upper gastrointestinal microbiota affects the development of the airway microbiota [117], which plays an immediate role in calibrating the alveolar immunity of COVID-19 patients [118]. Taken together, the facts listed in this section imply that the gut microbiota plays an active role in determining the clinical progression of COVID-19.
4. Direct Interaction and Immune Cross-Reactivity between SARS-CoV-2 and Commensal Bacteria
Available data have shown that the commensal bacteria and their products can interact directly with a variety of viruses to either promote or suppress viral infections [119,120,121,122]. Lipopolysaccharides (LPSs) and peptidoglycans (PGs) are the major microbial products that have been frequently observed to interact directly with viruses [119,123,124,125,126,127]. For example, the binding of an LPS to a poliovirus promotes virion stability and cell attachment [128]. The mouse mammary tumor virus (MMTV) can integrate a TLR-4 into its envelope to bind the bacterial LPS. The LPS bound to the MMTV stimulates the secretion of IL-10 through TLR-4 signaling, and IL-10 allows viral persistence through negative immune regulation [126,127]. The interaction between the SARS-CoV-2 S protein and the LPS has also been observed and proven to be able to boost cytokine responses in human peripheral blood mononuclear cells [129]. More intriguingly, a recent study demonstrates that the SARS-CoV-2 spike S1 subunit can inhibit the biofilm formation by Streptococcus pneumoniae and Staphylococcus aureus [130], suggesting that coronavirus infections may promote these opportunistic pathogens to resume a more virulent planktonic lifestyle. Moreover, although it has not been experimentally validated, an amino acid blast analysis suggests that proteobacteria may secrete homologues of the TMPRSS2 and the ACE2 peptidase domain [131], which may presumably inhibit SARS-CoV-2 infection via blocking the binding of the spike protein to the receptors.
Based on amino acid sequence analyses, another study indicates that common human pathogens and vaccines, such as Meningococcal B and combination vaccines for diphtheria, tetanus, and pertussis (DTP vaccine), may potentially induce cross-reactive immunity to SARS-CoV-2 [132]. This hypothesis is partly supported by a clinical observation that pre-existing antibodies acquired from childhood vaccinations or past infections of Rubella, Pneumococcus, and Bordetella pertussis may confer some protection against COVID-19 [133]. It has also been proven that commensal gut bacteria may facilitate the induction of neutralizing antibodies [134,135,136] and cross-reactive T cell responses [137,138] against viruses such as HIV-1. In a recent study, we provide the first experimental evidence that pre-existing antibodies targeting a conserved linear epitope on S2 (1147-SFKEELDKYFKNHT-1160) cross-react with commensal gut microbial antigens [139]. Specific monoclonal antibodies against the epitope are proven to cross-react with diverse antigens of gut bacteria, such as the HSP60 and HSP70 proteins derived from E. coli [139]. Our finding is corroborated by a subsequent study suggesting that this conserved spike epitope shares sequence homology to proteins in commensal gut microbiota and can prime immune responses in humans [140]. Of note, this is not a phenomenon only observed with respect to S2-specific antibodies; accumulating evidence suggests that RBD-specific antibodies [141] and T cell responses, cross-reactive to SARS-CoV-2 [142,143,144], can be primed by commensal gut bacteria. The pre-existing cross-reactive immunities elicited by commensal bacteria may shape the host’s immune responses after infection or vaccination; however, their exact role in controlling SARS-CoV-2 transmission and infection needs to be further specified.
5. The Microbiota Mediated Interventions for COVID-19
Microbiota-based interventions (such as diets, probiotics, Chinese herbs, and fecal microbiota transplantation) have been used in the clinical treatment of various human diseases (such as diabetes, ulcerative colitis, Crohn’s disease, and certain viral infections) [145,146]. Considering the significant impact of gut microbiota on the course of COVID-19, modulating the composition of gut microbiota is considered a possible method for treating SARS-CoV-2 [147,148].
Several approaches are exploited to achieve this goal. The first approach involves regulating the composition of the gut microbiota via dietary interventions. Diets have been shown to play an important role in shaping gut microbiota [146,149,150]. For example, glycated pea proteins increase the intestinal commensal bacteria (Bifidobacterium and Lactobacillus) [149], and the high-fiber diet can alter the ratio of Firmicutes to Bacteroidetes, which can exert anti-inflammatory effects by increasing short-chain fatty acids (SCFAs) [150]. The high-fiber diet has been shown to be able to improve gastrointestinal symptoms of COVID-19 by increasing the SCFAs-producing bacteria (such as Oscillibacter, Sellimonas, Bifidobacterium, Blautia, Lactobacillus, Faecalitalea, Anaerofustis, and Eubacterium) in the gut [151]. In addition, vitamin D supplements are shown to improve clinical symptoms by reducing inflammatory cytokine levels [152], which is partly because vitamin D can modulate the composition of gut microbes [153,154].
The second method involves regulating the gut microbiota via the supplementation of probiotics. The SARS-CoV-2 infection leads to a decrease in commensal bacteria such as Lactobacillus and Bifidobacterium, which can affect innate and adaptive immune responses to prevent and mitigate bacterial and viral infections [155,156,157]. Observations in mice infected with influenza A virus (H1N1) show that treatments with probiotic strains (Enterococcus faecalis and Bifidobacterium) can downregulate inflammatory cytokines by balancing the Th1/Th2 immune response and reducing the mortality of mice [158,159]. Clinical studies suggest that probiotics can be used to reduce inflammation by changing the composition of gut microbiota in COVID-19 patients [160], which includes the enrichment of intestinal commensal bacteria and the inhibition of opportunistic pathogens [161,162]. Moreover, it has also been found that drug therapy combined with probiotics can reduce gastrointestinal symptoms and mortalities in COVID-19 patients [163,164].
Third, regulating gut microbiota using traditional Chinese medicine (TCM) is a potential approach. It has been demonstrated that some Chinese herbs can regulate gut microbiota [165,166]. For example, extracts of Ginseng radix et rhizome rubra and Coicis semen promote the growth of probiotics (Lactobacillus and Bifidobacterium) and inhibit the growth of pathogenic bacteria (Escherichia, Staphylococcus, and Salmonella) [167]. Gegen Qinlian decocted can elevate the relative abundance of SCFA-producing bacteria, including Akkermansia, Bacteroides, Clostridium, Ruminococcus, and Phascolarctobacterium [168]. The treatment of COVID-19 with TCM via regulating gut microbiota has been proposed [166], but clinical and experimental evidence is needed to verify this notion.
Fourth, regulating gut microbiota via fecal microbiota transplantation (FMT) can be used to treat a variety of diseases related to gut dysbiosis [169,170]. It has been proven that FMT treatment can improve gut dysbiosis in recovered COVID-19 patients, especially in those with severe gastrointestinal symptoms [171].
6. Conclusions
The SARS-CoV-2 infection can cause gut dysbiosis and GI symptoms; conversely, gut microbiota can also impact the SARS-CoV-2 infection in the respiratory tract (Figure 1). Multiple mechanisms are involved in this mutual interaction. The gut–lung axis is usually believed to be the major bi-directional connection between the airway viral infection and the gut microbiota. In addition, a few recent studies characterized the cross-reactive antibody and T cell responses between SARS-CoV-2 and gut microbiota, demonstrating that there was an alternative bi-directional link between airway SARS-CoV-2 infections and the gut microbiota. Deeper insights into this phenomenon can expand the understanding of the entanglement between airway viral infections and the gut microbiota, thereby promoting the development of new treatments for COVID-19 and other severe respiratory viral infections.
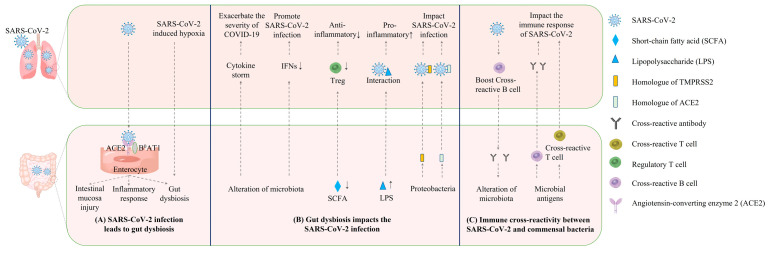
Figure 1.
