Abstract
Mongersen is a 21-mer antisense oligonucleotide designed to downregulate Mothers against decapentaplegic homolog 7 (SMAD7) expression to treat Crohn's disease. Mongersen was manufactured in numerous batches at different scales during several years of clinical development, which all appeared identical, using common physicochemical analytical techniques, while only phosphorous-31 nuclear magnetic resonance (31P-NMR) in solution showed marked differences. Close-up analysis of 27 mongersen batches revealed marked differences in SMAD7 downregulation in a cell-based assay. Principal component analysis of 31P-NMR profiles showed strong correlation with SMAD7 downregulation and, therefore, with pharmacological efficacy in vitro. Mongersen contains 20 phosphorothioate (PS) linkages, whose chirality (Rp/Sp) was not controlled during manufacturing. A different diastereomeric composition throughout batches would lead to superimposable analytical data, but to distinct 31P-NMR profiles, as indeed we found. We tentatively suggest that this may be the origin of different biological activity. As similar manifolds are expected for other PS-based oligonucleotides, the protocol described here provides a general method to identify PS chirality issues and a chemometric tool to score each preparation for this elusive feature.
Keywords: Crohn's disease, phosphorothioate, antisense oligonucleotides, 31P-NMR, mongersen
Introduction
Several synthetic oligonucleotides, including antisense oligonucleotides (ASOs) and small interfering RNAs (siRNAs), have recently gained approval as therapeutic agents and many are in clinical development [1–3]. Synthetic oligonucleotides used therapeutically are chemically modified to improve their pharmacokinetic and pharmacodynamic properties [4,5]. The replacement of phosphodiester (PO) with phosphorothioate (PS) linkages was the first chemical modification to be investigated and it is still the most extensively used, in particular, in RNase H-dependent ASOs [1,4,6,7]. In these ASOs, replacement of the PO with PS improves resistance to nucleases and facilitates cellular uptake and bioavailability in vivo without significantly impairing hybridization with the target RNA or the posthybridization RNase H activation [1,4,6,8].
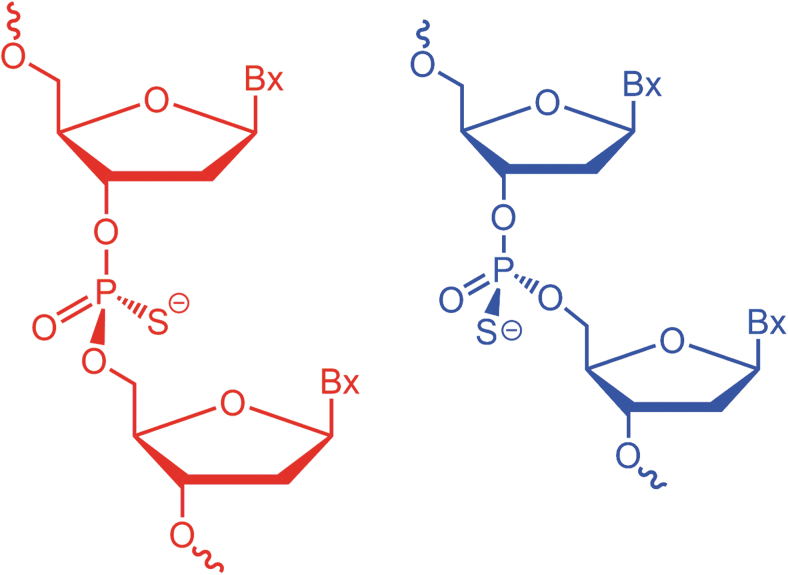
However, the PS modification results in a chiral center with either Sp or Rp configuration (Fig. 1). Such chiral centers coupled with the other stereogenic elements of the oligonucleotide chain lead to the formation of 2n diastereomers, with n being the number of PS linkages present in the PS-modified oligonucleotide [9,10]. Since common synthetic methods of PS-modified oligonucleotides do not allow for control of the chirality at the phosphorous atom, approved PS oligos are a mixture of diastereomers [4].
FIG. 1.
Schematic representation of phosphorothioate-modified oligonucleotides with Sp (red, left) and Rp (blue, right) configuration at the phosphorous atom.
It has been known for decades that these diastereomers have different biological activity, for example, the two PS nucleotides diastereomers have differential reactivity toward nucleases and kinases, and studies performed with ASOs where the stereochemistry of each PS linkage was predetermined, demonstrated that RNA binding properties, stability of sense/antisense hybrid, and posthybridization mechanisms can be affected by the stereochemistry [9,11–16].
In a study performed to explore the impact of stereochemistry on biological activity, mipomersen, an ASO approved by the Food and Drug Administration to treat homozygous familial hypercholesterolemia, was prepared in sets of Rp/Sp sequences across its 19 PS linkages, with the goal of determining the optimal configurations for RNase H1-mediated target cleavage [17]. This analysis demonstrated that the stereochemistry considerably modulates biological activity of the ASO. Stereochemical bias introduced during RNA synthesis has also been shown to impact the biological activity of siRNA [18].
However, as the overall efficacy of an ASO or siRNA is the result of a combination of events, where RNase H1 activity or RNA interference represents only one piece of the mosaic, it has also been reported that “ASOs with stereorandom PS linkages offer the optimal balance of activity and metabolic stability” [19], and that controlling PS chirality would not justify the added cost and complexity of the manufacturing process [20].
When stereochemistry is not controlled during oligonucleotide synthesis, random stereochemistry of a PS sequence is not ensured. Internal chiral induction is exerted by the nucleotide sugars and the growing chain itself. The bias toward Rp or Sp configurations depends on the conditions of preparation and purification of the ASO, and even small changes in the manufacturing can result in considerable differences in the chiral composition of PS linkages [21,22]. For this reason regulatory authorities require companies that manufacture oligonucleotides for therapeutic use ensure batch-to-batch consistency with regard to diastereomeric composition, which may represent a challenging aspect for most analytical methods, in the presence of a very large number of stereoisomers.
