Abstract
Simple Summary
Salmonellosis, an infection in humans and animals caused by Salmonella spp., poses a major concern to public health and food safety worldwide. Antibiotics are mostly prescribed to treat salmonellosis. Unfortunately, indiscriminate use of antibiotics leads to the emergence and transmission of multidrug-resistant Salmonella spp. As antibiotics are becoming increasingly ineffective, infections caused by MDR strains will be difficult to manage. The search for an alternative to antibiotics has led scientists to give renewed attention on phage therapy. Though commercial use of phages for controlling Salmonella in poultry is still in its early stage, the use of lytic phages is considered an environmentally friendly, cost-effective, and sustainable antimicrobial approach. Moreover, it provides advantages over antibiotics in terms of specificity, cost of development, resistance, and genetic amenability. Studies on laboratory and field scale use show promise on the effectiveness of phages against MDR Salmonella spp. However, inadequate data on safety of phage use, phage stability, and lack of regulatory framework remain major obstacles in the commercial application of phages. Our article provides a comprehensive overview on global prevalence and antimicrobial resistance of Salmonella in poultry, the efforts to control Salmonella using phage therapy, and challenges as well as future prospects of phage therapy.
Abstract
Salmonellosis is one of the most common bacterial infections that impacts both human health and poultry production. Although antibiotics are usually recommended for treating Salmonella infections, their misuse results in the evolution and spread of multidrug-resistant (MDR) bacteria. To minimize the health and economic burdens associated with antimicrobial resistance, a novel antibacterial strategy that can obliterate pathogens without any adverse effects on humans and animals is urgently required. Therefore, therapeutic supplementation of phages has gained renewed attention because of their unique ability to lyse specific hosts, cost-effective production, environmentally-friendly properties, and other potential advantages over antibiotics. In addition, the safety and efficacy of phage therapy for controlling poultry-associated Salmonella have already been proven through experimental studies. Phages can be applied at every stage of poultry production, processing, and distribution through different modes of application. Despite having a few limitations, the optimized and regulated use of phage cocktails may prove to be an effective option to combat infections caused by MDR pathogens in the post-antibiotic era. This article mainly focuses on the occurrence of salmonellosis in poultry and its reduction with the aid of bacteriophages. We particularly discuss the prevalence of Salmonella infections in poultry and poultry products; review the trends in antibiotic resistance; and summarize the application, challenges, and prospects of phage therapy in the poultry industry.
Keywords: bacteriophages, Salmonella, poultry, biocontrol, drug-resistant
1. Introduction
Salmonella, a rod-shaped, gram-negative, facultative anaerobic, and motile bacterium, is mostly known for its versatile ability to cause a wide spectrum of diseases in humans and animals, such as salmonellosis, typhoid fever, septicemia, and fowl typhoid [1,2]. Based on variations in somatic and flagellar antigens, 2500 serotypes of Salmonella enterica have been identified, representing approximately 99% of the pathogenic strains of Salmonella [1]. Salmonella belongs to the Enterobacteriaceae family and can be acquired from food, water, and environmental sources. It is widely prevalent in the intestinal tract of various animals, such as poultry, cattle, and pets [3]. The transmission of Salmonella from animals to humans can occur by both the consumption of food, water contaminated with animal waste, and by direct contact with Salmonella-infected animals (fecal–oral route) [4]. Salmonella can enter the food chain from animal feed and poultry processing sites, persistently contaminated livestock environments, contaminated hatcheries, vertical transmission, and can be disseminated to humans through the fecal–oral route. In humans, salmonellosis may develop 12–72 h after the consumption of food contaminated with Salmonella. It is characterized by fever, diarrhea, vomiting, and abdominal cramps. Globally, three million deaths have been reported among 1.3 billion estimated cases of Salmonella-associated gastroenteritis annually [5]. Moreover, the incidence of infections is higher in developing countries, posing a considerable burden to their health and economy [5,6]. In 2018, Salmonella was found to be responsible for 33% of the 5146 foodborne outbreaks, resulting in 48,365 illnesses in European Union (EU) member states [7]. The frequent occurrence of Salmonella in poultry and poultry products has been identified to be a potential threat to the growth and development of this industry worldwide. In the United States, contaminated poultry and red meats are responsible for one-third of Salmonella-associated infections and the annual economic losses have been estimated to be between $2.3 billion and $11.3 billion [8].
Antibiotics are permitted in poultry-producing countries for treating Salmonellosis and promoting growth [9]. Several efforts have been made to minimize antibiotic use in agriculture and poultry production in numerous countries. Sweden imposed a ban on the use of all growth promoting antibiotics in 1986. Denmark also outlawed the use of avoparcin and virginiamycin in 1995 and 1998, respectively. The European Union (EU) banned the use of avoparcin and four other antibiotics (bacitracin, spiramycin, tylosin, and virginiamycin) as growth promoters in 1997 and 1998, respectively [10]. One of the first nations in Africa to outlaw widespread use of antibiotics in livestock was Namibia [11]. In the United States, many antimicrobials were administered to livestock for growth promotion purposes, before the Food and Drug Administration (FDA) made those uses illegal at the start of 2017 [12]. Unfortunately, the misuse of antibiotics leads to the development and transmission of antibiotic-resistant pathogens, rendering antibiotics ineffective in the clinical management of infections. Moreover, resistant determinants can be transferred to other pathogens via horizontal gene transfer mechanisms, thereby promoting resistance in environmental pathogens. As per the ongoing antibiotic resistance trends, poultry production areas serve as an important reservoir of antimicrobial resistance (AMR) genes [13]. Alarmingly, poultry pathogens are now resistant to colistin, which is considered a last-resort drug for treating complicated bacterial infections in humans [14]. Considering the mortality, morbidity, and cost of treatment of diseases caused by multidrug-resistant (MDR) pathogens, different novel approaches are now under investigation to provide a sustainable solution.
Phages are viruses infecting bacteria; they can be classified as lytic (which kill the host at the end of replication) and lysogenic (which integrate their genome into the host genome) [15]. The application of bacteriophages is considered to be an emerging treatment option for preventing bacterial infections in humans and poultry. Because of their enormous bactericidal activity, host specificity, self-limiting capabilities, and ease of genetic manipulation, researchers now consider bacteriophages as potential alternatives to antibiotics [16]. A broad range of bacteria are susceptible to infection by bacteriophages that can tolerate a wide range of temperatures and pH levels. Moreover, high degree of host specificity allows lytic bacteriophage to kill only one species of bacteria. Hence, they are suitable candidates for therapeutic application. Many studies have reported the efficiency of host-specific phages in decreasing bacterial counts in different food items, such as meat, eggs, animal skin, vegetables, and processed foods [17,18,19,20]. The utilization of bacteriophages could be an effective intervention strategy to decrease the colonization of Salmonella in animals. Phage administration was found to reduce the Salmonella Enteritidis count in experimentally infected broiler chickens by 4.2 log10 CFU [21]. Further reduction could be achieved by the appropriate selection of bacteriophages, use of phage cocktails, and optimization of environmental conditions, among others [21]. Given the advantages of bacteriophages over antibiotics, the current explosion in research on the use of these bactericidal viruses in the food and poultry industries has resulted in the development of phage-based products that are now commercially available in developed countries. However, inactivation by harsh environmental conditions (temperature, pH, UV light), cost of large-scale production, and safety issues are major drawbacks that make phage application not suitable for all circumstances in controlling Salmonella [22].
2. Salmonellosis and Its Association with Poultry
Although the typical infectious dose of Salmonella for causing salmonellosis is 107 to 109 CFU/g, it may vary depending on the composition of foods and the health status of patients [23]. Lower infectious doses are linked to high fat content of the contaminated food products [24]. Salmonella Enteritidis has been reported to be the most frequently (65%) encountered serotype in nontyphoidal salmonellosis cases globally, followed by Salmonella Typhimurium (12%) and Salmonella Newport (4%) [25]. Moreover, Salmonella Enteritidis was found to be the dominant serotype in Asia, Latin America, Europe, and Africa, being detected in 38%, 31%, 87%, and 26% of clinical isolates, respectively [25]. Salmonella is also responsible for 93.8 million gastroenteritis cases worldwide, resulting in 155,000 deaths annually [26]. In total, 82,694 confirmed cases of salmonellosis were reported in 2013, making it the second most common zoonotic disease in Europe [27]. According to the CDC, Salmonella is responsible for approximately 1.35 million illnesses, resulting in 26,500 hospitalizations and 420 deaths annually in the USA [28].
Various foods, such as meat, eggs, vegetables, beef, pork, and milk, are often considered to be the major vehicles of Salmonella infections in humans [29]. Salmonella Enteritidis is typically found in numerous poultry products, whereas Salmonella Typhimurium is found in different animals, including pigs, cattle, and poultry [30]. Other serovars that are known to be associated with egg contamination are Salmonella Gallinarum, Salmonella Pullorum, and Salmonella Heidelberg [31]. In the EU, contaminated foodstuffs, especially table eggs and pig meat, act as a source of Salmonella infection in humans. However, the risks of consuming broiler and turkey meat are similar and around two-fold lower (EFSA, 2012). Between 1985 and 2002, egg contamination was identified as the primary source, accounting for 53% of Salmonella infections reported to the CDC in the United States [32]. Salmonella can contaminate eggs by two possible routes. Bacterial infection as a result of fecal material on the eggshell (trans-shell) or exposure during the hatching phase in commercial hatch cabinets, commonly known as horizontal transmission [33,34]. In the second mechanism, known as vertical transmission, Salmonella Enteritidis infects the reproductive organs, resulting in the contamination of the yolk, albumen, eggshell membranes, or eggshells before oviposition [35]. Table 1 showed the global prevalence of egg contamination by Salmonella spp.
Table 1.
Prevalence of Salmonella spp. in eggs in different countries.
| Country | Prevalence | Sample | Year | References |
|---|---|---|---|---|
| India | 4.82% | Eggs | 2006–2007 | [36] |
| Bangladesh | 28% and 83%, 3% | Eggs and eggshell, egg content | 2014–2015 and 2011–2012 | [37,38] |
| Ethiopia | 2.4%, 4.8%, and 5.3% | Egg content, eggshell and egg from market | 2018 and 2012–2013 | [39,40] |
| China | 6.6%, 5.5% | Eggs from poultry, eggs from marketplace | 2013–2014 | [41] |
| Guangdong, China | 5.4% | Eggs | 2017–2018 | [42] |
| Iran | 13.61% | Eggshell | 1996–2018 | [43] |
| Pakistan | 29.36% and 10.31% | Eggshell and egg content | 2011–2012 | [44] |
| Kuwait | 10% | Eggs | 2004–2005 | [45] |
| Iraq | 4.85% | Eggshell | 2016 | [46] |
| Thailand | 12.4%, 11% | Eggshell, egg content | 1992 | [47] |
| Nigeria | 7.3% | Eggs | 2019 | [48] |
| Brazil | 1.25% | Eggshell, egg content | n/a | [49] |
| Sri Lanka | 6.7% | Eggs | n/a | [50] |
| Zambia | 2.31% | Eggshell | 2018 | [51] |
| Uruguay | 0.0049% | Egg content | 2010 | [52] |
| Japan | 0.25% | Eggshell | 2007–2008 | [53] |
| South Korea | 7.4% | Egg content | 2010–2012 | [54] |
| Colombia | 2.93% | Eggshell | 2014 | [55] |
| Cameroon | 88.6% | Eggs | 2016 | [56] |
| Ireland | 0.04% | Egg contents | 2005–2006 | [57] |
The prevalence of Salmonella and predominant serotype in live poultry varies from country to country. Salmonella is carried by infected living birds and spreads to other birds by lateral transmission, which occurs mostly through feces, dirt, litter, food, water, dust, and feathers. The overall prevalence of different Salmonella serotypes among live birds ranges from 6% to 30% [58,59,60,61]. A study from the Republic of Ireland in 2006 found that 27.3% of 362 broiler flocks were infected with Salmonella [60]. In Kagoshima, Japan, 49% of 192 broiler flocks and 7.9% of 3071 cecal samples were found to be positive for Salmonella from 2009 to 2012 [62]. In the Shandong province, China, fecal swab analysis determined 12.7% prevalence of Salmonella in free range chickens in 2015 [63]. A 32% prevalence of Salmonella in cloacal swab was observed in Bangladesh during July 2014 to June 2015 [37]. Salmonella Mbandaka, Salmonella Infantis, and Salmonella Enteritidis were the predominant serotypes in the Republic of Ireland, Japan, and China, respectively. Such results indicate geographical differences in prevalence and serotype diversity of Salmonella in chicken flocks.
