Abstract
Background:
Recently, a plethora of events have affected the statin arena such as muscle-induced myalgia, myopathy, myositis, rare rhabdomyolysis, and new-onset diabetes. The latest statin pitavastatin has emerged with descent stamina (optimum efficacy and improved safety).
Objective:
The objective of the current review is to explore the pros and cons of pitavastatin as a novel second-generation statin in terms of efficacy and safety that delineate its clinical utility.
Methods:
The review was conducted via EBSCO hosted Medline search (AL Ain University, UAE subscription) for relevant English written literature articles containing “pitavastatin” as the primary search term “pitavastatin and safety;” “pitavastatin and efficacy” and “pitavastatin and safety and randomized clinical trials;” and “pitavastatin and efficacy and randomized clinical trials.”
Results:
The number of articles containing the word “pitavastatin” as the primary search term used was (n = 901). The next retrieves MeSH term was “pitavastatin and safety” (n = 99) and then “pitavastatin and efficacy” (n = 132). Furthermore, narrowing down the search by adding study design terms revealed: “pitavastatin and safety and randomized clinical trials,” (n = 10) and “pitavastatin and efficacy and randomized clinical trials” (n = 13). Combining the two main searches (safety and efficacy) has yielded 23 items, of which 15 articles were satisfying the current mini-review criteria. The prominent efficacy of pitavastatin was depicted by the increase in high-dense lipoprotein cholesterol and a decrease in low-dense lipoprotein cholesterol as illustrated by the clinical trials in the results and discussions section. The safety was enlightened with a very low propensity to cause new-onset diabetes and a low tendency for statin-induced muscular adverse events.
Conclusion:
Pitavastatin might be suitable for patients with the acute coronary syndrome (ACS), metabolic syndrome, and patients with diabetes. We highly recommend rational individualization for the selection of statin, especially in patients with diabetes and/or with ACS.
KEYWORDS: Pitavastatin and efficacy and randomized clinical trials, pitavastatin and efficacy, pitavastatin and safety and randomized clinical trials, pitavastatin and safety, pitavastatin
INTRODUCTION
The HMG-CoA reductase inhibitors, commonly known as statin, have contributed tremendously to improved clinical patient outcomes for both primary and secondary cardiovascular diseases (CVDs). Recently, a plethora of events have affected the statin arena such as muscle-induced myalgia, myopathy, myositis, rare rhabdomyolysis, and new-onset diabetes.
The new drug pitavastatin is an inhibitor of the enzyme HMG-CoA reductase that catalyzes the first step of cholesterol synthesis, resembles the new generation of statin as the latest to be launched.[1] It was first launched (Livazo®) in Japan in 2003, and promoted under license in South Korea and India (Zypitamag®). It is marketed in the United States of America under the trade name Livalo®. Pitavastatin was approved for use in hypercholesterolemia and for the prevention of CVDs outside South and Southeast Asia.[2] It has received Food and Drug Administration (FDA) approval in 2009 and Healthcare products Regulatory Agency in the United Kingdom in 2010.
Earlier in 2011, in patients with hypercholesterolemia (302), the multicenter randomized head-to-head (PATROL) trial has compared pitavastatin (2 mg/day), atorvastatin (10 mg/day), and rosuvastatin (2.5 mg/day) for safety and efficacy. The safety profile was assessed relevant to adverse event rates, (abnormal clinical laboratory variables related to liver and kidney function and skeletal muscle). The study reported no differences in adverse events rates. However, glycated hemoglobin (HbA1c) was increased, while uric acid was decreased in the atorvastatin and rosuvastatin groups. The efficacy profile was assessed by the changes in the levels and patterns of lipoproteins where by the three statin are equally reduced low-dense lipoprotein cholesterol (LDL-C) and LDL particles, as well as fast-migrating LDL (modified LDL) by 40%–45%.[3]
Pitavastatin causes increase in high-density lipoprotein cholesterol (HDL-C) where lower value is an important clinical risk factor for CVDs, has, on the other hand, it demonstrated lower effects on LDL-C and total triglycerides.[4,5,6]
Ethics approval
Not required in the current mini-review.
Objective
The objective of the current mini-review was to explore the pros and cons of pitavastatin as novel second-generation statin in terms of efficacy and safety that delineate its clinical utility.
METHODS
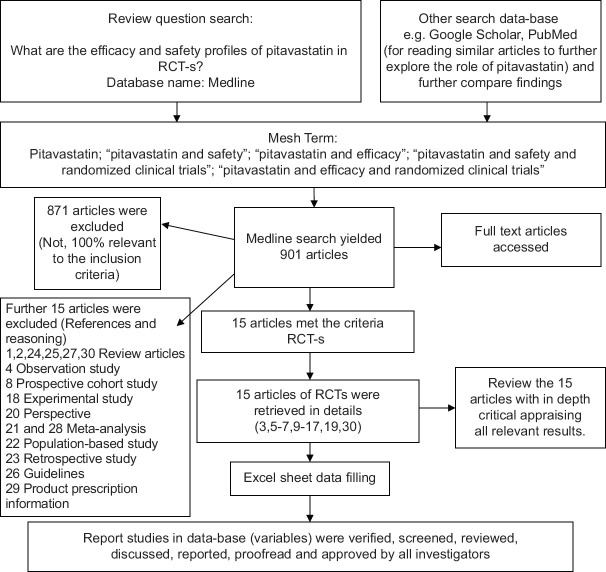
The current mini-review was conducted via EBESCO hosted Medline search (Gulf Medical University, UAE subscription) for relevant English written literature articles containing “pitavastatin” as the primary search term “pitavastatin and safety;” “pitavastatin and efficacy” and “pitavastatin and safety and randomized clinical trials;” and “pitavastatin and efficacy and randomized clinical trials.” The database was retrieved between the years 2001–2020 with the Medical Subject Headings (MeSH) terms.
