Abstract
Background and Objectives: Retrograde free venous flaps represent a separate entity among free venous flaps: their physiology is still unclear, but they provide an immediate visible refill after reconnection, with a similar behaviour to conventional flaps. Therefore, the dimensions and the indications of these flaps can be extended beyond what was previously believed, and they can be easily customized, including with respect to tendons and nerves. Nevertheless, they are still debated and regarded as unsafe. Materials and Methods: From 2012 to 2019, we performed 31 retrograde free venous flaps on 31 patients to reconstruct hands, digits, and in one case the heel. All the flaps were arterialized in a retrograde manner; the donor site was the forearm in 28 cases, the foot in 2 cases, and the calf in 1 case. We recorded the size, vein architecture, donor site, donor artery, donor morbidity, function for composite and non-composite flaps, immediate complications, late complications, survival rate, and the number of revisions. We recorded the hand function when appropriate. A total of 10 flaps were also intraoperatively studied with indocyanine green to monitor their hemodynamical behaviour. Results: All the patients were followed for an average of 8 months (6–15). The flap dimensions ranged from 6 cm2 to 136 cm2. All the flaps, except two that had complete necrosis, survived. Two flaps had partial necrosis. There was no correlation between necrosis and the size of the flap, with one case of necrosis and one of partial necrosis in the small flaps (<10 cm2). None of the cases with partial necrosis needed a new flap. Two flaps developed a late arterio-venous shunt that was ligated. Conclusions: The retrograde free venous flaps proved to be a useful tool for complex reconstructions of the hand and extremities. They can provide a large island of pliable skin and composite tissue with tendons and nerves, but surgeons must be aware of some caveats.
Keywords: hand reconstruction, extremities reconstruction, reconstructive microsurgery, flaps, free flaps, free venous flaps, venous flaps, composite flaps
1. Introduction
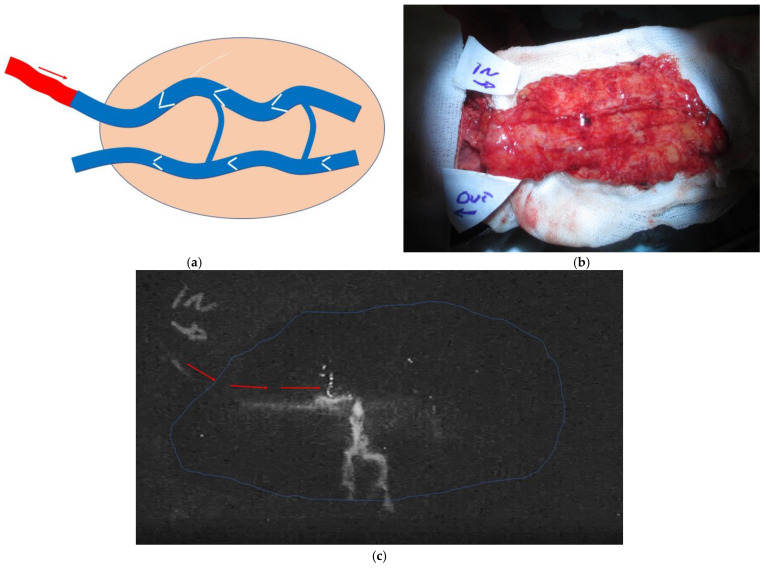
Venous flaps and free venous flaps use a vein as an afferent vessel rather than an artery. The blood drainage is obtained by the same input vein (flowing through the flaps) or by a different vein. These particular flaps have long been known and are mainly used in specific clinical situations such as difficult replantations as a source of acute coverage and as vessel carriers [1,2]. The dimensions of these flaps are normally reduced and they are generally considered unsafe and difficult to monitor as they tend not to show a clinical refill [3]. In general, venous flaps and free venous flaps have long been debated but it has been demonstrated that they can achieve physiological revascularization [4]. Nevertheless, venous flaps are appealing for the simplicity of the harvesting technique, the abundance of donor sites, and the possibility for harvesting these cutaneous flaps as composite flaps including tendons and nerves [2,5,6]. Retrograde free venous flaps represent a separate entity among free venous flaps: in these flaps, the input artery is anastomosed to the designated input vein of the flap in a retrograde manner, i.e., against the valves (Figure 1a). This specific configuration has been shown to be crucial to the behaviour of these flaps, as flaps with this design show an intraoperative visible refill as the incoming blood, once hitting the first valve in the flap, is pushed to the periphery of the flap itself [6,7,8,9] (Figure 1).
Figure 1.
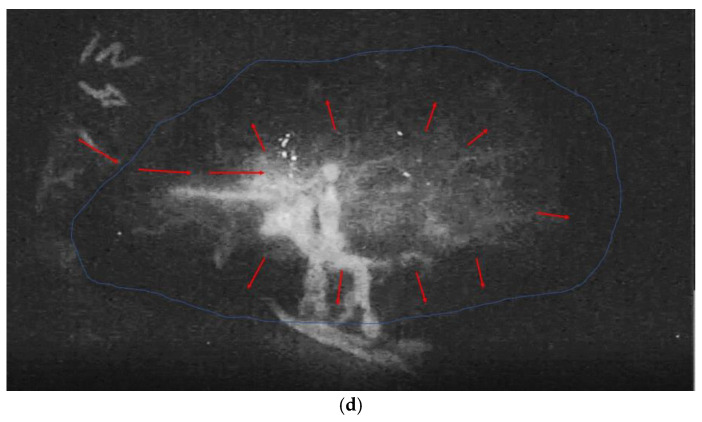
(a) In the retrograde free venous flaps, the input arterial flow (red arrow) is entering the flap through a vein against the valve; (b) In this case, more detailed in Figure 2, the flap was studied upside down with ICG green to document the revascularization pattern once the artery and the draining vein were sutured; (c) the same flap at time 0, when the ICG started flowing into the arterialized vein; (d) The flap after 5 min with diffuse revascularization. The strong stream signal in the centre is a leak from the arterialized vein.
We present our experience with 31 retrograde free venous flaps, including composite flaps, used for the reconstruction of the extremities, with a focus on the surgical technique. We report an extremely high survival rate in medium and large flaps with almost no difficulties in healing. We demonstrate how these flaps can be routinely used in elective situations and report that the survival of these flaps is much larger than was previously believed. Upon reporting our results, we highlight and analyse our cases with their complications in order to contribute to the further understanding of these flaps.
2. Materials and Methods
The Cantonal Ethics Committee of Zurich, Zurich, Switzerland, provided approval (BASEC-Nr. 2017-02001) for this study.
Between January 2012 and December 2020, 31 patients underwent reconstruction of soft tissue defects of the hand or the lower extremities with retrograde arterialized free venous flaps. The patients included 24 men and 7 women, with a mean age of 41 years (range 17 to 47). A total of 10 patients were overweight (body mass index between 25 and 30). There was 1 patient who was obese. A total of 6 patients were smokers. Diabetes and poor patient compliance were the only exclusion criteria.
