Abstract
Vitamin D is a critical regulator of calcium and bone homeostasis. While vitamin D has multiple effects on bone and calcium metabolism, the regulation of intestinal calcium (Ca) absorption efficiency is a critical function for vitamin D. This is necessary for optimal bone mineralization during growth, the protection of bone in adults, and the prevention of osteoporosis. Intestinal Ca absorption is regulated by 1,25 dihydroxyvitamin D (1,25(OH)2 D), a hormone that activates gene transcription following binding to the intestinal vitamin D receptor (VDR). When dietary Ca intake is low, Ca absorption follows a vitamin-D-regulated, saturable pathway, but when dietary Ca intake is high, Ca absorption is predominately through a paracellular diffusion pathway. Deletion of genes that mediate vitamin D action (i.e., VDR) or production (CYP27B1) eliminates basal Ca absorption and prevents the adaptation of mice to low-Ca diets. Various physiologic or disease states modify vitamin-D-regulated intestinal absorption of Ca (enhanced during late pregnancy, reduced due to menopause and aging).
Keywords: diet, transcellular, absorption, diffusion, intestine, homeostasis, parathyroid hormone
1. Introduction
It has now been 100 years since E.V. McCollum first identified a fat-soluble compound in food that supported bone growth and prevented rickets; he called this compound vitamin D. In the intervening century, many scientists have contributed to our understanding for how vitamin D regulates the physiology of calcium (Ca) metabolism. For example, in 1937 Nicolaysen showed that vitamin D is critical for intestinal Ca absorption [1] while later studies by Pansu et al. [2] and Sheikh et al. [3] showed that vitamin D deficiency significantly reduces intestinal Ca absorption. The critical breakthrough defining the mechanism used by vitamin D to regulate Ca metabolism came in the early 1970′s when research by Holick et al. [4] and Norman et al. [5] isolated the active metabolite of vitamin D, 1,25 dihydroxyvitamin D3 (1,25(OH)2D), from the intestine. Shortly thereafter, Brumbaugh and Haussler [6] discovered the nuclear receptor for 1,25(OH)2D, the vitamin D receptor (VDR), in intestinal mucosa as well. Since then, we’ve learned the detailed mechanism used by 1,25(OH)2D to regulate gene expression [7] while global and conditional VDR knockout mice have allow us to study the function of vitamin D in Ca/bone metabolism and in other physiologic systems. As part of this effort, my research group has shown that the single most important role for vitamin D during growth is the regulation of intestinal Ca absorption [8] but that 1,25(OH)2D signaling through the VDR has a broad array of target genes in the intestine [9]. Because of the central role that vitamin D plays in the regulation of intestinal Ca absorption, this review provides a critical starting point for anyone who wants to understand the physiologic importance of vitamin D.
2. Vitamin D Has a Critical Physiologic Role for Protecting Bone through the Regulation of Intestinal Ca Absorption
Bone mass is lost when dietary Ca intake is inadequate and so one usually thinks of bone when the term “Ca homeostasis” is used. However, Ca homeostasis is not regulated to maintain bone integrity. Instead, bone, the parathyroid gland, intestine, and kidney make up a multi-tissue axis that work together to maintain serum Ca within a narrow range (8.9–10.2 mg/dL). As a result, after a meal intestinal Ca absorption is a signal that disturbs and elevates serum Ca while bone formation and resorption, along with renal Ca excretion, respond to fluxes in serum Ca in an attempt to limit perturbations in serum Ca. This coordination was shown clearly by Bronner and Aubert who used Ca kinetics in rat models to show how the body adapts to habitual low Ca intake by increasing Ca absorption efficiency, reducing renal Ca loss, and mobilizing Ca from the bone (i.e., resorption) [10]. Pansu et al. [11] showed the impact of dietary Ca intake on intestinal Ca absorption directly when they found that feeding rats a 0.17%, low Ca diet for 5 weeks increased duodenal Ca absorption efficiency by increasing the saturable component of transport (Vmax increased 55%). Similarly, Norman et al. [12] found that in adult humans, feeding a diet with 300 mg of Ca/day for 8 weeks increased Ca absorption efficiency by 43% compared to subjects consuming 1600 mg Ca/day. These studies, and others like them, led to the identification of 1,25 dihydroxyvitamin D (1,25(OH)2 D) and parathyroid hormone (PTH) as the major hormonal regulators of Ca homeostasis.
When dietary Ca is habitually low, there is a transient reduction in serum Ca that is sensed at the parathyroid gland through the Ca sensing receptor (CaSR). This cell surface receptor mediates signals into the parathyroid gland to increase the production and release of PTH into the circulation—a condition called nutritional secondary hyperparathyroidism. PTH has several important functions in Ca metabolism. First, it regulates renal production of 1,25(OH)2 D by inducing the CYP27B1 gene that encodes the enzyme 25 hydroxyvitamin D-1α hydroxylase [13] and it suppresses expression of the CYP24A1 gene that encodes the 25 hydroxyvitamin D-24 hydroxylase [14,15]. 1,25(OH)2 D released from the kidney is the most important regulator of increased intestinal Ca absorption. Of course, this physiologic adaptation has limits so that if the degree of dietary Ca deprivation is too great, the adaptation of intestinal Ca absorption will not be sufficient to compensate. This case, 1,25(OH)2 D and PTH will both promote bone resorption by stimulating osteoclastic activity while also enhancing renal Ca reabsorption in the proximal renal tubule. Collectively, this physiological adaptation can protect serum Ca but at the expense of bone mass.
The central role for vitamin D as a regulator of intestinal Ca absorption has been known for more than 80 years [1,16]. In vitamin D deficient animals intestinal Ca absorption efficiency falls by >75% [2]. Similarly, dialysis patients with low circulating 1,25(OH)2 D levels also have low intestinal Ca absorption [3]. Finally, in elderly adults, secondary hyperparathyroidism can maintain serum 1,25(OH)2 D (and Ca absorption) until serum 25-hydroxyvitamin D (25(OH)D) levels fall to ≤10 nmol/L, at which point there is not enough substrate to convert to 1,25(OH)2 D [17].
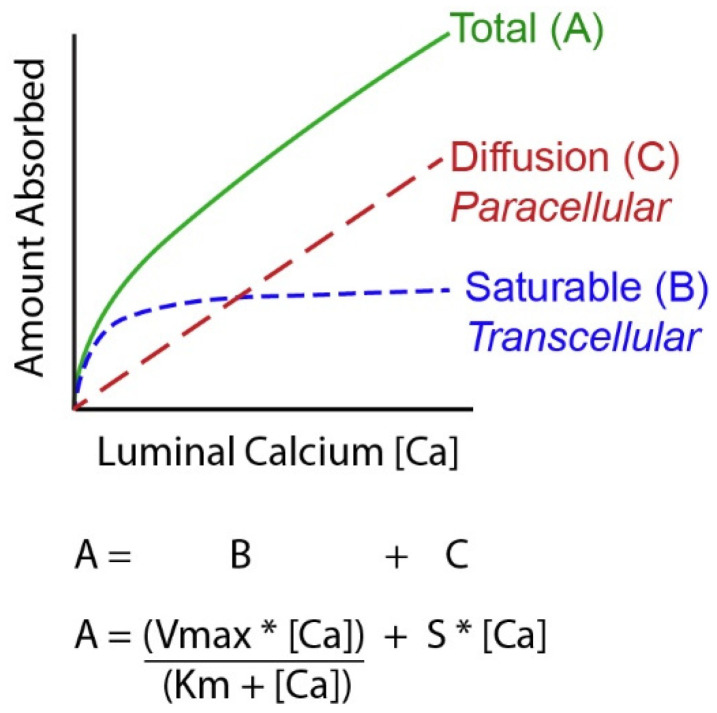
A challenging concept for many people examining Ca absorption is that it is not a single process but the sum of events that occur through two routes, a transcellular, saturable pathway and non-saturable, paracellular diffusion pathway [11,18,19,20] (see Figure 1). The relative importance of these two routes depends upon a person’s habitual Ca intake. When Ca intake is low, like most adult American women [21], the saturable pathways predominates while when Ca intake is high the bulk of absorption occurs through the diffusional route. Absorption through these two routes can be modeled mathematically using a Michaelis–Menten-like equation that contains a linear component that models diffusion (Figure 1). The saturable transport pathway comprises three parts, apical membrane Ca entry, transcellular diffusion, and basolateral membrane extrusion. The apical membrane transport occurs down a concentration gradient while basolateral membrane extrusion is against a concentration gradient and requires energy [22]. The saturable pathway is present in the proximal small intestine (duodenum and jejunum), cecum, and colon [23,24,25,26,27] but is absent in the ileum [2]. Studies in rat duodenum [2] and in differentiated monolayers of the human intestinal cell line Caco-2 [28], show that 1,25(OH)2 D acts on the saturable transport component where it increases the Vmax (maximal absorptive capacity) but not Km (the affinity of the process for Ca). This suggests that 1,25(OH)2 D increases the production of intestinal Ca transporters (which we’ll discuss below) but that this increase has limits. In contrast, the passive Ca movement across the intestinal barrier occurs at ~13% of the luminal Ca level per hour in humans [20]) and is seen in all segments of the intestine. There is some evidence that the non-saturable portion of Ca absorption in the human ileum is also vitamin D sensitive; the slope of the non-saturable transport pathway is reduced in chronic renal disease patients, and it returns to normal after 1,25(OH)2 D injection [20].
Figure 1.
A Mathematical model of intestinal calcium (Ca) absorption. By studying Ca absorption over a range of luminal Ca levels it has been shown that the total amount of Ca absorbed across the intestinal barrier can be described as a curvilinear function. Total transport (A) is the sum of a saturable component (likely transcellular, B) that can be defined by the Michaelis–Menten equation and a diffusional process (C) that is defined by a straight line. [Ca] = luminal Ca concentration; S = the slope of the diffusional component; Vmax = the maximum transport rate seen for the saturable transport component; Km = the luminal concentration of the mineral at ½ the Vmax.