The total of articles containing “pitavastatin” as the primary search term was (n = 901). The next retrieves MeSH was “pitavastatin and safety” (n = 99) and then “pitavastatin and efficacy” (n = 132). Furthermore, narrowing down the search by adding study design term revealed “pitavastatin and safety and efficacy and randomized clinical trials,” (n = 15). The current review was conducted on the 15 articles yield [Figure 1].
Figure 1.
Flow diagram of included and excluded RCT-s in the current review
RESULTS
The FDA labeled indications for pitavastatin include primary heterozygous familial hypercholesterolemia, and as an adjunct in mixed hyperlipidemia or primary hyperlipidemia.” The compelling indications of the five major statin and the approved FDA labeled use are shown in Table 1. The renal and hepatic dosing of the five major statin was illustrated in, Table 2. While the reported safety profile of the five major statin is depicted in, Table 3.
Table 1.
Comparison between five major statins against the food and drug administration approved compelling indication
| Pitavastatin | Atorvastatin | Simvastatin | Pravastatin | Rosuvastatin | |
|---|---|---|---|---|---|
| Efficacy data | |||||
| FDA approved indication | 1. Mixed hyperlipidemia; adjunct 2. Primary heterozygous familial hypercholesterolemia 3. Primary hyperlipidemia; adjunct | 1. Disorder of cardiovascular system, in patients with multiple risk factors for coronary heart disease; prophylaxis 2. Disorder of cardiovascular system, in patients with multiple risk factors for coronary heart disease; prophylaxis-Type 2 diabetes mellitus 3. Disorder of cardiovascular system, Secondary; prophylaxis 4. Familial hypercholesterolemia-homozygous, adjunct 5. Familial Type 3 hyperlipoproteinemia 6. Hypercholesterolemia, primary (heterozygous familial and nonfamilial) and mixed dyslipidemia (Fredrickson types IIa and IIb) 7. Hypertriglyceridemia | 1. Cardiovascular event risk, in patients with high coronary event risk; prophylaxis 2. Cerebrovascular accident, in patients with high coronary event risk; prophylaxis 3. Familial hypercholesterolemia-heterozygous 4. Familial hypercholesterolemia-homozygous 5. Familial Type 3 hyperlipoproteinemia 6. Hypertriglyceridemia 7. Mixed hyperlipidemia 8. Primary hypercholesterolemia | 1. Cerebrovascular accident, reduction of risk 2. Coronary arteriosclerosis, primary; prophylaxis 3. Coronary arteriosclerosis, secondary; prophylaxis 4. Familial hypercholesterolemia-heterozygous 5. Hyperlipidemia | 1. Disorder of cardiovascular system, primary; prophylaxis 2. Familial hypercholesterolemia-homozygous 3. Familial Type 3 hyperlipoproteinemia 4. Generalized atherosclerosis 5. Hyperlipidemia, primary 6. Hypertriglyceridemia 7. Mixed hyperlipidemia |
FDA: Food and drug administration
Table 2.
Comparison between the five major statins in renal and hepatic dosing
| Safety data (renal dosing/hepatic dosing) | ||||
|---|---|---|---|---|
|
| ||||
| Pitavastatin | Atorvastatin | Simvastatin | Pravastatin | Rosuvastatin |
| Renal impairment: (moderate, GFR 30-59 mL/min/1.73 m2): Initial, 1 mg orally once daily; MAX, 2 mg/day Renal impairment (severe, GFR 15–29 mL/min/1.73 m2, not receiving hemodialysis): Initial, 1 mg orally once daily; MAX, 2 mg/day | Renal impairment: No adjustment necessary | Renal impairment (mild or moderate): No adjustment necessary Renal impairment (severe): Initial, 5 mg orally once daily | Renal impairment (severe): Initial, 10 mg orally once daily | Renal impairment (severe): CrCl <30 mL/min/1.73 m2, nondialysis: Initial, 5 mg orally once daily; MAX 10 mg orally once daily |
| Hepatic impairment: Active liver disease (including unexplained persistent elevations of hepatic transaminases): Use is contraindicated Hemodialysis: Initial, 1 mg orally once daily; MAX, 2 mg/day Concomitant use of erythromycin: MAX, 1 mg/day Concomitant use of rifampin: MAX, 2 mg/day | Hepatic impairment: Contraindicated in patients with active liver disease which may include unexplained persistent elevations in hepatic transaminase levels. Geriatric: Use caution in elderly patients as advanced age is a predisposing factor for myopathy hemodialysis: No adjustment necessary. Concomitant cyclosporine, tipranavir/ritonavir, glecaprevir/pibrentasvir, letermovir with cyclosporine: Avoid use. Concomitant clarithromycin, itraconazole, elbasvir/grazoprevir, saquinavir/ritonavir, darunavir/ritonavir, fosamprenavir, fosamprenavir/ritonavir, or letermovir: Do not exceed atorvastatin doses of 20 mg daily; assess to ensure lowest possible dose is used. Concomitant nelfinavir: Do not exceed atorvastatin doses of 40 mg daily. Concomitant lopinavir/ritonavir: Use the lowest dose necessary of atorvastatin. Concomitant protease inhibitors other than tipranavir, saquinavir, darunavir, fosamprenavir, nelfinavir, or lopinavir: Use