Abstract
Background:
The benefits of performing open versus laparoscopic gubernaculum-sparing second-stage Fowler–Stephens orchiopexy (FSO) remain unclear. We compared the two techniques to answer this question.
Methods:
We retrospectively studied a cohort of patients who underwent laparoscopic first-stage FSO and open versus laparoscopic gubernaculum-sparing second-stage FSO at our institution between September 2004 and June 2020 (all patients underwent surgery by a single surgeon). We evaluated both procedures based on the incidence of testicular atrophy, testicular ascent, and other complications.
Results:
The age at initial surgery was 45.7 ± 28.2 months (median, 39). One hundred nine cases were treated with open second-stage gubernaculum-sparing FSO (OFSO), and 96 cases were treated with laparoscopic second-stage gubernaculum-sparing FSO (LFSO). The mean follow-up period was 59.8 months (median, 54; standard deviation, +35). The overall testicular atrophy rate was 1.5%. Atrophy was observed in 2 and 1 patient in the OFSO and LFSO groups, respectively (1.8% versus 1.0%, P > .05). There was no significant difference in the incidence of testicular ascent between both groups (2.8% versus 3.1%). Five and four complications were noted in the OFSO and LFSO groups, respectively (P > .05).
Conclusions:
Second-stage gubernaculum-sparing FSO achieved high testicular survival rates and satisfactory testicular positions. Neither the open nor laparoscopic approach appeared superior, because the overall testicular survival rates and incidence of testicular ascent and other complications were equivalent between both groups.
Keywords: cryptorchidism, orchiopexy, laparoscopy, open, testis
Introduction
The ideal surgical management for intra-abdominal testes (IAT) has been debated for many years. In most cases, the short spermatic vessel is the main limiting factor that prevents the proper positioning of the testis in the scrotum.1,2 To facilitate tension-free testicular mobilization into the scrotum, Fowler and Stephen were the first to describe a technique that divides the testicular vessels, which provides more length and allows the IAT to descend into the scrotum.3 Studies show that the two-stage Fowler–Stephens orchiopexy (FSO) is better than the one-stage FSO,4,5 such that the two-stage technique is now the recommended method.6,7
However, the overall atrophy rate for the two-stage FSO is still as high as 10%,5 and testicular loss usually occurs after the second-stage FSO.8 Moreover, the technique for the second-stage FSO is not standardized.9 There is still conflicting evidence on whether open or laparoscopic second-stage FSO provides better outcomes.9
Testicular survival after the second-stage FSO procedure is dependent on an adequate arterial supply10; preserving two sources of collateral blood supply (deferential artery and gubernaculum) is associated with better outcomes compared with preserving just one source (deferential artery).7 In theory, compared with open surgery, laparoscopic surgery may improve testicular survival rates, because it provides better visibility, which aids in the proper dissection of the spermatic cord in the retroperitoneum.11 We tested this hypothesis by comparing the postoperative testicular atrophy rates between open and laparoscopic second-stage FSO. We reviewed the outcomes of children with IAT who underwent open or laparoscopic second-stage gubernaculum-sparing FSO at our institution.
Materials and Methods
Study design and patient selection
The study was approved by the institutional review board of Shenzhen Children's Hospital. Written informed consent was obtained from the parents/guardians of all patients before treatment. We retrospectively reviewed cases of IAT who were treated with laparoscopic first-stage FSO and open versus laparoscopic second-stage gubernaculum-sparing FSO at our institution between September 2004 and June 2020. IAT was defined as testes lying >2 cm above the internal inguinal ring. In the IAT cases, testes may not reach the scrotum without division of the testicular vessels. A total of 6 patients were excluded, because they had missing data.
We also excluded 7 cases with <12 months of follow-up and 4 cases with bilateral ITA from the final analyses. In total, 205 boys with complete clinical records were included in our study and these patients underwent surgery by a single surgeon. The choice of open or laparoscopic gubernaculum-sparing second-stage FSO for IAT was based on surgeon's preference. A total of 109 patients underwent open second-stage gubernaculum-sparing FSO and 96 patients underwent laparoscopic second-stage gubernaculum-sparing FSO.
Surgical technique
Laparoscopic first-stage FSO
Laparoscopic first-stage FSO was performed in all patients with short spermatic vessels. In this procedure, the spermatic vessels were clipped and divided ∼2 cm proximal to the testis.
