Abstract
A locus close to one end of the linear N15 prophage closely resembles the sop operon which governs partition of the F plasmid; the promoter region contains similar operator sites, and the two putative gene products have extensive amino acid identity with the SopA and -B proteins of F. Our aim was to ascertain whether the N15 sop homologue functions in partition, to identify the centromere site, and to examine possible interchangeability of function with the F Sop system. When expressed at a moderate level, N15 SopA and -B proteins partly stabilize mini-F which lacks its own sop operon but retains the sopC centromere. The stabilization does not depend on increased copy number. Likewise, an N15 mutant with most of its sop operon deleted is partly stabilized by F Sop proteins and fully stabilized by its own. Four inverted repeat sequences similar to those of sopC were located in N15. They are distant from the sop operon and from each other. Two of these were shown to stabilize a mini-F sop deletion mutant when N15 Sop proteins were provided. Provision of the SopA homologue to plasmids with a sopA deletion resulted in further destabilization of the plasmid. The N15 Sop proteins exert effective, but incomplete, repression at the F sop promoter. We conclude that the N15 sop locus determines stable inheritance of the prophage by using dispersed centromere sites. The SopB-centromere and SopA-operator interactions show partial functional overlap between N15 and F. SopA of each plasmid appears to interact with SopB of the other, but in a way that is detrimental to plasmid maintenance.
The temperate coliphage N15 is in most respects a phage of the lambda type. Its DNA is similar to that of λ, 46.4 kb long and double stranded, with cohesive ends (33, 35, 39). Its lytic development resembles that of λ, resulting in virions with λ-like morphology, and it lysogenizes at similar frequencies (33). Its head and tail genes have extensive homology with those of λ, and the elements which control transcription and determine prophage immunity find their homologues in the repressor, operator, and antiterminator functions of λ and P22 (20). But the physical state of the N15 prophage is distinctive. Whereas the λ prophage is integrated into the chromosome, N15 prophage is a low-copy-number plasmid (35, 36). Moreover, unlike other prophage plasmids, such as P1, N15 is a linear double-stranded DNA molecule with closed, hairpin-like ends (39), the only replicon of Escherichia coli known to be stably maintained in this state.
The nucleotide sequence of N15 was published recently (13). Comparison of this sequence with those in the sequence databases revealed extensive homology with temperate-phage genomes, as indicated above. But perhaps the most remarkable similarity was that of a discrete region, close to one end of the N15 prophage, with the locus that governs partition of F plasmid copies to daughter cells (Fig. 1). This locus, sop, contains an operon of two genes (sopA and sopB) and a centromere-like site, sopC: all three elements are essential for stable inheritance of F in dividing cells (1, 28). The SopA protein binds to the sop promoter and, together with SopB, represses transcription of the operon, which thus is autoregulated (25). The SopB protein binds to sopC and, together with SopA and unknown host factors, orients F plasmid siblings and impels them towards the centers of the new daughter cells (8, 11, 25, 27).
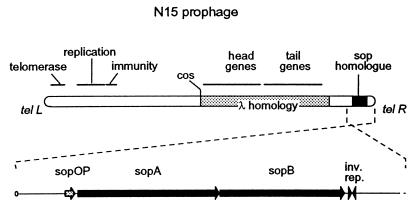
FIG. 1.
N15 prophage and the locus of homology with the F plasmid sop operon. The 46,375-bp bacteriophage sequence (13) was converted to the prophage form by in silico ligation of the ends followed by cutting at bp 24802 and -3. The prophage sketch is oriented following Lobocka et al. (19), with the enlargement of the sop homologue locus inverted to correspond to the usual representation of F sop. The rounded ends represent telomeric structures (telL and telR). Other loci are assigned on the basis of sequence data (13) and are drawn only approximately to scale.
The sop-like sequence of N15 includes the promoter region and two open reading frames encoding proteins similar to SopA and SopB, suggesting that the N15 sop homologue might specify a partition mechanism which ensures stable inheritance of the N15 prophage. Insertion of a transposon in this locus destabilizes plasmid maintenance (37). On the other hand, the N15 locus lacks an obvious counterpart of the 12 43-bp repeats which constitute the essential sopC site. Moreover, N15 was reported to be fully compatible with F (39). It was possible, therefore, that the sop homologue might be vestigial, as observed for certain replication loci in the F plasmid, or that it plays a role other than partition in maintenance and propagation of N15. The work reported in this paper was undertaken to determine whether the sop homologue in N15 is involved in partition and whether it interacts with the F plasmid partition system. (In a recently published review article [36a], the N15 sop region is referred to as par. Because the degree of homology of the proteins encoded by this region with the F Sop proteins is significantly higher than that with any other partition proteins, we will continue to use sop in this article to describe the components of the N15 partition system.)
MATERIALS AND METHODS
Bacteria and growth conditions.
The strains used in this study were derivatives of Escherichia coli K-12. MC1061 (5) and DH10B (9) were the primary recipients for transformation with DNA ligated in vitro. Strain DLT1127 was used for measurement of sop promoter activity: it was constructed by first preparing a lysate of a strain carrying the Kanr sopP::lacZ fusion plasmid, pZC450 (42), with λRS88 (38) and then selecting a Kanr Lac+ monolysogen from MC1061 (Δlac) cells infected with the λRS88 lysate. DH10B (recA1) was the host strain for plasmid stability measurements.
Cultures were grown with aeration at 37°C in Luria-Bertani (LB) broth (24), supplemented as appropriate with chloramphenicol (20 μg/ml) or kanamycin (50 μg/ml) and with 1.5% agar (Difco) for solid medium.
Plasmids. (i) Mini-F plasmids.
