Abstract
In Thailand, pesticides containing organophosphates (OP) are frequently applied to crops to suppress insects. School children can be exposed to OPs on a daily basis, from food consumption to breathing and touching pesticides drifted near classrooms. Living in an agricultural area can also be one of the causes. As a result, it is important to monitor OPs residues in the food chain and biomarkers of exposure. The Gas Chromatography–Flame Photometric Detector method was employed to examine the relationship between OPs residue and DAPs (Diakly phosphate) in four targeted locations in Thailand, as well as to examine the residues of OPs in vegetable samples and DAPs in 395 school children’s urine samples. Vegetables were found to contain at least one OP, with chlorpyrifos being the most prevalent. The OPs detected frequencies for Sakon Nakhon, Chiang Mai, Phang Nga, and Pathum Thani are 96.1%, 94%, 91.7%, and 83.3%, respectively. The overall centration level of OPs showed 0.3261 mg/kg, 0.0636 mg/kg, 0.0023 mg/kg, 0.0150 mg/kg, 0.2003 mg/kg, 0.0295 mg/kg, and 0.0034 mg/kg for diazinon, dimethoate, pirimiphos-methyl, chlorpyrifos, profenofos, ethion, and triazophos, respectively. Nearly 98% of school children were detected with at least one DAP. The overall level of dimethyl phosphate metabolites (5.258 µmole/mole creatinine) in urine samples is higher than diethyl phosphate metabolites (2.884 µmole/mole creatinine), especially in the case of Pathum Thani. Our findings show a consistent relationship between OPs in vegetables from wet markets and DAPs in urine samples of school children in various parts of Thailand.
Keywords: organophosphate, dialkyl phosphates metabolites, school children, pesticides exposure, pesticides residues
1. Introduction
Thai farmers frequently employ organophosphate (OP) pesticides to manage insects on their crops. Children exposed to pesticides are more likely to have cancer, respiratory issues, reproductive, endocrine, skin, and neurological disorders [1,2,3,4,5,6]. Since most of their organs are still developing, school children are more susceptible to pesticide exposure than adults are, as pesticides can affect the growth of different organs. They are also more vulnerable because of their distinct daily activity patterns and physiological traits [7,8,9]. They are more sensitive to the potential neurotoxic effects of pesticides because of their organ systems, such as the brain and central nervous system. These organs develop rapidly in those years of growth; therefore, they lack levels of pesticides that detoxify enzymes. It is well understood that acute effects of exposure to the OPs produce a wide range of neurological symptoms, and it can be monitored by clinical signs and inhibition of acetylcholinesterase activity [10]. Moderate or low exposure to pesticides is suspected of having adverse health effects as previous studies have reported that prolonged exposure to OPs can cause oxidative stress and damage DNA strands, resulting in an increased risk for chronic diseases such as cancer and neurological diseases [11].
School children can be exposed to various OPs daily, from daily intake, breathing, touching pesticides drift near classrooms, engaging in farm activity, or living nearby agricultural areas being sprayed. Common routes of exposure include contact with skin, inhalation, and dietary intake [12,13]. In addition, children’s health risks were affected by environmental injustice, especially among poor, minority, and marginalized populations for several reasons: toxic chemicals, contaminated air, contaminated water, unsafe workplaces, and other environmental hazards [14]. Prior research indicates that children are particularly susceptible to the potentially harmful health effects of pesticides are irreversible and may cause public health concerns. The public and policymakers must therefore have access to information on sources and exposure characteristics in order to lower exposure risks for children and safeguard their health. The government has allocated money for school lunches for students attending government schools, particularly in Thailand. The school is in charge of overseeing the administration of the kitchen and purchasing ingredients, primarily from nearby wet markets, including produce, fruit, and meat. Despite this, OP residues have been identified in fruit and vegetable samples from wet markets in various investigations [15,16,17]. Due to the fact that each DAP can be produced from multiple OP pesticides, dialkyl phosphates (DAPs) are metabolites that can be employed as non-specific urine biomarkers of OP pesticides. It was widely used for assessing exposure to OPs from several routes, that is, oral, dermal, and inhalation. Once OP pesticide exposure occurs, it is metabolized via dealkylation, hydrolysis, and isomerization. DAPs in the urine can be used indirectly to measure OP pesticide exposure [18,19]. In addition, dimethyl OP pesticides produce only dimethyl phosphate (DM) metabolites, while diethyl OP pesticides produce diethyl phosphate (DE) metabolites [20]. Therefore, most studies grouped the metabolites into these two groups (DM and DE) and indicated urine DAP concentrations as biomarkers of exposure to OPs within hours or days after sampling [21]. Studies reported the exposure in children using DAPs as the biomarkers of OPs and showed various risk factors of exposure. In the study by Bradman et al., they reported that children living in the agricultural area were more likely to expose to OPs from multiple sources, and urinary DAPs, particularly DMs levels increased with age, food, and pesticide used region [22]. Moreover, children from agricultural families in the northern part of Thailand were observed, and dietary sources were the likely contributors to pesticide exposure [23].
School children have the risk of exposure to pesticides for the reasons mentioned above, especially organophosphates, which are the most commonly used pesticides in Thailand. Therefore, the aim of this study was to investigate the relationship between the levels of OPs metabolites in urine samples of school children by using DAPs as the biomarker and the levels of OPs residues in vegetables from the market where the school is located in four target areas.
2. Materials and Methods
2.1. Study Design
A cross-sectional study was conducted on school children 5–15-year-old from 4 provinces in 4 parts of Thailand, i.e., Chiang Mai (northern), Sakon Nakhon (north-eastern), Pathum Thani (middle), and Phang Nga (southern) province. Demographic data were gathered. For age, data were collected through interviews, and for weight and height, data were collected through weight and height measurements.
The research project was approved by Human Experimentation Committee (HEC), Research Institute for Health Sciences, Chiang Mai University (Certificate no. 8/60, 18 October 2017).
2.2. Sample Collection
The OPs residues in vegetables were used as the contaminating markers, and DAP metabolites in urine were used as biomarkers of exposure among school children:
-
(1)
Vegetable samples: Two hundred and twenty-two vegetable samples were purchased from the same local wet markets where the cooker usually buys their ingredients, i.e., Chinese kale (Brassica alboglabra L.H. Bailey), cabbage (Brassica oleracea var. capitata L.), Chinese cabbage (Brassica rapa L. (Brassica pekinensis var. cylindrica Tsen and S.H.Lee)), Mockpak choi (Brassica rapa L.), carrot (Daucus carota), water convolvulus (Ipomoea aquatica Forsk. Var. reptan) yellow berried nightshade (Solanum xanthocarpum Schrad. and Wendl.), tomato (Lycopersicon esculentum Mill.), and yard long bean (Vigna unguiculata ssp. Sesquipedalis);
-
(2)
Urine samples: Three hundred ninety-five first-morning void urine samples were collected in the period of 5–8 AM from the children’s house and kept in polyethylene bottles with a unique identifying code of an individual. Furthermore, the bottles were secured in zip-lock plastic bags and kept in an ice box while transporting them for testing.