3. Antibiotic-Resistant Salmonella in Poultry
Antibiotics are utilized in animal farming at a rate of approximately 8 million kg per year, of which 70% is for nontherapeutic purposes (growth enhancement and disease control). In comparison, antibiotics are used at a rate of only 1.3 million kg per year for treating human infections [64]. Such high use of antibiotics in livestock leads to the emergence of resistant microbes in the native micro-biota of the animal and the local environment due to shedding in the feces [65]. The early development of MDR pathogens resulting from their unrestricted use makes infection management critical.
A high degree of AMR is frequently observed among Salmonella spp. isolated from eggs. Although several antibiotics are used in the poultry production chain, Salmonella exhibited the highest resistance to nalidixic acid and ampicillin [66]. Bacterial resistance to tetracycline, oxytetracycline, and nalidixic acid was found to be much higher in commercial layer hen eggs than in duck eggs in India [67]. In Bangladesh, Salmonella from chicken egg surfaces exhibited complete resistance to ampicillin and amoxicillin, followed by tetracycline, ciprofloxacin, and colistin [68]. In China, Xie et al. (2019) reported that Salmonella from eggs showed varying degrees of resistance to beta-lactam antibiotics, including amoxicillin, cefazolin, penicillin, and piperacillin, followed by aminoglycosides and tetracyclines, such as gentamicin, kanamycin, streptomycin, minocycline, and tetracycline [42]. Mobile genetic elements facilitate the transfer of AMR genes from one pathogen to another and thus cause the transmission of antibiotic resistance. IncA/C plasmids of Salmonella have carried genes that confer resistance to different classes of antibiotics such as aminoglycosides, β-lactams, chloramphenicol, trimethoprim, sulfisoxazole, and tetracyclines [69]. In addition, the transmission of antibiotic resistance genes through poultry litter has already been reported [65]. Antimicrobial resistance in Salmonella associated with other poultry products are outlined in Table 2.
Table 2.
Summary of the results of studies on antibiotic resistance among Salmonella isolates in poultry and poultry products in the last decades.
| Origin | Sample Type | Dominant Serotype | Phenotypic Resistance | Reference |
|---|---|---|---|---|
| Bangladesh | Cloacal swab, feed, litter | Salmonella Typhimurium | The percentage of resistance to tetracycline, chloramphenicol, ampicillin, and streptomycin were 97.14%, 94.28%, 82.85%, and 77.14%, respectively. | [70] |
| Bangladesh | Chicken samples (liver and intestine) | Salmonella spp. | High percentage of resistance were found against colistin (92.68%) and ciprofloxacin (73.17%), followed by tigecycline (62.20%), co-trimoxazole (60.98%). | [71] |
| Henan, China | Dead chicken | Salmonella Pullorum, Salmonella Enteritidis | 77%, 73%, 5.60% of isolates were resistant to ciprofloxacin, sulfisoxazole, and ampicillin, respectively; 69.64% were resistant to three or more antimicrobials. | [72] |
| Egypt | Broiler chicken | Salmonella Enteritidis, Salmonella Typhimurium | 76.7% isolates were multidrug resistant, resistant to sulfamethoxazole (100%), amoxicillin–clavulanic acid (68%), streptomycin (65%). | [73] |
| Iran | Fecal swab | Salmonella Enteritidis | Resistant to nitrofurantoin (90.2%), followed by nalidixic acid (67.2%), and cephalexin (37.7%). Multi-drug resistance characteristics were found in 57.4% isolates. | [74] |
| Pakistan | Poultry postmortem | Salmonella Infantis | Isolates showed maximum resistance against pefloxacin (94.4%), chloramphenicol (83.3%), and imipenem (77.7%). | [75] |
| Eastern region, China | Fecal swab |
Salmonella Indiana, Salmonella Enteritidis |
Isolates were resistant to sulfamethoxazole, ampicillin, tetracycline, doxycycline, and trimethoprim. | [76] |
| South Korea | Chicken meat, feces, and eggshells | Salmonella Enteritidis | All isolates were found to be resistant to at least 1 of 21 antibiotics, 65.2% were resistant to three or more antimicrobials, namely penicillins, sulfisoxazole, streptomycin, tetracyclines, quinolones. | [77] |
| India | Eggs, cloacal swabs, feces | Salmonella Typhimurium |
All the isolates showed resistance to clindamycin, oxacillin, penicillin, and vancomycin at varying degree. | [78] |
| Romania | Chicken meat | Salmonella Infantis | 66.6% of isolates were resistant to tetracycline, followed by nalidixic acid and sulfamethoxazole (64.3%), ciprofloxacin (61.9%), streptomycin (59.5%). | [79] |
| South Africa | Chicken carcass swabs, cloacal swabs | Salmonella Bovismorbificans, Salmonella Hadar, Salmonella Dublin, Salmonella Enteritidis | The frequency of MDR among the Salmonella isolates was 81.8%, highest to erythromycin (94.9%) and spectinomycin (82.7%). | [80] |
| Malaysia | Cloaca swab | Salmonella spp. | Resistance to erythromycin (100%), chloramphenicol (76.2%), tetracycline (62%), ampicillin (47.7%), sulfamethoxazole/trimethoprim (42.9%). | [81] |
| Thailand | Chicken meat | Salmonella spp. | Nalidixic acid had the highest rate of resistance (31%), followed by ampicillin (24%), tetracycline (19%), and sulfamethoxazole trimethoprim (8%). | [82] |
| Greece | Chicken carcass and liver | Salmonella Hadar, Salmonella Enteritidis, | The percentage of resistance to streptomycin, tetracycline, nalidixic acid, ampicillin, and rifampicin were 64.5%, 56.2%, 39.5%, and 33.3%, respectively. | [83] |
| Vietnam | Chicken carcasses | Salmonella Albany, | 73.3% isolates were resistant to at least one antibiotic with highest resistance to tetracycline (59.1%) and ampicillin (41.6%); 17.7% multidrug-resistance was also observed. | [84] |
| Singapore | Chicken meat | Salmonella Saintpaul | 59.6% isolates were multidrug-resistant. Phenotypic resistance to ampicillin, tetracycline and chloramphenicol, sulfamethoxazole-trimethoprim and nalidixic acid were 78.8%, 61.5%, 55.8%, and 30.5%, respectively. | [85] |
| Colombia | Chicken carcasses | Salmonella Paratyphi B | The percentage of Salmonella isolates resistant to 1–5, 6–10, and 11–15 antimicrobial agents were 35.2%, 24.6%, and 33.9%, respectively. | [86] |
| Turkey | Chicken meat | Salmonella spp. | High degree of resistance (≥89.2) to vancomycin, tetracycline, streptomycin was observed. | [87] |
| Myanmar | Chicken meat | Salmonella Albany | 52.2% isolates were multidrug-resistant. High frequency of resistance to trimethoprim-sulfamethoxazole (70.3%), tetracycline (54.3%), streptomycin (49.3%), ampicillin (47.1%) was found. | [88] |
| Canada | Chicken meat | Salmonella Hadar | About 21% of chicken isolates were resistant to amoxicillin–clavulanic acid, ceftiofur, and ceftriaxone. | [89] |
| Argentina | Chicken liver | Salmonella Schwarzengrund | All isolates were found to be sensitive to all tested antibiotics except 100% resistant to erythromycin. | [90] |
Infections caused by MDR bacteria result in prolonged hospitalization times, delayed treatment procedures, and increased medical costs. If this situation continues, food security, human health, and biodiversity will be threatened. Changes in husbandry, hygiene, disinfection, monitoring of breeding populations, legal controls, and enforcement of existing regulations have allowed major reductions in antimicrobial drug use for poultry in many parts of the world. However, a new, sustainable, and environmentally-friendly antimicrobial technology is needed to ensure safe health for all by reducing the dependence on antibiotics.
4. Bacteriophages an Alternative to Antibiotics in Controlling Pathogens
Bacteriophages, the most ubiquitous organisms on the earth, are commonly known as viruses of bacteria and archaea [91]. The number of phages in the biosphere (approximately 1031) is estimated to be 10-fold higher than the number of bacteria [92,93]. The morphological structure of phages consists of nucleic acids inside a protein coat, and the majority of phages have dsDNA as their nucleic acid [94]. Bacteriophages are classified into two different types based on their replication cycle. Lysogenic or temperate phages usually integrate their nucleic acid into the host DNA and are replicated with succeeding generations of hosts. They do not destroy the host at the end of replication. Lytic or virulent phages attach to the host, introduce their genome into the host genome, replicate with the aid of host replication machinery, assemble, and finally destroy the host cell using a phage-encoded enzyme. Lytic phages are getting renewed attention as a potential solution to the ever-increasing AMR crisis. The main reasons for choosing lytic phages for controlling bacterial pathogens include their enormous bacteriolytic activity and their inability to transduce or transfer genetic elements, which provide advantages over lysogenic phages [91]. Bacteriophages provide some unique advantages that make them an attractive and suitable alternative to antibiotics. First, unlike antibiotics, which have broad-spectrum activity, phages are highly specific to the host; thus, there is less chance of gut dysbiosis and secondary infections following phage therapy. Second, while it takes millions of dollars and a long time to develop a new antibiotic, the isolation, propagation, and large-scale production of phages are less expensive. Third, the other characteristics that make phages more advantageous are their ability to spread through the body upon systemic administration, their ability to cross the blood–brain barrier, and their biofilm inhibitory activity [95,96]. Finally, and most importantly, bacterial resistance to phage therapy is considered less significant in comparison with bacterial resistance to antibiotic therapy. If pathogens develop phage resistance, it is possible to counteract by utilizing modern genetic engineering tools because phages are amenable to genetic manipulation; however, this is quite impossible in the case of antibiotic resistance. Even pan-antimicrobial resistant bacteria are likely to remain fully susceptible to phage attack, provided a suitable phage can be found or with the development of genetically engineered phages.