Laparoscopic second-stage FSO
Laparoscopic second-stage FSO was performed after a mean period of 6.5 months (range 5.5–9). A peritoneal flap was created by dividing the peritoneum between the vas deferens and vessels, and the testis. The resulting peritoneal flap encompassed the vas deferens, testis, gubernaculum, and communicating branch of the blood vessel/s (Fig. 1A, B). The spermatic vessels were then divided between the endoclips that were applied during the first-stage FSO. Further dissection was performed proximal to the vas deferens, which provided generous mobilization of the peritoneal triangle that contained the collateral blood supply to the testis.
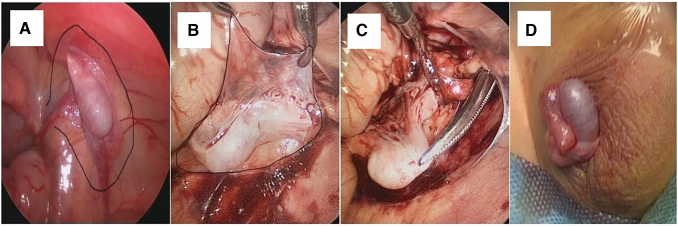
FIG. 1.
Intraoperative images. (A) This photograph shows the outline of the dissection plane for the peritoneal flap, which contains the collateral vessels; (B) this photograph shows the outline of the dissection plane of the actual peritoneal flap; (C) this photograph shows the mosquito artery forceps within the abdominal cavity and grasping the gubernaculum of the testis through the internal inguinal ring; (D) this photograph shows the well-vascularized testis positioned in the scrotum. Color images are available online.
A small scrotal incision was made, and a laparoscopic dissector was advanced through the internal inguinal ring into the scrotum. A mosquito artery forceps was introduced through the same path and used to grasp the testis at the gubernaculum (Fig. 1C) and reposition it into the dependent ipsilateral scrotum (Fig. 1D). If excessive testicular tension was noted, the peritoneum over the vas deferens was further dissected.
Open second-stage FSO
Open second-stage FSO was performed by retrieving the testis through an oblique inguinal incision that exposed the inguinal canal. The same steps as described previously for laparoscopic second-stage FSO were performed to dissect, mobilize, and deliver the testis into the Dartos pouch.
Follow-up
The patients were evaluated at 1, 6, and 12 months postoperatively and annually thereafter. An ultrasonography examination was performed at each evaluation and testicular volume and position were noted. Success was defined as a palpable testis in the scrotum. Ascent was defined as the location of the testis outside the scrotum, testicular atrophy was defined by the presence of an impalpable testis or a testicular nubbin, and was confirmed by ultrasonography.
Statistical analysis
Statistical analysis was performed using SPSS version 22.0. Categorical variables were evaluated using the chi-square test or logistic regression analysis, whereas continuous data were analyzed using the Student's t-test or covariance analysis. Statistical significance was set at P < .05.
Results
A total of 205 children were included in our study: 109 cases were treated with open gubernaculum-preserving FSO (OFSO) and 96 cases were treated with laparoscopic gubernaculum-sparing FSO (LFSO). None of the laparoscopic cases required conversion to open surgery. The mean age at initial diagnosis was 45.1 months (standard deviation [SD] ±27.5) in our institution. The mean age at initial surgery was 45.7 months (SD ±28.2). The patients' demographic characteristics and outcomes are summarized in Table 1.
Table 1.
Demographic and Clinical Characteristics and Outcomes of Patients Classified According to Group
| Variable | OFSO | LFSO | P |
|---|---|---|---|
| Number of patients | 109 | 96 | |
| Age (months) | 45.3 (range, 10–133) | 46.6 (range, 8–155) | >.05 |
| Follow-up (months) | 58.6 (range, 13–157) | 60.1 (range, 15–141) | >.05 |
| Success rate | 104 (95.4%) | 92 (95.8%) | >.05 |
| Testicular volume growth (mL) | 0.39 ± 0.66 | 0.38 ± 1.02 | >.05 |
| Complications | 5 (4.6%) | 4 (4.2%) | >.05 |
| Atrophy | 2 (1.8%) | 1 (1.0%) | >.05 |
| Testicular ascent | 3 (2.8%) | 3 (3.1%) | >.05 |
| Cost (CNY) | 6566 ± 1085 | 8414 ± 1255 | <.001 |
| Operating time (minute) | 66.8 ± 14.5 | 51.2 ± 10.3 | <.001 |
CNY, China Yuan; FSO, Fowler–Stephens orchiopexy; LFSO, laparoscopic gubernaculum-sparing FSO; OFSO, open gubernaculum-preserving FSO.
The mean follow-up time was 59.8 months (SD ±35). There was no significant difference in the success rates between the OFSO and LFSO groups (95.4% versus 95.8%, P > .05). Ultrasonography results showed no significant difference in the testicular volumes between the two groups (0.39 ± 0.66 mL versus 0.38 ± 1.02 mL, P > .05, Table 1).
