Abstract
This study aimed to identify ticks infesting dogs admitted to the Potchefstroom Animal Welfare Society (PAWS) and to detect tick-borne pathogens they are harbouring. A total of 592 ticks were collected from 61 stray dogs admitted to PAWS originating from several suburbs in and near Potchefstroom, South Africa. The dog ticks were identified as Haemaphysalis elliptica (39%) and Rhipicephalus sanguineus (61%) by both morphological and DNA analyses. Of these ticks, H. elliptica consisted of 67.5% (156/231) and 32.5% (75/231) female and male ticks, respectively, whilst R. sanguineus consisted of 48.5% (175/361) and 51.5% (186/361) female and male ticks, respectively. Microscopic examination of blood smears from engorged female ticks indicated overall occurrences of 0.5% (1/204) for Babesia spp. from R. sanguineus, 1% (2/204) of Anaplasma spp. from H. elliptica, and 22% (45/204) of Rickettsia spp. from both H. elliptica and R. sanguineus. Using pooled samples molecular detection of tick-borne pathogens indicated overall occurrences of 1% (1/104) for A. phagocytophilum in H. elliptica, 9.6% (10/104) of Rickettsia spp. in H. elliptica and R. sanguineus, 5.8% (6/104) of Ehrlichia canis in H. elliptica and R. sanguineus, and 13.5% (14/104) of Coxiella spp. in both H. elliptica and R. sanguineus. Additionally, PCR detected 6.5% (2/31) of Coxiella spp. DNA from H. elliptica eggs. Our data indicate that urban stray dogs admitted at PAWS are infested by H. elliptica and R. sanguineus ticks which are harbouring several pathogenic organisms known to cause tick-borne diseases.
Keywords: Anaplasma phagocytophilum, Babesia spp., Coxiella spp., Rickettsia spp., Ehrlichia canis, Haemaphysalis elliptica, Rhipicephalus sanguineus
1. Introduction
Ticks are blood feeding acarines with body sizes ranging from 2–30 mm infesting domestic and wild animals [1]. To date there are four described families of ticks including Ixodidae, Argasidae, Nuttalliellidae, and the extinct Deinocrotonidae [2,3,4]. Ticks are of great medical and veterinary significance due to their ability to transmit several pathogenic microorganisms to human and animal hosts [5,6]. These tick-borne pathogens are transmitted to other ticks and hosts associated with ticks by transovarial and transstadial transmission [7,8].
Ticks primarily feeding on companion animals may feed on humans in the absence of preferred hosts, resulting in the incidental transmission of tick-borne pathogens to humans [9]. In addition, these ticks are well adapted to urban environments, to the extent that female ticks deposit eggs in cracks of walls or inside dog bedding, resulting in tick infestations among human settlements [7]. Several tick-borne pathogens from ticks infesting dogs that are well-documented include Anaplasma phagocytophilum which is associated with human and canine granulocytic anaplasmosis, Babesia spp. causing canine babesiosis, Coxiella spp. causing Q-fever in humans, Ehrlichia canis associated with canine ehrlichiosis, as well as Rickettsia spp. causing African tick bite fever, Mediterranean spotted fever, and Astrakhan fever [7,10,11,12].
There is always a need to monitor and document ticks infesting urban dogs and their associated tick-borne pathogens due to their close association with human beings, particularly using modern DNA based techniques. This study aimed to identify ticks and their associated tick-borne pathogens, infesting stray dogs housed at the Potchefstroom Animal Welfare Society (PAWS) which originated from urban settlements. Ticks and tick-borne pathogens were identified by using a combination of morphological and molecular methods.
2. Results
2.1. Identification of Ticks
A total of 592 ticks were collected from 61 dogs admitted to PAWS originating from several suburbs in Potchefstroom (Table 1). These ticks were morphologically identified as H. elliptica (GTTM voucher accession number: OP5113) and R. sanguineus (GTTM voucher accession number: OP5078). The overall occurrence of H. elliptica was 39% (231/592), where R. sanguineus had an overall occurrence of 61% (361/592). In the respective species, 67.5% (156/231) and 48.5% (175/361) were female, and 32.5% (75/231) and 51.5% (186/361) were male. Of these ticks H. elliptica consisted of 43.7% (101/231) nymphs and 56.3% (130/231) adults, whereas R. sanguineus consisted of 60.9% (220/361) nymphs and 39.1% (141/361) adults. The H. elliptica species was most abundant in Miederpark (22.47%), whilst R. sanguineus was most abundant in both Miederpark (16.39%) and Ikageng (13.85%). There was a significant difference in tick species occurrence based on the geographical localities of R. sanguineus (X2 = 495.09, df = 9, p-value < 2.2 × 10−16) and H. elliptica (X2 = 629.3, df = 9, p-value < 2.2 × 10−16) ticks collected at various sampled sites.
Table 1.
Tick abundance from sampled dogs at PAWS and their various locations.
| Location | Tick Species | Total Number of Ticks per Location | Total Number of Dogs per Location | |
|---|---|---|---|---|
| H. elliptica (%) a | R. sanguineus (%) a | |||
| Potchefstroom b | 4 (3.31) | 117 (96.69) | 121 | 7 |
| Potchindustrie | 7 (21.88) | 25 (78.13) | 32 | 2 |
| Boskop | 21 (77.78) | 6 (22.22) | 27 | 4 |
| Die Bult | 11 (57.89) | 8 (42.11) | 19 | 6 |
| Baillie park | 4 (18.18) | 18 (81.82) | 22 | 3 |
| Miederpark | 133 (57.83) | 97 (42.17) | 230 | 27 |
| Boipatong c | 38 (88.37) | 5 (11.63) | 43 | 4 |
| Ikageng | 4 (4.65) | 82 (95.35) | 86 | 6 |
| Fochville c | 9 (100) | - | 9 | 1 |
| Kannonierspark | - | 3 (100) | 3 | 1 |
| Total number of ticks | 231 | 361 | 592 | 61 |
a: Indicates the occurrence of ticks from dogs originating from several locations in percentages; b: Ticks collected from dogs originating from Potchefstroom, but their exact locations of origin was unknown; c: Incidental tick samples from dogs not originating from Potchefstroom although admitted at PAWS Potchefstroom.
The BLASTn search results of the CO1 and ITS2 genes indicated that R. sanguineus sequences of this study (GenBank accession numbers: MK295614, MK295616, MK295617, and MK295618) were similar to other R. sanguineus sequences on the NCBI database with matching identifications of 99% for the CO1 gene and 94 to 97% for the ITS2 gene. For H. elliptica there was no reference sequence available on the NCBI database. However, BLASTn search indicated H. elliptica sequences from this study (GenBank accession numbers: MK295612, MK295613, and MK295615) were similar to several species among Haemaphysalis with pairwise identity between 87 to 99% for CO1, and 85 to 96% for ITS2. Phylogenetic analysis of CO1 (Supplementary Figure S1) and ITS2 (Supplementary Figure S2) revealed the presence of two major clades. In one of these clades, all Rhipicephalus species clustered together and in the other clade all Haemaphysalis species clustered together.
2.2. Molecular Detection of Tick-Borne Pathogens
The PCR agarose gel images are shown in Supplementary Figures S3, S5, S7 and S9. Out of the 104 tick pools screened for the presence of tick-borne pathogens there was an overall occurrence of 1% (1/104) for A. phagocytophilum, 9.6% (10/104) for Rickettsia, 5.8% (6/104) for Ehrlichia canis, and 13.5% (14/104) for Coxiella spp. Table 2 represents the occurrence of several tick-borne pathogens of H. elliptica and R. sanguineus tick pools of dogs originating from several suburbs in Potchefstroom, as well as from the Fochville and Boipatong suburbs. Of the 31 egg pools screened for the presence of tick-borne pathogens, only Coxiella spp. was detected with an overall occurrence of 6.5% (2/31). These pathogens were detected in 1 of 2 (50%) and 1 of 11 (9.1%) H. elliptica egg batches of dogs originating from Boipatong and Miederpark suburbs, respectively.