Potential mechanisms underlying the mutual impacts between intestinal microbiota and SARS-CoV-2. (A) SARS-CoV-2 can cause gut dysbiosis by infecting enterocytes or causing systematic hypoxia. (B) Gut dysbiosis can impact SARS-CoV-2 infection in the lungs via regulating immune responses in the lung or secreting homologues of ACE2 and TMPRSS2. (C) The microbial antigens may elicit cross-reactive antibody and T cell responses against SARS-CoV-2. SARS-CoV-2 infection may reinforce cross-reactive antibody responses against microbial antigens and thereby lead to the alteration of gut microbiota.
Author Contributions
Literature search and writing—original draft preparation, S.L. and Y.W.; writing—review and editing, Y.Z., D.Y. and Y.W. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data sharing not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This work was partly supported by the National Natural Science Foundation of China (Grant No. 81971559), the Science and Technology Commission of Shanghai Municipality (No. 21NL2600100), and the major project of Study on Pathogenesis and Epidemic Prevention Technology System (No.2021YFC2302500) by the Ministry of Science and Technology of China.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Gomaa E.Z. Human gut microbiota/microbiome in health and diseases: A review. Antonie Van Leeuwenhoek. 2020;113:2019–2040. doi: 10.1007/s10482-020-01474-7. [DOI] [PubMed] [Google Scholar]
- 2.Durack J., Lynch S.V. The gut microbiome: Relationships with disease and opportunities for therapy. J. Exp. Med. 2019;216:20–40. doi: 10.1084/jem.20180448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cong J., Zhou P., Zhang R. Intestinal Microbiota-Derived Short Chain Fatty Acids in Host Health and Disease. Nutrients. 2022;14:1977. doi: 10.3390/nu14091977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Young V.B., Britton R.A., Schmidt T.M. The human microbiome and infectious diseases: Beyond koch. Interdiscip. Perspect. Infect. Dis. 2008;2008:296873. doi: 10.1155/2008/296873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Honda K., Littman D.R. The microbiome in infectious disease and inflammation. Annu. Rev. Immunol. 2012;30:759–795. doi: 10.1146/annurev-immunol-020711-074937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harris V.C., Haak B.W., Boele van Hensbroek M., Wiersinga W.J. The Intestinal Microbiome in Infectious Diseases: The Clinical Relevance of a Rapidly Emerging Field. Open Forum Infect. Dis. 2017;4:ofx144. doi: 10.1093/ofid/ofx144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang F., Yang Y., Chen L., Zhang Z., Liu L., Zhang C., Mai Q., Chen Y., Chen Z., Lin T., et al. The gut microbiota mediates protective immunity against tuberculosis via modulation of lncRNA. Gut Microbes. 2022;14:2029997. doi: 10.1080/19490976.2022.2029997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ichinohe T., Pang I.K., Kumamoto Y., Peaper D.R., Ho J.H., Murray T.S., Iwasaki A. Microbiota regulates immune defense against respiratory tract influenza A virus infection. Proc. Natl. Acad. Sci. USA. 2011;108:5354–5359. doi: 10.1073/pnas.1019378108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yitbarek A., Alkie T., Taha-Abdelaziz K., Astill J., Rodriguez-Lecompte J.C., Parkinson J., Nagy E., Sharif S. Gut microbiota modulates type I interferon and antibody-mediated immune responses in chickens infected with influenza virus subtype H9N2. Benef. Microbes. 2018;9:417–427. doi: 10.3920/BM2017.0088. [DOI] [PubMed] [Google Scholar]
- 10.Dumas A., Bernard L., Poquet Y., Lugo-Villarino G., Neyrolles O. The role of the lung microbiota and the gut-lung axis in respiratory infectious diseases. Cell. Microbiol. 2018;20:e12966. doi: 10.1111/cmi.12966. [DOI] [PubMed] [Google Scholar]
- 11.Abt M.C., Osborne L.C., Monticelli L.A., Doering T.A., Alenghat T., Sonnenberg G.F., Paley M.A., Antenus M., Williams K.L., Erikson J., et al. Commensal bacteria calibrate the activation threshold of innate antiviral immunity. Immunity. 2012;37:158–170. doi: 10.1016/j.immuni.2012.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Antunes K.H., Fachi J.L., de Paula R., da Silva E.F., Pral L.P., Dos Santos A.A., Dias G.B.M., Vargas J.E., Puga R., Mayer F.Q., et al. Microbiota-derived acetate protects against respiratory syncytial virus infection through a GPR43-type 1 interferon response. Nat. Commun. 2019;10:3273. doi: 10.1038/s41467-019-11152-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Steed A.L., Christophi G.P., Kaiko G.E., Sun L., Goodwin V.M., Jain U., Esaulova E., Artyomov M.N., Morales D.J., Holtzman M.J., et al. The microbial metabolite desaminotyrosine protects from influenza through type I interferon. Science. 2017;357:498–502. doi: 10.1126/science.aam5336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bradley K.C., Finsterbusch K., Schnepf D., Crotta S., Llorian M., Davidson S., Fuchs S.Y., Staeheli P., Wack A. Microbiota-Driven Tonic Interferon Signals in Lung Stromal Cells Protect from Influenza Virus Infection. Cell Rep. 2019;28:245–256.e4. doi: 10.1016/j.celrep.2019.05.105. [DOI] [PubMed] [Google Scholar]
- 15.Wang J., Li F., Tian Z. Role of microbiota on lung homeostasis and diseases. Sci. China Life Sci. 2017;60:1407–1415. doi: 10.1007/s11427-017-9151-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Budden K.F., Shukla S.D., Rehman S.F., Bowerman K.L., Keely S., Hugenholtz P., Armstrong-James D.P.H., Adcock I.M., Chotirmall S.H., Chung K.F., et al. Functional effects of the microbiota in chronic respiratory disease. Lancet Respir. Med. 2019;7:907–920. doi: 10.1016/S2213-2600(18)30510-1. [DOI] [PubMed] [Google Scholar]
- 17.Ver Heul A., Planer J., Kau A.L. The Human Microbiota and Asthma. Clin. Rev. Allergy Immunol. 2019;57:350–363. doi: 10.1007/s12016-018-8719-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Russell S.L., Gold M.J., Hartmann M., Willing B.P., Thorson L., Wlodarska M., Gill N., Blanchet M.R., Mohn W.W., McNagny K.M., et al. Early life antibiotic-driven changes in microbiota enhance susceptibility to allergic asthma. EMBO Rep. 2012;13:440–447. doi: 10.1038/embor.2012.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lai H.C., Lin T.L., Chen T.W., Kuo Y.L., Chang C.J., Wu T.R., Shu C.C., Tsai Y.H., Swift S., Lu C.C. Gut microbiota modulates COPD pathogenesis: Role of anti-inflammatory Parabacteroides goldsteinii lipopolysaccharide. Gut. 2022;71:309–321. doi: 10.1136/gutjnl-2020-322599. [DOI] [PubMed] [Google Scholar]