2.1. Indications and Flap Size
All patients required soft tissue reconstruction to the hand or the distal forearm, except 1 who had an acute soft tissue defect to the ankle. A total of 14 patients had an acute trauma requiring early soft tissue reconstruction. A total of 4 patients were treated acutely for severe infections. The other 13 patients had an elective indication for soft tissue reconstruction. The mean surface area of the flaps was 40 cm2. A total of 16 flaps were small (less than 20 cm2), 9 flaps were medium-sized (24 to 70 cm2), and 6 were large (range 77 to 136 cm2). Details of the patients and defects are summarized in Table 1.
Table 1.
Population of our series, with age, mechanism of the soft tissue defect, size of the defect, and concomitant important structures missing.
| Case Number | Age | Sex | Mechanism | Side, Area to Be Covered | Defect Size cm2 | Concomitant Defects |
|---|---|---|---|---|---|---|
| 1 | 40 | Male | Crush-avulsion | Dorsum left hand | 102 | Extensor tendons dig 2 to 4 |
| 2 | 19 | Male | Infection | Left middle finger Dorsal and volar aspect |
60 | Ulnar digital nerve |
| 3 | 47 | Male | Burr Injury | Left little finger Dorsal aspect |
10 | |
| 4 | 60 | Male | Infection | Left Hand Dorsal aspect |
40 | |
| 5 | 23 | Male | Severe scarring after infection | Left hand 1st web space |
40 | 1st web branches of the radial nerve |
| 6 | 36 | Male | Post traumatic stiffness | Right hand Dorsal aspect index finger |
10 | Proximal interphalangeal joint severe arthritis |
| 7 | 32 | Male | Scar contracture | dorsum left index | 5 | Radial digital nerve |
| 8 | 64 | Female | Infection after cat bite | Left index, dorsal aspect | 9 | |
| 9 | 48 | Male | Degloving injury | Left little finger, circumferential | 84 | |
| 10 | 41 | Female | Open ulna fracture | Left ulna head | 50 | Ulnar artery |
| 11 | 52 | Male | Infection | Right ring finger, dorsal defect | 8 | |
| 12 | 35 | Female | Crush injury | Right hand, dorsal defect to the ring and little finger | 18 | Extensor tendon defect to the little finger withBone defect at the PIP joint |
| 13 | 45 | Female | Avulsion / Friction burn | Left hand, dorsal aspect 2nd and 3rd ray | 90 | Extensor tendon to the index finger |
| 14 | 23 | Male | Burr injury | Left little finger, all the palmar aspect | 21 | |
| 15 | 17 | Male | Explosion | Left middle finger, radial side | 8 | Radial digital nerve of the index finger |
| 16 | 29 | Male | Crush Injury | Right index finger dorsal and volar aspect | 15 | Extensor tendon |
| 17 | 40 | Male | Scar contracture after chemical Burn | Left hand dorsal aspect | 30 | |
| 18 | 27 | Male | Post traumatic scar contracture | Left finger, dorsal aspect | 14 | |
| 19 | 48 | Male | Pseudoarthrosis with unstable scar | Right thumb, dorsal aspect | 15 | Bone defect of the proximal phalanx of the thumb |
| 20 | 51 | Male | Post traumatic stiffness | Left little finger, dorsal aspect | 12 | |
| 21 | 52 | Female | Previous first ray amputation | Right thumb dorsal and vola aspect | 105 | Bone defect |
| 22 | 53 | Female | Infection | Left hand dorsal aspect | 63 | |
| 23 | 44 | Male | Post traumatic skin contracture | Left hand, 1st web space | 45 | 1st web branches of the radial nerve |
| 24 | 42 | Male | Thumb subamputation | Left thumb, dorsal aspect | 9 | Extensor pollicis longus |
| 25 | 32 | Male | Post traumatic stiffness | Right index, dorsal aspect | 10 | |
| 26 | 37 | Male | Burr injury | Left middle finger, radial aspect | 6 | |
| 27 | 47 | Male | Post traumatic stiffness and multiple operations | Left hand, 5th ray, dorsal aspect | 12 | |
| 28 | 47 | Female | Crush injury | Left ring finger, palmar aspect | 6 | |
| 29 | 67 | Male | Crush injury | Left ankle and heel | 76 | |
| 30 | 43 | Male | Soft tissue tumor | Right hand, palm | 12 | |
| 31 | 28 | Male | Explosion | Right thumb, all palmar surface | 21 | Ulnar digital nerve |
| average | 41 | 7f, 26m | 32 |
2.2. Surgical Technique
All patients underwent surgery under general anaesthesia with tourniquet control.
-
(1)
Preparation of the recipient site
In traumatic cases and infections, soft tissues were debrided before harvesting the flap. In extensor teno-arthrolysis, the tenolysis and joint procedures were performed firstly by simply incising the skin. Once this was completed, the affected digit was passively flexed fully, and a template of the missing skin was taken. Then, the feeding artery and the draining veins were prepared for skin reconstruction. A total of 9 flaps were anastomosed to a side branch of the dorsal radial artery and 2 flaps end-to-side to the main dorsal radial artery. A total of 1 flap was anastomosed to the ulnar artery, 5 flaps to a common digital artery, and 5 to a digital artery in the injured finger. The arterial feeding for 8 flaps was sourced from the digital artery harvested from the adjacent digit. One flap was anastomosed end-to-side to the tibial posterior (TP) artery. Dorsal draining veins were used in all cases but 3, where the palmar vein system was used.