Table 2.
Overall occurrence of tick-borne pathogens detected by PCR from tick pools.
| Location | Species | A. phagocytophilum (%) a | Rickettsia sp. (%) a | B. canis (%) a | B. vogeli (%) a | B. rossi (%) a | E. canis (%) a | Coxiella spp. (%) a | Total Pools Screened |
|---|---|---|---|---|---|---|---|---|---|
| Baillie park | H. elliptica | - | - | - | - | - | - | - | 1 |
| R. sanguineus | - | 2 (50) | - | - | - | - | 1 (25) | 4 | |
| Boipatong b | H. elliptica | 1 (33.3) | 1 (33.3) | - | - | - | - | - | 3 |
| R. sanguineus | - | - | - | - | - | - | - | 3 | |
| Die Bult | H. elliptica | - | - | - | - | - | - | - | 4 |
| R. sanguineus | - | - | - | - | - | - | - | 3 | |
| Fochville b | H. elliptica | - | - | - | - | - | - | 1 (50) | 2 |
| Ikageng | H. elliptica | - | - | - | - | - | - | - | 4 |
| R. sanguineus | - | - | - | - | - | - | - | 10 | |
| Kannonierspark | R. sanguineus | - | - | - | - | - | - | 1 (50) | 2 |
| Miederpark | H. elliptica | - | 6 (24) | - | - | - | 4 (16) | 9 (36) | 25 |
| R. sanguineus | - | - | - | - | - | 1 (4.3) | 1 (4.3) | 23 | |
| Boskop | H. elliptica | - | - | - | - | - | - | 1 (20) | 5 |
| R. sanguineus | - | - | - | - | - | 1 (50) | - | 2 | |
| Potchindustrie | H. elliptica | - | - | - | - | - | - | - | 1 |
| R. sanguineus | - | - | - | - | - | - | - | 2 | |
| Potchefstroom c | H. elliptica | - | - | - | - | - | - | - | 4 |
| R. sanguineus | - | 1 (16.7) | - | - | - | - | - | 6 | |
| Total | 1 (1) | 10 (9.6) | 0 (0) | 0 (0) | 0 (0) | 6 (5.8) | 14 (13.5) | 104 | |
a: Indicates the occurrence of ticks from dogs originating from several locations in percentages; b: incidental tick samples from dogs not originating from Potchefstroom; c: Ticks collected from dogs originating from Potchefstroom, but their exact locations of origin was unknown.
Anaplasma phagocytophilum were detected in 2% (1/49) of H. elliptica ticks. There was no A. phagocytophilum detected from R. sanguineus tick DNA extracts. There was no significant statistical difference (X2 = 9, df = 9, p-value = 0.4373) of A. phagocytophilum prevalence based on geographical localities. The E. canis was detected in 8.2% (4/49) of H. elliptica ticks and 3.6% (2/55) of R. sanguineus ticks. There was a significant statistical difference (X2 = 37.333, df = 9, p-value = 2.295 × 10−5) in prevalence of E. canis based on the geographical localities. Rickettsia spp. were detected in 14.3% (7/49) of H. elliptica ticks and 5.5% (3/55) of R. sanguineus ticks with significant difference of prevalence (X2 = 32, df = 9, p-value = 0.0001991) when geographical localities were compared. Coxiella spp. was detected in 10.5% (2/19) of H. elliptica eggs, 22.4% (11/49) of H. elliptica ticks and 5.5% (3/55) of R. sanguineus ticks with a significant statistical difference (X2 = 60.286, df = 9, p-value = 1.181 × 10−9) in the geographical localities in ticks, whereas there was no significant statistical difference (X2 = 5, df = 6, p-value = 0.5438) of Coxiella spp. prevalence of the 19 H. elliptica tick egg batches analysed. In addition, mixed infections of A. phagocytophilum and Rickettsia spp. were detected from ticks of dogs originating from Boipatong suburb as well as mixed Coxiella spp., E. canis, and Rickettsia spp. from dogs originating from Miederpark suburb.
The BLASTn results of 16s rRNA gene of A. phagocytophilum detected in this study (GenBank accession number: MK295611) confirmed that it matches with relevant species on the NCBI database (GenBank accession numbers: AY623650.1 and MF787270.1) with matching pairwise identity scores of 99% (Supplementary Figure S3).
The gltA gene results of Rickettsia from this study (GenBank accession numbers: MK295619, MK295620, MK295621, MK295622, MK295623, and MK295624) were similar to Rickettsia conorii sequences on the NCBI database (GenBank accession numbers: DQ821855.1, MF002509.1, and KY640399.1) with matching pairwise identity scores ranging between 98 and 100% (Supplementary Figure S4).
Similarly, the 16S rRNA gene of E. canis detected in the current study were similar to E. canis sequences on the NCBI database (GenBank accession numbers: DQ494536.1, JQ976640.1, and MF153965.1) with matching pairwise identity scores ranging between 85 and 100% (Supplementary Figure S5).
Furthermore, the results of IS1111 transposase gene of Coxiella spp. of this study were similar to Coxiella spp. sequences on the NCBI database (GenBank accession numbers: JF970261.1, MH394636.1, and CP014563.1) with matching pairwise identity ranging between 95 and 100%, respectively (Supplementary Figure S6).
3. Discussion
In this study, R. sanguineus were identified as the most abundant tick species infesting dogs admitted to PAWS, in the North West Province, as compared to H. elliptica. This was expected as findings of previous studies [13,14,15,16] suggested that the most abundant species infesting South African companion animals are R. sanguineus, H. elliptica, and R. simus. Results of this study were similar to studies conducted by Mtshali [17] with reported occurrences of 49.9% and 5% of R. sanguineus and H. elliptica, respectively, collected from companion animals in Mafikeng, North West Province. Furthermore, Bryson et al. [16] reported respective occurrences of 96.62% and 2.85% for R. sanguineus and H. elliptica in the North West Province. In the province of Mpumalanga, Kolo et al. [18] reported 27 R. sanguineus and 30 H. elliptica from a total of 103 ticks infesting dogs. In this study, the most ticks were collected from dogs originating from Miederpark (where H. elliptica was more abundant than R. sanguineus) and Ikageng (where R. sanguineus was more abundant) suburbs of Potchefstroom. These collections were from stray dogs housed at PAWS which is located in Miederpark, whilst Ikageng is a settlement where many strays occur. The R. sanguineus ticks are behaviourally adapted to survive in urban settlements and dog kennels for extended time periods [6]. If preferred hosts are absent, these ticks will readily infest other hosts such as other domestic animals, livestock, and humans [16,19,20]. Rautenbach et al. [14], Bechara et al. [21] and Little et al. [19] suggested that R. sanguineus are reported in higher abundance in settlements where strays are present, or in animal shelters where dogs are co-housed, because tick control measures are lacking. The H. elliptica ticks prefer to infest murid rodents during the larval and nymphal developmental stages, whereas the adult stage prefers to infest members of the Canidae family [13,16,22]. Horak [15] and Bryson et al. [16] suggested that H. elliptica are often reported in higher abundance from communities with access to modern veterinary services. This might explain their lower abundances in other suburbs of Potchefstroom [13,16,22]. In addition, Chong et al. [23] and Lebert et al. [24] suggested that the difference in the distribution of the locations of different tick species, as well as the difference in their numbers in the different developmental stages may be due to several factors, including but not limited to seasonality of the tick species, differences in climatic conditions and availability of preferred hosts.
The ITS2 and CO1 genes were used to supplement morphological identification of tick species. A study conducted by Fukunaga et al. [25] revealed that the use of ITS2 nucleotide sequences was able to distinguish between ticks sharing the same morphological features or synonymized tick species. The CO1 gene is often used as a standard barcode for animal identification [26,27,28,29]. Lv et al. [29] suggested that the combined use of the ITS2 and CO1 gene, along with other genes, including 12S rDNA, 16S rDNA, and 18S rDNA, give more reliable results.
