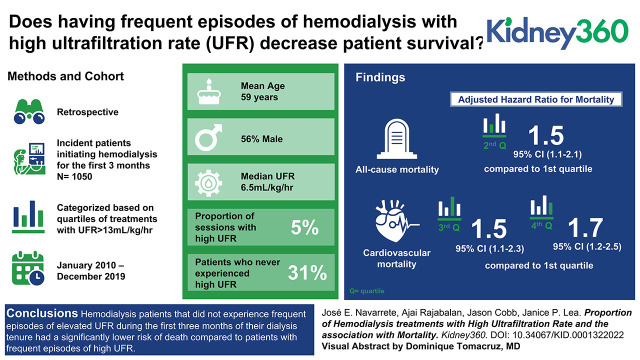
Key Points
Episodes of hemodialysis (HD) with high ultrafiltration rates (>13 ml/kg per hour) occurred frequently in 1050 incident dialysis patients.
Younger age, diabetes, heart failure, higher albumin, being a man, shorter treatment time, and lower weight were associated with high UFR.
Increasing numbers of dialysis sessions with high ultrafiltration rates were associated with higher all-cause and cardiovascular mortality.
Keywords: clinical nephrology, hemodialysis, mortality risk, ultrafiltration rate
Visual Abstract
Abstract
Background
Rapid fluid removal during hemodialysis has been associated with increased mortality. The limit of ultrafiltration rate (UFR) monitored by the Centers for Medicare & Medicaid Services is 13 ml/kg per hour. It is not clear if the proportion of treatments with high UFR is associated with higher mortality. We examined the association of proportion of dialysis treatments with high UFR and mortality in end stage kidney failure patients receiving hemodialysis.
Methods
This was a retrospective study of incident patients initiating hemodialysis between January 1, 2010, and December 31, 2019, at Emory dialysis centers. The proportion of treatments with high UFR (>13 ml/kg per hour) per patient was calculated using data from the initial 3 months of dialysis therapy. Patients were categorized on the basis of quartiles of proportion of dialysis sessions with high UFR. Risk of death and survival probabilities were calculated and compared for all quartiles.
Results
Of 1050 patients eligible, the median age was 59 years, 56% were men, and 91% were Black. The median UFR was 6.5 ml/kg per hour, and the proportion of sessions with high UFR was 5%. Thirty-one percent of patients never experienced high UFR. Being a man, younger age, shorter duration of hemodialysis sessions, lower weight, diabetic status, higher albumin, and history of heart failure were associated with a higher proportion of sessions with high UFR. Patients in the higher quartile (26% dialysis with high UFR, average UFR 9.8 ml/kg per hour, median survival of 5.6 years) had a higher risk of death (adjusted hazard ratio 1.54; 95% CI, 1.13 to 2.10) compared with those in the lower quartile (0% dialysis with high UFR, average UFR 4.7 ml/kg per hour, median survival 8.8 years).
Conclusions
Patients on hemodialysis who did not experience frequent episodes of elevated UFR during the first 3 months of their dialysis tenure had a significantly lower risk of death compared with patients with frequent episodes of high UFR.
Introduction
According to United States Renal Data System data, the adjusted all-cause mortality in prevalent patients receiving hemodialysis (HD) decreased from 191.5 per thousand patient-years in 2009 to 156.6 in 2019. The overall reduction in mortality from 2009 to 2019 was 18% for those receiving maintenance HD, 21% for those on peritoneal dialysis, and 11% for kidney transplant recipients (1), with the excess risk of death decreasing by 12%–27% over any 5-year interval between 1995 and 2013 (2). Despite this continued improvement in mortality, the rate of cardiovascular events and mortality remains several times above the general population, with 50% of deaths attributed to cardiovascular causes (1). For many years, the nephrology community and surveillance systems have focused on urea clearances as the most important metric in determining dialysis adequacy (3). The emergence of nontraditional risk factors (i.e., calcium metabolism and vascular calcifications, hyperparathyroidism, protein carbamylation, chronic inflammatory state, persistent acidosis, type of vascular access, etc.) (4) for morbidity and mortality in dialysis patients and attempts to control them likely have contributed to the recent improvements in mortality rates.
The Centers for Medicare & Medicaid Services (CMS) have recently included target weight achievement and ultrafiltration rate (UFR) as part of the ESKD quality metrics (5–7). Several studies have examined the increased risk of death as average UFR increases, and an upper limit of 13 ml/kg per hour has been suggested as a quality metric (8–17). Most studies place the inflection point for increased risk of death above 13 ml/kg per hour, and this finding is consistent across all groups of sex, race, dialysis session duration, and body size. Presumed mechanisms of higher mortality associated with high UFR are putative and include myocardial disease, increased risk of arrhythmias, sudden death, and intradialytic hypotension with associated consequences, among others (18–25). However, even patients in whom the average UFR is <10–13 ml/kg per hour may have dialysis sessions with high UFR, and the frequency of these events could also be associated with increased mortality risk in a prevalent cohort (12).
In this study, we examine the relationship between the frequency of episodes of HD with high UFR and patient survival in an incident cohort, predominantly Black, rather than focusing exclusively on the average UFR.
Materials and Methods
This study was a retrospective analysis approved by the Emory University Institutional Board Review. All incident patients on HD admitted to academic affiliated dialysis centers from January 1, 2010, to December 31, 2019, were identified. Only patients that remained on HD for at least 3 months were included. Nocturnal HD and home dialysis patients or patients receiving more than three HD sessions per week were not included. HD sessions with missing or inaccurate data or with a duration of <1 hour were excluded from further analysis. Patients were followed until death, transfer to another dialysis center, transplant, or censoring on December 31, 2020. Demographic, laboratory, and dialysis data were extracted from the electronical medical record. Deaths related to congestive heart failure (CHF), valvular disease, cerebrovascular accident, mesenteric ischemia, and coronary artery disease or acute myocardial infarction were categorized as cardiovascular deaths.