In this study, we focused on mongersen, a 21-mer PS-ASO investigational medicinal product developed for the treatment of Crohn's disease [23–29].
Crohn's disease is a chronic inflammatory bowel disease that affects a considerable portion of the population in Western countries, with increasing annual incidence and prevalence [30]. The Crohn's disease-associated inflammatory process is sustained by defects of normal counter-regulatory mechanisms [31]. One such mechanism involves transforming growth factor-β1 (TGF-β1), a multifunctional cytokine that inhibits multiple inflammatory signals in the gut and that contributes to the maintenance of the mucosal homeostasis [32,33]. In Crohn's disease, the defective activity of TGF-β1 is due to high levels of Mothers against decapentaplegic homolog 7 (SMAD7), an intracellular inhibitor of TGF-β1/SMAD signaling [23].
Consistent with this mechanism, reduction in SMAD7 levels using an ASO targeted to SMAD7 messenger RNA (mRNA) restored TGF-β1 activity, thereby suppressing inflammatory signals in human mucosal cells and in mouse models of colitis [34,35]. These studies paved the way for the development of an oral formulation of the SMAD7-targeted ASO now known as mongersen (previously GED-0301). A total of four phase 1 and phase 2 studies documented highly significant clinical and endoscopic benefit of mongersen in patients with active Crohn's disease [24,25,27,29].
Despite these very positive results, a multicenter randomized double-blind placebo-controlled phase 3 study was prematurely discontinued on the basis of a futility analysis showing that mongersen was not effective in Crohn's disease patients [28]. The reasons for the failure of the phase 3 study, despite the previous clinical trials documenting the efficacy of the drug, have not been addressed.
Mongersen has been prepared in numerous batches during several years of clinical development and, as is the case for most clinically evaluated PS-ASOs, it was synthesized without controlling PS stereochemistry. We had the unique opportunity to analyze samples from each batch to identify unpredicted and undesirable inhomogeneities, which are likely to affect other therapeutic oligonucleotides that contain PS linkages. From this point of view, mongersen constitutes a study case that represents a general situation.
Very recently, upon testing of a limited number of mongersen batches, Marafini et al. reported remarkable differences in their pharmacological activity [29].
Over the extended period of mongersen production, several conditions changed. The mere difference in manufacture scale can represent a variable, whose fallout on the composition of the mixture of the 220 possible diastereomers is difficult to evaluate. It should be borne in mind that current analytical techniques would fail to resolve and quantify this complex mixture.
With this study, we shall demonstrate that (1) phosphorous-31 nuclear magnetic resonance (31P-NMR) reveals inhomogeneity among the various batches and fingerprints them, (2) principal component analysis (PCA) can immediately group families of 31P-NMR spectra, and (3) the PCA families correlate with different biological activities in vitro. This enables us to rationalize one of the possible causes that could have contributed to the failure of the phase 3 study despite the previous positive scientific and clinical evidence. We tentatively assign this inhomogeneity to a variable diastereomeric composition throughout the different preparations.
The specific case of mongersen sheds light on a problem that is likely to be ubiquitous in the context of PS-modified oligonucleotides and validates a method to recognize it and an analytical target to direct manufacturing.
Materials and Methods
Mongersen samples
Mongersen was chemically synthesized using commercially available synthesizers in a process that included oligonucleotide synthesis on solid support, cleavage of the protected oligonucleotide from the solid support, deprotection, preparative chromatography purification, concentration, desalting, and lyophilization to yield the drug substance. Twenty-seven different batches of mongersen, prepared during the course of several years at different manufacturing scales, were studied (Supplementary Table S1). All batches (excluding E) were manufactured in compliance with current good manufacturing practice between 2008 and 2017.
Most of these batches were used in clinical trials. The exception is sample E, which was manufactured on laboratory scale (0.24 mmol, 150 mg) in 2020. Quality control testing performed at the time of manufacture was in line with standard requirements for ASOs and did not reveal significant chemical differences. The batches had a chemical purity of mean 92.1% ± 0.7%. The impurity profile, mainly represented by oligonucleotide-related impurities, was typical of this class of molecules. Of note, the impurity pattern was consistent across batches, as ascertained by reversed-phase high performance liquid chromatography (RP-HPLC) (Supplementary Fig. S1) and liquid chromatography mass spectrometry (LC-MS) (Supplementary Figs. S2 and S3) analyses. All the samples were stored at −20°C.
Quality and homogeneity in terms of purity and impurities profile were reconfirmed by RP-HPLC on all batches before 31P-NMR and in vitro testing performed for this study (Supplementary Fig. S1).
Moreover, representative batches were also characterized using a large array of state-of-the-art techniques aimed at detecting possible differences in the chemical composition and physical properties of the batches (ie, solid-state NMR, X-ray powder diffraction, Fourier transform (FT) infrared spectroscopy, FT-Raman spectroscopy, small-angle X-ray scattering, thermal gravimetric analysis evolved gases analysis, differential scanning calorimetry, dynamic vapor sorption, electronic circular dichroism [ECD], and variable temperature ECD). No significant variability between batches was detected (Supplementary Figs. S4–S15).
In vitro pharmacology experiments
The human colorectal cancer cell line HCT-116 was obtained from the American Type Culture Collection and maintained in McCoy's 5A (Lonza) supplemented with 10% fetal bovine serum (FBS) (Euroclone) and 1% penicillin/streptomycin (Lonza), in a 37°C 5% CO2 fully humidified incubator. The cell line was authenticated by short tandem repeat DNA fingerprinting using the PowerPlex 18D System kit according to the manufacturer's instructions (Promega); the profile matched the known DNA fingerprint. To perform transfection experiments, 1 mL of single-cell suspension (2 × 105 cells/mL) was added per well of six-well culture dishes.