Molecular detection of Anaplasma spp. detected in this study using species-specific primers was similar to observations made in previous studies conducted in South Africa. A study by Mtshali et al. [30] reported presence of Anaplasma-like organisms from dog and ticks. Inokuma et al. [31] also reported the presence of Anaplasma spp. infesting dogs from South Africa that are closely related to A. phagocytophilum and A. platys. The low occurrence may be due to A. phagocytophilum that activates cytopenias and reduces the amount of white and red blood cells which influences the infection ability of other haemoparasites [32].
Rickettsia species were detected from DNA of tick pools by PCR using genus specific Rickettsia primers. These were identified as R. conorii by sequencing. Fourie et al. [33] suggested that R. conorii infections in both R. sanguineus and H. elliptica ticks are possible. This pathogen has previously been detected in R. sanguineus ticks [34,35], and in H. elliptica [36]. Mtshali et al. [30] reported an overall occurrence of 38% for R. conorii and R. africae in dog ticks in the North West Province using PCR. In another study conducted by Kolo et al. [18], Rickettsia spp. infestation rates of 70% were reported from ticks and blood from dogs in the Mpumalanga province. Members of Rickettsia genus are known to be obligatory intracellular parasites or mutualists of arthropods [37] which explains their consistent positive detection from ticks. The European Food Safety Authority (EFSA) Panel on Animal Health and Welfare (AHAW) (2010) [38] and Uilenberg et al. [39] stated that although R. conorii mainly infect R. sanguineus, the transmission of this pathogen to humans is possible by H. elliptica as well. Rickettsia infections, due to tick bites from several species, were also reported by as well as from humans that either reside in or travelled to South Africa [40,41,42,43,44,45].
In the current study, Babesia spp. were not detected by the conventional PCR was used. Even though Babesia infections are commonly reported in companion animals in South Africa, infection rates seem to differ. Allan [46] reported the presence of B. rossi and B. vogeli with respective infection rates of 12.7% and 3.2% in Cape Town. Furthermore, the occurrence of B. rossi with respective infection rates of 75% and 32.1% and B. vogeli with 3% and 1.8% in dog blood was reported in the city of Pretoria, whilst these piroplasms were also detected from several tick species infesting dogs in the same city [15,16]. Schetters et al. [47], Horak [15] and Bryson et al. [16] suggested that although ticks are generally collected from dogs in South Africa which have been diagnosed with canine babesiosis, they are not essential vectors of this pathogen, possibly explaining lack of Babesia spp. detection in this study.
During the current study, the presence of E. canis was detected only by PCR from tick pools. Infections of E. canis in R. sanguineus ticks were previously reported by studies conducted by Murphy et al. [48], Aguiar et al. [49] and Harrus et al. [50]. Furthermore, H. elliptica are not recognised vectors of E. canis; however, Ogbu et al. [51] suggested that E. canis may be transmitted to other tick species after exposure to an infected host, possibly explaining the E. canis infection in H. elliptica observed during this study. Mtshali et al. [30] reported E. canis infections in the North West Province, whilst 16% were reported in Mpumalanga, 12.7% in Cape Town, 42% in Bloemfontein, and 17.2%, as well as 3% in Maboloka were reported by Kolo et al. [18], Allan [46], Pretorius and Kelly [52], Rautenbach et al. [14], and Matjila et al. [53], respectively. The E. canis infections are difficult to detect when using blood smears, due to low parasitaemia [14,52,54,55]. This may explain why this pathogen was absent in Giemsa-stained blood smears but could be detected by PCR.
Coxiella spp. was detected in the current study by PCR from tick pools as well as from egg batches. As suggested by Woldehiwet [56], de la Fuente et al. [11], as well as Angelakis and Raoult [57] Coxiella spp. may be transmitted by means of transstadial and transovarial transmission, thus suggesting the presence of Coxiella spp. in tick eggs as well as ticks during their different developmental stages. Mtshali et al. [30] reported an overall occurrence of 31% for Coxiella spp. from ticks infesting companion animals in the North West province. Duron et al. [58] also reported infections of Coxiella-like endosymbionts in several tick species, including R. decoloratus and R. microplus infesting wildlife in South Africa. Baca and Paretsky [59], Dupont et al. [40], Buhariwalla et al. [60], Zhang et al. [61], Loftis et al. [62] and Duron et al. [58] reported that Coxiella spp. infections in humans are quite common and globally reported, due to the inhalation of contaminated aerosol particles. This may be an indication that the majority of human infections are due to the association between humans and infected livestock. Heinzen et al. [63], Mediannikov et al. [64] and Duron et al. [58] suggests that arthropods, especially ticks, are not vital in maintaining transmission of Coxiella spp. to humans or other animals, but this pathogen may be transmitted by means of transstadial transmission during blood meals of infected ticks. This often results in Q-fever infections in reservoir hosts, including companion animals, and accidental hosts, including humans.
The study indicated mixed infections of several tick-borne pathogens. These include mixed infections of A. phagocytophilum and R. conorii, as well as R. conorii, E. canis, and Coxiella spp. Van Heerden [65] and Pennisi et al. [66] suggested that mixed infections are common, especially where domestic animals are co-housed. Matjila et al. [53,67] suggested that mixed infections may be attributed to R. sanguineus and H. elliptica ticks feeding on similar infected hosts in overlapping regions. Griffiths et al. [68] suggested that mixed infections are significant as pathogens present within the host interact with one another. These interactions may improve the transmission and progression of the associated diseases or cause disturbances in the colonization or virulence of other pathogens. Even though ticks are well known for their ability to transmit pathogenic organisms to their hosts the detection of medically and veterinary important tick-borne pathogens, associated with companion animals, in this study and previous studies [14,15,16,18,30,31,34,35,36,46,48,49,50,52,58,67] raises concern. These animal hosts often suffer due to illness caused by the pathogens. In several cases, infection of tick-borne pathogens may result in host mortality [20,69]. Several species of Ixodid ticks follow a three-host life cycle. This requires a blood meal during each developmental stage, of the ticks, from various hosts to enable life cycle completion. In the absence of companion animals, the ticks may feed on alternative hosts, including humans, resulting in zoonosis [11,16].
4. Materials and Methods
4.1. Sampling and Areas of Origin for the Dogs
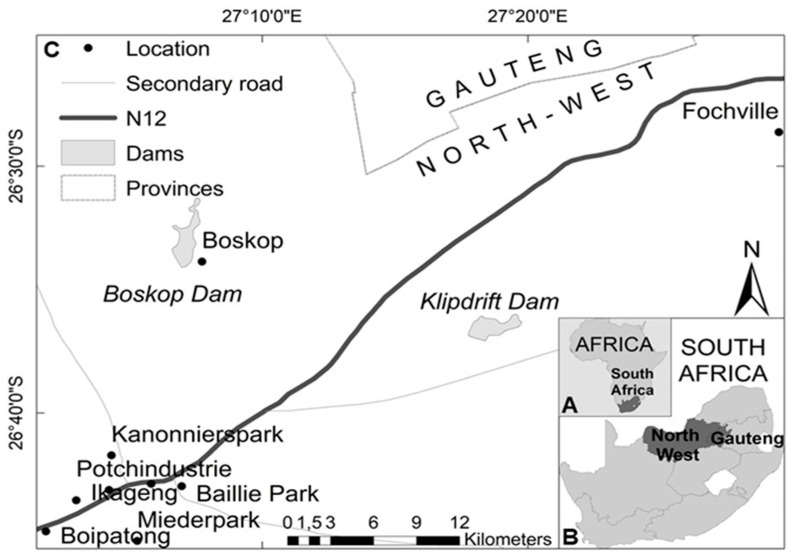
Tick specimens (N = 592) were collected by the veterinarian from rescued stray dogs (N = 61) on arrival for admission at PAWS in 2017–2020 (Table 1). The suburbs where dogs originated from before they were admitted to PAWS are Potchindustrie (26°43′7.37″ S, 27°4′14.0952″ E), Boskop (26°33′51.9836″ S, 27°7′44.0036″ E), Kanonnierspark (26°41′43.0631″ S, 27°4′19.3904″ E), Baillie Park (26°42′58.1173″ S, 27°6′58.2487″ E), Ikageng (26°43′32.3738″ S, 27°2′59.9492″ E), Die Bult (26°42′52.3069″ S, 27°5′49.371″ E), Miederpark (26°45′11.362″ S, 27°5′17.6428″ E), Boipatong (26°44′48.0476″ S, 27°1′51.492″ E) and Fochville (26°28′37.4059″ S, 27°29′27.1457″ E) (Figure 1).