On the basis of values reported in the literature, a UFR >13 ml/kg per hour was considered high (7–12). All HD sessions performed during the first 3 months of the dialysis tenure were analyzed, and the percentage of dialysis sessions with high UFR was recorded. Delivered UFR was calculated from electronic HD data and presented as milliliters per kilogram per hour using change in patient weight (pre-HD weight minus post-HD weight) as the measure of ultrafiltration. HD sessions with implausible values of pre- and post-HD weight, BP, or session duration were also excluded from analysis. Laboratory and HD data were averaged for the first 3 months of dialysis. Data are presented as the median (interquartile range) or mean±SD. All laboratory tests were analyzed via a single laboratory facility.
Factors associated with mortality were explored using a univariate model, and the most significant and clinically relevant factors were used for adjustments in a multivariate model that included age, presence of diabetes, BP post dialysis, type of vascular access, dialysis adequacy (adequate versus inadequate on the basis of average Kt/V ≥1.2), serum albumin, and history of CHF at dialysis initiation. Logistic regression models were used to examine factors associated with high UFR. To explore the association of high UFR frequency and mortality, patients were categorized on the basis of the quartiles of proportion of HD sessions with UFR >13 ml/kg per hour. Relative risk and Cox proportional hazard ratios were estimated for quartiles of proportions of HD sessions with high UFR. Restricted cubic splines models were constructed using a previously described methodology (26,27) to explore the association of proportion of dialyses with UFR >13 ml/kg per hour as a continuous variable with mortality. All data manipulation, statistical analyses, and graphs were performed using R statistical software (28). A P value of 0.05 was considered significant.
Results
Patient Characteristics
All patients who initiated HD between January 1, 2010, and December 31, 2019, and who remained on HD for at least 3 months were identified and followed until censored (lost to follow-up, death, transplant, or transfer to another dialysis facility) or up to December 31, 2020. A total of 1050 patients received 39,068 dialysis sessions during our analyses, with an average of 34.4 dialysis sessions/patient. The median follow-up time was 2.4 years (range 0.25–10.9 years; interquartile range 1.1–4.7).
Eight percent of dialysis sessions were considered to have missing, inaccurate, or implausible data or sessions were less than 1 hour long and were discarded from further analysis.
The median age of the cohort was 59 years, 92% were Black, 56% were men, and 80% were able to dialyze using a permanent vascular access at some point during their dialysis tenure. Fifty percent were diabetic, and 32% had a documented history of cardiovascular disease (CHF, coronary artery disease, peripheral vascular disease, atrial fibrillation, valvular heart disease, and/or cerebrovascular accident) before initiation of dialysis. Dialysis treatment parameters and laboratory values averaged during the first 3 months of dialysis are presented in Table 1.
Table 1.
Baseline demographics, comorbidities, and laboratory data
| Characteristic | N=1050 |
|---|---|
| Age, yr | 59 (49–68) |
| Sex | |
| Women | 470 (45) |
| Men | 580 (55) |
| Race | |
| Black | 958 (91) |
| White | 70 (7%) |
| Asian | 19 (2%) |
| Native Hawaiian or other Pacific Islander | 3 (0.3%) |
| Initial vascular access | |
| Fistula or graft | 438 (42%) |
| Catheter | 612 (58%) |
| Best vascular access achieved during follow-up | |
| Fistula or graft | 915 (87%) |
| Catheter | 135 (13%) |
| Diabetes | 584 (56%) |
| Chronic viral diseasea | 138 (13%) |
| Congestive heart failureb | 297 (28%) |
| Coronary artery diseasec | 125 (12%) |
| Cerebrovascular accidentd | 69 (7%) |
| Peripheral vascular diseasee | 74 (7%) |
| Arrythmiaf | 62 (6%) |
| Valvular diseaseg | 10 (1%) |
| Malignancy | 24 (2%) |
| Chronic lung disease | 24 (2%) |
| Chronic liver disease | 9 (0.9%) |
| Weight, kg | 77 (65–92) |
| Dialysis time, min | 209 (202–217) |
| Ultrafiltration, L/dialysis | 1.7 (1.3–2.3) |
| UFR, ml/kg per hour | 6.5 (4.9–8.5) |
| Percent of dialysis with UFR >13 ml/kg per hour | 5 (0–13) |
| Kt/V | 1.4 (1.2–1.6) |
| Mean arterial pressure pre dialysis, mm Hg | 105 (96–114) |
| Mean arterial pressure post dialysis, mm Hg | 102 (94–109) |
| Normalized protein catabolic rate | 0.9 (0.8–1.1) |
| Serum albumin, g/dl | 3.8 (3.5–4.1) |
| Hemoglobin, g/dl | 10.0 (9.1–10.9) |
| Phosphorus, mg/dl | 4.9 (4.2–5.6) |
| Calcium, mg/dl | 8.9 (8.4–9.2) |
| Parathyroid hormone, pg/ml | 387 (249–582) |
| Cholesterol, mg/dl | 146 (122–172) |
| Potassium, mEq/L | 4.2 (3.9–4.5) |
Data shown as median (interquartile range) or n (%). UFR, ultrafiltration rate.
Hepatitis B, hepatitis C, or HIV.
Systolic or diastolic.
History of coronary artery surgery, myocardial infarction, or coronary angina.
Ischemic or hemorrhagic stroke.
Claudication, documented arterial insufficiency, and/or leg amputation.
Atrial or ventricular arrythmias, bradycardia, and/or pacemaker presence.
Significant valvular abnormality or history of valvular surgery and/or replacement.
