Abstract
Low molecular weight chitosan (LMWC) has higher solubility and lower viscosity allowing for a wider pharmaceutical application compared to high molecular weight chitosan. LMWC chitosan can be obtained through a chitosan depolymerization process. This research aimed to produce LWMC using the combination of formic acid and ultrasonication method with the optimal condition of the depolymerization process. The chitosan depolymerization method was performed by combining formic acid and ultrasonication. The optimum conditions of the depolymerization process were obtained using the Box–Behnken design. The LMWC obtained from depolymerization was characterized to identify its yield, degree of deacetylation, the molecular weight, structure, morphology, thermal behavior, and crystallinity index. Results: The characterization results of LWMC obtained from the depolymerization process using the optimum conditions showed that the yield was 89.398%; the degree of deacetylation was 98.076%; the molecular weight was 32.814 kDa; there was no change in the chemical structure, LWMC had disorganized shape, there was no change in the thermal behavior, and LWMC had a more amorphous shape compared to native chitosan. Conclusion: The production of LWMC involving depolymerization in the presence of weak acid and ultrasonication can be developed by using the optimal condition of the depolymerization process.
Keywords: optimization, low molecular weight chitosan, depolymerization, Box-Behnken Design
1. Introduction
Chitosan is a deacetylated product from chitin [1]. Chitin is present in the exoskeleton of crustaceans, insects, fungi, bacteria, and some mushrooms [2,3]. Chitosan is a functional natural polymer because of its properties of biodegradability [4], biocompatibility [5], bio adhesivity, non-toxicity [6], and it is able to form a gel and cationic at a low pH [2,7,8]. The molecular weight of chitosan is related to its properties [9]. The high molecular weight of chitosan provides low solubility, thus limiting its use [3]. Molecular weight affects not only the solubility of chitosan [10], but also its biodegradability, biocompatibility, bioactivity, and toxicity. Low molecular weight chitosan has better biodegradability, biocompatibility, and bioactivity [11] as well as lower toxicity [12] compared to high molecular weight chitosan. In addition, low molecular weight chitosan also has a higher solubility [13] and lower viscosity [14] due to the shorter chain and the presence of the free amine group on the glucosamine unit [15], allowing for a wider pharmaceutical application [16]. Low molecular weight chitosan has been used for drug delivery systems [17], demonstrating an increased swelling property makes chitosan a perfect carrier for the drug. It was reported that reducing the molecular weight of chitosan leads to reduce the viscosity of the chitosan solution, obtaining particles with a smaller size [18].
LMWC chitosan can be obtained through a chitosan depolymerization process [19]. Depolymerization of chitosan into low molecular weight chitosan can be done by chemical [20], enzymatic [21], and physical methods [22]. One of the chemical depolymerization methods is followed by using acids [23,24]. The acid method has advantages: it can be used for large-scale production, has a low cost of production [19], can produce smaller fragments in large quantities, and can increase the rate of chitosan hydrolysis. However, the use of strong acids with a high concentration may damage the environment [3]. Besides, the use of an excessive amount of acids in the depolymerization process causes glucosamine degradation of chitosan [25]. Additionally, chitosan depolymerization by strong acid is excluded from medical application due to its toxicity [26]. Using weak acids in depolymerization comes as an alternative to using strong acids in depolymerization. Using weak acids is considered safer to produce LMWC for pharmaceutical application than using strong acids for chitosan depolymerization. The utilization of very low concentration of weak acid for the degradation of chitosan was reported by Savitri et al. (2014). Formic acid is a weak acid that can be used in depolymerization. Formic acid depolymerization with the combination of H2O2 significantly decreases the molecular weight of chitosan [27]. In addition to the acid methods, physical methods [28] using ultrasonication can also lower the molecular weight of chitosan [29]. Depolymerization by sonication is more environmentally friendly, energy efficient [30], and effective [31]. The principle of the sonication method is to provide energy to break chemical bonds [29]. Characterization results show that depolymerization using ultrasonication does not change chitosan structure [32].
Optimization of a depolymerization process can be a complicated task [33], particularly when using a combination of 2 methods, namely formic acid and ultrasonication which responses were strongly affected by chitosan concentration, temperature, and time of sonication. The depolymerization process should be optimized to obtain the optimal condition of the depolymerization process that affect the responses [34]. The depolymerization process can be optimized by using response surface method (RSM) [33]. RSM is an efficient way to optimize the depolymerization process [35,36]. RSM is a multivariate statistical technique used to predict the optimum conditions of a process that can discover the best possible responses in the investigated experimental area [34,37]. RSM can predict the quadratic effect of the independent variable on the response through a mathematical model of the resulting quadratic equation [38]. The Box–Behnken design (BBD) is the main type of RSM [33]. The BBD only has three levels per factor [39,40] which are low (−1), middle (0), and high level (+1), respectively, and produce a minimum number of runs then BBD is less expensive to run than other RSM design [33,41].
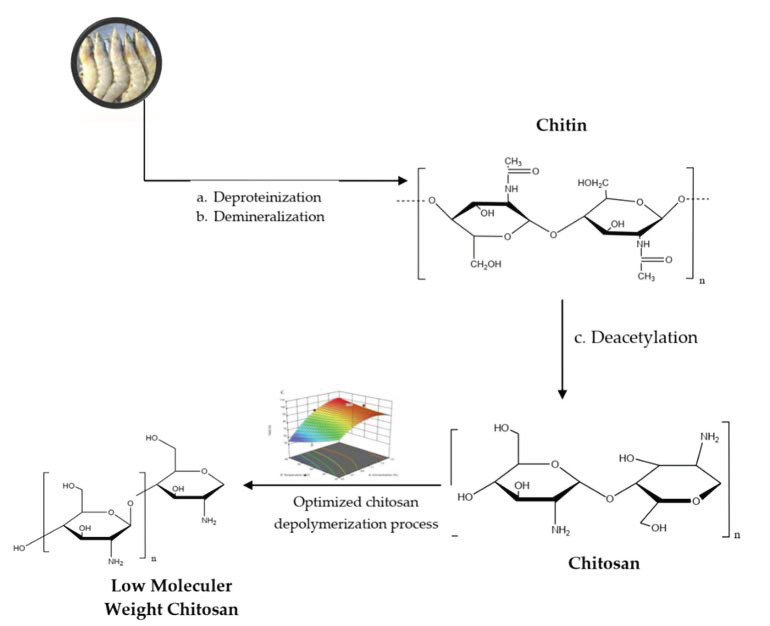
Our objective was to produce low molecular weight chitosan involving depolymerization in the presence of weak acid (formic acid) and ultrasonication. The Box–Behnken design was used to optimize the parameters of the depolymerization process. The strategy for producing a low molecular weight of chitosan using this method is shown in Figure 1.
Figure 1.
Strategy for production of low molecular weight chitosan by depolymerization.
2. Materials and Methods
2.1. Materials
The materials of this research are shown in Table 1.
Table 1.
