Key Points
Discovering new nephroprotectants may provide therapeutic strategies in AKI.
This study provides the first evidence that KLF11, a member of the Krüppel-like factor (KLF) family of proteins, protects against AKI.
In the absence of KLF11, exaggerated induction of endothelin-1 and IL-6 occurs after ischemic renal injury and may contribute to worse AKI.
Keywords: acute kidney injury and ICU nephrology, acute kidney injury, basic science, endothelin-1, heme oxygenase-1, IL-6, KLF11, protectant, reperfusion injury
AKI arises from the interplay of endothelial and vascular dysfunction, tubular epithelial cell injury and death, and inflammation, with modulation by systemic processes and circulating leukocytes (1). Rodent AKI models, including renal ischemia-reperfusion injury (IRI), elucidate new biomarkers, the pathogenesis of and recovery from AKI, nephroprotective pathways, and clinically relevant therapeutic strategies (1).
Certain members of the Krüppel-like factor (KLF) family of proteins (KLF2, 4, 5, 6, and 15) are determinants of renal injury induced by glomerulopathies, diabetes, and subtotal nephrectomy, with KLF4 protecting against renal IRI (2,3). KLFs possess a conserved C-terminal region, which houses three C2H2 zinc fingers that engage GC-rich sites in the promoters and enhancers of multiple target genes (2–4). The N-terminus of KLFs varies widely, houses transactivation and transrepression domains, and modulates the specificity of protein-protein and protein-DNA interactions; the N-terminus determines specificity of transcription. KLFs influence homeostasis; cell growth, differentiation, and death; vascular behavior; inflammation; and fibrosis. Broadly considered, KLFs determine adaptive and maladaptive responses to tissue injury (2–4).
This Brief Communication provides the first demonstration that another member of the KLF family, KLF11, protects against AKI. This study was predicated on the following considerations. First, as shown by the classic studies of Chen, Zhang, and colleagues, KLF11 is highly expressed in the endothelium where it prevents endothelial activation and inflammation by inhibiting NF-κB activation (5,6); endothelial activation underpins, in part, renal vasoconstriction and abnormal renal hemodynamics, which are proximate pathogenetic steps in IRI, whereas NF-κB activation contributes to renal inflammation and IRI (1). Second, KLF11 protects against brain ischemic injury, in part by suppressing IL-6 production (7), a cytokine that promotes renal IRI (1). Third, KLF11 suppresses the production of endothelin-1 (ET-1) (8), a vasoconstricting and proinflammatory peptide that also mediates AKI (9). We thus hypothesize that KLF11 protects against IRI because it suppresses renal production of ET-1 and inflammation. Interestingly, De Lorenzo et al. have just demonstrated that KLF11 reduces chronic kidney injury (unilateral ureteral obstruction) by inhibiting the TGF-β1/Smad3 pathway (10).
All studies were approved by the Institutional Animal Care and Use Committee of Mayo Clinic and performed in accordance with the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. KLF11+/+ and KLF11−/− mice were generated from a colony on a C57BL/6 strain background (backcrossed >20 generations) (11). Studies used male KLF11−/− mice and male littermate KLF11+/+ mice (16–24 weeks old). Bilateral IRI and sham procedures were employed, with measurement of serum creatinine and BUN at 1 day after 22 minutes of ischemia (12). Histologic examination was performed on formalin-fixed, paraffin-embedded kidney sections stained with hematoxylin and eosin (12). Gene and protein expression was assessed using quantitative real-time RT-PCR, Western blot analysis, and immunofluorescence, as described previously (12). Serum IL-6 levels were assayed by ELISA.