UFRs
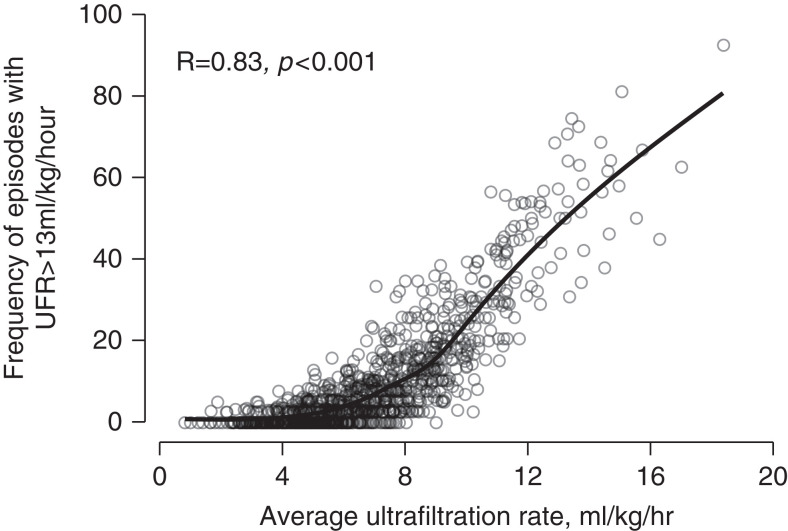
Figure 1 presents the association of average UFR per patient and percent of dialysis sessions with a UFR >13 ml/kg per hour (P<0.001). The median UFR of the entire cohort was 6.5 ml/kg per hour, and the proportion of HD sessions with a UFR >13 ml/kg per hour was 5%. In total, 321 (31%) patients never experienced a UFR >13 ml/kg per hour.
Figure 1.
Association of average ultrafiltration rate (UFR) with percent of hemodialysis (HD) sessions with a UFR >13 ml/kg per hour (P<0.001).
Not surprisingly, the first dialysis session of the week was associated with a higher number of episodes of high UFR, with 12% of sessions with a UFR >13 ml/kg per hour on the first, 9% on the second, and 8% on the third session of the week (P<0.001). The average UFR for the first HD session of the week was also higher at 7.4±4.9 ml/kg per hour compared with 6.7±4.8 and 6.5±4.7 ml/kg per hour for the second and third sessions of the week, respectively.
To evaluate clinical factors associated with episodes of high UFR and taking into consideration that 31% of patients never experienced episodes of high UFR, a zero-inflated regression model was constructed. Being a man, younger age, shorter duration of HD, lower weight, diabetic status, higher albumin, and a history of CHF were associated with higher proportion of HD sessions with elevated UFR (Table 2).
Table 2.
Factors associated with treatments with high UFR
| Characteristic | IRR | 95% CI | P Value |
|---|---|---|---|
| Age, every 5 yr | 0.9 | 0.89 to 0.91 | <0.001 |
| Men | 1.13 | 1.08 to 1.18 | <0.001 |
| Time of dialysis, every 15 min | 0.94 | 0.92 to 0.95 | <0.001 |
| Weight, for every 5 kg | 0.89 | 0.88 to 0.9 | <0.001 |
| Diabetes | 1.12 | 1.07 to 1.17 | <0.001 |
| Albumin, g/dl | 1.08 | 1.04 to 1.12 | <0.01 |
| Congestive heart failure | 1.05 | 1 to 1.11 | 0.05 |
UFR, ultrafiltration rate; IRR, incidence rate ratio; CI, confidence interval.
UFR and BP
When all dialysis sessions were analyzed, there was a weak correlation, albeit statistically significant, between BP drop (pre-dialysis systolic BP minus lowest systolic arterial BP) during dialysis and the intensity of fluid removal as judged by UFR (R=–0.08, P<0.001). Using a definition of dialysis-associated hypotension suggested elsewhere (29) of a pre-HD systolic BP ≥160 mm Hg and drop to <100 mm Hg for systolic BP or a drop to <90 mm Hg at any time during the dialysis session as an indicator of severe hypotension, 8.2% HD sessions with a UFR >13 ml/kg per hour were complicated by dialysis-associated hypotension compared with 7.8% of HD sessions with a UFR ≤13 ml/kg per hour (P=0.3).
Mortality
The all-cause mortality risk for the entire cohort was 9.4 deaths/100 patient-years.
To study the association of episodes of HD with high UFR with mortality, patients were categorized in four groups on the basis of quartiles of frequency of HD with high UFR. Clinical differences between these groups are presented in Table 3.
Table 3.