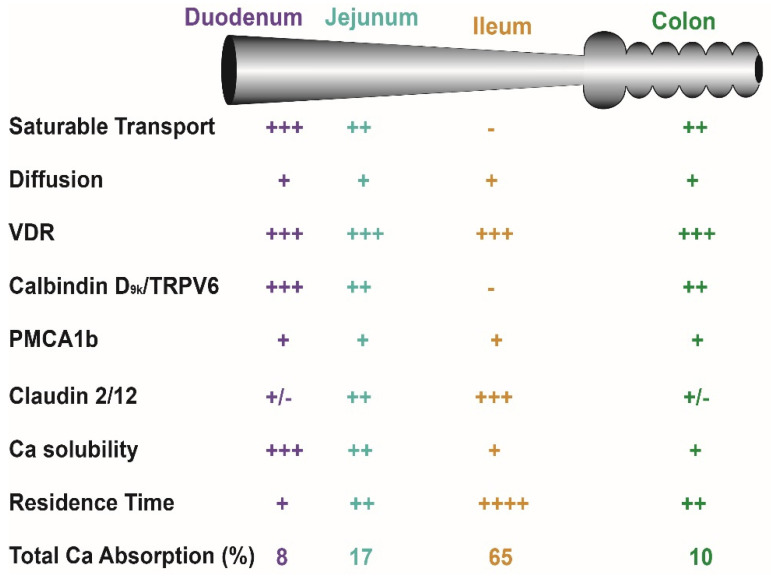
In normal healthy adults, the Km for the saturable component of Ca absorption from the small intestine of adults is 3.3 mM, a concentration met by 265 mg Ca in a meal (calculated from data in [19,20]). As a result, when a person eats a meal with ~400 mg (1/3 the RDA for Ca), saturable Ca transport is about 60% of total Ca absorption. However, the apparent efficiency of total Ca absorption falls as the meal Ca intake level is increased and the diffusional component of absorption takes a larger role. Normally, the amount of Ca absorbed in each intestinal segment is determined by: (a) the presence of the saturable and non-saturable pathways, (b) the residence time in the segment, and (c) the solubility of Ca within the segment (e.g., lower Ca solubility at higher pH [29,30,31]) (Figure 2). Although 1,25(OH)2 D clearly increases intestinal Ca absorption efficiency [2,32,33], some have argued that elevated serum 25(OH)D levels might also regulate intestinal Ca absorption. This is not supported by several large, well-designed studies that show no benefit of increasing serum 25(OH)D levels beyond 50 nmol/L [34,35,36,37].
Figure 2.
Critical factors that influence intestinal calcium (Ca) absorption. Many factors influence net Ca absorption and distinct Ca absorption mechanisms used in various intestinal segments. See Bronner and Pansu [38] for a discussion of solubility and transit time as factors affecting Ca absorption. The number of “+” signs reflects the magnitude of the parameter across tissues while a “-” sign indicates that the parameter is absent in a segment.
There are many studies that show adequate dietary Ca and vitamin D, and therefore total intestinal Ca absorption, is necessary for adequate bone growth. Deficiency of either Ca or vitamin D in growing children or animals causes nutritional rickets characterized by under-mineralized bone and low bone mass [39,40]. This is consistent with the concept that bone matrix cannot mineralize in the absence of mineral. However, net Ca absorption (which reflects both transport routes) is positively correlated to Ca balance in children, reflecting the critical role for Ca absorption in optimizing peak bone mass [41]. Consistent with this idea, we have shown that efficiency of Ca absorption through the saturable, vitamin-D-regulated pathway is significantly positively correlated with femoral trabecular bone volume/total volume in a genetically diverse population of 11 inbred mouse lines [42]. In addition, Patel et al. [43] reported that femur neck BMD was significantly positively correlated with Ca absorption efficiency in adult men. Several studies also indicate the high intestinal Ca absorption efficiency can protect against femoral bone loss in mice fed low Ca diets [44] or reduce the risk of osteoporotic hip fracture in women with low dietary Ca intake [45]. Collectively, these data show that both adequate Ca intake and genetically programed high intestinal Ca absorption efficiency are necessary to build and protect strong bones.
3. Vitamin D Effects on Intestinal Ca Absorption Are Mediated through the VDR
1,25(OH)2 D regulates Ca metabolism and intestinal Ca absorption by regulating gene transcription, a process that requires binding of the hormone to the Vitamin D Receptor (VDR), a nuclear receptor that is a ligand-activated transcription factor [7,46]. A number of studies have shown the critical importance of VDR for the regulation of Ca and bone metabolism. For example, children with inactivating mutations in the VDR gene (i.e., type II genetic rickets) have defects in Ca metabolism that include lower intestinal Ca absorption efficiency [47]. Similarly, VDR knockout mice have severe defects in bone growth and mineralization as well as a >70% reduction in Ca absorption efficiency [48,49]. While the gross phenotype of VDR knockout mice is abnormal bone, several lines of evidence indicate that the most important role for VDR in Ca/bone metabolism during growth is the control of intestinal Ca absorption. First, the phenotype of the intestine-specific VDR knockout mouse is identical to dietary Ca deficiency (osteomalacia, reduced serum Ca, elevated serum 1,25(OH)2 D levels) [50]. Conversely, feeding high Ca/high phosphate/high lactose “rescue” diets that promote passive/diffusional intestinal Ca absorption can prevent the abnormal bone and Ca metabolism phenotype of VDR knockout mice [51]. Finally, experiments from my lab showed that the VDR knockout mouse phenotype (e.g., hypocalcemia, elevated serum PTH, low bone mineral density) can be completely prevented by intestine epithelial cell-specific, transgenic expression of VDR that normalizes intestinal Ca absorption [8].
Several studies show that lower intestinal VDR levels disrupt the physiologic response to 1,25(OH)2D. In VDR KO mice, low level, intestine-specific transgenic VDR expression (10% of wild-type values) was insufficient to maintain normal intestinal Ca absorption [52]. Meanwhile, my lab has shown that a 50% reduction in intestinal VDR levels blunts 1,25(OH)2 D-regulated intestinal Ca absorption efficiency [53]. Consistent with the idea that reduced VDR function impairs intestinal Ca absorption, several studies have shown that Ca absorption efficiency is reduced in people with the longer, less transcriptionally active “f” allele of the Fok I restriction fragment length polymorphism [54,55,56]. Collectively these data support the hypothesis that variations in VDR level or function can influence vitamin-D-regulated intestinal Ca absorption as well as optimal intestinal responses to the increased serum 1,25(OH)2 D levels that accompany dietary Ca restriction.
4. Molecular Models of Ca Absorption
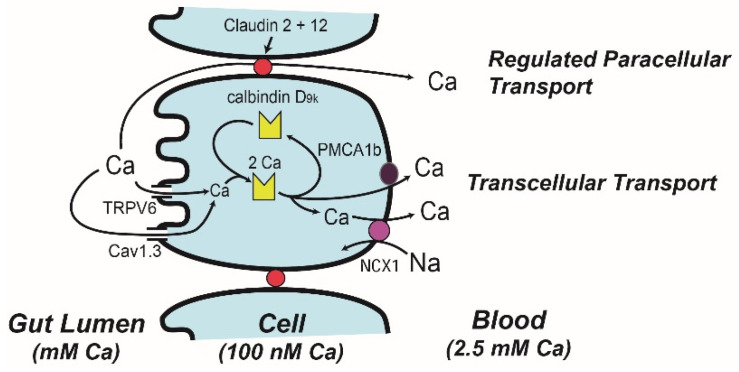
Ion microscopy reveals that Ca can enter at the apical membrane and flow through the absorptive epithelial cell in 20 min [57]. However, during vitamin D deficiency Ca becomes trapped in the region just below the microvilli. Treating vitamin D deficient chicks with 1,25(OH)2 D reverses this effect starting 2–4 h after treatment [58], consistent with the induction of gene expression mediated through the VDR. In 1986 Bronner et al. [59] critically reviewed transport data from a wide variety of well-controlled mechanistic studies, and from this analysis built the facilitated diffusion model (Figure 3, with protein distributions across the intestinal tract in Figure 2). In the first step of this model, brush border membrane uptake of Ca is mediated by an apical membrane Ca channel, which was later identified as the transient receptor potential cation channel vanilloid family member 6 (TRPV6, originally called CaT1 or ECAC2) [60]. TRPV6 gene expression is strongly regulated by 1,25(OH)2 D in the duodenum of mice [33,61] and in Caco-2 cells [62] and this induction is mediated by VDR binding enhancers upstream from the transcription start site [63,64]. Induction of TRPV6 mRNA precedes the increase in duodenal Ca absorption that occurs following 1,25(OH)2 D injection [33]. While initial studies suggested that 1,25(OH)2 D-mediated Ca absorption was not reduced in TRPV6 knockout mice [65,66], later studies showed that TRPV6 knockout mice [66], and mice with a D541A variant TRPV6 that inactivates Ca movement through the channel [67], had a blunted ability to increase Ca absorption in response to feeding a low Ca diet. In addition, my lab has shown that intestine-specific transgenic expression of TRPV6 increases Ca absorption efficiency and that this prevents the abnormalities in bone/Ca metabolism of VDR knockout mice [68].
Figure 3.
A Model describing intestinal calcium (Ca) absorption. The transcellular absorption pathway is described by the facilitated diffusion model while a regulated paracellular transport mechanisms mediated by claudin 2 and claudin 12 provides selectivity for Ca movement through the tight junction complex. For details of how vitamin D regulates various aspects of these models refer to the text.
An alternative model for apical membrane Ca uptake during Ca absorption is that Ca flows through the L-type Ca channel Cav1.3 (Figure 3), a transporter activated by glucose-induced membrane depolarization following a meal (reviewed in [69]). However, several studies do not support a physiologic role of Cav1.3 for vitamin-D-regulated Ca absorption in growing mice [70,71]. In contrast, other studies suggest that Cav1.3 may have a prolactin-regulated role in transcellular Ca transport during lactation [72] and contributes to Ca absorption prior to the development of vitamin-D-regulated Ca absorption in the neonatal mouse [73].
