Abstract
Polycystic ovary syndrome (PCOS) is the most common endocrine disorder in women of reproductive age, affecting approximately 10%. PCOS is diagnosed by the presence of at least two of these three criteria: hyperandrogenemia, oligo- or anovulation, and polycystic ovaries. The most common type (80%) of PCOS includes hyperandrogenemia. PCOS is also characterized by obesity or overweight (in 80% of US women with PCOS), insulin resistance with elevated plasma insulin but not necessarily hyperglycemia, dyslipidemia, proteinuria, and elevated BP. Although elevated compared with age-matched controls, BP may not reach levels considered treatable according to the current clinical hypertension guidelines. However, it is well known that elevated BP, even modestly so, increases the risk of cardiovascular disease. We have developed a model of hyperandrogenemia in rodents that mimics the characteristics of PCOS in women, with increases in body weight, insulin resistance, dyslipidemia, andproteinuria and elevated BP. This review discusses potential mechanisms responsible for the elevated BP in the adult and aging PCOS rat model that may be extrapolated to women with PCOS.
Keywords: hypertension, aging, basic science, blood pressure, hyperandrogenemia, obesity, polycystic ovary syndrome, pregnancy, renin-angiotensin system
Polycystic ovary syndrome (PCOS) is the most common endocrine disorder in women of reproductive age, affecting approximately 10% (1). On the basis of the Rotterdam criteria (2003) (2) and the Androgen Excess Society (2006) criteria for PCOS (3), two of three characteristics must be present for a diagnosis of PCOS: hyperandrogenemia, oligo- or anovulation, and polycystic ovaries. PCOS occurs as early as menarche but is rarely diagnosed until women are in their 20s. In fact, it often takes more than 2 years of consultation with physicians to receive a diagnosis of PCOS (4). One of the major reasons for women with PCOS to seek health care is issues with fertility, and assisted reproduction is common in women with PCOS (4).
In addition to fertility difficulties and hyperandrogenemia, women with PCOS also exhibit hirsutism, depending on the level of androgens, and metabolic characteristics that are associated with cardiovascular disease (CVD), such as obesity, insulin resistance (even in the absence of obesity), type 2 diabetes mellitus, dyslipidemia, endothelial dysfunction, and elevated BP (5,6). The mechanisms responsible for development of PCOS are not clear. Although a genetic component has been suggested, studies in daughters of women with PCOS are not consistent as to whether they develop PCOS themselves and whether similar metabolic and hypertensive phenotypes exist in daughters, or even sons, of women with PCOS (7–13).
Approximately 50% of women with PCOS exhibit increased microalbuminuria (14–17) that was found to be closely associated with increased diastolic BP and area under the curve for an oral glucose tolerance test. Unfortunately, there are minimal studies on kidney function in women with PCOS. Gozukara and colleagues reported that women with PCOS do not develop overt glomerular hyperfiltration, yet their GFR was significantly higher than controls (18). The increased GFR was also positively correlated with uric acid. The same study also found that women with PCOS have increased proteinuria (18). There are several caveats regarding this study, however. The women with PCOS who were included were young (approximately 25 years of age). In addition, GFR was not measured using tracer techniques but rather was estimated from serum creatinine levels. Finally, the PCOS cohort was not overweight or obese (body mass index [BMI] <25 kg/m2) (18), which is inconsistent with the majority of women with PCOS, especially in the United States.
In young women with PCOS, BP is typically only mildly elevated and often does not reach the level required for antihypertensive treatment according to the clinical guidelines (19). Interestingly, meta-analyses studies show that BP is elevated in women with PCOS compared with controls before menopause (relative risk=1.7-fold), but after menopause, there are no differences in the prevalence of hypertension (20), suggesting that BP increases in control postmenopausal women just as in women with PCOS (20). However, it is well known that increases in BP are associated with increased risk of CVD in the future (21), but whether women with PCOS have a higher prevalence of CVD after menopause is not clear.
Animal Model of PCOS
Over the past several years, we have studied an animal model of PCOS that was first described by Manneras and colleagues (22). The model uses female Sprague Dawley (SD) rats that are implanted with dihydrotestosterone (DHT) pellets (7.5 mg/90 days; Innovative Research, Inc., Novi, MI) beginning at 4 weeks of age; pellets are replaced every 85 days throughout the life of the rat (23). This dose results in an approximately three-fold increase in circulating DHT (23), similar to androgen levels in women with PCOS (24). DHT is used for the animal model because it cannot be converted to estradiol. Furthermore, this level of DHT is not sufficient to reduce the endogenous synthesis of testosterone and estradiol, such that estradiol levels are similar to age-matched control female SD rats (23). In the original studies by Manneras and colleagues, they compared the DHT female model with the letrozole model of PCOS (22). They found that the DHT model more closely resembled the phenotypes found in women with PCOS. However, they did not measure BP or proteinuria in the models because the investigators were mainly interested in whether the model would replicate the reproductive issues (22). As in women with PCOS, the DHT-treated female rats develop an increase in body weight due to an increase in food intake (22,23). Early in the development of obesity in the DHT-treated rats, computed tomography (CT) scans of adipose tissue show that there is an increase in subcutaneous adipose tissue (25) that is accompanied by increases in plasma leptin, insulin, area under the curve of an oral glucose tolerance test, and plasma cholesterol (23). The DHT-treated females remain normoglycemic, however. Similar to women with PCOS, DHT-treated rats have fertility difficulties with 6-day estrus cycles rather than 4-day cycles (23), and only 50%–60% become pregnant when they are placed with males compared with control SD females who become pregnant 99% of the time (26–28). As adults, the DHT-treated females also exhibit a modest (10 mm Hg) increase in BP similar to the small increases in BP in women with PCOS (23,25). Also, just as in women with PCOS, the DHT-treated female rats develop proteinuria (23).
As noted above, although the DHT female PCOS model is one of the most common, there are other animal models of PCOS. The letrozole PCOS model is produced by giving letrazole, a nonsteroidal aromatase inhibitor (given by oral gavage for at least 12 weeks (22). Vaginal smears in the letrozole model show they are anovulatory, and they have cysts in the ovary, increased body weight, elevated testosterone levels, abnormal glucose and lipid metabolism, and insulin resistance (22,29,30). Because the letrozole-treated rats or mice are anovulatory, this model cannot be used for PCOS reproductive studies. The dehydroepiandrosterone (DHEA) rat model of PCOS is induced with daily subcutaneous injections of the testosterone precursor (30,31). The DHEA model exhibits increases in androgens and ovarian cysts along with abnormal estrous cycling (30,31), and thus is used to study the reproductive consequences of PCOS. However, the DHEA model does not exhibit any metabolic phenotype, no adiposity, and no insulin resistance (30,31). Finally, there are other models of PCOS produced in simian and ovine female offspring by androgens or estrogens given to the dam during pregnancy, as noted in this review (32,33). These models have variable levels of insulin sensitivity and do not completely recapitulate all of the symptoms of PCOS in women. Thus, the DHT-treated female rat and murine models of PCOS most closely resemble the majority of the symptoms in women with PCOS and are the best in which to study the cardiovascular-renal and metabolic consequences of androgens in adult and aging females and the consequences of pregnancy on the DHT-treated dams throughout their pregnancy and as they age and on their offspring.