The materials of research.
| No | Materials | Source/Manufacturer |
|---|---|---|
| 1 | Vaname shrimp shell | PT Yanagi (Kendari, Indonesia) |
| 2 | Commercial chitosan | Sigma-Aldrich (Saint.Louis, MO, USA) |
| 3 | Formic acid | CV Multi Usaha Mandiri (Sidoarjo, Indonesia) |
| 4 | Acetic acid | CV Sumber Rejeki (Makassar-Indonesia) |
| 5 | Sodium hydroxide | CV Sumber Rejeki (Makassar-Indonesia) |
| 6 | Hydrochloric acid | CV Sumber Rejeki (Makassar-Indonesia) |
| 7 | ethanol 96% | CV Sumber Rejeki (Makassar-Indonesia) |
| 8 | deionized water | CV Sumber Rejeki (Makassar-Indonesia) |
| 9 | Potato Dextrose Agar | Merck KGaA (Darmstads, Germany) |
| 10 | Staphilococcus aureus ATCC25923 | Laboratory of Biomedic, Faculty of Medicine, Halu Oleo University, Kendari, Indonesia |
| 11 | Escherichia coli ATCC 35218 | Laboratory of Biomedic, Faculty of Medicine, Halu Oleo University, Kendari, Indonesia |
| 12 | Salmonella sp. ATCC 14028 | Laboratory of Biomedic, Faculty of Medicine, Halu Oleo University, Kendari, Indonesia |
2.2. Shrimp Shell Preparation
A total of 10 kg of shrimp shell waste was washed with 50 L of water at room temperature for 1 h, then dried at 50 °C for 24 h in an oven (memmert, Schwabach, Germany). Clean shrimp shell waste was then mashed into powder. The powder was then sieved using a 20-mesh sieve.
2.3. Characterization of Shrimp Shell Powder
Shrimp powder was characterized by organoleptic tests [42,43], solubility in water [14], solubility in 95% ethanol [14], ash content (SNI 01-2354.1-2006), fat content (SNI 01-2354.3-2006), protein content (SNI 01 2354.4-2006), and water content (SNI 2354.2:2015).
2.4. Chitosan Preparation
2.4.1. Deproteinization
A total of 500 g of shrimp shells were placed in a beaker glass equipped with a stirrer and thermometer (LT-12, Midwest Homebrewing, Philadelphia, PA, USA). Then, 3.5% NaOH was added with a ratio of shrimp shell mass to the solution of 1:10 (w/v) and heated on a hot plate (Stuart UC 152, Vernon Hills, IL, USA) for 2 h at a temperature of 65 °C with constant stirring. The precipitate was washed with distilled water until a neutral pH was achieved. The precipitate was dried in an oven at 60 °C for 24 h [44].
2.4.2. Demineralization
The chitin resulting from the deproteinization was placed in a beaker glass equipped with a stirrer and a thermometer and placed on a hot plate. Then 1N HCl was added with a ratio of 1:15 (w/v) for 1 h at room temperature, and the precipitate was dried in an oven at 60 °C for 24 h [44].
2.4.3. Deacetylation
The chitin obtained from the above processes was added to 50% NaOH solution with a ratio of chitin to the solution of 1:20 (w/v) at 120 °C while constantly stirred for 3 × 3 h. The deacetylation process aimed to produce a final product in the form of chitosan powder [44].
2.5. Chitosan Characterization Based on Pharmaceutical Requirement
The chitosan was then characterized in terms of its physicochemical properties to meet the requirements of pharmaceutical-grade chitosan, including organoleptic [16], particle size [17], pH [18], moisture content, solubility in water [14], solubility in 96% ethanol [14], molecular weight [19], deacetylation degree [20], loss of drying [43], ash content (SNI 01-2354.1-2006), heavy metal contamination [45], and microbial contamination [46].
2.6. Chitosan Depolymerization
Chitosan depolymerization was performed using a combination of weak acids, i.e., formic acid and ultrasonication. The chitosan was dissolved in 1% formic acid to obtain three concentrations, namely 0.5, 1, and 1.5%. Sonication was then performed on the chitosan solutions with temperature variations of 20, 40, and 60 °C and time variations of 10, 20, and 30 min (Table 1) using a sonicator (Kudos, Shanghai-China). Several studies have reported the effects of these conditions on the depolymerization of chitosan. The previous studies showed that chitosan was depolymerized faster in dilute acid solution and at lower temperatures [29]. It was reported that degradation of chitosan in very low concentration of weak acid [25]. The depolymerization temperature was varied at 40, 50, and 60 °C to study the influence of temperature on chitosan depolymerization, and the result showed that the molecular weight value was significantly reduced [27]. The degradation of chitosan was reported with the variations of sonication time of 30 to 120 min [25]. The depolymerization reaction was ceased by soaking the chitosan solution after the sonication was performed into ice. The samples were then collected and neutralized with 2 M NaOH until the pH reached 8–9. The solid formed was then filtered and washed with distilled water. Other contaminants were removed by rinsing the solids using 95% ethanol. The dried solids were then ground and characterized, including yield (%), degree of deacetylation, molecular weight using the viscometric method, structure analysis using FTIR and 1H-NMR, morphology, thermal analysis, and crystallinity index.
2.7. Optimization Process
Optimization of the depolymerization process was performed using the Box-Behnken Design using three independent variables, each of which used three levels (Table 2). There were 17 run orders with five center points, as shown in Table 3. The effects of each independent variable on the responses, i.e., molecular weight, degree of deacetylation, and yield, were measured using a second-order polynomial:
| (1) |
where y = predicted response, βi is a linear coefficient, βii is a quadratic coefficient, and βij is the interaction coefficient, Xi, Xj, and Xk are independent variables affecting the depolymerization processes, ε is random error. The success of the developed method was determined by coefficient R2. The significance of the regression coefficient model was evaluated using analysis of variance (ANOVA). After that, the 3-dimensional curve and response plot obtained were used to interpret the effect of the interaction between the independent variables on the response variables using design-expert version 13 [47].
Table 2.
Level of experimental variables.
| Level | Chitosan Concentration (%) | Temperature (°C) | Time (Minute) |
|---|---|---|---|
| Low (−1) | 0.5 | 20 | 10 |
| Middle (0) | 1 | 40 | 20 |
| High (+1) | 1.5 | 60 | 30 |
Table 3.