- 20.Qu L., Cheng Q., Wang Y., Mu H., Zhang Y. COPD and Gut-Lung Axis: How Microbiota and Host Inflammasome Influence COPD and Related Therapeutics. Front. Microbiol. 2022;13:868086. doi: 10.3389/fmicb.2022.868086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Francoise A., Hery-Arnaud G. The Microbiome in Cystic Fibrosis Pulmonary Disease. Genes. 2020;11:536. doi: 10.3390/genes11050536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang H., Wang H., Sun Y., Ren Z., Zhu W., Li A., Cui G. Potential Associations Between Microbiome and COVID-19. Front. Med. 2021;8:785496. doi: 10.3389/fmed.2021.785496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dhar D., Mohanty A. Gut microbiota and COVID-19- possible link and implications. Virus Res. 2020;285:198018. doi: 10.1016/j.virusres.2020.198018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zuo T., Zhang F., Lui G.C.Y., Yeoh Y.K., Li A.Y.L., Zhan H., Wan Y., Chung A.C.K., Cheung C.P., Chen N., et al. Alterations in Gut Microbiota of Patients With COVID-19 During Time of Hospitalization. Gastroenterology. 2020;159:944–955.e8. doi: 10.1053/j.gastro.2020.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yeoh Y.K., Zuo T., Lui G.C., Zhang F., Liu Q., Li A.Y., Chung A.C., Cheung C.P., Tso E.Y., Fung K.S., et al. Gut microbiota composition reflects disease severity and dysfunctional immune responses in patients with COVID-19. Gut. 2021;70:698–706. doi: 10.1136/gutjnl-2020-323020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tang L., Gu S., Gong Y., Li B., Lu H., Li Q., Zhang R., Gao X., Wu Z., Zhang J., et al. Clinical Significance of the Correlation between Changes in the Major Intestinal Bacteria Species and COVID-19 Severity. Engineering. 2020;6:1178–1184. doi: 10.1016/j.eng.2020.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rabaan A.A., Al-Ahmed S.H., Al-Malkey M., Alsubki R., Ezzikouri S., Al-Hababi F.H., Sah R., Al Mutair A., Alhumaid S., Al-Tawfiq J.A., et al. Airborne transmission of SARS-CoV-2 is the dominant route of transmission: Droplets and aerosols. Infez Med. 2021;29:10–19. [PubMed] [Google Scholar]
- 28.Abd E.W., Eassa S.M., Metwally M., Al-Hraishawi H., Omar S.R. SARS-CoV-2 Transmission Channels: A Review of the Literature. MEDICC Rev. 2020;22:51–69. doi: 10.37757/MR2020.V22.N4.3. [DOI] [PubMed] [Google Scholar]
- 29.Berdowska I., Matusiewicz M. Cathepsin L, transmembrane peptidase/serine subfamily member 2/4, and other host proteases in COVID-19 pathogenesis—With impact on gastrointestinal tract. World J. Gastroenterol. 2021;27:6590–6600. doi: 10.3748/wjg.v27.i39.6590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jackson C.B., Farzan M., Chen B., Choe H. Mechanisms of SARS-CoV-2 entry into cells. Nat. Rev. Mol. Cell Biol. 2022;23:3–20. doi: 10.1038/s41580-021-00418-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lamers M.M., Beumer J., van der Vaart J., Knoops K., Puschhof J., Breugem T.I., Ravelli R.B.G., Paul van Schayck J., Mykytyn A.Z., Duimel H.Q., et al. SARS-CoV-2 productively infects human gut enterocytes. Science. 2020;369:50–54. doi: 10.1126/science.abc1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chu H., Chan J.F., Wang Y., Yuen T.T., Chai Y., Shuai H., Yang D., Hu B., Huang X., Zhang X., et al. SARS-CoV-2 Induces a More Robust Innate Immune Response and Replicates Less Efficiently Than SARS-CoV in the Human Intestines: An Ex Vivo Study With Implications on Pathogenesis of COVID-19. Cell. Mol. Gastroenterol. Hepatol. 2021;11:771–781. doi: 10.1016/j.jcmgh.2020.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pascoal L.B., Rodrigues P.B., Genaro L.M., Gomes A., Toledo-Teixeira D.A., Parise P.L., Bispo-Dos-Santos K., Simeoni C.L., Guimaraes P.V., Buscaratti L.I., et al. Microbiota-derived short-chain fatty acids do not interfere with SARS-CoV-2 infection of human colonic samples. Gut Microbes. 2021;13:1–9. doi: 10.1080/19490976.2021.1874740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Qian Q., Fan L., Liu W., Li J., Yue J., Wang M., Ke X., Yin Y., Chen Q., Jiang C. Direct Evidence of Active SARS-CoV-2 Replication in the Intestine. Clin. Infect. Dis. 2021;73:361–366. doi: 10.1093/cid/ciaa925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lotfi M., Rezaei N. SARS-CoV-2: A comprehensive review from pathogenicity of the virus to clinical consequences. J. Med. Virol. 2020;92:1864–1874. doi: 10.1002/jmv.26123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ye Q., Wang B., Zhang T., Xu J., Shang S. The mechanism and treatment of gastrointestinal symptoms in patients with COVID-19. Am. J. Physiol. Gastrointest. Liver Physiol. 2020;319:G245–G252. doi: 10.1152/ajpgi.00148.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aguila E.J.T., Cua I.H.Y., Fontanilla J.A.C., Yabut V.L.M., Causing M.F.P. Gastrointestinal Manifestations of COVID-19: Impact on Nutrition Practices. Nutr. Clin. Pract. 2020;35:800–805. doi: 10.1002/ncp.10554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lin L., Jiang X., Zhang Z., Huang S., Zhang Z., Fang Z., Gu Z., Gao L., Shi H., Mai L., et al. Gastrointestinal symptoms of 95 cases with SARS-CoV-2 infection. Gut. 2020;69:997–1001. doi: 10.1136/gutjnl-2020-321013. [DOI] [PubMed] [Google Scholar]
- 39.Jin X., Lian J.S., Hu J.H., Gao J., Zheng L., Zhang Y.M., Hao S.R., Jia H.Y., Cai H., Zhang X.L., et al. Epidemiological, clinical and virological characteristics of 74 cases of coronavirus-infected disease 2019 (COVID-19) with gastrointestinal symptoms. Gut. 2020;69:1002–1009. doi: 10.1136/gutjnl-2020-320926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang J.J., Dong X., Cao Y.Y., Yuan Y.D., Yang Y.B., Yan Y.Q., Akdis C.A., Gao Y.D. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy. 2020;75:1730–1741. doi: 10.1111/all.14238. [DOI] [PubMed] [Google Scholar]
- 41.Buscarini E., Manfredi G., Brambilla G., Menozzi F., Londoni C., Alicante S., Iiritano E., Romeo S., Pedaci M., Benelli G., et al. GI symptoms as early signs of COVID-19 in hospitalised Italian patients. Gut. 2020;69:1547–1548. doi: 10.1136/gutjnl-2020-321434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kariyawasam J.C., Jayarajah U., Riza R., Abeysuriya V., Seneviratne S.L. Gastrointestinal manifestations in COVID-19. Trans. R. Soc. Trop. Med. Hyg. 2021;115:1362–1388. doi: 10.1093/trstmh/trab042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tang Q., Wang Y., Ou L., Li J., Zheng K., Zhan H., Gu J., Zhou G., Xie S., Zhang J., et al. Downregulation of ACE2 expression by SARS-CoV-2 worsens the prognosis of KIRC and KIRP patients via metabolism and immunoregulation. Int. J. Biol. Sci. 2021;17:1925–1939. doi: 10.7150/ijbs.57802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vaduganathan M., Vardeny O., Michel T., McMurray J.J.V., Pfeffer M.A., Solomon S.D. Renin-Angiotensin-Aldosterone System Inhibitors in Patients with COVID-19. N. Engl. J. Med. 2020;382:1653–1659. doi: 10.1056/NEJMsr2005760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hashimoto T., Perlot T., Rehman A., Trichereau J., Ishiguro H., Paolino M., Sigl V., Hanada T., Hanada R., Lipinski S., et al. ACE2 links amino acid malnutrition to microbial ecology and intestinal inflammation. Nature. 2012;487:477–481. doi: 10.1038/nature11228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Camargo S.M.R., Vuille-Dit-Bille R.N., Meier C.F., Verrey F. ACE2 and gut amino acid transport. Clin. Sci. 2020;134:2823–2833. doi: 10.1042/CS20200477. [DOI] [PubMed] [Google Scholar]