appropriate clinical assessment to ensure that the lowest dose necessary of atorvastatin is used | Hepatic impairment: Concomitant lomitapide in patients with homozygous familial hypercholesterolemia: Reduce simvastatin dose by 50%; MAX 20 mg/day, or 40 mg/day for patients who have previously taken simvastatin 80 mg/day chronically (i.e., for 12 months or more) without evidence of muscle toxicity Concomitant verapamil, diltiazem, or dronedarone: MAX 10 mg/day Concomitant amiodarone, amlodipine, or ranolazine: MAX 20 mg/day | Hepatic impairment (active liver disease or unexplained, persistent elevations of serum transaminases): Use is contraindicated Concomitant use of clarithromycin: Limit pravastatin dose to 40 mg orally once daily Concomitant use of cyclosporine: Initial pravastatin dose, 10 mg orally once a day at bedtime; limit pravastatin dose to 20 mg orally once daily | Hepatic Insufficiency: Contraindicated in patients with active liver disease Concomitant cyclosporine Maximum dosage: 5 mg orally once daily |
GFR: Glomerular filtration rate
Table 3.
Comparison between five major statins on common and serious adverse effects
| Safety data: Adverse effects | ||||
|---|---|---|---|---|
|
| ||||
| Pitavastatin | Atorvastatin | Simvastatin | Pravastatin | Rosuvastatin |
| Side effects Common Gastrointestinal: Constipation (1.5%-3.6%), diarrhea (1.5%-2.6%) Musculoskeletal: Backache (1.4%-3.9%), myalgia (1.9%-3.1%), pain in limb (0.6%-2.3%) Serious Endocrine metabolic: Disorder of glucose regulation Hepatic: ALT/SGPT level raised (0.5%), AST/SGOT level raised, increased liver enzymes, liver failure Musculoskeletal: Disorder of muscle, statin-associated, Increased creatine kinase level (1.9%), rhabdomyolysis | Common: Gastrointestinal: Diarrhea (up to 14.1%) Musculoskeletal: Arthralgia (up to 11.7%), myalgia (up to 8.4%) Renal: Urinary tract infectious disease (up to 8%) Respiratory: Nasopharyngitis (8.3%). Other: Pain, in extremity (up to 9.3%) Serious: Dermatologic: Dermatomyositis hepatic: Increased liver enzymes (0.2%-2.3%), liver failure. immunologic: Autoimmune disease, systemic lupus erythematosus. Musculoskeletal: Disorder of muscle, rhabdomyolysis, rupture of tendon. Neurologic: Hemorrhagic cerebral infarction (2.3%) | Common: Gastrointestinal: Abdominal pain (7.3%), Constipation (6.6%), Nausea (5.4%) Neurologic: Headache (2.5%-7.4%) Respiratory: Upper respiratory infection (9%) Serious Hepatic: Cholestatic hepatitis, increased liver enzymes (approximately 1%), jaundice, liver failure Musculoskeletal: Compartment syndrome of lower leg, disorder of muscle (20 mg/day, 0.02%-0.03%; 40 mg/day, 0.08%; 80 mg/day, 0.61%-0.9%), rhabdomyolysis (20 mg/day, 0%; 80 mg/day, 0.4%), rupture of tendon | Common Dermatologic: Rash (1.2%-7.2%) Gastrointestinal: Diarrhea (4.7%-8.5%), nausea and vomiting (4%-10.5%) *Endocrine metabolic: None reported Musculoskeletal: Musculoskeletal pain (3.9%-24.9%). Neurologic: Headache (3.5%-7.5%). Respiratory: Cough (1.2%-8.2%), rhinitis (1.2%-7%), upper respiratory infection (4.1%-21.2%) Serious Gastrointestinal: Pancreatitis. Hepatic: Increased liver enzymes (up to 1.2%) musculoskeletal: Disorder of muscle (<0.1%), rhabdomyolysis, rupture of tendon | Common Gastrointestinal: Abdominal pain (2.4% ), Nausea (2.4%-6.3%) Musculoskeletal: Myalgia (1.9%–12.7% ) Neurologic: Asthenia (0.9%–4.7% ), headache (3.1%-8.5%) Serious Endocrine metabolic Diabetes mellitus (2.8% ), High hemoglobin A1c level, Impaired fasting glucose Gastrointestinal: Pancreatitis Hepatic: Increased liver enzymes (1.1% ), Liver failure Musculoskeletal Disorder of muscle (<1%), rhabdomyolysis, rupture of tendon Renal Acute renal failure, hematuria (<2%), proteinuria (1.5%) |
*There are no reported endocrine disorders (diabetes) with pravastatin. Reference Miromedex. https://www.micromedexsolutions.com/ micromede×2/librarian/CS/DA6074/ND_PR/evidencexpert/ND_P/evidencexpert/DUPLICATIONSHIELDSYNC/D45403/ND_PG/ evidencexpert/ND_B/evidencexpert/ND_AppProduct/evidencexpert/ND_T/evidencexpert/PFActionId/evidencexpert.DoIntegratedSearch? SearchTerm=atorvastatin#. Accessed 10 October 2019. ALT: Alanine transaminase, SGPT: Serum glutamic pyruvic transaminase
The low- and high-intensity doses of statin and reduction in low-dense lipoprotein cholesterol
The high-intensity statin (rosuvastatin 10–20 mg and atorvastatin 40–80 mg) are able to decrease LDL-C by 50.0%–63.0% compared to LDL-C lowering with moderate-intensity statin (30%–49%) dependent on which statin is used. In regard to LDL-C lowering as a moderate-intensity statin pitavastatin in three different doses (1, 2, and 4 mg) does show a slight increase in LDL-C lowering compared to other moderate intensity statin (44.0%–45.0%). The comparisons of pitavastatin with three major statin (atorvastatin, pravastatin, and simvastatin) are depicted in, Table 4.
