Abstract
Acid-base disturbances in patients with cardiopulmonary or other disorders are common and are often misinterpreted or interpreted incompletely. Treating acid-base disorders in greater detail facilitates pathophysiologic understanding and improved therapeutic planning. Understanding the ratiometric relationship between the lungs, which excrete volatile acid as carbon dioxide, and the kidneys, which contribute to maintenance of plasma bicarbonate, allows precise identification of the dominant acid-base disturbance when more than a simple disorder is present and aids in executing a measured treatment response. Concordantly, mapping paired values of the partial pressure of carbon dioxide (PCO2) and the bicarbonate concentration ([HCO3–]) on a Cartesian coordinate system visually defines an acid-base disorder and validates the ratiometric methodology. We review and demonstrate the algebraic and logarithmic methods of arterial blood gas analysis through the example of a complex acid-base disorder, emphasizing examination of the PCO2-to-[HCO3–] ratio.
Keywords: acid/base and electrolyte disorders, acid-base disorders, arterial blood gas analysis, arterial blood gas interpretation, arterial blood gas measurement
Case
A 62-year-old man with a history of chronic obstructive pulmonary disease and congestive heart failure presented to an emergency department for dyspnea. He was tachypneic and fatigued and had left elbow swelling with erythema. He reported not having any fever, chills, nausea, or vomiting and was subsequently admitted for observation. The patient had a long-standing history of chronic obstructive pulmonary disease from long-term cigarette smoking. Two weeks before admission, the patient had developed bilateral lower extremity edema and an unspecified amount of weight gain. The patient’s primary care physician prescribed a thiazide diuretic to reduce the edema. After hospitalization, the patient’s plasma electrolytes were sodium 136 mM, potassium 1.8 mM, chloride 52 mM, total carbon dioxide (TCO2) 68 mM, BUN 46 mg/dl, and creatinine 1.21 mg/dL. An initial arterial blood gas (ABG) measurement revealed a pH of 7.5, partial pressure of oxygen (PO2) of 88.5 mm Hg, partial pressure of carbon dioxide (PCO2) of 93.7 mm Hg, and calculated a bicarbonate concentration [HCO3–] of 73.1 mM.
Introduction
This brief treatise aims to expose the mathematical intricacies of ABG analysis for greater comprehension of acid-base disturbances. Often, ABG interpretation is required to elucidate an underlying combination of disorders and their severity fully. Proper interpretation of ABG requires an appreciation of its measurement limitations and insight into the components that constitute an ABG. Adherence to a disciplined approach to ABG interpretation should precede examination of electrolytes (i.e., plasma/serum anion gap [AG]) (1,2). Mixed acid-base disorders should first be determined by accurate interpretation of ABG.
ABG Measurement
An ABG machine measures pH, PCO2, and PO2. The [HCO3–] is calculated. Arterial pH is normally 7.35–7.45, and PCO2 is normally 35–45 mm Hg (3,4) The pH, measured amperometrically as hydrogen (H+) activity, is accurate to within 0.01 pH units, and the PCO2, measured using bicarbonate electrode chemistry, is typically accurate to within ±2 mm Hg. The normal PO2 is age dependent, and the reader is referred to the published literature for these values. After acquisition of a specimen for ABG analysis, chilling the specimen on ice before laboratory measurement has been recommended. A 0°C-chilled sample may be analyzed up to 60 minutes later. However, if the specimen is transported to the laboratory within 5 minutes, no chilling is necessary. Unchilled samples must be analyzed rapidly because PCO2 increases by 3–10 mm Hg per hour. Consequently, the pH will decline, and the PO2 will decline. Air bubbles will usually lead to overestimation of PO2, but underestimation can occur among patients mechanically ventilated with PaO2 >150 mm Hg (5). Overheparinization of the sample will dilute [HCO3–], PCO2, and PO2 (6). This procedural error is obviated by preheparinized ABG sample collection syringes. Pain, either anticipatory or real from inaccurate needle entry during specimen acquisition, may lead to acute lowering of PCO2, with consequent respiratory alkalosis. Accordingly, less painful earlobe (heated) capillary blood may be used as a substitute for arterial blood, with excellent correlation between capillary and arterial ABG analyses for pH and PCO2 (7).
pH and [H+] Determinations
The concept of pH was originally defined in 1909 by Sørenson as the power (potenz) of [H+]10 (8). Currently, pH is defined as a function of H+ activity (a). Thus, pH equals –LOG aH+. Because the aH+ in physiologic fluids is 1, except in gastric fluids, pH becomes −LOG [H+] (nM), and [H+]=10(9 – pH). The concept of [H+] is easier to comprehend than logarithmic pH units and more easily manipulated because it is algebraically determined by the Henderson equation of 1908 (9,10):
| (1) |
Conventionally, the coefficient 23.9 is rounded to 24 for ease of calculation. After determining [H+] from the pH and measurement of PCO2, [HCO3] is calculated. The Henderson–Hasselbalch (H–H) equation of 1917 is a general equation that is applicable to the human bicarbonate-buffer system and is defined as Equation (2) (13) (11,13–15):
| (2) |
The H–H equation utilizes the apparent pKa (6.1) and the Bunsen coefficient (α, 0.0301 mmol/L⋅mm Hg) that defines the absorption of CO2 gas in plasma at 37.5°C at barometric pressure 760 mm Hg (14–16).
| (3) |
[H+] is determined to validate ABG [HCO3–] internally. [H+] can be estimated or calculated from pH. Internal validity of the ABG is accomplished by determining the [HCO3–] from the [H+] and PCO2 (1,2). Arterial [H+] is determined from pH and [HCO3–] from the Henderson equation. The Henderson equation–calculated [HCO3–] should closely approximate the [HCO3–] reported by the ABG machine.
Because of the near-linear relationship between pH and [H+] in the pH interval of 7.25–7.5, an estimated [H+] can be made by a “rule of 80” (Table 1) (1,2,16). Subtracting the last two digits of the pH from 80 approximates [H+]. Alternatively, one can successively multiply or divide [H+] from its normal baseline of 40 nM by 1.25 for each ΔpH of 0.1 units (Table 1). The multiplication/division method is the more accurate of these two estimation methods at pH >7.5 or <7.2 (1).