Box Bhenken design for three factors and three levels.
| No | Run Order | Chitosan Concentration (%) | Temperature (°C) | Time (Minute) |
|---|---|---|---|---|
| 1 | 11 | 0 | −1 | +1 |
| 2 | 7 | −1 | 0 | +1 |
| 3 | 8 | +1 | 0 | +1 |
| 4 | 16 | 0 | 0 | 0 |
| 5 | 14 | 0 | 0 | 0 |
| 6 | 6 | +1 | 0 | −1 |
| 7 | 2 | +1 | −1 | 0 |
| 8 | 15 | 0 | 0 | 0 |
| 9 | 17 | 0 | 0 | 0 |
| 10 | 9 | 0 | −1 | −1 |
| 11 | 12 | 0 | +1 | +1 |
| 12 | 13 | 0 | 0 | 0 |
| 13 | 3 | −1 | +1 | 0 |
| 14 | 4 | +1 | +1 | 0 |
| 15 | 10 | 0 | +1 | −1 |
| 16 | 1 | −1 | −1 | 0 |
| 17 | 5 | −1 | 0 | −1 |
2.8. Characterization of Low Molecular Weight Chitosan
2.8.1. Fourier Transform Infra-Red (FTIR) Spectroscopy
The low molecular weight chitosan obtained was read using a FTIR spectrophotometer (Shimadzu® IR-Prestige 21, Tokyo, Japan). The chitosan was made into pellets with KBr, then scanned at a frequency between 4000 cm−1 and 500 cm−1. The spectrum obtained was compared with those of chitin, initial chitosan, and standard chitosan [15,18].
2.8.2. H-NMR Spectroscopy
1H-NMR spectral measurements were performed using a 1H-NMR spectrophotometer (JEOL, Peabody, MA, USA). Ten milligrams of chitosan were suspended in 7.5 mL of D2O containing two drops of trifluoro acetic acid (TFA). The mixture was vortexed for 3 m. The spectra between 0 and 15 ppm were recorded [48]. The acquisition time was 1.745 s and the delay time was 5 s. The field strength was 11.747 T, and the frequency was 500 MHz.
2.8.3. Scanning Electron Microscope
The morphology of the obtained low molecular weight chitosan was observed under Scanning Electron Microscope (SEM) (Hitachi Series SU3500, Tokyo, Japan). A particular amount of chitosan was attached to the specimen holder with gold palladium plating, inserted into the specimen chamber, and run with a stereoscan microscope [49].
2.8.4. Differential Scanning Calorimetry
This was analyzed using differential scanning calorimetry (DSC) ((NEXTA DSC, Tokyo, Japan), of which the temperature had been set and the cell constant used indium. A total of 1–3 g of chitosan was placed in an aluminum container and analyzed at 50–500 °C with a heating rate of 10 °C/min. The samples were cleaned continuously with nitrogen at 50 mL/min [26].
2.8.5. X-ray Diffractometry
The crystallinity index was measured with XRD (X’Pert PRO PANatycal, Worcestershire, UK) using Cu-Kα radiation, where the detection was performed at 2θ = 5–50° at a speed of 2°/min and set at 40 kV and 30 mA. The chitosan powder was placed on the holder, and the crystallinity index was analyzed with the percentage of total area and crystal peak ratio [50].
3. Results and Discussion
3.1. Characterization of Shrimp Shell Powder
The result of the shrimp shell characterization is shown in Table 4.
Table 4.
Characteristics of shrimp shell powder.
| Specification | Shrimp Shell Powder |
|---|---|
| Organoleptic | Coarse powder, slightly yellowish, strong shrimp shell odour, less tasty |
| Solubility in water | 5% |
| Solubility in 96% ethanol | 3% |
| Ash content | 14.14% |
| Water content | 54.57% |
| Fat content | 1.81% |
| Protein content | 14.92% |
3.2. Chitosan Characterization Based on Pharmaceutical Requirement
The isolated chitosan was presented in Figure 2. Chitosan characteristics based on pharmaceutical requirements are shown in Table 5.
Figure 2.
The isolated chitosan from vaname shrimp shell.
Table 5.
Chitosan characteristics are based on the pharmaceutical requirement.
| Specification | Isolated Chitosan | Pharmaceutical Specification of Chitosan | Reference |
|---|---|---|---|
| Organoleptic properties | Odourless, creamy white powder | Odourless, white or creamy white, or flakes | Rowe, 2009 |
| Particle size | 4.3702 µm (PI = 0.679) | <30 µm | Rowe, 2009 |
| pH | 4.68 | 4.0–6.0 | Rowe, 2009 |
| Moisture content | 5.09% | ≤5% | USP-36, NF-31 |
| Solubility: | PhEur-5 | ||
| Water | Very slightly soluble | Sparingly Soluble | |
| Ethanol | Practically Insoluble | Practically Insoluble | |
| Molecular weight | 57,543.99 Dalton | ≤1,000,000 | USP-36, NF-31 |
| Degree of deacetylation | 95.95% | 70–95% | USP-36, NF-31 |
| Loss on drying | 0.37% | ≤10% | Rowe, 2009 |
| Ash content | 0.87% | <1% | Rowe, 2009 |
| Heavy metal Lead (Pb) Cadmium (Cd) Mercury (Hg) |
0.0002 mg/L 0.0004 mg/L <0.0001 mg/L |
≤40 ppm | Rowe, 2009 |
| Microbial contamination | SNI, 2013 | ||
| Escherecia coli | <3 MPM/g | <3 MPM/g | |
| Salmonella | - | ||
| Total plate count (Bacteria) | 270 colony/g | Maximum 1 × 103 colony/g | |
| Total plate count (fungi) | 350 colony/g | ||
| Yield | 26% | ||
| Nynhidrine test | Purple |
The results of the characterization of chitosan isolated from vanname shrimps showed that the chitosan produced met the pharmaceutical grade requirements, as seen in Table 5. However, there was a difference with the pharmaceutical grade standard in terms of the solubility in water. The solubility of chitosan isolated from vaname shrimps was very slightly soluble, while the pharmaceutical grade standard has set that the solubility should be sparingly soluble. Such differences can be due to several factors. The factors that affect the solubility of chitosan include the degree of deacetylation, molecular weight, and crystallinity [51]. The degree of deacetylation of the chitosan produced (95.95%) was also higher than the standard degree of deacetylation. The degree of deacetylation is significantly affected by the preparation processes during chitosan isolation. A high degree of deacetylation is caused by prolonged deacetylation processes by alkali treatment of chitin with intermittent washing by water.
The chitosan isolated from vanname shrimp produced a yield of 26%. Yield is significantly influenced by the source of chitosan. The yield of chitosan obtained from shrimp shells in a previous study was reported to be 15.40% [52]. Meanwhile, the chitosan obtained from the P.monodon shrimp shell reached 35% [53]. The yield of chitosan obtained from crab shells was 30–32% [54]. The chitosan isolated from fungi was approximately 17.6% [55]. In addition, the chitosan isolated from fungi generated a yield of 1.5–3.5% [56]. The chitosan isolated from various species of insects also varied, ranging between 3.1%–96.2%. The yield of chitosan is also highly dependent on the variables of the isolation method used.
Chitosan identification using ninhydrin reagent showed a purple color after the chitosan was sprayed with 1% ninhydrin reagent. Ninhydrin is an oxidizing agent that reacts with the amino groups found in chitosan in a pH range of 4–8.
3.3. Depolymerization Process
Depolymerization of chitosan isolated from vanname shrimp was performed using a combination of formic acids and ultrasonication. The depolymerization process was optimized using the Box–Behnken design (BBD) with three variables and three levels (Table 1). The responses were in the form of chitosan molecular weight, degree of deacetylation, and yield, as shown in Table 6.