The central player in the facilitated diffusion model is calbindin-D, a cytoplasmic Ca binding protein [59] found in intestine (the 9 kd form, calbindin D9k) and the kidney (the 28 kd form, calbindin D28k) [74] (Figure 2 and Figure 3). This was based on studies that show: (a) intestinal calbindin D9k protein levels are positively correlated to Ca absorption [59], (b) intestinal calbindin D9k levels are significantly reduced in vitamin D deficient animals and in VDR knockout mice [48,75], (c) 1,25(OH)2 D injections increase intestinal calbindin D9k levels [76], and (d) theophylline-mediated inhibition of Ca binding to calbindin D9k disrupts intestinal Ca absorption [77]. These observations led to the hypothesis that calbindin D acts as a ferry for intracellular Ca movement during Ca absorption [57,78]. In contrast, other studies indicate that calbindin D9k is not essential for intestinal Ca absorption but may instead act as an intracellular Ca buffer that protects cells from increases in intracellular Ca during Ca absorption. For example, neither basal nor 1,25(OH)2 D-induced Ca absorption are reduced in calbindin-D9k null mice [66,79]. In contrast, 1,25(OH)2 D-induced Ca absorption is reduced by 60% in calbindin-D9k/TRPV6 double knockout mice [66], suggesting the interaction of TRPV6 and calbindin-D9k has a special role in Ca absorption. Another observation that suggests elevated calbindin levels alone are not sufficient to drive intestinal Ca absorption is that calbindin-D protein remains elevated in the intestine even after 1,25(OH)2 D-induced Ca absorption returns to normal in chicks [80] and mice [33]. Finally, we have observed that intestinal calbindin-D9k levels increase in intestine-specific TRPV6 transgenic mice with elevated intestinal Ca absorption efficiency even in VDR knockout mice [68]. This suggests that calbindin-D9k is an intracellular Ca buffer that increases in response to elevated transcellular Ca absorption and that it is not induced to act as a facilitator of transcellular Ca movement.
The final step in the facilitated diffusion model is the extrusion of Ca from the cell. This is an energy dependent process [22] mediated by the plasma membrane Ca ATPase 1b (PMCA1b) [81,82] (Figure 2 and Figure 3). Deletion of the PMCA1b gene (Atp2b1), or the 4.1R protein that stabilizes PMCA1b in the basolateral membrane, reduces basal and 1,25(OH)2 D-induced intestinal Ca absorption [83,84]. While some suggest that the basolateral extrusion of Ca is also be mediated by a sodium-Ca exchanger [85], disrupting the sodium gradient necessary for sodium-Ca exchange did not block duodenal Ca transport [22].
Most intestinal Ca absorption research has focused on vitamin-D-regulated saturable Ca transport but several studies have shown that vitamin D signaling can increase Ca diffusion across the jejunum and ileum [20,86]. Tudpor et al. [87] found that 1,25(OH)2 D induced Ca ion movement across the intestinal barrier by a solvent drag mechanism that may involve charge selectivity of the tight junction. This is similar to the role of paracellin 1 (aka claudin 16), a tight junction protein that regulates ion-specific movement of magnesium and Ca in the kidney [88]. Consistent with this idea, 1,25(OH)2 D treatment significantly increased claudin-2 and 12 mRNA levels in Caco-2 cells and siRNA against these claudins reduced Ca permeability across Caco-2 cell monolayers [89]. In vivo, claudin 2/claudin 12 double knockout mice (but not single KO mice) have reduced Ca absorption across the colon but not small intestine [90]. Claudin-2 and -12 expression is highest in the distal small intestine [91] but essentially absent from the duodenum. A role for claudins in paracellular Ca transport may explain why the non-saturable component of ileal Ca absorption was reduced in chronic kidney disease patients with low serum 1,25(OH)2 D levels [20].
Two other models have been presented to explain vitamin D-mediated, transcellular intestinal Ca absorption: vesicular transport and transcaltachia. The vesicular transport model is an alternative to the role proposed for calbindin-D as a Ca ferry during transcellular intestinal Ca absorption. This is based on the observation that 1,25(OH)2 D treatment increased the activity and cycling of lysosomes [92,93], that Ca accumulates within brush border membrane endosomes [94] and in lysosomes [95] during Ca absorption, and that disrupting lysosomal pH prevents lysosomal Ca accumulation and blocks Ca absorption [95,96]. While these data suggest that vesicular movement may be a legitimate pathway for uptake and movement of Ca through the intestinal epithelial cell, it is not clear what makes the vesicular transport pathway specific for Ca. Transcaltachia is a mode of Ca transport that occurs within minutes of exposing the basolateral side of enterocytes to physiologic levels of 1,25(OH)2 D. Transcaltachia has been directly demonstrated in the perfused chick duodenum [97]. Some data suggests transcaltachia results from 1,25(OH)2 D binding to a unique, alternative ligand binding pocket [98,99] in VDR within caveolae [100], i.e., a novel non-nuclear role for the receptor. Other data suggest the basolateral membrane protein mediating transcaltachia is a multi-functional Membrane Associated Rapid Response Steroid receptor (MARRS). However, while intestine-specific deletion of MARRS in mice reduced cellular 1,25(OH)2 D binding, disrupted 1,25(OH)2 D regulated Ca and phosphate uptake into enterocytes [101,102] and reduced basal Ca absorption in by 30% [103], these reports have not reported the physiological impact of MARRS deletion on bone density. Additionally, the rapid fluxes in serum 1,25(OH)2 D needed for transcaltachia have not been reported during the consumption of Ca-rich meals when transcaltachia would have to occur for the physiologic benefit of the process to be realized. As such, transcaltachia is not a generally accepted mechanism for vitamin-D-regulated intestinal Ca absorption.
5. Physiologic Regulation of Vitamin D-Mediated Intestinal Ca Absorption
As it has been described above, the major physiologic condition where vitamin D signaling is engaged to regulate intestinal Ca absorption is the habitual consumption of a low Ca diet. However, there are a number of other physiologic states that affect vitamin D metabolism or action to influence intestinal Ca absorption, i.e., growth and development, pregnancy/lactation, and aging.
The bulk of mechanistic studies on intestinal Ca absorption have been conducted in growing 2–3-month-old rodents, but recent studies in mice indicate that vitamin D-mediated Ca absorption is not important prior to weaning [73]. This is not completely surprising as VDR is not expressed prior to 14d postnatally in rodents [104,105]. Ca absorption studies in premature infants also suggests that Ca absorption during late development is vitamin D independent [106]. Research suggests that during childhood and adolescence, growth hormone (GH) and its physiologic mediator insulin-like growth factor I (IGF-1) promote intestinal Ca absorption in two ways. The first effect is through activation of renal CYP27B1 and the elevation of serum 1,25(OH)2 D levels [107]. However, in adult animals, GH treatment increases intestinal Ca absorption without significantly increasing serum 1,25(OH)2 D levels [108]. It does so by modulating intestinal VDR levels and increasing cell sensitivity to 1,25(OH)2 D [109]. The effect of GH on Ca absorption is likely mediated through IGF-1 but the intestinal actions of IGF-1 may also be independent of vitamin D signaling [49,110].
Dietary Ca requirements increase significantly during the third trimester of pregnancy and during lactation to meet the needs of the fetus and term infant. While pregnancy causes a vitamin D-independent increase in Ca absorption whose mechanism is not clearly understood [111,112,113,114], during late pregnancy serum 1,25(OH)2 D levels and intestinal Ca absorption are both elevated [115]. This is because of PTH-independent 1,25(OH)2 D production by the placenta [116]. Ca absorption is also regulated during lactation in rodents (but not humans) but this is due to a prolactin-dependent mechanism [117,118]. However, prolactin cooperates with 1,25(OH)2D3 to regulate intestinal Ca transport and the expression of TRPV6 and calbindin-D9k in rats [119], suggesting prolactin acts together with 1,25(OH)2D3 to increase active intestinal Ca absorption.
Aging reduces Ca absorption efficiency [120,121,122,123,124,125]. Yet, despite the fact that age-associated Ca malabsorption was discovered 50 ago, we still do not know the molecular mechanism underlying this phenomenon. Lower serum 1,25(OH)2D levels in the elderly has been reported in some studies [126,127] but not others [128]. In fact, some research indicates that serum 1,25(OH)2 D is higher in older subjects even though fractional Ca absorption is not changed [128,129,130]. Similar age-associated intestinal resistance to 1,25(OH)2 D signaling has been formally demonstrated in rats [124] and humans [125]. Some evidence suggests that this phenomenon may be caused by lower intestinal VDR levels [130,131,132] but after adulthood is reached, age-related declines in intestinal VDR content are modest (−20%) [130,131] or non-existent [124]. Consistent with the lack of an impact of age on VDR expression, we recently reported that the open chromatin regions that control the expression of the intestinal VDR gene are not different between 3- and 21-mo-old mice, [104]. Thus, while my research group [53] has shown that a 50% reduction in intestinal VDR level blunts the intestinal response to elevated serum 1,25(OH)2D levels, the inconsistency in the reports on the impact of age on intestinal VDR levels suggests that other mechanisms may contribute to age-associated intestinal resistance to vitamin D.
Another aspect of aging that could negatively impact vitamin D metabolism or intestinal regulation of Ca absorption is the decline in sex hormone levels. Consistent with this, estrogen loss severely disrupts Ca metabolism in post-menopausal women, including reducing Ca absorption [133,134]. While estrogen signaling directly regulates intestinal Ca absorption [135,136,137], it also enhances the intestinal responsiveness to 1,25(OH)2 D [138]. Some [109,139,140], but not all [141], studies report that low estrogen levels reduce intestinal VDR levels and that this is responsible for intestinal vitamin D-resistance following estrogen loss. In prepubertal boys, testosterone therapy increased intestinal Ca absorption by 61% [142] and this was accompanied higher serum IGF-1 levels that might influence vitamin D metabolism. As men age, both Ca absorption efficiency and serum levels of the sulfated form of the testosterone prohormone DHEA, dehydroepiandrosterone sulphate (DHEAS), fall significantly [143]. However, the change in Ca absorption was independent of changes in serum 1,25(OH)2 D, suggesting changes in androgen signaling do not alter vitamin D metabolism. It is not known if testosterone regulates intestinal VDR levels.