Current investigations on phage therapy have revealed promising outcomes in treating infections caused by MDR, extensively drug-resistant (XDR), and pandrug-resistant (PDR) bacteria. Infections caused by ESKAPE pathogens (Enterococcus spp., Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter spp.) are currently posing challenges to healthcare management. The application of bacteriophages has been proven to be effective in controlling ESKAPE pathogens [97,98]. The number of XDR A. baumannii (XDRAB) populations was found to reduce from 108 to 103 CFU/mL within 30 min of application of the phage φkm18p. The phage also improved the survival rate of lung epithelial cells [99]. Anti-Salmonella phage cocktail treatment significantly lowered cecal Salmonella concentrations, while simultaneously reducing ileal Salmonella contents in swine [100]. Several studies have reported the successful application of bacteriophages in humans to treat septicemia caused by P. aeruginosa, prostatitis caused by E. faecalis, and MDR S. aureus-associated chronic rhinosinusitis [101,102,103]. Phage therapy was found to result in the prevention of infection and improvement of the patients’ condition. Moreover, 6 months following lytic phage treatment (through an eyedrop formulation) in a 65-year-old woman suffering from secondary eye infections caused by vancomycin-resistant S. aureus (VRSA), the results of the VRSA culture test were negative [104]. This indicates that phages can be delivered through different routes of administration. Commercially available phage products are now used to overcome bacterial contamination and to cure infections in humans. PhagoBioDerm, a polymeric bandage containing a phage cocktail, ciprofloxacin, and other ingredients, is used to heal wounds caused by S. aureus and P. aeruginosa [105]. Moreover, treatment with ListShield, a commercially available phage cocktail preparation, was found to reduce Listeria monocytogenes contamination in experimentally inoculated frozen entrees, lettuce, smoked salmon, and cheese by 99%, 91%, 90%, and 82%, respectively [106]. It is clear from the abovementioned examples that the optimized use of monophage or phage cocktails in humans, animals, and foods holds great promise to treat infections caused by antibiotic-resistant bacteria and can be a very good alternative to antibiotics. However, phage therapy has several limitations as a potential antibiotic alternative in terms of commercial use. These include lack of experimental procedures for maintaining quality and safety of phage formulation, lack of studies on stability of phage preparation, unclear evidence on the effectiveness on biofilm degradation in serving commercial purpose, development of phage resistance, lack of data on pharmacokinetics and immune response, etc. [107].
5. Application of Phages for Controlling Salmonella Infections in Poultry and Poultry Products
In the poultry industry, phage treatment of Salmonella serves two fundamental purposes. First, phage treatment minimizes the losses caused by the effects of bacterial pathogens on animal health and production. Second, phage-based biocontrol is considered a powerful tool to control the prevalence of foodborne infections in humans. Selecting the appropriate phages, phage titer, mode of application, and duration of application are the major factors that determine the therapeutic effectiveness of phages [21,108]. Phage cocktails can be administered through different approaches, e.g., by oral administration after mixing with water or as a feed additive, by spraying on eggs, or by direct addition of the phage suspension to contaminated products. Thus, bacteriophages can be a promising intervention strategy to curb the horizontal and vertical transmission of Salmonella. The use of phages as an aerosol spray during the transfer of eggs from incubators to hatchers could be a cost-effective way to reduce the horizontal transmission of Salmonella via eggs [109]. A study conducted to decrease Salmonella colonization in chickens by the oral inoculation of phage preparations have stated that the utilization of phages could pose an effective barrier to the vertical transmission of this pathogen [110]. Among all identified Salmonella phages, the most well-known are P22 and Felix-O1. Felix-O1, a broad-spectrum lytic phage, can lyse a wide number of Salmonella serotypes and is recognized as an efficient candidate for therapeutic and diagnostic applications [111]. The experimental studies that applied bacteriophage preparations (in monophage or cocktail form) on experimentally infected poultry and poultry products through different mode of administration and the outcomes of phage treatment are outlined in Table 3.
Table 3.
Summary of the experimental studies on phage treatment to reduce colonization of Salmonella spp. in poultry and poultry products.
| Experimental Model | Phage | Inoculation Dose | Phage Delivery Method | Outcomes (Compared with Control) |
Reference |
|---|---|---|---|---|---|
| Broiler chicken | Three Salmonella phage | 109–11 PFU | Oral | Phage reduced cecal colonization of Salmonella Enteritidis and Salmonella Typhimurium by ≥4.2 log10 CFU and ≥2.9 log10 CFU, respectively, within 24 h. | [21] |
| Leghorn chicken specific-pathogen-free (SPF) | Three-phage cocktail | 1010 PFU | Oral | When the bacteriophage cocktail was given 1 day before or immediately after bacterial infection, and then again on different days following infection, there was a decrease in Salmonella concentration in the chicken cecum. | [112] |
| Broiler chicken | Three-phage cocktail |
1011 PFU |
Oral | The colony-forming units of Salmonella Enteritidis PT4 per gram of cecal content were reduced by 3.5 orders of magnitude in the bacteriophage-treated group. | [113] |
| Chicken carcasses | Salmonella spp. phage | 10⁹ PFU/mL | Spraying | No Salmonella Enteritidis was detected in two trials and more than 70% reduction was achieved in the other two trials. | [114] |
| SPF chicks | Salmonella spp. phage | 1.18 × 1011 PFU–1.03 × 102 PFU | Oral | Cecal contents indicated a moderate decrease in Salmonella loads at 3 days post infection (dpi), with a greater reduction at 5 days post infection (dpi). All of the chicks were negative for Salmonella from 7 dpi through the completion of the trial at 15 dpi. | [115] |
| Broiler chicks | Mixture of bacteriophage | 2.5 × 109–7.5 × 109 PFU | Oral | Compared to untreated controls, Salmonella Enteritidis retrieved from cecal contents was reduced at 12 and 24 h following treatment. | [116] |
| One-day-old chicks | Bacteriophage ΦCJ07 |
105, 107 and 109 PFU | Oral | In challenged and contact chickens, all treatments reduced intestinal Salmonella colonization; after 3 weeks of treatment, no intestinal Salmonella was detected in 70% of contact hens treated with 109 PFU/g of bacteriophage. | [117] |
| Seven-day old chickens | Three different Salmonella-specific bacteriophages | 103 PFU | Spray | When competitive exclusion plus bacteriophage was used, the mean Salmonella Enteritidis cecal count decreased (1.6 × 102 CFU/g) compared to the control group (1.56 × 105 CFU/g). | [118] |
| Six-week-old chickens | Salmonella Gallinarum (SG)-specific bacteriophage | 106 PFU | Oral | In comparison to untreated contact hens, contact hens treated with the bacteriophage showed a considerable reduction in mortality. | [119] |
| Broiler chicks | Salmonella Enteritidis phage | 108 PFU | Oral | On day of trial 14, bacteriophage treatments significantly reduced the incidence of Salmonella Enteritidis in cloacal swabs. | [120] |
| Broiler chicks | P22hc-2, cPII and cI-7 and Felix 0 | 5 × 1011 PFU | Oral | In phage-treated hens, average cecal bacterial counts were 0.3–1.3 orders of magnitude lower than in untreated controls. | [121] |
| Ten-day old chickens | Three lytic phages | 103 PFU | Spray and Oral | Aerosol-spray of bacteriophages resulted in 72.7% decrease in the incidence of Salmonella Enteritidis infection. In addition, counts of Salmonella Enteritidis indicated that phage administration by coarse spray and drinking water decreased the bacteria′s colonization in the gut. | [122] |
| White Leghorn chicks | Φ st1 | 1012 PFU/mL | Intracloacal inoculation | Within 6 h of post-challenge, the Salmonella count had dropped to 2.9 log10 CFU/mL, and Salmonella Typhimurium was undetectable at and after 24 h. | [123] |
| Eggs | PSE5 | 4 × 107 PFU | Immersion | A reduction by 2 × 106 CFU/mL of Salmonella was achieved after phage treatment. | [110] |
| Liquid egg | Pu20 | 108 or 109 PFU/mL | Direct inoculation | At 4 °C and 25 °C for 24 h, the quantity of live bacteria in the treatment group reduced by up to 1.06 log10 CFU/mL and 1.12 log10 CFU/mL, respectively, and the highest antibacterial efficacy was 91.30% and 92.40%, respectively, when multiplicity of infection (MOI) = 1000. | [124] |
| Liquid whole egg | Two phages (OSY-STA and OSY-SHC) | n/a | Direct inoculation | 1.8 and >2.5 log CFU/mL reduction in Salmonella Typhimurium and Salmonella Enteritidis, respectively. | [125] |
| Chicken breasts and fresh eggs | UAB_Phi 20, UAB_Phi78, and UAB_Phi87 | 109 PFU/mL and 1 × 1010 PFU | Soaking in suspension and spraying | Salmonella reduction was >1 log10 CFU/g in chicken breasts. In fresh eggs, a reduction of 0.9 log10 CFU/cm2 in Salmonella was observed. | [126] |
| Raw chicken breast | Five Salmonella phages | 3 × 108 PFU | Suspension added on surface | The largest reductions in the number of Salmonella Enteritidis and Salmonella Typhimurium in phage-treated group were 3.06 and 2.21 log CFU/piece, respectively, when incubated at 25 °C. | [127] |
| Chicken breast | Two-phage cocktails | 4 × 109 PFU/mL | Added on surface | After 5 h, the Salmonella Enteritidis concentration on chicken breast was reduced by 2.5 log CFU/sample | [128] |
| Chicken breasts | SPHG1 and SPHG3 | 8.3 log10 PFU | Spotted | The phage cocktail was applied to chicken breasts at MOIs of 1000 or 100, and the viable count of Salmonella Typhimurium was significantly reduced. | [129] |
| Chicken breast meat | Four Salmonella phage | 108, 109, and 1010 PFU/mL | Directly added | When raw chicken breast samples were treated with a cocktail of all four bacteriophages at 4 °C for 7 days, viable cell counts of bacteria were considerably reduced. | [130] |
| Chicken breast fillets | Salmonella lytic bacteriophage preparation | 109 PFU/ml | Spraying | Salmonella reductions of 1.6–1.7 and 2.2–2.5 log CFU/cm2 were achieved with the use of chlorine and PAA followed by phage spray. | [131] |
| Chicken skin | Eϕ151, Tϕ7 phage suspension | 109 PFU | Spray | Salmonella reductions were 1.38 log10 MPN (Enteritidis) and 1.83 log10 MPN (Typhimurium) per skin area following phage treatment. | [17] |
| Chicken skin | vB_StyS-LmqsSP1 |
2.5 × 108 PFU/cm2 | Direct addition | Phage treatment of chicken skin resulted in about 2 log units reduction in Salmonella isolates from the first 3 h throughout a 1-week experiment at 4 ℃. | [132] |
| Raw chicken meat and chicken skin | SE-P3, P16, P37, and P47 | 10⁹ PFU | Direct inoculation | Throughout storage at 4 and 25 °C, phages reduced the number of viable Salmonella cells in samples containing 103 CFU/g to undetectable levels. | [133] |
| Chicken meat | Five bacteriophages | 109 PFU/mL | Direct inoculation | Compared to control, application of phage cocktail results in 1.4 logarithmic unit reduction at 10 ℃ at 48 h. | [134] |
| Chicken meat | Three lytic bacteriophages Ic_pst11, Is_pst22, and Is_pst24 |
108, 107, and 106 PFU/mL | Direct addition | At MOIs of 100, 1000, and 10,000, a substantial decline in the viable count of Salmonella Typhimurium was seen at 7 h after phage application with reductions of 1.17, 1.26, and 1.31 log10 CFU/g. | [135] |
| Chicken meat | STGO-35-1 | 4 × 106 PFU/mL | Direct addition | Phage treatment caused a significant 2.5 log10 reduction of Salmonella Enteritidis. | [136] |
| Chicken frankfurters | Felix O1 | 5.25 × 106 PFU | Direct addition of liquid | Suppression levels of 1.8 and 2.1 log units of Salmonella Typhimurium were achieved by two variants of phages. | [137] |
| Duck meat | fmb-p1 | 9.9 × 109 PFU | Direct inoculation | 4.52 log CFU/cm2 reduction in Salmonella Typhimurium counts in ready-to-eat duck meat was found. | [138] |
Although small-scale studies have demonstrated a desirable reduction in bacterial counts following phage treatment, its industrial-scale application needs adequate safety assessment. With an increase in the number of studies, more data will be available on the safety and efficacy of phage therapy. In a phage therapy trial including 34,680 broiler chickens at a commercial farm with a previous record of Salmonella outbreak, no significant mortality, productivity, and alteration in the gut microbiota were noted in the phage-treated group compared with the untreated control group, indicating the safety of the phage preparation. Here, the effect of phage treatment on gut microbiota was evaluated by 16s rRNA gene amplicon sequencing. So far, this is the largest trial to evaluate the safety and efficacy of Salmonella phages in a commercial setting [139,140].