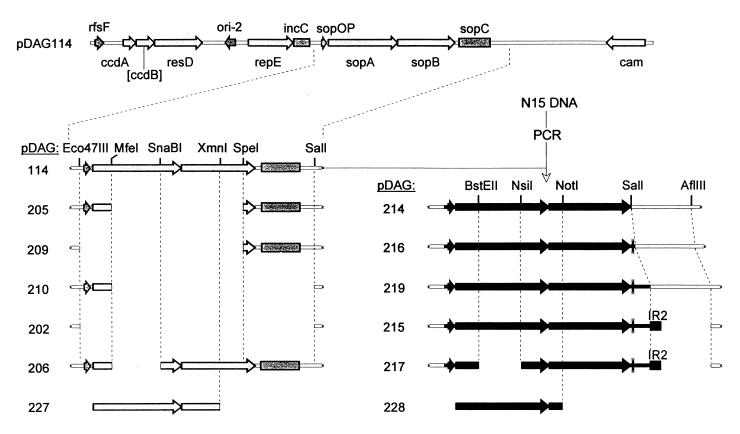
All mini-F plasmids were derived from pDAG114 by restriction fragment-mediated deletion and/or insertion of PCR fragments (Fig. 2). pDAG114 is an essentially wild-type mini-F which carries the resD-rfsF multimer resolution determinants and a Ccd system disabled by Klenow polymerase-mediated filling of the ApaLI site in ccdB. The N15 sop operon was amplified by PCR on N15 DNA using an upstream primer, 5′-GATGTTAACCTTAACTTTGCGTTTTC (PR1), with an HpaI site (underlined) and downstream primers with SalI sites (underlined), 5′-GGCGTCGACTATTGACC CTATTTTTTCTC (PR4; the double underline indicates the sopB stop codon), TGATTGTCGACTGTTGTAAAAAGCGA (PR6), and CATGTGTCGACTCGTTACTTGTTTC (PR9), and was inserted between the Eco47III and SalI sites of pDAG114, thus deleting the entire F sop locus and giving mini-Fs without (pDAG214) and with (pDAG216 and -219) the downstream inverted repeat. A fragment containing the IR2 sopC-like sequence was made by annealing two 46-mers (TCGAATAATCTGATATCGTGCGACCATGGTCGCACGGAATAGAAAA [PR23; the inverted repeat {IR2} sequence is underlined] and CACGTTTTCTATTCCGTGCGACCATGGTCGCACGATATCAGATTAT [PR24]) whose 5′ ends were complementary to the bases exposed by SalI and AflIII cleavage of pDAG219; the fragment was inserted between these sites to make pDAG215. All deletions were made, using the restriction endonucleases shown in Fig. 2, by repair of the ends with DNA polymerases and deoxynucleoside triphosphates (Klenow and T4 for 5′ and 3′ extensions, respectively) followed by ligation.
FIG. 2.
sop locus variants of mini-F. The pDAG114 plasmid is represented as a linear molecule; only the sop regions and restriction sites used in construction are shown below. ccdB is inactive, as indicated by square brackets. Light shading indicates genes and sites of mini-F, and dark shading indicates those of N15. Gaps show the extent of deleted DNA. The thin double line after the N15 sop operon is a natural IR. The block labelled IR2 is an inserted IR (see Materials and Methods). The sopB deletions at the bottom were introduced into sop operons inserted in the pZS*21 vector.
(ii) pZS*21 plasmids.
For provision of sop function in trans, sopA and -B genes were inserted as PCR fragments downstream from the PLtetO hybrid promoter in the low-copy-number vector pZS*21 (21). The sopABF fragment was obtained with the primers PR11, ATGGGGCCCATGAAACTCATGGAAACAC, and PR12, GTGGTCGACTCAGGGTGCTGGCT (underline, ApaI and SalI sites; double underline, sopA start codon and sopB stop codon); after treatment with ApaI and SalI it was inserted between the corresponding sites in the vector to form pDAG221. The same procedure was used to insert sopABN15, using primers PR10 (GGAGGGCCCATGTCGTTAATTAATTTGCTG; underlines as above) and PR4 (see above), to produce pDAG222. Derivatives of these plasmids lacking the sopB C terminus were made by deleting from the sites shown in Fig. 2 to the adjacent ClaI and SalI sites in the polylinker, and a 675-bp fragment carrying a chloramphenicol resistance gene was then inserted into the HindIII site in the polylinker of pZS*21, pDAG221, pDAG222, and the ΔsopB derivatives (see Fig. 2) to form pDAG10, -223, -224, -227, and -228, respectively. The Kanr sequence was deleted from pDAG223 with EagI and Ecl136II to produce pDAG241.
(iii) Other plasmids.
The midi-N15 plasmid, pG54, consists of the 20.1-kb BglII fragment of the N15 phage genome (23,699 to 43,800 bp) ligated to a BamHI fragment containing a Kanr gene from pUC4K (Pharmacia) (34). pZC450, a pBR322-based plasmid carrying the sopP::lacZ fusion (42), was provided by D. P. Biek.
DNA manipulations and related procedures.
Restriction endonuclease digestions, Klenow and T4 DNA polymerase reactions, and ligations with T4 DNA ligase (New England Biolabs) were carried out under conditions recommended by the supplier. Pfu DNA polymerase (Stratagene) was used for PCR amplification. DNA fragments from agarose gels and plasmid DNAs from cultured cells were purified using commercial kits (Qiagen). Standard procedures were used for agarose gel electrophoresis and for transformation with plasmid DNA.
Plasmid stability assays.
All experiments were started from colonies of cells freshly transformed with the plasmids under test. Two procedures were used; both gave the same results. In one, fresh overnight cultures of DH10B derivatives in LB medium containing selective antibiotics were diluted 200-fold into the same medium and grown to a cell density of 3 × 108 to 4 × 108/ml. Samples were diluted 103-fold into fresh LB medium without the antibiotic selective for the plasmid under test, and the new cultures were grown to at least the same cell density. The dilution and regrowth was repeated to allow the cells to go through 20 to 40 generations. To determine the fraction of cells that retained the plasmid, samples taken at the beginning and from each regrown culture were either plated on LB agar, followed by replica plating of the colonies to selective agar, or, where the fraction of plasmid-carrying cells was expected to be low, plated in parallel on nonselective and selective agar. In the other procedure, each fresh overnight culture grown as described above was diluted to various extents (usually 103, 106, and 109) in nonselective medium, and the diluted cells were regrown as separate cultures to early stationary phase and assayed for the fraction of plasmid-bearing cells as described above. Although loss rates were determined two or three times for most plasmids, the data shown in Results is representative rather than averaged because the true number of generations at sampling varied between experiments.
The percentage of plasmids lost per generation (L) was calculated from the equation L = 1 − (Ff/Fi)1/n × 100, where Fi is the fraction of cells carrying the plasmid initially and Ff is the plasmid-carrying fraction after n generations of nonselective growth.
Copy number measurement.
For the first demonstration that the N15 sop operon is involved in partition rather than replication, copy number was determined by quantifying radioactive plasmid and chromosomal probe DNAs hybridized to total DNA. Cultures grown as for the measurement of plasmid stability were sampled after 3.5 generations of nonselective growth for assay of the fraction of plasmid-bearing cells, as described above, and for preparation of DNA. DNA was extracted by lysis of the cells with lysozyme, sodium dodecyl sulfate (SDS), and proteinase K, then purified by extraction with phenol and chloroform, treatment with ribonuclease A, reextraction with phenol and chloroform, and precipitation with isopropanol. Redissolved DNA (0.25 μg) was digested to completion with PflMI, and the fragments were fractionated by electrophoresis in 0.8% agarose in Tris-borate-EDTA buffer and transferred by capillary blotting to uncharged nylon membrane (Hybond N; Amersham). After fixing of the DNA by UV cross-linking, the membrane was incubated in hybridization buffer consisting of 50% formamide, 0.75 M NaCl, 0.05 M sodium phosphate (pH 6.8), 0.1% SDS, and 0.1 mg of sonicated and denatured herring sperm DNA/ml at 42°C for 2 h. DNA fragments corresponding to portions of the repE gene of mini-F and the ldc gene (18) of E. coli were obtained by PCR and labelled with 32P by random primer extension (Megaprime kit; Amersham). The labelled fragments were added to the hybridization buffer at ≥40-fold molar excess over the expected amounts of the target sequences on the membrane. After overnight incubation at 42°C, the membrane was washed (final wash solution, 15 mM NaCl–0.05% SDS) at room temperature and exposed to a Fuji phosphorimager screen. The bands were quantitated with the TINA-PCBas program (Fuji Photo Film Co. Ltd.), and the figures were corrected for the fraction of cells that had lost the plasmid to give plasmid DNA per chromosome ratios. These ratios are not absolute, since the specific activities of the probes were not determined, but they allow accurate estimation of copy number differences between strains.