The vegetable and urine samples were transferred to the laboratory of Environmental and Occupational Health Sciences, Research Institute for Health Sciences (RIHES), Chiang Mai University, Chiang Mai, Thailand. The vegetable samples were finely chopped and stored at −20 °C in a freezer using a zip-lock plastic bag with individual code labeling. The urine samples were aliquoted in three tubes (10 mL in 15 mL-cryo-tube) for DAPs and creatinine analysis, then stored at −20 °C in a freezer prior to analysis.
2.3. Organophosphate Pesticide Analysis of Vegetables
Sample extraction and analysis were modified from the method of Koesukwiwat et al. [24] and Sapbamrer and Hongsibsong [15]. Briefly, 5 g of vegetable samples were added into a 50 mL centrifuge tube containing 10 mL acetonitrile (high-performance liquid chromatography [HPLC] grade; JT Baker, USA). The volume of 250 μL of 5 μg/mL triphenylphosphate (internal standard [IS]) was added, and centrifuging was performed at 2500 rpm for 5 min. The supernatant was transferred to a new 50 mL centrifuge tube that contained 6 g of MgSO4 and 3 g of NaCl and was subsequently centrifuged at 2500 rpm for 5 min. The evaporation was performed to complete the dryness of the extracted solution. A vacuum rotary evaporator (Buchi, Switzerland) with a water bath at 30 to 35 °C was used. The pellet was then resuspended with 5 mL of ethyl acetate (HPLC grade; JT Baker, Phillipsburg, NJ, USA). The volume of 1 mL of ethyl acetate phase was pipetted to a dispersive solid-phase extraction tube (carbon black, Vertical Chromatography Co., LTD, Nonthaburi, Thailand) and centrifuged at 2000 rpm for 3 min. The extract was evaporated with a gentle stream of nitrogen at room temperature and resuspended by 0.5 mL of ethyl acetate for gas chromatography (GC) analysis. The GC analysis consisted of a Hewlett-Packard model 6890 equipped with a flame photometric detector, a capillary column (DB-5 MS, 0.25 mm × I.D. × 30 m length × 0.25 μm film thickness (Agilent J and W column; Agilent Technologies, Santa Clara, CA, USA)), and a computerized data handling system (GC Chemstation A.10.02; Agilent Technologies, Santa Clara, CA, USA). The injection port had a temperature of 220 °C (splitless mode). The oven temperature was programmed as follows: initial temperature of 100 °C for 10 min, the first ramp of 15 °C/min to 180 °C (5 min), the second ramp of 5 °C/min to 250 °C (3 min), and final temperature of 290 °C for 4 min. The carrier gas was 99.999 % helium.
The method’s precision, accuracy, and overall reliability were assessed using quality control data for 7 types of OPs including diazinon (Dr. Ehrenstorfer GmbH, Augsburg, Germany), dimethoate (Dr. Ehrenstorfer GmbH, Augsburg, Germany), pirimiphos-methyl (Dr. Ehrenstorfer GmbH, Augsburg, Germany), chlorpyrifos (Dr. Ehrenstorfer GmbH, Augsburg, Germany), profenofos (Dr. Ehrenstorfer GmbH, Augsburg, Germany), ethion (Dr. Ehrenstorfer GmbH, Augsburg, Germany), and triazophos (Dr. Ehrenstorfer GmbH, Augsburg, Germany). Each set of three vegetable samples was subjected to spike the sample recovery for low (0.020 mg/kg), medium (0.080 mg/kg), and high (0.320 mg/kg) concentrations of OPs standard and blank analysis. The mean recovery of spiked Ops standard ranges from 91.31% to 106.53%, 84.45% to 95.85%, and 91.25% to 100.55% for low, medium, and high spiked concentration, respectively. The within-series imprecision (CV%) ranges from 6.05% to 8.6%. The limit of detection (LOD) and quantitative limit (LOQ) were 0.001 mg/kg and 0.004 mg/kg for dimethoate, 0.002 mg/kg and 0.009 mg/kg for diazinon, 0.007 mg/kg and 0.003 mg/kg for pirimiphos-methyl, 0.001 mg/kg and 0.002 for chlorpyrifos, 0.001 mg/kg and 0.003 mg/kg for profenofos, 0.0002 mg/kg and 0.001 mg/kg for ethion, 0.001 mg/kg and 0.005 mg/kg for triazophos
2.4. Urinary Dialkylphosphate Metabolites Analysis
A urine sample of 395 students from 4 provinces was randomized. The research was conducted by the Institute of Health Sciences Research. Chiang Mai Research for OP pesticide in 6 types of biomarkers, namely DMP, DMTP, DMDTP, DEP, DETP, and DEDTP. Urinary DAPs were measured using the method of Prapamontol et al. [25], and some were modified from Wongta et al. [17]. The DAPs were analyzed using a Hewlett-Packard 7890B-flame photometric detector (GC-FPD) and 7693 Autosampler (Agilent Technology, Santa Clara, CA, USA) (Agilent Technology, Santa Clara, CA, USA) equipped with HP-5 (30 m × 0.25 mm. id, 0.25 um film thickness) columns. The analysis included six nonspecific metabolites of organophosphates pesticides, that is, DAPs derivatives consisting of dimethyl phosphate (DMP, 98%, TRC canada, Toronto, Canada), diethyl phosphate (DEP, 99.5%, TRC canada, Toronto, Canada), dimethyl thiophosphate (DMTP, >90%, TRC canada, Toronto, Canada), dimethyl dithiophos phate (DMDTP, >90%, TRC canada, Toronto, Canada), diethyl dithiophosphate (DEDTP, 90%, TRC canada, Toronto, Canada), diethyl thiophosphate (DETP, 98%, TRC canada, Toronto, Canada). Sum variables were calculated: sumDes: DEP + DETP + DEDTP, sumDMs: DMP + DMTP + DMDTP, sumDAP: sumDEs + sumDMs. The DAPs concentrations were adjusted for creatinine concentrations and converted from μg/L to μg/g creatinine and μg/L to μmol/mol creatinine. The creatinine in urine was determined by Jaffe’s reaction.