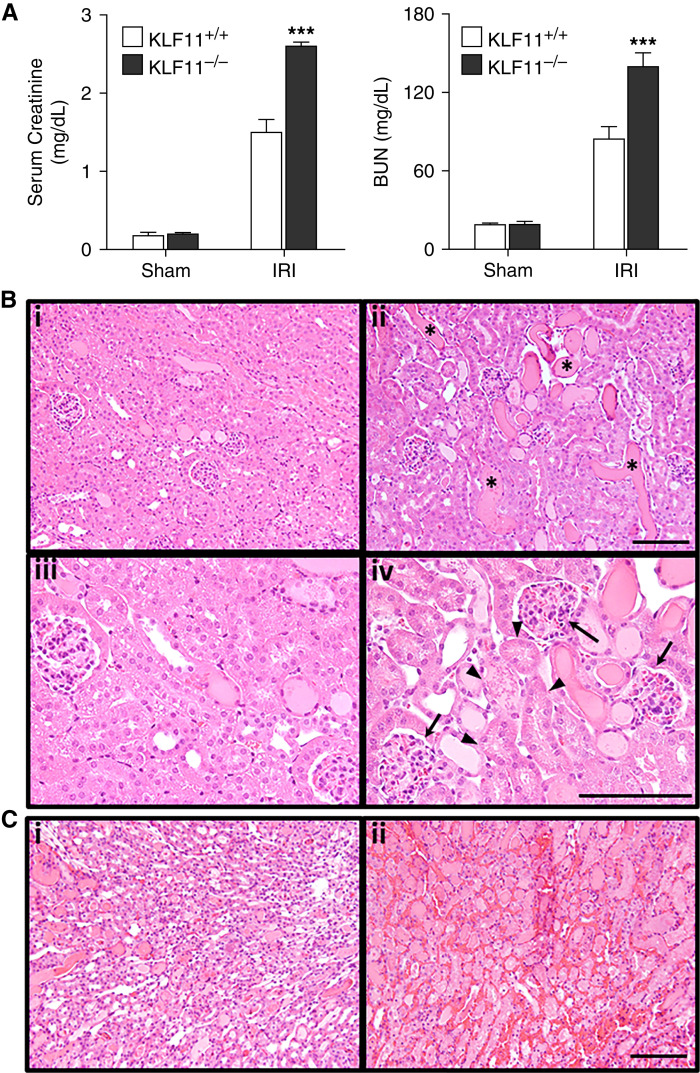
KLF11−/− mice compared with KLF11+/+ mice showed increased sensitivity to IRI as demonstrated by significantly higher serum creatinine and BUN (Figure 1A). Figure 1B shows representative kidney sections. Tubular epithelial cell injury and death were observed in the deep cortex and outer medulla of KLF11+/+ mice after IRI, whereas in KLF11−/− mice, such injury extended over full cortical thickness and was accompanied by prominent tubular cast formation, dilatation, and simplification. KLF11−/− mice exhibited prominent vascular congestion in the medullary and cortical vasculature and glomerular capillaries (Figure 1, B and C), whereas congestion in KLF11+/+ mice was milder and observed only in the medulla. Intravascular thrombosis was not observed in either group. Such exaggerated vascular congestion in KLF11−/− mice after IRI is likely significant because congestion exacerbates AKI via NF-κB-dependent mechanisms.
Figure 1.
Alterations in renal function and histology one day after renal ischemia-reperfusion injury (IRI) in Krüppel-like factor (KLF)11+/+ and KLF−/− mice. (A) KLF11−/− mice, as compared with KLF11+/+ mice, showed significantly higher levels of serum creatinine and BUN values after IRI. Values are the mean±SEM; n=5 for each sham group, and n=6 and 7 for KLF11+/+ and KLF11−/− mice subjected to IRI, respectively. For this and subsequent statistical analyses, a t test was used for parametric data, and the Mann–Whitney U test was used for nonparametric data. ***P<0.005, KLF11−/− IRI versus KLF11+/+ IRI. (B) Representative hematoxylin and eosin staining at 1 day after IRI for 22 minutes revealed increased necrosis (arrowheads), cast formation (asterisks), tubular dilatation, and glomerular congestion (arrows) in the renal cortex of KLF11−/− mice (ii and iv) when compared with KLF11+/+ mice (i and iii). (C) The renal medulla of KLF11−/− mice (ii) at 1 day after IRI for 22 minutes showed increased necrosis and vascular congestion compared with KLF11+/+ mice (i). Scale bar 100 μm.