For subsequent estimations of copy number, total plasmid DNA was isolated by the Qiagen miniprep procedure and samples were subjected to agarose gel electrophoresis followed by ethidium bromide staining. The bands on the digitized gel image were quantitated with PCBas, and the ratios of test plasmid (mini-F or pG54) to stable coresident plasmid (pZS*21 derivative) were corrected for plasmid size and plasmid loss to yield relative copy numbers, as described above.
Western immunoblotting.
Cells grown exponentially to an optical density at 600 nm (OD600) of ≈0.3 were centrifuged and resuspended in SDS-sample buffer (16) at 5 OD600 units/ml. The samples were incubated at 100°C for 5 min and chilled, and various volumes were applied to a 10% polyacrylamide gel in Tris-glycine buffer. The separated proteins were transferred to polyvinylidene difluoride membrane (Immobilon; Millipore) by electroblotting them in carbonate buffer (7). Immunodetection was performed essentially as described previously (3), using a 1:1,000 dilution of purified anti-SopA as the primary antibody and goat anti-rabbit immunoglobulin G (1:1,000) coupled to horseradish peroxidase (Sigma) as the secondary antibody. Bound peroxidase was detected with an ECL system (Amersham) and exposure to X-ray film. The digitized X-ray images were quantitated with the TINA-PCBas program. The antiserum was raised with a peptide corresponding to the C-terminal 12 amino acids of SopA (42) and was kindly provided by D. P. Biek.
Sop promoter repression assay.
Derivatives of DLT1127 were grown overnight from single colonies in LB medium with kanamycin and the antibiotic appropriate for the plasmid carrying the function to be tested. The cultures were diluted 100-fold into fresh medium with antibiotics and grown to an OD600 of ≈0.3. They were then sampled for determination of β-galactosidase specific activity (in triplicate) as described previously (17).
RESULTS
Sequence homology of sop with an N15 locus.
The F plasmid SopA and -B proteins show strong sequence homology with the predicted products of two open reading frames near the right-hand end of the linear N15 prophage (Fig. 1). The SopA homologues have 75% amino acid identity, and the SopB homologues have 49% (Fig. 3). The N15 open reading frames are preceded by a sequence similar to that of the sop promoter-operator region: four copies of a CTTTG motif, oriented and spaced identically to those in F and likewise interspersed with dA-dT runs, overlap the −35 and −10 promoter sequences. Nuclease protection experiments indicate that the CTTTG motifs in F are recognized and bound by SopA (25). In view of its strong homology with F sop, we use the term sop for the N15 locus.
FIG. 3.
Alignment of Sop homologues of N15 and F. In sopOP, the sop promoter-operator top-strand alignment is based on pairing the 5′-CTTTG SopA binding sites (dark shading) (24), whose relative orientation is indicated by arrows; the light shading denotes promoter and Shine-Dalgarno sequences and the sopA start codon. In SopA, amino acid sequences were aligned with the Multalin program. Shading denotes identical residues. The nucleotide and Mg binding sites are labelled ATP I (Walker A box) and II, respectively. Motifs 2 and 3 are conserved in members of the SopA/ParA partition protein family (25). The underlined sequence and that in SopB below are predicted helix-turn-helix structures (6). In SopB, aligned as for SopA, minor changes in parameters alter gap positions, most notably aligning the N15 C-terminal KNKEKK with the F C-terminal KELEKP. The large box (dotted outline) shows an F SopB peptide which protects sopC DNA; the small box (solid outline) indicates a C-terminal deletion which eliminates the protection (10).
Alignment of the SopA sequences shows that the homology is uniformly distributed throughout the protein. In particular, the motifs characteristic of the ParA/SopA family of partition proteins, ATP I (40) and ATP II, and motifs 2 and 3 (26) are strongly conserved in N15 SopA. In contrast, the similarity between the SopB sequences is strong only between residues 43 and 226 of the 323-amino-acid F SopB protein. A part of this homologous region lies within the extended C-terminal domain of F SopB which makes contact with DNA (10). It is not known which part of this domain in F SopB specifically recognizes the sopC site.
Sequence homology between N15 and F does not extend to the region downstream of sopB. Whereas in F this region is occupied by sopC, in N15 the only feature identified was an IR sequence of 24 bp which bears no obvious similarity to sopC.
Lack of stabilization by the N15 sop region.
To determine whether the sop-homologous region of N15 can act as a partition locus, we tested its ability to stabilize a mini-F plasmid which lacks its own partition functions. PCR fragments (Fig. 2) containing the sequences equivalent to the sop promoter, sopA and sopB, with or without the inverted repeat sequence downstream from the sopB open reading frame, were inserted into a mini-F Δsop plasmid. The stabilities of these plasmids were determined by measuring the rate at which plasmid-free cells accumulated during about 30 generations of growth in the absence of antibiotic selection. The result for the plasmid with the longest PCR insert, pDAG219, is shown in Fig. 4a; those for the other plasmids, which were lost at the same rate as pDAG219, are omitted for clarity. None of the plasmids was significantly more stable than the mini-F Δsop vector. This result indicated either that the sop open reading frames do not confer partition functions or that the downstream sequence is not sufficient for them to do so.
FIG. 4.