-
(2)
Preparation and harvesting of the flap
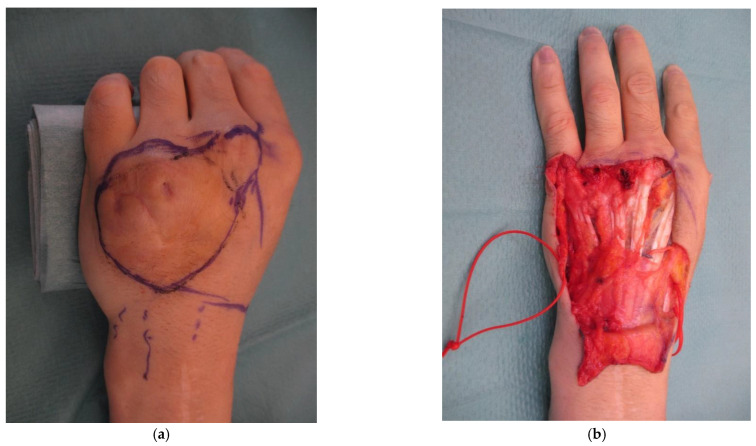
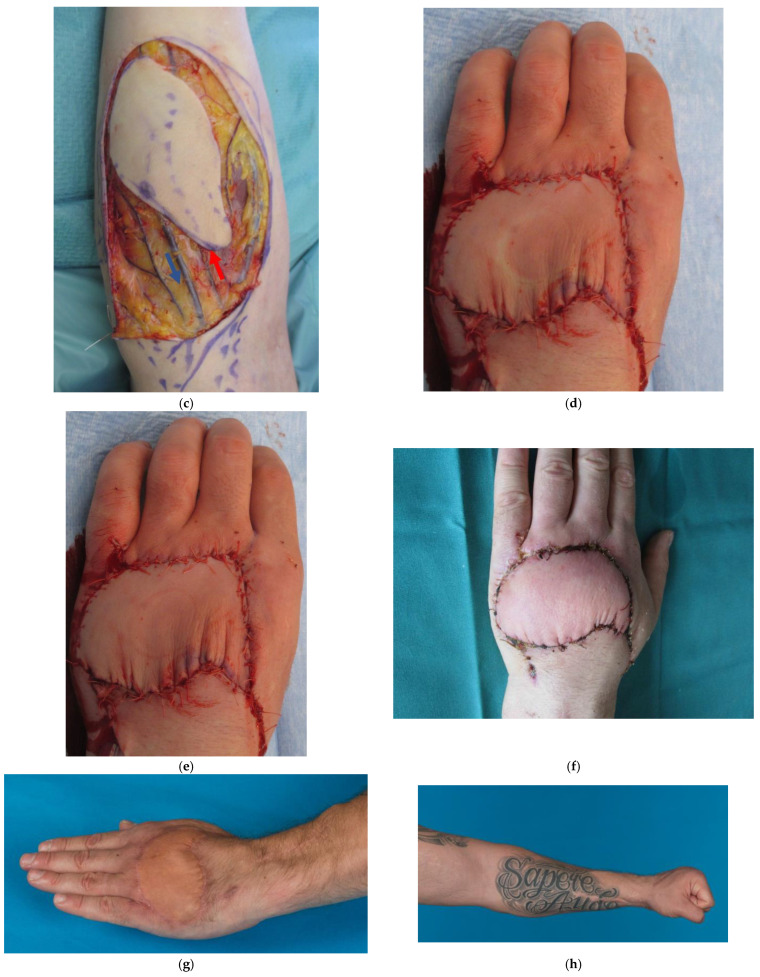
All visible veins were marked before draping the limb. In all cases, a template of the defect was made, and the defect drawn on the flexor aspect of the forearm in its distal third for small defects, on the middle third for medium defects, and on the proximal third for large defects. In 2 cases, glabrous skin from the thenar and hypothenar eminence was included to restore finger pulp. In 2 cases, the flaps were harvested from the foot and in 1 case from the calf. In 3 cases, the contralateral forearm of the injured hand was used as a donor site due to impeding local reasons: in 1 case there was an infection; in 1 case a large tattoo was present, which the patient did not want altered; and in 1 case there was a compromise of the soft tissue in the same forearm. The shape of the flap was always elliptical and oriented obliquely to the donor limb main axis. Such planning of the flap enables the inclusion of more veins and aids primary closure of the donor site. The position of the flap was also influenced by the necessity of harvesting a composite flap including tendons and/or nerves. When possible, 3 or more veins were included, and the most central vein intersecting the flap was selected to become the arterialized feeding vessel in medium and large flaps (Figure 2). Placement of the flap was influenced by the presence of hair, and in some cases, the need to include cutaneous nerves of the forearm and/or tendons in the flap. In this series, 12 flaps were composite flaps with 6 flaps including a tendon and 6 flaps including a nerve.
Figure 2.
(a) 40-year-old male patient with a full thickness chemical burn to the dorsum of the non-dominant left hand and extensor tendon adhesions; (b) Debridement of the burned skin and tenolysis with a resulting defect of 30 cm2 (6 cm × 5 cm); (c) retrograde free venous flap harvested from the contralateral forearm because of large tattoo on the left forearm. Red arrow indicates the vein chosen as the one to be arterialized. Blue arrows indicate the draining vein; (d) flap in place, anastomosed end-to end to a collateral branch of the dorsal radial artery and (e) immediate visible refill (ca. 5 min); (f) situation at 12 days, with no blistering, excessive swelling, or discolouring; (g) results at 6 months with full finger extension; (h) full flexion of the finger was also achieved.
-
(3)
Flap transfer and connection to the hand
When the same forearm was used for the flap donor site, the connecting veins of the flap were divided, and the flap was transferred to the hand without releasing the tourniquet. Haemostasis was then achieved after releasing the tourniquet. End-to-end anastomosis of the feeding and draining vessels was carried out with 10/0 nylon under the microscope for all flaps, except 3, where an end-to-side anastomosis to a main limb artery was performed: 1 tibia posterior artery and 2 radial arteries. The flap vein chosen as feeding vessel was always anastomosed to the feeding artery first, and then the clamps were released to facilitate immediate filling of the vascular tree of the flap. Anastomosis of the draining veins of the flap was then performed. In most cases, these veins were still empty when clamped before the anastomosis. Whenever possible, 2 draining veins were anastomosed for large flaps. After completion of the microsurgery, skin suture was completed. Either 1, 2, or 3 simple capillary drains were placed according to the dimensions of the flap.
A total of 10 patients were injected with indocyanine green intraoperatively (ICG) at 5 mg/mL (ICG Pulsion©, Pulsion Medical Systems, Munich, Germany) and the vascular tree of the flap was observed on the table—this occurred immediately after completing the arterial and venous anastomoses. Video recording with an infrared camera of the vascular tree pattern (Kodak, Rochester, NY, USA) was performed with the flap not yet sutured in place and “upside-down”. The patients were injected again with an additional 1 mL (5 mg) ICG intravenously after the final in-setting to record good flap vascularization.
In all cases, the time between arterial anastomosis and visible refill was recorded. The harvesting time was also recorded. All extra vascular procedures conducted in order to obtain visible refill and good vein drainage were recorded.
2.3. Postoperative Management
2.3.1. Immediate Postoperative Management
The hand, forearm, and elbow were wrapped in a bulky dressing and were elevated for 5 days while the patients remained hospitalized. Mobilization for personal needs was allowed after 72 h. All patients postoperatively received 10,000 u of heparin IV per 24 h for 4 days.
Flap colour, turgor, bleeding, swelling, and refill time were monitored every two hours by a trained nurse and every 4 h by a surgeon for the first 72 h. Any blistering or epidermolysis of the flaps was also recorded. Monitoring by the nurse continued at 3 hourly intervals for the next 2 days.
