Abstract
Immunocompromised patients with prolonged coronavirus disease 2019 symptoms present diagnostic and therapeutic challenges. We measured viral nucleocapsid antigenemia in 3 patients treated with anti-CD20 immunotherapy who acquired severe acute respiratory syndrome coronavirus 2 infection and experienced protracted symptoms. Our results support nucleocapsid antigenemia as a marker of persistent infection and therapeutic response.
Keywords: SARS-CoV-2, antigenemia, diagnostics, immunocompromised
Patients with immunocompromising conditions are at increased risk for adverse outcomes from coronavirus disease 2019 (COVID-19) [1]. Management of these patients is complicated by the potential for prolonged severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) replication [2–4] and limitations of current molecular methods in differentiating persistent viral replication from nonviable viral remnants [5]. Patients with impaired humoral immunity are especially vulnerable, particularly recipients of anti-CD20 immunotherapy [6]. Assays to determine the duration of viral replication and infectivity of SARS-CoV-2 in these individuals are needed [5–7]. The presence of SARS-CoV-2 nucleocapsid protein in blood (antigenemia) is a sensitive and specific marker for acute COVID-19 in unselected patients [8–11] but has not been previously described in immunocompromised patients.
We present a case series of 3 individuals receiving anti-CD20 therapy with prolonged symptoms consistent with persistent COVID-19 who had nucleocapsid antigenemia. Longitudinal data in 2 cases show a decline in antigenemia corresponding with treatment and symptomatic improvement.
METHODS
Case descriptions were adapted from the medical record. SARS-CoV-2 reverse transcription polymerase chain reaction (RT-PCR; performed on Roche Cobas, Applied Biosystems 7500, or Cepheid GeneXpert platforms) and a laboratory-developed test for immunoglobulin G (IgG) to SARS-CoV-2 spike protein were performed in routine care. Residual blood in venipuncture tubes with EDTA stored at 4°C were retrieved within 72 hours, and plasma was stored at 4°C until assays were performed. Plasma nucleocapsid was measured with the Quanterix HD-X SIMOA SARS-CoV-2 N Protein Antigen assay (Quanterix, Billerica, MA, USA), which has a lower limit of detection of 0.099 pg/mL [12]. Residual specimen analysis was approved by the Emory University Institutional Review Board (STUDY00000510).
RESULTS
Case 1
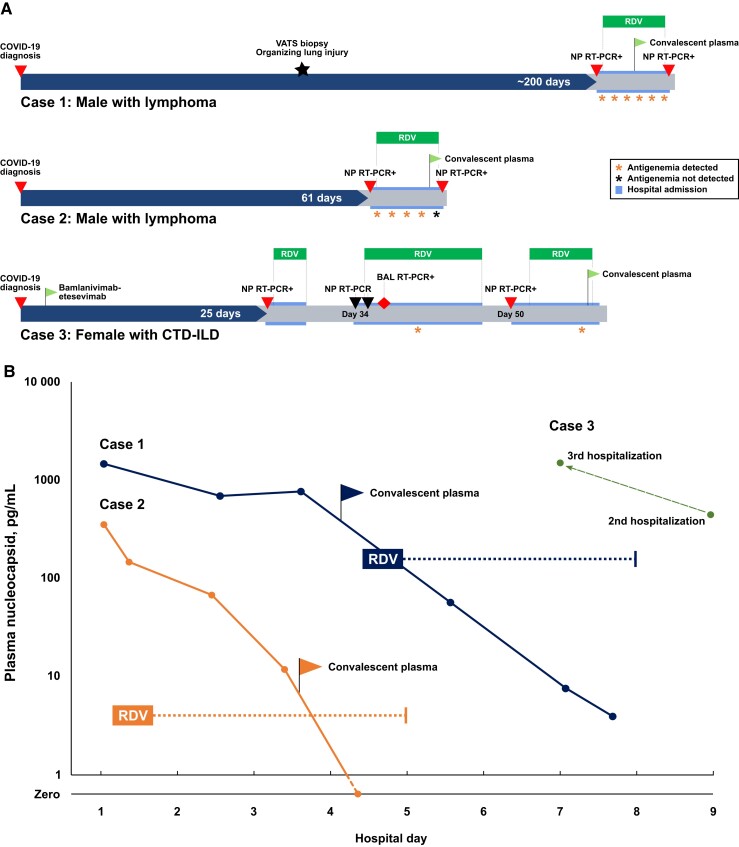
A man in his forties with lymphoma in remission receiving maintenance rituximab and not vaccinated against SARS-CoV-2 was diagnosed with COVID-19 in September 2020 ∼1 month after his last rituximab infusion. He did not initially require hospitalization but experienced progressive cough and shortness of breath for several months requiring home oxygen of up to 2 liters per minute (lpm). Nasopharyngeal SARS-CoV-2 RT-PCR was persistently positive. Approximately 3 months after COVID-19 diagnosis, a video-assisted thoracic surgery lung biopsy revealed organizing pneumonia and no evidence of bacterial, fungal, or mycobacterial infection or malignancy. Seven months