Ability of N15 sopAB to substitute for F sopAB. (a) Segregation of wild-type mini-F (pDAG114 [open circles]), mini-F ΔsopPABC (pDAG202 [solid circles]), and mini-F carrying the sop operon locus of N15 (pDAG219 [solid triangles]). (b) Segregation of mini-F ΔsopAB (pDAG205) from cells also carrying pDAG221 (sopABF [solid triangles]), pDAG222 (sopABN15 [open triangles]), or the vector (pZS*21 [solid circles]). (c) Relative pDAG205 copy number. In an experiment duplicating that shown in panel b, samples were withdrawn from cultures after three to four generations of growth for estimation of the fraction of cells still carrying pDAG205 (fr. cells mF+) and for measurement of copy number by hybridization of radioactively labelled mini-F and chromosomal probes to Southern blots of extracted DNA (see Materials and Methods). The PflMI site adjacent to repE is poorly cleaved (PflM), resulting in two mini-F fragments being detected; the radioactivity from both bands was summed for the calculation of miniF/chromosome ratios (mF/ldc). Correction for the fraction of each population that had lost the mini-F plasmid allowed calculation of the number of mini-F plasmids per cell relative to that in the strain with no sopAB (−) [mF/cell (rel.)]. This in turn allowed calculation of the contribution of the ∼10% copy number increase to stability: assuming random distribution of the partition-defective plasmid pDAG205 to daughter cells, the observed loss rate of 5.5% corresponds to a copy number of 4.2 at division (loss rate = 0.5copy-number), leading to an expected loss rate for the 1.1-fold-higher copy number of 4.1% in the absence of active partition. (d) Segregation of mini-F plasmids carrying sopC (pDAG209 [diamonds]) or sopP (pDAG210 [squares]) in the presence (open symbols) or absence (solid symbols) of a plasmid (pDAG222) carrying the N15 sop operon.
We next attempted to determine whether the N15 sop open reading frames specify partition functions by testing their ability to complement the partition deficiency of mini-F plasmids with partial sop deletions.
Complementation by the N15 sop region.
To provide a source of N15 SopA and SopB proteins for complementation studies, we placed the sopAB genes of N15 and F under control of a modified Ptet promoter in pZS*21, a vector derived from a low-copy-number mutant of pSC101 (21, 23). This strong promoter is normally subject to regulation by the TetR repressor, but we were able to obtain suitably modest levels of Sop proteins by placing the sopA ATG codon 18 bp from the vector Shine-Dalgarno sequence, thus diminishing the frequency of translation. The results of a Western blot analysis of extracts prepared from cells carrying these plasmids (pDAG221 [sopF] and pDAG222 [sopN15]), using antibodies raised against F SopA protein, are shown in Fig. 5. They show that the steady-state level of F SopA in cells carrying pDAG221 is about three times higher than that in cells carrying wild-type mini-F and that the corresponding N15 SopA is indeed produced. Because we have no measure of its affinity for anti-SopA we cannot estimate its concentration, but we assume it is similar to that of F SopA. We did not detect N15 SopB, since it does not cross-react with the anti-SopB antibody available to us.
FIG. 5.
Western blot analysis of SopA production. Exponential cultures of strains harboring wild-type mini-F (pDAG114), mini-F ΔsopAF (pDAG206), pZS*21 carrying the sop operon of F (pDAG221) or N15 (pDAG222), or no plasmid (−) were grown in LB broth with appropriate antibiotics and sampled for preparation of cell extracts. A series of twofold concentration increments of each sample was subjected to SDS-polyacrylamide gel electrophoresis and Western blot analysis with anti-SopA antibody. Lanes showing approximately the same level of cross-reacting host protein(s) were chosen. The band intensity was proportional to amounts applied to the gel for the film exposure times used.
To test the ability of N15 Sop proteins to substitute for those of F, we introduced pDAG221 and -222 into strain DH10B carrying pDAG205, a mini-F plasmid with a deletion of sopAB, and measured the stability of pDAG205 in the transformants. The plots of plasmid loss (Fig. 4b) show that the provision of N15 SopA and SopB proteins resulted in considerable stabilization of pDAG205. When accompanied only by the vector, the mini-F plasmid was lost at a rate of 5.0% per generation, whereas in the presence of pDAG222 the loss rate dropped to 0.5%. The F SopA and SopB proteins, produced from pDAG221, stabilized pDAG205 completely. To test the possibility that a copy number increase was responsible for the observed stabilization, we determined the copy numbers of pDAG205 per chloramphenicol-resistant cell by hybridization of radioactive mini-F and chromosomal (ldc) probes to total DNA extracted from cells in logarithmically growing cultures (Fig. 4c). The result showed that a slight (∼10%) increase in copy number occurred in the presence of the sop genes of F and N15. This accounts for only a small fraction of the stabilization (see the legend to Fig. 4), indicating that most of the stabilization results from active partition.
The only components of the F sop system remaining in pDAG205 are the promoter and sopC. Derivatives (pDAG209 and -210) lacking each of these were tested for stabilization by N15 SopA and SopB, as described above. Only pDAG209, which carries sopC, was stabilized (Fig. 4d). We conclude from these results that the SopA and SopB proteins of N15 can substitute for the F partition proteins in stabilizing a plasmid carrying sopC.
In light of these results it appeared that the N15 Sop proteins might also act in concert with a sopC-like sequence elsewhere on the N15 prophage to ensure correct partition of the plasmid.
Stabilization of a midi-N15.
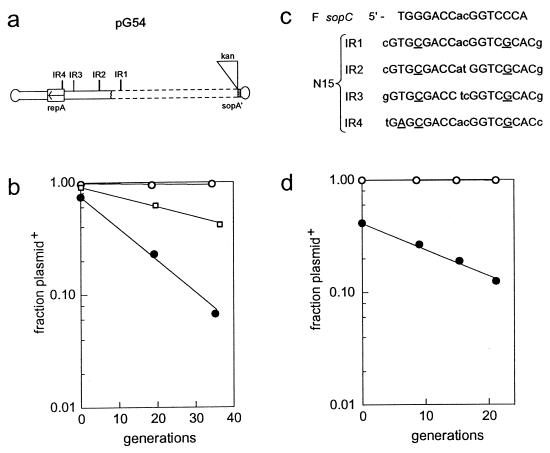
To test the possibility that a centromere site similar to sopC exists in N15, distant from sopAB, we made use of a plasmid formed by deletion from N15 of a 25.5-kb fragment that includes most of the sop locus. This unstable plasmid, pG54, carries N15 replication functions and a kanamycin resistance gene (34) (Fig. 6a). Cells carrying pG54 were transformed with mini-F carrying the N15 sop operon, pDAG216, and with the Δsop mini-F, pDAG202, and the stability of pG54 in the resulting strains was tested as described above (Fig. 6b). In the presence of pDAG216, pG54 was stable (0.06% loss per generation), whereas when accompanied by pDAG202 it was lost at 6.4% per generation. Analysis of extracted plasmid DNA by gel electrophoresis and ethidium bromide staining showed that the ratio of pG54 DNA (per plasmid-carrying cell) to the mini-F vector was the same in both strains (data not shown); stabilization is thus not a result of increased copy number.
FIG. 6.
Identification of sopC-like centromere sequences in N15 DNA. (a) The midi-N15 plasmid, pG54. The dashed box shows the 25.5-kb BglII deletion which has removed sopB and the 3′ end of sopA. IR1 to -4 denote sopC-like IRs detailed in panel c. (b) Segregation of pG54 from cells also carrying the mini-Fs pDAG202 [Δsop(PABC)F (solid circles)] and pDAG216 (sopPABN15 [open circles]) or the pZS*21-based plasmid pDAG241 (sopABF+ [open squares]). (c) Comparison of N15 IR sequences with the sopC IR. The underlined bases show differences from the sopC sequence. (d) Segregation of mini-F plasmids carrying the N15 sop operon without (pDAG219 [solid circles]) or with (pDAG215 [open circles]) IR2.