The first dressing change occurred 5- or 6-days following the operation (range 5 to 7), and compression bandaging and hand therapy was started at 9 days after surgery (range 7 to 12), unless any necrosis was seen. Hand therapy and flap training occurred with the limb at normal level for incremental periods and with the patient performing intermittent circular movements of the whole upper limb in order to change the blood pressure within the flap.
2.3.2. Long-Term Postoperative Management
All flaps were followed clinically for an average of 8 months (range 6 to 15). One flap was studied with indocyanine green at the 6-month follow up.
Assessment
Intra-operative and immediate postoperative assessment included parameters noted in Table 2.
Table 2.
Technical details of the flaps. * In all flaps with a visible refill under the minute, the time was considered as one minute.
| Case Number | Flap Size (cm2) | Length of Arterialized Vein (cm) |
Number of Draining Veins |
Structures Included Other Than Skin |
Harvesting Time (min) |
Time to Visible Refill (min) * |
% Survival | Early Complications | Donor Site Closure |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 105 | 5 | 1 | Palmaris longus Flexor carpi radialis |
102 | 4 | 100% | acute ischemia: switch arter. vein | Partial Skin graft |
| 2 | 77 | 3 | 2 | Lateral cutaneous nerve of the forearm | 60 | 7 | 100% | Direct | |
| 3 | 15 | 2 | 1 | Palmaris longus | 10 | 1 | 100% | Direct | |
| 4 | 55 | 3 | 1 | 40 | 4 | 100% | venous congestion: a-v shunt ligated | Direct | |
| 5 | 50 | 2 | 2 | Medial cutaneous nerve of the forearm | 40 | 10 | 100% | Direct | |
| 6 | 14 | 2 | 1 | 10 | 1 | 100% | intra-operative a-v shunt: immediate ligation |
Direct | |
| 7 | 6 | 1.5 | 1 | Peroneal nerve branch from the foot | 5 | 1 | 100% | Direct | |
| 8 | 14 | 2 | 1 | 9 | 1 | 100% | Direct | ||
| 9 | 110 | 3 | 2 | 84 | 1 | 100% | Skin graft | ||
| 10 | 55 | 2 | 1 | 50 | 15 | 100% | Direct | ||
| 11 | 12 | 1.5 | 1 | 8 | 4 | 100% | draining vein thrombosis: new anastomosis | Direct | |
| 12 | 24 | 3 | 1 | 18 | 1 | 100% | Direct | ||
| 13 | 100 | 3 | 1 | Palmaris longus | 90 | 9 | 100% | Skin graft | |
| 14 | 28 | 2 | 1 | 21 | 30 | 100% | Direct | ||
| 15 | 12 | 2 | 1 | Lateral cutaneous nerve of the forearm | 8 | 12 | 100% | Direct | |
| 16 | 18 | 2 | 1 | Palmaris longus | 15 | 1 | 100% | Direct | |
| 17 | 40 | 3 | 1 | 30 | 1 | 100% | Direct | ||
| 18 | 18 | 2 | 1 | 14 | 1 | 100% | blocking valve: excision and new arterial anastomosis | Direct | |
| 19 | 20 | 3 | 2 | 15 | 1 | 100% | Direct | ||
| 20 | 15 | 2 | 1 | 12 | 1 | 100% | Direct | ||
| 21 | 136 | 3 | 2 | 105 | 25 | 85% | Skin graft | ||
| 22 | 70 | 4 | 2 | 63 | 12 | 100% | Direct | ||
| 23 | 55 | 3 | 1 | Lateral cutaneous nerve of the forearm | 45 | 5 | 100% | Direct | |
| 24 | 11 | 2 | 1 | Extensor longus for the 4th toe | 9 | 1 | 100% | Direct | |
| 25 | 12 | 2 | 1 | 10 | 1 | 100% | Direct | ||
| 26 | 6 | 1 | 1 | 6 | 20 | 100% | Direct | ||
| 27 | 18 | 2 | 1 | 12 | 1 | 100% | Direct | ||
| 28 | 7 | 2 | 1 | 6 | 1 | 0% | venous congestion: draining vein new anastomosis | Direct | |
| 29 | 80 | 3 | 2 | 76 | 60 | 100% | Direct | ||
| 30 | 15 | 2 | 1 | Palmaris longus | 12 | 1 | 0% | venous congestion: draining vein new anastomosis | Direct |
| 31 | 28 | 3 | 1 | Lateral cutaneous nerve of the forearm | 21 | 1 | 95% | Direct | |
| average | 40 | 2.5 | 32 | 7.5 | 92% |
Late postoperative assessment included parameters noted in Table 3.
Table 3.
Late complications and further procedures.
| Case Number | Late Flap Correction |
Late A-V Fistula | Secondary Donor Site Correction | Static 2 Points Discrimination | Secondary Functional Procedures | End Function |
|---|---|---|---|---|---|---|
| 1 | Extensor tenolysis | Full | ||||
| 2 | 7 mm | Extensor tenolysis | MCP 0–80° PIP 15–50° DIP rigid |
|||
| 3 | Extensor tenolysis | MCP 0–90° PIP 5–90° DIP 0–40° |
||||
| 4 | None | Full | ||||
| 5 | 8 mm | None | Full 1st web opening | |||
| 6 | Late PIP Fusion | MCP 0–90° DIP 0–30° |
||||
| 7 | 10 mm | none | Full No pain |
|||
| 8 | Extensor and flexor tenolysis |
MCP 0–90 PIP 30–70 DIP 0–10 |
||||
| 9 | Yes | Shortening of the little finger and neurotomy of digital nerves | MCP 0–80° | |||
| 10 | ||||||
| 11 | None | Full | ||||
| 12 | Yes | Finger separation | D5: MCP 0–90, PIP 10–70°, DIP Rigid. D4: MCP 0–90, PIP 60–100, DIP 10–30 | |||
| 13 | Extensor tenolysis | D2: MCP 25–50°. PIP 0–50°, DIP 0–60° | ||||
| 14 | None | Full | ||||
| 15 | Yes | Yes | Yes | 7 mm | None | Full |
| 16 | Yes | None | Full | |||
| 17 | None | Full | ||||
| 18 | None | MCP 0–90°, PIP 30–80°, DIP 0–10 | ||||
| 19 | None | Kapandji 8 K-pinch 7 kg MCP 0–30. IP rigid |
||||
| 20 | None | MCP 0–90° PIP 30–90° DIP 0–40° |
||||
| 21 | New local flap for the tip of the thumb | Kapandji 7 K-Pinch 6 Kg |
||||
| 22 | Yes | None | Full | |||
| 23 | 7 mm | None | Full 1st web opening | |||
| 24 | None | Kapandji 7 Full 1st web opening |
||||
| 25 | None | MCP 0–90° PIP 20–70° DIP 0–15° |
||||
| 26 | None | Full | ||||
| 27 | None | MCP 0–80° PIP 0–100° DIP 0° 50° |
||||
| 28 | Flap necrosis: new flap | - | ||||
| 29 | None | Full | ||||
| 30 | Flap necrosis: new flap | - | ||||
| 31 | 7 mm | None | Kapandjy 8 MCP 0–40° IP 0–40° K-pinch 6 Kg |
3. Results
Survival rate and functional results are summarized in Table 2 and Table 3.