Figure 1.
Maps indicating the sampling locations (made with ARGIS). (A) indicates a map of Africa showing South Africa. (B) indicates a map of South Africa showing the different sampling provinces. (C) indicates the locations of origin of sampled dogs.
4.2. Morphological Identification of Ticks by Microscopy
Tick specimens were collected weekly from stray dogs from urban areas which were admitted to PAWS. Ticks were morphologically identified to species level by using the Nikon SMZ745 stereo microscope and identification keys of Horak et al. [70], Barker and Walker [71] and Walker et al. [72]. To confirm correct morphological identification, representatives of each tick species were submitted to the Gertrud Theiler Tick Museum, located at the Agricultural Research Council-Onderstepoort Veterinary Research (ARC-OVR) and voucher numbers were issued. All of the engorged female ticks (N = 204) collected during the study were kept, while they were still alive, in separate containers until eggs were laid. Afterwards the dead ticks and eggs were stored in 70% ethanol for a different study, however, the eggs were included for molecular detection of tick-borne pathogens.
4.3. Molecular Identification of Ticks
In preparation for molecular identification of the tick species, legs were removed from selected tick samples for DNA extraction. For molecular detection of tick-borne pathogens, ticks were pooled together according to the same species, host, the hosts location of origin, and life stage, from which they were collected. Tick pools consisted of three or four ticks, however, in cases where there were less than three specimens, the samples were not pooled but were treated separately as individual samples. Additionally, for the molecular detection of tick-borne pathogens, the tick egg batches (each made up of 50 eggs) originating from the same tick species and location were stored. In total there were 104 tick pools and 31 egg batches. Prior to DNA extraction, ticks and eggs were surface sterilized for 1 h with 10% Tween 20 and then rinsed twice with 70% ethanol and rinsed three times with double distilled water. Genomic DNA (gDNA) was extracted from legs, tick pools, and egg batches by the salting out method as described by Riveroa et al. [73] and stored at −35 °C until further use.
The cytochrome oxidase subunit 1 (CO1) and internal transcribed spacer 2 (ITS2) were the targeted gene regions for molecular identification of the collected tick samples. The PCR for the amplification of the CO1 gene was conducted using primers LCO1490 forward (GGT CAA CAA ATC ATA AAG ATA TTG G) and HCO2198 reverse (TAA ACT TCA GGG TGA CCA AAA AAT CA), and the ITS2 gene using primers ITS2F forward (YTG CGA RAC TTG GTG TGA AT) and ITS2R reverse (TAT GCT TAA RTT YAG SGG GT) described by Licari et al. [74] and Muruthi [3], respectively. For both gene regions, the PCR reaction mixture had a final volume of 25 µL which consisted of 12.5 μL of AmpliTaq Gold 360® Master Mix (Applied Biosystems, Woodlands, Singapore), 1 μL each of primer [each at 10 μM concentration], 2 μL of the template DNA, and 8.5 μL double distilled water. Haemaphysalis longicornis DNA (obtained from Obihiro University of Agriculture and Veterinary Medicine, Obihiro, Japan) was used as positive control, while distilled water was used as negative control. PCR conditions consisted of initial denaturation at 95 °C for 10 min, 35 cycles of denaturation at 95 °C for 30 s, annealing at 47 °C (CO1 gene) and 50 °C (ITS2 gene) for 30 s, and extension at 72 °C for 60 s, followed by a final extension at 72 °C for 7 min and final hold at 4 °C, using the ProFlex PCR System (Applied Biosystems, Woodlands, Singapore).
4.4. Molecular Detection of Tick-Borne Pathogens
Molecular detection of tick-borne pathogens, namely Anaplasma phagocytophilum, Babesia spp., Coxiella spp., Rickettsia spp. and Ehrlichia canis from 104 tick pools and 31 egg batches were analysed where the PCR mixture was prepared as described above using species specific PCR primers for the different pathogens. The PCR was conducted targeting the 16S rRNA gene to determine the presence of A. phagocytophilum [75] and E. canis [76] by, respectively, using the EHR-521 forward (TGT AGG CGG TTC GGT AAG TTA AAG) and EHR-747 reverse (GCA CTC ATC GTT TAC AGG GTG) primers, as well as the E.c 16S forward (TCG CTA TTA GAT GAG CCT ACG T) and E.c 16S reverse (GAG TCT GGA CCG TAT CTC AGT) primers. For Babesia spp., the 18S rRNA gene (Duarte et al., 2008) was targeted for the detection of B. canis, using the primers BAB1 forward (GTG AAC CTT ATC ACT TAA AGG) and BAB3 reverse (CTA CAC AGA GCA CAC AGC C), B. vogeli, using the primers BAB1 forward (GTG AAC CTT ATC ACT TAA AGG) and BAB4 reverse (CAA CTC CTC CAC GCA ATC G), as well as B. rossi, using the primers BAB1 forward (GTG AAC CTT ATC ACT TAA AGG) and BAB5 reverse (AGG AGT TGC TTA CGC ACT CA). For the detection of Rickettsia spp., the gltA gene [77] was targeted using the primers Rp877p forward (GGG GAC CTG CTC ACG GCG G) and Rp1258n reverse (ATT GCA AAA AGT ACA GTG AAC A). The detection of Coxiella spp. was performed targeting the IS1111 transposase gene [78] using the primers IS1111aF forward (CAT CAC ATT GCC GCG TTT AC) and IS1111aR reverse (GGT TGG TCC CTC GAC AAC AT). For positive controls, A. phagocytophilum DNA was obtained from a PCR positive horse from Northern Cape [79] while controls of E. canis, R. africae and Coxiella spp. DNA acquired from the Research Centre for Zoonosis Control, Hokkaido University, Japan were used. Babesia canis positive controls were synthesized from gBlock® gene fragments obtained from Whitehead Scientific (Pty) Ltd., Cape Town, South Africa. The PCR conditions consisted of initial denaturation at 95 °C for 30 s, 30 cycles of denaturation at 95 °C for 30 s, annealing at 50 °C (B. canis, B. rossi, and B. vogeli), 52 °C (Rickettsia spp.), 57 °C (Coxiella spp.), and 60 °C (A. phagocytophilum and E. canis) for 60 s and extension at 68 °C for 60 s followed by a final extension at 68 °C for 5 min and final hold at 4 °C, using the ProFlex PCR System (Applied Biosystems, Woodlands, Singapore).
The PCR products were purified using the QIAquick Gel Extraction Kit (Qiagen, DE, Hilden, Germany) by following the manufactures instructions (Qiagen, DE, Hilden, Germany). Purified PCR products were submitted for sequencing at Inqaba Biotechnological Industries (Pty) Ltd., Pretoria, South Africa. Sequence visualisation and editing was performed by using Molecular Evolutionary Genetics Analysis version 7.0 (MEGA7) software package [14]. The nucleotide Basic Local Alignment Search Tool (BLASTn) was used to confirm tick and tick-borne pathogen identification (https://blast.ncbi.nlm.nih.gov/Blast.cgi, accessed on 15 August 2017).