This result suggested strongly that a sequence with centromere function is present in the part of N15 that remains in pG54. The likelihood that this sequence resembles sopC was increased by the observation that the SopA and -B proteins of F, produced from the pZS*21-based plasmid pDAG241, were able to partly stabilize pG54 (2.1% loss per generation) (Fig. 6b).
SopC-like sequences of N15.
Within each 43-bp repeat of sopC, the sequence protected by SopB binding, presumably the specific recognition site, is an IR, 5′-TGGGACCnnGGTCCCA (n denotes variable bases). A search of the N15 genome for sequences with at least a five-of-seven match to one arm of this sequence uncovered four such IRs (IR1 to -4) (Fig. 6c). Three of these are present in pG54 (Fig. 6a). Apart from the 2-bp spacers, all have the same sequence, which differs from the F sopC repeat by a C↔G exchange at the fourth position in each arm and by a 1-bp increase in length (2 bp if degeneracy at the end position is allowed). The only exception is IR4, which has an A-to-T change at position 2 of one arm. Unlike the sopC repeats, the sequences flanking the IRs have no obvious similarity, and the IRs themselves are scattered on the N15 genome.
To determine whether these sequences can confer centromere function, complementary oligonucleotides corresponding to IR2 were inserted downstream of the N15 sopAB operon in pDAG219 (Fig. 2) to create pDAG215, and the stabilities of the two plasmids were measured (Fig. 6d). The plasmid containing IR2 was essentially stable (0.07% loss per generation), while the parental pDAG219 remained unstable (5.0% loss). Substitution of a short fragment carrying IR3 for IR2 gave a similar result (<0.02% loss per generation [data not shown]). We conclude that the IR2 and IR3 sopC-like sequences of N15 can act as centromeres. Presumably the other two IR sequences can as well.
These results (Fig. 6b), together with those of the reciprocal experiments shown in Fig. 4b and d, suggest that the SopB protein of each plasmid can interact functionally with the sopC sequences of the other. We next examined whether, in addition, a functional exchange of SopA alone is possible, that is, whether SopA of one plasmid can interact productively with SopB of the other.
Stabilization and destabilization by SopA.
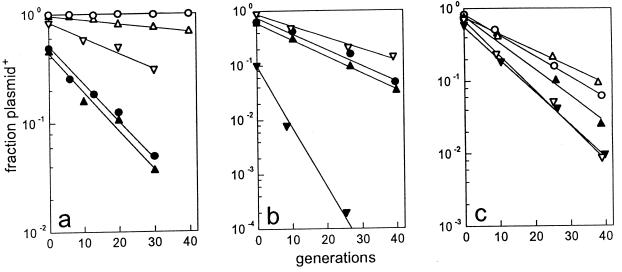
The bulk of the sopA gene was deleted from mini-F (pDAG114) carrying the wild-type F sop locus, producing plasmid pDAG206, and from mini-F (pDAG215) carrying the N15 sop operon and the IR2 centromere sequence to yield pDAG217. Elimination of SopA function is expected to derepress sop operon transcription, thus raising the SopB concentration and risking direct inhibition of plasmid maintenance (IncG incompatibility [15]). Therefore, we ensured that the deletions left the sopA 3′ end out of phase for translation, since maximal SopB production appears to require upstream coding capacity (43), presumably to allow translational coupling. In the event, the stability of the ΔsopA plasmids (6.5 to 7% loss per generation) (Fig. 7a and c) was marginally lower than that of the equivalent ΔsopC plasmids (5.5 to 6%) (Fig. 4a and 6b), indicating a moderate degree of SopB overproduction.
FIG. 7.
Effect of SopA on the stability of ΔsopA miniplasmids. For clarity, the rates of loss of pDAG206 (mini-F ΔsopAF) measured in separate experiments are shown in panels a and b, with the results for certain complementing plasmids shown in both. Note the different ranges on the ordinate of each panel. (a) Segregation of pDAG206 from cells which also carry pZS*21 (no sopAB [solid triangles]), pDAG221 (sopABF+ [open circles]), pDAG222 (sopABN15+ [open triangles]), pDAG225 (sopAF+ [open inverted triangles]), or no other plasmid (solid circles). (b) Same as panel a plus pDAG226 (sopAN15+ [solid inverted triangles]). (c) Segregation of pDAG217 (mini-F ΔsopAN15) from cells which also carry the plasmids shown in panels a and b.
The segregation of the mini-F sopA deletion derivatives in the presence of N15 and F Sop proteins produced from coresident pZS*21 derivatives is shown in Fig. 7. It is clear that although the two ΔsopA plasmids are lost at about the same rate (∼7%), they respond differently to Sop proteins supplied in trans. Whereas provision of SopA and SopB of either F or N15 results in substantial stabilization of pDAG206 (ΔsopAF), the increase in pDAG217 (ΔsopAN15) stability is only slight (Fig. 7a and c). Providing SopA alone gave surprising results. In the case of pDAG206, F SopA alone complemented the deletion mutant only weakly, presumably because of incomplete compensation for SopB-induced inhibition of replication, while the heterologous SopA of N15 severely destabilized the mutant (Fig. 7b). In the case of pDAG217, provision of either SopA protein resulted in moderate destabilization (Fig. 7c).
These results are not easy to interpret. The destabilization by heterologous SopA is presumably not due to increased repression of sopB transcription, since this would not increase plasmid loss beyond that seen for random segregation. It is clear, however, that in neither case is the plasmid with sopA deleted indifferent to the SopA protein of the other plasmid, suggesting that each SopA can interact with SopB of the other plasmid in an aberrant manner that leads to high rates of plasmid loss.
Sop promoter repression.
The similarity of the promoter sequences suggested that the N15 and F Sop proteins might be able to bind to each others’ operators to repress transcription. A fusion of the F sop promoter to the lacZ gene was integrated into the chromosome as part of a λ prophage (see Materials and Methods), and Sop proteins were provided in trans from pZS*21 derivatives (see Materials and Methods) (Fig. 2). The amounts of β-galactosidase made during exponential growth of the resulting strains were measured. The results in Table 1 show that, as expected, F SopA protein has a weak repressing effect on its own promoter but that repression is greatly enhanced in the presence of SopB. In the presence of N15 SopA protein, transcription is reduced just enough to indicate a very weak interaction with the sop promoter. When N15 SopB protein was also present, repression was greatly enhanced. The repressor and corepressor properties of the N15 Sop proteins thus appear to be equivalent to those of their F counterparts.
TABLE 1.