3.1. Survival, Revision, Early and Late Complications
Out of the 31 flaps, 29 survived with no need for further resurfacing procedures (93.5%).
Out of the 31 flaps, 4 flaps needed revision: 2 eventually survived and 2 failed. These four flaps dimensions were variable and comprised three small flaps and one medium flap.
The 2 flaps that were revised but eventually survived were one small (6 cm2) and one of medium dimensions (40 cm2). The single medium-flap had an early venous congestion and during revision we observed an A-V shunt with a patent draining vein. We ligated the A-V Shunt and then observed a good—not pulsating—flow through the draining vein. The flap had only one vein available for drainage and we did not perform any further procedure.
In the other case, which we revised and in which the flap eventually survived, we found a thrombosis at the level of the draining vein anastomosis. We performed a new anastomosis with an alternative dorsal recipient vein, with success. The flap was small (6 cm2) and there were no other veins available for drainage.
In both these two cases, we postoperatively observed a progressive complete superficial epidermolysis in the first few days. The epidermolysis progressed to superficial skin necrosis in the following weeks, but the fat tissue underneath the flap was clearly viable and survived. Both flaps re-epithelized spontaneously with a good aesthetic and functional result.
Of the two flaps that failed, both had an early venous congestion and were revised within 3 h from primary surgery. In both cases, the draining vein anastomosis was patent, but an A-V Shunt was observed with the draining vein displaying a pulsating arterial flow. In both cases, the A-V shunt was not ligated; however, we performed a new anastomosis of the same draining vein of the flap to a new dorsal and larger receiving vein. The congestion did not resolve, and as both flaps were of small dimensions, there were no other veins available for drainage. They both eventually failed, and we performed a second flap for resurfacing.
Of the 29 flaps that survived, 2 flaps had a partial marginal necrosis. In one case, which represents the largest flap of this series (136 cm2), we recorded a 15% superficial necrosis that eventually healed by secondary intention. The ischemic area of the flap was at the very distal end of the flap in a patient that had a base systolic pressure of 90 mm Hg. In the second case, we had a marginal necrosis of ca 5% that healed spontaneously (Figure 3). This was a large flap of 80 cm2.
Figure 3.
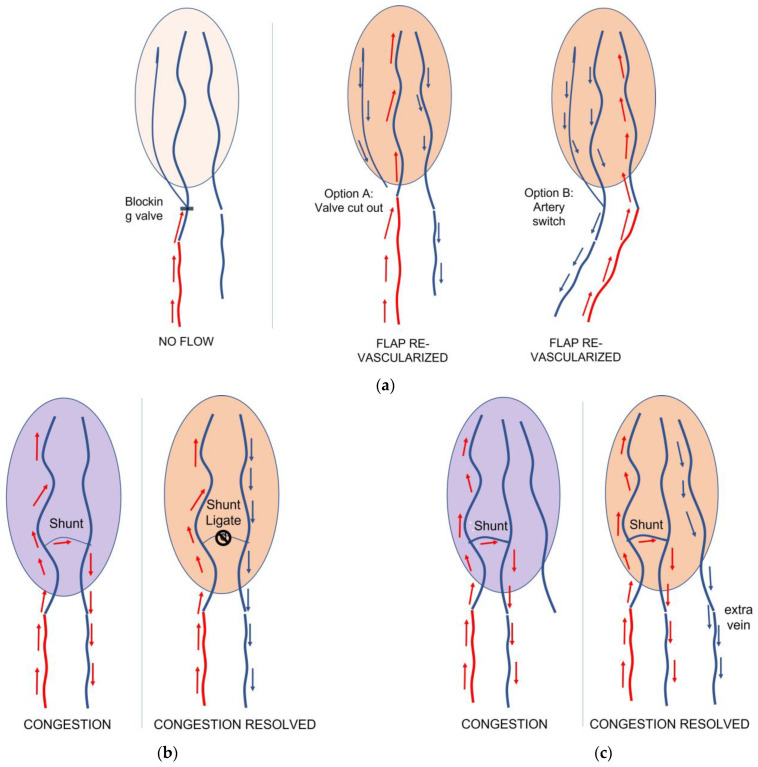
(a) In case of insufficient revascularization of the flap, a blocking valve before the flap can be the cause; accordingly, we suggest performing a more distal anastomosis to the same or to a different vein. Otherwise, we suggest switching the arterialized vein with the draining one as an alternative. (b) In case of congestion due to an Artero-Venous (AV) shunt, ligature is recommended; (c) alternatively, a second draining vein can be anastomosed in order to resolve the congestion.
In one case, the flap suddenly no longer showed visible refill at ca. 6 h postoperatively: at the bedside of the patient, a small hematoma was suspected, and three sutures were released. The hematoma was gently drained, and the small wound left open. The flap survived with no further incidents and the little opening healed by secondary intention.
In one elective case of the first web space, we had a postoperative infection that healed with antibiotics and no further procedures.
In two cases, we observed a late A-V shunt when the flap was already healed and the function was restored. In these two cases, with the pulsatile vein just under the skin paddle, we decided to ligate the A-V shunt to avoid major bleeding in case of future superficial injuries. The ligature performed 6 months from primary surgery did not alter the flap.
3.2. Harvesting Time and Time to Visible Refill
We recorded a harvesting time for this series with an average of 43 min (range 34–60). The flap that took the longest was the large flap harvested from the calf, as it included two vein systems (superficial and deep) of different calibres.
The time between arterial anastomosis and visible refill was an average of 7 min, from a range of 1–65. The median value was 1 min, showing a tendency for an early visible refill. The flap that showed a visible refill with 60 min was the one on the calf.
3.3. Intraoperative Extra Microvascular Procedures
In two cases, a valve in the feeding anastomosed vein was observed blocking the input arterial flow before entering the fat and skin paddle. This resulted in only part of the flap showing a slow visible refill. In one case, partial and insufficient vascularization was confirmed by the ICG. In these two cases, the vein chosen as a feeding vein was swept with the draining vein and two new anastomoses were performed. We then observed a full visible refill all over both flaps, and they survived without any further complications. In three cases, an immediate A-V shunt was observed intraoperatively after the venous anastomosis. In two cases, the A-V shunt was immediately ligated: we then observed a good, but not pulsating, flow through the draining vein. In the other case, we anastomosed a second vein with success and with no shunt. All flaps survived with no further complications.