Table 4.
The comparison between pitavastatin and other statin in reduction of low density lipoprotein-cholesterol
| Efficacy parameter | Pitavastatin 1 mg versus pravastatin 10 mg | Pitavastatin 2 mg versus pravastatin 20 mg | Pitavastatin 4 mg versus pravastatin 40 mg |
|---|---|---|---|
| Treatment differences (95% CI) in LDL-C | 9 (6-12), P<0.001 | 10 (7-13) P<0.001 | 10 (7-10) P<0.001 |
| Mean percent (%) change at week 12 (44% reduction) | |||
| Apo B | −25 | −31 | −37 |
| Triglycerides | −13 | −15 | −22 |
| HDL-C | +1 | +2 | +4 |
|
| |||
| Efficacy parameter | Pitavastatin 2 mg versus atorvastatin 10 mg | Pitavastatin 4 mg versus atorvastatin 20 mg | Pitavastatin was not studied against atorvastatin 40 mg and 80 mg doses |
|
| |||
| Treatment differences (95% CI) in LDL-C (P=NS) | 0 (−3-3) | 1 (−2-4) | - |
| Mean percent (%) change at week 12 (45% reduction) | |||
| Apo B | −30 | −35 | - |
| Triglycerides | −14 | −19 | - |
| HDL-C | +4 | +5 | - |
|
| |||
| Efficacy parameter | Pitavastatin 2 mg versus simvastatin 20 mg | Pitavastatin 4 mg versus simvastatin 40 mg | |
|
| |||
| Treatment differences (95% CI) in LDL-C | 4 (1-7), P=0.014 | 1 (−2-4) P=NS | - |
| Mean percent (%) change at week 12 (44% reduction) | |||
| Apo B | −30 | −35 | - |
| Triglycerides | −16 | −17 | - |
| HDL-C | +6 | +6 | - |
Pitavastatin has demonstrated statistically superior LDL-C reductions compared with pravastatin 10, 20 and 40 mg doses in patients ≥65 years of age. Pitavastatin was not studied against pravastatin 80 mg. Reference[7,8]. Pitavastatin has demonstrated comparable efficacy in lipid lowering to atorvastatin 10 and 20 mg doses. Reference[5,7]. Pitavastatin has demonstrated comparable efficacy in lipid lowering to simvastatin 20 and 40 mg doses. Reference.[6,7] Zypitamag is the Indian version of Livazo (Japan) and Levalo (USA). References.[5,6,7,8] ApoB: Lipoprotein B, CI: Confidence interval, LDL-C: Low-density lipoprotein-cholesterol, HDL-C: High-density lipoprotein-cholesterol, NS: Nonsignificant
Pitavastatin and the new-onset diabetes
The J-PERDICT trial (Japan Prevention Trial of Diabetes by Pitavastatin in Patients With Impaired Glucose Tolerance), pitavastatin has shown reduced risk of new-onset DM by 18.0% in patients with impaired glucose tolerance.[9] The LIVALO Effectiveness and Safety (LIVES) study of the Japanese long-term prospective postmarketing surveillance has demonstrated that pitavastatin reduced HbA1c (from 8.1% at baseline to 7.4% at 6 months) in poorly controlled subjects.[10]
Pitavastatin in major adverse cardiovascular events
The TOHO-LIP study
Recently, in 2020 a randomized, controlled, parallel, and multi-center clinical trial on 664 patients with hypercholesterolemia who had one or more cardiovascular risk factors followed for 240 weeks for major adverse cardiovascular events-MACE. The study reported that pitavastatin (2 mg/day) compared to atorvastatin (10 mg/day) significantly reduced the risk of MACE (2.9% versus 8.1%, HR, 0.366; 95% CI 0.170-0.787; P =0.01), respectively. In the secondary endpoints defined as composite of the primary endpoint event plus clinically indicated coronary revascularization for stable angina, pitavastatin compared to atorvastatin showed significant results (4.5% versus 12.9%, HR = 0.350; 95% CI 0.189-0.645, P = 0.001), respectively.[11]
Pitavastain in acute coronary syndrome
More recently in 2020, 1702 patients derived from the HIJ-PROPER study having acute coronary syndrome (ACS) (multi or single occluded vessel) and dyslipidemia were compared as randomized to either pitavastatin plus ezetimibe (targeting low-density lipoprotein cholesterol [LDL-C] <70 mg/dL) or pitavastatin monotherapy (targeting LDL-C <90 mg/dL). Findings of MACE incidence was significantly higher in the multi-vessel than in the single vessel group (43.7% vs. 25.9%, hazard ratio [HR], 1.95; 95% CI: 1.65-2.31, P <.001). In the single vessel group, pitavastatin + ezetimibe had significantly fewer MACE than pitavastatin mono-therapy (34.6% vs. 47.4%, HR, 0.72; 95% CI: 0.55-0.94, P =0.02).[12]
DISCUSSION
The clinical utility of pitavastatin in major trials
LIVALO effectiveness and safety study
The Japanese large scale (n = 20,279), long-term (104 weeks), prospective post-marketing surveillance study known as “The database of LIVALO effectiveness and safety (LIVES) Study,” reported pitavastatin's effect on HDL-C. This study was conducted in patients treated with pitavastatin for 2 years for hypercholesterolemia.[4]
A high percent (88.2%) of low-risk patients with LDL-C (<160 mg/dL = 4 mmol/L), 82.7% of intermediate-risk (<140 mg/dL = 3.5 mmol/L), 66.5% of high-risk (<120 mg/dL = 3 mmol/L), and 50.3% of secondary prevention patients (<100 mg/dL = 2.5 mmol/L), have achieved the LDL-C target rate as recommended by the Japanese Atherosclerosis Society. A significant reduction of total cholesterol (total cholesterol; −21.0%), LDL-C (−31.3%), and triglycerides (−6.1%) was seen at 104 weeks, in patients with low HDL-C.[5]