Repression of transcription from the F sop promotera
| Plasmid | sop genes | β-galactosidase (normalized) (± SD) | n |
|---|---|---|---|
| pDAG10 | 1 | 3 | |
| pDAG223 | sopAF+sopBF+ | 0.0063 ± 0.0005 | 4 |
| pDAG227 | sopAF+ ΔsopBF | 0.58 ± 0.04 | 2 |
| pDAG224 | sopAN15+sopBN15+ | 0.11 ± 0.01 | 3 |
| pDAG228 | sopAN15+ ΔsopBN15 | 0.96 ± 0.03 | 2 |
| −/no promoter | <0.0002 | 4 |
β-Galactosidase specific activities are expressed as fractions of the activity measured for the unrepressed sop promoter (3,180 ± 112 [standard deviation] Miller units). n, number of cultures assayed. The bottom line shows the level (essentially undetectable) in cells carrying the empty λRS88 vector prophage.
Our attempts to construct a fusion of the N15 sop promoter with lacZ were unsuccessful. However, preliminary slot blot hybridization experiments have shown that transcription initiated at the N15 sop promoter is repressed in the presence of N15 SopA and B proteins (data not shown), indicating that this promoter is subject to autoregulation, like sopP of F.
DISCUSSION
The main reason for undertaking this study was to exploit the sequence differences between the very similar sop genes of F and N15, with a view to locating determinants of specific interactions involved in partition. However, it was important first to identify all the components of the relatively unknown N15 partition system and to carry out a preliminary assessment of the extent to which the F and N15 systems interact. This we have now done. The results presented here show that the N15 locus identified by its homology with the F plasmid sop operon is functional for partition. The partition system specified by this locus is probably the only one capable of assuring stable inheritance of the N15 prophage, since transposon insertions in the locus cause instability (37). We have identified centromere-like sites with which the products of the N15 sop genes interact to stabilize plasmid maintenance. Results of a parallel study in the laboratory of M. Lobocka (Warsaw, Poland) also support a role for these sites in partition (19a). In addition, we have shown that the similarity to the F sop system is sufficient to allow some degree of functional complementation. Finally, our results show that SopA of N15 can recognize the F sop promoter and that its ability to impose effective repression depends on its cognate SopB.
Apart from the sequences of their IRs, the N15 and F centromeres appear to be notable more for their differences than their similarities. The difference in the number of repeats, 4 in N15 versus 12 in F, is probably of little significance, since a single 43-bp unit of sopC suffices to stabilize mini-F (4), as does a single IR of N15 (Fig. 5d). A more striking difference from the F IR, which is embedded in a longer repeated sequence, is the lack of extensive similarity among the sequences flanking the N15 IRs. This suggests that the repetition or otherwise of the flanking sequences is of little significance for present-day function, a proposal consistent with the observation of sequence-independent wrapping of DNA by SopB (4, 22). The presence or absence of flanking repetitions might rather reflect diversity in the mechanisms that originally amplified the F and N15 IRs.
Perhaps the most remarkable difference between the sopC-like sites is their arrangement. Whereas in F the sopC centromere site consists of a compact series of sequence repeats adjacent to the sop operon, in N15 the sopC-like sequences that function as centromeres are scattered over about 13 kb of N15, far from the sop locus: it is possible that others, more divergent from sopC, remain to be identified. Recent reports of other close sequence homologues of the F sop locus suggest that the separation and dispersal of sopC-like sites seen in N15 is unusual. The sop loci of Yersinia plasmids pCD1 and pYVe227 encode gene products with the same high degree of similarity to F SopAB as those of N15 (14, 30), but their sopC-like sequences are located within longer directly repeated sequences just downstream of sopB, as in F. Other examples of dispersed centromere sites are known, however: in RK2, sites which bind KorB (the SopB analogue), some of them apparently defective for partition, are scattered over several kilobases (41), and in Bacillus subtilis eight sites situated within a segment one-fifth the size of the chromosome bind Spo0J to assure chromosome partition (19).
How did the dispersal of sites arise in the ancestry of N15? One possibility is that an initially integrated locus was split by successive recombination events, possibly with the loss of some of the IRs. Alternatively, the operon may have separated from its original centromere during the recombination event that inserted it into an N15 ancestor, one which happened to carry sopC-like sequences that initially served other purposes. The presence of three of the IRs (IR1 to -3) at intergenic sites is consistent with either scenario.
The scattered arrangement of sopC-like sites also raises the questions of how many are normally involved in each partition event and of whether intramolecular coupling of partition complexes can impede the intermolecular pairing supposedly needed for faithful partition. Although we cannot yet resolve these issues, it is worth noting that they arise also in the case of dicentric derivatives of the plasmids NR1 and P1 and that these derivatives are maintained stably (2, 31).
It is clear from the results presented here that the sopC sequences in F and N15 are sufficiently similar to allow the SopA and -B proteins of each plasmid to substitute partly for those of the other. This interchangeability must rely primarily on the capacity of each SopB to bind appropriately to the other sopC site, implying that the binding domains of each SopB have several residues in common. Nevertheless, the interchangeability is far from total. Overproduction of Sop proteins is needed for even partial stabilization of plasmids carrying the sopC homologue (Fig. 5b), suggesting substantial differences in the affinity of each SopB for the two sopCs. The SopB domain that recognizes and binds to sopC has not yet been identified for any protein of the SopB/ParB family. Protein modification and protease protection experiments have indicated that multiple DNA contacts are made by the C-terminal two-thirds of F SopB (10). N15 and F SopB sequences show significant similarity only within the first part of this region, between residues 141/139 and 228/226 (Fig. 2), suggesting that sopC recognition specificity is located in this segment. A candidate for this determinant might be F SopB residues 179 to 198, which were predicted by Dodd and Egan (6) to form a helix-turn-helix DNA binding structure. This motif differs from the corresponding N15 sequence at several residues, consistent with reduced affinity for N15 sopC, as discussed above. However the role of the motif remains to be tested by direct experiment.
We expected that SopA produced from a coresident plasmid would at least partly stabilize a mini-F plasmid carrying the cognate partition system with a sopA deletion. This was so for F sop but, surprisingly, not for N15 (Fig. 7). The N15 SopA protein actually destabilized the ΔsopAN15 plasmid slightly. We cannot easily explain this behavior, since supplying both N15 Sop proteins in trans resulted in moderate stabilization. Ogura et al. (29) reported that an excess of SopA destabilizes mini-F. Under our conditions, a slight excess of F SopA stabilized the ΔsopAF plasmid, as noted above, but it is possible that N15 partition is more sensitive to an excess of its SopA protein and that supplying it together with SopB returns the ratio of the two proteins to within tolerable limits. Provision of F SopA protein to the ΔsopAN15 plasmid resulted in a similar slight destabilization. In this case it is possible that SopAF forms an abnormal complex with SopBN15 which prevents replication or the separation of paired plasmids, thus generating plasmid-free cells at a higher rate.