- 47.Guimarães Sousa S., Kleiton de Sousa A., Maria Carvalho Pereira C., Sofia Miranda Loiola Araújo A., de Aguiar Magalhães D., Vieira de Brito T., Barbosa A. SARS-CoV-2 infection causes intestinal cell damage: Role of interferon’s imbalance. Cytokine. 2022;152:155826. doi: 10.1016/j.cyto.2022.155826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.D’Amico F., Baumgart D.C., Danese S., Peyrin-Biroulet L. Diarrhea During COVID-19 Infection: Pathogenesis, Epidemiology, Prevention, and Management. Clin. Gastroenterol. Hepatol. 2020;18:1663–1672. doi: 10.1016/j.cgh.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Effenberger M., Grabherr F., Mayr L., Schwaerzler J., Nairz M., Seifert M., Hilbe R., Seiwald S., Scholl-Buergi S., Fritsche G., et al. Faecal calprotectin indicates intestinal inflammation in COVID-19. Gut. 2020;69:1543–1544. doi: 10.1136/gutjnl-2020-321388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xiao F., Tang M., Zheng X., Liu Y., Li X., Shan H. Evidence for Gastrointestinal Infection of SARS-CoV-2. Gastroenterology. 2020;158:1831–1833.e3. doi: 10.1053/j.gastro.2020.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Viana S.D., Nunes S., Reis F. ACE2 imbalance as a key player for the poor outcomes in COVID-19 patients with age-related comorbidities—Role of gut microbiota dysbiosis. Ageing Res. Rev. 2020;62:101123. doi: 10.1016/j.arr.2020.101123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Manosso L.M., Arent C.O., Borba L.A., Ceretta L.B., Quevedo J., Reus G.Z. Microbiota-Gut-Brain Communication in the SARS-CoV-2 Infection. Cells. 2021;10:1993. doi: 10.3390/cells10081993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.He Y., Wang J., Li F., Shi Y. Main Clinical Features of COVID-19 and Potential Prognostic and Therapeutic Value of the Microbiota in SARS-CoV-2 Infections. Front. Microbiol. 2020;11:1302. doi: 10.3389/fmicb.2020.01302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Penninger J.M., Grant M.B., Sung J.J.Y. The Role of Angiotensin Converting Enzyme 2 in Modulating Gut Microbiota, Intestinal Inflammation, and Coronavirus Infection. Gastroenterology. 2021;160:39–46. doi: 10.1053/j.gastro.2020.07.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang J., Li F., Wei H., Lian Z.X., Sun R., Tian Z. Respiratory influenza virus infection induces intestinal immune injury via microbiota-mediated Th17 cell-dependent inflammation. J. Exp. Med. 2014;211:2397–2410. doi: 10.1084/jem.20140625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Deriu E., Boxx G.M., He X., Pan C., Benavidez S.D., Cen L., Rozengurt N., Shi W., Cheng G. Influenza Virus Affects Intestinal Microbiota and Secondary Salmonella Infection in the Gut through Type I Interferons. PLoS Pathog. 2016;12:e1005572. doi: 10.1371/journal.ppat.1005572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sencio V., Machado M.G., Trottein F. The lung-gut axis during viral respiratory infections: The impact of gut dysbiosis on secondary disease outcomes. Mucosal Immunol. 2021;14:296–304. doi: 10.1038/s41385-020-00361-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Maslennikov R., Svistunov A., Ivashkin V., Ufimtseva A., Poluektova E., Efremova I., Ulyanin A., Okhlobystin A., Kardasheva S., Kurbatova A., et al. Early viral versus late antibiotic-associated diarrhea in novel coronavirus infection. Medicine. 2021;100:e27528. doi: 10.1097/MD.0000000000027528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sencio V., Barthelemy A., Tavares L.P., Machado M.G., Soulard D., Cuinat C., Queiroz-Junior C.M., Noordine M.L., Salome-Desnoulez S., Deryuter L., et al. Gut Dysbiosis during Influenza Contributes to Pulmonary Pneumococcal Superinfection through Altered Short-Chain Fatty Acid Production. Cell Rep. 2020;30:2934–2947.e6. doi: 10.1016/j.celrep.2020.02.013. [DOI] [PubMed] [Google Scholar]
- 60.Groves H.T., Higham S.L., Moffatt M.F., Cox M.J., Tregoning J.S. Respiratory Viral Infection Alters the Gut Microbiota by Inducing Inappetence. mBio. 2020;11:e03236-19. doi: 10.1128/mBio.03236-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zuo T., Zhan H., Zhang F., Liu Q., Tso E.Y.K., Lui G.C.Y., Chen N., Li A., Lu W., Chan F.K.L., et al. Alterations in Fecal Fungal Microbiome of Patients With COVID-19 During Time of Hospitalization until Discharge. Gastroenterology. 2020;159:1302–1310.e5. doi: 10.1053/j.gastro.2020.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cao J., Wang C., Zhang Y., Lei G., Xu K., Zhao N., Lu J., Meng F., Yu L., Yan J., et al. Integrated gut virome and bacteriome dynamics in COVID-19 patients. Gut Microbes. 2021;13:1–21. doi: 10.1080/19490976.2021.1887722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Seibert B., Caceres C.J., Cardenas-Garcia S., Carnaccini S., Geiger G., Rajao D.S., Ottesen E., Perez D.R. Mild and Severe SARS-CoV-2 Infection Induces Respiratory and Intestinal Microbiome Changes in the K18-hACE2 Transgenic Mouse Model. Microbiol. Spectr. 2021;9:e0053621. doi: 10.1128/Spectrum.00536-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sencio V., Machelart A., Robil C., Benech N., Hoffmann E., Galbert C., Deryuter L., Heumel S., Hantute-Ghesquier A., Flourens A., et al. Alteration of the gut microbiota following SARS-CoV-2 infection correlates with disease severity in hamsters. Gut Microbes. 2022;14:2018900. doi: 10.1080/19490976.2021.2018900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sencio V., Benech N., Robil C., Deruyter L., Heumel S., Machelart A., Sulpice T., Lamaziere A., Grangette C., Briand F., et al. Alteration of the gut microbiota’s composition and metabolic output correlates with COVID-19-like severity in obese NASH hamsters. Gut Microbes. 2022;14:2100200. doi: 10.1080/19490976.2022.2100200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sokol H., Contreras V., Maisonnasse P., Desmons A., Delache B., Sencio V., Machelart A., Brisebarre A., Humbert L., Deryuter L., et al. SARS-CoV-2 infection in nonhuman primates alters the composition and functional activity of the gut microbiota. Gut Microbes. 2021;13:1–19. doi: 10.1080/19490976.2021.1893113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Farsi Y., Tahvildari A., Arbabi M., Vazife F., Sechi L.A., Shahidi Bonjar A.H., Jamshidi P., Nasiri M.J., Mirsaeidi M. Diagnostic, Prognostic, and Therapeutic Roles of Gut Microbiota in COVID-19: A Comprehensive Systematic Review. Front. Cell. Infect. Microbiol. 2022;12:804644. doi: 10.3389/fcimb.2022.804644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gu S., Chen Y., Wu Z., Chen Y., Gao H., Lv L., Guo F., Zhang X., Luo R., Huang C., et al. Alterations of the Gut Microbiota in Patients With Coronavirus Disease 2019 or H1N1 Influenza. Clin. Infect. Dis. 2020;71:2669–2678. doi: 10.1093/cid/ciaa709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Liu Q., Mak J.W.Y., Su Q., Yeoh Y.K., Lui G.C., Ng S.S.S., Zhang F., Li A.Y.L., Lu W., Hui D.S., et al. Gut microbiota dynamics in a prospective cohort of patients with post-acute COVID-19 syndrome. Gut. 2022;71:544–552. doi: 10.1136/gutjnl-2021-325989. [DOI] [PubMed] [Google Scholar]
- 70.Kim H.S. Do an Altered Gut Microbiota and an Associated Leaky Gut Affect COVID-19 Severity? mBio. 2021;12:e03022-20. doi: 10.1128/mBio.03022-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Venzon M., Bernard-Raichon L., Klein J., Axelrad J., Hussey G., Sullivan A., Casanovas-Massana A., Noval M., Valero-Jimenez A., Gago J., et al. Gut microbiome dysbiosis during COVID-19 is associated with increased risk for bacteremia and microbial translocation. Res. Sq. 2021:rs.3.rs-726620. doi: 10.21203/rs.3.rs-726620/v1. [DOI] [Google Scholar]