A European randomized double-blind, phase III study (12-weeks), compared pitavastatin and simvastatin in regards to LDL-C reduction. Pitavastatin 2 mg/day reduced LDL-C by 39.0% (307), which was significantly greater than that with simvastatin 20 mg/day (35.0%, 107). Furthermore, pitavastatin 4 mg/day reduced LDL-C by 44.0% (319), which was comparable to simvastatin 40 mg/day (42.8%, 110). The major clinical efficacy of pitavastatin relies on low dose persisted long-term reduction in LDL-C and increase in HDL-C with superior effect than comparable statin.[6] Pitavastatin might be suitable for metabolic syndrome and diabetes population.[13] Another identical European study has reported that pitavastatin 2 mg/day reduced LDL-C by 37.9% (315), which was comparable to that of atorvastatin 10 mg/day (37.8%, 102), and that pitavastatin 4 mg/day reduced LDL-C by 44.6% (298) was comparable to atorvastatin 20 mg/day (43.5%, 102).[14]
CHIBA study
In the CHIBA study, the efficacy of pitavastatin was compared with that of atorvastatin in 204 Japanese patients with hypercholesterolemia (total cholesterol, 220 mg/dL or higher), in a short (12-weeks) randomized, multi-centered, open-label trial collaborative study on hypercholesterolemia drug intervention and their benefits for atherosclerosis prevention. Pitavastatin 2 mg/day and atorvastatin 10 mg/day significantly reduced serum LDL-C by 42.6% and 44.1%, and serum triglycerides by 17.3% and 10.7%, respectively. Pitavastatin treatment exhibited an increase of the serum HDL-C (3.2%, P <.033 versus baseline), which was not evident on atorvastatin treatment (1.7%, P <.221 versus baseline).[15]
Pitavastatin and acute coronary syndrome
The REAL-coronary artery disease
In the REAL-coronary artery disease (CAD), Japanese patients (13,054) with stable CAD in prospective, multicenter, randomized, open-label, blinded endpoint study, who achieved LDL-C <120 mg/dL, high-dose (4 mg/day) compared with low-dose (1 mg/day) pitavastatin significantly reduced cardiovascular events.[16]
The COMPACT-coronary artery disease study
The COMPACT-CAD study compared the effects of statin on HDL-C, pitavastatin 2–4 mg/day versus atorvastatin 10–20 mg/day in 129 patients with stable CAD, hypercholesterolemia, and hypo-HDL-cholesterolemia (HDL-C <50 mg/dL) followed for 30 months. The results indicated that treatment with pitavastatin exhibited greater increases in serum HDL-C and ApoAI levels without adverse effects on glucose metabolism, compared with atorvastatin. Treatment with pitavastatin, but not atorvastatin, significantly increased adiponectin levels.[14]
The PIAT study
In the PIAT study, the effects of pitavastatin (2 mg/day) or atorvastatin (10 mg/day) on HDL-C levels (the primary endpoint) in patients with hypercholesterolemia and glucose intolerance were compared in a multicenter, randomized, parallel-group comparison. The results revealed that the percentage change in HDL-C was greater in the pitavastatin than the atorvastatin arm (8.2% versus 2.9%, P = 0.031).[17]
Japanese dose-response trial
The Japanese dose-response trial reported that the LDL-C-lowering effect of pitavastatin after 12 weeks was 34.0% (81) at a dose of 1 mg, 42.0% (75) at a dose of 2 mg, and 47.0% (76) at a dose of 4 mg.[18] Another study reported that 6 months of Pitavastatin at 2 mg/day significantly reduced LDL-C, from 155 to 88 mg/dL, in 45 patients with diabetes.[19]
The safety profile of pitavastatin in major trials
The LIVES sub-analyses examined CVD-related conditions and the effect of pitavastatin on renal function. Patients with chronic kidney disease on pitavastatin for 2 years exhibited elevation from baseline in the estimated glomerular filtration rate (eGFR + 5.4 mL/min/1.73 m2; P <.001)”.[5] Furthermore, 1200 patients out of a total of 5000 patients were evaluated for HbA1c change, HbA1c, which was found to diminish by 0.28% from baseline.[5]
In Korea, 8-week, multicenter, prospective, randomized, open-label, clinical trial, the efficacy and safety of pitavastatin 2 mg/day versus simvastatin 20 mg/day was compared in patients with hypercholesterolemia.[20] Adverse drug reactions (ADRs) occurred in 15.4% of pitavastatin and 37.3% of simvastatin arm. However, no serious ADRs were noted in either group. Furthermore, more than 2-fold elevations over the upper limit of normal of serum creatine kinase were observed in 3.8% (2/52) of pitavastatin and in 9.8% (5/51) of simvastatin. The difference between the two groups was not statistically significant.[20]