6. Challenges of Using Phages in Poultry and Probable Solutions
Researchers face several limitations when using phages for the elimination of pathogens. The main challenges associated with the use of phage therapy against Salmonella in poultry can be divided into four categories: development of phage resistance in bacteria, selection of the candidate phage, delivery of the phage to the site of infection, and difficulties associated with the regulatory approval of phage products [141].
The mechanisms underlying the development of phage resistance in bacteria include host cell surface and extracellular modifications, such as receptor adaptations, outer membrane vesicles, and quorum sensing, as well as intracellular modifications, such as abortive infections, phage exclusion, restriction modification (RM) systems, and CRISPR/Cas systems [142]. RM is the most ubiquitous phage resistance mechanism present in bacteria and archaea; it is also known as the innate immune system of prokaryotes. The RM system identifies host DNA based on the methylation pattern and cleaves foreign DNA [143]. The phage exclusion mechanism, superinfection exclusion system, and abortive infection mechanism prevent phage DNA replication in the host cell and block the entry of phage genetic materials into the host. Thus, phage dissemination becomes limited due to premature bacterial death upon phage infection [144]. However, overcoming phage resistance is not an insurmountable problem because phages have counteracting mechanisms. Phages with the ability to acquire new receptors can change their receptor-binding proteins. Thus, when a host receptor changes to a mutant form, phages can recognize the changing receptor structure and counteract disruptions in phage adsorption receptors. To get around the wide range of RM systems, phages employ various active and passive anti restriction techniques [144].
Potential phage candidates must be virulent and propagate inside the host via the lytic cycle. Phages harboring virulence or AMR genes or carrying integrase or recombinase are not ideal for successful therapeutic applications. According to international experts, an ideal phage cocktail should include phages from different families or groups that have a broad host range, optimum adsorption ability, and the ability to withstand a wide range of physicochemical conditions [145]. Adherence to these criteria must be ensured during the primary phage selection process. Whole-genome sequencing is preferred to provide genomic insights and to confirm that the selected phages are unable to perform transduction and horizontal gene transfer [21,114,141]. Moreover, the incorporation of phages into cocktails boosts their potential for presumed usage. The higher the number of phages present in a certain formulation, the greater the likelihood of its long-term medical and commercial demand [146]. A study assessed four different methods (direct spot test, efficiency of plating, planktonic killing assay, and biofilm assay) to identify the most suitable one for formulating phage cocktails and concluded that the planktonic killing assay is a good choice when considering phage cocktails [147].
Delivering a phage at the site of infection is a major challenge during phage application, especially in live animals. To reduce Salmonella colonization on chicken meat, eggshell, or processed food, phage preparations can be sprayed or directly applied to the products. As Salmonella initially colonizes the chicken gut, a phage preparation needs to be administered orally to reduce bacterial colonization. In the gut, the phage will encounter acidic pH, resulting in a higher chance of inactivation if it cannot tolerate acidic pH levels. Alternative solutions, such as encapsulation (microencapsulation or liposomal encapsulation), dry formulation, or liquid formulation, can protect phages from acidic conditions [147]. The free phage Felix-O1 was found to be undetectable after 5 min of exposure to pH 3.7 because this phage is highly sensitive to acidic pH. However, this problem was overcome by delivering microencapsulated Felix-O1 through a chitosan–alginate–CaCl2 system that kept the phage viable for 1 h in simulated gastric juice (pH 2.4) and for 3 h in porcine bile extract [148].
For the commercialization of phages, specific regulatory pathways are necessary depending on their use as feed additives, disinfectants, or medicines. Developers should go through regulatory routes and present adequate data on the safety and efficacy of the products before marketing. It is obvious to establish basic safety issues to ensure confidence in using phages as antimicrobials. Examples of such issues include the impact of phage on microbiome, bacterial lysis-associated endotoxin release, immune and inflammatory response, biological and chemical contaminants in phage preparations, and others [149]. Adoption of a specific framework addressing safety criteria, safety endpoints, methods of safety assessments, quality assurance of phage preparations, etc., will advance commercialization of phage application. Besides, the regulatory issue related to phage therapy affects not only the market placement of phage-based products but also the conduct of clinical trials [150]. A significant obstacle to the veterinary use of phages in the EU is that bacteriophages do not fit into the existing EU regulations regarding the use of feed additives [151]. The EU found the current regulatory framework quite unsatisfactory and is looking for national solutions for the satisfactory regulation of phage therapy. The Food and Drug Administration (FDA) regulates phages in the United States, regardless of whether they are to be used in humans or animals. However, they go through distinct stages depending on how they will be utilized [141]. Nevertheless, the regulatory framework will undergo substantial changes in the future to ease the way of using phages for combatting MDR pathogens in poultry and poultry products, and to maintain adequate safety measures.
7. Future Prospect of Bacteriophage
Undoubtedly, phage therapy has huge potential in future medicine to tackle antibiotic resistance in humans, animals, and agriculture. The growing interest in phages as food antimicrobials has prompted more research on the efficiency of single or mixed phages against target bacteria while posing minimum concerns to human health. The bactericidal activity of phages and their advantages over antibiotics rapidly expand the research and development of introducing bacteriophage-based novel products into the global market. Approval and commercialization of AgriPhage developed by Omnilytics Inc(Sandy, UT, United States) for agricultural uses, EcoShield, and SalmoFresh by Intralytix Inc(Baltimore, MD, United States). to use against E. coli O157:H7 and Salmonella spp. occurring in ready-to-eat foods, poultry, and poultry products are examples of such development [152]. Synergistic application of phage and antibiotics, co-administration of phages with enzymes, genetic modification of phage to improve phage therapy outcomes, and utilizing engineered phages to deliver drugs are the emerging areas of phage therapy research that will bring substantial changes in medical and veterinary therapeutics.
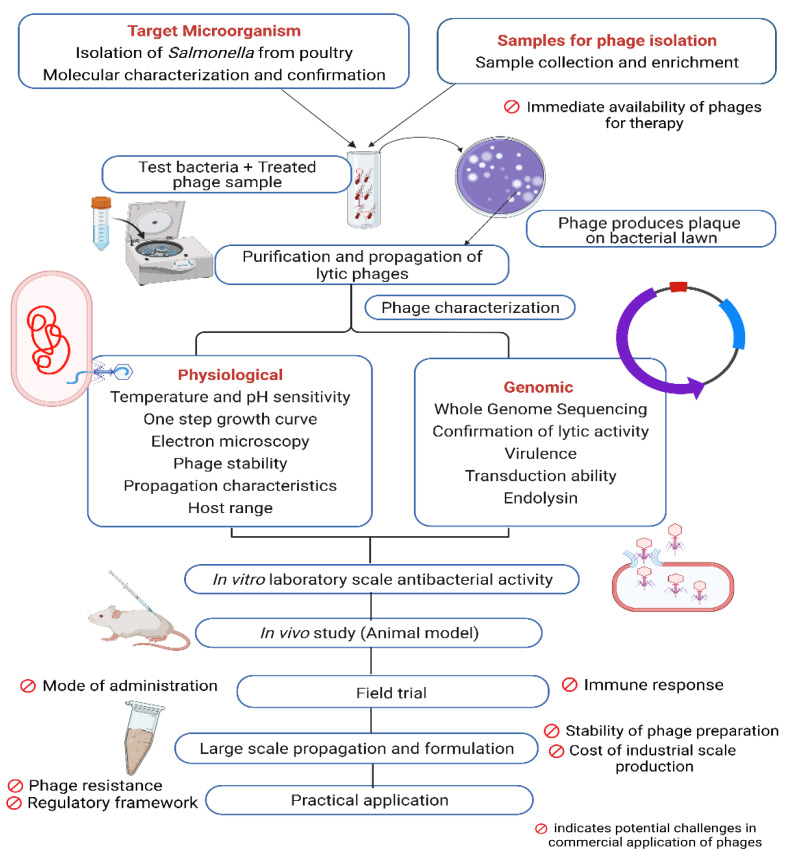
Antimicrobial resistance is more prevalent in developing nations due to inadequate infrastructure for healthcare, maintaining unregulated process in agricultural production, poor sanitation and hygiene, and widespread antibiotic overuse. Alternative treatment modalities are critically needed in the developing world due to public health and antibiotic resistance issues and, for various reasons, phage therapy has the potential to address the crisis. Lytic phages destroy specific host bacteria without harming gut microbes and eukaryotic cells. The ubiquity of phages keeps them available in wastewater, sewage, and excreta for isolation. Finally, the fast development, cost effectiveness, and environmentally-friendly characteristics of phage products make this a well-suited strategy to fight against MDR bacteria in developing countries [153]. Developing countries can also benefit economically from phage-mediated control of infectious diseases. Bacterial infections in poultry, cattle, and livestock result in huge financial losses each year. Moreover, the physiological and genetic makeup of causative agents vary between regions. Developing phage cocktails against local strains of bacteria may contribute to reduction in infections, thus decreasing economic losses. Establishment of phage-oriented biotech industries in those countries can address global crises by producing novel bio products, creating more job opportunities, and also helping to compete in a global market with new solutions to veterinary infectious diseases. A general procedure from isolation to application of phages to control Salmonella in poultry is illustrated in Figure 1.
Figure 1.
Steps in phage application process with potential challenges in poultry industry.
8. Conclusions
The identification of a new class of antimicrobials is of utmost importance to protect public health from the devastating effects of AMR. The emergence and transmission of antibiotic-resistant pathogens have opened a new window for phage therapy, which has a long history of use since its discovery. Current studies on phage therapy to reduce the prevalence of Salmonella in poultry have revealed promising outcomes that promote the development and use of bacteriophage-based products, not only to prevent the misuse of antibiotics but also to ensure food safety for the global population. Recent advancements in the fields of genomics and proteomics can help overcome the obstacles related to safety issues associated with the use of phages in food and animal production. Unlike antibiotics, with the current progression of phage research, the propagation, manipulation, and commercial-scale use of host-specific bacteriophages could serve as a sustainable technology that would drastically change the scenario and impact of AMR in the poultry industry, especially in developing countries.