Clinical characteristics on the basis of quartiles of proportion of dialysis with high UFR
| Characteristic | Q1, N=321 | Q2, N=207 | Q3, N=261 | Q4, N=261 |
|---|---|---|---|---|
| Age, yr | 59 (49–67) | 60 (49–67) | 60 (50–71) | 58 (44–66) |
| Sex | ||||
| Women | 146 (45) | 99 (48) | 107 (41) | 118 (45) |
| Men | 175 (55) | 108 (52) | 154 (59) | 143 (55) |
| Race | ||||
| Black | 289 (90) | 196 (95) | 237 (91) | 236 (90) |
| White | 28 (9) | 11 (5) | 16 (6.1) | 15 (6) |
| Native Hawaiian | 0 (0) | 0 (0) | 1 (0.4) | 2 (0.8) |
| Asian | 4 (1) | 0 (0) | 7 (3) | 8 (3) |
| Access at 3 months | ||||
| Fistula or graft | 146 (45) | 86 (42) | 112 (43) | 94 (36) |
| Catheter | 175 (55) | 121 (58) | 149 (57) | 167 (64) |
| Best access | ||||
| Fistula or graft | 291 (91) | 181 (87) | 224 (86) | 219 (84) |
| Catheter | 30 (9) | 26 (13) | 37 (14) | 42 (16) |
| Diabetes | 193 (60) | 118 (57) | 141 (54) | 132 (51) |
| Viral diseasea | 33 (10) | 27 (13) | 35 (13) | 43 (16) |
| Malignancy | 6 (2) | 4 (2) | 7 (3) | 7 (3) |
| Lung disease | 8 (3) | 5 (2) | 5 (2) | 6 (2) |
| Liver disease | 4 (1) | 1 (0.5) | 3 (1) | 1 (0.4) |
| Cerebrovascular accidentb | 20 (6) | 18 (9) | 16 (6) | 15 (6) |
| Peripheral vascular diseasec | 21 (7) | 10 (4.8%) | 21 (8) | 22 (8) |
| Coronary artery diseased | 45 (14) | 24 (12) | 28 (11) | 28 (11) |
| Congestive heart failuree | 99 (31) | 58 (28) | 83 (32) | 57 (22) |
| Arrhythmiaf | 21 (7) | 13 (6) | 18 (7) | 10 (4) |
| Valvular diseaseg | 4 (1%) | 2 (1) | 3 (1) | 1 (0.4) |
| Dialysis count, % | 11,208 (31) | 7212 (20) | 8988 (25) | 8764 (24) |
| UFR, ml/kg per hour | 4.7 (3.7–5.6) | 5.8 (4.6–6.9) | 7.1 (6.1–0.2) | 9.8 (8.7–11.3) |
| Ultrafiltration, L/dialysis | 1.5 (1.2–2) | 1.6 (1.1–2.2) | 1.8 (1.4–2.2) | 2.2 (1.7–2.6) |
| % HD with high UFR | 0 (0–0) | 3 (3–3) | 8 (6–10) | 26 (18–36) |
| % Patients with high UFR | 0 | 0 | 0 | 10 |
| Weight, kg | 92 (79–111) | 82 (67–96) | 72 (64–83) | 66 (57–74) |
| Dialysis time, min | 210 (206–225) | 209 (203–224) | 209 (202–214) | 206 (196–210) |
| KtV | 1.3 (1.2–1.5) | 1.3 (1.2–1.6) | 1.4 (1.23–1.53) | 1.4 (1.25–1.64) |
| Mean arterial pressure pre | 105 (96–113) | 106 (96–113) | 105 (95–113) | 107 (98–117) |
| Mean arterial pressure post | 101 (94–108) | 100 (94–108) | 101 (93–109) | 105 (96–112) |
| % HD with hypotensionh | 3 | 3 | 5 | 3 |
| Albumin, g/dl | 3.9 (3.6–4.1) | 3.8 (3.5–4.1) | 3.8 (3.5–4) | 3.8 (3.5–4.1) |
| Normalized protein catabolic rate | 0.9 (0.8–1.1) | 0.9 (0.8–1) | 0.9 (0.8–1.1) | 1 (0.8–1.2) |
| Calcium, mg/dl | 9 (8.5–9.3) | 8.9 (8.5–9.3) | 8.9 (8.4–9.2) | 8.8 (8.3–9.1) |
| Phosphorus, mg/dl | 4.9 (4.2–5.5) | 4.8(4.1–5.7) | 4.8 (4.2–5.5) | 5.2 (4.4–5) |
| Parathyroid hormone, pg/ml | 408 (259–618) | 394 (263–594) | 377 (239–542) | 366 (233–597) |
| Hemoglobin, g/dl | 10.1 (9.3–11) | 10.1 (9.2–10.9) | 9.9 (8.9–10.7) | 9.8 (8.8–10.9) |
| Cholesterol, mg/dl | 148 (126–174) | 148 (124–174) | 141 (117–172) | 146 (124–171) |
| Potassium, mEq/L | 4.2 (3.9–4.5) | 4.2 (3.8–4.5) | 4.2 (4–4.5) | 4.3 (4–4.6) |
Data shown as median (interquartile range) or n (%). UFR, ultrafiltration rate; HD, hemodialysis.
Hepatitis B, hepatitis C, or HIV.
Ischemic or hemorrhagic stroke.
Claudication, documented arterial insufficiency, and/or leg amputation.
History of coronary artery surgery, myocardial infarction, or coronary angina.
Systolic or diastolic.
Atrial or ventricular arrythmias, bradycardia, and/or pacemaker presence.
Significant valvular abnormality or history of valvular surgery and/or replacement.
Drop from pre-HD systolic BP ≥160 to <100 mm Hg or any drop to <90 mm Hg during dialysis session.
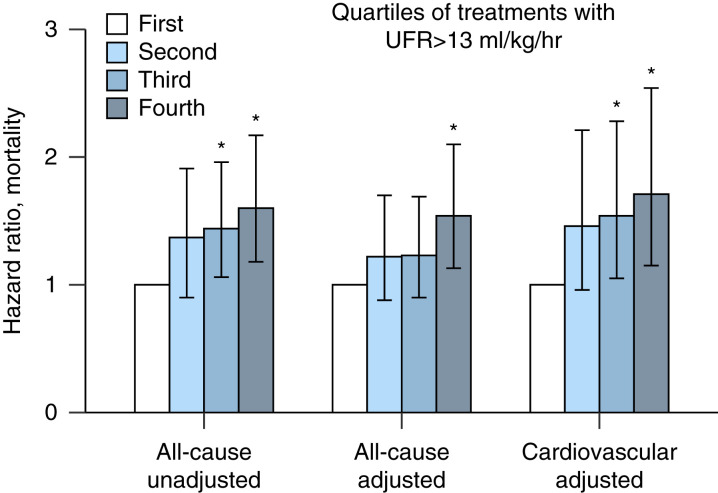
The all-cause mortality risk was 7.2, 9.8, 10.2, and 11.4 deaths/100 patient-years for the first, second, third, and fourth quartiles, respectively. Compared with the first quartile of proportion of HD sessions with high UFR, the relative risks for all-cause mortality were 1.36 (95% confidence interval [CI], 0.96 to 1.92), 1.42 (95% CI, 1.03 to 1.96), and 1.58 (95% CI, 1.16 to 2.17) for the second, third, and fourth quartiles, respectively. Meanwhile, the cardiovascular mortality risks were 4.38, 6.6, 7.32, and 7.11 cardiovascular deaths/100 patient-years, with resulting relative risks of 1.51 (95% CI, 0.97 to 2.32), 1.67 (95% CI, 1.12 to 2.50), and 1.62 (95% CI, 1.08 to 2.44) for the second, third, and fourth quartiles, respectively, compared with the first quartile of proportion of HD sessions with high UFR. In a model adjusted for patient age, best type vascular access during the HD tenure (catheter versus no catheter), diabetes, history of CHF at start of HD, dialysis Kt/V (adequate versus inadequate), mean arterial pressure post dialysis, and serum albumin, the upper quartile was associated with a higher hazard ratio of all-cause (1.56; 95% CI, 1.13 to 2.10) and cardiovascular mortality (1.71; 95% CI, 1.15 to 2.54; Figure 2).