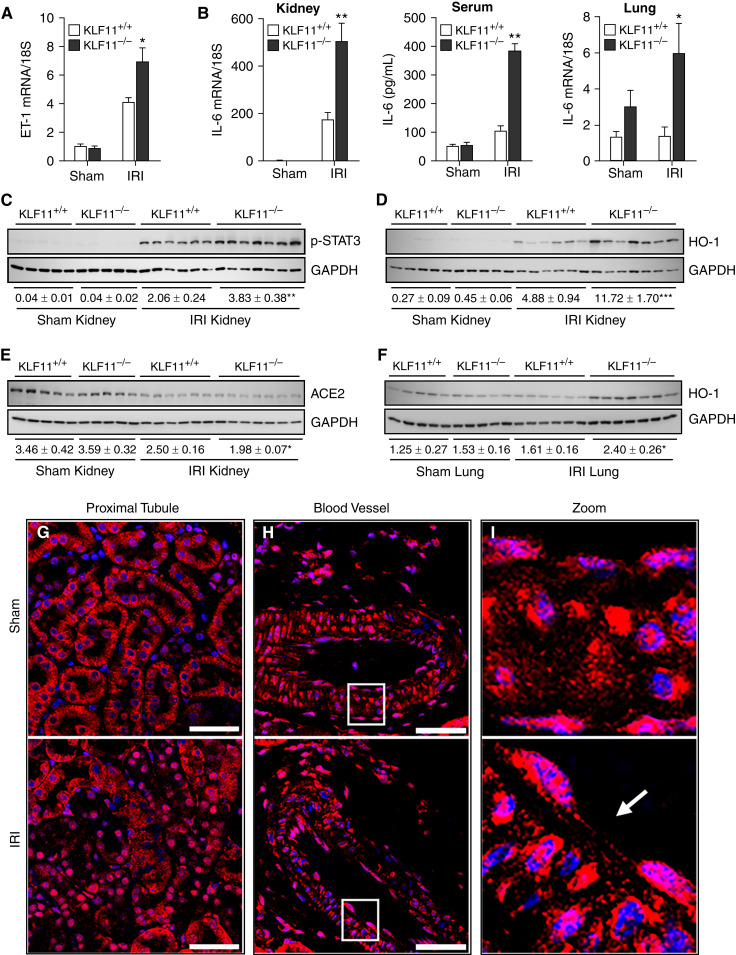
Renal ET-1 mRNA induction was markedly greater in KLF11−/− mice compared with KLF11+/+ mice after IRI (Figure 2A), as was renal induction of IL-6 mRNA (Figure 2B, left panel). Systemic IL-6 levels and increased IL-6 expression in distant vital organs (e.g., the lungs) are known to underlie morbidity and mortality observed in AKI. We thus assessed these parameters in KLF11+/+ and KLF11−/− mice following IRI. IRI elicited significantly higher plasma IL-6 levels and greater IL-6 mRNA expression in the lungs in KLF11−/− mice compared with KLF11+/+ mice (Figure 2B, middle and right panels). Renal expression of other genes relevant to IRI in KLF11+/+ and KLF11−/− mice are shown in Table 1. Notably, in KLF11−/− mice compared with KLF11+/+ mice after IRI, relevant gene expression was either exaggerated (MCP-1, PAI-1, SMAD3), unchanged (TNF-α, TGF-β1, ICAM1, IL-1β, and MMP-9), or decreased (PGC-1α). This latter finding suggests that KLF11 expression may influence mitochondrial integrity.
Figure 2.
Gene and protein expression in KLF11+/+ and KLF11−/− mice at 1 day after IRI for 22 minutes. (A) Quantitative real time RT-PCR analysis revealed enhanced induction of ET-1 mRNA in KLF11−/− mice compared with KLF11+/+ mice 1 day after IRI for 22 minutes. For this and subsequent statistical analyses, a t test was used for parametric data, and the Mann–Whitney U test was used for nonparametric data. *P<0.05, KLF11−/− IRI versus KLF11+/+ IRI. (B) Increased renal IL-6 mRNA expression (left panel), serum IL-6 protein (middle panel), and lung IL-6 mRNA expression (right panel) were observed in KLF11−/− mice; **P<0.001, *P<0.05, KLF11−/− IRI versus KLF11+/+ IRI. (C–E) Western blot analysis revealed significantly enhanced kidney p-STAT3 (C) and HO-1 protein expression (D), but decreased ACE2 protein expression (E) in KLF11−/− mice compared with KLF11+/+ mice at 1 day after IRI for 22 minutes. Normalized densitometry (GAPDH) is shown below the blots. *P<0.05, **P<0.01, ***P<0.001, KLF11−/− IRI versus KLF11+/+ IRI. (F) Western blot analysis also revealed significantly increased HO-1 protein abundance in the lung in KLF11−/− mice as compared with KLF11+/+ mice at 1 day after IRI for 22 minutes. Normalized densitometry (GAPDH) is shown below the blots. **P<0.01 is incorrect and should be replaced by *P<0.05, KLF11−/− IRI versus KLF11+/+ IRI. (G) Localization of kidney KLF11 protein expression 1 day after IRI for 22 minutes in renal tubules. This panel (G) and subsequent panels (H and I) display immunofluorescence staining for nuclei (DAPI, blue) and KLF11 (red). In kidneys after sham IRI, KLF11 is prominently expressed in the cytoplasm of renal proximal tubules, whereas after IRI, KLF11 is prominently expressed in nuclei with less prominent expression