Author Contributions
Conceptualization, M.A.S.K. and S.R.R.; methodology, S.R.R.; data curation, M.A.S.K.; writing—original draft preparation, M.A.S.K. and S.R.R.; writing—review and editing, M.A.S.K. and S.R.R. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data sharing not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kurtz J.R., Goggins J.A., McLachlan J.B. Salmonella infection: Interplay between the bacteria and host immune system. Immunol. Lett. 2017;190:42–50. doi: 10.1016/j.imlet.2017.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andino A., Hanning I. Salmonella enterica: Survival, colonization, and virulence differences among serovars. Sci. World J. 2015;2015:1–16. doi: 10.1155/2015/520179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jajere S.M. A review of Salmonella enterica with particular focus on the pathogenicity and virulence factors, host specificity and antimicrobial resistance including multidrug resistance. Vet. World. 2019;12:504–521. doi: 10.14202/vetworld.2019.504-521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eng S.-K., Pusparajah P., Ab Mutalib N.-S., Ser H.-L., Chan K.-G., Lee L.-H. Salmonella: A review on pathogenesis, epidemiology and antibiotic resistance. Front. Life Sci. 2015;8:284–293. doi: 10.1080/21553769.2015.1051243. [DOI] [Google Scholar]
- 5.Pui C.F., Wong W.C., Chai L.C., Tunung R., Jeyaletchumi P., Hidayah N., Ubong A., Farinazleen M.G., Cheah Y.K., Son R. Salmonella: A foodborne pathogen. Int. Food Res. J. 2011;18:465–473. [Google Scholar]
- 6.Angulo F.J., Mølbak K. Human health consequences of antimicrobial drug-resistant salmonella and other foodborne pathogens. Clin. Infect. Dis. 2005;41:1613–1620. doi: 10.1086/497599. [DOI] [PubMed] [Google Scholar]
- 7.Ehuwa O., Jaiswal A.K., Jaiswal S. Salmonella, food safety and food handling practices. Foods. 2021;10:907. doi: 10.3390/foods10050907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hsi D.J., Ebel E.D., Williams M.S., Golden N.J., Schlosser W.D. Comparing foodborne illness risks among meat commodities in the United States. Food Control. 2015;54:353–359. doi: 10.1016/j.foodcont.2015.02.018. [DOI] [Google Scholar]
- 9.Roth N., Käsbohrer A., Mayrhofer S., Zitz U., Hofacre C., Domig K.J. The application of antibiotics in broiler production and the resulting antibiotic resistance in Escherichia coli: A global overview. Poult. Sci. 2019;98:1791–1804. doi: 10.3382/ps/pey539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Casewell M., Friis C., Marco E., McMullin P., Phillips I. The European ban on growth-promoting antibiotics and emerging consequences for human and animal health. J. Antimicrob. Chemother. 2003;52:159–161. doi: 10.1093/jac/dkg313. [DOI] [PubMed] [Google Scholar]
- 11.Medina M., Legido-Quigley H., Hsu L.Y. Global Health Security. Springer; Cham, Germany: 2020. Antimicrobial resistance in one health; pp. 209–229. [Google Scholar]
- 12.Wallinga D., Smit L.A.M., Davis M.F., Casey J.A., Nachman K.E. A Review of the Effectiveness of Current US Policies on Antimicrobial Use in Meat and Poultry Production. Curr. Environ. Health Rep. 2022;9:339–354. doi: 10.1007/s40572-022-00351-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Agyare C., Boamah V.E., Zumbi C.N., Osei F.B. Antimicrobial Resistance—A Global Threat. IntechOpen; London, UK: 2018. Antibiotic use in poultry production and its effects on bacterial resistance; pp. 1–20. [Google Scholar]
- 14.Islam S., Urmi U.L., Rana M., Sultana F., Jahan N., Hossain B., Iqbal S., Hossain M., Mosaddek A.S., Nahar S. High abundance of the colistin resistance gene mcr-1 in chicken gut-bacteria in Bangladesh. Sci. Rep. 2020;10:1–11. doi: 10.1038/s41598-020-74402-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kasman L.M., Porter L.D. StatPearls [Internet] StatPearls Publishing; Treasure Island, FL, USA: 2020. Bacteriophages. [Google Scholar]
- 16.Mahony J., McAuliffe O., Ross R.P., Van Sinderen D. Bacteriophages as biocontrol agents of food pathogens. Curr. Opin. Biotechnol. 2011;22:157–163. doi: 10.1016/j.copbio.2010.10.008. [DOI] [PubMed] [Google Scholar]
- 17.Atterbury R.J., Gigante A.M., Rubio Lozano M.D.L.S., Méndez Medina R.D., Robinson G., Alloush H., Barrow P.A., Allen V.M. Reduction of Salmonella contamination on the surface of chicken skin using bacteriophage. Virol. J. 2020;17:1–8. doi: 10.1186/s12985-020-01368-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang C., Virk S.M., Shi J., Zhou Y., Willias S.P., Morsy M.K., Abdelnabby H.E., Liu J., Wang X., Li J. Isolation, characterization, and application of bacteriophage LPSE1 against Salmonella enterica in ready to eat (RTE) foods. Front. Microbiol. 2018;9:1046. doi: 10.3389/fmicb.2018.01046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hungaro H.M., Mendonça R.C.S., Gouvêa D.M., Vanetti M.C.D., de Oliveira Pinto C.L. Use of bacteriophages to reduce Salmonella in chicken skin in comparison with chemical agents. Food Res. Int. 2013;52:75–81. doi: 10.1016/j.foodres.2013.02.032. [DOI] [Google Scholar]
- 20.Sharma M., Patel J.R., Conway W.S., Ferguson S., Sulakvelidze A. Effectiveness of bacteriophages in reducing Escherichia coli O157: H7 on fresh-cut cantaloupes and lettuce. J. Food Prot. 2009;72:1481–1485. doi: 10.4315/0362-028X-72.7.1481. [DOI] [PubMed] [Google Scholar]
- 21.Atterbury R.J., Van Bergen M.A.P., Ortiz F., Lovell M.A., Harris J.A., De Boer A., Wagenaar J.A., Allen V.M., Barrow P.A. Bacteriophage therapy to reduce Salmonella colonization of broiler chickens. Appl. Environ. Microbiol. 2007;73:4543–4549. doi: 10.1128/AEM.00049-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li J., Zhao F., Zhan W., Li Z., Zou L., Zhao Q. Challenges for the application of bacteriophages as effective antibacterial agents in the food industry. J. Sci. Food Agric. 2022;102:461–471. doi: 10.1002/jsfa.11505. [DOI] [PubMed] [Google Scholar]
- 23.Chen H.-M., Wang Y., Su L.-H., Chiu C.-H. Nontyphoid Salmonella infection: Microbiology, clinical features, and antimicrobial therapy. Pediatr. Neonatol. 2013;54:147–152. doi: 10.1016/j.pedneo.2013.01.010. [DOI] [PubMed] [Google Scholar]
- 24.Vought K.J., Tatini S.R. Salmonella enteritidis contamination of ice cream associated with a 1994 multistate outbreak. J. Food Prot. 1998;61:5–10. doi: 10.4315/0362-028X-61.1.5. [DOI] [PubMed] [Google Scholar]
- 25.Galanis E., Wong D.M.A.L.F., Patrick M.E., Binsztein N., Cieslik A., Chalermchaikit T., Aidara-Kane A., Ellis A., Angulo F.J., Wegener H.C. Web-based surveillance and global Salmonella distribution, 2000–2002. Emerg. Infect. Dis. 2006;12:381. doi: 10.3201/eid1205.050854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Majowicz S.E., Musto J., Scallan E., Angulo F.J., Kirk M., O’Brien S.J., Jones T.F., Fazil A., Hoekstra R.M., International Collaboration on Enteric Disease ‘Burden of Illness’ Studies The global burden of nontyphoidal Salmonella gastroenteritis. Clin. Infect. Dis. 2010;50:882–889. doi: 10.1086/650733. [DOI] [PubMed] [Google Scholar]
- 27.Authority E.F.S. The European Union summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2016. EFSA J. 2017;15:5077. doi: 10.2903/j.efsa.2017.5077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.United States Department of Health and Human services, CDC . Antibiotic Resistance Threats in the United States, 2019. Department of Health and Human Services; Atlanta, GA, USA: 2019. [Google Scholar]
- 29.Mead G., Lammerding A.M., Cox N., Doyle M.P., Humbert F., Kulikovskiy A., Panin A., do Nascimento V.P., Wierup M., The Salmonella On Raw Poultry Writing Committee Scientific and technical factors affecting the setting of Salmonella criteria for raw poultry: A global perspective. J. Food Prot. 2010;73:1566–1590. doi: 10.4315/0362-028X-73.8.1566. [DOI] [PubMed] [Google Scholar]
- 30.Hugas M., Beloeil P.A. Controlling Salmonella along the food chain in the European Union-progress over the last ten years. Eurosurveillance. 2014;19:20804. doi: 10.2807/1560-7917.ES2014.19.19.20804. [DOI] [PubMed] [Google Scholar]
- 31.Guard-Petter J. The chicken, the egg and Salmonella enteritidis. Environ. Microbiol. 2001;3:421–430. doi: 10.1046/j.1462-2920.2001.00213.x. [DOI] [PubMed] [Google Scholar]
- 32.Food and Drug Administration, H.H.S. Prevention of Salmonella enteritidis in shell eggs during production, storage, and transportation. Final rule. Fed. Regist. 2009;74:33029–33101. [PubMed] [Google Scholar]
- 33.De Reu K., Grijspeerdt K., Messens W., Heyndrickx M., Uyttendaele M., Debevere J., Herman L. Eggshell factors influencing eggshell penetration and whole egg contamination by different bacteria, including Salmonella enteritidis. Int. J. Food Microbiol. 2006;112:253–260. doi: 10.1016/j.ijfoodmicro.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 34.Messens W., Grijspeerdt K., Herman L. Eggshell penetration by Salmonella: A review. World’s Poult. Sci. J. 2005;61:71–86. doi: 10.1079/WPS200443. [DOI] [PubMed] [Google Scholar]
- 35.Gantois I., Ducatelle R., Pasmans F., Haesebrouck F., Gast R., Humphrey T.J., Van Immerseel F. Mechanisms of egg contamination by Salmonella Enteritidis. FEMS Microbiol. Rev. 2009;33:718–738. doi: 10.1111/j.1574-6976.2008.00161.x. [DOI] [PubMed] [Google Scholar]
- 36.Singh S., Yadav A.S., Singh S.M., Bharti P. Prevalence of Salmonella in chicken eggs collected from poultry farms and marketing channels and their antimicrobial resistance. Food Res. Int. 2010;43:2027–2030. doi: 10.1016/j.foodres.2010.06.001. [DOI] [Google Scholar]
- 37.Karim M.R., Giasuddin M., Samad M.A., Mahmud M.S., Islam M.R., Rahman M.H., Yousuf M.A. Prevalence of Salmonella spp. in poultry and poultry products in Dhaka, Bangladesh. Int. J. Anim. Biol. 2017;3:18–22. [Google Scholar]
- 38.Mahmud M.S., Kabir M.L., Alam S.M.S., Ali M.M., Towhid S.T. Prevalence of Salmonella spp. in poultry eggs from different retail markets at Savar area, Bangladesh. Am. J. Food Sci. Health. 2015;1:27–31. [Google Scholar]
- 39.Taddese D., Tolosa T., Deresa B., Lakow M., Olani A., Shumi E. Antibiograms and risk factors of Salmonella isolates from laying hens and eggs in Jimma Town, South Western Ethiopia. BMC Res. Notes. 2019;12:1–7. doi: 10.1186/s13104-019-4516-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kemal J., Sibhat B., Menkir S., Beyene D. Prevalence, assessment, and antimicrobial resistance patterns of Salmonella from raw chicken eggs in Haramaya, Ethiopia. J. Infect. Dev. Ctries. 2016;10:1230–1235. doi: 10.3855/jidc.7885. [DOI] [PubMed] [Google Scholar]