Mechanisms Responsible for Elevated BP in Women with PCOS and the Rat Model
Role of Obesity in Women with PCOS
Obesity is a frequent clinical finding in women with PCOS (34) and exacerbates the increases in BP in women with PCOS. However, one should keep in mind that BP is also elevated in women with PCOS with a normal BMI (35,36). Obesity plays a major role in the clinical manifestations of the syndrome, and women with PCOS commented in a recent online survey that difficulty in losing weight was their most common clinical concern (4). Weight loss, the first-line therapeutic recommendation for women with PCOS, is associated with improvements in infertility, insulin resistance, and hyperandrogenism (37,38). However, whether weight loss in women with PCOS decreases their BP remains unclear.
Two major fat depots were characterized in women with PCOS: the subcutaneous adipose tissue (SAT), localized beneath the skin, and the visceral adipose tissue (VAT), which lines the internal organs. Computed tomography (CT), which clearly distinguishes fat from other tissues, allows the identification and quantification of VAT and SAT. When the SAT storage capacity is exceeded, due to either an inability to generate sufficient new adipocytes (hyperplasia) or an inability to expand existing adipocytes further (hypertrophy), adipose tissue begins to accumulate in the VAT. Women with PCOS have an expansion of both SAT and VAT, even after adjusting for BMI. Epidemiologic studies demonstrated that VAT accumulation is associated with insulin resistance in white women (39). A recent study showed that women with PCOS, independent of their BMI, had elevated VAT compared with controls (40). In the same study, VAT correlated positively with adverse cardiometabolic complications and elevated testosterone levels in women with PCOS (40). Associations between adipose tissue accumulation in specific depots and BP regulation have not been studied in PCOS. Furthermore, the molecular mechanism(s) by which obesity exacerbates the cardiometabolic complications in PCOS remains poorly understood. Because androgens are also increased in VAT with obesity in women who do not have PCOS (41,42), it is probable that the molecular mechanisms responsible for cardiometabolic complications in PCOS are in part mediated by adipose tissue and androgens, although the mechanisms are as yet unclear. Finally, to date, effective and safe therapeutic options to treat the obesity-associated cardiometabolic consequences in PCOS are limited to exercise, weight loss, and use of metformin to treat insulin resistance (43).
Obesity and Sympathetic Activation in a PCOS Rat Model
As mentioned above, the DHT-treated rat model exhibits increased food intake and body weight, and CT scans show that SAT, but not VAT, is increased by 14–16 weeks of age (23). By 12 months of age, CT scans show that both SAT and VAT (retroperitoneal depots) are increased in DHT-treated rats (23,44). Along with the increase in adipose tissue, plasma leptin levels, produced by adipose tissue, are also increased in DHT-treated females (23).
Because obesity is associated with sympathetic activation, the contribution of the sympathetic nervous system to the elevated BP in DHT-treated rats was tested. Rats were given terazosin and propranolol to block the α1, and β1,2-adrenergic receptors, and BP decreased to a greater extent in DHT-treated females than in controls, suggesting that the model did have sympathetic activation (25). Renal denervation also decreased the BP in DHT-treated females, suggesting that the renal nerves were involved in the elevated BP (25).
The melanocortin-3/4 receptor (MC4R) in pro-opiomelanocortin (POMC) neurons is thought to be upregulated in response to increased levels of leptin (45). The increased MC4R then activates the sympathetic nervous system, which is manifested by increases in BP or thermogenesis (45). Because plasma leptin is elevated in the DHT-treated rat model, the hypothesis was tested that the MC4R in POMC neurons may mediate the increased sympathetic activity in the PCOS model. Previous studies in female spontaneously hypertensive rats (SHR) that exhibited increased sympathetic activation and reduction in BP in response to adrenergic blockade and renal denervation did not exhibit a reduction in BP in response to the MC4R blocker SHU-9119 (45). In contrast, SHU-9119 reduced the BP in the DHT-treated female PCOS model, and interestingly, the protein expression of the MC4R in the hypothalamus was significantly increased (25). Furthermore, in the absence of an active MC4R in MC4R−/− rats, DHT was not able to increase BP (25). Taken together, the data strongly suggest that activation of the MC4R does increase sympathetic activity that is responsible at least in part for the elevated BP in the DHT-treated model. The mechanism responsible for the upregulation of the MC4R in the model is unclear; however, it could be due to either increased leptin or DHT, neither of which are upregulated in SHR females (45). Whether the MC4R plays a role in mediating the elevated BP in women with PCOS has not been investigated to our knowledge.
In summary, we hypothesize that sympathetic activation in the DHT-treated females is due to adipose-induced increases in leptin or to DHT-mediated upregulation of the MC4R in the POMC neurons that then stimulates the sympathetic nervous system (SNS) and activates the adrenergic receptors and the renal nerves to cause an increase in BP. It is possible also that the adipose tissue and/or sympathetic activation could upregulate the renin-angiotensin system (RAS), as discussed below.
Role of the RAS in PCOS
The RAS plays a major role in BP regulation, fluid homeostasis, and metabolism, which was extensively reviewed by Ferrario and colleagues (46). In the RAS system, the substrate angiotensinogen is hydrolyzed by renin to angiotensin (Ang) I, which in turn is converted to Ang II by the Ang I converting enzyme (ACE). Ang II binds and activates the Ang II type 1 receptor (AT1R) to elicit its bioactivity, thus making up the vasoconstrictor arm of the RAS. Ang II can also bind the AT2R or the ACE2 enzyme to generate the vasodilator Ang(1–7) that binds and activates the MAS receptor, making up the vasodilator arm of the RAS.
Young women with PCOS have increased plasma renin levels and activity (47,48) and increased plasma prorenin (49). Similarly, women with PCOS have higher levels of ACE and Ang II compared with control subjects. ACE gene polymorphism is also associated with increased metabolic comorbidities in women with PCOS (50,51). Furthermore, there is evidence that AT1R antagonists (ARBs) and AT2R activation normalize androgen levels in women with PCOS, although the studies have small numbers of participants (52,53) and the mechanisms responsible for how ARBs or AT2R activation could change androgen levels are not clear. Quigley and colleagues reported several years ago that losartan blocks the androgen-mediated increases in proximal tubule sodium reabsorption (54). As expected, a similar ARB to losartan, telmisartan, significantly reduced elevated BP in women with PCOS (53).
Angiotensinogen is highly expressed in adipose tissue and is constitutively secreted by mature adipocytes from separate adipose depots in animal models and humans (55). The concentration of androgens in adipose tissue from women who do not have PCOS is severalfold higher than in plasma (42). Overexpression of angiotensinogen in adipose tissue increases adiposity and BP in transgenic mice (56); therefore, it is possible that an elevated level of androgens in adipose tissue in women with PCOS activates the intra-adipose RAS in women with PCOS. Recently ACE2 deficiency in adipose tissue was shown to increase BP in female mice fed a high-fat diet but not in males (57). Whether androgen-mediated increases in Ang II and decreases in Ang(1–7) in adipose tissue play a role in mediating the elevated BP in obese women with PCOS remains to be determined.