For DAPs, each set of three samples was subjected to spike sample recovery for low (1–25 µg/L), medium (4–75 µg/L), and high (16–125 µg/L) concentrations of OPs standard and urine blank analysis. Pooled urine from a healthy volunteer who had not been given any medicines or directly exposed to chemicals was utilized as a blank for spike recoveries. The mean recovery of spiked DAPs standard ranges from 104.08% to 120.97%, 82.24% to 116.79%, and 80.34% to 102.34% for low, medium, and high spiked concentration, respectively. The within-series imprecision (CV%) ranges from 7.80% to 9.18%. The limit of detection (LOD) and quantitative limit (LOQ) were 2.25 and 20.0 µg/L for DMP, 0.20 and 5.50 µg/L for DMTP, 0.10 µg/L and 3.00 µg/L for DMDTP, 0.05 µg/L and 2.00 µg/L for DEP, 0.10 µg/L and 2.00 µg/L for DETP, 0.10 µg/L and 3.00 µg/L for DEDTP.
2.5. Data Analysis
The collected data were analyzed using SPSS Statistics Base 17.0, Thailand. All data were tested for normality before appropriate statistical analyses were performed. Mean, standard deviation (SD), and frequency was reported for variables associated with participant demographics and characteristics. An Independent T-Test was used to assess the difference between the demographics. Chi-square tests (χ2) were used for the comparison of categorical data between participant groups. The OPs residues and urinary metabolites were reported as means, SD, median and interquartile range (IQR). The Kruskal–Wallis test was used to compare the urinary OP metabolites in urine samples of school children between the provinces and for OPs residue in vegetable samples between the provinces at the significance level of 0.05.
3. Results
The demographic characteristics of the participants are displayed below. There are 395 participants, including 231 males and 164 females, both of whom are the same age (9.73 ± 2.74 and 9.50 ± 2.63, respectively). The BMI, height, and weight all fall within the same range as that displayed in Table 1.
Table 1.
Demographical data participated school children in rural areas.
| Sex | Male | Female | Total | Minimum | Maximum |
|---|---|---|---|---|---|
| n | 231 | 164 | 395 | - | - |
| Age, years | 9.73 ± 2.74 | 9.50 ± 2.63 | 9.601 ± 2.67 | 5 | 15 |
| Weight, kg | 32.79 ± 12.33 | 31.18 ± 13.88 | 31.90 ± 13.18 | 15 | 86 |
| Height, cm | 134.73 ± 18.05 | 132.95 ± 15.63 | 133.74 ± 16.85 | 100 | 175 |
| BMI, kg/m2 | 17.45 ± 3.04 | 16.93 ± 4.33 | 17.16 ± 3.80 | 12.4 | 37.22 |
3.1. Biomarkers of Organophosphate Pesticide Residues and Exposure
Organophosphate Residue in Vegetable Samples
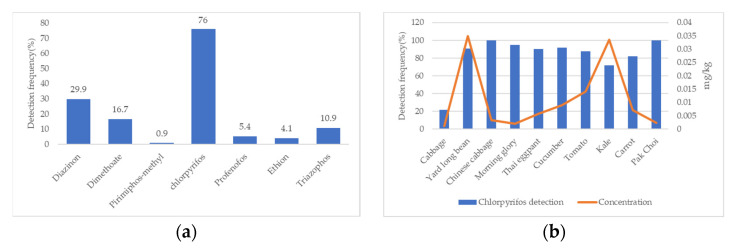
Two hundred twenty two vegetable samples were tested including cabbage (n = 42), yard long bean (n = 22), Chinese cabbage (n = 31), morning glory (n = 20), Thai eggpant (n = 20), cucumber (n = 12), tomato (n = 8), kale (n = 32), carrot (n = 22), and pak choi (n = 12). Chlorpyrifos, diazinon, and dimethoate were shown as the first (76%), second (29.9%), and third (16.7%) detected residues in this study (Figure 1a).
Figure 1.
Detection of OPs and concentration of residues in vegetables. Detection frequency (a), correlation of detection and concentration of residues; chlorpyrifos (b), dimethoate (c), and diazinon (d).
The correlation of detection and concentration of residues based on vegetable type revealed that chlorpyrifos had a high detection percentage in almost all vegetables. (81.8–100%) and showed low concentration except for yard long bean and kale (0.0035 and 0.0034 mg/kg) (Figure 1b). While dimethoate was found in Chinese cabbage (80.6%, 0.0308 mg/kg) (Figure 1c), high detection and concentration for diazinon was also found in just cabbage (64.4%, 0.3761 mg/kg) and kale (81.2%, 0.1229 mg/kg) (Figure 1d).
The province’s review of OPs residue results revealed a similar pattern to the overall detection result (Table 2). Briefly, the most detected is chlorpyrifos in any province, followed by diazinon and dimethoate. However, there is a difference between the kind of detected vegetables and the type of residue in each. The most detected OPs residues were found in Sakon Nakhon (96.1%), followed by Chiang Mai (94%), Phang Nga (91.7), and Pathum Thani (83.3%).
Table 2.
Comparison of residue detection frequency by province based on the vegetable.
| Province | Vegetables | N | Detection Frequency (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Diazinon | Dimethoate | Pirimiphos-Methyl | Chlorpyrifos | Profenofos | Ethion | Triazophos | Total OPs | |||
| CM | Cabbage | 10 | 60 | 0 | 0 | 10 | 0 | 0 | 40 | 70 |
| Yard long bean | 10 | 0 | 0 | 0 | 90 | 0 | 20 | 60 | 100 | |
| Chinese cabbage | 10 | 0 | 90 | 0 | 100 | 0 | 0 | 40 | 100 | |
| Kale | 10 | 70 | 0 | 0 | 90 | 10 | 0 | 20 | 100 | |
| Carrot | 10 | 10 | 0 | 0 | 100 | 10 | 10 | 0 | 100 | |
| Total | 50 | 28 | 18 | 0 | 78 | 4 | 6 | 32 | 94 | |
| PT | Cabbage | 12 | 66.7 | 0 | 0 | 8.3 | 0 | 0 | 0 | 66.7 |
| Morning glory | 12 | 33.3 | 33.3 | 0 | 91.7 | 8.3 | 0 | 0 | 100 | |
| Kale | 12 | 83.3 | 0 | 0 | 41.7 | 41.7 | 0 | 8.3 | 83.3 | |
| Carrot | 12 | 0 | 0 | 0 | 66.7 | 0 | 0 | 0 | 66.7 | |
| Pak Choi | 12 | 16.7 | 33.3 | 0 | 100 | 16.7 | 0 | 0 | 100 | |
| Total | 60 | 40 | 13.3 | 0 | 61.7 | 13.3 | 0 | 1.7 | 83.3 | |
| SK | Cabbage | 8 | 100 | 0 | 0 | 37.5 | 0 | 0 | 12.5 | 100 |
| Chinese cabbage | 9 | 33.3 | 77.8 | 0 | 100 | 11.1 | 0 | 33.3 | 100 | |
| Morning glory | 8 | 0 | 0 | 0 | 100 | 0 | 0 | 0 | 100 | |
| Thai eggpant | 8 | 0 | 0 | 12.5 | 100 | 0 | 0 | 0 | 100 | |
| Tomato | 8 | 0 | 0 | 0 | 87.5 | 0 | 12.5 | 0 | 87.5 | |
| Kale | 10 | 90 | 0 | 0 | 90 | 10 | 0 | 30 | 90 | |
| Total | 51 | 39.2 | 13.7 | 2 | 86.3 | 3.9 | 2 | 13.7 | 96.1 | |
| PNG | Cabbage | 12 | 41.7 | 0 | 0 | 33.3 | 0 | 0 | 0 | 66.7 |
| Yard long bean | 12 | 8.3 | 25 | 0 | 91.7 | 0 | 0 | 0 | 91.7 | |
| Chinese cabbage | 12 | 0 | 75 | 0 | 100 | 0 | 0 | 0 | 100 | |
| Thai eggpant | 12 | 16.7 | 0 | 0 | 83.3 | 0 | 16.7 | 0 | 100 | |
| Cucumber | 12 | 0 | 8.3 | 8.3 | 91.7 | 0 | 25 | 0 | 100 | |
| Total | 60 | 13.3 | 21.7 | 1.7 | 80 | 0 | 8.3 | 0 | 91.7 | |
Abbreviations: CM: Chiang Mai, PT: Pathum Thani, SK: Sakon Nakhon, PNG: Phang Nga and Total OPs: The sum of 7 types of organophosphate pesticide detection frequency (diazinone, dimethoate, pirimiphos-methyl, chorpyrifos, profenofos, ethion, and triazophos).