4.5. Blood Smears
Giemsa stained thin blood smears were prepared of engorged ticks by using a modified method described by Poostchi et al. [80].
4.6. Statistical Analysis
The significance relating to the geographic localities of ticks as well as tick-borne pathogens was determined in R-studio by using the Pearson’s chi-square test. Confidence interval (CI) of an average of 95% was used to determine tick and tick-borne pathogens occurrences. Phylogenetic trees were constructed using CO1 and ITS2 gene sequences obtained from this study, along with homologous sequences of closely related species obtained from the NCBI database. All sequences were added to the alignment explorer in MEGA7, aligned by ClustalW using default parameters and trimmed to be even length. Lowest Bayesian Information Criterion (BIC) score was used to determine the best nucleotide substitution model. Maximum likelihood method was used for construction of the phylogenetic trees with 10,000 bootstrap replications. During phylogenetic analysis, missing nucleotide data or gaps were removed and rates among sites were handled as uniform rates.
5. Conclusions
The ticks collected in this study were identified as R. sanguineus and H. elliptica in accordance with other studies and the literature. This is an indication that ticks flourish in environments where stray dogs are present, especially in the absence of tick control measures. This study also demonstrated the presence of tick-borne pathogens including, A. phagocytophilum, R. conorii, E. canis, and Coxiella spp., in ticks and their eggs indicating a cause for concern with regards to the health of companion animals and humans as most of these species are associated with zoonotic diseases.
Acknowledgments
The first author was sponsored by the National Research Foundation (NRF) Innovation Masters Scholarship. We thank the Potchefstroom Animal Welfare Society for their cooperation.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/pathogens11080862/s1. Figure S1. Phylogenetic analysis of tick CO1 gene sequences using the Maximum Likelihood (ML) method based on the General Time Reversible (NTR) model [81]. Bootstrap percentage of 10,000 replicates, in which the associated taxa are clustered together is displayed next to the branch nodes. Twelve nucleotide sequences were used for data analysis. Sequences of this study are indicated by a black bullet. Myialges spp. was used as an outgroup. Phylogenetic analysis was performed by using MEGA 7 [82]. Figure S2. Phylogenetic analysis of tick ITS2 gene using the Maximum Likelihood (ML) method based on the Kimura 2-parameter model [83]. Bootstrap percentage of 10,000 replicates, in which the associated taxa are clustered together is displayed next to the branch nodes. Fourteen nucleotide sequences were used for data analysis. Sequences of this study are indicated by a black bullet. Dermanyssus sp. was used as an outgroup. Phylogenetic analysis was performed by using MEGA 7 [81]. Figure S3: Fragment of the BLASTn alignment between A. phagocytophilum of this study and a corresponding sequence. First strand represents A. phagocytophilum detected from ticks collected from the JB Marks local municipality. Second strand represents a reference sequence from NCBI. Red arrows indicate where nucleotides mismatch. Figure S4: Fragment of the BLASTn alignment between R. conorii of this study and a corresponding sequence. First strand represents R. conorii detected from ticks collected from the JB Marks local municipality. Second strand represents a reference sequence from NCBI. Red arrows indicate where nucleotides mismatch. Figure S5: Fragment of the BLASTn alignment between E. canis of this study and a corresponding sequence. First strand represents E. canis detected from ticks collected from the JB Marks local municipality. Second strand represents a reference sequence from NCBI. Red arrows indicate where nucleotides mismatch. Figure S6: Fragment of the BLASTn alignment between C. burnetii of this study and a corresponding sequence. First strand represents C. burnetii detected from ticks collected from the JB Marks local municipality. Second strand represents a reference sequence from NCBI
Author Contributions
Conceptualization, O.T. and K.M.; methodology, C.-L.v.W.; formal analysis, C.-L.v.W. and M.O.T.; investigation, S.T., D.B. and C.-L.v.W.; resources, O.T. and X.X.; data curation, C.-L.v.W., M.O.T. and D.B.; writing—original draft preparation, C.-L.v.W.; writing—review and editing, S.T., K.M., M.O.T., T.R. and X.X.; supervision, O.T. and K.M.; funding acquisition, O.T. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
This study was approved by the scientific committee of the Integrated Pest Management, of the Unit for Environmental Sciences and Management, North-West University reference number: NWU-IPM-2017-009.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was funded by NRF Incentive grant for rated researchers (GUN: 118949) made available to OT. The Grantholder acknowledges that opinions, findings and conclusions or recommendations expressed in any publication generated by the NRF supported research is that of the author(s), and that the NRF accepts no liability whatsoever in this regard. This study was supported by a Grant-in-Aid for Scientific Research (18KK0188) and Japan Society for the Promotion of Science Core-to-Core program, both from the Ministry of Education, Culture, Sports, Science, and Technology of Japan, and a grant from the Strategic International Collaborative Research Project (JPJ008837) promoted by the Ministry of Agriculture, Forestry and Fisheries of Japan.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Parola P., Raoult D. Ticks and tickborne bacterial diseases in humans: An emerging infectious threat. Ticks Tick Borne Dis. 2001;32:897–928. doi: 10.1086/319347. [DOI] [PubMed] [Google Scholar]
- 2.Machado-Ferreira E., Vizzoni V.F., Piesman J., Gazeta G.S., Soares C.A.G. Bacteria associated with Amblyomma cajennense tick eggs. Genet. Mol. Biol. 2015;38:477–483. doi: 10.1590/S1415-475738420150040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Muruthi W.C. Master’s Thesis. Kenyatta University; Nakuru, Kenya: 2015. [(accessed on 12 January 2018)]. Phenotypic and Molecular Characterization of Hard Ticks (Acari: Ixodidae) Sampled from Wild Herbivores from Lake Nakuru and Tsavo National Parks in Kenya. Available online: https://ir-library.ku.ac.ke/handle/123456789/14328. [Google Scholar]
- 4.Dantas-Torres F. Species concepts: What about ticks? Trends Parasitol. 2018;34:1017–1026. doi: 10.1016/j.pt.2018.09.009. [DOI] [PubMed] [Google Scholar]
- 5.Barker S.C. Distinguishing species and populations of rhipicephaline ticks with its2 ribosomal RNA. J. Parasitol. 1998;84:887–892. doi: 10.2307/3284614. [DOI] [PubMed] [Google Scholar]