As mentioned, the RAS plays a role in the androgen-mediated increase in BP, and we demonstrated that the vasoconstrictor arm of the intrarenal RAS is activated by androgens in the DHT-treated female rat (23) and in male models of hypertension and renal injury, such as Dahl salt-sensitive rats and SHR (58). Intrarenal mRNA expression of angiotensinogen and ACE are increased and AT1R expression is decreased in DHT-treated females (23); enalapril (ACE inhibitor) reduces BP in the model (59). Interestingly, the body weight and BP in DHT-treated female rats remain elevated 6 months after DHT withdrawal (termed ex-DHT), and activation of the intrarenal RAS plays a major role in the remaining elevated BP in ex-DHT. These data are important because they suggest that chronic upregulation of the intrarenal and adipose tissue androgen receptor may explain the long-lasting effects of androgens (60), and that once androgen excess initiates obesity and upregulation of the RAS, the system remains upregulated.
Role of 20-Hydroxyeicosatetraenoic Acid in PCOS
20-hydroxyeicosatetraenoic acid (20-HETE) is a lipid produced from arachidonic acid, whose presence in the kidney can be either pro- or antihypertensive (61,62), depending on its cellular location. If 20-HETE is increased in the intrarenal microvaculature, it causes renal vasoconstriction, thus reducing GFR and causing increases in BP (62). If 20-HETE is increased in the renal tubules, it acts as a diuretic, reducing sodium reabsorption, and is thus antihypertensive (62,63). In collaboration with Dr. Richard Roman, we measured the levels of 20-HETE in renal microvessels of DHT-treated female rats and found that 20-HETE was increased, but not in renal tubules (63). In addition, we found that the intrarenal cytochrome P450 4A2 mRNA expression was increased 15-fold in the PCOS model (63). These data led us to studies in which a P450 4A2−/− rat was used to determine if DHT could increase the BP as in control females. In fact, we found that DHT was unable to increase the BP in 4A2−/− rats, unlike in DHT-treated control females (63). These data suggest that DHT-mediated 20-HETE increase in the renal microvasculature is at least in part responsible for the increased BP in the PCOS model.
As mentioned above, 20-HETE is a metabolite of arachidonic acid (61), and both Ang II and endothelin are able to increase the release of arachidonic acid, leading to increases in 20-HETE (61). However, whether Ang II or endothelin are elevated in this model and contribute to the elevated BP is not clear and will need to be studied. Furthermore, whether 20-HETE is elevated in kidneys of women with PCOS has not been studied to our knowledge.
PCOS, Menopause, and CVD
Few studies have been done in postmenopausal women to determine the age-related cardiovascular-renal consequences of having PCOS throughout their lives. Interestingly, meta-analyses studies show that BP is elevated in women with PCOS compared with controls before menopause (relative risk=1.7-fold), but after menopause, there are no differences in the prevalence of hypertension (20), suggesting that BP increases in control postmenopausal women just as in women with PCOS (20), and hypertension is a major risk factor for future CVD (21). Some reports show that women with PCOS may go through the menopause transition at a later age (53.3 years versus 51.2 years) than control women (64), suggesting that women with PCOS may have longer exposure to estrogens than control women. A recent review by Helvaci and Yildiz evaluated all studies published in English between 1990 and 2020 that used MeSH terms “polycystic ovary syndrome,” “menopause,” and other terms associated with CVD (65). Unfortunately, the diagnosis of PCOS was not specific for the Rotterdam or Androgen Excess-PCOS Society criteria in all of the studies included. The evaluation showed that menopause in the general population was associated with increased prevalence of obesity, glucose intolerance, hypertension, dyslipidemia, and metabolic syndrome (65). Premenopausal women with PCOS had a higher prevalence of these conditions than did premenopausal controls (66). However, despite the earlier exposure to CVD-promoting conditions, postmenopausal women with PCOS did not exhibit an increased risk of CVD events or mortality compared with age-matched controls (65). In another study, women with PCOS who were diagnosed according to the Rotterdam criteria and controls were studied at 70 years of age and compared with themselves at 50 years of age (67). At 70 years of age, there were few differences between control women and women with PCOS with regard to metabolic factors and BP (67). Again, as shown in other studies, at age 50 years, BP was significantly higher in women with PCOS than controls, but by 70 years of age, there were no differences between the groups (67). Whether postmenopausal women have increased proteinuria compared with premenopausal women was not studied, nor are there any studies to our knowledge in which kidney function has been reported in postmenopausal women with PCOS.
Aging in the PCOS Rat Model
Aging in the DHT-treated rat model of PCOS is associated with increases in BP compared with young adults and aging controls (26,44). The mechanisms for the further elevations in BP after the DHT-treated rats stop estrus cycling (at 10–12 months of age) are not clear. We recently reported that chronic administration of the glucagon-like peptide-1 receptor agonist (GLP-1 RA), liraglutide, in postestrus cycling DHT-treated rat model results in significant improvement in several cardiometabolic risk factors, such as insulin resistance, obesity (9% reduction in body weight), dyslipidemia, and leptin levels (68). Despite these improvements, the well-known BP-lowering effect of GLP-1 RA was not observed in DHT-treated rats, only in control rats (68). In contrast, there is a report that GLP-1 RAs did decrease BP in reproductive-age DHT-treated rats (69), suggesting differences in mechanisms of hypertension with aging in PCOS rat models. Similarly, administration of sodium-glucose cotransporter 2 inhibitor in women with PCOS causes a significant reduction in adipose tissue and leptin levels, with only modest (approximately 2 mm Hg) decreases in BP (70).
Aging control female SD rats are protected from hypertension (26). However, DHT-treated female rats, aged 22–24 months, exhibit a 20 mm Hg increase in BP compared with age-matched controls (controls: 110±5 mm Hg; DHT: 130±6 mm Hg; P=0.01) (71). Along with the increase in BP, the aging DHT-treated rats have renal hypertrophy, GFR is decreased by 60%, renal plasma flow is reduced by 50%, and proteinuria, factored for GFR, is increased by 20-fold (71). The kidneys of aging DHT-treated females also exhibit focal segmental glomerulosclerosis and interstitial fibrosis (71). Whether aging women with PCOS exhibit a higher incidence of CKD than control aging women is unknown.
As mentioned above, the therapeutic options for the cardiovascular-renal and metabolic consequences of PCOS are few. The use of anti-androgens in women with PCOS is not recommended by the US Food and Drug Administration due to the high prevalence of hepatotoxicity found in European studies of women with PCOS (72). However, on the basis of the data discussed above and as shown in our animal model, the increase in androgens certainly sets the cardiometabolic symptoms of the disease in motion. However, once established, normalization of androgens and reduction in body weight may not be enough to decrease BP in women with PCOS, as we found in our ex-DHT model (60). These data suggest then that the mechanisms responsible for the elevated BP in women with PCOS may be more complicated than just androgen excess and obesity. Future studies in women with PCOS will be necessary to determine the contributions of other hypertensive mechanisms, such as renal dysfunction, the RAS, endothelin, or 20-HETE, as we found in our rat model of PCOS, and whether these interactions change or synergize with aging.