after initial diagnosis, he was admitted to the hospital for evaluation and was experiencing stable mild hypoxia and exertional dyspnea. Nasopharyngeal SARS-CoV-2 RT-PCR was positive (Ct 22.6, Roche Cobas). SARS-CoV-2 spike IgG was not detected. Computed tomography (CT) of the chest revealed multifocal ground-glass opacities. He was treated with remdesivir, glucocorticoids, and 1 unit of high-titer convalescent plasma (CP) with symptomatic improvement (Figure 1A). Repeat nasopharyngeal RT-PCR after 5 days of remdesivir remained positive (Ct 31.3, Roche Cobas), and he was discharged with 20 mg daily of prednisone to be tapered over 5 weeks. Plasma nucleocapsid antigen was 1468 pg/mL on admission and declined to undetectable by discharge (Figure 1B). At the time of pulmonology clinic follow-up 10 weeks after discharge, he had tapered off steroids and supplemental oxygen with complete resolution of respiratory symptoms.
Figure 1.
A, Approximate timelines for 3 patients with impaired humoral immunity, clinical evidence of protracted SARS-CoV-2 infection, and nucleocapsid antigenemia during hospitalizations weeks to a month after initial diagnosis. B, Serial blood plasma nucleocapsid measurements during hospitalization for Case 1 and Case 2 show a decline in antigen concentration during antiviral therapy and following treatment with convalescent plasma. Dotted lines represent the approximate duration from first dose to final dose of remdesivir treatment. Available samples for Case 3 demonstrated antigenemia during both the second and third hospitalizations despite receiving 10 days of remdesivir during the second hospitalization. Abbreviations: BAL, bronchoalveolar lavage; CTD-ILD, connective tissue disease–associated interstitial lung disease; NP, Nasopharyngeal; RDV, remdesivir; RT-PCR, reverse transcription polymerase chain reaction; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; VATS, video-assisted thoracoscopic surgery.
Case 2
A man in his forties with lymphoma receiving maintenance rituximab who was not vaccinated against SARS-CoV-2 was diagnosed with COVID-19 in February 2021 ∼1 month following his last rituximab infusion. He did not require hospitalization. Over several weeks, he developed worsening cough and dyspnea and was treated with courses of levofloxacin, amoxicillin, and doxycycline, without improvement. Evaluation showed no evidence of pulmonary embolism or relapsed lymphoma. He presented to our center 61 days after initial COVID-19 diagnosis with progression of respiratory symptoms, mild hypoxia, and fever (38.5°C). Nasopharyngeal SARS-CoV-2 RT-PCR demonstrated a cycle threshold (Ct) of 26.5 (Cepheid GeneXpert), and SARS-CoV-2 spike IgG was not detected. Chest CT showed patchy bilateral ground-glass opacities and components of organization. Remdesivir and dexamethasone produced symptomatic improvement by hospital day 2 (Figure 1A). He received 5 days of remdesivir and 1 unit of high-titer CP. Plasma nucleocapsid was 355 pg/mL on admission and declined to 3.94 pg/mL (Figure 1B), although nasopharyngeal RT-PCR on the day of discharge remained positive (Ct 24.1, Roche Cobas). He did not have follow-up.