The next day, cells were washed with phosphate buffered saline (PBS) (Lonza) and transfected with different batches of mongersen using Opti-MEM I transfection medium plus Lipofectamine 3000 (Lipo) reagent (Thermo Fisher Scientific) according to the manufacturer's instructions. The transfection was performed in a final volume of 2 mL to result in a 1 μg/mL final concentration of each test item. Cells incubated with Opti-MEM I transfection medium and Lipo only were used as internal control. After 24 h, cells were washed with PBS and cultured with McCoy's 5A supplemented with 10% FBS and antibiotics for further 3 or 24 h to assess SMAD7 RNA transcripts or SMAD7 protein expression, respectively. Cells were then washed with PBS and either RNA or total proteins were extracted to perform reverse transcription-quantitative polymerase chain reaction (RT-qPCR) and Western blotting experiments.
RNA was extracted using PureLink mRNA mini kit (Thermo Fisher Scientific) according to the manufacturer's instructions. A constant amount of RNA (1 μg per sample) was reverse transcribed into complementary DNA (cDNA), and 1 μL of cDNA per sample was then amplified by RT-qPCR using iQ SYBR Green Supermix (Bio-Rad Laboratories, Milan, Italy) and the following primers: SMAD7: forward: 5′-GCCCGACTTCTTCATGGTGT-3′; reverse: 5′-TGCCGCTCCTTCAGTTTCTT-3′, β-actin: forward: 5′-AAGATGACCCAGATCATGTTTGAGACC-3′; reverse: 5′-AGCCAGTCCAGACGCAGGAT-3′. SMAD7 RNA expression was calculated relative to the housekeeping β-actin gene on the base of the ΔΔCt algorithm.
Total proteins were extracted using the following lysis buffer: 10 mmol/L N-2-hydroxyethylpiperazine-N'-2-ethanesulfonic (HEPES) pH 7.9, 1 mmol/L ethylenediaminetetra-acetic acid (EDTA), 60 mmol/L KCl, 0.2% Igepal CA-630, 1 mmol/L sodium fluoride, 10 μg/mL aprotinin, 10 μg/mL leupeptin, 1 mmol/L dithiothreitol (DTT), and 1 mmol/L phenylmethylsulfonyl fluoride (PMSF) (all from Sigma-Aldrich). Proteins were separated on a 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) gel, and blots were then incubated with a mouse antihuman monoclonal anti-SMAD7 antibody (0.5 μg/mL final dilution; R&D Systems) followed by incubation with a rabbit antimouse antibody conjugated to horseradish peroxidase (1:20,000 final dilution; Dako, Cat. No.: MAB2029).
After analysis for SMAD7, each blot was stripped and incubated with a mouse–antihuman monoclonal β-actin antibody (Sigma-Aldrich) to confirm equal loading of the lanes. Computer-assisted scanning densitometry (Image Lab Software) was used to analyze the intensity of the immunoreactive bands. Each batch of mongersen was tested in at least three independent experiments. Data were analyzed using the Student's t-test. Each experiment included the negative control (transfection reagent-only treated cells) and the mongersen reference batch H.
NMR experiments
In all cases, the ASOs were dissolved in D2O (∼9 mg in 0.5 mL) and immediately subjected to NMR (31P and 1H) analysis. The NMR experiments were conducted on an Agilent Inova 600 spectrometer operating at 14.1 T and at 25.0°C using standard 1H-NMR and 31P-NMR pulse sequences. For 1H-NMR, 128 transients were obtained. For 31P-NMR, 2,000 transients with 3-s relaxation times were obtained. The hydrogen deuterium oxide line width did not exceed 1.3 Hz. A line broadening of 2 Hz was applied to the 31P spectra.
Representative 1H- and 31P-NMR spectra are shown in Supplementary Figs. S16–S19. After recording a spectrum as already specified above, the tube containing sample F was heated to 85°C for 20 min. After this, it was cooled to room temperature again and measured a second time at 25°C; data from this experiment are shown in Supplementary Fig. S20.
31P-NMR PCA
PCA was performed using the freeware Chemometrics Agile Tool (http://www.gruppochemiometria.it/index.php/software/19-download-the-r-based-chemometric-software). All the 31P-NMR spectra were digitized between 53 and 57 ppm (a total of 2,175 points each). The samples C, F, G, H, L, M, Q, S, U, and W were chosen as the training set. To perform the PCA, all the NMR spectra were normalized on the area between 57 and 53 ppm. The data matrix was then prepared by arranging the variables in columns and the samples in rows. The PCA was performed on the data matrix by automatically scaling the intensity values through mean centering by column.
Results
In vitro pharmacology profile
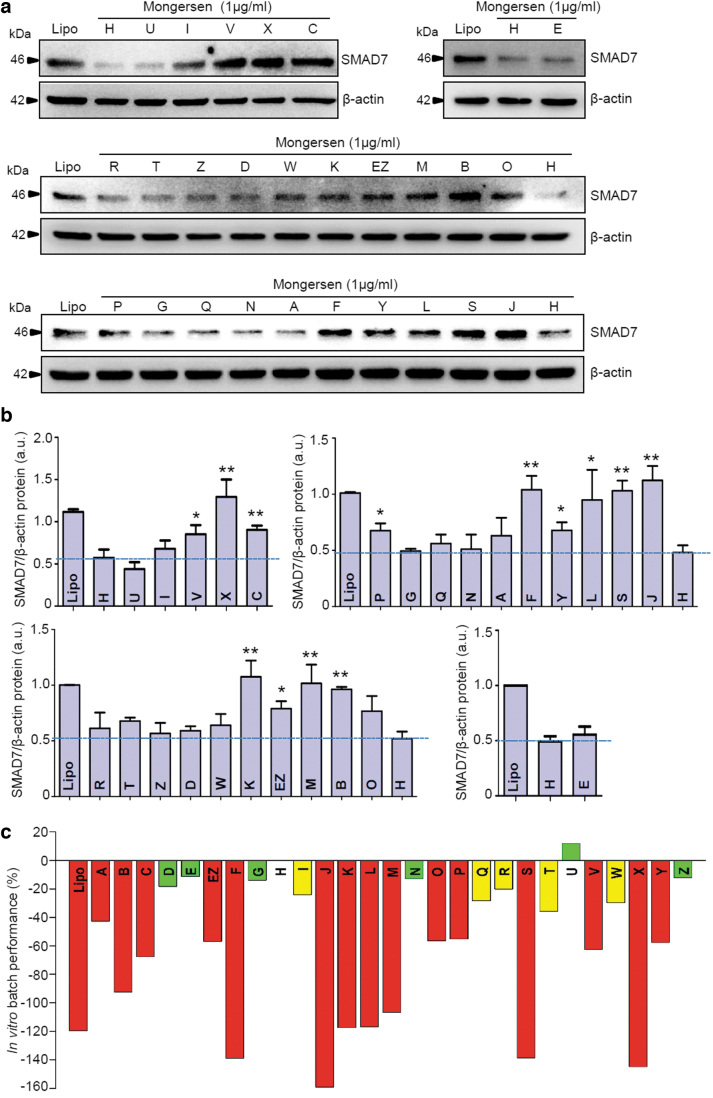
To identify differences in the pharmacological profiles of different clinical batches of mongersen drug substance (Supplementary Table S1), the downregulation of SMAD7 protein was tested in cells of the human colorectal cancer cell line HCT-116 treated with mongersen or not. This is a well-established bioassay, shown previously to give reproducible results [36]. Before in vitro testing, all samples were analyzed by RP-HPLC, which did not reveal significant differences among batches, confirming the considerable stability of mongersen over several years of storage. The batches differ in their ability to reduce the levels of SMAD7 target protein in cultured cells (Fig. 2a, b).
FIG. 2.
(a) Western blots for SMAD7 protein expression in cells treated with mongersen from different batches. HCT-116 cells were either incubated with Lipofectamine 3000 only (Lipo) or transfected with the indicated batches of drug substance. Whole-cell extracts were prepared and analyzed for SMAD7 expression by Western blotting. β-actin was used as a loading control. One of at least three representative experiments for each set of samples is shown. (b) Quantitative analysis of SMAD7/β-actin protein ratio in total extracts of HCT-116 cells treated as indicated in (a), as measured by densitometry scanning of Western blots. Values are expressed in a.u. and are the mean ± SEM of at least three experiments for each set of samples. The dashed lines represent the SMAD7/β-actin protein ratio in cells transfected with the reference batch H. “Lipo” indicates the negative control, with cells treated with transfection reagent only. Reference batch H versus the indicated mongersen batches, *P < 0.05, **P < 0.01. (c) In vitro capability of mongersen batches to downregulate SMAD7 protein expression compared with batch H. An arbitrary pharmacology performance score, defined as the mean percentage difference in SMAD7 protein expression between treatment with the indicated batch and the reference batch H, was assigned to the mongersen batches based on their capability to downregulate the SMAD7 protein. The pharmacology performance of the batches is represented by colored bars and is defined as follows: green—similar to batch H (efficacy within 20% of H), yellow—marginal decrease in performance compared with batch H (decrease in efficacy of 20–40% vs. H); red—poor performance (>40% decrease of efficacy compared with H). “Lipo” indicates the negative control, with cells treated with transfection reagent only. For each batch, the bar represents the mean of at least three independent experiments. a.u., arbitrary units; HCT-116; SEM, standard error of the mean; SMAD7, Mothers against decapentaplegic homolog 7.
An in vitro arbitrary pharmacology performance score was assigned to the batches based on their capability to downregulate the SMAD7 protein as compared with the capability of a reference batch H. The reference batch consistently reduced SMAD7 levels in this assay [29] and induced clinical remission in patients with active Crohn's disease when formulated as gastroresistant delayed release tablets used in the phase 1 and phase 2 clinical studies [24,25]. The in vitro pharmacology performance scores are plotted in Fig. 2c.
A color-coded score was assigned to the batches based on their in vitro pharmacology performance: green—good performance, yellow—medium performance, and red—poor performance. In addition, we evaluated the ability of some representative batches (ie, H, Z, K, and S) to affect SMAD7 RNA expression by RT-qPCR. Our data, depicted in Supplementary Fig. S21, clearly show the different capability of the tested batches in downregulating SMAD7 RNA transcripts and are in line with data obtained by Western blotting regarding the modulation of SMAD7 protein levels.
31P-NMR spectra of mongersen batches
After ascertaining that all other analytical data had revealed no differences among the various preparations (see Supplementary Data), all batches of drug substance were analyzed by 31P-NMR. Each phosphorous atom of the PS ASO results in a singlet in the 31P-NMR spectrum in the region between 54 and 56 ppm. The fine structure in this region is due to, among other effects, the distribution of Sp/Rp centers along the backbone. Most chemical impurities do not contribute to the 31P spectrum in the narrow chemical shift range of PS linkages and, therefore, the differences can arise from stereochemistry.
Wyrzykiewicz and Cole previously reported that the PS linkage formation is not stereorandom [21]. However, these authors suggested that the overall synthetic process is stereoreproducible. This will be correct, when synthesis is performed under rigorously identical conditions using the same starting materials. However, this may not be the case when synthesis scale differs and when drug substance is prepared over a long time span, during which there may be variations in the manufacturing protocols or in the starting materials. Moreover, owing to the diastereomeric nature of the Sp/Rp set of isomers, the chromatographic purification steps may further affect this composition [37,38].
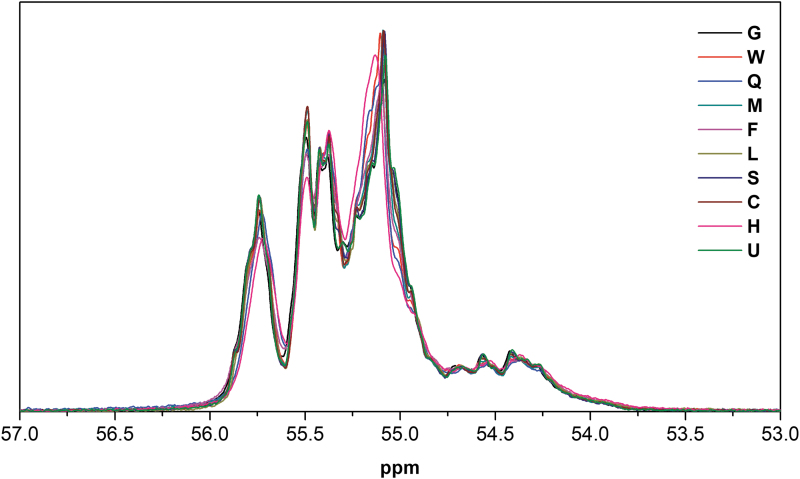
Provided the base sequence is the same, a 31P spectrum is further modulated by the sequence of Sp/Rp at each PS linkages. When there are 20 such linkages, there are 220 possible diastereomers, each leading to a different 31P spectrum. Thus, the signal between 54 and 56 ppm for mongersen batches is complex (we should hypothesize up to 20 × 220 = 20 × 106 singlets). For mongersen batches, we observed signals in the ranges of 54.8–55.3, 55.3–55.6, and 55.6–56.0 ppm; signals between 54 and 54.8 ppm were very weak. The fine structures within these regions differed from batch to batch as shown in Fig. 3.
FIG. 3.
The 31P-NMR spectra of a subset of mongersen batches, revealing differences in the fine structures of the 31P resonances.
Principal component analysis
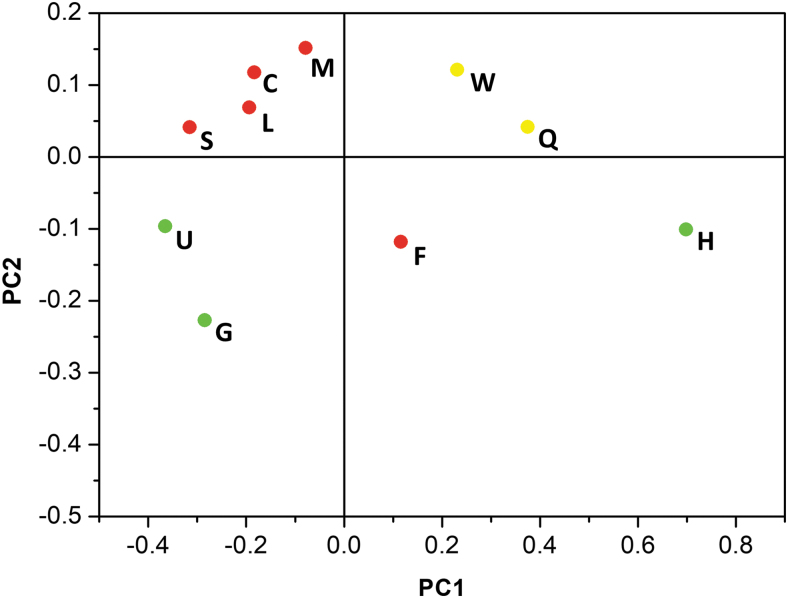
Because the 31P spectra are the convolution of a large number of contributions, it was necessary to resort to the application of chemometric analysis. Ten batches were selected that had spectra that appeared to be significantly different from one another (Fig. 3), and spectra of these batches were subjected to PCA using the Chemometric Agile Tool software. Six principal components accounted for >99% of variance of the 31P-NMR spectra, and only two components account for 90% of total variance. The score plot of this training set is shown in Fig. 4 and the scree plot is presented in Supplementary Fig. S22.
FIG. 4.
The score plot of the 31P spectra of the 10 batches of mongersen (M, C, L, S, U, G, W, Q, F, and H) used as a training set for the PCA. PCA, principal component analysis.
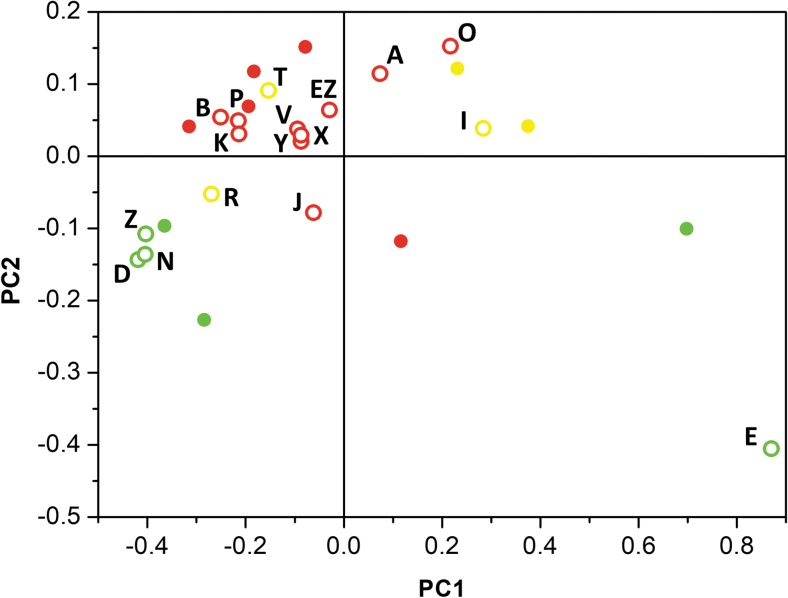
The remaining 17 spectra were treated as an external set and were projected onto the score plot of the training set (Fig. 5). Clusters of batches with almost identical PC1/PC2 were distinguished. The batches in the clusters had similar 31P-NMR spectra. A small number of batches are scattered and did not belong to any defined cluster.
FIG. 5.
The score plot of the 31P spectra of the 27 batches of mongersen analyzed in this study. The filled circles (batch number reported in Fig. 4) represent samples from the training set. The open circles represent the external set. The colors correspond to SMAD7 expression inhibition (as defined in Fig. 2 legend): green—similar to batch H, yellow—marginal decrease in performance versus H; and red—poor performance.
Besides the chirality of PS linkages, the 31P spectra would also respond to other stereochemical factors, the most critical one is represented by secondary structures. To exclude that mongersen batches are characterized by different secondary structures, ECD spectra were recorded. All samples had very similar ECD spectra and almost identical ECD versus temperature curves (Supplementary Figs. S14 and S15); all variations were within the experimental error. As a second experiment to exclude other factors, NMR spectra were obtained of batch F before and after heating at 85°C for 20 min. The spectra before and after heat treatment are superimposable (Supplementary Fig. S20), thus the oligonucleotide was not trapped in a folded conformation.
Correlation between SMAD7 inhibition and 31P-NMR PCA
In the score plot of Fig. 5, the cluster of batches centered at (−0.10, 0.05) corresponds to batches that had considerably lower in vitro SMAD7 inhibitory effect (“red cluster”) than the reference batch H. Outside this region, there is a transition zone, characterized by data corresponding to batches with marginal decrease in performance compared with batch H. A well-defined area around (−0.35, −0.15) is also distinguishable; in this region, batches with in vitro SMAD7 inhibitory performance similar to reference batch H are clustered (“green cluster”).
Two batches located around (0.7, −0.1) and (0.9, −0.45) are batch H (which was the reference for the in vitro pharmacology testing, and was the batch manufactured first) and batch E, prepared in 2020 in a laboratory-scale synthesis. Both batches H and E very efficiently inhibited SMAD7 expression in the in vitro assay.
Discussion
Mongersen is a PS-ASO designed to downregulate the expression of SMAD7.
Starting in 2008, a total of four phases 1 and 2 clinical studies documented highly significant clinical benefit in active Crohn's disease patients treated with mongersen [24,25,27,29], whereas a phase 3 study was discontinued in 2017 due to lack of efficacy [28].
The in vitro assay employing the human cell line HCT-116 provides an excellent model to test the pharmacological activity of this ASO. This assay demonstrated a large degree of variation in the ability of 27 different batches (most of which were used in mongersen clinical studies), to inhibit SMAD7. This variability indicates that there was a lack of reproducibility of the drug substance manufacture, which could not be detected during quality control testing, or by the employment of a large array of state-of-the-art techniques (ie, solid-state NMR, X-ray powder diffraction, FT infrared spectroscopy, FT-Raman spectroscopy, small-angle X-ray scattering, thermal gravimetric analysis, evolved gases analysis, differential scanning calorimetry, dynamic vapor sorption, and LC-MS impurity profiling, or by ECD analysis) aimed at thoroughly characterizing the composition and chemical/physical properties of the batches.
By using these analytical methods, one would have concluded that the product was homogeneous within the batches. It is also worth of note that LC-MS and 31P-NMR itself would reveal the presence of PO impurities. For a number n of PO replacing PS, one would expect an MS peak at mass M-(n × 16) and 31P resonances ∼0 ppm, which can be practically ruled out (below a few percentage of monophosphodiester-related substance detected by LC-MS in all batches) by considering the spectra in Supplementary Figs. S3, S6 and S17.
In contrast, 31P-NMR, which is a selective interrogation tool for the chemical environments of the phosphorous atoms, differed significantly from batch to batch. The remarkable differences in 31P-NMR point to an unexpected manifold, shedding light on the batch-to-batch differences observed in in vitro activity.
Of note, the batches of drug substance used in this study were manufactured over the course of several years, and batch size, equipment, parameters, and reagents were changed during this time period.
PCA allowed us to identify clusters of batches with similar 31P-NMR spectra. The spectral characteristics were strongly correlated with in vitro activity. Thus, the strategy we describe provides a chemometric tool to highlight similarities and differences in diastereomeric composition of batches of ASO. PC1 and PC2 explained ∼90% of total variance of these spectra and were used to provide a two-score characterization of each batch. It can be recognized that there are tight clusters around (−0.35, −0.15) and (−0.10, 0.05), several somewhat scattered points and two outliers (E and H).
These two batches, H and E, were unique with regard to their manufacture: the former is the first clinical batch of drug substance prepared in 2008, and the latter is the result of a very small laboratory-scale preparation from 2020. We can conclude that 31P-NMR in solution fingerprints the stereochemical identity of our specific drug substance mongersen. Noteworthy, preparations with the same 31P spectrum profile behave very similarly in biological assays.
Thus, 31P-NMR/PCA provided an analytical measure that correlated with in vitro activity of mongersen.
Among possible explanations of the differential biological activity of mongersen batches, it appeared to us that one matches the experimental observation of variable 31P-NMR profiles in the PS region, in the absence of other evidence of chemical contamination or by-product. Thus, we put forward that the common origin of the variances in pharmacological activity and in 31P-NMR is PS stereochemistry. Unfortunately, it is difficult to corroborate this hypothesis with more stringent experimental data and we leave it to further study and discussion.
This mongersen case study sheds light on a problem that is likely to be ubiquitous in the context of PS-modified oligonucleotides, which could have influenced the drug development of this specific ASO and may affect the future clinical advancement of these medical products.
Conclusions
Using solution 31P-NMR, a commonly available analytical tool, and PCA, we developed a protocol to fingerprint synthesis batches of oligonucleotide drug substance. The observed variation of 31P-NMR in the narrow range of PS resonances may be tentatively assigned to the diastereomeric composition of mongersen in terms of chirality at phosphorous. The 31P-NMR/PCA scores correlate with, and thus may predict, biological activity. We provide evidence that the mongersen batches administered in clinical studies were not homogeneous and presented a different ability to downregulate SMAD7 in cultured cells.
The simple 31P-NMR/PCA protocol described here provides a tool for assessing homogeneity of PS oligonucleotide preparations, particularly where the manufacturing conditions are altered, or where variations in synthetic or purification protocols occur. This tool will be even more valuable in critical situations such as process scale-up or following a change of drug substance manufacturer.
Supplementary Material
Author Disclosure Statement
M.Mc.N. is an employee of Nogra Pharma Ltd. F.V., S.D., and S.B. are employees of PPM Services SA, Swiss affiliate of Nogra Pharma Ltd. I.C. and A.F. are employees of Regulatory Pharma Net srl (regulatory consultants for the study). G.M., C.S., and L.D.B. have received fees for consultation from PPM Services.
Funding Information
This study was funded by Nogra Pharma Ltd, Dublin, Ireland.
Supplementary Material
References
- 1. Crooke ST, Baker BF, Crooke RM and Liang XH. (2021). Antisense technology: an overview and prospectus. Nat Rev Drug Discov 20:427–453. [DOI] [PubMed] [Google Scholar]
- 2. Roberts TC, Langer R and Wood MJA. (2020). Advances in oligonucleotide drug delivery. Nat Rev Drug Discov 19:673–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Crooke ST, Witztum JL, Bennett CF and Baker BF. (2018) RNA-targeted therapeutics. Cell Metabol 27:714–739. [DOI] [PubMed] [Google Scholar]
- 4. Khovarova A and Watts JK. (2017). The chemical evolution of oligonucleotide therapies of clinical utility. Nat Biotechnol 35:238–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wan WB and Seth PP. (2016). The medicinal chemistry of therapeutic oligonucleotides. J Med Chem 59:9645–9667. [DOI] [PubMed] [Google Scholar]
- 6. Eckstein F. (2000). Phosphorothioate oligodeoxynucleotides: what is their origin and what is unique about them? Antisense Nucleic Acid Drug Dev 10:117–121. [DOI] [PubMed] [Google Scholar]
- 7. Eckstein F. (2014). Phosphorothioates, essential components of therapeutic oligonucleotides. Nucleic Acid Ther 24:374–387. [DOI] [PubMed] [Google Scholar]
- 8. Kiełpiński LJ, Funder ED, Schmidt S and Hagedorn PH. (2021). Characterization of Escherichia coli RNase H discrimination of DNA phosphorothioate stereoisomers. Nucleic Acid Ther 31:383–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Eckstein F. (1985). Nucleoside phosphorothioates. Annu Rev Biochem 54:367–402. [DOI] [PubMed] [Google Scholar]
- 10. Eckstein F. (1970). Nucleoside phosphorothioates. J Am Chem Soc 92:4718–4723. [DOI] [PubMed] [Google Scholar]
- 11. Burgers PM, Eckstein F and Hunneman DH. (1979). Stereochemistry of hydrolysis by snake venom phosphodiesterase. J Biol Chem 254:7476–7478. [PubMed] [Google Scholar]
- 12. Koziołkiewicz M, Krakowiak A, Kwinkowski M, Boczkowska M and Stec WJ. (1995). Stereodifferentiation—the effect of P chirality of oligo(nucleoside phosphorothioates) on the activity of bacterial RNase H. Nucleic Acids Res 23:5000–5005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Koziołkiewicz M, Wójcik M, Kobylańska A, Karwowski B, R¸bowska B, Guga P and Stec WJ. (1997). Stability of stereoregular oligo(nucleoside phosphorothioate)s in human plasma: diastereoselectivity of plasma 3’-exonuclease. Antisense Nucleic Acid Drug Dev 7:43–48. [DOI] [PubMed] [Google Scholar]
- 14. Stec WJ, Cierniewski CS, Okruszek A, Kobylańska A, Pawłowska Z, Koziołkiewicz M, Pluskota E, Maciaszek A, Rebowska B and Stasiak M. (1997). Stereodependent inhibition of plasminogen activator inhibitor type 1 by phosphorothioate oligonucleotides: proof of sequence specificity in cell culture and in vivo rat experiments. Antisense Nucleic Acid Drug Dev 7:567–573. [DOI] [PubMed] [Google Scholar]
- 15. Wan WB, Migawa MT, Vasquez G, Murray HM, Nichols JG, Gaus H, Berdeja A, Lee S, Hart CE, et al. (2014). Synthesis, biophysical properties and biological activity of second generation antisense oligonucleotides containing chiral phosphorothioate linkages. Nucleic Acids Res 42:13456–13468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Li M, Lightfoot HL, Halloy F, Malinowska AL, Berk C, Behera A, Schümperli D and Hall J. (2017). Synthesis and cellular activity of stereochemically-pure 2′-O-(2-methoxyethyl)-phosphorothioate oligonucleotides. Chem Commun 53:541–544. [DOI] [PubMed] [Google Scholar]
- 17. Iwamoto N, Butler DCD, Svrzikapa N, Mohapatra S, Zlatev I, Sah DWY, Meena, Standley SM, Lu G, et al. (2017). Control of phosphorothioate stereochemistry substantially increases the efficacy of antisense oligonucleotides. Nat Biotechnol 35:845–851. [DOI] [PubMed] [Google Scholar]