Table 6.
Box Bhenken Design and Result.
| No | Factor | Response | ||||
|---|---|---|---|---|---|---|
| Concentration (%) | Temperature (°C) | Time (Minute) | Molecular Weight (kDa) | Deacetylation Degree (%) | Yield (%) | |
| 1 | 1 | 20 | 30 | 35.48 | 96.69 | 92.90 |
| 2 | 0.5 | 40 | 30 | 35.48 | 99.44 | 69.68 |
| 3 | 1.5 | 40 | 30 | 38.02 | 99.01 | 95.4 |
| 4 | 1 | 40 | 20 | 36.31 | 97.85 | 84.5 |
| 5 | 1 | 40 | 20 | 33.88 | 99.13 | 98 |
| 6 | 1.5 | 40 | 10 | 36.67 | 96.77 | 96.70 |
| 7 | 1.5 | 20 | 20 | 36.3 | 99.61 | 86.20 |
| 8 | 1 | 40 | 20 | 34.67 | 95.81 | 88.10 |
| 9 | 1 | 40 | 20 | 34.67 | 96.82 | 94.30 |
| 10 | 1 | 20 | 10 | 33.11 | 96.16 | 95.40 |
| 11 | 1 | 60 | 30 | 32.36 | 96.70 | 93.70 |
| 12 | 1 | 40 | 20 | 33.88 | 99.27 | 98 |
| 13 | 0.5 | 60 | 20 | 34.67 | 99.86 | 63.84 |
| 14 | 1.5 | 60 | 20 | 36.31 | 97.86 | 97.50 |
| 15 | 1 | 60 | 10 | 35.48 | 98.96 | 89.8 |
| 16 | 0.5 | 20 | 20 | 34.67 | 98.76 | 87.28 |
| 17 | 0.5 | 40 | 10 | 35.11 | 98.59 | 60.48 |
The results of the RSM calculation on the regression model of each predetermined parameter were seen from the lack of fit test. This model was used to determine and predict each response from each depolymerization parameter based on its significance. The accepted significance was p < 0.05. The lack of fit test results showed that the yield used the quadratic regression model while the molecular weight and degree of deacetylation used the linear regression. The ANOVA test showed that the regression model on the yield response could be used to determine the optimal condition of chitosan depolymerization with the combination of formic acids and ultrasonication methods. In contrast, the regression model for the other two responses could not be used. The response (yield) and the independent variables were correlated to a second order equation:
| Yield (%) = 92.8 + 11.82A − 2.12B + 1.16 C + 8.68 AB − 2.63AC +1.60BC − 10.63A2 + 1.75B2 − 1.38 C2. | (2) |
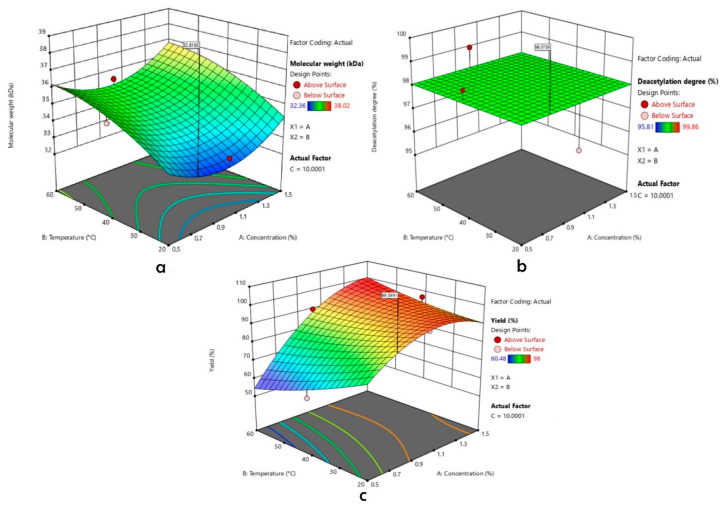
The interaction between all factors was evaluated, and the results are shown in Figure 3.
Figure 3.
Three-dimensional diagram of (a) molecular weight, (b) deacetylation degree, and (c) yield prediction of the developed model.
The solution offered by the Box–Behnken design for the depolymerization process was to use three variables, namely concentration, temperature, and time, i.e., 0.733%, 20 °C, and 10 min. The predicted responses in terms of molecular weight, degree of deacetylation, and yield were 32.814 kDa, 98.076%, and 89.398% respectively.
The verification of the optimum conditions recommended by the Box–Behnken design shows that the molecular weight, deacetylation degree, and yield values are consistent with the predicted values. The molecular weight, deacetylation degree, and yield values of low molecular weight chitosan at optimum conditions were 32.359 kDa, 98.86%, and 89.33%.
The optimal conditions were then used to make low molecular weight chitosan further characterized, including structure, particle shape and surface observation, thermal analysis and measurement of crystallinity index.
3.4. Low Molecular Wight Chitosan Characterization
3.4.1. Fourier Transform Infra-Red (FTIR) Spectroscopy
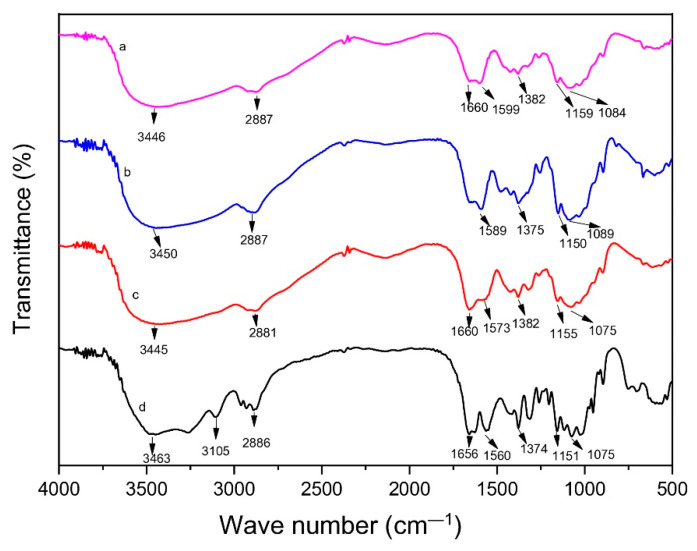
The FTIR spectrum of low molecular weight chitosan, native chitosan, commercial chitosan, and chitin is shown in Figure 4.
Figure 4.
FTIR spectrum of low molecular weight chitosan (a), native chitosan (b), commercial chitosan (c), and (d) chitin.