Table 1.
Estimated versus calculated [H+]
| pH | [H+], Rule of 80 | [H+], ×1.25, 0.8 | Actual [H+] |
|---|---|---|---|
| 7 | 80 | 97.7 | 100 |
| 7.05 | 75 | ×1.25 | 89.1 |
| 7.1 | 70 | 78.1 | 79.4 |
| 7.15 | 65 | ×1.25 | 70.8 |
| 7.2 | 60 | 62.5 | 63.1 |
| 7.25 | 55 | ×1.25 | 56.2 |
| 7.3 | 50 | 50 | 50.1 |
| 7.35 | 45 | ×0.8 | 44.7 |
| 7.4 | 40 | 40 | 39.8 |
| 7.45 | 35 | ×0.8 | 35.5 |
| 7.5 | 30 | 32 | 31.6 |
| 7.55 | 25 | ×0.8 | 28.2 |
| 7.6 | 20 | 25.6 | 25.1 |
In the “Rule of 80,” the last two digits of the pH plus [H+] equals 80 (column 2). An approximating method (column 3) that more closely reflects actual [H+] (column 4) successively multiplies the baseline [H+] (40 nM) by 1.25 or 0.8, respectively, for each 0.1 pH unit decrement (acidemia) or increment (alkalemia). [H+], hydrogen ion concentration (nM) (1,2,16).
Because hand-held calculators and smartphones have logarithmic functions, [H+] can be rapidly computed as [H+]=10(9–pH): in the above case, [H+]=10(9–7.5) or 31.6 nM. Determining [HCO3–] from [H+] rapidly yields a value of 71.2 mM (=24×93.7/31.6), equaling the calculated value of 70.6 mM and confirming internal validity of the data.
Previously, arterial [HCO3–] was determined from a nomogram after acquisition of pH and PCO2 (16–18). Because accuracy depended on the fastidiousness of the laboratory technologist, [HCO3–] was occasionally misinterpreted and reported in error, and an “impossible” ABG would be reported. Although [HCO3–] is now conveniently calculated by an autoanalyzer with embedded calculation of the H–H equation, the authors recommend performing a [HCO3–] validation step because ABG results are often transmitted by telephone in “stat” situations and transcription errors still occur.
Venous TCO2 and Blood Gas Bicarbonate Concentration
ABG autoanalyzers compute [HCO3–] from the H–H equation. The venous TCO2 or CO2 content is not a single analyte. It is composed of dissolved CO2, erythrocyte carbamino compounds, and carbonic acid (H2CO3) and has historically been determined by addition of strong alkali to plasma, electrolytic assay, or by an enzymatic technique, which was used in the index patient (12,15,16,19). The H–H equation denominator equals dCO2 plus short-lived carbonic acid (H2CO3): i.e., only 1H2CO3 per 340 molecules of CO2 (12,15,20). Thus, dCO2 as the anhydride of H2CO3 is 1.2 mmol/L at PCO2 40 mm Hg (1.2=0.0301×40) as described by the H–H equation [Equation (2)].
Depending on the institution’s practices, venous serum or plasma TCO2 concentration may be reported as “bicarbonate,” and bicarbonate represents 95% of TCO2 (0.95=[24/(24+0.0301×40]) (12,15,21). Substitution of the venous TCO2 for arterial [HCO3–] is a long-standing and errant practice, although espoused by some (22,23). Unless an ABG and venous sample are drawn simultaneously and processed rapidly, even correctly assayed values may differ. The practice of substituting venous TCO2 for arterial [HCO3–] practice is eschewed, particularly in critical care settings, where acid-base changes often transpire rapidly. Venous TCO2 usually exceeds arterial [HCO3–] because of higher venous PCO2 tensions. Under clinically stable circumstances, venous TCO2 is generally no greater than the arterial [HCO3–] by 2–4 mM (12,21), the difference representing the difference between venous and arterial blood and CO2 medicated H+ buffering by hemoglobin, with a negligible contribution of erythrocyte carbamino-CO2 (12,15,16).
Agreement between central venous and ABG parameters have been published: venous pH+0.03=arterial pH and venous [HCO3–]–arterial [HCO3–]=0.5 mM (24,25). Peripheral venous blood gas (VBG) versus ABG parameters of pH, PCO2, and [HCO3–] have been examined. The VBG pH is 0.03–0.04 less than from a corresponding ABG (26–32). The VBG PCO2 correlates with its arterial counterpart. The VBG PCO2 averages 4.4–8.6 mm Hg greater than the ABG PCO2, with large confidence intervals (23,26,29,31–33). The VBG [HCO3–] is approximately 0.52–1.5 mM greater than the ABG [HCO3–]. The aforementioned studies excluded patients in circulatory shock, and severe hemodynamic compromise where these correlations may not be applicable, and an ABG is warranted (23,26,29,30,32).
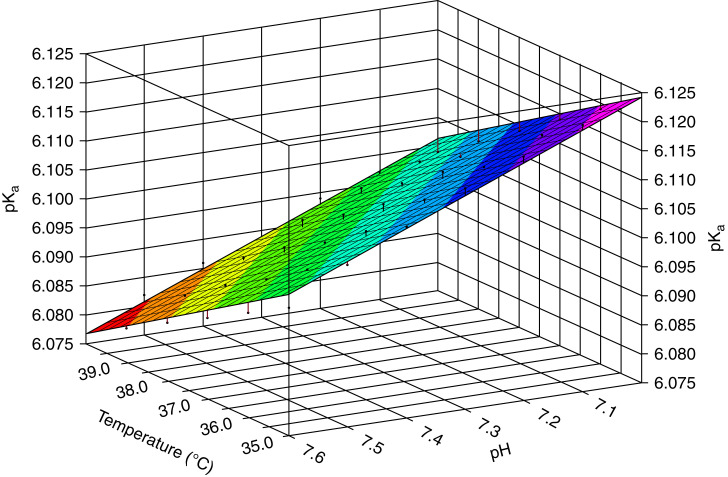
When venous TCO2 exceeds arterial [HCO3–] by >4 mM, considerations include venous CO2 accrual from tourniquet-induced stasis or dilution of the blood gas by heparin (34). Larger discrepancies are encountered in critically ill patients with extreme pH deviations from the norm, which may be attributable to blood gas autoanalyzer calculations using a constant pKa of 6.1 (12,15,34). The value of pKa is not only ionic strength and temperature dependent but also pH dependent (12,15,16,35,36). We reanalyzed the relationship between temperature, plasma pH, and pKa values. We conducted regression analysis of the originally published data (15) for the temperature range 10°C–40°C and pH range 7–7.6 (Datafit; Oakdale Engineering, Oakdale, PA). pKa is a linear function of temperature and pH with R2=0.9944: pKa=6.6605−0.0044⋅°C−0.0542⋅pH. This equation is concordant with that published by Kim (10) (Figure 1). For extreme pH values per se <7.10 or >7.80 or temperatures <32°C or >39°C, an adjusted pKa may be required to determine [HCO3–] accurately:
| (4a) |
Figure 1.