In Japan, statin routine clinical practice showed that the rate of muscle-related ADRs associated with pitavastatin was found to be lower than that with atorvastatin. For instance, atorvastatin was found to be associated with a higher risk of musculoskeletal ADRs, including elevation of the serum creatine kinase, as compared with pitavastatin (atorvastatin 144/4805, pitavastatin 154/7930; risk ratio 1.54, confidence interval 1.23–1.93; P =0.0001).[6]
Statin and new-onset diabetes
It has been reported in recent years the relationship between statin use and increased risk of new-onset diabetes mellitus. The FDA has issued a warning on the possible rise in glucose and HbA1c levels, via modifications in the labeling requirement for statin.[21,22,23,24,25,26] In addition, the European Medicines Agency has stated that statin could increase the risk of type 2 diabetes.[27] However, evidence for type 2 diabetes was from post hoc analyses of randomized controlled trials or meta-analysis synthesis derived from largely Western populations that is different from other ethnicities.
There are conflicting results regarding new-onset diabetes associated with statin use in patients without diabetes. In patients with diabetes, there is evidence for the effect of statin in glycemic control (reflected on fasting glucose and HbA1c). The currently available evidence from both clinical and experimental data revealed that statin influences glycemic control and bears the risk of new-onset diabetes.[28]
The first meta-analysis of 15 placebo/statin-randomized controlled trials with pitavastatin, comprising 4815 non-diabetic patients (∼1600 person-years), studying the effect of pitavastatin on glucose metabolism or new-onset diabetes has reported that pitavastatin did not: 1.(adversely affect glucose metabolism (fasting blood glucose and HbA1c) or 2. increase risk of developing diabetes compared to the controls (placebo/other statin).[29] The analyses by type of control, dose, or follow-up has not showed any significant results. Furthermore, the meta-analysis concluded that the long-term benefit was observed for pitavastatin.
The reported results in the current review showed optimum efficacy of pitavastatin that was depicted by the increase in HDL-C and decrease in LDL-C in the diverse population such as patients with cardiovascular risk and ACS. The safety of pitavastatin was enlightened with low propensity to cause new-onset diabetes and the low tendency for statin-induced muscular adverse events (unlike other lipophilic statin it undergoes limited metabolism via the CYP450 pathway).
Summary of the review
The current review has highlighted the efficacy and safety profile of pitavastatin. The emphasis coined the efficacy of pitavastatin in patients with ACS outcome studies. Pitavastatin has demonstrated dose-dependent reductions in LDL-C at lower doses than that exhibited by other low and moderate intensity statin (pravastatin, atorvastatin, and simvastatin). This emphasizes the use of high dose pitavastatin in high-risk subjects. The dose reduction in LDL-C was in the range of (40.0%–50.0%) and the dose increase in HDL-C was higher than other moderate intensity statin. The use of pitavastatin in patients with ACS has shown greater increases in serum HDL-C and lipoprotein A1 (ApoAI) levels without adverse effects on glucose metabolism, compared with atorvastatin. Treatment with pitavastatin, but not atorvastatin, significantly increased adiponectin levels. The safety profile of pitavastatin is indicated by reducing the incidences of new-onset diabetes.
Highlights, implications of key findings and impact on practice statements
Pitavastatin is indicated for adult patients with primary hypercholesterolemia, including heterozygous familial hypercholesterolemia, and blended dyslipidemia, when the response to dietary and other non-pharmacological measures are unsatisfactory.
There is a population of statin users who represent appropriate candidates for pitavastatin. Pitavastatin may be a suitable first-line treatment option for subjects, such as those with renal impairment, mild-to-moderate hepatic impairment, those with multiple medications, and elderly patients given altered pharmacokinetics.
Pitavastatin provides treatment option for patients with ACS, or metabolic syndrome and or diabetes. It is prudent to initiate screening for new-onset diabetes during the start of statin treatment.
Patients should be assessed and monitored thereafter for Type 2 diabetes before initiating statin, to enable risk reduction with lifestyle changes and informed risk.
The healthcare professionals may adopt the pharmacotherapy principles in the selection of statin based on individualized patient's evaluation and for better patient clinical outcomes.