Case 3
A woman in her thirties with connective tissue disease–associated interstitial lung disease on maintenance rituximab who had received a 2-dose primary SARS-CoV-2 vaccine series was diagnosed with COVID-19 within 2 weeks of her last rituximab infusion in December 2021. She did not initially require hospitalization and was treated with bamlanivimab-etesevimab. Approximately 3 weeks after diagnosis, she was admitted with persistent dyspnea and mild hypoxia. Nasopharyngeal SARS-CoV-2 RT-PCR was positive (Ct 35.9, Cepheid GeneXpert). SARS-CoV-2 spike IgG was detected, though confounded by prior bamlanivimab-etesevimab. Chest CT showed bilateral patchy ground-glass opacities. She was treated with remdesivir and dexamethasone for 3 days and discharged after symptomatic improvement. She returned to the hospital 6 days later with fever, worsening dyspnea, and hypoxia requiring supplemental oxygen up to 4 lpm. Repeat nasopharyngeal RT-PCR was negative on 2 consecutive days, and repeat chest CT showed progressive acute lung injury. She underwent diagnostic bronchoscopy; SARS-CoV-2 RT-PCR from bronchoalveolar lavage fluid was positive (Ct 16.8, Applied Biosystems 7500), and remdesivir was re-initiated. Plasma nucleocapsid antigen on hospital day 9 was 447 pg/mL. She completed 10 days of remdesivir and was discharged with 40 mg of prednisone daily and supplemental oxygen. Four days later, she returned to the hospital a third time with recurrent fevers and dyspnea. CT showed radiographic evolution of acute lung injury, and nasopharyngeal RT-PCR was positive for SARS-CoV-2 (Ct 26.3, Roche Cobas). Plasma nucleocapsid on hospital day 7 was 1504 pg/mL. She was treated again with remdesivir for 4 days and received 1 unit of high-titer CP, resulting in resolution of fever and symptomatic improvement. She was discharged on 40 mg daily of prednisone, which was tapered over 8 weeks to 10 mg daily. During pulmonology follow-up 8 weeks later, she reported sustained improvement on 10 mg of prednisone daily with only mild residual symptoms.
DISCUSSION
Immunocompromised individuals can present diagnostic and therapeutic dilemmas following the initial diagnosis of COVID-19. Secondary bacterial or fungal infections, organizing pneumonia, persistent SARS-CoV-2, and noninfectious and superimposed etiologies must be considered. The ability to arrive at a diagnosis accurately and rapidly is crucial given the breadth of potential therapies. When appropriate, choice of COVID-directed therapy and duration of therapy remain uncertain. Evaluation of antigenemia may be a valuable tool in these scenarios.
We describe 3 immunocompromised patients with clinical, virologic, and radiographic evidence of protracted SARS-CoV-2 infection. To our knowledge, this is the first report detailing nucleocapsid antigenemia in such cases, which was measured at levels similar to those observed in a large cohort with acute COVID-19 [8]. Because nucleocapsid antigenemia is a sensitive and specific marker of acute infection and resolves in most recovered patients [8, 9], it may have utility for diagnosis and monitoring therapeutic response in immunocompromised hosts. Blood sampling may also be easier to standardize relative to nasopharyngeal RT-PCR Ct value, which is limited by sampling variability and consistency across instruments and laboratories [7, 13]. For example, the Ct value in Case 2 at discharge was lower than at admission but performed on a different instrument, limiting interpretation. The positive BAL (bronchoalveolar lavage) RT-PCR despite 2 negative nasopharyngeal samples in Case 3 highlights an additional limitation of nasopharyngeal sampling and demonstrates that antigenemia may reveal compartmentalized infection without invasive procedures.
Although mechanisms have not been determined, antigenemia is likely protein shed into circulation from cells containing replicating virus that resolves in a short period following immune clearance of active virus [14]. Serial measurements in Cases 1 and 2 demonstrated a decline in antigenemia that correlated with antiviral therapy and symptomatic improvement. While antinucleocapsid antibodies in CP may decrease measurable antigenemia [8], a reduction was observed in Case 2 during antiviral therapy before administration of CP. This suggests that antigenemia should be considered as a monitoring tool to guide the management of immunocompromised patients with persistent infection. For example, patients with a rapid decline in antigenemia with antiviral therapy (Cases 1 and 2) may warrant monitoring, whereas patients with persistent antigenemia despite antiviral medication (Case 3) may need additional therapy such as monoclonal antibodies or CP [15]. All 3 patients in our cohort received CP with subsequent clinical improvement. For the 2 patients with follow-up in our health care system, this improvement was sustained for several months.