- 72.Tao W., Zhang G., Wang X., Guo M., Zeng W., Xu Z., Cao D., Pan A., Wang Y., Zhang K., et al. Analysis of the intestinal microbiota in COVID-19 patients and its correlation with the inflammatory factor IL-18. Med. Microecol. 2020;5:100023. doi: 10.1016/j.medmic.2020.100023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chhibber-Goel J., Gopinathan S., Sharma A. Interplay between severities of COVID-19 and the gut microbiome: Implications of bacterial co-infections? Gut Pathog. 2021;13:14. doi: 10.1186/s13099-021-00407-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Liu Y., Kuang D., Li D., Yang J., Yan J., Xia Y., Zhang F., Cao H. Roles of the gut microbiota in severe SARS-CoV-2 infection. Cytokine Growth Factor Rev. 2022;63:98–107. doi: 10.1016/j.cytogfr.2022.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Khan M., Mathew B.J., Gupta P., Garg G., Khadanga S., Vyas A.K., Singh A.K. Gut Dysbiosis and IL-21 Response in Patients with Severe COVID-19. Microorganisms. 2021;9:1292. doi: 10.3390/microorganisms9061292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kim H.N., Joo E.J., Lee C.W., Ahn K.S., Kim H.L., Park D.I., Park S.K. Reversion of Gut Microbiota during the Recovery Phase in Patients with Asymptomatic or Mild COVID-19: Longitudinal Study. Microorganisms. 2021;9:1237. doi: 10.3390/microorganisms9061237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ren Z., Wang H., Cui G., Lu H., Wang L., Luo H., Chen X., Ren H., Sun R., Liu W., et al. Alterations in the human oral and gut microbiomes and lipidomics in COVID-19. Gut. 2021;70:1253–1265. doi: 10.1136/gutjnl-2020-323826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J., Wang B., Xiang H., Cheng Z., Xiong Y., et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. J. Am. Med. Assoc. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wu C., Chen X., Cai Y., Xia J., Zhou X., Xu S., Huang H., Zhang L., Zhou X., Du C., et al. Risk Factors Associated With Acute Respiratory Distress Syndrome and Death in Patients With Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Intern. Med. 2020;180:934–943. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Garcia-Mena J., Corona-Cervantes K., Cuervo-Zanatta D., Benitez-Guerrero T., Velez-Ixta J.M., Zavala-Torres N.G., Villalobos-Flores L.E., Hernandez-Quiroz F., Perez-Cruz C., Murugesan S., et al. Gut microbiota in a population highly affected by obesity and type 2 diabetes and susceptibility to COVID-19. World J. Gastroenterol. 2021;27:7065–7079. doi: 10.3748/wjg.v27.i41.7065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Farshbafnadi M., Kamali Zonouzi S., Sabahi M., Dolatshahi M., Aarabi M.H. Aging & COVID-19 susceptibility, disease severity, and clinical outcomes: The role of entangled risk factors. Exp. Gerontol. 2021;154:111507. doi: 10.1016/j.exger.2021.111507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Nagpal R., Mainali R., Ahmadi S., Wang S., Singh R., Kavanagh K., Kitzman D.W., Kushugulova A., Marotta F., Yadav H. Gut microbiome and aging: Physiological and mechanistic insights. Nutr. Healthy Aging. 2018;4:267–285. doi: 10.3233/NHA-170030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Moreira-Rosario A., Marques C., Pinheiro H., Araujo J.R., Ribeiro P., Rocha R., Mota I., Pestana D., Ribeiro R., Pereira A., et al. Gut Microbiota Diversity and C-Reactive Protein Are Predictors of Disease Severity in COVID-19 Patients. Front. Microbiol. 2021;12:705020. doi: 10.3389/fmicb.2021.705020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Edwinson A., Yang L., Chen J., Grover M. Colonic expression of Ace2, the SARS-CoV-2 entry receptor, is suppressed by commensal human microbiota. Gut Microbes. 2021;13:1984105. doi: 10.1080/19490976.2021.1984105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gaibani P., D’Amico F., Bartoletti M., Lombardo D., Rampelli S., Fornaro G., Coladonato S., Siniscalchi A., Re M.C., Viale P., et al. The Gut Microbiota of Critically Ill Patients With COVID-19. Front. Cell. Infect. Microbiol. 2021;11:670424. doi: 10.3389/fcimb.2021.670424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Li S., Yang S., Zhou Y., Disoma C., Dong Z., Du A., Zhang Y., Chen Y., Huang W., Chen J., et al. Microbiome Profiling Using Shotgun Metagenomic Sequencing Identified Unique Microorganisms in COVID-19 Patients With Altered Gut Microbiota. Front. Microbiol. 2021;12:712081. doi: 10.3389/fmicb.2021.712081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hirayama M., Nishiwaki H., Hamaguchi T., Ito M., Ueyama J., Maeda T., Kashihara K., Tsuboi Y., Ohno K. Intestinal Collinsella may mitigate infection and exacerbation of COVID-19 by producing ursodeoxycholate. PLoS ONE. 2021;16:e0260451. doi: 10.1371/journal.pone.0260451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Baghbani T., Nikzad H., Azadbakht J., Izadpanah F., Haddad Kashani H. Dual and mutual interaction between microbiota and viral infections: A possible treat for COVID-19. Microb. Cell Fact. 2020;19:217. doi: 10.1186/s12934-020-01483-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wilks J., Beilinson H., Golovkina T.V. Dual role of commensal bacteria in viral infections. Immunol. Rev. 2013;255:222–229. doi: 10.1111/imr.12097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lima M.T., Andrade A.C.d.S.P., Oliveira G.P., Nicoli J.R., Martins F.d.S., Kroon E.G., Abrahão J.S. Virus and microbiota relationships in humans and other mammals: An evolutionary view. Hum. Microbiome J. 2019;11:100050. doi: 10.1016/j.humic.2018.11.001. [DOI] [Google Scholar]
- 91.Shreiner A.B., Kao J.Y., Young V.B. The gut microbiome in health and in disease. Curr. Opin. Gastroenterol. 2015;31:69–75. doi: 10.1097/MOG.0000000000000139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Vignesh R., Swathirajan C.R., Tun Z.H., Rameshkumar M.R., Solomon S.S., Balakrishnan P. Could Perturbation of Gut Microbiota Possibly Exacerbate the Severity of COVID-19 via Cytokine Storm? Front. Immunol. 2020;11:607734. doi: 10.3389/fimmu.2020.607734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Albrich W.C., Ghosh T.S., Ahearn-Ford S., Mikaeloff F., Lunjani N., Forde B., Suh N., Kleger G.R., Pietsch U., Frischknecht M., et al. A high-risk gut microbiota configuration associates with fatal hyperinflammatory immune and metabolic responses to SARS-CoV-2. Gut Microbes. 2022;14:2073131. doi: 10.1080/19490976.2022.2073131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhang F., Wan Y., Zuo T., Yeoh Y.K., Liu Q., Zhang L., Zhan H., Lu W., Xu W., Lui G.C.Y., et al. Prolonged Impairment of Short-Chain Fatty Acid and L-Isoleucine Biosynthesis in Gut Microbiome in Patients With COVID-19. Gastroenterology. 2022;162:548–561.e4. doi: 10.1053/j.gastro.2021.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wu J., Li J., Zhu G., Zhang Y., Bi Z., Yu Y., Huang B., Fu S., Tan Y., Sun J., et al. Clinical Features of Maintenance Hemodialysis Patients with 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. Clin. J. Am. Soc. Nephrol. 2020;15:1139–1145. doi: 10.2215/CJN.04160320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ruan Q., Yang K., Wang W., Jiang L., Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46:846–848. doi: 10.1007/s00134-020-05991-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Meduri G.U., Kohler G., Headley S., Tolley E., Stentz F., Postlethwaite A. Inflammatory cytokines in the BAL of patients with ARDS. Persistent elevation over time predicts poor outcome. Chest. 1995;108:1303–1314. doi: 10.1378/chest.108.5.1303. [DOI] [PubMed] [Google Scholar]