- 41.Li W., Li H., Zheng S., Wang Z., Sheng H., Shi C., Shi X., Niu Q., Yang B. Prevalence, serotype, antibiotic susceptibility, and genotype of Salmonella in eggs from poultry farms and marketplaces in Yangling, Shaanxi province, China. Front. Microbiol. 2020;11:1482. doi: 10.3389/fmicb.2020.01482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xie T., Wu G., He X., Lai Z., Zhang H., Zhao J. Antimicrobial resistance and genetic diversity of Salmonella enterica from eggs. Food Sci. Nutr. 2019;7:2847–2853. doi: 10.1002/fsn3.1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hosseininezhad B., Berizi E., Nader M., Mazloomi S.M., Hosseinzadeh S., Ebrahimi L., Zare M. Prevalence of Salmonella contamination in consumed eggs in Iran: A systematic review and meta-analysis study on published studies from 1996 to 2018. Vet. World. 2020;13:2743–2751. doi: 10.14202/vetworld.2020.2743-2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shahzad A., Mahmood M.S., Hussain I., Siddique F., Abbas R.Z. Prevalence of salmonella species in hen eggs and egg storing-trays collected from poultry farms and marketing outlets of Faisalabad, Pakistan. Pak. J. Agric. Sci. 2012;49:565–568. [Google Scholar]
- 45.Al-Zenki S., Al-Nasser A., Al-Safar A., Alomirah H., Al-Haddad A., Hendriksen R.S., Aarestrup F.M. Prevalence and antibiotic resistance of Salmonella isolated from a poultry farm and processing plant environment in the State of Kuwait. Foodborne Pathog. Dis. 2007;4:367–373. doi: 10.1089/fpd.2007.0017. [DOI] [PubMed] [Google Scholar]
- 46.Zubair A.I., Al-Berfkani M.I., Issa A.R. Prevalence of Salmonella species from poultry eggs of local stores in Duhok. Int. J. Res. Med. Sci. 2017;5:2468–2471. doi: 10.18203/2320-6012.ijrms20172430. [DOI] [Google Scholar]
- 47.Saitanu K., Jerngklinchan J., Koowatananukul C. Incidence of salmonellae in duck eggs in Thailand. Southeast Asian J. Trop. Med. Public Health. 1994;25:328. [PubMed] [Google Scholar]
- 48.Musawa A.I., Bashiru G., Al-Rasheed A., Yakubu Y., Jibril A.H., Ballah F.M., Sidi S., Lawal N., Bala J.A., Odhah M.N., et al. Prevalence and antimicrobial susceptibility profiling of salmonella isolated from poultry products sold in sokoto metropolis, nigeria. J. Anim. Health Prod. 2021;9:148–155. [Google Scholar]
- 49.Haubert L., Maia D.S.V., Rauber Würfel S.D.F., Vaniel C., da Silva W.P. Virulence genes and sanitizers resistance in Salmonella isolates from eggs in southern Brazil. J. Food Sci. Technol. 2021;59:1097–1103. doi: 10.1007/s13197-021-05113-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sharuni A.A.G.U., Sumaiya M.R.F., Ekanayake E.M.D.N., Chandrasiri N.S., Arachchi A.M.P.S. Contamination of chicken eggshell and egg contents with Salmonella species from selected farms in Kosgama, Colombo district. Sri Lankan J. Infect. Dis. 2021;11:S2–S6. doi: 10.4038/sljid.v11i0.8369. [DOI] [Google Scholar]
- 51.Kapena M.S., Muma J.B., Mubita C.M., Munyeme M. Antimicrobial resistance of Escherichia coli and Salmonella in raw retail table eggs in Lusaka, Zambia. Vet. World. 2020;13:2528. doi: 10.14202/vetworld.2020.2528-2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Betancor L., Pereira M., Martinez A., Giossa G., Fookes M., Flores K., Barrios P., Repiso V., Vignoli R., Cordeiro N., et al. Prevalence of Salmonella enterica in poultry and eggs in Uruguay during an epidemic due to Salmonella enterica serovar Enteritidis. J. Clin. Microbiol. 2010;48:2413–2423. doi: 10.1128/JCM.02137-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sasaki Y., Tsujiyama Y., Asai T., Noda Y., Katayama S., Yamada Y. Salmonella prevalence in commercial raw shell eggs in Japan: A survey. Epidemiol. Infect. 2011;139:1060–1064. doi: 10.1017/S0950268810002153. [DOI] [PubMed] [Google Scholar]
- 54.Lee S.-K., Chon J.-W., Song K.-Y., Hyeon J.-Y., Moon J.-S., Seo K.-H. Prevalence, characterization, and antimicrobial susceptibility of Salmonella Gallinarum isolated from eggs produced in conventional or organic farms in South Korea. Poult. Sci. 2013;92:2789–2797. doi: 10.3382/ps.2013-03175. [DOI] [PubMed] [Google Scholar]
- 55.Vergara D.C.M., Gutiérrez V.E.R., García N.V. Prevalence and molecular identification of Salmonella spp. isolated from commercialized eggs at Ibague, Colombia. Rev. Salud Anim. 2016;38:164–172. [Google Scholar]
- 56.Kouam M.K., Biekop M.H.F., Katte B., Teguia A. Salmonella status of table eggs in commercial layer farms in Menoua Division, West region of Cameroon. Food Control. 2018;85:345–349. doi: 10.1016/j.foodcont.2017.09.037. [DOI] [Google Scholar]
- 57.Murchie L., Whyte P., Xia B., Horrigan S., Kelly L., Madden R.H. Prevalence of Salmonella in grade A whole shell eggs in the island of Ireland. J. Food Prot. 2007;70:1238–1240. doi: 10.4315/0362-028X-70.5.1238. [DOI] [PubMed] [Google Scholar]
- 58.Liljebjelke K.A., Hofacre C.L., Liu T., White D.G., Ayers S., Young S., Maurer J.J. Vertical and horizontal transmission of Salmonella within integrated broiler production system. Foodb. Pathog. Dis. 2005;2:90–102. doi: 10.1089/fpd.2005.2.90. [DOI] [PubMed] [Google Scholar]
- 59.Van de Giessen A.W., Bouwknegt M., Dam-Deisz W.D.C., van Pelt W., Wannet W.J.B., Visser G. Surveillance of Salmonella spp. and Campylobacter spp. in poultry production flocks in The Netherlands. Epidemiol. Infect. 2006;134:1266–1275. doi: 10.1017/S0950268806005905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gutierrez M., Fanning J., Murphy A., Murray G., Griffin M., Flack A., Leonard N., Egan J. Salmonella in broiler flocks in the republic of Ireland. Foodborne Pathog. Dis. 2009;6:111–120. doi: 10.1089/fpd.2008.0163. [DOI] [PubMed] [Google Scholar]
- 61.Srinivasan P., Balasubramaniam G.A., Gopala T.R., Murthy K., Saravanan S., Balachandran P. Prevalence and Pathology of Salmonellosis in Commercial Layer Chicken from Namakkal, India. Pak. Vet. J. 2014;34:3241–3328. [Google Scholar]
- 62.Duc V.M., Nakamoto Y., Fujiwara A., Toyofuku H., Obi T., Chuma T. Prevalence of Salmonella in broiler chickens in Kagoshima, Japan in 2009 to 2012 and the relationship between serovars changing and antimicrobial resistance. BMC Vet. Res. 2019;15:1–8. doi: 10.1186/s12917-019-1836-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhao X., Gao Y., Ye C., Yang L., Wang T., Chang W. Prevalence and characteristics of Salmonella isolated from free-range chickens in Shandong Province, China. Biomed Res. Int. 2016;2016:1–6. doi: 10.1155/2016/8183931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Roe M.T., Pillai S.D. Monitoring and identifying antibiotic resistance mechanisms in bacteria. Poult. Sci. 2003;82:622–626. doi: 10.1093/ps/82.4.622. [DOI] [PubMed] [Google Scholar]
- 65.Dhanarani T.S., Shankar C., Park J., Dexilin M., Kumar R.R., Thamaraiselvi K. Study on acquisition of bacterial antibiotic resistance determinants in poultry litter. Poult. Sci. 2009;88:1381–1387. doi: 10.3382/ps.2008-00327. [DOI] [PubMed] [Google Scholar]
- 66.Castro-Vargas R.E., Herrera-Sánchez M.P., Rodríguez-Hernández R., Rondón-Barragán I.S. Antibiotic resistance in Salmonella spp. isolated from poultry: A global overview. Vet. World. 2020;13:2070. doi: 10.14202/vetworld.2020.2070-2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Harsha H.T., Reshmi R., Varghese R., Divya P.S., Rahiman K.M.M., Hatha A.A.M. Prevalence and antibiotic resistance of Salmonella from the eggs of commercial samples. J. Microbiol. Infect. Dis. 2011;1:93–100. doi: 10.5799/ahinjs.02.2011.03.0023. [DOI] [Google Scholar]
- 68.Mahmud T., Hassan M.M., Alam M., Khan M.M., Bari M.S., Islam A. Prevalence and multidrug-resistant pattern of Salmonella from the eggs and egg-storing trays of retail markets of Bangladesh. Int. J. One Health. 2016;2:7–11. doi: 10.14202/IJOH.2016.7-11. [DOI] [Google Scholar]
- 69.Hoffmann M., Pettengill J.B., Gonzalez-Escalona N., Miller J., Ayers S.L., Zhao S., Allard M.W., McDermott P.F., Brown E.W., Monday S.R. Comparative sequence analysis of multidrug-resistant IncA/C plasmids from Salmonella enterica. Front. Microbiol. 2017;8:1459. doi: 10.3389/fmicb.2017.01459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Alam S.B., Mahmud M., Akter R., Hasan M., Sobur A., Nazir K.H.M., Noreddin A., Rahman T., El Zowalaty M.E., Rahman M. Molecular detection of multidrug resistant Salmonella species isolated from broiler farm in Bangladesh. Pathogens. 2020;9:201. doi: 10.3390/pathogens9030201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Uddin M.B., Hossain S.M., Hasan M., Alam M.N., Debnath M., Begum R., Roy S., Harun-Al-Rashid A., Chowdhury M.S.R., Rahman M.M., et al. Multidrug antimicrobial resistance and molecular detection of MCR-1 gene in Salmonella species isolated from chicken. Animals. 2021;11:206. doi: 10.3390/ani11010206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Xu Y., Zhou X., Jiang Z., Qi Y., Ed-Dra A., Yue M. Epidemiological investigation and antimicrobial resistance profiles of Salmonella isolated from breeder chicken hatcheries in Henan, China. Front. Cell. Infect. Microbiol. 2020;10:497. doi: 10.3389/fcimb.2020.00497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Elkenany R., Elsayed M.M., Zakaria A.I., El-Sayed S.A.-E.-S., Rizk M.A. Antimicrobial resistance profiles and virulence genotyping of Salmonella enterica serovars recovered from broiler chickens and chicken carcasses in Egypt. BMC Vet. Res. 2019;15:1–9. doi: 10.1186/s12917-019-1867-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ghaderi R., Moradi Bidhendi S., Khaki P. Occurrence of multidrug-resistant Salmonella enterica serovar Enteritidis isolates from poultry in Iran. Arch. Razi Inst. 2016;71:43–49. [Google Scholar]
- 75.Wajid M., Saleemi M.K., Sarwar Y., Ali A. Detection and characterization of multidrug-resistant Salmonella enterica serovar Infantis as an emerging threat in poultry farms of Faisalabad, Pakistan. J. Appl. Microbiol. 2019;127:248–261. doi: 10.1111/jam.14282. [DOI] [PubMed] [Google Scholar]
- 76.Lu Y., Zhao H., Sun J., Liu Y., Zhou X., Beier R.C., Wu G., Hou X. Characterization of multidrug-resistant Salmonella enterica serovars Indiana and Enteritidis from chickens in Eastern China. PLoS ONE. 2014;9:e96050. doi: 10.1371/journal.pone.0096050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hur J., Kim J.H., Park J.H., Lee Y.-J., Lee J.H. Molecular and virulence characteristics of multi-drug resistant Salmonella Enteritidis strains isolated from poultry. Vet. J. 2011;189:306–311. doi: 10.1016/j.tvjl.2010.07.017. [DOI] [PubMed] [Google Scholar]