Temperature and pH dependency of pKa. Multiple regression analysis of original data from the relationship of pKa′ to pH and temperature revealed a linear equation: pKa′=6.6605−0.0044⋅°C−0.0542⋅pH; R2=0.99 (15).
A rapid calculation that determines whether the actual pKa has diverged from the canonical value of 6.1 is shown below (36). Measured [HCO3–]=TCO2–dCO2.
| (4b) |
This equation is used when TCO2 is significantly less than arterial [HCO3–]. Venous plasma TCO2 is determined by an enzymatic and spectrophotometric method and not by ABG machines. Thus, discrepancies can be a result of a number of reasons. In the index case, the patient’s temperature was normal, and the pH was 7.5. No pKa adjustments were made. Notably, the calculated [HCO3–] was greater than venous TCO2 by 5 mM. The possibility of an endogenous interferent of the TCO2 assay was considered, but this abnormality did not persist (34). A spurious loss of CO2 gas from plasma attributable to underfilling of a vacuum collection tube or during specimen processing may have also produced this atypical directional bias (34). More commonly, pseudohypobicarbonatemia from extremely elevated lipid levels may produce a much lower TCO2 than [HCO3–] than calculated (37). Alternatively, [HCO3–] can be determined as below via the Henderson equation or following equation, using Ka of 794 [antilog (9–6.1)] and deriving [H+] from pH [Equation (1)]. An adjusted Ka must be calculated when pKa is adjusted; i.e., Ka=10–pKa [Equation (2)]. This adjustment is rarely required clinically (12).
| (4c) |
If variations in pKa and elevated PCO2 do not provide adequate explanation for exceptionally significant differences between TCO2 and [HCO3–], troubleshooting is required (12,36). Analytical measurement errors of TCO2 should be ruled out. Errant input of patient temperature can affect the ABG autoanalyzer pH output. Misreporting of one of the ABG results is always possible. If no consistent source of error is elucidated by these steps, an independent [HCO3–] calculation using pKa of 6.1 [Equation (3)] is the last step.
Relationships: pH, [H+], and RpH
We define RpH as the ratio of PCO2 to [HCO3–], and this ratio defines a specific pH/[H+].
| (5) |
RpH qualitatively and quantitatively reflects the acid-base relationship between the regulation of CO2 by the lungs: i.e., PCO2 and the kidneys that control bicarbonate reabsorption, proton secretion, and bicarbonate regeneration as ammonium excretion. For convenience, the Henderson equation expresses the “lung” parameter (PCO2 numerator) divided by the “kidney” parameter ([HCO3–] denominator). Consequently, RpH is determined by [H+] in a linear fashion (Figure 2, Table 2) but in a curvilinear fashion when related to pH (Figure 3). In Equation 6a, RpH is inserted into a modified H–H equation that emphasizes the physiologic importance of the ratio of pulmonary ventilation to renal bicarbonate balancing. Equation 6b defines a “magical” pH of 7.62 when the PCO2 to [HCO3–] ratio equals 1 (38).
| (6a) |
| (6b) |
Figure 2.
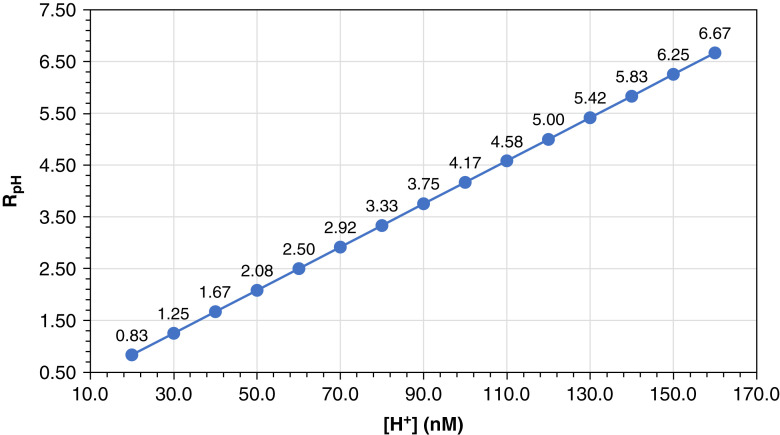
RpH versus [H+]. The relationship of the ratio of PCO2 to [HCO3–] (RpH) to [H+] () is linear and derived from the Henderson equation, [H+]=24×(PCO2/[HCO3–]). [H+], hydrogen ion concentration [H+] (nM); [HCO3–], bicarbonate concentration (mM); PCO2, partial pressure of carbon dioxide (mm Hg).
Table 2.
Relationships among pH, [H+], and RpH
| pH | [H+] | RpH |
|---|---|---|
| 7 | 100 | 4.17 |
| 7.1 | 79 | 3.33 |
| 7.2 | 63 | 2.63 |
| 7.3 | 50 | 2.09 |
| 7.4 | 40 | 1.67 |
| 7.5 | 32 | 1.32 |
| 7.6 | 25 | 1.05 |
| 7.7 | 20 | 0.83 |
As pH increases, the [H+] and RpH decrease in a linear and parallel fashion. With increases or decreases of 0.3 pH units, [H+] and RpH are halved or doubled, respectively. Calculations are determined from the Henderson equation. [H+], hydrogen ion concentration (nM); RpH, ratio of pCO2 to [HCO3–] (mm Hg⋅L/mmol) (9,10,36).
Figure 3.
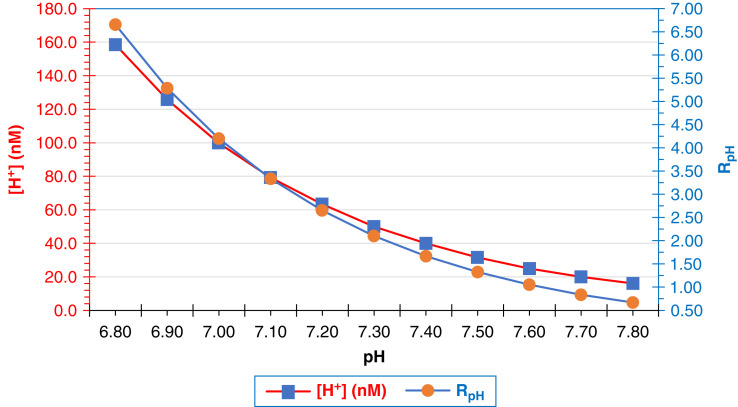
[H+] and RpH versus pH. The relationships of [H+] (left y axis, ) and RpH (right y axis, ), the ratio of PCO2 to [HCO3–] is logarithmically related to pH.
The index patient’s RpH, or R7.5, equals 1.33=93.7/70.6. This ratio is lower than the normal R7.4 of 1.67, or the normal ratio defined by PCO2 40 mm Hg and [HCO3–] 24 mM. As a corollary, any condition in which the PCO2 to [HCO3–] ratio equals 1.67 will have a pH of 7.4.
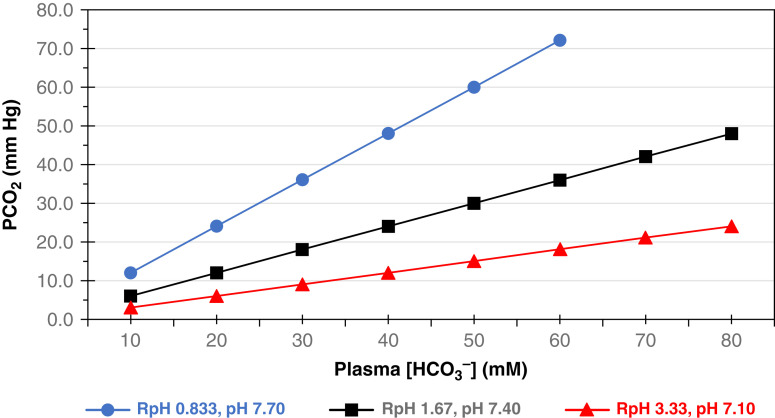
Specific relationships among the parameters pH, [H+], and RpH deserve mention. At a pH of 7.4, [H+]=40 nM and RpH=1.67. Because of the logarithmic relationship between pH and [H+], when the pH is 0.3 units more acidemic than normal (pH 7.1), the [H+] and RpH double. Moreover, when [H+] and RpH are halved, the plasma pH increases by 0.3 units to a severely alkalemic level of pH 7.7 (Figure 4). At any pH, a pH isopleth delineates the slope of the relationship between PCO2 and [HCO3–] (Figure 4). The pH isopleth represents the third dimension of a topological map of physiologic representations of pH, PCO2, and [HCO3–]. The figure is a remodeling of the original Davenport diagrams where pH isopleths are depicted instead of PCO2 isobars (16). The reconfiguration designates pH domains as functions of [HCO3–] and PCO2. Each pH isopleth represents a “state” of the relationship of PCO2 and [HCO3–]: i.e., RpH.
Figure 4.
Relationship of PCO2 to [HCO3–] at specific pH. Isopleths for pH 7.1 (), 7.4 (), and 7.7 () are plotted as functions of paired [HCO3–] and PCO2. Each isopleth slope equals the ratio of PCO2 to [HCO3–] (RpH). As pH varies in 0.3 increments, [H+] doubles or halves in concert with RpH. Accordingly, the respective slopes of RpH are 0.833, 1.67, and 3.33 at pH units of 7.7, 7.4, and 7.1. Corresponding, [H+] is a function of RpH.
ABG Interpretation
Determination of acid-base disorders requires a disciplined approach. This process involves three steps: (1) validation of the ABG; (2) delineation of primary disorders; and (3) determination of the dominant disorder. These steps can identify up to two primary disorders and should be conducted before examination of electrolytes.
After validating the ABG, identifying primary acid-base disturbances follows. There are four primary acid-base disorders: respiratory acidosis, respiratory alkalosis, metabolic acidosis, and metabolic alkalosis (1,2). Respiratory disorders can be subcategorized as acute or chronic disorders. Because one cannot simultaneously hypo- and hyperventilate, two primary respiratory disorders cannot occur simultaneously. In healthy individuals, a primary acid-base disturbance will invoke countervailing compensation by the “other” organ. For example, in metabolic acidosis, lowered [HCO3–] is compensated by increased “lung” ventilation (i.e., ↓PCO2). However, the respiratory compensation is incomplete, and arterial pH/[H+] is not returned to the baseline.
Simple or pure acid-base disturbances follow this rule: PCO2 and [HCO3–] are displaced in the same direction, and the resulting [H+] or pH equals that predicted by known data. Such data have been obtained empirically from dogs and humans (39–42). When the limitations of compensation formulas are exceeded, a second primary disturbance is present. Prediction rules for the six acid-base disorders are listed in Table 3. In chronic respiratory alkalosis, renal compensation is highly effective, and arterial pH may nearly normalize (2).
Table 3.