Statin is evident for reducing cardiovascular risk therefore physicians should continue to offer them for their patients who should continue using statin even if diabetes developed during statin therapy and in line with guidelines.
Limitations (strength and weakness)
The main strength of the current review relies on the outlined clinical therapeutic option for the use of pitavastatin in the diverse population such as patients with diabetes and in sub population such as those with coexisting ACS. The main weakness was relevant to the heterogeneity criteria of studies included in the review and the versatile pitavastatin users.
CONCLUSIONS
Pitavastatin provides effective management with increase in HDL-C and decrease in LDL-C. The molecule is well tolerated across a wide population of statin users. Pitavastatin might be suitable for patients with ACS, metabolic syndrome and patients with diabetes. We highly recommend rational individualization for the selection of statin, especially in patients with diabetes and/or with ACS.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Kajinami K, Takekoshi N, Saito Y. Pitavastatin: Efficacy and safety profiles of a novel synthetic HMG-CoA reductase inhibitor. Cardiovasc Drug Rev. 2003;21:199–215. doi: 10.1111/j.1527-3466.2003.tb00116.x. [DOI] [PubMed] [Google Scholar]
- 2.Mukhtar RY, Reid J, Reckless JP. Pitavastatin. Int J Clin Pract. 2005;59:239–52. doi: 10.1111/j.1742-1241.2005.00461.x. [DOI] [PubMed] [Google Scholar]
- 3.Saku K, Zhang B, Noda K, Saku K, Zhang B, Matsunaga A, et al. Randomized head-to-head comparison of pitavastatin, atorvastatin, and rosuvastatin for safety and efficacy (quantity and quality of LDL): The PATROL trial. Circ J. 2011;75:1493–505. doi: 10.1253/circj.cj-10-1281. [DOI] [PubMed] [Google Scholar]
- 4.Kurihara Y, Douzono T, Kawakita K, Nagasaka Y. A large-scale, long-term prospective post-marketing surveillance of pitavastatin (Livalo) – Livalo effectiveness and safety study (LIVES) Jpn Pharmacol Ther. 2008;36:709–31. [Google Scholar]
- 5.Budinski D, Arneson V, Hounslow N, Gratsiansky N. Pitavastatin compared with atorvastatin in primary hypercholesterolemia or combined dyslipidemia. Clin Lipidol. 2009;4:291–302. [Google Scholar]
- 6.Ose L, Budinski D, Hounslow N, Arneson V. Comparison of pitavastatin with simvastatin in primary hypercholesterolaemia or combined dyslipidaemia. Curr Med Res Opin. 2009;25:2755–64. doi: 10.1185/03007990903290886. [DOI] [PubMed] [Google Scholar]
- 7.Zypitamag™ [Prescribing Information] Ahmedabad, India: Cadila Healthcare Ltd; 2018. [Last accessed on 2020 Jan 20]. Available from: https://www.zypitamag.com/comparison-with-other-statins . [Google Scholar]
- 8.Stender S, Budinski D, Gosho M, Hounslow N. Pitavastatin shows greater lipid-lowering efficacy over 12 weeks than pravastatin in elderly patients with primary hypercholesterolaemia or combined (mixed) dyslipidaemia. Eur J Prev Cardiol. 2013;20:40–53. doi: 10.1177/2047487312451251. [DOI] [PubMed] [Google Scholar]
- 9.Odawara M, Yamazaki T, Kishimoto J, Ito C, Noda M, Terauchi Y, et al., editors. In: 73th American Diabetes Association Annual Meeting. Chicago: Illinois: Conference Paper; 2013. Pitavastatin for the delay or prevention of diabetes development in individuals with impaired glucose tolerance. [Google Scholar]
- 10.Huang CH, Huang YY, Hsu BR. Pitavastatin improves glycated hemoglobin in patients with poorly controlled type 2 diabetes. J Diabetes Investig. 2016;7:769–76. doi: 10.1111/jdi.12483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moroi M, Nagayama D, Hara F, Saiki A, Shimizu K, Takahashi M, et al. Outcome of pitavastatin versus atorvastatin therapy in patients with hypercholesterolemia at high risk for atherosclerotic cardiovascular disease. Int J Cardiol. 2020;305:139–46. doi: 10.1016/j.ijcard.2020.01.006. [DOI] [PubMed] [Google Scholar]
- 12.Ogiso M, Yamaguchi J, Kawada-Watanabe E, Koyanagi R, Sekiguchi H, Sakamoto T, et al. Effect of aggressive lipid-lowering therapy in single-vessel vs. Multivessel coronary artery disease patients with acute coronary syndrome – Heart institute of Japan-proper level of lipid lowering with pitavastatin and ezetimibe in acute coronary syndrome (HIJ-PROPER) substudy. Circ Rep. 2020;2:128–34. doi: 10.1253/circrep.CR-19-0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Teramoto T, Shimano H, Yokote K, Urashima M. Effects of pitavastatin (LIVALO Tablet) on high density lipoprotein cholesterol (HDL-C) in hypercholesterolemia. J Atheroscler Thromb. 2009;16:654–61. doi: 10.5551/jat.1719. [DOI] [PubMed] [Google Scholar]