There were five complications in the OFSO group (4.6%), including four inguinal or scrotal hematomas and one scrotal wound infection, compared with three complications in the LFSO group, including one umbilicus hematoma, one scrotal hematoma, and one scrotal wound infection. The overall atrophy rate was 1.5% (3/205) and included 2 and 1 patient from the OFSO and LFSO groups, respectively (1.8% versus 1.0%, P > .05, Table 1). Over the course of the follow-up, testicular ascent was noted in 3 and 3 patients in the OFSO and LFSO groups, respectively (2.8% versus 3.1%, P > .05, Table 1).
The OFSO group had significantly longer operating times (66.8 versus 51.2 minutes, P < .05, Table 1), but lower treatment costs (6566 ± 1085 China Yuan [CNY] versus 8414 ± 1255 CNY, P < .05, Table 1).
Discussion
Regardless of the surgical technique used to treat IAT, the success of orchiopexy is dependent on preserving adequate blood supply to the testis.4,12 Higher testicular atrophy rates have been observed in both open and laparoscopic second-stage FSO (following a laparoscopic first-stage procedure) when the gubernaculum was transected,7,13,14 because the gubernaculum provides an excellent blood supply.15 Ellis et al. demonstrated the presence of a rich arterial network between the gubernacular vessel and overlying spermatic and deferential vessels laterally. Preservation of the gubernaculum in the second-stage FSO procedure plays an important role in ensuring testicular survival.10
The two-stage gubernaculum-sparing FSO technique is associated with lower testicular atrophy rates. Since 2004, our unit has utilized this technique, through either the open or laparoscopic approaches, for all cases of IAT. Our results showed that preserving the gubernaculum and its collateral blood supply (cremasteric vessels) during staged FSO provided high success rates, because only 2 (1.8%) and 1 (1.0%) case of testicular atrophy were observed in the OFSO and LFSO groups, respectively.
The preliminary research on this technique is encouraging. Braga et al. reported that two-stage LFSO provided testis survival rates close to 99%.7 Dave et al. performed laparoscopic first-stage FSO with open second-stage FSO to treat IAT in 12 cases and demonstrated success rates of up to 100%.16 Therefore, the gubernaculum-sparing technique seems integral to improving the long-term outcomes of IAT.
The desired outcome after FSO is a palpable testis in the scrotum. The conventional second-stage FSO incises the gubernaculum and passes the testis medial to the inferior epigastric vessels to obtain the shortest route to the scrotum.17 Alagaratnam et al. retrospectively studied cases treated with staged FSO and reported an 8.8% rate of testicular ascent. They proposed that mobilization of the testes through the inguinal canal carries a greater risk of ascent versus passage through a new path medial to the inferior epigastric vessels.18 However, a recent study demonstrated that the incidence of testicular ascent was not significantly different with either technique.7 Interestingly, our study showed similar results, because only 2.9% of cases showed evidence of testicular ascent during follow-up.
Regardless of the technique used to position the testis within the scrotum, the length of the vas deferens is the main limiting factor that affects the final position of the testis during the second-stage FSO. Adequate dissection is often sufficient in our experience. In this study, adequate dissection allowed us to position the testes tension free within the scrotum in 95.6% of our cases. The peritoneum over the vas deferens was further dissected when necessary. We also agree that the testis has a tendency to descend spontaneously after the first-stage FSO. Robertson et al. reported that 10 of 25 testes were positioned lower after vessel ligation.15
In two small case series with retrospective study, the success rate of laparoscopic second-stage FSO was higher than that of open second-stage FSO,15,19 because the former provides magnification, which allows for detailed dissection and better preservation of the tiny collateral circulation. The laparoscopic approach also avoids the other collateral circulation otherwise damaged by the open approach.20 However, Casanova et al. reported the opposite results and argued that the open approach allows surgeons to handle the testis more delicately and better preserve the collateral vessels.9 Overall, there is still no consensus on whether the open or laparoscopic approach provides better outcomes for the second-stage FSO.
In this study, there was no statistically significant difference in the therapeutic efficacy between both groups. Our study analyzed the largest series of IAT cases treated with open versus laparoscopic second-stage FSO in the literature and followed these cases through a median period of >59 months. Our analysis demonstrated that overall success was more dependent on whether the collateral vessels were preserved rather than whether the open or laparoscopic approach was utilized. In our series, the vas deferens artery and gubernacular blood supply were clearly identified and easily preserved in both open and laparoscopic surgeries.
Ultrasonography examination also revealed that the testicular volume in both groups increased after surgery, but there was no significant difference in the testicular volume growth between the two groups. Our research and existing literature17,21 support the role of less dissection in improved testicular survival. We thus propose that preserving the gubernacular collaterals should be an indispensable step in the second-stage of FSO, regardless of whether an open or laparoscopic approach is taken.
Complications after staged FSO surgery have been reported, usually as individual case reports.5 The main complication found in our study was wound hematoma, which may have developed from injury to the small vessels and/or inadequate hemostasis. Although not statistically significant, the laparoscopic approach is associated with fewer complications than open surgery. This may be due to the advantages of a small wound and improved magnification during surgery.
Laparoscopy provides good mobilization of the testis and complete release of the vas deferens in the second-stage FSO.11 Consequently, satisfactory orchiopexy may be performed, because the pedicle provides sufficient length to reach the dependent part of the scrotum. Interestingly, there was no statistical difference in the incidence of testicular ascent between the OFSO and LFSO groups. Hence, satisfactory tension-free testicular position was achieved through adequate dissection and preservation of the collateral blood supply in both approaches of second-stage FSO.
We also examined the operative times and costs associated with both second-stage FOS approaches. LFSO required shorter operative times but more cost than OFSO.
The American Academy of Pediatrics recommend that orchiopexy should be performed before 1 year of age.11 In our study, only 23 (21.1%) and 20 (20.8%) of the children in the OFSO and LFSO groups, respectively, underwent the first-stage FSO before 1 year of age. This may be due to the following reasons: First, the majority of the children were referred to our department late. Second, some of the children were previously managed for cryptorchidism at other institutions before referral to our institution. Third, there is no consensus on the proper timing for orchiopexy.
However, FSO has been performed in younger and younger age groups, because parents are now more aware of cryptorchidism and the importance of timely treatment. The mean age of our patients was 45.7 months. The distance between the inner inguinal ring and scrotum increases as a child ages.22,23 The delay in treatment may explain the increased number of staged FSO in this series.
The advantage of this study is that it is the largest cohort to compare open versus laparoscopic second-stage FSO surgery. This study also conducted long-term follow-up, and an ultrasonography examination was performed at each follow-up.
This study has some potential limitations. First, this was not a randomized controlled study. Second, we lacked data on endocrine function and adult fertility outcomes. Our study population has to be followed up over a longer time period for these data to be evaluated.
Conclusion
Gubernaculum-sparing OFSO and LFSO achieved high testicular survival rates. Our findings suggested that preserving the gubernaculum to maximize collateral blood flow to the IAT provided excellent outcomes. However, excluding considerations for operative times and medical costs, the decision to perform the open or laparoscopic approach in the second-stage FSO should be based on the surgeon's comfort level and experience.
Acknowledgments
The authors thank Zhiling Yang and Yingtian Zhang for their refinement of this article.
Disclosure Statement
No competing financial interests exist.
Funding Information
This study is supported by Shenzhen Fund for Guangdong Provincial High-level Clinical Key specialties (SZXK035).
References
- 1. Wayne C, Chan E, Nasr A. Canadian Association of Paediatric Surgeons Evidence-Based Resource. What is the ideal surgical approach for intra-abdominal testes? a systematic review. Pediatr Surg Int 2015;31:327–338. [DOI] [PubMed] [Google Scholar]
- 2. Agrawal A, Joshi M, Mishra P, Gupta R, Sanghvi B, Parelkar S. Laparoscopic Stephen-Fowler stage procedure: Appropriate management for high intra-abdominal testes. J Laparoendosc Adv Surg Tech A 2010;20:183–185. [DOI] [PubMed] [Google Scholar]