in the cytoplasm. KLF11 staining in nuclei after IRI indicates nuclear translocation of KLF11 after IRI. (H) Localization of KLF11 protein expression after IRI for 22 minutes in intrarenal blood vessels. Intrarenal blood vessels stain prominently for KLF11 in both the sham and IRI groups in both endothelium and smooth-muscle cells. In the endothelium, staining for KLF11 was detected in both nuclear and cytoplasmic compartments in both the sham and IRI groups, whereas in smooth-muscle cells, KLF11 staining was primarily in the cytoplasm in the sham group and in both nuclei and cytoplasm for the IRI group. (I) Localization of KLF11 in the endothelium and smooth-muscle cells in intrarenal blood vessels after IRI for 22 minutes. Higher magnification demonstrates nuclear staining for KLF11 in the endothelium in both the sham and IRI groups, whereas in smooth-muscle cells, KLF11 staining was predominantly cytoplasmic in the sham groups but nuclear and cytoplasmic in the IRI groups. The vasculature shows structural injury in IRI as evidenced by endothelial denudation (arrow) and the rounding up of smooth-muscle cells (I, lower panel). Scale bar 50 μm.
Table 1.
Gene expression profile in the kidney of KLF11+/+ and KLF11−/− mice
| Gene | Sham | IRI | ||
|---|---|---|---|---|
| KLF11+/+ | KLF11−/− | KLF11+/+ | KLF11−/− | |
| KLF11 | 4.01±0.53 | ND | 4.82±0.35 | ND |
| MCP-1 | 2.47±0.2 | 2.23±0.17 | 17.49±1.56 | 26.11±2.72a |
| PAI-1 | 1.33±0.15 | 1.34±0.08 | 24.15±4.27 | 70.87±12.24b |
| SMAD3 | 6.14±0.31 | 5.87±0.49 | 9.22±0.6 | 12.37±0.62c |
| TNF-α | 3.07±0.42 | 2.12±0.17 | 16.63±3.29 | 15.36±2.09 |
| TGF-β1 | 4.7±0.31 | 4.06±0.22 | 13.51±1.23 | 16.16±1.04 |
| IL-1β | 1.07±0.12 | 1.22±0.26 | 4.25±0.83 | 4.75±0.81 |
| ICAM1 | 6.48±0.3 | 7.03±0.45 | 16.76±1.18 | 16.43±0.67 |
| MMP-9 | 1.5±0.2 | 1.41±0.2 | 11.19±3.26 | 17.2±2.19 |
| PGC-1α | 18.66±0.79 | 18.05±0.41 | 9.18±0.63 | 7.68±0.24a |
Quantitative real-time RT-PCR analysis was performed. Shown is mRNA expression of multiple genes in the kidney 1 day after IRI for 22 minutes or sham IRI. Values are the mean±SEM; n=5 for each sham group, and n=6 and 7 for KLF11+/+ and KLF11−/− IRI groups, respectively. Relative quantification was performed against a standard curve constructed for each mRNA target, normalized for expression of 18S rRNA, and expressed in arbitrary units. A t test was used for parametric data, and the Mann–Whitney U test was used for nonparametric data. KLF, Krüppel-like factor; ND, not detected; IRI, ischemia-reperfusion injury.
aP<0.05, bP<0.01, cP<0.005, KLF11−/− IRI versus KLF11+/+ IRI.
ET-1 induction was exaggerated in KLF11−/− mice after IRI. We did not measure BP in KLF11+/+ and KLF11−/− mice. Prior studies demonstrate that BP of KLF11−/− is not elevated under basal or stress conditions (6).