Cardiovascular-Renal Consequences of Previous Pregnancy in Women with PCOS and DHT-Treated Female Rats
To our knowledge, there are no studies reported in which CVD and BP in women with PCOS have been separated on the basis of previous pregnancy. As mentioned above, women with PCOS often have difficulty becoming pregnant and require reproductive assistance (73,74). Women with PCOS have a somewhat higher incidence of preeclampsia, gestational diabetes, and children born either small or large for gestational age (65–67), likely due to the extent of the gestational diabetes (75–78).
Just as in women with PCOS, the DHT-treated female rat model of PCOS also has difficulty becoming pregnant. However, the number of pups per litter of DHT-treated rats is similar to control pregnancies, although the pups are born small for gestational age (26–28). If left to age to 16–18 months, the DHT-treated dams have similar metabolic characteristics as virgin DHT-treated rats, including similar body weight, plasma insulin, leptin, and cholesterol, all of which are higher than age-matched control females (26). Surprisingly, the aged previously pregnant DHT-treated dams have significantly lower BP than the virgin DHT-treated rats (26), and in fact, the BP did not increase in the DHT-treated dams after 10 months of age, whereas BP continued to increase in virgin DHT-treated rats (26).
The mechanism(s) by which previous pregnancy protected aging DHT-treated rats from hypertension is not clear. There are increases in intrarenal mRNA expression of vasodilator systems in the aging, previously pregnant, DHT-treated dams, such as endothelial nitric oxide synthase and endothelin B receptor, and reductions in intrarenal vasoconstrictor systems, such as mRNA expression of renin, AT1R, and the endothelin ETA receptor, compared with DHT-treated virgins (26). Taken together, these data suggest that the previously pregnant DHT dams had an upregulation of intrarenal vasodilator systems and a downregulation of intrarenal vasoconstrictor systems.
As mentioned, to our knowledge, there are no studies on the extent of CVD in aging postmenopausal cohorts of women with PCOS who are separated on the basis of whether they have previously been pregnant. Thus, whether pregnancy protection against CVD and hypertension occurs in aging women with PCOS is not clear. Taken with the data described above in which comparison was made between PCOS cohorts who were 50 years of age compared with 70 years of age (20), the fact that BP was similar between women with PCOS and controls at 70 years of age is surprising on the basis of the early exposure to cardiometabolic dysfunction in women with PCOS. In addition, women with PCOS have a higher prevalence of gestational diabetes, preeclampsia, and superimposed preeclampsia than control women (78,79). Other studies have reported that women who have experienced preeclampsia during their pregnancies are at increased risk of CVD and hypertension later in life compared with control women (79). Thus, it is surprising that DHT-treated dams are protected from age-related CVD and hypertension. One caveat is that we do not know if the DHT-treated dams maintain elevated BP or even a further increase in BP during pregnancy because the DHT-treated rats have an elevated BP before pregnancy (23). Pregnancy in SHR, a model of essential hypertension, results in a reduction in BP in late pregnancy, just as in normotensive rats and humans (80,81). Another factor that may contribute is that although the DHT-treated rats have higher body weight than controls before pregnancy, by the end of the pregnancy, the dams have lower levels of fat mass than do age-matched virgin DHT-treated rats (26). It is surprising that the differences that occur at 4–5 months of age would have such profound effects when rats reach 16–18 months of age. In addition, it is surprising that these differences show up only after they stop estrous cycling because there were no differences in BP or metabolic factors between the previously pregnant and virgin DHT-treated rats at 10 months of age. It should be noted that adult male offspring of DHT-treated rats that were born with intrauterine growth restriction exhibit exaggerated responses to Ang II infusion (26,27), suggesting that there are perinatal factors occurring in the DHT-treated dams that affect the offspring later in life. Future studies in women with PCOS will be necessary to determine whether those who have been previously pregnant are actually protected against further increases in BP with aging.
Summary
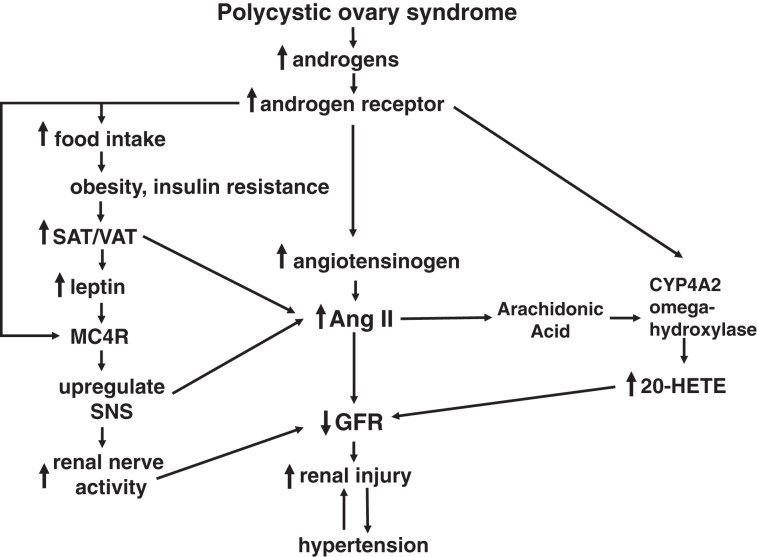
PCOS is the most common reproductive disorder in women, affecting approximately 10%. Although there are numerous studies on the pregnancy consequences of PCOS, there are significantly fewer studies on the cardiorenal consequences of PCOS, especially with aging. As shown in Figure 1, we hypothesize that an increase in androgens in PCOS via the androgen receptor increases food intake, leading to obesity and insulin resistance and to increases in subcutaneous and visceral fat depots that release leptin and, in combination with androgens and androgen receptor, upregulate the MC4R. Activation of the MC4R upregulates the SNS, which increases renal sympathetic nerve activity that decreases GFR. In addition, an increase in androgens and upregulation of the androgen receptor also increases synthesis of angiotensinogen to increase Ang II, also reducing GFR. Androgens via the androgen receptor and Ang II-mediated release of arachidonic acid upregulates and activates the ω-hydroxylase, respectively, which produces 20-HETE in the intrarenal microvasculature, which also reduces GFR. Taken together, the reduction in GFR leads to increased renal injury that then causes increases in BP, which in turn becomes a feed-forward mechanism to cause further renal injury and further increases in BP, especially with aging.
Figure 1.
Hypotheses regarding the mechanisms responsible for elevated BP in women with polycystic ovary syndrome.
