Summary
Even though coagulopathy is a familiar entity in trauma, its relationship to burn injury remains unclear. Literature appears inconsistent as to the conclusions of the use of coagulation assays, either routine methods or newer viscoelastic coagulation assays (VCAs), thromboelastography (TEG) and rotational thromboelastometry (ROTEM), for prediction of patients’ coagulation status and mortality. The use of diagnostic assays as mortality markers will be of great importance, since they would recognize at early stages patients with great medical demands and objectify burn injury severity. The aim of this study was to review the literature and evaluate burn patients’ characteristics and coagulation markers in the early post burn period. The secondary outcome was to investigate the role of different coagulation assays in mortality prognosis. Literature search was performed using PubMed, ScienceDirect, Wiley Online Library, Google Scholar, Proquest Dissertation and Theses Global, Scopus and Cochrane Library databases. All types of articles referring to adults with any type of burn injury admitted in the first 24h assessing coagulation and mortality were included. PRISMA guidelines ensured the evidence-based process. Eleven studies met the eligibility criteria. This review demonstrated the indubitable relationship of coagulopathy with burn injury and its significant impact on mortality. The rapid and dynamic process of coagulation makes standard coagulation assays unable to detect short-lived haemostatic changes. More susceptible markers such as VCAs need to be applied to the routine assessment of burn patients in order to obtain an overview on coagulopathy and standardize the gained knowledge.
Keywords: coagulation, burn, mortality, coagulopathy, systematic review
Abstract
Alors que la coagulopathie est bien décrite chez le traumatisé, sa relation avec la brûlure reste floue. La littérature reste élusive en ce qui concerne l’utilité des tests de routine, des tests viscoélastiques (TVE) et des thrombo- élastogrammes « ancien » (TEG) et optimisé (ROTEM) pour évaluer les troubles de coagulation et prédire la mortalité chez les brûlés. Hors, il s’agit d’une donnée importante pour prévoir la charge en soin (et l’éventuelle futilité- NDRLF) à prévoir. Cette étude a pour but de caractériser les patients brûlés et leurs troubles de coagulation, comme décrits dans la littérature, ainsi que d’évaluer la précision pronostique des différents tests de coagulation. La recherche bibliographique a été faite sur PubMed, Science Direct, Wiley Online Library, Google Scholar, Proquest Dissertation and Theses Global, Scopus et Cochrane. Toutes les études, pour peu qu’elles concernent des adultes, quel que soit le type de la brûlure, admis dans les 24 h, se penchant sur la coagulation et la mortalité étaient éligibles (11 au total). Les pré- requis PRISMA ont été utilisés pour juger de la pertinence. Il existe clairement une relation entre brûlure et coagulopathie, comme entre sa survenue et la mortalité. La rapidité des changements rend les tests standard peu utiles, si bien que les TVE ou les thrombo- élastogrammes sont nécessaires, ne serait- ce que pour préciser la coagulopathie des brûlés et, éventuellement, y remédier
Introduction
Burn patients are an incomparable population as their injury initiates inflammatory pathways which disturb the balance between the activity of coagulation factors and fibrinolytic activity.1,2 Since the first published article by Blonska and Kamienski in 1957, who described “coagulation disorders in burns”,3 lots of studies have been done focusing on the hematological profile of these patients. However, there is still significant research interest in this field.
Coagulopathy is a familiar entity in trauma patients, since 25% of severely injured patients are coagulopathic on admission.4 It seems that coagulopathy associated with burn injury is divided into two entities: traumatic coagulopathy due to systemic inflammatory response, hypothermia, platelet dysfunction and tissue injury, and iatrogenic coagulopathy due to resuscitation, hemodilution and blood loss after surgical excision.4-8 Both types may lead to serious venothromboembolic complications.9,10
Standard coagulation assays, such as prothrombin time (PT) and activated partial thromboplastin time (aPTT) are indirect indicators of extrinsic and intrinsic clotting pathway respectively. They are highly used in daily medical routine and direct critical interventions. 11,12 Laboratory signs of hypocoagulation are associated with mortality in critically ill patients.12 Likewise, a shortened aPTT on admission is associated with an increased risk of in‐hospital mortality.13 On the contrary, recent studies depict the inability of these assays to identify differences in coagulation profile, as they are small snapshots of an enormous and dynamic coagulation pathway.14,15
Viscoelastic coagulation assays (VCAs), such as thromboelastography (TEG) and rotational thromboelastometry (ROTEM) are gaining ground in the diagnosis of coagulopathy by analyzing all stages of hemostasis, including clotting time (CT) and clot structure/strength in whole blood samples.15,16 Information obtained from VCAs refer to clot formation, clot progress and its lysis, qualitative platelet function and fibrinolysis.17 Though a signature chart, TEG/ROTEM evaluate the three phases of haemostasis: initiation phase with R or CT value, that depicts the time from start to initial clot formation, depending on clotting factors, amplification phase though K value, which is the necessary time to achieve a certain level of clot strength and finally propagation phase though alpha angle (α-angle) assessing the rate of clot formation. Time to maximum amplitude (TMA – MA) estimates the stability of the clot regarding platelets and fibrin, amplitude at 30 / 60 min (A30, A60 or LY30, L60) refers to the fibrinolysis at 30 and 60 min post-MA, while CLT stands for clot lysis time.17 In critically ill patients they are considered the best perioperative monitoring tests, since they can detect both transfusion needs and bleeding disorders.12,18
Concerning burn injury, the existing literature appears inconsistent as to the conclusions of the use of different coagulation assays, either PT/PTT/INR or TEG/ROTEM, for prediction of patients’ coagulation status and their impact on overall survival. The potential use of routine assays as mortality markers will be of great importance, since they would recognize at early stages patients with great medical demands and objectify burn injury severity.
The aim of this study was to review the literature and evaluate burn patients’ characteristics and coagulation markers in the early post burn period. The secondary outcome was to investigate the role of different coagulation assays in mortality prognosis. The ultimate goal is to summarize the current state of research and identify future research needs in an effort to contribute in the future to drafting diagnostic and therapeutic guidelines.