- 18. Jahns H, Roos M, Imig J, Baumann F, Wang Y, Gilmour R and Hall. J (2015). Stereochemical bias introduced during RNA synthesis modulates the activity of phosphorothioate siRNAs. Nat Commun 6:6317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Østergaard ME, De Hoyos CL, Wan WB, Shen W, Low A, Berdeja A, Vasquez G, Murray S, Migawa MT, et al. (2020). Understanding the effect of controlling phosphorothioate chirality in the DNA gap on the potency and safety of gapmer antisense oligonucleotides. Nucleic Acids Res 48:1691–1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Crooke ST, Seth PP, Vickers TA and Liang XH. (2020). The interaction of phosphorothioate-containing RNA targeted drugs with proteins is a critical determinant of the therapeutic effects of these agents. J Am Chem Soc 142:14754–14771. [DOI] [PubMed] [Google Scholar]
- 21. Wyrzykiewicz TK and Cole DL. (1995). Stereo-reproducibility of the phosphoramidite method in the synthesis of oligonucleotide phosphorothioates. Bioorg Chem 23:33–41. [Google Scholar]
- 22. Ravikumar VT and Cole DL. (2002). Development of 2‘-O-methoxyethyl phosphorothioate oligonucleotides as antisense drugs under stereochemical control. Org Process Res Dev 6:798–806. [Google Scholar]
- 23. Monteleone G, Kumberova A, Croft NM, McKenzie C, Steer HW and MacDonald TT. (2001). Blocking Smad7 restores TGF-β1 signaling in chronic inflammatory bowel disease. J Clin Invest 108:601–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Monteleone G, Fantini MC, Onali S, Zorzi F, Sancesario G, Bernardini S, Calabrese E, Viti F, Monteleone I, Biancone L and Pallone F. (2012). Phase I clinical trial of Smad7 knockdown using antisense oligonucleotide in patients with active Crohn's disease. Mol Ther 20:870–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Monteleone G, Neurath MF, Ardizzone S, Di Sabatino A, Fantini MC, Castiglione F, Scribano ML, Armuzzi A, Caprioli F, et al. (2015). Mongersen, an oral Smad7 antisense oligonucleotide, and Crohn's disease. N Engl J Med 372:1104–1113. [DOI] [PubMed] [Google Scholar]
- 26. Monteleone G, Di Sabatino A, Ardizzone S, Pallone F, Usiskin K, Zhan X, Rossiter G and Neurath MF. (2016). Impact of patient characteristics on the clinical efficacy of mongersen (GED-0301), an oral Smad7 antisense oligonucleotide, in active Crohn's disease. Aliment Pharmacol Ther 43:717–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Feagan BG, Sands BE, Rossiter G, Li X, Usiskin K, Zhan X and Colombel JF. (2018). Effects of mongersen (GED-0301) on endoscopic and clinical outcomes in patients with active Crohn's disease. Gastroenterology 154:61–64 [DOI] [PubMed] [Google Scholar]
- 28. Sands BE, Feagan BG, Sandborn WJ, Schreiber S, Peyrin-Biroulet L, Colombel JF, Rossiter G, Usiskin K, Ather S, Zhan X and D'Haens G. (2020). Mongersen (GED-0301) for active Crohn's disease: results of a phase 3 study. Am J Gastroenterol 115:738–745. [DOI] [PubMed] [Google Scholar]
- 29. Marafini I, Stolfi C, Troncone E, Lolli E, Onali S, Paoluzi OA, Fantini MC, Biancone L, Calabrese E, et al. (2021). A pharmacological batch of mongersen that downregulates Smad7 is effective as induction therapy in active Crohn's disease: a phase II, open-label study. BioDrugs 35:325–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Abraham C and Cho JH. (2009). Inflammatory bowel disease. N Engl J Med 361:2066–2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. MacDonald TT, DiSabatino A and Gordon JN. (2005). Immunopathogenesis of Crohn's disease. J Parenter Enter Nutr 29:S118–S125. [DOI] [PubMed] [Google Scholar]
- 32. Feagins LA. (2010). Role of transforming growth factor-β in inflammatory bowel disease and colitis-associated colon cancer. Inflamm Bowel Dis 16:1963–1968. [DOI] [PubMed] [Google Scholar]
- 33. Ihara S, Hirata Y and Koike K. (2017). TGF-β in inflammatory bowel disease: a key regulator of immune cells, epithelium, and the intestinal microbiota. J Gastroenterol 52:777–787. [DOI] [PubMed] [Google Scholar]
- 34. Boirivant M, Pallone F, Di Giacinto C, Fina D, Monteleone I, Marinaro M, Caruso R, Colantoni A, Palmieri G, et al. (2006). Inhibition of Smad7 with a specific antisense oligonucleotide facilitates TGF-β1-mediated suppression of colitis. Gastroenterology 131:1786–1798. [DOI] [PubMed] [Google Scholar]
- 35. Garo LP, Ajay AK, Fujiwara M, Beynon V, Kuhn C, Gabriely G, Sadhukan S, Raheja R, Rubino S, Weiner HL and Murugaiyan G (2019). Smad7 controls immunoregulatory PDL2/1-PD1 signaling in intestinal inflammation and autoimmunity. Cell Rep 28:3353–3366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Stolfi C, De Simone V, Colantoni A, Franzè E, Ribichini E, Fantini MC, Caruso R, Monteleone I, Sica GS, et al. (2014). A functional role for Smad7 in sustaining colon cancer cell growth and survival. Cell Death Dis 5:e1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Thayer JR, Wu Y, Hansen E, Angelino MD and Rao S. (2011). Separation of oligonucleotide phosphorothioate diastereoisomers by pellicular anion-exchange chromatography. J Chromatogr A 1218:802–808. [DOI] [PubMed] [Google Scholar]
- 38. Enmark M, Rova M, Samuelsson J, E Örnskov, Schweikart F and Fornstedt T. (2019). Investigation of factors influencing the separation of diastereomers of phosphorothioated oligonucleotides. Anal Bioanal Chem 411:3383–3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.