The spectrum of low molecular weight chitosan was quite similar to native chitosan, indicating that the chitosan structure remains stable after the depolymerization process. A band around 3500 cm−1 in the FTIR spectra of low molecular weight chitosan and native chitosan indicates the presence of OH group. The band around 2887 cm−1 is attributed to C-H stretching. The band around 1660 cm−1 in the FTIR spectra of low molecular weight chitosan indicates the presence of the C=O group. The intensity of this peak was decreased compared to the native chitosan due to the decrease of acetyl group in low molecular weight chitosan compared to native chitosan. The presence of a band in 1559 cm−1 corresponds to N-H bending group. The bands around 1382 cm−1, 1159 cm−1 and 1084 cm−1 were attributed to C-O, C-N, and C-C groups, respectively. The results obtained were also compared with the commercial chitosan and chitin. Compared to FTIR spectra of chitin, there was a decrease of intensity peak around 1660 cm−1 due to the decrease of acetyl content because of the deacetylation process performed in chitin.
3.4.2. H-NMR Spectroscopy
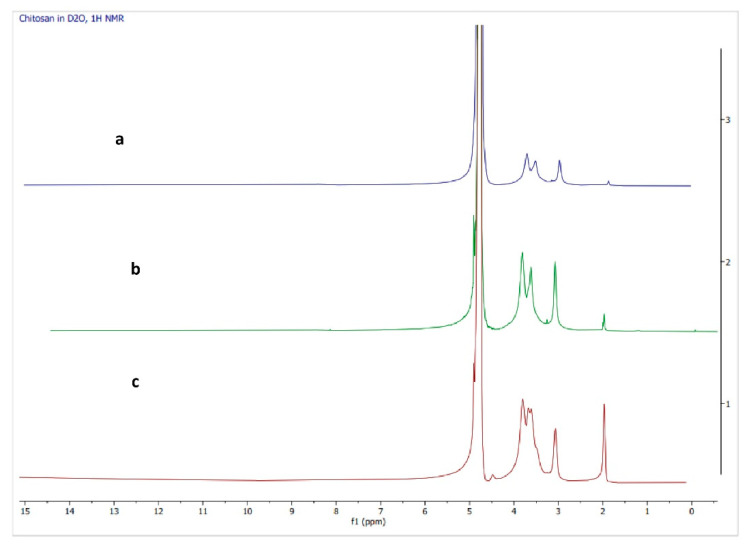
NMR spectra of low molecular weight chitosan, native chitosan, and commercial chitosan are shown in Figure 5.
Figure 5.
NMR spectrum of: (a) LMW chitosan, (b) native chitosan; (c) commercial chitosan.
Chitosan, N-glucosamine, is a result of deacetylation reaction of chitin. The basic structure of chitosan is similar to that of chitin, except that the acetyl groups in chitin have been partially removed to give an amine (NH2) group, thus constructing a polymer that comprises both N-glucosamine and N-acetylglucosamine as the monomers that make up the building block. The result of a conversion from chitin to chitosan through deacetylation reaction can be observed in an NMR spectrum. As in the case of chitin, the NMR spectrum of chitosan should show similar signals pattern. The only striking difference between chitin and chitosan in NMR spectrum lies in the presence of methyl proton signals. In chitin, the methyl signals should appear prominently at around 2.0 ppm. However, these signals should decrease in chitosan as some portions of the acetyl groups have been removed. The NMR spectrum of the native chitosan (Figure 5b) shows a signal pattern that corresponds to how a chitosan NMR spectrum should be performed. It can be observed in the spectrum that the methyl signals (around 2.0 ppm) are significantly lower than the other signals. The spectrum is comparable to commercial chitosan (Figure 5c), which exhibits similar signal patterns, suggesting a success in the deacetylation process that resulted in the native chitosan. Furthermore, the results of a depolymerization reaction carried out on the native chitosan to produce a low molecular weight chitosan (Figure 5a) indicated the presence of a product with a similar signal pattern in 1H NMR. However, the chain shortening needs to be observed using MS data and cannot be confirmed with NMR spectroscopy alone.
3.4.3. Scanning Electron Microscope
The morphology of the obtained low molecular weight chitosan and native chitosan is shown in Figure 6.
Figure 6.
SEM of native chitosan magnification at 1000× (A1), 500× (A2), 100× (A3) and SEM of low molecular weight chitosan magnification at 1000× (B1), 500× (B2), 100× (B3).
Observation using SEM showed that the low molecular weight chitosan had a more disorganized shape compared to native chitosan. The observation of the native chitosan showed an irregular shape, fibrous network, and smooth surface.
3.4.4. Thermal Analysis Using Differential Scanning Calorimetry
Thermal analysis using DSC is shown in Figure 7.
Figure 7.
DSC thermogram of (a) low molecular weight chitosan, (b) native chitosan, (c) comercial chitosan, and (d) chitin.
Thermal analysis using DSC on low molecular weight chitosan, native chitosan, and commercial chitosan showed a broad endothermic peak around 60–65° related to water dehydration. The exothermic peak of chitosan correspondent to amine group (GLcN) decomposition. Meanwhile, the DSC curves of chitin showed two endothermic peaks, whereas the peak around 50 °C correspondents to water loss, and the peak around 392 °C is related to chitin degradation. The endothermic enthalpies energy of low molecular weight of chitosan was decrease compared to native chitosan, indicating that these chitosan molecules differ in their strength of chitosan-water interaction and water-chitosan bonding capacity. Furthermore, the increase of exothermic enthalpies of low molecular weight chitosan compared to native chitosan indicates that these molecules differ in their strength of intramolecular bonding capacity due to the chain shortening.
3.4.5. X-ray Diffractometry
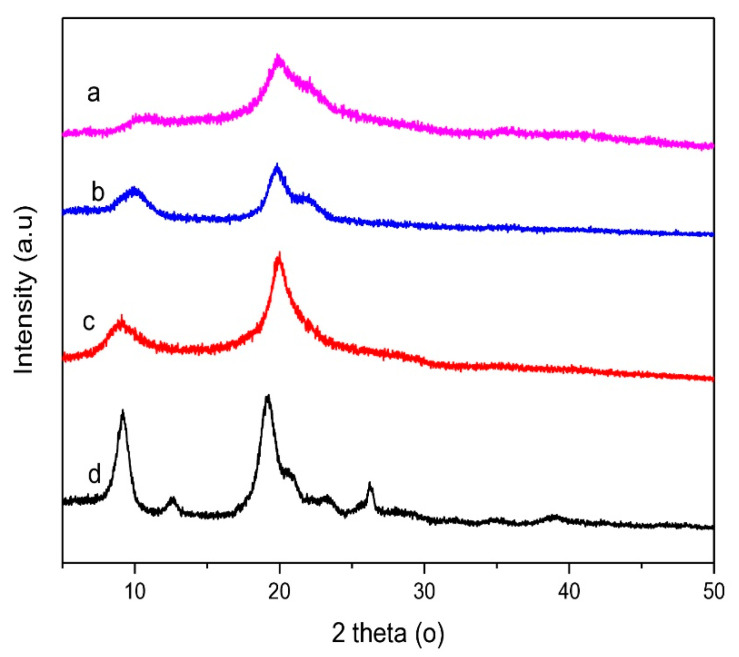
The crystallinity index and diffractogram of low molecular weight chitosan, native chitosan, commercial chitosan, and chitin are shown in Table 7 and Figure 8, respectively.