Materials and methods
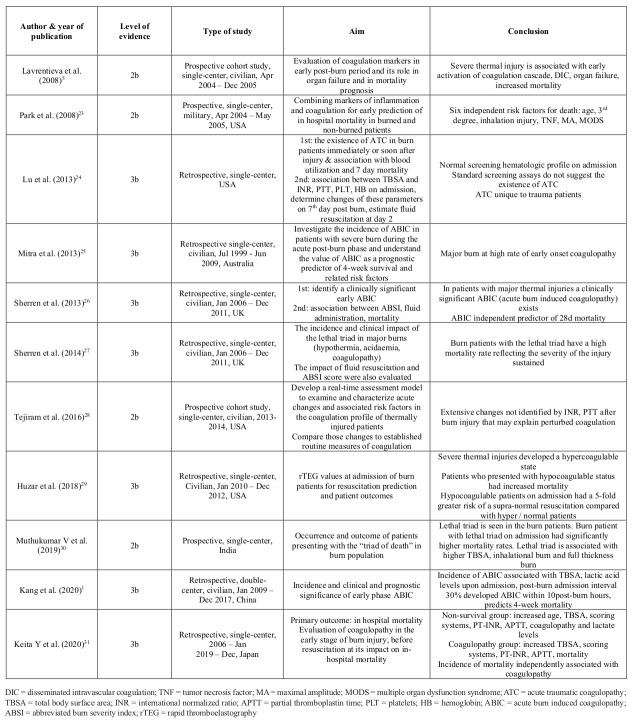
A manual search was conducted by one author in March 2021 in PubMed, ScienceDirect, Wiley Online Library, Google Scholar, Proquest Dissertation and Theses Global, Scopus and Cochrane Library databases. Using the terms: “burn” AND “coagulation” AND “mortality”, 360 articles were identified, in the period 1980-2020 (Table I). All types of articles in English, referring to adult patients with any type of burn injury, admitted the first 24h post burn, assessing coagulation and clinical outcome, were included. After removal of duplicates, the articles were evaluated. Animal studies, pediatric and trauma patients were not included. Lastly, abstracts of these articles were reviewed and evaluated according to inclusion and exclusion criteria. Titles and abstracts were assessed by two independent reviewers. Discussion solved any disagreements. Fifteen full-text articles were assessed, of which eleven studies met all inclusion criteria, and were thus selected for the qualitative synthesis. The procedure is presented in Fig. 1.
Table I. Search strategy used for each database with the corresponding results.
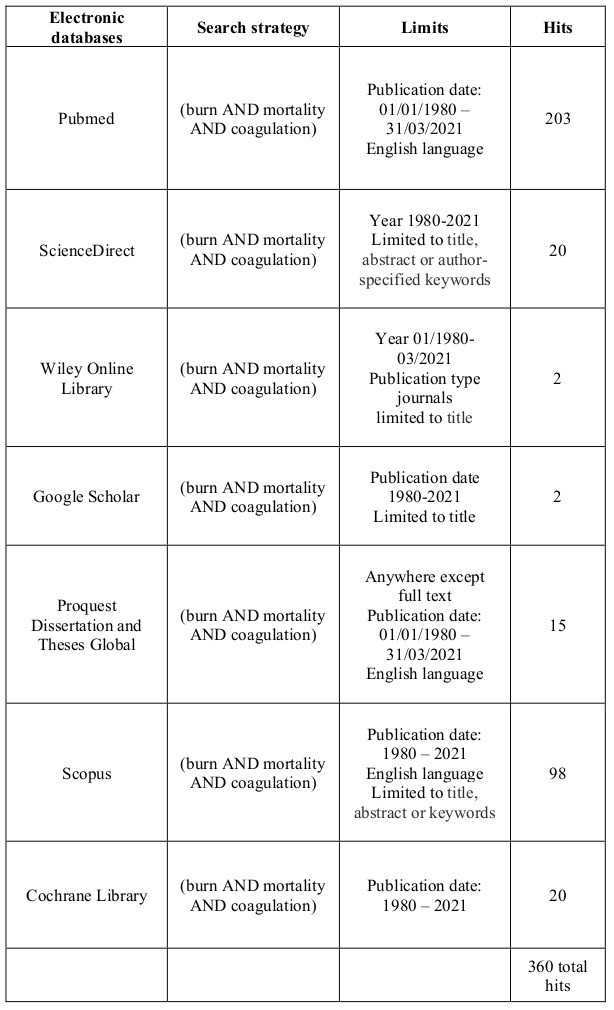
Fig. 1. Prisma 2009 Flaw Diagram.
Due to the novelty of the topic, no randomized controlled studies were reported. Since systematic reviews of cohort studies are acceptable,19 these types of studies which met the inclusion criteria were included.
PRISMA guidelines were applied in order to ensure the evidence-based process.20 Critical Appraisal Skills Programme (CASP) checklists evaluated studies for their validity and results.21 Table II summarizes the articles that met the inclusion criteria. Assessment of risk of bias relied on the New Castle-Ottawa.22 “High quality” studies were assigned 7–8 stars scale for cohorts, “moderate quality” 5–6 stars and “low quality” any number of stars below these, which were excluded by protocol.
Table II. Summary of article characteristics, aim and main conclusion.
Regarding synthesis results, data were summarized and considered suitable for pooling if similar groups were reported. Log Odds Ratios with 95% confidence intervals would be computed for dichotomous outcomes. A random–effects model was chosen, as proposed by DerSimonian and Laird. Between–study heterogeneity is calculated by the I2 statistic.
Results
Search results
The type of each study, date of publication, aim, conclusion endpoints, and level of evidence were data extracted and presented. A total of 360 related citations were found. Duplicates were removed. Title and abstract evaluation according to inclusion and exclusion criteria was conducted by two independent reviewers. Eleven full-text articles comprising 928 patients were included according to the eligibility criteria.
Study characteristics
From the critical appraisal of the 11 studies1,5,23-30,31 (Table II), we report four level 2b (282 patients)5,23,28,30 and seven level 3b (636 patients)1,24-27,29,31 studies.
There were no randomized controlled trials identified (Table II). Four studies were conducted in the USA,22,23,27,28 three in Europe,5,26,27 one in Australia25, one in India30, one in China1 and one in Japan.31 All but one study1 were single-centered.5,23-31 Despite the variety of geographical distribution, the coagulation tests were in accordance. The eligibility criteria were clearly reported, increasing the applicability of the results.
Participant characteristics
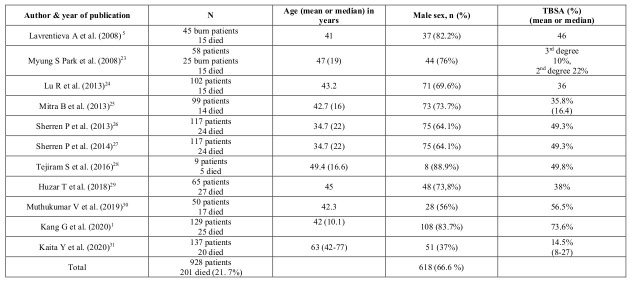
The review included 928 patients (Table II). A total of 201 deaths occurred (21.7%). Participants were selected during defined dates of admission. All studies involved the adult population, apart from Sherren et al. who also included children26,27 and Kang et al. who reduced the age limit to up to sixty years old.1 All studies referred to burn patients apart from Park et al.23 who used 25 burn and 33 non-burn trauma patients for the model development. Sherren et al.’s26,27 studies seemed to refer to the same population, since similar demographics and characteristics were identified. Nevertheless, both studies were included as different outcomes were measured. The sample size ranged from 9 patients to 137 patients. The median age of patients ranged from 34.7 years to 63 years. Of the 928 patients, 618 were males (66.6%), a fact that may affect the generalizability of the evidence. For the studies with recorded data,1,5,24-31 there were 125 coagulopathic non-survivors (Table III).
Table III. Patient characteristics and total body surface area (TBSA).
Extent and type of burn injury
Total burn surface area (TBSA) ranged from 14.5%31 to 100%(Table III).24 Apart from Park et al.23 and Kaita et al.31 who did not specify TBSA in the inclusion criteria, all other studies had a clear cut-off point. Two studies included burn patients with a TBSA higher than 15%.24,29 Mitra et al.5 used as a cut-off point a TBSA greater than 20%, which was characterized as severe burn injury. On the contrary, both Lavrentieva et al.5 and Tejiram et al.28 included burn patients with TBSA >25%. For Sherren et al.26,27 the threshold was 30%, whereas for Kang G. et al.1 and Muthukumar V et al.30 it reached 50%. Unfortunately, the lack of a clearly formulated definition of severe burn injury based on TBSA resulted in arbitrary interpretations that may affect consistency of the outcomes. Finally, seven studies gave an extensive report on full-thickness burn injuries.1,23,26,27,29,30,31 All eleven studies included inhalation injury. Two studies didn’t clarify the exact mechanism of burn injury.23,25 Six studies included only thermal injury patients,5,26,27-30 one study excluded patients with electrical injury,24 Kaita et al.31 excluded chemical and electrical injuries, whereas Kang et al.1 included all types of burn injury.
Coagulation markers and definition of coagulopathy
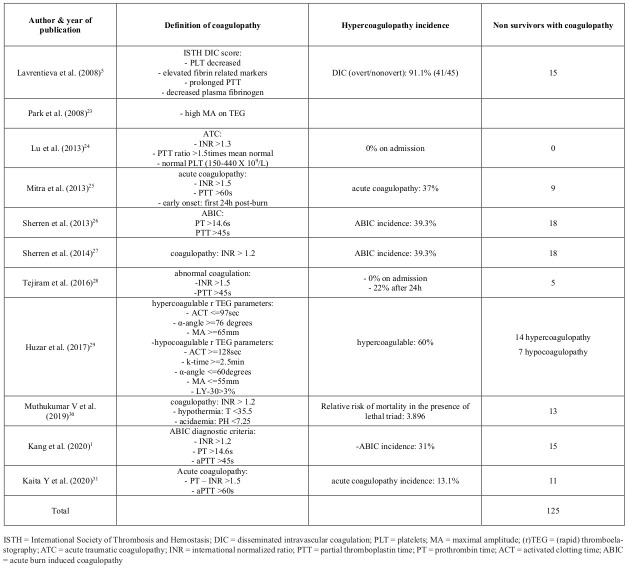
Every study analyzed the coagulation profile of the participants through standard coagulation assays, such as prothrombin time (PT), activated partial thromboplastin time (aPTT), international normalized ratio (INR)1,5,24,28 or newer viscoelastic coagulation assays (VCAs), such as thromboelastography (TEG).23,29 Throughout the eleven studies, different definitions of coagulopathy were used. They are summarized in Table IV. Lavrentieva et al.5 used the ISTH DIC scoring system. Eight studies preferred routine coagulation assays for the evaluation of the hemostatic status, with different cut-off points.1,24-28,30,31 Park et al.22 and Huzar et al.29 used TEG and r-TEG respectively.
Outcomes
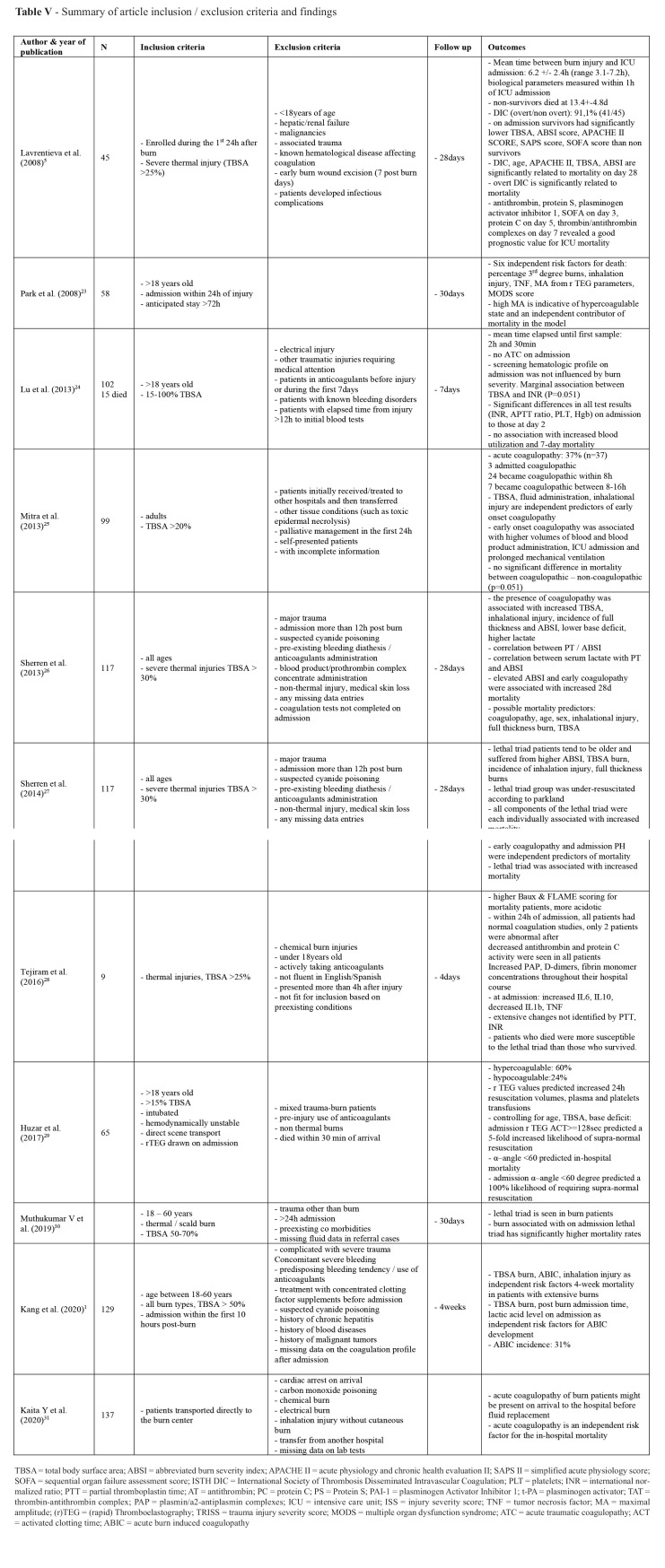
Clear research questions were stated to all eleven studies with relevant results responding to each of them (Table V). Apart from Lu et al.,24 all other studies depicted a hypercoagulopathy in the early post-burn period. Mitra et al.25 found no difference in mortality between coagulopathic – non-coagulopathic patients, with a marginal p (p=0.051). Eight studies concluded that coagulopathy is associated with mortality of burn patients alone, or as part of the lethal triad – acidosis, coagulopathy, hypothermia.1,5,23,26-28,30 Other parameters that are significantly related to mortality are: age,5,26,30,31 sex,26 APACHE II,5 TBSA,1,5,26,30,31 ABSI,5,26,30,31 percentage 3rd degree burns,23,26,30,31 inhalation injury,1,23,26,30 TNF,23 MA from r TEG parameters,23 MODS score,23 acidosis,27,28,30,31 hypothermia,27,28,30 Baux score,28,31 FLAME score28 BI, PBI and BOBI score,31 WBC.31 On the contrary, Huzar et al.29concluded that an α–angle <60 degrees on rTEG on admission, which stands for hypocoagulability, predicted in-hospital mortality. Predictors of coagulopathy are: TBSA,1,25,26,31 full thickness burn injury,26,31 fluid administration,25 inhalational injury,25,26,31 ABSI score,26,31 lower base deficit,26 higher lactate,1,26,31 and post burn admission time.1 Coagulation incidence ranged from 0%24,28 to 91.1%1 regarding mean time of measurements from admission. Early onset coagulopathy was associated with higher volumes of blood and blood product administration,25 ICU admission25 and prolonged mechanical ventilation.25 Despite the fact that Lu et al.24 did not find any relationship between coagulopathy and mortality, significant differences were noted in all test results (INR, APTT ratio, PLT, Hgb) from admission to those at day 2. Furthermore, supra-normal resuscitation was predicted from an α–angle <60 degree on admission1 and an ACT>=128s.29
Meta-analysis
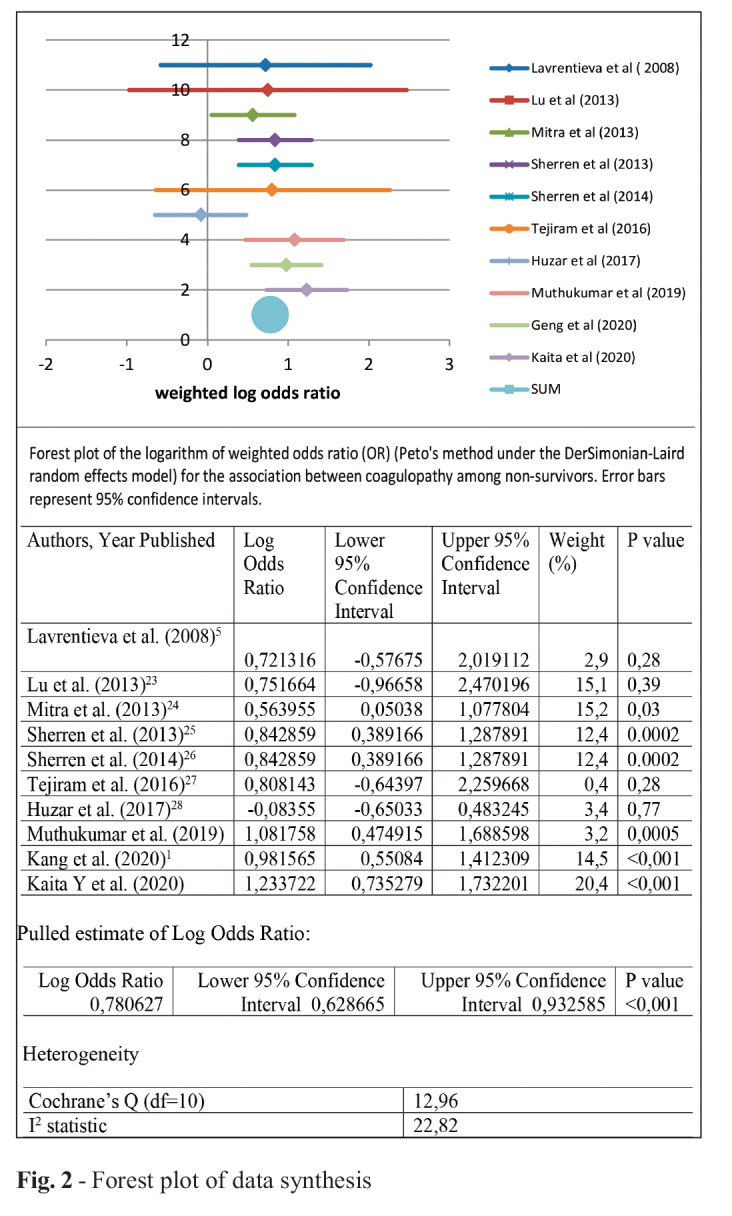
Coagulopathy was modeled as a risk factor for mortality. Logarithm of odds ratio and the corresponding 95% confidence intervals were calculated. The majority of the studies showed an OR for coagulopathy for non-survivors. Only one study showed negative log OR, contradicting the results of other studies. Yet this value was accompanied with patients with both hyper and hypo coagulation status, due to examination of their coagulation profile with rTEG. Other studies supported increased odds for coagulopathy. Summarized log OR under random effects model was 0,78 (95% CI=0,63-0.93). Heterogeneity based on Cochran’s Q for the studies was 12,96 (p=0,02), giving an I2 value of 22,82 (Fig. 2).
Table IV. Coagulopathy among included studies.
Table IV. Summary of article inclusion / exclusion criteria and findings.
Fig. 2. Forest plot of data synthesis.
Discussion
Burn patients in the ICU are high-risk patients with particular problems with coagulation. The upto-date published papers are limited but needed to be reviewed before beginning a new research. The primary outcome was to summarize burn patients’ characteristics and coagulation markers in the early post burn period and the secondary outcome was to investigate the coagulation profile of these patients in relation to mortality rates. This systematic review of the literature included eleven prospective and retrospective cohort studies. No randomized control studies were found.
Definition of coagulopathy & mortality
In order to detect coagulation abnormalities, routine assays were used from the majority of the included studies.1,5,24-28,30,31,32 For diagnosis of coagulopathy, several thresholds were used among them. One study referred to “disseminated intravascular coagulopathy” (DIC) in burns, according to the ISTH criteria for DIC, with an incidence of 91.1%.5 Another study borrowed from trauma patients the term “acute traumatic coagulopathy” in order to describe burn coagulation dysfunction.24 The thresholds were: INR greater than 1.3, aPTT ratio >1.5 times mean normal and normal PLT (150-440 X 109/L).24 In contrast with the previous study, no patient met these criteria on admission.24 On the other hand, Mitra et al.25 modified the thresholds of INR>1.5 and aPTT>60s according to recent studies of acute traumatic coagulopathies.30 The same rigorous thresholds were used by Kaita et al.31 “Early onset” was defined as coagulopathy in the first 24h post burns injury.25 In this study, 37% of patients developed early onset coagulopathy, 71% of them were coagulopathic within 8h and 20.6% between 8-16h post-burn. For Kaita et al. 18% were coagulopathic on admission, prior to fluid administration. 31 Sherren et al.,26,27 Kang et al.1 and Muthukumar et al.’s30 studies used the same cut-off points: INR>1.2, PT>14.6s, aPTT>45s for acute burn induced coagulopathy (ABIC). These cut-off values were consistent with the International Definition of Acute Traumatic Coagulopathy (ATC) by Davenport et al.33 The incidence was 39.3% and 31% respectively. For Tejiram et al.28 abnormal coagulation was defined as: INR>1.5 and PTT>45s, with 0% incidence on admission, 22% after. It is obvious that different thresholds in the diagnostic criteria are responsible for the inconsistency of the results. On the contrary, rTEG analysis of whole blood sample concluded that almost a quarter of patients were hypocoagulopathic on admission, which was also associated with higher mortality rates, even though the majority of the patients developed hypercoagulable status.29 For studies with given data, a total of 768 burn patients, 170 deaths occurred, with 124 fulfilling criteria of coagulopathy (72.9%). This means that the majority of non-survivors were coagulopathic. Despite the differences in definition of coagulopathy among the eleven studies, we cannot ignore this huge percentage of coagulopathy in non-survivors. This review is the first that gathers information on non-survivors’ hematological profile and exports a significant outcome that is difficult to be ignored, dismissing the arguments on burn coagulopathy. A prospective study on burn patients, comparing the accuracy of predicting coagulopathy throughout INR/PTT and TEG/ROTEM would be of great interest.
Severity of burn injury
The severity of the burn injury played an important role in the appearance and the onset time of coagulopathy. 25,26,33 In a retrospective study of 3331 patients, 14% of whom had burns >20% of TBSA,34 0.1% developed DIC, whereas in a prospective study of 45 patients, DIC was diagnosed in 91.1% with TBSA >25%.5 In another study of burn patients with TBSA >15%, no case of acute coagulopathy was found,24 another study with mean TBSA of 14.5% had 13.1% incidence of acute coagulopathy on admission, whereas in a study of patients with TBSA >30%, 39% met the defined criteria. In addition, the timing of the onset of coagulopathy was associated with the severity of the burn.25,26 King et al. concluded that burns greater than 6% TBSA induced a systemic hypercoagulability, whereas severe burns of TBSA over 40% induced consumptive coagulopathy.35 In a retrospective study, patients with TBSA >30%26 (mean TBSA 49.3%), became coagulopathic earlier than patients with lower mean TBSA of 35.8%.25 Also, a recent prospective study of patients with a mean TBSA of 49.8% concluded that 0% of admitted patients were coagulopathic and 22% became abnormal after.28 It is obvious that the degree of induction of coagulopathy seems to be proportional to the TBSA%.35 Also, the post burn admission time and the time of measurements greatly differentiated the result. Finally the existence of full thickness burn area23,26 and inhalational injury25,26 augmented the possibility of coagulopathy and an unfavorable outcome.
Acidosis, fluid resuscitation and hypothermia
Other parameters that seemed to influence the hematological status of burn patients were acidosis1,26,28,30,31, fluid resuscitation25,30,31 and hypothermia. 26,28,30 Base deficit and lactate are associated with tissue damage and according to previous studies are markers of morbidity and mortality.38,39-41 Furthermore, longer periods of acidosis and lower PH on admission are associated with activation of coagulation and mortality in burn populations.26,28 It is undeniable that resuscitation during the first 24 h post-burn is critical since it determines survival.36 Kaita et al.31 investigated the existence of coagulopathy before fluid administration. They concluded that acute coagulopathy might be present before fluid replacement and it is an independent risk factor for in-hospital mortality. 31 On the contrary, over-resuscitation was associated with augmented morbidity and mortality.36,37 Mitra et al. added to this knowledge that high volumes of crystalloid and colloid fluids were an independent predictor of early onset coagulopathy.25 It is obvious that the existence of a marker acting as a guide for fluid administration would be of great importance. Huzar et al. using the more sensitive to coagulation disorders marker, r-TEG, concluded that hypocoagulopathic burn patients on admission are at higher demand for resuscitation and transfusion requirements.29 Furthermore, Huzar et al. suggested that admission active clotting time (ACT) and α-angle could be the missing markers that could predict resuscitation needs since admission r TEG ACT>=128s predicted a 5-fold increased likelihood of supra-normal resuscitation and admission α–angle <60 degree predicted a 100% likelihood of requiring supra-normal resuscitation. Finally, it is well known that skin damage is associated with hypothermia leading to coagulation disorders, even from the first minutes post-burn. This hypothermia contributes to coagulopathy and as independent factor worsens acidosis.42-44
Surgical interventions
From escharotomies and fasciotomies for the avoidance of compartment syndrome, to debridement, excision and grafting, all types of surgical interventions affect the patient’s mutable status. In order to eliminate this confounding factor, Lavrentieva et al.5 chose to exclude patients who underwent any surgical intervention at the early post-burn period (first 7 days), whereas others chose to record them in association with blood product requirements. 23,24 For Lu et al.24 more than half of the patients had some kind of surgery (56.8%) within the first 7 days after burn injury, and almost half of them required blood products. Unfortunately, the effect of surgery could not be depicted. As an exogenous factor of bleeding with usually high demands of blood products, surgery has a great involvement on coagulation profile, probably by altering the burn injury per se needs. A recent study measuring blood transfusion in major burn injury in the operating room summarized that intraoperative monitoring and VCAs combined with a restricted transfusion strategy decreases by 50% transfusion needs, with no impact on morbidity–mortality compared to liberal transfusion policy, in accordance with similar studies. 45-50 Finally, Lu et al.24 chose to include ten patients who did not receive active resuscitation and were treated with palliative measures due to their critical situation. This antidiametric to surgery “treatment” speeded up the studied population to the upper limits, clearly influencing the results.
Other coagulation markers
Apart from standard coagulation assays and viscoelastic parameters, other coagulation markers were also analyzed from two studies.5,28 Anticoagulant activity was decreased through antithrombin and protein C in all patients. These parameters also revealed a good prognostic value for ICU mortality.5 Furthermore, an augmentation of clot formation and fibrinolytic activity was observed throughout the patient’s hospital course, since increased PAP, Ddimers, fibrin monomer concentrations were found.27 These extensive changes were not identified by PTT, INR. It seems that these changes alter clot microstructure, leading to a weaker, fibrin clot.51,52 A recent study measuring plasma fibrin degradation product (FDP) together with routine coagulative parameters and their relations with burn-severity concluded that the first increased significantly in burn patients, showing better and consistent correlation with burn-severity than routine coagulation parameters. 53
Limitations and strengths of the study
No randomized controlled studies on burn patients were found. Cohort studies had clear eligibility criteria but small n-designs. Only one study used a validated control group for model development.23 Studies also used different cut-off values and different tests, rendering the result interpretation and association difficult. Inclusion criteria differentiated between the included studies giving great heterogeneity of the studied population. From the age range, follow up time, to the burn type and the time of admission or enrollment, there were considerable deviations. Despite the heterogeneity of the studies, important information was gained with a great clinical impact, pointing the way for future well-designed studies and the establishment of clear guidelines. Through this review we demonstrate the indubitable relationship between coagulopathy with burn injury and its significant impact on mortality.
Conclusion
Burn coagulopathy is an indisputable fact, which should be taken seriously into consideration regarding mortality from burn injury. A clear definition of this entity should be given for burn patients and recorded guidelines ought to be institutionalized for resuscitation and blood product needs. The rapid and dynamic process of coagulation makes standard coagulation assays unable to detect short-lived hemostatic changes. As a result, their use may underestimate the severity of the situation. More susceptible markers such as viscoelastic coagulation assays (VCAs) need to be applied to the daily and routine assessment of burn patients in order to obtain an overview of burn coagulopathy and standardize the gained knowledge. Additional prospective studies with clear scientific questions are necessary to clarify the bleak but intriguing field of burn coagulopathy.
References
- 1.Kang G, Yonglin L, Yuting Y, Xiaobin D. Incidence and prognostic value of acute coagulopathy after extensive severe burns. J Burn Care Res. 2020;41(3):544–549. doi: 10.1093/jbcr/irz178. [DOI] [PubMed] [Google Scholar]
- 2.Lang TC, Zhao R, Kim A, Wijewardena A. Plasma protein C levels are directly associated with better outcomes in patients with severe burns. Burns. 2019;45:1659–1672. doi: 10.1016/j.burns.2019.05.001. [DOI] [PubMed] [Google Scholar]
- 3.Blonska X, Kamienski R. Coagulation disorders in burns. Pol Tyg Lek (Wars) 1957;12(30):1151–1153. [PubMed] [Google Scholar]
- 4.Wiegele M, Schaden E, Koch S, Bauer D. Thrombin generation in patients with severe thermal injury. Burns. 2019;45:54–62. doi: 10.1016/j.burns.2018.09.020. [DOI] [PubMed] [Google Scholar]
- 5.Lavrentieva A, Kontakiotis T, Bitzani M, Papaioannou-Gaki G. Early coagulation disorders after severe burn injury: impact on mortality. Intensive Care Med. 2008;34:700–706. doi: 10.1007/s00134-007-0976-5. [DOI] [PubMed] [Google Scholar]
- 6.Lippo G, Ippolito L, Cervellin G. Disseminated intravascular coagulation in burn injury. Semin Thromb Hemost. 2010;36:429–416. doi: 10.1055/s-0030-1254051. [DOI] [PubMed] [Google Scholar]
- 7.Glas GJ, Levi M, Schultz MJ. Coagulopathy and its management in patients with severe burns. J Thromb Haemost. 2016;14:865–874. doi: 10.1111/jth.13283. [DOI] [PubMed] [Google Scholar]
- 8.Midura EF, Kuethe JW, Rice TC, Veile R. Impact of platelets and platelet-derived microparticles on hypercoagulability following burn injury. Shock. 2016;45:82–87. doi: 10.1097/SHK.0000000000000460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Park MS, Martini WZ, Dubick MA, Salinas J. Thromboelastography as a better indicator of hypercoagulable state after injury than prothrombin time or activated partial thromboplastin time. J Trauma. 2009;67(2):266–276. doi: 10.1097/TA.0b013e3181ae6f1c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Geerts WH, Code KI, Jay RM, Chen E, Szalai JP. A prospective study of venous thromboembolism after major trauma. N Engl J Med. 1994;331(24):1601–1606. doi: 10.1056/NEJM199412153312401. [DOI] [PubMed] [Google Scholar]
- 11.Haas T, Fries D, Tanaka KA, Asmis L. Usefulness of standard plasma coagulation tests in the management of perioperative coagulopathic bleeding: is there any evidence? Br J Anaesth. 2015;114:217–224. doi: 10.1093/bja/aeu303. [DOI] [PubMed] [Google Scholar]
- 12.Larsson A, Tynngård N, Kander T, Bonnevier J, Schött U. Comparison of point-of-care hemostatic assays, routine coagulation tests, and outcome scores in critically ill patients. J Crit Care. 2015;30(5):1032–1038. doi: 10.1016/j.jcrc.2015.06.014. [DOI] [PubMed] [Google Scholar]
- 13.Ten Boekel E, de Kieviet W, Bartels PC. Subjects with a shortened activated partial thromboplastin time show increased in-hospitalmortality associated with elevated D-dimer, C-reactive protein and glucose levels. Scand J Clin Lab Invest. 2003;63(6):441–448. doi: 10.1080/00365510310002338. [DOI] [PubMed] [Google Scholar]
- 14.Veigas P, Callum J, Rizoli S, Nascimento B, da Luz LT. A systematic review on the rotational thrombelastometry (ROTEM) values for the diagnosis of coagulopathy, prediction and guidance of blood transfusion and prediction of mortality in trauma patients. Scand J Trauma Resusc Emerg Med. 2016;24(1):114–114. doi: 10.1186/s13049-016-0308-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wiegele M, Kozek-Langenecker S, Schaden E. Point-of-care testing in burn patients. Semin Thromb Hemost. 2017;43(4):433–438. doi: 10.1055/s-0037-1599155. [DOI] [PubMed] [Google Scholar]
- 16.Ganter MT, Hofer CK. Coagulation monitoring: current techniques and clinical use of viscoelastic point-of-care coagulation devices. Anesth Analg. 2008;106(5):1366–1375. doi: 10.1213/ane.0b013e318168b367. [DOI] [PubMed] [Google Scholar]
- 17.Wade CE, Baer LA, Cardenas JC, Folkerson LE. Upon admission coagulation and platelet function in patients with thermal and electrical injuries. Burns. 2016;42(8):1704–11711. doi: 10.1016/j.burns.2016.05.001. [DOI] [PubMed] [Google Scholar]
- 18.Bolliger D, Seeberger MD, Tanaka KA. Principles and practice of thromboelastography in clinical coagulation management and transfusion practice. Transfus Med Rev. 2012;26:1–13. doi: 10.1016/j.tmrv.2011.07.005. [DOI] [PubMed] [Google Scholar]
- 19.Greenhalgh T. How to read a paper: the basics of evidencebased medicine. John Wiley & Sons, Hoboken,; New Jersey, USA: 2011. [Google Scholar]
- 20.Moher D, Liberati A, Tetzlaff J, Altman DG. The PRISMA Group: Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e100009–e100009. [PMC free article] [PubMed] [Google Scholar]
- 21.Critical Appraisal Skills Programme (2018): [Accessed March 2021];CASP Systematic Review Checklist. Available at: https://casp-uk.bcdn.net/wp-content/uploads/2018/01/CASP-Systematic-Review-Checklist_2018.pdf . [Google Scholar]
- 22.Wells GA, Shea B, O’Connell D. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. [Accessed 30 Mar 2018]; available from: http://www. ohri.ca/programs/clinical_epidemiology/oxford.asp . [Google Scholar]
- 23.Park MS, Salinas J, Wade CE, Wang J. Combining early coagulation and inflammatory status improves prediction of mortality in burned and non-burned trauma patients. J Trauma. 2008;64:S188–S194. doi: 10.1097/TA.0b013e318160a5a3. [DOI] [PubMed] [Google Scholar]
- 24.Lu RP, Ni A, Lin FC, Ortiz-Pujols SM. Major injury is not associated with acute traumatic coagulopathy. J Trauma Acute Care Surg. 2013;74(6):1474–1479. doi: 10.1097/TA.0b013e3182923193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mitra B, Wasiak J, Cameron P, O’Reilly G. Early coagulopathy of major burns. Injury. 2013;44:40–43. doi: 10.1016/j.injury.2012.05.010. [DOI] [PubMed] [Google Scholar]
- 26.Sherren PB, Hussey J, Martin R, Kundishora T. Acute burn induced coagulopathy. Burns. 2013;39:1157–1161. doi: 10.1016/j.burns.2013.02.010. [DOI] [PubMed] [Google Scholar]
- 27.Sherren PB, Hussey J, Martin R, Kundishora T. Lethal triad in severe burns. Burns. 2014;40(8):1492–1496. doi: 10.1016/j.burns.2014.04.011. [DOI] [PubMed] [Google Scholar]
- 28.Tejiram S, Brummel-Ziedins KE, Orfeo T, Mete M. Indepth analysis of clotting dynamics in burn patients. J Surg Res. 2016;202(2):341–351. doi: 10.1016/j.jss.2016.01.006. [DOI] [PubMed] [Google Scholar]
- 29.Huzar TF, Martinez E, Love J, George TC. Admission rapid thrombelastography (Rteg) values predict resuscitation volumes and patient outcomes after thermal injury. J Burn Care Res. 2018;39(3):345–352. doi: 10.1097/BCR.0000000000000593. [DOI] [PubMed] [Google Scholar]
- 30.Muthukumar V, Karki D, Jatin B. Concept of lethal triad in critical care of severe burn injury. Indian J Crit Care Med. 2019;23(5):206–209. doi: 10.5005/jp-journals-10071-23161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kaita Y, Nishimura H, Tanaka Y, Suzuki J. Effect of acute coagulopathy before fluid administration in mortality for burned patients. Burns. 2020;47(4):805–811. doi: 10.1016/j.burns.2020.10.011. [DOI] [PubMed] [Google Scholar]
- 32.Brochi K, Cohen M, Davenport R. Acute coagulopathy of trauma: mechanism, identification and effect. Curr Opin Crit Care. 2007;13:680–685. doi: 10.1097/MCC.0b013e3282f1e78f. [DOI] [PubMed] [Google Scholar]
- 33.Davenport R, Manson J, De’Arth H, Platton S. Functional definition and characterization of acute traumatic coagulopathy. Crit Care Med. 2011;39(12):2652–2658. doi: 10.1097/CCM.0b013e3182281af5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Glas GJ, Levi M, Schultz MJ. Coagulopathy and its management in patients with severe burns. J Thromb Haemost. 2016;14(5):865–874. doi: 10.1111/jth.13283. [DOI] [PubMed] [Google Scholar]
- 35.Barret JP, Gomez PA. Disseminated intravascular coagulation: a rare entity in burn injury. Burns. 2005;31:354–357. doi: 10.1016/j.burns.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 36.King DR, Namias N, Andrews DM. Coagulation abnormalities following thermal injury. Blood Coagul Fibrinolysis. 2010;21(7):666–669. doi: 10.1097/MBC.0b013e32833ceb08. [DOI] [PubMed] [Google Scholar]
- 37.Fagan SP, Bilodau ML, Goverman J. Burn Intensive Care. Surg Clin North Am. 2014;94(4):765–779. doi: 10.1016/j.suc.2014.05.004. [DOI] [PubMed] [Google Scholar]
- 38.Chung KK, Wolf SE, Cancio LC, Alvarado R. Resuscitation of severely burned military casualties: fluid begets more fluid. J Trauma. 2009;67(2):231–237. doi: 10.1097/TA.0b013e3181ac68cf. [DOI] [PubMed] [Google Scholar]
- 39.Cartotto R, Choi J, Gomez M, Coper A. A prospective study on the implications of a base deficit during fluid resuscitation. J Burn Care Rehabil. 2003;24:75–84. doi: 10.1097/01.BCR.0000054177.24411.13. [DOI] [PubMed] [Google Scholar]
- 40.Kamolz LP, Andel H, Schramm W, Meissi G, Herndon DN, Frey M. Lactate: early predictor of morbidity and mortality in patients with severe burns. Burns. 2005;31(8):986–990. doi: 10.1016/j.burns.2005.06.019. [DOI] [PubMed] [Google Scholar]
- 41.Falcone R, Santanello S, Schulz M, Monk J. Correlation of metabolic acidosis with outcome following injury and its value as a scoring tool. World J Sur. 1993;17:575–579. doi: 10.1007/BF01659111. [DOI] [PubMed] [Google Scholar]
- 42.Van Haren RM, Thorson CM, Valle EJ, Busko AM. Hypercoagulability after burn injury. J Trauma Acute Care Surg. 2013;75(1):37–43. doi: 10.1097/TA.0b013e3182984911. [DOI] [PubMed] [Google Scholar]
- 43.Martin R, Kilgo P, Miller P, Hoth J. Injury-associated hypothermia: an analysis of the 2004 national trauma data bank. Shock. 2005;24:114–118. doi: 10.1097/01.shk.0000169726.25189.b1. [DOI] [PubMed] [Google Scholar]
- 44.Brohi K, Singh J, Heron M, Coats T. Acute traumatic coagulopathy. J Trauma. 2003;54:1127–1130. doi: 10.1097/01.TA.0000069184.82147.06. [DOI] [PubMed] [Google Scholar]
- 45.Palmieri T. Burn injury and blood transfusion. Curr Opin Anaesthesiol. 2019;32(2):247–251. doi: 10.1097/ACO.0000000000000701. [DOI] [PubMed] [Google Scholar]
- 46.Palmieri TL, Holmes JH 4th, Arnoldo B. Transfusion requirement in burn care evaluation (TRIBE): a multicenter randomized prospective trial of blood transfusion in major burn injury. Ann Surg. 2017;266:595–602. doi: 10.1097/SLA.0000000000002408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Welling H, Ostrowski SR, Stensballe J. Management of bleeding in major burn surgery. Burns. 2018 doi: 10.1016/j.burns.2018.08.024. pii: S0305-4179(18)30754-X . [DOI] [PubMed] [Google Scholar]
- 48.Voigt CD, Hundeshagen G, Malagaris I. Effects of a restrictive blood transfusion protocol on acute pediatric burn care: transfusion threshold in pediatric burns. J Trauma Acute Care Surg. 2018;85:1048–1054. doi: 10.1097/TA.0000000000002068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Palmieri TL, Holmes JH, Arnoldo B. Restrictive transfusion strategy is more effective in massive burns: results of the TRIBE multicenter prospective randomized trial. Mil Med. 2018 doi: 10.1093/milmed/usy279. doi: 10.1093/milmed/usy279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Foster JC, Sappenfield JW, Smith RS, Kiley SP. Initiation and termination of massive transfusion protocols: current strategies and future prospects. Anesth Analg. 2017;125:2045–2055. doi: 10.1213/ANE.0000000000002436. [DOI] [PubMed] [Google Scholar]
- 51.Marsden NJ, Lawrence M, Davies N, Davies G. The effect of the acute inflammatory response of burns and its treatment on clot characteristics and quality: a prospective case controlled study. Burns. 2020;46:1051–1059. doi: 10.1016/j.burns.2019.11.008. [DOI] [PubMed] [Google Scholar]
- 52.Lawrence MJ, Marsden N, Kaczynski J, Davies G. An investigation into the effects of in vitro dilution with different colloid resuscitation fluids on clot microstructure formation. Anesth Analg. 2016;123:1081–1088. doi: 10.1213/ANE.0000000000001536. [DOI] [PubMed] [Google Scholar]
- 53.Barua P, Iqbal MK, Haque M. Postburn elevation in fibrin degradation product is related to burn severity. Chattogram Maa-OShishu Hospital Medical College Journal. 2020;19:1–1. [Google Scholar]