- 6.Dantas-Torres F. Biology and ecology of the brown dog tick, Rhipicephalus sanguineus. Parasit Vectors. 2010;3:26. doi: 10.1186/1756-3305-3-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dantas-Torres F. The brown dog tick, Rhipicephalus sanguineus (Latreille, 1806) (Acari: Ixodidae): From taxonomy to control. Vet. Parasitol. 2008;152:173–185. doi: 10.1016/j.vetpar.2007.12.030. [DOI] [PubMed] [Google Scholar]
- 8.Rynkiewicz E.C., Hemmerich C., Rusch D.B., Fuqua C., Clay K. Concordance of bacterial communities of two tick species and blood of their shared rodent host. Mol. Ecol. 2015;24:2566–2579. doi: 10.1111/mec.13187. [DOI] [PubMed] [Google Scholar]
- 9.Jongejan F., Uilenberg G. The global importance of ticks. Parasitology. 2004;129:3–14. doi: 10.1017/S0031182004005967. [DOI] [PubMed] [Google Scholar]
- 10.Shaw S.E., Day M.J., Birtles R.J., Breitschwerdt E.B. Tick-borne infectious diseases of dogs. Trends Parasitol. 2001;17:74–80. doi: 10.1016/S1471-4922(00)01856-0. [DOI] [PubMed] [Google Scholar]
- 11.de la Fuente J., Estrada-Peña A., Venzal J.M., Kocan K.M., Sonenshine D.E. Overview: Ticks as vectors of pathogens that cause disease in humans and animals. Front. Biosci. 2008;13:6938–6946. doi: 10.2741/3200. [DOI] [PubMed] [Google Scholar]
- 12.Mtshali K., Khumalo Z.T.H., Nakao R., Grab D.J., Sugimoto C., Thekisoe O.M.M. Molecular detection of zoonotic tick-borne pathogens from ticks collected from ruminants in four South African provinces. J. Vet. Sci. 2015;77:1573–1579. doi: 10.1292/jvms.15-0170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Horak I.G., Guillarmod A.J., Moolman L.C., De Vos V. Parasites of domestic and wild animals in South Africa. XXII. Ixodid ticks on domestic dogs and wild carnivores. Onderstepoort J. Vet. Res. 1987;54:573–580. [PubMed] [Google Scholar]
- 14.Rautenbach G.H., Boomker J., De Villiers I.L. A descriptive study of the canine population in a rural town in Southern Africa. J. S Afr Vet. Assoc. 1991;62:158–162. doi: 10.4102/jsava.v62i4.1778. [DOI] [PubMed] [Google Scholar]
- 15.Horak I.G. Ixodid ticks collected at the faculty of veterinary science, Onderstepoort, from dogs diagnosed with Babesia canis infection. J. S Afr Vet. Assoc. 1995;66:170–171. [PubMed] [Google Scholar]
- 16.Bryson N.R., Horak I.G., Louw J.P. Ectoparasites of dogs belonging to people in resource-poor communities in North West Province, South Africa. J. S Afr Vet. Assoc. 2000;71:175–179. doi: 10.4102/jsava.v71i3.709. [DOI] [PubMed] [Google Scholar]
- 17.Mtshali K. Master’s Thesis. University of the Free State; Phuthaditjhaba, South Africa: 2012. [(accessed on 24 January 2018)]. Molecular detection of zoonotic tick-borne pathogens in livestock in different provinces of South Africa. Available online: http://scholar.ufs.ac.za:8080/xmlui/handle/11660/1715. [Google Scholar]
- 18.Kolo A.O., Sibeko-Matjila K.P., Maina A.N., Richards A.L., Knobel D.L., Matjila P.T. Molecular detection of zoonotic rickettsiae and Anaplasma spp. in domestic dogs and their ectoparasites in Bushbuckridge, South Africa. Vector Borne Zoonotic Dis. 2016;16:245–252. doi: 10.1089/vbz.2015.1849. [DOI] [PubMed] [Google Scholar]
- 19.Little S.E., Hostetler J., Kocan K.M. Movement of Rhipicephalus sanguineus adults between co-housed dogs during active feeding. Vet. Parasitol. 2007;150:139–145. doi: 10.1016/j.vetpar.2007.08.029. [DOI] [PubMed] [Google Scholar]
- 20.Dantas-Torres F., Otranto D. Further thoughts on the taxonomy and vector role of Rhipicephalus sanguineus group ticks. Vet. Parasitol. 2015;208:9–13. doi: 10.1016/j.vetpar.2014.12.014. [DOI] [PubMed] [Google Scholar]
- 21.Bechara G.H., Szabó M.P.J., Mukai L.S., Rosa P.C.S. Immunisation of dogs, hamsters and guinea pigs against Rhipicephalus sanguineus using crude unfed adult tick extracts. Vet. Parasitol. 1994;52:79–90. doi: 10.1016/0304-4017(94)90038-8. [DOI] [PubMed] [Google Scholar]
- 22.Apanaskevich D.A., Horak I.G., Camicas J.-L. Redescription of Haemaphysalis (Rhipistoma) elliptica (Koch, 1844), an old taxon of the Haemaphysalis (Rhipistoma) leachi group from East and southern Africa, and of Haemaphysalis (Rhipistoma) leachi (Audouin, 1826) (Ixodida, Ixodidae) Onderstepoort J. Vet. Res. 2007;74:181–208. doi: 10.4102/ojvr.v74i3.122. [DOI] [PubMed] [Google Scholar]
- 23.Chong S.T., Kim H.C., Lee I.Y., Kollars T.M., Jr., Sancho A.R., Sames W.J., Chae J.S., Klein T.A. Seasonal distribution of ticks in four habitats near the demilitarized zone, Gyeonggi-do (Province), Republic of Korea. Korean. J. Parasitol. 2013;51:319–325. doi: 10.3347/kjp.2013.51.3.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lebert I., Agoulon A., Bastian S., Butet A., Cargnelutti B., Cèbe N., Chastagner A., Léger E., Lourtet B., Masseglia S., et al. Distribution of ticks, tick-borne pathogens and the associated local environmental factors including small mammals and livestock, in two French agricultural sites: The OSCAR database. Biodivers. Data J. 2020;8:e50123. doi: 10.3897/BDJ.8.e50123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fukunaga M., Yabuki M., Hamase A., Oliver Jr J.H., Nakao M. Molecular phylogenetic analysis of Ixodid ticks based on the ribosomal DNA spacer, internal transcribed spacer 2, sequences. J. Parasitol. 2020;86:38–43. doi: 10.1645/0022-3395(2000)086[0038:MPAOIT]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 26.Erpenbeck D., Hooper J.N.A., Wörheide G. CO1 phylogenies in diploblasts and the “Barcoding of Life”-are we sequencing a suboptimal partition? Mol. Ecol. Notes. 2006;6:550–553. doi: 10.1111/j.1471-8286.2005.01259.x. [DOI] [Google Scholar]
- 27.Kress W.J., Erickson D.L. DNA barcodes: Genes, genomics, and bioinformatics. Proc. Natl. Acad. Sci. USA. 2008;105:2761–2762. doi: 10.1073/pnas.0800476105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stoeckle M.Y., Hebert P.D.N. Barcode of life. Sci. Am. 2008;299:82–89. doi: 10.1038/scientificamerican1008-82. [DOI] [PubMed] [Google Scholar]
- 29.Lv J., Wu S., Zhang Y., Chen Y., Feng C., Yuan X., Jia G., Deng J., Wang C., Wang Q., et al. Assessment of four DNA fragments (COI, 16S rDNA, ITS2, 12S rDNA) for species identification of the Ixodida (Acari: Ixodida) Parasit. Vectors. 2014;7:93. doi: 10.1186/1756-3305-7-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mtshali K., Nakao R., Sugimoto C., Thekisoe O.M.M. Occurence of Coxiella burnetti, Ehrlichia canis, Rickettsia species and Anaplasma phagocytophilum-like bacterium in ticks collected from dogs and cats in South Africa. J. S. Afr. Vet. Assoc. 2017;88:1390. doi: 10.4102/jsava.v88i0.1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Inokuma H., Oyamada M., Kelly P.J., Jacobson L.A., Fournier P.-E., Itamoto K., Okuda M., Brouqui P. Molecular detection of a new Anaplasma species closely related to Anaplasma phagocytophilum in canine blood from South Africa. J. Clin. Microbiol. 2005;43:2934–2937. doi: 10.1128/JCM.43.6.2934-2937.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Telfer S., Lambin X., Birtles R., Beldomenico P., Burthe S., Paterson S., Begon M. Species interactions in a parasite community drive infection risk in a wildlife population. Science. 2010;330:243–246. doi: 10.1126/science.1190333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fourie J.J., Fourie L.J., Horak I.G., Snyman M.G. The efficacy of a topically applied combination of cyphenothrin and pyriproxyfen against the southern African yellow dog tick, Haemaphysalis elliptica, and the cat flea, Ctenocephalides felis, on dogs. J. S Afr. Vet. Assoc. 2010;81:33–36. doi: 10.4102/jsava.v81i1.93. [DOI] [PubMed] [Google Scholar]