Figure 2.
All-cause and cardiovascular mortality hazard ratio according to quartiles of proportion of treatments with a UFR >13 ml/kg per hour. Compared with the first quartile (no episodes with a UFR >13 ml/kg per hour), patients in the higher quartile had a significantly higher risk of all-cause mortality, both unadjusted (1.60; 95% confidence interval [CI], 1.18 to 2.17) or adjusted (1.54; 95% CI, 1.13 to 2.10). Adjusted cardiovascular mortality was also higher for patients in the third (1.54; 95% CI, 1.05 to 2.25) and fourth quartiles compared with the first quartile (1.71; 95% CI, 1.15 to 2.54).
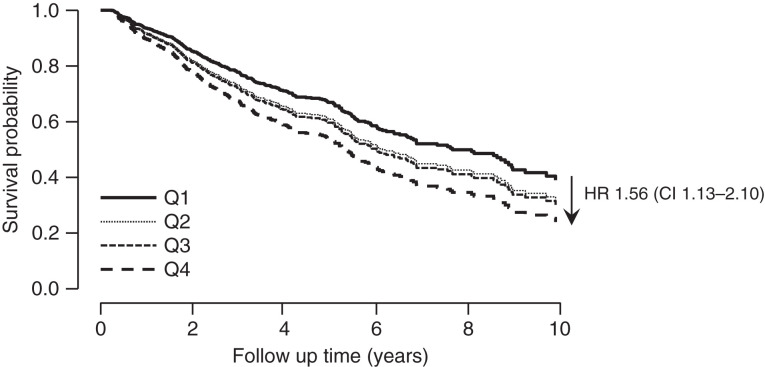
Survival rate differences between quartiles was analyzed using a Kaplan–Meier estimate. The median survival time for patients in the lowest quartile was significantly longer than patients in the upper quartile (8.8 years versus 5.6 years; P<0.001). The difference persisted after adjusting for age, best type of vascular access during the dialysis tenure (catheter versus no catheter), dialysis Kt/V, history of CHF, serum albumin, and mean arterial pressure post dialysis. Compared with the first quartile, patients in the higher quartile had a 56% higher risk of death (Figure 3).
Figure 3.
Survival probability according to quartiles of proportion of HD treatments with a UFR >13 ml/kg per hour. Compared with the lowest quartile (Q1), patients in the highest quartile (Q4) had a 56% higher risk of death, adjusted for age, best vascular access during dialysis tenure (catheter versus no catheter), dialysis Kt/V (adequate versus inadequate), history of congestive heart failure (CHF), serum albumin, and mean arterial pressure post dialysis.
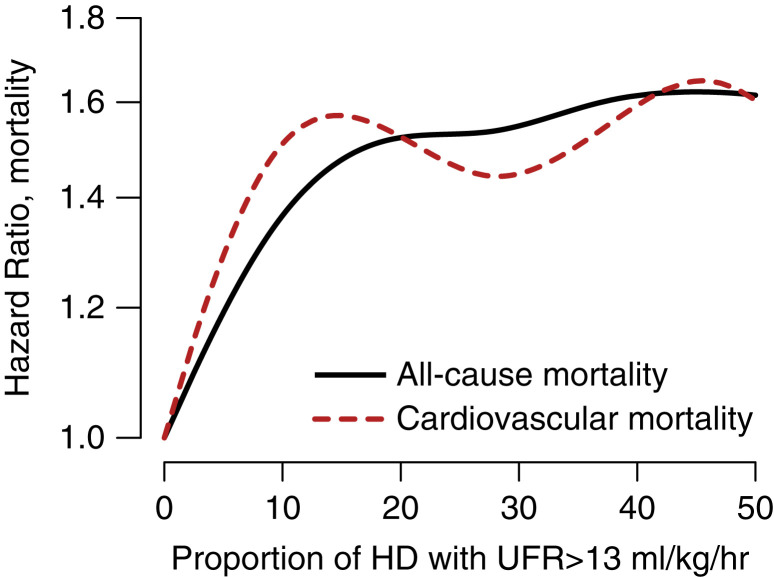
Additionally, cubic splines models were constructed using a previously described methodology (27) to explore the proportion of HD sessions with a UFR >13 ml/kg per hour and their associated all-cause and cardiovascular mortality. Adjusting for the same variables described previously, a higher proportion of dialysis sessions with high UFR were associated with increased mortality (Figure 4).
Figure 4.
All-cause and cardiovascular mortality continuous hazard ratio according to proportion of HD treatments with a UFR >13 ml/kg per hour, adjusted for age, best vascular access during dialysis tenure (catheter versus no catheter), diabetes, dialysis Kt/V (adequate versus inadequate), history of CHF, serum albumin, and mean arterial pressure post dialysis.