Table 7.
Crystallinity Index Value.
| Samples | The Cristalinity Index (%) |
|---|---|
| Low molecular weight chitosan | 36.64 |
| Native chitosan | 42.01 |
| Commercial chitosan | 42.90 |
| Chitin | 61.29 |
Figure 8.
Diffractogram of (a) low molecular weight chitosan, (b) native chitosan, (c) commercial chitosan and (d) chitin.
The diffractogram of low molecular weight chitosan showed how a chitosan diffractogram should be performed. There were diffraction peaks at 2θ = 10.62° and 2θ = 19.86°. The diffractogram was comparable to that of native chitosan and commercial chitosan, which exhibits a similar pattern. The intensity of low molecular weight chitosan diffraction peaks decreases correspondents to partial decrystallization of the chitosan. Crystallinity index decreased in the following order: low molecular weight chitosan > native chitosan > chitin due to increasing amorphous domains of chitosan. The increasing of amorphous domains of chitosan is correspondent to the sorption ability. The sorption mechanism is absorption of water molecules into the hydrophilic domain of amorphous chitosan [57].
4. Conclusions
The production of low molecular weight chitosan involving depolymerization in the presence of weak acid and ultrasonication can be developed commercially to produce low molecular weight chitosan. The optimal depolymerization process was studied by Box–Behnken design (BBD). The optimal condition and the best responses were obtained. The optimum concentration was 0.733%, the optimum temperature was 20 °C, and the optimum sonication time was 10 min. IR and NMR spectrum confirmed that there was no modified chemical structure of low molecular weight chitosan compared to native chitosan. There was no thermal behavior transformation in low molecular weight compared to native chitosan. The reduction of molecular weight of chitosan led to an increase in the amorphous domain of chitosan. However, data regarding the toxicity of low molecular weight of chitosan obtained by this method need to be evaluated because the chitosan will be applied in the future in pharmaceutical applications.
Acknowledgments
The authors would like to express gratitude to the Rector of Universitas Padjadjaran, by directorate research and community service for APC funding of this of this article. The authors acknowledge the facilities, scientific and technical support from Advanced Characterization Laboratories Yogyakarta, National Research and Innovation Agency through E- Layanan Sains, Badan Riset dan Inovasi Nasional.
Author Contributions
Conceptualization: S.S., L.O.A.N.R. and A.Y.C.; methodology: S.S., L.O.A.N.R. and S.H.S.; resources: I.M.J., R.R. and Y.W.W.; data curation: S.S., L.O.A.N.R., S.H.S. and Y.W.W.; Writing—original draft preparation: S.S.; writing—review and editing, S.S., A.Y.C. and Y.W.W.; project administration: R.R. All authors have read and agreed to the published version of manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
Rector of Universitas Padjajaran, Bandung, Indonesia via the Directorate of Research and Community Engagement for funding the publication fee of this article.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kou S.G., Peters L.M., Mucalo M.R. Chitosan: A Review of source and preparation methods. Int. J. Biol. Macromol. 2021;169:85–94. doi: 10.1016/j.ijbiomac.2020.12.005. [DOI] [PubMed] [Google Scholar]
- 2.Zargar V., Asghari M., Dashti A. A Review on Chitin and Chitosan Polymers: Structure, Chemistry, Solubility, Derivatives, and Applications. ChemBioEng Rev. 2015;2:204–226. doi: 10.1002/cben.201400025. [DOI] [Google Scholar]
- 3.Gonçalves C., Ferreira N., Lourenço L. Production of Low Molecular Weight Chitosan and Chitooligosaccharides (COS): A Review. Polymers. 2021;13:2466. doi: 10.3390/polym13152466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ahmad M., Mazoor K., Singh S., Ikram S. Chitosan centered bionanocamposites for medical specialty and curative applicatiobs: A review. Int. J. Pharm. 2017;529:200–217. doi: 10.1016/j.ijpharm.2017.06.079. [DOI] [PubMed] [Google Scholar]
- 5.Islam N., Dmour I., Taha M.O. Degradability of chitosan micro/nanoparticles for pulmonary drug delivery. Heliyon. 2019;5:e01684. doi: 10.1016/j.heliyon.2019.e01684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang Y., Zhang R., Qin W., Dai J., Zhang Q., Lee K., Liu Y. Physiochemical properties of gelatin fiilms containing tea polyphenol-loaded chitosan nanoparticle generated by electrospray. Mater. Des. 2020;185:108277. doi: 10.1016/j.matdes.2019.108277. [DOI] [Google Scholar]
- 7.Raorane C.J., Shastri D., Parveen A.S., Haldhar R., Raj V. Complex Inhibits Fluconazole-Resistant Candida albicans Biofilm Formation. Antibiotics. 2022;11:950. doi: 10.3390/antibiotics11070950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ra V., Kim K., Kim Y.-G., Lee J.-H., Lee J. Chitosan gum arabic embedded alizarin nanocarries inhibit biofilm formation of multispecies microorganisms. Carbohydr. Polym. 2022;284:118959. doi: 10.1016/j.carbpol.2021.118959. [DOI] [PubMed] [Google Scholar]
- 9.Jongsri P., Wangsomboondee T., Rojsitthisak P., Seraypheap K. Effect of molecular weights of chitosan coating on postharvest quality and physicochemical characteristics of mango fruit. LWT. 2016;73:28–36. doi: 10.1016/j.lwt.2016.05.038. [DOI] [Google Scholar]
- 10.Choi C., Nam J., Nah J. Appilcation of chitosan and chitosan derivatives as biomaterials. J. Ind. Eng. Chem. 2016;33:1–10. doi: 10.1016/j.jiec.2015.10.028. [DOI] [Google Scholar]
- 11.Chatterjee N.S., Dara P.K., Raman S.P., Vijayan D.K., Sadasivam J., Mathew S., Ravishankar C.N., Anandan R. Nanoencapsulation in low-molecular-weight chitosan improves in vivo antioxidant potential of black carrot anthocyanin. J. Sci. Food Agric. 2021;101:5264–5271. doi: 10.1002/jsfa.11175. [DOI] [PubMed] [Google Scholar]