Our findings are limited by a small sample size and restriction of time points to available residual specimens. We did not have access to respiratory specimens from the index COVID-19 diagnosis in each case or culture-based assays to verify the presence of replication-competent virus in subsequent specimens. Antigenemia should be correlated prospectively in larger studies with response to therapy, immune response biomarkers, and presence of culturable virus in the respiratory tract.
We postulate that nucleocapsid antigenemia may be a biomarker of active SARS-CoV-2 in immunocompromised hosts and that it could be used both to identify patients with persistent infection and to monitor response to therapy. Further investigation is needed in patients receiving anti-CD20 therapy and in individuals with other forms of immune compromise to define diagnostic performance, kinetics, and utility in guiding treatment decisions.
Acknowledgments
Financial support. This work was supported by NIH National Cancer Institute 1 U54 CA260563 01: “Immune Regulation of COVID-19 Infection in Cancer and Autoimmunity” to J.D.R. and NIH Grants U54 EB027690 03 and UL1TR002378. This work was also supported by funding from the Marcus Foundation to J.D.R.
Patient consent. This study was approved by the Institutional Review Board of Emory University for the use of residual clinical specimens (STUDY00000510). Written informed consent by the patients was not required.
Contributor Information
Eli Wilber, Division of Infectious Diseases, Department of Medicine, Emory University School of Medicine, Atlanta, Georgia, USA.
Anne Piantadosi, Division of Infectious Diseases, Department of Medicine, Emory University School of Medicine, Atlanta, Georgia, USA; Department of Pathology and Laboratory Medicine, Emory University School of Medicine, Atlanta, Georgia, USA.
Ahmed Babiker, Division of Infectious Diseases, Department of Medicine, Emory University School of Medicine, Atlanta, Georgia, USA; Department of Pathology and Laboratory Medicine, Emory University School of Medicine, Atlanta, Georgia, USA.
Kaleb McLendon, Department of Pathology and Laboratory Medicine, Emory University School of Medicine, Atlanta, Georgia, USA.
William O’Sick, Department of Pathology and Laboratory Medicine, Emory University School of Medicine, Atlanta, Georgia, USA.
Eric Fitts, Department of Pathology and Laboratory Medicine, Emory University School of Medicine, Atlanta, Georgia, USA.
Andrew S Webster, Division of Infectious Diseases, Department of Medicine, Emory University School of Medicine, Atlanta, Georgia, USA.
Hans Verkerke, Department of Pathology and Laboratory Medicine, Emory University School of Medicine, Atlanta, Georgia, USA; Department of Pathology, Brigham and Women’s Hospital, Harvard Medical School, Boston, Massachusetts, USA.
James S Kim, Division of Hospital Medicine, Department of Medicine, Emory University School of Medicine, Atlanta, Georgia, USA.
Varun K Phadke, Division of Infectious Diseases, Department of Medicine, Emory University School of Medicine, Atlanta, Georgia, USA.
Nadine Rouphael, Division of Infectious Diseases, Department of Medicine, Emory University School of Medicine, Atlanta, Georgia, USA.
Boghuma K Titanji, Division of Infectious Diseases, Department of Medicine, Emory University School of Medicine, Atlanta, Georgia, USA.
William T Blake, Division of Infectious Diseases, Department of Medicine, Emory University School of Medicine, Atlanta, Georgia, USA.
Jessica Howard-Anderson, Division of Infectious Diseases, Department of Medicine, Emory University School of Medicine, Atlanta, Georgia, USA.
John D Roback, Department of Pathology and Laboratory Medicine, Emory University School of Medicine, Atlanta, Georgia, USA.