Discussion
Our data show that in the group of patients with high numbers of HD sessions with a UFR >13 ml/kg per hour, there was a mortality risk, as others have shown (12); however, the average UFR of this group was only 9.8 ml/kg per hour, and thus most of these individuals likely would not have been flagged as high risk according to CMS guidelines. The unadjusted all-cause mortality hazard ratio for patients in the upper quartile of episodes of HD with UFR>13 ml/kg per hour was 1.58 (95% CI, 1.16 to 2.17) and persisted after adjustments for age, history of CHF, adequacy of HD, serum albumin, type of vascular access, and BP levels post-HD treatment (1.54; 95% CI, 1.13 to 2.10; Figure 2). The median survival of patients in the lower quartile was 8.8 years compared with 5.6 years for those in the upper quartile (P<0.001; Figure 3).
CMS measures use only one measurement of UFR done on the day of Kt/V assessments, which may vary across dialysis facilities and limit utility, particularly if day-of-week collection standards are not specified. Mean-based UFR measures, as proposed by the Kidney Care Quality Alliance (30–33), may best capture facility practices because they use the mean UFR for an entire week of HD sessions and thus account for variability of UFR across interdialytic intervals. Our study is unique in that it highlights the importance of careful fluid removal over the entirety of HD sessions in incident patients and in a predominantly vulnerable Black population. These data show that even in the first 3 months of the dialysis tenure, the frequency of dialysis sessions with high UFR affects mortality, and thus careful fluid management and consideration for longer dialysis times are important.
Similar findings were described by Assimon et al. (12) in a prevalent cohort where a higher percentage of dialysis treatments with a UFR >13 ml/kg per hour were associated with higher all-cause mortality. Compared with <25% of treatments above the threshold, greater proportions of treatments with a UFR >13 ml/kg per hour were associated with greater mortality. The more robust sampling of HD sessions in our study allowed a greater degree of granularity of the data, suggesting that an increasing number of HD sessions with high UFR are also associated with higher mortality, below the 25% reported by Assimon et al. (12).
The relationship between a patient’s average UFR and frequencies of dialysis sessions with high UFR is worth commenting on. As shown in Figure 1, patients with a UFR within a range considered “normal,” for example 5–10 ml/kg per hour, can experience a significant number of episodes of dialysis with high UFR, ranging from 0% to 38%.
Compared with other studies (8–12,14,15), the UFR noted in this cohort are lower than previously reported. The median UFR for the entire cohort was 6.5 ml/kg per hour (IQR 4.9–8.5 ml/kg per hour), and only 3% of patients had an average UFR >13 ml/kg per hour compared with 18% of patients in Assimon et al.’s study (12). The lower UFR in our study could be explained by it being an incident cohort, which is consistent with findings by Kim et al. (14). The higher UFR observed in prevalent cohorts could be explained by the loss of residual renal function and increased nutrient and fluid intake over time. Additionally, our patient population is predominantly Black (93%) as opposed to only 32% in Assimon et al.’s study (12). Similar to Kim et al. (14), we used delivered UFR to calculate the frequencies of dialysis sessions with high UFR. Sex, prevalence of CHF, and diabetic status were similar in our cohort compared with the studies by Assimon et al. (12) and Kim et al. (14). These studies were carried out with cohorts studied in early periods. Our more contemporary data may reflect changes in patterns on medical care aimed at limiting UFR. Despite the low overall UFR in this study, we show a linear and continuous increased risk of all-cause and cardiovascular mortality as the proportion of HD sessions with high UFR increases (Figure 4), with the lowest risk in patients who did not experience episodes of high UFR.
The risk of death on the basis of HD day of the week has been previously documented in ESKD patients (34,35), with risk for all-cause mortality, mortality from cardiac arrest, myocardial infarction, and dysrhythmia being higher during the first HD of the week, after the longer interdialytic interval. In our study, the first dialysis of the week resulted in a higher proportion of dialysis sessions with high UFR. Thus, adjustments in prescribed time or targeting a higher dry weight for the first dialysis session of the week could be a reasonable strategy to limit the frequency of episodes of dialysis with high UFR as long as volume status is not significantly compromised. In addition, other strategies to reduce UFR would be to emphasize the importance of dietary salt and fluid restriction to avoid excessive interdialytic weight gain and ultrafiltration, particularly in high-risk individuals, and for dialysis units to provide a more flexible schedule to allow longer session times or additional treatments for isolated ultrafiltration. Furthermore, adopting the practice of prescribing longer HD sessions, i.e., 3.5–4 hours at initiation of HD, instead of shorter times often prescribed due to perceived residual function may be prudent. This study identifies factors associated with episodes of high UFR, including being a man, younger age, shorter duration of dialysis sessions, lower weight, diabetic status, and a history of CHF. Thus, these higher risk individuals could be specifically targeted for the mitigation measures to avoid excessive ultrafiltration.
Dialysis time and weight directly influence the UFR, whereas the association with history of CHF is likely related to volume excess requiring higher UFR. Better nutritional status has been associated with higher UFR in previous studies (6,10,13) and likely related to higher food and fluid intake in patients with good appetites. We did not find a clear association between UFR and risk of severe hypotension during dialysis, with 8.2% of dialysis with a UFR >13 ml/kg per hour complicated by severe hypotension versus 7.8% of dialysis with a UFR ≤13 ml/kg per hour.
There are several limitations to our study, including the retrospective and observational nature of our analyses, which may not completely assess residual confounding, despite our robust comorbidity and laboratory covariate adjustments. Also, the lack of diverse patient populations may lessen the generalizability to the broader HD population, and the lack of standardization of BP measurements across dialysis facilities that may not accurately reflect BP changes, although it is consistent with real-world dialysis practices. We lack data on residual renal function that may affect the values of ultrafiltration presented and confound the outcomes. The major strengths of our study include the long-term follow-up of incident HD patients, a greater UFR sampling period, including the frequency of high UFR, detailed clinical and laboratory data for multivariate analyses, and uniform laboratory measurements from a single laboratory facility.