- 98.Mizutani T., Ishizaka A., Koga M., Ikeuchi K., Saito M., Adachi E., Yamayoshi S., Iwatsuki-Horimoto K., Yasuhara A., Kiyono H., et al. Correlation Analysis between Gut Microbiota Alterations and the Cytokine Response in Patients with Coronavirus Disease during Hospitalization. Microbiol. Spectr. 2022;10:e0168921. doi: 10.1128/spectrum.01689-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Arpaia N., Campbell C., Fan X., Dikiy S., van der Veeken J., deRoos P., Liu H., Cross J.R., Pfeffer K., Coffer P.J., et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature. 2013;504:451–455. doi: 10.1038/nature12726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Vieira C., Nery L., Martins L., Jabour L., Dias R., Simoes E.S.A.C. Downregulation of Membrane-bound Angiotensin Converting Enzyme 2 (ACE2) Receptor has a Pivotal Role in COVID-19 Immunopathology. Curr. Drug Targets. 2021;22:254–281. doi: 10.2174/1389450121666201020154033. [DOI] [PubMed] [Google Scholar]
- 101.Kuba K., Imai Y., Rao S., Gao H., Guo F., Guan B., Huan Y., Yang P., Zhang Y., Deng W., et al. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat. Med. 2005;11:875–879. doi: 10.1038/nm1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Baindara P., Chakraborty R., Holliday Z.M., Mandal S.M., Schrum A.G. Oral probiotics in coronavirus disease 2019: Connecting the gut-lung axis to viral pathogenesis, inflammation, secondary infection and clinical trials. New Microbes New Infect. 2021;40:100837. doi: 10.1016/j.nmni.2021.100837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Denny J.E., Powell W.L., Schmidt N.W. Local and Long-Distance Calling: Conversations between the Gut Microbiota and Intra- and Extra-Gastrointestinal Tract Infections. Front. Cell. Infect. Microbiol. 2016;6:41. doi: 10.3389/fcimb.2016.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yagi K., Asai N., Huffnagle G.B., Lukacs N.W., Fonseca W. Early-Life Lung and Gut Microbiota Development and Respiratory Syncytial Virus Infection. Front. Immunol. 2022;13:877771. doi: 10.3389/fimmu.2022.877771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Dang A.T., Marsland B.J. Microbes, metabolites, and the gut-lung axis. Mucosal Immunol. 2019;12:843–850. doi: 10.1038/s41385-019-0160-6. [DOI] [PubMed] [Google Scholar]
- 106.Keely S., Talley N.J., Hansbro P.M. Pulmonary-intestinal cross-talk in mucosal inflammatory disease. Mucosal Immunol. 2012;5:7–18. doi: 10.1038/mi.2011.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wang H., Liu J.S., Peng S.H., Deng X.Y., Zhu D.M., Javidiparsijani S., Wang G.R., Li D.Q., Li L.X., Wang Y.C., et al. Gut-lung crosstalk in pulmonary involvement with inflammatory bowel diseases. World J. Gastroenterol. 2013;19:6794–6804. doi: 10.3748/wjg.v19.i40.6794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Yazar A., Atis S., Konca K., Pata C., Akbay E., Calikoglu M., Hafta A. Respiratory symptoms and pulmonary functional changes in patients with irritable bowel syndrome. Am. J. Gastroenterol. 2001;96:1511–1516. doi: 10.1111/j.1572-0241.2001.03748.x. [DOI] [PubMed] [Google Scholar]
- 109.Bastard P., Rosen L.B., Zhang Q., Michailidis E., Hoffmann H.H., Zhang Y., Dorgham K., Philippot Q., Rosain J., Beziat V., et al. Autoantibodies against type I IFNs in patients with life-threatening COVID-19. Science. 2020;370:eabd4585. doi: 10.1126/science.abd4585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Arunachalam P.S., Wimmers F., Mok C.K.P., Perera R., Scott M., Hagan T., Sigal N., Feng Y., Bristow L., Tak-Yin Tsang O., et al. Systems biological assessment of immunity to mild versus severe COVID-19 infection in humans. Science. 2020;369:1210–1220. doi: 10.1126/science.abc6261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Busnadiego I., Fernbach S., Pohl M.O., Karakus U., Huber M., Trkola A., Stertz S., Hale B.G. Antiviral Activity of Type I, II, and III Interferons Counterbalances ACE2 Inducibility and Restricts SARS-CoV-2. mBio. 2020;11:e01928-20. doi: 10.1128/mBio.01928-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Tan J., McKenzie C., Potamitis M., Thorburn A.N., Mackay C.R., Macia L. The role of short-chain fatty acids in health and disease. Adv. Immunol. 2014;121:91–119. doi: 10.1016/B978-0-12-800100-4.00003-9. [DOI] [PubMed] [Google Scholar]
- 113.Li M., van Esch B., Wagenaar G.T.M., Garssen J., Folkerts G., Henricks P.A.J. Pro- and anti-inflammatory effects of short chain fatty acids on immune and endothelial cells. Eur. J. Pharmacol. 2018;831:52–59. doi: 10.1016/j.ejphar.2018.05.003. [DOI] [PubMed] [Google Scholar]
- 114.Nastasi C., Candela M., Bonefeld C.M., Geisler C., Hansen M., Krejsgaard T., Biagi E., Andersen M.H., Brigidi P., Odum N., et al. The effect of short-chain fatty acids on human monocyte-derived dendritic cells. Sci. Rep. 2015;5:16148. doi: 10.1038/srep16148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lee J.G., Lee J., Lee A.R., Jo S.V., Park C.H., Han D.S., Eun C.S. Impact of short-chain fatty acid supplementation on gut inflammation and microbiota composition in a murine colitis model. J. Nutr. Biochem. 2022;101:108926. doi: 10.1016/j.jnutbio.2021.108926. [DOI] [PubMed] [Google Scholar]
- 116.Trompette A., Gollwitzer E.S., Pattaroni C., Lopez-Mejia I.C., Riva E., Pernot J., Ubags N., Fajas L., Nicod L.P., Marsland B.J. Dietary Fiber Confers Protection against Flu by Shaping Ly6c(-) Patrolling Monocyte Hematopoiesis and CD8(+) T Cell Metabolism. Immunity. 2018;48:992–1005.e8. doi: 10.1016/j.immuni.2018.04.022. [DOI] [PubMed] [Google Scholar]
- 117.Marsland B.J., Trompette A., Gollwitzer E.S. The Gut-Lung Axis in Respiratory Disease. Ann. Am. Thorac. Soc. 2015;12((Suppl. 2)):S150–S156. doi: 10.1513/AnnalsATS.201503-133AW. [DOI] [PubMed] [Google Scholar]
- 118.Sulaiman I., Chung M., Angel L., Tsay J.J., Wu B.G., Yeung S.T., Krolikowski K., Li Y., Duerr R., Schluger R., et al. Microbial signatures in the lower airways of mechanically ventilated COVID-19 patients associated with poor clinical outcome. Nat. Microbiol. 2021;6:1245–1258. doi: 10.1038/s41564-021-00961-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kuss S.K., Best G.T., Etheredge C.A., Pruijssers A.J., Frierson J.M., Hooper L.V., Dermody T.S., Pfeiffer J.K. Intestinal microbiota promote enteric virus replication and systemic pathogenesis. Science. 2011;334:249–252. doi: 10.1126/science.1211057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Roth A.N., Grau K.R., Karst S.M. Diverse Mechanisms Underlie Enhancement of Enteric Viruses by the Mammalian Intestinal Microbiota. Viruses. 2019;11:760. doi: 10.3390/v11080760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.McCullers J.A. The co-pathogenesis of influenza viruses with bacteria in the lung. Nat. Rev. Microbiol. 2014;12:252–262. doi: 10.1038/nrmicro3231. [DOI] [PubMed] [Google Scholar]