Wilbur A Lam, Department of Pathology and Laboratory Medicine, Emory University School of Medicine, Atlanta, Georgia, USA; The Atlanta Center for Microsystems-Engineered Point-of-Care Technologies, Atlanta, Georgia, USA; Aflac Cancer & Blood Disorders Center at Children’s Healthcare of Atlanta, Atlanta, Georgia, USA; Wallace H. Coulter Department of Biomedical Engineering, Georgia Institute of Technology, Atlanta, Georgia, USA.
Gregory L Damhorst, Division of Infectious Diseases, Department of Medicine, Emory University School of Medicine, Atlanta, Georgia, USA; The Atlanta Center for Microsystems-Engineered Point-of-Care Technologies, Atlanta, Georgia, USA.
References
- 1. Izurieta HS, Graham DJ, Jiao Y, et al. . Natural history of coronavirus disease 2019: risk factors for hospitalizations and deaths among >26 million US Medicare beneficiaries. J Infect Dis 2021; 223:945–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Avanzato VA, Matson MJ, Seifert SN, et al. . Case study: prolonged infectious SARS-CoV-2 shedding from an asymptomatic immunocompromised individual with cancer. Cell 2020; 183:1901–12.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Aydillo T, Gonzalez-Reiche AS, Aslam S, et al. . Shedding of viable SARS-CoV-2 after immunosuppressive therapy for cancer. N Engl J Med 2020; 383:2586–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Corey L, Beyrer C, Cohen MS, Michael NL, Bedford T, Rolland M. SARS-CoV-2 variants in patients with immunosuppression. N Engl J Med 2021; 385:562–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Haidar G, Mellors JW. Improving the outcomes of immunocompromised patients with coronavirus disease 2019. Clin Infect Dis 2021; 73:e1397–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Calderón-Parra J, Múñez-Rubio E, Fernández-Cruz A, et al. . Incidence, clinical presentation, relapses and outcome of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in patients treated with anti-CD20 monoclonal antibodies. Clin Infect Dis 2022; 74:1786–94. [DOI] [PubMed] [Google Scholar]
- 7. Binnicker MJ. Can testing predict SARS-CoV-2 infectivity? The potential for certain methods to be surrogates for replication-competent virus. J Clin Microbiol 2021; 59:e0046921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Verkerke HP, Damhorst GL, Graciaa DS, et al. . Nucleocapsid antigenemia is a marker of acute SARS-CoV-2 infection. J Infect Dis 2022:jiac225. doi: 10.1093/infdis/jiac225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ahava MJ, Kurkela S, Kuivanen S, Lappalainen M, Jarva H, Jaaskelainen AJ. Detection of SARS-CoV-2 nucleocapsid antigen from serum can aid in timing of COVID-19 infection. J Virol Methods 2022; 302:114469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shan D, Johnson JM, Fernandes SC, et al. . N-protein presents early in blood, dried blood and saliva during asymptomatic and symptomatic SARS-CoV-2 infection. Nat Commun 2021; 12:1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhang Y, Ong CM, Yun C, et al. . Diagnostic value of nucleocapsid protein in blood for SARS-CoV-2 infection. Clin Chem 2021; 68:240–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Quanterix . Simoa® SARS CoV-2 N Protein Advantage Kit HD-X data sheet. 2022. Available at: https://www.quanterix.com/wp-content/uploads/2020/12/SARS-CoV-2-N-Protein-Advantage-Data-Sheet-for-HD-X.pdf. Accessed May 16, 2022.
- 13. Rhoads DD, Pinsky BA. The truth about SARS-CoV-2 cycle threshold values is rarely pure and never simple. Clin Chem 2021; 68:16–8. [DOI] [PubMed] [Google Scholar]
- 14. Costa R, Alberola J, Olea B, et al. . Combined kinetic analysis of SARS-CoV-2 RNAemia, N-antigenemia and virus-specific antibodies in critically ill adult COVID-19 patients. Sci Rep 2022; 12:8273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hueso T, Pouderoux C, Péré H, et al. . Convalescent plasma therapy for B-cell–depleted patients with protracted COVID-19. Blood 2020; 136:2290–5. [DOI] [PMC free article] [PubMed] [Google Scholar]