3.4. ICG Results
A total of 10 flaps were studied intraoperatively with ICG, firstly “upside-down” (Figure 1b) to visualize the immediate vascular tree and the optimal vascularization after the in-setting. In eight flaps, we observed the signal of the input arterial blood entering the arterialized vein through the first valve. At this point the signal was disappearing, but it reappeared shortly after at the dermal margins of the flap. These eight flaps showed a clinically visible refill on inspection after the operating room lights were switched back on. This behaviour might suggest the presence of a small artero-venous shunt between the venous and arterial system, probably at one of the two vascular plexuses: the deeper at the border between the subdermal fat and dermal layer, or the dermal plexus.
One flap showed peripheral revascularization at the dermal layer, but also a clear A-V shunt within the flap between the arterialized vein and the draining vein. This was a small flap, and no other veins were available in the flap for anastomosis. It was decided to reperform the draining vein anastomoses with a larger dorsal vein. The ICG behaviour did not alter. The flap eventaully failed.
One flap showed a very slow flow of the aterialized vein before entering the flap and an incomplete signal of the dermal layer. With the lights switched on, the flap showed a partially visible refill only in the part of the flap close to the entry point of the arterialized vein. We decided to switch the draining vein to the feeding artery, and we used a third vein as a draining vein. The ICG showed a good revascularization and survived with no further problems.
3.5. Donor Site Morbidity
Four flaps were closed partially using a skin graft. All of them were large flaps (>70 cm2). Two patients (case 13 and case 21) were not satisfied with the appearance of the donor site and one of them asked for a surgical correction while the other one (case 21) did not want any correction. One patient (case 22), with a relatively large flap that was harvested from the contralateral side, was not satisfied about the appearance of the donor site after direct closure because of a mild dog ear. This patient asked for surgical correction. None of the patients that had a composite flap including cutaneous nerves of the forearm developed neuropathic pain nor complained about the limited impaired sensation in the affected part of the forearm. None of the patients that had a composite flap including tendons complained about impaired postoperative hand or forearm function.
4. Discussion
Venous flaps and free venous flaps have long been known and debated. The main concern has always been the amount of unpredictability regarding the survival and difficulties in monitoring [10]. The literature is also not univocal, with several papers publishing good results and viability for these flaps but failing to explain their physiology in a satisfactory manner [10,11,12]. On top of this, in the last few years the hype has been mainly about the superficial circumflex inferior perforator (SCIP) flap. The SCIP flap is a very versatile flap, relatively easy to harvest, and one that can offer a pliable thin skin with minor donor site morbidity and the possibility of including a vascularized bone graft [13]. Nevertheless, retrograde free venous flaps still offer several advantages, such as the following:
-
(1)
They are easy and fast to harvest [14], with the possibility of easily including tendons and nerves for complex reconstructions. Differently from other configurations of free venous flaps, the input and output vessels are on the same side of the flap, crucially assisting the surgeon in the in-setting of the flap and in the set-up of the vessels’ configuration.
-
(2)
They offer pliable skin, especially when harvested from the forearm, the calf, or the dorsum of the foot. In case of patients with a very high BMI, they can be harvested from the foot as pliable and thin flaps that can also be composite. The need for pliable skin seems to be relevant in a Caucasian population.
-
(3)
They can be harvested from potentially every part of the body as counterpart flaps of their conventional version, making them easily available in specific situations in which a proximity-free tissue transfer is needed.
In retrograde arterialized free venous flaps, the input arterial flow is pushed by the closed valves to the periphery of the flap. This mechanism produces an interesting, and until now inexplicable, phenomenon, i.e., the visible revascularization of these flaps with a visible refill. (Figure 2)
We report in our series a relatively high rate of survival, and the success of the large retrograde venous flaps. We have summarized our finding in the following points, hoping to contribute to the understanding, and use, of these specific flaps.
Survival: Our rate of survival is 93%. We lost two flaps that both showed venous congestion, which was due to an A-V shunt that in these two cases was not ligated. In both these flaps, we re-anastomosed the draining vein to a new recipient vein. This solution has been shown to be inadequate. In all the other flaps that showed an A-V shunt that was ligated, or when we anastomosed an extra draining vein, the flaps survived. Arguably, if we could have performed an adequate ligature of the A-V shunt in the two flaps we lost, we could have potentially had an even higher survival rate. As already reported [15], the ligature of an immediate A-V shunt seems to be essential for these flaps to survive. As the difference between a proper and an unwanted A-V shunt and a fine vessel’s connection between the input and output system might be difficult to recognize intraoperatively, in the case of a true A-V shunt, we noticed that (a) the flap becomes rapidly congested, with a visible refill under 1 s; (b) the draining vein is strongly pulsating in a synchronized manner with the arterialized vein; and (c) by leaving the vessels connected and flipping the flap upside down, it is very easy to spot under magnification the A-V shunt.
Design: Analysing the different flap designs of our series, we established the following set of guidelines for the design of retrograde arterialized free venous flaps. The flap dimension should be slightly larger than the defect: the flaps tend to swell after surgery, regardless of their good venous drainage. An oblique design to the main axis of the donor limb is preferable for a better direct closure and at the same time allowing for the possibility of including more veins. The best possible configuration that helps direct skin closure is having the main axis of the flap oriented from proximal/ulnar to distal/radial. Preferably, the arterialized vein should be at the centre of the flap, but this is not mandatory. If the vein chosen to be arterialized has some side branch before entering the flap, beware, as it is most likely that a valve outside the flap will be present at that point, compromising the good vascularity of the flap. In case the flap does not include many veins, a connecting branch between the feeding arterialized vein and the draining vein should always been checked for an immediate A-V shunt and—as already suggested in the literature—ligated. In our experience, it is preferable to anastomose two draining veins, if possible.
Monitoring and Revision: The clinical monitoring process is identical to conventional free flaps: the clinical disappearance of the refill is correlated with a stop in the input flow. A congestion of the flap with swelling, darkening of the skin with darker spots at the margins, profuse marginal bleeding, and hypothermia are signs of a venous problem due to a thrombosis of the draining vein or to a high flow A-V shunt within the flap. These are indications for immediate revision, exactly as in conventional fascio cutaneous flaps. As obvious as this might sound, care should be taken not to judge a clinical venous congestion of a retrograde arterialized free venous flap as para-physiological: these flaps’ hemodynamic nature is very different from the more conventional anterograde arterialized venous flaps, and they closely reproduce the aspect and the behaviour of a conventional fascio cutaneous free flap. As a difference to conventional fascio cutaneous flaps, different refill speeds have been observed, from 1.5 to 3 s, with different refill speeds within large flaps. The refill speed per se did not seem to be a relevant prognostic sign for predicting congestion, but rather the change of refill speed over time. In Figure 3, we summarized our suggestions in case of insufficient blood input or in case of a A-V Shunt.