IL-6 induces countervailing pathways that may mitigate AKI, and this may occur through p-STAT3 and HO-1 (13,14). We thus assessed these downstream effects of IL-6. p-STAT3 was induced after IRI but more prominently in KLF11−/− mice (Figure 2C), as was the case for HO-1 mRNA expression in the kidney and lungs (Table 2); HO-2 mRNA expression was not significantly altered. Renal HO-1 protein expression was more strongly induced in KLF11−/− mice compared with KLF11+/+ mice (Figure 2D). To underscore the specificity of such exaggerated renal expression of HO-1 in KLF11−/− mice after IRI, expression of another potentially cytoprotective protein, ACE2, was lower in KLF11−/− mice after IRI (Figure 2E). Lung expression of HO-1 protein paralleled what occurred in the kidney (Figure 2F).
Table 2.
HO-1 gene expression after IRI in kidney and lung in KLF11+/+ and KLF11−/− mice
| HO-1 mRNA | Sham | IRI | ||
|---|---|---|---|---|
| KLF11+/+ | KLF11−/− | KLF11+/+ | KLF11−/− | |
| HO-1 (Kidney) | 6.08±0.42 | 6.8±0.83 | 28.14±4.86 | 58.24±4.35a |
| HO-1 (Lung) | 6.19±0.26 | 5.73±0.26 | 5.74±0.47 | 9.69±0.54a |
Quantitative real-time RT-PCR analysis revealed significantly increased HO-1 mRNA expression in KLF11−/− mice compared with KLF11+/+ mice at 1 day after 22 minutes of IRI or sham IRI in kidney and lung. Values are the mean±SEM; n=5 for each sham group, and n=6 and 7 for KLF11+/+ and KLF11−/− IRI groups, respectively. Relative quantification was performed against a standard curve constructed for each mRNA target, normalized for expression of 18S rRNA, and expressed in arbitrary units. A t test was used for parametric data, and the Mann–Whitney U test was used for nonparametric data. IRI, ischemia-reperfusion injury; KLF, Krüppel-like factor.
P<0.001, KLF11−/− IRI versus KLF11+/+ IRI.
Immunofluorescence staining for KLF11 in wild-type mice detected KLF11 in renal tubules (especially proximal), arterioles (endothelial and smooth-muscle cells), interstitial capillaries, and, weakly, glomeruli; overall, the intensity of KLF11 staining was comparable in sham and IRI groups. KLF11 staining in proximal tubules was largely cytoplasmic in sham IRI, whereas in IRI, KLF11 staining was prominent in nuclei (Figure 2G). In intrarenal blood vessels, the endothelium showed prominent nuclear and cytoplasmic KLF11 staining in both sham and IRI conditions; smooth-muscle cells showed more prominent nuclear KLF11 staining in IRI (Figure 2, H and I). Nuclear localization of KLF11 suggests an active transcription factor. We speculate that nuclear localization of KLF11 in the endothelium under basal conditions suggests active KLF11 behavior even under unstressed conditions, whereas such activation in the proximal tubules requires the stress of IRI.
KLF11 mRNA expression was not detected in KLF11−/− mice subjected to either IRI or sham IRI, whereas in KLF11+/+ mice, KLF11 mRNA expression did not significantly change with IRI (Table 1), findings consistent with KLF11 protein expression on immunofluorescence. As shown previously, endothelial KLF11 expression is strongly induced by lipopolysaccharide (LPS) (5). Similarly, we found that at 8 hours after LPS treatment, renal KLF11 mRNA expression was significantly increased (15.08±0.98 for LPS-treated versus 3.33±0.38 for saline-treated; P<0.001). IRI is recognized as a proinflammatory condition. Yet, whole-kidney KLF11 mRNA expression was unchanged after IRI in KLF11+/+ mice. We offer three considerations with regard to this finding. First, such unaltered KLF11 expression in wild-type mice may still be protective because unaltered “constitutive” systems can protect against IRI as their expression (and effects) are already present when the insult is imposed. For example, both inducible and constitutive HO isoforms, HO-1 and HO-2, protect against IRI. Second, ET-1 suppresses KLF11 in other tissues (15). We speculate that ET-1 induction that occurs in renal IRI suppresses KLF11 induction that may otherwise occur. We additionally speculate that this lack of KLF11 induction, and the consequent lack of counterregulatory inhibition by KLF11 on ET-1 expression (8), promotes ET-1 induction in renal IRI. Third, during IRI, although overall KLF11 immunofluorescence staining appeared unchanged, nuclear KLF11 was detected in tubular epithelial and endothelial cells, implying KLF11 activation.