Linear compensation formulas for the four primary acid-base disorders
| Acid-Base Disorder | Compensation Equation |
|---|---|
| Metabolic acidosis | PCO2=1.54×[HCO3–]+8.36±2.2 ↓ΔPCO2=1.1×↓Δ[HCO3–] |
| Metabolic alkalosis | PCO2=0.7×[HCO3–]+20±5 ↑ΔPCO2=0.75×↑Δ[HCO3–] |
| Acute respiratory acidosis | ↑Δ[HCO3–]=0.1×↑ΔPCO2 |
| Chronic respiratory acidosis | ↑Δ[HCO3–]=0.35×↑ΔPCO2 |
| Acute respiratory alkalosis | ↓Δ[HCO3–]=0.2×↓ΔPCO2 |
| Chronic respiratory alkalosis | [HCO3–]=0.41×PCO2+9.1 ↓Δ[HCO3–]=0.41×↓ΔPCO2 |
When compensation equations are not fulfilled in the face of a valid ABG analysis, a mixed acid-base problem is present, and the pH or the parameter that is most disproportionately displaced from its baseline value is the dominant disorder: i.e., the disorder that displaces pH most greatly. Alternatively, one may choose one of the two disorders and apply its compensation equation to determine what the pH (or [H+]) would be if there were only a single acid-base disturbance. The “distance” from pH 7.4 would indicate whether this disorder was dominant.
After an ABG has been analyzed to this point, the AG is examined. The AG may disclose a third acid-base disturbance and/or further characterize one of the disorders already identified. Electrolyte analysis detects the “triple disorder” of high AG metabolic acidosis, hyperchloremic metabolic acidosis, and an acute or chronic respiratory disorder.
Three general rules to follow when interpreting ABG analyses are as follows. First, if the pH is normal and either PCO2 or [HCO3–] has varied from its respective baseline level (PCO2, 40 mm Hg; [HCO3–], 24 mM), there are two offsetting disorders: metabolic acidosis/alkalosis and a respiratory alkalosis/acidosis. Second, if the pH is extremely displaced from normal (i.e., <7.2 or >7.55), the probability is that two acid-base disturbances are moving [H+] in the same direction (i.e., two acidoses or two alkaloses). Third, when there is a mixed acid-base disorder, the degree of variation of either PCO2 or [HCO3–] from its baseline level usually indicates which disorder is dominant (i.e., a respiratory one or a metabolic one). In all cases, findings established from these general qualitative rules should be verified by quantitative analysis.
The acid-base disorder of the index case is a mixed metabolic alkalosis with chronic respiratory acidosis. The ABG is quantitatively analyzed by a ratiometric analysis that delineates metabolic alkalosis as the dominant disorder, despite the severity of chronic respiratory acidosis. (Table 4) The RpH of 1.33=93.7/70.6 is lower than the normal ratio of 1.67, thereby defining an alkalemia of pH 7.5 or [H+] of 31.6 nM. The ratio of current [HCO3–] to normal [HCO3–]=2.94=70.6/24. This ratio is greater than the corresponding PCO2 to normal [PCO2] ratio of 2.34=93.7/40. The higher ratio of 2.94 exerts the greater effect of metabolic alkalosis on pH than the chronic respiratory acidosis. This ratiometric analysis of the numerator and denominator of RpH thus defines the respective severities of each component of a mixed acid-base disorder. The calculations are tabulated and additional examples of the ratiometric analytic approach to acid-base disorders are provided (Table 5).
Table 4.
Ratiometric analysis of arterial blood gas from index case
| Parameter | Patient Value | Normal Value | Ratio |
|---|---|---|---|
| pH | 7.5 | 7.4 | — |
| [H+] | 31.62 nM | 40 | — |
| PCO2 | 93.7 mm Hg | 40 | 2.34 |
| [HCO3] | 70.58 mM | 24 | 2.94 |
Both metabolic and primary respiratory alkalosis are present. The greater ratio for plasma [HCO3–] is consistent with metabolic alkalosis as the dominant acid-base disorder. [H+], hydrogen ion concentration (nM); PCO2, carbon dioxide partial pressure (mm Hg); [HCO3–], bicarbonate concentration (mM).
Table 5.
Mixed acid-base disturbance
| [H+] | pH | PCO2 | [HCO3–] | Acid-Base Disorders | Dominant Disorder |
|---|---|---|---|---|---|
| 39.8 | 7.4 | 21.6 (0.54) | 13 (0.54) | Metabolic acidosis, chronic respiratory alkalosis | — |
| 19.03 | 7.72 | 23.8 (0.6) | 30 (1.25) | Metabolic alkalosis, acute respiratory alkalosis | Acute respiratory alkalosis |
| 77.6 | 7.11 | 65 (1.63) | 20 (0.83) | Metabolic acidosis, acute respiratory acidosis | Acute respiratory acidosis |
| 63.1 | 7.2 | 35 (0.88) | 13.3 (0.55) | Metabolic acidosis, acute respiratory acidosis | Metabolic acidosis |
Each row represents a mixed acid-base disturbance comprising a metabolic disturbance and a respiratory one. Parenthetical numbers indicate the ratio of a parameter to its respective baseline, “ideal” value: pH 7.4, PCO2 40 mm Hg, and [HCO3–] 24 mM. The last column denotes a disturbance that altered the [H+]/pH to a greater degree: i.e., the dominant disorder. No inference is implied regarding the order of appearance of acid-base disturbances. These mixed acid-base disorders can be visualized on the acid-base map (Figure 5B). [H+], hydrogen ion concentration (nM); PCO2, carbon dioxide partial pressure (mm Hg); [HCO3–], bicarbonate concentration (mM).