The current CMS measures inventory tool monitors the number of adult ESKD patients at a dialysis facility with a UFR >13 ml/kg per hour, calculated on the basis of a single session per month—a tool that does not allow determination of frequency of episodes of high UFR per patient. We believe that the results of our study have important implications for the implementation of dialysis treatments in outpatient HD centers, where the emphasis and monitoring strategies are currently placed on average UFRs for the entire population of patients rather than on proportion of episodes of high UFR for individual patients. Our retrospective and observational study shows that higher proportions of HD treatments with a UFR >13 ml/kg per hour among incident dialysis patients are associated with increased all-cause and cardiovascular mortality irrespective of age, best vascular access during dialysis tenure, dialysis adequacy, history of CHF, serum albumin, and mean arterial pressure post dialysis. Future directions and applications of our findings are for clinical guidelines to take into account the number of HD sessions with high UFR and not just one session per month on laboratory day because it may not accurately reflect one’s absolute mortality risk. Frequency-based definitions of UFRs may better capture risk than single treatment or mean-based UFR definitions. More investigations into UFRs in HD patients and the associated predictors and outcomes are warranted and should include the proportion of HD sessions with high UFR.
Disclosures
J. Cobb reports research funding from Vertex and honoraria from Aurinia. All remaining authors have nothing to disclose.
Funding
None.
Footnotes
See related editorial, “High Ultrafiltration Rates and Mortality in Hemodialysis Patients: Current Evidence and Future Steps,” on pages 1293–1295.
Author Contributions
J. Cobb, J.P. Lea, and J.E. Navarrete reviewed and edited the manuscript; J.P. Lea and J.E. Navarrete were responsible for conceptualization, formal analysis, supervision, and visualization; J.E. Navarrete was responsible for data curation, project administration, resources, software, and validation, and all authors were responsible for the investigation and methodology, and wrote the original draft of the manuscript.
References
- 1.United States Renal Data System : 2021 USRDS Annual Data Report: Epidemiology of kidney disease in the United States, Bethesda, MD, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2021. https://sft.usrds.org/2020/end-stage-renal-disease/5-mortlaity. Accessed 02/08/2022 [Google Scholar]
- 2.Foster BJ, Mitsnefes MM, Dahhou M, Zhang X, Laskin BL: Changes in excess mortality from end stage renal disease in the United States from 1995 to 2013. Clin J Am Soc Nephrol 13: 91–99, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Twardowski ZJ: Treatment time and ultrafiltration rate are more important in dialysis prescription than small molecule clearance. Blood Purif 25: 90–98, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Cozzolino M, Mangano M, Stucchi A, Ciceri P, Conte F, Galassi A: Cardiovascular disease in dialysis patients. Nephrol Dial Transplant 33: iii28–iii34, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Centers for Medicare and Medicaid Services : Proposed Measure Specifications for the PY 2019 ESRD QIP. Available at: https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/ESRDQIP/Downloads/ESRD-QIP-Summary-Payment-Years-2014-2018.pdf. Accessed February 4, 2016
- 6.Centers for Medicare and Medicaid Services : Release of Fiscal Year 2016 End Stage Renal Disease Core Survey Data Worksheet. Available at: https://www.cms.gov/Medicare/ProviderEnrollment-and-Certification/Guidance-for-Laws-And-Regulations/Downloads/ESRD-Core-SurveyData-Worksheet.pdf. Accessed February 4, 2016
- 7.Flythe JE, Assimon MM, Wenger JB, Wang L: Ultrafiltration rates and the quality incentive program: Proposed measure definitions and their potential dialysis facility implications. Clin J Am Soc Nephrol 11: 1422–1433, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saran R, Bragg-Gresham JL, Levin NW, Twardowski ZJ, Wizemann V, Saito A, Kimata N, Gillespie BW, Combe C, Bommer J, Akiba T, Mapes DL, Young EW, Port FK: Longer treatment time and slower ultrafiltration in hemodialysis: Associations with reduced mortality in the DOPPS. Kidney Int 69: 1222–1228, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Movilli E, Gaggia P, Zubani R, Camerini C, Vizzardi V, Parrinello G, Savoldi S, Fischer MS, Londrino F, Cancarini G: Association between high ultrafiltration rates and mortality in uraemic patients on regular haemodialysis. A 5-year prospective observational multicentre study. Nephrol Dial Transplant 22: 3547–3552, 2007 [DOI] [PubMed] [Google Scholar]
- 10.Hussein WF, Arramreddy R, Sun SJ, Reiterman M, Schiller B: Higher ultrafiltration rate is associated with longer dialysis recovery time in patients undergoing conventional hemodialysis. Am J Nephrol 46: 3–10, 2017 [DOI] [PubMed] [Google Scholar]
- 11.Flythe JE, Kimmel SE, Brunelli SM: Rapid fluid removal during dialysis is associated with cardiovascular morbidity and mortality. Kidney Int 79: 250–257, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Assimon MM, Wenger JB, Wang L, Flythe JE: Ultrafiltration rate and mortality in maintenance hemodialysis patients. Am J Kidney Dis 68: 911–922, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kramer H, Yee J, Weiner DE, Bansal V, Choi MJ, Brereton L, Berns JS, Samaniego-Picota M, Scheel P Jr, Rocco M: Ultrafiltration rate thresholds in maintenance hemodialysis: An NKF-KDOQI controversies report. Am J Kidney Dis 68: 522–532, 2016 [DOI] [PubMed] [Google Scholar]