Specific human KLF11 gene mutations may lead to maturity-onset diabetes of the young (4). However, KLF11−/− mice compared with wild-type KLF11+/+ mice do not exhibit diabetes, diabetic nephropathy, increased age-related nephropathy, other pathologies, or a shortened lifespan in the unstressed state.
This demonstration of KLF11 as a protectant against IRI connects KLF11 to other established IRI protectants, specifically agonists of PPARγ and dopamine D2 receptor. PPARγ protects against ischemic stroke, and KLF11 is an essential co-regulator for PPARγ-mediated vasoprotection (16). PPARγ agonists protect against renal IRI. Additionally, KLF11 induces dopamine D2 receptor expression in nonkidney tissues. Dopamine D2 receptor deficiency worsens renal IRI, whereas induction of dopamine D2 receptor attenuates renal IRI (17). This raises the question of whether these and perhaps other IRI protectants involve KLF11.
We suggest that the protective effects of KLF11 involve the suppression of contributors to IRI (specifically ET-1 and IL-6), along with the recruitment of protective species. The protective species downstream of KLF11 are the focus of future studies. Tubules, the vasculature, and interstitial capillaries all express KLF11, and may all be protected by such expression. We speculate that the vascular endothelium represents a significant locus of nephroprotection because KLF11 serves, in part, as a guardian of the endothelium (5,6): KLF11 suppresses renal endothelial activation and lessens ET-1-dependent renal vasoconstriction, inflammation, and aberrant renal hemodynamics—early steps that culminate in IL-6 production, further inflammation, cell injury and death, and IRI.
Disclosures
J.P. Grande reports being a member of the editorial board for Biochemistry and Molecular Biology Education Journal. K.A. Nath reports an advisory or leadership role for the Journal of the American Society of Nephrology and Mayo Clinic Proceedings. All remaining authors have nothing to disclose.
Funding
This study was partially supported by DK11916 (K.A. Nath), HL134569 (Y.E. Chen), and HL138139 (J. Zhang).
Footnotes
See related editorial, “Endothelial KLF11 as a Nephroprotectant in AKI,” on pages 1302–1305.
Author Contributions
A.W. Ackerman, Y.E. Chen, A.J. Croatt, J.P. Grande, K. Khazaie, K.A. Nath, R.D. Singh, and J. Zhang reviewed and edited the manuscript; A.W. Ackerman, A.J. Croatt, J.P. Grande, K.A. Nath, and R.D. Singh were responsible for the formal analysis and validation; A.W. Ackerman, A.J. Croatt, K.A. Nath, and R.D. Singh were responsible for data curation; A.W. Ackerman, A.J. Croatt, and R.D. Singh were responsible for the investigation and the methodology; A.W. Ackerman and R.D. Singh were responsible for the software; Y.E. Chen, A.J. Croatt, K.A. Nath, R.D. Singh, and J. Zhang were responsible for conceptualization; A.J. Croatt and K.A. Nath were responsible for supervision; A.J. Croatt, K.A. Nath, and R.D. Singh were responsible for visualization and project administration, and wrote the original draft of the manuscript; K.A. Nath was responsible for funding acquisition and resources; and all authors approved the final version of the article and agree to be accountable for all aspects of the work pre and post publication.
Data Sharing Statement
All data are included in the manuscript and/or supporting information.