- 78.Singh R., Yadav A.S., Tripathi V., Singh R.P. Antimicrobial resistance profile of Salmonella present in poultry and poultry environment in north India. Food Control. 2013;33:545–548. doi: 10.1016/j.foodcont.2013.03.041. [DOI] [Google Scholar]
- 79.Tirziu E., Lazăr R., Sala C., Nichita I., Morar A., Şereş M., Imre K. Salmonella in raw chicken meat from the Romanian seaside: Frequency of isolation and antibiotic resistance. J. Food Prot. 2015;78:1003–1006. doi: 10.4315/0362-028X.JFP-14-460. [DOI] [PubMed] [Google Scholar]
- 80.Mokgophi T.M., Gcebe N., Fasina F., Adesiyun A.A. Antimicrobial resistance profiles of Salmonella isolates on chickens processed and retailed at outlets of the informal market in Gauteng Province, South Africa. Pathogens. 2021;10:273. doi: 10.3390/pathogens10030273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ibrahim S., Wei Hoong L., Lai Siong Y., Mustapha Z., CW Zalati C.W., Aklilu E., Mohamad M., Kamaruzzaman N.F. Prevalence of antimicrobial resistance (AMR) Salmonella spp. and Escherichia coli isolated from broilers in the East Coast of Peninsular Malaysia. Antibiotics. 2021;10:579. doi: 10.3390/antibiotics10050579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Vidayanti I.N., Sukon P., Khaengair S., Pulsrikarn C., Angkittitrakul S. Prevalence and antimicrobial resistance of Salmonella spp. isolated from chicken meat in upper northeastern Thailand. Vet. Integr. Sci. 2021;19:121–131. doi: 10.12982/VIS.2021.011. [DOI] [Google Scholar]
- 83.Zdragas A., Mazaraki K., Vafeas G., Giantzi V., Papadopoulos T., Ekateriniadou L. Prevalence, seasonal occurrence and antimicrobial resistance of Salmonella in poultry retail products in Greece. Lett. Appl. Microbiol. 2012;55:308–313. doi: 10.1111/j.1472-765X.2012.03298.x. [DOI] [PubMed] [Google Scholar]
- 84.Ta Y.T., Nguyen T.T., To P.B., Pham D.X., Le H.T.H., Thi G.N., Alali W.Q., Walls I., Doyle M.P. Quantification, serovars, and antibiotic resistance of Salmonella isolated from retail raw chicken meat in Vietnam. J. Food Prot. 2014;77:57–66. doi: 10.4315/0362-028X.JFP-13-221. [DOI] [PubMed] [Google Scholar]
- 85.Zwe Y.H., Tang V.C.Y., Aung K.T., Gutiérrez R.A., Ng L.C., Yuk H.-G. Prevalence, sequence types, antibiotic resistance and, gyrA mutations of Salmonella isolated from retail fresh chicken meat in Singapore. Food Control. 2018;90:233–240. doi: 10.1016/j.foodcont.2018.03.004. [DOI] [Google Scholar]
- 86.Donado-Godoy P., Clavijo V., León M., Arevalo A., Castellanos R., Bernal J., Tafur M.A., Ovalle M.V., Alali W.Q., Hume M., et al. Counts, serovars, and antimicrobial resistance phenotypes of Salmonella on raw chicken meat at retail in Colombia. J. Food Prot. 2014;77:227–235. doi: 10.4315/0362-028X.JFP-13-276. [DOI] [PubMed] [Google Scholar]
- 87.Siriken B., Türk H., Yildirim T., Durupinar B., Erol I. Prevalence and characterization of Salmonella isolated from chicken meat in Turkey. J. Food Sci. 2015;80:M1044–M1050. doi: 10.1111/1750-3841.12829. [DOI] [PubMed] [Google Scholar]
- 88.Moe A.Z., Paulsen P., Pichpol D., Fries R., Irsigler H., Baumann M.P.O., Oo K.N. Prevalence and antimicrobial resistance of Salmonella isolates from chicken carcasses in retail markets in Yangon, Myanmar. J. Food Prot. 2017;80:947–951. doi: 10.4315/0362-028X.JFP-16-407. [DOI] [PubMed] [Google Scholar]
- 89.Aslam M., Checkley S., Avery B., Chalmers G., Bohaychuk V., Gensler G., Reid-Smith R., Boerlin P. Phenotypic and genetic characterization of antimicrobial resistance in Salmonella serovars isolated from retail meats in Alberta, Canada. Food Microbiol. 2012;32:110–117. doi: 10.1016/j.fm.2012.04.017. [DOI] [PubMed] [Google Scholar]
- 90.Procura F., Bueno D.J., Bruno S.B., Rogé A.D. Prevalence, antimicrobial resistance profile and comparison of methods for the isolation of Salmonella in chicken liver from Argentina. Food Res. Int. 2019;119:541–546. doi: 10.1016/j.foodres.2017.08.008. [DOI] [PubMed] [Google Scholar]
- 91.Sulakvelidze A., Alavidze Z., Morris J.G., Jr. Bacteriophage therapy. Antimicrob. Agents Chemother. 2001;45:649–659. doi: 10.1128/AAC.45.3.649-659.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hendrix R.W., Hatfull G.F., Ford M.E., Smith M.C.M., Burns R.N. Evolutionary relationships among diverse bacteriophages and prophages: All the world’s a phage. Proc. Natl. Acad. Sci. USA. 1999;96:2192–2197. doi: 10.1073/pnas.96.5.2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Abedon S.T., Kuhl S.J., Blasdel B.G., Kutter E.M. Phage treatment of human infections. Bacteriophage. 2011;1:66–85. doi: 10.4161/bact.1.2.15845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ackermann H.-W. Bacteriophages. Springer; Cham, Switzerland: 2009. Phage classification and characterization; pp. 127–140. [DOI] [PubMed] [Google Scholar]
- 95.Wittebole X., De Roock S., Opal S.M. A historical overview of bacteriophage therapy as an alternative to antibiotics for the treatment of bacterial pathogens. Virulence. 2014;5:226–235. doi: 10.4161/viru.25991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Azeredo J., Sutherland I.W. The use of phages for the removal of infectious biofilms. Curr. Pharm. Biotechnol. 2008;9:261–266. doi: 10.2174/138920108785161604. [DOI] [PubMed] [Google Scholar]
- 97.Dvořáčková M., Růžička F., Benešík M., Pantůček R., Dvořáková-Heroldová M. Antimicrobial effect of commercial phage preparation Stafal® on biofilm and planktonic forms of methicillin-resistant Staphylococcus aureus. Folia Microbiol. 2019;64:121–126. doi: 10.1007/s12223-018-0622-3. [DOI] [PubMed] [Google Scholar]
- 98.Pallavali R.R., Degati V.L., Lomada D., Reddy M.C., Durbaka V.R.P. Isolation and in vitro evaluation of bacteriophages against MDR-bacterial isolates from septic wound infections. PLoS ONE. 2017;12:e0179245. doi: 10.1371/journal.pone.0179245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Shen G.-H., Wang J.-L., Wen F.-S., Chang K.-M., Kuo C.-F., Lin C.-H., Luo H.-R., Hung C.-H. Isolation and characterization of φkm18p, a novel lytic phage with therapeutic potential against extensively drug resistant Acinetobacter baumannii. PLoS ONE. 2012;7:e46537. doi: 10.1371/journal.pone.0046537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wall S.K., Zhang J., Rostagno M.H., Ebner P.D. Phage therapy to reduce preprocessing Salmonella infections in market-weight swine. Appl. Environ. Microbiol. 2010;76:48–53. doi: 10.1128/AEM.00785-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ooi M.L., Drilling A.J., Morales S., Fong S., Moraitis S., Macias-Valle L., Vreugde S., Psaltis A.J., Wormald P.-J. Safety and tolerability of bacteriophage therapy for chronic rhinosinusitis due to Staphylococcus aureus. JAMA Otolaryngol. Neck Surg. 2019;145:723–729. doi: 10.1001/jamaoto.2019.1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Letkiewicz S., Międzybrodzki R., Fortuna W., Weber-Dąbrowska B., Górski A. Eradication of Enterococcus faecalis by phage therapy in chronic bacterial prostatitis—Case report. Folia Microbiol. 2009;54:457–461. doi: 10.1007/s12223-009-0064-z. [DOI] [PubMed] [Google Scholar]
- 103.Jennes S., Merabishvili M., Soentjens P., Pang K.W., Rose T., Keersebilck E., Soete O., François P.-M., Teodorescu S., Verween G., et al. Use of bacteriophages in the treatment of colistin-only-sensitive Pseudomonas aeruginosa septicaemia in a patient with acute kidney injury—A case report. Crit. Care. 2017;21:1–3. doi: 10.1186/s13054-017-1709-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Fadlallah A., Chelala E., Legeais J.-M. Corneal infection therapy with topical bacteriophage administration. Open Ophthalmol. J. 2015;9:167–168. doi: 10.2174/1874364101509010167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Markoishvili K., Tsitlanadze G., Katsarava R., Glenn J., Morris M.D., Jr., Sulakvelidze A. A novel sustained-release matrix based on biodegradable poly (ester amide) s and impregnated with bacteriophages and an antibiotic shows promise in management of infected venous stasis ulcers and other poorly healing wounds. Int. J. Dermatol. 2002;41:453–458. doi: 10.1046/j.1365-4362.2002.01451.x. [DOI] [PubMed] [Google Scholar]
- 106.Perera M.N., Abuladze T., Li M., Woolston J., Sulakvelidze A. Bacteriophage cocktail significantly reduces or eliminates Listeria monocytogenes contamination on lettuce, apples, cheese, smoked salmon and frozen foods. Food Microbiol. 2015;52:42–48. doi: 10.1016/j.fm.2015.06.006. [DOI] [PubMed] [Google Scholar]
- 107.Pires D.P., Costa A.R., Pinto G., Meneses L., Azeredo J. Current challenges and future opportunities of phage therapy. FEMS Microbiol. Rev. 2020;44:684–700. doi: 10.1093/femsre/fuaa017. [DOI] [PubMed] [Google Scholar]
- 108.Wernicki A., Nowaczek A., Urban-Chmiel R. Bacteriophage therapy to combat bacterial infections in poultry. Virol. J. 2017;14:1–13. doi: 10.1186/s12985-017-0849-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Henriques A., Sereno R., Almeida A. Reducing Salmonella horizontal transmission during egg incubation by phage therapy. Foodborne Pathog. Dis. 2013;10:718–722. doi: 10.1089/fpd.2012.1363. [DOI] [PubMed] [Google Scholar]
- 110.Sonalika J., Srujana A.S., Akhila D.S., Juliet M.R., Santhosh K.S. Application of bacteriophages to control Salmonella Enteritidis in raw eggs. Iran. J. Vet. Res. 2020;21:221–225. [PMC free article] [PubMed] [Google Scholar]
- 111.Carvalho C.M., Santos S.B., Kropinski A.M., Ferreira E.C., Azeredo J. Bacteriophages. InTech; Rijeka, Croatia: 2012. Phages as therapeutic tools to control major foodborne pathogens: Campylobacter and Salmonella; pp. 179–214. [Google Scholar]
- 112.Bardina C., Spricigo D.A., Cortés P., Llagostera M. Significance of the bacteriophage treatment schedule in reducing Salmonella colonization of poultry. Appl. Environ. Microbiol. 2012;78:6600–6607. doi: 10.1128/AEM.01257-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Fiorentin L., Vieira N.D., Barioni W., Jr. Oral treatment with bacteriophages reduces the concentration of Salmonella Enteritidis PT4 in caecal contents of broilers. Avian Pathol. 2005;34:258–263. doi: 10.1080/01445340500112157. [DOI] [PubMed] [Google Scholar]
- 114.Bielke L.R., Higgins S.E., Donoghue A.M., Donoghue D.J., Hargis B.M., Tellez G. Use of wide-host-range bacteriophages to reduce Salmonella on poultry products. Int. J. Poult. Sci. 2007;6:754–757. [Google Scholar]