- 34.Socolovschi C., Bitam I., Raoult D., Parola P. Transmission of Rickettsia conorii conorii in naturally infected Rhipicephalus sanguineus. Clin. Microbiol. Infect. 2009;15:319–321. doi: 10.1111/j.1469-0691.2008.02257.x. [DOI] [PubMed] [Google Scholar]
- 35.Pacheco R.D.C., Moraes-Filho J., Guedes E., Silveira I., Richtzenhain L.J., Leite R.C., Labruna M.B. Rickettsial infections of dogs, horses, and ticks in Juiz de Fora, southeastern Brazil, and isolation of Rickettsia rickettsii from Rhipicephalus sanguineus ticks. Med. Vet. Entomol. 2011;25:148–155. doi: 10.1111/j.1365-2915.2010.00915.x. [DOI] [PubMed] [Google Scholar]
- 36.Kamani J., Baneth G., Mumcuoglu K.Y., Waziri N.E., Eyal O., Guthmann Y., Harrus S. Molecular detection and characterization of tick-borne pathogens in dogs and ticks from Nigeria. PLoS Negl Trop Dis. 2013;7:e2108. doi: 10.1371/journal.pntd.0002108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ogata H., Audic S., Renesto-Audiffren P., Fournier P.-E., Barbe V., Samson D., Roux V., Cossart P., Weissenbach J., Claverie J.-M., et al. Mechanisms of Evolution in Rickettsia conorii and R. prowazekii. Science. 2001;293:2093–2099. doi: 10.1126/science.1061471. [DOI] [PubMed] [Google Scholar]
- 38.EFSA Panel on Animal Health and Welfare (AHAW) Scientific opinion on geographic distribution of tick-borne infections and their vectors in Europe and the other regions of the Mediterranean Basin. EFSA J. 2010;8:1723. doi: 10.2903/j.efsa.2010.1723. [DOI] [Google Scholar]
- 39.Uilenberg G., Estrada-Peña A., Thal J. Ticks of the Central African Republic. Exp. Appl Acarol. 2013;60:1–40. doi: 10.1007/s10493-012-9605-2. [DOI] [PubMed] [Google Scholar]
- 40.Dupont H.T., Brouqui P., Faugere B., Raoult D., Dupont H.T., Brouqui P., Faugere B., Raoult D. Prevalence of antibodies to Coxiella burnetii, Rickettsia conorii, and Rickettsia typhi in seven African countries. Clin. Infect. Dis. 1995;21:1126–1133. doi: 10.1093/clinids/21.5.1126. [DOI] [PubMed] [Google Scholar]
- 41.Fournier P.E., Roux V., Caumes E., Donzel M., Raoult D. Outbreak of Rickettsia africae infections in participants of an adventure race in South Africa. Clin. Infect. Dis. 1998;27:316–323. doi: 10.1086/514664. [DOI] [PubMed] [Google Scholar]
- 42.Raoult D., Fournier P.E., Fenollar F., Jensenuis M., Prioe T., De Pina J.J., Caruso G., Jones N., Laferl H., Rosenblatt J.E., et al. Rickettsia africae, a tick-borne pathogen in travelers to Sub-Saharan Africa. N. Engl. J. Med. 2001;344:1504–1510. doi: 10.1056/NEJM200105173442003. [DOI] [PubMed] [Google Scholar]
- 43.Caruso G., Zasio C., Guzzo F., Granata C., Mondardini V., Guerra E., Macrì E., Benedetti P. Outbreak of African tick-bite fever in six Italian tourists returning from South Africa. Eur. J. Clin. Microbiol. Infect. Dis. 2002;21:133–136. doi: 10.1007/s10096-001-0663-3. [DOI] [PubMed] [Google Scholar]
- 44.Jensenius M., Fournier P.-E., Vene S., Hoel T., Hasle G., Henriksen A.Z., Hellum K.B., Raoult D., Myrvang B. African tick bite fever in travellers to rural sub-equatorial Africa. Clin. Infect. Dis. 2003;36:1411–1417. doi: 10.1086/375083. [DOI] [PubMed] [Google Scholar]
- 45.Pretorius A.-M., Birtles R.J. Rickettsia mongolotimonae infection in South Africa. Emerg Infect. Dis. 2004;10:125–126. doi: 10.3201/eid1001.020662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Allan R.E. Master’s Thesis. University of South Africa; Pretoria, South Africa: 2016. [(accessed on 12 January 2018)]. The occurrence of tick-borne pathogens, in dogs in welfare organisations and townships of Cape Town. Available online: https://uir.unisa.ac.za/handle/10500/22723. [Google Scholar]
- 47.Schetters T.P.M., Moubri K., Précigout E., Kleuskens J., Schlotes N.C., Gorenflot A. Different Babesia canis isolates, different diseases. Parasitology. 1997;115:485–493. doi: 10.1017/S0031182097001686. [DOI] [PubMed] [Google Scholar]
- 48.Murphy G.L., Ewing S.A., Whitworth L.C., Fox J.C., Kocan A.A. A molecular and serologic survey of Ehrlichia canis, E. chaffeensis, and E. ewingii in dogs and ticks from Oklahoma. Vet. Parasitol. 1998;79:325–339. doi: 10.1016/S0304-4017(98)00179-4. [DOI] [PubMed] [Google Scholar]
- 49.Aguiar D.M., Cavalcante G.T., Pinter A., Gennari S.M., Camargo L.M.A., Labruna M.B. Prevalence of Ehrlichia canis (Rickettsiales: Anaplasmataceae) in dogs and Rhipicephalus sanguineus (Acari: Ixodidae) ticks from Brazil. J. Med. Entomol. 2007;44:126–132. doi: 10.1093/jmedent/41.5.126. [DOI] [PubMed] [Google Scholar]
- 50.Harrus S., Perlman-Avrahami A., Mumcuoglu K.Y., Morick D., Eyal O., Baneth G. Molecular detection of Ehrlichia canis, Anaplasma bovis, Anaplasma platys, Candidatus Midichloria mitochondrii and Babesia canis vogeli in ticks from Israel. Clin. Microbiol. Infect. 2011;17:459–463. doi: 10.1111/j.1469-0691.2010.03316.x. [DOI] [PubMed] [Google Scholar]
- 51.Ogbu K.I., Olaolu O.S., Ochai S.O., Tion M.T. A review of some tick-borne pathogens of dogs. JASVM. 2018;3:140–153. doi: 10.31248/JASVM2018.106. [DOI] [Google Scholar]
- 52.Pretorius A.-M., Kelly P.J. Serological survey for antibodies reactive with Ehrlichia canis and E. chaffeensis in dogs from the Bloemfontein area, South Africa. J. S. Afr. Vet. Assoc. 1998;69:126–128. doi: 10.4102/jsava.v69i4.840. [DOI] [PubMed] [Google Scholar]
- 53.Matjila P.T., Leisewitz A.L., Jongejan F., Penzhorn B.L. Molecular detection of tick-borne protozoal and ehrlichial infections in domestic dogs in South Africa. Vet. Parasitol. 2008;155:152–157. doi: 10.1016/j.vetpar.2008.04.012. [DOI] [PubMed] [Google Scholar]
- 54.Allsopp M.T.E.P., Allsopp B.A. Novel Ehrlichia genotype detected in dogs in South Africa. J. Clin. Microbiol. 2001;39:4204–4207. doi: 10.1128/JCM.39.11.4204-4207.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Harrus S., Waner T. Diagnosis of canine monocytotropic ehrlichiosis (Ehrlichia canis): An overview. Vet. J. 2011;187:292–296. doi: 10.1016/j.tvjl.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 56.Woldehiwet Z. Q fever (coxiellosis): Epidemiology and pathogenesis. Res. Vet. Sci. 2004;77:93–100. doi: 10.1016/j.rvsc.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 57.Angelakis E., Raoult D. Q fever. Vet. Microbiol. 2010;140:297–309. doi: 10.1016/j.vetmic.2009.07.016. [DOI] [PubMed] [Google Scholar]