- 122.Li N., Ma W.T., Pang M., Fan Q.L., Hua J.L. The Commensal Microbiota and Viral Infection: A Comprehensive Review. Front. Immunol. 2019;10:1551. doi: 10.3389/fimmu.2019.01551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Robinson C.M., Jesudhasan P.R., Pfeiffer J.K. Bacterial lipopolysaccharide binding enhances virion stability and promotes environmental fitness of an enteric virus. Cell Host Microbe. 2014;15:36–46. doi: 10.1016/j.chom.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Aguilera E.R., Nguyen Y., Sasaki J., Pfeiffer J.K. Bacterial Stabilization of a Panel of Picornaviruses. mSphere. 2019;4:e00183-19. doi: 10.1128/mSphere.00183-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Karst S.M. The influence of commensal bacteria on infection with enteric viruses. Nat. Rev. Microbiol. 2016;14:197–204. doi: 10.1038/nrmicro.2015.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wilks J., Lien E., Jacobson A.N., Fischbach M.A., Qureshi N., Chervonsky A.V., Golovkina T.V. Mammalian Lipopolysaccharide Receptors Incorporated into the Retroviral Envelope Augment Virus Transmission. Cell Host Microbe. 2015;18:456–462. doi: 10.1016/j.chom.2015.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kane M., Case L.K., Kopaskie K., Kozlova A., MacDearmid C., Chervonsky A.V., Golovkina T.V. Successful transmission of a retrovirus depends on the commensal microbiota. Science. 2011;334:245–249. doi: 10.1126/science.1210718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Bowers J.R., Readler J.M., Sharma P., Excoffon K. Poliovirus Receptor: More than a simple viral receptor. Virus Res. 2017;242:1–6. doi: 10.1016/j.virusres.2017.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Petruk G., Puthia M., Petrlova J., Samsudin F., Stromdahl A.C., Cerps S., Uller L., Kjellstrom S., Bond P.J., Schmidtchen A.A. SARS-CoV-2 spike protein binds to bacterial lipopolysaccharide and boosts proinflammatory activity. J. Mol. Cell Biol. 2020;12:916–932. doi: 10.1093/jmcb/mjaa067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Loke M.F., Yadav I., Lim T.K., van der Maarel J.R.C., Sham L.T., Chow V.T. SARS-CoV-2 Spike Protein and Mouse Coronavirus Inhibit Biofilm Formation by Streptococcus pneumoniae and Staphylococcus aureus. Int. J. Mol. Sci. 2022;23:3291. doi: 10.3390/ijms23063291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Honarmand Ebrahimi K. SARS-CoV-2 spike glycoprotein-binding proteins expressed by upper respiratory tract bacteria may prevent severe viral infection. FEBS Lett. 2020;594:1651–1660. doi: 10.1002/1873-3468.13845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Reche P.A. Potential Cross-Reactive Immunity to SARS-CoV-2 From Common Human Pathogens and Vaccines. Front. Immunol. 2020;11:586984. doi: 10.3389/fimmu.2020.586984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Sumbul B., Sumbul H.E., Okyay R.A., Gulumsek E., Sahin A.R., Boral B., Kocyigit B.F., Alfishawy M., Gold J., Tasdogan A.M. Is there a link between pre-existing antibodies acquired due to childhood vaccinations or past infections and COVID-19? A case control study. PeerJ. 2021;9:e10910. doi: 10.7717/peerj.10910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Trama A.M., Moody M.A., Alam S.M., Jaeger F.H., Lockwood B., Parks R., Lloyd K.E., Stolarchuk C., Scearce R., Foulger A., et al. HIV-1 envelope gp41 antibodies can originate from terminal ileum B cells that share cross-reactivity with commensal bacteria. Cell Host Microbe. 2014;16:215–226. doi: 10.1016/j.chom.2014.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Williams W.B., Han Q., Haynes B.F. Cross-reactivity of HIV vaccine responses and the microbiome. Curr. Opin. HIV AIDS. 2018;13:9–14. doi: 10.1097/COH.0000000000000423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Liao H.X., Chen X., Munshaw S., Zhang R., Marshall D.J., Vandergrift N., Whitesides J.F., Lu X., Yu J.S., Hwang K.K., et al. Initial antibodies binding to HIV-1 gp41 in acutely infected subjects are polyreactive and highly mutated. J. Exp. Med. 2011;208:2237–2249. doi: 10.1084/jem.20110363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Su L.F., Kidd B.A., Han A., Kotzin J.J., Davis M.M. Virus-specific CD4(+) memory-phenotype T cells are abundant in unexposed adults. Immunity. 2013;38:373–383. doi: 10.1016/j.immuni.2012.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Campion S.L., Brodie T.M., Fischer W., Korber B.T., Rossetti A., Goonetilleke N., McMichael A.J., Sallusto F. Proteome-wide analysis of HIV-specific naive and memory CD4(+) T cells in unexposed blood donors. J. Exp. Med. 2014;211:1273–1280. doi: 10.1084/jem.20130555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Jia L., Weng S., Wu J., Tian X., Zhang Y., Wang X., Wang J., Yan D., Wang W., Fang F., et al. Pre-existing antibodies targeting a linear epitope on SARS-CoV-2 S2 cross-reacted with commensal gut bacteria and shaped vaccine induced immunity. medRxiv. 2022 doi: 10.1101/2021.07.13.21260404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Geanes E.S., LeMaster C., Fraley E.R., Khanal S., McLennan R., Grundberg E., Selvarangan R., Bradley T. Cross-reactive antibodies elicited to conserved epitopes on SARS-CoV-2 spike protein after infection and vaccination. Sci. Rep. 2022;12:6496. doi: 10.1038/s41598-022-10230-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Ninnemann J., Budzinski L., Bondareva M., Witkowski M., Angermair S., Kreye J., Durek P., Reincke S.M., Sánchez-Sendin E., Yilmaz S., et al. Induction of cross-reactive antibody responses against the RBD domain of the spike protein of SARS-CoV-2 by commensal microbiota. bioRxiv. 2021 doi: 10.1101/2021.08.08.455272. [DOI] [Google Scholar]
- 142.Tan C.C.S., Owen C.J., Tham C.Y.L., Bertoletti A., van Dorp L., Balloux F. Pre-existing T cell-mediated cross-reactivity to SARS-CoV-2 cannot solely be explained by prior exposure to endemic human coronaviruses. Infect. Genet. Evol. 2021;95:105075. doi: 10.1016/j.meegid.2021.105075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Bartolo L., Afroz S., Pan Y.G., Xu R., Williams L., Lin C.F., Tanes C., Bittinger K., Friedman E.S., Gimotty P.A., et al. SARS-CoV-2-specific T cells in unexposed adults display broad trafficking potential and cross-react with commensal antigens. Sci. Immunol. 2022:eabn3127. doi: 10.1126/sciimmunol.abn3127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Eggenhuizen P.J., Ng B.H., Chang J., Cheong R.M.Y., Yellapragada A., Wong W.Y., Ting Y.T., Monk J.A., Gan P.Y., Holdsworth S.R., et al. Heterologous Immunity Between SARS-CoV-2 and Pathogenic Bacteria. Front. Immunol. 2022;13:821595. doi: 10.3389/fimmu.2022.821595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Sorbara M.T., Pamer E.G. Microbiome-based therapeutics. Nat. Rev. Microbiol. 2022;20:365–380. doi: 10.1038/s41579-021-00667-9. [DOI] [PubMed] [Google Scholar]