- 14.Kurogi K, Sugiyama S, Sakamoto K, Tayama S, Nakamura S, Biwa T, et al. Comparison of pitavastatin with atorvastatin in increasing HDL-cholesterol and adiponectin in patients with dyslipidemia and coronary artery disease: The COMPACT-CAD study. J Cardiol. 2013;62:87–94. doi: 10.1016/j.jjcc.2013.03.008. [DOI] [PubMed] [Google Scholar]
- 15.Yokote K, Bujo H, Hanaoka H, Shinomiya M, Mikami K, Miyashita Y, et al. Multicenter collaborative randomized parallel group comparative study of pitavastatin and atorvastatin in Japanese hypercholesterolemic patients: Collaborative study on hypercholesterolemia drug intervention and their benefits for atherosclerosis prevention (CHIBA study) Atherosclerosis. 2008;201:345–52. doi: 10.1016/j.atherosclerosis.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 16.Taguchi I, Iimuro S, Iwata H, Takashima H, Abe M, Amiya E, et al. High-dose versus low-dose pitavastatin in Japanese patients with stable coronary artery disease (REAL-CAD): A randomized superiority trial. Circulation. 2018;137:1997–2009. doi: 10.1161/CIRCULATIONAHA.117.032615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Teramoto T, Shimano H, Yokote K, Urashima M. New evidence on pitavastatin: Efficacy and safety in clinical studies. Expert Opin Pharmacother. 2010;11:817–28. doi: 10.1517/14656561003641990. [DOI] [PubMed] [Google Scholar]
- 18.Saito Y, Teramoto T, Yamada N, Itakura H, Hata Y, Nakaya N, et al. Clinical efficacy of NK-104 (Pitavastatin), a new synthetic HMG-CoA reductase inhibitor, in the dose finding, double blind, three-group comparative study. J Clin Ther Med. 2001;17:829–55. [Google Scholar]
- 19.Nomura S, Shouzu A, Omoto S, Inami N, Tanaka A, Nanba M, et al. Correlation between adiponectin and reduction of cell adhesion molecules after pitavastatin treatment in hyperlipidemic patients with type 2 diabetes mellitus. Thromb Res. 2008;122:39–45. doi: 10.1016/j.thromres.2007.08.013. [DOI] [PubMed] [Google Scholar]
- 20.Park S, Kang HJ, Rim SJ, Ha JW, Oh BH, Chung N, et al. A randomized, open-label study to evaluate the efficacy and safety of pitavastatin compared with simvastatin in Korean patients with hypercholesterolemia. Clin Ther. 2005;27:1074–82. doi: 10.1016/j.clinthera.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 21.Goldfine AB. Statins: Is it really time to reassess benefits and risks? N Engl J Med. 2012;366:1752–5. doi: 10.1056/NEJMp1203020. [DOI] [PubMed] [Google Scholar]
- 22.Sattar N, Preiss D, Murray HM, Welsh P, Buckley BM, de Craen AJ, et al. Statins and risk of incident diabetes: A collaborative meta-analysis of randomised statin trials. Lancet. 2010;375:735–42. doi: 10.1016/S0140-6736(09)61965-6. [DOI] [PubMed] [Google Scholar]
- 23.Carter AA, Gomes T, Camacho X, Juurlink DN, Shah BR, Mamdani MM. Risk of incident diabetes among patients treated with statins: Population based study. BMJ. 2013;346:f2610. doi: 10.1136/bmj.f2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cho Y, Choe E, Lee YH, Seo JW, Choi Y, Yun Y, et al. Risk of diabetes in patients treated with HMG-CoA reductase inhibitors. Metabolism. 2015;64:482–8. doi: 10.1016/j.metabol.2014.09.008. [DOI] [PubMed] [Google Scholar]
- 25.Preiss D, Sattar N. Statins and the risk of new-onset diabetes: A review of recent evidence. Curr Opin Lipidol. 2011;22:460–6. doi: 10.1097/MOL.0b013e32834b4994. [DOI] [PubMed] [Google Scholar]
- 26.Navarese EP, Szczesniak A, Kolodziejczak M, Gorny B, Kubica J, Suryapranata H. Statins and risk of new-onset diabetes mellitus: Is there a rationale for individualized statin therapy? Am J Cardiovasc Drugs. 2014;14:79–87. doi: 10.1007/s40256-013-0053-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ryden L, Grant PJ, Anker SD, Berne C, Cosentino F, Danchin N, et al. ESC guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD: The task force on diabetes, pre-diabetes, and cardiovascular diseases of the European society of cardiology (ESC) and developed in collaboration with the European association for the study of diabetes (EASD) Eur Heart J. 2013;34:3035–87. doi: 10.1093/eurheartj/eht108. [DOI] [PubMed] [Google Scholar]
- 28.Betteridge DJ, Carmena R. The diabetogenic action of statins – Mechanisms and clinical implications. Nat Rev Endocrinol. 2016;12:99–110. doi: 10.1038/nrendo.2015.194. [DOI] [PubMed] [Google Scholar]
- 29.Vallejo-Vaz AJ, Kondapally Seshasai SR, Kurogi K, Michishita I, Nozue T, Sugiyama S, et al. Effect of pitavastatin on glucose, HbA1c and incident diabetes: A meta-analysis of randomized controlled clinical trials in individuals without diabetes. Atherosclerosis. 2015;241:409–18. doi: 10.1016/j.atherosclerosis.2015.06.001. [DOI] [PubMed] [Google Scholar]