- 12.Pandit A., Deshpande C., Patil S., Jain R., Dandekar P. Mechanistic insights into controlled depolymerization of Chitosan using H-Mordenite. Carbohydr. Polym. 2019;230:115600. doi: 10.1016/j.carbpol.2019.115600. [DOI] [PubMed] [Google Scholar]
- 13.Chen X., Yang H., Zhong Z., Yan N. Base-catalysed, one-step mechanochemical conversion of chitin and shrimp shells into low molecular weight chitosan. Green Chem. 2017;19:2783–2792. doi: 10.1039/C7GC00089H. [DOI] [Google Scholar]
- 14.Qandil A.M., Marji T.J., Al-Taani B.M., Khaled A.H., Badwan A.A. Depolymerization of HMW into a predicted LMW chitosan and determination of the degree of deacetylation to guarantee its quality for research use. J. Excipients Food Chem. 2018;9:51–63. [Google Scholar]
- 15.Hosseini-Ashtiani N., Tadjarodi A., Zare-Dorabei R. Low molecular weight chitosan-cyanocobalamin nanoparticles for controlled delivery of ciprofloxacin: Preparation and evaluation. Int. J. Biol. Macromol. 2021;176:459–467. doi: 10.1016/j.ijbiomac.2021.02.093. [DOI] [PubMed] [Google Scholar]
- 16.Kapadnis G., Dey A., Dandekar P., Jain R. Effect of degree of deacetylation on solubility of low-molecular-weight chitosan produced via enzymatic breakdown of chitosan. Polym. Int. 2019;68:1054–1063. doi: 10.1002/pi.5795. [DOI] [Google Scholar]
- 17.Soares P.I.P., Sousa A.I., Silva J.C., Ferreira I.M.M., Novo C.M.M., Borges J.P. Chitosan-based nanoparticles as drug delivery systems for doxorubicin: Optimization and modelling. Carbohydr. Polym. 2016;147:304–312. doi: 10.1016/j.carbpol.2016.03.028. [DOI] [PubMed] [Google Scholar]
- 18.Iacob A., Lupascu F., Apotrosoaei M., Vasincu I., Tauser R., Lupascu D., Giusca S., Caruntu I.-D., Profire L. Recent Biomedical Approaches for Chitosan Based Materials as Drug Delivery Nanocarriers. Pharmaceutics. 2021;13:587. doi: 10.3390/pharmaceutics13040587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Minh N.C., van Hoa N., Trung T.S. Handbook of Chitin and Chitosan. Elsevier; Amsterdam, The Netherlands: 2020. Preparation, properties and application of low-molecular-weight chitosan. [Google Scholar]
- 20.Doan V.K., Ly K.L., Tran N.M.P., Thi Ho T.P., Ho M.H., Ngoc-Dang N.T., Chang C.C., Thu Nguyen H.T., Ha P.T., Tran Q.N., et al. Characterizations and antibacterial efficacy of chitosan oligomers synthesized by microwace-assisted hydrogen peroxide oxidative depolymerization method for infectious wound application. Materials. 2021;14:16. doi: 10.3390/ma14164475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aranaz I., Alcántara A.R., Civera M.C., Arias C., Elorza B., Caballero A.H., Acosta N. Chitosan: An Overview of Its Properties and Applications. Polymers. 2021;13:3256. doi: 10.3390/polym13193256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Farias B.S., Grundmann D.D.R., Rizzi F.Z., Martins N.S.S., Junior T.R.S.C., Pinto L.A.D.A. Production of low molecular weight chitosan by acid and oxidative pathways: Effect on physicochemical properties. Food Res. Int. 2019;123:88–94. doi: 10.1016/j.foodres.2019.04.051. [DOI] [PubMed] [Google Scholar]
- 23.Tsao C.T., Chang C.H., Lin Y.Y., Wu M.F., Han J.L., Hsieh K.H. Kinetic study of acid depolymerization of chitosan and effects of low molecular weight chitosan on erythrocyte rouleau formation. Carbohydr. Res. 2011;346:94–102. doi: 10.1016/j.carres.2010.10.010. [DOI] [PubMed] [Google Scholar]
- 24.Aljbour N.D., Beg M.D., Gimbun J. Acid Hydrolysis of Chitosan to Oligomers Using Hydrochloric Acid. Chem. Eng. Technol. 2019;42:1741–1746. doi: 10.1002/ceat.201800527. [DOI] [Google Scholar]
- 25.Savitri E., Juliastuti S.R., Handaratri A., Sumarno. Roesyadi A. Degradation of chitosan by sonication in very-low-concentration acetid acid. Polym. Degrad. 2014;110:344–352. doi: 10.1016/j.polymdegradstab.2014.09.010. [DOI] [Google Scholar]
- 26.Schmitz C., Auza L.G., Koberidze D., Rasche S., Fischer R., Bortesi L. Conversion of Chitin to Defined Chitosan Oligomers: Current Status and Future Prospects. Mar. Drugs. 2019;17:452. doi: 10.3390/md17080452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Purwanto E., Connor J., Ngothai Y. The kinetics oxidative degradatiion of chitosan in formic acid with the presence of hydrogen peroxide. IOP Conf. Ser. Mater. Sci. Eng. 2019;703:012041. doi: 10.1088/1757-899X/703/1/012041. [DOI] [Google Scholar]
- 28.Alves H.J., Vieceli M., Alves C., Muñiz G.I.B., de Oliveira C.L.P., Feroldi M., Arantes M.K. Chitosan Depolymerization and Nanochitosan Production Using a Single Physical Procedure. J. Polym. Environ. 2018;26:3913–3923. doi: 10.1007/s10924-018-1267-7. [DOI] [Google Scholar]
- 29.Liu H., Bao J., Du Y., Zhou X., Kennedy J.F. Effect of ultrasonic treatment on the biochemphysical properties of chitosan. Carbohydr. Polym. 2006;64:553–559. doi: 10.1016/j.carbpol.2005.11.007. [DOI] [Google Scholar]
- 30.Kritchenkov A.S., Zhaliazniak N.V., Egorov A.R., Lobanov N.N., Volkova O.V., Zabodalova L.A., Suchkova E.P., Kurliuk A.V., Shakola T.V., Rubanik V.V., et al. Chitosan derivatives and their based nanoparticles: Ultrasonic approach to the synthesis, antimicrobial and transfection properties. Carbohydr. Polym. 2020;242:116478. doi: 10.1016/j.carbpol.2020.116478. [DOI] [PubMed] [Google Scholar]
- 31.Singh A., Benjakul S., Prodpran T. Ultrasound-Assisted Extraction of Chitosan from Squid Pen: Molecular Characterization and Fat Binding Capacity. J. Food Sci. 2019;84:224–234. doi: 10.1111/1750-3841.14439. [DOI] [PubMed] [Google Scholar]
- 32.Popa-Nita S., Lucas J.-M., Ladavière C., David L., Domard A. Mechanisms Involved During the Ultrasonically Induced Depolymerization of Chitosan: Characterization and Control. Biomacromolecules. 2009;10:1203–1211. doi: 10.1021/bm8014472. [DOI] [PubMed] [Google Scholar]