- 3. Fowler R, Stephens FD. The role of testicular vascular anatomy in the salvage of high undescended testes. Aust N Z J Surg 1959;29:92–106. [DOI] [PubMed] [Google Scholar]
- 4. Penson D, Krishnaswami S, Jules A, McPheeters ML. Effectiveness of hormonal and surgical therapies for cryptorchidism: A systematic review. Pediatrics 2013;131:e1897–e1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yu CJ, Long CL, Wei Y, et al. Evaluation of Fowler-Stephens orchiopexy for high-level intra-abdominal cryptorchidism: A systematic review and meta-analysis. Int J Surg 2018;60:74–87. [DOI] [PubMed] [Google Scholar]
- 6. Radmayr C, Oswald J, Schwentner C, Neururer R, Peschel R, Bartsch G. Long-term outcome of laparoscopically managed nonpalpable testes. J Urol 2003;170:2409–2411. [DOI] [PubMed] [Google Scholar]
- 7. Braga LH, Farrokhyar F, Mcgrath M, Lorenzo AJ. Gubernaculum testis and cremasteric vessel preservation during laparoscopic orchiopexy for intra-abdominal testes: Effect on testicular atrophy rates. J Urol 2019;201:378–385. [DOI] [PubMed] [Google Scholar]
- 8. Abdelhalim A, Chamberlin JD, Young I, et al. Testicular volume changes in laparoscopic staged Fowler-Stephens orchiopexy: Studying the impact of testicular vessel division. Urology 2019;127:113–118. [DOI] [PubMed] [Google Scholar]
- 9. Casanova NC, Johnson EK, Bowen DK, et al. Two-step Fowler-Stephens orchiopexy for intra-abdominal testes: A 28-year single institution experience. J Urol 2013;190:1371–1376. [DOI] [PubMed] [Google Scholar]
- 10. Ellis R, Lahiri R, Mahomed A. Mapping testicular blood supply in gubernaculum-sparing second-stage Fowler–Stephens procedure. Surg Endosc 2014;28:3158–3161. [DOI] [PubMed] [Google Scholar]
- 11. Moursy EE, Gamal W, Hussein MM. Laparoscopic orchiopexy for non-palpable testes: Outcome of two techniques. J Pediatr Urol 2011;7:178–181. [DOI] [PubMed] [Google Scholar]
- 12. Elzeneini WM, Mostafa MS, Dahab MM, Youssef A, Abouzeid AA. How far can one-stage laparoscopic Fowler Stephens orchiopexy be implemented in intraabdominal testes with short spermatic vessels? J Pediatr Urol 2020;16:197..e1–197.e7. [DOI] [PubMed] [Google Scholar]
- 13. Stec AA, Tanaka ST, Adams MC, Pope JC, Thomas JC, Brock JW. Orchiopexy for intra-abdominal testes: Factors predicting success. J Urol 2009;182:1917–1920. [DOI] [PubMed] [Google Scholar]
- 14. Taran I, Elder JS. Results of orchiopexy for the undescended testis. World J Urol 2006;24:231–239. [DOI] [PubMed] [Google Scholar]
- 15. Robertson SA, Munro FD, Mackinlay GA. Two-stage Fowler-Stephens orchidopexy preserving the gubernacular vessels and A purely laparoscopic second stage. J Laparoendosc Adv Surg Tech A 2007;17:101–107. [DOI] [PubMed] [Google Scholar]
- 16. Dave S, Manaboriboon N, Braga LHP, et al. Open versus laparoscopic staged Fowler-Stephens orchiopexy: Impact of long loop vas. J Urol 2009;182:2435–2439. [DOI] [PubMed] [Google Scholar]
- 17. Mahomed A, Adams S, Islam S. Initial success with gubernacular-sparing laparoscopic-assisted fowler-Stephens orchidopexy for intra-abdominal testes. J Laparoendosc Adv Surg Tech A 2012;22:192–194. [DOI] [PubMed] [Google Scholar]
- 18. Alagaratnam S, Nathaniel C, Cuckow P, et al. Testicular outcome following laparoscopic second stage Fowler-Stephens orchidopexy. J Pediatr Urol 2014;10:186–192. [DOI] [PubMed] [Google Scholar]
- 19. Merguerian PA, Mevorach RA, Shortliffe LD, Cendron M. Laparoscopy for the evaluation and management of the nonpalpable testicle. Urology 1998;51:3–6. [DOI] [PubMed] [Google Scholar]
- 20. Wang CY, Wang Y, Chen XH, Wei YX, Chen F, Zhong M. Efficacy of single-stage and two-stage Fowler-Stephens laparoscopic orchidopexy in the treatment of intraabdominal high testis. Asian J Surg 2017;40:490–494. [DOI] [PubMed] [Google Scholar]
- 21. Chang M, Franco I. Laparoscopic Fowler-Stephens orchiopexy: The Westchester Medical Center experience. J Endourol 2008;22:1315–1319. [DOI] [PubMed] [Google Scholar]
- 22. Bagga D, Teckchandani N, Kumar V, Grover SB, Yadav DK, Acharya SK. Predictive factors for successful vessel-intact laparoscopic orchiopexy for intra-abdominal testes. J Pediatr Urol 2013;9:453–457. [DOI] [PubMed] [Google Scholar]
- 23. Bagga D, Prasad A, Grover SB, Sugandhi N, Samie A. Evaluation of two-staged Fowler–Stephens laparoscopic orchidopexy (FSLO) for intra-abdominal testes (IAT). Pediatr Surg Int 2018;34:97–103. [DOI] [PubMed] [Google Scholar]