On the basis of this hypothesis, future studies are needed to determine the long-term consequences of PCOS on renal function. On the basis of animal studies, long-term exposure in women with PCOS to the cardiometabolic consequences of PCOS, including elevated BP, insulin resistance, hyperlipidemia, activation of the SNS, RAS, and 20-HETE systems, likely will have an adverse effect on renal function, especially with aging. Furthermore, whether women with PCOS who have been previously pregnant may be protected from age-related renal injury and hypertension and the mechanisms responsible also remain to be determined.
Disclosures
L.L. Yanes Cardozo reports research funding from Novo Nordisk, Inc., and an advisory or leadership role on the editorial board of Frontiers in Endocrinology and Biology of Sex Differences. All remaining authors have nothing to disclose.
Funding
This work was supported by the National Institutes of Health (NIH) (grants R01HL66072 to J.F. Reckelhoff, R01HL135089 to J.F. Reckelhoff and D.G. Romero, P01HL051971 to J.F. Reckelhoff, P20GM121334 to J.F. Reckelhoff, D.G. Romero, and L.L. Yanes Cardozo, and P20GM104357 to J.F. Reckelhoff) and the American Heart Association (grant 20POST35150001 to N.M. Shawky).
Author Contributions
J.F. Reckelhoff was responsible for funding acquisition and project administration; J.F. Reckelhoff and L.L. Yanes Cardozo were responsible for conceptualization and wrote the original draft of the manuscript; D.G. Romero, N.M. Shawky, and L.L. Yanes Cardozo were responsible for the methodology; N.M. Shawky was responsible for data curation; and all authors were responsible for the formal analysis and investigation and reviewed and edited the manuscript.
References
- 1.Azziz R, Woods KS, Reyna R, Key TJ, Knochenhauer ES, Yildiz BO: The prevalence and features of the polycystic ovary syndrome in an unselected population. J Clin Endocrinol Metab 89: 2745–2749, 2004. 10.1210/jc.2003-032046 [DOI] [PubMed] [Google Scholar]
- 2.Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group: Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum Reprod 19: 41–47, 2004. 10.1093/humrep/deh098 [DOI] [PubMed] [Google Scholar]
- 3.Azziz R, Carmina E, Dewailly D, Diamanti-Kandarakis E, Escobar-Morreale HF, Futterweit W, Janssen OE, Legro RS, Norman RJ, Taylor AE, Witchel SF; Androgen Excess Society : Positions statement: Criteria for defining polycystic ovary syndrome as a predominantly hyperandrogenic syndrome: An Androgen Excess Society guideline. J Clin Endocrinol Metab 91: 4237–4245, 2006. 10.1210/jc.2006-0178 [DOI] [PubMed] [Google Scholar]
- 4.Gibson-Helm M, Teede H, Dunaif A, Dokras A: Delayed diagnosis and a lack of information associated with dissatisfaction in women with polycystic ovary syndrome. J Clin Endocrinol Metab 102: 604–612, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ovalle F, Azziz R: Insulin resistance, polycystic ovary syndrome, and type 2 diabetes mellitus. Fertil Steril 77: 1095–1105, 2002. 10.1016/S0015-0282(02)03111-4 [DOI] [PubMed] [Google Scholar]
- 6.Wikiera B, Zubkiewicz-Kucharska A, Nocoń-Bohusz J, Noczyńska A: Metabolic disorders in polycystic ovary syndrome. Pediatr Endocrinol Diabetes Metab 23: 204–208, 2017. 10.18544/PEDM-23.04.0094 [DOI] [PubMed] [Google Scholar]
- 7.Kent SC, Gnatuk CL, Kunselman AR, Demers LM, Lee PA, Legro RS: Hyperandrogenism and hyperinsulinism in children of women with polycystic ovary syndrome: A controlled study. J Clin Endocrinol Metab 93: 1662–1669, 2008. 10.1210/jc.2007-1958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sir-Petermann T, Codner E, Pérez V, Echiburú B, Maliqueo M, Ladrón de Guevara A, Preisler J, Crisosto N, Sánchez F, Cassorla F, Bhasin S: Metabolic and reproductive features before and during puberty in daughters of women with polycystic ovary syndrome. J Clin Endocrinol Metab 94: 1923–1930, 2009. 10.1210/jc.2008-2836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sir-Petermann T, Maliqueo M, Codner E, Echiburú B, Crisosto N, Pérez V, Pérez-Bravo F, Cassorla F: Early metabolic derangements in daughters of women with polycystic ovary syndrome. J Clin Endocrinol Metab 92: 4637–4642, 2007. 10.1210/jc.2007-1036 [DOI] [PubMed] [Google Scholar]
- 10.Torchen LC, Legro RS, Dunaif A: Distinctive reproductive phenotypes in peripubertal girls at risk for polycystic ovary syndrome. J Clin Endocrinol Metab 104: 3355–3361, 2019. 10.1210/jc.2018-02313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Olszanecka-Glinianowicz M, Zachurzok A, Drosdzol-Cop A, Bożętowicz-Wikarek M, Owczarek A, Gawlik A, Chudek J, Skrzypulec-Plinta V, Małecka-Tendera E: Circulating anti-Müllerian hormone levels in daughters of women with and without polycystic ovary syndrome. Horm Res Paediatr 85: 372–378, 2016. 10.1159/000444637 [DOI] [PubMed] [Google Scholar]
- 12.Legro RS, Kunselman AR, Stetter CM, Gnatuk CL, Estes SJ, Brindle E, Vesper HW, Botelho JC, Lee PA, Dodson WC: Normal pubertal development in daughters of women with PCOS: A controlled study. J Clin Endocrinol Metab 102: 122–131, 2017. 10.1210/jc.2016-2707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Recabarren SE, Smith R, Rios R, Maliqueo M, Echiburú B, Codner E, Cassorla F, Rojas P, Sir-Petermann T: Metabolic profile in sons of women with polycystic ovary syndrome. J Clin Endocrinol Metab 93: 1820–1826, 2008. 10.1210/jc.2007-2256 [DOI] [PubMed] [Google Scholar]
- 14.Duleba AJ, Ahmed IM: Predictors of urinary albumin excretion in women with polycystic ovary syndrome. Fertil Steril 93: 2285–2290, 2010. 10.1016/j.fertnstert.2008.12.120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Patel AA, Bloomgarden ZT, Futterweit W: Premicroalbuminuria in women with polycystic ovary syndrome: A metabolic risk marker. Endocr Pract 14: 193–200, 2008. 10.4158/EP.14.2.193 [DOI] [PubMed] [Google Scholar]
- 16.Caglar GS, Oztas E, Karadag D, Pabuccu R, Eren AA: The association of urinary albumin excretion and metabolic complications in polycystic ovary syndrome. Eur J Obstet Gynecol Reprod Biol 154: 57–61, 2011. 10.1016/j.ejogrb.2010.08.024 [DOI] [PubMed] [Google Scholar]
- 17.Ziaee A, Oveisi S, Ghorbani A, Hashemipour S, Mirenayat M: Association between metabolic syndrome and premicroalbuminuria among Iranian women with polycystic ovary syndrome: A case control study. Glob J Health Sci 5: 187–192, 2012. 10.5539/gjhs.v5n1p187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gozukara IO, Gozukara KH, Kucur SK, Karakılıc EU, Keskin H, Akdeniz D, Aksoy AN, Carlıoglu A: Association of glomerular filtration rate with inflammation in polycystic ovary syndrome. Int J Fertil Steril 9: 176–182, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Holte J, Gennarelli G, Berne C, Bergh T, Lithell H: Elevated ambulatory day-time blood pressure in women with polycystic ovary syndrome: A sign of a pre-hypertensive state? Hum Reprod 11: 23–28, 1996. 10.1093/oxfordjournals.humrep.a019028 [DOI] [PubMed] [Google Scholar]
- 20.Amiri M, Ramezani Tehrani F, Behboudi-Gandevani S, Bidhendi-Yarandi R, Carmina E: Risk of hypertension in women with polycystic ovary syndrome: A systematic review, meta-analysis and meta-regression. Reprod Biol Endocrinol 18: 23, 2020. 10.1186/s12958-020-00576-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kshirsagar AV, Carpenter M, Bang H, Wyatt SB, Colindres RE: Blood pressure usually considered normal is associated with an elevated risk of cardiovascular disease. Am J Med 119: 133–141, 2006. 10.1016/j.amjmed.2005.08.023 [DOI] [PubMed] [Google Scholar]
- 22.Mannerås L, Cajander S, Holmäng A, Seleskovic Z, Lystig T, Lönn M, Stener-Victorin E: A new rat model exhibiting both ovarian and metabolic characteristics of polycystic ovary syndrome. Endocrinology 148: 3781–3791, 2007. 10.1210/en.2007-0168 [DOI] [PubMed] [Google Scholar]
- 23.Yanes LL, Romero DG, Moulana M, Lima R, Davis DD, Zhang H, Lockhart R, Racusen LC, Reckelhoff JF: Cardiovascular-renal and metabolic characterization of a rat model of polycystic ovary syndrome. Gend Med 8: 103–115, 2011. 10.1016/j.genm.2010.11.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Daan NMP, Jaspers L, Koster MPH, Broekmans FJM, de Rijke YB, Franco OH, Laven JSE, Kavousi M, Fauser BCJM: Androgen levels in women with various forms of ovarian dysfunction: Associations with cardiometabolic features. Hum Reprod 30: 2376–2386, 2015. 10.1093/humrep/dev195 [DOI] [PubMed] [Google Scholar]
- 25.Maranon R, Lima R, Spradley FT, do Carmo JM, Zhang H, Smith AD, Bui E, Thomas RL, Moulana M, Hall JE, Granger JP, Reckelhoff JF: Roles for the sympathetic nervous system, renal nerves, and CNS melanocortin-4 receptor in the elevated blood pressure in hyperandrogenemic female rats. Am J Physiol Regul Integr Comp Physiol 308: R708–R713, 2015. 10.1152/ajpregu.00411.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shawky NM, Patil CN, Dalmasso C, Maranon RO, Romero DG, Drummond H, Reckelhoff JF: Pregnancy protects hyperandrogenemic female rats from postmenopausal hypertension. Hypertension 76: 943–952, 2020. 10.1161/HYPERTENSIONAHA.120.15504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zuchowski Y, Dalmasso C, Shawky NM, Reckelhoff JF: Cardiometabolic consequences of maternal hyperandrogenemia in male offspring. Physiol Rep 9: e14941, 2021. 10.14814/phy2.14941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shawky NM, Dalmasso C, Ojeda NB, Zuchowski Y, Stachenfeld N, Alexander BT, Reckelhoff JF: Consequences of hyperandrogenemia during pregnancy in female offspring: Attenuated response to angiotensin II. J Hypertens 40: 712–722, 2022. 10.1097/HJH.0000000000003067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu J, Dun J, Yan J, Zhang J, Lin Q, Huang H, Ji F, Huang L, You X, Lin Y: Letrozole rat models mimics human polycystic ovary syndrome and changes in insulin signal pathways. Med Sci Monitor 26: e923073, 2020. 10.12659/MSM.923073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang Y, Xu L: Comparative study of DHEA and letrozole induced polycystic ovary syndrome in post-pubertal rats. Gynecol Endocrinol 38: 425–431, 2022. 10.1080/09513590.2022.2052843 [DOI] [PubMed] [Google Scholar]
- 31.Seow KM, Ting CH, Huang SW, Ho LT, Juan CC: The use of dehydroepiandrosterone-treated rats is not a good animal model for the study of metabolic abnormalities in polycystic ovary syndrome. Taiwan J Obstet Gynecol 57: 696–704, 2018. 10.1016/j.tjog.2018.08.015 [DOI] [PubMed] [Google Scholar]
- 32.Padmanabhan V, Veiga-Lopez A: Animal models of the polycystic ovary syndrome phenotype. Steroids 78: 734–740, 2013. 10.1016/j.steroids.2013.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Abbott DH, Rogers J, Dumesic DA, Levine JE: Naturally occurring and experimentally induced rhesus macaque models for polycystic ovary syndrome: Translational gateways to clinical application. Med Sci (Basel) 7: 107, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.El Hayek S, Bitar L, Hamdar LH, Mirza FG, Daoud G: Poly cystic ovarian syndrome: An updated overview. Front Physiol 7: 124, 2016. 10.3389/fphys.2016.00124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mellembakken JR, Mahmoudan A, Mørkrid L, Sundström-Poromaa I, Morin-Papunen L, Tapanainen JS, Piltonen TT, Hirschberg AL, Stener-Victorin E, Vanky E, Ravn P, Jensen RC, Andersen MS, Glintborg D: Higher blood pressure in normal weight women with PCOS compared to controls. Endocr Connect 10: 154–163, 2021. 10.1530/EC-20-0527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ollila ME, Kaikkonen K, Järvelin MR, Huikuri HV, Tapanainen JS, Franks S, Piltonen TT, Morin-Papunen L: Self-reported polycystic ovary syndrome is associated with hypertension: A northern Finland birth cohort 1966 study. J Clin Endocrinol Metab 104: 1221–1231, 2019. 10.1210/jc.2018-00570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Escobar-Morreale HF, Botella-Carretero JI, Alvarez-Blasco F, Sancho J, San Millán JL: The polycystic ovary syndrome associated with morbid obesity may resolve after weight loss induced by bariatric surgery. J Clin Endocrinol Metab 90: 6364–6369, 2005. 10.1210/jc.2005-1490 [DOI] [PubMed] [Google Scholar]
- 38.Kiddy DS, Hamilton-Fairley D, Bush A, Short F, Anyaoku V, Reed MJ, Franks S: Improvement in endocrine and ovarian function during dietary treatment of obese women with polycystic ovary syndrome. Clin Endocrinol (Oxf) 36: 105–111, 1992. 10.1111/j.1365-2265.1992.tb02909.x [DOI] [PubMed] [Google Scholar]
- 39.Zhang M, Hu T, Zhang S, Zhou L: Associations of different adipose tissue depots with insulin resistance: A systematic review and meta-analysis of observational studies. Sci Rep 5: 18495, 2015. 10.1038/srep18495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jena D, Choudhury AK, Mangaraj S, Singh M, Mohanty BK, Baliarsinha AK: Study of visceral and subcutaneous abdominal fat thickness and its correlation with cardiometabolic risk factors and hormonal parameters in polycystic ovary syndrome. Indian J Endocrinol Metab 22: 321–327, 2018. 10.4103/ijem.IJEM_646_17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wagner IV, Savchuk I, Sahlin L, Kulle A, Klöting N, Dietrich A, Holterhus P-M, Dötsch J, Blüher M, Söder O: De novo and depot-specific androgen production in human adipose tissue: A source of hyperandrogenism in women with obesity. Obes Facts 15: 281–291, 2022. 10.1159/000521571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Deslypere JP, Verdonck L, Vermeulen A: Fat tissue: A steroid reservoir and site of steroid metabolism. J Clin Endocrinol Metab 61: 564–570, 1985. 10.1210/jcem-61-3-564 [DOI] [PubMed] [Google Scholar]
- 43.Legro RS, Arslanian SA, Ehrmann DA, Hoeger KM, Murad MH, Pasquali R, Welt CK; Endocrine Society : Diagnosis and treatment of polycystic ovary syndrome: An Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 98: 4565–4592, 2013. 10.1210/jc.2013-2350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dalmasso C, Maranon R, Patil C, Bui E, Moulana M, Zhang H, Smith A, Yanes Cardozo LL, Reckelhoff JF: Cardiometabolic effects of chronic hyperandrogenemia in a new model of postmenopausal polycystic ovary syndrome. Endocrinology 157: 2920–2927, 2016. 10.1210/en.2015-1617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Maranon RO, Lima R, Mathbout M, do Carmo JM, Hall JE, Roman RJ, Reckelhoff JF: Postmenopausal hypertension: Role of the sympathetic nervous system in an animal model. Am J Physiol Regul Integr Comp Physiol 306: R248–R256, 2014. 10.1152/ajpregu.00490.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ferrario CM, Groban L, Wang H, Sun X, VonCannon JL, Wright KN, Ahmad S: The renin-angiotensin system biomolecular cascade: A 2022 update of newer insights and concepts. Kidney Int Suppl (2011) 12: 36–47, 2022. 10.1016/j.kisu.2021.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Alphan Z, Berberoglu Z, Gorar S, Candan Z, Aktas A, Aral Y, Ademoglu E: Increased total renin levels but not angiotensin-converting enzyme activity in obese patients with polycystic ovary syndrome. Med Princ Pract 22: 475–479, 2013. 10.1159/000351572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Uncu G, Sözer MC, Develioğlu O, Cengiz C: The role of plasma renin activity in distinguishing patients with polycystic ovary syndrome (PCOS) from oligomenorrheic patients without PCOS. Gynecol Endocrinol 16: 447–452, 2002. 10.1080/gye.16.6.447.452 [DOI] [PubMed] [Google Scholar]
- 49.Morris RS, Wong IL, Hatch IE, Gentschein E, Paulson RJ, Lobo RA: Prorenin is elevated in polycystic ovary syndrome and may reflect hyperandrogenism. Fertil Steril 64: 1099–1103, 1995. 10.1016/S0015-0282(16)57967-9 [DOI] [PubMed] [Google Scholar]
- 50.Celik O, Yesilada E, Hascalik S, Celik N, Sahin I, Keskin L, Ozerol E: Angiotensin-converting enzyme gene polymorphism and risk of insulin resistance in PCOS. Reprod Biomed Online 20: 492–498, 2010. 10.1016/j.rbmo.2009.12.014 [DOI] [PubMed] [Google Scholar]
- 51.Ożegowska K, Bogacz A, Bartkowiak-Wieczorek J, Seremak-Mrozikiewicz A, Pawelczyk L: Association between the angiotensin converting enzyme gene insertion/deletion polymorphism and metabolic disturbances in women with polycystic ovary syndrome. Mol Med Rep 14: 5401–5407, 2016. 10.3892/mmr.2016.5910 [DOI] [PubMed] [Google Scholar]
- 52.Leblanc S, Battista MC, Noll C, Hallberg A, Gallo-Payet N, Carpentier AC, Vine DF, Baillargeon JP: Angiotensin II type 2 receptor stimulation improves fatty acid ovarian uptake and hyperandrogenemia in an obese rat model of polycystic ovary syndrome. Endocrinology 155: 3684–3693, 2014. 10.1210/en.2014-1185 [DOI] [PubMed] [Google Scholar]
- 53.Jensterle M, Janez A, Vrtovec B, Meden-Vrtovec H, Pfeifer M, Prezelj J, Kocjan T: Decreased androgen levels and improved menstrual pattern after angiotensin II receptor antagonist telmisartan treatment in four hypertensive patients with polycystic ovary syndrome: Case series. Croat Med J 48: 864–870, 2007. 10.3325/cmj.2007.6.864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Quan A, Chakravarty S, Chen JK, Chen JC, Loleh S, Saini N, Harris RC, Capdevila J, Quigley R: Androgens augment proximal tubule transport. Am J Physiol Renal Physiol 287: F452–F459, 2004. 10.1152/ajprenal.00188.2003 [DOI] [PubMed] [Google Scholar]
- 55.Yasue S, Masuzaki H, Okada S, Ishii T, Kozuka C, Tanaka T, Fujikura J, Ebihara K, Hosoda K, Katsurada A, Ohashi N, Urushihara M, Kobori H, Morimoto N, Kawazoe T, Naitoh M, Okada M, Sakaue H, Suzuki S, Nakao K: Adipose tissue-specific regulation of angiotensinogen in obese humans and mice: Impact of nutritional status and adipocyte hypertrophy. Am J Hypertens 23: 425–431, 2010. 10.1038/ajh.2009.263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Massiéra F, Bloch-Faure M, Ceiler D, Murakami K, Fukamizu A, Gasc JM, Quignard-Boulange A, Negrel R, Ailhaud G, Seydoux J, Meneton P, Teboul M: Adipose angiotensinogen is involved in adipose tissue growth and blood pressure regulation. FASEB J 15: 2727–2729, 2001. 10.1096/fj.01-0457fje [DOI] [PubMed] [Google Scholar]
- 57.Shoemaker R, Tannock LR, Su W, Gong M, Gurley SB, Thatcher SE, Yiannikouris F, Ensor CM, Cassis LA: Adipocyte deficiency of ACE2 increases systolic blood pressures of obese female C57BL/6 mice. Biol Sex Differ 10: 45, 2019. 10.1186/s13293-019-0260-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yanes LL, Sartori-Valinotti JC, Iliescu R, Romero DG, Racusen LC, Zhang H, Reckelhoff JF: Testosterone-dependent hypertension and upregulation of intrarenal angiotensinogen in Dahl salt-sensitive rats. Am J Physiol Renal Physiol 296: F771–F779, 2009. 10.1152/ajprenal.90389.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yanes LL, Davis D, Romero DG, Reckelhoff JF: Activation of angiotensin II mediated hypertension in a rat model of polycystic ovary syndrome. FASEB J 24: 1041.14, 2010. 10.1096/fasebj.24.1_supplement.1041.14 [DOI] [Google Scholar]