According to the results of the detected vegetable tests, the vegetables with at least one residue were found in Sukhothai to be cabbage, Chinese cabbage, morning glory, and Thai eggplant; in Chiang Mai, it was yard long bean, Chinese cabbage, kale, and carrot; in Phang Nga, it was Chinese cabbage, Thai eggplant, and cucumber; and in Pathum Thani, it was morning glory and pak choi.
According to each province, the frequency of detection and the concentration of OP residues were determined for all vegetables in each province, including 50 samples for CM, 60 samples for PT, 51 samples for SK, and 60 samples for PNG, and the results were displayed in Table 3. The detection showed a similar pattern of vegetable results in each province. Chlorpyrifos was the most detected in all targeted areas at 78%, 61%, 86.3%, and 80% for Chiang Mai (CM), Sakon Nakhon (SK), Pathum Thani (PT), and Phang Nga (PNG) province, respectively. Diazinon was found to be the second in PT and SK (40% and 39.2%), while CM and PNG were triazophos and dimethoate (32% and 21.7%). However, some OPs residues could not be detected in each target area, including pirimiphos-methyl in CM and PT, ethion in PT, profenofos, and triazophos in PNG. The differences in the median of OPs residues between the province were observed; dimethoate was found to be significantly higher in PT (0.1637 mg/kg) than in CM and PNG (0.0114 and 0.0193 mg/kg). In contrast, chlorpyrifos in PT (0.0021 mg/kg) was found significantly lower than in CM, PNG and SK (0.0031, 0.0029 and 0.0025 mg/kg).
Table 3.
OPs residue detection frequency and concentrations by province.
| Province | OPs Residue | Detection | Mean | SD | Median | IQR (mg/kg) | ||
|---|---|---|---|---|---|---|---|---|
| (n) | % | (mg/kg) | (mg/kg) | 1st | 3rd | |||
| CM | Diazinon | 14 | 28 | 0.2216 | 0.2848 | 0.039 | 0.0028 | 0.5332 |
| Dimethoate | 9 | 18 | 0.0347 | 0.0567 | 0.0114 a | 0.0081 | 0.0355 | |
| Pirimiphos-methyl | 0 | 0 | - | - | - | - | - | |
| Chlorpyrifos | 39 | 78 | 0.0263 | 0.0734 | 0.0031 c | 0.0025 | 0.0061 | |
| Profenofos | 2 | 4 | 0.0061 | 0.0011 | 0.0061 | 0.0053 | - | |
| Ethion | 3 | 6 | 0.0103 | 0.0098 | 0.0056 | 0.0037 | - | |
| Triazophos | 16 | 32 | 0.0038 | 0.0016 | 0.0034 | 0.0027 | 0.0042 | |
| PT | Diazinon | 24 | 40 | 0.3525 | 0.4627 | 0.0912 | 0.0137 | 0.6669 |
| Dimethoate | 8 | 13.3 | 0.1623 | 0.1224 | 0.1637 a,b | 0.0609 | 0.2118 | |
| Pirimiphos-methyl | 0 | 0 | - | - | - | - | - | |
| Chlorpyrifos | 37 | 61.7 | 0.0202 | 0.0849 | 0.0021 c,d,e | 0.0018 | 0.0026 | |
| Profenofos | 8 | 13.3 | 0.2889 | 0.5554 | 0.0502 | 0.0031 | 0.4188 | |
| Ethion | 0 | 0 | - | - | - | - | - | |
| Triazophos | 1 | 1.7 | 0.0021 | - | 0.0021 | 0.0021 | 0.0021 | |
| SK | Diazinon | 20 | 39.2 | 0.2199 | 0.2625 | 0.0754 | 0.012 | 0.3976 |
| Dimethoate | 7 | 13.7 | 0.0354 | 0.0167 | 0.0413 | 0.0159 | 0.0499 | |
| Pirimiphos-methyl | 1 | 2 | 0.0021 | - | 0.0021 | 0.0021 | 0.0021 | |
| Chlorpyrifos | 44 | 86.3 | 0.0057 | 0.011 | 0.0025 d | 0.0021 | 0.0047 | |
| Profenofos | 2 | 3.9 | 0.0399 | 0.0516 | 0.0399 | 0.0034 | - | |
| Ethion | 1 | 2 | 0.0035 | - | 0.0035 | 0.0035 | 0.0035 | |
| Triazophos | 7 | 13.7 | 0.0027 | 0.0007 | 0.003 | 0.0022 | 0.0032 | |
| PNG | Diazinon | 8 | 13.3 | 0.6951 | 0.7643 | 0.4316 | 0.0606 | 1.2346 |
| Dimethoate | 13 | 21.7 | 0.0381 | 0.0468 | 0.0193 b | 0.0091 | 0.0502 | |
| Pirimiphos-methyl | 1 | 1.7 | 0.0024 | - | 0.0024 | 0.0024 | 0.0024 | |
| Chlorpyrifos | 48 | 80 | 0.0102 | 0.0322 | 0.0029 e | 0.0022 | 0.0038 | |
| Profenofos | 0 | 0 | - | - | - | - | - | |
| Ethion | 5 | 8.3 | 0.0462 | 0.0702 | 0.0085 | 0.0042 | 0.1071 | |
| Triazophos | 0 | 0 | - | - | - | - | - | |
Abbreviations: Interquartile range (IQR); 1st quartile–3rd quartile, value followed by the same letters (a–e) in the same column are significantly different at <0.05 by Kruskal–Wallis test, post hoc p-value, p-value adjusted with the Bonferroni method. CM: Chiang Mai, PT: Pathum Thani, SK: Sukhothai, and PNG: Phang Nga.
3.2. Dialkyphosphate Metabolites in Urine Samples
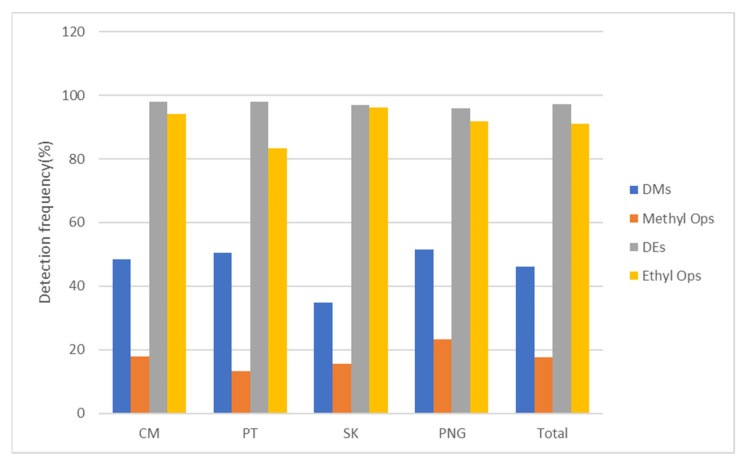
The results showed that about 98% of students detected pesticide residues in the urine (Table 4). DETP (92.8–95.0%) was found highest detected in any province followed by DEP (56.7–80.8%), DMTP (31.1–47.4%), DEDTP (8.2–19.4%), DMDTP (7.1–17.2%), and DMP (3.1–15.8%). The differences in the median of DAPs biomarkers between the province were observed; DMP in PT (16.376 µmole/mole creatinine) was found to be significantly higher than those found in CM (5.053 µmole/mole creatinine), DMDTP in SK (1.182 µmole/mole creatinine) was found higher than CM (0.231 µmole/mole creatinine), while the DEP level in CM (1.635 µmole/mole creatinine) showed higher than in SK (0.735 µmole/mole creatinine). For the sum of DAPs, the results showed just the DAPs level in CM (2.609 µmole/mole creatinine) higher than those in SK (1.596 µmole/mole creatinine).
Table 4.
DAPs detection frequency and concentration by province.
| Province | Biomarkers | Detection | Mean | SD | Median | IQR | ||
|---|---|---|---|---|---|---|---|---|
| (µmole/mole Creatinine) | ||||||||
| n | % | (µmole/mole Creatinine) |
(µmole/mole Creatinine) |
1st | 3rd | |||
| CM | DMP | 13 | 13.1 | 7.291 | 5.09 | 5.053 a | 3.419 | 9.92 |
| DMTP | 45 | 45.5 | 1.498 | 2.656 | 0.445 | 0.256 | 1.324 | |
| DMDTP | 17 | 17.2 | 0.407 | 0.382 | 0.231 b | 0.16 | 0.516 | |
| DEP | 80 | 80.8 | 2.413 | 2.682 | 1.635 c | 0.557 | 2.987 | |
| DETP | 94 | 94.9 | 1.48 | 2.942 | 0.535 | 0.313 | 1.185 | |
| DEDTP | 18 | 18.2 | 1.141 | 2.874 | 0.434 | 0.242 | 0.705 | |
| DMs | 48 | 48.5 | 3.523 | 6.363 | 0.786 | 0.276 | 3.787 | |
| DEs | 97 | 98 | 3.636 | 5.671 | 1.887 | 0.744 | 4.254 | |
| DAPs | 97 | 98 | 5.379 | 7.839 | 2.609 d | 0.804 | 6.187 | |
| PT | DMP | 16 | 15.8 | 24.26 | 22.944 | 16.376 a | 8.834 | 31.188 |
| DMTP | 44 | 43.6 | 4.451 | 10.989 | 0.659 | 0.381 | 1.773 | |
| DMDTP | 14 | 13.9 | 0.658 | 0.607 | 0.44 | 0.218 | 0.894 | |
| DEP | 80 | 79.2 | 2.08 | 2.769 | 0.853 | 0.363 | 2.248 | |
| DETP | 96 | 95 | 1.523 | 3.674 | 0.548 | 0.35 | 1.032 | |
| DEDTP | 13 | 12.9 | 0.628 | 0.676 | 0.422 | 0.25 | 0.741 | |
| DMs | 51 | 50.5 | 11.633 | 21.393 | 0.922 | 0.413 | 14.381 | |
| DEs | 99 | 98 | 3.24 | 5.301 | 1.19 | 0.725 | 2.918 | |
| DAPs | 99 | 98 | 9.233 | 18.317 | 1.557 | 0.992 | 6.659 | |
| SK | DMP | 3 | 3.1 | 5.924 | 6.617 | 2.747 | 1.495 | |
| DMTP | 31 | 31.6 | 1.993 | 5.346 | 0.471 | 0.234 | 0.714 | |
| DMDTP | 7 | 7.1 | 1.347 | 1.124 | 1.182 b | 0.531 | 1.551 | |
| DEP | 72 | 73.5 | 1.348 | 1.821 | 0.735 c | 0.302 | 1.632 | |
| DETP | 93 | 94.9 | 0.865 | 1.226 | 0.515 | 0.341 | 0.991 | |
| DEDTP | 19 | 19.4 | 0.405 | 0.173 | 0.361 | 0.266 | 0.453 | |
| DMs | 34 | 34.7 | 2.617 | 7.425 | 0.484 | 0.252 | 1.665 | |
| DEs | 95 | 96.9 | 1.949 | 2.458 | 1.158 | 0.511 | 2.433 | |
| DAPs | 97 | 99 | 2.826 | 6.032 | 1.596 d | 0.663 | 2.915 | |
| PNG | DMP | 4 | 4.1 | 10.221 | 5.597 | 9.322 | 5.43 | 15.912 |
| DMTP | 46 | 47.4 | 1.347 | 2.728 | 0.574 | 0.392 | 1.136 | |
| DMDTP | 15 | 15.5 | 0.536 | 0.379 | 0.495 | 0.239 | 0.603 | |
| DEP | 55 | 56.7 | 2.048 | 2.754 | 0.838 | 0.424 | 3.25 | |
| DETP | 90 | 92.8 | 1.464 | 2.958 | 0.563 | 0.399 | 1.058 | |
| DEDTP | 8 | 8.2 | 0.544 | 0.448 | 0.391 | 0.321 | 0.498 | |
| DMs | 50 | 51.5 | 2.218 | 4.061 | 0.706 | 0.418 | 1.775 | |
| DEs | 93 | 95.9 | 2.674 | 4.581 | 1.044 | 0.585 | 2.412 | |
| DAPs | 93 | 95.9 | 3.867 | 5.853 | 1.448 | 0.902 | 4.006 | |
Abbreviations: Interquartile range (IQR); 1st quartile–3rd quartile, value followed by the same letters (a–d) in the same column are significantly different at <0.05 by Kruskal–Wallis test, post-hoc p-value, p-value adjusted with the Bonferroni method. DMP: dimethyl phosphate, DMTP: dimethyl thiophosphate, DMDTP: dimethyl dithiophosphate, DEP: diethyl phosphate, DETP: diethyl thiophosphate, and DEDTP: diethyl dithiophosphate, DMs: DMP + DMTP + DMDPT, DEs: DEP + DETP + DEDTP, DAPs: DMs + DEs, CM: Chiang Mai, PT: Pathum Thani, SK: Sukhothai, and PNG: Phang Nga.
In order to understand the relation and consequences of OPs contamination and exposure to children in Thailand, the children in government schools were chosen as the school has the same lunch-supported policy. Additionally, almost all the schools buy the food materials from local wet markets, especially vegetables, which were reported of high pesticide residues. DAP metabolites can be produced from several intact forms of OPs, and they can be categorized into DM and DE forms. However, some OP does not produce any DAP metabolites. The detected OPs and DAPs degradation products in the urine are shown in Table 5.
Table 5.
Organophosphate pesticides and DAP degradation products in urine *.
| Organophosphate Pesticide | DAPs Metabolites in Urine |
|---|---|
| Chlorpyrifos | DEP, DETP |
| Diazinon | DEP, DETP |
| Dimethoate | DMP, DMTP, DMDTP |
| Ethion | DEP, DETP, DEDIP |
| Pirimiphos-methyl | DMP, DMTP |
| Triazophose | DEP |
| Profenophos | Not produce any DAPs |
* Quirós-Alcalá, L. et al., 2012 [26].
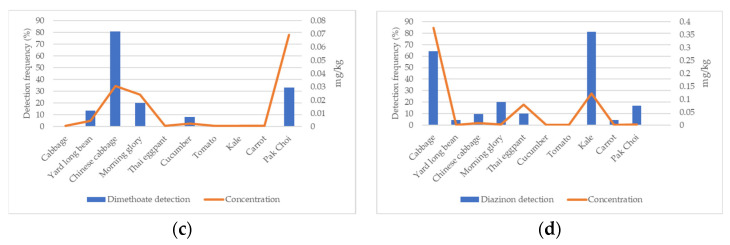
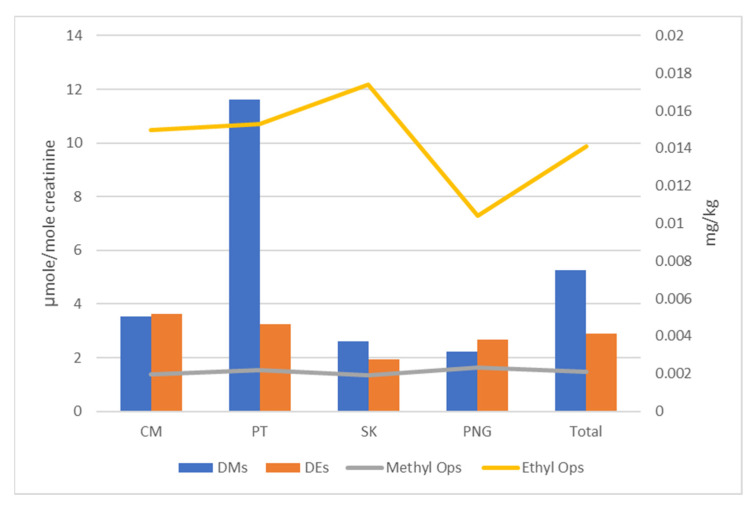
The relationship between OP residues in vegetables in local wet markets and DAPs in urine samples of school children was observed in this present study. The detection frequency of OP residues and DAPs were constructed in a bar chart to observe the relationship between OP residues and DAPs in each target area, as shown in Figure 2. The result confirmed the high detected DE metabolites consequences from high detected ethyl OP residues and low detected DM metabolites resulting from low detected methyl OP residues. While the overall concentration level of DM metabolites in urine samples is higher than DE metabolites even though the methyl OP residues levels are lower than ethyl OP residues, especially for PT, as shown in Figure 3.
Figure 2.
Detection frequency of OP residues and DAP metabolites by province.
Figure 3.
Comparison of DAPs levels in urine samples (bar chart) and OPs residues levels in vegetable samples (line chart) by province.
4. Discussion
In Thailand, during the past ten years, OPs have been the most widely used pesticides, particularly chlorpyrifos, until it was outlawed in 2020 [22]. However, it appears that today, over all of Thailand, chlorpyrifos and other OP group pesticides are the most often utilized. Several past studies reported OP residues in various types of vegetables, but most of them were studied just in one target area. The report on the finding of OP residues in vegetables from different sources such as farms, markets, and a supermarket in Phayao province, northern Thailand, showed that 59.3% of farms and 13.2% of markets vegetables contained OP residues at or above the maximum residue limits established by the European Union. The most common OP residues detected in farms, markets, and supermarket samples were chlorpyrifos at 50.0%, 33.9%, and 33.3%, respectively [15]. Moreover, in the study by Silipaunyo et al., they reported OP residues were detected at 21.43% in fruit samples and 31.50% in vegetable samples collected from wet markets and supermarkets in Ching Mai province, northern Thailand [27]. The study in Trang province, southern Thailand, reported that 41.58% of 190 samples were contaminated with OPs and carbamates such as cilantro, kale, Chinese cabbage, cabbage, cauliflower, chili, celery, spring onion, yard long bean, cucumber, tomato, Thai eggplant, white radish, and lemon [28].
The present study is the first study that collected vegetable samples from four provinces that represent four regions of Thailand, including Chiang Mai, Sakon Nakhon, Pathum Thani, and Krabi provinces, which represent the northern, north-eastern, middle, and southern parts, respectively. The results of this investigation on OP residues in vegetables show that all samples contained at least one OP residue and that the earlier studies had also revealed chlorpyrifos, diazinon, and dimethoate as the first (76 percent), second (29.9 percent), and third (16.7%) OP residues. The highest concentration of chlorpyrifos found in this study was 0.4936 mg/kg in cabbage from a wet market, which is lower than the levels found in studies by Sapbamrer and Hongsibsong that found 2.423 mg/kg in lemon balm from a farm and 7.785 mg/kg in market-purchased garlic but higher than 0.027 mg/kg in parsley from a grocery store. Most OPs residues were discovered in the province of SK (96.1%), followed by CM (94%), PNG (91.7%), and PT (83.3%). Additionally, 86.3%, 78%, 80%, and 61% for SK, CM, PNG, and PT, respectively, of all target regions were found to have chlorpyrifos. The results did not distinguish across the provinces in terms of total OP residue detection. The quantities of each residue do vary, though, and dimethoate was discovered to be substantially higher in PT than in CM and PNG. As opposed to CM, PNG, and SK, chlorpyrifos levels in PT were found to be substantially lower. That may confirm the explanation that the level of exposure to OP pesticides in consumers depends on the vegetable source, vegetable type, and treatment process [17,29].
The DAPs were used as urinary biomarkers of OP exposure in several previous studies [12,15,17,20,21,22]. In this study, at least one DAP was observed in almost all school children, and there were no differences in the detection between four target areas. However, a difference in concentration levels between the target areas was observed. The overall results found that the DAPs level of school children in SK province (median = 1.596 µmole/mole creatinine) is lower than the others province and significantly lower than those in CM province (median = 2.609 µmole/mole creatinine), especially for DEP metabolite. The DAPs levels in CM province were also reported to be higher levels than in other areas in the previous study [21], and the DAPs levels in SK were also reported to be lower than in children who lived in central Thailand [30,31].
According to a previous study conducted on American residents aged 6–59, DM levels were almost two times greater than DEs levels overall, and they noted that DAP concentrations in children aged 6 to 11 were significantly higher than in adults and adolescents [32]. The province of PT in central Thailand has the largest concentration of DMs. In line with a prior study in the same province that saw school-aged kids residing in a rice and aquaculture farming community [30]. The fact that this location is suburban and close to the capital, as opposed to other areas, where people live in rural settings, may be the source of the higher amount. It would be a good idea to look into this matter more in the future.
Few research discusses the connections between DAPs, particularly in children, and OP residues in local markets. The majority of them investigated contamination, exposure, or the danger of exposure and its repercussions. Several investigations [17,31,32] revealed residues in diverse target areas. In the study by Muoz-Quezada et al., which was conducted in Talca Province, Chile, more than 70% of children consume vegetables from the local market, which is primarily supplied by a large market in the city of Talca; it was reported that there is a relationship between chlorpyrifos residue and DAPs in school children. Similar to Thailand, free meals are provided to students in low-income public schools in Chile, and kids generally eat fresh fruit at school [33].
5. Conclusions
The correlation between OP residues in vegetables from local wet markets and DAPs in urine samples from school children in four major regions of Thailand is first reported in the present study. The findings demonstrated that OPs residue can be detected in veggies and that children were equally at risk of exposure in four parts of Thailand.
Acknowledgments
The authors are grateful to all the school children for participating in this study. S.H. would like to thank Research Institute for Health Science, Chiang Mai University, for its laboratory capacity and all the support.
Author Contributions
Conceptualization, S.H. and M.J.; methodology, A.W., N.S. and S.H.; validation, N.S. and S.H.; formal analysis, P.T.; investigation, S.H.; writing—original draft preparation, A.W.; writing—review and editing, S.H.; supervision, S.H. and M.J.; project administration, N.S.; funding acquisition, S.H. and M.J. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The research project was approved by Human Experimentation Committee (HEC), Research Institute for Health Sciences, Chiang Mai University (Certificate no. 8/60, 18 October 2017).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was funded by Chiang Mai University and The Field Alliance.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lerro C.C., Koutros S., Andreotti G., Friesen M.C., Alavanja M.C., Blair A., Hoppin J.A., Sandler D.P., Lubin J.H., Ma X., et al. Organophosphate insecticide use and cancer incidence among spouses of pesticide applicators in the Agricultural Health Study. Occup. Environ. Med. 2015;72:736–744. doi: 10.1136/oemed-2014-102798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hulse E.J., Davies J.O., Simpson A.J., Sciuto A.M., Eddleston M. Respiratory complications of organophosphorus nerve agent and insecticide poisoning. Implications for respiratory and critical care. Am. J. Respir. Crit. Care Med. 2014;190:1342–1354. doi: 10.1164/rccm.201406-1150CI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jamal F., Haque Q.S., Singh S., Rastogi S.K. The influence of organophosphate and carbamate on sperm chromatin and reproductive hormones among pesticide sprayers. Toxicol. Ind. Health. 2016;32:1527–1536. doi: 10.1177/0748233714568175. [DOI] [PubMed] [Google Scholar]
- 4.Yang F.W., Li Y.X., Ren F.Z., Luo J., Pang G.F. Assessment of the endocrine-disrupting effects of organophosphorus pesticide triazophos and its metabolites on endocrine hormones biosynthesis, transport and receptor binding in silico. Food Chem. Toxicol. 2019;133:110759. doi: 10.1016/j.fct.2019.110759. [DOI] [PubMed] [Google Scholar]
- 5.Peter J.V., Sudarsan T.I., Moran J.L. Clinical features of organophosphate poisoning: A review of different classification systems and approaches. Indian J. Crit. Care Med. 2014;18:735–745. doi: 10.4103/0972-5229.144017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rastogi S.K., Tripathi S., Ravishanker D. A study of neurologic symptoms on exposure to organophosphate pesticides in the children of agricultural workers. Indian J. Occup. Environ. Med. 2010;14:54–57. doi: 10.4103/0019-5278.72242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chalupka S., Chalupka A.N. The impact of environmental and occupational exposures on reproductive health. J. Obstet. Gynecol. Neonatal Nurs. 2010;39:84–100. doi: 10.1111/j.1552-6909.2009.01091.x. [DOI] [PubMed] [Google Scholar]
- 8.Landrigan P.J., Kimmel C.A., Correa A., Eskenazi B. Children’s health and the environment: Public health issues and challenges for risk assessment. Environ. Health Perspect. 2004;112:257–265. doi: 10.1289/ehp.6115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roberts J.R., Karr C.J., Council on Environmental Health Pesticide exposure in children. Pediatrics. 2012;130:e1765–e1788. doi: 10.1542/peds.2012-2758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Naughton S.X., Terry A.V., Jr. Neurotoxicity in acute and repeated organophosphate exposure. Toxicology. 2018;408:101–112. doi: 10.1016/j.tox.2018.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hongsibsong S., Sittitoon N., Sapbamrer R. Association of health symptoms with low-level exposure to organophosphates, DNA damage, AChE activity, and occupational knowledge and practice among rice, corn, and double-crop farmers. J. Occup. Health. 2017;59:165–176. doi: 10.1539/joh.16-0107-OA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Paulson J., Barnett C. Who’s in charge of children’s environmental health at school? New Solut. 2010;20:3–23. doi: 10.2190/NS.20.1.b. [DOI] [PubMed] [Google Scholar]
- 13.Damalas C.A., Koutroubas S.D. Farmers’ Exposure to Pesticides: Toxicity Types and Ways of Prevention. Toxics. 2016;4:1. doi: 10.3390/toxics4010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Landrigan P.J., Rauh V.A., Galvez M.P. Environmental justice and the health of children. Mt. Sinai J. Med. 2010;77:178–187. doi: 10.1002/msj.20173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sapbamrer R., Hongsibsong S. Organophosphorus pesticide residues in vegetables from farms, markets, and a supermarket around Kwan Phayao Lake of Northern Thailand. Arch. Environ. Contam. Toxicol. 2014;67:60–67. doi: 10.1007/s00244-014-0014-x. [DOI] [PubMed] [Google Scholar]
- 16.Jantasuwan R., Rithipukdee N., Thuethong C. Fruit and vegetable consumption, preventive behaviors against introduction of organophosphate and carbamate residues in vegetables and fruits into the body, and university students’ cholinesterase level in serum in southern Thailand. Dis. Control. J. 2021;47:333–342. doi: 10.14456/dcj.2021.30. [DOI] [Google Scholar]
- 17.Wongta A., Sawarng N., Tongchai P., Sutan K., Kerdnoi T., Prapamontol T., Hongsibsong S. The Pesticide Exposure of People Living in Agricultural Community, Northern Thailand. J. Toxicol. 2018;2018:4168034. doi: 10.1155/2018/4168034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.CDC (Centers for Disease Control and Prevention) Second National Report on Human Exposure to Environmental Chemicals. CDC, National Center Environmental Health; Atlanta, GA, USA: 2003. [(accessed on 15 May 2022)]. NCEH Pub. No. 02-0716. Available online: https://www.cdc.gov/exposurereport/index.html. [Google Scholar]
- 19.Fenske R.A., Bradman A., Whyatt R.M., Wolff M.S., Barr D.B. Lessons learned for the assessment of children’s pesticide exposure: Critical sampling and analytical issues for future studies. Environ. Health Perspect. 2005;113:1455–1462. doi: 10.1289/ehp.7674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weerasekera G., Smith K.D., Quirós-Alcalá L., Fernandez C., Bradman A., Eskenazi B., Needham L.L., Barr D.B. A mass spectrometry-based method to measure dialkylphosphate degradation products of organophosphorous insecticides in dust and orange juice. J. Environ. Monit. 2009;11:1345. doi: 10.1039/b821841b. [DOI] [PubMed] [Google Scholar]
- 21.Barr D.B., Angerer J. Potential uses of biomonitoring data: A case study using the organophosphorus pesticides chlorpyrifos and malathion. Environ. Health Perspect. 2006;114:1763–1769. doi: 10.1289/ehp.9062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bradman A., Castorina R., Barr D.B., Chevrier J., Harnly M.E., Eisen E.A., McKone T.E., Harley K., Holland N., Eskenazi B. Determinants of organophosphorus pesticide urinary metabolite levels in young children living in an agricultural community. Int. J. Environ. Res. Public Health. 2011;8:1061–1083. doi: 10.3390/ijerph8041061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Panuwet P., Prapamontol T., Chantara S., Barr D.B. Urinary pesticide metabolites in school students from northern Thailand. Int. J. Hyg. Environ. Health. 2009;212:288–297. doi: 10.1016/j.ijheh.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 24.Koesukwiwat U., Lehotay S.J., Mastovska K., Dorweiler K.J., Leepipatpiboon N. Extension of the QuEChERS method for pesticide residues in cereals to flaxseeds, peanuts, and doughs. J. Agric. Food Chem. 2010;58:5950–5958. doi: 10.1021/jf902988b. [DOI] [PubMed] [Google Scholar]
- 25.Prapamontol T., Sutan K., Laoyang S., Hongsibsong S., Lee G., Yano Y., Hunter R.E., Ryan P.B., Barr D.B., Panuwet P. Cross validation of gas chromatography-flame photometric detection and gas chromatography–mass spectrometry methods for measuring dialkylphosphate metabolites of organophosphate pesticides in human urine. Int. J. Hyg. Environ. Health. 2014;217:554–566. doi: 10.1016/j.ijheh.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 26.Quirós-Alcalá L., Bradman A., Smith K., Weerasekera G., Odetokun M., Barr D.B., Nishioka M., Castorina R., Hubbard A., Nicas M., et al. Organophosphorous pesticide breakdown products in house dust and children’s urine. J. Expo. Sci. Environ. Epidemiol. 2012;22:559–568. doi: 10.1038/jes.2012.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Silipunyo T., Hongsibsong S., Phalaraksh C., Laoyang S., Kerdnoi T., Patarasiriwong V., Prapamontol T. Determination of Organophosphate Pesticides Residues in Fruits, Vegetables and Health Risk Assessment Among Consumers in Chiang Mai Province, Northern Thailand. Res. J. Environ. Toxicol. 2017;11:20–27. doi: 10.3923/rjet.2017.20.27. [DOI] [Google Scholar]
- 28.Chaosuansreecharoen K.R., Dumtip P. Proceedings of the International Academic Conferences 3606106. International Institute of Social and Economic Sciences; London, UK: 2016. Organophosphate and Carbamate Residual Levels in Vegetables of Trang Municipality. [Google Scholar]
- 29.Bajwa U., Sandhu K.S. Effect of handling and processing on pesticide residues in food—A review. J. Food Sci. Technol. 2014;51:201–220. doi: 10.1007/s13197-011-0499-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rohitrattana J., Siriwong W., Tunsaringkarn T., Panuwet P., Ryan P.B., Barr D.B., Robson M.G., Fiedler N. Organophosphate pesticide exposure in school-aged children living in rice and aquacultural farming regions of Thailand. J. Agromedicine. 2014;19:406–416. doi: 10.1080/1059924X.2014.947457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sapbamrer R., Hongsibsong S., Khacha-Ananda S. Urinary organophosphate metabolites and oxidative stress in children living in agricultural and urban communities. Environ. Sci. Pollut. Res. 2020;27:25715–25726. doi: 10.1007/s11356-020-09037-z. [DOI] [PubMed] [Google Scholar]
- 32.Barr D.B., Bravo R., Weerasekera G., Caltabiano L.M., Whitehead R.D., Jr., Olsson A.O., Caudill S.P., Schober S.E., Pirkle J.L., Sampson E.J., et al. Concentrations of dialkyl phosphate metabolites of organophosphorus pesticides in the U.S. population. Environ. Health Perspect. 2014;112:186–200. doi: 10.1289/ehp.6503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Muñoz-Quezada M.T., Iglesias V., Lucero B., Steenland K., Barr D.B., Levy K., Ryan P.B., Alvarado S., Concha C. Predictors of exposure to organophosphate pesticides in school children in the Province of Talca, Chile. Environ. Int. 2012;47:28–36. doi: 10.1016/j.envint.2012.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.