Quantitation of Parallel Mixed Acid-Base Disorders as Serial Processes
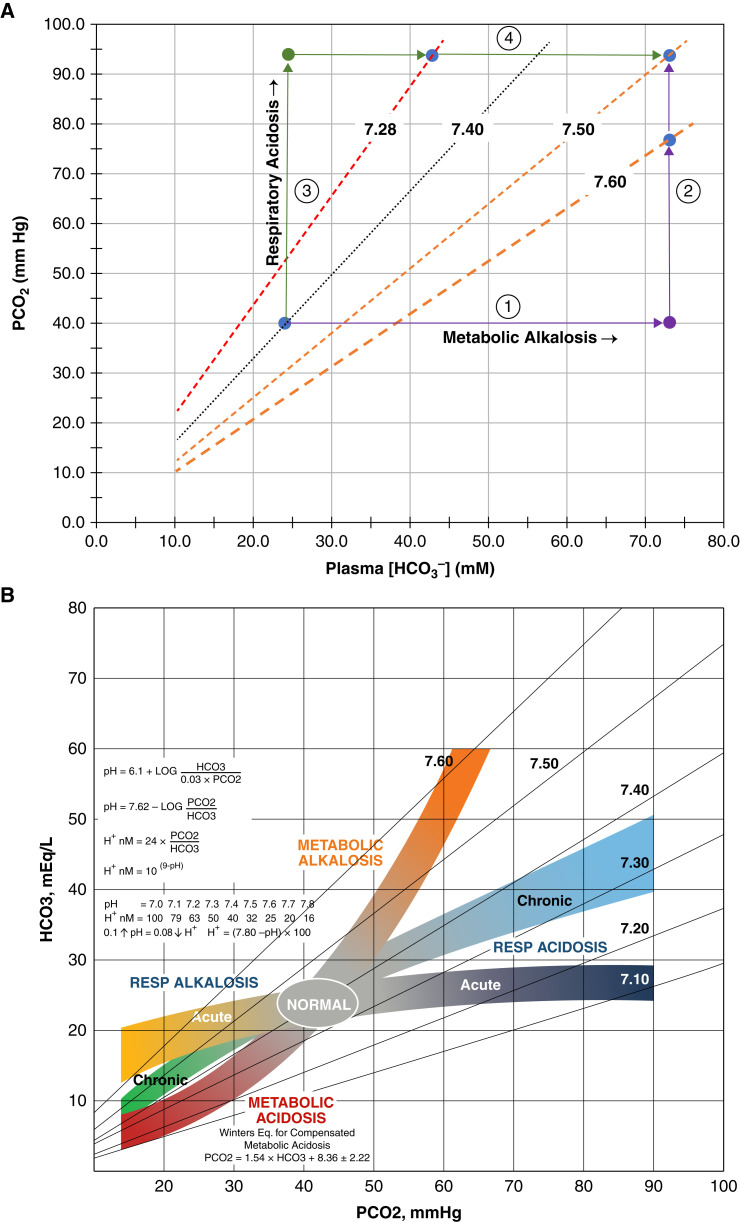
Mixed acid-base disorders that transpire in parallel can be treated as two serial disorders, virtually recapitulating prior canine experiments of mixed metabolic/respiratory acid-base disorders (43). If metabolic alkalosis were the only disorder and [HCO3–] increased from 24 to 73.1 mM, the compensatory increase in PCO2 is approximately 76.8 mm Hg (Figure 5A). The pH would increase to 7.6=7.62–log(76.8/73.1)—a displacement of +0.2 pH units from normal. The final pH of 7.5 is achieved by the addition of a chronic respiratory acidosis that increases PCO2 to 93.7 mm Hg while lowering the pH by 0.1 pH units. The greater change in pH that results from metabolic alkalosis confirms that it represents the dominant acid-base disorder, with twice the effect on pH.
Figure 5.
Quantitative analysis and clinical illustration of acid-base disorders. (A) Quantitative analysis of acid-base disorder provided from index case. The arterial blood gas of the index patient: pH 7.5, PCO2 93.7 mm Hg, and [HCO3–] 73.1 mM. The blue filled circle corresponds to the normal PCO2 of 40 mm Hg and plasma [HCO3–] of 24 mM at pH 7.4. The dotted lines are pH isopleths that bound pH values from 7.28 to 7.6. Conceptualization of a parallel-process, mixed acid-base disturbance developing as two distinct processes along two separate and convergent pathways: (A) metabolic alkalosis with respiratory compensation (arrow 1) followed by superimposed chronic respiratory acidosis (arrow 2) and (B) chronic respiratory acidosis with appropriate bicarbonate retention (arrow 3) followed by metabolic alkalosis (arrow 4). (B) Clinical illustration of mixed acid-base disorder provided from index case. Colored bands represent the known boundaries and compensatory responses of the six acid-base disturbances. The pathogenesis of the arterial blood gas of the index patient: pH 7.5, PCO2 93.7 mm Hg, and [HCO3–] 73.1 mM can occur by two separate and distinct pathways, both ending at a point of metabolic alkalosis and chronic respiratory acidosis. Chronic respiratory acidosis from the normal state (oval labeled “NORMAL”) increases PCO2 to 93.7 mm Hg () and followed by a metabolic alkalosis () with final [HCO3–] of 73.1 mM. Alternatively, metabolic alkalosis () precedes the chronic respiratory acidosis. [HCO3–] increases initially to 73.1 mM and followed by hypercapnia with final PCO2 of 93.7 mm Hg (). (Kidney Kard v4, © 2016. Courtesy, Jerry Yee and Mark L. Graeber).
On the other hand, if chronic respiratory acidosis had occurred first, the increase in PCO2 would have driven [HCO3–] to 42.8 mM, with a PCO2 of 93.7 mm Hg and pH of 7.28 (Figure 5A). A superimposed metabolic alkalosis that increases [HCO3–] from 42.8 to 73.1 mM, with unchanged PCO2, increases the pH by +0.22 units to 7.5. Again, the absolute difference in pH changes between the two acid-base disturbances is 0.2 pH units. On an acid-base map, we illustrate the separation of this dominant metabolic alkalosis–chronic respiratory acidosis disorder into separate pathways of two serial processes (Figure 5B).
Summary
The ABG remains the primary tool in analysis of acid-base disorders, and the following should be dealt with seriatim. Internal validation by the Henderson equation should be conducted on each blood gas, and its subsequent interpretation is conducted independently of the basic metabolic panel review. Significant differences between venous TCO2 and arterial [HCO3–] must be resolved. Appropriate quantitative blood gas analysis can reveal up to two acid-base disturbances. Subsequent ratiometric ABG analysis of [HCO3–] and PCO2 can reveal the dominant acid-base disturbances. Separation of mixed acid-base disturbances that proceed in parallel into its separately occurring serial components enhances pathophysiologic comprehension.
Disclosures
S. Frinak reports consultancy for Vasc-Alert; ownership interest in Vasc-Alert; and patents or royalties from Henry Ford Health System licensed to Vasc-Alert. J. Yee reports consultancy for AstraZeneca, Ardelyx, Bayer, EBSCO/Dynamed, Elsevier, and GLG; ownership interest in Vasc-Alert; honoraria from AlphaSights, Ardelyx, AstraZeneca, Fresenius Medical Corporation, North America, Gerson Lehman Group, and GLG; patents or royalties from Vasc-Alert; and other interests or relationships with American Journal of Nephrology (editorial board), BMC Nephrology (editorial board), EBSCO/DynaMed (editorial board), Elsevier (clinical key author and section editor), Ferri’s (clinical advisor 2022), Journal of OncoNephrology (editorial board); and Springer Heart Failure Reviews (editorial board). All remaining authors have nothing to disclose.
Funding
None.
Acknowledgments
The authors are grateful for the artistic rendering of Figure 5B by Gerard Zasuwa.
Author Contributions
S. Frinak and J. Yee were responsible for formal analysis, software, and supervision; J. Yee was responsible for conceptualization, methodology, resources, validation, and visualization; and all authors wrote the original draft of the manuscript and reviewed and edited the manuscript.
References
- 1.Narins RG, Emmett M: Simple and mixed acid-base disorders: A practical approach. Medicine (Baltimore) 59: 161–187, 1980. 10.1097/00005792-198005000-00001 [DOI] [PubMed] [Google Scholar]
- 2.Narins RG, Jones ER, Stom MC, Rudnick MR, Bastl CP: Diagnostic strategies in disorders of fluid, electrolyte and acid-base homeostasis. Am J Med 72: 496–520, 1982. 10.1016/0002-9343(82)90521-6 [DOI] [PubMed] [Google Scholar]
- 3.Cowley NJ, Owen A, Bion JF: Interpreting arterial blood gas results. BMJ 346: f16, 2013. 10.1136/bmj.f16 [DOI] [PubMed] [Google Scholar]
- 4.Larkin BG, Zimmanck RJ: Interpreting arterial blood gases successfully. AORN J 102: 343–354, quiz 355–357, 2015. 10.1016/j.aorn.2015.08.002 [DOI] [PubMed] [Google Scholar]
- 5.Biswas CK, Ramos JM, Agroyannis B, Kerr DN: Blood gas analysis: Effect of air bubbles in syringe and delay in estimation. Br Med J (Clin Res Ed) 284: 923–927, 1982. 10.1136/bmj.284.6320.923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gilbert HC, Vender JS: Arterial blood gas monitoring. Crit Care Clin 11: 233–248, 1995. 10.1016/S0749-0704(18)30094-0 [DOI] [PubMed] [Google Scholar]
- 7.Richter S, Kerry C, Hassan N, Chari A, Lunn D, Nickol A: Capillary blood gas as a substitute for arterial blood gas: A meta-analysis. Br J Hosp Med (Lond) 75: 136–142, 2014. 10.12968/hmed.2014.75.3.136 [DOI] [PubMed] [Google Scholar]
- 8.Siggaard-Andersen OS: Titratable acid or base of body fluids. Ann N Y Acad Sci 133: 41–58, 1966. 10.1111/j.1749-6632.1966.tb50707.x [DOI] [PubMed] [Google Scholar]
- 9.Henderson LJ: Concerning the relationship between the strength of acids and their capacity to preserve neutrality. Am J Physiol 21: 173–179, 1908. 10.1152/ajplegacy.1908.21.2.173 [DOI] [Google Scholar]
- 10.Henderson LJ: The theory of neutrality regulation in the animal organism. Am J Physiol 21: 427–448, 1908. 10.1152/ajplegacy.1908.21.4.427 [DOI] [Google Scholar]
- 11.Hasselbalch KA: Die berechnung der wasserstoffzahl des blutes aus der freien und gebundenen kohlensäure desselben, und die sauerstoffbindung des blutes als funktion der wasserstoffzahl. Biochem Z 78: 112–144, 1917 [Google Scholar]
- 12.Kim Y, Massie L, Murata GH, Tzamaloukas AH: Discrepancy between measured serum total carbon dioxide content and bicarbonate concentration calculated from arterial blood gases. Cureus 7: e398, 2015. 10.7759/cureus.398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Constable PD: Clinical acid-base chemistry. In: Critical Care Nephrology, edited by Ronco P, Bellomo R, Kellum JA, Philadelphia, PA, Saunders, 2009, pp 581–586 10.1016/B978-1-4160-4252-5.50116-7 [DOI] [Google Scholar]
- 14.Segel IH: Blood buffers. In: Biochemical Calculations: How to Solve Mathematical Problems in General Biochemistry, 2nd Ed., Milan, MI, John Wiley, 1976, pp 83–84 [Google Scholar]
- 15.Madias NE, Cohen JJ: Acid-base chemistry and buffering. In: Acid-Base, edited by Cohen JJ, Kassirer JP, Boston, MA, Little, Brown and Company, 1982, pp 3–24 [Google Scholar]
- 16.Davenport HW: The ABC of Acid-base Chemistry: The Elements of Physiological Blood-gas Chemistry for Medical Students and Physicians, 6th Ed. (revised), Chicago, IL, University of Chicago Press, 1974 [Google Scholar]
- 17.Andersen OS: Blood acid-base alignment nomogram. Scales for pH, pCO2 base excess of whole blood of different hemoglobin concentrations, plasma bicarbonate, and plasma total-CO2. Scand J Clin Lab Invest 15: 211–217, 1963. 10.3109/00365516309079734 [DOI] [PubMed] [Google Scholar]
- 18.Andersen OS: The pH-log pCO2 blood acid-base nomogram revised. Scand J Clin Lab Invest 14: 598–604, 1962. 10.1080/00365516209051290 [DOI] [PubMed] [Google Scholar]
- 19.Chittamma A, Vanavanan S: Comparative study of calculated and measured total carbon dioxide. Clin Chem Lab Med 46: 15–17, 2008. 10.1515/CCLM.2008.005 [DOI] [PubMed] [Google Scholar]
- 20.McLean FC: Application of the law of chemical equilibrium (law of mass action) to biological problems. Physiol Rev 18: 495–523, 1938. 10.1152/physrev.1938.18.4.495 [DOI] [Google Scholar]
- 21.Centor RM: Serum total carbon dioxide. In: Clinical Methods: The History, Physical, and Laboratory Examinations, edited by Walker HK, Hall WD, Hurst JW, 3rd Ed., Chapter 196, Boston, MA, Butterworths, 1990 [PubMed] [Google Scholar]
- 22.Kaynar AM: Arterial blood gas interpretation. In: Textbook of Critical Care, edited by Vincent JL, Abraham E, Moore FA, Kochanek PM, Fink PM, 7th Ed., Philadelphia, PA, Elsevier, 2017, pp 167–174 [Google Scholar]
- 23.Rang LC, Murray HE, Wells GA, Macgougan CK: Can peripheral venous blood gases replace arterial blood gases in emergency department patients? CJEM 4: 7–15, 2002. 10.1017/S1481803500006011 [DOI] [PubMed] [Google Scholar]
- 24.Middleton P, Kelly AM, Brown J, Robertson M: Agreement between arterial and central venous values for pH, bicarbonate, base excess, and lactate. Emerg Med J 23: 622–624, 2006. 10.1136/emj.2006.035915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chong WH, Saha BK, Medarov BI: Comparing central venous blood gas to arterial blood gas and determining its utility in critically ill patients: Narrative review. Anesth Analg 133: 374–378, 2021. 10.1213/ANE.0000000000005501 [DOI] [PubMed] [Google Scholar]
- 26.Kelly AM, McAlpine R, Kyle E: Venous pH can safely replace arterial pH in the initial evaluation of patients in the emergency department. Emerg Med J 18: 340–342, 2001. 10.1136/emj.18.5.340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Razi E, Nasiri O, Akbari H, Razi A: Correlation of arterial blood gas measurements with venous blood gas values in mechanically ventilated patients. Tanaffos 11: 30–35, 2012 [PMC free article] [PubMed] [Google Scholar]
- 28.Brandenburg MA, Dire DJ: Comparison of arterial and venous blood gas values in the initial emergency department evaluation of patients with diabetic ketoacidosis. Ann Emerg Med 31: 459–465, 1998. 10.1016/S0196-0644(98)70254-9 [DOI] [PubMed] [Google Scholar]
- 29.McCanny P, Bennett K, Staunton P, McMahon G: Venous vs arterial blood gases in the assessment of patients presenting with an exacerbation of chronic obstructive pulmonary disease. Am J Emerg Med 30: 896–900, 2012. 10.1016/j.ajem.2011.06.011 [DOI] [PubMed] [Google Scholar]
- 30.Byrne AL, Bennett M, Chatterji R, Symons R, Pace NL, Thomas PS: Peripheral venous and arterial blood gas analysis in adults: Are they comparable? A systematic review and meta-analysis. Respirology 19: 168–175, 2014. 10.1111/resp.12225 [DOI] [PubMed] [Google Scholar]
- 31.Malinoski DJ, Todd SR, Slone S, Mullins RJ, Schreiber MA: Correlation of central venous and arterial blood gas measurements in mechanically ventilated trauma patients. Arch Surg 140: 1122–1125, 2005. 10.1001/archsurg.140.11.1122 [DOI] [PubMed] [Google Scholar]
- 32.Malatesha G, Singh NK, Bharija A, Rehani B, Goel A: Comparison of arterial and venous pH, bicarbonate, PCO2 and PO2 in initial emergency department assessment. Emerg Med J 24: 569–571, 2007. 10.1136/emj.2007.046979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bloom BM, Grundlingh J, Bestwick JP, Harris T: The role of venous blood gas in the emergency department: A systematic review and meta-analysis. Eur J Emerg Med 21: 81–88, 2014. 10.1097/MEJ.0b013e32836437cf [DOI] [PubMed] [Google Scholar]
- 34.Goldwasser P, Manjappa NG, Luhrs CA, Barth RH: Pseudohypobicarbonatemia caused by an endogenous assay interferent: A new entity. Am J Kidney Dis 58: 617–620, 2011. 10.1053/j.ajkd.2011.06.003 [DOI] [PubMed] [Google Scholar]
- 35.Bradley AF, Severinghaus JW, Stupfel M: Variations of serum carbonic acid pK with pH and temperature. J Appl Physiol 9: 197–200, 1956. 10.1152/jappl.1956.9.2.197 [DOI] [PubMed] [Google Scholar]
- 36.O’Leary TD, Langton SR: Calculated bicarbonate or total carbon dioxide? Clin Chem 35: 1697–1700, 1989. 10.1093/clinchem/35.8.1697 [DOI] [PubMed] [Google Scholar]
- 37.Uduman J, Yee J: Pseudo-renal tubular acidosis: conditions mimicking renal tubular acidosis. Adv Chronic Kidney Dis 25: 358–365, 2018. 10.1053/j.ackd.2018.05.001 [DOI] [PubMed] [Google Scholar]
- 38.Albert MS, Dell RB, Winters RW: Quantitative displacement of acid-base equilibrium in metabolic acidosis. Ann Intern Med 66: 312–322, 1967. 10.7326/0003-4819-66-2-312 [DOI] [PubMed] [Google Scholar]
- 39.Javaheri S, Shore NS, Rose B, Kazemi H: Compensatory hypoventilation in metabolic alkalosis. Chest 81: 296–301, 1982. 10.1378/chest.81.3.296 [DOI] [PubMed] [Google Scholar]
- 40.Krapf R, Beeler I, Hertner D, Hulter HN: Chronic respiratory alkalosis. The effect of sustained hyperventilation on renal regulation of acid-base equilibrium. N Engl J Med 324: 1394–1401, 1991. 10.1056/NEJM199105163242003 [DOI] [PubMed] [Google Scholar]
- 41.Grogono AW: Acid-base reports need a text explanation. Anesthesiology 130: 668–669, 2019. 10.1097/ALN.0000000000002628 [DOI] [PubMed] [Google Scholar]
- 42.Morganroth ML: An analytic approach to diagnosing acid-base disorders. J Crit Illn 5: 138–150, 1990 [Google Scholar]
- 43.Bercovici M, Chen CB, Goldstein MB, Steinbaugh BJ, Halperin ML: Effect of acute changes in the PaCO2 on acid-base parameters in normal dogs and dogs with metabolic acidosis or alkalosis. Can J Physiol Pharmacol 61: 166–173, 1983. 10.1139/y83-025 [DOI] [PubMed] [Google Scholar]