- 146.Hu J., Zhang L., Lin W., Tang W., Chan F.K.L., Ng S.C. Review article: Probiotics, prebiotics and dietary approaches during COVID-19 pandemic. Trends Food Sci. Technol. 2021;108:187–196. doi: 10.1016/j.tifs.2020.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Daoust L., Pilon G., Marette A. Perspective: Nutritional Strategies Targeting the Gut Microbiome to Mitigate COVID-19 Outcomes. Adv. Nutr. 2021;12:1074–1086. doi: 10.1093/advances/nmab031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Chakraborty M., Munshi S.K. The prospects of employing probiotics in combating COVID-19. Tzu Chi Med. J. 2022;34:148–159. doi: 10.4103/tcmj.tcmj_104_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Swiatecka D., Narbad A., Ridgway K.P., Kostyra H. The study on the impact of glycated pea proteins on human intestinal bacteria. Int. J. Food Microbiol. 2011;145:267–272. doi: 10.1016/j.ijfoodmicro.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 150.Trompette A., Gollwitzer E.S., Yadava K., Sichelstiel A.K., Sprenger N., Ngom-Bru C., Blanchard C., Junt T., Nicod L.P., Harris N.L., et al. Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis. Nat. Med. 2014;20:159–166. doi: 10.1038/nm.3444. [DOI] [PubMed] [Google Scholar]
- 151.Wang Y., Wu G., Zhao L., Wang W. Nutritional Modulation of Gut Microbiota Alleviates Severe Gastrointestinal Symptoms in a Patient with Post-Acute COVID-19 Syndrome. mBio. 2022;13:e0380121. doi: 10.1128/mbio.03801-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Ohaegbulam K.C., Swalih M., Patel P., Smith M.A., Perrin R. Vitamin D Supplementation in COVID-19 Patients: A Clinical Case Series. Am. J. Ther. 2020;27:e485–e490. doi: 10.1097/MJT.0000000000001222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Ooi J.H., Li Y., Rogers C.J., Cantorna M.T. Vitamin D regulates the gut microbiome and protects mice from dextran sodium sulfate-induced colitis. J. Nutr. 2013;143:1679–1686. doi: 10.3945/jn.113.180794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Assa A., Vong L., Pinnell L.J., Avitzur N., Johnson-Henry K.C., Sherman P.M. Vitamin D deficiency promotes epithelial barrier dysfunction and intestinal inflammation. J. Infect. Dis. 2014;210:1296–1305. doi: 10.1093/infdis/jiu235. [DOI] [PubMed] [Google Scholar]
- 155.Lehtoranta L., Pitkaranta A., Korpela R. Probiotics in respiratory virus infections. Eur. J. Clin. Microbiol. Infect. Dis. 2014;33:1289–1302. doi: 10.1007/s10096-014-2086-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Kanauchi O., Andoh A., AbuBakar S., Yamamoto N. Probiotics and Paraprobiotics in Viral Infection: Clinical Application and Effects on the Innate and Acquired Immune Systems. Curr. Pharm. Des. 2018;24:710–717. doi: 10.2174/1381612824666180116163411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Baud D., Dimopoulou Agri V., Gibson G.R., Reid G., Giannoni E. Using Probiotics to Flatten the Curve of Coronavirus Disease COVID-2019 Pandemic. Front. Public Health. 2020;8:186. doi: 10.3389/fpubh.2020.00186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Ermolenko E.I., Desheva Y.A., Kolobov A.A., Kotyleva M.P., Sychev I.A., Suvorov A.N. Anti-Influenza Activity of Enterocin B In vitro and Protective Effect of Bacteriocinogenic Enterococcal Probiotic Strain on Influenza Infection in Mouse Model. Probiotics Antimicrob. Proteins. 2019;11:705–712. doi: 10.1007/s12602-018-9457-0. [DOI] [PubMed] [Google Scholar]
- 159.Mahooti M., Abdolalipour E., Salehzadeh A., Mohebbi S.R., Gorji A., Ghaemi A. Immunomodulatory and prophylactic effects of Bifidobacterium bifidum probiotic strain on influenza infection in mice. World Microbiol. Biotechnol. 2019;35:91. doi: 10.1007/s11274-019-2667-0. [DOI] [PubMed] [Google Scholar]
- 160.Wu C., Xu Q., Cao Z., Pan D., Zhu Y., Wang S., Liu D., Song Z., Jiang W., Ruan Y., et al. The volatile and heterogeneous gut microbiota shifts of COVID-19 patients over the course of a probiotics-assisted therapy. Clin. Transl. Med. 2021;11:e643. doi: 10.1002/ctm2.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Zhang L., Xu Z., Mak J.W.Y., Chow K.M., Lui G., Li T.C.M., Wong C.K., Chan P.K.S., Ching J.Y.L., Fujiwara Y., et al. Gut microbiota-derived synbiotic formula (SIM01) as a novel adjuvant therapy for COVID-19: An open-label pilot study. J. Gastroenterol. Hepatol. 2022;37:823–831. doi: 10.1111/jgh.15796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Peng J., Zhang M., Yao G., Kwok L.Y., Zhang W. Probiotics as Adjunctive Treatment for Patients Contracted COVID-19: Current Understanding and Future Needs. Front. Nutr. 2021;8:669808. doi: 10.3389/fnut.2021.669808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Ceccarelli G., Borrazzo C., Pinacchio C., Santinelli L., Innocenti G.P., Cavallari E.N., Celani L., Marazzato M., Alessandri F., Ruberto F., et al. Oral Bacteriotherapy in Patients With COVID-19: A Retrospective Cohort Study. Front. Nutr. 2020;7:613928. doi: 10.3389/fnut.2020.613928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.d’Ettorre G., Ceccarelli G., Marazzato M., Campagna G., Pinacchio C., Alessandri F., Ruberto F., Rossi G., Celani L., Scagnolari C., et al. Challenges in the Management of SARS-CoV-2 Infection: The Role of Oral Bacteriotherapy as Complementary Therapeutic Strategy to Avoid the Progression of COVID-19. Front. Med. 2020;7:389. doi: 10.3389/fmed.2020.00389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Liu Y.T., Qi S.L., Sun K.W. Traditional Chinese medicine, liver fibrosis, intestinal flora: Is there any connection?-a narrative review. Ann. Palliat. Med. 2021;10:4846–4857. doi: 10.21037/apm-20-2129. [DOI] [PubMed] [Google Scholar]
- 166.Yang Z., Liu Y., Wang L., Lin S., Dai X., Yan H., Ge Z., Ren Q., Wang H., Zhu F., et al. Traditional Chinese medicine against COVID-19: Role of the gut microbiota. Biomed. Pharmacother. 2022;149:112787. doi: 10.1016/j.biopha.2022.112787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Guo M., Ding S., Zhao C., Gu X., He X., Huang K., Luo Y., Liang Z., Tian H., Xu W. Red Ginseng and Semen Coicis can improve the structure of gut microbiota and relieve the symptoms of ulcerative colitis. J. Ethnopharmacol. 2015;162:7–13. doi: 10.1016/j.jep.2014.12.029. [DOI] [PubMed] [Google Scholar]
- 168.Liu C.S., Liang X., Wei X.H., Jin Z., Chen F.L., Tang Q.F., Tan X.M. Gegen Qinlian Decoction Treats Diarrhea in Piglets by Modulating Gut Microbiota and Short-Chain Fatty Acids. Front. Microbiol. 2019;10:825. doi: 10.3389/fmicb.2019.00825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Lam S., Bai X., Shkoporov A.N., Park H., Wu X., Lan P., Zuo T. Roles of the gut virome and mycobiome in faecal microbiota transplantation. Lancet Gastroenterol. Hepatol. 2022;7:472–484. doi: 10.1016/S2468-1253(21)00303-4. [DOI] [PubMed] [Google Scholar]
- 170.Qu Z., Tian P., Yang B., Zhao J., Wang G., Chen W. Fecal microbiota transplantation for diseases: Therapeutic potential, methodology, risk management in clinical practice. Life Sci. 2022;304:120719. doi: 10.1016/j.lfs.2022.120719. [DOI] [PubMed] [Google Scholar]
- 171.Liu F., Ye S., Zhu X., He X., Wang S., Li Y., Lin J., Wang J., Lin Y., Ren X., et al. Gastrointestinal disturbance and effect of fecal microbiota transplantation in discharged COVID-19 patients. Med. Case Rep. 2021;15:60. doi: 10.1186/s13256-020-02583-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable.