Abstract
Encoding a glutathione S-transferase (GST) and conferring resistance to Fusarium head blight (FHB), Fhb7 was successfully isolated from the newly assembled Thinopyrum elongatum genome by researchers, with blasting searches revealing that Thinopyrum gained Fhb7 through horizontal gene transfer from an endophytic Epichloë species. On the contrary, our molecular evidence reveals that the homologs of Fhb7 are distributed commonly in Triticeae. Other than Thinopyrum, the Fhb7 homologs were also detected in four other genera, Elymus, Leymus, Roegneria and Pseudoroegneria, respectively. Sequence comparisons revealed that the protein sequences were at least 94% identical across all of the Fhb7 homologs in Triticeae plants, which in turn suggested that the horizontal gene transfer of the Fhb7 might have occurred before Triticeae differentiation instead of Thinopyrum. The multiple Fhb7 homologs detected in some Triticeae accessions and wheat-Thinopyrum derivatives might be attributed to the alloploid nature and gene duplication during evolution. In addition, we discovered that some wheat-Thinopyrum derivatives carrying the Fhb7 homologs had a completely different reaction to Fusarium head blight, which made us question the ability of the GST-encoding Fhb7 to resist FHB.
Keywords: horizontal gene transfer, Fhb7, Triticeae, Thinopyrum, Fusarium head blight
1. Introduction
It is reported that beneficial plant-associated microbe and fungi possess widespread effects on plant growth and defense throughout their evolutionary history [1]. Researchers discovered that microbes influenced plant responses to global changes through at least four mechanisms: physical modification of the environment, secreting chemicals that mimic plant hormones, altering plant gene expression, and facilitating plant nutrient acquisition [2]. For example, in plants, bacteria in the genus Azospirillum have the capability of fixing nitrogen in the soil [3]. Other examples can be found in endophytes, organisms that inhabits the internal tissues of a plant without resulting in visible disease symptoms [4]. These include potentially beneficial micro-organisms for the plant, such as the endophytic fungal Colletotrichum tropicale, which induces the expression of hundreds of host defense-related genes in Theobroma cacao and resulting in stronger pathogen resistance [5].
Horizontal gene transfer (HGT) is the transmission of genetic material across the genome of biological organisms with reproductive barriers, which are particularly common in endophytic fungi species and their hosts [6,7]. On the one hand, HGT can facilitate microbial adaptation to adverse conditions in the environment and inside the host plant. The ciliate Tetrahymena thermophile acquired the genes involved in the catabolism of complex carbohydrates from bacteria and archaea, and these genes facilitated the Ciliates’ colonization of the rumen [8]. Eighteen Rhizophagus irregularis genes were found to be recently acquired from either plants or bacteria and these acquired genes may participate in diverse but fundamental biological processes such as regulation of gene expression, mitosis, and signal transduction [9]. On the other hand, the horizontal gene transfer and resultant integration of the transferred material might provide the host with adaptive advantages towards environment changes and acquisition of new traits and functions [7]. A study reported that a gene encoding β-1, 6-glucanase was transferred from fungal endophyte to a cool-season grass host and may function to protect against infection by other fungal pathogens [10]. Another example is that 128 genes of 57 families in the moss Physcomitrella patens were identified as derived from prokaryotes, fungi, or viruses. These acquired genes are involved in essential or plants specific activities such as xylem formation, plant defense, and nitrogen recycling, as well as the biosynthesis of starch, polyamines, hormones and glutathione, which played critical roles in the transition of plants from aquatic to terrestrial environments [11].
Different methods have been proposed to identify HGT events, such as relying on gene distribution patterns, unexpected phylogenetic tree topology, and similarity search between genomes [7,12]. The genome composition, including the base compositions, the patterns of codon usage, and the frequencies of di- and trinucleotides in DNA sequence, were also the clues to identifying HGT events [7,13]. Triticeae, one tribe of the grasses, comprises many economical important foods (wheat, barley, and rye) and some fine forage accessions, including the genera Thinopyrum. Previous reports indicate that endophytic Epichloë species were discovered among many grass genera in Triticeae, such as Elymus, Leymus, Roegneria and Agropyron [14]. With the increasing availability of genomic data, more and more horizontal gene transfer events from microbe to plants are being identified.
Fusarium head blight (FHB), mainly caused by the fungus Fusarium graminearum, is currently one of the most economically important wheat diseases in the world, which not only results in grain yield loses but also reduces grain quality due to mycotoxin contamination [15]. Fusarium species easily cloned on the head at the flowering stage and subsequent infection bleached the wheat spikes and shrank the kernels. FHB resistance has been controlled quantitatively and more than 432 quantitative trait loci (QTLs) have been mapped on all wheat chromosomes by linkage mapping or by association mapping [16]. Among these QTLs, researchers have formally named seven FHB-resistant ones: Fhb1 on chromosome 3BS, Fhb2 on chromosome 6BS, Fhb3 introgressed to chromosome 7AS from Leymus racemosus chromosome 7Lr#1, Fhb4 on chromosome 4BL, Fhb5 on chromosome 5AS, Fhb6 on chromosome 1A from 1Ets#1S of Elymus tsukushiensis and Fhb7 on chromosome 7E2 from Thinopyrum ponticum [17,18,19,20,21,22,23]. Using recombinant inbred lines derived from a cross between two Thatcher-Th. ponticum substitution lines, K11463 (7E1/7D) and K2620 (7E2/7D), the major FHB resistance locus FhbLoP (designated as Fhb7 now) was mapped to the very distal region of the long arm of chromosome 7E2 [23,24]. Wang et al. sequenced the genome of Th. elongatum (D-3458) and successfully mapped and cloned Fhb7, which encodes a glutathione S-transferase and confers wheat broad resistance to Fusarium head blight [25]. By blasting against the National Center for Biotechnology Information (NCBI) GenBank database, the authors did not find any homolog of Fhb7 in the Triticum genus or in the entire plant kingdom. They found that the Fhb7 homologs were distributed among Epichloë species, endophytic fungi of temperate grasses. Phylogenetic analyses suggested the Fhb7 in Thinopyrum wheatgrass was transferred from Epichloë species through horizontal gene transfer [25]. Based on these results, we were curious about why Fhb7 was transferred from Epichloë species only to Thinopyrum and whether there was a special mechanism for the transfer from Epichloë species to Thinopyrum.
In recent years, many genomes of Triticeae have been deciphered, including diploid, tetraploid, hexaploid wheat species, barley, and rye. However, due to lack of detailed genomic data for many other species in Triticeae, a single blast search may not accurately characterize the Fhb7 distribution in the entire plant kingdom. In this study, we checked the Fhb7 distribution among Triticeae by using specific molecular markers. Our results demonstrate that Fhb7 homologs are in fact distributed commonly in Triticeae and the horizontal gene transfer may have occurred before Triticeae differentiation instead of being unique to Thinopyrum.
2. Materials and Methods
2.1. Materials
The wheat-Th. elongatum addition line CS-7EL, the wheat-Th. elongatum substitution line 7E/7D and the common wheat Chinese Spring (CS) were provided by Mingcheng Luo (UC Davis Plant Sciences). The wheat-Th. ponticum partial amphiploids XY693 and XY784 were provided by Zhensheng Li (Institute of Genetics and Developmental Biology, Chinese Academy of Sciences). The wheat-Th. ponticum partial amphiploid SNTE122 was provided by Honggang Wang (Shandong Agricultural University) and the wheat-Th. ponticum translocation lines TNT-B provided by George Fedak (Plant Research Centre, Agriculture Canada, Ottawa, ON, Canada). Moshe Feldman (Weizmann Institute of Sciences) provided the Th. elonatum accessions Ae31 and Ae56. Other accessions in Triticeae (Table S1) were provided by Yiwen Li (Institute of Genetics and Developmental Biology, Chinese Academy of Sciences).
2.2. Methods
2.2.1. Sequence Amplification and Cloning of Fhb7 Homologs
A 3846-bp length sequence containing the Fhb7 coding region and its upstream 3 kb sequence were extracted from the released Th. elongatum (D-3458) genome. Primers that are specific for coding region and promoter region were designed by using the software Primer5 (Table 1). The primers 26102F and 26102R were used to amplify the coding region of Fhb7 homologs. The primers 26102ProF and 26102R were used to amplify the promoter and coding regions of Fhb7 homologs. About ten seeds for all accessions used in this study were germinated on moist filter paper in Petri dishes at room temperature. After five days, three to five seedlings for each accession were sampled for DNA extraction. The genomic DNA was extracted using the cetyltrimethylammonium bromide (CTAB) method. PCR was conducted in a 50 μL reaction volume containing 1 μL 100 ng/μL genomic DNA, 1 μL 10 μmol/L of each primer, 25 μL 2× KOD One™ PCR Master Mix (Toyobo Biotech Co., Ltd., Osaka, Japan) and 22 μL sterilized ddH2O. Amplified PCR products were separated on 1.5% agarose gels at 150 V for 20 min, stained with ethidium bromide and visualized using ultraviolet (UV) light. The amplified DNA product was purified by Universal DNA Purification Kit (Tiangen Biotech Co., Ltd., Beijing, China) and cloned into the pEASY®-Blunt Simple Cloning Vector (TransGen Biotech Co., Ltd., Beijing, China).
Table 1.
Primers used in this study.
| Primer Name | Sequence (5′-3′) | Tm (°C) | Amplified Region |
|---|---|---|---|
| 26102F | CGATAGAAGATAGCTTCAATCAACCCTTT | 60 | CDS |
| 26102R | CTACTTCACCTCGGCATACTTGTC | ||
| 26102ProF | TCCGCATTTCCCTTGCAGAT | 60 | Promoter and CDS |
| 26102RT-F | GGACTTCCCTTGGATCCTGC | 60 | CDS |
| 26102RT-R | ACCGACAATCATGTCCGCAT | ||
| Actin-F | CAACGAGCTCCGTGTCGCA | 60 | CDS |
| Actin-R | GAGGAAGCGTGTATCCCTCATAG |
CDS indicates the coding region sequence.
2.2.2. Chromosome Preparation and Fluorescence In Situ Hybridization (FISH)
The seeds of the line CS-7EL, 7E/7D, SNTE122, and TNT-B were germinated on moist filter paper in a petri dish at room temperature for two to three days. The main roots were cut from the seedlings and placed in nitrous oxide for 2 h. Subsequently the roots were fixed in 90% acetic acid for 5 min and then washed three times by using sterile water. The section containing dividing cells was cut and digested in 20 μL 1% pectolyase Y23 and 2% cellulase Onozuka R-10 solution for 1 h at 37 °C. After digestion, the root sections were washed in 75% ethanol two times briefly. The root sections were carefully broken by using a needle and collected by centrifugation. The sedimentation was resuspended in 100% acetic acid solution. The cell suspension was dropped onto glass slides in a wet box and dried slowly.
The probes were labeled using the nick translation method. 7EL-1 was obtained by Dop-PCR from the 7EL library constructed by chromosome microdissection. It was specific for the genome of Th. elongatum and Th. ponticum. The centromeric retrotransposon of wheat (CRW) clone 6C6 was labelled with Texas-red-5-dCTP. The 2846-bp Fhb7 homolog in the line CS-7EL and the probe 7EL-1 were labeled with Alexa Fluor-488-5-dUTP. The labeled probes were dissolved in 2 × SSC and 1× TE, dropped to the chromosome spreads. The slides were covered with a plastic sheet and denatured by heating at 100 °C for 5 min. After denaturing, the slides were placed into a moisturizing aluminum box with a lid and transferred into an incubator held at 55 °C overnight for hybridization. Then the slides were washed in 2× SSC buffer and the chromosomes were stained with DAPI (4′,6-diamidino-2-phenylindole).
2.2.3. FHB Resistance Evaluation on Wheat-Thinopyrum Derivatives
The FHB resistance evaluation was performed in field condition in Beijing (116°42′ E, 40°10′ N). To keep the flowering dates close, all plants were sown in stages. F. graminearum strains (Fg16-2, Fg16-5 and Fg16-11) and Fusarium asiaticum strain (Fa301) in Mung bean were mixed and 20 μL fungal suspension (1 × 106 conidia/mL) was injected into the central spikelet at early flowering stage. The wheat cultivar Jimai 22 was used as the susceptible control. For each of the lines, at least 10 spikes were inoculated with Fusarium species and the inoculated spikes were covered with a plastic bag for 2 days to keep moist for fungal infection. The number of diseased spikelets for each spike was recorded at 7, 14, and 21 days after inoculation. The statistical analysis was performed by the unpaired t test using the software GraphPad Prism 8.
2.2.4. The Expression Analysis of the Fhb7 Homolog in the Line TNT-B
Three spikelets around the inoculated one from at least three spikes from different plants of the line TNT-B were collected at 96 h post inoculation and grounded in liquid nitrogen for total RNA extraction using TRIzol® Reagent (Thermo Fisher Scientific lnc., Shanghai, China). First-strand cDNA synthesis from the total RNA was performed by using the FastKing RT kit (with gDNase) (TianGen Biotech Co., Beijing, China). The expression analyses were performed using the primers 26102RT-F and 26102RT-R (Table 1). The gene actin was used as an internal standard by the primers Actin-F and Actin-R. The relative expression of the Fhb7 homolog was calculated by the 2−ΔΔCT method.
3. Results
3.1. Fhb7 Is Not Unique to Thinopyrum in Triticeae
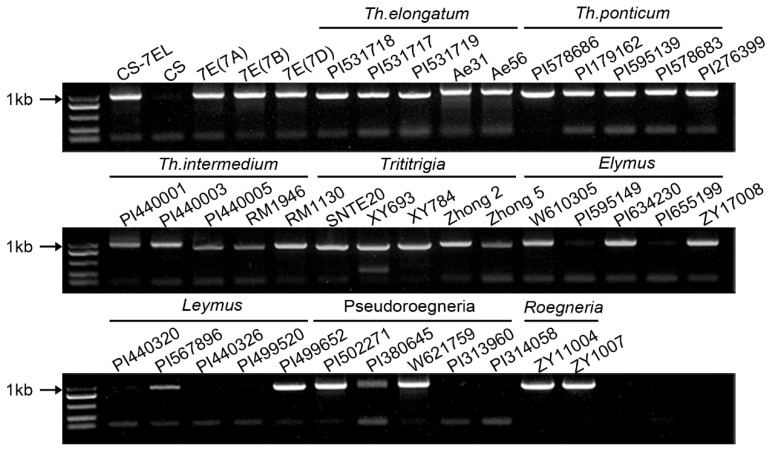
We collected 126 different accessions belonging to Triticeae to check the Fhb7 distribution via polymerase chain reaction (Table S1). Fhb7 homologs were indeed detected in the genera Thinopyrum, including Th. elongatum (2n = 2x = 14), Th. ponticum (2n = 10x = 70) and Th. intermedium (2n = 6x = 42) (Figure 1). These results were confirmed by detecting the Fhb7 homologs in some artificially synthesized wheat-Thinopyrum derivatives, such as the wheat-Th. elongatum addition line CS-7EL, wheat-Th. ponticum partial amphiploid XY693 and wheat-Th. intermedium partial amphiploid Zhong 2. However, other than the genera Thinopyrum, Fhb7 homologs were also discovered in some accessions among the genera Elymus, Leymus, Roegneria and Pseudoroegneria. What is more, unlike Thinopyrum, the other four genera all had some accessions that could not detect Fhb7 homologs, such as the Elymus accession PI 655199 and the Leymus accession PI 440326.
Figure 1.
Detection of the Fhb7 homologs among different species in Triticeae. Th. elongatum, diploid, 2n = 2x = 14. Th.ponticum, decaploid, 2n = 10x = 70. Th. intermedium, hexaploid, 2n = 6x = 42. Trititrigia means partial amphiploid produced from distant hybridization between common wheat and Thinopyrum. Elymus, Leymus, Pseudoregneria, and Roegneria represent four genera in Triticeae.
3.2. Polymorphism of Fhb7 Homologs in Triticeae
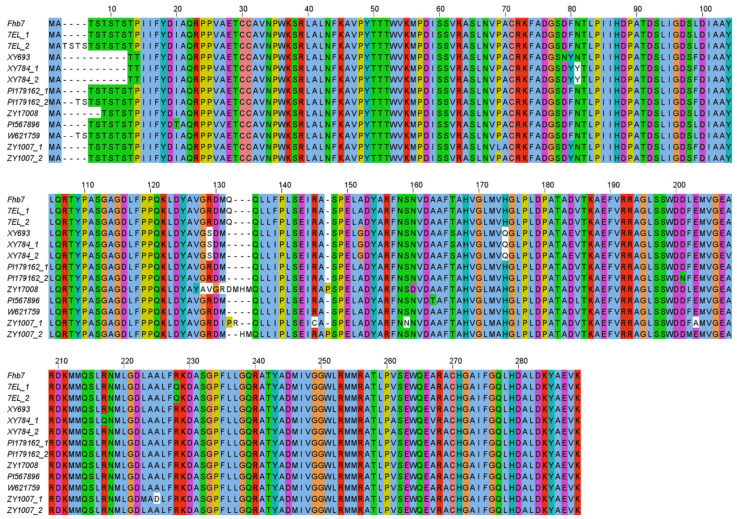
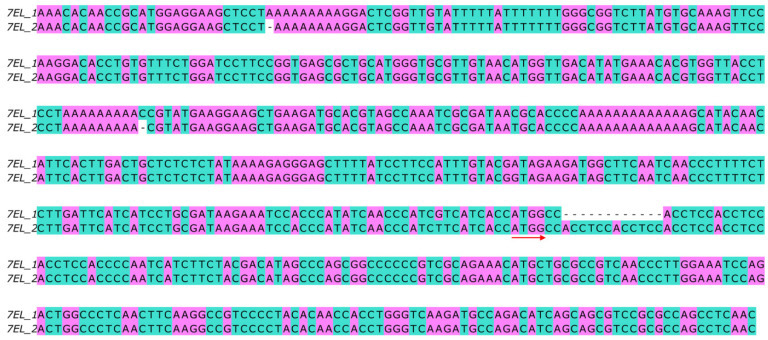
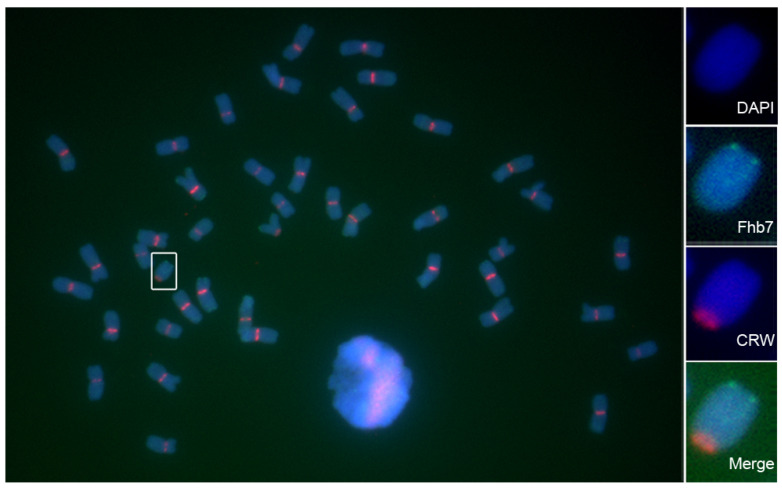
Fhb7 homologs were amplified from different accessions and inserted into the sequenced vector. Sequence comparisons revealed that the protein sequences were at least 94% identical across all of the Fhb7 homologs in Triticeae plants (Figure 2). Despite indel variation and amino acid substitution across all of the homologs of Fhb7, no premature termination and code-shifting mutations occurred in the protein sequences. The main variation was the number of Thr-Ser at the amino terminus of the protein sequence (Figure 2). We also noticed that more than one Fhb7 homologs were detected in some accessions, such as Th. ponticum PI 179162 and Roegneria kamoji ZY1007. These results were confirmed by detecting more than one Fhb7 homologs in some artificially synthetic wheat-Thinopyrum derivatives, such as the wheat-Th. elongatum addition line CS-7EL and the wheat-Th. ponticum partial amphiploid XY784. To exclude the possibility of endophytic Epichloë species contamination, we used the reference genome of Th. elongatum (D-3458) to design primers and tried to amplify the fragment that included Fhb7 homolog and its partial promoter region. Two similar fragments including a 2 kb promoter were isolated and sequenced. Further analysis revealed that the two sequences were corresponding to the two Fhb7 homologs detected in CS-7EL, respectively (Figure 3). These results were also confirmed by detecting two Fhb7 homologs by performing reverse transcription polymerase chain reaction in CS-7EL. To further analyze the distribution characterization of the two Fhb7 homologs in CS-7EL, fluorescence in situ hybridization was conducted on the metaphase chromosomes in CS-7EL by using a 2846-bp probe including the complete coding region of the Fhb7 homolog and its 2 kb promoter. It was obvious that only one signal was detected at the end of the alien chromosome 7EL (Figure 4). These results suggested that all Fhb7 homologs distributed proximal to the chromosome 7EL and each homolog might arrange in close proximity.
Figure 2.
Protein sequence alignments of the Fhb7 homologs in Triticeae. “_1” and “_2” means different Fhb7 homologs detected in one accession.
Figure 3.
Partial sequence comparison of two Fhb7 homologs coding region and promoter region in wheat-Th. elongatum addition line CS-7EL. The red arrow indicates the initiation codon.
Figure 4.
Fhb7 homolog distribution detected by fluorescence in situ hybridization in the line CS-7EL. The CRW is labeled in red. The 2846-bp Fhb7 homolog is labeled in green. DAPI staining is labeled in blue. The insets show high-magnification images of chromosomes 7EL in the white box.
3.3. Contrast Reactions of Wheat-Thinopyrum Derivatives Carrying Fhb7 Homologs to FHB
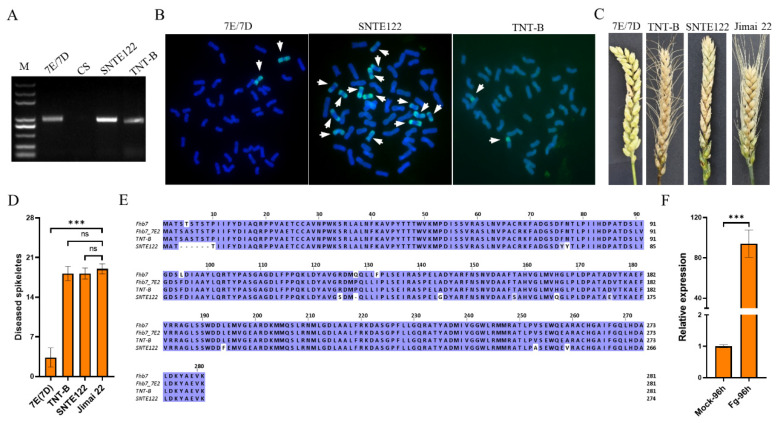
As mentioned above, the Fhb7 homologs were detected in some artificially synthesized wheat-Thinopyrum derivatives. We detected Fhb7 homologs in wheat-Th. elongatum substitution lines 7E/7D, wheat-Th. ponticum amphiploid SNTE122 and wheat-Th. ponticum translocation line TNT-B (Figure 5A,B). However, we found they have different reactions to FHB: the line 7E/7D showed high level resistance to FHB with only several spikelets bleached while SNTE122 and TNT-B were highly susceptible to FHB with nearly whole spike bleached (Figure 5C,D). To exclude the possibility of sequence variation, we cloned and sequenced the Fhb7 homologs in them. Compared with Fhb7, amino acid substitutions and deletions were discovered in the homolog of the line SNTE122 and only substitutions in that of the line TNT-B (Figure 5E). We also discovered that the protein sequence of the Fhb7 homolog in TNT-B is the same with that of the homolog in the wheat-Th. ponticum substitution line 7E2/7D (Figure 5E), which was used as the resistant parent in the Fhb7 mapping population [25]. Due to the contrast reaction to FHB, the expression pattern of the Fhb7 homolog in TNT-B was characterized by qRT-PCR. We surprisingly found that the Fhb7 homolog in TNT-B was dramatically induced 96 h after inoculation with Fusarium species (Figure 5F). Therefore, contrast reactions of wheat-Thinopyrum derivatives carrying Fhb7 homologs to FHB were discovered in our study.
Figure 5.
FHB resistance evaluation on wheat-Thinopyrum derivatives carrying Fhb7 homologs. (A) Detecting Fhb7 homolog in wheat-Thinopyrum derivatives. (B) Karyotype analysis on wheat-Thinopyrum derivatives. The white arrows indicate the alien chromosome. (C,D) FHB resistance evaluation on wheat-Thinopyrum derivatives. The spike pictures (C) were photographed and the number of diseased spikelets (D) were recorded 14 d after inoculation with Fusarium species. (E) Protein sequence comparison of the Fhb7 homologs among wheat-Thinopyrum derivatives. (F) Expression analysis of the Fhb7 homolog in the line TNT-B. Fg indicated Fusarium species. *** p < 0.001, ns, p > 0.05.
4. Discussion
Although several methods have been enumerated for identifying potential HGT events, there are some imperfections in each method [7]. Therefore, researchers suggested that one or more of the above-mentioned methods should be used in combination to properly identify identifying HGT events [7]. Although no Fhb7 homolog was discovered by blast searching the NCBI GenBank database, it may be inaccurate to draw the conclusion that Thinopyrum gained Fhb7 through the HGT from an endophytic Epichloë species [25,26]. Lacking detailed genomic information for many species in Triticeae, the true distribution of Fhb7 homologs in Triticeae was masked. In our study, the molecular evidence clearly shows that Fhb7 homologs are distributed commonly in Triticeae and is not exclusive to the genus Thinopyrum in the plant kingdom. Our results suggested that the HGT of the Fhb7 was not an accidental happening by chance only in the genera Thinopyrum but may have instead occurred before Triticeae differentiation. We wonder when Triticeae might have borrowed such an alien gene from Epichloë species during evolution. More puzzling is why horizontal gene transfer did not occur to the genera Triticum that are known to be seriously affected by Fusarium species.
As wheat relative species, Th. elongatum and Th. ponticum are important reservoirs of elite genes for wheat improvement and the genes for FHB resistance derived from them have been located on the homologous group seven, 7EL for Th. elongatum and 7E2 for Th. ponticum, respectively [23,27,28]. Although two genes were both located to the homologous group seven, no molecular evidence could verify the relationship between different genes or homologs. Referring to the newly assembled Th. elongatum (D-3458) genome, a glutathione S-transferase was identified as a candidate for Fhb7 by map-based cloning and conferred broad resistance to Fusarium species by detoxifying trichothecenes through de-epoxidation [25]. Unlike the results published, we firstly discovered that some wheat-Thinopyrum derivatives carrying Fhb7 homologs were highly susceptible to FHB. For the Fhb7 homolog in the line SNTE122, the similar protein sequence shared with that derived from the wheat-Thinopyrum substitution line 7E2/7D and the induction by Fusarium species lead us to suspect the function of Fhb7 on FHB resistance. Secondly, Fhb7 was proved by a single copy in the assembled Th. elongatum genome [25]. However, more than one Fhb7 homolog was detected in some Thinopyrum accessions and wheat-Thinopyrum derivatives in our study. By amplifying the promoter sequence of Fhb7 homolog, we excluded the possibility of contamination by the endophytic Epichloë species. The two expressed Fhb7 homologs in CS-7EL might be attributed to a recent burst of gene duplications in Triticeae [29]. The Fhb7 in an FHB-resistant substitution line 7E2/7D used for mapping was demonstrated to be semidominant and it was applied into wheat breeding by creating wheat-Th. ponticum translocation lines [25]. As wheat-alien chromosomes addition or substitution lines usually carried linkage drags between useful and undesirable genes, translocation lines were preferred to transfer alien genes to common wheat by breeders for its higher possibility of breaking linkage drag [30,31]. Based on the dosage effect of Fhb7 homolog, the translocation lines derived from the addition line CS-7EL may have better resistance to FHB than the substitution line 7E2/7D. Other than gene duplications, the alloploid nature might be another reason for the absence of more than one Fhb7 homologs in some accessions, such as Th. ponticum PI 179162 and R. kamoji ZY1007.
Acknowledgments
We thank Yiwen Li (Institute of Genetics and Developmental Biology, Chinese Academy of Sciences) and Moshe Feldman (Weizmann Institute of Sciences) for providing Triticeae accessions. We acknowledge Mingcheng Luo (UC Davis Plant Sciences) for providing the wheat-Th. elongatum addition line CS-7EL. We thank James A. Birchler (University of Missouri) and Nathan Han (Washington University, St. Louis) for critically reading and editing the manuscript, and for providing valuable suggestions.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/plants11162074/s1. Table S1: Distribution of the Fhb7 homologs in Triticeae.
Author Contributions
The experiments were designed by F.H., X.G. and F.H. wrote the manuscript. X.G. conducted DNA extraction, FISH and sequences clone. M.W. helped sequence comparison and date analysis. The manuscript was revised by H.K. and Y.Z. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This work was founded by the National Key Research and Development Program of China (2016YFD0102001).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Compant S., Samad A., Faist H., Sessitsch A. A review on the plant microbiome: Ecology, functions, and emerging trends in microbial application. J. Adv. Res. 2019;19:29–37. doi: 10.1016/j.jare.2019.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Angulo V., Beriot N., Garcia-Hernandez E., Li E., Masteling R., Lau J.A. Plant-microbe eco-evolutionary dynamics in a changing world. New Phytol. 2022;234:1919–1928. doi: 10.1111/nph.18015. [DOI] [PubMed] [Google Scholar]
- 3.Steenhoudt O., Vanderleyden J. Azospirillum, a free-living nitrogen-fixing bacterium closely associated with grasses: Genetic, biochemical and ecological aspects. FEMS Microbiol. Rev. 2000;24:487–506. doi: 10.1111/j.1574-6976.2000.tb00552.x. [DOI] [PubMed] [Google Scholar]
- 4.Goodwin P.H. The Endosphere Microbiome of Ginseng. Plants. 2022;11:415. doi: 10.3390/plants11030415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mejia L.C., Herre E.A., Sparks J.P., Winter K., Garcia M.N., Van Bael S.A., Stitt J., Shi Z., Zhang Y., Guiltinan M.J., et al. Pervasive effects of a dominant foliar endophytic fungus on host genetic and phenotypic expression in a tropical tree. Front. Microbiol. 2014;5:479. doi: 10.3389/fmicb.2014.00479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang J. Horizontal gene transfer in eukaryotes: The weak-link model. Bioessays. 2013;35:868–875. doi: 10.1002/bies.201300007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tiwari P., Bae H. Horizontal Gene Transfer and Endophytes: An Implication for the Acquisition of Novel Traits. Plants. 2020;9:305. doi: 10.3390/plants9030305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ricard G., McEwan N.R., Dutilh B.E., Jouany J.P., Macheboeuf D., Mitsumori M., McIntosh F.M., Michalowski T., Nagamine T., Nelson N., et al. Horizontal gene transfer from Bacteria to rumen Ciliates indicates adaptation to their anaerobic, carbohydrates-rich environment. BMC Genom. 2006;7:22. doi: 10.1186/1471-2164-7-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li M., Zhao J., Tang N., Sun H., Huang J. Horizontal gene transfer from bacteria and plants to the Arbuscular Mycorrhizal Fungus Rhizophagus irregularis. Front. Plant Sci. 2018;9:701. doi: 10.3389/fpls.2018.00701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shinozuka H., Hettiarachchige I.K., Shinozuka M., Cogan N.O.I., Spangenberg G.C., Cocks B.G., Forster J.W., Sawbridge T.I. Horizontal transfer of a ss-1,6-glucanase gene from an ancestral species of fungal endophyte to a cool-season grass host. Sci. Rep. 2017;7:9024. doi: 10.1038/s41598-017-07886-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yue J., Hu X., Sun H., Yang Y., Huang J. Widespread impact of horizontal gene transfer on plant colonization of land. Nat. Commun. 2012;3:1152. doi: 10.1038/ncomms2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koonin E.V., Makarova K.S., Aravind L. Horizontal gene transfer in prokaryotes: Quantification and classification. Annu. Rev. Microbiol. 2001;55:709–742. doi: 10.1146/annurev.micro.55.1.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ochman H., Lawrence J.G., Groisman E.A. Lateral gene transfer and the nature of bacterial innovation. Nature. 2000;405:299–304. doi: 10.1038/35012500. [DOI] [PubMed] [Google Scholar]
- 14.Song H., Nan Z., Song Q., Xia C., Li X., Yao X., Xu W., Kuang Y., Tian P., Zhang Q. Advances in research on Epichloë endophytes in Chinese native grasses. Front. Microbiol. 2016;7:1399. doi: 10.3389/fmicb.2016.01399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Timmusk S., Nevo E., Ayele F., Noe S., Niinemets Y. Fighting Fusarium pathogens in the era of climate change: A conceptual approach. Pathogens. 2020;9:419. doi: 10.3390/pathogens9060419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ma Z., Xie Q., Li G., Jia H., Zhou J., Kong Z., Li N., Yuan Y. Germplasms, genetics and genomics for better control of disastrous wheat Fusarium head blight. Theor. Appl. Genet. 2020;133:1541–1568. doi: 10.1007/s00122-019-03525-8. [DOI] [PubMed] [Google Scholar]
- 17.Cuthbert P.A., Somers D.J., Thomas J., Cloutier S., Brulé-Babel A. Fine mapping Fhb1, a major gene controlling Fusarium head blight resistance in bread wheat (Triticum aestivum L.) Theor. Appl. Genet. 2006;112:1465. doi: 10.1007/s00122-006-0249-7. [DOI] [PubMed] [Google Scholar]
- 18.Cuthbert P.A., Somers D.J., Brulé-Babel A. Mapping of Fhb2 on chromosome 6BS: A gene controlling Fusarium head blight field resistance in bread wheat (Triticum aestivum L.) Theor. Appl. Genet. 2007;114:429–437. doi: 10.1007/s00122-006-0439-3. [DOI] [PubMed] [Google Scholar]
- 19.Qi L.L., Pumphrey M.O., Friebe B., Chen P.D., Gill B.S. Molecular cytogenetic characterization of alien introgressions with gene Fhb3 for resistance to Fusarium head blight disease of wheat. Theor. Appl. Genet. 2008;117:1155–1166. doi: 10.1007/s00122-008-0853-9. [DOI] [PubMed] [Google Scholar]
- 20.Xue S., Li G., Jia H., Xu F., Lin F., Tang M., Wang Y., An X., Xu H., Zhang L., et al. Fine mapping Fhb4, a major QTL conditioning resistance to Fusarium infection in bread wheat (Triticum aestivum L.) Theor. Appl. Genet. 2010;121:147–156. doi: 10.1007/s00122-010-1298-5. [DOI] [PubMed] [Google Scholar]
- 21.Xue S., Xu F., Tang M., Zhou Y., Li G., An X., Lin F., Xu H., Jia H., Zhang L., et al. Precise mapping Fhb5, a major QTL conditioning resistance to Fusarium infection in bread wheat (Triticum aestivum L.) Theor. Appl. Genet. 2011;123:1055–1063. doi: 10.1007/s00122-011-1647-z. [DOI] [PubMed] [Google Scholar]
- 22.Cainong J.C., Bockus W.W., Feng Y., Chen P., Qi L., Sehgal S.K., Danilova T.V., Koo D.-H., Friebe B., Gill B.S. Chromosome engineering, mapping, and transferring of resistance to Fusarium head blight disease from Elymus tsukushiensis into wheat. Theor. Appl. Genet. 2015;128:1019–1027. doi: 10.1007/s00122-015-2485-1. [DOI] [PubMed] [Google Scholar]
- 23.Guo J., Zhang X., Hou Y., Cai J., Shen X., Zhou T., Xu H., Ohm H.W., Wang H., Li A. High-density mapping of the major FHB resistance gene Fhb7 derived from Thinopyrum ponticum and its pyramiding with Fhb1 by marker-assisted selection. Theor. Appl. Genet. 2015;128:2301–2316. doi: 10.1007/s00122-015-2586-x. [DOI] [PubMed] [Google Scholar]
- 24.Shen X., Ohm H. Molecular mapping of Thinopyrum-derived Fusarium head blight resistance in common wheat. Mol. Breed. 2007;20:131–140. doi: 10.1007/s11032-007-9079-9. [DOI] [Google Scholar]
- 25.Wang H., Sun S., Ge W., Zhao L., Kong L. Horizontal gene transfer of Fhb7 from fungus underlies Fusarium head blight resistance in wheat. Science. 2020;368:eaba5435. doi: 10.1126/science.aba5435. [DOI] [PubMed] [Google Scholar]
- 26.Wang Q., Huang J. Fungal genes in plants: Impact and potential applications. Trends Plant Sci. 2020;25:1064–1067. doi: 10.1016/j.tplants.2020.07.005. [DOI] [PubMed] [Google Scholar]
- 27.Fu S., Lv Z., Qi B., Guo X., Li J., Liu B., Han F. Molecular cytogenetic characterization of wheat-Thinopyrum elongatum addition, substitution and translocation lines with a novel source of resistance to wheat Fusarium head blight. J. Genet. Genom. 2012;39:103–110. doi: 10.1016/j.jgg.2011.11.008. [DOI] [PubMed] [Google Scholar]
- 28.Ceoloni C., Forte P., Kuzmanovic L., Tundo S., Moscetti I., De Vita P., Virili M.E., D’Ovidio R. Cytogenetic mapping of a major locus for resistance to Fusarium head blight and crown rot of wheat on Thinopyrum elongatum 7EL and its pyramiding with valuable genes from a Th. ponticum homoeologous arm onto bread wheat 7DL. Theor. Appl. Genet. 2017;130:2005–2024. doi: 10.1007/s00122-017-2939-8. [DOI] [PubMed] [Google Scholar]
- 29.Wang X., Yan X., Hu Y., Qin L., Wang D., Jia J., Jiao Y. A recent burst of gene duplications in Triticeae. Plant Com. 2021;3:100268. doi: 10.1016/j.xplc.2021.100268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Falke K.C., Susic Z., Wilde P., Wortmann H., Mohring J., Piepho H.P., Geiger H.H., Miedaner T. Testcross performance of rye introgression lines developed by marker-assisted backcrossing using an Iranian accession as donor. Theor. Appl. Genet. 2009;118:1225–1238. doi: 10.1007/s00122-009-0976-7. [DOI] [PubMed] [Google Scholar]
- 31.Jiang J., Friebe B., Gill B.S. Recent advances in alien gene transfer in wheat. Euphytica. 1993;73:199–212. doi: 10.1007/BF00036700. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.