References
- 1.Agarwal A, Dong Z, Harris R, Murray P, Parikh SM, Rosner MH, Kellum JA, Ronco C; Acute Dialysis Quality Initiative XIII Working Group : Cellular and molecular mechanisms of AKI. J Am Soc Nephrol 27: 1288–1299, 2016. 10.1681/asn.2015070740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mallipattu SK, Estrada CC, He JC: The critical role of Krüppel-like factors in kidney disease. Am J Physiol Renal Physiol 312: F259–F265, 2017. 10.1152/ajprenal.00550.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rane MJ, Zhao Y, Cai L: Krüppel-like factors (KLFs) in renal physiology and disease. EBioMedicine 40: 743–750, 2019. 10.1016/j.ebiom.2019.01.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fan Y, Lu H, Liang W, Hu W, Zhang J, Chen YE: Krüppel-like factors and vascular wall homeostasis. J Mol Cell Biol 9: 352–363, 2017. 10.1093/jmcb/mjx037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fan Y, Guo Y, Zhang J, Subramaniam M, Song CZ, Urrutia R, Chen YE: Krüppel-like factor-11, a transcription factor involved in diabetes mellitus, suppresses endothelial cell activation via the nuclear factor-κB signaling pathway. Arterioscler Thromb Vasc Biol 32: 2981–2988, 2012. 10.1161/atvbaha.112.300349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhao G, Chang Z, Zhao Y, Guo Y, Lu H, Liang W, Rom O, Wang H, Sun J, Zhu T, Fan Y, Chang L, Yang B, Garcia-Barrio MT, Chen YE, Zhang J: KLF11 protects against abdominal aortic aneurysm through inhibition of endothelial cell dysfunction. JCI Insight 6: 141673, 2021. 10.1172/jci.insight.141673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang X, Tang X, Ma F, Fan Y, Sun P, Zhu T, Zhang J, Hamblin MH, Chen YE, Yin KJ: Endothelium-targeted overexpression of Krüppel-like factor 11 protects the blood-brain barrier function after ischemic brain injury. Brain Pathol 30: 746–765, 2020. 10.1111/bpa.12831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Glineur C, Gross B, Neve B, Rommens C, Chew GT, Martin-Nizard F, Rodríguez-Pascual F, Lamas S, Watts GF, Staels B: Fenofibrate inhibits endothelin-1 expression by peroxisome proliferator-activated receptor α-dependent and independent mechanisms in human endothelial cells. Arterioscler Thromb Vasc Biol 33: 621–628, 2013. 10.1161/atvbaha.112.300665 [DOI] [PubMed] [Google Scholar]
- 9.Neuhofer W, Pittrow D: Role of endothelin and endothelin receptor antagonists in renal disease. Eur J Clin Invest 36 [Suppl 3]: 78–88, 2006. 10.1111/j.1365-2362.2006.01689.x [DOI] [PubMed] [Google Scholar]
- 10.De Lorenzo SB, Vrieze AM, Johnson RA, Lien KR, Nath KA, Garovic VD, Khazaie K, Grande JP: KLF11 deficiency enhances chemokine generation and fibrosis in murine unilateral ureteral obstruction. PLoS One 17: e0266454, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Song CZ, Gavriilidis G, Asano H, Stamatoyannopoulos G: Functional study of transcription factor KLF11 by targeted gene inactivation. Blood Cells Mol Dis 34: 53–59, 2005. 10.1016/j.bcmd.2004.08.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nath KA, Singh RD, Grande JP, Garovic VD, Croatt AJ, Ackerman AW, Barry MA, Agarwal A: Expression of ACE2 in the intact and acutely injured kidney. Kidney360 2: 1095–1106, 2021. 10.34067/KID.0001562021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Su H, Lei CT, Zhang C: Interleukin-6 signaling pathway and its role in kidney disease: An update. Front Immunol 8: 405, 2017. 10.3389/fimmu.2017.00405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nath KA: Heme oxygenase-1 and acute kidney injury. Curr Opin Nephrol Hypertens 23: 17–24, 2014. 10.1097/01.mnh.0000437613.88158.d3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cullingford TE, Butler MJ, Marshall AK, Tham L, Sugden PH, Clerk A: Differential regulation of Krüppel-like factor family transcription factor expression in neonatal rat cardiac myocytes: Effects of endothelin-1, oxidative stress and cytokines. Biochim Biophys Acta 1783: 1229–1236, 2008. 10.1016/j.bbamcr.2008.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yin KJ, Fan Y, Hamblin M, Zhang J, Zhu T, Li S, Hawse JR, Subramaniam M, Song CZ, Urrutia R, Lin JD, Chen YE: KLF11 mediates PPARγ cerebrovascular protection in ischaemic stroke. Brain 136: 1274–1287, 2013. 10.1093/brain/awt002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Konkalmatt PR, Asico LD, Zhang Y, Yang Y, Drachenberg C, Zheng X, Han F, Jose PA, Armando I: Renal rescue of dopamine D2 receptor function reverses renal injury and high blood pressure. JCI Insight 1: e85888, 2016. 10.1172/jci.insight.85888 [DOI] [PMC free article] [PubMed] [Google Scholar]