6. Conclusions
A large amount of data supports the conclusion that adequate vitamin D status and adequate production of the metabolite 1,25(OH)2 D are needed to support transcellular, saturable intestinal Ca absorption. 1,25(OH)2 D regulates intestinal biology by activating the VDR to stimulate gene expression. This increases the maximum capacity of the Ca transport system by increasing levels of a transporter that mediates saturable, transcellular Ca transport. The saturable, vitamin-D-regulated component of intestinal Ca absorption plays a significant role in maintaining Ca absorption efficiency because in most individuals, serum 1,25(OH)2 D levels are elevated by habitually low dietary Ca intake that is common in the general population. The exact mechanism that describes Ca movement through the enterocyte is still in question. The facilitated diffusion model is the best characterized mechanism but there are some inconsistencies in the model that must be resolved through additional research. In contrast, research supports a model for regulated paracellular Ca movement through tight junctions that predominates across the ileum and in the early postnatal period. Overall, the data suggest that vitamin D signaling regulates intestinal Ca absorption by different mechanisms that are segment specific.
Conflicts of Interest
The authors have no conflict to declare.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Nicolaysen R. Studies upon the mode of action of vitamin D. III. the influence of vitamin D on the absorption of calcium and phosphorus in the rat. Biochem. J. 1937;37:122–129. doi: 10.1042/bj0310122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pansu D., Bellaton C., Roche C., Bronner F. Duodenal and ileal calcium absorption in the rat and effects of vitamin D. Am. J. Physiol. 1983;244:G695–G700. doi: 10.1152/ajpgi.1983.244.6.G695. [DOI] [PubMed] [Google Scholar]
- 3.Sheikh M.S., Ramirez A., Emmett M., Santa A.C., Schiller L.R., Fordtran J.S. Role of vitamin D-dependent and vitamin D-independent mechanisms in absorption of food calcium. J. Clin. Investig. 1988;81:126–132. doi: 10.1172/JCI113283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Holick M.F., Schnoes H.K., DeLuca H.F., Suda T., Cousins R.J. Isolation and identification of 1,25-dihydroxycholecalciferol. A metabolite of vitamin D active in intestine. Biochemistry. 1971;10:2799–2804. doi: 10.1021/bi00790a023. [DOI] [PubMed] [Google Scholar]
- 5.Norman A.W., Myrtle J.F., Midgett R.J., Nowicki H.G., Williams V., Popjak G. 1,25-dihydroxycholecalciferol: Identification of the proposed active form of vitamin D3 in the intestine. Science. 1971;173:51–54. doi: 10.1126/science.173.3991.51. [DOI] [PubMed] [Google Scholar]
- 6.Brumbaugh P.F., Haussler M.R. Nuclear and cytoplasmic receptors for 1,25-dihydroxycholecalciferol in intestinal mucosa. Biochem. Biophys. Res. Commun. 1973;51:74–80. doi: 10.1016/0006-291X(73)90509-3. [DOI] [PubMed] [Google Scholar]
- 7.Haussler M.R., Whitfield G.K., Haussler C.A., Hsieh J.C., Thompson P.D., Selznick S.H., Dominguez C.E., Jurutka P.W. The nuclear vitamin D receptor: Biological and molecular regulatory properties revealed. J. Bone Miner. Res. 1998;13:325–349. doi: 10.1359/jbmr.1998.13.3.325. [DOI] [PubMed] [Google Scholar]
- 8.Xue Y.B., Fleet J.C. Intestinal Vitamin D Receptor Is Required for Normal Calcium and Bone Metabolism in Mice. Gastroenterology. 2009;136:1317–1327. doi: 10.1053/j.gastro.2008.12.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aita R.A.D., Hassan S., Hur J., Pellon-Cardenas O., Cohen E., Chen L., Shroyer N., Christakos S., Verzi M., Fleet J.C. Genomic Analysis of 1,25-dihydroxyvitamin D3 Action in Mouse Intestine Reveals Compartment and Segment-Specific Gene Regulatory Effects. J. Biol. Chem. 2022;298:102213. doi: 10.1016/j.jbc.2022.102213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bronner F., Aubert J.P. Bone metabolism and regulation of the blood calcium level in rats. Am. J. Physiol. 1965;209:887–890. doi: 10.1152/ajplegacy.1965.209.5.887. [DOI] [PubMed] [Google Scholar]
- 11.Pansu D., Bellaton C., Bronner F. Effect of Ca intake on saturable and nonsaturable components of duodenal Ca transport. Am. J. Physiol. 1981;240:32–37. doi: 10.1152/ajpgi.1981.240.1.G32. [DOI] [PubMed] [Google Scholar]
- 12.Norman D.A., Fordtran J.S., Brinkley L.J., Zerwekh J.E., Nicar M.J., Strowig S.M., Pak C.Y. Jejunal and ileal adaptation to alterations in dietary calcium: Changes in calcium and magnesium absorption and pathogenetic role of parathyroid hormone and 1,25-dihydroxyvitamin D. J. Clin. Investig. 1981;67:1599–1603. doi: 10.1172/JCI110194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Favus M.J., Walling M.W., Kimberg D.V. Effects of dietary calcium restriction and chronic thyroparathyroidectomy on the metabolism of (3H)25-hydroxyvitamin D3 and the active transport of calcium by rat intestine. J. Clin. Investig. 1974;53:1139–1148. doi: 10.1172/JCI107652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zierold C., Mings J.A., DeLuca H.F. Regulation of 25-hydroxyvitamin D3-24-hydroxylase mRNA by 1,25-dihydroxyvitamin D3 and parathyroid hormone. J. Cell. Biochem. 2003;88:234–237. doi: 10.1002/jcb.10341. [DOI] [PubMed] [Google Scholar]
- 15.Armbrecht H.J., Boltz M.A., Hodam T.L. PTH increases renal 25(OH)D3-1alpha -hydroxylase (CYP1alpha) mRNA but not renal 1,25(OH)2D3 production in adult rats. Am. J. Physiol. Ren. Physiol. 2003;284:F1032–F1036. doi: 10.1152/ajprenal.00306.2002. [DOI] [PubMed] [Google Scholar]
- 16.Gershoff S.N., Hegsted D.M. Effect of vitamin D and Ca:P ratios on chick gastrointestinal tract. Am. J. Physiol. 1956;187:203–206. doi: 10.1152/ajplegacy.1956.187.2.203. [DOI] [PubMed] [Google Scholar]
- 17.Need A.G., O’Loughlin P.D., Morris H.A., Coates P.S., Horowitz M., Nordin B.E. Vitamin D Metabolites and Calcium Absorption in Severe Vitamin D Deficiency. J. Bone Miner. Res. 2008;23:1859–1863. doi: 10.1359/jbmr.080607. [DOI] [PubMed] [Google Scholar]
- 18.Wasserman R.H., Taylor A.N. Some aspects of the intestinal absorption of calcium, with special reference to vitamin D. In: Comar C.L., Bronner F., editors. Mineral Metabolism, An Advanced Treatise. Volume 3. Academic Press; New York, NY, USA: 1969. pp. 321–403. [Google Scholar]
- 19.Heaney R.P., Saville P.D., Recker R.R. Calcium absorption as a function of calcium intake. J. Lab. Clin. Med. 1975;85:881–890. [PubMed] [Google Scholar]
- 20.Sheikh M.S., Schiller L.R., Fordtran J.S. In vivo intestinal absorption of calcium in humans. Miner. Electrolyte Metab. 1990;16:130–146. [PubMed] [Google Scholar]
- 21.Wallace T.C., Reider C., Fulgoni V.L., 3rd Calcium and vitamin D disparities are related to gender, age, race, household income level, and weight classification but not vegetarian status in the United States: Analysis of the NHANES 2001–2008 data set. J. Am. Coll. Nutr. 2013;32:321–330. doi: 10.1080/07315724.2013.839905. [DOI] [PubMed] [Google Scholar]
- 22.Favus M.J., Angeid-Backman E., Breyer M.D., Coe F.L. Effects of trifluoperazine, ouabain, and ethacrynic acid on intestinal calcium. Am. J. Physiol. 1983;244:G111–G115. doi: 10.1152/ajpgi.1983.244.2.G111. [DOI] [PubMed] [Google Scholar]
- 23.Favus M.J., Kathpalia S.C., Coe F.L. Kinetic characteristics of calcium absorption and secretion by rat colon. Am. J. Physiol. 1981;240:G350–G354. doi: 10.1152/ajpgi.1981.240.5.G350. [DOI] [PubMed] [Google Scholar]
- 24.Grinstead W.C., Pak C.Y.C., Krejs G.J. Effect of 1,25-Dihydroxyvitamin-D3 on Calcium-Absorption in the Colon of Healthy Humans. Am. J. Physiol. 1984;247:G189–G192. doi: 10.1152/ajpgi.1984.247.2.G189. [DOI] [PubMed] [Google Scholar]
- 25.Karbach U., Feldmeier H. The cecum is the site with the highest calcium absorption in rat intestine. Dig. Dis. Sci. 1993;38:1815–1824. doi: 10.1007/BF01296104. [DOI] [PubMed] [Google Scholar]
- 26.Dhawan P., Veldurthy V., Yehia G., Hsaio C., Porta A., Kim K.I., Patel N., Lieben L., Verlinden L., Carmeliet G., et al. Transgenic Expression of the Vitamin D Receptor Restricted to the Ileum, Cecum, and Colon of Vitamin D Receptor Knockout Mice Rescues Vitamin D Receptor-Dependent Rickets. Endocrinology. 2017;158:3792–3804. doi: 10.1210/en.2017-00258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jiang H., Horst R.L., Koszewski N.J., Goff J.P., Christakos S., Fleet J.C. Targeting 1,25(OH)2D-mediated calcium absorption machinery in proximal colon with calcitriol glycosides and glucuronides. J. Steroid Biochem. Mol. Biol. 2020;198:105574. doi: 10.1016/j.jsbmb.2019.105574. [DOI] [PubMed] [Google Scholar]
- 28.Giuliano A.R., Wood R.J. Vitamin D-regulated calcium transport in Caco-2 cells: Unique in vitro model. Am. J. Physiol. 1991;260:G207–G212. doi: 10.1152/ajpgi.1991.260.2.G207. [DOI] [PubMed] [Google Scholar]
- 29.Fordtran J.S., Locklear T.W. Ionic constituents and osmolality of gastric and small-intestinal fluids after eating. Am. J. Dig. Dis. 1966;11:503–521. doi: 10.1007/BF02233563. [DOI] [PubMed] [Google Scholar]
- 30.Marcus C.S., Lengemann F.W. Absorption of Ca 45 and Sr 85 from solid and liquid food at various levels of the alimentary tract of the rat. J. Nut. 1962;77:155–160. doi: 10.1093/jn/77.2.155. [DOI] [PubMed] [Google Scholar]
- 31.Duflos C., Bellaton C., Pansu D., Bronner F. Calcium solubility, intestinal sojourn time and paracellular permeability codetermine passive calcium absorption in rats. J. Nutr. 1995;125:2348–2355. doi: 10.1093/jn/125.9.2348. [DOI] [PubMed] [Google Scholar]
- 32.Boyle I.T., Gray R.W., Omdahl J.L., DeLuca H.F., Schilling R.F. The mechanism of adaptation of intestinal calcium absorption to low dietary calcium. J. Lab. Clin. Med. 1971;78:813. [PubMed] [Google Scholar]
- 33.Song Y., Peng X., Porta A., Takanaga H., Peng J.B., Hediger M.A., Fleet J.C., Christakos S. Calcium transporter 1 and epithelial calcium channel messenger ribonucleic acid are differentially regulated by 1,25 dihydroxyvitamin D3 in the intestine and kidney of mice. Endocrinology. 2003;144:3885–3894. doi: 10.1210/en.2003-0314. [DOI] [PubMed] [Google Scholar]
- 34.Abrams S.A., Hicks P.D., Hawthorne K.M. Higher serum 25-hydroxyvitamin D levels in school-age children are inconsistently associated with increased calcium absorption. J. Clin. Endocrinol. Metab. 2009;94:2421–2427. doi: 10.1210/jc.2008-2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Abrams S.A., Hawthorne K.M., Chen Z. Supplementation with 1000 IU vitamin D/d leads to parathyroid hormone suppression, but not increased fractional calcium absorption, in 4-8-y-old children: A double-blind randomized controlled trial. Am. J. Clin. Nutr. 2013;97:217–223. doi: 10.3945/ajcn.112.046102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lewis R.D., Laing E.M., Hill Gallant K.M., Hall D.B., McCabe G.P., Hausman D.B., Martin B.R., Warden S.J., Peacock M., Weaver C.M. A randomized trial of vitamin D(3) supplementation in children: Dose-response effects on vitamin D metabolites and calcium absorption. J. Clin. Endocrinol. Metab. 2013;98:4816–4825. doi: 10.1210/jc.2013-2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hansen K.E., Johnson R.E., Chambers K.R., Johnson M.G., Lemon C.C., Vo T.N., Marvdashti S. Treatment of Vitamin D Insufficiency in Postmenopausal Women: A Randomized Clinical Trial. JAMA Intern. Med. 2015;175:1612–1621. doi: 10.1001/jamainternmed.2015.3874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bronner F., Pansu D. Nutritional aspects of calcium absorption. J. Nutr. 1999;129:9–12. doi: 10.1093/jn/129.1.9. [DOI] [PubMed] [Google Scholar]
- 39.Sempos C.T., Durazo-Arvizu R.A., Fischer P.R., Munns C.F., Pettifor J.M., Thacher T.D. Serum 25-hydroxyvitamin D requirements to prevent nutritional rickets in Nigerian children on a low-calcium diet-a multivariable reanalysis. Am. J. Clin. Nutr. 2021;114:231–237. doi: 10.1093/ajcn/nqab048. [DOI] [PubMed] [Google Scholar]
- 40.Fischer P.R., Almasri N.I. Nutritional rickets-Vitamin D and beyond. J. Steroid Biochem. Mol. Biol. 2022;219:106070. doi: 10.1016/j.jsbmb.2022.106070. [DOI] [PubMed] [Google Scholar]
- 41.Matkovic V., Fontana D., Tominac C., Goel P., Chesnut C.H. Factors that influence peak bone mass formation: A study of calcium balance and the inheritance of bone mass in adolescent females. Am. J. Clin. Nutr. 1990;52:878–888. doi: 10.1093/ajcn/52.5.878. [DOI] [PubMed] [Google Scholar]
- 42.Replogle R.A., Li Q., Wang L., Zhang M., Fleet J.C. Gene-by-Diet Interactions Influence Calcium Absorption and Bone Density in Mice. J. Bone Miner. Res. 2014;29:657–665. doi: 10.1002/jbmr.2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Patel M.B., Makepeace A.E., Jameson K.A., Masterson L.M., Holt R.I., Swaminathan R., Javaid M.K., Cooper C., Arden N.K. Weight in infancy and adult calcium absorption as determinants of bone mineral density in adult men: The hertfordshire cohort study. Calcif. Tissue Int. 2012;91:416–422. doi: 10.1007/s00223-012-9648-8. [DOI] [PubMed] [Google Scholar]
- 44.Reyes Fernandez P.C., Replogle R.A., Wang L., Zhang M., Fleet J.C. Novel Genetic Loci Control Calcium Absorption and Femur Bone Mass as Well as Their Response to Low Calcium Intake in Male BXD Recombinant Inbred Mice. J. Bone Miner. Res. 2016;31:994–1002. doi: 10.1002/jbmr.2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ensrud K.E., Duong T., Cauley J.A., Heaney R.P., Wolf R.L., Harris E., Cummings S.R. Low fractional calcium absorption increases the risk for hip fracture in women with low calcium intake. Study of Osteoporotic Fractures Research Group. Ann. Intern. Med. 2000;132:345–353. doi: 10.7326/0003-4819-132-5-200003070-00003. [DOI] [PubMed] [Google Scholar]
- 46.Haussler M.R., Norman A.W. Chromosomal receptor for a vitamin D metabolite. Proc. Natl. Acad. Sci. USA. 1969;62:155–162. doi: 10.1073/pnas.62.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tiosano D., Hadad S., Chen Z., Nemirovsky A., Gepstein V., Militianu D., Weisman Y., Abrams S.A. Calcium absorption, kinetics, bone density, and bone structure in patients with hereditary vitamin D-resistant rickets. J. Clin. Endocrinol. Metab. 2011;96:3701–3709. doi: 10.1210/jc.2011-1432. [DOI] [PubMed] [Google Scholar]
- 48.Van Cromphaut S.J., Dewerchin M., Hoenderop J.G., Stockmans I., Van Herck E., Kato S., Bindels R.J., Collen D., Carmeliet P., Bouillon R., et al. Duodenal calcium absorption in vitamin D receptor-knockout mice: Functional and molecular aspects. Proc. Natl. Acad. Sci. USA. 2001;98:13324–13329. doi: 10.1073/pnas.231474698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Song Y., Kato S., Fleet J.C. Vitamin D Receptor (VDR) Knockout Mice Reveal VDR-Independent Regulation of Intestinal Calcium Absorption and ECaC2 and Calbindin D9k mRNA. J. Nutr. 2003;133:374–380. doi: 10.1093/jn/133.2.374. [DOI] [PubMed] [Google Scholar]
- 50.Lieben L., Masuyama R., Torrekens S., Van Looveren R., Schrooten J., Baatsen P., Lafage-Proust M.H., Dresselaers T., Feng J.Q., Bonewald L.F., et al. Normocalcemia is maintained in mice under conditions of calcium malabsorption by vitamin D-induced inhibition of bone mineralization. J. Clin. Investig. 2012;122:1803–1815. doi: 10.1172/JCI45890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Amling M., Priemel M., Holzmann T., Chapin K., Rueger J.M., Baron R., Demay M.B. Rescue of the skeletal phenotype of vitamin D receptor ablated mice in the setting of normal mineral ion homeostasis: Formal histomorphometric and biomechanical analysis. Endocrinology. 1999;140:4982–4987. doi: 10.1210/endo.140.11.7110. [DOI] [PubMed] [Google Scholar]
- 52.Lieben L., Verlinden L., Masuyama R., Torrekens S., Moermans K., Schoonjans L., Carmeliet P., Carmeliet G. Extra-intestinal calcium handling contributes to normal serum calcium levels when intestinal calcium absorption is suboptimal. Bone. 2015;81:502–512. doi: 10.1016/j.bone.2015.08.023. [DOI] [PubMed] [Google Scholar]
- 53.Song Y., Fleet J.C. Intestinal Resistance to 1,25 Dihydroxyvitamin D in Mice Heterozygous for the Vitamin D Receptor Knockout Allele. Endocrinology. 2007;148:1396–1402. doi: 10.1210/en.2006-1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ames S.K., Ellis K.J., Gunn S.K., Copeland K.C., Abrams S.A. Vitamin D receptor gene Fok1 polymorphisms predicts calcium absorption and bone mineral density in children. J. Bone Miner. Res. 1999;14:740–746. doi: 10.1359/jbmr.1999.14.5.740. [DOI] [PubMed] [Google Scholar]
- 55.Jurutka P.W., Remus L.S., Whitfield K., Thompson P.D., Hsieh J.C., Zitzer H., Tavakkoli P., Galligan M.A., Dang H.T.L., Haussler C.A., et al. The polymorphic N terminus in human vitamin D receptor isoforms influences trascriptional activity by modulating interaction with transcription factor IIB. Mol. Endocrinol. 2000;14:401–420. doi: 10.1210/mend.14.3.0435. [DOI] [PubMed] [Google Scholar]
- 56.Huang Z.W., Dong J., Piao J.H., Li W.D., Tian Y., Xu J., Yang X.G. Relationship between the absorption of dietary calcium and the Fok I polymorphism of VDR gene in young women. Zhonghua Yu Fang Yi Xue Za Zhi. 2006;40:75–78. [PubMed] [Google Scholar]
- 57.Chandra S., Fullmer C.S., Smith C.A., Wasserman R.H., Morrison G.H. Ion microscopic imaging of calcium transport in the intestinal tissue of vitamin D-deficient and vitamin D-replete chickens: A 44Ca stable isotope study. Proc. Natl. Acad. Sci. USA. 1990;87:5715–5719. doi: 10.1073/pnas.87.15.5715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fullmer C.S., Chandra S., Smith C.A., Morrison G.H., Wasserman R.H. Ion microscopic imaging of calcium during 1,25-dihydroxyvitamin D-mediated intestinal absorption. Histochem. Cell. Biol. 1996;106:215–222. doi: 10.1007/BF02484403. [DOI] [PubMed] [Google Scholar]
- 59.Bronner F., Pansu D., Stein W.D. An analysis of intestinal calcium transport across the rat intestine. Am. J. Physiol. 1986;250:G561–G569. doi: 10.1152/ajpgi.1986.250.5.G561. [DOI] [PubMed] [Google Scholar]
- 60.Peng J.B., Chen X.Z., Berger U.V., Vassilev P.M., Tsukaguchi H., Brown E.M., Hediger M.A. Molecular cloning and characterization of a channel-like transporter mediated intestinal calcium absorption. J. Biol. Chem. 1999;274:22739–22746. doi: 10.1074/jbc.274.32.22739. [DOI] [PubMed] [Google Scholar]
- 61.Meyer M.B., Zella L.A., Nerenz R.D., Pike J.W. Characterizing early events associated with the activation of target genes by 1,25-dihydroxyvitamin D3 in mouse kidney and intestine in vivo. J. Biol. Chem. 2007;282:22344–22352. doi: 10.1074/jbc.M703475200. [DOI] [PubMed] [Google Scholar]
- 62.Fleet J.C., Eksir F., Hance K.W., Wood R.J. Vitamin D-inducible calcium transport and gene expression in three Caco-2 cell lines. Am. J. Physiol. 2002;283:G618–G625. doi: 10.1152/ajpgi.00269.2001. [DOI] [PubMed] [Google Scholar]
- 63.Lee S.M., Riley E.M., Meyer M.B., Benkusky N.A., Plum L.A., DeLuca H.F., Pike J.W. 1,25-Dihydroxyvitamin D3 Controls a Cohort of Vitamin D Receptor Target Genes in the Proximal Intestine That Is Enriched for Calcium-regulating Components. J. Biol. Chem. 2015;290:18199–18215. doi: 10.1074/jbc.M115.665794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Meyer M.B., Watanuki M., Kim S., Shevde N.K., Pike J.W. The human transient receptor potential vanilloid type 6 distal promoter contains multiple vitamin D receptor binding sites that mediate activation by 1,25-dihydroxyvitamin D3 in intestinal cells. Mol. Endocrinol. 2006;20:1447–1461. doi: 10.1210/me.2006-0031. [DOI] [PubMed] [Google Scholar]
- 65.Kutuzova G.D., Sundersingh F., Vaughan J., Tadi B.P., Ansay S.E., Christakos S., DeLuca H.F. TRPV6 is not required for 1alpha,25-dihydroxyvitamin D3-induced intestinal calcium absorption in vivo. Proc. Natl. Acad. Sci. USA. 2008;105:19655–19659. doi: 10.1073/pnas.0810761105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Benn B.S., Ajibade D., Porta A., Dhawan P., Hediger M., Peng J.B., Jiang Y., Oh G.T., Jeung E.B., Lieben L., et al. Active intestinal calcium transport in the absence of transient receptor potential vanilloid type 6 and calbindin-D9k. Endocrinology. 2008;149:3196–3205. doi: 10.1210/en.2007-1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Woudenberg-Vrenken T.E., Lameris A.L., Weissgerber P., Olausson J., Flockerzi V., Bindels R.J., Freichel M., Hoenderop J.G. Functional TRPV6 channels are crucial for transepithelial Ca2+ absorption. Am. J. Physiol. Gastrointest. Liver Physiol. 2012;303:G879–G885. doi: 10.1152/ajpgi.00089.2012. [DOI] [PubMed] [Google Scholar]
- 68.Cui M., Li Q., Johnson R., Fleet J.C. Villin promoter-mediated transgenic expression of transient receptor potential cation channel, subfamily V, member 6 (TRPV6) increases intestinal calcium absorption in wild-type and vitamin D receptor knockout mice. J. Bone Miner. Res. 2012;27:2097–2107. doi: 10.1002/jbmr.1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kellett G.L. Alternative perspective on intestinal calcium absorption: Proposed complementary actions of Ca(v)1.3 and TRPV6. Nutr. Rev. 2011;69:347–370. doi: 10.1111/j.1753-4887.2011.00395.x. [DOI] [PubMed] [Google Scholar]
- 70.Reyes-Fernandez P.C., Fleet J.C. Luminal glucose does not enhance active intestinal calcium absorption in mice: Evidence against a role for Ca(v)1.3 as a mediator of calcium uptake during absorption. Nutr. Res. 2015;35:1009–1015. doi: 10.1016/j.nutres.2015.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Li J., Zhao L., Ferries I.K., Jiang L., Desta M.Z., Yu X., Yang Z., Duncan R.L., Turner C.H. Skeletal phenotype of mice with a null mutation in Cav 1.3 L-type calcium channel. J. Musculoskelet. Neuronal Interact. 2010;10:180–187. [PubMed] [Google Scholar]
- 72.Nakkrasae L.I., Thongon N., Thongbunchoo J., Krishnamra N., Charoenphandhu N. Transepithelial calcium transport in prolactin-exposed intestine-like Caco-2 monolayer after combinatorial knockdown of TRPV5, TRPV6 and Ca(v)1.3. J. Physiol. Sci. 2010;60:9–17. doi: 10.1007/s12576-009-0068-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Beggs M.R., Lee J.J., Busch K., Raza A., Dimke H., Weissgerber P., Engel J., Flockerzi V., Alexander R.T. TRPV6 and Cav1.3 Mediate Distal Small Intestine Calcium Absorption Before Weaning. Cell. Mol. Gastroenterol. Hepatol. 2019;8:625–642. doi: 10.1016/j.jcmgh.2019.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Christakos S., Gill R., Lee S., Li H. Molecular aspects of the calbindins. J. Nutr. 1992;122:678–682. doi: 10.1093/jn/122.suppl_3.678. [DOI] [PubMed] [Google Scholar]
- 75.Wasserman R.H., Taylor A.N. Vitamin D3-induced calcium-binding proteins in chick intestinal mucosa. Science. 1966;252:791–793. doi: 10.1126/science.152.3723.791. [DOI] [PubMed] [Google Scholar]
- 76.Bronner F., Buckley M. The molecular nature of 1,25-(OH)2-D3-induced calcium-binding protein biosynthesis in the rat. Adv. Exp. Med. Biol. 1982;151:355–360. doi: 10.1007/978-1-4684-4259-5_41. [DOI] [PubMed] [Google Scholar]
- 77.Pansu D., Bellaton C., Roche C., Bronner F. Theophylline inhibits active Ca transport in rat intestine by inhibiting Ca binding by CaBP. Prog. Clin. Biol. Res. 1988;252:115–120. [PubMed] [Google Scholar]
- 78.Feher J.J., Fullmer C.S., Wasserman R.H. Role of facilitated diffusion of calcium by calbindin in intestinal calcium absorption. Am. J. Physiol. 1992;262:C517–C526. doi: 10.1152/ajpcell.1992.262.2.C517. [DOI] [PubMed] [Google Scholar]
- 79.Akhter S., Kutuzova G.D., Christakos S., DeLuca H.F. Calbindin D9k is not required for 1,25-dihydroxyvitamin D3-mediated Ca2+ absorption in small intestine. Arch. Biochem. Biophys. 2007;460:227–232. doi: 10.1016/j.abb.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 80.Spencer R., Charman M., Wilson P.W., Lawson D.E.M. The relationship between vitamin D-stimulated calcium transport and intestinal calcium-binding protein in the chicken. Biochem. J. 1978;170:93–101. doi: 10.1042/bj1700093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wasserman R.H., Smith C.A., Brindak M.E., Detalamoni N., Fullmer C.S., Penniston J.T., Kumar R. Vitamin-D and mineral deficiencies increase the plasma membrane calcium pump of chicken intestine. Gastroenterology. 1992;102:886–894. doi: 10.1016/0016-5085(92)90174-W. [DOI] [PubMed] [Google Scholar]
- 82.Cai Q., Chandler J.S., Wasserman R.H., Kumar R., Penniston J.T. Vitamin D and adaptation to dietary calcium and phosphate deficiencies increase intestinal plasma membrane calcium pump gene expression. Proc. Natl. Acad. Sci. USA. 1993;90:1345–1349. doi: 10.1073/pnas.90.4.1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Liu C., Weng H., Chen L., Yang S., Wang H., Debnath G., Guo X., Wu L., Mohandas N., An X. Impaired intestinal calcium absorption in protein 4.1R-deficient mice due to altered expression of plasma membrane calcium ATPase 1b (PMCA1b) J. Biol. Chem. 2013;288:11407–11415. doi: 10.1074/jbc.M112.436659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ryan Z.C., Craig T.A., Filoteo A.G., Westendorf J.J., Cartwright E.J., Neyses L., Strehler E.E., Kumar R. Deletion of the intestinal plasma membrane calcium pump, isoform 1, Atp2b1, in mice is associated with decreased bone mineral density and impaired responsiveness to 1, 25-dihydroxyvitamin D3. Biochem. Biophys. Res. Commun. 2015;467:152–156. doi: 10.1016/j.bbrc.2015.09.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.van Corven E.J., Roche C., Van Os C.H. Distribution of Ca2+-ATPase, ATP-dependent Ca2+-transport, calmodulin and vitamin D-dependent Ca2+-binding protein along the villus-crypt axis in rat duodenum. Biochim. Biophys. Acta. 1985;820:274–282. doi: 10.1016/0005-2736(85)90121-X. [DOI] [PubMed] [Google Scholar]
- 86.Karbach U. Paracellular Calcium Transport Across the Small Intestine. J. Nutr. 1992;122:672–677. doi: 10.1093/jn/122.suppl_3.672. [DOI] [PubMed] [Google Scholar]
- 87.Tudpor K., Teerapornpuntakit J., Jantarajit W., Krishnamra N., Charoenphandhu N. 1,25-dihydroxyvitamin d(3) rapidly stimulates the solvent drag-induced paracellular calcium transport in the duodenum of female rats. J. Physiol. Sci. 2008;58:297–307. doi: 10.2170/physiolsci.RP002308. [DOI] [PubMed] [Google Scholar]
- 88.Simon D.B., Lu Y., Choate K.A., Velazquez H., Al-Sabban E., Praga M., Casari G., Bettinelli A., Colussi G., Rodriquez-Soriano J., et al. Paracellin-1, a renal tight junction protein required for paracellular Mg 2+ resorption. Science. 1999;285:103–106. doi: 10.1126/science.285.5424.103. [DOI] [PubMed] [Google Scholar]
- 89.Fujita H., Sugimoto K., Inatomi S., Maeda T., Osanai M., Uchiyama Y., Yamamoto Y., Wada T., Kojima T., Yokozaki H., et al. Tight junction proteins claudin-2 and-12 are critical for vitamin D-dependent Ca2+ absorption between enterocytes. Mol. Biol. Cell. 2008;19:1912–1921. doi: 10.1091/mbc.e07-09-0973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Beggs M.R., Young K., Pan W., O’Neill D.D., Saurette M., Plain A., Rievaj J., Doschak M.R., Cordat E., Dimke H., et al. Claudin-2 and claudin-12 form independent, complementary pores required to maintain calcium homeostasis. Proc. Natl. Acad. Sci. USA. 2021;118:e2111247118. doi: 10.1073/pnas.2111247118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Fujita H., Chiba H., Yokozaki H., Sakai N., Sugimoto K., Wada T., Kojima T., Yamashita T., Sawada N. Differential expression and subcellular localization of claudin-7, -8, -12, -13, and -15 along the mouse intestine. J. Histochem. Cytochem. 2006;54:933–944. doi: 10.1369/jhc.6A6944.2006. [DOI] [PubMed] [Google Scholar]
- 92.Davis W.L., Jones R.G. Lysosomal proliferation in rachitic avian intestinal absorptive cells following 1,25-dihydroxycholecalciferol. Tissue Cell. 1982;14:585–595. doi: 10.1016/0040-8166(82)90049-0. [DOI] [PubMed] [Google Scholar]
- 93.Nemere I., Szego C.M. Early actions of parathyroid hormone and 1,25-dihydroxycholecalciferol on isolated epithelial cells from rat intestine: 1. Limited lysosomal enzyme release and calcium uptake. Endocrinology. 1981;108:1450–1462. doi: 10.1210/endo-108-4-1450. [DOI] [PubMed] [Google Scholar]
- 94.Nemere I., Norman A.W. 1,25-Dihydroxyvitamin D3-mediated vesicular transport of calcium in intestine: Time-course studies. Endocrinology. 1988;122:2962–2969. doi: 10.1210/endo-122-6-2962. [DOI] [PubMed] [Google Scholar]
- 95.Nemere I., Leathers V., Norman A.W. 1, 25 dihydroxyvitamin D3-mediated intestinal calcium transport. Biochemical identification of lysozomes containing calcium and calcium-binding protein (calbindin-D 28k ) J. Biol. Chem. 1986;261:16106–16114. doi: 10.1016/S0021-9258(18)66684-0. [DOI] [PubMed] [Google Scholar]
- 96.Favus M.J., Tembe V., Tanklefsky M.D., Ambrosic K.A., Nellans H.N. Effects of quinacrine on calcium active transport by rat intestinal epithelium. Am. J. Physiol. 1989;257:G818–G822. doi: 10.1152/ajpgi.1989.257.5.G818. [DOI] [PubMed] [Google Scholar]
- 97.Nemere I., Yoshimoto Y., Norman A.W. Calcium transport in perfused duodena from normal chicks: Enhancement within fourteen minutes of exposure to 1,25 dihydroxyvitamin D3. Endocrinology. 1984;115:1476–1483. doi: 10.1210/endo-115-4-1476. [DOI] [PubMed] [Google Scholar]
- 98.Mizwicki M.T., Keidel D., Bula C.M., Bishop J.E., Zanello L.P., Wurtz J.M., Moras D., Norman A.W. Identification of an alternative ligand-binding pocket in the nuclear vitamin D receptor and its functional importance in 1alpha,25(OH)2-vitamin D3 signaling. Proc. Natl. Acad. Sci. USA. 2004;101:12876–12881. doi: 10.1073/pnas.0403606101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Norman A.W., Bishop J.E., Bula C.M., Olivera C.J., Mizwicki M.T., Zanello L.P., Ishida H., Okamura W.H. Molecular tools for study of genomic and rapid signal transduction responses initiated by 1 alpha,25(OH)(2)-vitamin D(3) Steroids. 2002;67:457–466. doi: 10.1016/S0039-128X(01)00167-2. [DOI] [PubMed] [Google Scholar]
- 100.Huhtakangas J.A., Olivera C.J., Bishop J.E., Zanello L.P., Norman A.W. The vitamin D receptor is present in caveolae-enriched plasma membranes and binds 1 alpha,25(OH)(2)-vitamin D-3 in vivo and in vitro. Mol. Endocrinol. 2004;18:2660–2671. doi: 10.1210/me.2004-0116. [DOI] [PubMed] [Google Scholar]
- 101.Nemere I., Garbi N., Hammerling G.J., Khanal R.C. Intestinal cell calcium uptake and the targeted knockout of the 1,25D3-MARRS (membrane-associated, rapid response steroid-binding) receptor/PDIA3/Erp57. J. Biol. Chem. 2010;285:31859–31866. doi: 10.1074/jbc.M110.116954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Nemere I., Garcia-Garbi N., Hammerling G.J., Winger Q. Intestinal cell phosphate uptake and the targeted knockout of the 1,25D(3)-MARRS receptor/PDIA3/ERp57. Endocrinology. 2012;153:1609–1615. doi: 10.1210/en.2011-1850. [DOI] [PubMed] [Google Scholar]
- 103.Nemere I., Garbi N., Hammerling G., Hintze K.J. Role of the 1,25D(3)-MARRS receptor in the 1,25(OH)(2)D(3)-stimulated uptake of calcium and phosphate in intestinal cells. Steroids. 2012;77:897–902. doi: 10.1016/j.steroids.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 104.Fleet J.C., Aldea D., Chen L., Christakos S., Verzi M. Regulatory domains controlling high intestinal vitamin D receptor gene expression are conserved in mouse and human. J. Biol. Chem. 2022;298:101616. doi: 10.1016/j.jbc.2022.101616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Pierce E.A., DeLuca H.F. Regulation of the intestinal 1,25-dihydroxyvitamin D3 receptor during neonatal development in the rat. Arch. Biochem. Biophys. 1988;261:241–249. doi: 10.1016/0003-9861(88)90338-4. [DOI] [PubMed] [Google Scholar]
- 106.Bronner F., Salle B.L., Putet G., Rigo J., Senterre J. Net calcium absorption in premature infants: Results of 103 metabolic balance studies. Am. J. Clin. Nutr. 1992;56:1037–1044. doi: 10.1093/ajcn/56.6.1037. [DOI] [PubMed] [Google Scholar]
- 107.Zoidis E., Gosteli-Peter M., Ghirlanda-Keller C., Meinel L., Zapf J., Schmid C. IGF-I and GH stimulate Phex mRNA expression in lungs and bones and 1,25-dihydroxyvitamin D(3) production in hypophysectomized rats. Eur. J. Endocrinol. 2002;146:97–105. doi: 10.1530/eje.0.1460097. [DOI] [PubMed] [Google Scholar]
- 108.Fleet J.C., Bruns M.E., Hock J.M., Wood R.J. Growth hormone and parathyroid hormone stimulate intestinal calcium absorption in aged female rats. Endocrinology. 1994;134:1755–1760. doi: 10.1210/endo.134.4.8137740. [DOI] [PubMed] [Google Scholar]
- 109.Chen C., Noland K.A., Kalu D.N. Modulation of intestinal vitamin D receptor by ovariectomy, estrogen and growth hormone. Mech. Ageing Dev. 1997;99:109–122. doi: 10.1016/S0047-6374(97)00094-8. [DOI] [PubMed] [Google Scholar]
- 110.Fatayerji D., Mawer E.B., Eastell R. The role of insulin-like growth factor I in age-related changes in calcium homeostasis in men. J. Clin. Endocrinol. Metab. 2000;85:4657–4662. doi: 10.1210/jc.85.12.4657. [DOI] [PubMed] [Google Scholar]
- 111.Quan-Sheng D., Miller S.C. Calciotrophic hormone levels and calcium absorption during pregnancy in rats. Am. J. Physiol. 1989;257:E118–E123. doi: 10.1152/ajpendo.1989.257.1.E118. [DOI] [PubMed] [Google Scholar]
- 112.Halloran B.P., DeLuca H.F. Calcium-Transport in Small-Intestine During Pregnancy and Lactation. Am. J. Physiol. 1980;239:E64–E68. doi: 10.1152/ajpendo.1980.239.1.E64. [DOI] [PubMed] [Google Scholar]
- 113.Brommage R., Baxter D.C., Gierke L.W. Vitamin D-independent intestinal calcium and phosphorus absorption during reproduction. Am. J. Physiol. 1990;259:631–638. doi: 10.1152/ajpgi.1990.259.4.G631. [DOI] [PubMed] [Google Scholar]
- 114.Fudge N.J., Kovacs C.S. Pregnancy up-regulates intestinal calcium absorption and skeletal mineralization independently of the vitamin D receptor. Endocrinology. 2010;151:886–895. doi: 10.1210/en.2009-1010. [DOI] [PubMed] [Google Scholar]
- 115.Ritchie L.D., Fung E.B., Halloran B.P., Turnlund J.R., Van Loan M.D., Cann C.E., King J.C. A longitudinal study of calcium homeostasis during human pregnancy and lactation and after resumption of menses. Am. J. Clin. Nutr. 1998;67:693–701. doi: 10.1093/ajcn/67.4.693. [DOI] [PubMed] [Google Scholar]
- 116.Breslau N.A., Zerwekh J.E. Relationship of estrogen and pregnancy to calcium homeostasis in pseudohypoparathyroidism. J. Clin. Endocrinol. Metab. 1986;62:45–51. doi: 10.1210/jcem-62-1-45. [DOI] [PubMed] [Google Scholar]
- 117.Robinson C.J., Spanos E., James M.F., Pike J.W., Haussler M.R., Makeen A.M., Hillyard C.J., MacIntyre I. Role of Prolactin in Vitamin-D Metabolism and Calcium-Absorption During Lactation in the Rat. J. Endocrinol. 1982;94:443–453. doi: 10.1677/joe.0.0940443. [DOI] [PubMed] [Google Scholar]
- 118.Pahuja D.N., DeLuca H.F. Stimulation of Intestinal Calcium-Transport and Bone Calcium Mobilization by Prolactin in Vitamin-D-Deficient Rats. Science. 1981;214:1038–1039. doi: 10.1126/science.7302575. [DOI] [PubMed] [Google Scholar]
- 119.Ajibade D.V., Dhawan P., Fechner A.J., Meyer M.B., Pike J.W., Christakos S. Evidence for a role of prolactin in calcium homeostasis: Regulation of intestinal transient receptor potential vanilloid type 6, intestinal calcium absorption, and the 25-hydroxyvitamin D(3) 1alpha hydroxylase gene by prolactin. Endocrinology. 2010;151:2974–2984. doi: 10.1210/en.2010-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Avioli L.V., McDonald J.E., Lee S.W. The influence of age on the intestinal absorption of 47Ca in women and its relation to 47Ca absorption in postmenopausal osteoporosis. J. Clin. Investig. 1965;44:1960–1967. doi: 10.1172/JCI105302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Bullamore J.R., Gallagher J.C., Wilkinson R., Nordin B.E.C., Marshall D.H. Effect of age on calcium absorption. Lancet. 1970;2:535–537. doi: 10.1016/S0140-6736(70)91344-9. [DOI] [PubMed] [Google Scholar]
- 122.Armbrecht H.J., Zenser T.V., Bruns M.E., Davis B.B. Effect of age on intestinal calcium absorption and adaptation to dietary calcium. Am. J. Physiol. 1979;236:E769–E774. doi: 10.1152/ajpendo.1979.236.6.E769. [DOI] [PubMed] [Google Scholar]
- 123.Ireland P., Fordtran J.S. Effect of dietary calcium and age on jejunal calcium absorption in humans studied by intestinal perfusion. J. Clin. Investig. 1973;52:2672–2681. doi: 10.1172/JCI107461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wood R.J., Fleet J.C., Cashman K., Bruns M.E., DeLuca H.F. Intestinal calcium absorption in the aged rat: Evidence of intestinal resistance to 1,25(OH)2 vitamin D. Endocrinology. 1998;139:3843–3848. doi: 10.1210/endo.139.9.6176. [DOI] [PubMed] [Google Scholar]
- 125.Pattanaungkul S., Riggs B.L., Yergey A.L., Vieira N.E., O’Fallon W.M., Khosla S. Relationship of intestinal calcium absorption to 1,25-dihydroxyvitamin D [1,25(OH)2D] levels in young versus elderly women: Evidence for age- related intestinal resistance to 1,25(OH)2D action. J. Clin. Endocrinol. Metab. 2000;85:4023–4027. doi: 10.1210/jc.85.11.4023. [DOI] [PubMed] [Google Scholar]
- 126.Gallagher J.C., Riggs B.L., Eisman J., Hamstra A., Arnaud S.B., DeLuca H.F. Intestinal calcium absorption and serum vitamin D metabolites in normal subjects and osteoporotic patients: Effect of age and dietary calcium. J. Clin. Investig. 1979;64:729–736. doi: 10.1172/JCI109516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Fujisawa Y., Kida K., Matsuda H. Role of change in vitamin D metabolism with age in calcium and phosphorus metabolism in normal human subjects. J. Clin. Endocrinol. Metab. 1984;59:719–726. doi: 10.1210/jcem-59-4-719. [DOI] [PubMed] [Google Scholar]
- 128.Scopacasa F., Wishart J.M., Horowitz M., Morris H.A., Need A.G. Relation between calcium absorption and serum calcitriol in normal men: Evidence for age-related intestinal resistance to calcitriol. Eur. J. Clin. Nutr. 2004;58:264–269. doi: 10.1038/sj.ejcn.1601777. [DOI] [PubMed] [Google Scholar]
- 129.Eastell R., Yergey A.L., Vieira N.E., Cedel S.L., Kumar R., Riggs B.L. Interrelationship among vitamin-D metabolism, true calcium absorption, parathyroid function, and age in women—Evidence of an age-related intestinal resistance to 1,25-dihydroxyvitamin-D action. J. Bone Min. Res. 1991;6:125–132. doi: 10.1002/jbmr.5650060205. [DOI] [PubMed] [Google Scholar]
- 130.Ebeling P.R., Sandgren M.E., Dimagno E.P., Lane A.W., DeLuca H.F., Riggs B.L. Evidence of an age-related decrease in intestinal responsiveness to vitamin-D—Relationship between serum 1,25-dihydroxyvitamin-D3 and intestinal vitamin-D receptor concentrations in normal women. J. Clin. Endocrinol. Metab. 1992;75:176–182. doi: 10.1210/jcem.75.1.1320048. [DOI] [PubMed] [Google Scholar]
- 131.Takamoto S., Seino Y., Sacktor B., Liang C.T. Effect of age on duodenal 1,25-dihydroxyvitamin D-3 receptors in Wistar rats. Biochim. Biophys. Acta. 1990;1034:22–28. doi: 10.1016/0304-4165(90)90148-P. [DOI] [PubMed] [Google Scholar]
- 132.Horst R.L., Goff J.P., Reinhardt T.A. Advancing age results in reduction of intestinal and bone 1,25 dihydroxyvitamin D receptor. Endocrinology. 1990;126:1053–1057. doi: 10.1210/endo-126-2-1053. [DOI] [PubMed] [Google Scholar]
- 133.Heaney R.P., Recker R.R., Saville P.D. Menopausal changes in calcium balance performance. J. Lab. Clin. Med. 1978;92:953–963. doi: 10.1111/j.1753-4887.1983.tb07709.x. [DOI] [PubMed] [Google Scholar]
- 134.Riggs B.L., Khosla S., Melton L.J., III Sex steroids and the construction and conservation of the adult skeleton. Endocr. Rev. 2002;23:279–302. doi: 10.1210/edrv.23.3.0465. [DOI] [PubMed] [Google Scholar]
- 135.Ten Bolscher M., Netelenbos J.C., Barto R., Van Buuren L.M., Van der vijgh W.J. Estrogen regulation of intestinal calcium absorption in the intact and ovariectomized adult rat. J. Bone Miner. Res. 1999;14:1197–1202. doi: 10.1359/jbmr.1999.14.7.1197. [DOI] [PubMed] [Google Scholar]
- 136.Van Abel M., Hoenderop J.G., van der Kemp A.W., van Leeuwen J.P., Bindels R.J. Regulation of the Epithelial Ca2+ Channels in Small Intestine as Studied by Quantitative mRNA Detection. Am. J. Physiol. Gastrointest. Liver Physiol. 2003;285:G78–G85. doi: 10.1152/ajpgi.00036.2003. [DOI] [PubMed] [Google Scholar]
- 137.Van Cromphaut S.J., Rummens K., Stockmans I., Van Herck E., Dijcks F.A., Ederveen A., Carmeliet P., Verhaeghe J., Bouillon R., Carmeliet G. Intestinal calcium transporter genes are upregulated by estrogens and the reproductive cycle through vitamin D receptor-independent mechanisms. J. Bone Miner. Res. 2003;18:1725–1736. doi: 10.1359/jbmr.2003.18.10.1725. [DOI] [PubMed] [Google Scholar]
- 138.Gennari C., Agnusdei D., Nardi P., Civitelli R. Estrogen preserves a normal intestinal responsiveness to 1,25-dihydroxyvitamin D3 in oophorectomized women. J. Clin. Endocrinol. Metab. 1990;71:1288–1293. doi: 10.1210/jcem-71-5-1288. [DOI] [PubMed] [Google Scholar]
- 139.Arjmandi B.H., Holis B.W., Kalu D.N. In vivo effect of 17 b-estradiol on intestinal calcium absorption in rats. Bone Miner. 1994;26:181–189. doi: 10.1016/S0169-6009(08)80062-1. [DOI] [PubMed] [Google Scholar]
- 140.Liel Y., Shany S., Smirnoff P., Schwartz B. Estrogen increases 1,25-dihydroxyvitamin D receptors expression and bioresponse in the rat duodenal mucosa. Endocrinology. 1999;140:280–285. doi: 10.1210/endo.140.1.6408. [DOI] [PubMed] [Google Scholar]
- 141.Colin E.M., Van Den Bemd G.J., Van Aken M., Christakos S., De Jonge H.R., Deluca H.F., Prahl J.M., Birkenhager J.C., Buurman C.J., Pols H.A., et al. Evidence for involvement of 17beta-estradiol in intestinal calcium absorption independent of 1,25-dihydroxyvitamin D3 level in the Rat. J. Bone Miner. Res. 1999;14:57–64. doi: 10.1359/jbmr.1999.14.1.57. [DOI] [PubMed] [Google Scholar]
- 142.Mauras N., Haymond M.W., Darmaun D., Vieira N.E., Abrams S.A., Yergey A.L. Calcium and protein kinetics in prepubertal boys. Positive effects of testosterone. J. Clin. Investig. 1994;93:1014–1019. doi: 10.1172/JCI117049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Chen R.Y., Nordin B.E., Need A.G., Scopacasa F., Wishart J., Morris H.A., Horowitz M. Relationship between calcium absorption and plasma dehydroepiandrosterone sulphate (DHEAS) in healthy males. Clin. Endocrinol. 2008;69:864–869. doi: 10.1111/j.1365-2265.2008.03272.x. [DOI] [PubMed] [Google Scholar]