- 14.Kim TW, Chang TI, Kim TH, Chou JA, Soohoo M, Ravel VA, Kovesdy CP, Kalantar-Zadeh K, Streja E: Association of ultrafiltration rate with mortality in incident hemodialysis patients. Nephron 139: 13–22, 2018 [DOI] [PubMed] [Google Scholar]
- 15.Lim Y, Yang G, Cho S, Kim SR, Lee YJ: Association between ultrafiltration rate and clinical outcome is modified by muscle mass in hemodialysis patients. Nephron 144: 447–452, 2020 [DOI] [PubMed] [Google Scholar]
- 16.Lee YJ, Okuda Y, Sy J, Lee YK, Obi Y, Cho S, Chen JLT, Jin A, Rhee CM, Kalantar-Zadeh K, Streja E: Ultrafiltration rate, residual kidney function, and survival among patients treated with reduced-frequency hemodialysis. Am J Kidney Dis 75: 342–350, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Agar JW: Personal viewpoint: Limiting maximum ultrafiltration rate as a potential new measure of dialysis adequacy. Hemodial Int 20: 15–21, 2016 [DOI] [PubMed] [Google Scholar]
- 18.Burton JO, Jefferies HJ, Selby NM, McIntyre CW: Hemodialysis-induced cardiac injury: Determinants and associated outcomes. Clin J Am Soc Nephrol 4: 914–920, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burton JO, Jefferies HJ, Selby NM, McIntyre CW: Hemodialysis-induced repetitive myocardial injury results in global and segmental reduction in systolic cardiac function. Clin J Am Soc Nephrol 4: 1925–1931, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nie Y, Zhang Z, Zou J, Liang Y, Cao X, Liu Z, Shen B, Chen X, Ding X: Hemodialysis-induced regional left ventricular systolic dysfunction. Hemodial Int 20: 564–572, 2016 [DOI] [PubMed] [Google Scholar]
- 21.Flythe JE, Mangione TW, Brunelli SM, Curhan GC: Patient-stated preferences regarding volume-related risk mitigation strategies for hemodialysis. Clin J Am Soc Nephrol 9: 1418–1425, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Assa S, Kuipers J, Ettema E, Gaillard CAJM, Krijnen WP, Hummel YM, Voors AA, van Melle JP, Westerhuis R, Willemsen A, Slart RHJA, Franssen CFM: Effect of isolated ultrafiltration and isovolemic dialysis on myocardial perfusion and left ventricular function assessed with 13N-NH3 positron emission tomography and echocardiography. Am J Physiol Renal Physiol 314: F445–F452, 2018 [DOI] [PubMed] [Google Scholar]
- 23.Yu J, Chen X, Li Y, Wang Y, Liu Z, Shen B, Teng J, Zou J, Ding X: High ultrafiltration rate induced intradialytic hypotension is a predictor for cardiac remodeling: A 5-year cohort study. Ren Fail 43: 40–48, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim JK, Song YR, Park G, Kim HJ, Kim SG: Impact of rapid ultrafiltration rate on changes in the echocardiographic left atrial volume index in patients undergoing haemodialysis: A longitudinal observational study. BMJ Open 7: e013990, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Flythe JE, Xue H, Lynch KE, Curhan GC, Brunelli SM: Association of mortality risk with various definitions of intradialytic hypotension. J Am Soc Nephrol 26: 724–734, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gauthier J, Wu QV, Gooley TA: Cubic splines to model relationships between continuous variables and outcomes: A guide for clinicians. Bone Marrow Transplant 55: 675–680, 2020 [DOI] [PubMed] [Google Scholar]
- 27.Therneau T: Spline Terms in a Cox Model. Available at: https://cran.r-project.org/web/packages/survival/vignettes/splines.pdf. Accessed 12/14/2021
- 28.RStudio Team : RStudio: Integrated Development Environment for R. RStudio, Boston, MA, PBC, 2021 [Google Scholar]
- 29.Flythe JE, Chang TI, Gallagher MP, Lindley E, Madero M, Sarafidis PA, Unruh ML, Wang AY, Weiner DE, Cheung M, Jadoul M, Winkelmayer WC, Polkinghorne KR; Conference Participants : Blood pressure and volume management in dialysis: Conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int 97: 861–876, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gotta V, Marsenic O, Atkinson A, Pfister M: Hemodialysis (HD) dose and ultrafiltration rate are associated with survival in pediatric and adolescent patients on chronic HD—A large observational study with follow-up to young adult age. Pediatr Nephrol 36: 2421–2432, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Murugan R, Bellomo R, Palevsky PM, Kellum JA: Ultrafiltration in critically ill patients treated with kidney replacement therapy. Nat Rev Nephrol 17: 262–276, 2021 [DOI] [PubMed] [Google Scholar]
- 32.Murugan R, Balakumar V, Kerti SJ, Priyanka P, Chang CH, Clermont G, Bellomo R, Palevsky PM, Kellum JA: Net ultrafiltration intensity and mortality in critically ill patients with fluid overload. Crit Care 22: 223, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.National Quality Forum : Renal, Fall 2020 Cycle CDP Report. September 13, 2021. qualityforum.org/Publications/2021/09/renal_Final_Report_Fall_2020_Cycle.aspx
- 34.Foley RN, Gilbertson DT, Murray T, Collins AJ: Long interdialytic interval and mortality among patients receiving hemodialysis. N Engl J Med 365: 1099–1107, 2011 [DOI] [PubMed] [Google Scholar]
- 35.Fotheringham J, Sajjad A, Stel VS, McCullough K, Karaboyas A, Wilkie M, Bieber B, Robinson BM, Massy ZA, Jager KJ: The association between longer haemodialysis treatment times and hospitalization and mortality after the two-day break in individuals receiving three times a week haemodialysis. Nephrol Dial Transplant 34: 1577–1584, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]