- 33.Masselin A., Rousseau A., Pradeaau S., Fort L., Gueret R., Buon L., Armand S., Cottaz S., Choisnard L., Fort S. Optimizing chitin depolymerization by lysozyme to long-chain oligasaccharides. Mar. Drugs. 2021;19:6. doi: 10.3390/md19060320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pratama H.B., Supijo M.C. Sutopo Experimental design and response surface method in geothermal energy: A comprehensive study in probabilistic resource assessment. Geothermics. 2020;87:101869. doi: 10.1016/j.geothermics.2020.101869. [DOI] [Google Scholar]
- 35.Damay J., Duret X., Ghislain T., Lalonde O., Lavoie J.-M. Steam explosion of sweet sorghum stems: Optimisation of the production of sugars by response surface methodology combined with the severity factor. Ind. Crop. Prod. 2018;111:482–493. doi: 10.1016/j.indcrop.2017.11.006. [DOI] [Google Scholar]
- 36.Zamri A.I., Latiff N.F., Abdullah Q.H., Ahmad F. Extraction and optimization of chitosa from razor clam (Ensis arcuatus) shell by using response surface methodology (RSM) Food Res. 2020;4:674–678. doi: 10.26656/fr.2017.4(3).308. [DOI] [Google Scholar]
- 37.Li Z., Gerdroodbary M.B., Valipour P., Moradi R., Babazadeh H. The optimization via response surface method for micro hydrogen gas actuator. Int. J. Hydrogen Energy. 2019;44:31633–31643. doi: 10.1016/j.ijhydene.2019.10.015. [DOI] [Google Scholar]
- 38.Li Z., Lu D., Gao X. Optimization of mixture proportions by statistical experimental design using response surface method—A review. J. Build. Eng. 2020;36:102101. doi: 10.1016/j.jobe.2020.102101. [DOI] [Google Scholar]
- 39.Solanki A.B., Parikh J.R., Parikh R.H. Formulation and optimization of piroxicam proniosomes by 3-factor, 3-level box-behnken design. AAPS PharmSciTech. 2007;8:43–49. doi: 10.1208/pt0804086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Beg S., Akhter S. Box–Behnken Designs and Their Applications in Pharmaceutical Product Development. In: Beg S., editor. Design of Experiments for Pharmaceutical Product Development. Springer; Singapore: 2021. pp. 77–85. [DOI] [Google Scholar]
- 41.Rai P., Pandey A., Pandey A. Optimization of sugar release from banana peel powder waste (BPPW) using box behnken design (BBD): BPPW to bioihydrogen conversion. Int. J. Hydrogen Energy. 2019;44:25505–25513. doi: 10.1016/j.ijhydene.2019.07.168. [DOI] [Google Scholar]
- 42.Utami Y.P., Sisang S., Burhan A. Pengukuran Parameter Simplisia Dan Ekstrak Etanol Daun Patikala (Etlingera elation (Jack) R. M. Sm) Asal Kabupaten Enrekang Sulawesi Selatan. Maj. Far. dan Farmakol. Sekol. Tinggi Farm. Makassar. 2020;24:5–10. [Google Scholar]
- 43.Rowe R.C., Sheskey P., Quinn M. Handbook of pharmaceutical excipients. Libros Digitales-Pharmaceutical Press; London, UK: 2009. [Google Scholar]
- 44.Ramadhan L.O.A.N., Radiman C.L., Wahyuningrum D., Suendo V., Ahmad L.O., Valiyaveetiil S. Deasetilasi Kitin secara Bertahap dan Pengaruhnya terhadap Derajat Deasetilasi serta Masa Molekul Kitosan. J. Kim. Indones. 2010;5:17–21. [Google Scholar]
- 45.Lubis H. Pemeriksaan Kandungan Logam Merkuri, Timbal, dan Kadmium dalam Daging Rajungan Segar yang Berasal dari TPI Gabion Belawan Secara Spektrofotometri Serapat Aton. Maj. Kedokt. Nusant. 2008;41:39–47. [Google Scholar]
- 46.Rismana E., Rosidah I., Bunga O., Yunianto P., Erna E. Pengujian Stabilitas Sediaan Luka Bakar Berbahan Baku Aktif Kitosan/Ekstral Pegagan (Centella Asiatica) J. Kim. Terap. Indones. 2015;17:27–37. doi: 10.14203/jkti.v17i1.20. [DOI] [Google Scholar]
- 47.Irnawati. Riyanto S., Martono S., Rohman A. Optimasi Metode Ultrasonik untuk Ekstraksi Minyak Biji Labu Kuning (Cucurbita maxima) The Optimization of Ultrasonic Method for the Extraction of Pumpkin seed (Cucurbita maxima) Indonesian J. Chemom. Pharm. Anal. 2021;1:42–51. [Google Scholar]
- 48.Kasaai M.R. Determination of the degree of N-acetylation for chitin and chitosan by various NMR spectroscopy techniques: A review. Carbohydr. Polym. 2010;79:801–810. doi: 10.1016/j.carbpol.2009.10.051. [DOI] [Google Scholar]
- 49.Vadackova P., Vranikova B., Petra S., Ales F., Jan E., Jan M. Evaluation and comparison of three types of spray dried coprocessed excipient Avicel® for direct compression. Biomed Res. Int. 2018;2018:2739428. doi: 10.1155/2018/2739428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Arjuna A., Natsir S., Khumaerah A.A., Yulianty R. Modifikasi Serat Limbah Kubis Menjadi Nanokristalin Selulosa Melalui Metode Hidrolisis Asam. J. Farm. Galen. 2018;4:119–125. doi: 10.22487/j24428744.2018.v4.i2.11093. [DOI] [Google Scholar]
- 51.Sogias I.A., Khutoryanskiy V.V., Williams A.C. Exploring the Factors Affecting the Solubility of Chitosan in Water. Macromol. Chem. Phys. 2009;211:426–433. doi: 10.1002/macp.200900385. [DOI] [Google Scholar]
- 52.Pakizeh M., Moradi A., Ghassemi T. Chemical extraction and modification of chitin and chitosan from shrimp shells. Eur. Polym. J. 2021;159:110709. doi: 10.1016/j.eurpolymj.2021.110709. [DOI] [Google Scholar]
- 53.Srinivasan H., Kanayairam V., Ravichandran R. Chitin and chitosan preparation from shrimp shells Penaerus monodon and its human ovarium cancar cell line, PA-1. Int. J. Biol. Macromol. 2018;107 Pt A:662–667. doi: 10.1016/j.ijbiomac.2017.09.035. [DOI] [PubMed] [Google Scholar]
- 54.Yen M.-T., Yang J.-H., Mau J.-L. Physicochemical characterization of chitin and chitosan from crab shells. Carbohydr. Polym. 2009;75:15–21. doi: 10.1016/j.carbpol.2008.06.006. [DOI] [Google Scholar]
- 55.Poverenov E., Rips H.A., Zaitev Y., Bar V., Danay O., Horev B., Saiz C.B., McHugh T., Rodov V. Potential of chitosan from mushroom waste to enhace quality and storability of fresh-cut-melons. Food Chem. 2018;268:233–241. doi: 10.1016/j.foodchem.2018.06.045. [DOI] [PubMed] [Google Scholar]
- 56.Ghormade V., Pathan E.K., Deshpande M.V. Can fungi compete with marine sources for chitosan production? Int. J. Biol. Macromol. 2017;104:1415–1421. doi: 10.1016/j.ijbiomac.2017.01.112. [DOI] [PubMed] [Google Scholar]
- 57.Ioelovich M. Crystallinity and Hydrophility of Chitin and Chitosan. Res. Rev. J. Chem. 2014;3:7–14. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.