A phenomenon of this kind is even more likely to be involved in the very strong destabilization of the mini-F ΔsopAF by SopA of N15 (Fig. 7b). A similar situation arose during an extensive study of specificity determinants in the Par proteins of P1 and a close relative, P7 (32). In a cell containing a hybrid operon with ParA from one plasmid and ParB from the other it was difficult to establish a plasmid that carried the parS centromere. An inappropriate interaction that prevented dissociation of the partition complex and so blocked plasmid propagation was proposed as a possible cause.
The properties of other P1-P7 hybrid proteins led Radnedge et al. (32) to propose that amino acids within 115 residues of the C terminus of ParA must interact specifically with amino acids within 28 residues of the ParB N terminus for partition to proceed. The overall homology relationships in the relevant parts of these proteins are similar to those in N15 and F SopA and -B: strongly conserved C-terminal sequences in the A proteins and little N-terminal sequence similarity in the B proteins. It is unlikely that the A protein C-terminal domain interacts only with the dissimilar N termini of the B proteins. The initial contact may be with a more strongly conserved region of the B protein and may position the proteins to allow the highly specific interactions with the B protein N-terminal domain that are needed for partition. If only the initial complex forms, as is likely when A protein finds the B protein homologue rather than its normal partner, it is blocked and disrupts normal maintenance.
Although the essential tools are now available for identifying the determinants of specificity in partition and autoregulation of the Sop system, some modification of the approach taken by Radnedge et al. for the P1-P7 Par system will be necessary. Unlike the components of the Sop systems studied here, those of the Par systems are not interchangeable (12, 32). This means that for P1 and P7 the distinction between partition and transcriptional repression phenotypes is virtually absolute, allowing the ready assignment of specificity to a given hybrid protein or operon. Distinguishing between partial Sop phenotypes of F-N15 hybrids will require precise measurements of plasmid stability and promoter repression. Inclusion of the recently reported Yersinia plasmid Sop systems (14, 30) may help refine the analysis.
ACKNOWLEDGMENTS
We thank Don Biek for kindly providing the promoter fusion construction and antibodies, Marc Lemonnier for bringing N15 to the attention of one of us (D.L.) and for assistance with the Western blot experiment, and the members of the group Dynamiques des replicons bactériens, as well as Valentin Rybchin and Malgorzata Lobocka, for their interest and for useful discussions.
N.R. was supported by a short-term EMBO fellowship, ASTF 9072, and by grant 96-1492 from the International Association for the Promotion of Cooperation with Scientists from the New Independent States of the Former Soviet Union (INTAS).
REFERENCES
- 1.Austin S, Wierzbecki A. Two mini-F encoded proteins are essential for equipartition. Plasmid. 1983;10:73–81. doi: 10.1016/0147-619x(83)90059-8. [DOI] [PubMed] [Google Scholar]
- 2.Austin S J. Bacterial plasmids that carry two functional centromere analogues are stable and are partitioned faithfully. J Bacteriol. 1984;158:742–745. doi: 10.1128/jb.158.2.742-745.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K. Current protocols in molecular biology. New York, N.Y: Wiley-Interscience; 1989. [Google Scholar]
- 4.Biek D P, Shi J. A single 43-bp sopC repeat of plasmid mini-F is sufficient to allow assembly of a functional nucleoprotein partition complex. Proc Natl Acad Sci USA. 1994;91:8027–8031. doi: 10.1073/pnas.91.17.8027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Casadaban M J, Cohen S N. Analysis of gene control signals by DNA fusion and cloning in Escherichia coli. J Mol Biol. 1980;138:179–207. doi: 10.1016/0022-2836(80)90283-1. [DOI] [PubMed] [Google Scholar]
- 6.Dodd I B, Egan J B. Improved detection of helix-turn-helix DNA-binding motifs in protein sequences. Nucleic Acids Res. 1990;18:5019–5026. doi: 10.1093/nar/18.17.5019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dunn S D. Effects of the modification of transfer buffer composition and the renaturation of proteins in gels on the recognition of proteins on Western blots by monoclonal antibodies. Anal Biochem. 1986;157:144–153. doi: 10.1016/0003-2697(86)90207-1. [DOI] [PubMed] [Google Scholar]
- 8.Gordon G S, Sitnikov D, Webb C D, Teleman A, Straight A, Losick R, Murray A W, Wright A. Chromosome and low copy plasmid segregation in E. coli: visual evidence for distinct mechanisms. Cell. 1997;90:1113–1121. doi: 10.1016/s0092-8674(00)80377-3. [DOI] [PubMed] [Google Scholar]
- 9.Grant S G, Jessee J, Bloom F R, Hanahan D. Differential plasmid rescue from transgenic mouse DNAs into Escherichia coli methylation-restriction mutants. Proc Natl Acad Sci USA. 1990;87:4645–4649. doi: 10.1073/pnas.87.12.4645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hanai R, Liu R, Benedetti P, Caron P R, Lynch A S, Wang J C. Molecular dissection of a protein SopB essential for Escherichia coli F plasmid partition. J Biol Chem. 1996;271:17469–17475. doi: 10.1074/jbc.271.29.17469. [DOI] [PubMed] [Google Scholar]
- 11.Hayakawa Y, Murotsu T, Matsubara K. Mini-F protein that binds to a unique region for partition of mini-F plasmid DNA. J Bacteriol. 1985;163:349–354. doi: 10.1128/jb.163.1.349-354.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hayes F, Radnedge L, Davis M A, Austin S J. The homologous operons for P1 and P7 plasmid partition are autoregulated from dissimilar operator sites. Mol Microbiol. 1994;11:249–260. doi: 10.1111/j.1365-2958.1994.tb00305.x. [DOI] [PubMed] [Google Scholar]
- 13.Hendrix R W, Ravin V K, Casjens S R, Ford M E, Ravin N V, Smirnov I K. Bacteriophage N15 complete sequence. GenBank accession no. AF064539. 1998. [Google Scholar]
- 14.Iriate M, Lambermont I, Kerbourch C, Cornelis G R. Yersinia enterocolitica plasmid pYVe227, complete sequence. GenBank accession no. AF102990. 1998. [Google Scholar]
- 15.Kusukawa N, Mori H, Kondo A, Hiraga S. Partitioning of the F plasmid: overproduction of an essential protein for partition inhibits plasmid maintenance. Mol Gen Genet. 1987;208:365–372. doi: 10.1007/BF00328125. [DOI] [PubMed] [Google Scholar]
- 16.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 17.Lane D, Cavaillé J, Chandler M. Induction of the SOS response by IS1 transposase. J Mol Biol. 1994;242:339–350. doi: 10.1006/jmbi.1994.1585. [DOI] [PubMed] [Google Scholar]
- 18.Lemonnier M, Lane D. Expression of the second lysine decarboxylase gene of Escherichia coli. Microbiology. 1998;144:751–760. doi: 10.1099/00221287-144-3-751. [DOI] [PubMed] [Google Scholar]
- 19.Lin D C-H, Grossman A D. Identification and characterization of a bacterial chromosome partition site. Cell. 1998;92:675–685. doi: 10.1016/s0092-8674(00)81135-6. [DOI] [PubMed] [Google Scholar]
- 19a.Lobocka, M. Personal communication.