Dimension: In the literature, free venous flaps are considered small if their surface is of 10 cm2 and large if their surface is 25 cm2 [2]. We slightly changed this definition and in our series we considered only nine flaps as large (77–136 cm2). If we were utilizing the classification used by Woo, our series would actually include 15 large flaps. This finding is in our opinion, of paramount importance, as most of the literature is focused on small venous flaps, with few examples of flaps of greater dimension [7]. In our series, none of the large flaps failed, and to us, larger flaps are safer than small flaps. This convinced us to use them in elective cases as well, and in our series of 31 flaps, 13 had an elective indication (Figure 4).
Figure 4.
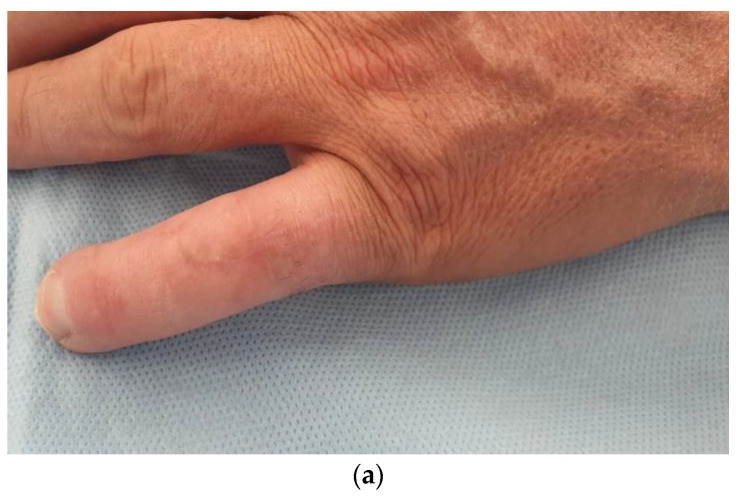
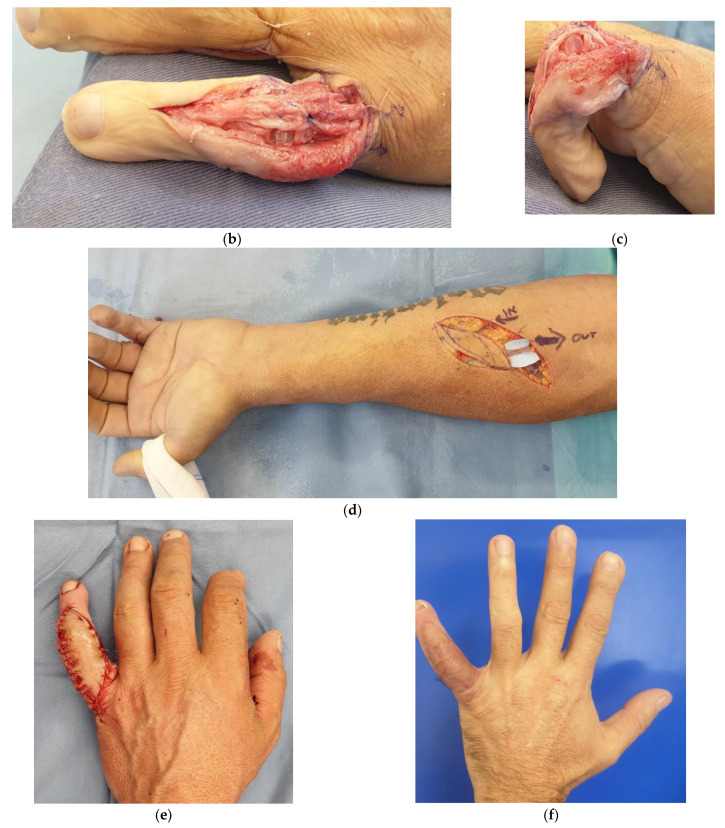
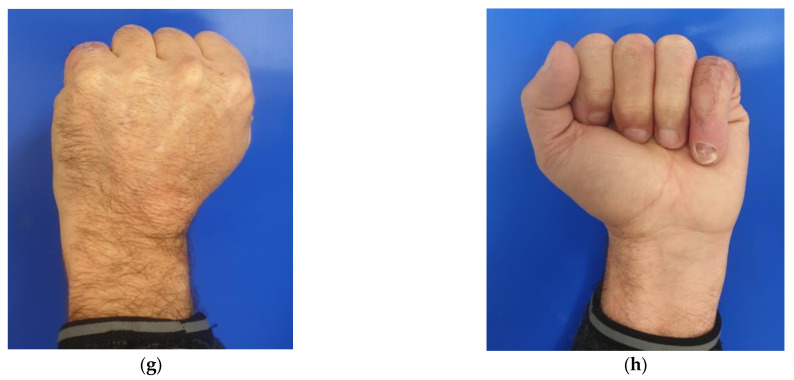
(a) 51 years old male patient with retracted skin, extensor tendon adhesions, and proximal interphalangeal joint post traumatic arthritis to the dorsum of the non-dominant left little finger; (b,c) tenoarthrolysis, joint replacement with a silastic implant, and reinforcement of the extensor tendon—the passive possible maximum flexion is shown; (d) harvesting of a retrograde free venous flap from the same forearm; (e) reconstruction of the skin of the dorsum of the finger—the arterialized vein is anastomosed end to end to the digital ulnar artery of the ring finger, dissected, and moved to the little finger; (f–h) result at 6 months with good finger extension and good flexion.
Physiology: The vascular supply to the venous flaps’ skin island has been reported in the published literature as coming mainly from the dermal plexus [16]. Nevertheless, the exact physiological behaviour of the retrograde free venous flaps has not yet been clearly demonstrated [4,8,11]. Several elements are worth consideration: recent studies have shown that the ligature of a vein in an anterograde free venous flap, where the input blood is running in favour of the valves, reproduces the same effect as in retrograde free venous flaps: a visible clinical refill [11]. In our flaps studied with ICG, we observed that the fluorescence of the incoming arterial blood was “disappearing” from the arterialized vein at the level of the first venous valve, and then “re-appearing” at the dermal layer from bleeding dermal spots. The reappearance of the fluorescence at the dermal layer was associated with the visible refill of the skin paddle of the flap. These two factors suggest that at the level of the subdermal or the dermal plexus, there is a possible switch of the incoming arterial blood from the vein system to the arterial system. Simplicity would suggest that this should happen at the level of the first encountered plexus, i.e., the deep plexus between the fat tissue and dermal layer. This interpretation is corroborated by two further elements we recorded: (1) The fastest refill time close to the entering point of the arterialized vein in large flaps suggests that close to that area, the venous draining system is somehow “congested”. To us, the simplest answer would be that close to the arterialized vein entering point the drainage is running through only one of the two systems, most likely the small veins of the dermal plexus. (2) In two flaps we revised because of venous congestion, we observed afterwards a progressive epidermolysis that progressed to skin necrosis, while the whole subcutaneous tissue of the flap survived. The two patients did not need any further operation as the defect re-epithelialized spontaneously. This differing behaviour between the dermal and subdermal layer suggests a dualism of hemodynamic function in these flaps.