- 60.Torres Fernandez ED, Adams KV, Syed M, Maranon RO, Romero DG, Yanes Cardozo LL: Long-lasting androgen-induced cardiometabolic effects in polycystic ovary syndrome. J Endocr Soc 2: 949–964, 2018. 10.1210/js.2018-00131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Roman RJ: P-450 metabolites of arachidonic acid in the control of cardiovascular function. Physiol Rev 82: 131–185, 2002. 10.1152/physrev.00021.2001 [DOI] [PubMed] [Google Scholar]
- 62.Williams JM, Murphy S, Burke M, Roman RJ: 20-hydroxyeicosatetraeonic acid: A new target for the treatment of hypertension. J Cardiovasc Pharmacol 56: 336–344, 2010. 10.1097/FJC.0b013e3181f04b1c [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dalmasso C, Maranon R, Patil C, Moulana M, Romero DG, Reckelhoff JF: 20-HETE and CYP4A2 ω-hydroxylase contribute to the elevated blood pressure in hyperandrogenemic female rats. Am J Physiol Renal Physiol 311: F71–F77, 2016. 10.1152/ajprenal.00458.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Forslund M, Landin-Wilhelmsen K, Schmidt J, Brännström M, Trimpou P, Dahlgren E: Higher menopausal age but no differences in parity in women with polycystic ovary syndrome compared with controls. Acta Obstet Gynecol Scand 98: 320–326, 2019. 10.1111/aogs.13489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Helvaci N, Yildiz BO: Cardiovascular health and menopause in aging women with polycystic ovary syndrome. Expert Rev Endocrinol Metab 15: 29–39, 2020. 10.1080/17446651.2020.1719067 [DOI] [PubMed] [Google Scholar]
- 66.Scicchitano P, Dentamaro I, Carbonara R, Bulzis G, Dachille A, Caputo P, Riccardi R, Locorotondo M, Mandurino C, Matteo Ciccone M: Cardiovascular risk in women with PCOS. Int J Endocrinol Metab 10: 611–618, 2012. 10.5812/ijem.4020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schmidt J, Landin-Wilhelmsen K, Brännström M, Dahlgren E: Cardiovascular disease and risk factors in PCOS women of postmenopausal age: A 21-year controlled follow-up study. J Clin Endocrinol Metab 96: 3794–3803, 2011. 10.1210/jc.2011-1677 [DOI] [PubMed] [Google Scholar]
- 68.Torres Fernandez ED, Huffman AM, Syed M, Romero DG, Yanes Cardozo LL: Effect of GLP-1 receptor agonists in the cardiometabolic complications in a rat model of postmenopausal PCOS. Endocrinology 160: 2787–2799, 2019. 10.1210/en.2019-00450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hoang V, Bi J, Mohankumar SM, Vyas AK: Liraglutide improves hypertension and metabolic perturbation in a rat model of polycystic ovarian syndrome. PLoS One 10: e0126119, 2015. 10.1371/journal.pone.0126119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pruett JE, Torres Fernandez ED, Everman SJ, Vinson RM, Davenport K, Logan MK, Ye SA, Romero DG, Yanes Cardozo LL: Impact of SGLT-2 inhibition on cardiometabolic abnormalities in a rat model of polycystic ovary syndrome. Int J Mol Sci 22: 2576, 2021. 10.3390/ijms22052576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Patil CN, Racusen LC, Reckelhoff JF: Consequences of advanced aging on renal function in chronic hyperandrogenemic female rat model: Implications for aging women with polycystic ovary syndrome. Physiol Rep 5: e13461, 2017. 10.14814/phy2.13461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Giorgetti R, di Muzio M, Giorgetti A, Girolami D, Borgia L, Tagliabracci A: Flutamide-induced hepatotoxicity: Ethical and scientific issues. Eur Rev Med Pharmacol Sci 21: 69–77, 2017 [PubMed] [Google Scholar]
- 73.Persson S, Elenis E, Turkmen S, Kramer MS, Yong EL, Sundström-Poromaa I: Fecundity among women with polycystic ovary syndrome (PCOS)—A population-based study. Hum Reprod 34: 2052–2060, 2019. 10.1093/humrep/dez159 [DOI] [PubMed] [Google Scholar]
- 74.He Y, Lu Y, Zhu Q, Wang Y, Lindheim SR, Qi J, Li X, Ding Y, Shi Y, Wei D, Chen ZJ, Sun Y: Influence of metabolic syndrome on female fertility and in vitro fertilization outcomes in PCOS women. Am J Obstet Gynecol 221: 138.e1–138.e12, 2019. 10.1016/j.ajog.2019.03.011 [DOI] [PubMed] [Google Scholar]
- 75.Roos N, Kieler H, Sahlin L, Ekman-Ordeberg G, Falconer H, Stephansson O: Risk of adverse pregnancy outcomes in women with polycystic ovary syndrome: Population based cohort study. BMJ 343: d6309, 2011. 10.1136/bmj.d6309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kjerulff LE, Sanchez-Ramos L, Duffy D: Pregnancy outcomes in women with polycystic ovary syndrome: A metaanalysis. Am J Obstet Gynecol 204: 558.e1–558.e6, 2011. 10.1016/j.ajog.2011.03.021 [DOI] [PubMed] [Google Scholar]
- 77.Sir-Petermann T, Hitchsfeld C, Maliqueo M, Codner E, Echiburú B, Gazitúa R, Recabarren S, Cassorla F: Birth weight in offspring of mothers with polycystic ovarian syndrome. Hum Reprod 20: 2122–2126, 2005. 10.1093/humrep/dei009 [DOI] [PubMed] [Google Scholar]
- 78.Mills G, Badeghiesh A, Suarthana E, Baghlaf H, Dahan MH: Polycystic ovary syndrome as an independent risk factor for gestational diabetes and hypertensive disorders of pregnancy: A population-based study on 9.1 million pregnancies. Hum Reprod 35: 1666–1674, 2020. 10.1093/humrep/deaa099 [DOI] [PubMed] [Google Scholar]
- 79.Craici I, Wagner S, Garovic VD: Preeclampsia and future cardiovascular risk: Formal risk factor or failed stress test? Ther Adv Cardiovasc Dis 2: 249–259, 2008. 10.1177/1753944708094227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Baylis C, Reckelhoff JF: Renal hemodynamics in normal and hypertensive pregnancy: Lessons from micropuncture. Am J Kidney Dis 17: 98–104, 1991. 10.1016/S0272-6386(12)81111-5 [DOI] [PubMed] [Google Scholar]
- 81.Gompf H, Luft FC, Morano I: Nitric oxide synthase upregulation and the predelivery blood pressure decrease in spontaneously hypertensive rats. J Hypertens 20: 255–261, 2002. 10.1097/00004872-200202000-00015 [DOI] [PubMed] [Google Scholar]