- 20.Lobocka M B, Svarchevsky A N, Rybchin V N, Yarmolinsky M B. Characterization of the primary immunity region of the Escherichia coli linear prophage N15. J Bacteriol. 1996;178:2902–2910. doi: 10.1128/jb.178.10.2902-2910.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lutz R, Bujard H. Independent and tight regulation of transcriptional units in Escherichia coli via the LacR/O, the TetR/O and AraC/I1-I2 regulatory elements. Nucleic Acids Res. 1997;25:1203–1210. doi: 10.1093/nar/25.6.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lynch A S, Wang J C. Use of an inducible site-specific recombinase to probe the structure of protein-DNA complexes involved in F plasmid partition in Escherichia coli. J Mol Biol. 1994;236:679–684. doi: 10.1006/jmbi.1994.1179. [DOI] [PubMed] [Google Scholar]
- 23.Manen D, Xia G, Caro L. A locus involved in the regulation of replication in plasmid pSC101. Mol Microbiol. 1994;11:875–884. doi: 10.1111/j.1365-2958.1994.tb00366.x. [DOI] [PubMed] [Google Scholar]
- 24.Miller J H. Experiments in molecular genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1972. [Google Scholar]
- 25.Mori H, Mori Y, Ichinose C, Niki H, Ogura T, Kato A, Hiraga S. Purification and characterization of SopA and SopB proteins essential for F plasmid partitioning. J Biol Chem. 1989;264:15535–15541. [PubMed] [Google Scholar]
- 26.Motallebi-Veshareh M, Rouch D A, Thomas C M. A family of ATPases involved in active partitioning of diverse bacterial plasmids. Mol Microbiol. 1990;4:1455–1463. doi: 10.1111/j.1365-2958.1990.tb02056.x. [DOI] [PubMed] [Google Scholar]
- 27.Niki H, Hiraga S. Subcellular distribution of actively partitioning F plasmid during the cell division cycle in E. coli. Cell. 1998;90:951–957. doi: 10.1016/s0092-8674(00)80359-1. [DOI] [PubMed] [Google Scholar]
- 28.Ogura T, Hiraga S. Partition mechanism of F plasmid: two plasmid gene-encoded products and a cis-acting site region are involved in partition. Cell. 1983;32:351–360. doi: 10.1016/0092-8674(83)90454-3. [DOI] [PubMed] [Google Scholar]
- 29.Ogura T, Niki H, Mori H, Morita H, Hasegawa M, Ichinose C, Hiraga S. Identification and characterization of gyrB mutants of Escherichia coli that are defective in partitioning of mini-F plasmids. J Bacteriol. 1990;172:1562–1568. doi: 10.1128/jb.172.3.1562-1568.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Perry R D, Straley S C, Fetherston J D, Rose D J, Gregor J, Blattner F R. DNA sequencing and analysis of the low-Ca2+-response plasmid pCD1 of Yersinia pestis KIM5. Infect Immun. 1998;66:4611–4623. doi: 10.1128/iai.66.10.4611-4623.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Peterson B C, Hashimoto H, Rownd R H. Cointegrate formation between homologous plasmids in Escherichia coli. J Bacteriol. 1982;151:1086–1094. doi: 10.1128/jb.151.3.1086-1094.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Radnedge L, Youngren B, Davis M, Austin S. Probing the structure of complex macromolecular interactions by homolog specificity scanning: the P1 and P7 plasmid partition systems. EMBO J. 1998;17:6076–6085. doi: 10.1093/emboj/17.20.6076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ravin N V, Doroshenko O I, Ravin V K. Cos region of the temperate bacteriophage N15. Mol Gen Mikrobiol Virusol. 1998;2:17–20. . (In Russian.) [PubMed] [Google Scholar]
- 34.Ravin N V, Ravin V K. An ultrahigh-copy plasmid based on the replicon of the temperate bacteriophage N15. Mol Gen Mikrobiol Virusol. 1994;1:37–39. . (In Russian.) [PubMed] [Google Scholar]
- 35.Ravin V K. Lysogeny. Moscow, Russia: Nauka Press; 1971. p. 106. . (In Russian.) [Google Scholar]
- 36.Ravin V K, Shul’ga M G. Evidence for extrachromosomal location of prophage N15. Virology. 1970;40:800–807. doi: 10.1016/0042-6822(70)90125-x. [DOI] [PubMed] [Google Scholar]
- 36a.Rybchin V N, Svarchevsky A N. The plasmid prophage N15: a linear DNA with covalently closed ends. Mol Microbiol. 1999;33:895–903. doi: 10.1046/j.1365-2958.1999.01533.x. [DOI] [PubMed] [Google Scholar]
- 37.Sankova T P, Svarchevsky A N, Rybchin V N. Isolation, characterization and mapping of the N15 plasmid insertion mutations. Genetika. 1992;28:66–76. . (In Russian.) [PubMed] [Google Scholar]
- 38.Simons R W, Houman F, Kleckner N. Improved single and multicopy lac-based cloning vector for protein and operon fusions. Gene. 1987;53:85–96. doi: 10.1016/0378-1119(87)90095-3. [DOI] [PubMed] [Google Scholar]
- 39.Svarchevsky A N, Rybchin V N. Characteristics of plasmid properties of bacteriophage N15. Mol Gen Mikrobiol Virusol. 1984;N4:34–39. . (In Russian.) [Google Scholar]
- 40.Walker J E, Saraste M, Gay N J. E. coli F1-ATPase interacts with a membrane protein component of a proton channel. Nature. 1982;298:867–869. doi: 10.1038/298867a0. [DOI] [PubMed] [Google Scholar]
- 41.Williams D R, Macartney D P, Thomas C M. The partitioning activity of the RK2 central control region requires only incC, korB and KorB-binding site OB3 but other KorB-binding sites form destabilizing complexes in the absence of OB3. Microbiology. 1998;144:3369–3378. doi: 10.1099/00221287-144-12-3369. [DOI] [PubMed] [Google Scholar]
- 42.Yates P, Lane D, Biek D P. The F plasmid centromere, sopC, is required for full repression of the sopAB operon. J Mol Biol. 1999;290:627–638. doi: 10.1006/jmbi.1999.2909. [DOI] [PubMed] [Google Scholar]
- 43.Yates P A. Studies of mutations affecting the replication and partition of the mini-F plasmid in Escherichia coli. Ph.D. thesis. Lexington: University of Kentucky; 1997. [Google Scholar]