Indications: Our indications ranged from acute traumatic, to acute infection, and to elective scar contractures and 1st web contractures. Arguably, the most interesting and novel indications were the elective ones, i.e., the possibility of performing large 1st web reconstruction with sensate skin, the possibility of performing dorsal teno-arthrolysis of the digits with pliable and thin skin, and the possibility of including tendons at ease.
5. Conclusions
Arterialized retrograde free venous flaps have been shown to behave differently from other configurations of venous flaps and to be safe. They are a useful tool in the hand of the microsurgeon and can be considered as a primary tool for reconstructing complex composite defects in the extremities. Nevertheless, their physiology is still unclear and further research is needed to help further understand their behaviour
Author Contributions
Conceptualization, methodology, original draft preparation, T.G.; validation and writing—review and editing, O.P.; resources and supervision, M.C. and I.T. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Cantonal Ethics Committee of Zurich (BASEC-Nr. 2017-02001, date of approval 23 November 2017) for further use of human clinical data.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study. Written informed consent has been obtained from the patient(s) to publish this paper.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Tsai T.M., Matiko J.D., Breidenbach W., Kutz J.E. Venous flaps in digital revascularization and replantation. J. Reconstr. Microsurg. 1987;3:113–119. doi: 10.1055/s-2007-1006973. [DOI] [PubMed] [Google Scholar]
- 2.Woo S.H., Kim K.C., Lee G.J., Ha S.H., Kim K.H., Dhawan V., Lee K.S. A retrospective analysis of 154 arterialized venous flaps for hand reconstruction: An 11-year experience. Plast. Reconstr. Surg. 2007;119:1823–1838. doi: 10.1097/01.prs.0000259094.68803.3d. [DOI] [PubMed] [Google Scholar]
- 3.De Lorenzi F., van der Hulst R.R., den Dunnen W.F., Vranckx J.J., Vandenhof B., Francois C., Boeckx W.D. Arterialized venous free flaps for soft-tissue reconstruction of digits: A 40-case series. J. Reconstr. Microsurg. 2002;18:569–574. Discussion 575–567. doi: 10.1055/s-2002-35093. [DOI] [PubMed] [Google Scholar]
- 4.Pittet B., Quinodoz P., Alizadeh N., Schlaudraff K.U., Mahajan A.L. Optimizing the arterialized venous flap. Plast. Reconstr. Surg. 2008;122:1681–1689. doi: 10.1097/PRS.0b013e31818cbef1. [DOI] [PubMed] [Google Scholar]
- 5.Woo S.H., Lee Y.K., Kim J.Y., Kim Y.W., Cheon H.J. Palmaris longus tendocutaneous arterialized venous free flap to reconstruct the interphalangeal collateral ligament in composite defects. J. Hand Surg. Eur. Vol. 2018;43:518–523. doi: 10.1177/1753193417735455. [DOI] [PubMed] [Google Scholar]
- 6.Giesen T., Forster N., Kunzi W., Giovanoli P., Calcagni M. Retrograde arterialized free venous flaps for the reconstruction of the hand: Review of 14 cases. J. Hand Surg. Am. 2014;39:511–523. doi: 10.1016/j.jhsa.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 7.Koch H., Scharnagl E., Schwarzl F.X., Haas F.M., Hubmer M., Moshammer H.E. Clinical application of the retrograde arterialized venous flap. Microsurgery. 2004;24:118–124. doi: 10.1002/micr.20011. [DOI] [PubMed] [Google Scholar]
- 8.Moshammer H.E., Schwarzl F.X., Haas F.M., Maechler H., Pierer G., Wiltgen M., Koch H. Retrograde arterialized venous flap: An experimental study. Microsurgery. 2003;23:130–134. doi: 10.1002/micr.10108. [DOI] [PubMed] [Google Scholar]
- 9.Kong B.S., Kim Y.J., Suh Y.S., Jawa A., Nazzal A., Lee S.G. Finger soft tissue reconstruction using arterialized venous free flaps having 2 parallel veins. J. Hand. Surg. Am. 2008;33:1802–1806. doi: 10.1016/j.jhsa.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 10.Yan H., Brooks D., Ladner R., Jackson W.D., Gao W., Angel M.F. Arterialized venous flaps: A review of the literature. Microsurgery. 2010;30:472–478. doi: 10.1002/micr.20769. [DOI] [PubMed] [Google Scholar]
- 11.Lin Y.T., Henry S.L., Lin C.H., Lee H.Y., Lin W.N., Lin C.H., Wei F.C. The shunt-restricted arterialized venous flap for hand/digit reconstruction: Enhanced perfusion, decreased congestion, and improved reliability. J. Trauma. 2010;69:399–404. doi: 10.1097/TA.0b013e3181bee6ad. [DOI] [PubMed] [Google Scholar]
- 12.Yan H., Fan C., Zhang F., Gao W., Li Z., Zhang X. Reconstruction of large dorsal digital defects with arterialized venous flaps: Our experience and comprehensive review of literature. Ann. Plast. Surg. 2013;70:666–671. doi: 10.1097/SAP.0b013e3182433575. [DOI] [PubMed] [Google Scholar]
- 13.Yamashita S., Hattori Y., Tomioka Y., Kurita M., Miyamoto S., Iida T., Okazaki M. Superficial Circumflex Iliac Perforator-Osteocutaneous Flap for Reconstruction of Extensive Composite Defects in the Forefoot. Plast. Reconstr. Surg. Glob. Open. 2020;8:e3076. doi: 10.1097/GOX.0000000000003076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Walle L., Vollbach F.H., Fansa H. Arterialized venous free flaps for resurfacing hand and finger defects. Handchir. Mikrochir. Plast. Chir. 2013;45:160–166. doi: 10.1055/s-0033-1349102. [DOI] [PubMed] [Google Scholar]
- 15.Lombardo G.A.G., Tamburino S., Tarico M.S., Perrotta R.E. Reverse Flow Shunt Restricted Arterialized Venous Free Flap. J. Hand Surg. Am. 2018;43:492.e1–492.e5. doi: 10.1016/j.jhsa.2018.02.023. [DOI] [PubMed] [Google Scholar]
- 16.Galumbeck M.A., Freeman B.G. Arterialized venous flaps for reconstructing soft-tissue defects of the extremities. Plast. Reconstr. Surg. 1994;94:997–1002. doi: 10.1097/00006534-199412000-00013. [DOI] [PubMed] [Google Scholar]