- 58.Duron O., Noël V., McCoy K.D., Bonazzi M., Sidi-Boumedine K., Morel O., Vavre F., Zenner L., Jourdain E., Durand P., et al. The recent evolution of a maternally-inherited endosymbiont of ticks led to the emergence of the Q fever pathogen, Coxiella burnetii. PLoS Pathog. 2015;11:e1004892. doi: 10.1371/journal.ppat.1004892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Baca O.G., Paretsky D. Q fever and Coxiella burnetii: A model for host-parasite interactions. Microbiol. Rev. 1983;47:127–149. doi: 10.1128/mr.47.2.127-149.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Buhariwalla F., Cann B., Marrie T.J. A dog-related outbreak of Q fever. J. Clin. Microbiol. 1996;23:753–755. doi: 10.1093/clinids/23.4.753. [DOI] [PubMed] [Google Scholar]
- 61.Zhang G.Q., Nguyen S.V., To H., Ogawa M., Hotta A., Yamaguchi T., Kim H.J., Fukushi H., Hirai K. Clinical evaluation of a new PCR assay for detection of Coxiella burnetii in human serum samples. J. Clin. Microbiol. 1998;36:77–80. doi: 10.1128/JCM.36.1.77-80.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Loftis A.D., Reeves W.K., Szumlas D.E., Abbassy M.M., Helmy I.M., Moriarity J.R., Dasch G.A. Rickettsial agents in Egyptian ticks collected from domestic animals. Exp. Appl Acarol. 2006;40:67–81. doi: 10.1007/s10493-006-9025-2. [DOI] [PubMed] [Google Scholar]
- 63.Heinzen R.A., Scidmore M.A., Rockey D.D., Hackstadt T. Differential interaction with endocytic and exocytic pathways distinguish parasitophorous vacuoles of Coxiella burnetii and Chlamydia trachomatis. Infect. Immun. 1996;64:796–809. doi: 10.1128/iai.64.3.796-809.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mediannikov O., Fenollar F., Socolovschi C., Diatta G., Bassene B., Molez J.-F., Sokhna C., Trape J.-F., Raoult D. Coxiella burnetii in humans and ticks in rural Senegal. PLoS Negl. Trop. Dis. 2010;4:e654. doi: 10.1371/journal.pntd.0000654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.van Heerden J. A retrospective study on 120 natural cases of canine ehrlichiosis. J. S. Afr. Vet. Assoc. 1982;22:17–22. [PubMed] [Google Scholar]
- 66.Pennisi M.G., Caprì A., Solano-Gallego L., Lombardo G., Torina A., Masucci M. Prevalence of antibodies against Rickettsia conorii, Babesia canis, Ehrlichia canis, and Anaplasma phagocytophilum antigens in dogs from the Stretto di Messina area (Italy) Ticks Tick Borne Dis. 2012;3:315–318. doi: 10.1016/j.ttbdis.2012.10.026. [DOI] [PubMed] [Google Scholar]
- 67.Matjila P.T., Penzhorn B.L., Bekker C.P.J., Nijhof A.M., Jongejanm F. Confirmation of occurrence of Babesia canis vogeli in domestic dogs in South Africa. Vet. Parasitol. 2004;122:119–125. doi: 10.1016/j.vetpar.2004.03.019. [DOI] [PubMed] [Google Scholar]
- 68.Griffiths E.C., Pedersen A.B., Fenton A., Petchey O.L. The nature and consequences of coinfection in humans. J. Infect. 2011;63:200–206. doi: 10.1016/j.jinf.2011.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Azad A.F., Beard C.B. Rickettsial pathogens and their arthropod vectors. Emerg Infect. Dis. 1998;4:179–186. doi: 10.3201/eid0402.980205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Horak I.G., Heyne H., Williams R., Gallivan G.J., Spickett A.M., Bezuidenhout J.D., Estrada-Peña A. The Genus Amblyomma Koch, 1844, The Ixodid Ticks (Acari: Ixodidae) of Southern Africa. Springer International Publishing; Springer; Cham, Switzerland: 2018. pp. 67–140. [Google Scholar]
- 71.Barker S.C., Walker A.R. Ticks of Australia. The species that infest domestic animals and humans. Zootaxa. 2014;3816:1–144. doi: 10.11646/zootaxa.3816.1.1. [DOI] [PubMed] [Google Scholar]
- 72.Walker A.R., Bouattour A., Camicas J.-L., Estrada-peña A., Horak I.G., Latif A.A., Pegram R.G., Preston P.M. Ticks of Domestic Animals in Africa: A Guide to Identification of Species. Bioscience Reports; Edinburgh, UK: 2003. p. 114. [Google Scholar]
- 73.Riveroa E.R.C., Neves A.C., Silva-Valenzuela M.G., Sousa S.O.M., Nunes F.D. Simple salting-out method for DNA extraction from formalin-fixed, paraffin-embedded tissues. Pathol. Res. Pract. 2006;202:523–529. doi: 10.1016/j.prp.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 74.Licari E., Takács N., Solymosi N., Farkas R. First detection of tick-borne pathogens of dogs from Malta. Ticks Tick Borne Dis. 2017;8:396–399. doi: 10.1016/j.ttbdis.2017.01.002. [DOI] [PubMed] [Google Scholar]
- 75.Welc-Falęciak R., Rodo A., Siński E., Bajer A. Babesia canis and other tick-borne infections in dogs in Central Poland. Vet. Parasitol. 2009;166:191–198. doi: 10.1016/j.vetpar.2009.09.038. [DOI] [PubMed] [Google Scholar]
- 76.Peleg O., Baneth G., Eyal O., Inbar J., Harrus S. Multiplex real-time qPCR for the detection of Ehrlichia canis and Babesia canis vogeli. Vet. Parasitol. 2010;173:292–299. doi: 10.1016/j.vetpar.2010.06.039. [DOI] [PubMed] [Google Scholar]
- 77.Parola P., Cornet J.-P., Sanogo Y.O., Miller R.S., Van Thien H., Gonzalez J.-P., Raoult D., Telford S.R., III., Wongsrichanalai C. Detection of Ehrlichia spp., Anaplasma spp., Rickettsia spp., and other eubacteria in ticks from the Thai-Myanmar border and Vietnam. J. Clin. Microbiol. 2003;41:1600–1608. doi: 10.1128/JCM.41.4.1600-1608.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Roest H.I.J., Ruuls R.C., Tilburg J.J.H.C., Nabuurs-Franssen M.H., Klaassen C.H.W., Vellema P., van den Brom R., Dercksen D., Wouda W., Spierenburg M.A.H., et al. Molecular epidemiology of Coxiella burnetii from ruminants in Q fever outbreak, the Netherlands. Emerg. Infect. Dis. 2011;17:668–675. doi: 10.3201/eid1704.101562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mlangeni M.A. Master’s Thesis. North-West University; Potchefstroom, South Africa: 2016. [(accessed on 5 April 2018)]. Molecular Epidemiology of Dourine, Equine Piroplasmosis and Ehrlichiosis from Donkeys and Horses in South Africa. Available online: https://repository.nwu.ac.za/handle/10394/21093. [Google Scholar]
- 80.Poostchi M., Silamut K., Maude R., Jaeger S., Thoma G. Image analysis and machine learning for detecting malaria. Trans. Res. 2016;194:36–55. doi: 10.1016/j.trsl.2017.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nei M., Kumar S. Molecular Evolution and Phylogenetics. Oxford University Press; New York, NY, USA: 2000. pp. 87–103. [Google Scholar]
- 82.Kumar S., Stecher G., Tamura K. MEGA7: Molecular Evolutionary Genetics Analysis version 7. 0 for bigger datasets. Mol Biol Evol. 2016;33:1870–1874. doi: 10.1093/molbev/msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kimura M. A simple method for estimating evolutionary rate of base substitutions through comparative studies of nucleotide sequences. J. Mol. Evol. 1980;16:111–120. doi: 10.1007/BF01731581. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.