- 115.Nabil N.M., Tawakol M.M., Hassan H.M. Assessing the impact of bacteriophages in the treatment of Salmonella in broiler chickens. Infect. Ecol. Epidemiol. 2018;8:1539056. doi: 10.1080/20008686.2018.1539056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Higgins S.E., Higgins J.P., Bielke L.R., Hargis B.M. Selection and application of bacteriophages for treating Salmonella enteritidis infections in poultry. Int. J. Poult. Sci. 2007;6:163–168. doi: 10.3923/ijps.2007.163.168. [DOI] [Google Scholar]
- 117.Lim T.-H., Kim M.-S., Lee D.-H., Lee Y.-N., Park J.-K., Youn H.-N., Lee H.-J., Yang S.-Y., Cho Y.-W., Lee J.-B., et al. Use of bacteriophage for biological control of Salmonella Enteritidis infection in chicken. Res. Vet. Sci. 2012;93:1173–1178. doi: 10.1016/j.rvsc.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 118.Borie C., Sánchez M.L., Navarro C., Ramírez S., Morales M.A., Retamales J., Robeson J. Aerosol spray treatment with bacteriophages and competitive exclusion reduces Salmonella enteritidis infection in chickens. Avian Dis. 2009;53:250–254. doi: 10.1637/8406-071008-Reg.1. [DOI] [PubMed] [Google Scholar]
- 119.Lim T.-H., Lee D.-H., Lee Y.-N., Park J.-K., Youn H.-N., Kim M.-S., Lee H.-J., Yang S.-Y., Cho Y.-W., Lee J.-B., et al. Efficacy of bacteriophage therapy on horizontal transmission of Salmonella Gallinarum on commercial layer chickens. Avian Dis. 2011;55:435–438. doi: 10.1637/9599-111210-Reg.1. [DOI] [PubMed] [Google Scholar]
- 120.Kimminau E.A., Russo K.N., Karnezos T.P., Oh H.G., Lee J.J., Tate C.C., Baxter J.A., Berghaus R.D., Hofacre C.L. Bacteriophage in-feed application: A novel approach to preventing Salmonella Enteritidis colonization in chicks fed experimentally contaminated feed. J. Appl. Poult. Res. 2020;29:930–936. doi: 10.1016/j.japr.2020.09.003. [DOI] [Google Scholar]
- 121.SKLAR I.A.N.B., JOERGER R.D. Attempts to utilize bacteriophage to combat Salmonella Enterica serovar entemtidis infection in chickens. J. Food Saf. 2001;21:15–29. doi: 10.1111/j.1745-4565.2001.tb00305.x. [DOI] [Google Scholar]
- 122.Borie C., Albala I., Sánchez P., Sánchez M.L., Ramírez S., Navarro C., Morales M.A., Retamales J., Robeson J. Bacteriophage treatment reduces Salmonella colonization of infected chickens. Avian Dis. 2008;52:64–67. doi: 10.1637/8091-082007-Reg. [DOI] [PubMed] [Google Scholar]
- 123.Wong C.L., Sieo C.C., Tan W.S., Abdullah N., Hair-Bejo M., Abu J., Ho Y.W. Evaluation of a lytic bacteriophage, Φ st1, for biocontrol of Salmonella enterica serovar Typhimurium in chickens. Int. J. Food Microbiol. 2014;172:92–101. doi: 10.1016/j.ijfoodmicro.2013.11.034. [DOI] [PubMed] [Google Scholar]
- 124.Zhang Y., Ding Y., Li W., Zhu W., Wang J., Wang X. Application of a novel lytic podoviridae phage Pu20 for biological control of drug-resistant Salmonella in liquid eggs. Pathogens. 2021;10:34. doi: 10.3390/pathogens10010034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Yi Y., Abdelhamid A.G., Xu Y., Yousef A.E. Characterization of broad-host lytic Salmonella phages isolated from livestock farms and application against Salmonella Enteritidis in liquid whole egg. LWT. 2021;144:111269. doi: 10.1016/j.lwt.2021.111269. [DOI] [Google Scholar]
- 126.Spricigo D.A., Bardina C., Cortés P., Llagostera M. Use of a bacteriophage cocktail to control Salmonella in food and the food industry. Int. J. Food Microbiol. 2013;165:169–174. doi: 10.1016/j.ijfoodmicro.2013.05.009. [DOI] [PubMed] [Google Scholar]
- 127.Duc H.M., Son H.M., Honjoh K., Miyamoto T. Isolation and application of bacteriophages to reduce Salmonella contamination in raw chicken meat. LWT. 2018;91:353–360. doi: 10.1016/j.lwt.2018.01.072. [DOI] [Google Scholar]
- 128.Bao H., Zhang P., Zhang H., Zhou Y., Zhang L., Wang R. Bio-control of Salmonella Enteritidis in foods using bacteriophages. Viruses. 2015;7:4836–4853. doi: 10.3390/v7082847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Esmael A., Azab E., Gobouri A.A., Nasr-Eldin M.A., Moustafa M., Mohamed S.A., Badr O.A., Abdelatty A.M. Isolation and characterization of two lytic bacteriophages infecting a multi-drug resistant Salmonella Typhimurium and their efficacy to combat salmonellosis in ready-to-use foods. Microorganisms. 2021;9:423. doi: 10.3390/microorganisms9020423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kim J.H., Kim H.J., Jung S.J., Mizan M.F.R., Park S.H., Ha S. Characterization of Salmonella spp.-specific bacteriophages and their biocontrol application in chicken breast meat. J. Food Sci. 2020;85:526–534. doi: 10.1111/1750-3841.15042. [DOI] [PubMed] [Google Scholar]
- 131.Sukumaran A.T., Nannapaneni R., Kiess A., Sharma C.S. Reduction of Salmonella on chicken meat and chicken skin by combined or sequential application of lytic bacteriophage with chemical antimicrobials. Int. J. Food Microbiol. 2015;207:8–15. doi: 10.1016/j.ijfoodmicro.2015.04.025. [DOI] [PubMed] [Google Scholar]
- 132.Shakeri G., Hammerl J.A., Jamshidi A., Ghazvini K., Rohde M., Szabo I., Kehrenberg C., Plötz M., Kittler S. The Lytic Siphophage vB_StyS-LmqsSP1 Reduces the Number of Salmonella enterica Serovar Typhimurium Isolates on Chicken Skin. Appl. Environ. Microbiol. 2021;87:e0142421. doi: 10.1128/AEM.01424-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Demirarslan Ö.A., Alasalvar H., Yildirim Z. Biocontrol of Salmonella Enteritidis on chicken meat and skin using lytic SE-P3, P16, P37, and P47 bacteriophages. LWT. 2021;137:110469. doi: 10.1016/j.lwt.2020.110469. [DOI] [Google Scholar]
- 134.Aguilera M., Martínez S., Tello M., Gallardo M.J., García V. Use of Cocktail of Bacteriophage for Salmonella Typhimurium Control in Chicken Meat. Foods. 2022;11:1164. doi: 10.3390/foods11081164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kumar A., Malik H., Dubal Z.B., Jaiswal R.K., Kumar S., Kumar B., Agarwal R.K. Isolation and characterization of Salmonella phages and phage cocktail mediated biocontrol of Salmonella enterica serovar Typhimurium in chicken meat. LWT. 2022;155:112957. [Google Scholar]
- 136.Rivera D., Moreno-Switt A.I., Denes T.G., Hudson L.K., Peters T.L., Samir R., Aziz R.K., Noben J.-P., Wagemans J., Dueñas F. Novel Salmonella Phage, vB_Sen_STGO-35-1, Characterization and Evaluation in Chicken Meat. Microorganisms. 2022;10:606. doi: 10.3390/microorganisms10030606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Whichard J.M., Sriranganathan N., Pierson F.W. Suppression of Salmonella growth by wild-type and large-plaque variants of bacteriophage Felix O1 in liquid culture and on chicken frankfurters. J. Food Prot. 2003;66:220–225. doi: 10.4315/0362-028X-66.2.220. [DOI] [PubMed] [Google Scholar]
- 138.Wang C., Chen Q., Zhang C., Yang J., Lu Z., Lu F., Bie X. Characterization of a broad host-spectrum virulent Salmonella bacteriophage fmb-p1 and its application on duck meat. Virus Res. 2017;236:14–23. doi: 10.1016/j.virusres.2017.05.001. [DOI] [PubMed] [Google Scholar]
- 139.Clavijo V., Baquero D., Hernandez S., Farfan J.C., Arias J., Arévalo A., Donado-Godoy P., Vives-Flores M. Phage cocktail SalmoFREE® reduces Salmonella on a commercial broiler farm. Poult. Sci. 2019;98:5054–5063. doi: 10.3382/ps/pez251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Clavijo V., Morales L.T., Flores M.J.V., Reyes A. The gut microbiota of chickens in a commercial farm treated with a Salmonella phage cocktail. Sci. Rep. 2022;12:1–16. doi: 10.1038/s41598-021-04679-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Thanki A.M., Hooton S., Gigante A.M., Atterbury R.J., Clokie M.R.J. Potential Roles for Bacteriophages in Reducing Salmonella from Poultry and Swine. IntechOpen Limited; London, UK: 2021. [Google Scholar]
- 142.Caflisch K.M., Suh G.A., Patel R. Biological challenges of phage therapy and proposed solutions: A literature review. Expert Rev. Anti-Infect. Ther. 2019;17:1011–1041. doi: 10.1080/14787210.2019.1694905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Sneppen K., Semsey S., Seshasayee A.S.N., Krishna S. Restriction modification systems as engines of diversity. Front. Microbiol. 2015;6:528. doi: 10.3389/fmicb.2015.00528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Moghadam M.T., Amirmozafari N., Shariati A., Hallajzadeh M., Mirkalantari S., Khoshbayan A., Jazi F.M. How phages overcome the challenges of drug resistant bacteria in clinical infections. Infect. Drug Resist. 2020;13:45–61. doi: 10.2147/IDR.S234353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Mulani M.S., Kamble E.E., Kumkar S.N., Tawre M.S., Pardesi K.R. Emerging strategies to combat ESKAPE pathogens in the era of antimicrobial resistance: A review. Front. Microbiol. 2019;10:539. doi: 10.3389/fmicb.2019.00539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Chan B.K., Abedon S.T., Loc-Carrillo C. Phage cocktails and the future of phage therapy. Future Microbiol. 2013;8:769–783. doi: 10.2217/fmb.13.47. [DOI] [PubMed] [Google Scholar]
- 147.Haines M.E.K., Hodges F.E., Nale J.Y., Mahony J., Van Sinderen D., Kaczorowska J., Alrashid B., Akter M., Brown N., Sauvageau D., et al. Analysis of selection methods to develop novel phage therapy cocktails against antimicrobial resistant clinical isolates of bacteria. Front. Microbiol. 2021;12:564. doi: 10.3389/fmicb.2021.613529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Ma Y., Pacan J.C., Wang Q., Xu Y., Huang X., Korenevsky A., Sabour P.M. Microencapsulation of bacteriophage Felix O1 into chitosan-alginate microspheres for oral delivery. Appl. Environ. Microbiol. 2008;74:4799–4805. doi: 10.1128/AEM.00246-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Liu D., Van Belleghem J.D., de Vries C.R., Burgener E., Chen Q., Manasherob R., Aronson J.R., Amanatullah D.F., Tamma P.D., Suh G.A. The safety and toxicity of phage therapy: A review of animal and clinical studies. Viruses. 2021;13:1268. doi: 10.3390/v13071268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Fauconnier A. Phage therapy regulation: From night to dawn. Viruses. 2019;11:352. doi: 10.3390/v11040352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Gigante A., Atterbury R.J. Veterinary use of bacteriophage therapy in intensively-reared livestock. Virol. J. 2019;16:1–9. doi: 10.1186/s12985-019-1260-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Moye Z.D., Woolston J., Sulakvelidze A. Bacteriophage applications for food production and processing. Viruses. 2018;10:205. doi: 10.3390/v10040205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Nagel T.E., Chan B.K., De Vos D., El-Shibiny A., Kang’Ethe E.K., Makumi A., Pirnay J.-P. The developing world urgently needs phages to combat pathogenic bacteria. Front. Microbiol. 2016;7:882. doi: 10.3389/fmicb.2016.00882. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable.