Abstract
The present study describes the use of a leaf extract from Ficus carica as a source of natural antioxidants for the surface alteration of bulk titanium dioxide (TiO2) in two steps. First, the hydro-thermal treatment of the bulk TiO2 material was carried out and followed by thermal annealing at 300 °C for 3 h in air. The role of the leaf extract of Ficus carica on the performance of the bulk TiO2 material for the removal of methylene blue (MB) was also studied. Various analytical techniques such as powder X-ray diffraction (XRD), scanning electron microscopy (SEM), and energy dispersive spectroscopy (EDS) were used to explore the crystalline structure, morphology, and composition. The bulk TiO2 material after the leaf-extract treatment exhibited mixed anatase and rutile phases, a flower-like morphology, and Ti, O, and C were its main elements. The average crystallite size was also calculated, and the obtained values for the bulk TiO2 material, 18.11 nm, and the treated bulk TiO2 material with various amounts, 5, 10, and 15 mL, of leaf extract were 16.4, 13.16, and 10.29 nm respectively. Moreover, Fourier-transform infrared spectroscopy validated the typical metal–oxygen bonds and strengthened the XRD results. The bulk TiO2 material chemically treated with Ficus carica has shown outstanding activity towards the degradation of MB under sunlight. The 15 mL of Ficus carica extract significantly enhanced the photocatalytic activity of the bulk TiO2 material towards the degradation of MB. The dye degradation efficiency was found to be 98.8%, which was experimentally proven by the Fourier Transform Infrared spectroscopoyy (FTIR) analysis. The obtained performance of the bulk TiO2 material with Ficus carica revealed excellent surface modifying properties for poorly-performing photocatalysts towards the degradation of synthetic dyes when used in their pristine form. The presented approach suggests that Ficus carica could be of great interest for tuning the surface properties of materials, either in the form of nano-size or bulk-phase in a particular application.
Keywords: Ficus carica, bulk TiO2 material, methylene blue
1. Introduction
The release of wastewater from industries, including pharmaceuticals, textile, chemical, sugar, beverages, paper, and so forth, is accompanied by several organic and inorganic pollutants, which severely deteriorate the resources of natural water [1,2]. The published works confirm that many of the synthetic dyes, such as methylene blue, acid black 234, mordant black 1, acid black 210, etc., are life-threatening to us [3]. It has been found that the degradation of synthetic dyes is a big challenge to less toxic compounds [4]. Hence, it is an immediate task for scientists and environmentalists to develop either new or modified methods for the efficient degradation of synthetic dyes [5]. The existing commercial methods, including biological and physiochemical for wastewater treatment, have a performance for the removal of organic pollutants. These methods have certain demerits, such as high cost, the consumption of a lot of energy, poor performance at low concentrations of dye, and the poor decolorization of dyes [6,7]. The use of advanced oxidation processes mainly depends on the generation of active OH radicals, which efficiently degrade organic pollutants [8,9]. Photocatalysis is among the advanced oxidation processes; it is highly studied due to its potentiality for the mineralization of organic pollutants into carbon dioxide and water [10,11]. For this purpose, various photocatalysts such as titanium dioxide (TiO2), ZnO, ZnS, Fe2O3, and graphene composite have been investigated. The use of TiO2, compared to other materials used as photocatalysts, is studied intensively because of the low toxicity, cost, and strong oxidizing capability [12]. In contrast to this, the wide band gap of pristine TiO2 has limited its performance up to the UV light, which is only 5% of the solar spectrum and does not capitalize the visible light, approximately 45% of the solar light. Furthermore, the recombination of the rate of electron and hole pairs is high in the bulk TiO2 material, which impairs the degradation activity. Therefore, it is very important to fabricate the visible light photosensitive materials in order to capitalize the full solar spectrum and enhance the photocatalytic efficiency [13,14]. Different methodologies for the surface tuning of TiO2 towards visible light functionality, such as metal dopants [15], nonmetal dopants [14], hybrid composites [16,17], dye sensitizing methods [18], and dyes [19], have been used. However, there has been little attention on the use of natural antioxidants for the surface modification of bulkTiO2 nanoparticles, thus increasing the degradation performance in the visible region. Ficus carica (FC) grows in tropical and subtropical climates around the world. It is a member of the Moraceae family and is a rich source of many bioactive substances (phenolic compounds, vitamins, minerals, carbohydrates, dietary fibers, sugars, and organic acids) that are low in fat, cholesterol, and have high amino acid content. Leucoderma and ringworms were traditionally treated using the root of Ficus carica plant. The fruit is sweet and has antipyretic properties of aphrodisiac, and has proven useful in paralysis and inflammations. The FC leaves are used to cure jaundice [20]. The chemical components of FC include fatty acids, stigmasterol, fucosterol, campesterol, and β-sitosterol [21], as well as psoralen, umbelliferone, and bergapten [22,23], calotrophenyl, and lupeol acetate [24,25,26], and 6-O-acyl-d glucosyl-sitosterol as an antiproliferative agent The anticancer compounds 9,9-cycloarlane triterpenoid and 6-(2-methoxy-Z-vinyl)-7-methyl-pyranocoumarin glucosyl-sitosterol [26,27]. Among the natural oxidants, fig (Ficus carica) leaf extract is rich with polyphenols, flavonoids, and a high density of antioxidants [28]. The leaf extract of Ficus carica is neither utilized for the surface alteration of bulk TiO2 material nor is it used for the degra dation purposes of MB under natural sunlight to date. As a result, we looked into how Ficus carica (FC) leaf extract affected the ability of bulk TiO2 to degrade MB when exposed to sunlight naturally. The advantages of the present study are the simplicity, low cost, and the surface modification of the bulk TiO2 material is performed by an abundant natural source of antioxidants from Ficus carica. Moreover, the present approach is very simple, and it prevents the toxic effects of nanostructured materials just by using the bulk TiO2 material with excellent degradation efficiency towards MB in an aqueous solution.
In this study, we have used bulk TiO2 material and chemically treated it with a leaf extract of Ficus carica (FC) via a hydrothermal process followed by thermal annealing at 300 °C in air. The material characterization was carried out through SEM, EDS, XRD, and UV–visible spectroscopy techniques. The photocatalytic activity of the bulk TiO2 chemically treated with Ficus carica (FC) was studied with the degradation of MB under natural sunlight. The obtained degradation efficiency was around 98.8% at 240 min.
2. Materials and Methods
2.1. Chemical Reagents
The FC leaves were obtained from the garden of the Institute of Chemistry, University of Sindh Jamshoro. The model dye methylene blue (C16H18ClN3S, Mm = 319.85 g/mL), Titanium dioxide (Mm = 79.866) as bulk, ammonium hydroxide (Mm = 35.04, 25% NH₄OH) were purchased from Merck). Excellent analytical grade chemicals were utilized in a received condition. For material synthesis and the preparation of the stock solution of methylene blue, deionized (DI) water was used thoroughly.
2.2. Preparation of Leaf Extract
At first, the leaves obtained from the FC plants were thoroughly washed with tap water followed by distilled water to ensure the removal of the dirt. About 15 g of leaves were ground by a mortar and pestle, resulting in a paste. Next, the paste was then placed in 50 mL of DI and annealed for 25 min at 65 °C. The mass was then filtered by using filter paper. Finally, the resultant leaf extract was applied as an agent for surface modification for the TiO2.
2.3. The Surface Alteration of Bulk TiO2 Material by Ficus Carica (FC) Leaf Extract
At first, four beakers were prepared to bear a solution composed of (2.22 g) of titania powder and 5 mL of 25% ammonium hydroxide. Then, 100 mL of DI water and four different compositions of FC plant leaf extract (5 mL, 10 mL, and 15 mL) were added to three beakers. The designation “pristine titanium dioxide” was given to the fourth beaker, which was left in its prepared state (TiO2). The aluminum foil was used to completely enclose the beakers, which was performed after 5 h of preheating the oven to 95 °C. After annealing, the bulk TiO2 appeared as a white powder on the filter paper and was ready for further characterization.
2.4. Characterization
At first, the morphological investigation of modified TiO2 was performed by SEM at an accelerating voltage of 10 kV. In contrast, the elemental composition of treated TiO2 was performed by Energy dispersive X-ray spectroscopy (EDS) (model Jeol JSM-6380 A, Tokyo, Japan). At 45 kV, CuK radiation (=1.54050), and 45 mA, a powder X-ray diffractometer (XRD) (PANlytical, Netherland) was used to study the purity and phase analysis of extract-treated bulk TiO2 material. The T–O bond was examined using FTIR equipment (Spectrum two PerkinElmer, Waltham, MA, USA), and dye degradation using the changed bulk TiO2 material and Ficus carica (FC) leaf extract was demonstrated [29].
2.5. Photo Catalytic Measurements
Methylene Blue was selected as a model pollutant for examining its photocatalytic degradation by using the chemically treated bulk TiO2 material. The photocatalytic activity was performed under natural sunlight irradiation. At first, 50 mL of 5 × 10−5 M solution of Methylene Blue dye with a dye concentration of 1 mg/50 mL and 2 mg/50 mL of pristine TiO2 and surface treated TiO2 in conjunction with FC leaf extract 5, 10, and 15 mL were introduced in four distinct glass beakers. The mixture was then sonicated for five minutes in the dark to guarantee uniformity. Next, the solution was exposed to sunlight at predetermined intervals, such as 30–360 min (for 6 h). The UV–vis spectrophotometer (PE Lamada 356) was used in the optical experiment to look at the dye absorption spectra during the photocatalytic degradation process. According to the analysis, the reaction mixture of absorbance reduced as the exposure duration increased, which indicates that the concentration of MB dye decreased as well. The photocatalytic degradation was carried out, and the calculations were made under the following formula:
| Dye elimination % (C0 − Ct)/C0 × 100 | (1) |
where, C0 stands for the dye’s initial concentration and Ct for the dye’s concentration after following the application of sunlight, respectively.
3. Results and Discussion
3.1. Analysis of Structure, Phase and Composition of the Surface Modified Bulk TiO2 Material after Treated with FC Leaf Extract
3.1.1. Morphological Investigation
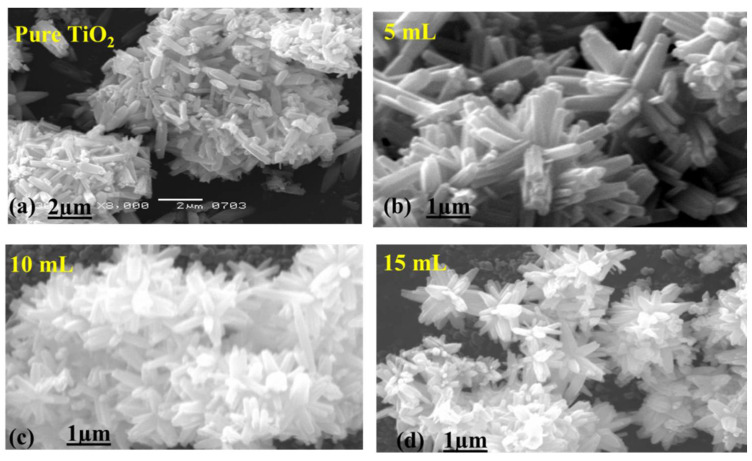
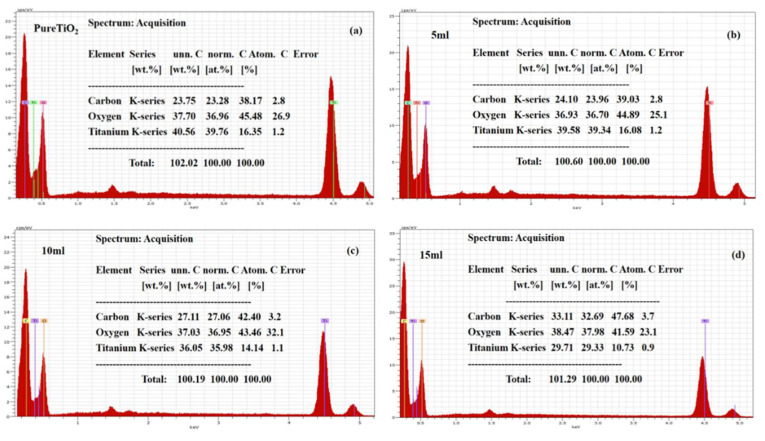
Using the SEM technique, the morphology of the surface-modified and pure TiO2 was studied. It was observed that the pristine bulk TiO2 was bearing a rod-like structure having a dimension in microns, as given in Figure 1a. Whereas the samples treated with 5 mL of leaf extract seemed to bear little variation in shape with a flower-like appearance [30], next, the samples with 10 and 15 mL of leaf extract followed a similar trend of bearing a flower shape with a slightly thinner surface, as presented in Figure 1c,d. A little difference in morphology appeared at a certain value after the addition of leaf extract to TiO2 material, which may be corresponding to the formation of electrical charges. The chemical composition for pristine TiO2 material and bulk leaf extract treated TiO2 material is enclosed in Figure 2a–d. The samples throughout revealed that the composition was mostly dominated by Ti and O, as depicted in Figure 2a–d.
Figure 1.
SEM images of bulk TiO2 that has been hydrothermally treated (a) and treated with 5, 10, and 15 mL of FC leaf extracts (b–d).
Figure 2.
EDX analysis of bulk TiO2 that has been hydrothermally treated (a) and treated with 5, 10, and 15 mL of FC leaf extracts (b–d).
The use of the FC extract has shown a significant effect on the morphology, as can be seen from SEM images, and it can play a vital role in dye degradation kinetics. We have observed that the surface of bulk TiO2 became thinner with the use of higher amounts of FC extract, and it can further expose more surface area with catalytic sites for light interaction. Consequently, a high-density generation of electrons and holes further adds high-value oxidizing radicals such as hydroxyl and superoxide. Then, these oxidizing radicals participate effectively in reducing the MB at high degradation effectively.
3.1.2. XRD Investigation
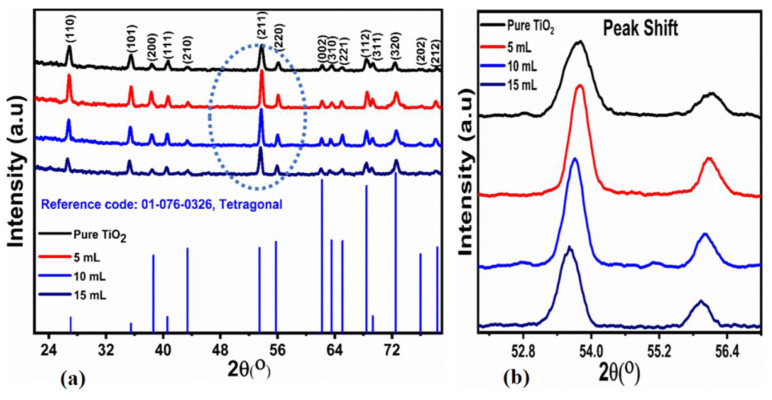
The diffraction peaks of the pure and leaf-extract-treated samples were examined by the XRD technique. The resultant peaks were in good agreement with the reference ICSD card no.01-076-0326, bearing a tetragonal crystalline structure with a mixed phase of anatase and rutile. As illustrated in Figure 3a, the appearance of various diffraction patterns at two specific theta angles are observed at 27.05° (110), at 35.48° (101), at 38.63° (200), at 40.57° (111), at 43.41° (210), at 53.46° (211), at 55.78° (220), at 61.58° (002), at 62.21° (310), at 65.09° (221), at 68.43° (112), at 69.31° (311), at 72.52° (320), at 76.00° (202) and at 78.33° (212). These reflection peaks indicate the excellent crystalline aspects of the treated samples with FC extract.
Figure 3.
XRD patterns bulk TiO2 that has been hydrothermally treated (a) and treated with 5, 10, and 15 mL of FC leaf extracts (a) and peak shift analysis at (211, 220) with increasing amount of Ficus carica (FC) (b).
The resultant diffraction peaks at 2θ were recorded at 27.04° (110) and 43.41° (210) corresponded to the rutile phase of bulk titania. The rest of the peaks corresponded to the anatase phase belonging to bulk TiO2. These observations suggest that the material is mainly characterized by mixed phases of anatase and rutile. The presence of mixed phases with rutile and anatase bulk TiO2 material corresponding to leaf extract might be assigned to the variation in the oxygen concentration. It was further noticed that oxygen was significantly impressed by the synthesis technique and the resultant crystalline phase [31]. At the plane (211), the peaks are highly intense, showing the predominantly-favored crystal formation leading to a countable shift of reflection peak could be observed in Figure 3b. The reason for the shift might be assigned to the smaller ionic radii of titanium dioxide. According to Sagneetha et al., another possible cause of the minor shift to the lower angle is a change in crystal size and morphological disorder [32].
The average crystalline size for the pristine and leaf-extract-treated samples was estimated by using Debye–Scherrer’s Equation (2) at (211) an intense diffraction peak. The crystalline diameters of all of the samples, whether untreated or treated, were found to range between 10.29 and 18.11 nm, as indicated in Table 1. The structural details of each sample are also listed in Table 1.
| (2) |
Table 1.
Structural properties of bulk TiO2 i-e hydrothermally treated and altered with 5, 10, and 15 mL of FC leaf extract.
| Sample | 2θ (°) (211) | FWHM | Height | Crystalline Size (nm) |
Constant (K) 1 mg | Constant (K) 2 mg |
|---|---|---|---|---|---|---|
| Pure TiO2 | 53.81 | 0.55333 | 36.55 | 18.1 | - | 2.27 × 10−3 min−1 |
| 5 mL | 53.78 | 0.37074 | 51.55 | 16.1 | 6.84 × 10−3 min−1 | 6.13 × 10−3 min−1 |
| 10 mL | 53.71 | 0.38377 | 51.33 | 13.1 | 9.99 × 10−3 min−1 | 1.23 × 10−2 min−1 |
| 15 mL | 53.62 | 0.41464 | 38.77 | 10.2 | 1.14 × 10−2 min−1 | 1.55 × 10−2 min−1 |
Here, Dp denotes the mean crystalline domain, which can be greater or smaller than a particle size of less than 100 nm, λ indicates the X-ray wavelength of CuKα radiation (1.54 Å), β1/2 represents full width and half maximum. According to Table 1, the bulk TiO2 material treated with various quantities of FC leaf extract had an average crystallite size of 18.11 nm, 16.14 nm, 13.16 nm, and 10.29 nm.
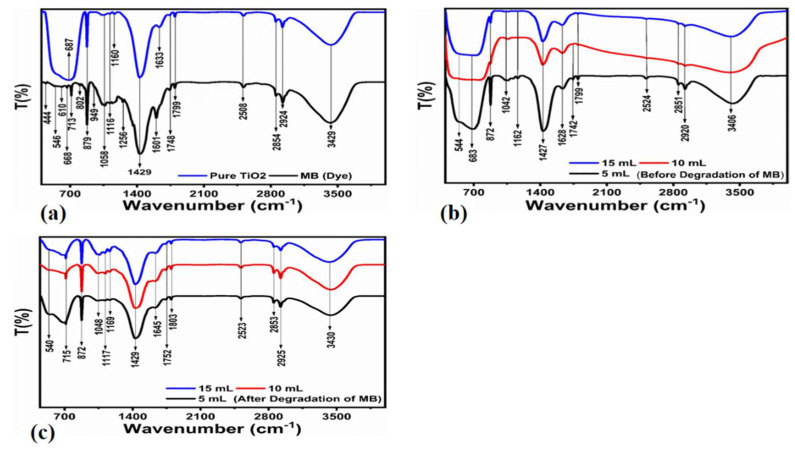
Table 1 clearly shows that the treated leaf extract TiO2 material has a smaller size than untreated TiO2. Furthermore, the catalyst effect on photocatalytic activity is assigned to the crystalline size and surface area. Therefore, if the crystalline size is smaller, the surface area will be larger, and photodegradation activity will be higher [33]. The FTIR technique was used to identify functional groups and to investigate the residual organic compounds in the extract-treated samples, as given in Figure 4. The FTIR spectrums for the untreated TiO2 as well as methylene blue are depicted in Figure 4a. Corresponding peaks in the range of 3429 cm−1 and 2508 cm−1 represent the vibrational modes of the –OH group. Matching frequencies for the bulk untreated TiO2 were detected at 687 cm−1 to confirm the typical metal–oxygen vibration modes, as in our investigation, and peaks at 1633 cm−1 to demonstrate the unique Ti–O–Ti bonding characteristics of the bulk TiO2 crystal [34]. As shown in Figure 4b, FTIR analysis of TiO2 treated with different leaf extract concentrations was also performed. The majority of the metal–oxygen peaks were discovered to have persisted in all samples with only a minor shift in frequency, confirming the high purity of the samples and were in good agreement with the XRD findings. Furthermore, the breakdown of dye by the leaf-extract-altered material was affirmed by the FTIR results, as given in Figure 4c. It is explicit that most of the vibration modes for the pollutant vanished during the degradation process. This proves the excellent efficiency of the treated samples. The variations at 715 cm−1 and 2853–2925 m−1 could be connected to the C–H bending vibrations that emerged from the possible negligible number of MB molecules after the degradation process.
Figure 4.
FTIR spectrum of bulk TiO2 that has been hydrothermally treated and MB (a), FTIR spectrum of TiO2 treated with 5, 10, and 15 mL of FC leaf extract before degradation of MB (b) and FTIR spectrum of TiO2 treated with 5, 10, and 15 mL of FC leaf extracts before degradation of MB (c).
Figure 4b,c include the IR spectra before and after the degradation of MB, respectively. The spectra are closely the same, and we aim to show through IR study the successful degradation of MB near the surface of the presented photocatalysts. However, the confirmation of the modification to bulk TiO2 should be verified before, which is difficult to show in the presented work. Therefore, new studies are required to investigate this part of the presented photocatalysts in the future.
3.2. Photocatalytic Efficiency
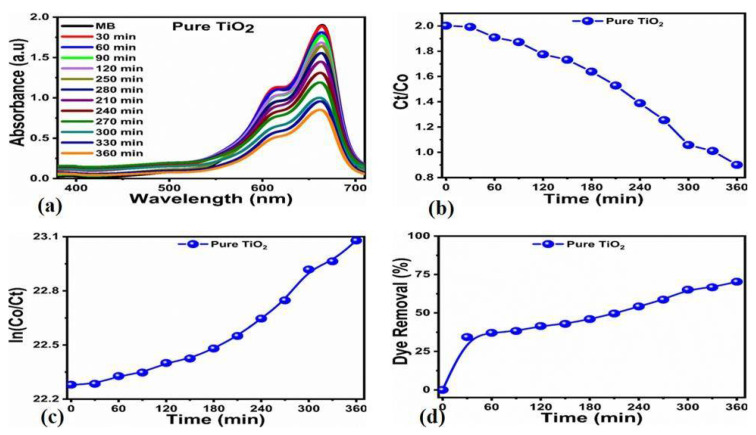
From the reaction mixture, an aliquot of 3 mL was taken at certain intervals, followed by a measurement of the absorbance of MB at 664 nm. A decrease in the intensity of the absorption was observed continuously, corresponding to the degradation of MB. The UV–vis spectra for the untreated TiO2 with a concentration of 2 mg in MB dye (5 × 10−5 M) under sunlight for various time periods from 30 min to 360 min at an equal interval of 30 min is displayed in Figure 5a.
Figure 5.
Changing in UV–vis spectra of pure TiO2 (a), plot of Ct. Co verses irradiation of photodegradation of MB at various times (b), pseudo-first order plot for photodegradation of MB (c) and shows the calibration curve of dye removal (%) under natural light illumination of only hydrothermally treated bulk TiO2 material (d).
The results showed that for the samples without the leaf-extract treatment, the dye degradation occurred on the surface. The concentration (Ct./Co) for MB is displayed in Figure 5b. The untreated TiO2 followed reaction kinetics in good agreement with the pseudo-first-order, as shown in Figure 5c. The dye removal percentage (%) results are given in Figure 5d. A poor degradation efficiency trend was noticed for the untreated TiO2. The maximum degradation efficiency of 70.31% was recorded for the degradation time of 360 min (Figure 5d).
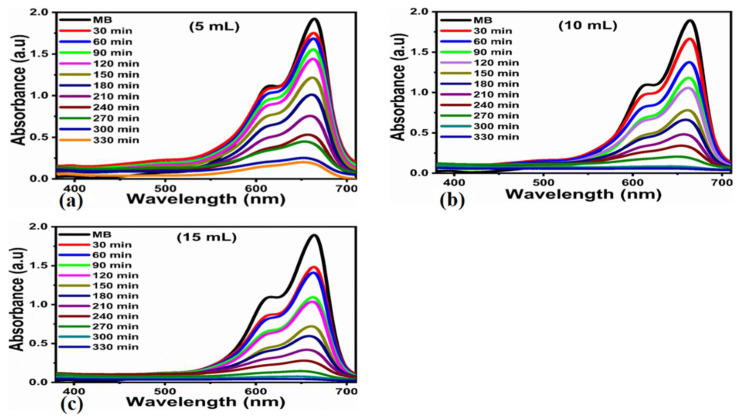
The poor efficiency could be attributed to fast electron-hole pairs recombination and the lack of catalytic sites for the untreated TiO2 material. The UV–vis spectrum of 1 mg of the TiO2 material with various doping concentrations of FC leaf extract of 5, 10, and 15 mg were examined in MB dye (5 × 10−5 M) under sunlight irradiation for times ranging from 30 min to 33 min at an equal interval of 30 min up to the maximum time of 330 min, as depicted in Figure 6a–c.
Figure 6.
(a–c) change in Uv–Vis spectrum for photodegradtion of MB under the natural light illumination over bulk TiO2 that has been hydrothermally treated with 5, 10, and 15 mL of FC leaf extracts at 30–330 min.
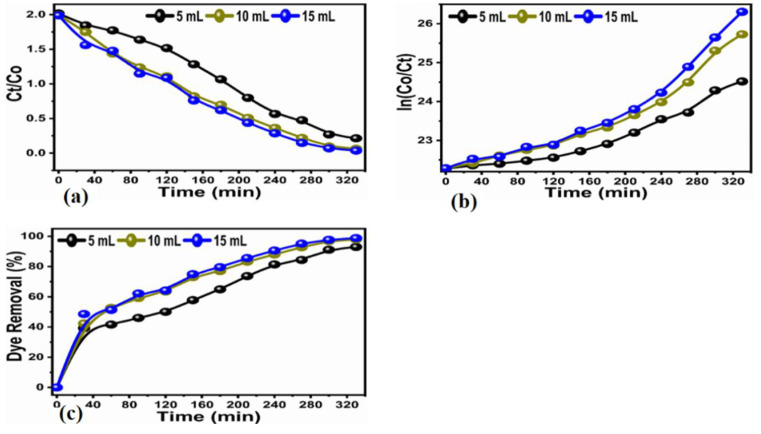
The kinetics of photodegradation for FC leaf-extract-treated TiO2 seemed to be following pseudo-first order reaction kinetics, as shown in Figure 7a. This is because the rate of reaction is related to the concentration of the dye, and the reaction degradation was performed in an aqueous solution. The entry of water tells us that the rate of the reaction is not dependent on it; this is the reason we believe that reaction kinetics are mainly governed by pseudo-first-order reaction kinetics.
Figure 7.
Plot of Ct. Co verses irradiation of photodegradation of MB at various times (a), pseudo-first order plot for photodegradation of MB (b) and the calibration curve of dye removal (%) undernatural light illumination of bulk TiO2 material that has been hydrothermally treated with 5, 10, and 15 mL of FC leaf extracts at 30–330 min (c).
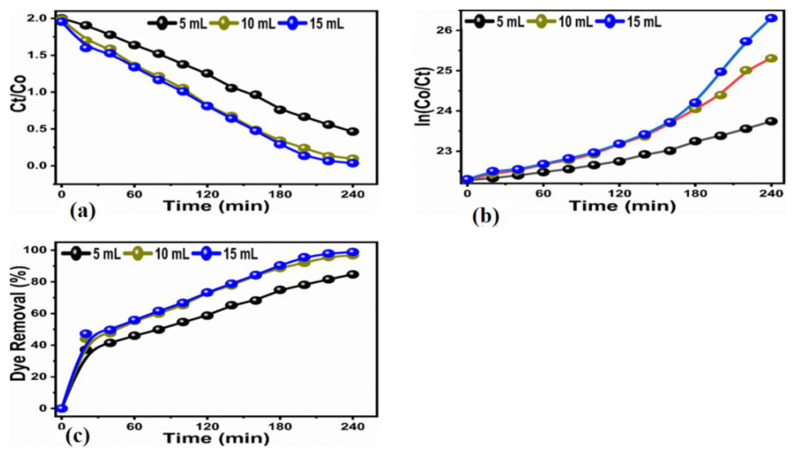
With respect to time, a rising tendency of deterioration efficiency was seen. For 5 mL of the FC leaf-extract-treated TiO2, an excellent maximum efficiency of 92.95% was achieved. The concentration was increased to 10 mL for the next sample, an increase in the efficiency trend was observed, and enhanced efficiency of up to 97.89% was recorded. The next sample with an increased FC leaf extract of up to 15 mL in TiO2 resulted in a further increased efficiency for the removal of MB of up to 98.87%, as depicted in Figure 7c.
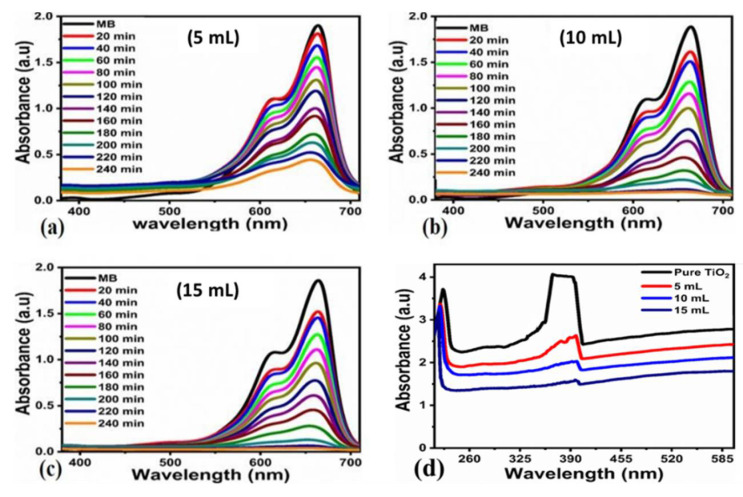
It is very evident from the results that the pollutant MB was almost 100% removed by using a minute amount (1 mg) of bulk titania treated with FC leaf extract with time variations. Comparatively, varying amounts (2 mg) of bulk titania treated with varying amounts of FC leaf extract, namely (5, 10, and 15 mL), were measured using the availability of sunlight treatment for time variations of 20 to 240 min at equal intervals of 20 min, as shown in Figure 8a–c. The absorbance spectra for pristine and modified TiO2 is also presented in Figure 8d, which corresponds a variation in the absorbance with respect to the increase in dopant dose.
Figure 8.
(a–c) change in Uv–Vis spectrum for photodegradtion of MB under the natural light illumination over bulk TiO2 that has been hydrothermally treated with 5, 10, and 15 mL of FC leaf extracts at 20–240 min. (d) A comparison of absorbance of pristine Titania with doped ones.
Pseudo-first and second-order schemes, as shown in Figure 9a,b, were explored to better understand the kinetics of the degradation of the bulk TiO2 that had been treated using the FC leaf extract. The efficiency of pollutant removal was examined with respect to varying time intervals for up to 240 min. The minimum efficiency of 37.20% was recorded with an increasing trend up to the maximum efficiency of 84.70% was recorded for the 5 mL of leaf-extract altered TiO2, 96.80% was achieved for 10 mL and 98.82% for 15 mL, as depicted in Figure 9c. Therefore, it is clear from the obtained results that adding more FC leaf extract into the synthesized material will significantly increase the photocatalytic activity of bulk TiO2 for the removal of the organic dye contaminant MB. The density of the antioxidants was significantly altered by the addition of a concentration of the FC leaf extract for the degradation of Methylene blue. Increasing the FC leaf extract affects the density of antioxidants highly [35]; therefore, it has highly impressed the surface properties of TiO2 with respect to morphology, crystal size, as well as the number of catalytic sites on the material surface that favored a superior efficiency of degradation. The result of the 15 mL leaf-extract-treated bulk TiO2 provided superior degradation behavior for the successive removal of pollutants. Different FC extract amounts have shown a certain effect on the change in morphology, and with a larger amount of FC extract, the surface of TiO2 became much sharper, suggesting that its larger surface area favored photocatalytic properties.
Figure 9.
(a) Plot of Ct. Co verses irradiation of photodegradation of MB at various times (b), pseudo-first order plot for photodegradtion of MB (c) and the calibration curve of dye removal (%) under natural light illumination of bulk TiO2 material that has been hydrothermally treated with 5, 10, and 15 mL of FC leaf extracts at 20–240 min.
Finally, the kinetic study for the concentration of the dye was observed by a Langmuir–Hinshelwood (L-H) model [36,37], as shown in (Figure 7a and Figure 9a).
The efficiency of dye degradation efficiency was calculated by:
| C/C0 = A/A0 | (3) |
where A0 as well as A represents absorbance recorded before and after the reaction for the degradation of methylene blue at 664 nm. The following model may describe the statistics more significantly.
| Ln(C/C0) = −Kappt | (4) |
In this case, Kapp stands for apparent velocity constant for the dye degradation process. Where as C and C0 shows the initial dye concentration and dye concentration after certain intervals of time. As enclosed in (Figure 8b and Figure 10b). The correspond rate constants for the two different dye concentrations and various amounts of FC extract, such as 5 mL (K = 6.8410−3 min−1, K = 6.13 × 10−3 min−1), 10 mL (K = 9.99 × 10−3 min−1, K = 1.23 × 10−2 min−1), and 20 mL (K = 9.99 × 10−3 min−1, K = 1.23 × 10−2 min−1).
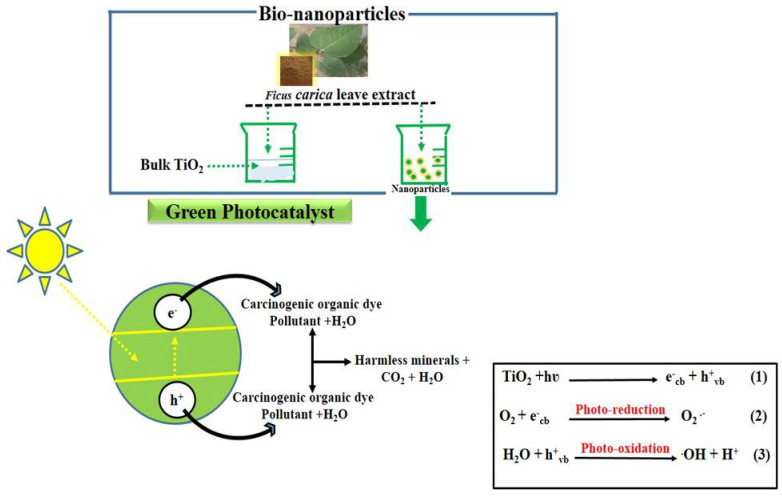
Figure 10.
Projected mechanism of photocatalytic degradation of dyes using bulk TiO2 material treated with various Ficus carica leaf extract of 5, 10, and 15 mL.
As a typical semiconductor metal oxide photocatalyst, TiO2 produces (e− and h+) when illuminated by light energy greater/equal to the band gap width. Some of these pairs are migratory, and the redox (reduction–oxidation) reaction involves interactions with absorbed species on the surface of the nanocatalyst. The MB degradation is mediated by the radicals produced by the generated electron and hole pairs after the interaction of solar light with the surface of TiO2. Radicals such as hydroxyl and superoxide are generated by the interaction of electron and hole pairs with water and oxygen from an aqueous solution of the dye. Additionally, as shown in the equations, the absorbed organic molecules are removed when h+ vb interacts with H2O and O2 from the aqueous solution of MB to form hydroxyl (OH) and (e− cb) continuously consumes oxygen to form a superoxide radical (O2−) anion as given (Figure 10). In the degradation of MB under sunlight, the hydroxyl and superoxide radicals are the main radicals which favor the conversion of MB into mineralized products, including carbon dioxide.
Figure 10 depicts a proposed mechanism for the breakdown of dye methylene blue when different concentrations of FC leaf extract are mixed with the bulk TiO2 during exposure to sunlight. As is customary, hazardous organic pollutants are broken down into innocuous byproducts such as (CO2, H2O) or other organic ions by diverse catalytic pathways in nanostructured materials [38]. The acquired findings were also compared, and the observed performance of the proposed photocatalyst is included in Table 2. The performance evaluation confirms that the proposed photocatalyst could be considered for wastewater treatment as it is associated with superior or equal performance to recently reported photocatalysts [39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56], and the material fabrication is low cost and simple, which enables large-scale production.
Table 2.
The comparative analysis of presented results with published works.
| Catalysts | Weight | Light Source | Dye Concentrati on | Time (min) | Dye Removal (%) | Method | Ref. | |
|---|---|---|---|---|---|---|---|---|
| TiO2@Bi2O3 | 35 mg | Sun light | 2 mg of catalyst (50 mL of dye solution) | 250 | 94% | Green synthesis | [39] | |
| TiO2 NPs |
100 mg | UV irradiation |
100 mL MB | 120 | 92% | Chemical and green synthesis methods |
[40] | |
| TiOSO4 | 0.1 g | UV irradiation |
20 ppm MO | 150 | 94% | Sol-gel method | [41] | |
| MnTiO3 | 5 mg | Sun light | MB 1 × 10−5 M |
250 | 75% | Sol-gel | [42] | |
| Fe2TiO5 | 50 mg | Sunlight | MB 10 mg L−1 |
250 | 97% | Sol-gel | [43] | |
| porous TiO2 ceramic s |
eighty pieces (20 mg each piece) | UV light | RhB 10 mg L−1 | 300 min | 99.3% | camphene-based freeze-casting process | [44] | |
| macro/mesop orous anatase TiO2 ceramic | eighty pieces (20 mg each piece) | UV light | RhB 10 mg mL−1 | 180 | 99.4% | a camphene-based freeze-casting process | [45] | |
| Fe–N- codoped TiO2/fly ashecnospheres |
0.2 g | Sun light | RhB mg L−1 | 4 h | 89% | Sol-gel method | [46] | |
| hollow TiO2/fly ashecnospheres | 1.5 g | Sun light | MB 30 mg L−1 |
9 h | 0.0922 (min−1) |
Sol-gel | [47] | |
| Ag+-doped TiO2/ploystyrene | 0.05 g | Sun light | MB 5 mg L−1 |
5 h | 83% | Impregnation and strewing based on a simple solvent-cast method | [48] | |
| TiO2/polyuret hane foam | forty pieces | Sun light | MB + CrVI 1 0 mg L−1 | 150 | 90% | a low-temperature ultrasonic and deposition approach | [49] | |
| TiO2/low density polyethylene | 1 g | Sun light | MB 0.16 mmol L−1 |
225 | 30% | [50] | ||
| C,N- TiO2/polytetrafluoroethylene |
1 gL−2 | Visible light | MO 20 mg L−1 |
4 h | 96.9% | y a simple high-energy ball-milling process | [51] | |
| B–N– TiO2/expanded perlite |
(6 mg/g, 24 wt% TiO2 | Solar light | RhB | 5 h | 99.1% | Sol-gel | [52] | |
| W doped ZnO | 20 mg | UV light | MB | 3 h | 96.9% | Low temperature aqueous chemical growth method | [53] | |
| TiO2- graphene |
0.2 g | UV light | RhB 0.02 mM | 2–4 h | 95% | Sol-gel | [54] | |
| TiO2/polypro pylene | 15– 20 mg |
UV light | MO 15 mg L−1 |
4 h | 65% | Low temperature Hydrothermal method | [55] | |
| TiO2/polypro pylene fabric | a piece (d = 47 mm) | UV light | MO 15 mg L−1 |
240 | 100% | Hydrothermal Process | [56] | |
| BC-TiO2 | --------- | UV light | MB 10 mg/L |
180 | 90% | Low temperature Pyrolysis | [57] | |
| TiO2/AC | 10 g | Sun light | 25 mg dm−3 | 90 | 98.1% | in situ immobilization process | [58] | |
| TiO2/CRFs | 10 mg | Solar light | 10 mg L−1 | 80 | 98.1% | Impregnation and Calcination method | [59] | |
| TiO2 | Ficus | 2 mg | Solar Light | 5 × 10−5 M (MB) | 330 | 98.82% | Simple Hydrothermal Process | Present Work |
| carica leave extract | ||||||||
4. Conclusions
In summary, we have used the leaf extract of Ficus carica for the surface modification of bulk TiO2 material by a two-step methodology. First, the bulk TiO2 was treated with different amounts of leaf extract of Ficus carica using the hydrothermal method. Second, the hydrothermal leaf extract treated with bulk TiO2 material was combusted at 300 °C for 3 h in order to remove the residual products from the sample.
There was little effect on the morphology of the material; however, with the increasing amount of leaf extract of Ficus carica, the top surface of the material became sharp. The crystalline aspects were found in the mixed phase of anatase and rutile for TiO2. The average crystallite size of TiO2 was noticed to be smaller than bulk TiO2 without treatment. The Ficus carica treated bulk TiO2 material was found to be highly active towards the degradation of MB under natural sunlight, and a degradation efficiency of 98.8% was observed for 240 min. The enhanced degradation performance of the leaf-extract treated bulk TiO2 material is attributed to the minimum charge recombination rate, tuned surface features, and decrease in crystallite size. These findings confirm that the Ficus carica leaf extract containing a natural source of antioxidants can be of high privilege as a surface-modifying agent for a wide range of photosensitive materials.
Acknowledgments
This research project is supported by Princess Nourah bint Abdulrahman University Researchers Supporting Project number (PNURSP2022R108), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia.
Author Contributions
M.A.B., Investigation, Methodology, Writing—original draft; S.J.G., Formal analysis, Project Administration; A.A.S., Data curation, Methodology, Writing—review & editing; I.A.C., Formal analysis, Methodology, K.F.A.; Project administration, Resources; A.D.C., Project administration; I.A.H., Data curation, Formal analysis; A.T., Validation, Writing—review & editing; M.N.B.J., Project administration, Resources; Z.H.I., Project administration, Resources. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
Authors declare no conflicts of interest in this study.
Funding Statement
This research was funded by Princess Nourah Bint Abdulrahman University Researchers Supporting Project number (PNURSP2022R108), Princess Nourah Bint Abdulrahman University, Riyadh, Saudi Arabia.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Benjamin S., Vaya D., Punjabi P.B., Ameta S.C. Enhancing photocatalytic activity of zinc oxide by coating with some natural Pigments. Arab. J. Chem. 2011;4:205–209. doi: 10.1016/j.arabjc.2010.06.038. [DOI] [Google Scholar]
- 2.Rauf M.A., Meetani M.A., Khaleel A., Ahmed A. Photocatalytic degradation of methylene blue using a mixed catalyst and product analysis by LC/MS. Chem. Eng. J. 2010;157:373–378. doi: 10.1016/j.cej.2009.11.017. [DOI] [Google Scholar]
- 3.Khan S.A., Shahid S., Nazir M., Kanwal S., Zaman S., Sarwar M.N., Haroon S.M. Efficient template based synthesis of Ni nanorods by etching porous alumina for their enhanced photocatalytic activities against Methyl Red and Methyl Orange dyes. J. Mol. Struct. 2019;1184:316–323. doi: 10.1016/j.molstruc.2019.02.038. [DOI] [Google Scholar]
- 4.Coughlin M.F., Kinkle B.K., Bishop P.L. Degradation of acid orange 7 in an aerobic biofilm. Chemosphere. 2002;46:11–19. doi: 10.1016/S0045-6535(01)00096-0. [DOI] [PubMed] [Google Scholar]
- 5.Khan S.A., Noreen F., Kanwal S., Hussain G. Comparative synthesis, characterization of Cu-doped ZnO nanoparticles and their antioxidant, antibacterial, antifungal and photocatalytic dye degradation activities. Dig. J. Nanomater. Biostruct. 2017;12:877–889. [Google Scholar]
- 6.Ijaz F., Shahid S., Khan S.A., Ahmad W., Zaman S. Green synthesis of copper oxide nanoparticles using Abutilon indicum leaf extract: Antimicrobial, antioxidant and photocatalytic dye degradation activitie. Trop. J. Pharm. Res. 2017;16:743–753. doi: 10.4314/tjpr.v16i4.2. [DOI] [Google Scholar]
- 7.Khan S.A., Noreen F., Kanwal S., Iqbal A., Hussain G. Green synthesis of ZnO and Cu-doped ZnO nanoparticles from leaf extracts of Abutilon indicum, Clerodendrum infortunatum, Clerodendrum inerme and investigation of their biological and photocatalytic activities. Mater. Sci. Eng. C. 2018;82:46–59. doi: 10.1016/j.msec.2017.08.071. [DOI] [PubMed] [Google Scholar]
- 8.Tijani J.O., Fatoba O.O., Madzivire G., Petrik L.F. A review of combined advanced oxidation technologies for the removal of organic pollutants from water. Water Air Soil Pollut. 2014;225:2102. doi: 10.1007/s11270-014-2102-y. [DOI] [Google Scholar]
- 9.Hisaindee S., Meetani M.A., Rauf M.A. Application of LC-MS to the analysis of advanced oxidation process (AOP) degradation of dye products and reaction mechanisms. TrAC Trends Anal. Chem. 2013;49:31–44. doi: 10.1016/j.trac.2013.03.011. [DOI] [Google Scholar]
- 10.Zhang L., Jin Z., Huang S., Huang X., Xu B., Hu L., Cui H., Ruan S., Zeng Y.J. Bio-inspired carbon doped graphitic carbon nitride with booming photocatalytic hydrogen evolution. Appl. Catal. B Environ. 2019;246:61–71. doi: 10.1016/j.apcatb.2019.01.040. [DOI] [Google Scholar]
- 11.He Y., Peng G., Jiang Y., Zhao M., Wang X., Chen M., Lin S. Environmental Hazard Potential of Nano-Photocatalysts Determined by Nano-Bio Interactions and Exposure Conditions. Small. 2020;16:1907690. doi: 10.1002/smll.201907690. [DOI] [PubMed] [Google Scholar]
- 12.Byrne C., Subramanian G., Pillai S.C. Recent advances in photocatalysis for environmental applications. J. Environ. Chem. Eng. 2018;6:3531–3555. doi: 10.1016/j.jece.2017.07.080. [DOI] [Google Scholar]
- 13.Bora L.V., Mewada R.K. Visible/solar light active photocatalysts for organic effluent treatment: Fundamentals, mechanisms and parametric review. Renew. Sustain. Energy Rev. 2017;76:1393–1421. doi: 10.1016/j.rser.2017.01.130. [DOI] [Google Scholar]
- 14.Saien J., Mesgari Z. Highly efficient visible-light photocatalyst of nitrogen-doped TiO2 nanoparticles sensitized by Hematoporphyrin. J. Mol. Catal. A Chem. 2016;14:108–115. doi: 10.1016/j.molcata.2015.12.027. [DOI] [Google Scholar]
- 15.Khairy M., Zakaria W. Effect of metal-doping of TiO2 nanoparticles on their photocatalytic activities toward removal of organic dyes. Egypt. J. Pet. 2014;23:419–426. doi: 10.1016/j.ejpe.2014.09.010. [DOI] [Google Scholar]
- 16.Li X., Chen X., Niu H., Han X., Zhang T., Liu J., Lin H., Qu F. The synthesis of CdS/TiO2 hetero-nanofibers with enhanced visible photocatalytic activity. J. Colloid Interface Sci. 2015;452:89–97. doi: 10.1016/j.jcis.2015.04.034. [DOI] [PubMed] [Google Scholar]
- 17.Kanagaraj T., Thiripuranthagan S. Photocatalytic activities of novel SrTiO3–BiOBr heterojunction catalysts towards the degradation of reactive dyes. Appl. Catal. B Environ. 2017;207:218–232. doi: 10.1016/j.apcatb.2017.01.084. [DOI] [Google Scholar]
- 18.Chatterjee D., Dasgupta S., Rao N.N. Visible light assisted photodegradation of halocarbons on the dye modified TiO2 surface using visible light. Sol. Energy Mater. Sol. Cells. 2006;90:1013–1020. doi: 10.1016/j.solmat.2005.05.016. [DOI] [Google Scholar]
- 19.Swarnkar A.K., Sahare S., Chander N., Gangwar R.K., Bhoraskar S.V., Bhave T.M. Nanocrystalline titanium dioxide sensitised with natural dyes for eco-friendly solar cell application. J. Exp. Nanosci. 2015;10:1001–1011. doi: 10.1080/17458080.2014.951410. [DOI] [Google Scholar]
- 20.Barolo M.I., Mostacero N.R., Lopez S.N. Ficus carica, L. (Moraceae): An ancient source of food and health. Food Chem. 2014;164:119–127. doi: 10.1016/j.foodchem.2014.04.112. [DOI] [PubMed] [Google Scholar]
- 21.Jeong W.S., Lachance P.A. Phytosterols and fatty acids in fig (Ficus carica, var. Mission) fruit and tree components. J. Food Sci. 2001;66:278–281. doi: 10.1111/j.1365-2621.2001.tb11332.x. [DOI] [Google Scholar]
- 22.Seong-Kuk K., Dong-Ok C., Hee-Jong C. Purification and identification of antimicrobial substances in phenolic fraction of fig leaves. Appl. Biol. Chem. 1995;38:293–296. [Google Scholar]
- 23.Khodarahmi G.A., Ghasemi N., Hassanzadeh F., Safaie M. Cytotoxic effects of different extracts and latex of Ficus carica L. on HeLa cell line. Iran. J. Pharm. Res. IJPR. 2011;10:273. [PMC free article] [PubMed] [Google Scholar]
- 24.Saeed M.A., Sabir A.W. Irritant potential of triterpenoids from Ficus carica leaves. Fitoterapia. 2002;73:417–420. doi: 10.1016/S0367-326X(02)00127-2. [DOI] [PubMed] [Google Scholar]
- 25.Shai R., Yoel K., Ruth R., Michael S., Raphael M. Suppressors of cancer cell proliferation from fig (Ficus carica) resin: Isolation and structure elucidation. J. Nat. Prod. 2001;64:993–996. doi: 10.1021/np000592z. [DOI] [PubMed] [Google Scholar]
- 26.Weiping Y., Hongming C., Tianxin W., Mengshen C. A new coumarin compound with anticancer activity. Chin. Trad. Herb. Drug. 1997;28:3–4. [Google Scholar]
- 27.Weiping Y., Hongming C., Tianxin W., Mengshen C. Research on the chemical structure and anticancer activity of 9,19-cyclopropane-24, 25 ethyleneoxide-5-en-3β-spirostol. Chin. J. Med. Chem. 1997;7:46–47. [Google Scholar]
- 28.Solomon A., Golubowicz S., Yablowicz Z., Grossman S., Bergman M., Gottlieb H.E., Altman A., Kerem Z., Flaishman M.A. Antioxidant activities and anthocyanin content of fresh fruits of common fig (Ficus carica L.) J. Agric. Food Chem. 2006;54:7717–7723. doi: 10.1021/jf060497h. [DOI] [PubMed] [Google Scholar]
- 29.Bhatti M.A., Tahira A., dad Chandio A., Almani K.F., Bhatti A.L., Waryani B., Nafady A., Ibupoto Z.H. Enzymes and phytochemicals from neem extract robustly tuned the photocatalytic activity of ZnO for the degradation of malachite green (MG) in aqueous media. Res. Chem. Intermed. 2021;47:1581–1599. doi: 10.1007/s11164-020-04391-6. [DOI] [Google Scholar]
- 30.Sagadevan S., Podder J. Investigations on structural, optical, morphological and electrical properties of nickel oxide nanoparticles. Int. J. Nanopart. 2015;8:289–301. doi: 10.1504/IJNP.2015.073731. [DOI] [Google Scholar]
- 31.Banerjee I., Karmakar S., Kulkarni N.V., Nawale A.B., Mathe V.L., Das A.K., Bhoraskar S.V. Effect of ambient pressure on the crystalline phase of nano TiO2 particles synthesized by a dc thermal plasma reactor. J. Nanopart. Res. 2010;12:581–590. doi: 10.1007/s11051-009-9627-9. [DOI] [Google Scholar]
- 32.Tauc J., Menth A. States in the gap. J. Non-Cryst. Solids. 1972;8:569–585. doi: 10.1016/0022-3093(72)90194-9. [DOI] [Google Scholar]
- 33.Avciata O., Benli Y., Gorduk S., Koyun O. Ag doped TiO2 nanoparticles prepared by hydrothermal method and coating of the nanoparticles on the ceramic pellets for photocatalytic study: Surface properties and photoactivity. J. Eng. Technol. Appl. Sci. 2016;1:34–50. doi: 10.30931/jetas.281381. [DOI] [Google Scholar]
- 34.Tripathi A.K., Mathpal M.C., Kumar P., Singh M.K., Mishra S.K., Srivastava R.K., Chung J.S., Verma G., Ahmad M.M., Agarwal A. Synthesis based structural and optical behavior of anatase TiO2 nanoparticles. Mater. Sci. Semicond. Process. 2014;23:136–143. doi: 10.1016/j.mssp.2014.02.041. [DOI] [Google Scholar]
- 35.Vaya J., Mahmood S. Flavonoid content in leaf extracts of the fig (Ficus carica L.), carob (Ceratonia siliqua L.) and pistachio (Pistacia lentiscus L.) Biofactors. 2006;28:169–175. doi: 10.1002/biof.5520280303. [DOI] [PubMed] [Google Scholar]
- 36.Turchi C.S., Ollis D.F. Photocatalytic degradation of organic water contaminants: Mechanisms involving hydroxyl radical attack. J. Catal. 1990;122:178–192. doi: 10.1016/0021-9517(90)90269-P. [DOI] [Google Scholar]
- 37.Al-Sayyed G., D’Oliveira J.C., Pichat P. Semiconductor-sensitized photodegradation of 4-chlorophenol in water. J. Photochem. Photobiol. A Chem. 1991;58:99–114. doi: 10.1016/1010-6030(91)87101-Z. [DOI] [Google Scholar]
- 38.Yaqoob A.A., Parveen T., Umar K., Mohamad Ibrahim M.N. Role of nanomaterials in the treatment of wastewater: A review. Water. 2020;12:495. doi: 10.3390/w12020495. [DOI] [Google Scholar]
- 39.Rani M., Shanker U. Efficient degradation of organic pollutants by novel titanium dioxide coupled bismuth oxide nanocomposite: Green synthesis, kinetics and photoactivity. J. Environ. Manag. 2021;300:113777. doi: 10.1016/j.jenvman.2021.113777. [DOI] [PubMed] [Google Scholar]
- 40.Aravind M., Amalanathan M., Mary M.S.M. Synthesis of TiO2 nanoparticles by chemical and green synthesis methods and their multifaceted properties. SN Appl. Sci. 2021;3:409. doi: 10.1007/s42452-021-04281-5. [DOI] [Google Scholar]
- 41.Khade G.V., Suwarnkar M.B., Gavade N.L., Garadkar K.M. Green synthesis of TiO2 and its photocatalytic activity. J. Mater. Sci. Mater. Electron. 2015;26:3309–3315. doi: 10.1007/s10854-015-2832-7. [DOI] [Google Scholar]
- 42.Alkaykh S., Mbarek A., Ali-Shattle E.E. Photocatalytic degradation of methylene blue dye in aqueous solution by MnTiO3 nanoparticles under sunlight irradiation. Heliyon. 2020;6:e03663. doi: 10.1016/j.heliyon.2020.e03663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vasiljevic Z.Z., Dojcinovic M.P., Vujancevic J.D., Jankovic-Castvan I., Ognjanovic M., Tadic N.B., Stojadinovic S., Brankovic G.O., Nikolic M.V. Photocatalytic degradation of methylene blue under natural sunlight using iron titanate nanoparticles prepared by a modified sol–gel method. R. Soc. Open Sci. 2020;7:200708. doi: 10.1098/rsos.200708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xing Z., Li J., Wang Q., Zhou W., Tian G., Pan K., Tian C., Zou J., Fu H. A floating porous crystalline TiO2 ceramic with enhanced photocatalytic performance for wastewater decontamination. Eur. J. Inorg. Chem. 2013;13:2411–2417. doi: 10.1002/ejic.201201494. [DOI] [Google Scholar]
- 45.Xing Z., Zhou W., Du F., Qu Y., Tian G., Pan K., Tian C., Fu H. A floating macro/mesoporous crystalline anatase TiO2 ceramic with enhanced photocatalytic performance for recalcitrant wastewater degradation. Dalton Trans. 2014;43:790–798. doi: 10.1039/C3DT52433G. [DOI] [PubMed] [Google Scholar]
- 46.Song J., Wang X., Bu Y., Zhang J., Wang X., Huang J., Chen J., Zhao J. Preparation, characterization, and photocatalytic activity evaluation of Fe–N-codoped TiO2/fly ash cenospheres floating photocatalyst. Environ. Sci. Pollut. Res. 2016;23:22793–22802. doi: 10.1007/s11356-016-7353-2. [DOI] [PubMed] [Google Scholar]
- 47.Wang B., Li C., Pang J., Qing X., Zhai J., Li Q. Novel polypyrrole-sensitized hollow TiO2/fly ash cenospheres: Synthesis, characterization, and photocatalytic ability under visible light. Appl. Surf. Sci. 2012;258:9989–9996. doi: 10.1016/j.apsusc.2012.06.061. [DOI] [Google Scholar]
- 48.Singh S., Singh P.K., Mahalingam H. Novel floating Ag+-doped TiO2/polystyrene photocatalysts for the treatment of dye wastewater. Ind. Eng. Chem. Res. 2014;53:16332–16340. doi: 10.1021/ie502911a. [DOI] [Google Scholar]
- 49.Zhang L., Xing Z., Zhang H., Li Z., Zhang X., Zhang Y., Li L., Zhou W. Multifunctional floating titania-coated macro/mesoporous photocatalyst for efficient contaminant removal. ChemPlusChem. 2015;80:623. doi: 10.1002/cplu.201402327. [DOI] [PubMed] [Google Scholar]
- 50.Magalhães F., Moura F.C., Lago R.M. TiO2/LDPE composites: A new floating photocatalyst for solar degradation of organ iccontaminants. Desalination. 2011;276:266–271. doi: 10.1016/j.desal.2011.03.061. [DOI] [Google Scholar]
- 51.Zhong W., Yu Y., Du C., Li W., Wang Y., He G., Xie Y., He Q. Characterization and high pollutant removal ability of buoyant (C, N)–TiO2/PTFE flakes prepared by high-energy ball-milling. RSC Adv. 2014;4:40019–40028. doi: 10.1039/C4RA06496H. [DOI] [Google Scholar]
- 52.Xue H., Jiang Y., Yuan K., Yang T., Hou J., Cao C., Feng K., Wang X. Floating photocatalyst of B–N–TiO2/expanded perlite: A sol–gel synthesis with optimized mesoporous and high photocatalytic activity. Sci. Rep. 2016;6:29902. doi: 10.1038/srep29902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bhatti M.A., Almaani K.F., Shah A.A., Tahira A., Chandio A.D., Mugheri A.Q., Bhatti L.L., Waryani B., Medany S.S., Nafady A., et al. Low Temperature Aqueous Chemical Growth Method for the Doping of W into ZnO Nanostructures and Their Photocatalytic Role in the Degradration of Methylene Blue. J. Clust. Sci. 2022;33:1445–1456. doi: 10.1007/s10876-021-02069-6. [DOI] [Google Scholar]
- 54.Modestov A., Glezer V., Marjasin I., Lev O. Photocatalytic degradation of chlorinated phenoxyacetic acids by a new buoyant titania-exfoliated graphite composite photocatalyst. J. Phys. Chem. B. 1997;101:623–4629. doi: 10.1021/jp970132n. [DOI] [Google Scholar]
- 55.Han H., Bai R. Buoyant photocatalyst with greatly enhanced visible-light activity prepared through a low temperature hydrothermal method. Ind. Eng. Chem. Res. 2009;48:2891–2898. doi: 10.1021/ie801362a. [DOI] [Google Scholar]
- 56.Han H., Bai R. Highly effective buoyant photocatalyst prepared with a novel layered-TiO2 configuration on polypropylene fabric and the degradation performance for methyl orange dye under UV–Vis and Vis lights. Sep. Purif. Technol. 2010;73:142–150. doi: 10.1016/j.seppur.2010.03.017. [DOI] [Google Scholar]
- 57.Pinna M., Binda G., Altomare M., Marelli M., Dossi C., Monticelli D., Spanu D., Recchia S. Biochar nanoparticles over TiO2 nanotube arrays: A green co-catalyst to boost the photocatalytic degradation of organic pollutants. Catalysts. 2021;11:1048. doi: 10.3390/catal11091048. [DOI] [Google Scholar]
- 58.Dalto F., Kuźniarska-Biernacka I., Pereira C., Mesquita E., Soares O.S.G., Pereira M.F.R., Rosa M.J., Mestre A.S., Carvalho A.P., Freire C. Solar light-induced methylene blue removal over TiO2/AC composites and photocatalytic regeneration. Nanomaterials. 2021;11:3016. doi: 10.3390/nano11113016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chen Y., Wang C., Chen J., Wang S., Ju J., Kang W. Preparing Biomass Carbon Fiber Derived from Waste Rabbit Hair as a Carrier of TiO2 for Photocatalytic Degradation of Methylene Blue. Polymers. 2022;14:1593. doi: 10.3390/polym14081593. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.